Abstract
The elastic pericardium exerts a compressive contact force on the surface of the myocardium that becomes more substantial when heart volume increases, as in patients with various forms of heart failure. Pericardial restraint plays an important role in determining hemodynamics and ventricular function in both health and disease. In this review, we will discuss the physiology of pericardial restraint in heart failure, and explore it can be targeted indirectly through medical interventions and directly through a number of existing and future therapies.
Keywords: pericardium, heart failure, hemodynamics
Introduction
The pericardium is a membranous sac that surrounds the heart consisting of visceral and parietal layers, which exerts a compressive contact force on the surface of the heart that is amplified when heart volume increases. Even in the absence of pericardial disease, the pericardium exerts important biological effects on ventricular function, pressures, and volumes, particularly in patients with heart failure (HF). In this review, we will discuss the pathophysiologic impact of pericardial restraint in various forms of HF, and then review how this restraint may be targeted therapeutically.
Pericardial contribution to intracavitary pressure
Ventricular filling is restrained by the pericardium.(1–9) When a catheter is inserted into the left ventricle (LV), the measured end diastolic pressure (LVEDP) is often assumed to be a valid estimate of ventricular preload. However, LV end diastolic volume (LVEDV) rather than LVEDP determines the degree of end-diastolic myocyte stretch, which determines the force and velocity of contraction according to the Frank-Starling principle, and is therefore the more accurate measure of preload.(10) LVEDP varies directly with LVEDV up to a point, but as venous return is increased beyond this point, further increases in LVEDP do not reflect an increase in distending pressure but rather an increase in extrinsic restraint. For example, above an LVEDP of 9 mmHg, 65% of any further increase in intracavitary pressure is caused by pericardial restraint rather than viscoelastic muscle properties.(6)
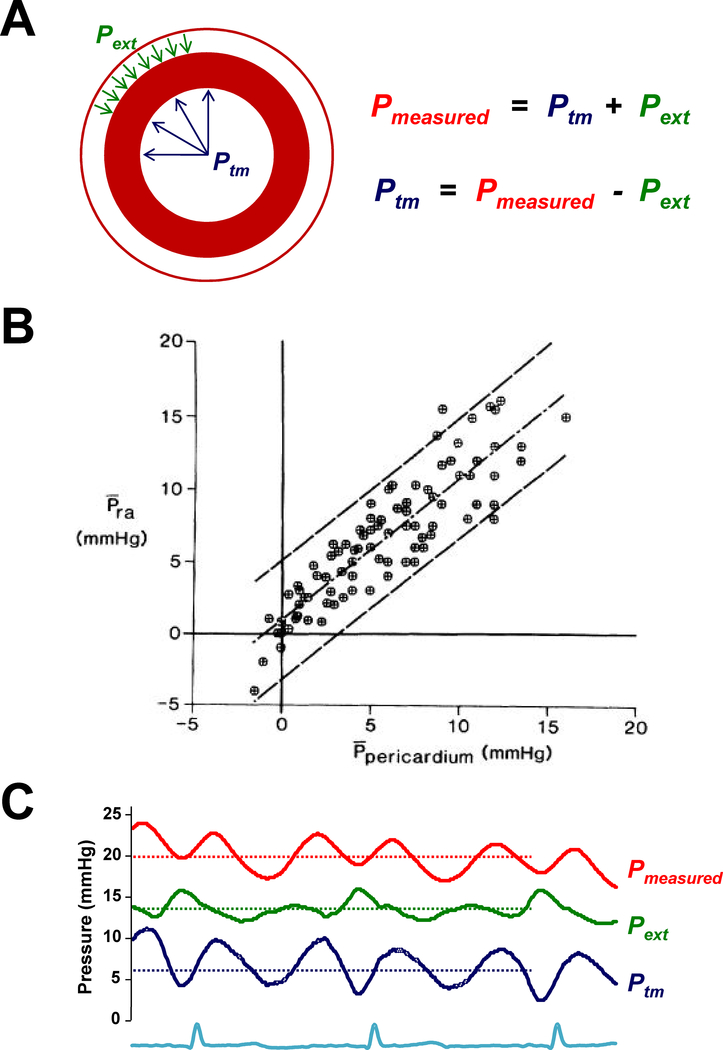
The measured intracavitary pressure in the LV represents the sum of two opposing vector forces. There is an effective distending pressure (transmural pressure) related to the volume of blood present in the chamber and the stiffness and relaxation properties of the myocardium. This transmural pressure exerts force outwards from within, favoring LV chamber distention (Figure 1A). There is also an external compressive force acting on the LV due to the right ventricle (RV) and pericardium. This force exerts a contact pressure that increases measured intracavitary pressure but (when positive) favors a decrease in LV volume. Between 20–40% of intracavitary LV pressure is related to this extrinsic restraint.(11)
Figure 1:
[A] Pressure measured is related equal to the sum of the transmural pressure (Ptm) and the external pressure (Pext) applied outside. [B] Pericardial pressure, a measure of Pext, varies directly with right atrial pressure (Pra). [C] Example showing estimation of LV Ptm as the difference between measured pulmonary capillary wedge pressure (red) and pericardial Pext, estimated by right atrial pressure (green). Panel B adapted with permission from reference #12.
Because measured LVEDP is equal to the sum of external and LV transmural pressure, one can define transmural pressure by their difference (Figure 1A). Measuring pericardial pressure directly is difficult and impractical.(3) Tyberg and colleagues demonstrated through an elegant series of open and closed chest experiments that right atrial (RA) pressure is a very reliable estimate of pericardial pressure (Figure 1B).(12–15) Therefore, by subtracting right atrial pressure from the LVEDP (or pulmonary capillary wedge pressure, PCWP), one can clinically estimate the transmural distending pressure in the LV (Figure 1C).
The Pericardium, Ventricular Interdependence, and Frank-Starling’s Law of the Heart
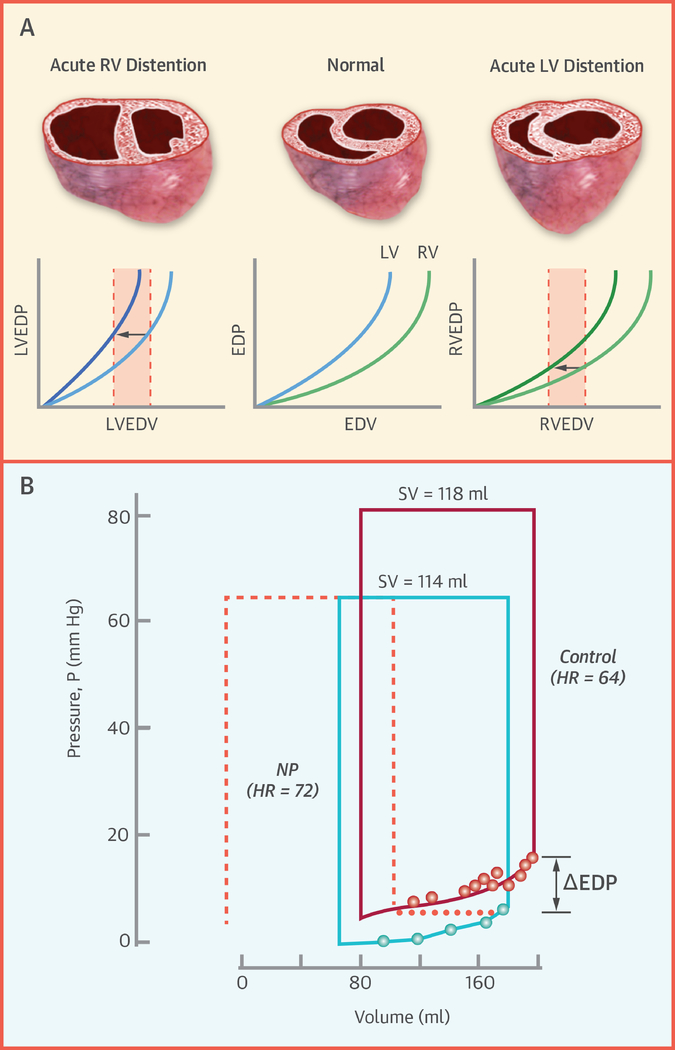
The pericardial stress-strain relationship is bilinear. Pericardial pressure increases minimally at lower heart volumes, but rises strikingly as heart volume increases beyond a critical inflection point where the heart abuts the parietal pericardium.(1,2,5,6,8,16) The pericardium dilates in response to chronic increases in cardiac volume, but the intrinsic stiffness properties do not change with remodeling.(17) At normal heart volumes, pericardial restraint is minimal but measurable.(18) However, when heart volume increases acutely, as during exercise, there is a rapid rise in pericardial restraint, particularly in patients with cardiomegaly.(19) Because the thinner walled RV is more compliant than the LV, right heart dilation is the more commonly observed cause. Acute increases in RV volume shift the apparent LV pressure volume relationship upward, even as LV properties have not changed, because the external pressure from the right heart and pericardium increase LVEDP, even as LV transmural pressure is unchanged (Figure 2A–D), a phenomenon termed diastolic ventricular interaction. Removal of the pericardium shifts the diastolic pressure volume relationship down and to the right; because the external contact pressure is removed and ventricular interaction becomes minimal (Figure 2C).(1–9)
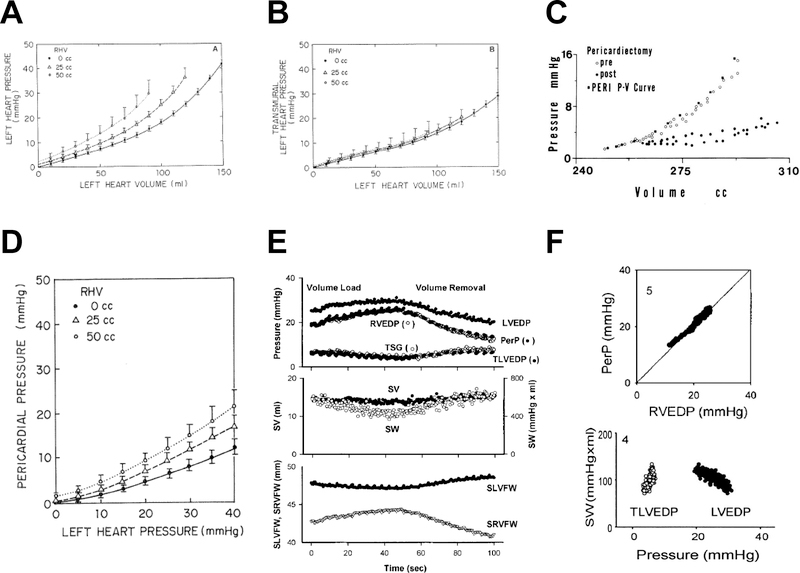
Figure 2:
[A] At higher right heart volumes (RHV), the left heart diastolic pressure-volume relationship shifts upward, even as transmural pressures [B] are unaffected. [C] Removal of the pericardium shifts the diastolic pressure-volume relationship down and to the right. [D] Pericardial pressure increases more steeply with left heart filling at higher RHV, demonstrating how RV-LV interdependence alters apparent chamber stiffness. [E] Volume loading in a dog with HFrEF increases LVEDP and RVEDP, but because RVEDP (approximating pericardial pressure) increases more than LVEDP, the LV transmural pressure (TLVEDP) and LV septolateral free wall dimension (SLVFW) decrease, while the corresponding RV dimension (SRVFW) increases, leading to a reduction in stroke work (SW). [F] Because RVEDP approximates pericardial pressure (PerP), the apparent violation of Starling’s Law in panel E (decreasing SW with increasing LVEDP) is no longer present when plotting SW versus TLVEDP. Adapted with permission from references #21 (A, B, D), #9 (C), and #10 (E, F).
In models of HFrEF, acute volume loading increases pericardial pressure and therefore LVEDP, even as LVEDV and transmural pressures are reduced (Figure 2E).(10) This suggests an apparent violation of Frank-Starling’s law. In reality, there is no violation; this finding is explained by an uncoupling between LVEDP and LVEDV due to ventricular interdependence and enhanced extrinsic restraint. If LV performance (stroke work) is plotted against LVEDP with volume loading in this setting, a negative relationship is observed, suggesting a “descending limb” (Figure 2F). In contrast, a plot of stroke work against transmural distending pressure, the relationship is positive and linear, in keeping with Starling’s Law of the heart.(10)
Any increase in pericardial restraint augments the parallel interaction between the right and left sides of the heart. At pericardial pressures <5 mmHg the pericardium is slack and compliant, but above this cutpoint the pericardium behaves like an elastic band limiting expansion of the cross sectional area of the heart.(20) This serves to regulate beat-to-beat coupling of left and right-sided stroke volumes during acute changes in preload. When pericardial restraint is amplified, any increase in right heart volume must cause a corresponding decrease in left heart volume, or an increase in left heart pressure to maintain its volume (Figure 2A). For example, during squatting, there is an acute increase in venous return to the RV, causing an increased RVEDV, RVEDP, RA pressure and pericardial pressure. The increase in right-sided venous return is accommodated by a leftward septal shift, which decreases LVEDV and LV stroke volume.(20) However, the ensuing increase in RV stroke volume eventually reaches the left heart after traversing the lung, leading to a larger LVEDV. In this way, the pericardium helps to maintain equilibrium between RV and LV cardiac output.(18,20)
Contributors to Heightened External Restraint
Any condition that increases total epicardial heart volume can enhance interaction of the heart and pericardium, increasing pericardial pressure and decreasing LV transmural pressure and LVEDV for any LVEDP or PCWP (Figures 2, 3). Even with compromise in LV preload, elevation in intracavitary LVEDP due to increased external pressure still acts to increase pulmonary capillary hydrostatic pressures to promote dyspnea.(21) Stated differently, it is possible to develop lung congestion and dyspnea even as the LV is underfilled due to low transmural pressure when right heart and pericardial pressures are very high.(22,23)
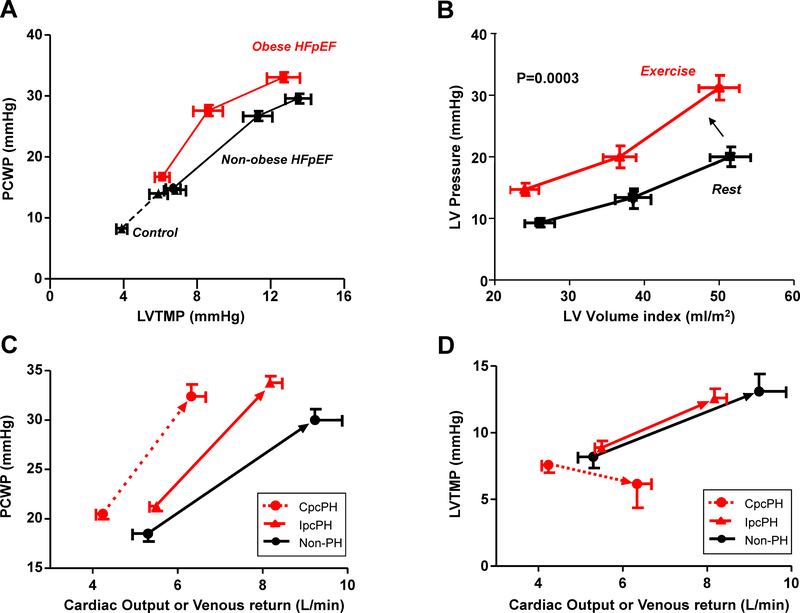
Figure 3:
Increases in pericardial restraint leads to [A] higher pulmonary capillary wedge pressure (PCWP) relative to LV transmural pressure (LVTMP) in obese HFpEF, and [B] upward and leftward shift in the left ventricular diastolic pressure volume relationship during exercise. [C] Patients with HFpEF and no pulmonary hypertension (Non-PH), isolated post-capillary PH (IpcPH), and combined pre-and post-capillary PH all develop similar elevations in PCWP as cardiac output increases during exercise, but patients with Cpc-PH HFpEF develop a reduction in LVTMP, indicating LV underdistention despite high filling pressures, causing an impairment in cardiac output reserve [D]. Adapted with permission from references #29 (A), #27 (B), and #25 (C,D).
This explains how patients with acute pericarditis or large pericardial effusions commonly complain of exertional dyspnea, even in the absence of tamponade, due to heightened external restraint, which develops due to acute changes in pericardial distensibility or accumulation of pericardial fluid. An increase in pericardial restraint can be caused by (i) enlarged epicardial heart volume (ventricles, atria), displacing even the normal pericardium to the steeper portion of its pressure-volume relationship, (ii) alteration of the viscoelastic properties of the pericardium by radiation, aging, or inflammation, (iii) accumulation of pericardial fluid or epicardial fat. External restraint on the heart may also be driven by structures outside the pericardium, as with mediastinal or chest wall pathologies, lung hyperinflation, or increased pleural pressure.
Estimation of pericardial restraint
Because RA pressure is roughly equal to pericardial pressure, (10,12–15) simultaneous measurement of RA pressure and PCWP can provide important information about the degree of pericardial restraint (Figure 1). In some patients with isolated right HF, RA pressure may exceed LVEDP, leading to an apparent negative transmural pressure that is not physiologic. It is likely best to consider these patients as having a near-zero LV transmural pressure. Higher RA pressure and higher ratio of RA to PCWP suggest increased pericardial restraint, often triggered by RV dilation. As extrinsic constraint increases, there must be higher PCWP to achieve a given transmural pressure (Figure 3A). This causes a parallel upward and leftward shift of the LV pressure-volume relationship (Figure 2A). This phenomenon likely explains the parallel shift upward in the LV diastolic pressure volume relationship during exercise in patients with HFpEF (Figure 3B).(24)
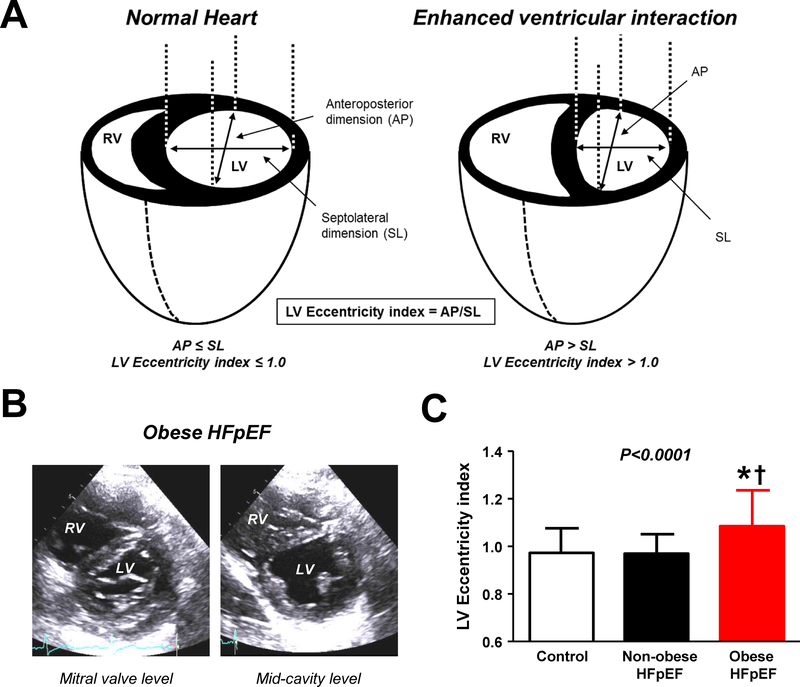
Evaluation of the configuration and shape of the interventricular septum by echocardiography provides a noninvasive means to evaluate pericardial restraint (Figure 4). When restraint is increased, there is greater competition between the RV and LV for filling. The ratio of RV to LV diastolic pressure approaches unity, and the transeptal gradient (difference between LVEDP and RVEDP) decreases to zero. This causes the septum to shift from its normal position of being concave toward the RV to flat, with the LV becoming increasingly “D-shaped” in its short axis (Figure 4). The degree of restraint can be quantified by the eccentricity index, where higher values connote greater pericardial restraint. This is commonly seen in patients with PH, right heart failure, and in patients with the obese phenotype of HFpEF.(25)
Figure 4:
[A-C] Enhanced ventricular interdependence is identified by an increase in the LV eccentricity index, which is increased in patients with the obese phenotype of HFpEF. Adapted with permission from reference #29.
Causes of HF Prominently Affected by Pericardial Restraint
HFrEF
Janicki first described the role of the pericardium in human HFrEF during exercise.(19) In patients where stroke volume plateaued during exercise, there was an equal increase in the right and left sided filling pressures and reduction in transeptal gradient, presumably reflective of pericardial restraint limiting LVEDV. In contrast, HF patients with preserved stroke volume reserve during exercise demonstrated an increase in PCWP that was 2–3 times that of the RA pressure (suggesting greater ability to increase LV transmural pressure and thus LVEDV), associated with greater cardiac output reserve and exercise capacity.(19)
This contribution of pericardial restraint to the pathophysiology of chronic HFrEF was further proven by seminal human experiments using mechanical unloading to modulate RV preload.(11,26) In 6 patients with dilated cardiomyopathy, acute RV unloading from transient caval occlusion caused a 20% drop in LVEDP over the first several beats following occlusion, without reduction in LV end diastolic volume, reflecting relief of pericardial restraint.(11) In another study, Atherton and colleagues applied more sustained mechanical unloading with lower body negative suction in patients with HFrEF, incredibly demonstrating an increase in LVEDV in 9 out of 21 patients with reduction in venous return to the heart.(26) Importantly, the increase in LVEDV was nearly equal to the reduction in RVEDV, and proportional to the degree of PCWP elevation prior to lower body suction (presumably identifying patients with higher baseline pericardial pressure).
HFpEF
Pericardial restraint plays an important role in two common phenotypes of HFpEF: obesity related HFpEF and HFpEF associated with pulmonary vascular disease.(22,25) Obese HFpEF patients display unique pathophysiological features, including greater hypervolemia, abnormal RV-PA coupling, greater biventricular hypertrophy and increased epicardial fat mass.(25) The combination of increased heart volume and epicardial fat exacerbate pericardial restraint in this population results in a heightened RA/PCWP ratio during rest and exercise, an increased LV eccentricity index (Figure 4) and a higher PCWP to achieve a given transmural pressure (Figure 3). The degree of pericardial restraint is synergistically exacerbated by worsening pulmonary hypertension and right heart dilation during exercise.(25)
Enhanced pericardial restraint in obese HFpEF partially explains the lower natriuretic peptides observed in this cohort.(25) Obokata et al. found that PCWP was higher for any given NTproBNP level in obese HFpEF compared to non-obese HFpEF, but this upward shift was no longer apparent when NTproBNP levels were plotted against LV transmural pressure.(25) This is not surprising when considering that the stimulus for natriuretic peptide release is diastolic wall stress, which is determined by transmural pressure and chamber dimension.(25) Similar physiology underlies the lower natriuretic peptide levels seen in constrictive pericarditis.
A substantial number of HFpEF patients display pulmonary hypertension (PH) that is “out of proportion” to the degree of left heart disease due to coexisting pulmonary vascular disease, referred to as combined pre- and post-capillary PH. These patients demonstrate evidence of greater pericardial restraint and ventricular interdependence during exercise due to abnormal RV-PA coupling responses.(22) Despite marked PCWP elevation, these patients develop a decrease in LV transmural pressure during exercise due to enhanced interdependence and right heart dilation, which combined with impaired RV systolic reserve, severely compromises cardiac output reserve (Figure 3). The reduction in LV transmural pressure in HFpEF with pulmonary vascular disease is correlated with the degree of elevation of pulmonary vascular resistance, supporting this mechanistic relationship.(22) Right heart dysfunction and dilation also develop in the absence of severe PH, yet pericardial restraint may still be observed.(27)
Right-sided HF
Patients with severe tricuspid regurgitation (TR) often present with dilated right heart chambers causing heightened pericardial restraint. The situation is worsened during exercise because of increases in venous return and regurgitant volume as PA pressures increase.(23) Excessive right heart dilation then compromises LV filling due to septal shift and ventricular interaction, limiting the increase in LVEDV and cardiac output reserve. Despite the underfilled LV, these patients often display marked elevation in PCWP elevation that is transmitted back to the pulmonary capillaries, contributing to dyspnea, even as LV transmural pressures decrease.(23)
Atrial fibrillation is intimately linked with TR and the development of incident RV dysfunction and RV dilation in patients with HFpEF.(27) In animal preparations, development of atrial fibrillation or loss of atrioventricular synchrony worsens pericardial restraint due to atrial enlargement, which stretches the pericardium to compromise LV filling.(28) Atrial dilation contributing to restraint likely occurs in human HF as well, but has not been well-characterized.
Acute right-sided HF can cause some of the most dramatic examples of enhanced restraint and ventricular interdependence. Belenkie and colleagues demonstrated that experimental pulmonary artery embolization and constriction lead to increases in LVEDP even as LVEDV and transmural filling pressure decrease.(29) Similar physiology occurs in acute RV infarction, where experimental pericardiotomy relieves the hemodynamic derangements.
In contrast to the acute situation, chronic right HF due to PH involves gradual RV remodeling, allowing more time for pericardial dilatation.(17) In patients with right HF due to PH, there is clinical evidence of ventricular interdependence mediated by pericardial restraint with effective LV underfilling that improves with RV preload reduction.(30) Curvature of the septum and LVEDV correlate best with stroke volume in these patients, in contrast to RV volumes, emphasizing the importance of LV underfilling from relative pericardial restraint.(31)
Targeting Pericardial Restraint for Treatment of HF
Indirect Treatments
By reducing plasma volume, diuretics alleviate pericardial restraint by reducing RV volumes to improve LVEDV and stroke volume.(26) A number of commonly-used HF drugs have acute effects that are modulated in part by changes in pericardial pressures. Nitrates lower LVEDP largely by reducing pericardial restraint owing to venodilation in the mesenteric circulation and periphery.(2,32–34) This acutely shifts the diastolic pressure volume relationship downward, leading to lower LVEDP (Central Illustration). Conversely, angiotensin causes venoconstriction, which favors translocation of blood from the mesenteric veins to the right heart, increases pericardial pressure, shifting the pressure-volume relationship upward.(33) Konstam demonstrated that part of the acute benefit of ACE inhibitors was likely related to acute reduction in RV volumes through this mechanism.(35) Cardiac resynchronization mitigates ventricular interdependence through LV preexcitation, allowing for earlier LV filling during the time of lowest extrinsic restraint.(36)
Central Illustration:
[A] Excessive loading of the RV (top) or LV (bottom) reduces effective diastolic chamber compliance in the contralateral ventricle. [B] Conversely, acute unloading of the RV, accomplished through venodilation induced by infusion of nitroprusside (NP), reduces LV diastolic pressures (dots) without a significant change in volume, mediated by acute reduction of pericardial restraint. Adapted with permission from references #17 and #45.
Chronically, any intervention that reduces heart size may improve pericardial restraint. This may be achieved though hypertrophy regression and reverse remodeling, as with neurohormonal antagonists or cardiac resynchronization therapy. Regression of epicardial fat with weight loss or other means may also be useful to mitigate excessive restraint.(25) Because RV structure and function are potently related to afterload in HF,(37) therapies targeting the pulmonary vascular dysfunction may lead to reduction in RV size and favorable effects on RV-PA coupling. In a recent double blind, placebo-controlled trial, acute treatment with the inhaled beta-2 agonist albuterol reduced pulmonary vascular resistance during exercise, improving RV-PA coupling.(38) This was associated with an improvement in LV transmural pressure, despite no change in PCWP, indicating enhanced LV preload, and the degree of improvement in LV transmural pressure was correlated with the reduction in pulmonary vascular resistance. Albuterol increased exercise cardiac output by 20%, presumably related in part to better utilization of the Frank-Starling reserve.(38)
Direct Treatments
In acute animal experiments, resection of the pericardium improves LV compliance, even as muscle properties remain unchanged, since the external restraint of the pericardium is eliminated (Figure 2C).(1–9) Chronic experiments in dogs and pigs have shown that pericardiectomy increases LVEDV and improves Frank-Starling reserve, leading to increases in exercise stroke volume, cardiac output, and aerobic capacity.(4,7) LeWinter and colleagues observed that pericardiotomy for coronary bypass surgery (not full resection) resulted in ~20% increases in LV volume and mass at 6 weeks, which was sustained out to 7 months, with no further remodeling and no impairment in systolic function.(39) Humans with congenital absence of the left pericardium display higher LVEDV and RVEDV at similar filling pressures when lying on the left side but not the right, providing further evidence that removal of the pericardium could improve diastolic filling.(40)
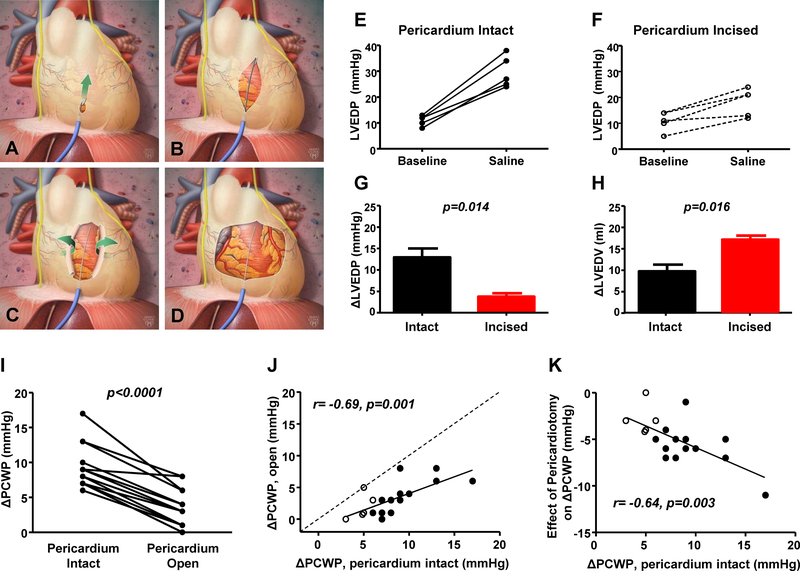
Patients with HFpEF develop symptoms in large part due to elevation in filling pressures, which impairs exercise capacity.(21,41) During exercise, the LV diastolic pressure-volume relationship shifts upward, indicating that at least part of the increase in LVEDP is mediated by an increase in external restraint (Figure 3B).(24) These observations, coupled with the prior animal and human data above, led us to hypothesize that anterior pericardiotomy might be a novel treatment for patients with HFpEF (Figure 5).(42,43) In earlier animal experiments, we found that fluoroscopically-guided anterior pericardiotomy substantially mitigated the increase in LVEDP with volume loading, both in normal dogs and pigs with features of human HFpEF.(42) This effect was coupled with a greater increase in LVEDV indicating improved Frank-Starling reserve, and importantly was achieved without opening the chest, suggesting that the technique might be applicable in a catheterization lab setting without the need for open-heart surgery (Figure 5).
Figure 5:
[A-D] Minimally invasive pericardiotomy to decrease external restraint. Animal data showing that as compared to pericardium intact [E], opening of the pericardium abrogates the rise in LVEDP [F, G] while enabling a greater increase in LVEDV [H], enhancing FrankStarling reserve. [I-K] In humans undergoing cardiac surgery, the increase in PCWP with volume loading is reduced after opening the pericardium, with greater unloading effects at the highest baseline pressures. Adapted with permission from Borlaug BA, Carter RE, Melenovsky V, et al. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10:e003612 and Borlaug BA, Schaff HV, Pochettino A, et al. Pericardiotomy Enhances Left Ventricular Diastolic Reserve With Volume Loading in Humans. Circulation. 2018;138:2295–2297.
We subsequently confirmed this finding in humans undergoing aortic valve replacement or coronary artery bypass grafting, where the increase in PCWP with pericardium open was reduced by 67% simply by opening the anterior pericardium (Figure 5).(43) Notably, the reduction in PCWP with pericardiotomy was greatest in patients with the largest PCWP at baseline, suggesting greater efficacy in patients with the most chamber distention, who would be predicted to display more pericardial restraint. Pericardiotomy could also have untoward effects, including impairments in longitudinal RV function and LV torsion (44,45), or an increase in LV mass and volume,(39) and these will need to be carefully evaluated for moving forward. A pilot trial testing this treatment approach for patients with refractory HFpEF will soon begin enrollment.
Patients Unlikely to Benefit from Targeting the Pericardium
While direct removal of excessive pericardial restraint may be beneficial for many patients by lowering diastolic pressures and improving chamber filling, others could theoretically be harmed. Since pericardiotomy modestly increases cardiac volumes,(4,7,39) it might be harmful in patients where eccentric remodeling is already present, including patients with dilated cardiomyopathy or severe right HF. In these cases, chamber dilation could worsen, resulting in greater systolic dysfunction and valve regurgitation. Indeed, prior studies in HFrEF patients have taken the opposite approach, either by attempting to reduce heart size using external restraining devices(46) or through surgical right latissimus dorsi cardiomyoplasty, which function like an elastic girdle that enables reverse remodeling in dilated ventricles.(47)
Future directions
Multiple studies spanning the past 40 years have clearly delineated an important but still underappreciated role of the pericardium in modulating hemodynamics, cardiac function and response to drug therapy in patients with heart failure. Pathophysiologic studies have identified a number of HF patients who would be poised to respond favorably to treatments to mitigate excessive pericardial restraint. Future research is needed to determine whether medical and surgical modifications of pericardial restraint, using both direct and indirect approaches, can be used to improve clinical status and eventually outcomes for patients with heart failure.
Highlights:
The pericardium exerts a compressive contact force on the surface of the heart that becomes exaggerated in various forms of heart failure where cardiac volumes increase.
The resulting increase in pericardial restraint contributes importantly to determine ventricular function, the hemodynamic changes that occur in heart failure, and the responses to vasodilation and decongestion in clinical practice
The right atrial pressure is a reliable surrogate for pericardial pressure and provides a clinically-relevant estimate the degree of pericardial restraint.
A number of therapies in heart failure work in part through relief of relative pericardial restraint, and direct therapies including anterior pericardiotomy are undergoing investigation
Acknowledgement
BAB is supported by R01 HL128526, R01 HL 126638, U01 HL125205 and U10 HL110262, all from the National Institute of Health.
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glantz SA, Misbach GA, Moores WY, et al. The pericardium substantially affects the left ventricular diastolic pressure-volume relationship in the dog. Circ Res. 1978;42:433–41. [DOI] [PubMed] [Google Scholar]
- 2.Shirato K, Shabetai R, Bhargava V, Franklin D, Ross J Jr. Alteration of the left ventricular diastolic pressure-segment length relation produced by the pericardium. Effects of cardiac distension and afterload reduction in conscious dogs. Circulation. 1978;57:1191–8. [DOI] [PubMed] [Google Scholar]
- 3.Smiseth OA, Frais MA, Kingma I, Smith ER, Tyberg JV. Assessment of pericardial constraint in dogs. Circulation. 1985;71:158–64. [DOI] [PubMed] [Google Scholar]
- 4.Stray-Gundersen J, Musch TI, Haidet GC, Swain DP, Ordway GA, Mitchell JH. The effect of pericardiectomy on maximal oxygen consumption and maximal cardiac output in untrained dogs. Circ Res. 1986;58:523–30. [DOI] [PubMed] [Google Scholar]
- 5.Assanelli D, Lew WY, Shabetai R, LeWinter MM. Influence of the pericardium on right and left ventricular filling in the dog. J Appl Physiol. 1987;63:1025–32. [DOI] [PubMed] [Google Scholar]
- 6.Applegate RJ, Santamore WP, Klopfenstein HS, Little WC. External pressure of undisturbed left ventricle. Am J Physiol 1990;258:H1079–86. [DOI] [PubMed] [Google Scholar]
- 7.Hammond HK, White FC, Bhargava V, Shabetai R. Heart size and maximal cardiac output are limited by the pericardium. Am J Physiol 1992;263:H1675–81. [DOI] [PubMed] [Google Scholar]
- 8.Applegate RJ, Johnston WE, Vinten-Johansen J, Klopfenstein HS, Little WC. Restraining effect of intact pericardium during acute volume loading. Am J Physiol. 1992;262:H1725–33. [DOI] [PubMed] [Google Scholar]
- 9.Janicki JS, Weber KT. The pericardium and ventricular interaction, distensibility, and function. Am J Physiol 1980;238:H494–503. [DOI] [PubMed] [Google Scholar]
- 10.Moore TD, Frenneaux MP, Sas R, et al. Ventricular interaction and external constraint account for decreased stroke work during volume loading in CHF. Am J Physiol Heart Circ Physiol 2001;281:H2385–91. [DOI] [PubMed] [Google Scholar]
- 11.Dauterman K, Pak PH, Maughan WL, et al. Contribution of external forces to left ventricular diastolic pressure. Implications for the clinical use of the Starling law. Ann Intern Med. 1995;122:737–42. [DOI] [PubMed] [Google Scholar]
- 12.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–32. [DOI] [PubMed] [Google Scholar]
- 13.Smiseth OA, Frais MA, Kingma I, et al. Assessment of pericardial constraint: the relation between right ventricular filling pressure and pericardial pressure measured after pericardiocentesis. J Am Coll Cardiol. 1986;7:307–14. [DOI] [PubMed] [Google Scholar]
- 14.Refsum H, Junemann M, Lipton MJ, Skioldebrand C, Carlsson E, Tyberg JV. Ventricular diastolic pressure-volume relations and the pericardium. Effects of changes in blood volume and pericardial effusion in dogs. Circulation. 1981;64:997–1004. [DOI] [PubMed] [Google Scholar]
- 15.Smiseth OA, Refsum H, Tyberg JV. Pericardial pressure assessed by right atrial pressure: a basis for calculation of left ventricular transmural pressure. Am Heart J. 1984;108:603–5. [DOI] [PubMed] [Google Scholar]
- 16.Weber KT, Janicki JS, Shroff S, Fishman AP. Contractile mechanics and interaction of the right and left ventricles. Am J Cardiol. 1981;47:686–95. [DOI] [PubMed] [Google Scholar]
- 17.Freeman GL, LeWinter MM. Pericardial adaptations during chronic cardiac dilation in dogs. Circ Res 1984;54:294–300. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto N, Shibata S, Hastings JL, et al. Effects of pericardial constraint and ventricular interaction on left ventricular hemodynamics in the unloaded heart. Am J Physiol. 2011;300:H1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janicki JS. Influence of the pericardium and ventricular interdependence on left ventricular diastolic and systolic function in patients with heart failure. Circulation. 1990;81:III15–20. [PubMed] [Google Scholar]
- 20.Kroeker CA, Shrive NG, Belenkie I, Tyberg JV. Pericardium modulates left and right ventricular stroke volumes to compensate for sudden changes in atrial volume. Am J Physiol Heart Circ Physiol. 2003;284:H2247–54. [DOI] [PubMed] [Google Scholar]
- 21.Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, Dyspnea, and Pulmonary Reserve in Heart Failure with Preserved Ejection Fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail. 2014;7:911–7. [DOI] [PubMed] [Google Scholar]
- 24.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349:1720–4. [DOI] [PubMed] [Google Scholar]
- 27.Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linderer T, Chatterjee K, Parmley WW, Sievers RE, Glantz SA, Tyberg JV. Influence of atrial systole on the Frank-Starling relation and the end-diastolic pressure-diameter relation of the left ventricle. Circulation. 1983;67:1045–53. [DOI] [PubMed] [Google Scholar]
- 29.Belenkie I, Dani R, Smith ER, Tyberg JV. Effects of volume loading during experimental acute pulmonary embolism. Circulation. 1989;80:178–88. [DOI] [PubMed] [Google Scholar]
- 30.Kasner M, Westermann D, Steendijk P, et al. Left ventricular dysfunction induced by nonsevere idiopathic pulmonary arterial hypertension: a pressure-volume relationship study. Am J Respir Crit Care Med. 2012;186:181–9. [DOI] [PubMed] [Google Scholar]
- 31.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, et al. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;119:1761–5. [DOI] [PubMed] [Google Scholar]
- 32.Kingma I, Smiseth OA, Belenkie I, et al. A mechanism for the nitroglycerin-induced downward shift of the left ventricular diastolic pressure-diameter relation. Am J Cardiol. 1986;57:673–7. [DOI] [PubMed] [Google Scholar]
- 33.Smiseth OA, Manyari DE, Lima JA, et al. Modulation of vascular capacitance by angiotensin and nitroprusside: a mechanism of changes in pericardial pressure. Circulation. 1987;76:875–83. [DOI] [PubMed] [Google Scholar]
- 34.Smith ER, Smiseth OA, Kingma I, Manyari D, Belenkie I, Tyberg JV. Mechanism of action of nitrates. Role of changes in venous capacitance and in the left ventricular diastolic pressure-volume relation. Am J Med. 1984;76:14–21. [DOI] [PubMed] [Google Scholar]
- 35.Konstam MA, Kronenberg MW, Udelson JE, et al. Effect of acute angiotensin converting enzyme inhibition on left ventricular filling in patients with congestive heart failure. Relation to right ventricular volumes. Circulation. 1990;81:III115–22. [PubMed] [Google Scholar]
- 36.Bleasdale RA, Turner MS, Mumford CE, et al. Left ventricular pacing minimizes diastolic ventricular interaction, allowing improved preload-dependent systolic performance. Circulation. 2004;110:2395–400. [DOI] [PubMed] [Google Scholar]
- 37.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B, Borlaug BA. The beta-Adrenergic Agonist Albuterol Improves Pulmonary Vascular Reserve in Heart Failure With Preserved Ejection Fraction. Circ Res. 2019;124:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tischler MD, Rowan M, LeWinter MM. Increased left ventricular mass after thoracotomy and pericardiotomy. A role for relief of pericardial constraint? Circulation 1993;87:1921–7. [DOI] [PubMed] [Google Scholar]
- 40.Beppu S, Naito H, Matsuhisa M, Miyatake K, Nimura Y. The effects of lying position on ventricular volume in congenital absence of the pericardium. Am Heart J. 1990;120:1159–66. [DOI] [PubMed] [Google Scholar]
- 41.Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borlaug BA, Carter RE, Melenovsky V, et al. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017;10:e003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borlaug BA, Schaff HV, Pochettino A, et al. Pericardiotomy Enhances Left Ventricular Diastolic Reserve With Volume Loading in Humans. Circulation. 2018;138:2295–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raina A, Vaidya A, Gertz ZM, Susan C, Forfia PR. Marked changes in right ventricular contractile pattern after cardiothoracic surgery: implications for post-surgical assessment of right ventricular function. J Heart Lung Transplant. 2013;32:777–83. [DOI] [PubMed] [Google Scholar]
- 45.Houston BA, Shah KB, Mehra MR, Tedford RJ. A new “twist” on right heart failure with left ventricular assist systems. J Heart Lung Transplant. 2017;36:701–707. [DOI] [PubMed] [Google Scholar]
- 46.Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH. Clinical evaluation of the CorCap Cardiac Support Device in patients with dilated cardiomyopathy. Ann Thoracic Surg. 2007;84:1226–35. [DOI] [PubMed] [Google Scholar]
- 47.Kass DA, Baughman KL, Pak PH, et al. Reverse remodeling from cardiomyoplasty in human heart failure. External constraint versus active assist. Circulation. 1995;91:2314–8. [DOI] [PubMed] [Google Scholar]