Abstract
Background
Melanoma is one of the most aggressive forms of skin cancer, with the potential to metastasise to other parts of the body via the lymphatic system and the bloodstream. Melanoma accounts for a small percentage of skin cancer cases but is responsible for the majority of skin cancer deaths. Various imaging tests can be used with the aim of detecting metastatic spread of disease following a primary diagnosis of melanoma (primary staging) or on clinical suspicion of disease recurrence (re‐staging). Accurate staging is crucial to ensuring that patients are directed to the most appropriate and effective treatment at different points on the clinical pathway. Establishing the comparative accuracy of ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)‐CT imaging for detection of nodal or distant metastases, or both, is critical to understanding if, how, and where on the pathway these tests might be used.
Objectives
Primary objectives
We estimated accuracy separately according to the point in the clinical pathway at which imaging tests were used. Our objectives were:
• to determine the diagnostic accuracy of ultrasound or PET‐CT for detection of nodal metastases before sentinel lymph node biopsy in adults with confirmed cutaneous invasive melanoma; and
• to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for whole body imaging in adults with cutaneous invasive melanoma:
○ for detection of any metastasis in adults with a primary diagnosis of melanoma (i.e. primary staging at presentation); and
○ for detection of any metastasis in adults undergoing staging of recurrence of melanoma (i.e. re‐staging prompted by findings on routine follow‐up).
We undertook separate analyses according to whether accuracy data were reported per patient or per lesion.
Secondary objectives
We sought to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for whole body imaging (detection of any metastasis) in mixed or not clearly described populations of adults with cutaneous invasive melanoma.
For study participants undergoing primary staging or re‐staging (for possible recurrence), and for mixed or unclear populations, our objectives were:
• to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of nodal metastases;
• to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of distant metastases; and
• to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of distant metastases according to metastatic site.
Search methods
We undertook a comprehensive search of the following databases from inception up to August 2016: Cochrane Central Register of Controlled Trials; MEDLINE; Embase; CINAHL; CPCI; Zetoc; Science Citation Index; US National Institutes of Health Ongoing Trials Register; NIHR Clinical Research Network Portfolio Database; and the World Health Organization International Clinical Trials Registry Platform. We studied reference lists as well as published systematic review articles.
Selection criteria
We included studies of any design that evaluated ultrasound (with or without the use of fine needle aspiration cytology (FNAC)), CT, MRI, or PET‐CT for staging of cutaneous melanoma in adults, compared with a reference standard of histological confirmation or imaging with clinical follow‐up of at least three months' duration. We excluded studies reporting multiple applications of the same test in more than 10% of study participants.
Data collection and analysis
Two review authors independently extracted all data using a standardised data extraction and quality assessment form (based on the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2)). We estimated accuracy using the bivariate hierarchical method to produce summary sensitivities and specificities with 95% confidence and prediction regions. We undertook analysis of studies allowing direct and indirect comparison between tests. We examined heterogeneity between studies by visually inspecting the forest plots of sensitivity and specificity and summary receiver operating characteristic (ROC) plots. Numbers of identified studies were insufficient to allow formal investigation of potential sources of heterogeneity.
Main results
We included a total of 39 publications reporting on 5204 study participants; 34 studies reporting data per patient included 4980 study participants with 1265 cases of metastatic disease, and seven studies reporting data per lesion included 417 study participants with 1846 potentially metastatic lesions, 1061 of which were confirmed metastases. The risk of bias was low or unclear for all domains apart from participant flow. Concerns regarding applicability of the evidence were high or unclear for almost all domains. Participant selection from mixed or not clearly defined populations and poorly described application and interpretation of index tests were particularly problematic.
The accuracy of imaging for detection of regional nodal metastases before sentinel lymph node biopsy (SLNB) was evaluated in 18 studies. In 11 studies (2614 participants; 542 cases), the summary sensitivity of ultrasound alone was 35.4% (95% confidence interval (CI) 17.0% to 59.4%) and specificity was 93.9% (95% CI 86.1% to 97.5%). Combining pre‐SLNB ultrasound with FNAC revealed summary sensitivity of 18.0% (95% CI 3.58% to 56.5%) and specificity of 99.8% (95% CI 99.1% to 99.9%) (1164 participants; 259 cases). Four studies demonstrated lower sensitivity (10.2%, 95% CI 4.31% to 22.3%) and specificity (96.5%,95% CI 87.1% to 99.1%) for PET‐CT before SLNB (170 participants, 49 cases). When these data are translated to a hypothetical cohort of 1000 people eligible for SLNB, 237 of whom have nodal metastases (median prevalence), the combination of ultrasound with FNAC potentially allows 43 people with nodal metastases to be triaged directly to adjuvant therapy rather than having SLNB first, at a cost of two people with false positive results (who are incorrectly managed). Those with a false negative ultrasound will be identified on subsequent SLNB.
Limited test accuracy data were available for whole body imaging via PET‐CT for primary staging or re‐staging for disease recurrence, and none evaluated MRI. Twenty‐four studies evaluated whole body imaging. Six of these studies explored primary staging following a confirmed diagnosis of melanoma (492 participants), three evaluated re‐staging of disease following some clinical indication of recurrence (589 participants), and 15 included mixed or not clearly described population groups comprising participants at a number of different points on the clinical pathway and at varying stages of disease (1265 participants). Results for whole body imaging could not be translated to a hypothetical cohort of people due to paucity of data.
Most of the studies (6/9) of primary disease or re‐staging of disease considered PET‐CT, two in comparison to CT alone, and three studies examined the use of ultrasound. No eligible evaluations of MRI in these groups were identified. All studies used histological reference standards combined with follow‐up, and two included FNAC for some participants. Observed accuracy for detection of any metastases for PET‐CT was higher for re‐staging of disease (summary sensitivity from two studies: 92.6%, 95% CI 85.3% to 96.4%; specificity: 89.7%, 95% CI 78.8% to 95.3%; 153 participants; 95 cases) compared to primary staging (sensitivities from individual studies ranged from 30% to 47% and specificities from 73% to 88%), and was more sensitive than CT alone in both population groups, but participant numbers were very small.
No conclusions can be drawn regarding routine imaging of the brain via MRI or CT.
Authors' conclusions
Review authors found a disappointing lack of evidence on the accuracy of imaging in people with a diagnosis of melanoma at different points on the clinical pathway. Studies were small and often reported data according to the number of lesions rather than the number of study participants. Imaging with ultrasound combined with FNAC before SLNB may identify around one‐fifth of those with nodal disease, but confidence intervals are wide and further work is needed to establish cost‐effectiveness. Much of the evidence for whole body imaging for primary staging or re‐staging of disease is focused on PET‐CT, and comparative data with CT or MRI are lacking. Future studies should go beyond diagnostic accuracy and consider the effects of different imaging tests on disease management. The increasing availability of adjuvant therapies for people with melanoma at high risk of disease spread at presentation will have a considerable impact on imaging services, yet evidence for the relative diagnostic accuracy of available tests is limited.
Keywords: Adult; Humans; Diagnosis, Computer-Assisted; Diagnosis, Computer-Assisted/methods; Magnetic Resonance Imaging; Melanoma; Melanoma/diagnostic imaging; Neoplasm Metastasis; Neoplasm Recurrence, Local; Neoplasm Recurrence, Local/diagnostic imaging; Neoplasm Staging; Positron Emission Tomography Computed Tomography; Randomized Controlled Trials as Topic; Sensitivity and Specificity; Skin Neoplasms; Skin Neoplasms/diagnostic imaging; Tomography, X-Ray Computed; Ultrasonography
Plain language summary
How good are ultrasound, CT, MRI, and PET‐CT for identifying spread of disease in the body among people with melanoma?
What is the aim of the review?
We wanted to find out which imaging tests are better for identifying spread of disease among people with a first diagnosis of melanoma (primary staging) and among people with possible recurrence of melanoma (re‐staging). We looked at the evidence for ultrasound, CT, MRI, and PET‐CT and included 39 studies to answer these questions.
Why are imaging tests for melanoma important?
Melanoma is one of the most aggressive forms of skin cancer, with potential for metastases (cancer cells) to spread to the lymph nodes and other organs of the body. To make sure that people with melanoma receive the most appropriate and effective treatment, it is important to identify whether the disease has spread and to which parts of the body it has spread. This is called 'staging of disease'. Staging is done to find out if a melanoma has spread to regional lymph nodes or to lymph nodes close to the original melanoma, and to determine if the melanoma has spread to lymph nodes in other parts of the body or to organs of the body such as the liver or the brain (distant metastases). Imaging tests are tools that can be used to help find out how much the disease has spread. Several new treatments are now available for reducing the risk of spread of melanoma and for treating melanoma when it has spread.
What was studied in the review?
The review includes four imaging tests that create images of the body in different ways. Ultrasound uses high‐frequency sound waves to create images, CT scans use ionising radiation in the form of X‐rays (a very low dose of radiation), and MRI uses large magnets and non‐ionising radiation in the form of radio waves (which are not harmful) to generate images of the body. PET‐CT requires injection of a weakly radioactive substance (FDG). The PET part of the scan identifies areas of the body that take up a lot of FDG (indicating possibly cancerous cells), and the CT part of the scan helps to improve image quality and to more accurately pinpoint areas using more FDG. Ultrasound can also be performed along with a fairly simple procedure called 'fine needle aspiration cytology' (FNAC), by which a very fine needle is used to take a small sample of cells from a lymph node that looks suspicious on ultrasound. A microscope is then used to identify whether or not the cells are malignant.
Imaging can be used at different time points after diagnosis of melanoma. Healthcare providers can use imaging to look at the regional lymph nodes closest to the melanoma before a type of surgery called sentinel lymph node biopsy is performed. Sentinel lymph node biopsy takes out the lymph nodes that are most likely to have metastases inside them so they can be tested in a laboratory. Imaging can also be used after sentinel lymph node biopsy or in people with higher‐risk melanoma to look for any spread of disease. Imaging can be used in people who were treated for melanoma at an earlier point and who might be having a recurrence of their disease.
What are the main results of the review?
Ultrasound of regional lymph nodes before sentinel lymph node biopsy
We found 11 relevant studies including 2614 people. Three of these studies compared ultrasound on its own to ultrasound combined with FNAC. Results suggest that the combined procedure correctly identifies around one‐fifth of people with metastases in the lymph nodes with very few false positive results (people with incorrect diagnosis of metastasis). These results can be illustrated by imagining a group of 1000 people with melanoma who are going to have sentinel lymph node biopsy, of whom 237 (24%) have metastases in the lymph nodes. The combination of ultrasound with FNAC potentially allows 43 people with lymph node metastases to be identified and avoid a sentinel lymph node biopsy, at a cost of two people with false positive results who might go on to have the wrong treatment. Those with metastases in the lymph nodes that are missed on ultrasound (false negatives) will be identified on subsequent SLNB.
Whole body imaging (detection of any metastases)
We found 24 studies, but only nine were clear about the point in the time course of disease that imaging was carried out. Six studies including 492 people looked at imaging for primary staging following a confirmed diagnosis of melanoma, and three studies in 589 people evaluated re‐staging of disease in people with possible recurrence of disease.
Most of the studies (6/9) considered PET‐CT, two in comparison to CT alone, and three studies examined the use of ultrasound. We did not find any suitable studies of MRI in these groups.
Overall results suggest that PET‐CT is better for correctly identifying people with metastatic spread of disease who might be having a recurrence of disease (re‐staging) than people who have a new diagnosis of melanoma (primary staging). PET‐CT also seems to be better than CT for identifying spread of disease in both groups of people, but studies were very small and results might not be reliable.
How reliable are the results of the studies included in this review?
In most of our studies, a reliable diagnosis of spread of disease (or reference standard) was made by performing biopsy and by following up with people over time using clinical assessment and imaging. There was often a lack of detail on how patients were followed up and which tests were used. Lots of studies did not include people at clearly defined time points in the disease process, making it difficult to assess the relevance of their results. Reporting of application and interpretation of tests was poor.
To whom do the results of this review apply?
Thirty‐three studies were done in Europe (85%), and the rest in North America (n = 4), Asia (n = 1), or Oceania (n = 1). The average age of people in the studies was between 50 and 67 years, and around half were men. Studies mostly included people with melanoma on any part of the body, but two included only people with melanoma on the head or neck. Studies often included people at different stages of disease, and we were not able to look at the accuracy of tests for people at any particular disease stage. Studies were small, and their results might not match what happens in real life.
What are the implications of this review?
Reviewers found some evidence to support the use of imaging with ultrasound combined with FNAC before sentinel lymph node biopsy, but further work is needed to establish cost‐effectiveness. Limited evidence is available for whole body imaging for primary staging or re‐staging of disease. Available evidence is focused on PET‐CT; there are few comparisons with CT and no comparisons with MRI. Future research needs to look at more than test accuracy and must consider the effects of different imaging tests on treatment decisions for patients.
How up‐to‐date is this review?
The reviewers searched for and included studies published up to August 2016.*
*In these studies, biopsy and clinical or imaging follow‐up were the reference standards (methods of establishing the final diagnosis).
Summary of findings
Summary of findings'. 'Summary of findings table.
| Question | How accurate is ultrasound, CT, MRI, or PET‐CT for staging or re‐staging of cutaneous invasive melanoma in adults? | ||||||
| Population: | Adults with a confirmed diagnosis of melanoma undergoing imaging for staging purposes:
|
||||||
| Index test(s): | Ultrasound with or without fine needle aspiration cytology (FNAC) Computed tomography (CT) Magnetic resonance imaging (MRI) Positron emission tomography–computed tomography (PET‐CT) |
||||||
| Comparator test: | All of the index tests may be used in comparison to each other | ||||||
| Target condition: | For pre‐SLNB imaging: detection of nodal metastases For all other imaging: detection of any metastases |
||||||
| Reference standard: | Histology plus clinical or imaging follow‐up | ||||||
| Action: | If accurate, positive results of imaging before SLNB in some circumstances could allow patients with nodal metastases to proceed directly to commence adjuvant therapy and avoid an additional invasive procedure (SLNB). Accurate whole body imaging will allow appropriate locoregional and systemic therapies to be initiated in a timely manner | ||||||
| Quantity of evidence (n = 39 studies) | Number of studies | Number of participants | Number of cases | ||||
| Per patient data: | 34 | 4980 | 1265 | ||||
| Per lesion data: | 7 | 417 (1846 lesions) | 1061 metastases | ||||
| Limitations | |||||||
| Risk of bias: | Some concerns due to poor reporting across almost all domains. Unclear risk for participant selection method (11/39) or exclusions not clearly described (3/39). High risk from exclusions on the basis of index test results (4/39). Low risk for the index test for pre‐SLNB ultrasound (6/11), other ultrasound evaluation (3/5), CT (7/10), and MRI (4/4). For PET‐CT, unclear risk from lack of description of blinded case note review to ascertain imaging results for retrospective studies (13/23) and high risk from data driven selection of test threshold (1/23). Unclear risk for reference standard from lack of detail on participant follow‐up schedules (12/39). Lack of blinding of the histological diagnosis (2/39) or data collection on follow‐up (3/39) to the index result. High risk from differential verification (20/39) and participant exclusions (13/39). Low risk for comparisons between tests (6/9) | ||||||
| Applicability of evidence to question: | High or unclear concern for applicability for almost all domains. High concern for participant selection from mixed populations (11/39) or data presented per lesion (5/39). Unclear concern from lack of clarity regarding study population. High concern for index tests from poor description of test thresholds (pre‐SLNB ultrasound (1/11), other ultrasound (1/5), CT (5/10), MRI (3/4), PET‐CT (4/23)) or consensus test interpretation (CT (6/10), MRI (2/4), PET‐CT (11/23)). Unclear concern for application and interpretation of the index test (pre‐SLNB US (10/11), CT (3/10), MRI (2/4), PET‐CT (6/23)) or unclear observer expertise (pre‐SLNB ultrasound (6/11), CT (3), MRI (2/4), PET‐CT (6/23)). Unclear concern for applicability of the reference standard from lack of description of the target condition or no breakdown of cases according to nodal or distant metastases. Expertise of the histopathologist poorly described (6/39) | ||||||
| Findings | |||||||
| Thirty‐nine studies reporting accuracy data for pre‐SLNB imaging (n = 18) or for whole body imaging (n = 24) were included. The 24 studies of whole body imaging were of primary staging (n = 6) or staging for potential recurrence of disease (n = 3), or were conducted in mixed or not clearly described populations (n = 15). As we are unable to make clear statements regarding the expected accuracy of imaging at any particular point on the clinical pathway for the mixed population group, the findings presented are based on results for pre‐SLNB imaging, and for primary staging and re‐staging of melanoma only. | |||||||
| Test: pre‐SLNB imaging | |||||||
| Test |
Studies: patients (cases) |
Sensitivity (95% CI) |
Specificity (95% CI) |
Numbers in a cohort of 1000 lesions at a median prevalence of 23.7%a | |||
|
TP (95% CI) |
FN (95% CI) |
FP (95% CI) |
TN (95% CI) |
||||
| US | 11: 2614 (542) |
35.4 (17.0 to 59.4) |
93.9 (86.1 to 97.5) |
84 (40 to 141) |
153 (197 to 96) |
47 (106 to 19) |
716 (657 to 744) |
| US + FNAC | 3: 1164 (259) |
18.0 (3.58 to 56.5) |
99.8 (99.1 to 99.9) |
43 (8 to 134) |
194 (229 to 103) |
2 (7 to 1) |
761 (756 to 762) |
| PET‐CT | 4: 170 (49) |
10.2 (4.31 to 22.3) |
96.5 (87.1 to 99.1) |
24 (10 to 53) |
213 (227 to 184) |
27 (98 to 7) |
736 (665 to 756) |
| Whole bodyimaging for primary staging of melanoma | |||||||
|
Quantity of evidence (n = 6 studies) |
Number of studies | Number of participants | Number of cases | ||||
| Any metastases | 3 | 81 | 51 | ||||
| Nodal metastases | 3 | 373 | 68 | ||||
| Distant metastases | 2 | 112 | 17 | ||||
| Findings | |||||||
Four of the six studies evaluated PET‐CT, one in comparison to CT.
No data for MRI were identified. Results for ultrasound for the detection of nodal metastases (2 studies) were highly variable and likely subject to bias. | |||||||
| Whole bodyimaging for re‐staging of melanoma | |||||||
| Quantity of evidence (n = 3 studies) | Number of studies | Number of participants (lesions) | Number of cases (metastases) | ||||
| Any metastases: | 2 (1) | 153 (139) | 95 (87) | ||||
| Nodal metastases: | 1 | 460 | 37 | ||||
| Distant metastases: | 0 | N/A | N/A | ||||
| Findings: | |||||||
No data for MRI were identified. | |||||||
aMedian prevalence observed across 11 studies of pre‐SLNB ultrasound (interquartile range: 25th percentile 20.5%, 75th percentile 25.4%).
CT: computed tomography; FN: false negative; FNAC: fine needle aspiration cytology; FP: false positive; MRI: magnetic resonance imaging; PET: positron emission tomography; SLNB: sentinel lymph node biopsy; TN: true negative; TP: true positive.
Background
This review is one of a series of Cochrane Diagnostic Test Accuracy (DTA) reviews on the diagnosis and staging of melanoma and keratinocyte skin cancers conducted for the National Institute for Health Research (NIHR) Cochrane Systematic Reviews Programme. Appendix 1 shows the content and structure of the programme. Appendix 2 provides a glossary of terms used, and Appendix 3 presents a table of acronyms used.
Target condition being diagnosed
Melanoma is one of the most aggressive forms of skin cancer, with the potential to metastasise to other parts of the body via the lymphatic system and the bloodstream. Melanoma accounts for a small percentage of skin cancer cases but is responsible for up to 75% of skin cancer deaths (Boring 1994; Cancer Research UK 2017). Melanoma arises from uncontrolled proliferation of melanocytes ‐ the epidermal cells that produce pigment or melanin. It most commonly arises in the skin but can occur in any organ that contains melanocytes, including mucosal surfaces, the back of the eye, and the lining around the spinal cord and brain. 'Cutaneous melanoma' refers to a skin lesion with malignant melanocytes present in the dermis, and includes superficial spreading and nodular, acral lentiginous, and lentigo maligna melanoma variants (Figure 1).
1.
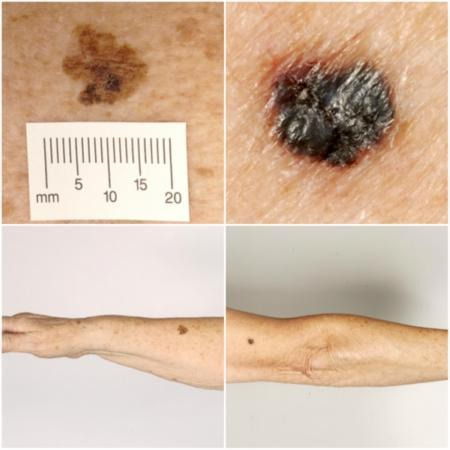
Sample photographs of superficial spreading melanoma (left) and nodular melanoma (right). Copyright © 2010 Dr. Rubeta Matin: reproduced with permission.
The incidence of melanoma rose to over 200,000 newly diagnosed cases worldwide in 2012 (Erdmann 2013; Ferlay 2015), with an estimated 55,000 deaths (Ferlay 2015). The highest incidence is observed in Australia, with 11,405 new cases of melanoma of the skin (ACIM 2014), and in New Zealand, with 2341 registered cases in 2010 (Cancer Society of New Zealand 2013). In the USA for 2014, the predicted incidence was 73,870 per annum, and the predicted number of deaths 9940 (Siegel 2015). The highest rates in Europe are seen in northwestern Europe and the Scandinavian countries, with highest incidence reported in Switzerland of 25.8 per 100,000 in 2012. Rates in the UK trebled from 4.6 and 6.0 per 100,000 in men and women, respectively, in England in 1990, to 18.6 and 19.6 per 100,000 in 2012 (EUCAN 2012). In the UK, melanoma has one of the fastest rising incidence rates of any cancer, and it shows the biggest projected increase in incidence between 2007 and 2030 (Mistry 2011). In the decade leading up to 2013, age standardised incidence increased by 46%, with 14,500 new cases in 2013 and 2459 deaths in 2014 (Cancer Research UK 2017a). Although overall incidence rates are higher in women than in men, the rate of incidence in men is increasing faster than in women (Arnold 2014).
The rising incidence of melanoma is thought to be primarily related to rising recreational sun exposure and tanning bed use, along with an increasingly ageing population with higher lifetime recreational ultraviolet (UV) exposure (Boniol 2012; Gandini 2005), in conjunction with possible earlier detection (Belbasis 2016; Linos 2009). Putative risk factors are reviewed in detail elsewhere (Belbasis 2016), but they can be broadly divided into host and environmental factors. Host factors include fair skin and light hair or eye colour; older age (Geller 2002); male sex (Geller 2002); previous skin cancer history (Tucker 1985); predisposing skin lesions (e.g. high melanocytic naevus counts) (Gandini 2005), clinically atypical naevi (Gandini 2005), and large congenital naevi (Swerdlow 1995)); genetically inherited skin disorders (e.g. xeroderma pigmentosum) (Lehmann 2011); and a family history of melanoma (Gandini 2005). Environmental factors include recreational and occupational exposure to sunlight (both cumulative and episodic burning) (Armstrong 1977; Gandini 2005); artificial tanning (Boniol 2012); and immunosuppression (e.g. in organ transplant recipients or human immunodeficiency virus (HIV)‐positive individuals) (DePry 2011). Lower socioeconomic class may be associated with delayed presentation and thus more advanced disease at diagnosis (Reyes‐Ortiz 2006).
The main prognostic indicators following diagnosis of cutaneous melanoma can be divided into histological and clinical factors. Histologically, Breslow thickness is the single most important predictor of survival, as it is a quantitative measure of tumour invasion or volume, and thus propensity to metastasise (Balch 2001). Other factors associated with poorer prognosis histologically include microscopic ulceration, mitotic rate, microscopic satellites, regression, lymphovascular invasion, and nodular (rapidly growing) or amelanotic (lacking in melanin pigment) subtypes (Moreau 2013; Shaikh 2012). Independent of tumour thickness, prognosis is worse in older people, males, and those with locally recurrent lesions, regional lymph node involvement, or primary lesion location on the scalp or neck (Zemelman 2014).
Following histological confirmation of diagnosis, the lesion is staged according to the American Joint Committee on Cancer (AJCC) Staging System to inform treatment strategy (the eighth version of the Staging System ‐ AJCC 8 ‐ is outlined in Gershenwald 2017). Stage 0 refers to melanoma in situ; stages I to II localised melanoma; stage III regional metastasis (spread to the lymph nodes, usually but not always those nearest to the primary tumour); and stage IV distant metastasis. A preliminary stage is assigned based on histological evaluation (thickness of primary lesion and presence of ulceration) and clinical (and sometimes radiological) assessment of regional lymph nodes. A pathological stage is then confirmed based on histology of the primary lesion and of the regional lymph nodes (if the patient has sentinel lymph node biopsy (SLNB) or completion lymphadenectomy (CLND) for those with clinically palpable lymph nodes) and imaging to confirm the presence or absence of disseminated disease, where indicated.
An American database of over 40,000 patients from 1998 onwards, which assisted the development of AJCC 8, indicated five‐year survival of 99% for very early‐stage melanoma, dropping to anything between 32% and 93% in stage III disease, depending on tumour thickness, the presence of ulceration, and the number of involved nodes (Gershenwald 2017). Before the advent of targeted therapy and immunotherapies, disseminated melanoma (to distant sites/visceral organs) was associated with median survival of six to nine months, one‐year survival of 25%, and three‐year survival of 15% (Balch 2009; Korn 2008).
Between 1975 and 2010, five‐year relative survival for melanoma (i.e. not including death from other causes) in the United States increased from 80% to 94%, with survival for localised, regional, and distant disease estimated at 99%, 70%, and 18%, respectively, in 2010 (Cho 2014). However, mortality rates showed little change, at 2.1 per 100,000 deaths in 1975, and 2.7 per 100,000 in 2010 (Cho 2014). Increasing incidence of localised disease over the same period (from 5.7 to 21 per 100,000) suggests that much of the observed improvement in survival may be due to earlier detection and heightened vigilance (Cho 2014). New targeted therapies for advanced (stage IV) melanoma (e.g. BRAF inhibitors) have improved survival, and immunotherapies are evolving such that long‐term survival is being documented (Rozeman 2018). No new data regarding survival prospects for patients with stage IV disease were analysed for the AJCC 8 staging guidelines because of lack of contemporary data (Gershenwald 2017).
Treatment of melanoma
Treatment of melanoma varies to some extent, according to the stage of disease upon diagnosis. For primary melanoma, the mainstay of treatment is complete lesion excision, with a safety margin some distance from the borders of the primary tumour to remove both the tumour and any malignant cells that might have spread into the surrounding skin (Garbe 2016; Marsden 2010; NICE 2015a; SIGN 2017; Sladden 2009). Recommended surgical margins vary according to tumour thickness ‐ Garbe 2016 ‐ and stage of disease at presentation ‐ NICE 2015a. Evidence for further local or regional interventions such as wider surgical margins is limited (Sladden 2009; Wheatley 2016), although further trials in this area are planned.
Sentinel lymph node biopsy has been offered to those without clinically palpable lymph nodes as a means of providing prognostic information for several years, with the option of CLND in the event of a positive result (metastases identified on SLNB). Recent data (MLST II ‐ Kyrgidis 2015 and Morton 2014 ‐ and DeCOG ‐ Leiter 2016 and Leiter 2018 ‐ trials) show no survival benefit from CLND for this patient group, and the procedure is no longer a standard of care for most patients. Recent advances demonstrating longer recurrence‐free survival for patients with stage III melanoma receiving BRAF‐directed therapy or immunotherapies have resulted in use of SLNB as a test to identify patients who should be offered adjuvant treatment (Eggermont 2016; Eggermont 2018; Long 2017; Weber 2017). Currently available guidelines do not, as yet, reflect this recent change in practice (Garbe 2016; NICE 2015a). In the UK, the National Institute for Health and Care Excellence (NICE) has already approved dabrafenib and trametinib for adjuvant treatment of resected BRAF V600 mutation positive melanoma (NICE 2018a), with further appraisals of pembrolizumab for adjuvant treatment of melanoma with high risk of recurrence (NICE 2018b), as well as ongoing appraisals of nivolumab for adjuvant treatment of resected stage III and IV melanoma (NICE 2019a).
For stage IV melanoma, dacarbazine was the only drug approved worldwide for many years, with fotemustine used in some European countries (Avril 2004), and interleukin (IL)‐2 given in the USA (Atkins 1999). Temozolomide has also been used, especially for people with brain metastases, because of its strong ability to pass the blood‐brain barrier (Lukas 2014; Zhu 2014). This landscape has changed dramatically, with two distinct therapeutic approaches suggesting survival benefit in metastatic melanoma: (1) targeting mutations in tumour cells, and (2) providing immunomodulation (Chapman 2011; Chapman 2012; Dummer 2014; Hamid 2013; Hodi 2010; Larkin 2014; Robert 2015; Villanueva 2010). Several different therapies have now shown high response rates and, most important, have demonstrated for the first time in the treatment of melanoma the potential for a durable clinical response (Chapman 2011; Hamid 2013; Hodi 2010; Hodi 2016; Larkin 2015; Maio 2015; Sznol 2013). Several therapies are now recommended for use alone or in combination for particular subgroups of patients with metastatic melanoma, both in the UK ‐ NICE 2018a ‐ and beyond ‐ Garbe 2016 ‐ and have recently been the topic of a Cochrane Review (Pasquali 2018). An appraisal of encorafenib with binimetinib for advanced BRAF V600 mutation positive melanoma is under way (NICE 2019b), and several other treatments are currently suspended pending marketing authorisation applications from the companies concerned (NICE 2018c).
Psychosocial interventions to improve quality of life and general psychological distress after diagnosis for patients with cancer are also available. However, a Cochrane Review found considerable variation in the evidence to support such interventions (Galway 2012).
Index test(s)
Accurate staging of melanoma is more important than ever, in part to avoid unnecessary treatment and associated morbidity in those with early‐stage disease, and in part to ensure that potentially effective therapies are initiated in a timely manner for those with nodal or distant metastatic disease.
Imaging techniques such as ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) scans can be undertaken at several points along the clinical pathway, including on initial presentation of disease (primary staging), on development of recurrence (re‐staging), and on follow‐up after previous treatment for those who are asymptomatic for recurrence. The use of imaging during follow‐up with no specific clinical indication for imaging (i.e. as a monitoring test for disease surveillance) is not the focus of our reviews. Historically, most staging in terms of imaging has been undertaken in people with clinical stage III and IV disease (see Clinical pathway). However, this landscape is changing as more adjuvant systemic therapies for melanoma are becoming available.
Imaging tests are typically undertaken and interpreted by radiologists, with decisions about patient management following imaging or SLNB made at multi‐disciplinary team meetings that include oncologists, dermatologists, and surgeons (Clinical pathway).
Ultrasound
Ultrasound uses high‐frequency sound waves to create images of the body. Ultrasound can be used to assist in detection of diseased lymph nodes with clinically node negative melanoma; in treatment of patients who have a positive imaging result, proceeding to fine needle aspiration cytology (FNAC) or core biopsy; and in treatment of patients who are negative on ultrasound alone or on ultrasound combined with FNAC proceeding to SLNB. A 2011 systematic review identified 21 studies of ultrasound for primary lymph node staging or surveillance; for primary staging, sensitivity was 60% for detection of diseased lymph nodes, with specificity of 97% (the number of studies that considered staging vs surveillance is unclear) (Xing 2011).
Computed tomography (CT) (non‐contrast‐enhanced or contrast‐enhanced)
Computed tomography scans use ionising radiation in the form of X‐rays to take cross‐sectional images of the body (Bluemm 1983; van Waes 1983). This procedure involves varying amounts of radiation according to the area of the body to be scanned (Mahesh 2017), and it can be conducted with an intravenous contrast agent (contrast‐enhanced) to increase the sensitivity of metastasis detection in solid organs.
Mohr 2009 describes contrast‐enhanced CT as the best method of identifying intrathoracic metastases and as superior to X‐ray for detection of mediastinal and hilar adenopathy associated with lymphatic spread and for assessment of lesions in the bone. Computed tomography can also be used for assessment of metastatic spread to the brain, but magnetic resonance imaging (MRI) is considered more sensitive (Goulart 2011). Overall specificity is reportedly high for detection of regional nodal and distant disease, but sensitivity varies from 23% to 85% for detection of lymph node metastases, and from 25% to 74% for assessment of distant spread (Xing 2011).
Magnetic resonance imaging (MRI) (non‐contrast‐enhanced or contrast‐enhanced)
Magnetic resonance imaging scans use large magnets and non‐ionising radiation in the form of radio waves to generate images of the body (Ai 2012). These scans are more expensive and take longer to carry out compared to CT scans (Whaley 2016b).
We did not identify any systematic reviews of MRI for melanoma staging through our scoping searches; however, several studies have considered whole body MRI (Jouvet 2014; Mosavi 2013), as well as MRI for detection of brain or hepatic metastases (Aukema 2010a; Sofue 2012). Because melanoma is one of the top three cancers responsible for brain metastases (Cagney 2017), the body of evidence for the incremental accuracy of MRI compared with other imaging tests must be considered.
PET‐CT (positron emission tomography‐computed tomography)
Positron emission tomography‐computed tomography is a hybrid imaging technique that provides both functional and anatomical information. It involves injection of a weakly radioactive positron‐emitting radiopharmaceutical, which is usually 2‐deoxy‐2‐[18F]fluoro‐D‐glucose (FDG), for the purposes of oncological imaging. The distribution of FDG throughout the body is represented on images, with malignant tissue usually demonstrating greater levels of FDG uptake than normal tissue (Lammertsma 2017). The low‐dose CT component of the study generates attenuation factors that improve the quality of PET images and allows accurate anatomical localisation of areas of FDG uptake (IAEA 2016). Although initially, PET scanners were stand‐alone devices, since 2004, all modern scanners have been integrated PET‐CT scanners (Jones 2017). A systematic review of the added value of integrated PET‐CT compared to PET alone across a range of cancers suggested a 10% increase in sensitivity of PET‐CT compared to PET alone from a meta‐analysis of 10 comparative studies (Gao 2013). For these reasons, PET alone has not been considered as an index test for this review.
In comparison to CT alone, PET‐CT is generally considered to be a more sensitive test (Xing 2011); however, increases in sensitivity must be linked to any patient benefit in terms of changes in management and ultimately in patient outcomes (Schroer‐Gunther 2012; Subesinghe 2013). It may be that PET‐CT has the greatest added value for metastases in areas that are difficult to image with CT or other imaging modalities (Tan 2012), or for indeterminate metastases in areas such as the lung. Whether these assumptions are supported by current evidence has yet to be established. The evidence report for the NICE guideline in 2015 found no evidence "to suggest that earlier treatment of metastatic disease improves survival and therefore increased sensitivity was viewed currently as not an important issue" (NICE 2015d). With adjuvant therapy now an increasing option for melanoma, this conclusion seems likely to be revised in a future guideline update.
Clinical pathway
Staging of confirmed melanoma takes place in secondary and tertiary care settings only (NICE 2015a). Recommendations on the management of melanoma following diagnosis, published in the 2015 NICE Guideline (NICE 2015a), as well as in other UK guideline documents (Burkill 2014; Marsden 2010; Melanoma Taskforce 2011), are summarised in Figure 2 and are outlined below; however, practice varies across the UK. It is important to note that clinical practice is changing as more adjuvant therapies are licensed for the treatment of melanoma, and this is not adequately reflected by current guidelines. However, a consensus statement reflecting changes in decision thresholds for the use of SLNB for staging of melanoma has been published (Melanoma Focus 2018). Any key variations in practice recommended in European or US guidelines (ESMO 2019; Swetter 2019), or under consideration in a current Australian guideline update (Cancer Council Australia 2019; Gyorki 2018; Millward 2018; Morton 2018; Saw 2018), are also reflected below.
2.
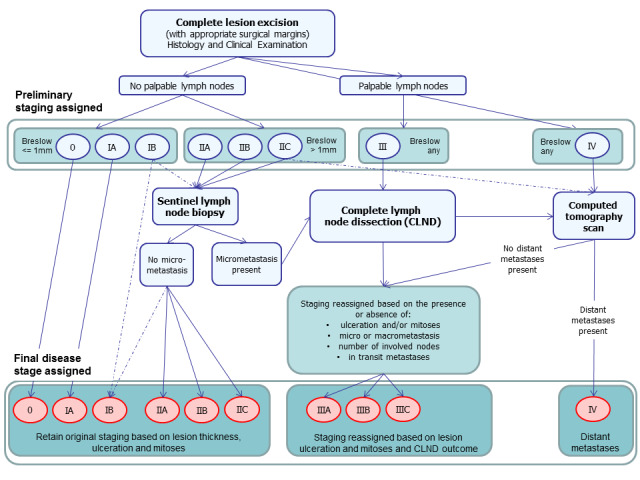
Summary of 2015 NICE guideline recommendations for the management of cutaneous melanoma following primary diagnosis (NICE 2015a); not necessarily reflective of current practice.
Following complete excision of the primary lesion, all patients should undergo preliminary staging. This involves a detailed clinical history to determine if there are any symptoms such as weight loss suggesting metastatic spread of disease, followed by a thorough clinical examination, including whole body skin examination, palpation of the lymph nodes, and full abdominal and chest examination (Figure 2). A preliminary stage is assigned on the basis of histopathology results for the primary lesion(s). Those with palpable lymph nodes are automatically assigned to clinical stage III or IV, and those with no palpable lymph nodes are assigned a stage between 0 and IIC, according to the thickness of the tumour (Breslow) and the presence of ulceration (Gershenwald 2017).
The results of all investigations carried out during the process of diagnosis are discussed at a multi‐disciplinary team meeting (Melanoma Taskforce 2011), where decisions regarding further staging procedures are made. This could be a local skin multi‐disciplinary team or, for those with stage IIB disease and above, a specialist skin multi‐disciplinary team (Marsden 2010). Teams should include dermatologists, surgeons (including plastic surgeons), medical and clinical oncologists, radiologists, histopathologists, skin cancer nurse specialists, physiotherapists, psychologists, lymphoedema service providers, occupational therapists, and cosmetic camouflage advisors (Melanoma Taskforce 2011).
On current UK guidance (based on AJCC version 7 (Balch 2009)), no further staging investigations beyond a full clinical examination are recommended for people with thin melanomas (≤ 1 mm) without ulceration or mitoses, and SLNB is reserved for those with stage IB or stage II disease (NICE 2015a). Current practice is now based on staging according to AJCC version 8, for example, with ‘thin’ melanomas now defined as < 0.8 mm in thickness without evidence of ulceration (Gershenwald 2017). Furthermore, with the advent of new adjuvant therapies, SLNB is now considered essential in determining eligibility for systemic adjuvant therapy (Gyorki 2018; Melanoma Focus 2018; Swetter 2019), and imaging is used in sentinel node positive patients to confirm absence of further disease spread (ESMO 2019; Swetter 2019). SLNB is recommended for those with primary melanoma greater than 1.0 mm and should be considered for some patients with thinner melanomas (i.e. melanomas < 0.8 mm with ulceration, and melanomas 0.8 to 1.0 mm with or without ulceration), especially in the presence of lymphovascular invasion or a mitotic rate of at least 2 per mm² (Melanoma Focus 2018). Those with clinically palpable lymph nodes or with significant nodal disease identified on imaging are likely to undergo CLND, with the option of adjuvant therapy for those with no evidence of distant metastases.
Available recommendations on the optimal choice of imaging tests vary to some extent, even within the UK (Burkill 2014; Melanoma Focus 2014; NICE 2015a). Computed tomography is generally the imaging test of choice; however, some centres additionally offer high‐resolution ultrasound, MRI, or PET‐CT scans. The National Institute for Health and Care Excellence recommends CT staging to identify those who may benefit from systemic therapy among those with stage IIC, stage III, or suspected stage IV disease (NICE 2015a), as well as imaging of the brain (with CT for adults and MRI for children and young adults) only if metastatic disease outside the central nervous system is suspected (NICE 2015a). However, the Melanoma Focus position paper recommends that all ‘high‐risk’ patients should undergo CT of the chest, abdomen, and pelvis (or whole body PET‐CT), plus MRI of the head, as standard treatment (Melanoma Focus 2014). In current clinical practice, eligibility for imaging is likely to diverge from both of these target groups; however, the emergence of new treatment options is not likely to impact the choice of imaging tests performed nor body sites imaged.
European guidelines recommend pre‐SLNB baseline lymph node (LN) ultrasound for stage IB to IIA disease, and CT or PET for stage IIB and upwards (ESMO 2019). Australian guidelines in Morton 2018 and US guidelines in Swetter 2019 recommend against baseline imaging for all asymptomatic and clinically node negative patients. In the United States, CT or PET‐CT may be considered for sentinel lymph node (SLN) positive disease but otherwise should be reserved for investigation of specific signs or symptoms or nodal or distant metastases (Swetter 2019). In Australia, US and FNAC are recommended to identify the extent of regional LN involvement in clinically node positive melanoma (Saw 2018), as well as whole body PET‐CT with CT or MRI of the brain for clinical stage III or IV disease (Saw 2018; Millward 2018).
The Royal College of Radiologists guideline recommends that scans should be tailored according to the site of the primary lesion and most likely the regional lymph node basin. In general, CT imaging of the head, chest, abdomen, and pelvis should be employed for lower limb and lower body wall lesions, with CT of the neck added for upper limb, scalp, neck, and upper torso primary tumours (Burkill 2014). Magnetic resonance imaging may be more appropriate for imaging the central nervous system (Burkill 2014). Although PET‐CT has been suggested to have a role in imaging the lower limbs, further evidence is required (Burkill 2014).
Genotyping is also now offered to identify BRAF mutations to allow further planning of systemic treatment (Melanoma Taskforce 2011; NICE 2018a; NICE 2019b).
Prior test(s)
Consideration of the degree of prior testing that study participants have undergone is key to interpretation of resulting test accuracy indices, which are known to vary according to the spectrum or case mix of included participants (Lachs 1992; Leeflang 2013; Moons 1997; Usher‐Smith 2016). Prior testing can be considered in two ways. First, the results of any tests undertaken around the time of application of the index test may contribute to the decision to undertake the index test in any particular study participant. For example, PET‐CT may be undertaken because of the presence of high‐risk primary melanoma characteristics or because of abnormal findings on abdominal ultrasound or chest X‐ray; the likelihood of abnormal findings on PET‐CT, and therefore sensitivity or specificity, may be influenced by the results of any tests previously undergone.
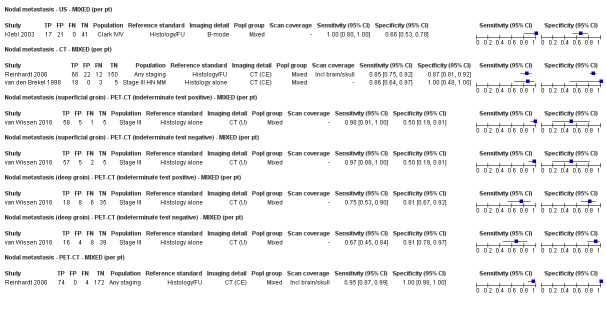
Second, prior testing can be considered in terms of the place on the clinical pathway or the time course of disease that patients have reached. People undergoing imaging for staging following a primary diagnosis of melanoma are less likely to have metastatic spread of disease compared to those for whom imaging is prompted by signs of recurrence, and the nature of any disease spread is likely to vary between a primary staging population and patients undergoing follow‐up, who may have already undergone previous treatment such as complete lymphadenectomy. Reinhardt 2006 evaluated the accuracy of CT, PET, and PET‐CT in 250 participants with melanoma "at different time points in the course of disease", including primary staging after sentinel node biopsy (n = 75); therapy control after chemotherapy for metastatic disease (n = 42); staging of clinically suspected recurrent disease (n = 65); and assessment during follow‐up within five years of primary treatment (n = 68). For both nodal and distant staging, the overall sensitivity and specificity of each test masked likely variations in accuracy between subgroups. For example, the overall sensitivity and specificity of CT for detection of nodal metastases were 85% and 87%, but when estimated for each subgroup of participants, the sensitivity of CT ranged from 67% for those undergoing follow‐up to 93% for those having imaging for treatment evaluation, and specificities ranged from 73% for the treatment evaluation group to 93% for those having primary staging (Reinhardt 2006). The overall pooled analysis suggested statistically significant differences in sensitivities (CT 73% vs PET‐CT 99%; P < 0.0001) and in specificities (CT 88% vs PET‐CT 98%; P < 0.0001) for detection of distant metastases, but for the primary staging subgroup, no difference in sensitivities was observed (93.8% for both tests) and the difference in specificities was non‐significant (CT 94.9% vs PET‐CT 98.3%) (Reinhardt 2006). For the re‐staging subgroup, differences in both sensitivities (CT 85% vs PET‐CT 100%) and specificities (CT 79% vs PET‐CT 96%) between tests were observed (Reinhardt 2006). Although subgroup numbers were relatively small, these findings lend support to the hypothesis that the clinical pathway does affect test accuracy in this context, although as for other tests and diseases, the mechanisms of action can be complex and difficult to identify (Leeflang 2013).
Role of index test(s)
Ultrasound with FNAC as a triage test before SLNB was originally promoted as having a role in fast‐tracking those with positive cytology results (micro‐metastases identified) to CLND, while those with negative cytology may proceed to SLNB, as required (Voit 2014). With the changing clinical pathway and lack of evidence for survival benefit from CLND (Leiter 2018; Morton 2014), the only potential role for ultrasound and FNAC in the UK is considered to be seen at centres where SLNB is not immediately available (with a positive cytology result indicating that adjuvant therapy should be initiated); however this approach is still recommended for use following primary melanoma diagnosis in Europe (ESMO 2019), as well as for clinically node positive melanoma in Australia (Saw 2018).
No role has been recommended for imaging tests in early‐stage disease. The need to rule out distant metastases among those who are otherwise eligible for adjuvant therapy suggests that imaging might now be used in a much more broadly defined patient group than previously. To date, CT has been recommended as the imaging approach of choice for detection of nodal and distant spread for those with stage III or IV disease (and for those with stage IIC if no SLNB has been performed) (NICE 2015a). Positron emission tomography‐computed tomography is increasingly used; however, practice varies across the country, primarily according to availability. The advantages of disease management derived from PET‐CT are not yet known. The most appropriate role for MRI in staging melanoma in adults, other than for central nervous system disease, remains unclear.
Alternative test(s)
Several other tests may be used to inform disease management following a diagnosis of melanoma.
Sentinel lymph node biopsy, which allows detection of metastatic spread to the regional lymph node basins, is the topic of another review in this series of reviews (Ferrante di Ruffano 2019).
Core needle biopsy of the lymph nodes, as in Whaley 2016a, or FNAC, as in Hall 2013, to confirm the presence of macro‐metastases can be guided by simple palpation or, for more deep‐seated lesions, via image‐based guidance to identify micro‐metastases (requiring use of a microscope for visualisation) (Bohelay 2015). Although the accuracy of core needle biopsy compared to fine needle aspiration has been identified as a key clinical question for investigation, this topic is beyond the scope of these reviews, which focus primarily on detection of non‐palpable metastatic disease.
Genetic testing of primary melanoma specimens, for BRAF mutations for example, is used increasingly (NICE 2015a), particularly with the emergence of systemic treatments for BRAF V600 mutation positive melanoma (Chapman 2011; Chapman 2012; Larkin 2014; Larkin 2015). However, its purpose is to inform systemic treatment decisions rather than to serve as an integral part of the staging procedure itself. Biomarkers, such as S100, are used in countries such as Germany as a marker of prognosis (Gray 2014), or of early disease relapse (Peric 2011), rather than for staging purposes per se (Egberts 2010; Pirpiris 2010), and lactate dehydrogenase (LDH) is part of AJCC staging for stage IV (Pirpiris 2010); however, these approaches are beyond the scope of our reviews.
Rationale
Appropriate staging of melanoma is crucial for ensuring that patients are directed to the most appropriate and effective treatment. Several tests are available to assist in the staging of melanoma; however, their comparative accuracy for detection of nodal or distant metastases, or both, according to histological stage at presentation is unclear.
The NICE guideline recommendations for staging (see Clinical pathway) were based on available systematic reviews of both SLNB and imaging tests (Hall 2013; Jimenez‐Requena 2010; Krug 2008; Rodriguez 2014; Valsecchi 2011; Xing 2011), with some supplementary data derived from primary studies (NICE 2015d). Most reviews are limited in terms of currency (de Rosa 2011; Jimenez‐Requena 2010; Krug 2008; Valsecchi 2011; Warycha 2009; Xing 2011), with literature searches in most cases extending only as recently as 2009 (Jimenez‐Requena 2010; Krug 2008; Valsecchi 2011; Xing 2011). Furthermore, the only review that compared accuracy across imaging tests did not consider histological stage (Xing 2011). Two reviews provide a more recent evaluation of PET and PET‐CT (search dates up to 2012 and 2011, respectively) (Rodriguez 2014; Schroer‐Gunther 2012); however, the Schroer‐Gunther 2012 review also relied on previously published reviews (Jimenez‐Requena 2010; Krug 2008), with supplementary searching for more recently published studies, and the Rodriguez 2014 review included only stage III melanoma. The Schroer‐Gunther 2012 review relied on quality assessment that was carried out for the original systematic reviews, and only a small number of studies were eventually included; the review authors themselves recommend that future reviews should include a broader range of study designs (Schroer‐Gunther 2012).
The comparative accuracy of imaging tests according to stage of disease therefore remains to be determined. Furthermore, any evidence for or against the routine use of brain scanning in stage III melanoma with either CT or MRI remains to be identified. Positron emission tomography‐computed tomography is increasingly used, but any additional role of this test compared with CT or MRI needs to be examined according to particular patient groups.
This review follows a generic Cochrane DTA protocol for staging of melanoma (Dinnes 2017). The Background and Methods sections of this review therefore include some text that was originally published in the protocol (Dinnes 2017), along with text that overlaps some of our other reviews for the diagnosis or staging of melanoma (e.g. Dinnes 2018; Ferrante di Ruffano 2019).
Objectives
Primary objectives
We estimated accuracy separately according to the point in the clinical pathway at which imaging tests were used. Our objectives were:
to determine the diagnostic accuracy of ultrasound or PET‐CT for detection of nodal metastases before sentinel lymph node biopsy in adults with confirmed cutaneous invasive melanoma; and
-
to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for whole body imaging in adults with cutaneous invasive melanoma:
for detection of any metastasis in adults with a primary diagnosis of melanoma (i.e. primary staging at presentation); and
for detection of any metastasis in adults undergoing staging of recurrence of melanoma (i.e. re‐staging prompted by findings on routine follow‐up).
We undertook separate analyses according to whether accuracy data were reported per patient or per lesion.
Secondary objectives
We sought to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for whole body imaging (detection of any metastasis) in mixed or not clearly described populations of adults with cutaneous invasive melanoma.
For study participants undergoing primary staging or re‐staging (for possible recurrence), and for mixed or unclear populations, our objectives were:
to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of nodal metastases;
to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of distant metastases; and
to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of distant metastases according to metastatic site.
Investigation of sources of heterogeneity
We aimed to consider a range of potential sources of heterogeneity for investigation, as outlined in our generic protocol and described in Appendix 4, but insufficient data were identified to allow any heterogeneity investigations to be undertaken.
Methods
Criteria for considering studies for this review
Types of studies
We included test accuracy studies that allow comparison of results of the index test versus a reference standard, including:
prospective and retrospective studies;
studies where all participants receive a single index test and a reference standard;
studies where all participants receive more than one index test(s) (concurrently) and a reference standard;
studies where participants are allocated (by any method) to receive different index tests or combinations of index tests and all receive a reference standard (between‐person comparative studies);
studies that recruit a series of participants unselected by true disease status; and
diagnostic case‐control studies that separately recruit diseased and non‐diseased groups (Rutjes 2005).
We excluded follow‐up and surveillance studies using repeated imaging tests to detect disease recurrence, as defining the most appropriate follow‐up schedule for melanoma patients is not the primary objective of these reviews.
We excluded studies if it was not possible to derive the numbers of true positives, false positives, false negatives, and true negatives from data provided in the paper, and we excluded small studies with fewer than five disease positive or fewer than five disease negative participants or lesions identified on imaging. Although the size threshold of five is arbitrary, such small studies are likely to yield unreliable estimates of sensitivity or specificity, and are unlikely to add precision to estimates of accuracy.
We included studies reporting either lesion‐based or participant‐based analyses; however, we accorded more weight to those reporting data on a per participant basis as detection of multiple metastatic sites in an individual patient may have a disproportionate effect on estimates of test accuracy based on per lesion data. Furthermore, treatment following staging is generally directed to the patient rather than to the individual metastatic lesion, making the patient the more appropriate unit of analysis.
We excluded studies available only as conference abstracts.
Participants
We included studies in adults with cutaneous melanoma at any primary site who were undergoing staging, either following primary presentation of disease or following recurrence of disease. We included for completeness studies that included mixed populations of patients, or where the clinical pathway could not be determined, but we undertook no statistical pooling. We included studies if up to 10% of participants had other forms of melanoma such as ocular or mucosal melanoma. We included studies with greater proportions of participants with non‐cutaneous melanoma and studies including participants with other forms of cancer only if test results for participants with cutaneous melanoma could be differentiated.
Index tests
Studies reporting accuracy data for a single application of one or more of the following tests were eligible for inclusion.
Ultrasound (with or without subsequent FNAC or core biopsy).
CT (non‐contrast‐enhanced or contrast‐enhanced).
PET‐CT (¹⁸FDG only).
MRI (non‐contrast‐enhanced or contrast‐enhanced).
We included any threshold for deciding test positivity, either qualitative or quantitative.
We excluded studies reporting multiple applications of the same test in more than 10% of study participants because of anticipated effects on test accuracy (multiple tests increasing the chance of detection of metastases, thereby increasing test sensitivity and reducing specificity). The threshold of 10% is arbitrary but allows for inclusion of studies primarily focused on evaluating the accuracy of a single test application for staging of disease. We excluded studies of surveillance imaging following initial definitive treatment.
Target conditions
Primary target conditions were defined as detection of:
nodal metastases in participants scheduled for SLNB (to identify those who should proceed directly to CLND); and
any metastases for all other staging.
Two additional definitions of the target condition were considered in secondary analyses, namely, detection of:
any nodal metastases; and
any distant metastases (combined or by metastatic site).
Reference standards
Acceptable reference standards include:
histology of lymph node or distant specimens, with samples obtained by core biopsy, SLNB, or lymph node dissection;
cytology of lymph node specimens, with samples obtained by core biopsy or fine needle aspiration;
clinical or radiological follow‐up to identify nodal or distant recurrence of at least three months; and
any combination of the above.
We excluded studies using cross‐sectional imaging‐based reference standards (i.e. direct comparison of the index test vs an alternative reference standard imaging test).
Search methods for identification of studies
Electronic searches
The Information Specialist (SB) carried out a comprehensive search for published and unpublished studies. A single large literature search was conducted to cover all topics in the programme grant (see Appendix 1 for a summary of reviews included in the programme grant). This allowed screening of search results for potentially relevant papers for all reviews at the same time. A search combining disease‐related terms with terms related to test names, using both text words and subject headings, was formulated. The search strategy was designed to capture studies evaluating tests for the diagnosis or staging of skin cancer. As a majority of records were related to searches for tests for staging of disease, a filter using terms related to cancer staging and to accuracy indices was applied to the staging test search to try to eliminate irrelevant studies, for example, those using imaging tests to assess treatment effectiveness. A sample of 300 records that would be missed by applying this filter was screened and the filter adjusted to include potentially relevant studies. When piloted on MEDLINE, inclusion of the filter for staging tests reduced the overall numbers by around 6000. The final search strategy, incorporating the filter, was subsequently applied to all bibliographic databases as listed below (Appendix 5). The final search result was cross‐checked against the list of studies included in five systematic reviews; our search identified all but one of these studies, and this study was not indexed on MEDLINE. The Information Specialist devised the search strategy, with input from the Information Specialist from Cochrane Skin. No additional limits were used.
We searched the following bibliographic databases to 29 August 2016 for relevant published studies.
MEDLINE via OVID (from 1946).
MEDLINE In‐Process & Other Non‐Indexed Citations via OVID.
Embase via OVID (from 1980).
We searched the following bibliographic databases to 30 August 2016 for relevant published studies.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 7), in the Cochrane Library.
Cochrane Database of Systematic Reviews (CDSR; 2016, Issue 8), in the Cochrane Library.
Cochrane Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2).
CRD HTA (Health Technology Assessment) database (2016, Issue 3).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO from 1960.
We searched the following databases for relevant unpublished studies using a strategy based on the MEDLINE search.
Conference Proceedings Citation Index (CPCI), via Web of Science™ (from 1990; searched 28 August 2016).
Science Citation Index (SCI) Expanded™ via Web of Science™ (from 1900, using the 'Proceedings and Meetings Abstracts' Limit function; searched 29 August 2016).
We searched the following trials registers using the search terms 'melanoma', 'squamous cell', 'basal cell', and 'skin cancer' combined with 'diagnosis'.
Zetoc (from 1993; searched 28 August 2016).
US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov; searched 29 August 2016).
NIHR Clinical Research Network Portfolio Database (www.nihr.ac.uk/research‐and‐impact/nihr‐clinical‐research‐network‐portfolio/; searched 29 August 2016).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/; searched 29 August 2016).
We aimed to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or in progress), but because of time constraints, we were unable to follow up on potentially relevant studies identified from conference abstracts. We applied no date limits.
Searching other resources
We screened relevant systematic reviews identified by the searches for their included primary studies, and we included any missed by our searches. We checked the reference lists of all included papers, and subject experts within the author team reviewed the final list of included studies. We conducted no electronic citation searching.
Data collection and analysis
Selection of studies
At least one review author (JDi or NC) screened titles and abstracts and discussed and resolved any queries by consensus. A pilot screen of 539 MEDLINE references showed good agreement (89% with a kappa of 0.77) between screeners. Primary test accuracy studies and test accuracy reviews (for scanning of reference lists) of any test used to investigate suspected melanoma, basal cell carcinoma (BCC), or cutaneous squamous cell carcinoma (cSCC) were included at initial screening. Inclusion criteria were applied independently by both a clinical review author (from one of a team of 12 clinician reviewers) and a methodologist review author (JDi, NC, or LFR) to all full‐text articles, and disagreements were resolved by consensus or by a third party (JDe, CD, HW, RM) (Appendix 6). No study authors were contacted in regard to study eligibility because of the volume of data retrieved. Authors of eligible studies were contacted when insufficient data were presented to allow for construction of 2×2 contingency tables.
The study selection process is described in a PRISMA‐DTA flowchart (McInnes 2018).
Data extraction and management
One clinical (SAC, AD, AG, LP) and at least one methodologist review author (LFR, JDi) extracted data concerning details of study design, participants, index test(s) or test combinations, criteria for index test positivity, reference standards, and data required to populate a 2×2 diagnostic contingency table for each index test using a piloted data extraction form. Disagreements were resolved through discussion or by a third party (JDe, CD, HW, RM).
Dealing with multiple publications and companion papers
In the event of multiple reports of a primary study, the most complete and up‐to‐date data source available was used to contribute 2×2 contingency table data to eliminate double‐counting of datasets. When possible, yield of information regarding study methods and participants was maximised by extracting relevant data from multiple publications.
Assessment of methodological quality
We assessed risk of bias and applicability of included studies using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) checklist (Whiting 2011), which had been tailored to the review topic (Appendix 7). We piloted the modified QUADAS‐2 tool on a small number of included full‐text articles. One clinical (as detailed above) and at least one methodologist review author (LFR, JDi, BH, or SB) independently assessed quality for the remaining studies; any disagreement was resolved by consensus or by a third party when necessary (JDe, CD, HW, RM).
Statistical analysis and data synthesis
We conducted separate analyses first according to whether study participants were recruited on primary presentation of melanoma or with a disease recurrence, and second according to our primary and secondary objectives (i.e. detection of any metastasis (which must include both nodal and distant recurrence) and detection of nodal metastasis alone or detection of any distant metastasis, as defined under Target condition being diagnosed).
Studies may report test accuracy per lesion or per patient. Our unit of analysis for primary analyses was the patient, as study participants may have multiple metastatic sites at any one time, such that a per lesion analysis may overestimate test accuracy.
We initially explored the data by plotting estimates of sensitivity and specificity on coupled forest plots and in receiver operating characteristic (ROC) space for each index test. We performed meta‐analyses using the bivariate method to produce summary operating points (summary sensitivities and specificities) with 95% confidence and prediction regions (Chu 2006; Macaskill 2010; Reitsma 2005). When few studies were available for a meta‐analysis, we simplified the bivariate model to univariate fixed‐effect or random‐effects logistical regression models depending on whether or not heterogeneity was observed on forest plots and in ROC space (Takwoingi 2015). If there were only two or three studies and we observed heterogeneity on the plots, we did not pool the data, as a fixed‐effect approach would be inappropriate and the number of studies too small to reliably estimate random effects.
To compare the accuracy of the index tests, we performed both direct and indirect test comparisons, as comparative studies are scarce (Takwoingi 2013). To formally compare index tests, we added a co‐variate for test type to a bivariate model (i.e. bivariate meta‐regression). We used likelihood ratio tests to assess the statistical significance of differences in sensitivity and specificity by comparing models without the co‐variate terms versus models containing the co‐variate terms. Using parameter estimates from bivariate meta‐regression models, we calculated absolute differences in sensitivity and specificity. We obtained 95% confidence intervals and P values for these differences using the delta method and the Wald test, respectively. When the number of studies in a direct comparison was insufficient for meta‐regression, we examined individual study results and computed absolute differences in sensitivity and specificity for each comparative study. We calculated 95% confidence intervals (CIs) for these differences using the Newcombe‐Wilson method without continuity correction (Newcombe 1998).
We conducted analyses using Review Manager 5 (Review Manager 2014), along with the meqrlogit command in the statistical software STATA version 15 (STATA 2017).
Investigations of heterogeneity
We initially examined heterogeneity between studies by visually inspecting forest plots of sensitivity and specificity and summary ROC plots. We identified insufficient numbers of studies to allow meta‐regression to formally investigate potential sources of heterogeneity.
Sensitivity analyses
We performed no sensitivity analyses because limited data were available.
Assessment of reporting bias
Because of uncertainty about the determinants of publication bias for diagnostic accuracy studies and the inadequacy of tests for detecting funnel plot asymmetry (Deeks 2005), we did not assess publication bias.
Results
Results of the search
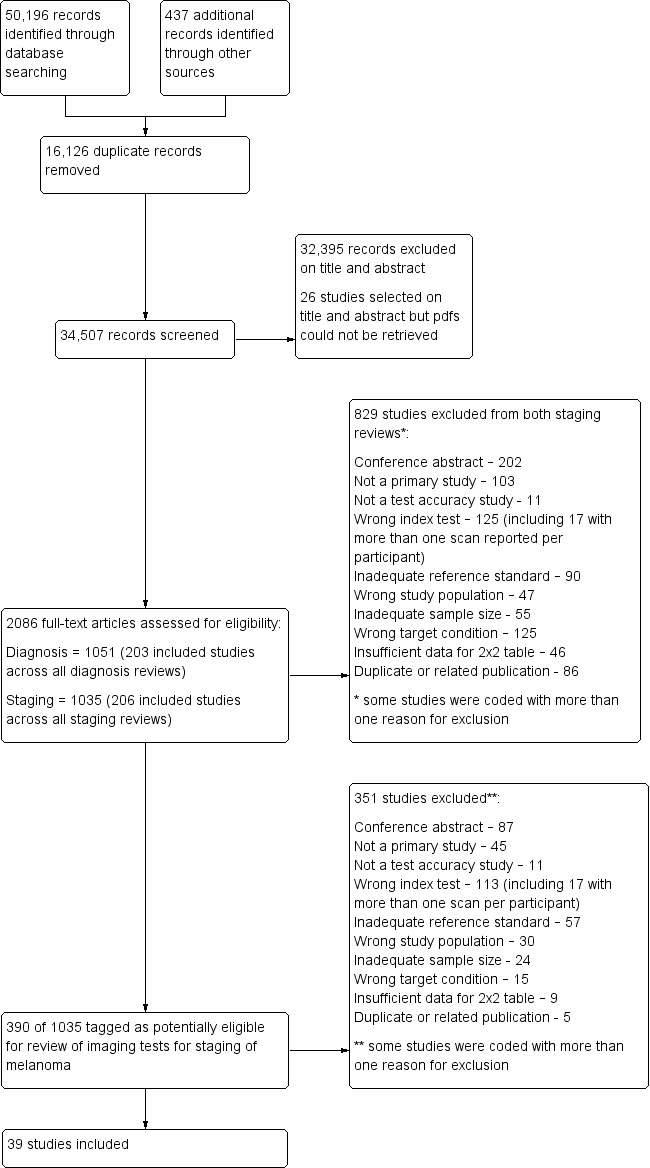
We identified and screened for inclusion a total of 34,507 unique references. Of these, we reviewed 1035 full‐text papers for eligibility for any one of the reviews of tests for staging of melanoma or cSCC. Of the 1035 full‐text papers assessed, we excluded 829 from all reviews in our series (see Figure 3 PRISMA flow diagram of search and eligibility results).
3.
PRISMA flow diagram.
Of the 390 studies tagged as potentially eligible for this review of imaging tests for staging of melanoma, we included 39 publications. Exclusions were due to publication as a conference abstract (n = 202), not a primary study (n = 103), not a test accuracy study (no index test and or reference standard reported) (n = 11), wrong index test (n = 125; including 17 studies with more than one scan reported per participant), inadequate reference standard (n = 90), wrong study population (n = 47), inadequate sample size (n = 55), wrong target condition (n = 125), missing data to complete 2×2 contingency table (n = 46), and duplicate or related publication (n = 86). We have provided a list of the 351 publications excluded from this review with reasons for exclusion in Characteristics of excluded studies. We contacted the authors of four included studies for further details of study methods (Chai 2012; Reinhardt 2006; Stoffels 2012; Voit 2014). We received a response in regard to one study (Reinhardt 2006), but study authors did not provide the additional data requested.
The 39 included study publications provide 195 contingency table datasets for a total of 5204 study participants. Thirty‐four studies reported data on a per patient basis, including two that also reported data per lesion identified on imaging (Cachin 2014; Iagaru 2007), and five reported data only on a per lesion basis (Dellestable 2011; Hausmann 2011; Jouvet 2014; Pfannenberg 2007; Pfluger 2011). The 34 studies that reported data per patient included 4980 study participants, 1265 of whom had confirmed metastatic disease. The seven studies that reported data per lesion included 417 study participants with 1846 potentially metastatic lesions identified on imaging, 1061 of which were confirmed metastases.
Table 2 cross‐tabulates the index tests evaluated and the population groups and target conditions considered in the 39 included studies. Eighteen studies considered the use of imaging for nodal metastases before SLNB; 11 of these studies considered the use of ultrasound, and eight evaluated PET‐CT. Twenty‐four studies evaluated the use of imaging as a staging tool in study participants undergoing primary staging on diagnosis of melanoma (n = 6) or re‐staging for recurrence of disease (n = 3), or inclusion of mixed (n = 11) or not clearly described populations (n = 4). The imaging tests evaluated included ultrasound (n = 5), CT (n = 10), MRI (n = 4), and PET‐CT (n = 15) for detection of any metastases (n = 14), nodal metastases (n = 14), or distant metastases (n = 9). Five studies also reported data separately by metastatic site.
1. Cross‐tabulation of studies by index test, population group, and target condition.
| Study | US | US‐ FNAC | CT | MRI | PET‐CT | Population group | Population detail | Reference standard | Any metastases | Distant metastases | Nodal metastases | Other sites |
| PRIMARY STAGING | ||||||||||||
| Arrangoiz 2012 | ‐ | ‐ | ‐ | ‐ | X | Primary (any); primary (pre‐SLNB) | BT > 4 mm | SLNB/CLND/FU | Per patient | Per patient | Per patient/ Pre‐SLNB | ‐ |
| Chai 2012 | X | ‐ | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB/CLND ± FU | ‐ | ‐ | Pre‐SLNB | ‐ |
| Hafner 2004 | X | ‐ | ‐ | ‐ | (X) | Primary (pre‐SLNB); primary | Standard SLNB Any (incl N+) | SLNB/CLND | ‐ | ‐ | Per patient/ Pre‐SLNB | ‐ |
| Hinz 2011 | X | ‐ | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| Hinz 2013 | X | ‐ | ‐ | ‐ | X | Primary (pre‐SLNB) | High risk (BT ≥ 2.0 mm or other RF) | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| Hocevar 2004 | X | X | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB/CLND | ‐ | ‐ | Pre‐SLNB | ‐ |
| Kang 2011 | ‐ | ‐ | ‐ | ‐ | X | Primary (any) | All staging (incl N+) | Histology/FU | per patient | ‐ | ‐ | ‐ |
| Kell 2007 | X | Primary (pre‐SLNB) | Standard SLNB | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ | ||||
| Klode 2010 | ‐ | ‐ | ‐ | ‐ | X | Primary (pre‐SLNB) | Standard SLNB | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| Kunte 2009 | X | ‐ | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| Maubec 2007 | ‐ | ‐ | ‐ | ‐ | X | Primary (any); primary (pre‐SLNB) | BT > 4 mm | SLNB/CLND ± FU | Per patient | ‐ | Pre‐SLNB | ‐ |
| Prayer 1990 | X | ‐ | ‐ | ‐ | ‐ | Primary (any) | All staging (incl N+) | CLND/FU | ‐ | ‐ | Per patient | ‐ |
| Radzhabova 2009 | X | ‐ | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB; any (incl N+) | SLNB ± FU | ‐ | ‐ | Pre‐SLNB | ‐ |
| Revel 2010 | ‐ | ‐ | ‐ | ‐ | X | Primary (pre‐SLNB) | HN MM | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| Sanki 2009 | X | ‐ | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB | Pre‐SLNB | |||
| Sibon 2007 | X | ‐ | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| Singh 2008 | ‐ | ‐ | ‐ | ‐ | X | Primary (pre‐SLNB) | Standard SLNB/BT > 4 mm | SLNB | ‐ | ‐ | Pre‐SLNB | ‐ |
| van Rijk 2006 | X | X | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB/CLND | ‐ | ‐ | pre‐SLNB | ‐ |
| Veit‐Haibach 2009 | ‐ | ‐ | X | ‐ | X | Primary (any) | All staging (incl N+) | Histology/FU | ‐ | Per patient | Per patient | ‐ |
| Voit 2014 | X | X | ‐ | ‐ | ‐ | Primary (pre‐SLNB) | Standard SLNB | SLNB/CLND | ‐ | ‐ | Pre‐SLNB | ‐ |
| Wagner 2012 | ‐ | ‐ | ‐ | ‐ | X | Primary (pre‐SLNB) | High risk (BT ≥ 4 mm or > 1 mm and ulcerated) | SLNB/CLND | ‐ | ‐ | Pre‐SLNB | ‐ |
| RE‐STAGING | ||||||||||||
| Iagaru 2007 | ‐ | ‐ | X | ‐ | X | Re‐staging | Any re‐staging | Histology/FU | Per patient /Per lesion |
‐ | ‐ | ‐ |
| Rubaltelli 2011 | X | ‐ | ‐ | ‐ | ‐ | Re‐staging | Any FU and suspicious on B‐mode US | FNAC/Histology/FU | ‐ | ‐ | Per patient | ‐ |
| Strobel 2007a | ‐ | ‐ | ‐ | ‐ | X | Re‐staging | High risk (BT > 4 mm, etc.), elevated S100 | Histology/Cytology/FU | Per patient | ‐ | ‐ | ‐ |
| MIXED OR UNCLEARLY REPORTED | ||||||||||||
| Abbott 2011 | ‐ | ‐ | ‐ | ‐ | X | Mixed | Stage III | Histology/FU | Per patient | ‐ | ‐ | ‐ |
| Aukema 2010a | ‐ | ‐ | ‐ | (X ‐ Brain) | X | Mixed | S100 positive | FNAC/Histology/Imaging FU | Per patient | ‐ | ‐ | ‐ |
| Aukema 2010b | ‐ | ‐ | ‐ | (X ‐ Brain) | X | Unclear | Node positive | FNAC/Histology/FU | Per patient | ‐ | ‐ | ‐ |
| Bastiaannet 2009 | ‐ | ‐ | X | ‐ | (X) | Mixed | All node positive | Histology/FU | ‐ | Per patient | ‐ | ‐ |
| Cachin 2014 | ‐ | ‐ | ‐ | ‐ | X | Mixed | Stage III | Histology/Imaging/FU | Per patient/Per lesion | Per lesion | Per lesion | Per lesion |
| Dellestable 2011 | ‐ | ‐ | X | X | X | Mixed | All staging | Histology/FU | Per lesion | Per lesion | Per lesion | Per lesion |
| Hausmann 2011 | ‐ | ‐ | X | X | ‐ | Unclear | Stage III/IV | Histology/FU | Per lesion | Per lesion | Per lesion | Per lesion |
| Jouvet 2014 | X | ‐ | X | X | X | Unclear | Stage IV | FNAC/FU | Per lesion | Per lesion | Per lesion | Per lesion |
| Klebl 2003 | X | ‐ | ‐ | ‐ | ‐ | Mixed | Clark IV/V in FU | Histology/FU | ‐ | ‐ | per patient | ‐ |
| Pfannenberg 2007 | ‐ | ‐ | X | X | X | Mixed | Stage III/IV | Histology/Imaging/FU | Per lesion | Per patient | Per lesion | Per lesion |
| Pfluger 2011 | ‐ | ‐ | X | ‐ | X | Mixed | All stage III | Histology/FU | Per patient | ‐ | ‐ | ‐ |
| Reinhardt 2006 | ‐ | ‐ | X | ‐ | X | Mixed | All staging (incl N+) | Histology/FU | Per patient | Per patient | Per patient | ‐ |
| Strobel 2007b | ‐ | ‐ | ‐ | ‐ | X | Unclear | High risk (BT > 4 mm, etc.) | Histology/Cytology/FU | Per patient | ‐ | ‐ | ‐ |
| van den Brekel 1998 | X | ‐ | ‐ | Mixed | HN MM and N+ | Histology | ‐ | ‐ | Per patient | ‐ | ||
| van Wissen 2016 | ‐ | ‐ | ‐ | ‐ | X | Mixed | Stage IIIB/IIIC palpable groin mets | Histology (combined groin dissection) | ‐ | ‐ | Per patient | ‐ |
BT: Breslow thickness; CLND: complete lymph node dissection; CT: computed tomography; FNAC: fine needle aspiration cytology; FU: follow‐up; HN: head and neck; MM: malignant melanoma; MRI: magnetic resonance imaging; mm: millimetre; N+: node positive; PET: positron emission tomography; RF: risk factor; SLNB: sentinel lymph node biopsy; US: ultrasound.
Methodological quality of included studies
The overall methodological quality of all included study cohorts is summarised in Figure 4 and Figure 5. Studies were generally at low or unclear risk of bias and of high or unclear concern regarding applicability of the evidence.
4.
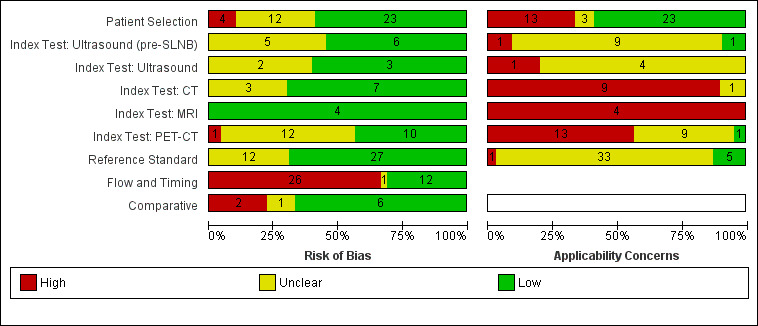
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
5.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Over half of studies (23; 59%) were at low risk of bias for participant selection. High risk of bias was observed in four studies (10%) because of inappropriate participant exclusions; all excluded study participants on the basis of findings on the index test (ultrasound in all cases) (Hinz 2011; Hinz 2013; Radzhabova 2009; Rubaltelli 2011). Those at unclear risk of bias (n = 12) did not clearly describe participant recruitment as random or consecutive (n = 11) (all except Iagaru 2007) (Abbott 2011; Aukema 2010b; Cachin 2014; Hocevar 2004; Iagaru 2007; Jouvet 2014; Kang 2011; Klebl 2003; Pfluger 2011; Prayer 1990; Sanki 2009; Singh 2008), or did not clearly report participant exclusions (n = 3) (Iagaru 2007; Kang 2011; Pfluger 2011).
Over half of evaluations were considered at low risk of bias for the index test (55% (6/11) for pre‐SLNB ultrasound; 60% (3/5) for other uses of ultrasound; 70% (7/10) for CT; 100% (4/4) for MRI; and 43% (10/23) for PET‐CT). Across the 11 evaluations of pre‐SLNB ultrasound, five (45%) studies were retrospective or unclear in the nature of their design and did not describe blinded case note review to ascertain imaging test results (Hinz 2013; Hocevar 2004; Radzhabova 2009; Sanki 2009; van Rijk 2006). The same rationale for unclear risk of bias was made for two of the five (40%) other evaluations of ultrasound (Prayer 1990; Rubaltelli 2011), three evaluations of CT (30%) (Iagaru 2007; Pfluger 2011; van den Brekel 1998), and 13 (57%) evaluations of PET‐CT (Abbott 2011; Arrangoiz 2012; Aukema 2010a; Hinz 2013; Iagaru 2007; Kang 2011; Klode 2010; Pfluger 2011; Revel 2010; Singh 2008; Strobel 2007a; van Wissen 2016; Wagner 2012). One evaluation of pre‐SLNB ultrasound ‐ Radzhabova 2009 ‐ and one of PET‐CT ‐ Iagaru 2007 ‐ did not clearly prespecify the index test threshold. One study of PET‐CT ‐ Kang 2011 ‐ retrospectively selected the maximum standardised uptake value (SUVmax) threshold for PET‐CT using ROC analysis and therefore scored high risk of bias for this domain.
Most studies (27/39) were judged at low risk of bias for the reference standard; the 12 studies at unclear risk of bias provided no information on the follow‐up schedule used to determine final disease status (Arrangoiz 2012; Aukema 2010a; Aukema 2010b; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Iagaru 2007; Jouvet 2014; Pfluger 2011; Rubaltelli 2011; Strobel 2007b; Veit‐Haibach 2009). Although blinding of the reference standard diagnosis did not contribute to overall risk of bias, two studies clearly reported blinding of the histological diagnosis (Pfannenberg 2007; Sibon 2007), and three reported blinding of data collection on follow‐up (Hausmann 2011; Pfannenberg 2007; Reinhardt 2006). Two studies reported no blinding of histological diagnosis (Cachin 2014; Singh 2008), and three reported no blinding to the original imaging result during follow‐up (Abbott 2011; Cachin 2014; Jouvet 2014).
Two‐thirds of studies were at high risk of bias for participant flow and timing (26/39), and one was judged as having unclear risk. High risk of bias was considered in one study because of performance of the imaging test (PET‐CT) up to four months after the reference standard (SLNB) (Maubec 2007); in 19 studies (49%) because of differential verification (Abbott 2011; Arrangoiz 2012; Aukema 2010a; Aukema 2010b; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Hausmann 2011; Iagaru 2007; Jouvet 2014; Kang 2011; Klebl 2003; Pfannenberg 2007; Pfluger 2011; Prayer 1990; Reinhardt 2006; Strobel 2007a; Strobel 2007b; Veit‐Haibach 2009); and in 13 studies (33%) because of exclusion of participants from the analysis (Bastiaannet 2009; Cachin 2014; Chai 2012; Dellestable 2011; Hafner 2004; Hausmann 2011; Klebl 2003; Pfannenberg 2007; Pfluger 2011; Radzhabova 2009; Rubaltelli 2011; van Wissen 2016; Wagner 2012).
Among the nine studies providing direct comparisons of index tests, six were judged at low risk of bias for the comparative domain. Pfluger 2011 was considered at high risk of bias, as PET‐CT and CT images were interpreted side by side, and in Hinz 2013, only a subgroup of those with US also underwent PET‐CT. In two studies, blinding between tests was not clearly described (Hinz 2013; Iagaru 2007).
In terms of applicability of evidence to the review question, 40% (n = 16) of studies were of high or unclear concern due to participant selection (Figure 4). High concern was primarily due to inclusion of participants from mixed population groups (including primary staging, re‐staging, or patient follow‐up) (Abbott 2011; Aukema 2010a; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Klebl 2003; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; van den Brekel 1998; van Wissen 2016), or it was due to the presentation of only per lesion rather than per patient data (Dellestable 2011; Hausmann 2011; Jouvet 2014; Pfannenberg 2007; Pfluger 2011). Three studies were of unclear concern due to lack of clear description of the indication for imaging (Aukema 2010b; Iagaru 2007; Strobel 2007b).
Almost all test evaluations were considered at high or unclear concern around applicability of the index test. For pre‐SLNB ultrasound, there was high concern from lack of detail regarding the threshold used (n = 1) (Hafner 2004), and unclear concern resulted from lack of information on application and interpretation of the index test (n = 9) (Chai 2012; Hafner 2004; Hinz 2011; Hocevar 2004; Kunte 2009; Radzhabova 2009; Sibon 2007; van Rijk 2006; Voit 2014), or regarding the expertise of the observer performing the ultrasound examination (n = 6) (Chai 2012; Hinz 2011; Kunte 2009; Radzhabova 2009; Sanki 2009; van Rijk 2006).
For CT, six evaluations were of high concern due to use of consensus test interpretation (Iagaru 2007; Jouvet 2014; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Veit‐Haibach 2009), two for MRI (Jouvet 2014; Pfannenberg 2007), and 11 for PET‐CT (Aukema 2010a; Aukema 2010b; Iagaru 2007; Jouvet 2014; Kang 2011; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Revel 2010; Strobel 2007a; Strobel 2007b). Only five CT evaluations described the provision of usual clinical information to test interpreters (Jouvet 2014; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Veit‐Haibach 2009), one evaluation of MRI (Pfannenberg 2007), and four for PET‐CT (Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Revel 2010). Three CT evaluations were unclear on the information provided to assist test interpretation (Bastiaannet 2009; Dellestable 2011; van den Brekel 1998), two for MRI (Dellestable 2011; Hausmann 2011), and six for PET‐CT (Dellestable 2011; Kell 2007; Klode 2010; Maubec 2007; Singh 2008; Veit‐Haibach 2009).
Inadequate details of test threshold were provided in five evaluations of CT (Bastiaannet 2009; Dellestable 2011; Iagaru 2007; Jouvet 2014; Reinhardt 2006), three for MRI (Dellestable 2011; Hausmann 2011; Jouvet 2014), and four for PET‐CT (Abbott 2011; Hinz 2013; Jouvet 2014; Reinhardt 2006). Threshold details were unclear for one study for both CT and MRI (Pfannenberg 2007), as were six for PET‐CT (Aukema 2010a; Aukema 2010b; Dellestable 2011; Klode 2010; Maubec 2007; Wagner 2012). Two CT evaluations were unclear with regard to observer expertise (Dellestable 2011; van den Brekel 1998), one for MRI (Dellestable 2011), and seven for PET‐CT (Arrangoiz 2012; Dellestable 2011; Hinz 2013; Kell 2007; Klode 2010; Maubec 2007; Revel 2010).
For applicability of the reference standard, five studies were considered of low concern (Hafner 2004; Hinz 2011; Hinz 2013; Kang 2011; van Wissen 2016), and one was rated of high concern because it did not present data for the primary target condition of any metastasis (nodal plus distant metastases) (Veit‐Haibach 2009). The remaining 33 studies were considered at unclear concern for applicability because they did not clearly define the target condition or provide a breakdown according to nodal or distant metastases. Only five studies described the expertise of the histopathologist (Hafner 2004; Hinz 2011; Hinz 2013; Kang 2011; van Wissen 2016); the remaining studies were rated of unclear concern.
Findings
1. Imaging for detection of nodal metastases before SLNB
Imaging before SLNB can be used to identify patients with nodal metastatic disease that is not detectable clinically such that they can bypass the SLNB procedure and undergo complete lymph node dissection. Eighteen studies were included, 10 of which considered the use of pre‐SLNB ultrasound (Chai 2012; Hafner 2004; Hinz 2011; Hocevar 2004; Kunte 2009; Radzhabova 2009; Sanki 2009; Sibon 2007; van Rijk 2006; Voit 2014); seven evaluated PET‐CT (Arrangoiz 2012; Kell 2007; Klode 2010; Maubec 2007; Revel 2010; Singh 2008; Wagner 2012), and one evaluated both tests (Hinz 2013). Three studies of ultrasound also presented accuracy data for ultrasound combined with FNAC (i.e. complete lymph node dissection recommended only if both ultrasound and FNAC were positive for metastases) (Hocevar 2004; van Rijk 2006; Voit 2014).
Forest plots of study data are provided in Figure 6. Summary estimates for indirect and direct comparisons of tests are presented in Table 3 and Figure 7. Summary details of all studies in this section are presented alphabetically in Appendix 8.
6.

Forest plot of all data for pre‐SLNB ultrasound, ultrasound plus FNAC, or PET‐CT for the detection of nodal metastasis. (HN MM ‐ head and neck only malignant melanoma.)
2. Summary results from studies of imaging for primary staging or re‐staging.
| Test | Studies | Participants (cases) | Sensitivity (95% CI), % | Specificity (95% CI), % |
| Comparison of imaging tests before SLNB | ||||
| Indirect comparison of imaging tests for detection of nodal metastasis (per patient data) | ||||
| US | 11 | 2614 (542) | 35.4 (17.0 to 59.4) | 93.9 (86.1 to 97.5) |
| US‐FNAC | 3 | 1164 (259) | 18.0 (3.58 to 56.5) | 99.8 (99.1 to 99.9) |
| PET‐CT | 4 | 170 (49) | 10.2 (4.31 to 22.3) | 96.5 (87.1 to 99.1) |
| Difference | P = 0.07 | P < 0.001 | ||
| Direct comparison of imaging tests for detection of nodal metastasis (per patient data) | ||||
| US | 3 | 1164 (259) | 58.7 (36.5 to 77.9) | 79.4 (70.0 to 86.4) |
| US‐FNAC | 3 | 1164 (259) | 18.0 (3.58 to 56.5) | 99.8 (99.1 to 99.9) |
| Difference | ‐40.7 (‐75.0 to ‐6.50), P = 0.02 | +20.4 (+12.2 to +28.6), P < 0.001 | ||
| Whole body imaging | ||||
| Imaging for re‐staging for the detection of any metastasis (per patient data) | ||||
| PET‐CT | 2a | 153 (95) | 92.6 (85.3 to 96.4) | 89.7 (78.8 to 95.3) |
CI: confidence interval; CT: computed tomography; FNAC: fine needle aspiration cytology; PET: positron emission tomography; SLNB: sentinel lymph node biopsy; US: ultrasound.
aWhere there were only two studies, estimates of summary sensitivity and summary specificity were obtained by using univariate fixed‐effect logistic regression models to pool sensitivities and specificities separately.
7.

Summary ROC plot comparing pre‐SLNB ultrasound vs ultrasound plus FNAC vs PET‐CT.
Description of studies
Study design and setting. Four of the 18 studies (22%) were prospective case series (Hafner 2004; Hinz 2011; Kunte 2009; Maubec 2007), nine (50%) were retrospective (Arrangoiz 2012; Chai 2012; Hinz 2013; Kell 2007; Klode 2010; Revel 2010; Sibon 2007; van Rijk 2006; Wagner 2012), and five (28%) did not clearly report the design (Hocevar 2004; Radzhabova 2009; Sanki 2009; Singh 2008; Voit 2014). Studies were conducted in Europe (n = 14), Australia (n = 1; Sanki 2009), and the USA (n = 3; Arrangoiz 2012; Chai 2012; Kell 2007).
Participants. Thirteen of the 18 studies (72%) were considered to have been conducted in ‘standard’ SLNB populations, either reporting the inclusion of participants with primary melanomas with Breslow thickness of at least 0.76 mm or 1 mm unless other adverse prognostic factors were present such as Clark level of at least IV, ulceration, or regression (Chai 2012; Hafner 2004; Hinz 2011; Kell 2007; Klode 2010; Kunte 2009; Sanki 2009; Sibon 2007; Singh 2008; van Rijk 2006; Voit 2014), or reporting including ‘candidates for SLNB’ with no further detail (Hocevar 2004; Radzhabova 2009). One study restricted inclusion to participants with head and neck melanoma only (Revel 2010), and the remaining four studies included only higher‐risk participants, with Breslow thickness of at least 2 mm (Hinz 2013), or 4 mm (Arrangoiz 2012; Maubec 2007; Wagner 2012).
A total of 2894 participants were included, with 640 nodal metastases identified (prevalence 22%, ranging from 10% in Hinz 2011 to 60% in Hinz 2013). Sample sizes ranged from 20 participants in Hinz 2013 and Maubec 2007 to 1000 in Voit 2014. When reported (n = 11), the ages of included participants ranged from one year in Hocevar 2004 to 94 years in Voit 2014. The mean age of included participants was reported in 11 studies (the median of reported means was 57.5 years, range 50 to 67 years), and the median age was reported in five studies (the median of reported means was 58 years, range 55 to 62 years); five studies reported neither the mean nor median age of included study participants (Hinz 2013; Hocevar 2004; Radzhabova 2009; Sanki 2009; Wagner 2012). When reported (n = 15), 48% of included participants were male. Of 11 studies reporting the site of the primary melanoma lesion (excluding Revel 2010, which included head and neck melanomas only), the percentage of participants with head and neck melanoma ranged from 0% in Hinz 2013 to 36% in Maubec 2007 (median 14%), and melanoma of the extremities, including the hands or feet where documented, from 32% in Maubec 2007 to 56% in Kunte 2009 (median 50%).
Ultrasound. The 11 studies of pre‐SLNB ultrasound were all conducted in standard SLNB populations, although Hinz 2013 restricted inclusion to participants with melanomas at least 2 mm thick or with risk factors such as ulceration or regression. The two studies by Hinz and colleagues excluded participants with classic sonographic signs of lymphatic metastasis (Hinz 2011; Hinz 2013), whereas Radzhabova 2009 included only those who were positive on US or in whom metastases could not be excluded.
Studies employed mainly B‐mode ultrasound, with two studies also employing Doppler ultrasound in all participants (Hinz 2011; Voit 2014). B‐mode ultrasound frequencies were variable, mainly ranging from 5 or 6 MHz to 10 or 12 MHz in each study, apart from Voit 2014, which used three transducers ranging from 1 to 18 MHz in frequency. Ultrasound was performed before lymphoscintigraphy in five studies (Chai 2012; Hafner 2004; Hinz 2011; Hinz 2013; Sibon 2007), after lymphoscintigraphy in two studies (Sanki 2009; Voit 2014), and both before and after lymphoscintigraphy in four studies (Hocevar 2004; Kunte 2009; Radzhabova 2009; van Rijk 2006). Lymph node basins were imaged according to the site of the primary melanoma (Chai 2012; Hafner 2004; Hinz 2011; Hinz 2013; Sibon 2007; Voit 2014), according to the site marked following lymphoscintigraphy (Sanki 2009; Voit 2014), or this information was not reported (Hocevar 2004; Kunte 2009; Radzhabova 2009; van Rijk 2006). Criteria for detection of nodal metastases were clearly described in all studies apart from Hafner 2004 (Appendix 8). Ultrasound was reported to be performed by dermatologists (Kunte 2009), sonographers (Voit 2014), radiologists (Hafner 2004; Hocevar 2004; Sibon 2007), or nuclear medicine physicians (Sanki 2009), or this was not reported.
PET‐CT. Of the eight studies of PET‐CT before SLNB, four were conducted in any participant eligible for SLNB (Hinz 2013; Kell 2007; Klode 2010; Singh 2008); one in those with head and neck melanoma (Revel 2010); and three in higher‐risk melanoma populations (Arrangoiz 2012; Maubec 2007; Wagner 2012). Singh 2008 also reported data for the subgroup of participants with higher‐risk melanoma (Breslow thickness > 4 mm). When reported (n = 3), studies employed two‐dimensional (2D) PET (Wagner 2012), three‐dimensional (3D) PET (Maubec 2007), or either 2D or 3D PET (Arrangoiz 2012). PET was combined with unenhanced ‐ in Arrangoiz 2012,Kell 2007, and Maubec 2007 ‐ or contrast‐enhanced ‐ in Hinz 2013,Klode 2010, and Singh 2008 ‐ CT scans (use of contrast not reported in Revel 2010 and Wagner 2012). When reported, CT was used for attenuation correction (Arrangoiz 2012; Hinz 2013; Revel 2010; Singh 2008; Wagner 2012), as well as for anatomical localisation (Revel 2010; Wagner 2012).
Criteria for the detection of nodal metastases were not reported in Hinz 2013, were based on a qualitative assessment of increased 18FDG uptake in six studies (Kell 2007; Klode 2010; Maubec 2007; Revel 2010; Singh 2008; Wagner 2012), and were based on a quantitative assessment of focal uptake in Arrangoiz 2012 (SUV ≥ 2.5) (see Appendix 8). Performance and interpretation of PET‐CT were not clearly described. For example, Wagner 2012 reported interpretation by a nuclear medicine specialist, while two others mentioned an in‐house medical physicist ‐ Singh 2008 ‐ and a team of radiologists and nuclear physicians ‐ Arrangoiz 2012. Only Revel 2010 and Wagner 2012 reported the provision of clinical or other radiological findings to assist PET‐CT interpretation.
Reference standard. Ten studies (56%) evaluated the accuracy of imaging in comparison to histology from SLNB alone (Hinz 2011; Hinz 2013; Kell 2007; Klode 2010; Kunte 2009; Radzhabova 2009; Revel 2010; Sanki 2009; Sibon 2007; Singh 2008), seven studies (39%) included histology results from participants proceeding directly to CLND as well as SLNB results as a reference standard (Chai 2012; Hafner 2004; Hocevar 2004; Maubec 2007; van Rijk 2006; Voit 2014; Wagner 2012), and one study reported only data for histology based on CLND or SLNB combined with follow‐up to determine any false negative results on PET‐CT as the reference standard (Arrangoiz 2012).
Participant exclusions. Five studies reported the exclusion of between two and eight participants primarily due to technical failure of SLNB (sentinel node not identified or SLNB not performed) (Chai 2012; Hafner 2004; Maubec 2007; Revel 2010; Wagner 2012), and in one study (Radzhabova 2009), 100 participants did not undergo SLNB on the basis of a negative ultrasound finding.
Results: ultrasound for detection of nodal metastases
Across the 11 ultrasound evaluations, sensitivity for detection of nodal metastasis in comparison to a histological reference standard (SLNB or LCND) ranged from 0% in Hafner 2004 to 33% in Chai 2012 and Kunte 2009 in eight studies, and from 71% in Hocevar 2004 and Voit 2014 to 100% in Radzhabova 2009 in three. Specificity ranged from 73% in Voit 2014 to 100% in Kunte 2009,Hinz 2011,Hinz 2013, and Radzhabova 2009) (Figure 6). Radzhabova 2009 included a highly selected group of study participants, which likely explains the perfect sensitivity and specificity observed. The particularly low sensitivity in Hafner 2004 (0%) may be related to the use of only a 5‐MHz ultrasound transducer, but this study was poorly reported and other explanations may be possible. The relatively high sensitivities (both 71%) in Hocevar 2004 and Voit 2014 are also difficult to explain based on the information reported. In terms of specificity, Kunte 2009, Hinz 2011, and Hinz 2013 all applied ultrasound before and after the use of lymphoscintigraphy, which is likely to have contributed to the 100% specificity observed.
The summary sensitivity of ultrasound across the 11 studies was 35.4% (95% CI 17.0% to 59.4%) and summary specificity was 93.9% (86.1% to 97.5%) for 2614 participants and 542 confirmed cases of nodal metastasis (Table 3; Figure 7).
The three studies that reported the accuracy of ultrasound combined with FNAC reported decreased sensitivity but increased specificity in comparison to ultrasound alone. Sensitivities ranged from 3% (95% CI 0% to 14%) in van Rijk 2006 to 51% (95% CI 44% to 58%) in Voit 2014, and specificities ranged from 99% (95% CI 92% to 100%) in van Rijk 2006 to 100% (95% CI 99% to 100%) in Voit 2014 (Figure 6). The summary sensitivity was 18.0% (95% CI 3.58% to 56.5%), and summary specificity was 99.8% (95% CI 99.1% to 99.9%), based on 1164 participants and 259 cases (Table 3; Figure 7).
Results: PET‐CT for detection of nodal metastases
The four studies comparing PET‐CT to histology based on SLNB in standard SLNB populations reported sensitivities ranging from 0% (95% CI 0% to 26%) in Hinz 2013 to 22% (95% CI 3% to 60%) in Kell 2007, and specificities from 89% (95% CI 72% to 98%) in Kell 2007 to 100% (95% CI 63% to 100%) in Hinz 2013 and 100% (95% CI 92% to 100%) in Klode 2010 (Figure 6). The summary sensitivity was 10.2% (95% CI 4.31% to 22.3%) and summary specificity was 96.5% (95% CI 87.1% to 99.1%) for 170 participants and 49 confirmed cases of nodal metastasis (Table 3; Figure 7).
Data from the three studies in higher‐risk melanoma populations (75 participants with 28 cases) that compared PET‐CT to histology based on SLNB alone could not be pooled because of substantial heterogeneity likely resulting from small sample sizes (Maubec 2007; Singh 2008; Wagner 2012). Sensitivities ranged from 0% (95% CI 0% to 41%) in Maubec 2007 to 43% (95% CI 18% to 71%) in Wagner 2012, and specificities from 92% (95% CI 64% to 100%) in Maubec 2007 to 100% (95% CI 48% to 100%) in Singh 2008 and 100% (95% CI 88% to 100%) in Wagner 2012 (Figure 6).
One of these studies ‐ Maubec 2007 ‐ and Arrangoiz 2012 reported data for PET‐CT compared to histology based on SLNB plus follow‐up to identify false negatives. Maubec 2007 identified one additional false negative result on follow‐up, but sensitivity (0%) and specificity (92%) remained the same with marginal changes to CIs (95% CI 0% to 37% for sensitivity and 62% to 100% for specificity). Arrangoiz 2012 reported sensitivity of PET‐CT as 41% (95% CI 24% to 61%) and specificity as 89% (95% CI 71% to 98%) (Figure 6).
Revel 2010 reported the sensitivity of PET‐CT as 20% (95% CI 3% to 56%) and 100% (95% CI 69% to 100%) for 20 participants with head and neck melanoma when compared to SLNB alone as a reference standard. Adding data for a follow‐up reference standard identified two additional nodal metastases missed on PET‐CT for sensitivity of 17% (95% CI 2% to 48%) and specificity of 100% (95% CI 69% to 100%) (Figure 6).
Results: comparison between tests
Upon comparison of ultrasound alone, ultrasound plus FNAC, and PET‐CT, summary sensitivities were not statistically significantly different (P = 0.07), and summary specificities were significantly higher for ultrasound plus FNAC compared to the other two modalities (P < 0.001) (Table 3; Figure 7).
The direct comparison of ultrasound alone versus ultrasound plus FNAC suggested higher sensitivity (58.7%, 95% CI 36.5% to 77.9%) but lower specificity (79.4%, 95% CI 70.0% to 86.4%) (3 studies; 1164 participants; 259 cases of nodal metastases) for ultrasound alone compared to the overall pooled result. Requiring both ultrasound and FNAC to be positive for nodal metastases (as an indicator for CLND instead of SLNB) reduced sensitivity by 40.7% (95% CI 6.50% to 75.0%; P = 0.02) but increased specificity by 20.4% (95% CI 12.2% to 28.6%; P < 0.001) (Table 3).
2. Whole body imaging for detection of any metastases, nodal metastases, and distant metastases
Twenty‐four studies evaluated whole body imaging. Summary characteristics of all studies are tabulated alphabetically in Appendix 9, and a narrative description is provided in Appendix 10. Results are presented below according to the population group studied, the target condition and imaging test, and the presentation of data per patient and per lesion.
Primary staging
Six studies recruited participants undergoing primary staging following a confirmed diagnosis of melanoma (Arrangoiz 2012; Hafner 2004; Kang 2011; Maubec 2007; Prayer 1990; Veit‐Haibach 2009). Two studies included any participant following diagnosis of melanoma (Kang 2011; Veit‐Haibach 2009); two excluded those with distant metastases on diagnosis (Hafner 2004; Maubec 2007). Maubec 2007 restricted data to those with melanomas at least 4 mm in thickness; one study included clinically node positive participants but did not report exclusion of those with distant metastases (Prayer 1990), and one included only clinically node negative participants with melanomas of at least 4 mm Breslow thickness (Arrangoiz 2012). Three studies also reported data for pre‐SLNB imaging (Arrangoiz 2012; Hafner 2004; Maubec 2007), two of which reported subgroup data for clinically node negative participants who underwent SLNB (Hafner 2004; Maubec 2007). All six studies reported accuracy data on a per patient basis; no per lesion data were identified.
Forest plots of all available study data are presented in Figure 8. Summary details of all studies in this section are presented alphabetically in Appendix 9. Sensitivities and specificities from studies evaluating more than one target condition (any metastasis, nodal metastasis, or distant metastasis) are tabulated in Appendix 11.
8.
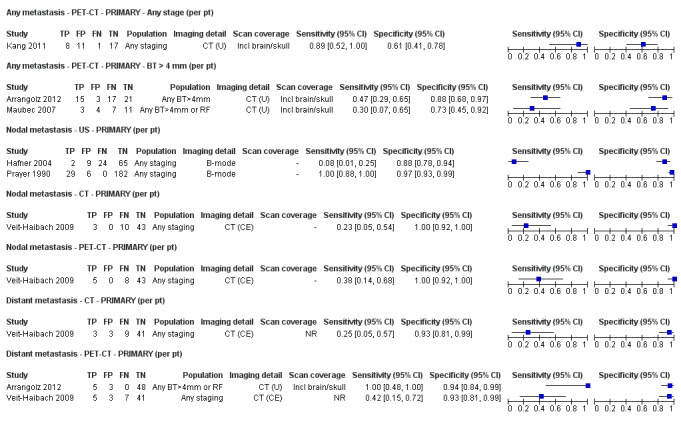
Forest plot of imaging for primary staging, for the detection of any metastases, nodal metastases, and distant metastases (per patient and per lesion data).
Results: detection of any metastases
Three studies presented data for detection of any metastasis in 118 study participants with 51 cases of metastatic disease (Figure 8) (Arrangoiz 2012; Kang 2011; Maubec 2007); the prevalence of metastases ranged from 24% in Kang 2011 to 57% in Arrangoiz 2012.
CT. No data on CT were identified for participants undergoing primary staging of melanoma.
MRI. No data on MRI were identified for participants undergoing primary staging of melanoma.
PET‐CT. Three studies presented per patient data for PET‐CT for detection of any metastasis; no per lesion data were identified.
Two studies evaluated PET‐CT in participants with melanomas ≥ 4 mm in thickness: one reported sensitivity and specificity for detection of any metastases of 47% (95% CI 29% to 65%) and 88% (95% CI 68% to 97%) (56 participants; 32 cases of metastatic disease) (Arrangoiz 2012); and in the other (Maubec 2007), sensitivity was 30% (95% CI 7% to 65%) and specificity 73% (95% CI 45% to 92%) (25 participants; 10 cases of metastatic disease) (Figure 8). Arrangoiz 2012 identified four patients with distant metastases whose disease would have been missed without PET‐CT imaging (prevalence of distant metastases 4/56; 7%). In the other study (Maubec 2007), no distant metastases were identified, but all three false positive results suggested possible distant metastases.
The third study evaluated PET‐CT for the prediction of subsequent recurrence in any participant following diagnosis of melanoma (Kang 2011). Stage of disease on presentation was reported as stage 0 (n = 7), stage I or II (n = 23), stage III (n = 6), and stage IV (n = 1); all patients underwent curative surgery for primary and metastatic lesions. The sensitivity of PET‐CT for the prediction of later recurrence at an SUVmax ≥ 2.2 at baseline was 89% (95% CI 52% to 100%) and specificity 61% (95% CI 41% to 78%) (37 participants; nine cases of metastatic disease) (Figure 8). The accuracy of PET‐CT for initial staging was not reported. Three of the nine patients who developed recurrence during follow‐up were stage III or greater at presentation.
Results: detection of nodal metastases
Three studies presented data for the detection of nodal metastases in 373 participants with 68 cases of nodal metastases (Hafner 2004; Prayer 1990; Veit‐Haibach 2009) (Figure 8); the prevalence of nodal metastases ranged from 13% in Prayer 1990 to 26% in Hafner 2004.
Ultrasound. Two studies evaluated the use of ultrasound in any participant following the diagnosis of melanoma, including those who were clinically node positive (Hafner 2004; Prayer 1990). Hafner 2004 restricted inclusion to those with melanomas ≥ 1 mm in thickness, and all underwent SLNB including three with clinically detectable nodal metastases (data for clinically node negative are reported in the pre‐SLNB imaging section above). The sensitivity of ultrasound was 8% (95% CI 1% to 25%) and specificity 88% (95% CI 78% to 94%) (100 participants, 26 with nodal metastases) (Figure 8); the only true positive results were both detected on physical examination. In Prayer 1990, the sensitivity of ultrasound was 100% (95% CI 88% to 100%) and specificity 97% (95% CI 93% to 99%) (217 participants, 29 with nodal metastases) (Figure 8). These results are likely to be influenced by incorporation bias and inadequate follow‐up to identify false negatives on ultrasound. Of 217 included participants, 15% (35/217) had suspicious findings on palpation; however, among these, only those who were also found to have suspicious nodes on ultrasound had histological verification (n = 15). This left 17 who were positive on palpation but negative on ultrasound, along with 165 who were negative on both palpation and ultrasound, with a six‐month follow‐up reference standard.
Other imaging tests. One study presented data comparing CT with PET‐CT for the detection of nodal metastases in all participants referred for PET‐CT after primary melanoma resection (Veit‐Haibach 2009). No false positive results were obtained with either test (specificity 100%, 95% CI 92% to 100%), but sensitivity was higher for PET‐CT (38%, 95% CI 14% to 68%) compared to CT (23%, 95% CI 5% to 54%) (56 participants, 13 with nodal metastases) (Figure 8). Initial staging procedures including histology of the primary lesion, SLNB, and all imaging procedures apart from PET‐CT identified four of the 13 participants with nodal metastases, two of whom were identified only on SLNB and were missed by all imaging tests (Veit‐Haibach 2009). Both CT and PET‐CT correctly identified additional participants with nodal metastases (one and three, respectively) that were not picked up by other staging procedures at the time of melanoma diagnosis.
No data on MRI to detect nodal disease were identified for participants undergoing primary staging of melanoma.
Results: detection of distant metastases
Two studies presented data for the detection of distant metastases in 112 participants with 17 cases of distant metastases (Arrangoiz 2012; Veit‐Haibach 2009) (Figure 8); the prevalence of distant metastases was 9% in Arrangoiz 2012 and 21% in Veit‐Haibach 2009.
CT. One study presented data for CT: sensitivity for the detection of 12 distant metastases was 25% (95% CI 5% to 57%) and specificity 93% (95% CI 81% to 99%) (56 participants) (Veit‐Haibach 2009).
MRI. No per patient data on MRI were identified for participants undergoing primary staging of melanoma.
PET‐CT.Veit‐Haibach 2009 provided a direct comparison of CT with PET‐CT; two additional cases of distant metastases were detected in comparison to CT (sensitivity 42%, 95% CI 15% to 72%), with no difference in specificity (93%, 95% CI 81% to 99%) (Figure 8).
Arrangoiz 2012 evaluated the use of PET‐CT in clinically node negative participants with primary melanoma greater than 4 mm in thickness and no indications for distant metastases; all five distant metastases were detected by PET‐CT (sensitivity 100%, 95% CI 48% to 100%), with three false positive results (specificity 94%, 95% CI 84% to 99%) (56 participants).
Results: detection of distant metastases by metastatic site
No data were identified for the detection of metastatic disease according to metastatic site in participants undergoing imaging for primary staging of melanoma.
In three of six studies, scan coverage was reported to include the skull (Arrangoiz 2012; Kang 2011; Maubec 2007), but the detection of brain metastases was not separately documented.
Re‐staging
Three studies recruited participants undergoing imaging for re‐staging of disease following a clinical indication of recurrence (Iagaru 2007; Rubaltelli 2011; Strobel 2007a). One study included any participant having imaging for re‐staging purposes (Iagaru 2007), and two included clinically node negative participants either undergoing ultrasound of the regional lymph nodes as part of a follow‐up program (Rubaltelli 2011), or with raised serum S100 (> 0.2 μg/L) during follow‐up (Strobel 2007a).
Forest plots of all available study data are presented in Figure 9. Summary estimates of sensitivity and specificity are presented in Table 3. Summary details of all studies in this section are presented alphabetically in Appendix 9.
9.

Forest plot of imaging for re‐staging of melanoma, for the detection of any metastases or nodal metastases (per patient and per lesion data).
Results: detection of any metastases
Two studies provided per patient data for the detection of any metastasis in 153 participants with 95 cases of metastatic disease (Figure 9); the prevalence of any metastasis was 53% in Iagaru 2007 and 83% in Strobel 2007a.
CT. In one study, the sensitivity of CT for detection of any metastasis on a per patient basis was 68% (95% CI 54% to 80%) and specificity 94% (95% CI 83% to 99%) (106 participants; 56 cases of metastatic disease) (Iagaru 2007).
MRI. No data on MRI were identified for participants undergoing re‐staging of melanoma.
PET‐CT. Two studies evaluated PET‐CT on a per‐patient basis in 153 participants, 95 of whom had confirmed metastases (Iagaru 2007; Strobel 2007a); summary sensitivity was 92.6% (95% CI 85.3% to 96.4%) and specificity 89.7% (95% CI 78.8% to 95.3%) (Table 3).
Comparison of PET‐CT with CT in Iagaru 2007 demonstrated PET‐CT to be more sensitive (89%, 95% CI 78% to 96%) than CT alone (increase of 21%), with similar specificity (88%, 95% CI 76% to 95%). Similar results were observed on a per lesion basis (Figure 9). Although numbers were small, PET‐CT was more sensitive in the subgroup with stage IIIc to IV disease who underwent PET‐CT for re‐staging after therapy (100%, 95% CI 81% to 100%) (n = 32; 18 with metastatic disease) than in those with less advanced disease (84%, 95% CI 69% to 94%).
Results: detection of nodal metastases
One study presented per patient data for the detection of nodal recurrence after primary treatment in 460 participants with 37 cases of nodal metastases (prevalence 8%) (Rubaltelli 2011) (Figure 9).
Ultrasound. Considering participants with 'common signs of malignancy' or with focal hypoechoic cortical thickening as positive for metastases detected all participants with nodal metastases (sensitivity 100%, 95% CI 91% to 100%) with a specificity of 93% (95% CI 90% to 95%) (460 participants, 37 with nodal metastases) (Rubaltelli 2011). The combination of contrast‐enhanced ultrasound with B‐mode ultrasound for participants with focal cortical thickening (presence of perfusion defects corresponding to the cortical focal thickening required for a positive test result) increased specificity to 100% (95% CI 98% to 100%).
Other imaging tests. No data on CT, MRI, or PET‐CT for the detection of nodal metastases were identified for participants undergoing re‐staging for recurrence of melanoma.
Results: detection of distant metastases
No data were identified for the detection of distant metastases in participants undergoing re‐staging for disease recurrence.
Results: detection of distant metastases by metastatic site
Two of three studies conducted in participants undergoing imaging for re‐staging of melanoma included imaging of the brain and documented some results for the detection of brain metastases.
In Iagaru 2007, one of the nine lesions classified as a false negative on PET‐CT was a brain lesion that was identified by MRI during follow‐up; the total number of brain metastases identified in the study was not reported.
In Strobel 2007a, two brain metastases were identified on PET‐CT, both of which were confirmed to be malignant on the reference standard.
3. Staging in mixed or not clearly described populations
Studies in mixed and not clearly described populations have been considered together on the basis that we would be unable to make clear statements regarding the expected accuracy of imaging at any particular point on the clinical pathway for either subset of studies. Table 4 describes variability in the clinical pathway and indications for imaging, inclusion criteria, and stage of disease of participants included in these studies.
3. Characteristics of studies conducted in mixed or unclear population groups.
|
Study Population group |
Participant inclusion criteria and reported indications for imaging | Stage of disease on presentation | Imaging tests |
Patients/cases (prevalence) [lesions/metastases (prevalence)] |
Average no. metastases per patient |
| PER PATIENT DATA | |||||
|
Abbott 2011 Mixed – primary or follow‐up |
Undergoing FU after prior SLNB/CLND for micro‐metastases or presenting with clinically detectable nodal disease at or subsequent to initial diagnosis | Stage: IIIA 18, 53% IIIB 10, 29% IIIC 6, 18% |
PET‐CT (NR) | 34/7 (21%) | N/A |
|
Aukema 2010a Mixed – primary or re‐staging |
Asymptomatic S100 positive. Previously treated for locoregional recurrence (n = 15) or distant metastases (n = 5); or with unfavourable primary tumour (n = 6), primary melanoma with simultaneous nodal metastases (n = 20) | Any (stage NR) | PET‐CT (U) | 46/23 (50%) | N/A |
|
Aukema 2010b Unclear |
Palpable and pathology proven lymph node metastases and no signs of distant metastases. Imaging to identify further ‘undetected’ disease | Stage III: 100% | PET‐CT (U) | 70/30 (43%) | N/A |
|
Bastiaannet 2009 Mixed – primary or re‐staging |
Node positive (clinical or histology/cytology proven) candidates for CLND; imaging to identify further disease. Includes those with LN mets diagnosed at time of primary diagnosis 39, 15.5%; LN metastases identified ≤ 3 years since primary diagnosis 145, 57.8%; recurrence > 3 years since primary diagnosis 67, 26.7% | Stage III (100%) | CT (CE) | 251/78 (31%) distant metastases | N/A |
|
Cachin 2014 Mixed ‐ staging or re‐staging |
Any primary MM, visceral metastases, or cutaneous metastases from unknown primary | Any; 51% with metastases | PET‐CT (NR) | 67/39 (58%) [176/85 (48%)] |
N/A |
|
Klebl 2003 Mixed |
Clark level IV or V undergoing FU after primary surgery. Reports primary (n = 8) and imaging during follow‐up (n = 75) | Any (NR) | US | 79/17 (22%) nodal | N/A |
|
Reinhardt 2006 Mixed – primary, re‐staging, FU, disease response |
All with PET‐CT for primary staging after sentinel node biopsy (n = 75); therapy control after chemotherapy of metastatic disease (n = 42); staging of clinically suspected recurrent disease (n = 65); during follow‐up within 5 years of primary treatment (n = 68) | Stage I 22, 9% Stage II 88, 35% Stage III 108, 43% Stage IV 32, 13% |
CT (CE) PET‐CT (CE) |
250/116 (46%) | N/A |
|
Strobel 2007b Unclear |
High risk melanoma (BT > 4 mm, or Clark level III or IV, or known resected metastases) with PET‐CT for depiction or exclusion of metastases | Any (NR) | PET‐CT (CE) | 124/53 (43%) | N/A |
|
van den Brekel 1998 Mixed ‐ primary and recurrence |
Head and neck MM with CT before neck dissection, including therapeutic and elective (negative on palpation). "Interval between the treatment of the primary and the neck dissection ranged from 0 to 8.8 years (mean: 21 months)" | Stage I to II 8, 31% Stage III 18, 69% |
CT (CE) | 26/21 (81%) nodal | N/A |
|
van Wissen 2016 Mixed ‐ primary and recurrence |
Stage IIIB or IIIC MM with palpable groin metastases; selected for therapeutic combined groin dissection. Discussion states: "large proportion of our patients were initially treated for their primary tumour at other hospitals, and sometimes years prior to the current groin dissection" | All stage IIIB and C | PET‐CT (U) | 69/59 (superficial nodes 86%) 67/24 (deep nodes 36%) |
N/A |
| PER LESION DATA | |||||
|
Cachin 2014 Mixed ‐ staging or re‐staging |
Any primary MM, visceral metastases, or cutaneous metastases from unknown primary. Lesions with equivocal focal uptake considered test positive Only 1 eligible index test |
Any: 51% with metastases | CT (NR) | 67/39 (58%) [176/85 (48%)] |
1 (85/67) |
|
Dellestable 2011 Mixed ‐ primary or follow‐up |
All with PET‐CT regardless of AJCC stage or indication for examination Number of lesions included varies per test |
Stage I to II: 27.5% Stage III to IV: 72.5% |
CT (CE) MRI (DW) PET‐CT |
40 [108/66 (61%)] [117/70 (60%)] [119/72 (61%)] |
2 (72/40) |
|
Hausmann 2011 Unclear |
AJCC stage III or IV with positive SLNB or suspicious lesions on ultrasound or X‐ray studies Number of lesions included same per test |
Stage III 38% Stage IV 62% |
CT (CE) MRI (NR) |
33 All tests [824/455 (55%)] |
14 (455/33) |
|
Jouvet 2014 Unclear |
AJCC stage IV. Number of lesions included varies per test |
Stage IV: 100% | CT (CE) MRI (DW) MRI (DW + ultrafast GE) PET‐CT |
37 (218 lesions) [209/115 (55%)] [218/125 (57%)] [191/104 excl brain (54%)] |
3 (125/37) |
|
Pfannenberg 2007 Mixed – incl primary, FU, and NR |
Stage III or IV imaged before surgery due to abnormal radiological, clinical, and laboratory findings, or routine surveillance in high risk Number of lesions included same per test |
Stage III: 39% Stage IV: 61% |
CT (CE) MRI (DW + ultrafast GE) PET‐CT |
64 All tests [420/297 (71%)] |
5 (297/64) |
|
Pfluger 2011 Mixed ‐ primary or follow‐up |
Melanoma with regional lymph node metastases; excluded any lesions newly arising during follow‐up Number of lesions included same per test |
Stage III: 100% | CT (CE); CT (U) PET‐CT (CE); (U) |
50 All tests [232/151 (65%)] |
3 (151/50) |
AJCC: American Joint Cancer Committee; BT: Breslow thickness; CE: contrast enhanced; CLND: complete lymph node dissection; CT: computed tomography; DW: diffusion weighted; FNAC: fine needle aspiration cytology; FU: follow‐up; GE: gradient echo; HN: head and neck; LN: lymph node; MM: malignant melanoma; MRI: magnetic resonance imaging; mm: millimetre; N+: node positive; N/A: not applicable; NR: not reported; PET: positron emission tomography; SLNB: sentinel lymph node biopsy; U: unenhanced; US: ultrasound; VIBE: MRI sequence.
Fifteen studies were conducted in mixed population groups (n = 11), including participants undergoing primary staging, re‐staging, and follow‐up imaging (i.e. at more than one point in the clinical pathway) (Abbott 2011; Aukema 2010a; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Klebl 2003; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; van den Brekel 1998; van Wissen 2016), or did not clearly describe the clinical pathway in included participants (n = 4) (Aukema 2010b; Hausmann 2011; Jouvet 2014; Strobel 2007b).
Stage of disease on recruitment was not reported in four studies (Aukema 2010a; Cachin 2014; Klebl 2003; Strobel 2007b), two studies included any stage of disease (with 56% (Reinhardt 2006) and 73% (Dellestable 2011) at stage III or stage IV), six included only stage III melanoma (Abbott 2011; Aukema 2010b; Bastiaannet 2009; Pfluger 2011; van den Brekel 1998; van Wissen 2016), one included stage IV only (Jouvet 2014), and two included either stage III or IV melanoma (both with just under 40% with stage III disease) (Hausmann 2011; Pfannenberg 2007).
Nine of the fifteen studies reported accuracy data only on a per patient basis (Abbott 2011; Aukema 2010a; Aukema 2010b; Bastiaannet 2009; Klebl 2003; Reinhardt 2006; Strobel 2007b; van den Brekel 1998; van Wissen 2016), five reported data per lesion (Dellestable 2011; Hausmann 2011; Jouvet 2014; Pfannenberg 2007; Pfluger 2011), and one reported both per patient and per lesion data (Cachin 2014). Of those reporting per lesion data, two studies reported accuracy data only for those imaging abnormalities identified by each test (Dellestable 2011; Jouvet 2014), such that comparative studies reported different numbers of lesions and confirmed metastases per index test evaluated (Dellestable 2011; Jouvet 2014). Three studies included all lesions detected by any index test so that the number of lesions included in each 2×2 contingency table was the same for every test (Hausmann 2011; Pfannenberg 2007; Pfluger 2011); two included all lesions considered suspicious by any one index test (Hausmann 2011; Pfannenberg 2007); and one reported including only lesions considered positive for melanoma on at least one index test (Pfluger 2011). Variation in lesion inclusion has the potential to reduce sensitivity in studies that included all lesions detected by any index test, as any metastases missed by any one test would count as false negative results in the contingency table; any missed benign lesions would be considered true negative results, but a large number of lesions would need to be missed to have any detectable effect on specificity.
The considerable clinical heterogeneity between studies in terms of population groups, stages of disease, lesion selection, differences between tests, and definitions of target conditions (either including or excluding imaging of the brain) means that no conclusions can be drawn from studies in mixed and not clearly described populations (Table 4; Appendix 9). Study results are therefore described narratively in Appendix 12.
Discussion
Summary of main results
There is a considerable volume of literature evaluating the accuracy of imaging tests for staging of melanoma, but other than the specific use of imaging to identify nodal metastases before sentinel lymph node biopsy (SLNB), only a small number of identified studies were eligible for our review and were conducted in well‐defined populations of participants undergoing primary staging or re‐staging for disease recurrence. In terms of methodological quality, studies were generally at low or unclear risk of bias, with methods of participant selection and blinded case note review particularly poorly reported. Studies were of high or unclear concern regarding the applicability of evidence due to participant selection from mixed or not clearly defined populations, poorly described application and interpretation of the index test or observer expertise, and lack of definition of the target condition (e.g. including or excluding the detection of metastases of the brain, no breakdown of cases according to nodal or distant metastases). Because few studies compared eligible tests and because available data for magnetic resonance imaging (MRI) were limited and information regarding the stage of disease at diagnosis was lacking, we could not fully answer the review question.
Four main findings can be drawn from our review.
1. Pre‐SLNB ultrasound combined with fine needle aspiration cytology (FNAC) allows around a fifth of patients with nodal metastases to be identified with few false positive results.
Half of all included studies (18/39) considered the use of imaging before SLNB to identify people with nodal metastases, particularly the use of ultrasound with or without FNAC (n = 11). Study populations were well defined and included people likely to be considered eligible for SLNB in routine practice. Studies primarily used B‐mode ultrasound, although two also used Doppler ultrasound in all participants, and the use of ultrasound before or after lymphoscintigraphy to identify sentinel nodes varied.
Table 1 presents key results and translates summary estimates to a hypothetical cohort of 1000 lesions. Given that completion lymphadenectomy (CLND) is no longer a standard of care for patients who are eligible for SLNB, the previously postulated benefit from ultrasound and FNAC of triaging those with nodal metastases on cytology directly to CLND is no longer relevant. We have therefore framed the potential benefit from ultrasound and FNAC in terms of access to adjuvant therapy, but any benefit would be incurred only if a result from imaging and cytology could be obtained significantly more quickly than an SLNB result, or, if SLNB was not available or was contraindicated.
All imaging tests had poor sensitivity, detecting at best a third of people with nodal metastases that were not clinically detectable (sensitivity of 35.4% for ultrasound alone); however, all summary specificities were higher than 90%.
With use of the median prevalence of nodal metastases observed across the 11 studies of ultrasound, a test sensitivity of 35.4% would correctly identify 84 of 237 people with nodal metastases but with 47 false positive results, or people who would be incorrectly considered for adjuvant therapy. Combining ultrasound with FNAC, such that only those positive on both ultrasound and subsequent FNAC would be considered to have nodal disease (i.e. a more narrowly defined threshold for test positivity), reduces by 41 the number of people with nodal metastases who are correctly identified (from 84 to 43) but also reduces to two the number with false positive results. In other words, for every 1000 people eligible for SLNB, ultrasound with FNAC has the potential to allow around a fifth of those with nodal metastases to be considered for adjuvant therapy without the need for a more invasive procedure (SLNB), at a cost of two people being inappropriately managed (false negatives).
However, considerable between‐study differences were observed, such that the number of people with false positive results could range between one and seven, and the number of people with false negative results could range between 8 and 134. Results were also dominated by a single large study of 1000 participants from an expert group (Voit 2014), and it is difficult to determine whether these results could be replicated.
2. Limited test accuracy data were available for whole body imaging via positron emission tomography‐computed tomography (PET‐CT) for primary staging or re‐staging for disease recurrence and none evaluated MRI.
Of 24 studies meeting the inclusion criteria for this review, only six clearly recruited participants who were undergoing primary staging following a confirmed diagnosis of melanoma and three recruited participants undergoing imaging for re‐staging of disease following some clinical indication of recurrence. Most of the studies (6/9) considered PET‐CT, two in comparison to CT alone, and three studies examined the use of ultrasound. None of the studies included in these groups evaluated MRI. Observed sensitivities and specificities for the detection of any metastases for PET‐CT appeared to be higher for those having re‐staging of disease (summary sensitivity from two studies was 92.6% and specificity 89.7%) compared to primary staging (sensitivities ranged from 30% to 47% and specificities from 73% to 88%) and were more sensitive than CT alone in both population groups, but participant numbers were very small.
3. No conclusions can be drawn regarding routine imaging of the brain with either MRI or CT.
We excluded from this review a number of studies that reported data for ‘conventional imaging’ including CT or MRI because they did not have clearly defined imaging protocols whereby all included participants underwent both tests (Finkelstein 2004; Fuster 2004; Gulec 2003; Oehr 1999; Paquet 2000; Rinne 1998). Furthermore we identified no eligible studies reporting data for MRI in primary or re‐staging populations.
Of the studies conducted in mixed populations, scan coverage variably included the brain such that the definition of the target condition of any metastasis could either include or specifically exclude the detection of brain metastases. Generally speaking, studies were too small to include significant numbers of brain lesions. Only two studies in mixed population groups identified a sufficient number of brain metastases to allow sensitivities to be estimated. Jouvet 2014 showed CT with intravenous (IV) contrast and MRI with ultrafast gradient echo sequences to have sensitivities of 95% (CT) or more (100% for MRI) compared to 65% sensitivity for MRI without ultrafast gradient echo sequences. In Cachin 2014, PET‐CT detected one of seven confirmed metastases of the brain (sensitivity 14%, 95% CI 0% to 58%).
4. There are high concerns regarding the applicability of the evidence, although risk of bias is generally low.
Study quality was moderate in terms of risk of bias, and there are real concerns regarding the applicability of the evidence to the review question. Much of this concern is due to the inclusion of mixed and not clearly defined participant groups. There was a tendency to include participants based on the availability of results for a particular test, but more careful consideration of the indication for imaging is needed before the comparative accuracy of tests at different points on the clinical pathway can be established. Although there is an understandable temptation to translate results from mixed populations to a primary staging or re‐staging setting, there is at least some evidence that accuracy varies by pathway and in different ways for different tests (Reinhardt 2006), and this is supported by work in other fields (Leeflang 2013).
Further concerns around applicability relate to reporting of data per lesion as opposed to per patient, not only potentially impacting estimates of test sensitivity and specificity but making it more difficult to consider the implications of testing for patient management unless further information is provided in the papers. Although one might expect sensitivity to be inflated by per lesion data, effects on accuracy are not always clear cut. Cachin 2014 was one of the few studies reporting data both per patient and per lesion; both the sensitivity and specificity of PET‐CT were higher for data reported per patient (87% and 71%) compared to those reported per lesion (80% and 54%). The detection of additional metastatic lesions by any one test is of limited benefit if there is no resulting change in stage of disease classification or in patient management options. For example, in Pfluger 2011, the five lesions found to be false negative on unenhanced PET‐CT were all identified in patients with multiple metastases, such that there would have been no impact on TNM stage; on the other hand, all six false positive lesions were identified in otherwise metastasis‐free patients who were falsely upstaged from M0 to M1.
We also noted variations in the scan coverage between studies, which will impact the definition of the target condition, and limited information was provided on the thresholds used to identify the presence of metastases.
Strengths and weaknesses of the review
The strengths of this review include an in‐depth and comprehensive electronic literature search, systematic review methods including double extraction of papers by both clinicians and methodologists, and attempts to contact study authors to clarify data. A detailed and replicable analysis of methodological quality was undertaken and a clear analysis structure was adopted.
In comparison to other available systematic reviews of imaging tests (e.g. Catalano 2011; Danielsen 2014; El‐Maraghi 2008; Rodriguez 2014; Sadigh 2014; Schroer‐Gunther 2012; Xing 2011), our review covers a more recent search period and a broader review question, including both primary staging and re‐staging of melanoma, as opposed to one or the other (Danielsen 2014; Schroer‐Gunther 2012), and this review considers the comparative accuracy of different tests as opposed to reviewing a single test (as in all other reviews apart from Xing 2011). We have also separately considered data according to reporting of study data per patient as opposed to per lesion ‐ an approach not taken by any of the other identified systematic reviews.
Our stringent application of review inclusion criteria means that we excluded a considerable proportion of studies included in previous reviews. For example, across a selection of four reviews considering PET, we included only 3 of 12 (El‐Maraghi 2008), 7 of 12 (Xing 2011), 3 of 9 (Rodriguez 2014), and 1 of 7 studies included in those reviews (Danielsen 2014). We excluded studies on the basis of having evaluated PET alone rather than PET‐CT (Acland 2000; Acland 2001; Belhocine 2002; Fink 2004; Havenga 2003; Koskivuo 2007; Longo 2003; Nguyen 1999; Steinert 1998; Tyler 2000; Vereecken 2005; Wagner 1999; Wagner 2005), a combination of PET and PET‐CT, which could not be differentiated from each other (Horn 2006; Wagner 2011), use of PET for treatment response (Beasley 2012; Raymond 2011), use of an inadequate reference standard (e.g. minimum follow‐up period was not reported) (Peric 2011), inadequate sample size (Libberecht 2005), or inability to estimate the 2×2 data (Mottaghy 2007). We included all five PET‐CT studies included by Schroer‐Gunther and colleagues for primary staging of melanoma (Schroer‐Gunther 2012), but we considered two of the five to have been conducted in mixed rather than primary staging populations (Aukema 2010b; Strobel 2007b).
A similar picture was observed for other tests. We included only 7 of 22 studies of ultrasound and 4 of 13 studies of CT alone that were included by Xing 2011, 6 of 24 studies of ultrasound from Catalano 2011, and 2 of 8 studies of CT in Sadigh 2014. The most common reason for exclusion of studies of ultrasound from this review was the reporting of more than one ultrasound scan per patient (e.g. Binder 1997; Brountzos 2003; Schmid‐Wendtner 2003; Tregnaghi 1997; Voit 2001). For CT, it was the reporting of accuracy data for CT combined with other imaging tests such as MRI in Finkelstein 2004 and Fuster 2004 or reporting of more than one CT scan per patient in Sawyer 2009 and Swetter 2002, inadequate reference standards (Buzaid 1993; Buzaid 1995; Chomyn 1992; Holder 1998), or the inclusion of more than 10% of participants with non‐cutaneous melanoma (Brady 2006; Sofue 2012).
The main concerns for this review result from the poor reporting of primary studies, in particular, limiting assessment of studies according to clinical pathway, by stage of disease on diagnosis, and by varying definitions of the target condition. This review is also somewhat limited by the date of the last search (2016); however, imaging of melanoma has not been a particularly fast‐moving field (with only 7 of 39 included studies published in the five years before the search); furthermore, we are not aware of publication of any landmark studies in the interim period.
Applicability of findings to the review question
Varying definitions of eligible study populations and lack of clarity regarding the patient pathway and any prior testing restrict the extent to which our findings can be applied in the clinical setting.
Authors' conclusions
Implications for practice.
We identified a disappointing lack of evidence for imaging in populations of participants that were clearly defined according to the clinical pathway. Studies were generally small and reported data according to the number of lesions identified as opposed to the number of study participants included. We identified some evidence to suggest that imaging with ultrasound combined with fine needle aspiration cytology (FNAC) before sentinel lymph node biopsy (SLNB) may have a limited role, but further work is needed to identify whether the suggested benefit in terms of avoiding SLNB is cost‐effective. Much of the evidence for whole body imaging for primary staging or re‐staging of disease is focused on positron emission tomography‐computed tomography (PET‐CT), and comparative data with CT or magnetic resonance imaging (MRI) are lacking. Increasing availability of adjuvant therapies for melanoma is bound to have a considerable impact on imaging services, but the evidence base to support any increase in imaging for primary staging of disease is limited.
Implications for research.
Although there are challenges in designing studies that will remain relevant at the point of publication of findings in such a rapidly changing landscape, and despite potential limitations in service provision in terms of access to different imaging modalities, key questions remain around the most appropriate use of imaging tests for melanoma. In particular, studies need to go beyond diagnostic accuracy and must consider the effects of different imaging tests on patient management.
First, the role of ultrasound of the regional lymph nodes following a primary diagnosis of melanoma is unclear. When combined with FNAC, ultrasound has the potential to avoid SLNB in around a quarter of people with lymph node metastases, allowing them to proceed directly to adjuvant therapy; however, whether this approach would actually be implemented in clinical practice, and for which patients or at which centres of care, needs to be determined. An economic model using currently available data, potentially incorporating downstream consequences as more adjuvant therapies become available, would determine the circumstances under which this pathway saves resources and is worthwhile.
For whole body staging, comparative accuracy studies that incorporate changes to patient management as a result of imaging are needed both for those undergoing primary staging following a confirmed diagnosis of melanoma and for those undergoing re‐staging of disease on clinical suspicion of recurrence. For primary staging in particular, more clarity is needed regarding who should undergo whole body staging in terms of whether this should be restricted to those with confirmed stage III or IV disease (identified clinically or following SLNB), or whether there is a role for more widespread imaging in high‐risk groups, for example. A survey of current practice in the UK would be useful, to identify which imaging tests are being used in which patient groups across the country and why.
Studies should carry out blinded comparisons of routine imaging using contrast‐enhanced CT of the neck, chest, abdomen, and pelvis and contrast‐enhanced CT of the head, with full body 18FDG PET‐CT, and with post‐contrast whole body MRI, or MRI of the head alone, in all participants. The final diagnosis should be established by histology and with the implementation of a clearly defined imaging follow‐up schedule in all study participants. Study results in terms of accuracy should be reported per lesion and per patient and should be reported by metastatic site to allow an assessment of the success and failure of contrast‐enhanced CT and 18FDG PET‐CT in different areas of the body. Imaging of the brain with contrast‐enhanced CT versus MRI could also be performed.
It is essential that future research studies be clear about the diagnostic pathway followed by study participants, and they should conform to the updated Standards for Reporting of Diagnostic Accuracy (STARD) guideline (Bossuyt 2015).
Acknowledgements
Members of the Cochrane Skin Cancer Diagnostic Test Accuracy Group include the following.
The full project team (Susan Bayliss, Naomi Chuchu, Clare Davenport, Jonathan Deeks, Jacqueline Dinnes, Lavinia Ferrante di Ruffano, Kathie Godfrey, Rubeta Matin, Colette O'Sullivan, Yemisi Takwoingi, Hywel Williams).
Our 12 clinical reviewers (Rachel Abbott, Ben Aldridge, Oliver Bassett, Sue Ann Chan, Alana Durack, Monica Fawzy, Abha Gulati, Jacqui Moreau, Lopa Patel, Daniel Saleh, David Thompson, Kai Yuen Wong) and two methodologists (Lavinia Ferrante di Ruffano and Louise Johnston), who assisted with full‐text screening, data extraction, and quality assessment across the entire suite of reviews of diagnosis and staging and skin cancer.
Our expert advisor and co‐author Manil Subesinghe.
All members of our Advisory Group (Jonathan Bowling, Seau Tak Cheung, Colin Fleming, Matthew Gardiner, Abhilash Jain, Susan O'Connell, Pat Lawton, John Lear, Mariska Leeflang, Richard Motley, Paul Nathan, Julia Newton‐Bishop, Miranda Payne, Rachael Robinson, Simon Rodwell, Julia Schofield, Neil Shroff, Hamid Tehrani, Zoe Traill, Fiona Walter, Angela Webster).
We wish to thank Dr. Olga Sphadaruk for her assistance in assessing the eligibility of a Russian paper and members of the University of Birmingham Test Evaluation Research Group for assistance with data extraction and quality assessment for this review (Sophie Beese, Rita Champaneria, Bella Harris, John O'Rourke, Claire Smith).
The Cochrane Skin editorial base wishes to thank Robert Dellavalle, who was the Dermatology Editor for this review; the clinical referees Clare Barlow and Fergus Macbeth; the Cochrane Diagnostic Test Accuracy Editorial Team; and Dolores Matthews, who copy‐edited the review.
Appendices
Appendix 1. Current content and structure of the Programme Grant
| LIST OF REVIEWS | Number of studies | |
| Diagnosis of melanoma | ||
| 1 | Visual inspection | 49 |
| 2 | Dermoscopy ± visual inspection | 104 |
| 3 | Teledermatology | 22 |
| 4 | Smartphone applications | 2 |
| 5a | Computer‐aided diagnosis – dermoscopy‐based techniques | 42 |
| 5b | Computer‐aided diagnosis – spectroscopy‐based techniques | Review amalgamated into 5a |
| 6 | Reflectance confocal microscopy | 18 |
| 7 | High frequency ultrasound | 5 |
| Diagnosis of keratinocyte skin cancer (BCC and cSCC) | ||
| 8 | Visual inspection ± Dermoscopy | 24 |
| 5c | Computer‐aided diagnosis – dermoscopy‐based techniques | Review amalgamated into 5a |
| 5d | Computer‐aided diagnosis – spectroscopy‐based techniques | Review amalgamated into 5a |
| 9 | Optical coherence tomography | 5 |
| 10 | Reflectance confocal microscopy | 10 |
| 11 | Exfoliative cytology | 9 |
| Staging of melanoma | ||
| 12 | Imaging tests (ultrasound, CT, MRI, PET‐CT) | 39 |
| 13 | Sentinel lymph node biopsy | 155 |
| Staging of cSCC | ||
| 14 | Imaging tests review | Review dropped; only 1 study identified |
| 15 | Sentinel lymph node biopsy | Review amalgamated into 13 above (n = 15 studies) |
Appendix 2. Glossary of terms
| Term | Definition |
| Adjuvant therapy or treatment | A treatment given after the main treatment for cancer to reduce the risk of recurrence. |
| Adverse event | Detrimental change in health occurring in a person receiving the treatment whether or not it has been caused by the treatment. |
| Axillary | In the armpit. |
| Biopsy | Removal of a sample of tissue from the body to assist in diagnosis or inform the choice of treatment of a disease. |
| BRAF V600 mutation | BRAF is a human gene that makes a protein called B‐Raf, which is involved in the control of cell growth. BRAF mutations (damaged DNA) occur in around 40% of melanomas, which can then be treated with particular drugs. |
| BRAF inhibitors | Therapeutic agents that inhibit the serine‐threonine protein kinase BRAF mutated metastatic melanoma. |
| Breslow thickness | A scale for measuring the thickness of melanomas by the pathologist using a microscope, measured in mm from the top layer of skin to the bottom of the tumour. |
| Cervical (lymph nodes) | Lymph nodes found in the neck area of the body. |
| Computed tomography (CT) | Imaging technique in which the person lies on a table within an X‐ray gantry. The images are acquired using a spiral (helical) path and banks of detectors, allowing presentation of the internal organs and blood vessels in different projections including 3D views. |
| Coronal | Frontal plane dividing the body into front and back. |
| False negative | An individual who is truly positive for a disease, but whom a diagnostic test classifies as disease‐free. |
| False positive | An individual who is truly disease‐free, but whom a diagnostic test classifies as having the disease. |
| Histopathology | The study of tissue, usually obtained by biopsy or excision, for example under a microscope. |
| Incidence | The number of new cases of a disease in a given time period. |
| Inguinal | Lymph nodes in or just above or just below the groin. |
| Isolated limb perfusion | A medical procedure that directly delivers a drug through the bloodstream in a limb to the site affected by melanoma. |
| Local recurrence | Re‐growth of a tumour in the area from which it was originally removed. |
| Locoregional recurrence | Re‐growth of a tumour in the area from which it was originally removed or in the regional lymph nodes (usually nearest to the original tumour site). |
| Lymph node | Lymph nodes filter the lymphatic fluid (clear fluid containing white blood cells) that travels around the body to help fight disease; they are located throughout the body often in clusters (nodal basins). |
| Lymph node dissection | Surgical removal or 1 or more lymph nodes in the absence of proven involvement with melanoma. |
| Lymphadenectomy | Lymphadenectomy or lymph node dissection is a surgical operation to remove 1 or more groups of lymph nodes. |
| Lymphoscintigraphy | An imaging technique used to identify the lymph drainage basin, determine the number of sentinel nodes, differentiate sentinel nodes from subsequent nodes, locate the sentinel node in an unexpected location, and mark the sentinel node over the skin for biopsy. It requires the injection of a radioisotope into the skin around the biopsy scar and a scan some hours later to determine to which lymph nodes the tracer has travelled. |
| Lymphovascular invasion | Tumour cells that have spread to involve the blood vessels and lymphatic vessels within the skin. |
| Magnetic resonance imaging (MRI) | A type of scan that uses a magnetic field and radio waves to produce images of sections of the body. |
| Mediastinal and hilar adenopathy | Enlargement of the pulmonary lymph nodes. |
| MEK inhibitors | Drugs that inhibit the mitogen‐activated protein kinase enzymes, which are often upregulated in melanoma. |
| Meta‐analysis | A form of statistical analysis used to synthesise results from a collection of individual studies. |
| Metastases/metastatic disease | Spread of cancer away from the primary site to somewhere else through the bloodstream or the lymphatic system. |
| Micro‐metastases | Micro‐metastases are metastases so small that they can be seen only under a microscope. |
| Mitotic rate | Microscopic evaluation of the number of cells actively dividing in a tumour. |
| Morbidity | Detrimental effects on health. |
| Mortality | Either (1) the condition of being subject to death; or (2) the death rate, which reflects the number of deaths per unit of population in relation to any specific region, age group, disease, treatment, or other classification, usually expressed as deaths per 100, 1000, 10,000, or 100,000 people. |
| Multi‐disciplinary team | A team with members from different healthcare professions and specialties (e.g. urology, oncology, pathology, radiology, nursing). Cancer care in the National Health Service (NHS) uses this system to ensure that all relevant health professionals are engaged to discuss the best possible care for a patient. |
| Nodal basin | Cluster of lymph nodes that filter lymphatic fluid as it travels around the body; clusters are located under the arm (axilla) and in the groin, neck, chest, and abdomen. |
| Oncology | The study of cancers. This term also refers to the medical specialty of cancer care, with particular reference to the use of radiotherapy or drugs to treat cancer. The medical specialty is often split into clinical oncology (doctors who use radiotherapy and drug treatment) and medical oncology (doctors who use drug treatment). |
| Palpation | Feeling with the fingers or hands as part of a clinical examination of the body. |
| Positron emission tomography (PET) | A nuclear medicine imaging technique whereby a radioactive glucose (usually 18FDG) is administered intravenously before a scan is conducted to create an image using colours to show where the FDG (or other radioactive tracer) has been taken up in the body. |
| Prevalence | The proportion of a population found to have a condition. |
| Prognostic factors/indicators | Specific characteristics of a cancer or the person who has it that might affect the patient’s prognosis. |
| Radiotherapy | The use of radiation, usually high‐energy X‐rays, to control the growth of cancer cells. |
| RAS‐RAF‐MEK‐ERK signalling pathway | A chain of proteins that allow signals from a receptor on the surface of a cell to be sent to the DNA in the cell nucleus; a mutation in one of the proteins in the pathway is associated with the development of many cancers. |
| Recurrence | Recurrence occurs when new cancer cells are detected following treatment. This can occur either at the site of the original tumour or at other sites in the body. |
| Relapse | Where cancer starts to grow again after treatment. |
| Sagittal | Median plane dividing the body into left and right. |
| Sensitivity | In this context, the term is used to mean the proportion of individuals with a disease who have that disease correctly identified by the study test. |
| Sentinel lymph node biopsy (SLNB) | A radioactive tracer and blue dye are injected into the skin surrounding the primary lesion and the 'sentinel' lymph nodes to which the tracer drains are located by imaging (usually lymphoscintigraphy) and then are removed and examined for nodal metastatic spread that cannot be detected clinically or on imaging. |
| Signal transduction | Occurs when extracellular signalling molecules activate a specific receptor, which then triggers cellular pathways. |
| Staging | Clinical description of the size and spread of a patient’s tumour, fitting into internationally agreed categories. |
| Stereotactic radiotherapy | A technique for delivering high‐dose radiotherapy very accurately to small areas inside the body, which reduces damage done by radiotherapy to adjacent healthy tissues. |
| Subclinical (disease) | Disease that usually is asymptomatic and is not easily observable (e.g. by clinical or physical examination). |
| Systemic treatment | Treatment, usually given by mouth or by injection, that reaches and affects cancer cells throughout the body rather than targeting one specific area. |
| Ultrasound | A type of scan in which high‐frequency sound waves are used to outline a part of the body. |
Appendix 3. Table of acronyms
| Acronym | Definition |
| μm | micrometre |
| AK | actinic keratosis |
| ANN | artificial neural network |
| BCC | basal cell carcinoma |
| BD | Bowen’s disease |
| BPC | between‐person comparison (of tests) |
| CAD | computer‐assisted diagnosis |
| CCS | case‐control study |
| CS | case series |
| cSCC | cutaneous squamous cell carcinoma |
| D‐ | disease negative |
| D+ | disease positive |
| Derm–CAD | digital dermoscopy‐based computer‐assisted diagnosis |
| DF | dermatofibroma |
| DRS | diffuse reflectance spectroscopy |
| DRSi | diffuse reflectance spectroscopy imaging |
| Dx | diagnosis |
| EIS | electrical impedance spectroscopy |
| FN | false negative |
| FP | false positive |
| FU | follow‐ up |
| GP | general practitioner |
| H&E | haematoxylin and eosin stain |
| HFUS | high‐frequency ultrasound |
| Hz | hertz |
| KHz | kilohertz |
| K–NN | k nearest neighbour |
| MHz | megahertz |
| MiS | melanoma in situ (or lentigo maligna) |
| MM | malignant melanoma |
| mm | millimetre |
| MSI | multi‐spectral imaging |
| N/A | not applicable |
| NC | non‐comparative |
| nm | nanometre |
| NPV | negative predictive value |
| NR | not reported |
| P | prospective |
| PPV | positive predictive value |
| PSL | pigmented skin lesion |
| R | retrospective |
| RCM | reflectance confocal microscopy |
| RCT | randomised controlled trial |
| SCC | squamous cell carcinoma |
| SD | standard deviation |
| se | sensitivity |
| sp | specificity |
| spectro–CAD | spectroscopy‐based computer–assisted diagnosis |
| SK | seborrhoeic keratosis |
| SSM | superficial spreading melanoma |
| SVM | support vector machine |
| TN | true negative |
| TS | telespectrophotometry system |
| VI | visual inspection |
| UNREF | unreferred population |
| WPC | within‐person comparison (of tests) |
| WPC‐algs | within‐person comparison (of algorithms) |
Appendix 4. Proposed sources of heterogeneity
These may vary between reviews but may include the following.
i. Population characteristics
AJCC stage of disease
Sentinel lymph node status (for imaging studies only)
Clinical nodal status (for imaging studies only)
Primary tumour site (head and neck, trunk, limb, and other)
ii. Index test characteristics
Differences in test positivity thresholds (e.g. for SLNB, the tracer threshold for a 'hot' vs 'cold' node)
Other relevant test characteristics as appropriate to the test under consideration
iii. Reference standard characteristics
Reference standard used (histology, clinical, or imaging‐based follow‐up; concurrent imaging‐based reference standard)
iv. Study quality
Consecutive or random sample of participants recruited
Index test interpreted, blinded to the reference standard result
Index test interpreted, blinded to the result of any other index test
Presence of partial or differential verification bias (whereby only a sample of those subject to the index test are verified by the reference test or by the same reference test, with selection dependent on the index test result)
Use of an adequate reference standard
Overall risk of bias
Appendix 5. Final search strategies
Melanoma search strategies to August 2016
Database: Ovid MEDLINE(R) 1946 to August Week 3 2016
Search strategy:
1 exp melanoma/
2 exp skin cancer/
3 exp basal cell carcinoma/
4 basalioma$1.ti,ab.
5 ((basal cell or skin) adj2 (cancer$1 or carcinoma$1 or mass or masses or tumour$1 or tumor$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or lesion$1 or malignan$ or nodule$1)).ti,ab.
6 (pigmented adj2 (lesion$1 or mole$ or nevus or nevi or naevus or naevi or skin)).ti,ab.
7 (melanom$1 or nonmelanoma$1 or non‐melanoma$1 or melanocyt$ or non‐melanocyt$ or nonmelanocyt$ or keratinocyt$).ti,ab.
8 nmsc.ti,ab.
9 (squamous cell adj2 (cancer$1 or carcinoma$1 or mass or masses or tumor$1 or tumour$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or epithelial or lesion$1 or malignan$ or nodule$1) adj2 (skin or epiderm$ or cutaneous)).ti,ab.
10 (BCC or CSCC or NMSC).ti,ab.
11 keratinocy$.ti,ab.
12 Keratinocytes/
13 or/1‐12
14 dermoscop$.ti,ab.
15 dermatoscop$.ti,ab.
16 photomicrograph$.ti,ab.
17 exp epiluminescence microscopy/
18 (epiluminescence adj2 microscop$).ti,ab.
19 (confocal adj2 microscop$).ti,ab.
20 (incident light adj2 microscop$).ti,ab.
21 (surface adj2 microscop$).ti,ab.
22 (visual adj (inspect$ or examin$)).ti,ab.
23 ((clinical or physical) adj examin$).ti,ab.
24 3 point.ti,ab.
25 three point.ti,ab.
26 pattern analys$.ti,ab.
27 ABCD$.ti,ab.
28 menzies.ti,ab.
29 7 point.ti,ab.
30 seven point.ti,ab.
31 (digital adj2 (dermoscop$ or dermatoscop$)).ti,ab.
32 artificial intelligence.ti,ab.
33 AI.ti,ab.
34 computer assisted.ti,ab.
35 computer aided.ti,ab.
36 neural network$.ti,ab.
37 exp diagnosis, computer‐assisted/
38 MoleMax.ti,ab.
39 image process$.ti,ab.
40 automatic classif$.ti,ab.
41 image analysis.ti,ab.
42 SIAscop$.ti,ab.
43 Aura.ti,ab.
44 (optical adj2 scan$).ti,ab.
45 MelaFind.ti,ab.
46 SIMSYS.ti,ab.
47 MoleMate.ti,ab.
48 SolarScan.ti,ab.
49 VivaScope.ti,ab.
50 (high adj3 ultraso$).ti,ab.
51 (canine adj2 detect$).ti,ab.
52 ((mobile or cell or cellular or smart) adj ((phone$1 adj2 app$1) or application$1)).ti,ab.
53 smartphone$.ti,ab.
54 (DermoScan or SkinVision or DermLink or SpotCheck).ti,ab.
55 Mole Detective.ti,ab.
56 Spot Check.ti,ab.
57 (mole$1 adj2 map$).ti,ab.
58 (total adj2 body).ti,ab.
59 exfoliative cytolog$.ti,ab.
60 digital analys$.ti,ab.
61 (image$1 adj3 software).ti,ab.
62 (teledermatolog$ or tele‐dermatolog$ or telederm or tele‐derm or teledermoscop$ or tele‐dermoscop$ or teledermatoscop$ or tele‐dermatoscop$).ti,ab.
63 (optical coherence adj (technolog$ or tomog$)).ti,ab.
64 (computer adj2 diagnos$).ti,ab.
65 exp sentinel lymph node biopsy/
66 (sentinel adj2 node).ti,ab.
67 nevisense.mp. or HFUS.ti,ab.
68 electrical impedance spectroscopy.ti,ab.
69 history taking.ti,ab.
70 patient history.ti,ab.
71 (naked eye adj (exam$ or assess$)).ti,ab.
72 (skin adj exam$).ti,ab.
73 physical examination/
74 ugly duckling.mp. or UD.ti,ab.
75 ((physician$ or clinical or physical) adj (exam$ or triage or recog$)).ti,ab.
76 ABCDE.mp. or VOC.ti,ab.
77 clinical accuracy.ti,ab.
78 Family Practice/ or Physicians, Family/ or clinical competence/
79 (confocal adj2 microscop$).ti,ab.
80 diagnostic algorithm$1.ti,ab.
81 checklist$.ti,ab.
82 virtual imag$1.ti,ab.
83 volatile organic compound$1.ti,ab.
84 dog$1.ti,ab.
85 gene expression analy$.ti,ab.
86 reflex transmission imag$.ti,ab.
87 thermal imaging.ti,ab.
88 elastography.ti,ab.
89 or/14‐88
90 (CT or PET).ti,ab.
91 PET‐CT.ti,ab.
92 (FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical$).ti,ab.
93 exp Deoxyglucose/
94 deoxy‐glucose.ti,ab.
95 deoxyglucose.ti,ab.
96 CATSCAN.ti,ab.
97 exp Tomography, Emission‐Computed/
98 exp Tomography, X‐ray computed/
99 positron emission tomograph$.ti,ab.
100 exp magnetic resonance imaging/
101 (MRI or fMRI or NMRI or scintigraph$).ti,ab.
102 exp echography/
103 Doppler echography.ti,ab.
104 sonograph$.ti,ab.
105 ultraso$.ti,ab.
106 doppler.ti,ab.
107 magnetic resonance imag$.ti,ab.
108 or/90‐107
109 (stage$ or staging or metasta$ or recurrence or sensitivity or specificity or false negative$ or thickness$).ti,ab.
110 "Sensitivity and Specificity"/
111 exp cancer staging/
112 or/109‐111
113 108 and 112
114 89 or 113
115 13 and 114
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations August 29, 2016
Search strategy:
1 basalioma$1.ti,ab.
2 ((basal cell or skin) adj2 (cancer$1 or carcinoma$1 or mass or masses or tumour$1 or tumor$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or lesion$1 or malignan$ or nodule$1)).ti,ab.
3 (pigmented adj2 (lesion$1 or mole$ or nevus or nevi or naevus or naevi or skin)).ti,ab.
4 (melanom$1 or nonmelanoma$1 or non‐melanoma$1 or melanocyt$ or non‐melanocyt$ or nonmelanocyt$ or keratinocyt$).ti,ab.
5 nmsc.ti,ab.
6 (squamous cell adj2 (cancer$1 or carcinoma$1 or mass or masses or tumor$1 or tumour$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or epithelial or lesion$1 or malignan$ or nodule$1) adj2 (skin or epiderm$ or cutaneous)).ti,ab.
7 (BCC or CSCC or NMSC).ti,ab.
8 keratinocy$.ti,ab.
9 or/1‐8
10 dermoscop$.ti,ab.
11 dermatoscop$.ti,ab.
12 photomicrograph$.ti,ab.
13 (epiluminescence adj2 microscop$).ti,ab.
14 (confocal adj2 microscop$).ti,ab.
15 (incident light adj2 microscop$).ti,ab.
16 (surface adj2 microscop$).ti,ab.
17 (visual adj (inspect$ or examin$)).ti,ab.
18 ((clinical or physical) adj examin$).ti,ab.
19 3 point.ti,ab.
20 three point.ti,ab.
21 pattern analys$.ti,ab.
22 ABCD$.ti,ab.
23 menzies.ti,ab.
24 7 point.ti,ab.
25 seven point.ti,ab.
26 (digital adj2 (dermoscop$ or dermatoscop$)).ti,ab.
27 artificial intelligence.ti,ab.
28 AI.ti,ab.
29 computer assisted.ti,ab.
30 computer aided.ti,ab.
31 neural network$.ti,ab.
32 MoleMax.ti,ab.
33 image process$.ti,ab.
34 automatic classif$.ti,ab.
35 image analysis.ti,ab.
36 SIAscop$.ti,ab.
37 Aura.ti,ab.
38 (optical adj2 scan$).ti,ab.
39 MelaFind.ti,ab.
40 SIMSYS.ti,ab.
41 MoleMate.ti,ab.
42 SolarScan.ti,ab.
43 VivaScope.ti,ab.
44 (high adj3 ultraso$).ti,ab.
45 (canine adj2 detect$).ti,ab.
46 ((mobile or cell or cellular or smart) adj ((phone$1 adj2 app$1) or application$1)).ti,ab.
47 smartphone$.ti,ab.
48 (DermoScan or SkinVision or DermLink or SpotCheck).ti,ab.
49 Mole Detective.ti,ab.
50 Spot Check.ti,ab.
51 (mole$1 adj2 map$).ti,ab.
52 (total adj2 body).ti,ab.
53 exfoliative cytolog$.ti,ab.
54 digital analys$.ti,ab.
55 (image$1 adj3 software).ti,ab.
56 (teledermatolog$ or tele‐dermatolog$ or telederm or tele‐derm or teledermoscop$ or tele‐dermoscop$ or teledermatoscop$ or tele‐dermatoscop$).ti,ab.
57 (optical coherence adj (technolog$ or tomog$)).ti,ab.
58 (computer adj2 diagnos$).ti,ab.
59 (sentinel adj2 node).ti,ab.
60 nevisense.mp. or HFUS.ti,ab.
61 electrical impedance spectroscopy.ti,ab.
62 history taking.ti,ab.
63 patient history.ti,ab.
64 (naked eye adj (exam$ or assess$)).ti,ab.
65 (skin adj exam$).ti,ab.
66 ugly duckling.mp. or UD.ti,ab.
67 ((physician$ or clinical or physical) adj (exam$ or triage or recog$)).ti,ab.
68 ABCDE.mp. or VOC.ti,ab.
69 clinical accuracy.ti,ab.
70 (Family adj (Practice or Physicians)).ti,ab.
71 (confocal adj2 microscop$).ti,ab.
72 clinical competence.ti,ab.
73 diagnostic algorithm$1.ti,ab.
74 checklist$.ti,ab.
75 virtual imag$1.ti,ab.
76 volatile organic compound$1.ti,ab.
77 dog$1.ti,ab.
78 gene expression analy$.ti,ab.
79 reflex transmission imag$.ti,ab.
80 thermal imaging.ti,ab.
81 elastography.ti,ab.
82 or/10‐81
83 (CT or PET).ti,ab.
84 PET‐CT.ti,ab.
85 (FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical$).ti,ab.
86 deoxy‐glucose.ti,ab.
87 deoxyglucose.ti,ab.
88 CATSCAN.ti,ab.
89 positron emission tomograph$.ti,ab.
90 (MRI or fMRI or NMRI or scintigraph$).ti,ab.
91 Doppler echography.ti,ab.
92 sonograph$.ti,ab.
93 ultraso$.ti,ab.
94 doppler.ti,ab.
95 magnetic resonance imag$.ti,ab.
96 or/83‐95
97 (stage$ or staging or metasta$ or recurrence or sensitivity or specificity or false negative$ or thickness$).ti,ab.
98 96 and 97
99 82 or 98
100 9 and 99
Database: Embase 1974 to 2016 August 29
Search strategy:
1 *melanoma/
2 *skin cancer/
3 *basal cell carcinoma/
4 basalioma$.ti,ab.
5 ((basal cell or skin) adj2 (cancer$1 or carcinoma$1 or mass or masses or tumour$1 or tumor$1 or neoplasm$ or adenoma$ or epithelioma$ or lesion$ or malignan$ or nodule$)).ti,ab.
6 (pigmented adj2 (lesion$1 or mole$ or nevus or nevi or naevus or naevi or skin)).ti,ab.
7 (melanom$1 or nonmelanoma$1 or non‐melanoma$1 or melanocyt$ or non‐melanocyt$ or nonmelanocyt$ or keratinocyt$).ti,ab.
8 nmsc.ti,ab.
9 (squamous cell adj2 (cancer$1 or carcinoma$1 or mass or tumor$1 or tumour$1 or neoplasm$1 or adenoma$1 or epithelioma$1 or epithelial or lesion$1 or malignan$ or nodule$1) adj2 (skin or epiderm$ or cutaneous)).ti,ab.
10 (BCC or cscc).mp. or NMSC.ti,ab.
11 keratinocyte.ti,ab.
12 keratinocy$.ti,ab.
13 or/1‐12
14 dermoscop$.ti,ab.
15 dermatoscop$.ti,ab.
16 photomicrograph$.ti,ab.
17 *epiluminescence microscopy/
18 (epiluminescence adj2 microscop$).ti,ab.
19 (confocal adj2 microscop$).ti,ab.
20 (incident light adj2 microscop$).ti,ab.
21 (surface adj2 microscop$).ti,ab.
22 (visual adj (inspect$ or examin$)).ti,ab.
23 ((clinical or physical) adj examin$).ti,ab.
24 3 point.ti,ab.
25 three point.ti,ab.
26 pattern analys$.ti,ab.
27 ABCD$.ti,ab.
28 menzies.ti,ab.
29 7 point.ti,ab.
30 seven point.ti,ab.
31 (digital adj2 (dermoscop$ or dermatoscop$)).ti,ab.
32 artificial intelligence.ti,ab.
33 AI.ti,ab.
34 computer assisted.ti,ab.
35 computer aided.ti,ab.
36 neural network$.ti,ab.
37 MoleMax.ti,ab.
38 exp diagnosis, computer‐assisted/
39 image process$.ti,ab.
40 automatic classif$.ti,ab.
41 image analysis.ti,ab.
42 SIAscop$.ti,ab.
43 (optical adj2 scan$).ti,ab.
44 Aura.ti,ab.
45 MelaFind.ti,ab.
46 SIMSYS.ti,ab.
47 MoleMate.ti,ab.
48 SolarScan.ti,ab.
49 VivaScope.ti,ab.
50 confocal microscop$.ti,ab.
51 (high adj3 ultraso$).ti,ab.
52 (canine adj2 detect$).ti,ab.
53 ((mobile or cell$ or cellular or smart) adj ((phone$1 adj2 app$1) or application$1)).ti,ab.
54 smartphone$.ti,ab.
55 (DermoScan or SkinVision or DermLink or SpotCheck).ti,ab.
56 Spot Check.ti,ab.
57 Mole Detective.ti,ab.
58 (mole$1 adj2 map$).ti,ab.
59 (total adj2 body).ti,ab.
60 exfoliative cytolog$.ti,ab.
61 digital analys$.ti,ab.
62 (image$1 adj3 software).ti,ab.
63 (optical coherence adj (technolog$ or tomog$)).ti,ab.
64 (teledermatolog$ or tele‐dermatolog$ or telederm or tele‐derm or teledermoscop$ or tele‐dermoscop$ or teledermatoscop$).mp. or tele‐dermatoscop$.ti,ab.
65 (computer adj2 diagnos$).ti,ab.
66 *sentinel lymph node biopsy/
67 (sentinel adj2 node).ti,ab.
68 nevisense.ti,ab.
69 HFUS.ti,ab.
70 electrical impedance spectroscopy.ti,ab.
71 history taking.ti,ab.
72 patient history.ti,ab.
73 (naked eye adj (exam$ or assess$)).ti,ab.
74 (skin adj exam$).ti,ab.
75 *physical examination/
76 ugly duckling.ti,ab.
77 UD sign$.ti,ab.
78 ((physician$ or clinical or physical) adj (exam$ or recog$ or triage)).ti,ab.
79 ABCDE.ti,ab.
80 clinical accuracy.ti,ab.
81 *general practice/
82 (confocal adj2 microscop$).ti,ab.
83 clinical competence/
84 diagnostic algorithm$.ti,ab.
85 checklist$1.ti,ab.
86 virtual image$1.ti,ab.
87 volatile organic compound$1.ti,ab.
88 VOC.ti,ab.
89 dog$1.ti,ab.
90 gene expression analys$.ti,ab.
91 reflex transmission imaging.ti,ab.
92 thermal imaging.ti,ab.
93 elastography.ti,ab.
94 dog$1.ti,ab.
95 gene expression analys$.ti,ab.
96 reflex transmission imaging.ti,ab.
97 thermal imaging.ti,ab.
98 elastography.ti,ab.
99 or/14‐93
100 PET‐CT.ti,ab.
101 (CT or PET).ti,ab.
102 (FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical$).ti,ab.
103 exp Deoxyglucose/
104 CATSCAN.ti,ab.
105 deoxyglucose.ti,ab.
106 deoxy‐glucose.ti,ab.
107 *positron emission tomography/
108 *computer assisted tomography/
109 positron emission tomograph$.ti,ab.
110 *nuclear magnetic resonance imaging/
111 (MRI or fMRI or NMRI or scintigraph$).ti,ab.
112 *echography/
113 Doppler.ti,ab.
114 sonograph$.ti,ab.
115 ultraso$.ti,ab.
116 magnetic resonance imag$.ti,ab.
117 or/100‐116
118 (stage$ or staging or metasta$ or recurrence or sensitivity or specificity or false negative$ or thickness$).ti,ab.
119 "Sensitivity and Specificity"/
120 *cancer staging/
121 or/118‐120
122 117 and 121
123 99 or 122
124 13 and 123
Database: Cochrane Library (Wiley) 2016 searched 30 August 2016 CDSR issue 8 of 12 2016 CENTRAL Issue 7 of 12 2016 HTA Issue 3 of 4 July 2016 DARE Issue 3 of 4 2015
Search strategy:
#1 melanoma* or nonmelanoma* or non‐melanoma* or melanocyt* or non‐melanocyt* or nonmelanocyt* or keratinocyte*
#2 MeSH descriptor: [Melanoma] explode all trees
#3 "skin cancer*"
#4 MeSH descriptor: [Skin Neoplasms] explode all trees
#5 skin near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*)
#6 nmsc
#7 "squamous cell" near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*) near/2 (skin or epiderm* or cutaneous)
#8 "basal cell" near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*)
#9 pigmented near/2 (lesion* or nevus or mole* or naevi or naevus or nevi or skin)
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 dermoscop*
#12 dermatoscop*
#13 Photomicrograph*
#14 MeSH descriptor: [Dermoscopy] explode all trees
#15 confocal near/2 microscop*
#16 epiluminescence near/2 microscop*
#17 incident next light near/2 microscop*
#18 surface near/2 microscop*
#19 "visual inspect*"
#20 "visual exam*"
#21 (clinical or physical) next (exam*)
#22 "3 point"
#23 "three point"
#24 "pattern analys*"
#25 ABDC
#26 menzies
#27 "7 point"
#28 "seven point"
#29 digital near/2 (dermoscop* or dermatoscop*)
#30 "artificial intelligence"
#31 "AI"
#32 "computer assisted"
#33 "computer aided"
#34 AI
#35 "neural network*"
#36 MoleMax
#37 "computer diagnosis"
#38 "image process*"
#39 "automatic classif*"
#40 SIAscope
#41 "image analysis"
#42 "optical near/2 scan*"
#43 Aura
#44 MelaFind
#45 SIMSYS
#46 MoleMate
#47 SolarScan
#48 Vivascope
#49 "confocal microscopy"
#50 high near/3 ultraso*
#51 canine near/2 detect*
#52 Mole* near/2 map*
#53 total near/2 body
#54 mobile* or smart near/2 phone*
#55 cell next phone*
#56 smartphone*
#57 "mitotic index"
#58 DermoScan or SkinVision or DermLink or SpotCheck
#59 "Mole Detective"
#60 "Spot Check"
#61 mole* near/2 map*
#62 total near/2 body
#63 "exfoliative cytolog*"
#64 "digital analys*"
#65 image near/3 software
#66 teledermatolog* or tele‐dermatolog* or telederm or tele‐derm or teledermoscop* or tele‐dermoscop* or teledermatoscop* or tele‐dermatolog*
#67 "optical coherence" next (technolog* or tomog*)
#68 computer near/2 diagnos*
#69 sentinel near/2 node*
#70 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69
#71 ultraso*
#72 sonograph*
#73 MeSH descriptor: [Ultrasonography] explode all trees
#74 Doppler
#75 CT or PET or PET‐CT
#76 "CAT SCAN" or "CATSCAN"
#77 MeSH descriptor: [Positron‐Emission Tomography] explode all trees
#78 MeSH descriptor: [Tomography, X‐Ray Computed] explode all trees
#79 MRI
#80 MeSH descriptor: [Magnetic Resonance Imaging] explode all trees
#81 MRI or fMRI or NMRI or scintigraph*
#82 "magnetic resonance imag*"
#83 MeSH descriptor: [Deoxyglucose] explode all trees
#84 deoxyglucose or deoxy‐glucose
#85 "positron emission tomograph*"
#86 #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85
#87 stage* or staging or metasta* or recurrence or sensitivity or specificity or "false negative*" or thickness*
#88 MeSH descriptor: [Neoplasm Staging] explode all trees
#89 #87 or #88
#90 #89 and #86
#91 #70 or #90
#92 #10 and #91
#93 BCC or CSCC or NMCS
#94 keratinocy*
#95 #93 or #94
#96 #10 or #95
#97 nevisense
#98 HFUS
#99 "electrical impedance spectroscopy"
#100 "history taking"
#101 "patient history"
#102 naked next eye near/1 (exam* or assess*)
#103 skin next exam*
#104 "ugly duckling" or (UD sign*)
#105 MeSH descriptor: [Physical Examination] explode all trees
#106 (physician* or clinical or physical) near/1 (exam* or recog* or triage*)
#107 ABCDE
#108 "clinical accuracy"
#109 MeSH descriptor: [General Practice] explode all trees
#110 confocal near microscop*
#111 "diagnostic algorithm*"
#112 MeSH descriptor: [Clinical Competence] explode all trees
#113 checklist*
#114 "virtual image*"
#115 "volatile organic compound*"
#116 dog or dogs
#117 VOC
#118 "gene expression analys*"
#119 "reflex transmission imaging"
#120 "thermal imaging"
#121 elastography
#122 #97 or #98 or #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108 or #109 or #110 or #111 or #112 or #113 or #114 or #115 or #116 or #117 or #118 or #119 or #120 or #121
#123 #70 or #122
#124 #96 and #123
#125 #96 and #90
#126 #125 or #124
#127 #10 and #126
Database: CINAHL Plus (EBSCO) 1937 to 30 August 2016
Search strategy:
S1 (MH "Melanoma") OR (MH "Nevi and Melanomas+")
S2 (MH "Skin Neoplasms+")
S3 (MH "Carcinoma, Basal Cell+")
S4 basalioma*
S5 (basal cell) N2 (cancer* or carcinoma* or mass or masses or tumor* or tumour* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*)
S6 (pigmented) N2 (lesion* or mole* or nevus or nevi or naevus or naevi or skin)
S7 melanom* or nonmelanoma* or non‐melanoma* or melanocyt* or non‐melanocyt* or nonmelanocyt*
S8 nmsc
S9 TX BCC or cscc or NMSC
S10 (MH "Keratinocytes")
S11 keratinocyt*
S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
S13 dermoscop* or dermatoscop* or photomicrograph* or (3 point) or (three point) or ABCD* or menzies or (7 point) or (seven point) or AI or Molemax or SIASCOP* or Aura or MelaFind or SIMSYS or MoleMate or SolarScan or smartphone* or DermoScan or SkinVision or DermLink or SpotCheck
S14 (epiluminescence or confocal or incident or surface) N2 (microscop*)
S15 visual N1 (inspect* or examin*)
S16 (clinical or physical) N1 (examin*)
S17 pattern analys*
S18 (digital) N2 (dermoscop* or dermatoscop*)
S19 (artificial intelligence)
S20 (computer) N2 (assisted or aided)
S21 (neural network*)
S22 (MH "Diagnosis, Computer Assisted+")
S23 (image process*)
S24 (automatic classif*)
S25 (image analysis)
S26 SIAScop*
S27 (optical) N2 (scan*)
S28 (high) N3 (ultraso*)
S29 elastography
S30 (mobile or cell or cellular or smart) N2 (phone*) N2 (app or application*)
S31 (mole*) N2 (map*)
S32 total N2 body
S33 exfoliative cytolog*
S34 digital analys*
S35 image N3 software
S36 teledermatolog* or tele‐dermatolog* or telederm or tele‐derm or teledermoscop* or tele‐dermoscop* or teledermatoscop* or tele‐dermatoscop* teledermatolog* or tele‐dermatolog* or telederm or tele‐derm or teledermoscop*
S37 (optical coherence) N1 (technolog* or tomog*)
S38 computer N2 diagnos*
S39 sentinel N2 node
S40 (MH "Sentinel Lymph Node Biopsy")
S41 nevisense or HFUS or checklist* or VOC or dog*
S42 electrical impedance spectroscopy
S43 history taking
S44 "Patient history"
S45 naked eye
S46 skin exam*
S47 physical exam*
S48 ugly duckling
S49 UD sign*
S50 (physician* or clinical or physical) N1 (exam*)
S51 clinical accuracy
S52 general practice
S53 (physician* or clinical or physical) N1 (recog* or triage)
S54 confocal microscop*
S55 clinical competence
S56 diagnostic algorithm*
S57 checklist*
S58 virtual image*
S59 volatile organic compound*
S60 gene expression analys*
S61 reflex transmission imag*
S62 thermal imaging
S63 S13 or S14 or S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62
S64 CT or PET
S65 PET‐CT
S66 FDG or F18 or Fluorodeoxyglucose or radiopharmaceutical*
S67 (MH "Deoxyglucose+")
S68 deoxy‐glucose or deoxyglucose
S69 CATSCAN
S70 CAT‐SCAN
S71 (MH "Deoxyglucose+")
S72 (MH "Tomography, Emission‐Computed+")
S73 (MH "Tomography, X‐Ray Computed")
S74 positron emission tomograph*
S75 (MH "Magnetic Resonance Imaging+")
S76 MRI or fMRI or NMRI or scintigraph*
S77 echography
S78 doppler
S79 sonograph*
S80 ultraso*
S81 magnetic resonance imag*
S82 S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81
S83 stage* or staging or metasta* or recurrence or sensitivity or specificity or (false negative*) or thickness
S84 (MH "Neoplasm Staging")
S85 S83 OR S84
S86 S82 AND S85
S87 S63 OR S86
S88 S12 AND S87
Database: Science Citation Index SCI Expanded (Web of Science) 1900 to 30 August 2016
Conference Proceedings Citation Index (Web of Science) 1900 to 1 September 2016
Search strategy:
#1 (melanom* or nonmelanom* or non‐melanoma* or melanocyt* or non‐melanocyt* or nonmelanocyt* or keratinocyt*)
#2 (basalioma*)
#3 ((skin) near/2 (cancer* or carcinoma or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*))
#4 ((basal) near/2 (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*))
#5 ((pigmented) near/2 (lesion* or mole* or nevus or nevi or naevus or naevi or skin))
#6 (nmsc or BCC or NMSC or keratinocy*)
#7 ((squamous cell (cancer* or carcinoma* or mass or masses or tumour* or tumor* or neoplasm* or adenoma* or epithelioma* or lesion* or malignan* or nodule*))
#8 (skin or epiderm* or cutaneous)
#9 #8 AND #7
#10 #9 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1
#11 ((dermoscop* or dermatoscop* or photomicrograph* or epiluminescence or confocal or "incident light" or "surface microscop*" or "visual inspect*" or "physical exam*" or 3 point or three point or pattern analy* or ABCDE or menzies or 7 point or seven point or dermoscop* or dermatoscop* or AI or artificial or computer aided or computer assisted or neural network* or Molemax or image process* or automatic classif* or image analysis or siascope or optical scan* or Aura or melafind or simsys or molemate or solarscan or vivascope or confocal microscop* or high ultraso* or canine detect* or cellphone* or mobile* or phone* or smartphone or dermoscan or skinvision or dermlink or spotcheck or spot check or mole detective or mole map* or total body or exfoliative psychology or digital or image software or optical coherence or teledermatology or telederm* or teledermoscop* or teledermatoscop* or computer diagnos* or sentinel))
#12 ((nevisense or HFUS or impedance spectroscopy or history taking or patient history or naked eye or skin exam* or physical exam* or ugly duckling or UD sign* or physician* exam* or physical exam* or ABCDE or clinical accuracy or general practice or confocal microscop* or clinical competence or diagnostic algorithm* or checklist* or virtual image* or volatile organic or VOC or dog* or gene expression or reflex transmission or thermal imag* or elastography))
#13 #11 or #12
#14 ((PET or CT or FDG or deoxyglucose or deoxy‐glucose or fluorodeoxy* or radiopharma* or CATSCAN or positron emission or computer assisted or nuclear magnetic or MRI or FMRI or NMRI or scintigraph* or echograph* or Doppler or sonograph* or ultraso* or magnetic reson*))
#15 ((stage* or staging or metast* or recurrence or sensitivity or specificity or false negative* or thickness*))
#16 #14 AND #15
#17 #16 OR #13
#18 #10 AND #17
Refined by: DOCUMENT TYPES: (MEETING ABSTRACT OR PROCEEDINGS PAPER)
Appendix 6. Full text inclusion criteria
| Criterion | Inclusion | Exclusion |
| Study design |
For diagnostic and staging reviews
|
|
| Target condition |
|
|
| Population |
For diagnostic reviews
For staging reviews
|
|
| Index tests |
For diagnosis
For staging
Any test combination and in any order Any test positivity threshold Any variation in testing procedure (e.g. radioisotope used) |
|
| Reference standard |
For diagnostic studies
For studies of imaging tests for staging: Histopathology (via LND or SLMB) Clinical/radiological follow‐up A combination of the above For studies of SLNB accuracy for staging: LND of both SLN+ and SLn participants to identify all diseased nodes LND of SLN+ participants and follow‐up of SLN participants to identify a subsequent nodal recurrence in a previously investigated nodal basin |
For diagnostic studies
|
| BCC: basal cell carcinoma; cSCC: cutaneous squamous cell carcinoma; CT: computed tomography; FNAC: fine needle aspiration cytology; LND: lymph node dissection; MRI: magnetic resonance imaging; PET: positron emission tomography; PET‐CT: positron emission tomography‐computed tomography; RCT: randomised controlled trial; SCC: squamous cell carcinoma; SLN+: positive sentinel lymph node; SLn: negative sentinel lymph node; SLNB: sentinel lymph node biopsy. | ||
Appendix 7. QUADAS interpretation
| Item | Response (delete as required) |
| PARTICIPANT SELECTION (1) ‐ RISK OF BIAS | |
| 1) Was a consecutive or random sample of participants or images enrolled? |
Yes ‐ if paper states consecutive or random No – if paper describes other method of sampling Unclear – if participant sampling not described |
| 2) Was a case‐control design avoided? |
Yes ‐ if consecutive or random or case‐control design clearly not used No – if study described as case‐control or describes sampling specific numbers of participants with particular diagnoses Unclear – if not described |
| 3) Did the study avoid inappropriate exclusions both for melanoma and for cutaneous squamous cell carcinoma (cSCC) staging? |
Yes ‐ if inappropriate exclusions were avoided No – if lesions were excluded that might affect test accuracy, e.g. indeterminate results or where disagreement between evaluators was observed Unclear – if not clearly reported |
| 4) For between‐person comparative (BPC) studies only (i.e. allocating different tests to different study participants such as randomised controlled trials (RCTs)): | |
|
Yes ‐ if same selection criteria were used for each index test No – if different selection criteria were used for each index test Unclear – if selection criteria per test were not described N/A – if only 1 index test was evaluated or all participants received all tests |
|
Yes ‐ if adequate randomisation procedures are described No – if inadequate randomisation procedures are described Unclear – if the method of allocation to groups is not described (a description of ‘random’ or ‘randomised’ is insufficient) N/A – if only 1 index test was evaluated or all participants received all tests |
|
Yes ‐ if appropriate methods of allocation concealment are described No – if appropriate methods of allocation concealment are not described Unclear – if the method of allocation concealment is not described (sufficient detail to allow a definite judgement is required) N/A – if only 1 index test was evaluated |
| Could the selection of participants have introduced bias? | |
| v FOR NON‐COMPARATIVE (NC) STUDIES | |
| If answers to all of questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk Unclear |
| v FOR BETWEEN‐PERSON COMPARATIVE STUDIES | |
| If answers to all of questions 1) and 2) and 3) and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) or 4) was ‘Unclear’: | Risk Unclear |
| PARTICIPANT SELECTION (1) ‐ CONCERNS REGARDING APPLICABILITY | |
| For sentinel lymph node biopsy and imaging tests: | |
| 1) Does the study report results for participants unselected by stage of disease or site of primary lesion, i.e. the study does not focus solely on those with a particular stage of disease such as American Joint Committee on Cancer (AJCC) stage I or melanoma ≤ 1 mm in thickness? |
Yes ‐ if an unrestricted group of participants have been included No ‐ if a selected group of study participants have been included, e.g. those with clinical stage I disease or only those with thin melanoma Unclear – if insufficient details are provided to determine the spectrum of included participants |
| 2) Did the study report data on a per patient rather than per lesion basis? |
Yes – if a per patient analysis was reported No – if a per lesion analysis only was reported Unclear – if it is not possible to assess whether data are presented on a per patient or per lesion basis |
| For imaging tests only: | |
| 3) Does the study focus primarily on participants undergoing primary staging or those undergoing staging for disease recurrence? |
Yes ‐ if at least 80% of study participants are undergoing primary staging following diagnosis of a primary cutaneous melanoma or staging of recurrence No ‐ if less than 80% of study participants are undergoing primary staging following diagnosis of a cutaneous melanoma or staging of recurrence Unclear – if insufficient details are provided to determine the proportion of patients undergoing primary staging vs those undergoing staging of recurrence |
| Is there concern that the included participants do not match the review question? | |
| If the answer to question 1) or 2) (and 3)) was ‘Yes’: | Concern is Low |
| If the answer to question 1) or 2) (and 3)) was ‘No’: | Concern is High |
| If the answer to question 1) or 2) (and 3)) was ‘Unclear’: | Concern is Unclear |
| INDEX TEST (2) ‐ RISK OF BIAS (to be completed per test evaluated) | |
| 1) Was the index test or testing strategy result interpreted without knowledge of the results of the reference standard? |
Yes ‐ if index test described as interpreted without knowledge of reference standard result, or for prospective studies, if index test is always conducted and interpreted before the reference standard No – if index test described as interpreted in knowledge of reference standard result Unclear – if index test blinding is not described |
| 2) Was the diagnostic threshold at which the test was considered positive prespecified? |
Yes ‐ if threshold was prespecified (i.e. before analysing study results) No ‐ if threshold was not prespecified Unclear ‐ if not possible to tell whether or not diagnostic threshold was prespecified |
| For imaging tests only: | |
| 3) For studies reporting the accuracy of multiple diagnostic thresholds (tumour characteristic or parameter) for the same index test, was each threshold interpreted without knowledge of the results of the others? |
Yes ‐ if thresholds were selected prospectively and each was interpreted by a different reader, or if study implements a retrospective (or no) cutoff No ‐ if study uses prospective threshold and report states reported by same reader Unclear ‐ if no mention of number of readers for each threshold or if prespecification of threshold not reported N/A ‐ multiple diagnostic thresholds not reported for the same index test |
| 4) For within‐person comparison (WPC) of index tests or testing strategies (i.e. > 1 index test applied per participant), was each index test result interpreted without knowledge of the results of other index tests or testing strategies? |
Yes ‐ if all index tests were described as interpreted without knowledge of the results of the others No ‐ if the index tests were described as interpreted in the knowledge of the results of the others Unclear – if it is not possible to tell whether knowledge of other index tests could have influenced test interpretation N/A – if only 1 index test was evaluated |
| Could the conduct or interpretation of the index test have introduced bias? | |
| v FOR NC and BPC STUDIES item 3) / 4) to be added | |
| If answers to questions 1) and 2) was ‘Yes’: | Risk is Low |
| If answers to either questions 1) or 2) was ‘No’: | Risk is High |
| If answers to either questions 1) or 2) was ‘Unclear’: | Risk is Unclear |
| v FOR WPC STUDIES | |
| If answers to all questions 1), 2) for any index test and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) for any index test or 3) was ‘Unclear’: | Risk is Unclear |
| INDEX TEST (2) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? This item applies equally to studies using objective and more subjective approaches to test interpretation. For sentinel lymph node biopsy (SLNB) studies, this requires description of the tracer threshold for identification of the SLN and the histological assessment. |
Yes – if the criteria for diagnosis of the target disorder were reported in sufficient detail to allow replication No – if the criteria for diagnosis of the target disorder were not reported in sufficient detail to allow replication Unclear – if some but not sufficient information on criteria for diagnosis to allow replication were provided |
| 2) Was the test interpretation carried out by an experienced examiner? |
Yes – if the test was interpreted by an experienced examiner as defined in the review protocol No – if the test was not interpreted by an experienced examiner (see above) Unclear – if the experience of the examiner(s) was not reported in sufficient detail to judge or if examiners described as 'Expert' with no further detail given |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | |
| If answers to questions 1) and 2) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 2) was ‘No’: | Concern is High |
| If answers to questions 1) or 2) was ‘Unclear’: | Concern is Unclear |
| REFERENCE STANDARD (3) ‐ RISK OF BIAS | |
| 1) Is the reference standard likely to correctly classify the target condition? | |
|
a) DISEASE POSITIVE ‐ 1 or more of: ‐ Histological confirmation of metastases following lymph node dissection (or SLNB or core biopsy for imaging studies) ‐ Clinical/radiological follow‐up to identify clinically detectable disease in a mapped nodal basin (SLNB studies) ‐ Clinical/radiological follow‐up to identify any metastases (imaging studies) subsequently confirmed on histology |
Yes – if all disease positive participants underwent 1 of the listed reference standards No – if a final diagnosis for any disease positive participant was reached without histopathology Unclear – if the method of final diagnosis was not reported for any disease positive participant |
|
b) DISEASE NEGATIVE ‐ 1 or more of: ‐ Histological confirmation of absence of disease in a mapped nodal basin following lymph node dissection (or following SLNB for imaging studies) ‐ Clinical/radiological follow‐up of test negative participants |
Yes – if at least 90% of disease negative participants underwent 1 of the listed reference standards No – if more than 10% of benign diagnoses were reached by concurrent imaging test Unclear – if the method of final diagnosis was not reported for any participant with benign or disease negative diagnosis |
| 2) Were the histology‐based reference standard results interpreted without knowledge of the results of the index test? |
Yes – if the histopathologist was described as blinded to the index test result No – if the histopathologist was described as having knowledge of the index test result Unclear – if blinded histology interpretation was not clearly reported |
| 3) Were the reference standard results based on patient follow‐up interpreted without knowledge of the results of the index test? |
Yes – if the clinician or radiologist was described as blinded to the index test result No – if the clinician or radiologist was described as having knowledge of the index test result Unclear – if blinded interpretation was not clearly reported |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| REFERENCE STANDARD (3) ‐ CONCERN ABOUT APPLICABILITY | |
| 1) Does the study use the same definition of disease positive as the primary review question, or is it possible to fully disaggregate data such that data matching the review question can be extracted? |
Yes – same definition of disease positive used, or patients can be disaggregated and re‐grouped according to review definition No – some patients cannot be disaggregated For SLNB review – disease positive includes participants with any nodal recurrence (not restricted to clinical recurrence in same nodal basin) For imaging reviews – participants with nodal vs distant recurrences cannot be disaggregated Unclear – definition of disease positive not clearly reported |
| For studies of imaging tests: | |
| 2) The result of another imaging test (without patient follow‐up to determine later emergence of disease) was not used as a reference standard |
Yes – if imaging‐based diagnosis was not used as a reference standard for any participant No – if imaging‐based diagnosis was used as a reference standard for any participant Unclear – if not clearly reported |
| 3) Item on observer experience could be included? Is there concern that the target condition as defined by the reference standard does not match the review question? |
|
| If answers to all questions 1), 2) and 3) was ‘Yes’: | Concern is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Concern is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Concern is Unclear |
| ***For teledermatology studies only: | |
| If answers to questions 1) and 3) was ‘Yes’: | Concern is Low |
| If answers to questions 1) or 3) was ‘No’: | Concern is High |
| If answers to questions 1) or 3) was ‘Unclear’: | Concern is Unclear |
| FLOW AND TIMING (4): RISK OF BIAS | |
| 1) Was there an appropriate interval between index test and reference standard? | |
|
Yes – if study reports ≤ 1 month between index and histological reference standard No – if study reports > 1 month between index and histological reference standard Unclear – if study does not report interval between index and histological reference standard |
|
Yes – if study reports a follow‐up visit within 6 months of application of the index test No – if study reports the first follow‐up visit beyond 6 months of the index test Unclear – if study does not report timing of follow‐up visits |
| 2) Did all participants receive the same reference standard? |
Yes – if all participants underwent the same reference standard No – if more than 1 reference standard was used Unclear – if not clearly reported |
| 3) Were all participants included in the analysis? |
Yes – if all participants were included in the analysis No – if some participants were excluded from the analysis Unclear – if not clearly reported |
| 4) For WITHIN‐PERSON COMPARISON (WPC) of index tests: Was the interval between application of index tests ≤ 1 month? Could the participant flow have introduced bias? |
Yes – if study reports ≤ 1 month between index tests No – if study reports > 1 month between index tests Unclear – if study does not report interval between index tests |
| v FOR NON‐COMPARATIVE and BPC STUDIES | |
| If answers to questions 1) and 2) and 3) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1) or 2) or 3) was ‘No’: | Risk is High |
| If answers to any one of questions 1) or 2) or 3) was ‘Unclear’: | Risk is Unclear |
| v FOR WITHIN‐PERSON COMPARATIVE STUDIES (WPCs) | |
| If answers to all questions 1), 2), 3), and 4) was ‘Yes’: | Risk is Low |
| If answers to any one of questions 1), 2), 3), or 4) was ‘No’: | Risk is High |
| If answers to any one of questions 1), 2), 3), or 4) was ‘Unclear’: | Risk is Unclear |
Appendix 8. Summary characteristics of studies for pre‐SLNB imaging
| Study Country Pt/lesion number | Study design Outcome: prevalence | Presentation Inclusion criteria Imaging eligibility | Age, gender, site, BT, Clark | Index test | Threshold Observers |
Reference Exclusions |
|
Arrangoiz 2012 USA Patients: 56 Primary lesions: 56 LNBs/Metastases: NR |
NC Retrospective (medical record review not described) Data: per pt Nodal mets: 29/56 = 52% | Primary (pre‐SLNB) Stage of disease: all T4 and clinically node negative and negative for distant metastases Inclusion: node negative; BT > 4 mm |
Mean age: 67; Median age: NR; Range: 26 to 89 years
Male: 32 (57%) Site: trunk 16, 29%; extremities 28, 50%; HN 12, 21% BT median 6 mm; mean 9 mm; range 4.1 to 40 mm |
PET‐CT. 2D or 3D; CT (U, helical, low dose) Scan coverage: WB; vertex of the head down to feet for all patients Contrast: U CT parameters: Discovery LS ‐ 140 kVp, 90mA; Siemens Biograph ‐ 130 kVp, 100 mA; 5 mm 18FDG: 15 mCi (IV) Breath hold: normal breathing CT used for: attenuation correction; co‐registered images Reconstruction: Discovery LS ‐ OSEM algorithm; Siemens Biograph ‐ TrueX (iterative reconstruction) algorithm | SUV of 2.5 Info provided: NR No. observers: NR; 'in‐house medical physicist' mentioned Diagnosis: unclear |
Histology (54, 96% (48 SLNB and 6 LND))
FNAC (n NR)
FU (n NR): no details
Histology interval: NR; states that 6 "proceeded directly to therapeutic lymph node dissection" after PET
FU interval: NR Exclusions: n = 0; N/A |
|
Chai 2012 USA Patients: 325 Primary lesions: 325 LNBs/Metastases: 347 |
NC Retrospective (prospective database reported) Data: per pt Nodal mets: 64/317 = 20% | Primary (pre‐SLNB) Stage of disease: NR Inclusion: node negative, BT > 0.76 mm or < 0.76 mm with high‐risk features such as ulceration, high mitotic rate, or a positive deep margin |
Mean age: NR; Median age: 58; Range: 18 to 86
Male: 189 (58%) Site: HN 34 (10.5%) Trunk 129 (39.7%) Upper extremity 101 (31.1%) Lower extremity 61 (18.8%) BT median (range) 1.78 (0.42 to 14.4) Clark’s level III 24 (7.4%), IV 275 (84.6%), V 20 (6.2%), unknown 6 (1.8%) |
US. B‐mode; linear array (9 or 12 MHz); US before LS
Scan coverage: according to primary MM site and discretion of attending surgeon Contrast: N/A |
US ‐ classed as ‘‘abnormal,’’ ‘‘suspicious,’’ or ‘‘indeterminate recommending a short‐term follow‐up’’ were considered positive (criteria described in detail) Info provided: NR No. observers: NR (NR) Diagnosis: NR |
Histology (325, 100%)
FNAC (6, 1.8%)
FU (NR; presume 100%): NR; FU for SLNB negatives is reported but no description is given
Histology interval: US performed either immediately or several days before LS
FU interval: NR Exclusions: n = 8; 1 draining basin identified by LS was not examined with US; plus 7 SLN positive who did not get US |
| Hafner 2004 Switzerland Patients: 101 Primary lesions: 101 LNBs/Metastases: 105 LNBs; 136 SLNs | WPC Prospective Data: per pt Nodal mets: 23/97 = 24% | Primary (pre‐SLNB) Stage of disease: NR; stage IV (evidence of distant mets) excluded Inclusion: any cutaneous MM with BT ≥ 1 mm without evidence of detectable distant metastasis (includes clinically palpable) Excluded if < 1 mm |
Median age: 55; Range: 18 to 79
Male: 55 (55%) Site: limbs 49, 49%, trunk 35, 35%, HN 16, 16% BT: 1.01 to 2 mm 38; 2.01 to 4 mm 43; > 4.0 mm 19 |
US. B‐mode (5 Mhz); US before LS Scan coverage: regional lymph nodes of the groins, axillae, and neck (abdominal US also performed) Contrast: N/A | NR; 'radiologically suspect' Info provided: unclear; clinical exam by dermatologist and US by radiologist No. observers: 1; radiologist (NR) Diagnosis: single |
Histology (100; 100%)
FNAC (NR (abstract reports 3 LN mets identified on physical exam, 2 of which were detected by US)):
FU (n NR): 20 months (8 to 39)
Histology interval: 2 weeks
FU interval: 6 months Exclusions: n = 4; 1 sentinel node was not found intraoperatively; 3 clinically node positive excluded by Bham team |
|
Hinz 2013
Germany Patients: 20 Primary lesions: 20 LNBs/Metastases: 59 SLN removed |
WPC
Retrospective (retrospective computer‐aided search of preoperatively performed staging procedures) Data: per pt Nodal mets: 12/20 = 60% |
Primary (pre‐SLNB) Stage of disease: NR Inclusion: high risk cutaneous MM; implies BT ≥ 2.0 mm or RF such as ulceration or regression Excluded if 'further risk factors' or classic sonographic signs of lymphatic metastasis | Mean age: full sample: 55.2; Median age: NR; Range: Full sample: SD 13.3 years Male: 9 (45%). Site: trunk n = 10 (50%); upper extremity n = 3(15%); lower extremity n = 4 (20%); acral n = 3(15%) BT: 1.01 to 2 mm n = 3 (15%), 2.10 to 4 mm n = 9 (45%), > 4 mm n = 8 (40%); Clark’s level III n = 1(5%); IV n = 16(80%); V n = 3 (15%) |
PET‐CT. 2D/3D NR; CE‐CT, helical. Reinhardt 2006 states helical, dual detector (N/A)
Scan coverage: WB; Reinhardt 2006: "base of the skull to the apex of the lungs, ... from the shoulders to upper thighs, ... from the proximal femur to the tip of the toes"
Contrast: Reinhardt 2006: iodinated oral contrast agent (Peritrast‐oral‐GI; Köhler Chemie GmbH, Alsbach, Germany)
CT parameters: 130 kV, 40 mAs (Reinhardt 2006); 5 mm (Reinhardt 2006)
18FDG: 371 ± 41 MBq (Reinhardt 2006)
Breath hold: limited breath hold technique for CT and shallow breathing for PET
CT used for: Reinhardt 2006: attenuation correction based on re‐scaling of the CT image
Reconstruction: iterative as described elsewhere (Kinahan 2003) US. B‐mode (6.0‐ to 11.0‐MHz linear transducer); US pre‐ and post‐LS Scan coverage: all relevant regional LN basins depending on localisation of the primary melanoma Contrast: N/A |
PET‐CT: NR
Info provided: NR
No. observers: unclear; no details
Diagnosis: unclear US: morphology criteria (Solbiati 1988; Vassalo 1992; Voit 2010d); suspicious LNs were re‐examined with US after LS Info provided: clinical exam/US performed by same clinician No. observers: unclear; physicians with broad experience in dermato‐oncology (NR) Diagnosis: unclear; appears as though single observer |
Histology (20 (100%))
FNAC (0): histology interval: NR
FU interval: N/A Exclusions: n = 0 |
| Hinz 2011 Germany Patients: 81 Primary lesions: 81 LNBs/Metastases: NR; 170 SLNs | NC Prospective Data: per pt Nodal mets: 8/81 = 10% | Primary (pre‐SLNB) (? 1 secondary nodular SSM) Stage of disease: NR Inclusion: clinically node negative, BT ≥ 1 mm or < 1 mm with risk factors such as ulceration or regression | Mean age: 52.8; Median age: NR; Range: SD 15.4; node positive given (36 to 62) Male: 48 (0.5925%). Site: HN 2,2.5%; Trunk 36, 44.4%; Upper extremity 14, 17.2%; Lower extremity 23, 28.4%; Acral 6, 7.4% Median BT 1.68 mm (0.76 to 6.00 mm) Clark’s level: 1 1, 1.4%; 2 26, 35.6%; 3 39, 53.4%; 4 7, 9.6% | US. B‐mode (linear array); Doppler (6.0 to 11.0 MHz linear transducer); US pre‐ and post‐LS Scan coverage: LN areas predicted by sites of melanoma Contrast: N/A | Positive radiological findings according to published criteria Info provided: NR; likely full info available No. observers: 1 of 4 clinicians trained in USS imaging; NR; broad experience in dermato‐oncology and special ultrasound skills (NR) Diagnosis: unclear; appears as though single observer |
Histology (1)
FNAC (n NR)
FU (N/A): not stated
Histology interval: NR
FU interval: NR Exclusions: n = 0 |
|
Hocevar 2004
Slovenia Patients: 57 Primary lesions: 57 LNBs/Metastases: 61 |
WPC
Design unclear Data: per pt Nodal mets: 14/57 = 25% |
Primary (pre‐SLNB) Stage of disease: NR Inclusion: MM candidates for SLNB | Mean age: NR; Median age: NR; Range: 1 to 93 Male: 21 (0.37%). Site: 14, 25% head, 19, 38% trunk, 24, 42% extremity BT < 1 mm 2, 4%, BT 1 to 2 mm 23, 40%, BT 2.01 to 4 mm 20, 35%, BT > 4 mm 12, 21% Clark’s level unknown ‐ 2, 4%, 3 23; 42%, 4 26; 44%, 5 6, 10% |
US. B‐mode; linear array transducer with small parts probe (12 and 15 MHz); US before LS
Scan coverage: NR
Contrast: N/A
Breath hold: regional lymph nodes US + FNAC for those positive on US |
Rounded appearance of the LN, Ioss of the hilar echogenic reflex, and deformed radial nodal vascularity Info provided: NR No. observers: 1; oncological radiologist (NR) Diagnosis: single |
Histology (CLND; SLNB)
FNAC (14/17 US positive underwent FNAC)
FU (n NR): no details
Histology interval: NR
FU interval: NR Exclusions: n = 0 |
|
Kell 2007
USA Patients: 37 Primary lesions: NR LNBs/Metastases: NR |
NC Retrospective (prospective database reported) Data: per pt Nodal mets: 9/37 = 24% | Primary (pre‐SLNB) Stage of disease: NR Inclusion: MM, BT > 0.75 mm, candidates for SLNB | Mean age: 61.4 years; Median age: NR; Range: NR Male: NR (0%). Site: NR Mean BT: 2.4 mm | PET‐CT. 2D/3D NR; CT (U) Scan coverage: base of skull to feet Contrast: U CT parameters: NR 18FDG: NR Breath hold: NR CT used for: NR Reconstruction: NR | Quantitative for areas of abnormally increased 18FDG uptake Info provided: NR No. observers: NR; no details. Diagnosis: NR |
Histology (37, 100%)
FNAC (0):
FU (n NR): no details
Histology interval: NR
FU interval: NR Exclusions: n = 0; 46 with SLNB but no PET‐CT could not be included |
| Klode 2010 Germany Patients: 61 Primary lesions: NR LNBs/Metastases: NR | NC Retrospective Data: per pt Nodal mets: 14/61 = 23% | Primary (pre‐SLNB) Stage of disease: NR (I or II) Inclusion: primary MM AJCC stage I or II (BT > 1 mm) | Mean age: 58.8; Median age: 61; Range: 31 to 82 Male: 36 (0.5901%). Site: trunk and lower limbs 26, 42.6%; upper extremities 9, 14.8%; NR for remaining 27 lesions BT: mean 2.62 mm, median 2.0 mm, range 1 to 8 mm | PET‐CT. 2D/3D NR; CE‐CT Scan coverage: cranial base to mid femur; additional views according to melanoma localisation Contrast: iodine‐containing contrast agent CT parameters: NR 18FDG: 349 mBq Breath hold: breath hold instructions NR CT used for: NR Reconstruction: NR | NR; hypermetabolic tumour focus Info provided: NR No. observers: NR; no details Diagnosis: NR |
Histology (61, 100%)
FNAC (N/A)
FU (61, 100%): median 38 months, 13 to 55 months
Histology interval: median 14 days PET to SLNB
FU interval: NR Exclusions: n = 0; 60 patients with SLNB did not agree to pre‐op PET |
| Kunte 2009 Germany Patients: 25 Primary lesions: 25 LNBs/Metastases: 68 LNBs; 35 SLNs |
NC
Prospective (Prosp database: N/A)
Data: per pt
Nodal mets: 6/35 = 17% Data: per SLN Nodal mets: 6/35 = 17% |
Primary (pre‐SLNB) (NR). Stage of disease: NR Inclusion: cutaneous MM SLNB candidates; 'mainly' ≥ 1.0 mm BT or RF (ulceration or regression or Clark level IV and V) | Mean age: 54; Median age: NR; Range: NR Male: 15 (60%). Site: Limbs 14, 56%; HN 2, 8%; trunk 9 36% BT: ≤ 1 mm 8, 32%; 1.01 to 2 mm 11, 44%; 2.01 to 4 mm 5 20%; > 4.0 mm 1, 4% | US. B‐mode; linear transducer (7.5 to 10MHz); US pre‐ and post‐LS Scan coverage: regional lymphatic basins Contrast: N/A Breath hold: regional LN basins | Qualitative presence of morphological features (described) Info provided: unclear; may be same dermatologists as for clinical exam No. observers: 2; dermatologists (experienced). Diagnosis: unclear |
Histology (51% n = 35)
FNAC (0):
FU (0): –
Histology interval: < 24 hours
FU interval: N/A Exclusions: n = NR |
|
Maubec 2007
France
Patients: 25 Primary lesions: 26 LNBs/Metastases: 20 from 19 pts |
NC
Prospective (Prosp database: N/A) Data: per pt Nodal mets: 7/20 = 35%; 1 FN identified on FU |
Primary (pre‐SLNB) Stage of disease: all T4; post surgery AJCC stage IIB 10, 40%; IIC 4, 16%; IIIA 4, 16%; IIIB 6, 24%; IIIC in 1, 4% Inclusion: any MM BT > 4 mm; SLNB planned if clinically node negative | Mean age: 60; Range: 14 to 87 Male: 15 (0.6%). Site: trunk 8, 32%; limbs 8; 32%; head and neck 9, 36% Mean BT 6.6 mm, range 4.8 to 12.5 mm | PET‐CT. 3D; CT (U) Scan coverage: WB; "top of the head to the mid‐thigh and included if necessary, the lower limbs" Contrast: U CT parameters: 110 kV; 80 mA; 5 mm 18FDG: 5 MBq/kg Breath hold: normal breathing; "no breath hold instructions" CT used for: NR; integrated system Reconstruction: iterative algorithm (FORE and AWOSEM) | Uptake site suspicious for malignancy or not clearly explained by a benign etiology (SUV estimated but does not appear to formally contribute to diagnosis) Info provided: NR No. observers: NR; no details. Diagnosis: NR |
Histology (22, 88%; 3 node positive underwent CLND; 19 had SLNB; 3 no surgery)
FNAC (N/A)
FU (25, 100%): mean 11 months (2 to 19 months)
Histology interval: NR
FU interval: NR Exclusions: n = 6; 3 clinically N+ underwent CLND (all PET+ and N+); 3 did not undergo any surgery |
|
Radzhabova 2009
Russia Patients: 152 Primary lesions: NR LNBs/Metastases: NR |
NC Design unclear Data: per pt Nodal mets: 11/52 = 21%; 2 FNS identified on FU | Primary (pre‐SLNB) Stage of disease: NR Inclusion: clinically node negative MM and SLNB (based on US result) | Mean age: NR; Median age: NR; Range: NR Male: NR (0%). Site: NR NR | US. B‐mode; sectoral and linear (7 to 10 MHz); pre‐LS Scan coverage: NR Contrast: N/A Breath hold: N/A | High PSV, EDV, Stuart index, and PI < 1000. Mets could not be excluded if PSV and PI were high but EDV = 0, Stuart index was undetectable (PI = pulse index, PSV = peak systolic volume, EDV = end‐diastolic volume, Stuart index) Info provided: NR No. observers: NR; no details. Diagnosis: NR |
Histology (52, 100%)
FNAC (0):
FU (NR):
Histology interval: NR
FU interval: NR Exclusions: n = 100; benign on US did not get SLNB |
|
Revel 2010
France Patients: 22 Primary lesions: 22 LNBs/Metastases: 21 |
WPC Retrospective Data: per pt Nodal mets: 10/20 = 50%; 2 FN identified on FU | Primary (pre‐SLNB) Stage of disease: stage I or II Inclusion: clinically node negative HN MM with pre‐SLNB PET‐CT Excluded if > 1 month between PET‐CT and SLNB | Mean age: 60. Range: 18 to 88 Male: 16 (73%). Site: scalp 5, 23%; cheek 3, 14%; cervical or neck 3, 14%; atrial region (ear, mastoid, temples) 6, 27%; palpebral or periorbital 4, 18%; frontal 1, 5% BT: 4.5 mm (0.26 to 10 mm) | PET‐CT. 2D/3D NR Scan coverage: WB; vertex to the toes Contrast: NR CT parameters: Biograph 2: 130 kV, 80 mAs Biograph 6: 130 kV, 4D Care Dose; Biograph 2: 5 mm Biograph 6: 4 mm 18FDG: 5.5 MBq/kg for Biograph 2; 4 MBq/kg for Biograph 6 True V; Flucis1, Schering, Cisbio International Breath hold: no breath hold instructions reported CT used for: appears to be used for attenuation correction; also describes anatomical localisation on fused images Reconstruction: OSEM 3D | Any hypermetabolic focus more intense than the surrounding background, including equivocal foci, compared with the corresponding anatomical structure on coupled CT Info provided: localisation of the initial tumour and the standard clinical and radiological assessment were known No. observers: 2; no details. Diagnosis: consensus of 2 |
Histology (22, 100%) FNAC (N/A) FU (22/22, 100%): mean 17 months (range 1 to 44)^ Histology interval: 12 days FU interval: NR Exclusions: n = 2; 2 test fails (no SN detected) |
| Sanki 2009 Australia Patients: 716 Primary lesions: NR LNBs/Metastases: 871 |
NC
Design unclear Data: per pt Nodal mets: 125/716 = 17% |
Primary (pre‐SLNB) Stage of disease: NR Inclusion: SLNB; BT > 1 mm or < 1 mm with adverse histological features, such as Clark’s level IV to V invasion, ulceration, or high mitotic rate | Mean age: NR; Median age: NR; Range: NR Male: NR (0%). Site: NR NR | US. B‐mode US; linear array transducer with high‐resolution small‐parts probe 5 to 10 MHz (linear transducer); 10 to 14 MHz (small parts probe); LS before US Scan coverage: sites marked by nuclear medicine physician during LS Contrast: N/A Breath hold: N/A | Re‐classification of original report as suspicious, or highly probable, e.g. increased vascular signature, rounding of the normal ovoid shape of the nodes, loss of normal hilar echoes, presence of focal low‐level subcapsular space echoes Info provided: NR No. observers: NR; nuclear medicine physician (NR) Diagnosis: single |
Histology (716, 100%)
FNAC (0)
FU (100% (SLNB; not ref standard for US)): 13.5 months (mean, 18.4 months)
Histology interval: SLN performed within 24 hours of LS and US
FU interval: NR Exclusions: n = 0 |
| Sibon 2007 France Patients: 131 Primary lesions: 132 LNBs/Metastases: NR; 189 SLNs | NC Retrospective (prospective database reported; plus prospective re‐interpretation of US images) Data: per pt Nodal mets: 35/133 = 26% |
Primary (pre‐SLNB) Stage of disease: NR Inclusion: SLNB; BT > 1 mm or < 1 mm with adverse histological features, such as Clark’s level IV to V invasion, ulceration, or high mitotic rate |
Mean age: 56; Range: 17 to 92 years
Male: 70 (53.4%). Site: arms 18, 13.6%, legs 43, 33%; trunk 48, 32%; hands/feet 10, 8%; HN 18, 14%
Mean BT 2.60 ± 2.91 mm Clark’s level II 8, 6%; III 30, 23%; IV 88, 66%, V 7, 5%; unknown 1, 1% |
US. B‐mode; linear transducer (6 to 12 MHz); US before LS Scan coverage: site of the excised primary melanoma scar and followed the paths of the lymphatic vessels to the lymph node area(s) Contrast: N/A Breath hold: N/A | Stringent criteria: circular/oval hypoechoic lymph node with a Solbiati index < 1.5 and no hyperechoic hilum; Non‐stringent criteria included the presence of 1 or 2 stringent criteria Info provided: NR for original interpretation or for re‐interpretation No. observers: NR; 1 radiologist reviewed all images; radiologist (high) Diagnosis: single |
Histology (131, 100%)
FNAC (‐):
FU (n NR): no details
Histology interval: NR
FU interval: NR Exclusions: n = 0; using stringent criteria, US detected 1/24 micro‐metastases < 2 mm (as measured by US) and 2/11 macro‐metastases ≥ 2 mm (both > 5 mm) |
| Singh 2008 Germany Patients: 52 Primary lesions: NR LNBs/Metastases: 67 LNBs; 111 SLNs |
NC
Unclear
Data: per pt
Nodal mets: 14/52 = 27% > 4 mm BT: 7/12 = 58% ≤ 4 mm BT: 7/40 = 18% |
Primary (pre‐SLNB) Stage of disease: all I or II Inclusion: primary MM undergoing SLNB (all > 1 mm) | Mean age: 55; Median age: 61; Range: 17 to 76 Male: 36 (69 %). Site: extremities 23, 44%; trunk 16, 31%; HN 13, 25% Mean BT 3.46 mm, range 1.0 mm to 12.0 mm | PET‐CT. Helical, CT (CE, dual detector) Scan coverage: WB; base of skull to tip of toes in 3 parts Contrast: Peritrast‐oral‐GI; Kohler Chemie GmbH, Alsbach, Germany CT parameters: 130 kV, 40 mAs; 5 mm 18FDG: 370 ± 40 MBq 18FDG through an anterior cubital vein Breath hold: limited breath hold for CT and shallow breathing for PET CT used for: attenuation correction based on re‐scaling of CT image; image fusion Reconstruction: iterative (not further detailed) | Any focal uptake more than background unless it was found to be a false positive focus (physiological accumulation or brown fat tissue) in fusion imaging Info provided: NR No. observers: 2; two experienced observers assessed 18FDG PET‐CT fusion imaging independently; also refers to team of radiologists and nuclear physicians (experienced) Diagnosis: consensus |
Histology (52, 100%)
FNAC (N/A)
FU (n NR): no details
Histology interval: PET before LS before SLNB
FU interval: NR Exclusions: n = 0; 2 TP both BT ≥ 4 mm and FPs < 4 mm |
| van Rijk 2006 Netherlands Patients: 107 Primary lesions: 107 LNBs/Metastases: NR; 37 D+ in 42 LNBs | WPC Retrospective Data: per pt Nodal mets: 37/107 = 35% | Primary (pre‐SLNB) Stage of disease: NR Inclusion: SLNB candidates; cutaneous MM BT > 1 mm or Clark ≥ level IV | Mean age: 50; Range: 15 to 52 Male: 57 (53%). Site: HN 6, 6%; trunk 43, 40%; arm 24, 22%; leg 34, 32% median BT 2.0 mm (0.6 to 12.5 mm) Clark’s level II 1, 1%; III 37, 35%; IV 55, 51%; V 9, 8%; undeterminable 5, 5% |
US. B‐mode linear array (7.5 MHz; 6 to 12 MHz); US before LS
Scan coverage: NR
Contrast: N/A
Breath hold: N/A US + FNAC for US positive |
Suspicious ‐ length–depth ratio < 2, conversion of a fatty hilum to a hypoechoic hilum, substantial cortical asymmetry or a focal area of low‐level echoes in the subcapsular sinus of the node and diameter > 5 mm for LN of the neck Info provided: NR No. observers: NR; no details Diagnosis: NR |
Histology (107, 100%)
FNAC (22; not part of ref standard)
FU (2/107; 2% (reported only for 2 positive on FNAC)): NR
Histology interval: NR
FU interval: NR Exclusions: n = 0; FU of 2 FNAC positive participants is reported but no further reference is made to any recurrences |
| Voit 2014 Germany Patients: 1000 Primary lesions: 1000 LNBs/Metastases: NR | WPC Design unclear Data: per pt Nodal mets: 208/1000 = 21% | Primary (pre‐SLNB) Stage of disease: NR Inclusion: SLNB candidates; BT > 1 mm thickness, or Clark IV/V, ulcerated and/or regressed | Mean age: 59; Median age: 62; Range: 15 to 94 Male: 567 (57%) Site: NR Mean BT 2.58 mm; median BT 1.57 mm BT < 1 mm 288 29%; 1 to 2 mm 308 31%; 2 to 4 mm 231 23%; > 4 mm 173 17% Clarks II 32 3%; III 341 34%; IV 554 56%; V 54 6%, unknown 13 1% |
US. B‐mode & Doppler (1 to 18 MHz); LS before US
Scan coverage: LNBs; patients first underwent a lymphoscintigraphy, which assists the ultrasonographist to better focus the examination
Contrast: NA US + FNAC for US positive |
Malignant if total loss of central echoes (LCE) or LN enlarged and balloon shaped (BS); suspicious if peripheral perfusion present or central echo wandering towards the rim Info provided: NR No. observers: 3; ultrasonographist (mixed; 1 expert and 2 trained but less expert) Diagnosis: unclear; likely single? |
Histology (1000, 100%)
FNAC (332, 33%; authors report as 342, including 10 US malignant as FNAC positive even though no FNAC was undertaken):
FU (1000; 100%): mean 56 m; median 53 m; range 1 to 132 m
Histology interval: NR
FU interval: NR Exclusions: n = 0 |
| Wagner 2012 France Patients: 48 Primary lesions: 48 LNBs/Metastases: NR | NC Retrospective Data: per pt Nodal mets: 14/43 = 33% | Primary (pre‐SLNB) Stage of disease: stage IIA 8, 16.7%; stage IIB 19, 39.6%; stage IIC 19, 39.6%; 2, 4.2% NR Inclusion: SLNB candidates; BT ≥ 4 mm or BT > 1 mm with ulceration | Mean age: NR; Median age: NR; Range: NR Male: 25 (52%). Site: NR Mean BT 7.6 mm (±4.5) (range 1.1 to 18 mm) | PET‐CT. 2D; CT (NR) Scan coverage: WB; not further described Contrast: NR CT parameters: 140 kV, 200 mA (attenuation correction and anatomical correlation); 7.5 mm 18FDG: 370MBq (Glucotep Cyclopharma, St Beauzire, France) Breath hold: normal breathing; "remain rested, to refrain from speaking, and to minimize swallowing" CT used for: attenuation correction and anatomical correlation Reconstruction: iterative OSEM (Ordered Subset Expectation Maximization) algorithm (3 iterations; 10 subsets) | Abnormally increased 18FDG uptake in a lymph node in the drainage territory of the melanoma Info provided: aware of all clinical findings No. observers: NR; nuclear medicine specialist (high) Diagnosis: unclear; 'at least one' |
Histology (43, 89.6%; 2 CLND only, 1 SLNB + CLND, 40 SLNB only)
FNAC (N/A)
FU (1): min 12 months
Histology interval: NR
FU interval: NR Exclusions: n = 5; SLNB not performed for technical reasons |
| +: positive; AJCC: American Joint Cancer Committee; AWOSEM: attenuation weighted ordered subsets expectation maximisation; BT: Breslow thickness; CE: contrast enhanced; CLND: complete lymph node dissection; CT: computed tomography; 2D: two‐dimensional; 3D: three‐dimensional; EDV: end‐diastolic volume; 18FDG: 2‐deoxy‐2‐[18F]fluoro‐D‐glucose; FNAC: fine needle aspiration cytology; FORE: Fourier rebinning; FU: follow‐up; HN: head and neck; LN: lymph node; LNB: lymph node basin; LND: lymph node dissection; LS: lymphoscintigraphy; mA: measure of tube current; mets: metastases; MM: malignant melanoma; NC: non‐comparative; OSEM: ordered subsets expectation maximisation algorithm; PET: positron emission tomography; PI: pulse index; PSV: peak systolic volume; prosp: prospective; RF: risk factor; SD: standard deviation; SLN: sentinel lymph node; SLNB: sentinel lymph node biopsy; SSM: superficial spreading melanoma; SUV: standardised uptake value; U: unenhanced; US: ultrasound; WB: whole body. | ||||||
Appendix 9. Characteristics of studies of whole body imaging by population group (primary staging, re‐staging, and mixed or unclear populations)
| Study Country Pt/lesion numbers | Study design Outcome Prevalence | Presentation Inclusion criteria Imaging eligibility | Age, gender, site, BT, Clark | Index test | Threshold Observers |
Reference Exclusions Other result |
| PRIMARY STAGING OF DISEASE | ||||||
| Arrangoiz 2012 USA Patients: 56 Primary lesions: 56 LNBs/Metastases: NR | NC
Retrospective (Prosp database: NR) Data: per pt Any mets (NR; scan incl head): 32/56 = 57% Nodal: 29/56 = 52% Distant mets : 5/56 = 9% |
Primary (N/A) Stage of disease: all T4 and clinically node negative and negative for distant metastases Inclusion: node negative; BT > 4 mm |
Mean age: 67; Median age: NR; Range: 26 to 89
Male: 32 (57.1428571428571%) Site: trunk 16, 29%; extremities 28, 50%; head and neck 12, 21% BT median 6 mm; mean 9 mm; range 4.1 to 40 mm |
PET‐CT. 2D or 3D; CT (U, helical, low dose) Scan coverage: WB; vertex of the head down to feet for all patients Contrast: U CT parameters: Discovery LS ‐ 140 kVp, 90 mA; Siemens Biograph ‐ 130 kVp, 100 mA; 5 mm FDG: 15 mCi (IV) Breath hold: normal breathing CT used for: attenuation correction; co‐registered images Reconstruction: Discovery LS ‐ OSEM algorithm with 28 subsets and 2 iterations Siemens Biograph ‐ TrueX algorithm with 21 subsets and 2 iterations | SUV of 2.5 Info provided: NR No. observers: NR; 'in‐house medical physicist' mentioned; NR; 'in‐house medical physicist' mentioned (NR) Diagnosis: unclear |
Histology (54, 96% (48 SNB and 6 LND))
FNAC (NR):
FU (NR): NR
Histology interval: NR; states that 6 "proceeded directly to therapeutic lymph node dissection" after PET
FU interval: NR Exclusions: n = 0; N/A |
|
Hafner 2004 Switzerland Patients: 101 Primary lesions: 101 LNBs/Metastases: 105 LNBs; 136 SLNs |
WPC
Prospective Data: per pt Nodal mets: 26/100 = 26% |
Primary (N/A). Stage of disease: NR; stage IV (evidence of distant mets) excluded Inclusion: any cutaneous MM with BT ≥ 1 mm without evidence of detectable distant metastasis (includes clinically palpable) |
Mean age: NR; Median age: 55; Range: 18 to 79
Male: 55 (55%) Site: limbs 49, 49%, trunk 35, 35%, HN 16, 16% BT: 1.01 to 2 mm 38; 2.01 to 4 mm 43; > 4.0 mm 19 |
US. B‐mode (5 Mhz); US before LS Scan coverage: regional lymph nodes of the groins, axillae, and neck (abdominal US also performed) | NR; 'radiologically suspect' Info provided: unclear No. observers: 1; radiology resident (NR) Diagnosis: single (supervision by senior staff radiologist) |
Histology (100; 100%)
FNAC (NR (abstract reports 3 LN mets identified on physical exam, 2 of which were detected by US)):
FU (NR): 20 months (8 to 39)
Histology interval: 2 weeks
FU interval: 6 months Exclusions: n = 1; sentinel node was not found intraoperatively No confirmed distant mets detected at time of imaging; 9 patients with suspicious findings on imaging were negative for progression/recurrence at 12 months |
|
Kang 2011 S Korea Patients: 37 Primary lesions: 37 LNBs/Metastases: NR |
NC
Retrospective (Prosp database: no; medical record review) Data: per pt Any mets (incl brain): 9/37 = 24% |
Primary (N/A) Stage of disease: stage 0: 7 (18.9%); stage I: 6 (16.2%); stage II: 17 (45.9%); stage III: 6 (16.2%); stage IV: 1 (2.7%) Inclusion: newly diagnosed cutaneous MM undergoing staging work‐up with PET‐CT (any stage, including clinically node positive) |
Mean age: 61.7 years; Median age: NR; Range: 48.1 to 75.3; ± 13.6 years
Male: 17 (45.9%) Site: hand/foot 23 (62.1%), trunk 6 (16.2), head/neck 4 (10.8%), extremity 4 (10.8%) BT < 1.0 mm 8, 22%; ≥ 1 mm 15, 41%; NR 14, 38% |
PET‐CT. CT (U, 6 slice or 16 slice) (N/A) Scan coverage: vertex of skull to knees; plus lower limbs if with lower leg MM Contrast: U CT parameters: Reveal RT‐HiRez: 130 kV, 95 mA Discovery ST: 140 kV, 160 mA; Reveal RT‐HiRez: 2.5 mm Discovery ST: 3.75 mm FDG: 350 to 400 MBq Breath hold: NR; 'standard protocol' CT used for: unclear; combined PET‐CT unit, mentions identification of anatomical location on fused PET‐CT image Reconstruction: ordered subset expectation‐maximisation | SUVmax ≥ 2.2 Info provided: NR No. observers: 2; nuclear physicians (experienced) Diagnosis: consensus of 2 |
Histology (6 (16.2%))
FNAC (0)
FU (37 (100%)): median followup 24.3 ± l l.7 months (range 8 to 55 months)
Histology interval: NR
FU interval: 3 months Exclusions: n = 0 |
|
Maubec 2007 France Patients: 25 Primary lesions: 26 LNBs/Metastases: NR |
WPC
Prospective Data: per pt Any (incl brain): 7/25 = 28% |
Primary (N/A). Stage of disease: all T4; 3 clinically node positive Post surgery: AJCC stage IIB 10, 40%; IIC 4, 16%; IIIA 4, 16%; IIIB 6, 24%; IIIC in 1, 4% Inclusion: any MM BT > 4 mm; SLNB planned if clinically node negative | Mean age: 60; Range: 14 to 87 Male: 15 (0.6%). Site: trunk 8, 32%; limbs 8; 32%; head and neck 9, 36% Mean BT 6.6 mm, range 4.8 to 12.5 mm | PET‐CT. 3D; CT (U) Scan coverage: WB; "top of the head to the mid‐thigh and included if necessary, the lower limbs" Contrast: U CT parameters: 80 mA; 110 kV; 5 mm FDG: 5 MBq/kg Breath hold: normal breathing; "no breath hold instructions" CT used for: NR; integrated system Reconstruction: iterative algorithm (FORE and AWOSEM) with 2 iterations, 8 subsets, and a 5 mm full‐width half maximum (FWHM) Gaussian postfilter | Uptake site suspicious for malignancy or not clearly explained by a benign etiology (SUV estimated but does not appear to formally contribute to diagnosis) Info provided: NR No. observers: NR; NR (NR) Diagnosis: NR |
Histology (22, 88%; 3 node positive underwent CLND; 19 had SLNB; 3 no surgery)
FNAC (N/A)
FU (25, 100%): mean 11 months (2 to 19 months)
Histology interval: NR
FU interval: NR Exclusions: n = 0; all recruited pts can be included in analysis for any mets. 3 PET +ve for distant mets (1 orbital, 1 thyroid, 1 liver) |
|
Prayer 1990 Austria Patients: 217 Primary lesions: NR LNBs/Metastases: NR |
WPC
Unclear (Prosp database: NR) Data: per pt Nodal mets: 29/217 = 13% |
Primary Stage of disease: NR Inclusion: primary MM investigated either before or after removal of the primary melanoma in postoperative follow‐up |
Mean age: 56; Median age: NR; Range: 25 to 82
Male: 104 (47.926267281106%) Site: HN 42, 19%; arm 61, 28%; shoulder 23, 11%; leg 91, 42% BT < 0.75 mm 25, 12%; 0.75 to 1.5 mm 96, 44%; 1.5 to 3.00 mm 79, 36%; > 3 mm 17, 8% Clark level II 936, III 89, IV 33 |
US. B‐mode (7.5 MHz); N/A Scan coverage: primary LNs depending on tumour localisation. Cervical (42); axillary (84); inguinal (91) Contrast: N/A | Suspicious ‐ circular and oval masses with poor echo; longitudinally configurated LNs with echogenic eccentric hilum regarded as “enlarged reactively” Info provided: unclear; different clinicians for palpation (dermatologist) and for US (radiologist) No. observers: 1; radiologist (NR) Diagnosis: single |
Histology (29, 13%)
FNAC (0)
FU (188, 87%): 6 months
Histology interval: NR
FU interval: 2 months Exclusions: n = 0 There were no false‐negative sonographic results i.e. melanoma metastases did not occur within the following 6 months in any of the patients classified as having no suspect regional lymph nodes. The smallest metastasis detected by ultrasound was 11 mm in diameter |
| Veit‐Haibach 2009 Germany Patients: 56 Primary lesions: 56 LNBs/Metastases: NR |
WPC
Prospective Data: per pt Nodal: 13/56 = 23% Distant (brain NR): 12/56 = 21% |
Primary (N/A) Stage of disease: on presentation: stage I or II 44, 79%; stage III or IV 12, 21% Inclusion: any primary MM referred for PET‐CT Excluded if insufficient FU |
Mean age: 62 years; Median age: Range: 23 to 86 years
Male: 27 (48.2142857142857%) Site: trunk 26, 46%; upper extremities 10, 18%; lower extremity 18, 32%; HN 2, 4% NR |
CT. CE; two slice (N/A); NR Scan coverage: WB; no further detail, just states caudocranial direction Contrast: dual phase injection of 140 mL of 300 mmol/mL iodinated contrast agent (90 mL at a rate of 3 mL/s, and 50 mL at a rate of 1.5 mL/s; dual phase used to ensure fully diagnostic (portal venous phase) CT data in the abdomen) CT parameters: NR; NR Breath hold: NR PET‐CT. full ring‐CT (CE; 2 slice) (N/A); NR Scan coverage: WB; no further detail, just states caudocranial direction Contrast: 140 mL of 300 mmol/mL iodinated contrast agent CT parameters: NR; NR FDG: 330 to 350 MBq Breath hold: NR CT used for: attenuation correction Reconstruction: reconstructed iteratively (FORE‐OSEM, 2 iterations, 8 subsets, 128×128 matrix with 5 mm gaussian smoothing) |
CT: Lesion size and central necrosis for malignancy; fatty hilum and calcifications for benign. For size: short‐axis diameter threshold of 1.5 cm for jugulodigastric and precarinal LNs and threshold of 1 cm for all other LNs of the neck, thorax, and abdomen [16] PET‐CT: increased glucose metabolism and independent of their size Info provided: provided patient‐specific clinical background (first diagnosis of melanoma, postsurgical resection status, location of the resection site) but blinded to clinical exam and histopathology of primary tumour No. observers: 2; radiologists (NR). Diagnosis: consensus of 2 |
Histology (unclear; 14 with SLNB, 25%)
FNAC (0)
FU (56, 100%): mean 780 days (range 102 to 1390 days); roughly equivalent to 25.6 months (3.3 to 45.7 months)
Histology interval: 4 weeks for SLNB
FU interval: NR Exclusions: n = 0 |
| RE‐STAGING OF DISEASE | ||||||
|
Iagaru 2007 USA Patients: 106 Primary lesions: NR LNBs/Metastases: 139 |
WPC
Retrospective (Prosp database: NR) Data: per pt Any mets (incl skin and brain): 56/106 = 53% (all tests) Data: per lesion Any mets (incl skin and brain): 87/139 = 63% |
Re‐staging (all patients had the study requested for disease re‐staging) Stage of disease: NR; 76 stage I to IIIc and 30 stage IIIb to IV) Inclusion: PET‐CT for MM re‐staging Excluded if NR ORNR PET‐CT for MM re‐staging |
Mean age: 56.8 ± 15.9 Median age: nr; Range: 20 to 87
Male: 68 (64.1%) Site: NR BT at initial diagnosis (n = 76): mean 3.56 mm, 0.4 to 25 mm; < 1 mm in 6 (8%), 1 to 4.0 mm 58 (76%), > 4 mm 12 (16%) Clark's level (n = 70): 3 (4%), level II; 13 (19%), level III; 43 (61%), level IV; 11 (16%), level V |
CT. U, multislice helical (N/A)
Scan coverage: WB; top of the head to the ankles
Contrast: N/A
CT parameters: 140 kV, 40 mA; 5 mm
Breath hold: no breath hold instructions reported PET‐CT. 2D; CT (U, multi‐slice helical) (N/A) Scan coverage: WB ; top of the head to the ankles Contrast: U CT parameters: 140 kV, 40 mA, 5 mm FDG: 15 mCi Breath hold: no breath hold instructions reported CT used for: attenuation correction and anatomical localisation Reconstruction: OSEM, 2 iterative steps, 28 subsets |
CT: NR PET‐CT: SUVmax ≥ 2.5 Info provided: NR for original interpretation or for re‐interpretation No. observers: NR; board‐certified nuclear medicine physicians and radiologists (board certified) Diagnosis: consensus |
Histology (97, 91.5%)
FNAC (N/A)
FU (9, 8.5%): NR
Histology interval: NR
FU interval: NR Exclusions: n = 0; N/A |
| Rubaltelli 2011 Italy Patients: 436 Primary lesions: NR LNBs/Metastases: NR |
WPC
Unclear Data: per pt Nodal mets: 13/436 = 3% |
Re‐staging (all undergoing postoperative follow‐up designed to ensure the early identification of lymph node metastases) Stage of disease: NR Inclusion: cutaneous MM with US of regional LNs as part of a follow‐up; those with 'common signs of malignancy' on B‐mode US were excluded |
Mean age: 54; Median age: 58; Range: 27 to 81 years
Male: Full sample: 240 (52%) Site: NR |
US. B‐mode; linear array transducers (7.5 to 13 MHz)
Scan coverage: variable: axillary lymph nodes for MM of the upper limbs, inguinal lymph nodes for MM of the lower limbs, both axillary and inguinal lymph nodes for MM of the trunk, and cervical and supraclavicular lymph nodes for MM of the head and neck (72 neck, 248 axillary, and 354 inguinal LNBs were examined)
Contrast: N/A US. contrast‐enhanced US (7.5 to 13 MHz) Scan coverage: LNBs identified on B‐mode US Contrast: sulfur hexafluoride microbubbles (SonoVue, Bracco) |
US: focal hypoechoic cortical thickening ‐ a focal area of cortex at least twice as thick as the cortex in the remainder of the same lymph node CE‐US: perfusion defects corresponding to the cortical focal thickening; homogeneous intense enhancement of the cortex considered benign Info provided: NR No. observers: 1 of 3; sonologist (high) Diagnosis: single |
Histology (13, 3%)
FNAC (436, 100%)
FU (31/44 FNAC negative, 70%): 6 to 16 months (median, 10 months)
Histology interval: NR
FU interval: NR Exclusions: n = 24; definite signs of malignancy on B‐mode US |
|
Strobel 2007a Switzerland Patients: 47 Primary lesions: 47 LNBs/Metastases: NR |
NC
Retrospective (Prosp database: NR) Data: per pt Any (incl brain): 39/47 = 83% |
Re‐staging (all pts followed up according to updated Swiss melanoma guidelines) Stage of disease: NR Inclusion: high risk melanoma (BT > 4 mm, or Clark level III or IV, or known resected metastases) and raised S100 (> 0.2 μg/L) undergoing follow‐up after primary treatment Excluded if PET‐CT and S100B measurement > 2 weeks apart; treatment initiated between PET‐CT and tumour marker measurement; or systemic therapy before the PET‐CT investigation | Mean age: 58.4 years; Median age: Range: 20 to 83 years Male: 20 (42.5531914893617%) Site: NR BT 1.02 mm to 15 mm; unknown in 9 | PET‐CT. 2D PET, CT (CE, multi‐slice, helical) Scan coverage: head to the knees with scanning of the lower legs for patients with primary tumours of the lower extremities Contrast: oral CT contrast agent given 15 minutes before injection of 18F‐FDG CT parameters: 140 kV, 40 mAs, 4.25 mm FDG: 370 to 400 MBq Breath hold: CT: breath holding in the normal expiratory position CT used for: attenuation correction, fused Reconstruction: standard iterative algorithm (OSEM) | FDG uptake clearly greater than background and established morphological CT criteria; if a focal FDG‐active lesion was detected, the exact anatomical localisation was determined on the fused PET‐CT images. Lesions with 18F‐FDG uptake in physiological sites or benign variants, e.g. muscles, brown fatty tissue or pulmonary infiltrations, were determined as benign Info provided: blinded to serum S100B No. observers: 2; nuclear radiology physicians (experienced) Diagnosis: consensus of 2 |
Histology (29, 62%; 20 distant mets and 9 LN mets)
FNAC (4, 8.5%)
FU (47, 100%): minimum of 6 months (range 6 to 18 months in all patients
Histology interval:
FU interval: 3 months Exclusions: n = 0; N/A Reports characteristics of those with elevated S100 but not mets detected on imaging |
| STAGING IN MIXED OR UNCLEAR POPULATIONS | ||||||
| Abbott 2011 UK Patients: 34 (microscopic group 20; macroscopic group 14) Primary lesions: 34 LNBs/Metastases: NR | NC
Retrospective (Prosp. database used) Data: per pt Any (excl brain): 7/34 = 21% |
Mixed (primary/FU) Stage of disease: IIIA 18, 53%; IIIB 10, 29%; IIIC 6, 18% Inclusion: stage III: micro‐metastases on SLNB or clinically detectable nodal metastases on diagnosis or FU | Mean age: NR; Median age: microscopic group: 50; macroscopic group: 63 Range: microscopic group: 19 to 74 years Macroscopic group: 48 to 79 years Male: microscopic group: 14 Macroscopic group: 6 (Microscopic group: 70% Macroscopic group: 43%%) Site: HN 1 (3%), upper extremity 3 (9%), trunk 20 (59%), lower extremity 10 (29%) BT (mean): microscopic group 2.27 mm (1.2 to 9.7 mm); macroscopic group 2.01 mm (1.0 to 13 mm) | PET‐CT. 2D; CT (NR) Scan coverage: skull base to upper thigh Contrast: NR CT parameters: NR; NR FDG: 400 MBq Breath hold: NR CT used for: attenuation correction and lesion localisation Reconstruction: iterative technique using an OSEM algorithm | Clearly indicative/highly suspicious for malignancy considered positive Info provided: clinical ‐ NR; other tests ‐ NR No. observers: NR; nuclear medicine consultants (experienced) Diagnosis: NR | Histology (5, 15%)
FNAC (0)
FU (34, 100%): microscopic mean 38 months (21 to 54 months); macroscopic mean 34 months (15 to 52 months)
Histology interval: NR
FU interval: 3 months Exclusions: n = 0 |
|
Aukema 2010a Netherlands Patients: 46 Primary lesions: NR LNBs/Metastases: NR |
NC
Retrospective (Prosp database: NR) Data: per pt Any (brain NR): 23/46 = 50% |
Mixed (imaged on recurrence or after primary melanoma treatment) Stage of disease: NR; unfavorable primary tumour (n = 6); primary melanoma with simultaneous nodal metastases (n = 18); unknown primary melanoma with nodal metastasis (n = 2); locoregional recurrence (n = 15); distant recurrence (n = 5) Inclusion: raised S100 during FU after resection of nodal or distant metastases or with high risk primary tumour |
Mean age: 59; Median age: ; Range: 25 to 93 years
Male: NR (0%) Site: NR NR |
PET‐CT. NR; CT (U) (N/A) Scan coverage: whole body; not described Contrast: U CT parameters: kV NR; 40 mAs, 5 mm FDG: 180 to 240 MBq (4.9 to 6.5 mCi) Breath hold: No breath hold instructions reported CT used for: attenuation correction; PET fused to low‐dose CT Reconstruction: NR | NR; "hypermetabolic lesions" Info provided: NR No. observers: 3; nuclear medicine physicians (experienced) Diagnosis: consensus of 3 |
Histology (13, 28.3%)
FNAC (0)
FU (33, 71.7%): for D negative only (n = 19): median 12 months (4 to 32 months); NR for full sample
Histology interval: NR
FU interval Exclusions: n = NR Other result: "MRI revealed 2 brain metastases of 2 and 4 mm in 1 patient (2%). This patient also had other distant metastases that were detected by PET‐CT" |
|
Aukema 2010b Netherlands Patients: 70 Primary lesions: 70 LNBs/Metastases: 73 |
NC
Prospective Data: per pt Any mets: 30/70 = 43% |
Unclear (N). Stage of disease: ≥ stage IIIb (all with clinically palpable nodes) Inclusion: clinically node positive with no sign of distant metastases; primary/re‐staging NR |
Mean age: 58; Median age: NR; Range: NR
Male: 37 (0.54%) Site: upper extremity 4, 6%; lower extremity 37, 53%; trunk 19, 27%; head/neck 9, 13%; unknown primary 1, 1% BT: median 3 mm |
PET‐CT. 2D ; CT (U) (N/A) Scan coverage: WB according to primary lesion site (i.e. IRT inclusion of cranium or lower extremities) Contrast: U CT parameters: kV NR; 40 mAs, 5 mm FDG: 180 to 240 MBq Breath hold: no breath hold instructions reported CT used for: attenuation correction; PET fused to low‐dose CT Reconstruction: PET was fused with the low‐dose CT after correction for attenuation | NR; "metabolically active" Info provided: NR No. observers: 3; nuclear medicine physicians (NR) Diagnosis: consensus of 3 |
Histology (NR; 11 with histology or cytology)
FNAC (NR; 11 with histology or cytology):
FU (59; 84%): NR; ≥ 6 months
Histology interval: N/A
FU interval: NR Exclusions: n = 0 Other result: MRI detected brain mets in 5 pts, no reference standard reported |
| Bastiaannet 2009 Netherlands Patients: 251 Primary lesions: 251 LNBs/Metastases: NR |
WPC
Prospective Data: per pt Distant mets: 78/251 = 31% |
Mixed (primary (LN mets diagnosed at time of primary diagnosis) 39, 15.5%; recurrence (LN mets identified ≤ 3 years since primary dx) 145, 57.8%; recurrence > 3 years since primary dx 67, 26.7%) Stage of disease: III (100%) Inclusion: node positive (clinical or histology/cytology proven) candidates for CLND; imaging to id further disease |
Mean age: 56.9 years (n = 253); Median age: NR; Range: 19 to 93 years (reported in Bastiannet 2012)
76 (30.3%) < 50 years; 99 (39.4%) 50 to 65 years; 76 (30.3%) > 65
Male: 152 (0.606%) Site: HN 29, 11.6%; upper extremities 26, 10.4%; trunk 93, 37.0%; lower extremities 88, 35.0%; unknown primary 15, 6.0% BT: ≤ 1 mm 32, 12.8%; 1.0 to 2.0 73, 29.1%; ≥ 2.0 129, 51.4%; unknown primary 15, 5.9%; missing 2, 0.8% Clark level: I/II/III (n = 84; 33.5%), IV/V (n = 144; 57.4%), unknown primary (n = 15; 5.9%), missing (n = 8; 3.2%) |
CT. CE, spiral, multi‐slice Scan coverage: chest, abdomen plus neck for those with LN in the neck Contrast: oral and IV CT parameters: NR; NR; 'multi‐slice' Breath hold: no breath hold instructions reported | NR (presence/absence of mets) Info provided: NR No. observers: NR; attending staff nuclear medicine physicians (NR) Diagnosis: NR |
Histology (NR)
FNAC (NR)
FU (251, 100%): median 13.7 months; minimum 6 months stated for index test positive, NR for index test negative
Histology interval: NR
FU interval: NR Exclusions: n = 8; excluded due to follicular structure (n = 1), > 13 years between primary and lymph nodes (n = 3), incidence abroad (n = 1), mucosal melanoma (n = 2), and primary melanoma treated as benign lesion (n = 1) (1) accuracy of PET alone, (2) change in treatment resulting from PET and/or CT |
|
Cachin 2014 France Patients: 87 Primary lesions: 176 LNBs/Metastases: check entry |
NC
Prospective Data: per pt Any (incl brain, subcut mets): 39/67 = 58% Data: per lesion Any (incl brain): 85/176 = 48% Nodal: 20/39 = 51% Distant (incl brain): 65/137 = 47% Bone: 14/34 = 41% Lung: 10/27 = 37% Soft tissue: 16/25 = 64% Skin: 7/9 = 78% Brain: 7/9 = 78% |
Mixed (states imaging was for staging or for re‐staging ). Stage of disease: NR; 45 (51% were diagnosed with melanoma mets on study Inclusion) Inclusion: prior history of cutaneous or ocular MM undergoing staging or restaging including: (a) newly diagnosed at any TNM stage, (b) known visceral or cutaneous MM metastases with unknown primary tumour, or (c) MM without metastases (included to assess test specificity) |
Mean age: NR; Median age: NR; Range: NR
Male: 42 (48.3%) Site: NR Breslow thickness (mm): < 1.0: 12, 13.8%; 1.0 to 2.0: 34, 39.1%; ≥ 2.0, 41, 47.1% Clark level: I 3, 3.4%; II 2, 2.3%; III 20, 23.0%; IV 46, 52.9%; V 3, 3.4%; not known 13, 14.9% |
PET‐CT. NR; SPECT used in 4 of 8 centres Scan coverage: WB (not further described) Contrast: NR CT parameters: SPECT; N/A FDG: 3 to 5 MBq/kg Breath hold: NR CT used for: PET 'correlated' with CT abnormalities Reconstruction: iterative in 6 of 8 centres; filtered backprojection in 2 of 8 centres | PET. Positive if there was focal uptake greater than mediastinal or liver uptake that could not clearly be related to physiological processes. Negative when a normal distribution of tracer was observed, even if the CT scan showed abnormalities. Bone accumulations were considered positive when the uptake was higher than in normal bone marrow. Any instance of equivocal PET uptake was considered positive Info provided: NR No. observers: NR; nuclear physician (experienced) Diagnosis: single |
Histology (25; 28.7%)
FNAC (N/A)
FU (87, 100%): at least 6 months
Histology interval: NR
FU interval: NR Exclusions: n = 20; 12 did not undergo FDG PET due to imaging cancellation; 8 are unaccounted for (text describes 75 having PET but reports results for only 67) |
| Dellestable 2011 France Patients: 40 Primary lesions: 40 LNBs/Metastases: NR; 72 lesions |
WPC
Prospective
Data: per lesion
Any (incl brain): 72/119 = 61% (CT) 70/117 = 60% (MRI) 72/119 = 61% (PET‐CT) Nodal: 31/39 = 79% (CT) 31/40 = 78% (MRI) 31/38 = 82% (PET‐CT) Bone: 14/17 = 82% (CT) 14/16 = 88% (MRI) 14/17 = 82% (PET‐CT) Liver: 4/21 = 19% (CT) 4/26 = 15% (MRI) 4/25 = 16% (PET‐CT) Lung: 13/16 = 81% (CT) 13/14 = 93% (MRI) 13/15 = 87% (PET‐CT) |
Mixed (both primary staging and FU; breakdown reported but not legible on pdf)
Stage of disease: AJCC I to II 11, 27.5%; AJCC III to IV 29, 72.5% Inclusion: PET‐CT for primary staging or follow‐up of MM, regardless of AJCC stage or indication for examination |
Mean age: 57 years; Median age: Range: 27 to 85 years Male: 20 (0.5%). Site: NR BT mean: 3.2 mm, median 2.7 mm, range 0.6 to 11 mm |
CT. CT (N/A); NR
Scan coverage: skull, neck, thorax, abdomen, and pelvis
Contrast: iodised injection was administered by the same venous route as for the previous examinations
CT parameters: NR; NR
Breath hold: no breath hold instructions reported MRI. WB, DW, T2STIR, CE 3D gradient echo (N/A); NR Scan coverage: WB; head to lower limbs Contrast: gadolinium injection MRI parameters: T2STIR, T1, diffusion and 3D gradient echo T1 after gadolinium injection; 1.5 T Breath hold: no breath hold instructions reported PET‐CT. NR; CT (CE) (N/A); NR Scan coverage: WB; top of the skull to the feet Contrast: unclear; contrast is reported for CT; however CT component of PET‐CT is not clear CT parameters: NR; NR FDG: 5.5 MBq/kg Breath hold: no breath hold instructions reported CT used for: attenuation correction and anatomical registration Reconstruction: NR |
CT: NR MRI: NR PET‐CT: focal uptake; unusual location or visual or quantitative intensity (SUV measurement) Info provided: NR No. observers: 3; NR (NR) Diagnosis: single with consensus if the results of any modality disagreed |
Histology (36 lesions, 28% of 128)
FNAC (0)
FU (72, 56%): > 4 months
Histology interval: NR
FU interval: NR Exclusions: n = 20 lesions; 4 lesions with indeterminate reference and 16 not picked up by CT |
|
Hausmann 2011 Germany Patients: 50 eligible; 33 included Primary lesions: 50 LNBs/Metastases: NR |
WPC
Prospective Data: per lesion Any mets (excl brain): 455/824 = 55% (all tests) Nodal: 192/379 = 51% Distant: 263/445 = 59% Liver: 33/67 = 49% Lung: 145/197 = 74% Subcutaneous: 33/46 = 72% Other (authors' 'Other' category plus Adrenal, Kidney, Muscle and spleen sites): 51/118 = 43% Adrenal: 2/5 = 40% Bone: 1/17 = 6% Kidney: 2/32 = 6% Muscle: 22/26 = 85% Spleen: 4/24 = 17% |
Unclear (Pts described as having undergone a previous assessment of tumour spread based on ADO (German) guidelines but staging/re‐staging not described Stage of disease: full sample only: stage III (19); stage IV (31) Inclusion: AJCC stage III or IV MM; clinical indication for imaging was positive sentinel‐node biopsy or suspicious lesions on ultrasound or X‐ray studies |
Mean age: full sample only: 59.6; Median age: Range: full sample only: 26 to 86
Male: full sample only: 32 (64%) Site: NR NR |
CT. U + CE, multi‐detector (N/A)
Scan coverage: skull base to pelvis; CT and MR compared for "neck to the pelvis" only; sites imaged included lungs, liver, spleen, kidneys, adrenal glands, subcutaneous tissue, lymph nodes, muscle, bone marrow, and “other”
Contrast: U + CE
CT parameters: NR; NR
Breath hold: no breath hold instructions reported MRI. U + CE; 'standard sequences' (N/A) Scan coverage: WB; as above Contrast: U + CE MRI parameters: standard sequences with parallel imaging techniques; 1.5 T Breath hold: no breath hold instructions reported |
CT: NR (presence/absence of mets) MRI: NR (presence/absence of mets) Info provided: diagnosis/age/sex No. observers: 4 (2 included); radiologist (high) Diagnosis: single |
Histology (NR)
FNAC (0)
FU (33, 100%): ≥ 3 months
Histology interval: N/A
FU interval: minimum 3 months Exclusions: n = 17; no WB‐CT follow‐up undertaken. Results presented by region and for less experienced observers 3 and 4; also presented no. Mets detected by cranial MR but no 2×2 extractable |
| Jouvet 2014 France Patients: 37 Primary lesions: LNBs/Metastases: 209 lesions (n varies per test) |
WPC
Prospective
Data: per lesion
Any mets (incl brain): 115/209 = 55% (CT) 125/218 = 57% (MRI) Any mets (excl brain): 95/186 = 51% (CT) 105/195 = 54% (MRI) 104/191 = 54% (PET‐CT) Nodal: 23/53 = 43% (all tests) Bone: 15/33 = 45% (CT/MRI) 16/35 = 46% (PET‐CT) Liver: 12/27 = 44% (all tests) Lung: 31/45 = 69% (all tests) Subcutaneous: 2/15 = 13% (CT) 10/22 = 45% (MRI) 7/15 = 47% (PET‐CT) |
Unclear (NR)
Stage of disease: stage IV: 37 (100%) Inclusion: AJCC stage IV cutaneous MM referred for simultaneous staging by PET‐CT, CT, superficial lymph node US, and MRI |
Mean age: NR; Median age: NR; Range: NR
Male: NR (0%) Site: NR NR |
CT. CE; Helical; 16 row (N/A)
Scan coverage: neck/chest/ abdomen/pelvis; "cervico‐thoraco‐abdomino‐pelvic helicoidal acquisition"; then skull
Contrast: iodinated IV injection
CT parameters: 120 kV, 250 mAs (neck to pelvis); 140 kV, 120 mAs (skull), 1.25 mm (neck to pelvis), 2.5 mm (skull)
Breath hold: no breath hold instructions reported
MRI. (1) DW alone (N/A); (2) DW, VIBE ‐ 3D echo gradient CE, T1 ‐ skull (N/A) Scan coverage: WB; top of skull to feet Contrast: U MRI parameters: echo‐planar DW alone; 1.5 T Breath hold: no breath hold instructions reported |
CT and MRI: NR (presence/absence of mets) Info provided: NR No. observers: 1; radiologist (experienced). Diagnosis: consensus of 2 (all images interp independently by 2 examiners, discordant results resolved by consensus) |
Histology (0)
FNAC (5, 13.5%)
FU (32; 86.5%): > 9 months
Histology interval: NR
FU interval: NR Exclusions: n = 0; N/A Results are also presented by metastatic site. Provides K values for inter‐ and intra‐observer agreements, but not the 2×2 tables for each observer |
|
Klebl 2003 Germany Patients: 83 Primary lesions: 83 LNBs/Metastases: NR; 653 LNs examined |
WPC
Prospective Data: per pt Nodal mets: 17/79 = 22% |
Mixed (primary (n = 8), follow‐up (n = 75)) Stage of disease: NR Inclusion: MM Clark level IV or V undergoing FU after primary surgery |
Mean age: NR; Median age: NR; Range: NR
Male: 46 (55.421686746988%) Site: NR Clark level IV 68, 82%; level V 15, 18% |
US. B‐mode US; high resolution linear array (5 to 10 MHz); N/A Scan coverage: cervical, axillary, and inguinal LNBs Contrast: N/A | Suspicious/indeterminate/benign based on diameter, shape, echogenicity, and vascularisation pattern Info provided: unclear; could be same examiner as for LN palpation No. observers: NR; NR (NR) Diagnosis: NR |
Histology (17, 20%)
FNAC (0)
FU (62, 75%): minimum 1 year; mean time since primary surgery 2.6 ± 2.3 years
Histology interval: NR
FU interval: 6 to 8 weeks for control visit, 6 to 12 months for FU visit Exclusions: n = 4; 4 were indeterminate on follow‐up so that a final diagnosis could not be made No |
| Pfannenberg 2007 2007 Germany Patients: 64 Primary lesions: 420 LNBs/Metastases: NR |
WPC
Prospective Data: per lesion Any metastases (excl brain): 297/420 = 71% (all tests) Nodal: 102/158 = 65% (CT) Distant (excl brain): 195/262 = 74% (all tests) Bone: 35/50 = 70% (all tests) Liver: 35/37 = 95% (all tests) Lung: 59/80 = 74% (all tests) Local: 53/70 = 76% (all tests) Other viscera: 13/25 = 52% (all tests) |
Mixed (pre‐surgery; investigation of abnormal findings; surveillance) Stage of disease: Stage III (25, 39%); Stage IV (39, 61%) Inclusion: stage III or IV cutaneous MM undergoing imaging for exclusion of widespread disease and confirmation of local disease before surgical resection (n = 9); characterisation of abnormal radiological, clinical and laboratory findings (n = 48); routine melanoma surveillance in high risk patients (n = 7) |
Mean age: 57.8 years; Median age: Range: 23.3 to 79.1 years
Male: 41 (64.0625%) Site: NR Mean BT: 2.69 mm (0.6 to 12 mm) |
CT. CT (CE, 16 row multi‐slice) (NA); N/A
Scan coverage: base of the skull to the lower legs
Contrast: Ultravist 370, Schering GmbH, Berlin, Germany, plus 1000 mL Mannitol 2% as a negative oral contrast agent before CT
CT parameters: 120 kV, 120 to 160 mAs; 5 mm (axial, with an increment of 5 mm) and 3 mm (coronal with an increment of 2 mm)
Breath hold: CT: patients were asked to stop breathing in normal expiration during the contrast‐enhanced CT scans for optimal co‐registration MRI. CE; multiple phased‐array; axial and coronal (NA); N/A Scan coverage: head to toe Contrast: yes PET‐CT. 3D; CT (CE, 16 row multi‐slice) (NA); N/A Scan coverage: base of the skull to the lower legs Contrast: Ultravist 370, Schering GmbH, Berlin, Germany, plus 1000 mL mannitol 2% as a negative oral contrast agent before CT CT parameters: 120 kV, 120 to 160 mAs, 5 mm (axial, with an increment of 5 mm) and 3 mm (coronal with an increment of 2 mm) FDG: 370 MBq F‐FDG IV 55 to 65 minutes before scanning Breath hold: CT: patients were asked to stop breathing in normal expiration during the contrast‐enhanced CT scans for optimal co‐registration CT used for: attenuation corrected and co‐registered Reconstruction: iteratively reconstructed using commercial software (eSoft; Siemens., Erlangen, Germany) |
CT: based on morphological characteristics and enhancement pattern; region‐specific nodal size criteria based on measurement of the small axis diameter MRI: based on morphological characteristics and enhancement pattern; detected lymph nodes smaller than 10 mm but with brighter signal on T1 sequences, due to the paramagnetic effect of melanin, also were rated as suspicious PET: any focal tracer uptake exceeding normal regional tracer accumulation was assessed as a malignant lesion. Lesions rated malignant or probably malignant were considered to be malignant Info provided: aware of the clinical status No. observers: 6; 2 dermato‐oncologists; 2 radiologists (2 specialists in nuclear medicine, 2 CT radiologists, and 2 MRI radiologists) Diagnosis: consensus of 2 or 4 |
Histology (65 (15%))
FNAC (N/A)
FU (267 (64%) lesions by imaging follow‐up, 88 (21%) lesions by clinical follow‐up): mean 252.5 days (range, 99 to 474 days)
Histology interval: NR
FU interval: every 3 months Exclusions: n = 36; no wbMRI (n = 25; due to metallic implants or claustrophobia (5 patients), refuse of a second whole body examination on the same day (17 patients) or abortion of the examination (3 patients); no evidence of tumour spread (3 patients) or lack of follow‐up data for lesion characterisation (8 patients) |
| Pfluger 2011 Germany Patients: 50 Primary lesions: NR LNBs/Metastases: NR; 232 lesions |
WPC
Retrospective (Prosp database: NR) Data: per lesion Any (incl brain): 151/232 = 65% (CT) |
Mixed (PET‐CT was done for primary staging and for follow‐up) Stage of disease: NR Inclusion: MM with regional LN metastases (NR if clinically detectable or micro‐metastases) undergoing PET‐CT either for primary staging or during follow‐up. Only included lesions considered malignant by at least 1 of the 3 modalities (NECT, CECT, 18F‐FDG PET) |
Mean age: 57; Median age: Range: 29 to 85 years
Male: 36 (72%) Site: NR NR |
CT. (1) CE, dual slice, helical (N/A); NR (2) U, dual slice, helical (N/A); NR
Scan coverage: WB; from the skull including the legs
Contrast: 120 mL (2.5 mL/s) of iodine‐containing contrast medium
CT parameters: 120 kV, 145 mAs, 2.5 mm
Breath hold: CT expiration protocols for shallow free breathing during the emission scan PET‐CT (1) 3D; CT (CE, dual slice, helical) (N/A); NR (2) 3D‐ CT (U, dual slice, helical) (N/A); NR Scan coverage: WB; from the skull including the legs Contrast: 120 mL (2.5 mL/s) of iodine‐containing contrast medium CT parameters: 120 kV, 145 mAs, 2.5 mm FDG: 200 MBq Breath hold: CT expiration protocols for shallow free breathing during the emission scan CT used for: unclear; reports side by side PET‐CT display with spatially synchronised images Reconstruction: NR; PET and CT interpreted side by side with spatially synchronised images to ensure that the identical lesion was assessed in both modalities |
CT ‐ abnormal soft tissue masses and/or enlarged LNs (diameter > 1.0 cm) plus degree of contrast enhancement for CE CT only PET alone ‐ non‐physiologically increased uptake of FDG with SUVmax > 2.5. For lesions with discrepant findings on both modalities, the finding of the modality with the higher diagnostic confidence score was accepted. If results from both modalities were discrepant and had the same diagnostic confidence score value, the lesion was judged positive Info provided: knowledge of clinical data No. observers: 2; NR (experienced) Diagnosis: consensus |
Histology (41, 17.7%)
FNAC (0)
FU (191, 82.3%): ≥ 6 months; no further detail
Histology interval: NR
FU interval: NR n = NR; ** In cases of new tumour lesions during the follow‐up period, these lesions were not included in the study. The reason given for not including these lesions was the fact that non‐detectable lesions in CT or 18F‐FDG PET cannot be distinguished from non‐existent lesions in the case of a newly detected tumour lesion during follow‐up |
|
Reinhardt 2006 Germany Patients: 250 Primary lesions: 250 LNBs/Metastases: NR; 670 lesions identified |
WPC
Retrospective (Prosp database: NR) Data: per pt Any (excl brain): 116/250 = 46% Nodal mets: 78/250 = 31% Distant mets (excl brain): 84/250 = 34% |
Mixed (primary staging after sentinel node biopsy (n = 75); therapy control after chemotherapy of metastatic disease (n = 42), staging of clinically suspected recurrent disease (n = 65), during follow‐up within 5 years of primary treatment (n = 68)
Stage of disease: initial pathology: stage I 22, 9%; stage II 88, 35%; stage III 108, 43%; stage IV 32, 13%
Inclusion: cutaneous MM referred for PET‐CT Excluded if inadequate reference standard (no histology or FU < 1 year) |
Mean age: 58; Median age: Range: ±16
Male: 145 (58%) Site: NR Tumor depth: ≤ 1.0 mm 29, 12%; 1.01 to 2.0 mm 68, 27%; 2.01 to 4.0 mm 66, 26%; > 4.0 mm 64, 26% |
CT. CE, helical, dual detector (N/A); N/A
Scan coverage: WB; base of skull to tip of toes in 3 parts
Contrast: Peritrast‐oral‐GI; Kohler Chemie GmbH, Alsbach, Germany
CT parameters: 130 kV, 40 mAs, 5 mm
Breath hold: limited breath hold for CT and shallow breathing for PET PET‐CT. CT (CE), helical, dual detector (N/A); N/A Scan coverage: WB; base of skull to tip of toes in 3 parts Contrast: Peritrast‐oral‐GI; Kohler Chemie GmbH, Alsbach, Germany CT parameters: 130 kV, 40 mAs, 5 mm FDG: 371 ± 40 MBq FDG through an anterior cubital vein Breath hold: limited breath hold for CT and shallow breathing for PET CT used for: attenuation correction based on re‐scaling of the CT image Reconstruction: iteratively reconstructed with attenuation correction on the basis of a re‐scaling of the CT image as described elsewhere (Kinahan 2003) |
NR for any test; states only that accuracy was assessed according to according to the current AJCC staging classification Info provided: routine clinical fashion; same clinical information about each patient No. observers: NR; NR; consensus by each of 2 experienced investigators (experienced) Diagnosis: consensus (of 2?) |
Histology (100, 40% for N‐staging (including 15 with SLNB)
20, 8% for M‐staging)
FNAC (N/A)
FU (250, 100%): NR; ≥ 1 year
Histology interval: NR
FU interval: NR Exclusions: n = 0 Reports data for differentiation by metastatic sites (M1A to M1C) and for detection of visceral and non‐visceral mets. Gives Se/Sp by population group but prevalence per group not given to allow 2×2 to be included |
|
Strobel 2007b Switzerland Patients: 124 Primary lesions: NR LNBs/Metastases: NR |
NC
Prospective Data: per pt Any (incl brain): 53/124 = 43% |
Unclear (NR; PET‐CT for depiction or exclusion of metastases) Stage of disease: NR Inclusion: high risk melanoma (BT > 4 mm, or Clark level III or IV, or known resected metastases) and raised S100 (> 0.2 μg/L) undergoing follow‐up after primary treatment Excluded if systemic therapy before the PET‐CT investigation | Mean age: 54.4 years; Median age: Range: 15 to 82 years Male: 59 (47.5806451612903%) Site: NR NR | PET‐CT. CT (CE, multi‐slice, helical) (N/A); NR Scan coverage: head to the knees with scanning of the lower legs for patients with primary tumours of the lower extremities Contrast: oral CT contrast agent given 15 minutes before injection of 18F‐FDG CT parameters: 140 kV, 40 mAs, 4.25 mm FDG: 350 to 400 MBq Breath hold: CT: breath holding in the normal expiratory position CT used for: attenuation correction, fused Reconstruction: standard iterative algorithm (OSEM) | Mets present if detected by 1 or both readers. FDG uptake clearly greater than background; if a focal FDG‐active lesion was detected, the exact anatomical localisation was determined on the fused PET‐CT images. Lesions with 18F‐FDG uptake in physiological sites or benign variants, e.g. muscles, brown fatty tissue or pulmonary infiltrations, were determined as benign. Semi‐quatitative analysis of FDG uptake in terms of SUVmax also conducted Info provided: blinded to serum S100B No. observers: 2; nuclear radiology physicians (experienced (13 years and 7 years)) Diagnosis: consensus of 2 |
Histology (20, 16.1%)
FNAC (21, 16.9%)
FU (124, 100%, 18 D+ and 61 D‐ had status confirmed by PET‐CT or clinical FU, 4 D‐ had MRI to confirm absence of mets and 10/53 D+): minimum of 6 months (range 6 to 18 months in all patients
Histology interval: NR
FU interval: NR Exclusions: n = 3; chemotherapy before PET‐CT |
|
van den Brekel 1998 Netherlands Patients: 26 Primary lesions: 26 LNBs/Metastases: NR |
NC
Retrospective (Prosp database: NR) Data: per pt Nodal (neck): 21/26 = 81% |
Mixed (NR; but interval between treatment of the primary and neck dissection ranged from 0 to 8.8 years (mean 21 months)) Stage of disease: stage III (palpable LN) 18, 69%; stage I and II 8, 31% Inclusion: HN MM with CT before neck dissection, including therapeutic and elective (negative on palpation). Also included primary and recurrence | Mean age: 54.5 years; Median age: Range: 55 to 83 years Male: 18 (0.692307692307692%) Site: scalp 6, 23%; temporal 3, 12%; ear 4, 15%; anterior face 4, 15%; neck 1, 4%; shoulder 1, 4%; upper limb 1, 4%; nasal mucosa 1, 4%; unknown primary 5, 19% BT 0.8 mm to 22 mm | CT. CE (N/A); NR Scan coverage: neck Contrast: IV bolus plus drip infusion of iodine contrast CT parameters: NR; 5 mm for 24 pts; 2 mm for 2 pts (both FN) Breath hold: NR | Presence of necrosis or axial diameter > 10 or > 11 mm Info provided: NR No. observers: 2; NR; co‐authors (NR) Diagnosis: unclear |
Histology (26, 100%)
FNAC (0)
FU (0): N/A
Histology interval: 4 weeks
FU interval: N/A Exclusions: n = 0 Both FN on CT used 8 mm CT thickness |
| van Wissen 2016 Netherlands Patients: 70 Primary lesions: 70 LNBs/Metastases: NR | NC
Retrospective (Prosp database: no) Data: per pt Nodal (superficial groin mets only): 59/69 = 86% Nodal (deep groin mets only): 24/67 = 36% |
Mixed (NR. Discussion states "large proportion of our patients were initially treated for their primary tumour at other hospitals, and sometimes years prior to the current groin dissection") Stage of disease: only stage III B & C Inclusion: stage IIIB or IIIC MM with palpable groin metastases; selected for therapeutic CGD |
Mean age: NR; Median age: 58; Range: 24 to 83
Male: 35 (0.5%) Site: leg 58, 83%; trunk 6, 9%; arm 0, 0%; unknown 6, 9% BT mm: ≤ 1.00 6 (9%); ≤ 2.00 15 (21%); 2.01 to 4.00 15 (21%); > 4.00 12 (17%); missing/unknown 22 (31%) |
PET‐CT. CT (U) (NA) Scan coverage: WB; not further described Contrast: none CT parameters: Kv NR; 40 mAs, 2 to 5 mm FDG: 180 to 240 MBq Breath hold: standard acquisition protocols CT used for: attenuation correction; fused images Reconstruction: NR | FDG uptake (qualitative assessment) Info provided: NR No. observers: 1; nuclear medicine (nr) Diagnosis: single |
Histology (70, 100%)
FNAC (NA)
FU (not for ref purposes): median 16 months (0 to 71 months)
Histology interval: NR
FU interval: NR Exclusions: n = 1; missing pathology 7/10 disease negative had a diagnostic resection of a lymph node before lymph node dissection potentially leading to FPs |
| + ‐ positive; AJCC: American Joint Cancer Committee; AWOSEM: attenuation weighted ordered subsets expectation maximisation; BT: Breslow thickness; CE: contrast enhanced; CLND: complete lymph node dissection; CT: computed tomography; 2D: two‐dimensional; 3D: three‐dimensional; DW: diffusion weighted; EDV: end‐diastolic volume; 18FDG: 2‐deoxy‐2‐[18F]fluoro‐D‐glucose; FNAC: fine needle aspiration cytology; FORE: Fourier rebinning; FU: follow‐up; GE: gradient echo; HN: head and neck; LN: lymph node; LNB: lymph node basin; LND: lymph node dissection; LS: lymphoscintigraphy; mA: measure of tube current; mets: metastases; MM: malignant melanoma; MRI: magnetic resonance imaging; NC: non‐comparative; OSEM: ordered subsets expectation maximisation algorithm; PET: positron emission tomography; PI: pulse index; PSV: peak systolic volume; prosp: RF: risk factor; SD: standard deviation; SLN: sentinel lymph node; SLNB: sentinel lymph node biopsy; SSM: superficial spreading melanoma; SUV: standardised uptake value; SUVmax: maximum standardised uptake value; U: unenhanced; US: ultrasound; WB: whole body. | ||||||
Appendix 10. Descriptive synthesis of all included studies of whole body imaging
Study design and setting
Twelve of the 24 studies (50%) were prospective case series (Aukema 2010b; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Hafner 2004; Hausmann 2011; Jouvet 2014; Klebl 2003; Maubec 2007; Pfannenberg 2007; Strobel 2007b; Veit‐Haibach 2009), ten (40%) were retrospective in design (Abbott 2011; Arrangoiz 2012; Aukema 2010a; Iagaru 2007; Kang 2011; Pfluger 2011; Reinhardt 2006; Strobel 2007a; van den Brekel 1998; van Wissen 2016), and in two, the direction of the design was not clear (Prayer 1990; Rubaltelli 2011). All studies were conducted in Europe apart from two US‐based studies ‐ Arrangoiz 2012; Iagaru 2007 ‐ and one conducted in South Korea (Kang 2011).
Participants
Primary staging. Of the six studies conducted in participants undergoing primary staging, two included any participant following diagnosis of melanoma (Kang 2011; Veit‐Haibach 2009); two excluded those with distant metastases on diagnosis (Hafner 2004; Maubec 2007) (Maubec 2007 was restricted to those with melanomas at least 4 mm in thickness); one included clinically node positive participants but did not report exclusion of those with distant metastases (Prayer 1990); and one included only clinically node negative participants with melanomas of at least 4 mm Breslow thickness (Arrangoiz 2012). Three studies also reported data for pre‐SLNB imaging (Arrangoiz 2012; Hafner 2004; Maubec 2007), two of which reported subgroup data for clinically node negative participants who underwent SLNB (Hafner 2004; Maubec 2007). All six studies reported accuracy data on a per patient basis; no per lesion data were identified.
A total of 492 participants were included with sample sizes ranging from 25 in Maubec 2007 to 217 in Prayer 1990. When reported (n = 5), the ages of included participants ranged from 18 years in Hafner 2004 to 89 years in Arrangoiz 2012. The mean age of included participants was reported in five studies (the median of reported means was 61 years, range 56 to 67 years) and median age in one study (median 55 years in Hafner 2004). Fifty‐two per cent of included participants were male. The percentage of participants with head and neck melanoma ranged from 4% in Veit‐Haibach 2009 to 36% in Maubec 2007 (median 15%) and melanoma of the extremities, including the hands or feet where documented, from 32% in Maubec 2007 to 73% in Kang 2011 (median 50%).
Re‐staging. Of the three studies conducted in participants undergoing re‐staging of disease, one included any participant having imaging for re‐staging purposes (Iagaru 2007); and two included clinically node negative participants either undergoing ultrasound of the regional lymph nodes as part of a follow‐up program, as in Rubaltelli 2011, or with raised S100 during follow‐up, as in Strobel 2007a.
A total of 589 participants were included with sample sizes of 47 in Strobel 2007a, 106 in Iagaru 2007, and 460 in Rubaltelli 2011. The ages of included participants ranged from 20 years to 87 years. The median of reported mean ages was 55 years. Fifty‐three per cent of included participants were male. The site of the primary melanoma was not reported in any study. All three studies reported accuracy data on a per patient basis, and one study also reported data per lesion (139 lesions identified in 30 participants; Iagaru 2007).
Mixed or unclear. The 15 studies conducted in mixed or not clearly described population groups are described in Table 4 according to the reported indication for imaging and participant stage of disease on recruitment.
Two studies clearly included participants at any stage of disease (Dellestable 2011; Reinhardt 2006). In Dellestable 2011, 27% of participants had stage I or II melanoma and 73% had stage III or IV disease; imaging was undertaken for primary staging or follow‐up. In Reinhardt 2006, 44% of participants had stage I or II melanoma and the remaining participants had stage III or IV disease. Imaging was undertaken for primary staging after SLNB (30%); therapy control after chemotherapy of metastatic disease (17%), staging of clinically suspected recurrent disease (26%), and imaging during follow‐up within five years of primary treatment (27%). Insufficient data were available from this study to allow 2×2 contingency tables to be estimated for each subgroup of participants, despite author contact.
Stage of disease on recruitment was not reported in four studies, and these were judged to have included ‘any’ stage of disease (Aukema 2010a; Cachin 2014; Klebl 2003; Strobel 2007b). Aukema 2010a included asymptomatic patients with raised S100 either judged to be high risk after primary melanoma treatment (56%) or undergoing follow‐up after surgical treatment of regional (33%) or distant (11%) metastases. Cachin 2014 described imaging for staging or for re‐staging but did not give a breakdown of the number of participants in each group. Klebl 2003 restricted inclusion to those with Clark level IV or V melanomas, with 10% of participants having primary staging and 90% undergoing follow‐up, and Strobel 2007b included those with melanomas at least 4 mm in thickness, Clark level III or IV, or known resected metastases, further reporting only that imaging was used for depiction or exclusion of metastases.
The remaining nine studies in mixed or not clearly described population groups included only participants with stage III disease (Abbott 2011; Aukema 2010b; Bastiaannet 2009; Pfluger 2011; van den Brekel 1998; van Wissen 2016), stage IV disease (Jouvet 2014), or both (Hausmann 2011; Pfannenberg 2007) (Table 4). In the two studies including participants with stage III and IV melanoma, the percentage with stage III disease was 38% in Hausmann 2011 and 39% in Pfannenberg 2007. Four studies in mixed population groups included those having primary staging or follow‐up but did not report the number of participants with each indication for imaging (Abbott 2011; Pfluger 2011; van den Brekel 1998; van Wissen 2016). Bastiaannet 2009 included those with nodal disease identified at the time of primary diagnosis (15%), or with recurrence up to three years from diagnosis (58%) or more than three years since primary diagnosis (27%). In Pfannenberg 2007, imaging was undertaken to exclude widespread disease and before surgical resection (14%); to characterise abnormal radiological, clinical, and laboratory findings (75%); or as part of routine surveillance in high‐risk patients (11%). The remaining three studies did not clearly describe the indication for imaging and were conducted in patients with palpable and pathology proven lymph node metastases and no signs of distant metastases (Aukema 2010b); participants with positive sentinel node biopsy or suspicious lesions on ultrasound or X‐ray studies (Hausmann 2011); or patients with stage IV melanoma (Jouvet 2014).
A total of 1265 participants were included in the 15 studies with sample sizes ranging from 26 in van den Brekel 1998 to 251 in Bastiaannet 2009. When reported (n = 10), the ages of included participants ranged from 15 years in Strobel 2007b to 93 years in Aukema 2010a. The mean age of included participants was reported in nine studies (the median of reported means was 57 years, range 54 to 59 years) and median age in two studies (Abbott 2011 reporting a median age of 50 for those with microscopic disease and 63 for those with macroscopic disease; and van Wissen 2016 reporting a median age of 58 years). Forty‐eight per cent of included participants were male. The site of the primary melanoma was reported in only five of the 16 studies (Abbott 2011; Aukema 2010b; Bastiaannet 2009; van den Brekel 1998; van Wissen 2016), one of which included only head and neck melanoma (van den Brekel 1998), and one of which included only those undergoing combined groin dissection for melanomas of the trunk (17%) or extremities (83%) (van Wissen 2016). Excluding van den Brekel 1998 and van Wissen 2016, the percentage of participants with head and neck melanoma ranged from 3% in Abbott 2011 to 13% in Aukema 2010b, and melanoma of the extremities, including the hands or feet where documented from 38% in Abbott 2011 to 59% in Aukema 2010b.
Index tests
Sixteen studies contributed data for a single index test (Abbott 2011; Arrangoiz 2012; Aukema 2010a; Aukema 2010b; Bastiaannet 2009; Cachin 2014; Hafner 2004; Kang 2011; Klebl 2003; Maubec 2007; Prayer 1990; Rubaltelli 2011; Strobel 2007a; Strobel 2007b; van den Brekel 1998; van Wissen 2016), and eight compared the accuracy of one or more index tests (Dellestable 2011; Hausmann 2011; Iagaru 2007; Jouvet 2014; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Veit‐Haibach 2009) (Table 2). Two studies also provided data for PET alone (ineligible index test) (Bastiaannet 2009; Hafner 2004), and two reported data for MRI of the brain in all patients but 2×2 contingency table data could not be included because of small patient or lesion numbers (Aukema 2010a; Aukema 2010b). However, available information on MRI of the brain has been separately summarised as an additional result.
Ultrasound. Five studies evaluated ultrasound as a staging tool (Hafner 2004; Jouvet 2014; Klebl 2003; Prayer 1990; Rubaltelli 2011). All studies employed B‐mode ultrasound, three at single frequencies of 5 MHz (Hafner 2004), 7.5 MHz (Prayer 1990), and 12.5 MHz (Jouvet 2014), and two using variable frequencies of 5 to 10 MHz (Klebl 2003), and 7.5 to 13 MHz (Rubaltelli 2011). One study of ultrasound used in potential SLNB candidates performed ultrasound before lymphoscintigraphy. Lymph node basins were imaged according to the site of the primary melanoma in all studies. The criteria for the detection of nodal metastases were described in all studies apart from Hafner 2004 (Appendix 9). Ultrasound was performed by radiologists (Hafner 2004; Jouvet 2014; Prayer 1990), was performed by a sonologist (Rubaltelli 2011), or was not reported (Klebl 2003).
CT. Ten studies evaluated CT ‐ unenhanced (Iagaru 2007), contrast enhanced (Bastiaannet 2009; Dellestable 2011; Jouvet 2014; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; van den Brekel 1998; Veit‐Haibach 2009), or both (Hausmann 2011). CT parameters (tube current (mA), tube voltage (kV), and slice thickness (mm)) were not reported in four studies (Bastiaannet 2009; Dellestable 2011; Hausmann 2011; Veit‐Haibach 2009), and ranged from 40 mA in Iagaru 2007 and Reinhardt 2006; to 250 mA in Jouvet 2014; 120 kV in Jouvet 2014,Pfannenberg 2007, and Pfluger 2011; to 140 kV in Iagaru 2007, with slice thicknesses from 1.25 mm in Jouvet 2014 to 5 mm in Iagaru 2007,Pfannenberg 2007,Reinhardt 2006, and van den Brekel 1998 (reported per study in Appendix 9).
Scan coverage included the skull (Dellestable 2011; Iagaru 2007; Jouvet 2014; Pfluger 2011), or specifically excluded the skull (Bastiaannet 2009; Hausmann 2011; Pfannenberg 2007; Reinhardt 2006), and it extended to the abdominal or pelvic area (Bastiaannet 2009; Dellestable 2011; Jouvet 2014; Pfluger 2011), or it also included the lower limbs (Iagaru 2007; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006). van den Brekel 1998 imaged the neck area only, and Veit‐Haibach 2009 did not clearly document the scan coverage, describing whole body imaging in a caudocranial direction. The criteria for the detection of metastases were not reported in six studies (Bastiaannet 2009; Dellestable 2011; Hausmann 2011; Iagaru 2007; Jouvet 2014; Reinhardt 2006). Four studies reported the use of morphological characteristics (Pfannenberg 2007; van den Brekel 1998), soft tissue masses (Pfluger 2011; Veit‐Haibach 2009), contrast enhancement (Pfannenberg 2007; Pfluger 2011; Veit‐Haibach 2009), and nodal size criteria (Pfannenberg 2007; Pfluger 2011; van den Brekel 1998).
Test interpretation was provided by radiologists (Hausmann 2011; Jouvet 2014; Veit‐Haibach 2009), nuclear medicine physicians (Bastiaannet 2009), or both (Iagaru 2007), or by dermato‐oncologists and radiologists (Pfannenberg 2007). Four studies did not report observer qualifications (Dellestable 2011; Pfluger 2011; Reinhardt 2006; van den Brekel 1998). Half of studies reported providing test interpreters with clinical information including the diagnosis, age, and sex of the patient (Hausmann 2011), clinical status (Pfannenberg 2007), clinical data (Pfluger 2011), routine clinical information (Reinhardt 2006), or patient‐specific clinical background (Veit‐Haibach 2009). All studies apart from two ‐ Iagaru 2007 and van den Brekel 1998 ‐ reported blinding to the results of other imaging tests.
MRI. Four studies evaluated 1.5 T MRI using a variety of different sequences before and after gadolinium contrast enhancement (Dellestable 2011; Hausmann 2011; Jouvet 2014; Pfannenberg 2007), including diffusion weighting (Dellestable 2011; Jouvet 2014), as well as ultrafast gradient echo (described as VIBE in the study reports) sequences (Jouvet 2014;Pfannenberg 2007). Scan coverage in three studies was from the head to the feet (Jouvet 2014; Pfannenberg 2007; Laurent 2010), and from the neck to the pelvis only in Hausmann 2011. Two studies did not report the criteria used to assess the presence of metastases (Hausmann 2011; Jouvet 2014); one reported a qualitative assessment of signal intensity (Dellestable 2011), and one reported use of morphological characteristics, enhancement pattern, and lymph node size and signal (Pfannenberg 2007). Four studies reported test interpretation by radiologists (Dellestable 2011; Hausmann 2011; Jouvet 2014; Pfannenberg 2007). Two studies reported providing test interpreters with clinical information including the diagnosis, age, and sex of the patient (Hausmann 2011), or clinical status (Pfannenberg 2007). All studies reported blinding to the results of other imaging tests.
PET‐CT. Seventeen studies examined the use of PET‐CT for staging purposes, combining PET with unenhanced CT (Arrangoiz 2012; Aukema 2010a; Aukema 2010b; Dellestable 2011; Iagaru 2007; Kang 2011; Maubec 2007; van Wissen 2016), contrast enhanced CT (Jouvet 2014; Pfannenberg 2007; Reinhardt 2006; Strobel 2007a; Strobel 2007b; Veit‐Haibach 2009), or evaluating both (Pfluger 2011). Two studies did not report whether or not the CT component was contrast enhanced (Abbott 2011; Cachin 2014). CT was clearly described as used for attenuation correction (Arrangoiz 2012; Aukema 2010a; Aukema 2010b; Pfannenberg 2007; Reinhardt 2006; Strobel 2007a; Strobel 2007b; van Wissen 2016; Veit‐Haibach 2009), or for anatomical localisation (Cachin 2014; Kang 2011), or for both (Abbott 2011; Dellestable 2011; Iagaru 2007), or it was not clearly described (Jouvet 2014; Maubec 2007; Pfluger 2011).
Where reported (n = 8), studies employed 2D PET (Abbott 2011; Aukema 2010b; Iagaru 2007; Strobel 2007a), 3D PET (Maubec 2007; Pfannenberg 2007; Pfluger 2011), or either 2D or 3D PET (Arrangoiz 2012). CT parameters were not reported in four studies (Abbott 2011; Cachin 2014; Dellestable 2011; Veit‐Haibach 2009). In 14 studies, parameters ranged from 40 mA ‐ Aukema 2010a; Aukema 2010b; Iagaru 2007; Reinhardt 2006; Strobel 2007a; Strobel 2007b; van Wissen 2016 ‐ to 160 mA ‐ Kang 2011; Pfannenberg 2007, or from 110 kV ‐ Maubec 2007 ‐ to 140 kV ‐ Arrangoiz 2012; Iagaru 2007; Jouvet 2014; Kang 2011; Strobel 2007a; Strobel 2007b ‐ and slice thickness from 2.5 mm ‐ Kang 2011; Pfluger 2011 ‐ to 6.5 mm ‐ Jouvet 2014 ‐ and are reported in Appendix 9.
Scan coverage included the skull (Arrangoiz 2012; Dellestable 2011; Iagaru 2007; Kang 2011; Maubec 2007; Pfluger 2011; Strobel 2007a; Strobel 2007b), or specifically excluded the skull (Abbott 2011; Jouvet 2014; Pfannenberg 2007; Reinhardt 2006), and it extended to the upper thigh (Abbott 2011), or to the lower limbs (Arrangoiz 2012; Dellestable 2011; Iagaru 2007; Jouvet 2014; Kang 2011; Maubec 2007; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Strobel 2007a; Strobel 2007b). Five studies did not clearly document the scan coverage, describing whole body imaging (Aukema 2010a; Cachin 2014; van Wissen 2016), imaging according to the primary lesion site (Aukema 2010b), or imaging in a caudocranial direction (Veit‐Haibach 2009).
The criteria for the detection of metastases were not reported in three studies (Abbott 2011; Jouvet 2014; Reinhardt 2006), or they were described as the presence of metabolically active lesions with no further detail in two (Aukema 2010a; Aukema 2010b). Six studies reported assessment of focal FDG uptake relative to background (Cachin 2014; Strobel 2007a), as supported by SUVmax assessment (Dellestable 2011; Maubec 2007; Pfannenberg 2007; Pfluger 2011; Strobel 2007b; van Wissen 2016; Veit‐Haibach 2009). Three studies reported the use of SUVmax alone (≥ 2.2 in Kang 2011 and ≥ 2.5 in Arrangoiz 2012 and Iagaru 2007).
Test interpretation was provided by nuclear medicine physicians alone (Abbott 2011; Arrangoiz 2012; Aukema 2010a; Aukema 2010b; Cachin 2014; Jouvet 2014; Kang 2011; Strobel 2007a; Strobel 2007b; van Wissen 2016), or teamed with radiologists (Iagaru 2007; Veit‐Haibach 2009), or by dermato‐oncologists and radiologists (Pfannenberg 2007). Four studies did not report observer qualifications (Dellestable 2011; Maubec 2007; Pfluger 2011; Reinhardt 2006). Four studies reported providing test interpreters with some form of clinical patient information (Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Veit‐Haibach 2009). Seven studies reported blinding to the results of other imaging tests (Dellestable 2011; Jouvet 2014; Pfannenberg 2007; Reinhardt 2006; Strobel 2007a; Strobel 2007b; Veit‐Haibach 2009).
Reference standards
Four of the 24 studies (17%) evaluated the accuracy of imaging in comparison to histology alone, using samples from SLNB or CLND (Hafner 2004;Maubec 2007), or from neck (van den Brekel 1998), or from groin (van Wissen 2016) dissection, and in two studies, the reference standard combined histology based on CLND or SLNB with follow‐up to determine any false negative results on imaging (Arrangoiz 2012; Prayer 1990). The remaining studies used a combination of histology or follow‐up (Abbott 2011; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Hausmann 2011; Iagaru 2007; Kang 2011; Klebl 2003; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Veit‐Haibach 2009), FNAC or follow‐up (Jouvet 2014), or histology, FNAC, or follow‐up as a reference standard (Aukema 2010a; Aukema 2010b; Rubaltelli 2011; Strobel 2007a; Strobel 2007b).
Across the 20 studies reporting some form of follow‐up, two did not report the length of follow‐up, but more than 90% of included participants had a histological reference standard reported (Arrangoiz 2012; Iagaru 2007). Eighteen studies reported or required minimum follow‐up periods of at least three months (n = 11) or reported the mean or median follow‐up with a range that was at least three months (n = 7). Minimum follow‐up was between three and six months (Aukema 2010a; Dellestable 2011; Hausmann 2011; Pfannenberg 2007; Veit‐Haibach 2009), from six months to a year (Aukema 2010b; Bastiaannet 2009; Cachin 2014; Jouvet 2014; Kang 2011; Pfluger 2011; Prayer 1990; Rubaltelli 2011; Strobel 2007a; Strobel 2007b), or one year or longer (Abbott 2011; Klebl 2003; Reinhardt 2006). Where reported, median follow‐up times ranged from 10 months in Rubaltelli 2011 to 24.3 months in Kang 2011, and mean follow‐up from 8.3 months to 34 months (Abbott 2011).
Follow‐up schedules were documented in eight studies (Abbott 2011; Hausmann 2011; Kang 2011; Klebl 2003; Pfannenberg 2007; Prayer 1990; Reinhardt 2006; Strobel 2007a). Tests used during follow‐up were mentioned in 16 studies (Abbott 2011; Aukema 2010a; Aukema 2010b; Bastiaannet 2009; Cachin 2014; Dellestable 2011; Hausmann 2011; Jouvet 2014; Kang 2011; Pfannenberg 2007; Pfluger 2011; Reinhardt 2006; Rubaltelli 2011; Strobel 2007a; Strobel 2007b; Veit‐Haibach 2009), although the detail provided varied considerably, for example from ‘clinical or radiological follow‐up’ in Dellestable 2011 to ‘physical examination, blood tests, ultrasound studies, X‐rays, and CT scans of the body from the neck to the pelvis (WB‐CT) as well as an MRI of the head (MRI‐CR)’ in Hausmann 2011 (Appendix 9).
Exclusions
Ten studies reported the exclusion of between 1 and 36 study participants (Bastiaannet 2009; Cachin 2014; Hausmann 2011; Klebl 2003; Pfannenberg 2007; Rubaltelli 2011; Strobel 2007b; van Wissen 2016), or lesions (Dellestable 2011). Pfluger 2011 further reported that new lesions detected during the follow‐up period were not included as false negative on imaging on the basis that they may have been newly emergent lesions.
Appendix 11. Sensitivities and specificities of imaging tests from studies reporting data for more than one target condition
|
Test Study Population group No. patients/cases a [Lesions/cases] |
Imaging detail | Sensitivity [95% CI] % TP/Diseased | Specificity [95% CI] % TN/Non‐Diseased | ||||||||
| Any metastasis | Nodal metastasis | Distant metastasis | Any metastasis | Nodal metastasis | Distant metastasis | ||||||
| Including brain | Excluding brain | Including brain | Excluding brain | Including brain | Excluding brain | Including brain | Excluding brain | ||||
| CT | |||||||||||
|
Veit‐Haibach 2009 Primary Per patient data 56 |
CT (CE) | Not assessed | Not assessed |
23 [5 to 54] 3/13 |
Not assessed |
Brain NR 25 [5 to 57] 3/12 |
Not assessed | Not assessed |
100 [92 to 100] 43/43 |
Not assessed |
Brain NR 93 [81 to 99] 41/46 |
|
Reinhardt 2006 Mixed Per patient data 250/116 |
CT (CE) |
81 [73 to 88] 94/116 |
Not assessed |
85 [75 to 92] 66/78 |
74 [63 to 83] 62/84 |
Not assessed |
77
[69 to 84] 103/134 |
Not assessed |
87
[81 to 92] 150/172 |
88
[82 to 92] 146/166 |
Not assessed |
|
Dellestable 2011 Mixed Per lesion data 40 [118 / 72] |
CT (CE) | 80 [69 to 89] 53/66 |
Not assessed | 94 [79 to 99] 29/31 |
59 [42 to 74] 24/41 |
Not assessed | 95
[84 to 99] 40/42 |
Not assessed | 100
[63 to 100] 8/8 |
87
[73 to 96] 34/39 |
Not assessed |
|
Hausmann 2011 Unclear Per lesion data 33 [824 /455] |
CT (CE) | Not assessed | 78 [74 to 82] 356/455 |
86 [81 to 91] 166 /192 |
Not assessed | 71 [65 to 76] 186/263 |
Not assessed | 50
[44 to 55] 183/369 |
29
[22 to 36] 54/187 |
Not assessed | 71
[64 to 77] 129/182 |
|
Jouvet 2014 Unclear Per lesion data 37 [218 / 125] |
CT (CE) | 90 [82 to 94] 103/115 |
88 [80 to 94] 84/95 |
96 [78 to 100] 22/23 |
88 [80 to 94] 81/92 |
86 [76 to 93] 62/72 |
70
[60 to 79] 66/94 |
69
[59 to 78] 63/91 |
63
[44 to 80] 19/30 |
73
[61 to 84] 47/63 |
72
[59 to 83] 44/61 |
|
Pfannenberg 2007 Mixed Per lesion data 64 [420/297] |
CT (CE) | Not assessed | 77 [72 to 82] 229/297 |
76 [67 to 84] 78/102 |
Not assessed | 77 [71 to 83] 151/195 |
Not assessed | 70
[61 to 78] 86/123 |
77
[64 to 87] 43/56 |
Not assessed | 64
[52 to 76] 43/67 |
| MRI | |||||||||||
|
Dellestable 2011 Mixed Per lesion data 40 [118 / 72] |
MRI (DW) | 83
[72 to 91] 58/70 |
Not assessed | 90
[74 to 98] 28/31 |
77
[61 to 89] 30/39 |
Not assessed | 96
[85 to 99] 45/47 |
Not assessed | 89
[52 to 100] 8/9 |
97
[86 to 100] 37/38 |
Not assessed |
|
Hausmann 2011 Unclear Per lesion data 33 [824 / 455 ] |
MRI (NR) | Not assessed | 73
[69 to 77] 334/455 |
82
[76 to 87] 157/192 |
Not assessed | 67
[61 to 73] 177/263 |
Not assessed | 84
[80 to 87] 309/369 |
77
[70 to 83] 144/187 |
Not assessed | 91
[85 to 94] 165/182 |
|
Jouvet 2014 Unclear Per lesion data 37 [218 / 125 ] |
MRI (DW) | 68
[59 to 76] 85/125 |
69
[59 to 77] 72/105 |
96
[78 to 100] 22/23 |
62
[52 to 71] 63/102 |
61
[50 to 72] 50/82 |
73
[63 to 82] 68/93 |
72
[62 to 81] 65/90 |
80
[61 to 92] 24/30 |
70
[57 to 81] 44/63 |
68
[55 to 80] 41/60 |
| MRI (DW+ VIBE) | 84
[76 to 90] 105/125 |
81
[72 to 88] 85/105 |
87
[66 to 97] 20/23 |
83
[75 to 90] 85/102 |
79
[69 to 87] 65/82 |
87
[79 to 93] 81/93 |
87
[78 to 93] 78/90 |
100
[88 to 100] 30/30 |
81
[69 to 90] 51/63 |
80
[68 to 89] 48/60 |
|
|
Pfannenberg 2007 Mixed Per lesion data 64 [420/297 ] |
MRI (DW+ VIBE) | Not assessed | 80
[75 to 84] 237/297 |
66
[56 to 75] 67/102 |
Not assessed | 87
[82 to 92] 170/195 |
Not assessed | 76
[68 to 84] 94/123 |
77
[64 to 87] 43/56 |
Not assessed | 76
[64 to 86] 51/67 |
| PET‐CT | |||||||||||
|
Arrangoiz 2012 Primary 56/32 |
CT (NR) |
47 [29 to 65] 15/32 |
Not assessed | Not assessed |
100 [48 to 100] 5/0 |
Not assessed |
88 [68 to 97] 21/24 |
Not assessed | Not assessed |
94 [84 to 99] 48/51 |
Not assessed |
|
Reinhardt 2006 Mixed Per patient data 250/116 |
CT (CE) |
97 [91 to 99] 112/116 |
Not assessed |
95 [87 to 99] 74/78 |
99 [94 to 100] 83/84 |
Not assessed |
98
[94 to 100] 131/134 |
Not assessed |
100
[98 to 100] 172/172 |
98
[94 to 99] 162/166 |
Not assessed |
|
Veit‐Haibach 2009 Primary Per patient data 56/13 Nodal; 12 Distant |
CT (CE) |
38 [14 to 68] 5/13 |
Brain NR 42 [15 to 72] 5/12 |
100 [92 to 100] 43/43 |
Brain NR 93 [81 to 99] 41/44 |
||||||
|
Cachin 2014 Mixed Per lesion data 87 [176 / 85] |
CT (NR) | 80
[70 to 88] 68/85 |
Not assessed | 85
[62 to 97] 17/20 |
78
[67 to 88] 51/65 |
Not assessed | 54
[43 to 64] 49/91 |
Not assessed | 37
[16 to 62] 7/19 |
58
[46 to 70] 42/72 |
Not assessed |
|
Dellestable 2011 Mixed Per lesion data 40 [118 / 72] |
CT (CE) | 74
[62 to 83] 53/72 |
Not assessed | 84
[66 to 95] 26/31 |
66
[49 to 80] 27/45 |
Not assessed | 89
[77 to 96] 42/47 |
Not assessed | 100
[59 to 100] 7/7 |
88
[73 to 96] 35/40 |
Not assessed |
|
Jouvet 2014 Unclear Per lesion data 37 [218 / 125] |
CT (CE) | Not assessed | 80
[71 to 87] 83/104 |
96
[78 to 100] 22/23 |
PET‐CT did not cover skull | 75
[64 to 84] 61/81 |
Not assessed | 93
[86 to 97] 81/87 |
97
[83 to 100] 29/30 |
PET‐CT did not cover skull | 91
[81 to 97] 52/57 |
|
Pfannenberg 2007 Mixed Per lesion data 64 [420/297] |
CT (CE) | Not assessed | 91
[87 to 94] 269/297 |
85
[77 to 92] 87/102 |
PET‐CT did not cover skull | 93
[89 to 96] 182/195 |
Not assessed | 77
[69 to 84] 95/123 |
89
[78 to 96] 50/56 |
PET‐CT did not cover skull | 67
[55 to 78] 45/67 |
| a studies with per patient data denoted in bold type CE: contrast enhanced; CT: computed tomography; DW: diffusion weighted; GE: gradient echo; MRI: magnetic resonance imaging; NR: not reported; PET: positron emission tomography; SLNB: sentinel lymph node biopsy; TN: true negative; TP: true positive; U: unenhanced; US: ultrasound; WB: whole body. | |||||||||||
Appendix 12. Findings from studies conducted in mixed or not clearly reported populations
Sensitivities and specificities from studies evaluating more than one target condition (any metastasis, nodal metastasis or distant metastasis) are tabulated in Appendix 11. Summary estimates of sensitivities and specificities are presented in Appendix 13.
Results: detection of any metastases
Eleven studies reported accuracy data for the detection of any metastasis in mixed study populations (Abbott 2011;Aukema 2010a;Aukema 2010b;Cachin 2014;Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007;Pfluger 2011;Reinhardt 2006;Strobel 2007b) (Table 1).
Forest plots of study data are provided in Figure 99 (per patient) and Figure 100 (per lesion). Summary estimates for indirect and direct comparisons of tests are presented in Appendix 13 and ROC plots of direct comparisons between tests in Figure 101, Figure 102, and Figure 103 (per lesion data only).
10.
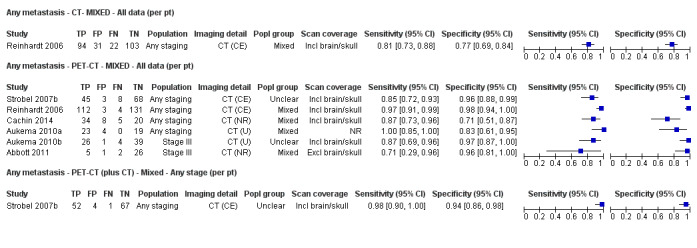
Forest plot of tests for the detection of any metastases (mixed populations ‐ per patient data).
11.
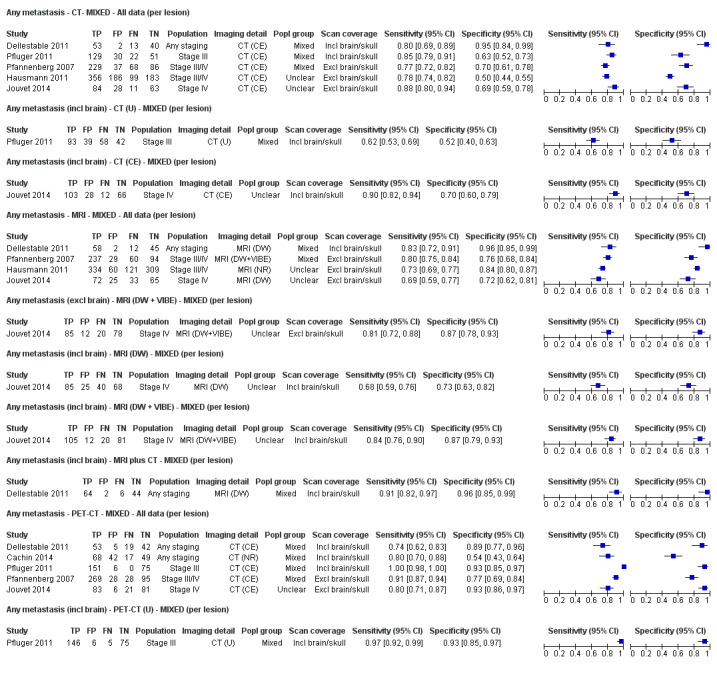
Forest plot of tests for the detection of any metastases (mixed populations ‐ per lesion data).
12.
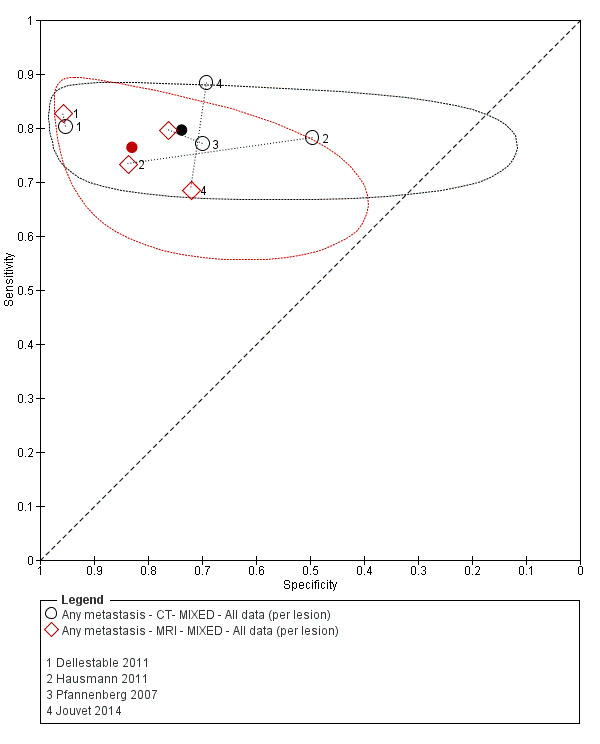
ROC plot of direct comparisons between CT and MRI for the detection of any metastases in mixed population group studies (per lesion data).
13.
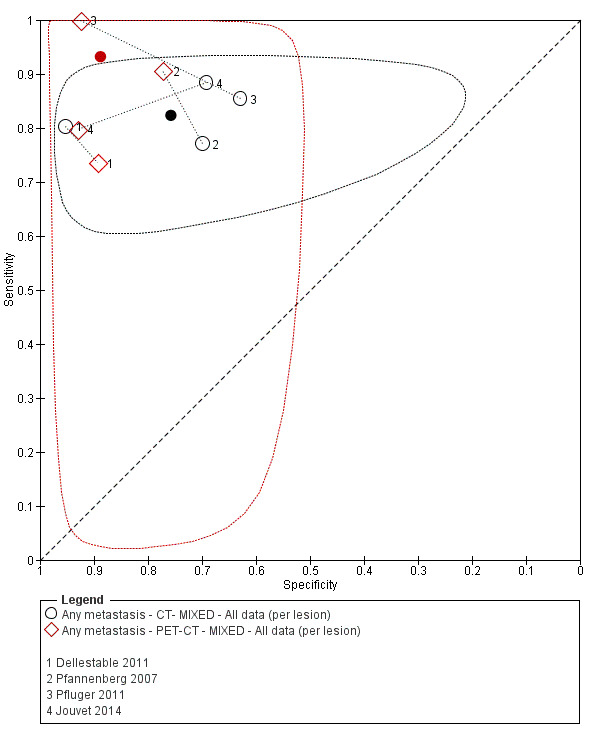
ROC plot of direct comparisons between CT and PET‐CT for the detection of any metastases in mixed population group studies (per lesion data).
14.
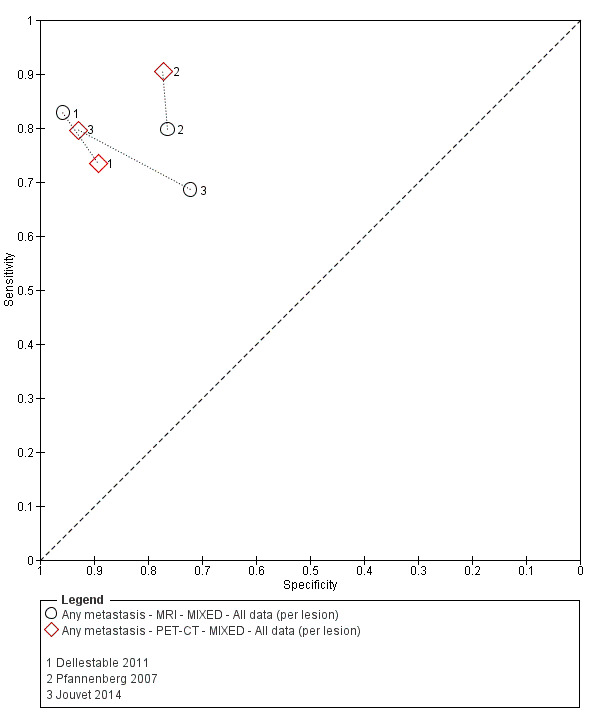
ROC plot of direct comparisons between MRI and PET‐CT for the detection of any metastases in mixed population group studies (per lesion data).
Per patient data
Six studies reported per patient data for a total of 553 study participants and 268 cases of metastases (Abbott 2011;Aukema 2010a;Aukema 2010bCachin 2014;Reinhardt 2006;Strobel 2007b) (Figure 99); prevalence ranged from 21% in Abbott 2011) to 58% in Cachin 2014.
CT. CT was evaluated in one study of 250 participants with mixed indications for imaging, including over 40% with stage I or II disease on presentation (Reinhardt 2006); scan coverage did not include the skull in this study. Observed sensitivity was 81% (95% CI 73, 88%) and specificity 77% (95% CI 69, 84%) (250 participants; 166 cases).
MRI. No per patient data for MRI were identified.
PET‐CT. Six studies provided per patient data for PET‐CT for the detection of any metastasis (Abbott 2011;Aukema 2010a;Aukema 2010bCachin 2014;Reinhardt 2006;Strobel 2007b). The sensitivity of PET‐CT ranged from 71% (95% CI 29% to 96%) in Abbott 2011 to 100% (95% CI 85% to 100%) in Aukema 2010a and specificity from 71% (95% CI 41% to 87%) in Cachin 2014 to 98% (95% CI 94% to 100%) in Reinhardt 2006.
Summary sensitivity from the six studies was 91.1% (95% CI 83.6% to 95.3%) and specificity 93.8% (95% CI 85.1% to 97.6%) (591 patients, 268 cases) (Appendix 13; Table A).
Observed sensitivity in Strobel 2007b increased from 85% (95% CI 72% to 93%) to 98% (95% CI 90% to 100%) (seven additional metastases detected) when PET‐CT interpretation was combined with a separate dedicated CT interpretation, with one additional false positive result (specificities 96% and 94%, respectively).
Reinhardt 2006 provided a direct comparison of the accuracy of contrast enhanced CT with PET‐CT, which found PET‐CT to be significantly more sensitive (97%, 95% CI 91% to 99%) and specific (98%, 95% CI 94% to 100%) in comparison to CT alone (increases of 16% and 22%, respectively).
Per lesion data
Six studies reported per lesion data for a total of 311 study participants, 1989 lesions, and 1185 confirmed metastases (Cachin 2014;Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007;Pfluger 2011) (Figure 100). The prevalence of metastases on a lesion basis ranged from 48% in Cachin 2014 to 71% in Pfannenberg 2007. The average number of confirmed metastatic lesions per study participant ranged from 1 in Cachin 2014 to 14 in Hausmann 2011, with a median of 3.
CT. Five studies presented data for contrast enhanced CT for the detection of any metastases (Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007;Pfluger 2011). Sensitivity ranged from 77% (95% CI 72% to 82%) in Pfannenberg 2007 to 88% (95% CI 80% to 94%) in Jouvet 2014, and specificity from 50% (95% CI 44% to 55%) in Hausmann 2011 to 95% (95% CI 84% to 99%) in Dellestable 2011.
Summary sensitivity from the five studies was 81.3% (95% CI 76.8% to 85.1%) and specificity 71.2% (95% CI 53.9% to 83.9%) (1770 lesions, 1064 metastases) (Appendix 13; Table B).
A single study providing a direct comparison of the accuracy of contrast enhanced CT with unenhanced CT found contrast enhanced CT to be significantly more sensitive (85%, 95% CI 79% to 85%) compared to unenhanced CT (62%, 95% CI 53% to 69%), with a smaller decrease (11%) in specificity for unenhanced CT (52%, 95% CI 40% to 63%) (232 lesions, 151 confirmed metastases) (Figure 100) (Pfluger 2011).
MRI. Four studies presented data for MRI for the detection of any metastases (Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007). Sensitivity ranged from 69% (95% CI 59% to 77%) in Jouvet 2014 to 83% (95% CI 72% to 91%) in Dellestable 2011, and specificity from 72% (95% CI 62% to 81%) in Jouvet 2014 to 96% (95% CI 85% to 99%) in Dellestable 2011.
Summary sensitivity from the four studies was 76.4% (95% CI 70.6% to 81.4%) and specificity 83.0% (95% CI 71.9% to 90.3%) (1556 lesions, 927 metastases) (Appendix 13; Table B). Sensitivity and specificity in Jouvet 2014 were both increased (by 13% and 15%, respectively) with the addition of ultrafast gradient echo (VIBE) sequences to the MRI protocol.
PET‐CT. Five studies evaluated PET‐CT for the detection of any metastasis (Cachin 2014;Dellestable 2011;Jouvet 2014;Pfluger 2011;Pfannenberg 2007). Sensitivity ranged from 74% (95% CI 62% to 83%) in Dellestable 2011 to 100% (95% CI 98% to 100%) in Pfluger 2011, and specificity from 54% (95% CI 43% to 64%) in Cachin 2014 to 93% (95% CI 86% to 97%) in Jouvet 2014.
Summary sensitivity from the five studies was 90.7% (95% CI 69.0% to 97.7%) and specificity 84.5% (95% CI 69.7% to 92.9%) (1138 lesions, 709 metastases) (Appendix 13; Table B).
Pfluger 2011 showed only marginal differences in accuracy between PET‐CT using contrast enhanced CT versus unenhanced CT; sensitivity for unenhanced PET‐CT (97%, 95% CI 92% to 99%) compared to enhanced PET‐CT (100%, 95% CI 98% to 100%) (232 lesions; 151 confirmed metastases).
Comparisons between tests. The statistical model comparing the three sets of pooled estimates showed no statistically significant differences in sensitivity (P = 0.17) or specificity (P = 0.29) between tests (Appendix 13; Table B).
Three of the studies provided a direct comparison of CT, MRI, and PET‐CT (Dellestable 2011;Jouvet 2014;Pfannenberg 2007), Hausmann 2011 compared CT and MRI, and Pfluger 2011 compared CT and PET‐CT. The direct comparisons between tests in these studies are plotted ROC space in Figure 101,Figure 102, and Figure 103. None of the differences in sensitivity and specificity between tests reached statistical significance (Appendix 13; Table C).
Results: detection of nodal metastases
Ten studies reported accuracy data for the detection of nodal metastases (Cachin 2014; Dellestable 2011; Hausmann 2011; Jouvet 2014; Klebl 2003; Pfannenberg 2007; Reinhardt 2006; Rubaltelli 2011; van den Brekel 1998; van Wissen 2016).
Forest plots of study data are provided in Figure 104 (per patient) and Figure 105 (per lesion).
15.
Forest plot of tests for the detection of nodal metastases (mixed populations ‐ per patient data).
16.
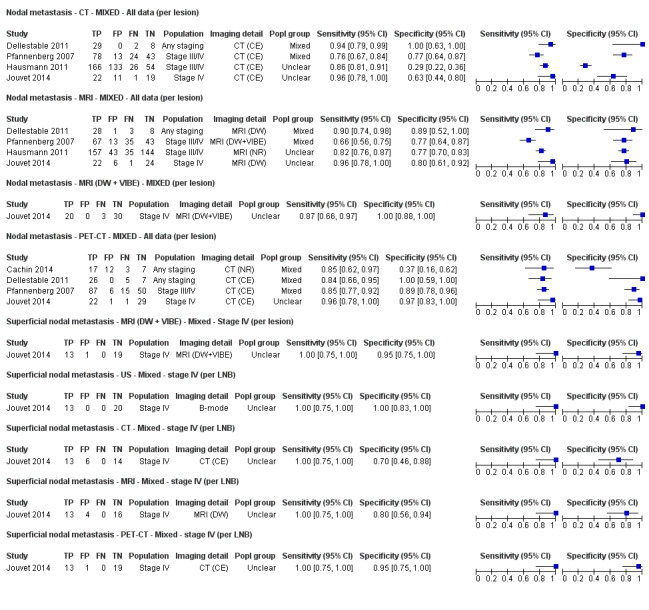
Forest plot of tests for the detection of nodal metastases (mixed populations ‐ per lesion data).
Per patient data
Four studies reported per patient data for a total of 355 study participants and 175 cases of nodal metastases (Klebl 2003; Reinhardt 2006; Van den Brekel 1998; van Wissen 2016) (Figure 104); the prevalence of nodal metastases ranged from 22% in Klebl 2003 to 86% in van Wissen 2016.
Ultrasound. One study evaluated ultrasound for nodal metastases in participants with Clark level IV or V melanoma following primary treatment (n = 8) or during follow‐up (n = 75) (Klebl 2003). All 17 participants with nodal metastases were identified on ultrasound (sensitivity 100%, 95% CI 80% to 100%) with 21 false positives (specificity 66%, 95% CI 53% to 78%); 11 of the 17 true positive results were also detected on palpation, with a total of 12 false positive results (Klebl 2003).
CT. CT was evaluated for the detection of nodal metastases in two studies. In Reinhardt 2006, 78 of the 166 participants with confirmed metastatic disease had nodal metastases (prevalence 78/250; 31%). Sensitivity was 85% (95% CI 75% to 92%) and specificity 87% (95% CI 81% to 92%). Similarly high sensitivity was reported in a high prevalence study of CT before therapeutic or elective dissection of the lymph nodes of the neck in participants with head and neck melanoma (86%, 95% CI 64% to 97%), with specificity of 100% (95% CI 48% to 100%) (26 participants; 21 cases of nodal metastases) (van den Brekel 1998).
MRI. No per patient data were identified for MRI in this patient group.
PET‐CT. PET‐CT was evaluated for the detection of nodal metastases in two studies. In a direct comparison with CT alone, PET‐CT was more sensitive (95%, 95% CI 87% to 99%) than CT alone but with overlapping confidence intervals, and was significantly more specific (100%, 95% CI 98% to 100%) (250 participants; 78 cases of nodal metastases) (Reinhardt 2006).
van Wissen 2016 evaluated the use of PET‐CT in 69 participants scheduled for combined superficial and deep groin dissection due to palpable groin metastases. Results showed that although PET‐CT was highly sensitive for the detection of superficial groin metastases (98%, 95% CI 91% to 100%) (59 cases), six participants with deep groin metastases were missed by PET‐CT even when indeterminate PET‐CT results were considered test positive (sensitivity 75%, 95% CI 53% to 90%) (24 cases). Specificity was 81% (95% CI 76% to 92%), with eight false positive results.
Per lesion data
Per lesion data were reported in five studies for a total of 241 study participants, 669 lesions, and 338 confirmed metastases(Cachin 2014;Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007) (Figure 105). The prevalence of metastases on a lesion basis ranged from 43% in Hausmann 2011 to 78% in Dellestable 2011. Summary estimates for indirect and direct comparisons of tests are presented in Appendix 13, and ROC plots of direct comparisons between tests in Figure 106, Figure 107, and Figure 108 (per lesion data only).
17.
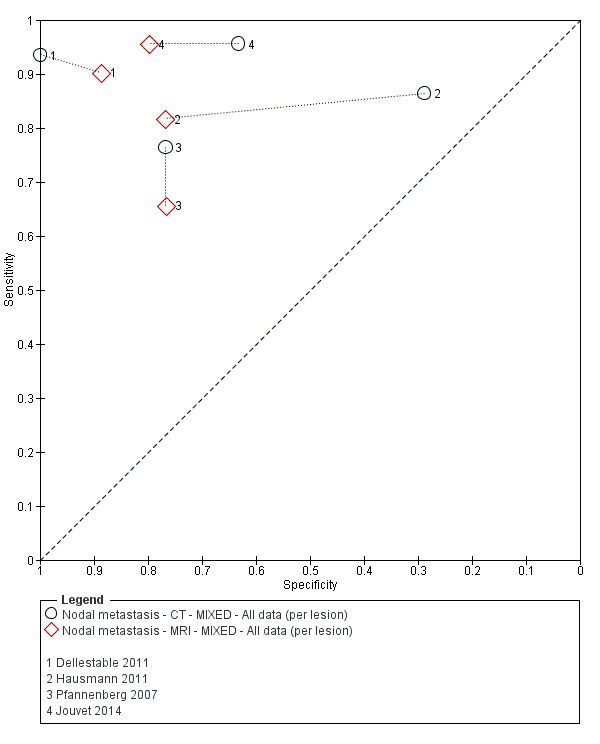
ROC plot of direct comparisons between CT and MRI for the detection of nodal metastases in mixed population group studies (per lesion data).
18.

ROC plot of direct comparisons between CT and PET‐CT for the detection of nodal metastases in mixed population group studies (per lesion data).
19.

ROC plot of direct comparisons between MRI and PET‐CT for the detection of nodal metastases in mixed population group studies (per lesion data).
CT. Four studies evaluated contrast enhanced CT for the detection of nodal metastasis (Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007). Sensitivity ranged from 76% (95% CI 67% to 84%) in Pfannenberg 2007 to 96% (95% CI 78% to 100%) in Jouvet 2014, and specificity from 29% (95% CI 22% to 36%) in Hausmann 2011 to 100% (95% CI 63% to 100%) in Dellestable 2011.
Summary sensitivity from the four studies was 87.2% (95% CI 76.5% to 93.4%) and specificity 69.2% (95% CI 34.6% to 90.5%) (629 lesions, 348 metastases) (Appendix 13; Table B).
MRI. The same four studies considered MRI for the detection of nodal metastasis using a number of different MRI protocols (Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007). Sensitivity ranged from 66% (95% CI 56% to 75%) in Pfannenberg 2007 to 96% (95% CI 78% to 100%) in Jouvet 2014, and specificity from 77% (95% CI 64% to 87%) in Pfannenberg 2007 to 77% (95% CI 70% to 83%) in Hausmann 2011 to 89% (95% CI 52% to 100%) in Dellestable 2011. Summary sensitivity from the four studies was 83.9% (95% CI 68.9% to 92.5%) and specificity 78.1% (95% CI 72.1% to 83.1%) (630 lesions, 348 metastases) (Appendix 13; Table B).
The direct comparison of diffusion weighted MRI compared with diffusion weighted plus VIBE sequences in Jouvet 2014 found the addition of VIBE to be less sensitive but more specific, but with small lesion numbers (53 nodal lesions and 23 malignancies), the differences were not statistically significant.
PET‐CT. Four studies evaluated PET‐CT for the detection of nodal metastasis (Cachin 2014;Dellestable 2011;Jouvet 2014;Pfannenberg 2007). Sensitivities ranged from 84% (95% CI 66% to 95%) in Dellestable 2011 to 96% (95% CI 83% to 100%) in Jouvet 2014, and specificities from 37% (95% CI 16% to 62%) in Cachin 2014 to 100% (95% CI 59% to 100%) in Dellestable 2011.
Summary sensitivity from the four studies was 86.4% (95% CI 80.5% to 90.7%) and specificity 89.1% (95% CI 53.1% to 98.3%) (288 lesions, 176 metastases) (Appendix 13; Table B).
Comparison between tests. The statistical model comparing the three sets of pooled estimates showed no statistically significant differences in sensitivity (P = 0.22) or specificity (P = 0.89) between tests (Appendix 13; Table B).
Three studies in mixed population groups provided a direct comparison of CT, MRI, and PET‐CT (Dellestable 2011;Jouvet 2014;Pfannenberg 2007); Hausmann 2011 also compared CT and MRI. Three studies included the same total numbers of nodal lesions and metastases per test, while the number detected per test varied for Dellestable 2011. ROC plots show direct comparisons between tests in Figure 106, Figure 107, and Figure 108 (per lesion data only). No statistically significant differences in sensitivity were observed in any of the direct comparisons, but the specificity of PET‐CT (92.5%, 95% CI 85.0% to 96.4%) was significantly higher than both MRI (by 13.5%, 95% CI 3.73% to 23.3%; P = 0.007) and CT alone (by 18.0%, 95% CI 7.69% to 28.3%; P = 0.001) (Appendix 13; Table C).
Results: detection of distant metastases
Nine studies considered the detection of distant metastases (Arrangoiz 2012;Bastiaannet 2009;Cachin 2014;Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007;Reinhardt 2006;Veit‐Haibach 2009).
Forest plots of study data are provided in Figure 109 (per patient) and Figure 110 (per lesion).
20.
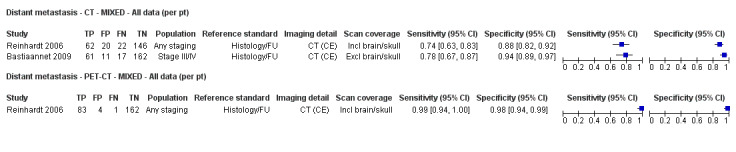
Forest plot of tests for the detection of distant metastases (mixed populations ‐ per patient data only).
21.
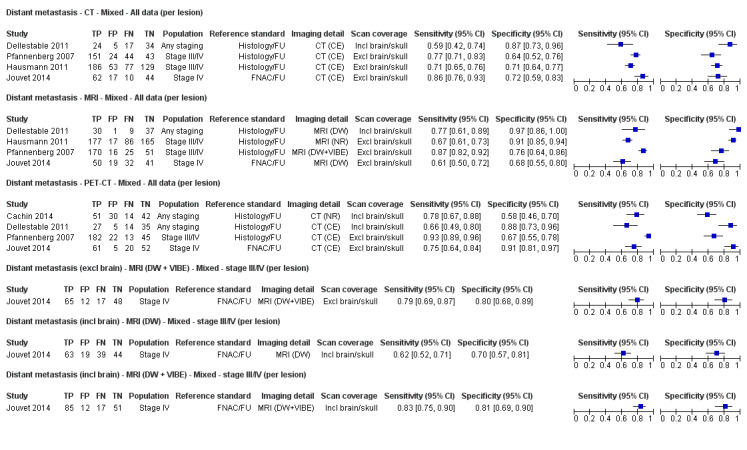
Forest plot of tests for the detection of distant metastases (per lesion data).
Per patient data
Two studies reported per patient data for a total of 501 study participants and 162 cases of distant metastases (Bastiaannet 2009;Reinhardt 2006) (Figure 109); the prevalence of nodal metastases was 31% (Bastiaannet 2009) and 34% (Reinhardt 2006).
CT.Reinhardt 2006 reported sensitivity of 74% (95% CI 63% to 83%) and specificity of 88% (95% CI 84% to 99%) in participants at any stage of disease and with mixed indications for imaging (250 participants; 84 cases of distant metastases). Bastiaannet 2009 included participants with palpable, confirmed lymph node metastases who were considered candidates for regional lymph node dissection. Sensitivity was 78% (95% CI 67% to 87%) and specificity 94% (95% CI 89% to 97%) (251 participants; 78 cases of distant metastases).
MRI. No per patient data were identified for MRI in this patient group.
PET‐CT.Reinhardt 2006 reported a direct comparison of CT with PET‐CT (Figure 109). Both sensitivity and specificity increased significantly with PET‐CT (sensitivity 99%, 95% CI 94% to 100% and specificity 98%, 95% CI 94% to 99%).
Per lesion data
Per lesion data were reported in five studies for a total of 501 study participants, 1090 lesions, and 666 confirmed metastases (Cachin 2014;Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007) (Figure 110). The prevalence of distant metastases on a lesion basis ranged from 47% in Cachin 2014 to 74% in Pfannenberg 2007.
CT. Four studies evaluated contrast enhanced CT for the detection of distant metastasis (Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007). Sensitivity ranged from 59% (95% CI 42% to 74%) in Dellestable 2011 to 86% (95% CI 76% to 93%) in Jouvet 2014, and specificity from 64% (95% CI 52% to 76%) in Pfannenberg 2007 to 87% (95% CI 73% to 96%) in Dellestable 2011 (Figure 110).
Summary sensitivity from the four studies was 73.4% (95% CI 63.6% to 81.3%) and specificity 71.9% (95% CI 64.3% to 78.5%) (920 lesions, 571 metastases) (Appendix 13; Table B).
MRI. The same four studies considered MRI for the detection of distant metastasis (Dellestable 2011;Hausmann 2011;Jouvet 2014;Pfannenberg 2007). Sensitivity ranged from 61% (95% CI 50% to 72%) in Jouvet 2014, to 87% (95% CI 82% to 92%) in Pfannenberg 2007, and specificity from 68% (95% CI 64% to 87%) in Jouvet 2014 to 97% (95% CI 70% to 83%) in Dellestable 2011 (Figure 110).
Summary sensitivity from the four studies was 74.5% (95% CI 62.1% to 83.9%) and specificity 85.8% (95% CI 70.4% to 93.9%) (926 lesions, 579 metastases) (Appendix 13; Table B).
The low sensitivity and specificity observed in Jouvet 2014 were improved to 79% (95% CI 69% to 87%) and 80% (95% CI 68% to 89%) with the addition of VIBE sequences but with overlapping confidence intervals.
PET‐CT. Four studies evaluated PET‐CT for the detection of nodal metastasis (Cachin 2014;Dellestable 2011;Jouvet 2014;Pfannenberg 2007). Sensitivities ranged from 66% (95% CI 49% to 80%) in Dellestable 2011 to 93% (95% CI 89% to 96%) in Pfannenberg 2007, and specificities from 58% (95% CI 46% to 70%) in Cachin 2014 to 91% (95% CI 81% to 97%) in Jouvet 2014 (Figure 110).
Summary sensitivity from the four studies was 81.0% (95% CI 67.5% to 90.0%) and specificity 78.5% (95% CI 61.0% to 89.5%) (618 lesions, 382 metastases) (Appendix 13; Table B).
Comparison between tests. The statistical model comparing the three sets of pooled estimates showed no statistically significant differences in sensitivity (P = 0.22) or specificity (P = 0.89) between tests (Appendix 13; Table B).
Three studies in mixed population groups provided a direct comparison of CT, MRI, and PET‐CT (Dellestable 2011;Jouvet 2014;Pfannenberg 2007); Hausmann 2011 also compared CT and MRI. Two studies included the same total numbers of lesions and metastases per test (Hausmann 2011;Pfannenberg 2007), and two included only those lesions detected by each test so that the number of lesions varied per test (Dellestable 2011;Jouvet 2014). The direct comparisons between tests in these studies are plotted as ROC space in Figure 111,Figure 112, and Figure 113. No statistically significant differences in sensitivity were observed in any of the direct comparisons, but the specificity of MRI (85.8%, 95% CI 70.4% to 93.9%) was significantly higher than CT (by 13.9%, 95% CI 0.43% to 27.3%; P = 0.043) (Appendix 13; Table C).
22.

ROC plot of direct comparisons between CT and MRI for the detection of distant metastases in mixed population group studies (per lesion data).
23.
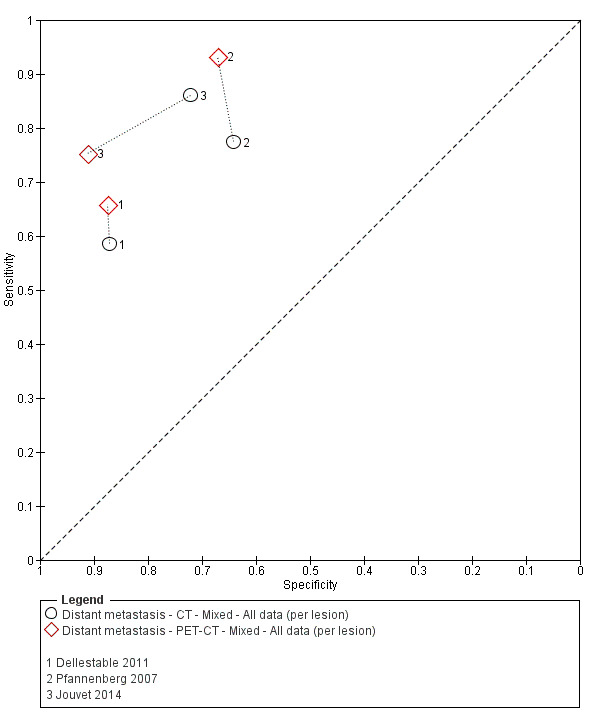
ROC plot of direct comparisons between CT and PET‐CT for the detection of distant metastases in mixed population group studies (per lesion data).
24.
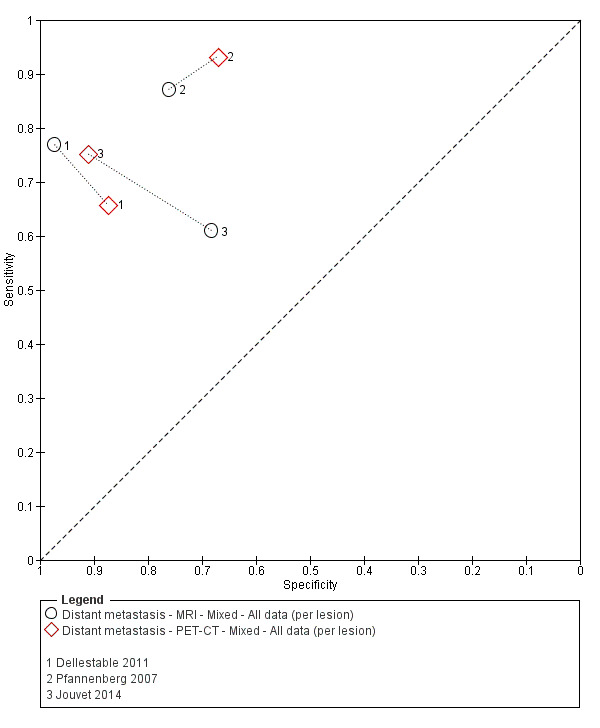
ROC plot of direct comparisons between MRI and PET‐CT for the detection of distant metastases in mixed population group studies (per lesion data).
Results: detection of distant metastases by metastatic site
Four studies conducted in mixed or not clearly described population groups reported per lesion data according to metastatic site (Cachin 2014; Dellestable 2011; Jouvet 2014; Pfannenberg 2007). Appendix 14 presents sensitivities and specificities for all metastatic sites according to test for ease of comparison of accuracy across different sites. Sensitivity and specificity were not estimated for sites with fewer than five malignant or benign lesions. Forest plots of study data for each test by metastatic site are presented in Figure 114, Figure 115, Figure 116, and Figure 117. Summary estimates for indirect and direct comparisons of tests are presented in Appendix 13.
25.

Forest plot of tests for the detection of bone metastasis in mixed population groups (per lesion data).
26.
Forest plot of tests for the detection of lung metastasis in mixed population groups (per lesion data).
27.
Forest plot of tests for the detection of liver metastasis in mixed population groups (per lesion data).
28.
Forest plot of tests: 75 soft tissue metastasis ‐ PET‐CT ‐ MIXED (per lesion), 76 local/subcutaneous metastasis ‐ CT ‐ MIXED (per lesion), 77 local/subcutaneous metastasis ‐ MRI ‐ MIXED (per lesion), 78 local/subcutaneous metastasis ‐ MRI (DW + VIBE) ‐ MIXED (per lesion), 79 local/subcutaneous metastasis ‐ PET‐CT ‐ MIXED (per lesion).
Bone metastases
For the detection of metastases in the bone, CT performed the poorest in terms of sensitivity, which ranged from 50% (95% CI 23% to 77%) in Dellestable 2011 to 67% (95% CI 38% to 88%) in Jouvet 2014 in three studies, compared to 93% (95% CI 66% to 100%) in Dellestable 2011 to 100% in Jouvet 2014 and Pfannenberg 2007 for MRI, and 71% (95% CI 42% to 92%) in Dellestable 2011 to 91% (95% CI 77% to 98%) in Pfannenberg 2007 for PET‐CT (Figure 114).
Data could be pooled for CT and PET‐CT for two studies with more than five metastases and more than five benign lesions (Jouvet 2014;Pfannenberg 2007). For PET‐CT (85 lesions and 51 metastases), summary sensitivity was 90.2% (95% CI 78.5% to 95.9%) and specificity 88.2% (95% CI 72.5% to 95.5%) (Appendix 13). Summary sensitivity for CT was 26.2% lower (P = 0.001) at 64.0% (95% CI 49.9% to 76.0%) and specificity non‐significantly higher at 94.0% (95% CI 49.5% to 99.6%), (P = 0.56).
Lung metastases
For the detection of lung metastases (four studies), CT performed the best in terms of sensitivity, which ranged from 78% (95% CI 27% to 84%) in Hausmann 2011 to 100% (95% CI 75% to 100%) in Dellestable 2011 compared to 47% (95% CI 39% to 55%) in Hausmann 2011 to 87% (95% CI 75% to 95%) in Pfannenberg 2007 for MRI and 31% (95% CI 09% to 61%) in Dellestable 2011 to 100% (95% CI 69% to 100%) in Cachin 2014 for PET‐CT (Figure 115). For those studies with more than five disease negative lesions identified, specificities were consistently poor for CT compared to MRI or PET‐CT.
Data were pooled for CT and for MRI for three studies with more than five metastases and more than five benign lesions (Jouvet 2014;Pfannenberg 2007). For CT (312 lesions and 229 metastases), summary sensitivity was 90.6% (95% CI 75.7% to 96.8%) and specificity 43.8% (29.5% to 59.1%) (Appendix 13). Summary sensitivity for MRI was 34.9% lower (P = 0.054) at 55.7% (95% CI 24.0% to 83.4%) and specificity significantly higher at 91.3% (95% CI 77.3% to 97.0%) (P < 0.001).
Liver metastases
For liver metastases, only three studies included more than five metastatic lesions to allow comparison of sensitivities (Hausmann 2011;Jouvet 2014;Pfannenberg 2007). Both MRI ‐ Hausmann 2011;Jouvet 2014;Pfannenberg 2007 ‐ and PET‐CT ‐ Jouvet 2014;Pfannenberg 2007 ‐ had higher sensitivities compared to CT, but differences were significant only for Hausmann 2011 due to small numbers (Figure 116).
Three studies included more than five benign lesions to allow comparison of specificities. Specificities were 90% or more for CT, MRI, and PET‐CT in Dellestable 2011, but the number of benign lesions detected by each test varied from 17 (for CT) to 22 (for MRI). Hausmann 2011 reported specificity to be higher for MRI (100%) compared to CT (50%), but specificities were consistently high for CT, MRI, and PET‐CT (87% to 100%) in Jouvet 2014.
No statistical pooling could be undertaken for this target condition.
Local or subcutaneous metastases and soft tissue metastases
The detection of local or subcutaneous metastases was reported in three studies. Overall PET‐CT appeared more sensitive than MRI (sensitivities 90% ‐ Pfannenberg 2007 ‐ and 100% ‐ Jouvet 2014 ‐ compared to 70% and 78% for MRI, respectively) and MRI more sensitive in comparison to CT (sensitivities 78% ‐ Pfannenberg 2007 ‐ and 100% ‐ Hausmann 2011 ‐ compared to CT (sensitivities 64% ‐ Pfannenberg 2007 ‐ and 82% ‐ Hausmann 2011), but lesion numbers were small and confidence intervals overlapping. No clear differences in specificities were observed (Figure 117).
Brain metastases
Only two studies included sufficient numbers of imaging abnormalities of the brain to allow sensitivity to be estimated for CT and MRI (Jouvet 2014), and for PET‐CT (Cachin 2014). The lowest sensitivity was observed for diffusion weighted MRI (65%, 95% CI 41% to 85%); however the addition of VIBE sequences increased sensitivity to 100% (95% CI 83% to 100%) (23 lesions identified, 20 confirmed metastases). In comparison, the sensitivity of CT was 95% (95% CI 75% to 100%).
In Cachin 2014, the sensitivity of PET‐CT for detection of brain metastases was 22% (95% CI 3% to 60%) (nine lesions identified, seven confirmed metastases).
Three additional studies conducted in mixed or unclear populations reported some data on the detection of brain metastases, but numbers were insufficient to include 2×2 contingency tables. In Strobel 2007b, a single confirmed brain metastasis was described as detected on PET‐CT. Two studies evaluated whole body PET‐CT in combination with MRI of the brain (Aukema 2010a;Aukema 2010b). In Aukema 2010a, MRI detected two confirmed brain metastases in one patient, and in Aukema 2010b, five confirmed brain metastases were detected ‐ four in patients with multiple metastases detected by PET‐CT and one solitary brain metastasis. Neither study reported the detection of any benign imaging abnormalities.
Appendix 13. Summary estimates of sensitivities and specificities from mixed or unclear population studies
Table A Summary estimates for tests evaluated in mixed study populations, per patient data
|
Test Target condition |
Studies | Participants (cases) | Sensitivity (95% CI) % | Specificity (95% CI) % |
| Any metastasis | ||||
| PET‐CT | 6 | 591 (268) | 91.1 (83.6 to 95.3) | 93.8 (85.1 to 97.6) |
Table B Indirect comparison of imaging tests from mixed study populations, per lesion data
|
Test Target condition |
Studies | Participants (cases) | Sensitivity (95% CI) % | Specificity (95% CI) % |
| Detection of any metastasis | ||||
| CT | 5 | 1770 (1064) | 81.3 (76.8 to 85.1) | 71.2 (53.9 to 83.9) |
| MRI | 4 | 1556 (927) | 76.4 (70.6 to 81.4) | 83.0 (71.9 to 90.3) |
| PET‐CT | 5 | 1138 (709) | 90.7 (69.0 to 97.7) | 84.5 (69.7 to 92.9) |
| Difference (P value) | 0.17 | 0.29 | ||
| Detection of nodal metastasis | ||||
| CT | 4 | 629 (348) | 87.2 (76.5 to 93.4) | 69.2 (34.6 to 90.5) |
| MRI | 4 | 630 (348) | 83.9 (68.9 to 92.5) | 78.1 (72.1 to 83.1) |
| PET‐CT | 4 | 288 (176) | 86.4 (80.5 to 90.7) | 89.1 (53.1 to 98.3) |
| Difference (P value) | 0.22 | 0.89 | ||
| Detection of distant metastasis | ||||
| CT | 4 | 920 (571) | 73.4 (63.6 to 81.3) | 71.9 (64.3 to 78.5) |
| MRI | 4 | 926 (579) | 74.5 (62.1 to 83.9) | 85.8 (70.4 to 93.9) |
| PET‐CT | 4 | 618 (382) | 81.0 (67.5 to 90.0) | 78.5 (61.0 to 89.5) |
| Difference (P value) | 0.58 | 0.21 | ||
Table C Direct comparisons of imaging tests from mixed study populations, per lesion data
|
Test Target condition |
Studies | Participants (cases) | Sensitivity (95% CI) % | Specificity (95% CI) % |
| Detection of any metastasis | ||||
| CT | 4 | 1538 (913) | 79.6 (76.0 to 82.8) | 73.8 (51.5 to 88.2) |
| MRI | 4 | 1556 (927) | 76.4 (70.6 to 81.4) | 83.0 (71.9 to 90.3) |
| Difference % (95% CI), P value | 3.19 (‐3.25 to 9.64), P = 0.33 |
‐9.21 (‐30.1 to 11.7), P = 0.39 |
||
| Detection of any metastasis | ||||
| PET‐CT | 4 | 962 (624) | 93.2 (63.9 to 99.1) | 88.8 (80.6 to 93.8) |
| CT | 4 | 946 (609) | 82.3 (76.6 to 86.9) | 75.8 (58.9 to 87.2) |
| Difference % (95% CI), P value | 10.9 (‐3.08 to 24.8), P = 0.13 |
13.0 (‐2.66 to 28.7), P = 0.10 |
||
| Detection of any metastasis | ||||
| PET‐CT | 3 | 730 (473) | 83.1 (65.3 to 92.8) | 87.3 (76.7 to 93.5) |
| MRI | 3 | 732 (472) | 77.4 (70.6 to 82.9) | 83.1 (72.9 to 90.0) |
| Difference % (95% CI), P value | 5.79 (‐4.67 to 16.3), P = 0.28 |
4.20 (‐11.6 to 20.0), P = 0.60 |
||
| Detection of nodal metastasis | ||||
| CT | 4 | 629 (348) | 87.2 (76.5 to 93.4) | 69.2 (34.6 to 90.5) |
| MRI | 4 | 630 (348) | 83.7 (68.8 to 92.3) | 77.7 (72.4 to 82.1) |
| Difference % (95% CI), P value | 3.41 (‐10.8 to 17.6), P = 0.64 |
‐8.45 (‐39.7 to 22.8), P = 0.60 |
||
| Detection of nodal metastasis | ||||
| PET‐CT | 3 | 249 (156) | 86.5 (80.2 to 91.1) | 92.5 (85.0 to 96.4) |
| CT | 3 | 250 (156) | 89.0 (71.9 to 96.2) | 74.5 (64.7 to 82.3) |
| Difference % (95% CI), P value | ‐2.44 (‐14.9 to 10.0), P = 0.70 |
18.0 (7.69 to 28.3), P = 0.001 |
||
| Detection of nodal metastasis | ||||
| PET‐CT | 3 | 249 (156) | 86.5 (80.2 to 91.1) | 92.5 (85.0 to 96.4) |
| MRI | 3 | 251 (156) | 86.1 (63.1 to 95.7) | 78.9 (69.6 to 86.0) |
| Difference % (95% CI), P value | 0.48 (‐15.8 to 16.8), P = 0.95 |
13.5 (3.73 to 23.3), P = 0.007 |
||
| Detection of distant metastasis | ||||
| CT | 4 | 920 (571) | 73.4 (63.6 to 81.3) | 72.0 (64.3 to 78.5) |
| MRI | 4 | 926 (579) | 74.5 (62.1 to 83.9) | 85.8 (70.4 to 93.9) |
| Difference % (95% CI), P value | ‐1.10 (‐15.2 to 13.0), P = 0.88 |
‐13.9 (‐27.3 to ‐0.43), P = 0.043 |
||
| Detection of distant metastasis | ||||
| PET‐CT | 3 | 481 (317) | 81.8 (63.1 to 92.2) | 83.5 (68.0 to 92.3) |
| CT | 3 | 475 (308) | 76.0 (62.6 to 85.7) | 74.2 (61.9 to 83.6) |
| Difference % (95% CI), P value | 5.77 (‐12.7 to 24.2), P = 0.54 |
9.35 (‐6.85 to 25.5), P = 0.26 |
||
| Detection of distant metastasis | ||||
| PET‐CT | 3 | 481 (317) | 81.8 (63.1 to 92.2) | 83.5 (68.0 to 92.3) |
| MRI | 3 | 481 (316) | 77.0 (61.7 to 87.4) | 83.8 (59.8 to 94.8) |
| Difference % (95% CI), P value | 4.78 (‐14.6 to 24.1), P = 0.63 |
‐0.33 (‐21.1 to 20.4), P = 0.98 |
||
Table D Direct comparisons of tests by metastatic site
| Test | Studies | Participants (cases) | Sensitivity (95% CI) % | Specificity (95% CI) % |
| Detection of bone metastasis | ||||
| PET‐CT | 2 | 85 (51) | 90.2(78.5 to 95.9) | 88.2 (72.5 to 95.5) |
| CT | 2 | 83 (50) | 64.0 (49.9 to 76.0) | 94.0 (49.5 to 99.6) |
| Difference % (95% CI), P value | 26.2 (10.6 to 41.8), P=.001 | ‐5.73(‐24.8 to 13.3), P=0.56 | ||
| Detection of lung metastasis | ||||
| CT | 3 | 312 (229) | 90.6 (75.7 to 96.8) | 43.8 (29.5 to 59.1) |
| MRI | 3 | 312 (229) | 55.7 (24.0 to 83.4) | 91.3 (77.3 to 97.0) |
| Difference % (95% CI), P value | 34.9 (‐0.61 to 70.4 ), P=0.054 | ‐47.5 (‐65.2 to ‐29.8), P<0.001 | ||
| Detection of local or subcutaneous metastasis | ||||
| CT | 2 | 126 (92) | 71.8 (57.6 to.82.7 ) | 64.7 (47.6 to 78.7) |
| MRI | 2 | 126 (92) | 96.2 (31.1 to 99.9) | 70.6 (53.4 to 83.3) |
| Difference % (95% CI), P value | ‐24.4 (‐43.9 to ‐4.86), P=0.01 | ‐5.88 (‐28.1 to 16.3), P=0.60 | ||
| Detection of local or subcutaneous metastasis | ||||
| PET‐CT | 2 | 95 (66) | 90.9 (81.2 to 95.9) | 65.5 (46.9 to 80.3) |
| MRI | 2 | 102 (69) | 76.8 (65.4 to 85.2) | 69.7 (52.3 to 82.9) |
| Difference % (95% CI), P value | 14.1 (1.96 to 26.2), P=0.02 | ‐4.18 (‐27.5 to 19.2), P=0.73 | ||
| Detection of local or subcutaneous metastasis | ||||
| MRI | 3 | 148 (102) | 89.7 (53.7–98.5) | 71.7 (57.2–82.8) |
Appendix 14. Sensitivity and specificity of imaging tests by metastatic site
|
Study Population group |
No. pts Lesions/ cases |
Test | Distant | Bone | Lung | Liver | Skin/subcutaneous | Other | ||||
|
Sensitivity: [95% CI]% TP/Dis Specificity: [95% CI]% TN/No Dis |
Sensitivity [95% CI] % TP/Dis |
Specificity [95% CI] % TN/No Dis |
Sensitivity [95% CI] % TP/Dis |
Specificity [95% CI] % TN/No Dis |
Sensitivity [95% CI] % TP/Dis |
Specificity [95% CI] % TN/No Dis |
Sensitivity [95% CI] % TP/Dis |
Specificity [95% CI] % TN/No Dis |
Sensitivity: [95% CI] %
TP/Dis Specificity: [95% CI]% TN/No Dis |
|||
| CT | ||||||||||||
|
Dellestable 2011 Mixed |
40 118/72 (no. varies per test) |
CT (CE) | Incl brain: Se: 59 [42 to 74] 24/41 Sp: 87 [73 to 96] 34/39 |
50 [23 to 77] 7/14 |
Insufficient data (3/3 detected) | 100 [75 to 100] 13/13 |
Insufficient data (2/3 detected) | Insufficient data (2/4 detected) | 100
[80 to 100] 17/17 |
Distant minus bone, lung, liver mets Se: 20 [3 to 56] 2/10 Sp: 75 [48 to 93] 12/16 |
||
|
Hausmann 2011 Unclear |
33 824/455 (all detected by ≥ 1 test) |
CT (CE) | Excl brain: Se: 71 [65 to 76] 186/263 Sp: 71 [64 to 77] 129/182 |
(n = 1) | (n = 1) | 78 [70 to 84] 113/145 |
52
[38 to 66] 27/52 |
39 [23 to 58] 13/33 |
50
[32 to 68] 17/34 |
Subcutaneous: 82 [65 to 93] 27/33 |
Subcutaneous: 54
[25 to 81] 7/13 |
Adrenal, spleen, muscle, kidney plus ‘other’a: Se: 63 [49 to 76] 33/52 Sp: 94 [86 to 98] 78/83 |
|
Jouvet 2014 Unclear |
37 218/125 (no. varies per test) |
CT (CE) | Incl brain: Se: 88 [80 to 94] 81/92 Sp: 73 [61 to 84] 47/63 Excl brain: Se: 86 [76 to 93] 62/72 Sp: 72 [59 to 83] 44/61 |
67 [38 to 88] 10/15 |
100
[81 to 100] 18/18 |
94 [79 to 99] 29/31 |
36
[13 to 65] 5/14 |
83 [52 to 98] 10/12 |
87
[60 to 98] 13/15 |
Subcutaneous: 2/2 | Subcutaneous: 62 [32 to 86] 8/13 |
Brain: Se: 95 [75 to 100] 19/20 Sp: 3/3 lesions correctly identified as benign Otherb: 13/13 correctly identified; 1 (small bowel) missed by CT |
| Pfannenberg 2007 Mixed |
64 420/297 (all suspicious on ≥ 1 test) |
CT (CE) | Excl brain: Se: 77 [71 to 83] 151/195 Sp: 64 [52 to 76] 43/67 |
63 [45 to 79] 22/35 |
80
[52 to 96] 12/15 |
96 [87 to 100] 51/53 |
29
[10 to 56] 5/17 |
80 [63 to 92] 28/34 |
Insufficient data (1/2 detected) | 64 [51 to 76] 38/59 |
71
[48 to 89] 15/21 |
‘Other viscera’c: Se: 92 [64 to 100] 12/13 Sp: 83 [52 to 98] 10/12 Brain metastases excludedd |
| MRI | ||||||||||||
|
Dellestable 2011 Mixed |
40 118/72 (no. varies per test) |
MRI (DW) | Incl brain: Se: 77 [61 to 89] 30/39 Sp: 97 [86 to 100] 37/38 |
93
[66 to 100] 13/14 |
Insufficient data (2/0 detected) | 62
[32 to 86] 8/13 |
Insufficient data (1/1 detected) | Insufficient data (4/4 detected) | 100
[85 to 100] 22/22 |
Distant minus bone, lung, liver mets Se: 63 [24 to 91] 5/8 Sp: 92 [64 to 100] 12/13 |
||
|
Hausmann 2011 Unclear |
33 824/455 (all detected by ≥ 1 test) |
MRI (NR) | Excl brain: Se: 67 [61 to 73] 177/263 Sp: 91 [85 to 94] 165/182 |
Insufficient data (1/1 detected) | Insufficient data (1/1 detected) | 47
[39 to 55] 68/145 |
96
[87 to 100] 50/52 |
85
[68 to 95] 28/33 |
100
[90 to 100] 34/34 |
Subcutaneous: 100
[89 to 100] 33/33 |
Subcutaneous: 77
[46 to 95] 10/13 |
Adrenal, spleen, muscle, kidney plus ‘other’a: sensitivity: Se: 92 [81 to 98] 48/52 Sp: 86 [76 to 92] 71/83 |
|
Jouvet 2014 Unclear |
37 218/125 (no. varies per test) |
MRI (DW) | Incl brain: Se: 62 [52 to 71] 63/102 Sp: 70 [57 to 81] 44/63 Excl brain: Se: 61[50 to 72] 50/82 Sp: 68 [55 to 80] 41/60 |
100
[79 to 100] 16/16 |
47
[24 to 71] 9/19 |
26
[12 to 45] 8/31 |
93
[66 to 100] 13/14 |
92
[62 to 100] 11/12 |
67
[38 to 88] 10/15 |
Subcutaneous: 70
[35 to 93] 7/10 |
Subcutaneous: 75 [43 to 95] 9/12 |
Brain: Se: 65 [41 to 85] 13/20 Sp: 3/3 benign correctly identified Other 2: 13/13 correctly identified; 1 (small bowel) missed by MRI |
| MRI (DW + VIBE) | Incl brain: Se: 83 [75 to 90] 85/102 Sp: 81 [69 to 90] 51/63 Excl brain: Se: 79 [69 to 87] 65/82 Sp: 80 [68 to 89] 48/60 |
100
[79 to 100] 16/16 |
74
[49 to 91] 14/19 |
52
[33 to 70] 16/31 |
79
[49 to 95] 11/14 |
100
[74 to 100] 12/12 |
93
[68 to 100] 14/15 |
Subcutaneous: 90 [55 to 100] 9/10 |
Subcutaneous: 75 [43 to 95] 9/12 |
Brain: Se: 100 [83 to 100] 20/20 Sp: 3/3 benign correctly identified Otherb: 13/13 correctly identified; 1 (small bowel) missed by MRI |
||
|
Pfannenberg 2007 Mixed |
64 420/297 (all suspicious on ≥ 1 test) |
MRI (DW + VIBE) | Excl brain: Se: 87 [82 to 92] 170/195 Sp: 76 [64 to 86] 51/67 |
100
[90 to 100] 35/35 |
73
[45 to 92] 11/15 |
87
[75 to 95] 46/53 |
76
[50 to 93] 13/17 |
100 [90 to 100] 35/35 |
Insufficient data (2/2 detected) | 78
[65 to 88] 46/59 |
67
[43 to 85] 14/21 |
‘Other viscera: sensitivity: 62 [32 to 86] 8/13 Sp: 92 [62 to 100] 11/12 Brain metastases excludedd |
| PET‐CT | ||||||||||||
|
Cachin 2014 Mixed |
87 176/85 |
PET‐CT (NR) | Incl brain: Se: 78 [67 to 88] 51/65 Sp: 58 [46 to 70] 42/72 |
86
[57 to 98] 12/14 |
86
[57 to 98] 12/14 |
100
[69 to 100] 10/10 |
100
[69 to 100] 10/10 |
2/2 | 71 [29 to 96] 5/7 |
Skin: 86 [42 to 100] 6/7 |
0/2 | Brain: Se: 22 [3 to 60] 2/7 Sp: 2/7 Soft tissue metastasis: Sp: 75 [48 to 93] 12/16 Sp: 6/7 |
|
Dellestable 2011 Mixed |
40 118/72 (no. varies per test) |
PET‐CT (CE) | Incl brain: Se: 66 [49 to 80] 27/45 Sp: 88 [73 to 96] 35/40 |
71
[42 to 92] 10/14 |
3/3 | 31
[9 to 61] 4/13 |
2/2 | 2/4 | 90
[70 to 99] 19/21 |
Distant minus bone, lung, liver mets: Se: 92 [62 to 100] 11/12 Sp: 79 [49 to 95] 11/14 |
||
|
Jouvet 2014 Unclear |
37 218/125 (no. varies per test) |
PET‐CT (CE) | Excl brain: Se: 75 [64 to 84] 61/81 Sp: 91 [81 to 97] 52/57 |
88
[62 to 98] 14/16 |
75
[43 to 95] 18/19 |
48
[30 to 67] 15/31 |
100
[77 to 100] 14/14 |
100
[74 to 100] 12/12 |
100
[78 to 100] 15/15 |
100 [59 to 100] | 63
[24 to 91] 5/8 |
Other2: 13/13 correctly identified; 1 (small bowel) missed by CT |
|
Pfannenberg 2007 Mixed |
64 420/297 (all suspicious on ≥ 1 test) |
PET‐CT (CE) | Excl brain: Se: 93[89 to 96] 182/195 Sp: 67 [55 to 78] 45/67 |
91
[77 to 98] 32/35 |
80
[52 to 96] 12/15 |
96
[87 to 100] 51/53 |
35
[14 to 62] 6/17 |
94 [81 to 99] 33/35 |
2/2 | 90
[79 to 96] 53/59 |
67
[43 to 85] 14/21 |
‘Other viscera’c: Se: 100 [75 to 100 13/13 Sp: 92 [62 to 100] 11/12 Brain metastases excludedd |
|
a‘Other’ not further defined (Hausmann 2011). bFourteen ‘other metastatic sites described’, all assumed (by us) to be malignant adrenal (4), heart (2), spleen (2), peritoneal carcinosis (2), breast (1), pleura (1), vagina (1), and small intestine (1). cother visceral metastases such as bowel or peritoneal lesion (Pfannenberg 2007). dBrain metastases excluded from comparison of accuracy; reports 15 patients with cerebral metastases, “exclusively diagnosed by wbMRI” (Pfannenberg 2007). CE: contrast enhanced; CI: confidence interval; CT: computed tomography; Dis: diseased group; DW: diffusion weighted; excl: excluding; GE: gradient echo; incl: including; MRI: magnetic resonance imaging; No Dis: non‐diseased group; NR: not reported; PET: positron emission tomography; Se: sensitivity; Sp: specificity; TN: true negative; TP: true positive; U: unenhanced; US: ultrasound; WB: whole body. | ||||||||||||
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
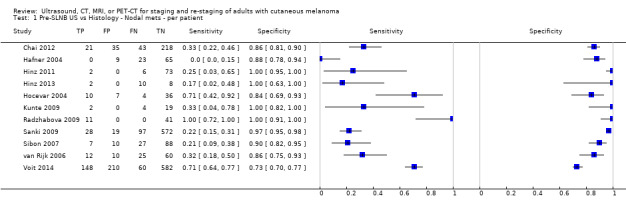
Pre‐SLNB US vs Histology ‐ Nodal mets ‐ per patient.
2. Test.

Pre‐SLNB US (stringent US criteria) vs Histology ‐ Nodal mets ‐ per patient.
3. Test.
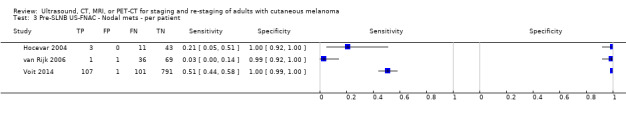
Pre‐SLNB US‐FNAC ‐ Nodal mets ‐ per patient.
4. Test.
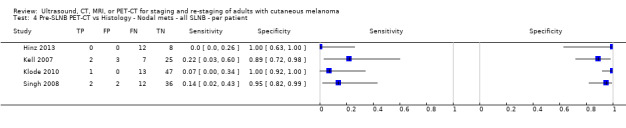
Pre‐SLNB PET‐CT vs Histology ‐ Nodal mets ‐ all SLNB ‐ per patient.
5. Test.
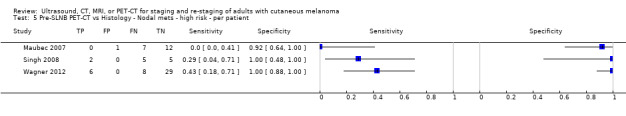
Pre‐SLNB PET‐CT vs Histology ‐ Nodal mets ‐ high risk ‐ per patient.
6. Test.

Pre‐SLNB PET‐CT vs Histology ‐ Nodal mets ‐ head and neck only ‐ per patient.
7. Test.
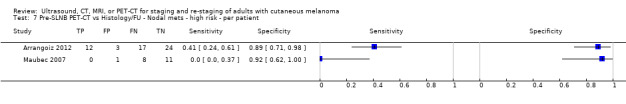
Pre‐SLNB PET‐CT vs Histology/FU ‐ Nodal mets ‐ high risk ‐ per patient.
8. Test.

Pre‐SLNB PET‐CT vs Histology/FU ‐ Nodal mets ‐ head and neck only ‐ per patient.
9. Test.

Any metastasis ‐ PET‐CT ‐ PRIMARY ‐ Any stage (per pt).
10. Test.

Any metastasis ‐ PET‐CT ‐ PRIMARY ‐ BT > 4 mm (per pt).
11. Test.

Any metastasis ‐ CT ‐ RE‐STAGING ‐ Any stage (per pt).
12. Test.

Any metastasis ‐ PET‐CT ‐ RE‐STAGING ‐ Any stage (per pt).
13. Test.

Any metastasis ‐ PET‐CT ‐ RE‐STAGING ‐ Stage IIIb or less (per pt).
14. Test.

Any metastasis ‐ PET‐CT ‐ RE‐STAGING ‐ Stage IIIc to IV (per pt).
15. Test.

Any metastasis ‐ CT‐ MIXED ‐ All data (per pt).
16. Test.
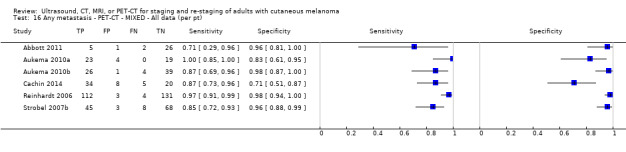
Any metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per pt).
17. Test.

Any metastasis ‐ PET‐CT (plus CT) ‐ Mixed ‐ Any stage (per pt).
18. Test.

Any metastasis ‐ PET‐CT ‐ RE‐STAGING ‐ Any stage (per lesion).
19. Test.
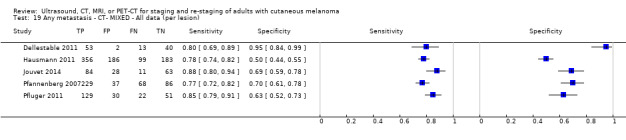
Any metastasis ‐ CT‐ MIXED ‐ All data (per lesion).
20. Test.

Any metastasis (incl brain) ‐ CT (U) ‐ MIXED (per lesion).
21. Test.

Any metastasis (incl brain) ‐ CT (CE) ‐ MIXED (per lesion).
22. Test.

Any metastasis ‐ MRI ‐ MIXED ‐ All data (per lesion).
23. Test.

Any metastasis (excl brain) ‐ MRI (DW + VIBE) ‐ MIXED (per lesion).
24. Test.

Any metastasis (incl brain) ‐ MRI (DW) ‐ MIXED (per lesion).
25. Test.

Any metastasis (incl brain) ‐ MRI (DW + VIBE) ‐ MIXED (per lesion).
26. Test.

Any metastasis (incl brain) ‐ MRI plus CT ‐ MIXED (per lesion).
27. Test.
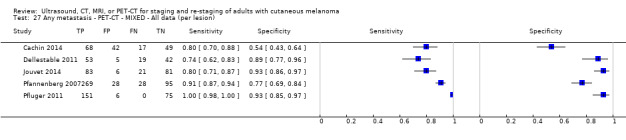
Any metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per lesion).
28. Test.

Any metastasis (incl brain) ‐ PET‐CT (U) ‐ MIXED (per lesion).
29. Test.

Any metastasis (direct test comparisons) ‐ CT ‐ Mixed ‐ Stage III/IV (per lesion).
30. Test.
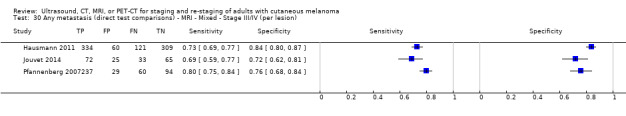
Any metastasis (direct test comparisons) ‐ MRI ‐ Mixed ‐ Stage III/IV (per lesion).
31. Test.
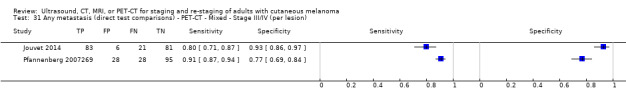
Any metastasis (direct test comparisons) ‐ PET‐CT ‐ Mixed ‐ Stage III/IV (per lesion).
32. Test.
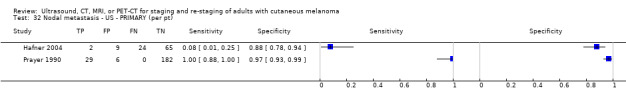
Nodal metastasis ‐ US ‐ PRIMARY (per pt).
33. Test.

Nodal metastasis ‐ CT ‐ PRIMARY (per pt).
34. Test.

Nodal metastasis ‐ PET‐CT ‐ PRIMARY (per pt).
35. Test.

Nodal metastasis ‐ US ‐ RE‐STAGING (per pt).
36. Test.

Nodal metastasis ‐ US plus US (CE) ‐ RE‐STAGING (per pt).
37. Test.

Nodal metastasis ‐ US ‐ MIXED (per pt).
38. Test.
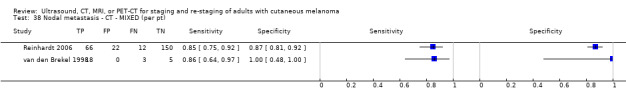
Nodal metastasis ‐ CT ‐ MIXED (per pt).
39. Test.

Nodal metastasis (superficial groin) ‐ PET‐CT (indeterminate test positive) ‐ MIXED (per pt).
40. Test.

Nodal metastasis (superficial groin) ‐ PET‐CT (indeterminate test negative) ‐ MIXED (per pt).
41. Test.

Nodal metastasis (deep groin) ‐ PET‐CT (indeterminate test positive) ‐ MIXED (per pt).
42. Test.

Nodal metastasis (deep groin) ‐ PET‐CT (indeterminate test negative) ‐ MIXED (per pt).
43. Test.

Nodal metastasis ‐ PET‐CT ‐ MIXED (per pt).
44. Test.

Nodal metastasis ‐ CT ‐ MIXED ‐ All data (per lesion).
45. Test.

Nodal metastasis ‐ MRI ‐ MIXED ‐ All data (per lesion).
46. Test.

Nodal metastasis ‐ MRI (DW + VIBE) ‐ MIXED (per lesion).
47. Test.

Nodal metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per lesion).
48. Test.

Superficial nodal metastasis ‐ US ‐ Mixed ‐ stage IV (per LNB).
49. Test.

Superficial nodal metastasis ‐ CT ‐ Mixed ‐ stage IV (per LNB).
50. Test.

Superficial nodal metastasis ‐ MRI ‐ Mixed ‐ stage IV (per LNB).
51. Test.

Superficial nodal metastasis ‐ MRI (DW + VIBE) ‐ Mixed ‐ Stage IV (per lesion).
52. Test.

Superficial nodal metastasis ‐ PET‐CT ‐ Mixed ‐ stage IV (per LNB).
53. Test.

Distant metastasis ‐ CT ‐ PRIMARY (per pt).
54. Test.

Distant metastasis ‐ PET‐CT ‐ PRIMARY (per pt).
55. Test.

Distant metastasis ‐ CT ‐ MIXED ‐ All data (per pt).
56. Test.

Distant metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per pt).
57. Test.

Distant metastasis ‐ CT ‐ Mixed ‐ All data (per lesion).
58. Test.

Distant metastasis ‐ MRI ‐ Mixed ‐ All data (per lesion).
59. Test.

Distant metastasis ‐ PET‐CT ‐ Mixed ‐ All data (per lesion).
60. Test.

Distant metastasis (excl brain) ‐ MRI (DW + VIBE) ‐ Mixed ‐ stage III/IV (per lesion).
61. Test.

Distant metastasis (incl brain) ‐ MRI (DW) ‐ Mixed ‐ stage III/IV (per lesion).
62. Test.

Distant metastasis (incl brain) ‐ MRI (DW + VIBE) ‐ Mixed ‐ stage III/IV (per lesion).
63. Test.

Bone metastasis ‐ CT‐ MIXED ‐ All data (per lesion).
64. Test.

Bone metastasis ‐ MRI ‐ MIXED ‐ All data (per lesion).
65. Test.

Bone metastasis ‐ MRI (DW + VIBE) ‐ MIXED ‐ All data (per lesion).
66. Test.

Bone metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per lesion).
67. Test.

Liver metastasis ‐ CT‐ MIXED ‐ All data (per lesion).
68. Test.

Liver metastasis ‐ MRI ‐ MIXED ‐ All data (per lesion).
69. Test.

Liver metastasis ‐ MRI (DW + VIBE) ‐ Mixed ‐ stage III/IV (per lesion).
70. Test.
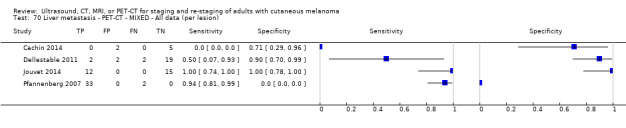
Liver metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per lesion).
71. Test.

Lung metastasis ‐ CT ‐ MIXED ‐ All data (per lesion).
72. Test.
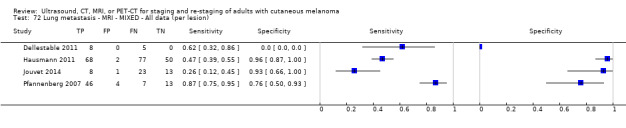
Lung metastasis ‐ MRI ‐ MIXED ‐ All data (per lesion).
73. Test.

Lung metastasis ‐ MRI (DW + VIBE) ‐ Mixed ‐ stage III/IV (per lesion).
74. Test.
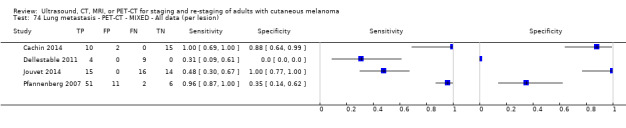
Lung metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per lesion).
75. Test.

Soft tissue metastasis ‐ PET‐CT ‐ MIXED (per lesion).
76. Test.
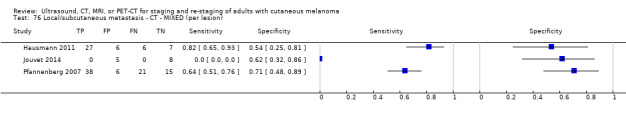
Local/subcutaneous metastasis ‐ CT ‐ MIXED (per lesion).
77. Test.
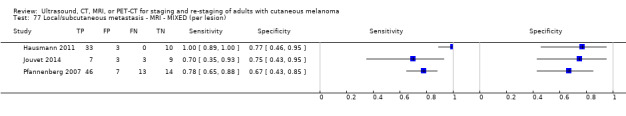
Local/subcutaneous metastasis ‐ MRI ‐ MIXED (per lesion).
78. Test.

Local/subcutaneous metastasis ‐ MRI (DW + VIBE) ‐ MIXED (per lesion).
79. Test.

Local/subcutaneous metastasis ‐ PET‐CT ‐ MIXED (per lesion).
80. Test.

Brain metastasis ‐ CT‐ MIXED ‐ All data (per lesion).
81. Test.

Brain metastasis ‐ MRI (DW) ‐ MIXED ‐ All data (per lesion).
82. Test.

Brain metastasis ‐ MRI (DW + VIBE) ‐ MIXED ‐ All data (per lesion).
83. Test.

Brain metastasis ‐ PET‐CT ‐ MIXED ‐ All data (per lesion).
93. Test.

'Other' metastasis ‐ CT ‐ Mixed ‐ Any stage (per lesion).
94. Test.

'Other' metastasis ‐ MRI ‐ Mixed ‐ Any stage (per lesion).
95. Test.

'Other' metastasis ‐ PET‐CT ‐ Mixed ‐ Any stage (per lesion).
96. Test.

'Other' metastasis ‐ CT ‐ Mixed ‐ stage III/IV (per lesion).
97. Test.
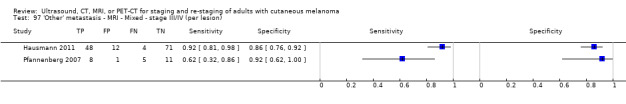
'Other' metastasis ‐ MRI ‐ Mixed ‐ stage III/IV (per lesion).
98. Test.

'Other' metastasis ‐ PET‐CT ‐ Mixed ‐ stage III/IV (per lesion).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbott 2011.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (Prosp. database: yes) Country: UK Data collection: NR; up to May 08 Inclusion criteria: stage III: micro‐metastases on SLNB or clinically detectable nodal metastases on diagnosis or FU | ||
| Patient characteristics and setting |
Presentation: mixed (either undergoing FU after prior SLNB/LND for micro‐metastases or presenting with clinically detectable nodal disease at or subsequent to initial diagnosis (primary/FU))
Number patients: 34 (microscopic group 20; macroscopic group 14)
Number primary lesions: 34
Number LNBs/metastases: NR
Stage of disease: IIIA 18, 53%; IIIB 10, 29%; IIIC 6, 18%
Median age: microscopic group 50 y ‐ macroscopic group 63 y Range: microscopic group 19 74 y; macroscopic group 48 79 y Male: microscopic group 14, 70%; macroscopic group 6, 43% Primary lesion site: HN 1, 3%; upper extremity 3, 9%; trunk 20, 59%; lower extremity 10, 29% Breslow/Clark: microscopic group mean BT 2.27 mm (1.2 to 9.7 mm) Macroscopic group: mean BT 2.01 mm (1.0 to 13 mm) Ulceration: NR Other: NR |
||
| Index tests |
PET‐CT: 2D; CT (NR)
Machine: General Electric ST, Wisconsin, USA
Scan coverage: skull base to upper thigh
Contrast: NR
CT parameters: NR
FDG: 400 MBq
Breath hold: NR
CT used for: attenuation correction and lesion localisation
Reconstruction: iterative technique using an ordered subset expectation‐maximisation algorithm Threshold: clearly indicative/highly suspicious for malignancy considered positive Number observers: NR Qualification (experience): nuclear medicine consultants (experienced) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical NR; other tests NR |
||
| Target condition and reference standard(s) |
Histology/Imaging FU
Histological detail (n, %): NR, mixture of excisions and LND (5, 15%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): clinical and/or radiological FU (incl PET‐CT) (34, 100%)
FU schedule: every 3 months for clinical examination; annual PET (second annual PET reported for 15/34 (44%) and third annual for 4/34 (12%)). All FU clinically ≥ 6 months following each surveillance PET‐CT FU duration: microscopic mean 38 months (21 to 54 months); macroscopic mean 34 months (15 to 52 months) Reference blinding: aware of prior PET‐CT results during FU # Target condition Data: per pt Definition: any (excl brain; including local, ITM) Prevalence: 7/34 = 21%; 4 local or ITM, 2 nodal, 1 distant metastasis |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: 3 months Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other results: 3 recurrences occurred in microscopic group (1 ITM and 1 pulmonary detected by PET‐CT plus 1 local recurrence missed on first annual PET‐CT); 4 clinically undetected recurrences occurred in macroscopic group (2 LN, 1 local detected by PET‐CT, and 1 ITM missed by staging PET‐CT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | No | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Arrangoiz 2012.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (medical record review) Country: USA Data collection: Jan 03 to Jan 09 Inclusion criteria: node negative; BT > 4 mm | ||
| Patient characteristics and setting | Presentation: primary Number patients: 56 Number primary lesions: 56 Number LNBs/metastases: NR Stage of disease: all T4, clinically node negative, and negative for distant metastases Mean age: 67 years; Median age: NR; Range: 26 to 89 years Male: 32 (57%) Primary lesion site: trunk 16, 29%; extremities 28, 50%; head and neck 12, 21% Breslow/Clark: BT median 6 mm; mean 9 mm; range 4.1 to 40 mm Ulceration: 34, 61% Other: satellitosis: 25, 45% | ||
| Index tests |
PET‐CT: 2D or 3D; CT (U, helical, low dose)
Machine: GE Discovery LS PET/CT Scanner (from 2003 to 10/2010) or a Siemens Biograph 16 PET/CT Scanner (from 10/2010 onwards)
SUV values reportedly comparable with cross‐calibration by manufacturer‐trained field engineers and in‐house medical physicist
Scan coverage: WB; vertex of the head down to feet for all patients
Contrast: U
CT parameters: Discovery LS ‐ 140 kVp, 90 mA; Siemens Biograph ‐ 130 kVp, 100 mA; 5 mm
FDG: 15 mCi (IV)
Breath hold: normal breathing
CT used for: attenuation correction; co‐registered images
Reconstruction: Discovery LS ‐ ordered subsets expectation maximisation (OSEM) algorithm with 28 subsets and 2 iterations. Siemens Biograph ‐ rueX algorithm with 21 subsets and 2 iterations Threshold: SUV 2.5 Number observers: NR; 'in‐house medical physicist' mentioned Qualification (experience): NR; 'in‐house medical physicist' mentioned (NR) Diagnosis (single, consensus, etc.): unclear Info provided during test interpretation: clinical NR; other tests NR |
||
| Target condition and reference standard(s) | Histology (SLNB, CLND, biopsy); FU
Histological detail (n, %): NR (54, 96% (48 SNB and 6 LND)). Histopathologist: NR
FNAC (n, %): NR (NR)
Follow‐up (n, %): NR; 2/56 had no SLNB or LND reported so must have had some follow‐up to confirm absence of disease. Also the number D+ reported by authors in Table 4 does not add up to combined SLNB/CLND numbers D+; presume that 4 of SLNB negative must have recurred with regional disease at some point (NR)
FU schedule: NR FU duration: NR Reference blinding: NR Target condition Data: per pt Definition: any mets (NR; scan incl head); Prevalence: 32/56 = 57% Definition: nodal mets; Prevalence: 29/56 = 52% Definition: distant mets (not documented; scan incl head); Prevalence: 5/56 = 9% |
||
| Flow and timing | Index to histology interval: NR; states that 6 "proceeded directly to therapeutic lymph node dissection" after PET Index to FU interval: NR Exclusions: n = 0; N/A | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Aukema 2010a.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (Prosp. database: NR) Country: Netherlands Data collection: Aug 2006 to Mar 2009 Inclusion criteria: raised S100 during FU after resection of nodal or distant metastases or with high‐risk primary tumour | ||
| Patient characteristics and setting | Presentation: mixed (15 treated for locoregional recurrence and 5 for distant mets; remaining 26 followed up after primary melanoma treatment) Number patients: 46 Number primary lesions: NR Number LNBs/metastases: NR Stage of disease: NR; unfavorable primary tumour (n = 6); primary melanoma with simultaneous nodal metastases (n = 18); unknown primary melanoma with nodal metastasis (n = 2); locoregional recurrence (n = 15); distant recurrence (n = 5) Mean age: 59 years; Range: 25 to 93 years Male: NR Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
PET‐CT: NR; CT (U)
Machine: Gemini II, Philips, Eindhoven, The Netherlands
Scan coverage: whole body; not described
Contrast: U
CT parameters: kV NR; 40 mAs; 5 mm
FDG: 180 to 240 MBq (4.9 to 6.5 mCi)
Breath hold: no breath hold instructions reported
CT used for: attenuation correction; PET fused to low‐dose CT
Reconstruction: NR Threshold: NR; "hypermetabolic lesions" # MRI: patients underwent MRI of the brain; insufficient data to include separate 2×2 Machine: Achieva, Philips, Eindhoven, The Netherlands Scan coverage: brain Contrast: yes, not documented MRI parameters: transversal T2‐weighted; axial fluid attenuated inversion recovery (FLAIR) imaging, diffusion‐weighted imaging and pre‐ and post‐contrast coronal T1‐weighted 3D‐FFE imaging Tesla: 3.0 Number observers: 3 Qualification (experience): nuclear medicine physicians (experienced') Diagnosis (single, consensus,etc.): consensus of 3 Info provided during test interpretation: clinical NR; other tests NR; MRI brain also conducted |
||
| Target condition and reference standard(s) |
FNAC/histology/imaging FU
Histological detail (n, %): NR (13, 28.3%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): clinical exam; CT (33, 71.7%)
FU schedule: NR FU duration: for disease negative only (n = 19): median 12 months (4 to 32 months); NR for full sample Reference blinding: NR Target condition Data: per pt Definition: any (not documented; brain NR) Prevalence: 23/46 = 50% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: Exclusions: n = NR | ||
| Comparative | |||
| Notes | Other result: "MRI revealed 2 brain metastases of 2 and 4 mm in 1 patient (2%). This patient also had other distant metastases that were detected by PET‐CT" | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Unclear | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Aukema 2010b.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; prospective Country: Netherlands Data collection: Oct 06 to Mar 09 Inclusion criteria: clinically node positive with no sign of distant metastases; primary/re‐staging NR | ||
| Patient characteristics and setting | Presentation: unclear (NR; all have palpable and proven LN metastases) Number patients: 70 Number primary lesions: 70 Number LNBs/metastases: 73 Stage of disease: ≥ stage IIIb (all with clinically palpable nodes) Mean age: 58 y; Median age: NR; Range: NR Male: 37 (54%) Primary lesion site: upper extremity 4, 6%; lower extremity 37, 53%; trunk 19, 27%; head/neck 9, 13%; unknown primary 1, 1% Breslow/Clark: Breslow: median 3 mm Ulceration: NR Other: NR | ||
| Index tests |
PET‐CT: 2D; CT (U)
Machine: Gemini II, Philips, Eindhoven, The Netherlands
Scan coverage: WB according to primary lesion site (i.e. with regard to inclusion of cranium or lower extremities)
Contrast: U
CT parameters: kV NR; 40 mAs; 5 mm
FDG: 180 to 240 MBq
Breath hold: no breath hold instructions reported
CT used for: attenuation correction; PET fused to low‐dose CT
Reconstruction: PET was fused with low‐dose CT after correction for attenuation Threshold: NR; "metabolically active" # MRI: patients underwent MRI of the brain; insufficient data to include separate 2×2 Machine: Achieva, Philips, Eindhoven, The Netherlands Scan coverage: brain Contrast: yes, not documented MRI parameters: transversal T2‐weighted; axial fluid attenuated inversion recovery (FLAIR) imaging, diffusion‐weighted imaging and pre‐ and post‐contrast coronal T1‐weighted 3D‐FFE imaging Tesla: 3.0 # Number observers: 3 Qualification (experience): nuclear medicine physicians (NR) Diagnosis (single, consensus,etc.): consensus of 3 Info provided during test interpretation: clinical NR; other tests NR; MRI brain also conducted |
||
| Target condition and reference standard(s) |
FNAC/histology/imaging FU
Histological detail (n, %): NR (NR; 11 with histology or cytology). Histopathologist: NR
FNAC (n, %): NR (NR; 11 with histology or cytology)
Follow‐up (n, %): CT, ultrasound, or clinical follow‐up for TP cases (59; 84%)
FU schedule: NR FU duration: ≥ 6 months Reference blinding: NR # Target condition Data: per pt Definition: any mets (incl in transit mets and skull according to primary lesion site); Prevalence: 30/70 = 43% Metastases: PET‐CT detected additional involved LNBs (3) and 'distant' metastases (20); false negative results included ITM (2), liver metastases (1), extensive metastases 3 months post PET‐CT (1) |
||
| Flow and timing | Index to histology interval: N/A Index to FU interval: NR Exclusions: n = 0; N/A | ||
| Comparative | |||
| Notes | Other result: MRI: detected brain mets in 5 pts, 4 with multiple other metastases detected by PET‐CT and 1 with solitary brain metastases. Outcome: 2 received dexamethasone and radiotherapy of the brain, 1 was treated with temozolomide, and 1 received supportive care; solitary brain metastasis removed surgically and underwent adjuvant whole brain radiotherapy; no signs of recurrent disease at 15 months | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Unclear | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Bastiaannet 2009.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: Netherlands Data collection: Jul 2003 to Dec 2007 Inclusion criteria: node positive (clinical or histology/cytology proven) candidates for CLND | ||
| Patient characteristics and setting | Presentation: mixed (primary (LN mets diagnosed at time of primary diagnosis) 39, 15.5%; recurrence (LN mets identified ≤ 3 years since primary dx) 145, 57.8%; recurrence (> 3 years since primary dx) 67, 26.7%) Number patients: 251 Number primary lesions: 251 Number LNBs/metastases: NR Stage of disease: III (100%) Mean age: reported in Bastiannet 2012 as 56.9 years (n = 253); Range: 19 to 93 years ‐ 76 (30.3%) < 50 years; 99 (39.4%) 50 to 65 years; 76 (30.3%) > 65 years Male: 152 (61%) Primary lesion site: HN 29, 11.6%; upper extremities 26, 10.4%; trunk 93, 37.0%; lower extremities 88, 35.0%; unknown primary 15, 6.0% Breslow/Clark: Breslow: ≤ 1 mm 32, 12.8%; 1.0 to 2.0 mm 73, 29.1%; ≥ 2.0 129, 51.4%; unknown primary 15, 5.9%; missing 2, 0.8% Clark level: I/II/III (n = 84; 33.5%), IV/V (n = 144; 57.4%), unknown primary (n = 15; 5.9%), missing (n = 8; 3.2%) Ulceration: yes 53, 21.1%; unknown 15, 6% Other: localisation of SLN: neck 43, 17.1%; axilla 94, 37.5%; inguinal 114, 45.4% | ||
| Index tests |
CT: CE, spiral, multi‐slice
Machine: NR
Scan coverage: chest, abdomen plus neck for those with LN in the neck
Contrast: oral and IV
CT parameters: NR; 'multi‐slice'
Breath hold: no breath hold instructions reported Threshold: NR (presence/absence of mets) Number observers: NR Qualification (experience): attending staff nuclear medicine physicians (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical NR; other tests blinded to PET |
||
| Target condition and reference standard(s) |
Histology/FU
Histological detail (n, %): cytopathology, histopathology (NR). Histopathologist: NR
FNAC (n, %): NR (NR)
Follow‐up (n, %): bone scan, MRI, 'follow‐up' (251, 100%)
FU schedule: NR FU duration: median 13.7 months; minimum 6 months stated for index test positive, NR for index test negative Reference blinding: NR Target condition Data: per pt Definition: distant mets (including lymph nodes beyond regional LNs) Prevalence: 78/251 = 31% Metastases: 120 TP metastatic sites identified by CT included liver (20), lung (41), abdomen (13), bone (10), subcutaneous (5), other (11); 16 patients FN on CT had metastases in the bone (5), lung (5), multiple sites (2), liver (2), sternal (1), leg (1) Presenting LN metastases were correctly identified by CT in 231/151 patients and by PET alone in 229/251 |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 8; excluded due to follicular structure (n = 1), > 13 years between primary and lymph nodes (n = 3), incidence abroad (n = 1), mucosal melanoma (n = 2), primary melanoma treated as benign lesion (n = 1) | ||
| Comparative | |||
| Notes | Other result: (1) accuracy of PET alone, (2) change in treatment resulting from PET and/or CT | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Cachin 2014.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; prospective Country: France Data collection: Aug 2008 to Sep 2010 Inclusion criteria: prior history of cutaneous or ocular MM undergoing staging or re‐staging including (a) newly diagnosed at any TNM stage, (b) known visceral or cutaneous MM metastases with unknown primary tumour, or (c) MM without metastases (included to assess test specificity) | ||
| Patient characteristics and setting | Presentation: mixed (melanoma status at inclusion was one of the following: "newly diagnosed cutaneous or ocular melanoma at any TNM stage, presence of known visceral melanoma metastases, or cutaneous melanoma metastases with unknown primary tumour. Patients with melanoma without metastases were also included, principally to assess the specificity of the imaging. Also states imaging was for staging or for re‐staging") Number patients: 87 Number primary lesions: NR Number LNBs/metastases: 85 Stage of disease: NR; 45 (51% were diagnosed with melanoma mets on study Inclusion) Mean age: NR; Median age: NR; Range: NR Male: 42 (48.3%) Primary lesion site: NR Breslow/Clark: Breslow thickness (mm): < 1.0: 12, 13.8%; 1.0 to 2.0: 34, 39.1%; ≥ 2.0, 41, 47.1% Clark level: I 3, 3.4%; II 2, 2.3%; III 20, 23.0%; IV 46, 52.9%; V 3, 3.4%; not known 13, 14.9% Ulceration: NR Other: cutaneous melanoma pigmentation: pigmented 51, 58.6%; achromic 7, 8.0%; not known 29, 33.3% | ||
| Index tests |
PET‐CT: NR; SPECT used in 4 of 8 centres
Machine: Discovery ST2, GE; Biograph 6, Siemens; Biograph HIREZ True Point, Siemens; Discovery ST4, GE; Gemini Dual, Philips; Gemini, Philips
Scan coverage: WB (not further described)
Contrast: NR
CT parameters: SPECT; N/A
FDG: 3 to 5 MBq/kg
Breath hold: NR
CT used for: PET 'correlated' with CT abnormalities
Reconstruction: iterative in 6 of 8 centres; filtered back‐projection in 2 of 8 centres Threshold: PET positive if there was focal uptake greater than mediastinal or liver uptake that could not clearly be related to physiological processes; negative when a normal distribution of tracer was observed, even if the CT scan showed abnormalities. Bone accumulations were considered positive when the uptake was higher than in normal bone marrow. Any instance of equivocal PET uptake was considered positive Number observers: NR Qualification (experience): nuclear physician (experienced) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical NR; other tests NR; PET interpretation independent of CT and then correlated with CT |
||
| Target condition and reference standard(s) |
Histology/Imaging FU/FU
Histological detail (n, %): NR; "a total of 25 biopsies (1 per patient) were performed." (25; 28.7%). Histopathologist: NR
FNAC (n, %): N/A (N/A)
Follow‐up (n, %): could include CT scan, biopsy, pathology, clinical follow‐up (87, 100%)
FU schedule: NR FU duration: ≥ 6 months Reference blinding: NR Target condition Data: per pt Definition: any (incl brain, subcutaneous mets); Prevalence: 39/67 = 58% Data: per lesion Definition: any (incl brain, subcutaneous); Prevalence: 85/176 = 48% Definition: nodal; Prevalence: 20/39 = 51% Definition: distant (incl brain and skin); Prevalence: 65/137 = 47% Definition: bone; Prevalence: 14/34 = 41% Definition: lung; Prevalence: 10/27 = 37% Definition: soft tissue; Prevalence: 16/25 = 64% Definition: skin; Prevalence: 7/9 = 78% Definition: brain; Prevalence: 7/9 = 78% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 20; 12 did not undergo FDG PET due to imaging cancellation; 8 are unaccounted for (text describes 75 having PET but reports results for only 67) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | No | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Chai 2012.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (Prosp. database: yes) Country: USA Data collection: Jun 2005 to Sep 2009 Inclusion criteria: node negative, BT > 0.76 mm or < 0.76 mm with high‐risk features such as ulceration, high mitotic rate, or positive deep margin | ||
| Patient characteristics and setting |
Presentation: primary (pre‐SLNB)
Number patients: 325
Number primary lesions: 325
Number LNBs/metastases: 347 LNBs
Stage of disease: NR
Mean age: NR; Median age: 58 years; Range: 18 to 86 years
Male: 189 (58%)
Primary lesion site: head and neck 34 (10.5%), trunk 129 (39.7%), upper extremity 101 (31.1%), lower extremity 61 (18.8%)
Breslow/Clark: BT median (range) 1.78 (0.42 to 14.4); BT ≤ 1.00 56 (17.2%), 1.01 to 2.00 136 (41.8%), 2.01 to 4.00 88 (27.1%), 4.00 44 (13.5%), unknown 1 (0.3%)
Clark level: III 24 (7.4%), IV 275 (84.6%), V 20 (6.2%), unknown 6 (1.8%)
Ulceration: 97, 29.8%; unknown 16, 4.9%
Other: regression present 26 (8.0%), unknown 15 (4.6%). Growth phase: radial 20 (6.2%), vertical 283 (87.1%), unknown 22 (6.7%) Angiolymphatic invasion: present 15 (4.6%), unknown 20 (6.2%) Mitotic rate: 0 9 (2.8%), C1 303 (93.2%), unknown 23 (7.1%) |
||
| Index tests |
US: B mode; linear array
Machine: NR
Scan coverage: acc to primary MM site and discretion of attending surgeon (extremity melanomas ‐ ipsilateral groin or axilla, MM of hand or forearm also had epitrochlear US and of lower leg had popliteal US; HN MM ‐ ipsilateral neck, parotid, and supraclavicular US; MM on trunk according to Sappey’s line ‐ at or above the beltline included axillary ultrasound, at or below included groin ultrasound, lesions close to the midline had bilateral US)
Contrast: N/A
FNAC: If US performed the day before SLNB, US‐guided FNAC was offered; FNAC +ve proceeded to CLND, FNAC‐ to SLNB as planned Threshold: US ‐ classed as "abnormal," "suspicious," or "indeterminate ‐ recommending a short‐term follow‐up" were considered positive (criteria described in detail) Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical NR; other tests NR |
||
| Target condition and reference standard(s) |
Histology (CLND/SLNB)
Histological detail (n, %): H&E (serial section); IHC (NR) (325, 100%). Histopathologist: NR
FNAC (n, %): NR; all positive on CLND (6, 1.8%)
Follow‐up (n, %): NR (NR; presume 100%)
FU schedule: NR FU duration: NR; FU for SLNB negatives mentioned but no description given Reference blinding: NR Target condition Data: per pt Definition: nodal mets; Prevalence: 64/317 = 20% |
||
| Flow and timing | Index to histology interval: US performed either immediately or several days before LS Index to FU interval: NR Exclusions: n = 8; 1 patient had ultrasound of a non‐draining nodal basin, while the actual draining basin identified by lymphoscintigraphy was not examined with ultrasound; this patient was not included in further analysis for comparison between ultrasound and SLNB. Plus 7 SLN positive who did not get US | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Dellestable 2011.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: France Data collection: Aug 2006 to May 2007 Inclusion criteria: PET‐CT for primary staging or follow‐up of MM, regardless of AJCC stage or indication for examination Excluded if contraindications to MRI or iodine injection | ||
| Patient characteristics and setting | Presentation: mixed (both primary staging and FU; breakdown reported but not legible on scanned pdf) Number patients: 40 Number primary lesions: 40 Number LNBs/metastases: NR; 72 lesions Stage of disease: AJCC I to II 11, 27.5%; AJCC III to IV 29, 72.5% Mean age: 57 years; Range: 27 to 85 years Male: 20 (50%) Primary lesion site: NR Breslow/Clark: BT mean 3.2 mm, median 2.7 mm, range 0.6 to 11 mm Ulceration: NR Other: NR | ||
| Index tests |
CT
Machine: VCT (General Electric Healthcare, Wisconsin, USA)
Scan coverage: skull, neck, thorax, abdomen, pelvis
Contrast: iodised injection was administered by the same venous route as for previous examinations
CT parameters: NR
Breath hold: no breath hold instructions reported Threshold: NR MRI: WB, DW, T2STIR, CE 3D gradient echo Machine: Signa Excite HD MRI (General Electric Healthcare, Milwaukee, United States) Scan coverage: WB; head to lower limbs MRI parameters: T2STIR, T1, diffusion, 3D gradient echo T1 after gadolinium injection Magnet: 1.5 T Threshold: NR # PET‐CT: NR; CT (CE) Machine: Biograph "coupled to an X‐ray scanner for attenuation correction and anatomical registration" Scan coverage: WB; top of the skull to the feet Contrast: unclear; contrast is reported for CT; however CT component of PET‐CT is not clear CT parameters: NR FDG: 5.5 MBq/kg Breath hold: no breath hold instructions reported CT used for: attenuation correction and anatomical registration Reconstruction: NR Threshold: focal uptake; unusual location or visual or quantitative intensity (SUV measurement) Number observers: 3 Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): single with consensus if results of any modality disagree Info provided during test interpretation: clinical NR; other tests ‐ each of the 3 exams was interpreted by a different reader, who had no knowledge of results of the other 2 |
||
| Target condition and reference standard(s) |
Histology/Imaging or clin FU
Histological detail (n, %): NR (36 lesions, 28% of 128). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): clinical or radiological (72, 56%)
FU schedule: NR FU duration: > 4 months Reference blinding: N/A Target condition Data: per lesion Definition: any (incl brain); Prevalence: CT 72/119 = 61%; MRI 70/117 = 60%; PET‐CT 72/119 = 61% Definition: nodal; Prevalence: CT 31/39 = 79%; MRI 31/40 = 78%; PET‐CT 31/38 = 82% Definition: site specific (bone); Prevalence: CT 14/17 = 82%; MRI 14/16 = 88%; PET‐CT 14/17 = 82% Definition: site specific (liver); Prevalence: CT 4/21 = 19%; MRI 4/26 = 15%; PET‐CT 4/25 = 16% Definition: site specific (lung); Prevalence: CT 13/16 = 81%; MRI 13/14 = 93%; PET‐CT 13/15 = 87% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: CT n = 20 lesions; 4 lesions with indeterminate reference and 16 not picked up by CT; MRI n = 9 lesions; 4 lesions with indeterminate reference and 7 not picked up by MRI; PET‐CT n = 9 lesions; 4 lesions with indeterminate reference and 5 not picked up by PET | ||
| Comparative | (1) Each of the three exams was interpreted by a different reader, who had no knowledge of results of the other 2 (2) Tests were consecutively applied, same day (3) Prospective study included all patients scheduled for PET‐CT |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | No | ||
| Low | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Low | |||
Hafner 2004.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: Switzerland Data collection: Aug 1999 to Mar 2002 Inclusion criteria: any cutaneous MM with BT ≥ 1 mm without evidence of detectable distant metastasis (includes clinically palpable) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB/any primary) Number patients: 101 Number primary lesions: 101 Number LNBs/metastases: 105 LNBs; 136 SLNs Stage of disease: NR; stage IV (evidence of distant mets) excluded Median age: 55 years; Range: 18 to 79 years Male: 55 (55%) Primary lesion site: limbs 49, 49%; trunk 35, 35%; H&N 16, 16% Breslow/Clark: Breslow: 1.01 to 2 mm 38; 2.01 to 4 mm 43; > 4.0 mm 19 Ulceration: NR Other: NR | ||
| Index tests |
US: B‐mode
Machine: Acuson Sequoia 512 or General Electric Logiq 700 Experty, with dedicated 5‐MHz curved array probes
Scan coverage: regional lymph nodes of the groins, axillae, and neck (abdominal US also performed)
Contrast: N/A
FNAC: clinically or radiologically suspect LN mets underwent FNAC; FNAC+ underwent SLNB and CLND in same procedure Threshold: NR; 'radiologically suspect' Number observers: 1 Qualification (experience): radiologist (NR) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical unclear; clinical exam by dermatologist and US by radiologist; other tests NR |
||
| Target condition and reference standard(s) |
Histology (CLND/SLNB)
Histological detail (n, %): SLN id ‐ hot or blue node; SLN positive based on EORTC and UICC recommendations (100; 100%). Histopathologist: all specimens were examined by an experienced pathologist
FNAC (n, %): appears that some had FNAC before SLNB but not clearly reported: "In the presence of a clinically or radiologically suspect lymph node metastasis, fine‐needle aspiration was performed. If the lymph node proved to be cytologically positive for melanoma metastasis, SN biopsy was performed" (n NR; abstract reports 3 LN mets identified on physical exam, 2 of which were detected by US)
Follow‐up (n, %): NR; implies CT but could include any of study tests (chest X‐ray, US, PET, CT) (NR)
FU schedule: NR FU duration: 20 months (8 to 39) Reference blinding: NR # Target condition Data: per pt Definition: nodal mets Prevalence: 23/97 = 24%, including 3 clinically node positive 26/100 = 26% |
||
| Flow and timing | Index to histology interval: 2 weeks Index to FU interval: 6 months Exclusions: n = 4; 1 sentinel node was not found intraoperatively; 3 clinically node positive were excluded by Bham team for pre‐SLNB analysis | ||
| Comparative | |||
| Notes | Other result: no confirmed distant mets detected at time of imaging; 9 patients with suspicious findings on imaging were negative for progression/recurrence at 12 months; PET (2), abdominal US (3), chest X‐ray (4) 5/26 SLNB positive and 4/74 SLNB negative patients had recurrence OR progression (no breakdown by US result was given). Recurrences in the SLN positive group were 1 nodal and 4 distant, and in the SLN negative group were 4 nodal plus 1 ITM and 1 distant mets in 2 patients with nodal recurrences | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Hausmann 2011.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: Germany Data collection: NR; 18‐month period Inclusion criteria: AJCC stage III or IV MM; clinical indication for imaging was positive sentinel node biopsy or suspicious lesions on ultrasound or X‐ray studies Excluded if second tumours | ||
| Patient characteristics and setting | Presentation: unclear (pts described as having undergone previous assessment of tumour spread based on ADO (German) guidelines but staging/re‐staging not described; indication for imaging was SLN+ or suspicious lesions were identified on ultrasound or X‐ray) Number patients: 50 eligible; 33 included Number primary lesions: 50 Number LNBs/metastases: NR Stage of disease: full sample only: stage III (19); stage IV (31) Mean age: full sample only: 59.6 years; Range: full sample only: 26 to 86 years Male: full sample only: 32 (64%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
CT: U + CE, multi‐detector
Machine: multi‐detector CT (Somatom Volume Zoom, Siemens Healthcare Sector, Erlangen)
Scan coverage: skull base to pelvis; CT and MR compared for "neck to the pelvis" only; sites imaged included lungs, liver, spleen, kidneys, adrenal glands, subcutaneous tissue, lymph nodes, muscle, bone marrow, and "other"
Contrast: U + CE
CT parameters: NR
Breath hold: no breath hold instructions reported Threshold: NR (presence/absence of mets) # MRI: U + CE; 'standard sequences' Machine: Magnetom Avanto, Siemens Healthcare Sector, Erlangen Scan coverage: WB; NR. CT and MR compared for "neck to the pelvis" only; sites imaged included lungs, liver, spleen, kidneys, adrenal glands, subcutaneous tissue, lymph nodes, muscle, bone marrow, and "other" MRI parameters: standard sequences with parallel imaging techniques Magnet: 1.5 T Threshold: NR (presence/absence of mets) # Number observers: 4 (results for 2 included) Qualification (experience): radiologist (high) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical diagnosis/age/sex; other tests blinded to MRI/CT |
||
| Target condition and reference standard(s) |
Histology or Imaging FU
Histological detail (n, %): NR (NR). Histopathologist:
FNAC (n, %): (0)
Follow‐up (n, %): physical examination, blood tests, ultrasound studies, X‐rays, CT scans of the body from the neck to the pelvis (WB‐CT) as well as MRI of the head (MRI‐CR) (33, 100%)
FU schedule: 3 to 12 months FU duration: ≥ 3 months Reference blinding: FU by an independent radiologist Target condition Data: per lesion Definition: any mets (excl brain); Prevalence: 455/824 = 55% Definition: nodal: 192/379 = 51% Definition: site specific (liver): 33/67 = 49% Definition: site specific (lung): 145/197 = 74%a Definition: site specific (subcutaneous): 33/46 = 72% Definition: site specific (other): 51/118 = 43% (estimated by adding individual 2×2s for originally reported 'Other' category plus adrenal, kidney, muscle, and spleen sites) |
||
| Flow and timing | Index to histology interval: N/A Index to FU interval: minimum 3 months Exclusions: n = 17; no WB‐CT follow‐up undertaken | ||
| Comparative | (1) Test interpretation blinded (2) Within 14 days (3) Prospective study; indication for testing was positive SLNB or findings on US or X‐ray |
||
| Notes | Other result: results presented by region and for less experienced observers, 3 and 4; also presented number of mets detected by cranial MR but no 2×2 extractable | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Unclear | ||
| Did the study report data on a per patient rather than per lesion basis? | No | ||
| Low | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Yes | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Low | |||
Hinz 2011.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; prospective Country: Germany Data collection: Oct 2007 to Feb 2009 Inclusion criteria: clinically node negative, BT ≥ 1 mm or < 1 mm with risk factors such as ulceration or regression Excluded if sono‐morphological criteria for lymph node metastases | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB but includes a secondary nodular SSM) Number patients: 81 Number primary lesions: 81 Number LNBs/metastases: NR; 170 SLNs Stage of disease: NR Mean age: 52.8 years; Median age: NR; Range: SD 15.4 years; range reported for node positive only (36 to 62 years) Male: 48 (59%) Primary lesion site: head 2, 2.5%; trunk 36, 44.4%; upper ext 14, 17.2%; lower ext 23, 28.4%; acral 6, 7.4% Breslow/Clark: median BT 1.68 mm (0.76 to 6.00 mm); 0.75 to 1.00 mm 20, 25%; 1.01 to 1.50 mm 24, 30%; 1.51 to 2.00 mm 12, 15%; 2.01 to 4.00 mm 18, 22%; > 4 mm 7, 9% Clark levels: II 1, 1%; III 26, 32%; IV 47, 58%; V 7, 9% Ulceration: 14, 17.3% Other: NR | ||
| Index tests |
US: B‐mode (linear array); Doppler
Machine: Nemio SSA‐550A; Toshiba Diagnostic Ultrasound System, Neuss, Germany
Scan coverage: LN areas predicted by sites of melanoma
Contrast: N/A
FNAC: N/A Threshold: positive radiological findings according to published criteria plus PD signs of accessory peripheral vessels or displacement of intranodal vessels or asymmetrical avascular areas or aberrant course of central vessels Number observers: 1 of 4 clinicians trained in USS imaging Qualification (experience): NR; broad experience in dermato‐oncology and special ultrasound skills (NR) Diagnosis (single, consensus, etc.): unclear; appears as though single observer Info provided during test interpretation: clinical NR; likely full info available; other tests NR |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): H&E (serial section); IHC (S‐100, HMB 45, and Melan A); mets categorised as macro or micro mets or as cluster of cells (10 to 30 grouped cells) in the subcapsular space or interfollicular zone, or isolated melanoma cells (1 to ≤ 20 individual cells) (ref Starz 2001) (1). Histopathologist: 2 experienced dermatopathologists
FNAC (n, %): NR (NR)
Follow‐up (n, %): not stated (N/A)
FU schedule: NR FU duration: not stated Reference blinding: NR Target condition Data: per pt Definition: nodal mets; Prevalence: 8/81 = 10% |
||
| Flow and timing |
Index to histology interval: NR Index to FU interval: NR Exclusions: n = 0 |
||
| Comparative | |||
| Notes | Other result: of 7 FN LNBs, 3 were classified as reactive on US and 4 were not visualised; the 2 TPs were both correctly classified pre‐LS and post‐LS. Of 8 SLN positive, all described in text as micro‐mets, but Table 2 describes 5 as > 2 mm and 3 as ≤ 2 mm; both TPs were > 2 mm | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hinz 2013.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; retrospective (retrospective computer‐aided search of preoperatively performed staging procedures) Country: Germany Data collection: Jan 2009 to Jan 2011 Inclusion criteria: high risk cutaneous MM; implies BT ≥ 2.0 mm or RF such as ulceration or regression Excluded if classic sonographic signs of lymphatic metastasis | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 20 Number primary lesions: 20 Number LNBs/metastases: 59 SLN Stage of disease: NR Mean age: full sample 55.2 years; Median age: NR; Range: full sample SD 13.3 years Male: 9 (45%) Primary lesion site: trunk n = 10 (50%); upper extremity n = 3 (15%); lower extremity n = 4 (20%); acral n = 3 (15%) Breslow/Clark: BT 1.01 to 2 mm n = 3 (15%), 2.10 to 4 mm n = 9 (45%), > 4 mm n = 8 (40%) Clark level: III n = 1 (5%); IV n = 16 (80%); V n = 3 (15%) Ulceration: 7, 35% Other: | ||
| Index tests |
US: B‐mode
Machine: Nemio SSA‐550A; Toshiba Diagnostic Ultrasound System, Neuss, Germany
Scan coverage: all relevant regional LN basins depending on localisation of the primary melanoma
Contrast: N/A
FNAC: N/A Threshold: morphology criteria of Solbiati 1988, Vassalo 1992, and Voit 2010; suspicious LNs were re‐examined with US after LS PET‐CT: 2D/3D NR; CE‐CT, helical. Reinhardt 2006 states helical, dual detector Machine: Biograph; Siemens Medical Solutions Inc., Erlangen, Germany Scan coverage: WB; Reinhardt 2006: "base of the skull to the apex of the lungs, ... from the shoulders to upper thighs, ... from the proximal femura to the tip of the toes" Contrast: Reinhardt 2006: iodinated oral contrast agent (Peritrast‐oral‐GI; Köhler Chemie GmbH, Alsbach, Germany) CT parameters: 130 kV, 40 mAs (Reinhardt 2006); 5 mm (Reinhardt 2006) FDG: 371 ± 41 MBq (Reinhardt 2006) Breath hold: limited breath hold technique for CT and shallow breathing for PET CT used for:Reinhardt 2006: attenuation correction based on re‐scaling of the CT image Reconstruction: iterative reconstruction with attenuation correction based on re‐scaling of the CT image as described elsewhere (Kinahan 2003) Threshold: NR Number observers: unclear Qualification (experience): US by physicians with broad experience in dermato‐oncology (NR); NR for PET‐CT Diagnosis (single, consensus, etc.): unclear; appears as though single observer for US, NR for PET‐CT Info provided during test interpretation: clinical: clinical exam/US performed by same clinician; other tests: before PET‐CT |
||
| Target condition and reference standard(s) |
Histology (SLNB) Histological detail (n, %): H&E (Serial); IHC (S‐100, HMB 45, and Melan A). Mets were classified according to Carlson et al (2003) ‐ macro‐metastasis (> 2 mm), micro‐metastasis (≤ 2 mm), cluster of cells (10 to 30 grouped cells) in the subcapsular space or interfollicular zone, or isolated melanoma cells (1 to ≤ 20 individual cells) in subcapsular sinuses. Histopathologist: 2 experienced FNAC (n, %): – (0) Follow‐up (n, %): – (0) FU schedule: N/A FU duration: N/A Reference blinding: NR Target condition Data: per pt Definition: nodal mets; Prevalence: 12/20 = 60% (17/59 SLN = 29%) |
||
| Flow and timing | Index to histology interval: before lymphoscintigraphy Index to FU interval: N/A Exclusions: n = 0 | ||
| Comparative | (1) Blinding unclear; US undertaken before CT (2) Tests undertaken consecutively (3) Only subgroup of those with US had PET‐CT; reason NR |
||
| Notes | Other result: no FU for FNs reported; all 17 disease positive were micro‐metastases | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Unclear | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | No | ||
| High | |||
Hocevar 2004.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; unclear design Data collection: Jun 2002 to Aug 2003 Inclusion criteria: MM candidates for SLNB (SLNB eligibility NR) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 57 Number primary lesions: 57 Number LNBs/metastases: 61 Stage of disease: NR Mean age: NR; Median age: NR; Range: 1 to 93 years Male: 21 (37%) Primary lesion site: 14, 25% head; 19, 38% trunk; 24, 42% extremity Breslow/Clark: BT < 1 mm 2, 4%; BT 1 to 2 mm 23, 40%; BT 2.01 to 4 mm 20, 35%; BT > 4 mm 12, 21% Clark level: unknown 2, 4%; III 23, 42%; IV 26, 44%; V 6, 10% Ulceration: 21, 37%; unknown 3, 5% | ||
| Index tests |
US: B‐mode; linear array transducer with small parts probe
Machine: Power Vision 800, Toshiba Corporation, Ottawa, Japan
Scan coverage: NR
Contrast: N/A
FNAC: US + ve; Giemsma stained according to Papanicolaou method, and if necessary, immunocytochemical reaction with monoclonal antibody HMB45 and S100 on an automatic immunostainer Threshold: rounded appearance of the LN, Ioss of the hilar echogenic reflex, and deformed radial nodal vascularity Number observers: 1 Qualification (experience): oncological radiologist (NR) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical NR; other tests NR |
||
| Target condition and reference standard(s) |
Histology (CLND or SLNB)
Histological detail (n, %): H&E (step sectioned); IHC (S100). If negative, additional sections stained with S100 and HMB45 (CLND; SLNB). Histopathologist: NR
FNAC (n, %): melanoma cells in lymph node sample from FNA (14/17 US + ve underwent FNAC)
Follow‐up (n, %): NR (NR)
FU schedule: NR FU duration: NR Reference blinding: NR Target condition Data: per pt Definition: nodal mets; Prevalence: 14/57 = 25% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: no FU to identify FNs; 10/14 disease positive were macro‐metastases; US alone correctly picked up 2/4 micro‐metastases | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Iagaru 2007.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; retrospective (Prosp. database: NR) Country: USA Data collection: Jan 2003 to Jun 2005 Inclusion criteria: PET‐CT for MM re‐staging | ||
| Patient characteristics and setting | Presentation: re‐staging (all patients had the study requested for disease re‐staging) Number patients: 106 Number primary lesions: NR Number LNBs/metastases: 139 metastatic lesions Stage of disease: 76 stage I to IIIc; 30 stage IIIb to IV Mean age: 56.8 years ± 15.9 years; Median age: NR; Range: 20 to 87 years Male: 68 (64.1%) Primary lesion site: NR Breslow/Clark: BT at initial diagnosis (n = 76): mean 3.56 mm, 0.4 to 25 mm; < 1 mm in 6 (8%), 1 to 4.0 mm in 58 (76%), > 4 mm in 12 (16%) Clark level (n = 70): 3 (4%), level II; 13 (19%), level III; 43 (61%), level IV; 11 (16%), level V Ulceration: NR Other: NR | ||
| Index tests |
CT: U, multi‐slice helical
Machine: Discovery LS PET/CT unit (GE Medical Systems, Milwaukee, WI)
Scan coverage: WB; top of the head to the ankles
Contrast: N/A
CT parameters: 140 kV, 40 mA; 5 mm
Breath hold: no breath hold instructions reported Threshold: NR # PET‐CT: 2D; CT (U, multi‐slice helical) Machine: Discovery LS PET/CT unit (GE Medical Systems, Milwaukee, WI) Scan coverage: WB; top of the head to the ankles Contrast: U CT parameters: 140 kV, 40 mA; 5 mm FDG: 15 mCi Breath hold: no breath hold instructions reported CT used for: attenuation correction and anatomical localisation Reconstruction: standard iterative algorithm (OSEM, 2 iterative steps, 28 subsets) using GE software release 5.0 Threshold: SUVmax ≥ 2.5 # Number observers: NR Qualification (experience): nuclear medicine physicians and radiologists (board certified) Diagnosis (single, consensus, etc.): consensus Info provided during test interpretation: clinical ‐ NR for original interpretation or for re‐interpretation; other tests ‐ NR for original interpretation or for re‐interpretation |
||
| Target condition and reference standard(s) |
Histology/FU
Histological detail (n, %): NR (97, 91.5%). Histopathologist: NR
FNAC (n, %): N/A
Follow‐up (n, %): NR (9, 8.5%)
FU schedule: NR FU duration: NR Reference blinding: PET‐CT and pathology reported were 'reviewed'; no blinding described Target condition Data: per pt Definition: any mets (incl skin and brain) Prevalence per patient: 56/106 = 53% (stage I to IIIc 38/76 = 50%; stage IIIb to IV 18/30 = 60%) Prevalence per lesion: 87/139 = 63% Metastases: of the 50 patients TP on PET‐CT: 7 were residual MM, 34 'metastases', and 9 'widespread metastases'. FN on PET‐CT documented only per lesion: 6 recurrences at the resection site, 2 sub‐centimetre soft tissue nodules, and 1 brain lesion (identified by MRI presumably during follow‐up) |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 0; N/A | ||
| Comparative | (1) Blinding between tests unclear (2) Test interval consecutive; same scanner (3) Retrospective; all had PET‐CT with separate interpretation of CT alone |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Unclear | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Unclear | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Unclear | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Unclear | |||
Jouvet 2014.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: France Data collection: Mar 2009 to Jan 2012 Inclusion criteria: AJCC stage IV cutaneous MM referred for simultaneous staging by PET‐CT, CT, superficial lymph node US, and MRI | ||
| Patient characteristics and setting | Presentation: unclear (no details; referred for simultaneous staging) Number patients: 37 Number primary lesions: NR Number LNBs/metastases: 209 lesions (n varies per test) Stage of disease: stage IV: 37 (100%) Mean age: NR; Median age: NR; Range: NR Male: NR (0%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
US: B‐mode
Machine: NR; 12.5‐MHz surface probe
Scan coverage: putative lymphatic drainage area of the primary melanoma
Contrast: N/A
FNAC: N/A Threshold: circular/ovoid hypoechoic lymph node and no hyperechoic hilum # CT: CE; helical; 16 row Machine: CT Philips Scanner (Philips Medical System, Eindhoven, The Netherlands) Scan coverage: neck/chest/abdomen/pelvis; "cervico‐thoraco‐abdomino‐pelvic helicoidal acquisition"; then skull Contrast: iodinated IV injection CT parameters: 120 kV, 250 mAs (neck to pelvis); 140 kV, 120 mAs (skull); 1.25 mm (neck to pelvis); 2.5 mm (skull) Breath hold: no breath hold instructions reported Threshold: NR (presence/absence of mets) # MRI (DW) and MRI (DW + VIBE): DW, VIBE ‐ 3D echo gradient CE, T1 ‐ skull Machine: AVANTO (33 mT, 120 mT/m, Siemns, Erlangen, Germany) Scan coverage: WB; top of skull to feet MRI parameters: echo‐planar DW; axial with coronal reconstruction; VIBE (3D gradient echo w CE); T1 axial on skull Magnet: 1.5 T Threshold: NR (presence/absence of mets) # PET‐CT: 3D GSO; CT (CE, helical; 2 row) Machine: Gemini PET‐CT (Philips Medical System, Eindhoven, The Netherlands) Scan coverage: skull base to the feet (lower limb MM); skull to thighs (MM head, upper limbs, and trunk) Contrast: CE CT parameters: 120 to 140 kV, 100 mAs; 6.5 mm FDG: 5.2 MBq/kg 1 hour before scanning Breath hold: no breath hold instructions reported CT used for: unclear; PET was attenuation corrected but does not state using CT, PET images superimposed with CT data Reconstruction: attenuation corrected PET data were iteratively reconstructed and superimposed with CT data Threshold: NR (presence/absence of mets) # US, CT, MRI: Number observers: 1 Qualification (experience): radiologist (experienced). Diagnosis (single, consensus, etc.): consensus of 2 (all images interpreted independently by 2 examiners; discordant results resolved by consensus) Presume ultrasound also undertaken by radiologist Info provided during test interpretation: clinical ‐ NR; other tests ‐ blinded PET‐CT: Number observers: 2 Qualification (experience): nuclear medicine specialist (experienced) Diagnosis (single, consensus, etc.): consensus of 2 (all images interpreted independently by 2 examiners; discordant results resolved by consensus) Info provided during test interpretation: clinical ‐ NR; other tests ‐ blinded |
||
| Target condition and reference standard(s) |
FNAC, FU:
Histological detail (n, %): N/A (0). Histopathologist: NR
FNAC (n, %): no details; FNAC was performed in 5 cases, and all other positive cases have been diagnosed on the basis of progression of the target (5, 13.5%)
Follow‐up (n, %): 'sequential imaging'; not further described (32; 86.5%)
FU schedule: NR FU duration: > 9 months Reference blinding: N/A Target condition Data: per lesion Definition: any mets (incl brain, subcut); Prevalence: CT 115/209 = 55%; MRI 125/218 = 57% Definition: any (excl brain mets); Prevalence: CT 95/186 = 51%; MRI 105/195 = 54%; PET‐CT 104/191 = 54% Definition: nodal; Prevalence: all tests 23/53 = 43% Definition: nodal (superficial); Prevalence: all tests 13/33 = 39% (per LNB) Definition: site specific (bone); Prevalence: CT 15/33 = 45%; MRI 16/35 = 46%; PET‐CT 16/35 = 46% Definition: site specific (liver); Prevalence: all tests 12/27 = 44% Definition: site specific (lung); Prevalence: all tests 31/45 = 69% Definition: site specific (local); Prevalence: MRI, PET‐CT only 10/22 = 45% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 0; N/A | ||
| Comparative | (1) "All the examiners were unaware to the results of the other imaging techniques" (2) "All examinations were performed within a mean interval of 7 days" (3) Prospective; "referred for simultaneous staging" |
||
| Notes | Other result: provides K values for inter‐ and intra‐observer agreements, but not the 2×2 tables for each observer | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Unclear | ||
| Did the study report data on a per patient rather than per lesion basis? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | No | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Low | |||
Kang 2011.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (medical record review) Country: S Korea Data collection: Mar 2005 to Sep 2009 Inclusion criteria: newly diagnosed cutaneous MM undergoing staging work‐up with PET‐CT (any stage, including clinically node positive) | ||
| Patient characteristics and setting | Presentation: primary (any) Number patients: 37 Number primary lesions: 37 Number LNBs/metastases: NR Stage of disease: stage 0: 7 (18.9%); stage I: 6 (16.2%); stage II: 17 (45.9%); stage III: 6 (16.2%); stage IV: 1 (2.7%) Mean age: 61.7y ± 13.6 years; Median age: NR; Range: 48.1 to 75.3 years Male: 17 (45.9%) Primary lesion site: hand/foot 23 (62.1%), trunk 6 (16.2), head/neck 4 (10.8%), extremity 4 (10.8%) Breslow/Clark: BT < 1.0 mm 8, 22%; ≥ 1 mm 15, 41%; NR 14, 38% Ulceration: present 7, 19%; absent 30, 81% Other: mean SUVmax 2.8 ± 2.3 | ||
| Index tests |
PET‐CT: CT (U, 6 slice or 16 slice)
Machine: Reveal RT‐HiRez CTIMI (Knoxville, TN, USA), a 6‐slice CT; or Discovery ST (GE Health Systems, Milwaukee, Wl, USA), a 16‐slice CT
Scan coverage: vertex of skull to knees; plus lower limbs if with lower leg MM
Contrast: U
CT parameters: Reveal RT‐HiRez 130 kV, 95 mA; Discovery ST 140 kV, 160 mA; Reveal RT‐HiRez 2.5 mm; Discovery ST 3.75 mm
FDG: 350 to 400 MBq
Breath hold: NR; 'standard protocol'
CT used for: unclear; combined PET‐CT unit; mentions identification of anatomical location on fused PET‐CT image
Reconstruction: ordered subset expectation‐maximisation Threshold: SUVmax ≥ 2.2 (set using ROC analysis) # Number observers: 2 Qualification (experience): nuclear physicians (experienced) Diagnosis (single, consensus, etc.): consensus of 2 Info provided during test interpretation: clinical ‐ NR; other tests ‐ N/A |
||
| Target condition and reference standard(s) |
Histology/Imaging FU
Histological detail (n, %): reported for only 6 of disease positive group (6 (16.2%)). Histopathologist: experienced dermatopathologist and pathologist
FNAC (n, %): N/A (0)
Follow‐up (n, %): clinical, CT, PET‐CT (37 (100%))
FU schedule: physical examination every 3 months for 1 to 2 years, then every 6 months; imaging every 6 to 12 months and/or when clinically indicated FU duration: median followup 24.3 ± l l.7 months (range 8 to 55 months) Reference blinding: NR # Target condition Data: per pt Definition: any mets (incl brain, local/skin); Prevalence: 9/37 = 24% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: 3 months Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: sites of recurrence were LN (3); distant (5; lung or liver); 'local' (2); skin (1); 3 patients died related to CMM | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Kell 2007.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (prospective database) Country: USA Data collection: NR; 12‐month period Inclusion criteria: MM, BT ≥ 0.76 mm, candidates for SLNB who underwent PET‐CT (46/83 with SLNB) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 37 Number primary lesions: NR Number LNBs/metastases: NR Stage of disease: NR Mean age: 61.4 years; Median age: NR; Range: NR Male: NR (0%) Primary lesion site: NR Breslow/Clark: mean BT 2.4 mm Ulceration: NR Other: NR | ||
| Index tests |
PET‐CT: CT (U)
Machine: NR
Scan coverage: base of skull to feet
Contrast: U
CT parameters: NR
FDG: NR
Breath hold: NR; standard protocols
CT used for: NR
Reconstruction: NR; combined PET‐CT images Threshold: quantitative for areas of abnormally increased 18‐FDG uptake relative to surrounding normal tissues and areas of increased physiological uptake # Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): NR (37, 100%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): NR (NR)
FU schedule: NR FU duration: NR Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 9/37 = 24% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: N/A Exclusions: n = 0; 46 with SLNB but no PET‐CT could not be included | ||
| Comparative | |||
| Notes | Other result: PET‐CT revealed no unheralded metastatic disease but did identify a second occult malignancy in 4 (10.8%) patients undergoing therapy for melanoma | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Klebl 2003.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison (US vs palpation) Country: Germany Data collection: Aug 1997 to Dec 1998 Inclusion criteria: MM Clark level IV or V undergoing FU after primary surgery | ||
| Patient characteristics and setting | Presentation: mixed (primary (n = 8), follow‐up (n = 75)) Number patients: 83 Number primary lesions: 83 Number LNBs/metastases: NR; 653 LNs examined Stage of disease: NR Mean age: NR; Median age: NR; Range: NR Male: 46 (55%) Primary lesion site: NR Breslow/Clark: Clark level IV 68, 82%; level V 15, 18% Ulceration: NR | ||
| Index tests |
US: B‐mode US; high‐resolution linear array
Machine: HDI Ultramark 9 using a high‐resolution 5‐ to 10‐MHz linear sonicator
Scan coverage: cervical, axillary, and inguinal LNBs
Contrast: N/A
FNAC: no Threshold: suspicious/indeterminate/benign based on diameter, shape, echogenicity, and vascularisation pattern # Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical ‐ unclear; could be same examiner as for LN palpation; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (NR), FU
Histological detail (n, %): NR (17, 20%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): NR (62, 75%)
FU schedule: suspicious, but not clearly malignant findings were reviewed at intervals of 6 to 8 weeks. For unremarkable findings, a check was carried out after 6 to 12 months as part of the tumour follow‐up FU duration: minimum 1 year; mean time since primary surgery 2.6 ± 2.3 years Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 17/79 = 22% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: 6 to 8 weeks for control visit, 6 to 12 months for FU visit Exclusions: n = 4; 4 were indeterminate on follow‐up so that a final diagnosis could not be made | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Klode 2010.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (prospective database NR) Country: Germany Data collection: Jan 2004 to Dec 2006 Inclusion criteria: primary MM AJCC stage I or II (BT > 1 mm) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 61 Number primary lesions: NR Number LNBs/metastases: 174 SLNs Stage of disease: NR (I or II) Mean age: 58.8; Median age: 61; Range: 31 to 82 Male: 36 (0.5901%) Primary lesion site: trunk and lower limbs 26, 42.6%; upper extremities 9, 14.8%; NR for remaining 27 lesions Breslow/Clark: BT mean 2.62 mm, median 2.0 mm, range 1 to 8 mm Ulceration: 15, 24.6% Other: NR | ||
| Index tests |
PET‐CT: 2D/3D NR; CE‐CT
Machine: Siemens Biograph Duo PET‐CT scanner (Siemens, Erlangen)
Scan coverage: cranial base to mid‐femur; additional views according to melanoma localisation
Contrast: iodine‐containing contrast agent
CT parameters: NR
FDG: 349 mBq
Breath hold: breath hold instructions NR
CT used for: NR
Reconstruction: NR Threshold: NR; hypermetabolic tumour focus # Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): H&E (serial); IHC (S100, MElanA, HMB45). Tumour focus < 2.0 mm defined as micro‐metastasis; ≥ 2.0 mm macro‐metastasis (61, 100%) Histopathologist: NR
FNAC (n, %): N/A (N/A)
Follow‐up (n, %): NR (61, 100%)
FU schedule: NR FU duration: median 38 months, 13 to 55 months Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 14/61 = 23% (17/174 SLNs = 10%) |
||
| Flow and timing | Index to histology interval: median 14 days PET to SLNB Index to FU interval: NR Exclusions: n = 0; 60 patients with SLNB did not agree to preop PET | ||
| Comparative | |||
| Notes | Other result: 174 SLNs removed from 68 lymphatic drainage areas. The TP result was a macro‐mets > 10 mm; of the 16 FNs on PET‐CT, 2 were macro‐mets (5.5 mm and 10 mm) and 14 were micro‐mets. On FU, disease progression observed in 6 patients (3 of whom died), 3 of whom were SLN positive (PET‐CT result NR) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kunte 2009.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; prospective Data collection: Dec 2002 to Mar 2003 Inclusion criteria: cutaneous MM SLNB candidates; reported as 'mainly' ≥ 1.0 mm BT or risk factors (ulceration or regression or Clark level IV and V) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 25 Number primary lesions: 25 Number LNBs/metastases: 68 LNBs; 35 SLNs Stage of disease: NR Mean age: 54 years; Median age: NR; Range: NR Male: 15 (60%) Primary lesion site: limbs 14, 56%; head and neck 2, 8%; trunk 9, 36% Breslow/Clark: Breslow ≤ 1 mm 8, 32%; 1.01 to 2 mm 11, 44%; 2.01 to 4 mm 5, 20%; > 4.0 mm 1, 4% Ulceration: 6, 24% Other: regression 0, 0% | ||
| Index tests |
US: B‐mode; linear transducer
Machine: SSA‐340 A; Toshiba Medical Systems, Neuss, Germany
Scan coverage: regional lymphatic basins
Contrast: N/A
FNAC: no Threshold: qualitative presence of morphological features (described) # Number observers: 2 Qualification (experience): dermatologists (experienced) Diagnosis (single, consensus, etc.): unclear Info provided during test interpretation: clinical ‐ unclear; may be same dermatologists as for clinical exam; other tests ‐ pre and post lymphoscintigraphy ultrasound |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): H&E (serial section); IHC (S‐100, HMB 45, NKiC3, Melan A). LNs with histologically proven tumour deposits were considered metastatic except when fewer than 4 isolated tumour cells were present. The metastatic deposit was documented for each SLN concerning location within the LN and size (micro‐metastasis and macro‐metastasis) (25, 100%). Histopathologist: NR
FNAC (n, %): – (0)
Follow‐up (n, %): – (0)
FU schedule: N/A FU duration: N/A Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 6/25 = 24% (6/35 SLN; 17%) |
||
| Flow and timing | Index to histology interval: < 24 hours Index to FU interval: N/A Exclusions: n = NR; NR | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Maubec 2007.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; prospective Country: France Data collection: Jan 2004 to Jun 2005 Inclusion criteria: any MM BT > 4 mm; SLNB planned if clinically node negative Excluded if presence of distant mets (those with clinically palpable nodes were included but no SLNB was given and no 2×2 can be estimated) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) and primary (any) Number patients: 25 Number primary lesions: 26 Number LNBs/metastases: 20 from 19 pts Stage of disease: all T4; 3 clinically node positive; post surgery: AJCC stage IIB 10, 40%; IIC 4, 16%; IIIA 4, 16%; IIIB 6, 24%; IIIC in 1, 4% Mean age: 60 years; Range: 14 to 87 years Male: 15 (0.6%) Primary lesion site: trunk 8, 32%; limbs 8; 32%; head and neck 9, 36% Breslow/Clark: mean BT 6.6 mm, range 4.8 to 12.5 mm Ulceration: 9, 36% | ||
| Index tests |
PET‐CT: 3D; CT (U)
Machine: Biograph, LSO System, Siemens Medical Systems, Germany; full‐ring tomograph (ECAT ACCEL, CPS Innovation, Knoxville, Tennesee), single‐slice spiral CT (Somatom Emotion, Siemens Medical Solutions)
Scan coverage: WB; "top of the head to the mid‐thigh and included if necessary, the lower limbs"
Contrast: U
CT parameters: 110 kV; 80 mA; 5 mm
FDG: 5 MBq/kg
Breath hold: normal breathing; "no breath hold instructions"
CT used for: NR; integrated system
Reconstruction: iterative algorithm (FORE and AWOSEM) with 2 iterations, 8 subsets, and a 5‐mm full‐width half maximum (FWHM) Gaussian post filter Threshold: uptake site suspicious for malignancy or not clearly explained by a benign aetiology (SUV estimated but does not appear to formally contribute to diagnosis) # Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (SLNB, CLND)
Histological detail (n, %): H&E (serial); IHC (S100, HMB45, Melan A). Processed according to EORTC melanoma group (22, 88%; 3 node positive underwent CLND; 19 had SLNB; 3 no surgery). Histopathologist: NR
FNAC (n, %): N/A (N/A)
Follow‐up (n, %): NR (25, 100%)
FU schedule: mean 11 months (2 to 19 months)
Reference blinding: NR # Target condition Data: per pt (data per LNB but counted as per patient as 20 LNBs examined in 19 patients) Definition: nodal mets (pre‐SLNB population); Prevalence: 7/20 = 35%; 1 FN identified on FU Definition: any mets (full population); Prevalence: 7/25 = 28% (no distant metastases identified) |
||
| Flow and timing | Index to histology interval: NR; some PET performed up to 4 months after SLNB Index to FU interval: NR Exclusions: n = 6; 3 clinically node positive underwent CLND (all PET+ and N+); 3 did not undergo any surgery | ||
| Comparative | |||
| Notes | Other result: 3 PET +ve for distant mets; all found to to be FP | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Pfannenberg 2007.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: Germany Data collection: Sep 2004 to Sep 2005 Inclusion criteria: stage III or IV cutaneous MM undergoing imaging for exclusion of widespread disease; confirmation of local disease before surgical resection; further characterisation of abnormal radiological, clinical, and laboratory (S100 protein, lactic dehydrogenase) findings; routine melanoma surveillance of high‐risk MM | ||
| Patient characteristics and setting | Presentation: mixed (included exclusion of widespread disease and confirmation of local disease before surgical resection (n = 9); characterisation of abnormal radiological, clinical, and laboratory findings (n = 48); routine melanoma surveillance in high‐risk patients (n = 7)) Number patients: 64 Number primary lesions: NR Number LNBs/metastases: 420 Stage of disease: stage III (25, 39%); stage IV (39, 61%) Mean age: 57.8 years; Range: 23.3 to 79.1 years Male: 41 (64%) Primary lesion site: NR Breslow/Clark: mean BT 2.69 mm (0.6, 12 mm) Ulceration: NR Other: NaR | ||
| Index tests |
CT: CT (CE, 16 row multi‐slice)
Machine: Hi‐Rez Biograph 16 (Siemens Medical Solutions, Knoxville, TN)
Scan coverage: base of the skull to the lower legs
Contrast: Ultravist 370, Schering GmbH, Berlin, Germany, plus 1000 ml Mannitol 2% as a negative oral contrast agent before CT
CT parameters: 120 kV, 120 to 160 mAs; 5 mm (axial, with an increment of 5 mm) and 3 mm (coronal with an increment of 2 mm)
Breath hold: CT: patients were asked to stop breathing in normal expiration during contrast‐enhanced CT scans for optimal co‐registration Threshold: based on morphological characteristics and enhancement pattern; region‐specific nodal size criteria based on measurement of the small axis diameter # MRI: CE; multiple phased‐array; axial and coronal Machine: Avanto, Siemens AG, Erlangen, Germany Scan coverage: head to toe MRI parameters: N/A Magnet: N/A Threshold: based on morphological characteristics and enhancement pattern; detected lymph nodes smaller than 10 mm but with brighter signal on T1 sequences due to the paramagnetic effect of melanin; also were rated as suspicious # PET‐CT: 3D; CT (CE, 16 row multi‐slice) Machine: Hi‐Rez Biograph 16 (Siemens Medical Solutions, Knoxville, TN) Scan coverage: base of the skull to the lower legs Contrast: Ultravist 370, Schering GmbH, Berlin, Germany, plus 1000 mL Mannitol 2% as a negative oral contrast agent before CT CT parameters: 120 kV, 120 to 160 mAs; 5 mm (axial, with an increment of 5 mm) and 3 mm (coronal with an increment of 2 mm) FDG: 370 MBq F‐FDG IV 55 to 65 minutes before scanning Breath hold: CT: patients were asked to stop breathing in normal expiration during contrast‐enhanced CT scans for optimal co‐registration CT used for: attenuation corrected and co‐registered Reconstruction: iteratively reconstructed using commercial software (eSoft; Siemens, Erlangen, Germany) Threshold: for PET: any focal tracer uptake exceeding normal regional tracer accumulation was assessed as a malignant lesion. Lesions rated malignant or probably malignant were considered to be malignant # Number observers: 6 Qualification (experience): 2 dermato‐oncologists; 2 radiologists (2 specialists in nuclear medicine, 2 CT radiologists, and 2 MRI radiologists) Diagnosis (single, consensus, etc.): consensus of 2 or 4 Info provided during test interpretation: clinical ‐ aware of clinical status; other tests ‐ blinded to results of other imaging studies and previous tests |
||
| Target condition and reference standard(s) |
Histology/Imaging/FU
Histological detail (n, %): NR; confirmed by histology after resection; 65 (15%). Histopathologist: NR
FNAC (n, %): N/A (N/A)
Follow‐up (n, %): PET‐CT, CT, dedicated MRI, ultrasound, bone scan or radiography, tumour markers (S100, lactic dehydrogenase), other laboratory and clinical tests (267 (64%) lesions by imaging follow‐up, 88 (21%) lesions by clinical follow‐up)
FU schedule: regular 3‐month interval follow‐up schedule FU duration: mean 252.5 days (range 99 to 474 days) Reference blinding: N/A # Target condition Data: per lesion Definition: any metastases (excl brain); Prevalence: 297/420 = 71% Definition: nodal; Prevalence: 102/158 = 65% Definition: distant (excl local); Prevalence: 136/182 = 75% Definition: site specific (bone); Prevalence: 35/50 = 70% Definition: site specific (lung); Prevalence: 53/70 = 76% Definition: site specific (local); Prevalence: 59/80 = 74% Definition: site specific (other); Prevalence: 13/25 = 52% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: every 3 months Exclusions: n = 36; no wbMRI (n = 25; due to metallic implants or claustrophobia (5 patients); refusal of a second whole body examination on the same day (17 patients) or abortion of the examination (3 patients); no evidence of tumour spread (3 patients); lack of follow‐up data for lesion characterisation (8 patients)) | ||
| Comparative | (1) Blinded to the results of other imaging studies and previous tests (2) 24‐hour to 72‐hour interval (3) prospective; consecutively referred for staging |
||
| Notes |
Other result: when changes in the treatment schedule were analysed for the influence of different imaging procedures, PET/ CT performed best; 90.2% of the changes could be motivated by PET‐CT alone, 87.8% by wbMRI alone (cerebral metastases excluded), 75.6% by PET alone, and 73.2% by CT alone # Text states that MRI sensitivity increased from 79.8% to 86.9% on retrospective review of images not blinded to the other imaging tests (i.e. FNs reduced from 60 to 39) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | No | ||
| Low | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test MRI | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Yes | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Low | |||
Pfluger 2011.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; retrospective (Prosp. database NR) Country: Germany Data collection: NR; 3.5‐year period Inclusion criteria: MM with regional LN metastases (NR if clinically detectable or micro‐metastases) undergoing PET‐CT for primary staging or during follow‐up. Included only lesions considered malignant by at least 1 of the 3 modalities | ||
| Patient characteristics and setting | Presentation: mixed (PET‐CT was done for primary staging and for follow‐up) Number patients: 50 Number primary lesions: NR Number LNBs/metastases: 232 lesions Stage of disease: NR Mean age: 57 years; Range: 29 to 85 years Male: 36 (72%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
CT: U & CE, dual‐slice, helical
Machine: Philips Gemini PET/CT System (Philips, Hamburg, Germany), consisting of a dedicated GSO full‐ring PET scanner and a dual‐slice helical CT scanner
Scan coverage: WB; from the skull including the legs
Contrast: reports for unenhanced and CE using 120 mL (2.5 mL/s) of iodine‐containing contrast medium
CT parameters: U ‐ 140 kV, 20 mAs, 5 mm; CE ‐ 120 kV, 145 mAs, 2.5 mm
Breath hold: CT expiration protocols for shallow free breathing during the emission scan for CE only Threshold: unenhanced ‐ abnormal soft tissue masses and/or enlarged LNs (diameter > 1.0 cm); contrast enhanced ‐ same plus degree of contrast enhancement # PET‐CT: 3D; CT (U and CE, dual‐slice, helical) Machine: Philips Gemini PET/CT System (Philips, Hamburg, Germany), consisting of a dedicated GSO full‐ring PET scanner and a dual‐slice helical CT scanner Scan coverage: WB; from the skull including the legs Contrast: 120 mL (2.5 mL/s) of iodine‐containing contrast medium CT parameters: 120 kV, 145 mAs, 2.5 mm FDG: 200 MBq Breath hold: CT expiration protocols for shallow free breathing during the emission scan CT used for: unclear; reports side‐by‐side PET‐CT display with spatially synchronised images Reconstruction: NR Threshold: non‐physiologically increased uptake of FDG with SUVmax > 2.5. CT (U and CE) and PET alone first analysed separately, followed by combined PET‐CT analysis using a side‐by‐side display with spatially synchronised images to ensure the same lesion was assessed on both modalities. For lesions with discrepant results on CT and PET, the finding of the modality with the higher diagnostic confidence score was accepted. If results from both modalities were discrepant and had the same diagnostic confidence score value, the lesion was judged positive.Confidence scores were assigned as follows: (1) both observers uncertain about positive or negative findings, (2) one observer uncertain and one observer certain and (3) both observers certain. If there were no signs of an active tumour lesion or physiological changes in one modality, the diagnostic confidence score “3” was assigned to this “lesion” that was suspicious for melanoma involvement in another modality # Number observers: 2 Qualification (experience): NR (experienced); consensus Info provided during test interpretation: clinical ‐ knowledge of clinical data but blinded to any imaging. Other tests ‐ PET‐CT viewed side by side |
||
| Target condition and reference standard(s) |
Histology/FU
Histological detail (n, %): NR (41, 17.7%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): used an imaging method 'appropriate to the respective lesion (38 PET‐CT scans, 8 CT scans, 4 ultrasound examinations)' (191, 82.3%)
FU schedule: NR FU duration: ≥ 6 months; no further detail Reference blinding: NR # Target condition Data: per pt Definition: any (incl subcutaneous, brain); Prevalence: 151/232 = 65% Metastases: only FP and FN results were documented by anatomical site. FNs on CE CT included subcutaneous (6), bone or bone marrow (5), muscular (4), LN (4), liver (3). FNs on unenhanced PET‐CT included 3 liver, 1 LN, 1 spleen (no FNs on CE, PET‐CT) |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = NR; in cases of new tumour lesions during the follow‐up period, these lesions were not included in the study. The reason for not including these lesions was the fact that non‐detectable lesions in CT or 18F‐FDG PET cannot be distinguished from non‐existent lesions in the case of a newly detected tumour lesion during follow‐up | ||
| Comparative | (1) Combined PET‐CT analysis using a side‐by‐side display with spatially synchronised images to ensure the same lesion was assessed on both modalities (2) Same scanner (3) Retrospective; all with PET‐CT |
||
| Notes | Other result: all 6 FPs on PET‐CT would have affected TNM classification as they would have been single metastatic lesions in otherwise metastasis‐free patients. The 5 FNs on unenhanced PET‐CT did not affect TNM classification as all were identified in patients with multiple metastases (stage M1c) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Unclear | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | No | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| High | |||
Prayer 1990.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison (US vs palpation); unclear Country: Austria Data collection: NR; 18‐month period Inclusion criteria: primary MM investigated before or after removal of the primary melanoma in postoperative follow‐up | ||
| Patient characteristics and setting | Presentation: primary (LNs investigated before or after removal of the primary melanoma in postoperative follow‐up) Number patients: 217 Number primary lesions: NR Number LNBs/metastases: NR Stage of disease: NR Mean age: 56 years; Median age: NR; Range: 25 to 82 years Male: 104 (48%) Primary lesion site: HN 42, 19%; arm 61, 28%; shoulder 23, 11%; leg 91, 42% Breslow/Clark: BT < 0.75 mm 25, 12%; 0.75 to 1.5 mm 96, 44%; 1.5 to 3.00 mm 79, 36%; > 3 mm 17, 8% Clark level: II 93; III 89; IV 33 Ulceration: NR Other: NR | ||
| Index tests |
US: B‐mode
Machine: ATL ‘Ultramark 8’ with an anular array and detachable elastomere
Scan coverage: primary LNs depending on tumour localisation. Cervical (42); axillary (84); inguinal (91)
Contrast: N/A
FNAC: N/A Threshold: suspicious ‐ circular and oval masses with poor echo; longitudinally configurated LNs with echogenic eccentric hilum regarded as “enlarged reactively” # Number observers: 1 Qualification (experience): radiologist (NR) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical ‐ unclear; different clinicians for palpation (dermatologist) and for US (radiologist); other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (presume LND), FU for US negative
Histological detail (n, %): NR (29, 13%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): NR (188, 87%)
FU schedule: every 2 months FU duration: 6 months Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 29/217 = 13% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: 2 months Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: there were no false‐negative US results (i.e. melanoma metastases did not occur within the following 6 months in any of the patients classified as having no suspect regional lymph nodes). The smallest metastasis detected by ultrasound was 11 mm in diameter | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Radzhabova 2009.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; unclear Country: Russia Data collection: NR Inclusion criteria: clinically node negative MM and SLNB (based on US result) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 152 Number primary lesions: NR Number LNBs/metastases: NR Stage of disease: NR Mean age: NR; Median age: NR; Range: NR Male: NR (0%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
US: B‐mode; sectoral and linear
Machine: NR
Scan coverage: NR
Contrast: N/A
FNAC: N/A Threshold: test positive considered as high PSV, EDV, S/D, and PI < 1000. Mets could not be excluded if PSV and PI were high but EDV = 0, S/D was undetectable (PI – pulse index, PSV – peak systolic volume, EDV ‐ end‐diastolic volume, Stuart index) # Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histo (SLNB); FU
Histological detail (n, %): NR (52, 100%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): NR (0)
FU schedule: NR FU duration: NR Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 11/52 = 21% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 100; benign on US did not get SLNB | ||
| Comparative | |||
| Notes | 2 FN on SLNB identified during FU; all 100 with no SLNB reportedly disease free on FU | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Reinhardt 2006.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; retrospective (Prosp. database NR) Country: Germany Data collection: Nov 2002 to Jun 2004 Inclusion criteria: cutaneous MM referred for PET‐CT for primary staging after sentinel node biopsy, for therapy control after chemotherapy of metastatic disease, for staging of clinically suspected recurrent disease, and during follow‐up within 5 years of primary treatment Excluded if inadequate reference standard (no histology or FU < 1 year) | ||
| Patient characteristics and setting | Presentation: mixed (primary staging after sentinel node biopsy (n = 75); therapy control after chemotherapy of metastatic disease (n = 42), staging of clinically suspected recurrent disease (n = 65), during follow‐up within 5 years of primary treatment (n = 68)) Number patients: 250 Number primary lesions: 250 Number LNBs/metastases: NR; 670 lesions identified Stage of disease: initial pathology: stage I 22, 9%; stage II 88, 35%; stage III 108, 43%; stage IV 32, 13% Mean age: 58 years ± 16 years Male: 145 (58%) Primary lesion site: NR Breslow/Clark: tumour depth ≤ 1.0 mm 29, 12%; 1.01 to 2.0 mm 68, 27%; 2.01 to 4.0 mm 66, 26%; > 4.0 mm 64, 26% Ulceration: NR Other: NR | ||
| Index tests |
CT: CE, helical, dual detector
Machine: Biograph; Siemens Medical Solutions Inc., Hoffman Estates, Illinois, USA
Scan coverage: WB; base of skull to tip of toes in 3 parts
Contrast: Peritrast‐oral‐GI; Kohler Chemie GmbH, Alsbach, Germany
CT parameters: 130 kV, 40 mAs, 5 mm
Breath hold: limited breath hold for CT and shallow breathing for PET Threshold: NR; states only that accuracy was assessed according to current AJCC staging classification # PET‐CT: CT (CE), helical, dual detector Machine: Biograph; Siemens Medical Solutions Inc., Hoffman Estates, Illinois, USA Scan coverage: WB; base of skull to tip of toes in 3 parts Contrast: Peritrast‐oral‐GI; Kohler Chemie GmbH, Alsbach, Germany CT parameters: 130 kV, 40 mAs, 5 mm FDG: 371 ± 40 MBq FDG through an anterior cubital vein Breath hold: limited breath hold for CT and shallow breathing for PET CT used for: attenuation correction based on re‐scaling of the CT image Reconstruction: iteratively reconstructed with attenuation correction on the basis of re‐scaling of the CT image as described elsewhere (Kinahan 2003) # Threshold: NR; states only that accuracy was assessed according to current AJCC staging classification Number observers: NR Qualification (experience): NR; consensus by each of 2 experienced investigators Diagnosis (single, consensus, etc.): consensus (of 2) Info provided during test interpretation: clinical ‐ routine clinical fashion ‐ same clinical clinical information about each patient; other tests ‐ blinded to competitive imaging procedure |
||
| Target condition and reference standard(s) |
Histology (SLNB or other biopsy), FU
Histological detail (n, %): no details; 100, 40% for N‐staging (including 15 with SLNB); 20, 8% for M‐staging. Histopathologist: NR
FNAC (n, %): N/A (N/A)
Follow‐up (n, %): all available clinical information, laboratory tests, radiologic and nuclear medicine imaging studies such as MRI, contrast‐enhanced CT, ultrasound, and bone scans (250, 100%)
FU schedule: every 3 months FU duration: ≥ 1 year Reference blinding: blinded to standard of reference; data collection for the reference standard was done by a physician unaware of the results of PET‐CT imaging # Target condition Data: per pt Definition: any (excl brain); Prevalence: 116/250 = 46% Definition: nodal; Prevalence: 78/250 = 31% Definition: distant; Prevalence: 84/250 = 34% Metastases: distant metastases included distant LN, lungs, and other organs (numbers per group NR and not further differentiated by anatomical site) |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: 3 months Exclusions: n = 0 | ||
| Comparative | (1) Blinded to competitive imaging procedure (2) Same scanner; CT performed 1 minute before PET (3) All undergoing PET‐CT |
||
| Notes | Other result: data reported by clinical setting, for differentiation by metastatic sites (M1A to M1C), and for detection of visceral and non‐visceral metastases, but number diseased per group is not given such that 2×2 cannot be estimated | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Yes | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Low | |||
Revel 2010.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (Prosp. database NR) Country: France Data collection: Jan 2005 to Sep 2008 Inclusion criteria: clinically node negative HN MM qithpre‐SLNB PET‐CT Excluded if or > 1 month between PET‐CT and SLNB | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 22 Number primary lesions: 22 Number LNBs/metastases: 21 Stage of disease: stage I or II Mean age: 60 years; Range: 18 to 88 years Male: 16 (73%) Primary lesion site: scalp 5, 23%; cheek 3, 14%; cervical or neck 3, 14%; atrial region (ear, mastoid, temples) 6, 27%; palpebral or periorbital 4, 18%; frontal 1, 5% Breslow/Clark: 4.5 mm (0.26 to 10 mm) Ulceration: unknown | ||
| Index tests |
PET‐CT:
Machine: Biograph 2 (Siemens1 Germany) (2003 to 2007); Biograph 6 True V imager (Siemens1) (2007 onwards)
Scan coverage: WB; vertex to the toes
Contrast: NR
CT parameters: Biograph 2: 130 kV, 80 mAs; Biograph 6: 130 kV, 4D Care Dose; Biograph 2: 5 mm Biograph 6: 4 mm
FDG: 5.5 MBq/kg for Biograph 2; 4 MBq/kg for Biograph 6 True V; Flucis1, Schering, Cisbio International
Breath hold: no breath hold instructions reported
CT used for: appears to be used for attenuation correction; also describes anatomical localisation on fused images
Reconstruction: iterative reconstruction algorithms using Osem 3D, with correction of scatter and attenuation Threshold: any hypermetabolic focus more intense than the surrounding background, including equivocal foci, was systematically compared with the corresponding anatomical structure on the coupled CT, after accuracy of registration on merged PET‐CT images was verified. An FN was considered present if a patient was SLN positive and PET‐CT for the same basin was negative, regardless of whether PET was positive for a different LNB # Number observers: 2 Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): consensus of 2 Info provided during test interpretation: clinical ‐ localisation of the initial tumor and standard clinical and radiological assessment were known during image interpretation; other tests ‐ standard radiological assessment ‐ known but blinded to review of PET alone |
||
| Target condition and reference standard(s) |
Histology (SLNB); FU
Histological detail (n, %): H&E; IHC (S100, HMB45, melanA antibodies) (22, 100%). Histopathologist: NR
FNAC (n, %): N/A (N/A)
Follow‐up (n, %): NR (22/22, 100%)
FU schedule: NR FU duration: mean 17 months (range 1 to 44) Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 10/20 = 50% (excluding 2 SLNB failures (histo only reference standard)); 12/22 = 55% (including 2 SLNB failures (histo + FU reference standard)) |
||
| Flow and timing | Index to histology interval: 12 days; PET undergone in month before surgery Index to FU interval: NR Exclusions: n = 2; 2 test fails (no SN detected; however data can be extracted excluding these) | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | No | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Rubaltelli 2011.
| Study characteristics | |||
| Patient sampling |
Design: NC; unclear
Country: Italy
Data collection: Jun 2008 to Dec 2009
Inclusion criteria: cutaneous MM with US of regional LNs as part of follow‐up Excluded if or malignant on B‐mode US as assessed by usual US features |
||
| Patient characteristics and setting | Presentation: re‐staging (all undergoing postoperative follow‐up designed to ensure early identification of lymph node metastases) Number patients: 436 Number primary lesions: NR Number LNBs/metastases: NR Stage of disease: NR Mean age: 54 years; Median age: 58 years; Range: 27 to 81 years Male: full sample: 240 (52%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
US: B‐mode plus contrast‐enhanced US for subgroup; linear array transducers
Machine: Sonoline Elegra Scanner (Siemens Healthcare)
Scan coverage: variable: axillary lymph nodes for MM of the upper limbs, inguinal lymph nodes for MM of the lower limbs, both axillary and inguinal lymph nodes for MM of the trunk, and cervical and supraclavicular lymph nodes for MM of the head and neck (72 neck, 248 axillary, and 354 inguinal LNBs were examined). LNBs identified on B‐mode US were examined with CE US
Contrast: sulfur hexafluoride microbubbles (SonoVue, Bracco)
FNAC: yes as ref standard Threshold: B‐mode ‐ focal hypoechoic cortical thickening ‐ focal area of cortex at least twice as thick as the cortex in the remainder of the same lymph node. CE ‐ perfusion defects corresponding to cortical focal thickening; homogeneous intense enhancement of the cortex considered benign # Number observers: 1 of 3 Qualification (experience): sonologist (high) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
FNAC; histo in FNAC+, FU in some FNAC‐
FNAC (n, %): no details (436, 100%) Histological detail (n, %): no details (13, 3%). Histopathologist: NR Follow‐up (n, %): US, clinical exam (31/44 negative on CE‐US, 70%) FU schedule: NR FU duration: 6 to 16 months (median, 10 months) Reference blinding: NR Target condition Data: per pt Definition: nodal mets; Prevalence: 13/436 = 3% |
||
| Flow and timing | Index to histology interval: US and FNAC consecutive Index to FU interval: NR Exclusions: n = 24; definite signs of malignancy on B‐mode US | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test Ultrasound | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Sanki 2009.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; unclear (cites ethics approval for MSLT‐I and MSLT‐II trials, so likely prospective database at a minimum; text states that US findings were extracted from original reports, however, which implies retrospective) Country: Australia Data collection: Jan 2001 to Aug 2005 Inclusion criteria: SLNB; BT > 1 mm or < 1 mm with adverse histological features, such as Clark level IV to V invasion, ulceration, or high mitotic rate | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 716 Number primary lesions: NR Number LNBs/metastases: 871 LNBs Stage of disease: NR Mean age: NR; Median age: NR; Range: NR Male: NR (0%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
US: B‐mode US; linear array transducer with high‐resolution small‐parts probe
Machine: ATL Ultramark‐9 HDI with a linear array L10‐5 transducer (Advanced Technology Laboratories Australia Pty Ltd, New South Wales, Australia); Toshiba Aplio US System (Toshiba, Otawara‐Shi, Japan) with PLT‐1204AT probe (Toshiba)
Scan coverage: sites marked by nuclear medicine physician during LS
Contrast: N/A
FNAC: N/A Threshold: reclassification of original report as suspicious, or highly probable (e.g. increased vascular signature, rounding of the normal ovoid shape of the nodes, loss of normal hilar echoes, presence of focal low‐level subcapsular space echoes) # Number observers: NR Qualification (experience): nuclear medicine physician (NR) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical ‐ NR; other tests ‐ result of lymphoscintigraphy known |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): H&E (serial section); IHC (S100, HMB45); (716, 100%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): NR (100% (not ref standard for US)
FU schedule: NR FU duration: 13.5 months (mean, 18.4 months) Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 125/716 = 17% (144/871 LNBs = 17%) |
||
| Flow and timing | Index to histology interval: SLN performed within 24 hours of LS and US Index to FU interval: NR Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: 24 FNs on SLNB were reported; not broken down by US result | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sibon 2007.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (prospective database with prospective re‐interpretation of US images) Country: France Data collection: Jan 1999 to May 2005 Inclusion criteria: SLNB; BT > 1 mm or < 1 mm with adverse histological features, such as Clark level IV to V invasion, ulceration, or high mitotic rate | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 131 Number primary lesions: 132 Number LNBs/metastases: NR; 189 SLNs Stage of disease: NR Mean age: 56 years; Range: 17 to 92 years Male: 70 (53%) Primary lesion site: arms 18, 13.6%; legs 43, 33%; trunk 48, 32%; hands/feet 10, 8%; HN 18, 14% Breslow/Clark: mean BT 2.60 ± 2.91 mm; ≤ 1 mm 12, 9%; 1.01 to 2.00 mm 67, 51%; 2.01 to 4.00 mm 16, 27%; unknown 1, 1% Clark level: II 8, 6%; III 30, 23%; IV 88, 66%, V 7, 5%; unknown 1, 1% Ulceration: 37, 28% Other: regression 13, 10% | ||
| Index tests |
US: B‐mode; linear transducer
Machine: Power Vision 6000 (Toshiba Medical France SA, Puteaux, France)
Scan coverage: site of the excised primary melanoma scar and followed paths of the lymphatic vessels to lymph node area(s)
Contrast: N/A
FNAC: N/A Threshold: stringent criteria: circular/oval hypoechoic lymph node with Solbiati index < 1.5 and no hyperechoic hilum; non‐stringent criteria included presence of 1 or 2 of stringent criteria and/or 1 or 2 minor criteria (nodular hypoechoic focus within a lymph node with an irregular lymph node margin) # Number observers: unclear how many undertook the original examination but 1 radiologist reviewed all images Qualification (experience): radiologist (high) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical ‐ NR for original interpretation or for re‐interpretation; other tests ‐ radiologist reviewed original radiology reports and images |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): H&E (serial section); IHC (S‐100 and HMB45) for H&E negative only. Any size of tumour deposit was considered metastatic unless < 5 isolated tumour cells present (131, 100%). Histopathologist: NR
FNAC (n, %): N/A (‐)
Follow‐up (n, %): NR (NR)
FU schedule: NR FU duration: NR Reference blinding: re‐interpretaion blinded to patient outcomes # Target condition Data: per pt Definition: nodal mets; Prevalence: 35/133 = 26% |
||
| Flow and timing | Index to histology interval: US 24 hours before LS Index to FU interval: NR Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: US detected 1/24 micro‐metastases < 2 mm and 2/11 macro‐metastases ≥ 2 mm (both > 5 mm) identified on SLNB | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Singh 2008.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; unclear Country: Germany Data collection: NR Inclusion criteria: primary MM undergoing SLNB (all > 1 mm) | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 52 Number primary lesions: NR Number LNBs/metastases: 67 LNBs; 111 SLNs Stage of disease: all stage I or II Mean age: 55 years; Median age: 61 years; Range: 17 to 76 years Male: 36 (69%) Primary lesion site: extremities 23, 44%; trunk 16, 31%; HN 13, 25% Breslow/Clark: mean 3.46 mm, range 1.0 to 12.0 mm Ulceration: NR Other: NR | ||
| Index tests |
PET‐CT: helical, CT (CE, dual detector)
Machine: Biograph; Siemens Medical Solutions Inc., Hoffman Estates, Illinois, USA
Scan coverage: WB; base of skull to tip of toes in 3 parts
Contrast: Peritrast‐oral‐GI; Kohler Chemie GmbH, Alsbach, Germany
CT parameters: 130 kV, 40 mAs, 5 mm
FDG: 370 ± 40 MBq FDG through an anterior cubital vein
Breath hold: limited breath hold for CT and shallow breathing for PET
CT used for: attenuation correction based on re‐scaling of CT image; image fusion
Reconstruction: iterative (not further detailed) Threshold: any focal uptake more than background unless it was found to be a false positive focus (physiological accumulation or brown fat tissue) in fusion imaging # Number observers: 2 Qualification (experience): 2 experienced observers assessed FDG PET‐CT fusion imaging independently; also refers to team of radiologists and nuclear physicians (experienced) Diagnosis (single, consensus, etc.): consensus Info provided during test interpretation: clinical ‐ NR; other tests ‐ PET before LS |
||
| Target condition and reference standard(s) |
Histology (SLNB)
Histological detail (n, %): "the surgeons knew the FDG‐PET findings"; H&E with IHC only in H&E negative (52, 100%). Histopathologist: NR
FNAC (n, %): N/A
Follow‐up (n, %): N/A
FU schedule: N/A FU duration: N/A Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 14/52 = 27% (BT > 4 mm 7/12 = 58%; BT ≤ 4 mm 7/40 = 17%) |
||
| Flow and timing | Index to histology interval: PET before LS before SLNB Index to FU interval: NR Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: 2 TPs; both BT ≥ 4 mm; FPs < 4 mm | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Strobel 2007a.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (Prosp. database NR) Country: Switzerland Data collection: Jan 2005 to Jan 2006 Inclusion criteria: high‐risk melanoma (BT > 4 mm, or Clark level III or IV, or known resected metastases) and raised S‐100 (> 0.2 μg/L) undergoing follow‐up after primary treatment Excluded if FDG PET/ CT and S‐100B measurement > 2 weeks apart; treatment initiated between PET‐CT and tumour marker measurement; or systemic therapy before PET‐CT investigation | ||
| Patient characteristics and setting | Presentation: re‐staging (all patients followed up according to updated Swiss melanoma guidelines) Number patients: 47 Number primary lesions: 47 Number LNBs/metastases: NR Stage of disease: NR Mean age: 58.4 years; Range: 20 to 83 years Male: 20 (43%) Primary lesion site: NR Breslow/Clark: BT 1.02 to 15 mm; unknown in 9 Ulceration: NR Other: NR | ||
| Index tests |
PET‐CT: 2D PET, CT (CE, multi‐slice, helical)
Machine: Discovery LS or Discovery ST (GE Health Systems, Milwaukee, WI); integrated PET scanner (GE Advance Nxi, GE Health Systems, Milwaukee, WI) with a multi‐slice helical CT (LightSpeed Plus or Lightspeed 16; GE Health Systems, Milwaukee, WI)
Scan coverage: head to knees with scanning of lower legs for patients with primary tumours of the lower extremities
Contrast: oral CT contrast agent given 15 minutes before injection of 18F‐FDG
CT parameters: 140 kV, 40 mAs, 4.25 mm
FDG: 370 to 400 MBq
Breath hold: CT: breath holding in the normal expiratory position
CT used for: attenuation correction, fused
Reconstruction: standard iterative algorithm (OSEM) Threshold: FDG uptake clearly greater than background and established morphological CT criteria; if a focal FDG‐active lesion was detected, the exact anatomical localisation was determined on fused PET‐CT images. Lesions with 18F‐FDG uptake in physiological sites or benign variants (e.g. muscles, brown fatty tissue, pulmonary infiltrations) were determined as benign # Number observers: 2 Qualification (experience): nuclear radiology physicians (experienced) Diagnosis (single, consensus, etc.): consensus of 2 Info provided during test interpretation: clinical ‐ blinded to serum S‐100B; other tests ‐ blinded |
||
| Target condition and reference standard(s) |
Histology/cytology/imaging/FU
Histological detail (n, %): no details (29, 62%; 20 distant mets and 9 LN mets). Histopathologist: NR
FNAC (n, %): no details (4, 8.5%)
Follow‐up (n, %): MRI, PET‐CT follow‐up, clinical follow‐up (47, 100%)
FU schedule: follow‐up PET‐CT examinations 3 or 6 months later; no clinical suspicion of metastases arose > 6 months after the scan FU duration: minimum 6 months (range 6 to 18 months in all patients) Reference blinding: NR Target condition Data: per pt Definition: any (incl brain, subcut); Prevalence: 39/47 = 83%; included 9 regional LN metastases and 30 distant metastases, including 12 with lung metastases and 2 with brain metastases (not further documented) |
||
| Flow and timing |
Index to histology interval: NR Index to FU interval: 3 months Exclusions: n = 0 |
||
| Comparative | |||
| Notes |
Other result: reports characteristics of those with elevated S‐100 but no mets detected on imaging Two brain metastases detected on PET‐CT ‐ elevated FDG uptake compared with normal brain tissue or additional bleeding. Both confirmed on reference standard; method not documented; however both showed perifocal vasogenic oedema on CT |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
Strobel 2007b.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; prospective Country: Switzerland Data collection: Aug 2004 to Apr 2005 Inclusion criteria: high‐risk melanoma (BT > 4 mm, or Clark level III or IV, or known resected metastases) and raised S‐100 (> 0.2 μg/L) undergoing follow‐up after primary treatment Excluded if systemic therapy before PET‐CT investigation | ||
| Patient characteristics and setting | Presentation: unclear (NR; PET‐CT for depiction or exclusion of metastases) Number patients: 124 Number primary lesions: NR Number LNBs/metastases: NR Stage of disease: NR Mean age: 54.4 years; Range: 15 to 82 years Male: 59 (48%) Primary lesion site: NR Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
PET‐CT: CT (CE, multi‐slice, helical)
Machine: Discovery LS or Discovery ST (GE Health Systems, Milwaukee, WI)
Scan coverage: head to knees with scanning of lower legs for patients with primary tumours of lower extremities
Contrast: oral CT contrast agent given 15 minutes before injection of 18F‐FDG
CT parameters: 140 kV, 40 mAs, 4.25 mm
FDG: 350 to 400 MBq
Breath hold: CT: breath holding in normal expiratory position
CT used for: attenuation correction, fused
Reconstruction: standard iterative algorithm (OSEM) Threshold: results presented based on co‐registered PET‐CT alone and on PET‐CT with separate interpretation of CT component. Mets present if detected by 1 or both readers. FDG uptake clearly greater than background (plus established morphological CT criteria for separate CT interpretation); if a focal FDG‐active lesion was detected, the exact anatomical localisation was determined on fused PET‐CT images. Lesions with 18F‐FDG uptake in physiological sites or benign variants (e.g. muscles, brown fatty tissue, pulmonary infiltrations) were determined as benign. Semi‐quantitative analysis of FDG uptake in terms of SUVmax also conducted # Number observers: 2 Qualification (experience): nuclear radiology physicians (experienced (13 years and 7 years)) Diagnosis (single, consensus, etc.): consensus of 2 Info provided during test interpretation: clinical ‐ blinded to serum S‐100B; other tests ‐ blinded |
||
| Target condition and reference standard(s) |
Histology/cytology/imaging/FU
Histological detail (n, %): no details (20, 16.1%). Histopathologist: NR
FNAC (n, %): no details (21, 16.9%)
Follow‐up (n, %): MRI, PET‐CT follow‐up, clinical follow‐up (124, 100%, 18 D+ and 61 D‐ had status confirmed by PET‐CT or clinical FU; 4 D‐ had MRI to confirm absence of mets and 10/53 D+)
FU schedule: NR FU duration: minimum 6 months (range 6 to 18 months in all patients) Reference blinding: N/A # Target condition Data: per pt Definition: any (incl brain, subcut); Prevalence: 53/124 = 43% Metastases: documented only for FNs; 7 patients with metastases were missed by PET‐CT without a dedicated CT readout, including in the lungs (4), iliac LNs (1), or gluteal subcutaneous tissue (1) and the psoas muscle (1) |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 3; chemotherapy before PET‐CT | ||
| Comparative | |||
| Notes | Other result: text describes detection of brain metastases on initial PET‐CT; lesion confirmed by MRI 3 days later | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Unclear | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Unclear | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
van den Brekel 1998.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (prospective database NR) Country: Netherlands Data collection: Jan 1989 to May 1995 Inclusion criteria: HN MM with CT before neck dissection, including therapeutic and elective (i.e. negative on palpation). Also included primary and recurrent | ||
| Patient characteristics and setting | Presentation: mixed (interval between treatment of primary and neck dissection ranged from 0 to 8.8 years (mean 21 months)) Number patients: 26 Number primary lesions: 26 Number LNBs/metastases: NR Stage of disease: stage III (palpable LN) 18, 69%; stageI I and II 8, 31% Mean age: 54.5 years; Range: 55 to 83 years Male: 18 (69%) Primary lesion site: scalp 6, 23%; temporal 3, 12%; ear 4, 15%; anterior face 4, 15%; neck 1, 4%; shoulder 1, 4%; upper limb 1, 4%; nasal mucosa 1, 4%; unknown primary 5, 19% Breslow/Clark: BT 0.8 to 22 mm Ulceration: NR Other: NR | ||
| Index tests |
CT: CE
Machine: NR
Scan coverage: neck
Contrast: IV bolus plus drip infusion of iodine contrast
CT parameters: NR; 5 mm for 24 pts; 2 mm for 2 pts (both FN)
Breath hold: NR Threshold: presence of necrosis or axial diameter > 10 or > 11 mm # Number observers: 2 Qualification (experience): NR; co‐authors (NR) Diagnosis (single, consensus, etc.): unclear Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (LND)
Histological detail (n, %): no details (26, 100%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): N/A (0)
FU schedule: N/A
Reference blinding: CT scored blinded to histopathological outcome; NR for record review # Target condition Data: per pt Definition: nodal (neck); Prevalence: 21/26 = 81% |
||
| Flow and timing | Index to histology interval: 4 weeks Index to FU interval: N/A Exclusions: n = 0 | ||
| Comparative | |||
| Notes | Other result: both FNs on CT were with 8‐mm CT slice thickness | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
van Rijk 2006.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; retrospective (prospective database NR) Country: Netherlands Data collection: Nov 2000 to Dec 2004 Inclusion criteria: SLNB candidates; cutaneous MM BT > 1 mm or Clark ≥ level IV | ||
| Patient characteristics and setting |
Presentation: primary (pre‐SLNB)
Number patients: 107
Number primary lesions: 107
Number LNBs/metastases: NR; 37 with metastases in 42 LNBs
Stage of disease: NR
Mean age: 50 years; Median age: NR; Range: 15 to 52 years
Male: 57 (53%)
Primary lesion site: HN 6, 6%; trunk 43, 40%; arm 24, 22%; leg 34, 32%
Breslow/Clark: median BT 2.0 mm (0.6 to 12.5 mm) Clark level: II 1, 1%; III 37, 35%; IV 55, 51%; V 9, 8%; undeterminable 5, 5% Ulceration: 32, 30% |
||
| Index tests |
US and US plus FNAC: B‐mode linear array
Machine: Siemens Elegra (Erlangen, Germany) or a Kretz Voluson 730 Expert (GE Medical Systems, Zipf, Austria)
Scan coverage: NR
Contrast: N/A
FNAC: US positive (suspicious) underwent FNAC 21‐ or 22‐gauge needle (Figure 1), aspirated material air dried, methanol fixated and stained (May‐Grunwald‐Giemsa). FNAC+ underwent CLND Threshold: US alone suspicious ‐ length–depth ratio < 2, conversion of a fatty hilum to a hypoechoic hilum, substantial cortical asymmetry or focal area of low‐level echoes in the subcapsular sinus of the node, and diameter > 5 mm for LN of the neck. US + FNAC ‐ US positive and metastases on FNAC # Number observers: NR Qualification (experience): NR (NR) Diagnosis (single, consensus, etc.): NR Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (SLNB; CLND)
Histological detail (n, %): CLND not described; SLNB H&E (minimum 6 levels); IHC (S100, HMB45). Metastases were classified as > 2 mm in diameter or < 2 mm, as 2 mm is the current spatial resolution of ultrasonography according to Rossi et al (107, 100%). Histopathologist: NR
FNAC (n, %): N/A (22 but not as part of reference standard)
Follow‐up (n, %): NR (2/107; 2% (reported only for 2 positive on FNAC))
FU schedule: NR FU duration: NR Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 37/107 = 35% |
||
| Flow and timing | Index to histology interval: 1 to several days Index to FU interval: N/A Exclusions: n = 0 | ||
| Comparative | |||
| Notes |
Other result: FU of 2 FNAC positive participants is reported but no further reference to any recurrences. A breakdown of micro‐ vs macro‐metastases is also reported for those positive on histology. Of the 12 TPs on ultrasound, 7 (58%) were macro‐metastases and 5 (42%) were micro‐metastases; of the 25 FNs on US, 8 (32%) were macro‐metastases and 17 (68%) micro‐metastases The single patient who was TP on US & FNAC had macro‐metastasis; of the 36 who were FN on US & FNAC, 14 (39%) were macro‐metastases and 22 (61%) were micro‐metastases |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
van Wissen 2016.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective Country: Netherlands Data collection: 2003 to 2013 Inclusion criteria: stage IIIB or IIIC MM with palpable groin metastases; selected for therapeutic combined groin dissection (CGD) | ||
| Patient characteristics and setting |
Presentation: mixed (discussion states: "large proportion of our patients were initially treated for their primary tumour at other hospitals, and sometimes years prior to the current groin dissection")
Number patients: 70
Number primary lesions: 70
Number LNBs/metastases: NR
Stage of disease: only stage III B & C
Mean age: NR; Median age: 58 years; Range: 24 to 83 years
Male: 35 (50%) Primary lesion site: leg 58, 83%; trunk 6, 9%; arm 0, 0%; unknown 6, 9% Breslow/Clark: BT, mm: ≤ 1.00 6 (9%); ≤ 2.00 15 (21%); 2.01 to 4.00 15 (21%); > 4.00 12 (17%); missing/unknown 22 (31%) Ulceration: yes 11 (16%); missing/unknown 40 (57%) Other: extracapsular invasion 14 (19%) |
||
| Index tests |
PET‐CT: CT (U)
Machine: Gemini II; Philips, Eindhoven, The Netherlands
Scan coverage: WB; not further described
Contrast: none
CT parameters: Kv NR, 40 mAs, 2 to 5 MM
FDG: 180 to 240 MBq
Breath hold: standard acquisition protocols
CT used for: attenuation correction; fused images
Reconstruction: NR Threshold: FDG uptake (qualitative assessment); indeterminate on PET‐CT considered negative by study authors but have been extracted as both test positive and test negative for this review # Number observers: 1 Qualification (experience): nuclear medicine (NR) Diagnosis (single, consensus, etc.): single Info provided during test interpretation: clinical ‐ NR; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (CGD)
Histological detail (n, %): no details (70, 100%). Histopathologist: originally different pathologists; reports reviewed by single expert pathologist for study purposes
FNAC (n, %): N/A
Follow‐up (n, %): NR (not for ref purposes)
FU schedule: NR FU duration: median 16 months (0 to 71 months) Reference blinding: NR # Target condition Data: per pt Definition: nodal (superficial groin mets only); Prevalence: 59/69 = 86% Definition: nodal (deep groin mets only); Prevalence: 24/67 = 36% |
||
| Flow and timing | Index to histology interval: NR Index to FU interval: NR Exclusions: n = 4; missing pathology ‐ 1 excluded from superficial LN analysis and 3 from deep node analysis | ||
| Comparative | |||
| Notes | Other result: 7/10 disease negative had diagnostic resection of a lymph node before lymph node dissection. PET‐CT is likely to have shown inflammation after this resection rather than residual disease in the groin Also reports 30‐day complications and DFS and OS according to pathology positive/negative iliac nodal status | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | No | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Veit‐Haibach 2009.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison; prospective Country: Germany Data collection: NR Inclusion criteria: any primary MM referred for PET‐CT Excluded if insufficient FU | ||
| Patient characteristics and setting | Presentation: primary (any); any primary MM referred for PET‐CT Number patients: 56 Number primary lesions: 56 Number LNBs/metastases: NR Stage of disease: presentation stage I or II 44, 79%; stage III or IV 12, 21% Mean age: 62 years; Median age: NR; Range: 23 to 86 years Male: 27 (48.2%) Primary lesion site: trunk 26, 46%; upper extremities 10, 18%; lower extremity 18, 32%; HN 2, 4% Breslow/Clark: NR Ulceration: NR Other: NR | ||
| Index tests |
CT: CE; 2‐slice
Machine: Biograph Duo PET/CT System (Siemens Molecular Imaging, Hoffman Estates, IL); integrates a dual‐slice CT scanner (Somatom Emotion, Siemens Medical Solutions, Forchheim, Germany) and a full‐ring, BGO‐based PET Tomograph (Siemens Molecular Imaging)
Scan coverage: WB; no further detail, just states caudocranial direction
Contrast: dual‐phase injection of 140 mL of 300 mmol/mL iodinated contrast agent (90 mL at a rate of 3 mL/s, and 50 mL at a rate of 1.5 mL/s; dual‐phase used to ensure fully diagnostic (portal venous phase) CT data in the abdomen)
CT parameters: NR
Breath hold: NR Threshold: nodal mets ‐ lesion size and central necrosis for malignancy; fatty hilum and calcifications for benign. For size: short‐axis diameter threshold of 1.5 cm for jugulodigastric and pre‐carinal LNs and threshold of 1 cm for all other LNs of the neck, thorax, and abdomen. Distant mets ‐ detection of soft tissue masses (or focal cutaneous thickening) with contrast enhancement # PET‐CT: full‐ring CT (CE; 2‐slice) Machine: Biograph Duo PET/CT System (Siemens Molecular Imaging, Hoffman Estates, IL); integrates a dual‐slice CT scanner (Somatom Emotion, Siemens Medical Solutions, Forchheim, Germany) and a full‐ring, BGO‐based PET tomograph (Siemens Molecular Imaging) Scan coverage: WB; no further detail, just states caudocranial direction Contrast: 140 mL of 300 mmol/mL iodinated contrast agent CT parameters: NR FDG: 330 to 350 MBq Breath hold: NR CT used for: attenuation correction Reconstruction: reconstructed iteratively (FORE‐OSEM, 2 iterations, 8 subsets, 128×128 matrix with 5‐mm gaussian smoothing) Threshold: nodal mets ‐ increased glucose metabolism and independent of size. Diatant mets ‐ qualitative + SUV; detection of soft tissue masses (or focal cutaneous thickening) with contrast enhancement in different body compartments and in conjunction with focally increased glucose metabolism above the surrounding tissue level on FDG PET/ CT; supported by SUVmax ≥ 1.5 for cutaneous lesions, ≥ 2.5 for other extrahepatic lesions, and ≥ 3.5 for intrahepatic lesions # Number observers: 2 Qualification (experience): radiologists and and nuclear medicine specialist for PET‐CT (NR) Diagnosis (single, consensus, etc.): consensus of 2 Info provided during test interpretation: clinical ‐ provided patient‐specific clinical background (first diagnosis of melanoma, postsurgical resection status, location of resection site) but blinded to clinical exam and histopathology of primary tumour; other tests ‐ blinded to other imaging procedures |
||
| Target condition and reference standard(s) |
Histology/FU
Histological detail (n, %): all patients with suspected metastases on imaging, histopathological evaluation of at least 1 metastatic site served as the standard of reference for both N‐stage and M‐stage during the clinical course. Total of 14 patients had SLNB within 4 weeks of the initial PET‐CT procedure (unclear; 14 with SLNB, 25%). Histopathologist: NR
FNAC (n, %): N/A (0)
Follow‐up (n, %): imaging, tumour markers, physical examination (56, 100%)
FU schedule: NR FU duration: mean 780 days (range 102 to 1390 days); roughly equivalent to 25.6 months (3.3 to 45.7 months) Reference blinding: N/A # Target condition Data: per pt Definition: nodal; Prevalence: 13/56 = 23% Definition: distant; Prevalence: 12/56 = 21% (no breakdown by anatomical site) Metastases: 12 patients with nodal and/or distant mets reported as detected on initial staging; 4 patients with nodal mets (stage III) and 8 with distant (stage IV). PET‐CT correctly classified 6/12 and CT correctly classified 3/12. A further 6 patients had metastases detected on follow‐up for a total of 18 patients with any metastases Of the 8 FNs on PET‐CT and 10 FNs on CT alone, 2 were micro‐metastases identified by SLNB |
||
| Flow and timing | Index to histology interval: 4 weeks for SLNB Index to FU interval: NR Exclusions: n = 0 | ||
| Comparative | (1) Blinded to other imaging procedures (2) Same scanner (3) All referred for PET‐CT |
||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | No | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | Unclear | ||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | No | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
| DOMAIN 5: Comparative | |||
| 1) was each index test result interpreted without knowledge of the results of other index tests or testing strategies? | Yes | ||
| 2) Was the interval between application of index tests | Yes | ||
| 3) Was it predetermined that all index tests should be given to all study participants? | Yes | ||
| Low | |||
Voit 2014.
| Study characteristics | |||
| Patient sampling | Design: within‐person comparison (US vs US + FNAC); unclear ('prospective database') Country: Germany Data collection: July 2001 to Nov 2010 Inclusion criteria: SLNB candidates; BT > 1 mm thickness or Clark IV/V, ulcerated, and/or regressed | ||
| Patient characteristics and setting |
Presentation: primary (pre‐SLNB)
Number patients: 1000
Number primary lesions: 1000
Number LNBs/metastases: NR
Stage of disease: NR
Mean age: 59 years; Median age: 62 years; Range: 15 to 94 years
Male: 567 (57%)
Primary lesion site: NR
Breslow/Clark: mean BT 2.58 mm; median BT 1.57 mm. BT < 1 mm 288 29%; 1 to 2 mm 308 31%; 2 to 4mm 231 23%; > 4 mm 173 17% Clark level: II 32, 3%; III 341, 34%; IV 554, 56%; V 54, 6%, unknown 13, 1% Ulceration: 242, 24% Other: regression absent 633, 63%; present 300, 30%, unknown 67, 7% |
||
| Index tests |
US and US + FNAC. B mode & Doppler
Machine: NR
Scan coverage: LNBs; patients first underwent a lymphoscintigraphy, which assists the ultrasonographist to better focus their examination
Contrast: N/A FNAC: US positive underwent FNAC with 26 gauge needle; smears considered adequate if around 100 cells present. Cyto results reported to the surgeon, who decided whether to proceed with SLNB or direct to LND Threshold: malignant on US if total loss of central echoes (LCE) or LN enlarged and balloon shaped (BS); suspicious if peripheral perfusion present or central echo wandering towards the rim. NR for FNAC # Number observers: 3 Qualification (experience): ultrasonographist (mixed; 1 expert and 2 trained but less expert) Diagnosis (single, consensus, etc.): unclear; likely single Info provided during test interpretation: clinical ‐ NR; other tests ‐ LS result available |
||
| Target condition and reference standard(s) |
Histology (SLNB or CLND)
Histological detail (n, %): H&E (serial); IHC (S100, HMB45); microanatomical location of metastases and SN tumour burden were assessed according to Dewar and Rotterdam criteria, respectively (1). Histopathologist: NR
FNAC (n, %): not as reference
Follow‐up (n, %): no details (1000; 100%)
FU schedule: NR FU duration: mean 56 m; median 53 m; range 1 to 132 m Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 208/1000 = 21% |
||
| Flow and timing | Index to histology interval: preoperative Index to FU interval: NR Exclusions: n = 0 | ||
| Comparative | |||
| Notes |
Other result: 332 patients underwent FNAC; however authors report as 342 (including 10 US malignant as FNAC positive even though no FNAC was undertaken) There were 198 patients (20%) with recurrences and 81 melanoma‐related deaths (8%) during this follow‐up (not broken down by index test) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test Ultrasound (pre‐SLNB) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Unclear | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Yes | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wagner 2012.
| Study characteristics | |||
| Patient sampling | Design: non‐comparative; retrospective (Prospective database NR) Country: France Data collection: Sep 2003 ‐ Sep 2006 Inclusion criteria: SLNB candidates; BT ≥ 4 mm or BT > 1 mm with ulceration | ||
| Patient characteristics and setting | Presentation: primary (pre‐SLNB) Number patients: 48 Number primary lesions: 48 Number LNBs/Metastases: NR Stage of disease: stage IIA 8, 16.7%; stage IIB 19, 39.6%; stage IIC 19, 39.6%; stage NR 2, 4.2% (both BT > 4 mm) Mean age: NR; Median age: NR; Range: NR Male: 25 (52%) Primary lesion site: NR Breslow/Clark: mean BT 7.6 mm (±4.5) (range 1.1 to 18 mm) Ulceration: 19, 39.6%; NR 2, 4.1% Other: NR | ||
| Index tests |
PET‐CT. 2D; CT (NR)
Machine: Discovery ST; General Electric Healthcare, Waukesha, WI, USA)
Scan coverage: WB; not further described
Contrast: NR
CT parameters: 140 kV, 200 mA, 7.5 mm
FDG: 370 MBq (Glucotep Cyclopharma, St Beauzire, France)
Breath hold: normal breathing; "remain rested, to refrain from speaking, and to minimize swallowing"
CT used for: attenuation correction and anatomical correlation
Reconstruction: iterative OSEM (Ordered Subset Expectation Maximisation) algorithm (3 iterations; 10 subsets) Threshold: abnormally increased FDG uptake in a lymph node in the drainage territory of the melanoma # Number observers: NR Qualification (experience): nuclear medicine specialist (high) Diagnosis (single, consensus, etc.): unclear; 'at least one' Info provided during test interpretation: clinical ‐ aware of all clinical findings; other tests ‐ NR |
||
| Target condition and reference standard(s) |
Histology (SLNB, CLND)
Histological detail (n, %): NR; describes tumoural deposit < 200 Um (n = 4), > 200 Um (n = 5), and perinodal tumoural deposit (n = 1) (43, 89.6%; 2 CLND only, 1 SLNB + CLND, 40 SLNB only). Histopathologist: NR
FNAC (n, %): N/A
Follow‐up (n, %): FU reported but only for possible distant mets and not nodal
FU schedule: NR FU duration: minimum 12 months Reference blinding: NR # Target condition Data: per pt Definition: nodal mets; Prevalence: 14/43 = 33% |
||
| Flow and timing | Index to histology interval: before SLNB Index to FU interval: NR Exclusions: n = 5; SLNB not performed for technical reasons | ||
| Comparative | |||
| Notes | Other result: result presented for detection of distant mets but only 1 D+ so does not meet sample size restrictions (2×2 0, 6, 1, 41) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Does the study report results for participants at the same point in the clinical pathway and who would be eligible for imaging in normal practice? | Yes | ||
| Did the study report data on a per patient rather than per lesion basis? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test PET‐CT | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was the imaging test applied and interpreted in a clinically applicable manner? | Yes | ||
| Were thresholds or criteria for diagnosis reported in sufficient detail to allow replication? | Unclear | ||
| Was the test interpreted by an experienced examiner? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Were the reference standard results based on patient follow‐up interpreted without knowledge of the original imaging test result? | |||
| Does the study use the same definition of disease positive as the primary review question (i.e. any mets) OR is it possible to disaggregate or regroup data such that data matching the review question can be extracted? | Yes | ||
| Was histology or cytology interpretation carried out by an experienced histopathologist or by a dermatopathologist? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
2D: two‐dimensional; 3D: three‐dimensional; AJCC: American Joint Committee on Cancer; AWOSEM: attenuation weighted ordered subsets expectation maximization: BT: Breslow thickness; CE: contrast enhanced; CLND: completion lymphadenectomy; CMM: cutaneous malignant melanoma; CT: computed tomography; DFS: disease‐free survival; DW: diffusion weighted; EDV: end‐diastolic volume; EORTC: European Organisation for Research and Treatment of Cancer; FDG: fluorodeoxyglucose; FFE: fast field echo; FLAIR: fluid attenuated inversion recovery; FN: false negative; FNAC: fine needle aspiration cytology; FORE: Fourier rebinned; FP: false positive; FU: follow‐up; FWHM: full‐width half maximum; H&N: head and neck; HD: high definition; HN: head and neck; IHC: immunohistochemistry; ITM: in‐transit metastases; LN: lymph node; LNB: lymph node biopsy; LND: lymphadenectomy; MM: malignant melanoma; MRI: magnetic resonance imaging; N/A: not applicable; NR: not reported; OS: overall survival; OSEM: ordered subsets expectation‐maximization; PD: Power Doppler; PET‐CT: positron emission tomography‐computed tomography; PI: pulse index; PSV: peak systolic volume; RF: risk factors; SLNB: sentinel lymph node biopsy; SPECT: single‐photon emission computed tomography; SSM: superficial spreading melanoma; SUVmax: maximum standardised uptake volume; T2STIR: T2‐weighted short tau inversion recovery; TNM: tumour node metastasis; TP: true positive; UICC: Union for International Cancer Control; US: ultrasound; WB: whole body.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2009 | Conference abstract |
| Abdi 1988 | Inadequate reference standard |
| Abella‐Columna 2002 | Not a primary study |
| Acland 2000 | Wrong index test |
| Acland 2001 | Wrong index test |
| Agarwal 2008 | Not a primary study |
| Ahmed 2015 | Conference abstract |
| Akcali 2007 | Inadequate reference standard |
| Aldridge 2010 | Conference abstract |
| Aloia 2006 | Inadequate sample size; wrong index test; inadequate reference standard |
| Alvarado 2007 | Not a primary study |
| Angeles 2014 | Conference abstract |
| Ardizzoni 1987 | Inadequate sample size; wrong index test |
| Arrangoiz 2011 | Conference abstract |
| Ashour 2016 | Conference abstract |
| Bafounta 2004 | Systematic review |
| Baker 2011 | Conference abstract |
| Baker 2012 | Conference abstract |
| Baker 2014 | Multiple scans reported per participant |
| Balagula 2012 | Wrong index test |
| Ban 2013 | Conference abstract |
| Barsky 2014 | Inadequate reference standard |
| Bastiaannet 2006 | Not test accuracy |
| Bastiaannet 2008 | Conference abstract |
| Bastiaannet 2008a | Conference abstract |
| Bastiaannet 2008b | Conference abstract |
| Bastiaannet 2009a | Conference abstract |
| Bastiaannet 2010 | Conference abstract |
| Bastiaannet 2012 | Duplicate or related publication |
| Beasley 2010 | Conference abstract |
| Beasley 2012 | Wrong study population |
| Beitollahi 2013 | Conference abstract |
| Belhocine 2002 | Inadequate sample size; wrong index test |
| Ben Lakhdar 2011 | Conference abstract |
| Bernabo 2015 | Conference abstract; wrong index test |
| Beyeler 2006 | Wrong study population |
| Bhatia 2012 | Wrong study population |
| Bier 2016 | Inadequate reference standard |
| Biersack 1987 | Wrong target condition; wrong index test |
| Bikhchandani 2014 | Wrong index test |
| Binder 1997 | Multiple scans reported per participant |
| Binns 2012 | Conference abstract |
| Blend 1992 | Wrong index test |
| Blessing 1995 | Wrong index test |
| Blum 2000 | Multiple scans reported per participant; exclusion on 2×2 |
| Blum 2006 | Wrong study population; wrong target condition |
| Bode 2011 | Conference abstract; wrong index test |
| Bohelay 2014 | Conference abstract |
| Bohuslavizki 2000 | Wrong index test; inadequate reference standard |
| Boni 1995 | Wrong index test; inadequate reference standard |
| Boni 1996 | Not a primary study |
| Boni 1996a | Inadequate sample size |
| Borrego 2006 | Wrong index test |
| Boy 2011 | Conference abstract; wrong index test |
| Brady 2006 | Wrong study population |
| Breitenbauch 2015 | Inadequate sample size; inadequate reference standard |
| Brenner 1999 | Wrong index test |
| Bronstein 2012 | Wrong study population |
| Brountzos 2003 | Multiple scans reported per participant |
| Buckle 2016 | Wrong target condition; wrong index test |
| Bude 2004 | Not a primary study |
| Buerke 2011 | Wrong target population; inadequate reference standard |
| Buzaid 1993 | Inadequate reference standard |
| Buzaid 1995 | Inadequate reference standard; exclusion on 2×2 |
| Bydder 1981 | Inadequate reference standard |
| Cachin 2012 | Conference abstract |
| Catalano 2010 | Not a primary study |
| Catalano 2010a | Not a primary study |
| Catalano 2010b | Not a primary study |
| Catalano 2011 | Systematic review |
| Catalano 2015 | Wrong index test |
| Chai 2010 | Conference abstract |
| Cho 2005 | Inadequate reference standard |
| Chomyn 1992 | Inadequate reference standard |
| Clark 2006 | Wrong index test |
| Clement 1998 | Wrong target condition |
| Clement 2001 | Wrong target condition |
| Clemente‐Ruiz 2012 | Wrong target condition; wrong index test |
| Cobben 2003 | Wrong index test |
| Connell 2003 | Not a primary study |
| Constantinidou 2008 | Wrong index test |
| Cordova 2006 | Wrong index test |
| Cousen 2014 | Inadequate reference standard |
| Crippa 2000 | Wrong index test |
| Curtis 1982 | Inadequate sample size; inadequate reference standard |
| Dalle 2006 | Not test accuracy; wrong study population |
| Damian 1996 | Wrong index test |
| Danielsen 2013 | Systematic review |
| Davidson 2011 | Conference abstract |
| Davis 1991 | Inadequate sample size |
| De Giorgi 2010 | Wrong index test |
| De Rosa 2010 | Not test accuracy; inadequate reference standard |
| DeRose 2010 | Conference abstract |
| Dietlein 1999 | Wrong target condition; wrong index test |
| Diodato 2015 | Conference abstract |
| Doiron 1981 | Wrong index test; inadequate reference standard |
| Dresel 2003 | Wrong study population |
| Drzezga 2012 | Inadequate sample size |
| Eigtved 2000 | Wrong index test |
| El‐Maraghi 2008 | Not a primary study; systematic review |
| Emmett 2012 | Not a primary study |
| Facius 2002 | Wrong index test |
| Fakhry 2009 | Inadequate sample size; wrong index test |
| Falk 2007 | Multiple scans reported per participant |
| Faries 2010 | Wrong index test |
| Ferrandiz 2016 | Not test accuracy; inadequate reference standard |
| Fink 2004 | Wrong index test |
| Finkelstein 2004 | Wrong index test |
| Fletcher 2008 | Not a primary study; systematic review |
| Fogarty 2006 | Inadequate reference standard |
| Fohne 2015 | Conference abstract |
| Friedman 2004 | Not a primary study; systematic review |
| Fuster 2003 | Conference abstract |
| Fuster 2004 | Wrong study population |
| Garbe 2003 | Not test accuracy |
| Gellen 2015 | Multiple scans reported per participant |
| Ghanem 2005 | Inadequate reference standard |
| Giles 2014 | Conference abstract; wrong index test |
| Ginaldi 1981 | Inadequate reference standard |
| Giovagnorio 2003 | Wrong target condition |
| Gold 2007 | Wrong target condition; wrong index test |
| Grigolato 2011 | Conference abstract |
| Gritters 1993 | Wrong index test |
| Gulec 2003 | Inadequate reference standard |
| Gupta 2012 | Conference abstract; inadequate sample size |
| Haddad 2013 | Conference abstract |
| Haddad 2013a | Inadequate sample size |
| Hall 2013 | Not a primary study; systematic review |
| Harlan 2010 | Inadequate sample size |
| Harris 2005 | Wrong index test |
| Havenga 2003 | Wrong index test |
| Heaston 1983 | Inadequate reference standard |
| Herceg 2012 | Conference abstract |
| Herceg 2013 | Conference abstract |
| Herceg 2014 | Conference abstract |
| Herceg 2015 | Conference abstract |
| Heusner 2011 | Inadequate sample size |
| Hinz 2010 | Inadequate sample size |
| Ho Shon 2008 | Not a primary study |
| Hofmann 2002 | Multiple scans reported per participant; inadequate reference standard |
| Hofmann 2011 | Wrong index test |
| Hoh 1993 | Wrong target condition |
| Holder 1998 | Wrong index test; inadequate reference standard |
| Holtas 1981 | Inadequate reference standard |
| Horn 2006 | Inadequate sample size; wrong index test; inadequate reference standard |
| Horn 2010 | Wrong index test |
| Hu 2009 | Inadequate reference standard |
| Hughes 2013 | Not test accuracy |
| Hunyadi 2002 | Wrong index test; inadequate reference standard |
| Iscoe 1987 | Inadequate sample size |
| Ismaheel 2016 | Inadequate reference standard |
| Jackson 2014 | Not test accuracy |
| Jadvar 2000 | Wrong index test; inadequate reference standard |
| Jenicke 2001 | Wrong index test; inadequate reference standard |
| Jennings 2009 | Not a primary study |
| Jimenez‐Requena 2010 | Not a primary study; systematic review |
| Johnson 1997 | Inadequate reference standard; exclusion on 2×2 |
| Jones 2014 | Conference abstract |
| Kader 2016 | Wrong study population; wrong index test |
| Kelly 2013 | Not a primary study |
| Knappe 2000 | Wrong study population |
| Koskivuo 2007 | Wrong index test |
| Krug 2000 | Wrong index test; inadequate reference standard |
| Krug 2008 | Systematic review |
| Krug 2009 | Conference abstract; not a primary study |
| Krug 2010 | Not a primary study |
| Kuvshinoff 1997 | Inadequate reference standard |
| Lanka 2005 | Wrong study population |
| Laurent 2010 | Duplicate or related publication (see Dellestable 2011) |
| Leon‐Ferre 2015 | Conference abstract |
| Lewin 2015 | Conference abstract |
| Liszkay 2010 | Conference abstract |
| Loffler 2003 | Inadequate reference standard |
| Longo 2003 | Wrong index test; exclusion on 2×2 |
| Loose 1990 | Multiple scans reported per participant |
| Macfarlane 1998 | Wrong index test |
| Machet 2005 | Multiple scans reported per participant |
| Majchrzak 2013 | Inadequate sample size |
| Mayerhoefer 2012 | Wrong study population |
| McIvor 2014 | Wrong index test |
| McNamara 2005 | Conference abstract; wrong index test |
| Medina‐Quiroz 2010 | Conference abstract |
| Mendenhall 2012 | Not a primary study |
| Mercier 2001 | Wrong index test |
| Meyers 2009 | Inadequate reference standard |
| Meyers 2009a | Wrong index test |
| Mijnhout 2001 | Systematic review |
| Mijnhout 2002 | Wrong index test |
| Miner 2011 | Conference abstract |
| Minn 2011 | Not a primary study |
| Miranda 2004 | Inadequate sample size |
| Miranda 2006 | Not a primary study |
| Mocellin 2007 | Not a primary study; systematic review |
| Moehrle 1999 | Wrong study population |
| Morton 2007 | Not a primary study |
| Mosavi 2013 | Inadequate reference standard |
| Mottaghy 2007 | Exclusion on 2×2 data |
| Mozzillo 2013 | Wrong study population; wrong index test |
| Mruck 1999 | Wrong index test |
| Muller 2006 | Not a primary study |
| Muller‐Horvat 2006 | Wrong study population |
| Nazarian 1996 | Inadequate reference standard |
| Nazarian 1998 | Wrong study population |
| Niebling 2013a | Conference abstract |
| Niebling 2013b | Wrong index test; duplicate or related publication |
| Niederkohr 2007 | Inadequate reference standard |
| Novikov 2012 | Wrong index test |
| Oehr 1999 | Wrong index test |
| Ogata 2014 | Inadequate sample size |
| Omlor 1996 | Multiple scans reported per participant |
| Orfaniotis 2012 | Multiple scans reported per participant; inadequate reference standard |
| Ortega‐Candil 2016 | Not test accuracy; exclusion on 2×2 |
| Padovano 2013 | Conference abstract |
| Panagiotou 2001 | Wrong index test |
| Pandalai 2011 | Inadequate reference standard |
| Paquet 2000 | Wrong index test |
| Pecegueiro 2005 | Wrong index test; inadequate reference standard |
| Pellacani 2006 | Not test accuracy |
| Peric 2011 | Inadequate reference standard |
| Petersen 2016 | Systematic review |
| Pleiss 2007 | Wrong index test; inadequate reference standard |
| Poduje 2012 | Inadequate sample size |
| Poyraz 2012 | Inadequate reference standard |
| Prakoso 2007 | Wrong index test |
| Prakoso 2011 | Inadequate reference standard |
| Prichard 2002 | Systematic review |
| Punjabi 2006 | Not a primary study |
| Querellou 2010 | Multiple scans reported per participant |
| Querellou 2011 | Not a primary study |
| Ramirez 2015 | Conference abstract |
| Renna 2015 | Conference abstract |
| Rep 2011 | Conference abstract; wrong index test |
| Rinne 1998 | Wrong index test |
| Roarke 2008 | Inadequate sample size |
| Roh 2008 | Wrong study population; wrong index test |
| Rossi 1997 | Wrong target condition; inadequate sample size |
| Rossi 1999 | Conference abstract |
| Rossi 2000 | Duplicate or related publication |
| Rossi 2003 | Exclusion on 2×2 data |
| Rossi 2008 | Not a primary study |
| Rudolph 2010 | Conference abstract |
| Sadigh 2014 | Systematic review |
| Saiag 2005 | Multiple scans reported per participant |
| Saiag 2010 | Conference abstract |
| Samimi 2010 | Wrong study population; wrong target condition |
| Samples 2012 | Conference abstract |
| Sanli 2010 | Conference abstract |
| Santha 2011 | Conference abstract |
| Sarandi 2008 | Not a primary study |
| Sawyer 2009 | Multiple scans reported per participant |
| Schafer‐Hesterberg 2007 | Not a primary study |
| Schafer‐Hesterberg 2008 | Not a primary study |
| Schauwecker 2003 | Wrong index test |
| Scheier 2015 | Conference abstract |
| Scheier 2016 | Inadequate reference standard |
| Schmid‐Wendtner 2002 | Inadequate reference standard |
| Schmid‐Wendtner 2003 | Multiple scans reported per participant |
| Schmid‐Wendtner 2004 | Inadequate reference standard |
| Schule 2016 | Inadequate reference standard |
| Schwimmer 2000 | Systematic review |
| Sergieva 2012 | Conference abstract |
| Serra‐Arbeloa 2015 | Conference abstract; systematic review |
| Seshadri 2006 | Inadequate sample size; wrong index test |
| Shah 2015 | Conference abstract |
| Shintani 2008 | Inadequate sample size |
| Sigmund 1985 | Wrong study population |
| Sijan 2010 | Wrong study population |
| Singnurkar 2016 | Inadequate reference standard |
| Smith 2011 | Conference abstract |
| Sofue 2012 | Wrong study population |
| Soler 1997 | Wrong index test |
| Solivetti 2006 | Wrong target condition; inadequate reference standard |
| Solivetti 2012 | Not test accuracy |
| Solivetti 2014 | Wrong study population; wrong target condition |
| Solomon 2004 | Wrong study population; wrong target condition; wrong index test |
| Son 2016 | Inadequate sample size |
| Srivastava 2012 | Wrong index test |
| Starritt 2005 | Inadequate sample size |
| Stas 2002 | Wrong index test |
| Stecco 2016 | Wrong study population |
| Steinert 1998 | Wrong index test; inadequate reference standard |
| Stoffels 2012 | Exclusion on 2×2 data |
| Stoffels 2014 | Wrong index test; inadequate reference standard |
| Stoffels 2016 | Conference abstract; wrong target condition |
| Stretch 2005 | Wrong index test |
| Stucker 2002 | Wrong study population; wrong index test |
| Subesinghe 2012 | Conference abstract |
| Subesinghe 2013 | Wrong study population; multiple scans reported per participant |
| Supriya 2014 | Wrong study population |
| Swetter 2002 | Multiple scans reported per participant |
| Tejera‐Vaquerizo 2007 | Wrong index test |
| Testori 2005 | Exclusion on 2×2 data |
| Thompson 2002 | Not a primary study |
| Thompson 2011 | Conference abstract |
| Tomaszewski 2014 | Wrong study population |
| Tregnaghi 1997 | Multiple scans reported per participant |
| Tyler 2000 | Wrong index test |
| Ulrich 2015 | Conference abstract |
| Uren 1999 | Inadequate reference standard |
| Valdes 2011 | Wrong index test |
| Valk 1996 | Not a primary study |
| Van Akkooi 2012 | Conference abstract |
| Van Akkooi 2013 | Conference abstract |
| Van Akkooi 2014 | Conference abstract |
| Van Akkooi 2015 | Conference abstract |
| Van den Broucke 2010 | Conference abstract |
| Van der Ploeg 2007 | Wrong index test |
| Van der Ploeg 2009a | Wrong index test |
| Van der Ploeg 2009b | Wrong index test |
| Van der Ploeg 2011 | Wrong study population |
| Vereecken 2005 | Wrong index test |
| Vidal‐Sicart 2010 | Conference abstract |
| Voit 1999 | Wrong index test; multiple scans reported per participant |
| Voit 2000 | Wrong index test |
| Voit 2001 | Wrong index test |
| Voit 2005 | Conference abstract |
| Voit 2006 | Conference abstract; duplicate or related publication |
| Voit 2009a | Conference abstract; duplicate or related publication |
| Voit 2009b | Conference abstract (overlaps Voit 2014) |
| Voit 2009c | Conference abstract |
| Voit 2010a | Not a primary study |
| Voit 2010b | Not a primary study |
| Voit 2010c | Duplicate or related publication |
| Voit 2010d | Conference abstract; duplicate or related publication |
| Voit 2011a | Conference abstract |
| Voit 2011b | Wrong index test |
| Voit 2011c | Conference abstract |
| Voit 2013 | Conference abstract |
| Voit 2016 | Wrong index test; duplicate or related publication (overlaps Voit 2014) |
| Von Schulthess 1998 | Wrong index test |
| Wagner 1997 | Wrong index test |
| Wagner 1999 | Wrong index test |
| Wagner 2001 | Wrong index test |
| Wagner 2005 | Wrong index test |
| Wagner 2009a | Conference abstract |
| Wagner 2009b | Conference abstract |
| Wagner 2011 | Wrong index test |
| Wasif 2013 | Conference abstract |
| Webb 2012 | Conference abstract |
| Weisinger 1998 | Conference abstract |
| Weiss 1995 | Wrong index test; not test accuracy |
| Windorbska 2007 | Inadequate reference standard |
| Winkler 2013 | Wrong study population |
| Wong 2011 | Conference abstract |
| Xing 2010 | Conference abstract |
| Xing 2011 | Systematic review |
| Yancovitz 2007 | Inadequate reference standard |
| Yang 2003 | Inadequate reference standard |
| Zender 2014 | Wrong index test |
| Zimmermann 2000 | Wrong index test |
| Zukauskaite 2013 | Inadequate reference standard |
Differences between protocol and review
We set out to separately review the evidence for ultrasound, CT, MRI, and PET‐CT for staging of melanoma, and to bring the reviews together in a Cochrane Overview review; however, as our main focus is on the comparative accuracy of different imaging tests, the reviews were brought together into a single review.
A new primary objective was added: to determine the diagnostic accuracy of ultrasound or PET‐CT for detection of nodal metastases before SLNB in adults with confirmed cutaneous invasive melanoma.
A new secondary objective was added: to determine the diagnostic accuracy of ultrasound, CT, MRI, or PET‐CT for detection of any metastasis in the staging of disease in mixed or not clearly described populations of adults with cutaneous invasive melanoma. According to the protocol, the effect of mixed or not clearly reported populations was to be considered as a subgroup analysis.
We clarified that the primary objectives refer to adults with melanoma.
We amended the text to clarify that studies available only as conference abstracts would be excluded from the review; studies available only as conference abstracts do not allow a comprehensive assessment of study methods or methodological quality.
Sources of heterogeneity could not be formally investigated because of lack of data.
We allowed the inclusion of up to 10% of participants having non‐cutaneous melanoma.
We excluded studies of PET alone as the technology is now considered obsolete, instead including only those that examined PET combined with CT.
Studies reporting multiple applications of the same test in more than 10% of study participants were excluded because of anticipated effects on test accuracy (multiple tests increasing the chance of metastases being detected), thereby increasing test sensitivity and reducing specificity.
Reference standard inclusion criteria were amended to allow malignancy to be confirmed by imaging follow‐up (growth or regression of suspicious lesion on imaging) and to recognise that histology may be available for index negative (e.g. SLNB may be conducted in all those with ultrasound regardless of positive or negative). The minimum follow‐up required was also dropped from six months to three months in accordance with the minimum required in diagnosis reviews.
We proposed to supplement the database searches by searching the annual meetings of appropriate organisations (e.g. British Association of Dermatologists' Annual Meeting, American Academy of Dermatology Annual Meeting, European Academy of Dermatology and Venereology Meeting, Society for Melanoma Research Congress, World Congress of Dermatology, European Association of Dermato Oncology), but because of the volume of evidence retrieved from database searches and time restrictions, we were unable to do this.
For quality assessment, we tailored the QUADAS‐2 tool according to the review topic. In terms of analysis, we did not restrict analysis to per patient data due to lack of data.
Contributions of authors
JD was the contact person with the editorial base. JD co‐ordinated contributions from the co‐authors and wrote the final draft of the review. SEB conducted the literature searches. JD, NC, LFR, AD, AG, LP, and SAC screened papers against eligibility criteria. JD, LFR, AD, AG, LP, and SAC appraised the quality of papers. JD, LFR, AD, AG, LP, and SAC extracted data for the review and sought additional information about papers. JD entered data into Review Manager 5 (Review Manager 2014). JD and YT analysed and interpreted data. JD, JJD, NC, LFR, YT, and CD worked on the methods sections. JD, JNB, STC, PN, MS, ZT, RNM, and HCW drafted the clinical sections of the background and responded to clinical comments of the referees. JD, JJD, CD, and YT responded to the methodology and statistics comments of the referees. KG was the consumer co‐author and checked the review for readability and clarity and ensured that outcomes were relevant to consumers. JD is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Skin and Cochrane Programme Grant funding. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
This review presents independent research supported by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the author(s) and are not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Systematic Review Programme, UK.
This project was funded by an NIHR Cochrane Systematic Reviews Programme Grant (13‐89‐15)
-
National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of Cochrane Skin
-
NIHR Birmingham Biomedical Research Centre, UK.
JD, JJD, and YT receive support from the NIHR Birmingham Biomedical Research Centre
Declarations of interest
Jacqueline Dinnes: I am employed by the University of Birmingham under an NIHR Cochrane Programme Grant (13‐89‐15) to produce the review. Lavinia Ferrante di Ruffano: nothing to declare. Yemisi Takwoingi: nothing to declare. Seau Tak Cheung: nothing to declare. Paul Nathan: I have received consultancy fees from Bristol Myers Squibb (BMS), Pfizer, Merck Sharp Dohme (MSD), Merck, and Immunocore to sit on advisory boards. I have received payment from BMS and Novartis for lectures at satellite symposia; payment from BMS for webcasts; payment of travel, accommodations, and meeting expenses from BMS and MSD for attending conferences of the American Society of Clinical Oncology, the Society for Melanoma Research, and the European Society of Medical Oncology. Rubeta N Matin: my institution received a grant for a Barco NV commercially sponsored study to evaluate digital dermoscopy in the skin cancer clinic. My institution also received Oxfordshire Health Services Research Charitable Funds for carrying out a study of feasibility of using the Skin Cancer Quality of Life Impact Tool (SCQOLIT) in non‐melanoma skin cancer, and ScARF funding for the melanoma unmet needs study. I have received payment from Public Health England for the "Be Clear on Cancer Skin Cancer" report; payment for development of educational presentations on skin toxicity for BMS; and royalties for the Oxford Handbook of Medical Dermatology (Oxford University Press). I have no conflicts of interest to declare that directly relate to the publication of this work. Naomi Chuchu: nothing to declare. Sue Ann Chan: nothing to declare. Alana Durack: nothing to declare. Susan E Bayliss: nothing to declare. Abha Gulati: nothing to declare. Lopa Patel: nothing to declare. Clare Davenport: my employer (University of Birmingham) received funding for my participation in this review as part of an NIHR programme grant awarded to Jac Dinnes, the PI. Kathie Godfrey: nothing to declare. Manil Subsesinghe: nothing to declare. Zoe Traill: nothing to declare. Jonathan J Deeks: my employer (University of Birmingham) received funding for my participation in this review as part of an NIHR programme grant awarded to Jac Dinnes, the PI. Hywel C Williams: I am director of the NIHR HTA Programme. HTA is part of the NIHR, which also supports the NIHR systematic reviews programme from which this work is funded.
New
References
References to studies included in this review
Abbott 2011 {published data only}
- Abbott RA, Acland KM, Harries M, O'Doherty M. The role of positron emission tomography with computed tomography in the follow‐up of asymptomatic cutaneous malignant melanoma patients with a high risk of disease recurrence. Melanoma Research 2011;21(5):446‐9. [PUBMED: 21849913] [DOI] [PubMed] [Google Scholar]
Arrangoiz 2012 {published data only}
- Arrangoiz R, Papavasiliou P, Stransky CA, Yu JQ, Tianyu Li, Sigurdson ER, et al. Preoperative FDG‐PET/CT is an important tool in the management of patients with thick (T4) melanoma. Dermatology Research and Practice 2012;2012:614349. [PUBMED: 22654898] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aukema 2010a {published data only}
- Aukema TS, Olmos RA, Korse CM, Kroon BB, Wouters MW, Vogel WV, et al. Utility of FDG PET/CT and brain MRI in melanoma patients with increased serum S‐100B level during follow‐up. Annals of Surgical Oncology 2010;17(6):1657‐61. [PUBMED: 20151211] [DOI] [PubMed] [Google Scholar]
Aukema 2010b {published data only}
- Aukema TS, Valdes Olmos RA, Wouters MW, Klop WM, Kroon BB, Vogel WV, et al. Utility of preoperative 18F‐FDG PET/CT and brain MRI in melanoma patients with palpable lymph node metastases. Annals of Surgical Oncology 2010;17(10):2773‐8. [PUBMED: 20422454] [DOI] [PubMed] [Google Scholar]
Bastiaannet 2009 {published data only}
- Bastiaannet E, Wobbes T, Hoekstra OS, Jagt EJ, Brouwers AH, Koelemij R, et al. Prospective comparison of [18F]fluorodeoxyglucose positron emission tomography and computed tomography in patients with melanoma with palpable lymph node metastases: diagnostic accuracy and impact on treatment. Journal of Clinical Oncology 2009;27(28):4774‐80. [PUBMED: 19720925] [DOI] [PubMed] [Google Scholar]
Cachin 2014 {published data only}
- Cachin F, Miot‐Noirault E, Gillet B, Isnardi V, Labeille B, Payoux P, et al. (123)I‐BZA2 as a melanin‐targeted radiotracer for the identification of melanoma metastases: results and perspectives of a multicenter phase III clinical trial. Journal of Nuclear Medicine 2014;55(1):15‐22. [PUBMED: 24263087] [DOI] [PubMed] [Google Scholar]
Chai 2012 {published data only}
- Chai CY, Zager JS, Szabunio MM, Marzban SS, Chau A, Rossi RM, et al. Preoperative ultrasound is not useful for identifying nodal metastasis in melanoma patients undergoing sentinel node biopsy: preoperative ultrasound in clinically node‐negative melanoma. Annals of Surgical Oncology 2012;19(4):1100‐6. [PUBMED: 22193886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellestable 2011 {published data only}
- Dellestable P, Granel‐Brocard F, Rat AC, Olivier P, Regent D, Schmutz JL. Impact of whole body magnetic resonance imaging (MRI) in the management of melanoma patients, in comparison with positron emission tomography/computed tomography (TEP/CT) and CT. Annales de Dermatologie et de Venereologie 2011;138(5):377‐83. [PUBMED: 21570561] [DOI] [PubMed] [Google Scholar]
Hafner 2004 {published data only}
- Hafner J, Schmid MH, Kempf W, Burg G, Kunzi W, Meuli‐Simmen C, et al. Baseline staging in cutaneous malignant melanoma. British Journal of Dermatology 2004;150(4):677‐86. [PUBMED: 15099363] [DOI] [PubMed] [Google Scholar]
Hausmann 2011 {published data only}
- Hausmann D, Jochum S, Utikal J, Hoffmann RC, Zechmann C, Neff KW, et al. Comparison of the diagnostic accuracy of whole‐body MRI and whole‐body CT in stage III/IV malignant melanoma. Journal der Deutschen Dermatologischen Gesellschaft 2011;9(3):212‐22. [PUBMED: 21352483] [DOI] [PubMed] [Google Scholar]
Hinz 2011 {published data only}
- Hinz T, Wilsmann‐Theis D, Buchner A, Wenzel J, Wendtner CM, Bieber T, et al. High‐resolution ultrasound combined with power Doppler sonography can reduce the number of sentinel lymph node biopsies in cutaneous melanoma. Dermatology 2011;222(2):180‐8. [PUBMED: 21464558] [DOI] [PubMed] [Google Scholar]
Hinz 2013 {published data only}
- Hinz T, Voth H, Ahmadzadehfar H, Hoeller T, Wenzel J, Bieber T, et al. Role of high‐resolution ultrasound and PET/CT imaging for preoperative characterization of sentinel lymph nodes in cutaneous melanoma. Ultrasound in Medicine & Biology 2013;39(1):30‐6. [PUBMED: 23122637] [DOI] [PubMed] [Google Scholar]
Hocevar 2004 {published data only}
- Hocevar M, Bracko M, Pogacnik A, Vidergar‐Kralj B, Besic N, Zgajnar J, et al. The role of preoperative ultrasonography in reducing the number of sentinel lymph node procedures in melanoma. Melanoma Research 2004;14(6):533‐6. [PUBMED: 15577326] [DOI] [PubMed] [Google Scholar]
Iagaru 2007 {published data only}
- Iagaru A, Quon A, Johnson D, Gambhir SS, McDougall IR. 2‐Deoxy‐2‐[F‐18]fluoro‐D‐glucose positron emission tomography/computed tomography in the management of melanoma. Molecular Imaging & Biology 2007;9(1):50‐7. [PUBMED: 17051322] [DOI] [PubMed] [Google Scholar]
Jouvet 2014 {published data only}
- Jouvet JC, Thomas L, Thomson V, Yanes M, Journe C, Morelec I, et al. Whole‐body MRI with diffusion‐weighted sequences compared with 18 FDG PET‐CT, CT and superficial lymph node ultrasonography in the staging of advanced cutaneous melanoma: a prospective study. Journal of the European Academy of Dermatology and Venereology 2014;28(2):176‐85. [PUBMED: 23331931] [DOI] [PubMed] [Google Scholar]
Kang 2011 {published data only}
- Kang S, Ahn BC, Hong CM, Song BI, Lee HJ, Jeong SY, et al. Can (18)F‐FDG PET/CT predict recurrence in patients with cutaneous malignant melanoma?. Nuclear‐Medizin 2011;50(3):116‐21. [PUBMED: 21246162] [DOI] [PubMed] [Google Scholar]
Kell 2007 {published data only}
- Kell MR, Ridge JA, Joseph N, Sigurdson ER. PET CT imaging in patients undergoing sentinel node biopsy for melanoma. European Journal of Cancer Surgery 2007;33(7):911‐3. [DOI: 10.1016/j.ejso.2006.11.016; PUBMED: 17207956] [DOI] [PubMed] [Google Scholar]
Klebl 2003 {published data only}
- Klebl FH, Gelbmann CM, Lammert I, Bogenrieder T, Stolz W, Scholmerich J, et al. Detection of lymph node metastases of malignant melanoma by palpation and ultrasound. Medizinische Klinik 2003;98(12):783‐7. [PUBMED: 14685681] [DOI] [PubMed] [Google Scholar]
Klode 2010 {published data only}
- Klode J, Dissemond J, Grabbe S, Hillen U, Poeppel T, Boeing C. Sentinel lymph node excision and PET‐CT in the initial stage of malignant melanoma: a retrospective analysis of 61 patients with malignant melanoma in American Joint Committee on Cancer stages I and II. Dermatologic Surgery 2010;36(4):439‐45. [PUBMED: 20187901] [DOI] [PubMed] [Google Scholar]
Kunte 2009 {published data only}
- Kunte C, Schuh T, Eberle JY, Baumert J, Konz B, Volkenandt M, et al. The use of high‐resolution ultrasonography for preoperative detection of metastases in sentinel lymph nodes of patients with cutaneous melanoma. Dermatologic Surgery 2009;35(11):1757‐65. [PUBMED: 19660025] [DOI] [PubMed] [Google Scholar]
Maubec 2007 {published data only}
- Maubec E, Lumbroso J, Masson F, Suciu V, Kolb F, Mamelle G, et al. F‐18 fluorodeoxy‐D‐glucose positron emission tomography scan in the initial evaluation of patients with a primary melanoma thicker than 4 mm. Melanoma Research 2007;17(3):147‐54. [PUBMED: 17505260] [DOI] [PubMed] [Google Scholar]
Pfannenberg 2007 {published data only}
- Pfannenberg C, Aschoff P, Schanz S, Eschmann SM, Plathow C, Eigentler TK, et al. Prospective comparison of 18F‐fluorodeoxyglucose positron emission tomography/computed tomography and whole‐body magnetic resonance imaging in staging of advanced malignant melanoma. European Journal of Cancer 2007;43(3):557‐64. [PUBMED: 17224266] [DOI] [PubMed] [Google Scholar]
Pfluger 2011 {published data only}
- Pfluger T, Melzer HI, Schneider V, Fougere C, Coppenrath E, Berking C, et al. PET/CT in malignant melanoma: contrast‐enhanced CT versus plain low‐dose CT. European Journal of Nuclear Medicine & Molecular Imaging 2011;38(5):822‐31. [PUBMED: 21210112] [DOI] [PubMed] [Google Scholar]
Prayer 1990 {published data only}
- Prayer L, Winkelbauer H, Gritzmann N, Winkelbauer F, Helmer M, Pehamberger H. Sonography versus palpation in the detection of regional lymph‐node metastases in patients with malignant melanoma. European Journal of Cancer 1990;26(7):827‐30. [PUBMED: 2145905] [DOI] [PubMed] [Google Scholar]
Radzhabova 2009 {published data only}
- Radzhabova ZA, Barchuk AS, Kostromina EV, Anisimov VV. [The detection of early regional metastases in patients with skin melanoma by dopplerography]. Vestnik Khirurgii Imeni I. I. Grekova 2009;168(1):50‐53. [PUBMED: 19432146] [PubMed] [Google Scholar]
Reinhardt 2006 {published data only}
- Reinhardt MJ, Joe AY, Jaeger U, Huber A, Matthies A, Bucerius J, et al. Diagnostic performance of whole body dual modality 18F‐FDG PET/CT imaging for N‐ and M‐staging of malignant melanoma: experience with 250 consecutive patients. Journal of Clinical Oncology 2006;24(7):1178‐87. [PUBMED: 16505438] [DOI] [PubMed] [Google Scholar]
Revel 2010 {published data only}
- Revel A, Revel C, Dolivet G, Gillet N, Didot N, Meneroux B, et al. Is 18FDG PET‐CT useful for detecting occult nodal metastases in patients with cutaneous head and neck melanoma, in addition to sentinel lymph node biopsy? [La TEP‐TDM au 18FDG a‐t‐elle un interet dans la stadification ganglionnaire des melanomes malins cutanes cervicofaciaux beneficiant de la technique du ganglion sentinelle? A propos de 22 cas]. Medecine Nucleaire 2010;34(9):528‐39. [DOI: 10.1016/j.mednuc.2010.06.007] [DOI] [Google Scholar]
Rubaltelli 2011 {published data only}
- Rubaltelli L, Beltrame V, Tregnaghi A, Scagliori E, Frigo AC, Stramare R. Contrast‐enhanced ultrasound for characterizing lymph nodes with focal cortical thickening in patients with cutaneous melanoma. AJR. American Journal of Roentgenology 2011;196(1):W8‐12. [PUBMED: 21178038] [DOI] [PubMed] [Google Scholar]
Sanki 2009 {published data only}
- Sanki A, Uren RF, Moncrieff M, Tran KL, Scolyer RA, Lin HY, et al. Targeted high‐resolution ultrasound is not an effective substitute for sentinel lymph node biopsy in patients with primary cutaneous melanoma. Journal of Clinical Oncology 2009;27(33):5614‐9. [PUBMED: 19786669] [DOI] [PubMed] [Google Scholar]
Sibon 2007 {published data only}
- Sibon C, Chagnon S, Tchakerian A, Bafounta ML, Longvert C, Clerici T, et al. The contribution of high‐resolution ultrasonography in preoperatively detecting sentinel‐node metastases in melanoma patients. Melanoma Research 2007;17(4):233‐7. [PUBMED: 17625453] [DOI] [PubMed] [Google Scholar]
Singh 2008 {published data only}
- Singh B, Ezziddin S, Palmedo H, Reinhardt M, Strunk H, Tuting T, et al. Preoperative 18F‐FDG‐PET/CT imaging and sentinel node biopsy in the detection of regional lymph node metastases in malignant melanoma. Melanoma Research 2008;18(5):346‐52. [PUBMED: 18781133] [DOI] [PubMed] [Google Scholar]
Strobel 2007a {published data only}
- Strobel K, Skalsky J, Kalff V, Baumann K, Seifert B, Joller‐Jemelka H, et al. Tumour assessment in advanced melanoma: value of FDG‐PET/CT in patients with elevated serum S‐100B. European Journal of Nuclear Medicine & Molecular Imaging 2007;34(9):1366‐75. [PUBMED: 17390135] [DOI] [PubMed] [Google Scholar]
Strobel 2007b {published data only}
- Strobel K, Dummer R, Husarik DB, Perez Lago M, Hany TF, Steinert HC. High‐risk melanoma: accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. Radiology 2007;244(2):566‐74. [PUBMED: 17641374] [DOI] [PubMed] [Google Scholar]
van den Brekel 1998 {published data only}
- Brekel MW, Pameijer FA, Koops W, Hilgers FJ, Kroon BB, Balm AJ. Computed tomography for the detection of neck node metastases in melanoma patients. European Journal of Surgical Oncology 1998;24(1):51‐4. [PUBMED: 9542517] [DOI] [PubMed] [Google Scholar]
van Rijk 2006 {published data only}
- Rijk MC, Teertstra HJ, Peterse JL, Nieweg OE, Olmos RA, Hoefnagel CA, et al. Ultrasonography and fine‐needle aspiration cytology in the preoperative evaluation of melanoma patients eligible for sentinel node biopsy. Annals of Surgical Oncology 2006;13(11):1511‐6. [PUBMED: 17009151] [DOI] [PubMed] [Google Scholar]
van Wissen 2016 {published data only}
- Wissen J, Hiel B, Hage JA, Wiel BA, Wouters MW, Akkooi AC. The diagnostic value of PET/CT imaging in melanoma groin metastases. Annals of Surgical Oncology 2016;23(7):2323‐9. [PUBMED: 26920386] [DOI] [PubMed] [Google Scholar]
Veit‐Haibach 2009 {published data only}
- Veit‐Haibach P, Vogt FM, Jablonka R, Kuehl H, Bockisch A, Beyer T, et al. Diagnostic accuracy of contrast‐enhanced FDG‐PET/CT in primary staging of cutaneous malignant melanoma. European Journal of Nuclear Medicine & Molecular Imaging 2009;36(6):910‐8. [PUBMED: 19156409] [DOI] [PubMed] [Google Scholar]
Voit 2014 {published data only}
- Voit CA, Gooskens SL, Siegel P, Schaefer G, Schoengen A, Rowert J, et al. Ultrasound‐guided fine needle aspiration cytology as an addendum to sentinel lymph node biopsy can perfect the staging strategy in melanoma patients. European Journal of Cancer 2014;50(13):2280‐8. [PUBMED: 24999208] [DOI] [PubMed] [Google Scholar]
Wagner 2012 {published data only}
- Wagner T, Chevreau C, Meyer N, Mourey L, Courbon F, Zerdoud S. Routine FDG PET‐CT in patients with a high‐risk localized melanoma has a high predictive positive value for nodal disease and high negative predictive value for the presence of distant metastases. Journal of the European Academy of Dermatology & Venereology 2012;26(11):1431‐5. [PUBMED: 22017492] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbott 2009 {published data only}
- Abbott R, Harries M, Acland KM, O'Doherty M. Positron‐emission tomography with computed tomography (PET/CT) in melanoma follow‐up. British Journal of Dermatology 2009;161(Suppl 1):36. [Google Scholar]
Abdi 1988 {published data only}
- Abdi EA, Terry T. Lymphography and computed tomography in lymph node metastases from malignant melanoma. Acta Radiologica 1988;29(4):391‐4. [PubMed] [Google Scholar]
Abella‐Columna 2002 {published data only}
- Abella‐Columna E, Valk PE. Positron emission tomography imaging in melanoma and lymphoma. Seminars in Roentgenology 2002;37(2):129‐39. [DOI] [PubMed] [Google Scholar]
Acland 2000 {published data only}
- Acland KM, O'Doherty MJ, Russell‐Jones R. The value of positron emission tomography scanning in the detection of subclinical metastatic melanoma. Journal of the American Academy of Dermatology 2000;42(4):606‐11. [PubMed] [Google Scholar]
Acland 2001 {published data only}
- Acland KM, Healy C, Calonje E, O'Doherty M, Nunan T, Page C, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of micrometastases of primary cutaneous malignant melanoma. Journal of Clinical Oncology 2001;19(10):2674‐8. [DOI] [PubMed] [Google Scholar]
Agarwal 2008 {published data only}
- Agarwal V, Branstetter BF 4th, Johnson JT. Indications for PET/CT in the head and neck. Otolaryngologic Clinics of North America 2008;41(1):23‐49. [DOI] [PubMed] [Google Scholar]
Ahmed 2015 {published data only}
- Ahmed F, Fohne L, Muzaffar R, Kelly P, Fernandes H, Tu Y, et al. Bone metastasis as detected by FDG PET with negative CT of the PET/CT: Frequency in 2000 scans. Journal of Nuclear Medicine. Conference: Society of Nuclear Medicine and Molecular Imaging Annual Meeting, SNMMI 2015;56(3):550. [Google Scholar]
Akcali 2007 {published data only}
- Akcali C, Zincirkeser S, Erbagcy Z, Akcali A, Halac M, Durak G, et al. Detection of metastases in patients with cutaneous melanoma using FDG‐PET/CT. Journal of International Medical Research 2007;35(4):547‐53. [DOI] [PubMed] [Google Scholar]
Aldridge 2010 {published data only}
- Aldridge BA, Beatty JS, Kruse EJ, Lind DS, Williams HT, McLoughlin JM. Ulcerated melanoma: patients may benefit from PET/CT at initial staging. Journal of Surgical Research 2010;158(2):261. [Google Scholar]
Aloia 2006 {published data only}
- Aloia TA, Gershenwald JE, Andtbacka RH, Johnson MM, Schacherer CW, Ng CS, et al. Utility of computed tomography and magnetic resonance imaging staging before completion lymphadenectomy in patients with sentinel lymph node‐positive melanoma. Journal of Clinical Oncology 2006;24(18):2858‐65. [DOI] [PubMed] [Google Scholar]
Alvarado 2007 {published data only}
- Alvarado M, Sondak V, Leong PL. The value of sentinel lymph node biopsy in the management of head and neck melanoma. Journal of Surgical Oncology 2007;95(6):524‐5; author reply 523. [DOI] [PubMed] [Google Scholar]
Angeles 2014 {published data only}
- Angeles CV, Blasi D, Brady MS, Ariyan CE, Coit DG. Yield of radiologic studies for identifying distant disease in clinical stage IIB and IIC melanoma. Annals of Surgical Oncology 2014;21(1 Suppl):S111. [Google Scholar]
Ardizzoni 1987 {published data only}
- Ardizzoni A, Grimaldi A, Repetto L, Bruzzone M, Sertoli MR, Rosso R. Stage I‐II melanoma: the value of metastatic work‐up. Oncology 1987;44(2):87‐9. [DOI] [PubMed] [Google Scholar]
Arrangoiz 2011 {published data only}
- Arrangoiz R, Papavasiliou P, Houssock C, Berger AC, Kairys JC, Mastrangelo MJ, et al. Preoperative F‐18 fluorodeoxy‐D‐glucose‐positron emission tomography is an important tool in the management of patients with thick (T4) melanoma. Annals of Surgical Oncology 2011;18(1 Suppl):S115. [Google Scholar]
Ashour 2016 {published data only}
- Ashour O, Dhayihi T, Jaara E, Sadik K, Coombs R, Lewis T, et al. Variation of radiologist efficiency for oncologic PET/CT interpretation relative to training. Journal of Nuclear Medicine. Conference: Society of Nuclear Medicine and Molecular Imaging Annual Meeting, SNMMI 2016;57(2):595. [Google Scholar]
Bafounta 2004 {published data only}
- Bafounta ML, Beauchet A, Chagnon S, Saiag P. Ultrasonography or palpation for detection of melanoma nodal invasion: a meta‐analysis. Lancet Oncology 2004;5(11):673‐80. [DOI] [PubMed] [Google Scholar]
Baker 2011 {published data only}
- Baker JJ, Meyers MO, Yeh JJ, Frank J, Amos KD, Stitzenberg KB, et al. Routine restaging PET/CT and detection of recurrence in sentinel lymph node positive stage III melanoma. Annals of Surgical Oncology 2011;18(1 Suppl):S114. [Google Scholar]
Baker 2012 {published data only}
- Baker JJ, Newman NA, Levine EA, Deal AM, Frank JS, Stewart JH, et al. Utility of routine PET/CT for initial staging of patients with sentinel lymph node positive melanoma. Annals of Surgical Oncology 2012;19(1 Suppl):S127. [Google Scholar]
Baker 2014 {published data only}
- Baker JJ, Meyers MO, Frank J, Amos KD, Stitzenberg KB, Ollila DW. Routine restaging PET/CT and detection of initial recurrence in sentinel lymph node positive stage III melanoma. American Journal of Surgery 2014;207(4):549‐54. [DOI] [PubMed] [Google Scholar]
Balagula 2012 {published data only}
- Balagula Y, Braun RP, Rabinovitz HS, Dusza SW, Scope A, Liebman TN, et al. The significance of crystalline/chrysalis structures in the diagnosis of melanocytic and nonmelanocytic lesions. Journal of the American Academy of Dermatology 2012;67(2):194.e1‐8. [DOI] [PubMed] [Google Scholar]
Ban 2013 {published data only}
- Ban JN, Ramkumar DP, Tariq M, Mustafa S. The role of ultrasound scanning of the neck in pre‐operative staging of cutaneous squamous‐cell carcinoma of the head and neck. International Journal of Oral and Maxillofacial Surgery 2013;42(10):1351. [Google Scholar]
Barsky 2014 {published data only}
- Barsky M, Cherkassky L, Vezeridis M, Miner TJ. The role of preoperative positron emission tomography/computed tomography (PET/CT) in patients with high‐risk melanoma. Journal of Surgical Oncology 2014;109(7):726‐9. [DOI] [PubMed] [Google Scholar]
Bastiaannet 2006 {published data only}
- Bastiaannet E, Hoekstra OS, Oyen WJ, Jager PL, Wobbes T, Hoekstra HJ. Level of fluorodeoxyglucose uptake predicts risk for recurrence in melanoma patients presenting with lymph node metastases. Annals of Surgical Oncology 2006;13(7):919‐26. [DOI] [PubMed] [Google Scholar]
Bastiaannet 2008 {published data only}
- Bastiaannet E, Wobbes T, Hoekstra OS, Brouwers AH, Oyen WJ, Meijer S, et al. Diagnostic performance of FDG‐PET and CT in the upstaging of melanoma patients with lymph node metastases and the clinical consequences. Pigment Cell & Melanoma Research 2008;21(2):332. [Google Scholar]
Bastiaannet 2008a {published data only}
- Bastiaannet E, Wobbes T, Hoekstra OS, Brouwers AH, Jagt EJ, Oyen W, et al. Detection of distant metastases with FDG‐PET and CT in 251 clinically stage III melanoma patients. Annals of Oncology 2008;19(Suppl 8):viii240‐1. [Google Scholar]
Bastiaannet 2008b {published data only}
- Bastiaannet E, Wobbes T, Meijer S, Hoekstra HJ. Change in treatment as result of FDG‐PET and CT in clinically stage III melanoma patients: a prospective study in 221 patients. Journal of Clinical Oncology 2008;26(15 Suppl):9069. [Google Scholar]
Bastiaannet 2009a {published data only}
- Bastiaannet E, Jong JR, Brouwers AH, Suurmeijer AJ, Hoekstra HJ. The prognostic value of FDG‐PET measured by standardized uptake value in patients with melanoma stage III evaluated in a prospective study. Journal of Clinical Oncology 2009;27(15_suppl):e20000. [Google Scholar]
Bastiaannet 2010 {published data only}
- Bastiaannet E, Uyl C, Brouwers AH, Jagt EJ, Hoekstr OS, Thompson JF, et al. Cost‐effectiveness of adding FDG‐PET or CT to the diagnostic work‐up of melanoma patients stage III. Pigment Cell and Melanoma Research 2010;23(6):941. [Google Scholar]
Bastiaannet 2012 {published data only}
- Bastiaannet E, Uyl‐de Groot CA, Brouwers AH, Jagt EJ, Hoekstra OS, Oyen W, et al. Cost‐effectiveness of adding FDG‐PET or CT to the diagnostic work‐up of patients with stage III melanoma. Annals of Surgery 2012;255(4):771‐6. [DOI] [PubMed] [Google Scholar]
Beasley 2010 {published data only}
- Beasley GM, Selim A, McMahon NS, Coleman A, Abernethy AP, Nelson K, et al. Prospective evaluation of PET/CT as a surveillance tool to define response to therapy and identify new recurrent disease in patients with locally advanced melanoma undergoing regional chemotherapy treatment with melphalan. Annals of Surgical Oncology 2010;17(1 Suppl):S115. [Google Scholar]
Beasley 2012 {published data only}
- Beasley GM, Parsons C, Broadwater G, Selim MA, Marzban S, Abernethy AP, et al. A multicenter prospective evaluation of the clinical utility of F‐18 FDG‐PET/CT in patients with AJCC stage IIIB or IIIC extremity melanoma. Annals of Surgery 2012;256(2):350‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beitollahi 2013 {published data only}
- Beitollahi H, Jaap K, Hunsinger M, Woll N, Shabahang M, Blansfield J. Advanced imaging for the detection of occult metastatic disease in patients with American joint committee on cancer stage III melanoma. Annals of Surgical Oncology 2013;20(1 Suppl):S96. [Google Scholar]
Belhocine 2002 {published data only}
- Belhocine T, Pierard G, Labrassinne M, Lahaye T, Rigo P. Staging of regional nodes in AJCC stage I and II melanoma: 18FDG PET imaging versus sentinel node detection. Oncologist 2002;7(4):271‐8. [DOI] [PubMed] [Google Scholar]
Ben Lakhdar 2011 {published data only}
- Ben Lakhdar A, Ilie M, Tomasic G, Chami L, Robert C, Vielh P. Benefits of ultrasound‐guided fine needle aspiration cytology before lymph node biopsy in melanoma patients. Virchows Archiv 2011;459(1 Suppl):S10. [Google Scholar]
Bernabo 2015 {published data only}
- Bernabo JL, Herrera‐Acosta E, Mendiola‐Fernandez M, Lopez‐Navarro N, Herrera‐Ceballos E. Epidemiologic study and survival analysis of head and neck melanoma: a 17‐year study in Malaga, Spain. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB168. [Google Scholar]
Beyeler 2006 {published data only}
- Beyeler M, Waldispuhl S, Strobel K, Joller‐Jemelka HI, Burg G, Dummer R. Detection of melanoma relapse: first comparative analysis on imaging techniques versus S100 protein. Dermatology 2006;213(3):187‐91. [DOI] [PubMed] [Google Scholar]
Bhatia 2012 {published data only}
- Bhatia KS, Yuen EH, Cho CC, Tong CS, Lee YY, Ahuja AT. A pilot study evaluating real‐time shear wave ultrasound elastography of miscellaneous non‐nodal neck masses in a routine head and neck ultrasound clinic. Ultrasound in Medicine & Biology 2012;38(6):933‐42. [DOI] [PubMed] [Google Scholar]
Bier 2016 {published data only}
- Bier G, Hoffmann V, Kloth C, Othman AE, Eigentler T, Garbe C, et al. CT imaging of bone and bone marrow infiltration in malignant melanoma ‐ Challenges and limitations for clinical staging in comparison to 18FDG‐PET/CT. European Journal of Radiology 2016;85(4):732‐8. [DOI] [PubMed] [Google Scholar]
Biersack 1987 {published data only}
- Biersack HJ, Bockisch A, Vogel J. Scintigraphic detection of malignancies with radiolabeled tumor antibodies [Szintigraphischer Malignomnachweis Mit Radioaktiv Markierten Tumorantikorpern. Klinische Ergebnisse Auf Der Basis Immunhistochemischer Untersuchungen]. Deutsche Medizinische Wochenschrift 1987;112(9):341‐4. [DOI] [PubMed] [Google Scholar]
Bikhchandani 2014 {published data only}
- Bikhchandani J, Wood J, Richards AT, Smith RB. No benefit in staging fluorodeoxyglucose‐positron emission tomography in clinically node‐negative head and neck cutaneous melanoma. Head & Neck 2014;36(9):1313‐6. [DOI] [PubMed] [Google Scholar]
Binder 1997 {published data only}
- Binder M, Kittler H, Steiner A, Dorffner R, Wolff K, Pehamberger H. Lymph node sonography versus palpation for detecting recurrent disease in patients with malignant melanoma. European Journal of Cancer 1997;33(11):1805‐8. [DOI] [PubMed] [Google Scholar]
Binns 2012 {published data only}
- Binns D, Hong E, Lau E. Optimal detection of metastatic malignant melanoma with true whole body FDG‐PET/CT. Internal Medicine Journal 2012;42:22. [Google Scholar]
Blend 1992 {published data only}
- Blend MJ, Ronan SG, Salk DJ, Gupta TK. Role of technetium 99m‐labeled monoclonal antibody in the management of melanoma patients. Journal of Clinical Oncology 1992;10(8):1330‐7. [DOI] [PubMed] [Google Scholar]
Blessing 1995 {published data only}
- Blessing C, Feine U, Geiger L, Carl M, Rassner G, Fierlbeck G. Positron emission tomography and ultrasonography. A comparative retrospective study assessing the diagnostic validity in lymph node metastases of malignant melanoma. Archives of Dermatology 1995;131(12):1394‐8. [DOI] [PubMed] [Google Scholar]
Blum 2000 {published data only}
- Blum A, Schlagenhauff B, Stroebel W, Breuninger H, Rassner G, Garbe C. Ultrasound examination of regional lymph nodes significantly improves early detection of locoregional metastases during the follow‐up of patients with cutaneous melanoma: results of a prospective study of 1288 patients. Cancer 2000;88(11):2534‐9. [DOI] [PubMed] [Google Scholar]
Blum 2006 {published data only}
- Blum A, Schmid‐Wendtner MH, Mauss‐Kiefer V, Eberle JY, Kuchelmeister C, Dill‐Muller D. Ultrasound mapping of lymph node and subcutaneous metastases in patients with cutaneous melanoma: results of a prospective multicenter study. Dermatology 2006;212(1):47‐52. [DOI] [PubMed] [Google Scholar]
Bode 2011 {published data only}
- Bode B, Schaefer N. Ultrasound‐guided fine needle aspirations of PET‐CT findings during staging of malignancies. Cytopathology 2011;22:51. [Google Scholar]
Bohelay 2014 {published data only}
- Bohelay G, Battistella M, Pages C, Basset‐Seguin N, Viguier M, Kerob D, et al. Ultrasound‐guided core needle biopsy (US‐CNB) of superficial lymph nodes: an alternative to fine‐needle aspiration cytology (FNAC) for the diagnosis of lymph node metastasis in melanoma. Journal of Clinical Oncology 2014;32(15 Suppl):e20026. [DOI: 10.1200/jco.2014.32.15_suppl.e20026] [DOI] [PubMed] [Google Scholar]
Bohuslavizki 2000 {published data only}
- Bohuslavizki KH, Klutmann S, Neuber K, Wedler J, Altenhoff J, Kroger S, et al. Correlation of 18F‐FDG‐PET and histopathology in patients with malignant melanoma. Radiology and Oncology 2000;34(1):1‐9. [Google Scholar]
Boni 1995 {published data only}
- Boni R, Boni RA, Steinert H, Burg G, Buck A, Marincek B, et al. Staging of metastatic melanoma by whole‐body positron emission tomography using 2‐fluorine‐18‐fluoro‐2‐deoxy‐D‐glucose. British Journal of Dermatology 1995;132(4):556‐62. [DOI] [PubMed] [Google Scholar]
Boni 1996 {published data only}
- Boni R. Whole‐body positron emission tomography: an accurate staging modality for metastatic melanoma. Archives of Dermatology 1996;132(7):833‐4. [DOI] [PubMed] [Google Scholar]
Boni 1996a {published data only}
- Boni R, Huch‐Boni RA, Steinert H, Schulthess GK, Burg G. Early detection of melanoma metastasis using fludeoxyglucose F 18 positron emission tomography. Archives of Dermatology 1996;132(8):875‐6. [DOI] [PubMed] [Google Scholar]
Borrego 2006 {published data only}
- Borrego Dorado I, Vazquez Albertino R, Lopez Martin J, Alvarez Perez RM. Evaluation of efficacy and clinical impact of FDG‐PET on patients with suspicion of recurrent cutaneous melanoma. Revista Espanola de Medicina Nuclear 2006;25(5):301‐11. [DOI] [PubMed] [Google Scholar]
Boy 2011 {published data only}
- Boy C, Poeppel TD, Stoffels I, Kuhn J, Dissemond J, Rosenbaum‐Krumme S, et al. Preoperative SPECT/CT in detection of sentinel lymph nodes in melanoma: an analysis of 406 patients. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(Suppl 2):S168. [Google Scholar]
Brady 2006 {published data only}
- Brady MS, Akhurst T, Spanknebel K, Hilton S, Gonen M, Patel A, et al. Utility of preoperative [(18)]f fluorodeoxyglucose‐positron emission tomography scanning in high‐risk melanoma patients. Annals of Surgical Oncology 2006;13(4):525‐32. [DOI] [PubMed] [Google Scholar]
Breitenbauch 2015 {published data only}
- Breitenbauch MT, Holm J, Rodgaard JC, Stolle LB. Utility of chest X‐ray and abdominal ultrasound for stage III cutaneous malignant melanoma. European Journal of Plastic Surgery 2015;38(3):189‐92. [Google Scholar]
Brenner 1999 {published data only}
- Brenner W, Klomp HJ, Bohuslavizki KH, Szonn B, Kampen WU, Henze E. Limited sensitivity of iodine‐123‐2‐hydroxy‐3‐iodo‐6‐methoxy‐N‐[(1‐ethyl‐2‐pyrrolidinyl)methyl ] benzamide whole‐body scintigraphy in patients with malignant melanoma: a comparison with thallium‐201 imaging. European Journal of Nuclear Medicine 1999;26(12):1567‐71. [DOI] [PubMed] [Google Scholar]
Bronstein 2012 {published data only}
- Bronstein Y, Ng CS, Rohren E, Ross MI, Lee JE, Cormier J, et al. PET/CT in the management of patients with stage IIIC and IV metastatic melanoma considered candidates for surgery: evaluation of the additive value after conventional imaging. AJR. American Journal of Roentgenology 2012;198(4):902‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brountzos 2003 {published data only}
- Brountzos EN, Panagiotou IE, Bafaloukos DI, Kelekis DA. Ultrasonographic detection of regional lymph node metastases in patients with intermediate or thick malignant melanoma. Oncology Reports 2003;10(2):505‐10. [PubMed] [Google Scholar]
Buckle 2016 {published data only}
- Buckle T, KleinJan GH, Engelen T, Berg NS, DeRuiter MC, Heide U, et al. Diffusion‐weighted‐preparation (D‐prep) MRI as a future extension of SPECT/CT based surgical planning for sentinel node procedures in the head and neck area?. Oral Oncology 2016;60:48‐54. [DOI] [PubMed] [Google Scholar]
Bude 2004 {published data only}
- Bude RO. Does contrast‐enhanced US have potential for sentinel lymph node detection?. Radiology 2004;230(3):603‐4. [DOI] [PubMed] [Google Scholar]
Buerke 2011 {published data only}
- Buerke B, Gerss J, Puesken M, Weckesser M, Heindel W, Wessling J. Usefulness of semi‐automatic volumetry compared to established linear measurements in predicting lymph node metastases in MSCT. Acta Radiologica 2011;52(5):540‐6. [DOI] [PubMed] [Google Scholar]
Buzaid 1993 {published data only}
- Buzaid AC, Sandler AB, Mani S, Curtis AM, Poo WJ, Bolognia JL, et al. Role of computed tomography in the staging of primary melanoma. Journal of Clinical Oncology 1993;11(4):638‐43. [DOI] [PubMed] [Google Scholar]
Buzaid 1995 {published data only}
- Buzaid AC, Tinoco L, Ross MI, Legha SS, Benjamin RS. Role of computed tomography in the staging of patients with local‐regional metastases of melanoma. Journal of Clinical Oncology 1995;13(8):2104‐8. [DOI] [PubMed] [Google Scholar]
Bydder 1981 {published data only}
- Bydder GM, Kreel L. Body computed tomography in the diagnosis of malignant melanoma metastases. Journal of Computed Tomography 1981;5(1):21‐4. [DOI] [PubMed] [Google Scholar]
Cachin 2012 {published data only}
- Cachin F, Gillet B, Isnardi V, Labeille B, Payoux P, Meyer N, et al. Phase III clinical trial comparing the value of 18FDG TEP/CT and 123I‐BZA2 as a melanin tracer for diagnosis of melanoma metastases: results and perspectives. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(Suppl 2):S190. [Google Scholar]
Catalano 2010 {published data only}
- Catalano O, Caraco C, Mozzillo N, Siani A. Locoregional spread of cutaneous melanoma: sonography findings. AJR. American Journal of Roentgenology 2010;194(3):735‐45. [DOI] [PubMed] [Google Scholar]
Catalano 2010a {published data only}
- Catalano O, Setola SV, Vallone P, Raso MM, D'Errico AG. Sonography for locoregional staging and follow‐up of cutaneous melanoma: how we do it. Journal of Ultrasound in Medicine 2010;29(5):791‐802. [DOI] [PubMed] [Google Scholar]
Catalano 2010b {published data only}
- Catalano O, Siani A. Cutaneous melanoma: role of ultrasound in the assessment of locoregional spread. Current Problems in Diagnostic Radiology 2010;39(1):30‐6. [DOI] [PubMed] [Google Scholar]
Catalano 2011 {published data only}
- Catalano O. Critical analysis of the ultrasonographic criteria for diagnosing lymph node metastasis in patients with cutaneous melanoma: a systematic review. Journal of Ultrasound in Medicine 2011;30(4):547‐60. [DOI] [PubMed] [Google Scholar]
Catalano 2015 {published data only}
- Catalano O, Caraco C, Nunziata A, Mozzillo N, Petrillo A. Sentinel lymph node (SLN) melanoma micrometastasis managed conservatively: sonography (US) patterns of recurrence. Ultrasound in Medicine and Biology 2015;41(4 Suppl):S147. [Google Scholar]
Chai 2010 {published data only}
- Chai CY, Zager JS, Marzban SS, Rossi RM, Szabunio M, Sondak VK. Preoperative ultrasound is not useful for identifying nodal metastasis in melanoma patients undergoing sentinel lymph node biopsy. Annals of Surgical Oncology 2010;17(1 Suppl):S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2005 {published data only}
- Cho SB, Chung WG, Yun M, Lee JD, Lee MG, Chung KY. Fluorodeoxyglucose positron emission tomography in cutaneous squamous cell carcinoma: retrospective analysis of 12 patients. Dermatologic Surgery 2005;31(4):442‐6; discussion 446‐7. [DOI] [PubMed] [Google Scholar]
Chomyn 1992 {published data only}
- Chomyn JJ, Stamm ER, Thickman D. CT of melanoma liver metastases: is the examination without contrast media superfluous?. Journal of Computer Assisted Tomography 1992;16(4):568‐71. [DOI] [PubMed] [Google Scholar]
Clark 2006 {published data only}
- Clark PB, Soo V, Kraas J, Shen P, Levine EA. Futility of fluorodeoxyglucose F 18 positron emission tomography in initial evaluation of patients with T2 to T4 melanoma. Archives of Surgery 2006;141(3):284‐8. [DOI] [PubMed] [Google Scholar]
Clement 1998 {published data only}
- Clement A, Fayet P, Hoeffel C, Oudjit A, Hazebroucq V, Zavarro A, et al. Value of high‐frequency (20 MHz) in the diagnosis of cutaneous tumors. Journal de Radiologie 1998;79(4):313‐7. [PubMed] [Google Scholar]
Clement 2001 {published data only}
- Clement A, Hoeffel C, Fayet P, Benkanoun S, Sahut D'izarn J, Oudjit A, et al. Value of high frequency (20mhZ) and doppler ultrasound in the diagnosis of pigmented cutaneous tumors. Journal de Radiologie 2001;82(5):563‐71. [PubMed] [Google Scholar]
Clemente‐Ruiz 2012 {published data only}
- Clemente‐Ruiz de Almiron A, Serrano‐Ortega S. Risk factors for in‐transit metastasis in patients with cutaneous melanoma. Actas Dermo‐Sifiliograficas 2012;103(3):207‐13. [DOI] [PubMed] [Google Scholar]
Cobben 2003 {published data only}
- Cobben DC, Jager PL, Elsinga PH, Maas B, Suurmeijer AJ, Hoekstra HJ. 3'‐18F‐fluoro‐3'‐deoxy‐L‐thymidine: a new tracer for staging metastatic melanoma?. Journal of Nuclear Medicine 2003;44(12):1927‐32. [PubMed] [Google Scholar]
Connell 2003 {published data only}
- Connell LE, Verheyden CN. The efficacy of laboratory studies in the detection of recurrent melanoma. Plastic & Reconstructive Surgery 2003;111(1):502‐3. [DOI] [PubMed] [Google Scholar]
Constantinidou 2008 {published data only}
- Constantinidou A, Hofman M, O'Doherty M, Acland KM, Healy C, Harries M. Routine positron emission tomography and positron emission tomography/computed tomography in melanoma staging with positive sentinel node biopsy is of limited benefit. Melanoma Research 2008;18(1):56‐60. [DOI] [PubMed] [Google Scholar]
Cordova 2006 {published data only}
- Cordova A, Napoli P, Costa R, Giambona C, Tripoli N, Moschella F. 18Fluoro‐2‐deoxy‐D‐glucose‐positron emission tomography (FDG‐PET) imaging versus sentinel lymph node biopsy (SLNB) in the staging of cutaneous melanoma in AJCC stage I and II. Chirurgia 2006;19(3):189‐91. [Google Scholar]
Cousen 2014 {published data only}
- Cousen P, Chow K, Colver GB. What is the role of ultrasound evaluation of lymph nodes in patients with high‐grade squamous cell carcinoma of the head and neck?. British Journal of Dermatology 2014;171(Suppl 1):76‐7. [DOI] [PubMed] [Google Scholar]
Crippa 2000 {published data only}
- Crippa F, Leutner M, Belli F, Gallino F, Greco M, Pilotti S, et al. Which kinds of lymph node metastases can FDG PET detect? A clinical study in melanoma. Journal of Nuclear Medicine 2000;41(9):1491‐4. [PubMed] [Google Scholar]
Curtis 1982 {published data only}
- Curtis AM, Ravin CE, Deering TF, Putman CE, McLoud TC, Greenspan RH. The efficacy of full‐lung tomography in the detection of early metastatic disease from melanoma. Radiology 1982;144(1):27‐9. [DOI] [PubMed] [Google Scholar]
Dalle 2006 {published data only}
- Dalle S, Paulin C, Lapras V, Balme B, Ronger‐Savle S, Thomas L. Fine‐needle aspiration biopsy with ultrasound guidance in patients with malignant melanoma and palpable lymph nodes. British Journal of Dermatology 2006;155(3):552‐6. [DOI] [PubMed] [Google Scholar]
Damian 1996 {published data only}
- Damian DL, Fulham MJ, Thompson E, Thompson JF. Positron emission tomography in the detection and management of metastatic melanoma. Melanoma Research 1996;6(4):325‐9. [DOI] [PubMed] [Google Scholar]
Danielsen 2013 {published data only}
- Danielsen M, Hojgaard L, Kjaer A, Fischer BM. Positron emission tomography in the follow‐up of cutaneous malignant melanoma patients: a systematic review. American Journal of Nuclear Medicine and Molecular Imaging 2013;4(1):17‐28. [PMC free article] [PubMed] [Google Scholar]
Davidson 2011 {published data only}
- Davidson J, King A, Mitra I, Johnson M, Rao S, Sundram FX. Whole versus half body PET‐CT imaging in patients with malignant melanoma. Nuclear Medicine Communications 2011;32(5):437. [Google Scholar]
Davis 1991 {published data only}
- Davis PC, Hudgins PA, Peterman SB, Hoffman JC Jr. Diagnosis of cerebral metastases: double‐dose delayed CT vs contrast‐enhanced MR imaging. AJNR: American Journal of Neuroradiology 1991;12(2):293‐300. [PMC free article] [PubMed] [Google Scholar]
De Giorgi 2010 {published data only}
- Giorgi V, Gori A, Grazzini M, Rossari S, Marino G, D'Elia G, et al. Contrast‐enhanced ultrasound: a filter role in AJCC stage I/II melanoma patients. Oncology 2010;79(5‐6):370‐5. [DOI] [PubMed] [Google Scholar]
De Rosa 2010 {published data only}
- Rosa N, Herndon JE, Marcello J, Tyler DS, Scheri RP, Pruitt SK, et al. Patterns of recurrence in melanoma and the impact on survival. Annals of Surgical Oncology 2010;17(1 Suppl):S104‐5. [Google Scholar]
DeRose 2010 {published data only}
- DeRose ER, Pleet A, Seery VJ, Lee M, Renzi S, Sullivan RJ, et al. Utility of 3‐year torso CT and head imaging in asymptomatic patients with high‐risk melanoma. Journal of Clinical Oncology 2010;28(15 Suppl):8594. [DOI: 10.1200/jco.2010.28.15_suppl.8594] [DOI] [Google Scholar]
Dietlein 1999 {published data only}
- Dietlein M, Krug B, Groth W, Smolarz K, Scheidhauer K, Psaras T, et al. Positron emission tomography using 18F‐fluorodeoxyglucose in advanced stages of malignant melanoma: a comparison of ultrasonographic and radiological methods of diagnosis. Nuclear Medicine Communications 1999;20(3):255‐61. [DOI] [PubMed] [Google Scholar]
Diodato 2015 {published data only}
- Diodato S, Vivo S, Baraldi C, Veronesi G, Dika E, Fanti S, et al. Diagnostic accuracy of 18F‐FDG PET/CT in primary staging of cutaneous malignant melanoma according to Breslow thickness: a preliminary study. European Journal of Nuclear Medicine and Molecular Imaging 2015;42(Suppl 1):S322‐3. [Google Scholar]
Doiron 1981 {published data only}
- Doiron MJ, Bernardino ME. A comparison of noninvasive imaging modalities in the melanoma patient. Cancer 1981;47(11):2581‐4. [DOI] [PubMed] [Google Scholar]
Dresel 2003 {published data only}
- Dresel S, Grammerstorff J, Schwenzer K, Brinkbaumer K, Schmid R, Pfluger T, et al. [18F]FDG imaging of head and neck tumours: comparison of hybrid PET and morphological methods. European Journal of Nuclear Medicine & Molecular Imaging 2003;30(7):995‐1003. [DOI] [PubMed] [Google Scholar]
Drzezga 2012 {published data only}
- Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez‐Moller A, et al. First clinical experience with integrated whole‐body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. Journal of Nuclear Medicine 2012;53(6):845‐55. [DOI: 10.2967/jnumed.111.098608] [DOI] [PubMed] [Google Scholar]
Eigtved 2000 {published data only}
- Eigtved A, Andersson AP, Dahlstrom K, Rabol A, Jensen M, Holm S, et al. Use of fluorine‐18 fluorodeoxyglucose positron emission tomography in the detection of silent metastases from malignant melanoma. European Journal of Nuclear Medicine 2000;27(1):70‐5. [DOI] [PubMed] [Google Scholar]
El‐Maraghi 2008 {published data only}
- El‐Maraghi RH, Kielar AZ. PET vs sentinel lymph node biopsy for staging melanoma: a patient intervention, comparison, outcome analysis. Journal of the American College of Radiology 2008;5(8):924‐31. [DOI] [PubMed] [Google Scholar]
Emmett 2012 {published data only}
- Emmett L, Ho B. Imaging for melanoma and non‐melanoma skin cancers. Cancer Forum 2012;36(3):134‐7. [ISSN 0311‐306X] [Google Scholar]
Facius 2002 {published data only}
- Facius M, Malich A, Schneider G, Boehm T, Anderson R, Kaiser WA. Electrical impedance scanning used in addition to ultrasound for the verification of submandibular and parotid lesions: initial results. Investigative Radiology 2002;37(8):421‐7. [DOI] [PubMed] [Google Scholar]
Fakhry 2009 {published data only}
- Fakhry N, Tessonnier L, Cohen F, Gras R, Grob JJ, Giovanni A, et al. Management of cervical lymph node recurrence of melanoma of the head and neck. Revue de Laryngologie Otologie Rhinologie 2009;130(4‐5):211‐4. [PubMed] [Google Scholar]
Falk 2007 {published data only}
- Falk MS, Truitt AK, Coakley FV, Kashani‐Sabet M, Hawkins RA, Franc B. Interpretation, accuracy and management implications of FDG PET/CT in cutaneous malignant melanoma. Nuclear Medicine Communications 2007;28(4):273‐80. [DOI] [PubMed] [Google Scholar]
Faries 2010 {published data only}
- Faries MB, Wanek LA, Elashoff D, Wright BE, Morton DL. Predictors of occult nodal metastasis in patients with thin melanoma. Archives of Surgery 2010;145(2):137‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrandiz 2016 {published data only}
- Ferrandiz L, Silla‐Prosper M, Garcia‐de‐la‐Oliva A, Mendonca FM, Ojeda‐Vila T, Moreno‐Ramirez D. Yield of computed tomography at baseline staging of melanoma. Actas Dermo‐Sifiliograficas 2016;107(1):55‐61. [DOI] [PubMed] [Google Scholar]
Fink 2004 {published data only}
- Fink AM, Holle‐Robatsch S, Herzog N, Mirzaei S, Rappersberger K, Lilgenau N, et al. Positron emission tomography is not useful in detecting metastasis in the sentinel lymph node in patients with primary malignant melanoma stage I and II. Melanoma Research 2004;14(2):141‐5. [DOI] [PubMed] [Google Scholar]
Finkelstein 2004 {published data only}
- Finkelstein SE, Carrasquillo JA, Hoffman JM, Galen B, Choyke P, White DE, et al. A prospective analysis of positron emission tomography and conventional imaging for detection of stage IV metastatic melanoma in patients undergoing metastasectomy. Annals of Surgical Oncology 2004;11(8):731‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fletcher 2008 {published data only}
- Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F‐FDG PET in oncology. Journal of Nuclear Medicine 2008;49(3):480‐508. [DOI] [PubMed] [Google Scholar]
Fogarty 2006 {published data only}
- Fogarty GB, Tartaguia C. The utility of magnetic resonance imaging in the detection of brain metastases in the staging of cutaneous melanoma. Clinical Oncology (Royal College of Radiologists) 2006;18(4):360‐2. [DOI] [PubMed] [Google Scholar]
Fohne 2015 {published data only}
- Fohne L, Ahmed F, Botkin C, Hubble W, Osman M. Identification of bone lesions on PET/CT imaging: a comparison of PET to CT detection capabilities. Journal of Nuclear Medicine 2015;56(3):2723. [Google Scholar]
Friedman 2004 {published data only}
- Friedman KP, Wahl RL. Clinical use of positron emission tomography in the management of cutaneous melanoma. Seminars in Nuclear Medicine 2004;34(4):242‐53. [DOI] [PubMed] [Google Scholar]
Fuster 2003 {published data only}
- Fuster D, Schuchter LM, Zhuang H, Johnson G, Alavi A. Comparison of fluorine‐18F‐fluorodeoxyglucose positron emission tomography and computed tomography in the detection of recurrent or metastatic melanoma. Journal of Nuclear Medicine 2003;44(5):384P. [Google Scholar]
Fuster 2004 {published data only}
- Fuster D, Chiang S, Johnson G, Schuchter LM, Zhuang H, Alavi A. Is 18F‐FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma?. Journal of Nuclear Medicine 2004;45(8):1323‐7. [PubMed] [Google Scholar]
Garbe 2003 {published data only}
- Garbe C, Paul A, Kohler‐Spath H, Ellwanger U, Stroebel W, Schwarz M, et al. Prospective evaluation of a follow‐up schedule in cutaneous melanoma patients: recommendations for an effective follow‐up strategy. Journal of Clinical Oncology 2003;21(3):520‐9. [DOI] [PubMed] [Google Scholar]
Gellen 2015 {published data only}
- Gellen E, Santha O, Janka E, Juhasz I, Peter Z, Erdei I, et al. Diagnostic accuracy of (18)F‐FDG‐PET/CT in early and late stages of high‐risk cutaneous malignant melanoma. Journal of the European Academy of Dermatology & Venereology 2015;29(10):1938‐44. [DOI] [PubMed] [Google Scholar]
Ghanem 2005 {published data only}
- Ghanem N, Altehoefer C, Hogerle S, Nitzsche E, Lohrmann C, Schafer O, et al. Detectability of liver metastases in malignant melanoma: prospective comparison of magnetic resonance imaging and positron emission tomography. European Journal of Radiology 2005;54(2):264‐70. [DOI] [PubMed] [Google Scholar]
Giles 2014 {published data only}
- Giles B, Wasif N, Rawal B, Bagaria S. Does increasing wait time to surgery for cutaneous melanoma increase the risk for nodal metastases?. Annals of Surgical Oncology 2014;21(1 Suppl):S110. [Google Scholar]
Ginaldi 1981 {published data only}
- Ginaldi S, Wallace S, Shalen P, Luna M, Handel S. Cranial computed tomography of malignant melanoma. AJR. American Journal of Roentgenology 1981;136(1):145‐9. [DOI] [PubMed] [Google Scholar]
Giovagnorio 2003 {published data only}
- Giovagnorio F, Valentini C, Paonessa A. High‐resolution and color doppler sonography in the evaluation of skin metastases. Journal of Ultrasound in Medicine 2003;22(10):1017‐22; quiz 1023‐5. [DOI] [PubMed] [Google Scholar]
Gold 2007 {published data only}
- Gold JS, Jaques DP, Busam KJ, Brady MS, Coit DG. Yield and predictors of radiologic studies for identifying distant metastases in melanoma patients with a positive sentinel lymph node biopsy. Annals of Surgical Oncology 2007;14(7):2133‐40. [DOI] [PubMed] [Google Scholar]
Grigolato 2011 {published data only}
- Grigolato D, Zuffante M, Fiorio E, Caruso B, Etta LE, Bosco F, et al. Malignant melanoma: role of PET/CT imaging and correlation with protein S‐100B. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(Suppl 2):S386. [Google Scholar]
Gritters 1993 {published data only}
- Gritters LS, Francis IR, Zasadny KR, Wahl RL. Initial assessment of positron emission tomography using 2‐fluorine‐18‐fluoro‐2‐deoxy‐D‐glucose in the imaging of malignant melanoma. Journal of Nuclear Medicine 1993;34(9):1420‐7. [PubMed] [Google Scholar]
Gulec 2003 {published data only}
- Gulec SA, Faries MB, Lee CC, Kirgan D, Glass C, Morton DL, et al. The role of fluorine‐18 deoxyglucose positron emission tomography in the management of patients with metastatic melanoma: impact on surgical decision making. Clinical Nuclear Medicine 2003;28(12):961‐5. [DOI] [PubMed] [Google Scholar]
Gupta 2012 {published data only}
- Gupta S, Rogers K, Kearney N, Allen L. Diffuse cutaneous uptake of 18FDG is associated with adverse prognosis in patients with melanoma: a case series. Internal Medicine Journal 2012;42(Suppl 3):24. [Google Scholar]
Haddad 2013 {published data only}
- Haddad D, Etzioni D, Pockaj BA, Gray RJ, Wasif N. Preoperative imaging for staging of cutaneous melanoma in the United States: a population‐based analysis. Journal of Clinical Oncology 2013;31(15 Suppl):9034. [DOI: 10.1200/jco.2013.31.15_suppl.9034] [DOI] [Google Scholar]
Haddad 2013a {published data only}
- Haddad D, Garvey EM, Mihalik L, Pockaj BA, Gray RJ, Wasif N. Preoperative imaging for early‐stage cutaneous melanoma: predictors, usage, and utility at a single institution. American Journal of Surgery 2013;206(6):979‐85; discussion 985‐6. [DOI] [PubMed] [Google Scholar]
Hall 2013 {published data only}
- Hall BJ, Schmidt RL, Sharma RR, Layfield LJ. Fine‐needle aspiration cytology for the diagnosis of metastatic melanoma: systematic review and meta‐analysis. American Journal of Clinical Pathology 2013;140(5):635‐42. [DOI] [PubMed] [Google Scholar]
Harlan 2010 {published data only}
- Harlan E, Davis MD, Pittelkow MR. Positron emission tomography/computed tomography: use for initial staging of malignant melanoma. International Journal of Dermatology 2010;49(9):1056‐8. [DOI] [PubMed] [Google Scholar]
Harris 2005 {published data only}
- Harris MT, Berlangieri SU, Cebon JS, Davis ID, Scott AM. Impact of 2‐deoxy‐2[F‐18]fluoro‐D‐glucose positron emission tomography on the management of patients with advanced melanoma. Molecular Imaging & Biology 2005;7(4):304‐8. [DOI] [PubMed] [Google Scholar]
Havenga 2003 {published data only}
- Havenga K, Cobben DC, Oyen WJ, Nienhuijs S, Hoekstra HJ, Ruers TJ, et al. Fluorodeoxyglucose‐positron emission tomography and sentinel lymph node biopsy in staging primary cutaneous melanoma. European Journal of Surgical Oncology 2003;29(8):662‐4. [DOI] [PubMed] [Google Scholar]
Heaston 1983 {published data only}
- Heaston DK, Putman CE, Rodan BA, Nicholson E, Ravin CE, Korobkin M, et al. Solitary pulmonary metastases in high‐risk melanoma patients: a prospective comparison of conventional and computed tomography. AJR. American Journal of Roentgenology 1983;141(1):169‐74. [DOI] [PubMed] [Google Scholar]
Herceg 2012 {published data only}
- Herceg GH, Bracic I, Kusacic‐Kuna S, Herceg D, Mutvar A, Dodig D. Ultrasound and US‐guided FNAC can reduce the number of sentinel lymph node biopsies in cutaneous melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(Suppl 2):S591. [Google Scholar]
Herceg 2013 {published data only}
- Herceg GH, Bracic I, Kusacic‐Kuna S, Mutvar A, Antulov J, Herceg D. Introduction of US‐guided FNAC in preoperative staging prior to sentinel lymph node biopsy: benefit for patients with cutaneous melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2013;40(Suppl 2):S483. [Google Scholar]
Herceg 2014 {published data only}
- Herceg GH, Bracic I, Kusacic‐Kuna S, Mutvar A, Antulov J, Herceg D. Introduction of US‐guided FNAC in preoperative staging prior to sentinel lymph node biopsy: benefit for patients with cutaneous melanoma. Nuklearmedizin 2014;53(2):A132. [Google Scholar]
Herceg 2015 {published data only}
- Herceg GH, Bracic I, Kralik M, Herceg D, Kusacic‐Kuna S, Antulov J. Benefits of performing ultrasound and US‐guided FNAC in melanoma patient scheduled for sentinel lymph node biopsy. European Journal of Nuclear Medicine and Molecular Imaging 2015;42(1 Suppl 1):S699‐700. [Google Scholar]
Heusner 2011 {published data only}
- Heusner T, Golitz P, Hamami M, Eberhardt W, Esser S, Forsting M, et al. "One‐stop‐shop" staging: should we prefer FDG‐PET/CT or MRI for the detection of bone metastases?. European Journal of Radiology 2011;78(3):430‐5. [DOI] [PubMed] [Google Scholar]
Hinz 2010 {published data only}
- Hinz T, Buchner A, Ahmadzadehfar H, Wenzel J, Bieber T, Schmid‐Wendtner MH. Ultrasound detection of a PET/CT negative lymph node metastasis in cutaneous melanoma. European Journal of Dermatology 2010;20(6):835‐6. [DOI] [PubMed] [Google Scholar]
Hofmann 2002 {published data only}
- Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow‐up in melanoma patients: monocenter evaluation of methods, costs and patient survival. British Journal of Cancer 2002; Vol. 87, issue 2:151‐7. [DOI: 10.1038/sj.bjc.6600428] [DOI] [PMC free article] [PubMed]
Hofmann 2011 {published data only}
- Hofmann MA, Schicke B, Fritsch A, Biesold S, Gussmann F, Kuchler I, et al. Impact of lymph node metastases on serum level of melanoma inhibitory activity in stage III melanoma patients. Journal of Dermatology 2011;38(9):880‐6. [DOI] [PubMed] [Google Scholar]
Hoh 1993 {published data only}
- Hoh CK, Hawkins RA, Glaspy JA, Dahlbom M, Tse NY, Hoffman EJ, et al. Cancer detection with whole‐body PET using 2‐[18F]fluoro‐2‐deoxy‐D‐glucose. Journal of Computer Assisted Tomography 1993;17(4):582‐9. [DOI] [PubMed] [Google Scholar]
Holder 1998 {published data only}
- Holder WD Jr, White RL Jr, Zuger JH, Easton EJ Jr, Greene FL. Effectiveness of positron emission tomography for the detection of melanoma metastases. Annals of Surgery 1998;227(5):764‐9; discussion 769‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holtas 1981 {published data only}
- Holtas S, Cronqvist S. Cranial computed tomography of patients with malignant melanoma. Neuroradiology 1981;22(3):123‐7. [DOI] [PubMed] [Google Scholar]
Horn 2006 {published data only}
- Horn J, Lock‐Andersen J, Sjostrand H, Loft A. Routine use of FDG‐PET scans in melanoma patients with positive sentinel node biopsy. European Journal of Nuclear Medicine & Molecular Imaging 2006;33(8):887‐92. [DOI] [PubMed] [Google Scholar]
Horn 2010 {published data only}
- Horn J, Sjostrand H, Lock‐Andersen J, Loft A. PET scanning for malignant melanoma and positive sentinel node diagnostics. Ugeskrift for Laeger 2010;172(15):1126‐30. [PubMed] [Google Scholar]
Ho Shon 2008 {published data only}
- Ho Shon IA, Chung DK, Saw RP, Thompson JF. Imaging in cutaneous melanoma. Nuclear Medicine Communications 2008;29(10):847‐76. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu YY, Lin XP, Liang PY, Zhang X, Zhang WG, Fan W. Application of 18F‐FDG PET/CT in diagnosis and staging of malignant melanoma. Chinese Journal of Medical Imaging Technology 2009;25(4):685‐8. [Google Scholar]
Hughes 2013 {published data only}
- Hughes MC, Wright A, Barbour A, Thomas J, Smithers BM, Green AC, et al. Patients undergoing lymphadenectomy for stage III melanomas of known or unknown primary site do not differ in outcome. International Journal of Cancer 2013;133(12):3000‐7. [DOI] [PubMed] [Google Scholar]
Hunyadi 2002 {published data only}
- Hunyadi J, Szakall Jr S, Gilde K, Begany A, Esik O, Tron L, et al. The role of PET scan in the diagnosis of malignant melanoma [A PET jelentosege a melanoma malignum diagnosztikajaban]. Orvosi Hetilap 2002;143(21 Suppl 3):1272‐5. [PubMed] [Google Scholar]
Iscoe 1987 {published data only}
- Iscoe N, Kersey P, Gapski J, Osoba D, From L, DeBoer G, et al. Predictive value of staging investigations in patients with clinical stage I malignant melanoma. Plastic & Reconstructive Surgery 1987;80(2):233‐9. [DOI] [PubMed] [Google Scholar]
Ismaheel 2016 {published data only}
- Ismaheel L, Lengana T, Vorster M, Sathekge M. 18F‐FDG PET/CT in the evaluation of recurrent malignant melanoma. Journal of Nuclear Medicine. Conference: Society of Nuclear Medicine and Molecular Imaging Annual Meeting, SNMMI 2016;57(Suppl 2):1569. [Google Scholar]
Jackson 2014 {published data only}
- Jackson JE, Burmeister BH, Burmeister EA, Foote MC, Thomas JM, Meakin JA, et al. Melanoma brain metastases: the impact of nodal disease. Clinical & Experimental Metastasis 2014;31(1):81‐5. [DOI] [PubMed] [Google Scholar]
Jadvar 2000 {published data only}
- Jadvar H, Johnson DL, Segall GM. The effect of fluorine‐18 fluorodeoxyglucose positron emission tomography on the management of cutaneous malignant melanoma. Clinical Nuclear Medicine 2000;25(1):48‐51. [DOI] [PubMed] [Google Scholar]
Jenicke 2001 {published data only}
- Jenicke L, Klutmann S, Bohuslavizki KH, Neuber K, Altenhoff J, Wedler J, et al. Conventional staging and 18F‐FDG‐PET staging of malignant melanoma. Radiology and Oncology 2001;35(2):95‐103+150. [Google Scholar]
Jennings 2009 {published data only}
- Jennings L, Murphy GM. Predicting outcome in melanoma: where are we now?. British Journal of Dermatology 2009;161(3):496‐503. [DOI] [PubMed] [Google Scholar]
Jimenez‐Requena 2010 {published data only}
- Jimenez‐Requena F, Delgado‐Bolton RC, Fernandez‐Perez C, Gambhir SS, Schwimmer J, Perez‐Vazquez JM, et al. Meta‐analysis of the performance of 18F‐FDG PET in cutaneous melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2010; Vol. 37, issue 2:284‐300. [DOI] [PMC free article] [PubMed]
Johnson 1997 {published data only}
- Johnson TM, Fader DJ, Chang AE, Yahanda A, Smith JW 2nd, Hamlet KR, et al. Computed tomography in staging of patients with melanoma metastatic to the regional nodes. Annals of Surgical Oncology 1997;4(5):396‐402. [DOI] [PubMed] [Google Scholar]
Jones 2014 {published data only}
- Jones MS, Guzman M, Rivera M, Baynosa JL, Hill CR, Kirgan DM. PET‐CT identifies regional nodal metastasis in cutaneous T4 melanoma. Annals of Surgical Oncology 2014;21(1 Suppl):S122. [Google Scholar]
Kader 2016 {published data only}
- Kader I, Leavers B, Shashinder S, Wylie B, Chi KK, Sundaresan P. Synchronous or metachronous lymphoma and metastatic cutaneous squamous cell carcinoma in the head and neck region: a diagnostic and management dilemma. Journal of Laryngology & Otology 2016;130(Suppl 4):S45‐9. [DOI] [PubMed] [Google Scholar]
Kelly 2013 {published data only}
- Kelly J. Does the addition of positron emission tomography/computed tomography (PET/CT) to the routine investigation and assessment of patients with melanoma yield clinical and economic benefits?. www.healthcareimprovementscotland.org/our_work/medicines_and_technology/shtg_‐_evidence_notes/evidence_note_48.aspx. NHS Quality Improvement Scotland (NHS QIS), (accessed before 11 February 2019).
Knappe 2000 {published data only}
- Knappe M, Louw M, Gregor RT. Ultrasonography‐guided fine‐needle aspiration for the assessment of cervical metastases. Archives of Otolaryngology‐Head & Neck Surgery 2000;126(9):1091‐6. [DOI] [PubMed] [Google Scholar]
Koskivuo 2007 {published data only}
- Koskivuo IO, Seppänen MP, Suominen EA, Minn HR. Whole body positron emission tomography in follow‐up of high risk melanoma. Acta Oncologica 2007;46(5):685‐90. [DOI] [PubMed] [Google Scholar]
Krug 2000 {published data only}
- Krug B, Dietlein M, Groth W, Stutzer H, Psaras T, Gossmann A, et al. Fluor‐18‐fluorodeoxyglucose positron emission tomography (FDG‐PET) in malignant melanoma. Diagnostic comparison with conventional imaging methods. Acta Radiologica 2000;41(5):446‐52. [DOI] [PubMed] [Google Scholar]
Krug 2008 {published data only}
- Krug B, Crott R, Lonneux M, Baurain JF, Pirson AS, Vander Borght T. Role of PET in the initial staging of cutaneous malignant melanoma: systematic review. Radiology 2008;249(3):836‐44. [DOI] [PubMed] [Google Scholar]
Krug 2009 {published data only}
- Krug B, Crott R, Roch I, Beguin C, Baurain J, Lonneux M, et al. The economic impact of PET‐CT in the management of malignant melanoma patients with pulmonary metastases. European Journal of Nuclear Medicine and Molecular Imaging 2009;36(2 Suppl):S354. [Google Scholar]
Krug 2010 {published data only}
Kuvshinoff 1997 {published data only}
- Kuvshinoff BW, Kurtz C, Coit DG. Computed tomography in evaluation of patients with stage III melanoma. Annals of Surgical Oncology 1997;4(3):252‐8. [DOI] [PubMed] [Google Scholar]
Lanka 2005 {published data only}
- Lanka B, Turner M, Orton C, Carrington BM. Cross‐sectional imaging in non‐melanoma skin cancer of the head and neck. Clinical Radiology 2005;60(8):869‐77. [DOI] [PubMed] [Google Scholar]
Laurent 2010 {published data only}
- Laurent V, Trausch G, Bruot O, Olivier P, Felblinger J, Regent D. Comparative study of two whole‐body imaging techniques in the case of melanoma metastases: advantages of multi‐contrast MRI examination including a diffusion‐weighted sequence in comparison with PET‐CT. European Journal of Radiology 2010;75(3):376‐83. [DOI] [PubMed] [Google Scholar]
Leon‐Ferre 2015 {published data only}
- Leon‐Ferre RA, Kottschade LA, Lowe VJ, Markovic S. Utility of PET/CT for surveillance of asymptomatic patients with resected stage III or IV melanoma. Journal of Clinical Oncology 2015;33(15 Suppl):9051. [Google Scholar]
Lewin 2015 {published data only}
- Lewin JH, Sanelli A, Walpole I, Kee D, Henderson MA, Speakman D, et al. Surveillance imaging with FDG‐PET in the follow‐up of melanoma patients at high risk of relapse. Journal of Clinical Oncology 2015;33(15 Suppl):9003. [DOI: 10.1200/jco.2015.33.15_suppl.9003] [DOI] [Google Scholar]
Liszkay 2010 {published data only}
- Liszkay G, Kasler M, Gilde K, Fejos Z, Lengyel Z, Borbola K, et al. False negative and false positive findings with 18F‐FDG PET/CT in malignant melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2010;37(Suppl 2):S442. [Google Scholar]
Loffler 2003 {published data only}
- Loffler M, Weckesser M, Franzius Ch, Nashan D, Schober O. Malignant melanoma and (18)F‐FDG‐PET: should the whole body scan include the legs?. Nuclear‐Medizin 2003;42(4):167‐72. [PubMed] [Google Scholar]
Longo 2003 {published data only}
- Longo MI, Lazaro P, Bueno C, Carreras JL, Montz R. Fluorodeoxyglucose‐positron emission tomography imaging versus sentinel node biopsy in the primary staging of melanoma patients. Dermatologic Surgery 2003;29(3):245‐8. [DOI] [PubMed] [Google Scholar]
Loose 1990 {published data only}
- Loose R, Weiss J, Simon R, Kuhn W, Teubner J. Detection and differential diagnosis of metastatic peripheral lymph nodes of the malignant melanoma: comparison of high resolution real‐time sonography and clinical findings [Erkennbarkeit Und Differentialdiagnose Metastatischer Peripherer Lymphknoten Des Malignen Melanoms. Ein Vergleich Von Hochauflosender Real‐Time‐Sonographie Und Klinischem Befund]. Aktuelle Dermatologie 1990;16(9‐10):262‐5. [Google Scholar]
Macfarlane 1998 {published data only}
- Macfarlane DJ, Sondak V, Johnson T, Wahl RL. Prospective evaluation of 2‐[18F]‐2‐deoxy‐D‐glucose positron emission tomography in staging of regional lymph nodes in patients with cutaneous malignant melanoma. Journal of Clinical Oncology 1998;16(5):1770‐6. [DOI] [PubMed] [Google Scholar]
Machet 2005 {published data only}
- Machet L, Nemeth‐Normand F, Giraudeau B, Perrinaud A, Tiguemounine J, Ayoub J, et al. Is ultrasound lymph node examination superior to clinical examination in melanoma follow‐up? A monocentre cohort study of 373 patients. British Journal of Dermatology 2005;152(1):66‐70. [DOI] [PubMed] [Google Scholar]
Majchrzak 2013 {published data only}
- Majchrzak E, Cholewinski W, Szybiak B, Luczewski L, Sowka M, Golusinski P, et al. Evaluation of the effectiveness of 8F‐FDG‐PET/CT examination of head and neck cancer ‐ own experience. Otolaryngologia Polska 2013;67(1):18‐24. [DOI] [PubMed] [Google Scholar]
Mayerhoefer 2012 {published data only}
- Mayerhoefer ME, Prosch H, Herold CJ, Weber M, Karanikas G. Assessment of pulmonary melanoma metastases with 18F‐FDG PET/CT: which PET‐negative patients require additional tests for definitive staging?. European Radiology 2012;22(11):2451‐7. [DOI] [PubMed] [Google Scholar]
McIvor 2014 {published data only}
- McIvor J, Siew T, Campbell A, McCarthy M. FDG PET in early stage cutaneous malignant melanoma. Journal of Medical Imaging & Radiation Oncology 2014;58(2):149‐54; quiz 266. [DOI] [PubMed] [Google Scholar]
McNamara 2005 {published data only}
- McNamara D, Chevreau C, Zerdoud S, Caselles O, Girault S, Gancel M, et al. Clinical utility of FDG‐PET in staging and assessment of therapy in melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2005;32(1 Suppl):S151. [Google Scholar]
Medina‐Quiroz 2010 {published data only}
- Medina‐Quiroz P, Martinez‐Rodriguez I, Banzo I, Quirce R, Jimenez‐Bonilla J, Arcocha M, et al. Clinical impact of FDG‐PET/CT scan in the initial staging and restaging of malignant melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2010;37(2 Suppl):S442. [Google Scholar]
Mendenhall 2012 {published data only}
- Mendenhall WM, Ferlito A, Takes RP, Bradford CR, Corry J, Fagan JJ, et al. Cutaneous head and neck basal and squamous cell carcinomas with perineural invasion. Oral Oncology 2012;48(10):918‐22. [DOI] [PubMed] [Google Scholar]
Mercier 2001 {published data only}
- Mercier GA, Alavi A, Fraker DL. FDG positron emission tomography in isolated limb perfusion therapy in patients with locally advanced melanoma: preliminary results. Clinical Nuclear Medicine 2001;26(10):832‐6. [DOI] [PubMed] [Google Scholar]
Meyers 2009 {published data only}
- Meyers MO, Yeh JJ, Amos KD, Long P, Frank J, Ollila DW. Utility of routine PET/CT and MRI staging in patients with sentinel lymph node positive melanoma. Journal of Clinical Oncology 2009;27(15 Suppl 1):9071. [Google Scholar]
Meyers 2009a {published data only}
- Meyers MO, Yeh JJ, Frank J, Long P, Deal AM, Amos KD, et al. Method of detection of initial recurrence of stage II/III cutaneous melanoma: analysis of the utility of follow‐up staging. Annals of Surgical Oncology 2009;16(4):941‐7. [DOI] [PubMed] [Google Scholar]
Mijnhout 2001 {published data only}
- Mijnhout GS, Hoekstra OS, Tulder MW, Teule GJ, Deville WL. Systematic review of the diagnostic accuracy of (18)F‐fluorodeoxyglucose positron emission tomography in melanoma patients. Cancer 2001;91(8):1530‐42. [PubMed] [Google Scholar]
Mijnhout 2002 {published data only}
- Mijnhout GS, Comans EF, Raijmakers P, Hoekstra OS, Teule GJ, Boers M, et al. Reproducibility and clinical value of 18F‐fluorodeoxyglucose positron emission tomography in recurrent melanoma. Nuclear Medicine Communications 2002;23(5):475‐81. [DOI] [PubMed] [Google Scholar]
Miner 2011 {published data only}
- Miner MT, Barsky M, Cherkassky L, Vezeridis M. The value of preoperative positron emission tomography/computed tomography (PET/CT) in patients with high‐risk. Annals of Surgical Oncology 2011;18(Suppl 1):S115. [DOI] [PubMed] [Google Scholar]
Minn 2011 {published data only}
- Minn H, Vihinen P. Melanoma imaging with highly specific PET probes: ready for prime time?. Journal of Nuclear Medicine 2011;52(1):5‐7. [DOI] [PubMed] [Google Scholar]
Miranda 2004 {published data only}
- Miranda EP, Gertner M, Wall J, Grace E, Kashani‐Sabet M, Allen R, et al. Routine imaging of asymptomatic melanoma patients with metastasis to sentinel lymph nodes rarely identifies systemic disease. Archives of Surgery 2004;139(8):831‐6; discussion 836‐7. [DOI] [PubMed] [Google Scholar]
Miranda 2006 {published data only}
- Miranda EP. Utility of computed tomography and magnetic resonance imaging staging before completion lymphadenectomy in sentinel lymph node‐positive melanoma. Journal of Clinical Oncology 2006;24(32):5178; author reply 5178. [DOI] [PubMed] [Google Scholar]
Mocellin 2007 {published data only}
- Mocellin S, Hoon DS, Pilati P, Rossi CR, Nitti D. Sentinel lymph node molecular ultrastaging in patients with melanoma: a systematic review and meta‐analysis of prognosis. Journal of Clinical Oncology 2007;25(12):1588‐95. [DOI] [PubMed] [Google Scholar]
Moehrle 1999 {published data only}
- Moehrle M, Blum A, Rassner G, Juenger M. Lymph node metastases of cutaneous melanoma: diagnosis by B‐scan and color Doppler sonography. Journal of the American Academy of Dermatology 1999;41(5 Pt 1):703‐9. [DOI] [PubMed] [Google Scholar]
Morton 2007 {published data only}
- Morton DL, Scheri RP, Balch CM. Can completion lymph node dissection be avoided for a positive sentinel node in melanoma?. Annals of Surgical Oncology 2007;14(9):2437‐9. [DOI] [PubMed] [Google Scholar]
Mosavi 2013 {published data only}
- Mosavi F, Ullenhag G, Ahlstrom H. Whole‐body MRI including diffusion‐weighted imaging compared to CT for staging of malignant melanoma. Upsala Journal of Medical Sciences 2013;118(2):91‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mottaghy 2007 {published data only}
- Mottaghy FM, Sunderkotter C, Schubert R, Wohlfart P, Blumstein NM, Neumaier B, et al. Direct comparison of [18F]FDG PET/CT with PET alone and with side‐by‐side PET and CT in patients with malignant melanoma.[Erratum appears in Eur J Nucl Med Mol Imaging. 2007 Sep;34(9):1365]. European Journal of Nuclear Medicine & Molecular Imaging 2007;34(9):1355‐64. [DOI] [PubMed] [Google Scholar]
Mozzillo 2013 {published data only}
- Mozzillo N, Caraco C, Marone U, Monta G, Crispo A, Botti G, et al. Superficial and deep lymph node dissection for stage III cutaneous melanoma: clinical outcome and prognostic factors. World Journal of Surgical Oncology 2013;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mruck 1999 {published data only}
- Mruck S, Baum RP, Rinne D, Hor G. Diagnostic accuracy and predictive value of the tumor‐associated antigen S100 in malignant melanomas: validation by whole body FDG‐PET and conventional diagnostics. Anticancer Research 1999;19(4A):2685‐90. [PubMed] [Google Scholar]
Muller 2006 {published data only}
- Muller SP. Malignant melanoma: PET/CT as a staging procedure. Frontiers of Radiation Therapy & Oncology 2006;39:159‐70. [DOI] [PubMed] [Google Scholar]
Muller‐Horvat 2006 {published data only}
- Muller‐Horvat C, Radny P, Eigentler TK, Schafer J, Pfannenberg C, Horger M, et al. Prospective comparison of the impact on treatment decisions of whole‐body magnetic resonance imaging and computed tomography in patients with metastatic malignant melanoma. European Journal of Cancer 2006;42(3):342‐50. [DOI] [PubMed] [Google Scholar]
Nazarian 1996 {published data only}
- Nazarian LN, Alexander AA, Rawool NM, Kurtz AB, Maguire HC, Mastrangelo MJ. Malignant melanoma: impact of superficial US on management. Radiology 1996;199(1):273‐7. [DOI] [PubMed] [Google Scholar]
Nazarian 1998 {published data only}
- Nazarian LN, Alexander AA, Kurtz AB, Capuzzi DM Jr, Rawool NM, Gilbert KR, et al. Superficial melanoma metastases: appearances on gray‐scale and color Doppler sonography. AJR. American Journal of Roentgenology 1998;170(2):459‐63. [DOI] [PubMed] [Google Scholar]
Niebling 2013a {published data only}
- Niebling M, Bastiaannet E, Hoekstra O, Bonenkamp H, Koelemij R, Hoekstra HJ. Survival and recurrence in clinical stage III melanoma patients with whole body FDG‐PET and CT added to the diagnostic work‐up. Annals of Surgical Oncology 2013;20(1 Suppl):S100. [DOI] [PubMed] [Google Scholar]
Niebling 2013b {published data only}
- Niebling MG, Bastiaannet E, Hoekstra OS, Bonenkamp JJ, Koelemij R, Hoekstra HJ. Outcome of clinical stage III melanoma patients with FDG‐PET and whole‐body CT added to the diagnostic workup. Annals of Surgical Oncology 2013;20(9):3098‐105. [DOI] [PubMed] [Google Scholar]
Niederkohr 2007 {published data only}
- Niederkohr RD, Rosenberg J, Shabo G, Quon A. Clinical value of including the head and lower extremities in 18F‐FDG PET/CT imaging for patients with malignant melanoma. Nuclear Medicine Communications 2007;28(9):688‐95. [DOI] [PubMed] [Google Scholar]
Novikov 2012 {published data only}
- Novikov SN, Kanaev SV, Gotovchikova MU, Jukova LA, Anisimov VV, Gafton GI. Diagnostic value of melanoma visualization with 99mTc‐MIBI. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(2 Suppl):S475. [Google Scholar]
Oehr 1999 {published data only}
- Oehr P, Stegemann G, Steen K, Ruhlmann J. The value of FDG‐PET whole body imaging, conventional imaging, and serum S‐100 determinations in metastatic malignant melanoma. Clinical Laboratory 1999;45(9‐10):523‐8. [Google Scholar]
Ogata 2014 {published data only}
- Ogata D, Uematsu T, Yoshikawa S, Kiyohara Y. Accuracy of real‐time ultrasound elastography in the differential diagnosis of lymph nodes in cutaneous malignant melanoma (CMM): a pilot study. International Journal of Clinical Oncology 2014;19(4):716‐21. [DOI] [PubMed] [Google Scholar]
Omlor 1996 {published data only}
- Omlor G, Dill‐Muller D, Gross G, Kautz G, Schuder G, Zaun H, et al. Elective lymph node dissection in malignant melanoma ‐ status of color Doppler findings. Zentralblatt fur Chirurgie 1996;121(6):469‐73. [PubMed] [Google Scholar]
Orfaniotis 2012 {published data only}
- Orfaniotis G, Mennie JC, Fairbairn N, Butterworth M. Findings of computed tomography in stage IIB and IIC melanoma: a six‐year retrospective study in the South‐East of Scotland. Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS 2012;65(9):1216‐9. [DOI] [PubMed] [Google Scholar]
Ortega‐Candil 2016 {published data only}
- Ortega‐Candil A, Rodriguez‐Rey C, Cano‐Carrizal R, Cala‐Zuluaga E, Gonzalez Larriba JL, Jimenez‐Ballve A, et al. Breslow thickness and (18)F‐FDG PET‐CT result in initial staging of cutaneous melanoma: can a cut‐off point be established?. Revista Espanola de Medicina Nuclear e Imagen Molecular 2016;35(2):96‐101. [DOI] [PubMed] [Google Scholar]
Padovano 2013 {published data only}
- Padovano B, Alessi A, Maurichi A, Serafini G, Bampo C, Santinami M, et al. F‐18 FDG‐PET/CT in the follow‐up of patients (pts) with stage II‐III malignant melanoma (MM): the experience of the National Cancer Institute of Milano. European Journal of Nuclear Medicine and Molecular Imaging 2013;40(2 Suppl):S111. [Google Scholar]
Panagiotou 2001 {published data only}
- Panagiotou IE, Brountzos EN, Bafaloukos D, Tsavaris N, Mylonakis N, Karabelis A, et al. Evaluation of imaging studies at the initial staging and during follow‐up of patients with local‐regional malignant melanoma. Journal of B.U.ON. 2001;6(4):411‐4. [Google Scholar]
Pandalai 2011 {published data only}
- Pandalai PK, Dominguez FJ, Michaelson J, Tanabe KK. Clinical value of radiographic staging in patients diagnosed with AJCC stage III melanoma. Annals of Surgical Oncology 2011;18(2):506‐13. [DOI] [PubMed] [Google Scholar]
Paquet 2000 {published data only}
- Paquet P, Henry F, Belhocine T, Hustinx R, Najjar F, Pierard‐Franchimont C, et al. An appraisal of 18‐fluorodeoxyglucose positron emission tomography for melanoma staging. Dermatology 2000;200(2):167‐9. [DOI] [PubMed] [Google Scholar]
Pecegueiro 2005 {published data only}
- Pecegueiro MM, Salgado L, Moura C, Sachse MF, Rafael M, Vieira MR, et al. Comparative study to evaluate the benefits of positron emission tomography (PET) versus computed tomography (CT) in malignant melanoma patients. Skin Cancer 2005;20(4):197‐202. [Google Scholar]
Pellacani 2006 {published data only}
- Pellacani G, Giannelli P, Longo C, Bassoli S, Seidenari S. Perspective evaluation of a four‐year period of application of a follow‐up protocol for melanoma patient management. Giornale Italiano di Dermatologia e Venereologia 2006;141(2):107‐15. [Google Scholar]
Peric 2011 {published data only}
- Peric B, Zagar I, Novakovic S, Zgajnar J, Hocevar M. Role of serum S100B and PET‐CT in follow‐up of patients with cutaneous melanoma. BMC Cancer 2011;11:328. [PUBMED: 21810220] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2016 {published data only}
- Petersen H, Holdgaard PC, Madsen PH, Knudsen LM, Gad D, Gravergaard AE, et al. FDG PET/CT in cancer: comparison of actual use with literature‐based recommendations. European Journal of Nuclear Medicine and Molecular Imaging 2016;43(4):695‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pleiss 2007 {published data only}
- Pleiss C, Risse JH, Biersack HJ, Bender H. Role of FDG‐PET in the assessment of survival prognosis in melanoma. Cancer Biotherapy & Radiopharmaceuticals 2007;22(6):740‐7. [DOI] [PubMed] [Google Scholar]
Poduje 2012 {published data only}
- Poduje S, Huljev D, Cubrilovic Z, Bosnjak J. Squamous cell carcinoma ‐ case report. Acta Medica Croatica 2012;66(Suppl 1):123‐6. [PubMed] [Google Scholar]
Poyraz 2012 {published data only}
- Poyraz NY, Ozdemir E, Kandemir Z, Keskin M, Dede DS, Turkolmez S. The contribution of 18‐F FDG PET/CT in assessment of the extent of disease and management strategy in cutaneous malignant melanoma [Kutanoz malign melanomda hastaligin yayginligini belirlemede ve tedavi seciminde 18‐F FDG PET/BT'nin katkisi]. Gazi Medical Journal 2012;23(3):62‐5. [Google Scholar]
Prakoso 2007 {published data only}
- Prakoso E, Selby WS. Capsule endoscopy in patients with malignant melanoma. American Journal of Gastroenterology 2007;102(6):1204‐8. [DOI] [PubMed] [Google Scholar]
Prakoso 2011 {published data only}
- Prakoso E, Fulham M, Thompson JF, Selby WS. Capsule endoscopy versus positron emission tomography for detection of small‐bowel metastatic melanoma: a pilot study. Gastrointestinal Endoscopy 2011;73(4):750‐6. [DOI] [PubMed] [Google Scholar]
Prichard 2002 {published data only}
- Prichard RS, Hill AD, Skehan SJ, O'Higgins NJ. Positron emission tomography for staging and management of malignant melanoma. British Journal of Surgery 2002;89(4):389‐96. [DOI] [PubMed] [Google Scholar]
Punjabi 2006 {published data only}
- Punjabi SP, Blomley MJ, Cosgrove D, Teixeira F, Chu AC. Microbubble ultrasound: how can it help detect melanoma metastasis?. International Journal of Dermatology 2006;45(8):1004‐6. [DOI] [PubMed] [Google Scholar]
Querellou 2010 {published data only}
- Querellou S, Keromnes N, Abgral R, Sassolas B, Roux PY, Cavarec MB, et al. Clinical and therapeutic impact of 18F‐FDG PET/CT whole‐body acquisition including lower limbs in patients with malignant melanoma. Nuclear Medicine Communications 2010;31(9):766‐72. [DOI] [PubMed] [Google Scholar]
Querellou 2011 {published data only}
- Querellou S. Clinical and therapeutic impact of 18F‐FDG PET/CT whole‐body acquisition including lower limbs on patients with malignant melanoma. Nuclear Medicine Communications 2011;32(9):873. [DOI] [PubMed] [Google Scholar]
Ramirez 2015 {published data only}
- Ramirez YE, Santos I, Martinez A, Rodado S, Hernandez I, Escabias C, et al. Does 18F‐FDG PET‐CT field of view that includes vertex have an impact on oncological patients management. European Journal of Nuclear Medicine and Molecular Imaging 2015;42(1 Suppl):S719. [Google Scholar]
Renna 2015 {published data only}
- Renna MA, Niccoli Asabella A, Loseto V, Iuele F, Simone F, Rubini G. The role of radio‐guided sentinel node biopsy and <sup>18</sup>F‐FDG‐PET in the management of cutaneous head‐neck melanoma patients. Clinical and Translational Imaging 2015;3(1 Suppl):S111. [Google Scholar]
Rep 2011 {published data only}
- Rep S, Fettich J. SPECT/CT imaging for sentinel node mapping. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(2 Suppl):S458. [Google Scholar]
Rinne 1998 {published data only}
- Rinne D, Baum RP, Hor G, Kaufmann R. Primary staging and follow‐up of high risk melanoma patients with whole‐body 18F‐fluorodeoxyglucose positron emission tomography: results of a prospective study of 100 patients. Cancer 1998;82(9):1664‐71. [DOI] [PubMed] [Google Scholar]
Roarke 2008 {published data only}
- Roarke M, Nguyen BD, Pockaj BA. Desmoplastic melanoma: true positive and false negative findings on F‐18 FDG‐PET/CT. Clinical Nuclear Medicine 2008;33(8):562‐4. [DOI] [PubMed] [Google Scholar]
Roh 2008 {published data only}
- Roh JL, Moon BJ, Kim JS, Lee JH, Cho KJ, Choi SH, et al. Use of 18F‐fluorodeoxyglucose positron emission tomography in patients with rare head and neck cancers. Clinical & Experimental Otorhinolaryngology 2008;1(2):103‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossi 1997 {published data only}
- Rossi CR, Seno A, Vecchiato A, Foletto M, Tregnaghi A, Candia A, et al. The impact of ultrasound scanning in the staging and follow‐up of patients with clinical stage I cutaneous melanoma. European Journal of Cancer 1997;33(2):200‐3. [DOI] [PubMed] [Google Scholar]
Rossi 1999 {published data only}
- Rossi CR, Scagnet B, Vecchiato A, Casara D, Rubaltelli L, Montesco MC, et al. Sentinel node biopsy (SNB) and ultrasound (US) scanning in cutaneous melanoma: technical and clinical considerations. European Journal of Nuclear Medicine 1999;26(4):S57. [DOI] [PubMed] [Google Scholar]
Rossi 2000 {published data only}
- Rossi CR, Scagnet B, Vecchiato A, Mocellin S, Pilati P, Foletto M, et al. Sentinel node biopsy and ultrasound scanning in cutaneous melanoma: clinical and technical considerations. European Journal of Cancer 2000;36(7):895‐900. [DOI] [PubMed] [Google Scholar]
Rossi 2003 {published data only}
- Rossi CR, Mocellin S, Scagnet B, Foletto M, Vecchiato A, Pilati P, et al. The role of preoperative ultrasound scan in detecting lymph node metastasis before sentinel node biopsy in melanoma patients. Journal of Surgical Oncology 2003;83(2):80‐4. [DOI] [PubMed] [Google Scholar]
Rossi 2008 {published data only}
- Rossi CR, Pasquali S, Mocellin S. Actual false‐negative rate prompts the routine use of ultrasound scan before and after sentinel node biopsy in melanoma. Annals of Surgical Oncology 2008;15(10):2976‐7. [DOI] [PubMed] [Google Scholar]
Rudolph 2010 {published data only}
- Rudolph N, Spillane J, Speakman D. Ineffectiveness of FDG‐PET in the upfront staging of primary melanoma thicker than 4 mm. Pigment Cell and Melanoma Research 2010;23(6):971. [Google Scholar]
Sadigh 2014 {published data only}
- Sadigh G, Applegate KE, Baumgarten DA. Comparative accuracy of intravenous contrast‐enhanced CT versus noncontrast CT plus intravenous contrast‐enhanced CT in the detection and characterization of patients with hypervascular liver metastases: a critically appraised topic. Academic Radiology 2014;21(1):113‐25. [DOI] [PubMed] [Google Scholar]
Saiag 2005 {published data only}
- Saiag P, Bernard M, Beauchet A, Bafounta ML, Bourgault‐Villada I, Chagnon S. Ultrasonography using simple diagnostic criteria vs palpation for the detection of regional lymph node metastases of melanoma. Archives of Dermatology 2005;141(2):183‐9. [DOI] [PubMed] [Google Scholar]
Saiag 2010 {published data only}
- Saiag P, Lebbe C, Basset Seguin N, Wolkenstein P, Dupin N, Descamps V, et al. Role of lymph‐node ultrasonography (US) in the follow‐up of melanoma patients to detect nodal recurrence after sentinel lymph node biopsy (SNLB): a prospective cohort study. Journal of Clinical Oncology 2010;28(15 Suppl):8576. [Google Scholar]
Samimi 2010 {published data only}
- Samimi M, Perrinaud A, Naouri M, Maruani A, Perrodeau E, Vaillant L, et al. High‐resolution ultrasonography assists the differential diagnosis of blue naevi and cutaneous metastases of melanoma. British Journal of Dermatology 2010;163(3):550‐6. [DOI] [PubMed] [Google Scholar]
Samples 2012 {published data only}
- Samples J, Meyers MO, Deal AM, Baker JJ, Frank JS, Ollila DW. Impact of PET/CT at the time of clinically detected regionally recurrent cutaneous melanoma. Annals of Surgical Oncology 2012;19(1 Suppl):S124‐5. [Google Scholar]
Sanli 2010 {published data only}
- Sanli Y, Has D, Gecer F, Yilmaz E, Ekmekci S, Turkmen C, et al. The use of PET‐CT for staging and restaging purpose in malign melanoma: preliminary results. European Journal of Nuclear Medicine and Molecular Imaging 2010;37(2 Suppl):S443. [Google Scholar]
Santha 2011 {published data only}
- Santha O, Varga J, Olajos J, Garai I. Prognostic value of staging 18FDG PET/CT in patients with malignant melanoma in correlation with clarke level. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(2 Suppl):S385. [Google Scholar]
Sarandi 2008 {published data only}
- Sarandi F, Hindie E, Kerob D, Basset‐Seguin N, Lebbe C, Toubert ME, et al. Use of fluorine‐18‐FDG PET‐CT scans in initial management and follow‐up of patients with cutaneous melanoma [Role de la TEP‐TDM au fluor18‐FDG dans la prise en charge initiale et le suivi des melanomes cutanes]. Annales de Dermatologie et de Venereologie 2008;135(10):691‐9. [DOI] [PubMed] [Google Scholar]
Sawyer 2009 {published data only}
- Sawyer A, McGoldrick RB, Mackey SP, Allan R, Powell B. Does staging computered tomography change management in thick malignant melanoma?. Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS 2009;62(4):453‐6. [DOI] [PubMed] [Google Scholar]
Schafer‐Hesterberg 2007 {published data only}
- Schafer‐Hesterberg G, Schoengen A, Sterry W, Voit C. Use of ultrasound to early identify, diagnose and localize metastases in melanoma patients. Expert Review of Anticancer Therapy 2007;7(12):1707‐16. [DOI] [PubMed] [Google Scholar]
Schafer‐Hesterberg 2008 {published data only}
- Schafer‐Hesterberg G, Voit C. Ultrasound pre sentinel node dissection. Melanoma Research 2008;18(1):68‐9. [DOI] [PubMed] [Google Scholar]
Schauwecker 2003 {published data only}
- Schauwecker DS, Siddiqui AR, Wagner JD, Davidson D, Jung SH, Carlson KA, et al. Melanoma patients evaluated by four different positron emission tomography reconstruction techniques. Nuclear Medicine Communications 2003;24(3):281‐9. [DOI] [PubMed] [Google Scholar]
Scheier 2015 {published data only}
- Scheier B, Lao CD, Kidwell KM, Redman BG. Utility of pre‐operative PET/CT staging in sentinel lymph node‐positive melanoma. Journal of Clinical Oncology. Conference 2015;33(15 Suppl):6566. [DOI: 10.1200/jco.2015.33.15_suppl.6566] [DOI] [Google Scholar]
Scheier 2016 {published data only}
- Scheier BY, Lao CD, Kidwell KM, Redman BG. Use of preoperative PET/CT staging in sentinel lymph node‐positive melanoma. JAMA Oncology 2016;2(1):136‐7. [DOI] [PubMed] [Google Scholar]
Schmid‐Wendtner 2002 {published data only}
- Schmid‐Wendtner MH, Partscht K, Korting HC, Volkenandt M. Improved differentiation of benign and malignant lymphadenopathy in patients with cutaneous melanoma by contrast‐enhanced color Doppler sonography. Archives of Dermatology 2002;138(4):491‐7. [DOI] [PubMed] [Google Scholar]
Schmid‐Wendtner 2003 {published data only}
- Schmid‐Wendtner MH, Paerschke G, Baumert J, Plewig G, Volkenandt M. Value of ultrasonography compared with physical examination for the detection of locoregional metastases in patients with cutaneous melanoma. Melanoma Research 2003;13(2):183‐8. [DOI] [PubMed] [Google Scholar]
Schmid‐Wendtner 2004 {published data only}
- Schmid‐Wendtner MH, Dill‐Muller D, Baumert J, Wagner A, Eberle J, Tilgen W, et al. Lymph node metastases in patients with cutaneous melanoma: improvements in diagnosis by signal‐enhanced color Doppler sonography. Melanoma Research 2004;14(4):269‐76. [DOI] [PubMed] [Google Scholar]
Schule 2016 {published data only}
- Schule SC, Eigentler TK, Garbe C, Fougere C, Nikolaou K, Pfannenberg C. Influence of 18F‐FDG PET/CT on therapy management in patients with stage III/IV malignant melanoma. European Journal of Nuclear Medicine and Molecular Imaging 2016;43(3):482‐8. [DOI] [PubMed] [Google Scholar]
Schwimmer 2000 {published data only}
- Schwimmer J, Essner R, Patel A, Jahan SA, Shepherd JE, Park K, et al. A review of the literature for whole‐body FDG PET in the management of patients with melanoma. Quarterly Journal of Nuclear Medicine 2000;44(2):153‐67. [PubMed] [Google Scholar]
Sergieva 2012 {published data only}
- Sergieva SB, Alexandrova E, Baichev G, Dimcheva M, Troianova P, Parvanova V, et al. Role of SPECT‐CT in detection of sentinel lymph nodes in breast cancer and melanoma patients. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(2 Suppl):S176. [Google Scholar]
Serra‐Arbeloa 2015 {published data only}
- Serra‐Arbeloa P, Rabines‐Juarez AO, Alvarez‐Ruiz S, Guillen‐Grima F. The role of preoperative lymph node ultrasound in patients with primary cutaneous melanoma: a meta‐analysis. European Journal of Nuclear Medicine and Molecular Imaging 2015;42(1 Suppl):S155. [Google Scholar]
Seshadri 2006 {published data only}
- Seshadri N, Wat J, Balan K. Bilateral adrenal metastases from malignant melanoma: concordant findings on (18)F‐FDG and (18)F‐FDOPA PET. European Journal of Nuclear Medicine & Molecular Imaging 2006;33(7):854‐5. [DOI] [PubMed] [Google Scholar]
Shah 2015 {published data only}
- Shah R, Johnson J, Rohren E, Schellingerhout D. Accuracy of screening for brain metastases with whole‐body FDG PET in initial staging of non‐central nervous system malignancy. Neuro‐Oncology 2015;17:v51. [Google Scholar]
Shintani 2008 {published data only}
- Shintani SA, Foote RL, Lowe VJ, Brown PD, Garces YI, Kasperbauer JL. Utility of PET/CT imaging performed early after surgical resection in the adjuvant treatment planning for head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 2008;70(2):322‐9. [DOI] [PubMed] [Google Scholar]
Sigmund 1985 {published data only}
- Sigmund G, Bahren W, Ranzinger G, Haase S. Value of computerized tomography in the diagnosis of recurrence in malignant head and neck tumors. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 1985;143(4):398‐407. [DOI] [PubMed] [Google Scholar]
Sijan 2010 {published data only}
- Sijan G, Kozarski J, Stefanovic D, Lalkovic M, Milicevic S, Stankovic G. Ultrasonographic findings validity in the identification of metastatic regional lymph nodes in patients with cutaneous melanoma. Vojnosanitetski Pregled 2010;67(1):25‐31. [DOI] [PubMed] [Google Scholar]
Singnurkar 2016 {published data only}
- Singnurkar A, Wang J, Joshua AM, Langer DL, Metser U. 18F‐FDG‐PET/CT in the staging and management of melanoma: a prospective multicenter Ontario PET registry study. Clinical Nuclear Medicine 2016;41(3):189‐93. [DOI] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith E, Robson Y, Bhatia S, Morris A. Lymph node imaging in high‐risk cutaneous squamous cell carcinoma. British Journal of Dermatology 2011;165(Suppl 1):101. [Google Scholar]
Sofue 2012 {published data only}
- Sofue K, Tateishi U, Tsurusaki M, Arai Y, Yamazaki N, Sugimura K. MR imaging of hepatic metastasis in patients with malignant melanoma: evaluation of suspected lesions screened at contrast‐enhanced CT. European Journal of Radiology 2012;81(4):714‐8. [DOI] [PubMed] [Google Scholar]
Soler 1997 {published data only}
- Soler C, Perrot JL, Thiffet O, Beauchesne P, Lanthier K, Boucheron S, et al. The role of technetium‐99m sestamibi single photon emission tomography in the follow‐up of malignant melanoma and the detection of lymph node metastases. European Journal of Nuclear Medicine 1997;24(12):1522‐5. [DOI] [PubMed] [Google Scholar]
Solivetti 2006 {published data only}
- Solivetti FM, Luca Sidozzi A, Pirozzi G, Coscarella G, Brigida R, Eibenshutz L. Sonographic evaluation of clinically occult in‐transit and satellite metastases from cutaneous malignant melanoma. Radiologia Medica 2006;111(5):702‐8. [DOI] [PubMed] [Google Scholar]
Solivetti 2012 {published data only}
- Solivetti FM, Elia F, Graceffa D, Carlo A. Ultrasound morphology of inguinal lymph nodes may not herald an associated pathology. Journal of Experimental & Clinical Cancer Research 2012;31:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solivetti 2014 {published data only}
- Solivetti FM, Elia F, Santaguida MG, Guerrisi A, Visca P, Cercato MC, et al. The role of ultrasound and ultrasound‐guided fine needle aspiration biopsy of lymph nodes in patients with skin tumours. Radiology & Oncology 2014;48(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2004 {published data only}
- Solomon J, Mavinkurve S, Cox D, Summers RM. Computer‐assisted detection of subcutaneous melanomas: feasibility assessment. Academic Radiology 2004;11(6):678‐85. [DOI] [PubMed] [Google Scholar]
Son 2016 {published data only}
- Son SH, Kang SM, Jeong SY, Lee SW, Lee SJ, Lee J, et al. Prognostic value of volumetric parameters measured by pretreatment 18F FDG PET/CT in patients with cutaneous malignant melanoma. Clinical Nuclear Medicine 2016;41(6):e266‐73. [DOI] [PubMed] [Google Scholar]
Srivastava 2012 {published data only}
- Srivastava A, Woodcock JP, Mansel RE, Webster DJ, Laidler P, Hughes LE, et al. Doppler ultrasound flowmetry predicts 15 year outcome in patients with skin melanoma. Indian Journal of Surgery 2012;74(4):278‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Starritt 2005 {published data only}
- Starritt EC, Uren RF, Scolyer RA, Quinn MJ, Thompson JF. Ultrasound examination of sentinel nodes in the initial assessment of patients with primary cutaneous melanoma. Annals of Surgical Oncology 2005;12(1):18‐23. [DOI] [PubMed] [Google Scholar]
Stas 2002 {published data only}
- Stas M, Stroobants S, Dupont P, Gysen M, Hoe L Van, Garmyn M, et al. 18‐FDG PET scan in the staging of recurrent melanoma: additional value and therapeutic impact. Melanoma Research 2002;12(5):479‐90. [DOI] [PubMed] [Google Scholar]
Stecco 2016 {published data only}
- Stecco A, Ciolfi S, Buemi F, Cassarà A, Sacchetti GM, Brambilla M, et al. Combined multimodal co‐registration of PET/CT and MRI images increases diagnostic accuracy in squamous cell carcinoma staging. Radiologia Medica 2016;121(6):502‐9. [DOI] [PubMed] [Google Scholar]
Steinert 1998 {published data only}
- Steinert HC, Voellmy DR, Trachsel C, Bicik I, Buck A, Huch RA, et al. Planar coincidence scintigraphy and PET in staging malignant melanoma. Journal of Nuclear Medicine 1998;39(11):1892‐7. [PubMed] [Google Scholar]
Stoffels 2012 {published data only}
- Stoffels I, Dissemond J, Poeppel T, Klotgen K, Hillen U, Korber A, et al. Advantages of preoperative ultrasound in conjunction with lymphoscintigraphy in detecting malignant melanoma metastases in sentinel lymph nodes: a retrospective analysis in 221 patients with malignant melanoma AJCC Stages I and II. Journal of the European Academy of Dermatology & Venereology 2012;26(1):79‐85. [DOI] [PubMed] [Google Scholar]
Stoffels 2014 {published data only}
- Stoffels I, Gunzer M, Leyh J, Hillen U, Helfrich I, Schadendorf D, et al. The photo optoacoustic tomography for the non‐invasive and non‐radioactive Identification of the sentinel lymph node status in melanoma patients. Journal Der Deutschen Dermatologischen Gesellschaft 2014;12:5‐6. [Google Scholar]
Stoffels 2016 {published data only}
- Stoffels I, Petri M, Morscher S, Burton N, Schadendorf D, Gunzer M, et al. Clinical application of noninvasive and nonradioactive determination of microscopic lymph node tumor status by multispectral optoacoustic imaging. Journal of Nuclear Medicine. Conference: Society of Nuclear Medicine and Molecular Imaging Annual Meeting, SNMMI 2016;56(3):40. [Google Scholar]
Stretch 2005 {published data only}
- Stretch JR, Somorjai R, Bourne R, Hsiao E, Scolyer RA, Dolenko B, et al. Melanoma metastases in regional lymph nodes are accurately detected by proton magnetic resonance spectroscopy of fine‐needle aspirate biopsy samples. Annals of Surgical Oncology 2005;12(11):943‐9. [DOI] [PubMed] [Google Scholar]
Stucker 2002 {published data only}
- Stucker M, Esser M, Hoffmann M, Memmel U, Hirschmuller A, Bormann C, et al. High‐resolution laser Doppler perfusion imaging aids in differentiating between benign and malignant melanocytic skin tumours. Acta Dermato‐Venereologica 2002;82(1):25‐9. [DOI] [PubMed] [Google Scholar]
Subesinghe 2012 {published data only}
- Subesinghe M, Marples M, Scarsbrook AF, Smith JT. Clinical impact of 18F‐FDG PET‐CT on management decisions in patients with malignant melanoma. Nuclear Medicine Communications 2012;33(5):546. [Google Scholar]
Subesinghe 2013 {published data only}
- Subesinghe M, Marples M, Scarsbrook AF, Smith JT. Clinical impact of (18)F‐FDG PET‐CT in recurrent stage III/IV melanoma: a tertiary centre Specialist Skin Cancer Multidisciplinary Team (SSMDT) experience. Insights Into Imaging 2013;4(5):701‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supriya 2014 {published data only}
- Supriya M, Suat‐Chin N, Sizeland A. Use of positron emission tomography scanning in metastatic head and neck cutaneous squamous cell cancer: does it add to patient management?. American Journal of Otolaryngology 2014;35(3):347‐52. [DOI] [PubMed] [Google Scholar]
Swetter 2002 {published data only}
- Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Annals of Surgical Oncology 2002;9(7):646‐53. [DOI] [PubMed] [Google Scholar]
Tejera‐Vaquerizo 2007 {published data only}
- Tejera‐Vaquerizo A, Barrera‐Vigo MV, Fernandez‐Canedo I, Blazquez‐Sanchez N, Mendiola‐Fernandez M, Fernandez‐Orland A, et al. Longitudinal study of different metastatic patterns in the progression of cutaneous melanoma. Actas Dermo‐Sifiliograficas 2007;98(8):531‐8. [PubMed] [Google Scholar]
Testori 2005 {published data only}
- Testori A, Lazzaro G, Baldini F, Tosti G, Mosconi M, Lovati E, et al. The role of ultrasound of sentinel nodes in the pre‐ and post‐operative evaluation of stage I melanoma patients. Melanoma Research 2005;15(3):191‐8. [DOI] [PubMed] [Google Scholar]
Thompson 2002 {published data only}
- Thompson JF, Shaw HM. The prognosis of patients with thick primary melanomas: is regional lymph node status relevant, and does removing positive regional nodes influence outcome?. Annals of Surgical Oncology 2002;9(8):719‐22. [DOI] [PubMed] [Google Scholar]
Thompson 2011 {published data only}
- Thompson JF, Haydu LE, Uren RF, Cochran AJ, We DR, Morton DM. Preoperative ultrasound assessment of sentinel nodes in melanoma patients does not provide reliable staging: results from a large multicenter trial. Annals of Surgical Oncology 2011;18(1 Suppl):S21. [Google Scholar]
Tomaszewski 2014 {published data only}
- Tomaszewski JM, Lau E, Corry J. Utility of positron emission tomography/computed tomography for nodal staging of cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. American Journal of Otolaryngology 2014;35(1):66‐9. [DOI] [PubMed] [Google Scholar]
Tregnaghi 1997 {published data only}
- Tregnaghi A, Candia A, Calderone M, Cellini L, Rossi CR, Talenti E, et al. Ultrasonographic evaluation of superficial lymph node metastases in melanoma. European Journal of Radiology 1997;24(3):216‐21. [DOI] [PubMed] [Google Scholar]
Tyler 2000 {published data only}
- Tyler DS, Onaitis M, Kherani A, Hata A, Nicholson E, Keogan M, et al. Positron emission tomography scanning in malignant melanoma. Cancer 2000;89(5):1019‐25. [PubMed] [Google Scholar]
Ulrich 2015 {published data only}
- Ulrich J, Akkooi AC, Eggermont AM, Voit CA. Sonographic criteria for diagnosing sentinel node metastases in melanoma patients. Ultraschall in der Medizin 2015;36(2):149‐53. [DOI] [PubMed] [Google Scholar]
Uren 1999 {published data only}
- Uren RF, Howman‐Giles R, Thompson JF, Shaw HM, Roberts JM, Bernard E, et al. High‐resolution ultrasound to diagnose melanoma metastases in patients with clinically palpable lymph nodes. Australasian Radiology 1999;43(2):148‐52. [DOI] [PubMed] [Google Scholar]
Valdes 2011 {published data only}
- Valdes Olmos RA, Brouwer O, Klop M, Balm AJ, Brekel MW. SPECT/CT and concomitant low dose CT for anatomical sentinel node localisation in head and neck malignancies. European Journal of Nuclear Medicine and Molecular Imaging 2011;38(2 Suppl):S126. [Google Scholar]
Valk 1996 {published data only}
- Valk PE, Pounds TR, Tesar RD, Hopkins DM, Haseman MK. Cost‐effectiveness of PET imaging in clinical oncology. Nuclear Medicine & Biology 1996;23(6):737‐43. [DOI] [PubMed] [Google Scholar]
Van Akkooi 2012 {published data only}
- Akkooi AC, Gooskens S, Siegel P, Schaefer‐Hesterberg G, Schoengen A, Roewert‐Huber J, et al. Sensitivity rate of ultrasound (US)‐guided fine‐needle aspiration cytology (FNAC) using the Berlin morphology criteria for lymph node metastases to reduce the need for surgical sentinel node (SN) staging in melanoma. Journal of Clinical Oncology 2012;30(15 Suppl):8535. [DOI: 10.1200/jco.2012.30.15_suppl.8535] [DOI] [Google Scholar]
Van Akkooi 2013 {published data only}
- Akkooi AC, Siegel P, Gooskens S, Schoengen A, Sterry W, Eggermont AM, et al. Use of preoperative ultrasound (US)‐guided fine needle aspiration cytology (FNAC) to identify positive sentinel nodes (SN) in melanoma. Journal of Clinical Oncology 2013;31(15 Suppl):e20035. [DOI: 10.1200/jco.2013.31.15_suppl.e20035] [Google Scholar]
Van Akkooi 2014 {published data only}
- Akkooi AC, Gooskens S, Siegel P, Schaefer G, Schoengen A, Sterry W, et al. Interobserver variability in ultrasound (US) guided fine needle aspiration cytology (FNAC) of sentinel nodes (SN): experience in 1,000 melanoma patients. Journal of Clinical Oncology 2014;32(15 Suppl):9097. [DOI: 10.1200/jco.2014.32.15_suppl.9097] [DOI] [Google Scholar]
Van Akkooi 2015 {published data only}
- Akkooi AC, Siegel P, Schoengen A, Roewert‐Huber J, Eggermont AM, Voit CA. Long‐term results of ultrasound (US)‐guided fine needle aspiration cytology (FNAC) in conjunction with sentinel node biopsy (SNB) to support step‐wise approach in melanoma. Journal of Clinical Oncology. Conference 2015;33(15 SUPPL):9067. [DOI: 10.1200/jco.2015.33.15_suppl.9067] [DOI] [Google Scholar]
Van den Broucke 2010 {published data only}
- Vandenbroucke F, Neyns B, Deryk S, Vanbinst A, Wilgenhof S, Pierret L, et al. Efficiency of total body 18F PET/CT in the detection of cerebral metastases in patients with advanced melanoma. Melanoma Research 2010;20:e31‐2. [Google Scholar]
Van der Ploeg 2007 {published data only}
- Ploeg IM, Valdes Olmos RA, Nieweg OE, Rutgers EJ, Kroon BB, Hoefnagel CA. The additional value of SPECT/CT in lymphatic mapping in breast cancer and melanoma. Journal of Nuclear Medicine 2007;48(11):1756‐60. [DOI] [PubMed] [Google Scholar]
Van der Ploeg 2009a {published data only}
- Ploeg IM, Olmos RA, Kroon BB, Vogel WV, Hoefnagel CA, Nieweg OE. Three‐dimensional imaging of sentinel nodes in melanoma using a novel SPECT/CT technique. Annals of Surgical Oncology 2009;16(1 Suppl):100‐1. [Google Scholar]
Van der Ploeg 2009b {published data only}
- Ploeg IM, Valdes Olmos RA, Kroon BB, Wouters MW, Brekel MW, Vogel WV, et al. The yield of SPECT/CT for anatomical lymphatic mapping in patients with melanoma. Annals of Surgical Oncology 2009;16(6):1537‐42. [DOI] [PubMed] [Google Scholar]
Van der Ploeg 2011 {published data only}
- Ploeg AP, Akkooi AC, Schmitz PI, Geel AN, Wilt JH, Eggermont AM, et al. Therapeutic surgical management of palpable melanoma groin metastases: superficial or combined superficial and deep groin lymph node dissection. Annals of Surgical Oncology 2011;18(12):3300‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vereecken 2005 {published data only}
- Vereecken P, Laporte M, Petein M, Steels E, Heenen M. Evaluation of extensive initial staging procedure in intermediate/high‐risk melanoma patients. Journal of the European Academy of Dermatology & Venereology 2005;19(1):66‐73. [DOI] [PubMed] [Google Scholar]
Vidal‐Sicart 2010 {published data only}
- Vidal‐Sicart S, Vilalta A, Rull R, Carrera C, Puig S, Malvehy J. SPECT/CT and portable gamma camera to refine the sentinel node localization. Pigment Cell and Melanoma Research 2010;23(6):983. [Google Scholar]
Voit 1999 {published data only}
- Voit C, Schoengen A, Schwurzer M, Weber L, Mayer T, Proebstle TM. Detection of regional melanoma metastases by ultrasound B‐scan, cytology or tyrosinase RT‐PCR of fine‐needle aspirates. British Journal of Cancer 1999;80(10):1672‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voit 2000 {published data only}
- Voit C, Mayer T, Proebstle TM, Weber L, Kron M, Krupienski M, et al. Ultrasound‐guided fine‐needle aspiration cytology in the early detection of melanoma metastases. Cancer 2000;90(3):186‐93. [PubMed] [Google Scholar]
Voit 2001 {published data only}
- Voit C, Mayer T, Kron M, Schoengen A, Sterry W, Weber L, et al. Efficacy of ultrasound B‐scan compared with physical examination in follow‐up of melanoma patients. Cancer 2001;91(12):2409‐16. [PubMed] [Google Scholar]
Voit 2005 {published data only}
- Voit CA, Mayer T, Schafer G, Gellrich S, Kron M, Sterry W, et al. Ultrasound (US) and ultrasound guided fine needle aspiration cytology (FNAC) reduces surgical procedures, sentinel node (SN) dissection in melanoma patients and reliably predicts lymph node involvement in cutaneous lymphoma patients. Journal of Clinical Oncology 2005;23(16):719S. [Google Scholar]
Voit 2006 {published data only}
- Voit C, Kron M, Schafer G, Schoengen A, Audring H, Lukowsky A, et al. Ultrasound‐guided fine needle aspiration cytology prior to sentinel lymph node biopsy in melanoma patients. Annals of Surgical Oncology 2006;13(12):1682‐9. [DOI] [PubMed] [Google Scholar]
Voit 2009a {published data only}
- Voit CA, Akkooi AC, Schafer‐Hesterberg G, Schoengen A, Schmitz PI, Sterry W, et al. Rotterdam Criteria for sentinel node (SN) tumor burden and the accuracy of ultrasound (US)‐guided fine‐needle aspiration cytology (FNAC): can US‐guided FNAC replace SN staging in patients with melanoma?. Journal of Clinical Oncology 2009;27(30):4994‐5000. [DOI] [PubMed] [Google Scholar]
Voit 2009b {published data only}
- Voit C, Akkooi Van A, Schaefer‐Hesterberg G, Schoengen A, Sterry W, Eggermont AM. New ultrasound morphology criteria can predict melanoma metastases in the sentinel lymph node (SN) and correlate with tumour burden and survival. European Journal of Cancer, Supplement 2009;7(2‐3):577. [Google Scholar]
Voit 2009c {published data only}
- Voit CA, Akkooi AC, Schaefer‐Hesterberg G, Schoengen A, Sterry W, Eggermont AM. Correlation of ultrasound criteria for detection of melanoma metastases in the sentinel lymph node (SN) with tumor burden and survival. Journal of Clinical Oncology 2009;27(15 Suppl):9015. [Google Scholar]
Voit 2010a {published data only}
- Voit CA, Akkooi ACJ, Eggermont AM. Role of ultrasound in the assessment of the sentinel node of melanoma patients. AJR. American Journal of Roentgenology 2010;195(6):W474‐5; author reply W476. [DOI] [PubMed] [Google Scholar]
Voit 2010b {published data only}
- Voit CA, Akkooi AJ, Schafer‐Hesterberg G, Sterry W, Eggermont AM. The value of preoperative ultrasound (after lymphoscintigraphy) in conjunction with pre‐sentinel lymph node biopsy fine‐needle aspiration outweighs the usage of ultrasound alone in conjunction with lymphoscintigraphy: the need for an algorithm. Melanoma Research 2010;20(4):357‐9. [DOI] [PubMed] [Google Scholar]
Voit 2010c {published data only}
- Voit C, Akkooi AC, Schafer‐Hesterberg G, Schoengen A, Kowalczyk K, Roewert JC, et al. Ultrasound morphology criteria predict metastatic disease of the sentinel nodes in patients with melanoma. Journal of Clinical Oncology 2010;28(5):847‐52. [DOI] [PubMed] [Google Scholar]
Voit 2010d {published data only}
- Voit C, Akkooi AC, Schaefer G, Schoengen A, Sterry W, Eggermont AM. Early ultrasound criteria drive sensitivity for detection of sentinel node metastases in melanoma patients: a prospective study in 800 patients. Pigment Cell and Melanoma Research 2010;23(6):984. [Google Scholar]
Voit 2011a {published data only}
- Voit C, Akkooi AJ, Siegel P, Schaefer‐Hesterberg G, Sterry W, Schoengen A, et al. Ultrasound (US) guided fine needle aspiration cytology (FNAC) predicts sentinel node (SN) metastases and improves the nomogram for melanoma patients. European Journal of Cancer 2011;47(Suppl 1):S652. [Google Scholar]
Voit 2011b {published data only}
- Voit CA, Akkooi AC, Eggermont AM, Schafer‐Hesterberg G, Kron M, Ulrich J, et al. Fine needle aspiration cytology of palpable and nonpalpable lymph nodes to detect metastatic melanoma. Journal of the National Cancer Institute 2011;103(23):1771‐7. [DOI] [PubMed] [Google Scholar]
Voit 2011c {published data only}
- Voit CA, Akkooi AC, Siegel P, Sterry W, Schoengen A, Schaefer‐Hesterberg G, et al. Ultrasound (US)‐guided fine‐needle aspiration cytology (FNAC) for the prediction of sentinel node (SN) metastases and its effect on the nomogram for melanoma patients. Journal of Clinical Oncology 2011;29(15 Suppl):8550. [DOI: 10.1200/jco.2011.29.15_suppl.8550] [DOI] [Google Scholar]
Voit 2013 {published data only}
- Voit CA, Akkooi AC, Siegel P, Schoengen A, Sterry W, Eggermont AM. High sensitivity rate of ultrasound (US) guided fine needle aspiration cytology (FNAC) using the Berlin morphology criteria for lymph node metastases significantly reduces need for surgical sentinel node (SN) staging in melanoma. Skin Research and Technology 2013;19(1):e574‐5. [Google Scholar]
Voit 2016 {published data only}
- Voit CA, Oude Ophuis CM, Ulrich J, Akkooi AC, Eggermont AM. Ultrasound of the sentinel node in melanoma patients: echo‐free island is a discriminatory morphologic feature for node positivity. Melanoma Research 2016;26(3):267‐71. [DOI] [PubMed] [Google Scholar]
Von Schulthess 1998 {published data only}
- Schulthess GK, Steinert HC, Dummer R, Weder W. Cost‐effectiveness of whole‐body PET imaging in non‐small cell lung cancer and malignant melanoma. Academic Radiology 1998;5(9 Suppl):S300‐2. [DOI] [PubMed] [Google Scholar]
Wagner 1997 {published data only}
- Wagner JD, Schauwecker D, Hutchins G, Coleman JJ 3rd. Initial assessment of positron emission tomography for detection of nonpalpable regional lymphatic metastases in melanoma. Journal of Surgical Oncology 1997;64(3):181‐9. [DOI] [PubMed] [Google Scholar]
Wagner 1999 {published data only}
- Wagner JD, Schauwecker D, Davidson D, Coleman JJ 3rd, Saxman S, Hutchins G, et al. Prospective study of fluorodeoxyglucose‐positron emission tomography imaging of lymph node basins in melanoma patients undergoing sentinel node biopsy. Journal of Clinical Oncology 1999;17(5):1508‐15. [DOI] [PubMed] [Google Scholar]
Wagner 2001 {published data only}
- Wagner JD, Schauwecker DS, Davidson D, Wenck S, Jung SH, Hutchins G. FDG‐PET sensitivity for melanoma lymph node metastases is dependent on tumor volume. Journal of Surgical Oncology 2001;77(4):237‐42. [DOI] [PubMed] [Google Scholar]
Wagner 2005 {published data only}
- Wagner JD, Schauwecker D, Davidson D, Logan T, Coleman JJ 3rd, Hutchins G, et al. Inefficacy of F‐18 fluorodeoxy‐D‐glucose‐positron emission tomography scans for initial evaluation in early‐stage cutaneous melanoma. Cancer 2005;104(3):570‐9. [DOI] [PubMed] [Google Scholar]
Wagner 2009a {published data only}
- Wagner TL, Zerdoud S, Courbon F. Effectiveness of FDG PET‐CT for the detection of distant metastases at initial staging of localized high risk melanomas. European Journal of Nuclear Medicine and Molecular Imaging 2009;36(2 Suppl):S340. [Google Scholar]
Wagner 2009b {published data only}
- Wagner TL, Julian A, Zerdoud S, Payoux P, Courbon F. Effectiveness of FDG PET for the detection of distant metastases at initial staging of melanoma patients with a positive sentinel lymph node biopsy. European Journal of Nuclear Medicine and Molecular Imaging 2009;36(2 Suppl):S177. [Google Scholar]
Wagner 2011 {published data only}
- Wagner T, Meyer N, Zerdoud S, Julian A, Chevreau C, Payoux P, et al. Fluorodeoxyglucose positron emission tomography fails to detect distant metastases at initial staging of melanoma patients with metastatic involvement of sentinel lymph node. British Journal of Dermatology 2011;164(6):1235‐40. [DOI] [PubMed] [Google Scholar]
Wasif 2013 {published data only}
- Wasif N, Haddad D, Pockaj BA, Gray R, Bagaria S, Etzioni D. Positron emission tomography (PET) for staging of cutaneous melanoma in the United States: a population‐based analysis. Annals of Surgical Oncology 2013;20(1 Suppl):S97‐8. [DOI] [PubMed] [Google Scholar]
Webb 2012 {published data only}
- Webb H, Latifi H, Grossman S, Oza U, Griffeth L, Joyner K, et al. Use of whole‐body ("head‐to‐toe") PET/CT in the evaluation of melanoma and sarcoma patients. American Journal of Roentgenology 2012;198(5):1891. [Google Scholar]
Weisinger 1998 {published data only}
- Weisinger K, Blake S P, Atkins MB, Raptopoulos VD. Utility of triphasic contrast enhanced CT in the diagnosis of liver metastases from malignant melanoma. Radiology 1998;209P:215. [Google Scholar]
Weiss 1995 {published data only}
- Weiss M, Loprinzi CL, Creagan ET, Dalton RJ, Novotny P, O'Fallon JR. Utility of follow‐up tests for detecting recurrent disease in patients with malignant melanomas. JAMA 1995;274(21):1703‐5. [PubMed] [Google Scholar]
Windorbska 2007 {published data only}
- Windorbska W, Partyka A, Lewandowska A, Malkowki B. Evaluation of diagnostic value of FDG‐PET/CT study in detection of metastases in malignant melanoma patients [Ocena wartosci diagnostycznej badania FDG‐PET/CT w rozpoznawaniu przerzutow u chorych na czerniaka]. Wspolczesna Onkologia 2007;11(4):200‐3. [Google Scholar]
Winkler 2013 {published data only}
- Winkler N, Rezvani M, Heilbrun M, Shaaban A. Utility of dual phase liver CT for metastatic melanoma staging and surveillance. European Journal of Radiology 2013;82(12):2189‐93. [DOI] [PubMed] [Google Scholar]
Wong 2011 {published data only}
- Wong F. The clinical impact of FDG PET/CT in the assessment of cutaneous malignant melanoma. Internal Medicine Journal 2011;41:18. [Google Scholar]
Xing 2010 {published data only}
- Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, et al. Diagnostic imaging modalities for the surveillance of melanoma patients: a meta‐analysis. Journal of Clinical Oncology 2010;28(15 Suppl):8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xing 2011 {published data only}
- Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta‐analysis. Journal of the National Cancer Institute 2011;103(2):129‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yancovitz 2007 {published data only}
- Yancovitz M, Finelt N, Warycha MA, Christos PJ, Mazumdar M, Shapiro RL, et al. Role of radiologic imaging at the time of initial diagnosis of stage T1b‐T3b melanoma. Cancer 2007;110(5):1107‐14. [DOI] [PubMed] [Google Scholar]
Yang 2003 {published data only}
- Yang M, Martin DR, Karabulut N, Frick MP. Comparison of MR and PET imaging for the evaluation of liver metastases. Journal of Magnetic Resonance Imaging 2003;17(3):343‐9. [DOI] [PubMed] [Google Scholar]
Zender 2014 {published data only}
- Zender C, Guo T, Weng C, Faulhaber P, Rezaee R. Utility of SPECT/CT for periparotid sentinel lymph node mapping in the surgical management of head and neck melanoma. American Journal of Otolaryngology 2014;35(1):12‐8. [DOI] [PubMed] [Google Scholar]
Zimmermann 2000 {published data only}
- Zimmermann T, Schmitt H, Saadeh N, Padberg W. A twenty year balance of elective lymph node dissection in malignant melanoma of the trunc [20‐Jahres‐bilanz der elektiven lymphknotendissektion beim korperstamm‐melanom]. Acta Chirurgica Austriaca 2000;32(4):201‐3. [Google Scholar]
Zukauskaite 2013 {published data only}
- Zukauskaite R, Schmidt H, Asmussen JT, Hansen O, Bastholt L. Asymptomatic brain metastases in patients with cutaneous metastatic malignant melanoma. Melanoma Research 2013;23(1):21‐6. [PUBMED: 23117880] [DOI] [PubMed] [Google Scholar]
Additional references
ACIM 2014
- Australian Cancer Database. Melanoma of the skin for Australia (ICD10 C43). Australian Cancer Incidence and Mortality (ACIM) Books (www.aihw.gov.au/acim‐books/). Canberra: Australian Institute of Health and Welfare, 2011. [Google Scholar]
Ai 2012
- Ai T, Morelli JN, Hu X, Hao D, Goerner FL, Ager B, et al. A historical overview of magnetic resonance imaging, focusing on technological innovations. Investigative Radiology 2012;47(12):725‐41. [PUBMED: 23070095] [DOI] [PubMed] [Google Scholar]
Armstrong 1977
- Armstrong BK, Cust AE. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma: a perspective on Fears et al. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. American Journal of Epidemiology 1977;105:420‐7. [DOI] [PubMed] [Google Scholar]
Arnold 2014
- Arnold M, Holterhues C, Hollestein LM, Coebergh JW, Nijsten T, Pukkala E, et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. Journal of the European Academy of Dermatology & Venereology 2014;28(9):1170‐8. [PUBMED: 23962170] [DOI] [PubMed] [Google Scholar]
Atkins 1999
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High‐dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of Clinical Oncology 1999;17(7):2105‐16. [PUBMED: 10561265] [DOI] [PubMed] [Google Scholar]
Avril 2004
- Avril MF, Aamdal S, Grob JJ, Hauschild A, Mohr P, Bonerandi JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. Journal of Clinical Oncology 2004;22(6):1118‐25. [PUBMED: 15020614] [DOI] [PubMed] [Google Scholar]
Balch 2001
- Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. Journal of Clinical Oncology 2001;19(16):3622‐34. [PUBMED: 11504744] [DOI] [PubMed] [Google Scholar]
Balch 2009
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology 2009;27(36):6199‐206. [PUBMED: 19917835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belbasis 2016
- Belbasis L, Stefanaki I, Stratigos AJ, Evangelou E. Non‐genetic risk factors for cutaneous melanoma and keratinocyte skin cancers: an umbrella review of meta‐analyses. Journal of Dermatological Science 2016;84(3):330‐9. [DOI] [PubMed] [Google Scholar]
Bluemm 1983
- Bluemm RG. Direct intracranial sagittal and coronal CT scanning: anatomy and pathology. AJNR. American Journal of Neuroradiology 1983;4(3):484‐7. [PUBMED: 6410778] [PMC free article] [PubMed] [Google Scholar]
Bohelay 2015
- Bohelay G, Battistella M, Pages C, Margerie‐Mellon C, Basset‐Seguin N, Viguier M, et al. Ultrasound‐guided core needle biopsy of superficial lymph nodes: an alternative to fine‐needle aspiration cytology for the diagnosis of lymph node metastasis in cutaneous melanoma. Melanoma Research 2015;25(6):519‐27. [PUBMED: 25933210] [DOI] [PubMed] [Google Scholar]
Boniol 2012
- Boniol M, Autier P, Boyle P, Gandini S. Cutaneous melanoma attributable to sunbed use: systematic review and meta‐analysis. British Medical Journal 2012;345:e4757. [PUBMED: 22833605] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boring 1994
- Boring CC, Squires TS, Tong T, Montgomery S. Cancer statistics, 1994. CA: a Cancer Journal for Clinicians 1994;44(1):7‐26. [PUBMED: 8281473] [DOI] [PubMed] [Google Scholar]
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI: 10.1136/bmj.h5527; PUBMED: 26511519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burkill 2014
- Burkill G. Melanoma. In: Nicholson T editor(s). Recommendations for Cross‐Sectional Imaging in Cancer Management. 2. London: The Royal College of Radiologists, 2014. [Google Scholar]
Cagney 2017
- Cagney DN, Martin AM, Catalano PJ, Redig AJ, Lin NU, Lee EQ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population‐based study. Neuro‐oncology 2017; Vol. 19, issue 11:1511‐21. [DOI: 10.1093/neuonc/nox077] [DOI] [PMC free article] [PubMed]
Cancer Council Australia 2019
- Cancer Council Australia. Clinical practice guidelines for the diagnosis and management of melanoma. wiki.cancer.org.au/australia/Guidelines:Melanoma (accessed before 28 February 2019).
Cancer Research UK 2017
- Cancer Research UK. Skin cancer statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/skin‐cancer (accessed before 24 August 2017).
Cancer Research UK 2017a
- Cancer Research UK. Skin cancer incidence statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/skin‐cancer/incidence (accessed before 19 July 2017).
Cancer Society of New Zealand 2013
- Cancer Society of New Zealand. Skin Cancer Facts and Figures. www.cancernz.org.nz/reducing‐your‐cancer‐risk/sunsmart/about‐skin‐cancer/skin‐cancer‐facts‐and‐figures/. Cancer Society of New Zealand, (accessed before 14 February 2019).
Chapman 2011
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New England Journal of Medicine 2011;364(26):2507‐16. [PUBMED: 21639808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 2012
- Chapman PB, Hauschild A, Robert C, Larkin J, Haanen JB, Ribas A, et al. Updated overall survival (OS) results for BRIM‐3, a phase III randomized, open‐label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAFV600E‐mutated melanoma. Journal of Clinical Oncology 2012;30(15 Suppl 1):8502. [EMBASE: 71004853] [Google Scholar]
Cho 2014
- Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. Journal of the National Cancer Institute. Monographs 2014;2014(49):187‐97. [PUBMED: 25417232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis for sensitivity and specificity with sparse data: a generalized linear mixed model approach (comment). Journal of Clinical Epidemiology 2006;59(12):1331‐2. [PUBMED: 17098577] [DOI] [PubMed] [Google Scholar]
Danielsen 2014
- Danielsen M, Højgaard L, Kjær A, Fischer BM. Positron emission tomography in the follow‐up of cutaneous malignant melanoma patients: a systematic review. American Journal of Nuclear Medicine and Molecular Imaging 2014;4(1):17‐28. [PMC free article] [PubMed] [Google Scholar]
de Rosa 2011
- Rosa N, Lyman GH, Silbermins D, Valsecchi ME, Pruitt SK, Tyler DM, et al. Sentinel node biopsy for head and neck melanoma: a systematic review. Otolaryngology ‐ Head and Neck Surgery 2011;145(3):375‐82. [PUBMED: 21540313] [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [PUBMED: 16085191] [DOI] [PubMed] [Google Scholar]
DePry 2011
- DePry JL, Reed KB, Cook‐Norris RH, Brewer JD. Iatrogenic immunosuppression and cutaneous malignancy. Clinics in Dermatology 2011;29(6):602‐13. [PUBMED: 22014982] [DOI] [PubMed] [Google Scholar]
Dinnes 2018
- Dinnes J, Deeks JJ, Chuchu N, Ferrante di Ruffano L, Matin RN, Thomson DR, et al. Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults. Cochrane Database of Systematic Reviews 2018, Issue 12. [DOI: 10.1002/14651858.CD011902.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dummer 2014
- Dummer R, Arenberger P, Ascierto PA, Groot JW, Hallmeyer S, Lotem M, et al. 1130TiP‐NEMO: a phase 3 trial of binimetinib (MEK162) versus dacarbazine in patients with advanced NRAS‐mutant melanoma who are untreated or have progressed after any number of immunotherapy regimens. Annals of Oncology 2014;25(Suppl_4):iv392. [DOI: 10.1093/annonc/mdu344.46; PUBMED: 28171154] [DOI] [Google Scholar]
Egberts 2010
- Egberts F, Momkvist A, Egberts JH, Kaehler KC, Hauschild A. Serum S100B and LDH are not useful in predicting the sentinel node status in melanoma patients. Anticancer Research 2010;30(5):1799‐805. [PUBMED: 20592382] [PubMed] [Google Scholar]
Eggermont 2016
- Eggermont AM, Chiarion‐Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Ipilimumab (IPI) vs placebo (PBO) after complete resection of stage III melanoma: final overall survival results from the EORTC 18071 randomized, double‐blind, phase 3 trial. Annals of Oncology 2016;27(Suppl_6):LBA2_PR. [DOI: 10.1093/annonc/mdw435.35; EMBASE: 613911278] [DOI] [Google Scholar]
Eggermont 2018
Erdmann 2013
- Erdmann F, Lortet‐Tieulent J, Schuz J, Zeeb H, Greinert R, Breitbart EW, et al. International trends in the incidence of malignant melanoma 1953‐2008 ‐ are recent generations at higher or lower risk?. International Journal of Cancer 2013;132(2):385‐400. [PUBMED: 22532371] [DOI] [PubMed] [Google Scholar]
ESMO 2019
- Dummer R, Keilholz U, ESMO Guidelines Committee. eUpdate – Cutaneous Melanoma Algorithms. www.esmo.org/Guidelines/Melanoma/Cutaneous‐Melanoma/eUpdate‐Algorithms. Canberra: Australian Institute of Health and Welfare, (accessed before 18 March 2019).
EUCAN 2012
- EUCAN, International Agency for Research on Cancer. Malignant melanoma of skin: estimated incidence, mortality & prevalence for both sexes, 2012. eco.iarc.fr/eucan/Cancer.aspx?Cancer=20. International Agency for Research on Cancer, (accessed before 14 February 2019).
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [PUBMED: 25220842] [DOI] [PubMed] [Google Scholar]
Ferrante di Ruffano 2019
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, Matin RN, Chuchu N, Saleh D, et al. Sentinel lymph node biopsy for staging cutaneous melanoma and cutaneous squamous cell carcinoma in adults. Cochrane Database of Systematic Reviews (in press).
Galway 2012
- Galway K, Black A, Cantwell M, Cardwell CR, Mills M, Donnelly M. Psychosocial interventions to improve quality of life and emotional wellbeing for recently diagnosed cancer patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007064.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandini 2005
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta‐analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. European Journal of Cancer 2005;41(1):28‐44. [PUBMED: 15617989] [DOI] [PubMed] [Google Scholar]
Gao 2013
- Gao G, Gong B, Shen W. Meta‐analysis of the additional value of integrated 18FDG PET–CT for tumor distant metastasis staging: comparison with 18FDG PET alone and CT alone. Surgical Oncology 2013;22(3):195‐200. [DOI] [PubMed] [Google Scholar]
Garbe 2016
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Bastholt L, et al. Diagnosis and treatment of melanoma. European consensus‐based interdisciplinary guideline ‐ Update 2016. European Journal of Cancer 2016;63:201‐17. [PUBMED: 27367293] [DOI] [PubMed] [Google Scholar]
Geller 2002
- Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969‐1999. JAMA 2002;288(14):1719‐20. [PUBMED: 12365954] [DOI] [PubMed] [Google Scholar]
Gershenwald 2017
- Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross, MI, et al. Melanoma staging: evidence‐based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA: A Cancer Journal for Clinicians 2017;67(6):472‐92. [PUBMED: 29028110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goulart 2011
- Goulart CR, Mattei TA, Ramina R. Cerebral melanoma metastases: a critical review on diagnostic methods and therapeutic options. ISRN Surgery 2011 May 24 [Epub ahead of print]. [DOI: 10.5402/2011/276908; PUBMED: 22084751] [DOI] [PMC free article] [PubMed]
Gray 2014
- Gray MR, Martin del Campo S, Zhang X, Zhang H, Souza FF, Carson WE 3rd, et al. Metastatic melanoma: lactate dehydrogenase levels and CT imaging findings of tumor devascularization allow accurate prediction of survival in patients treated with bevacizumab. Radiology 2014;270(2):425‐34. [PUBMED: 24072776] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gyorki 2018
- Gyorki D, Teddy L, Barbour A, Mar V, Sandhu S, Hanikeri M, et al. When is a sentinel node biopsy indicated? In: Cancer Council Australia Melanoma Guidelines Working Party. Clinical practice guidelines for the diagnosis and management of melanoma. wiki.cancer.org.au/australiawiki/index.php?oldid=186218. Sydney: Cancer Council Australia, (accessed 27 February 2019).
Hamid 2013
- Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD‐L1 antibody in patients with locally advanced or metastatic melanoma (mM). Journal of Clinical Oncology 2013;31(15 Suppl 1):9010. [EMBASE: 71099860] [Google Scholar]
Hodi 2010
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine 2010;363(8):711‐23. [PUBMED: 20525992] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodi 2016
- Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2‐year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncology 2016;17(11):1558‐68. [DOI: 10.1016/S1470-2045(16)30366-7; PUBMED: 27622997] [DOI] [PMC free article] [PubMed] [Google Scholar]
IAEA 2016
- International Atomic Energy Agency. Radiation protection of patients. www.rpop.iaea.org/RPOP/RPoP/Content/AdditionalResources/Training/1_TrainingMaterial/PETCT.htm (accessed November 2016).
Jones 2017
- Jones T, Townsend DW. History and future technical innovation in positron emission tomography. Journal of Medical Imaging 2017;4(1):011013. [DOI: 10.1117/1.JMI.4.1.011013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korn 2008
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta‐analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression‐free and overall survival benchmarks for future phase II trials. Journal of Clinical Oncology 2008;26(4):527‐34. [PUBMED: 18235113] [DOI] [PubMed] [Google Scholar]
Kyrgidis 2015
- Kyrgidis A, Tzellos T, Mocellin S, Apalla Z, Lallas A, Pilati P, et al. Sentinel lymph node biopsy followed by lymph node dissection for localised primary cutaneous melanoma. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD010307.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lachs 1992
- Lachs MS, Nachamkin I, Edelstein PH, Goldman J, Feinstein AR, Schwartz JS. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Annals of Internal Medicine 1992;117(2):135‐40. [PUBMED: 1605428] [DOI] [PubMed] [Google Scholar]
Lammertsma 2017
- Lammertsma AA. Forward to the past: the case for quantitative PET imaging. Journal of Nuclear Medicine 2017;58(7):1019‐24. [PUBMED: 28522743] [DOI] [PubMed] [Google Scholar]
Larkin 2014
- Larkin J, Ascierto PA, Dréno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF‐mutated melanoma. New England Journal of Medicine 2014;371(20):1867‐76. [PUBMED: 25265494] [DOI] [PubMed] [Google Scholar]
Larkin 2015
- Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J, et al. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild‐type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA (Oncology) 2015;1(4):433‐40. [PUBMED: 26181250] [DOI] [PubMed] [Google Scholar]
Leeflang 2013
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. Canadian Medical Association Journal 2013;185(11):E537‐44. [PUBMED: 23798453] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehmann 2011
- Lehmann AR, McGibbon D, Stefanini M. Xeroderma pigmentosum. Orphanet Journal of Rare Diseases 2011;6:70. [PUBMED: 22044607] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leiter 2016
- Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG‐SLT): a multicentre, randomised, phase 3 trial. Lancet Oncology 2016;17(6):757‐67. [DOI: 10.1016/S1470-2045(16)00141-8] [DOI] [PubMed] [Google Scholar]
Leiter 2018
- Leiter UM, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, et al. Final analysis of DECOG‐SLT trial: survival outcomes of complete lymph node dissection in melanoma patients with positive sentinel node. Journal of Clinical Oncology 2018;36(15 Suppl):9501. [DOI: 10.1200/JCO.2018.36.15_suppl.9501] [DOI] [PubMed] [Google Scholar]
Libberecht 2005
- Libberecht K, Husada G, Peeters T, Michiels P, Gys T, Molderez C. Initial staging of malignant melanoma by positron emission tomography and sentinel node biopsy. Acta Chirurgica Belgica 2005;105(6):621‐5. [DOI: 10.1080/00015458.2005.11679789] [DOI] [PubMed] [Google Scholar]
Linos 2009
- Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. Journal of Investigative Dermatology 2009;129(7):1666‐74. [PUBMED: 19131946] [DOI] [PMC free article] [PubMed] [Google Scholar]
Long 2017
Lukas 2014
- Lukas RV, Gabikian P, Garza M, Chmura SJ. Treatment of brain metastases. Oncology 2014;87(6):321‐9. [PUBMED: 25227433] [DOI] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from methods.cochrane.org/sdt/handbook‐dta‐reviews.
Mahesh 2017
- Mahesh M. Computed tomography dose (CT dose). www.radiologyinfo.org/en/info.cfm?pg=safety‐xray (accessed before 24 August 2017).
Maio 2015
- Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, et al. Five‐year survival rates for treatment‐naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. Journal of Clinical Oncology 2015;33(10):1191‐6. [PUBMED: 25713437] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsden 2010
- Marsden JR, Newton‐Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. BAD guidelines: revised UK guidelines for the management of cutaneous melanoma 2010. British Journal of Dermatology 2010;163(2):238‐56. [PUBMED: 20608932] [DOI] [PubMed] [Google Scholar]
McInnes 2018
- McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the PRISMA‐DTA Group, et al. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies: the PRISMA‐DTA statement. JAMA 2018;319(4):388‐96. [DOI: 10.1001/jama.2017.19163]; PUBMED: 29362800] [DOI] [PubMed] [Google Scholar]
Melanoma Focus 2014
- Acland K, Algurafi H, Allan R, Barlow C, Board R, Brown E, et al. Melanoma Focus. 2013 position paper: follow‐up of high risk cutaneous melanoma in the UK. www.melanomafocus.com/wp‐content/uploads/2014/02/Cutaneous‐Melanoma‐Follow‐Up‐Position‐Paper‐30Jan14.pdf. London: Melanoma Focus, (accessed before 19 September 2017).
Melanoma Focus 2018
- Melanoma Focus. Sentinel node biopsy guideline based on a multi‐disciplinary consensus meeting held in Cambridge, UK, on 17 May 2018. melanomafocus.com/information‐portal/snb‐guideline/ (accessed before 05 December 2018).
Melanoma Taskforce 2011
- Melanoma Taskforce. Quality in Melanoma Care: a best practice pathway. www.londoncancer.org/media/59993/melanoma‐taskforce‐2011.pdf. London: The Melanoma Taskforce, (accessed before 19 September 2017).
Millward 2018
- Millward M, Menzies A, Atkinson V, Brown M, Haydon A, Cancer Cancer Council Australia Melanoma Guidelines Working Party. What investigations should be performed when Stage IV melanoma is diagnosed? In: Cancer Council Australia Melanoma Guidelines Working Party. Clinical practice guidelines for the diagnosis and management of melanoma. wiki.cancer.org.au/australiawiki/index.php?oldid=186478. Sydney: Cancer Council Australia, (accessed before 18 March 2019).
Mistry 2011
- Mistry M, Parkin DM, Ahmad AS, Sasieni P. Cancer incidence in the United Kingdom: projections to the year 2030. British Journal of Cancer 2011;105(11):1795‐803. [PUBMED: 22033277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohr 2009
- Mohr P, Eggermont AMM, Hauschild A, Buzaid A. Staging of cutaneous melanoma. Annals of Oncology 2009;20(Suppl 6):vi14‐21. [PUBMED: 19617293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moons 1997
- Moons KG, Es GA, Deckers JW, Habbema JD, Grobbee DE. Limitations of sensitivity, specificity, likelihood ratio, and bayes' theorem in assessing diagnostic probabilities: a clinical example. Epidemiology 1997;8(1):12‐7. [PUBMED: 9116087] [DOI] [PubMed] [Google Scholar]
Moreau 2013
- Moreau JF, Weissfeld JL, Ferris LK. Characteristics and survival of patients with invasive amelanotic melanoma in the USA. Melanoma Research 2013;23(5):408‐13. [DOI: 10.1097/CMR.0b013e32836410fe; PUBMED: 23883947] [DOI] [PubMed] [Google Scholar]
Morton 2014
- Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, et al. MSLT Group. Final trial report of sentinel‐node biopsy versus nodal observation in melanoma. New England Journal of Medicine 2014;370(7):599‐609. [PUBMED: 24521106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morton 2018
- Morton R, Barbour A, Bell C, Mar V, Smithers M, Cancer Cancer Council Australia Melanoma Guidelines Working Party. What investigations should be performed following a diagnosis of primary cutaneous melanoma for asymptomatic stage I and stage II patients? In: Cancer Council Australia Melanoma Guidelines Working Party. Clinical practice guidelines for the diagnosis and management of melanoma. wiki.cancer.org.au/australiawiki/index.php?oldid=186474. Sydney: Cancer Council Australia, (accessed before 18 March 2019).
Newcombe 1998
- Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine 1998;17(8):857‐72. [DOI] [PubMed] [Google Scholar]
Nguyen 1999
- Nguyen AT, Akhurst T, Larson SM, Coit DG, Brady MS. PET scanning with (18)F 2‐fluoro‐2‐deoxy‐D‐glucose (FDG) in patients with melanoma: benefits and limitations. Clinical Positron Imaging 1999;2(2):93‐8. [PUBMED: 14516545] [DOI] [PubMed] [Google Scholar]
NICE 2015a
- National Institute for Health and Care Excellence. Melanoma: assessment and management. www.nice.org.uk/guidance/ng14. London: National Institute for Health and Care Excellence, (accessed before 19 September 2017).
NICE 2015d
- National Institute for Health and Care Excellence. Melanoma: assessment and management. Evidence review. www.nice.org.uk/guidance/ng14/documents/melanoma‐evidence‐review. London: National Institute for Health and Care Excellence, (accessed before 19 September 2017).
NICE 2018a
- National Institute for Health and Care Excellence. Dabrafenib with traetinib for adjuvant treatment of resected BRAF V600 mutation‐positive melanoma. www.nice.org.uk/Guidance/TA544. London: National Institute of Health and Care Excellence, (accessed before 03 December 2018).
NICE 2018b
- National Institute for Health and Care Excellence. Pembrolizumab for adjuvant treatment of melanoma with high risk of recurrence [ID1266]. www.nice.org.uk/Guidance/indevelopment/gid‐ta10247. London: National Institute of Health and Care Excellence, (accessed before 03 December 2018).
NICE 2018c
- National Institute for Health and Care Excellence. Skin cancer. www.nice.org.uk/guidance/conditions‐and‐diseases/cancer/skin‐cancer#panel‐in‐development (accessed 05 October 2018).
NICE 2019a
- National Institute for Health and Care Excellence. Nivolumab for adjuvant treatment of resected stage III and IV melanoma [ID1316]. www.nice.org.uk/Guidance/indevelopment/gid‐ta10286. London: National Institute of Health and Care Excellence, (accessed before 03 December 2018).
NICE 2019b
- National Institute for Health and Care Excellence. Encorafenib with binimetinib for treating advanced (unresectable or metastatic) BRAF V600 mutation‐positive melanoma [ID923]. www.nice.org.uk/Guidance/indevelopment/gid‐ta10217. London: National Institute of Health and Care Excellence, (accessed before 03 December 2018).
Pasquali 2018
- Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD011123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pirpiris 2010
- Pirpiris A, Saw R, Hersey P, Thompson JF. The relationship between serum LDH and PET scans in patients with stage III and IV melanoma. Pigment Cell and Melanoma Research 2010;23(6):906‐7. [DOI: 10.1111/j.1755-148X.2010.00767.x] [DOI] [Google Scholar]
Raymond 2011
- Raymond AK, Beasley GM, Broadwater G, Augustine CK, Padussis JC, Turley R, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. Journal of the American College of Surgeons 2011;213(2):306‐16. [DOI: 10.1016/j.jamcollsurg.2011.03.013; PUBMED: 21493111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [PUBMED: 16168343] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes‐Ortiz 2006
- Reyes‐Ortiz CA, Goodwin JS, Freeman JL, Kuo YF. Socioeconomic status and survival in older patients with melanoma. Journal of the American Geriatrics Society 2006;54(11):1758‐64. [PUBMED: 17087705] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robert 2015
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. New England Journal of Medicine 2015;372(1):30‐9. [DOI: 10.1056/NEJMoa1412690; PUBMED: 25399551] [DOI] [PubMed] [Google Scholar]
Rodriguez 2014
- Rodriguez Rivera AM, Alabbas H, Ramjaun A, Meguerditchian A‐N. Value of positron emission tomography scan in stage III cutaneous melanoma: a systematic review and meta‐analysis. Surgical Oncology 2014;23(1):11‐6. [PUBMED: 24556310] [DOI] [PubMed] [Google Scholar]
Rozeman 2018
Rutjes 2005
- Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM. Case‐control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51(8):1335‐41. [PUBMED: 15961549] [DOI] [PubMed] [Google Scholar]
Saw 2018
- Saw R, Menzies A, McArthur G, Spillane J, Haydon A, Cancer Cancer Council Australia Melanoma Guidelines Working Party. What investigations should be performed when in transit and/or regional node disease (Stage III melanoma) is diagnosed? In: Cancer Council Australia Melanoma Guidelines Working Party. Clinical practice guidelines for the diagnosis and management of melanoma. wiki.cancer.org.au/australiawiki/index.php?oldid=186475. Sydney: Cancer Council Australia, (accessed before 18 March 2019).
Schroer‐Gunther 2012
- Schroer‐Gunther MA, Wolff RF, Westwood ME, Scheibler FJ, Schurmann C, Baumert BG, et al. F‐18‐fluoro‐2‐deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: a systematic review. Systematic Reviews 2012;1:62. [PUBMED: 23237499] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaikh 2012
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology 2012;148(1):30‐6. [PUBMED: 21931016] [DOI] [PubMed] [Google Scholar]
Siegel 2015
- Siegel R, Miller K, Jemal A. Cancer statistics, 2015. CA: a Cancer Journal for Clinicians 2014;65(1):5‐29. [DOI] [PubMed] [Google Scholar]
SIGN 2017
- Scottish Intercollegiate Guidelines Network. Cutaneous melanoma. www.sign.ac.uk/sign‐146‐melanoma.html. Scotland: SIGN, (accessed before 19 July 2017).
Sladden 2009
- Sladden MJ, Balch C, Barzilai DA, Berg D, Freiman A, Handiside T, et al. Surgical excision margins for primary cutaneous melanoma. Cochrane Database of Systematic Reviews 2009, Issue 10. [DOI: 10.1002/14651858.CD004835.pub2] [DOI] [PubMed] [Google Scholar]
Solbiati 1988
- Solbiati L, Rizzatto G, Belotti E. High‐resolution sonography of cervical lymph nodes in head and neck cancer: criteria for differentiation of reactive versus malignant nodes. Radiology 1988;169:113. [Google Scholar]
STATA 2017 [Computer program]
- StataCorp LLC. 2017 STATA Statistical Software. Version 15. College Station, TX, USA: StataCorp LLC, 2017.
Swerdlow 1995
- Swerdlow AJ, English JS, Qiao Z. The risk of melanoma in patients with congenital nevi: a cohort study. Journal of the American Academy of Dermatology 1995;32(4):595‐9. [PUBMED: 7896948] [DOI] [PubMed] [Google Scholar]
Swetter 2019
- Swetter S, Tsao H, Bichakjian CK, Curiel‐Lewandrowski C, Elder DE, Gershenwald JE, et al. Guidelines of care for the management of primary cutaneous melanoma. Journal of the American Academy of Dermatology 2019;80(1):208‐50. [DOI] [PubMed] [Google Scholar]
Sznol 2013
- Sznol M, Chen L. Antagonist antibodies to PD‐1 and B7‐H1 (PD‐L1) in the treatment of advanced human cancer. Clinical Cancer Research 2013;19(5):1021‐34. [PUBMED: 23460533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2013
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544‐54. [PUBMED: 23546566] [DOI] [PubMed] [Google Scholar]
Takwoingi 2015
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2015;24:1‐19. [DOI: 10.1177/0962280215592269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2012
- Tan JC, Chatterton BE. Is there an added clinical value of "true" whole body(18)F‐FDG PET/CT imaging in patients with malignant melanoma?. Hellenic Journal of Nuclear Medicine 2012;15(3):202‐5. [PUBMED: 23106051] [DOI] [PubMed] [Google Scholar]
Tucker 1985
- Tucker MA, Boice JD Jr, Hoffman DA. Second cancer following cutaneous melanoma and cancers of the brain, thyroid, connective tissue, bone, and eye in Connecticut, 1935‐82. National Cancer Institute Monographs 1985;68:161‐89. [PUBMED: 4088297] [PubMed] [Google Scholar]
Usher‐Smith 2016
- Usher‐Smith JA, Sharp SJ, Griffin SJ. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ 2016;353:i3139. [DOI: 10.1136/bmj.i3139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valsecchi 2011
- Valsecchi ME, Silbermins D, Rosa N, Wong SL, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in patients with melanoma: a meta‐analysis. Journal of Clinical Oncology 2011;29(11):1479‐87. [PUBMED: 21383281] [DOI] [PubMed] [Google Scholar]
van Waes 1983
- Waes PFGM, Zonneveld FW, Feldberg MAM. Direct coronal and direct sagittal whole body computed tomography. In: Heuck Friedrich HW, Donner MW editor(s). Radiology Today. Berlin, Heidelberg: Springer Berlin Heidelberg, 1983:76‐84. [Google Scholar]
Vassalo 1992
- Vassallo P, Wernecke K, Ross N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high resolution US. Radiology 1992;183:215‐20. [DOI] [PubMed] [Google Scholar]
Villanueva 2010
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga‐Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF‐1R/PI3K. Cancer Cell 2010;18(6):683‐95. [PUBMED: 21156289] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warycha 2009
- Warycha MA, Zakrzewski J, Ni Q, Shapiro RL, Berman RS, Pavlick AC, et al. Meta‐analysis of sentinel lymph node positivity in thin melanoma (< or = 1 mm). Cancer 2009;115(4):869‐79. [PUBMED: 19117354] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2017
Whaley 2016a
- Whaley JT, Oncolink. Core needle biopsy. www.oncolink.org/cancer‐treatment/procedures‐diagnostic‐tests/biopsy‐procedures/core‐needle‐biopsy (accessed before 19 September 2017).
Whaley 2016b
- Whaley JT, Oncolink. MRI (magnetic resonance imaging). www.oncolink.org/cancer‐treatment/procedures‐diagnostic‐tests/radiology‐tests/mri‐magnetic‐resonance‐imaging (accessed before 19 September 2017).
Wheatley 2016
- Wheatley K, Wilson JS, Gaunt P, Marsden JR. Surgical excision margins in primary cutaneous melanoma: a meta‐analysis and Bayesian probability evaluation. Cancer Treatment Reviews 2016;42:73‐81. [PUBMED: 26563920] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
Zemelman 2014
- Zemelman VB, Valenzuela CY, Sazunic I, Araya I. Malignant melanoma in Chile: different site distribution between private and state patients. Biological Research 2014;47(1):34. [PUBMED: 25204018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2014
- Zhu W, Zhou L, Qian JQ, Qiu TZ, Shu YQ, Liu P. Temozolomide for treatment of brain metastases: a review of 21 clinical trials. World Journal of Clinical Oncology 2014;5(1):19‐27. [PUBMED: 24527399] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Dinnes 2017
- Dinnes J, Saleh D, Newton‐Bishop J, Cheung ST, Nathan P, Matin RN, et al. Tests to assist in the staging of cutaneous melanoma: a generic protocol. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD012806] [DOI] [Google Scholar]