Treatment with cipemastat, a selective matrix metalloproteinase inhibitor, paradoxically worsens disease in a mouse model of tuberculosis. Our investigation demonstrates that host-directed therapies for tuberculosis could have unpredicted deleterious effects. Cipemastat will require careful preclinical evaluation before undergoing clinical trials.
Keywords: Tuberculosis, matrix metalloproteinases, cavities, cipemastat, mouse
Abstract
Matrix metalloproteinases (MMPs) degrade extracellular matrix and are implicated in tuberculosis pathogenesis and cavitation. In particular, MMP-7 is induced by hypoxia and highly expressed around pulmonary cavities of Mycobacterium tuberculosis–infected C3HeB/FeJ mice. In this study, we evaluated whether administration of cipemastat, an orally available potent inhibitor of MMP-7, could reduce pulmonary cavitation in M. tuberculosis–infected C3HeB/FeJ mice. We demonstrate that, compared with untreated controls, cipemastat treatment paradoxically increases the frequency of cavitation (32% vs 7%; P = .029), immunopathology, and mortality. Further studies are needed to understand the role of MMP inhibitors as adjunctive treatments for pulmonary tuberculosis.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases that degrade collagen and remodel the extracellular matrix. Multiple MMPs have been associated with tuberculosis pathogenesis [1, 2], and MMP-1 (interstitial collagenase), MMP-9 (gelatinase B), and MMP-7 (matrilysin) in particular have been associated with active tuberculosis and cavitation [3]. MMP-7 is induced by hypoxia [4] and highly expressed in cavitary and hypoxic pulmonary lesions of Mycobacterium tuberculosis–infected C3HeB/FeJ mice [5].
Cipemastat (Trocade; Ro 32–3555) is an orally available drug that inhibits several MMPs, including MMP-7, with high potency [6]. In this study, we evaluated whether administration of cipemastat could alter the development of pulmonary cavities in M. tuberculosis–infected C3HeB/FeJ mice.
METHODS
All protocols were approved by the Johns Hopkins Biosafety, Radiation Safety, and Animal Care and Use Committees.
In Vivo Aerosol Infection
Female C3HeB/FeJ mice (Jackson Laboratory) aged 4–6 weeks were infected by aerosol with frozen stocks of M. tuberculosis H37Rv, using the Middlebrook Inhalation Exposure System (Glas-Col). Three mice were euthanized using an isoflurane (Henry Schein) overdose 1 day after infection to determine the number of bacilli implanted in the lungs. Mice were randomized into the cipemastat treatment group or the control untreated group. At each time point, a subset of 4 infected mice from each group were euthanized to determine the bacillary burden, specified in log10 colony-forming units (CFU). The entire lungs were harvested, homogenized in phosphate-buffered saline (PBS), and then plated by serial dilution in triplicate onto Middlebrook 7H11 selective plates (Becton Dickinson). All plates were incubated at 37°C for 4 weeks before colonies were counted.
Chemotherapy
Cipemastat (F. Hoffmann–La Roche) was administered via oral gavage at 100 mg/kg daily (divided over 2 equal doses) for 10 weeks. The control group received sham treatment with PBS twice daily via oral gavage.
Biocontainment and Computed Tomography (CT)
At 4, 8, and 10 weeks after infection, M. tuberculosis–infected animals were serially imaged within a sealed biocontainment bed (Minerve) modified in-house to be compliant with biosafety level 3 containment, as described previously [5]. CT was performed using the NanoSPECT/CT (Bioscan) in vivo animal imager; images were reconstructed and visualized using VivoQuant 2.5 (Invicro). A cavity was defined as a macroscopic region of air (density, less than −900 Hounsfield units) within the diseased lung parenchyma.
Histopathological Analysis
The lungs were harvested after systemic perfusion with PBS under deep anesthesia, fixed in 4% paraformaldehyde, and sectioned to 5-μm thickness. Hematoxylin-eosin staining was performed following standard procedures. The slides were scanned using the Apeiro digital scanner (Leica). The total area involved with disease (including granulomas, and cavities, and pneumonia-like lesions) was measured and compared to total lung area, using ImageJ (NIH). Briefly, we utilized the free-hand tool in Image J and manually demarcated the diseased areas. The ROI manager tool was used to quantify diseased areas and dividing each diseased area by the total area for a given section analyzed.
Immunohistochemical Analysis
The lungs from M. tuberculosis–infected mice (untreated control) were processed as described above and paraffin-embedded sections were rehydrated in graded alcohols, steamed in citrate buffer at pH 6, probed at room temperature for 2 hours for detection of MMP-7, using a rabbit polyclonal antibody (dilution, 1:250; Abcam), and processed with a polymer–horseradish peroxidase kit (BioGenex) with diaminobenzidine development and Mayer hematoxylin counterstaining.
Statistical Analysis
Statistical analysis using the 2-tailed Fisher exact test, 2-tailed Student t test, or log-rank test was performed as indicated, using Prism 6, version 6.07 (GraphPad). Data are presented as means ± standard deviations on a logarithmic (for CFU counts) or linear scale.
RESULTS
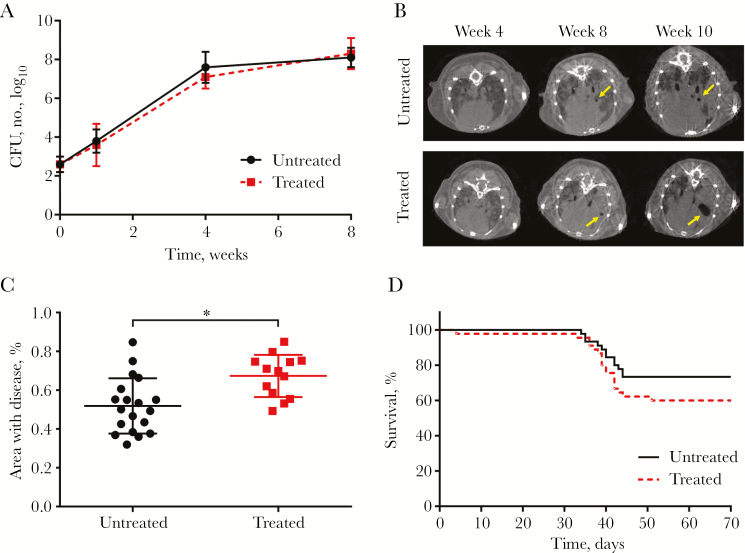
The mean pulmonary bacterial burden (±SD) 1 day after infection was 2.6 ± 0.4 log10 CFU. MMP-7 was highly expressed in M. tuberculosis–infected pulmonary lesions (Supplementary Figure 1). There were no differences in the pulmonary bacterial burden between animals with and those without cipemastat treatment over the course of the study (Figure 1A). However, animals receiving cipemastat had a significant increase in the proportion of cavitation 10 weeks after infection (32% vs 7%; P = .029, by the 2-tailed Fisher exact test; Figure 1B and Table 1). Details on location and size of each cavitary lesion are provided in Supplementary Table 1, demonstrating a trend toward an increased size of cavities in the cipemastat-treated mice, compared with the untreated mice (3.65 mm3 vs 1.48 mm3). Postmortem gross pathological lung samples collected 10 weeks after infection are shown in Supplementary Figure 2, showing extensive pathology in all infected mice. Postmortem histopathological analysis also demonstrated significantly increased disease severity in cipemastat-treated mice (P = .003, by the 2-tailed Student t test; Figure 1C). Representative hematoxylin-eosin–stained sections are shown in Supplementary Figure 3. Both treated and untreated groups had a similar percentage area of granulomas, but cipemastat-treated mice had a significantly larger area of pneumonia-like disease, compared with controls (P = .004, by the 2-tailed Student t test; Supplementary Figure 4). Finally, there was also a trend demonstrating increased mortality among the cipemastat-treated mice, compared with the control group (P = .17, by the log-rank test; Figure 1D).
Figure 1.
Effect of cipemastat in Mycobacterium tuberculosis–infected mice. A, Pulmonary bacterial burden, represented as the number of colony-forming units (CFU), in treated and untreated animals after infection. B, Transverse computed tomographic images of representative mice that developed cavitary lesions (yellow arrows) 4, 8, and 10 weeks after infection. C, Disease severity 10 weeks after infection, quantified as the percentage of lung involved with disease, as seen on hematoxylin-eosin stained sections, was significantly higher in 13 treated mice, compared with 19 untreated controls. *P = .003. D, Survival of infected mice treated with cipemastat was lower than that among controls. Data are means ± standard deviations.
Table 1.
Proportion of Mycobacterium tuberculosis–Infected Mice With Cavitations 4, 8, and 10 Weeks After Infection, by Cipemastat Treatment Group
| Group | Time After Infection, Proportion (%)a | ||
|---|---|---|---|
| Week 4 | Week 8 | Week 10b | |
| Treated | 0/45 (0) | 2/29 (7) | 7/22 (32) |
| Untreated | 0/45 (0) | 1/34 (3) | 2/29 (7) |
aData are no. of mice with cavitary lesions/total no. of scanned mice at each time point (%).
b P = .029, by the 2-tailed Fisher exact test.
DISCUSSION
Destruction of lung extracellular matrix is a prerequisite for cavity formation in tuberculosis and is associated with collagenase activity [6–8]. The presence of cavitary lesions in patients with tuberculosis correlates with worse outcomes, infectiousness, and higher rates of drug-resistance [9]. These observations have led to an interest in developing adjuvant therapies that could modulate collagen remodeling and reduce cavitary disease. Cipemastat is an orally available selective MMP inhibitor, originally developed by Roche Pharmaceuticals as an antiarthritis agent. Cipemastat inhibits several collagenases (MMP-1, MMP-8, and MMP-13), as well as matrilysin (MMP-7), all of which are upregulated in M. tuberculosis infection [6–8]. Although mice do not express an ortholog of MMP-1 [10], MMP-1 does not seem to be essential for cavity formation in this model. Mice do express MMP-7, as well as other collagenases (MMP-8 and MMP-13), and both MMP-7 and MMP-13 are potently inhibited (half maximal inhibitory concentration, <9 nm) by cipemastat [6, 7].
We therefore used a murine model of pulmonary tuberculosis that develops well-organized, hypoxic tuberculosis granulomas, as well as cavitary lesions after aerosol-based infection [5, 11], and evaluated whether cipemastat treatment could reduce cavitary formation and immunopathology. Unexpectedly, we found that cipemastat-treated animals developed more cavities, worse histopathological findings, and a trend toward increased mortality. Rabbits express MMP-1 and other Key MMPs, and these data are consistent with those reported by Urbanowski et al, who found that cipemastat monotherapy in a rabbit model of cavitary tuberculosis increased both the frequency and volume of cavitary disease, compared with control animals [12]. Furthermore, prior studies have demonstrated that daily administration of 10–25 mg/kg of cipemastat provides high plasma levels in rodents (Supplementary Table 2), and a dose-ranging study in mice demonstrated that treatment with 10, 25, or 50 mg/kg cipemastat were effective in preventing tissue damage and reduce disease progression in experimentally induced arthritis [7]. However, while we used an adequately high dose of 100 mg/kg, we did not measure intralesional concentrations of cipemastat. In addition, it is also possible that other MMPs or processes that are involved with cavitation were not affected by cipemastat treatment.
Interestingly, Xu et al have recently demonstrated that administration of marimastat (a selective MMP-2 and MMP-9 inhibitor) alone was not protective in M. tuberculosis–infected C57BL/6J mice. However, when administered as adjunctive treatment with either rifampin or isoniazid, marimastat increased drug exposures in infected lung tissues and led to a reduction (by 0.5–1 log10) in the pulmonary bacterial burden, compared with animals treated with rifampin or isoniazid alone [13]. Similarly, we have also recently demonstrated improved pulmonary bacterial burden or stable (relapse-free) cure in C3HeB/FeJ mice receiving adjunctive anti–MMP-9 antibody in combination with multidrug first-line tuberculosis treatment, compared with standard therapy alone, although these results were not different from those for control animals receiving adjunctive isotype control antibody [14]. Consequently, MMP inhibition in tuberculosis seems to have divergent effects when administered alone or in combination with effective tuberculosis treatment. These findings have parallels in the cancer field, where MMP inhibitors were proposed as adjunctive therapy, but findings of subsequent clinical trials were disappointing [15], an outcome ascribed to the complexity of protease pathways and diversity of MMP function in normal physiology.
In summary, we demonstrate that treatment with cipemastat, a selective MMP inhibitor, paradoxically worsens disease and increases cavitation in a mouse model of tuberculosis. Our investigation of monotherapy with an MMP inhibitor demonstrates that host-directed therapy in tuberculosis, which would be coadministered with antibiotics, may have unpredicted deleterious effects. Consequently, careful preclinical evaluation is required before progression to clinical trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. A. A. O., S. P., P. T. E., and S. K. J. designed the study. A. A. O., S. P., J. S.-B., and M. H. K. performed the studies. M. E. U., A. K., and W. R. B. obtained cipemastat and helped plan animal dosing. A. A. O., S. P., P. T. E., and S. K. J. analyzed the data. A. A. O. and S. K. J. wrote the initial draft. All authors edited the manuscript.
Financial support. This work was supported by the National Institutes of Health (Director’s Transformative Research Award R01-EB-020539 and New Innovator Award DP2-OD-006492 to S. K. J.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Salgame P. MMPs in tuberculosis: granuloma creators and tissue destroyers. J Clin Invest 2011; 121:1686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med 2014; 190:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh S, Kubler A, Singh UK, et al. Antimycobacterial drugs modulate immunopathogenic matrix metalloproteinases in a cellular model of pulmonary tuberculosis. Antimicrob Agents Chemother 2014; 58:4657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke B, Giannoudis A, Corke KP, et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol 2003; 163:1233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ordonez AA, Tasneen R, Pokkali S, et al. Mouse model of pulmonary cavitary tuberculosis and expression of matrix metalloproteinase-9. Dis Model Mech 2016; 9:779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujisawa T, Igeta K, Odake S, Morita Y, Yasuda J, Morikawa T. Highly water-soluble matrix metalloproteinases inhibitors and their effects in a rat adjuvant-induced arthritis model. Bioorg Med Chem 2002; 10:2569–81. [DOI] [PubMed] [Google Scholar]
- 7. Brewster M, Lewis EJ, Wilson KL, Greenham AK, Bottomley KM. Ro 32-3555, an orally active collagenase selective inhibitor, prevents structural damage in the STR/ORT mouse model of osteoarthritis. Arthritis Rheum 1998; 41:1639–44. [DOI] [PubMed] [Google Scholar]
- 8. Elkington PT, Nuttall RK, Boyle JJ, et al. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med 2005; 172:1596–604. [DOI] [PubMed] [Google Scholar]
- 9. Benator D, Bhattacharya M, Bozeman L, et al. ; Tuberculosis Trials Consortium Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002; 360:528–34. [DOI] [PubMed] [Google Scholar]
- 10. Elkington P, Shiomi T, Breen R, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest 2011; 121:1827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harper J, Skerry C, Davis SL, et al. Mouse model of necrotic tuberculosis granulomas develops hypoxic lesions. J Infect Dis 2012; 205:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Urbanowski ME, Ihms EA, Bigelow K, Kübler A, Elkington PT, Bishai WR. Repetitive aerosol exposure promotes cavitary tuberculosis and enables screening for targeted inhibitors of extensive lung destruction. J Infect Dis 2018; 218:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Y, Wang L, Zimmerman MD, et al. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog 2018; 14:e1006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ordonez AA, Pokkali S, Kim S, et al. Adjunct antibody administration with standard treatment reduces relapse rates in a murine tuberculosis model of necrotic granulomas. PLoS One 2018; 13:e0197474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore MJ, Hamm J, Dancey J, et al. ; National Cancer Institute of Canada Clinical Trials Group Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2003; 21:3296–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.