ABSTRACT
Poor solubility is a common challenge encountered during the development of high concentration monoclonal antibody (mAb) formulations, but there are currently no methods that can provide predictive information on high-concentration behavior of mAbs in early discovery. We explored the utility of methodologies used for determining extrapolated solubility as a way to rank-order mAbs based on their relative solubility properties. We devised two approaches to accomplish this: 1) vapor diffusion technique utilized in traditional protein crystallization practice, and 2) polyethylene glycol (PEG)-induced precipitation and quantitation by turbidity. Using a variety of in-house mAbs with known high-concentration behavior, we demonstrated that both approaches exhibited reliable predictability of the relative solubility properties of these mAbs. Optimizing the latter approach, we developed a format that is capable of screening a large panel of mAbs in multiple pH and buffer conditions. This simple, material-saving, high-throughput approach enables the selection of superior molecules and optimal formulation conditions much earlier in the antibody discovery process, prior to time-consuming and material intensive high-concentration studies.
KEYWORDS: Solubility screening, high-throughput, mAb developability assessment, formulation
Introduction
Poor solubility can plague downstream monoclonal antibody (mAb) development. mAbs need to be sufficiently stable and soluble at high concentration in order to withstand manufacturing, storage, and administration processes to support their intended therapeutic activity.1 For instance, subcutaneous self-injection is the preferred route of administration for patients’ convenience. The dose volume is typically 1 mL, but may be up to 2.25 mL in some cases to achieve the desired biological efficacy. As a result, antibody solutions often need to be formulated at concentrations greater than 100 mg/mL. Such requirements place a high demand for superior antibody solubility. Therefore, the ability to confirm that antibody candidates possess desired solubility properties as early as possible during discovery can substantially improve the likelihood of successful development of therapeutic mAbs.
Solubility issues, among other colloidal instability concerns such as opalescence and high viscosity, tend to manifest only at higher mAb concentrations (i.e., >50 mg/mL).2,3 Most techniques for characterizing high-concentration behaviors are often tedious and low throughput, requiring large quantities of protein. For example, conventional solubility assessment (e.g., concentrating by ultrafiltration) typically consumes hundreds of milligrams of purified mAb for rigorous assessment. This material requirement essentially prohibits screening of solubility properties at early mAb discovery for all but a few selected molecules. Similarly, it undermines the use of powerful protein engineering approaches for solubility improvement due to limited screening capacity.
Often, during either antibody discovery or antibody engineering efforts, hundreds, or even thousands, of mAbs are available to be evaluated, yet only small quantities of material are available for each mAb. Although there are many high-throughput methods available for assessing binding-related activity or conformational stability of mAbs, only a few methods exist for assessing protein solubility or colloidal stability. There has been considerable interest in developing techniques that are able to predict solubility properties with small quantities of protein. Progress has been made with the introduction of several techniques, such as cross-interaction chromatography (CIC),4,5 affinity-capture self-interaction nanoparticle spectroscopy (AC-SINS),6-9 or clone self-interaction by bio-layer interferometry (CSI-BLI).10 These approaches evaluate mAbs on their potential for cross- or self-interaction at low protein concentration, thus attempting to predict mAb high-concentration behavior.
The tools mentioned above are useful for identifying troublesome molecules. They require only microgram quantities of protein, and some approaches can be implemented in a high-throughput fashion. However, there are a number of considerations that limit the utility of these approaches in predicting mAb solubility: 1) some of these approaches require the use of added, non-specific antibody (i.e., polyclonal human IgG for CIC, or polyclonal capture antibody in AC-SINS); 2) nearly all involve non-typical surfaces (i.e., gold-particles, chromatography resins), thus introducing the potential for non-specific surface interaction to confound assay results; and 3) the approaches have limited capacity in exploring more than one buffer condition for all of the assay formats.
The high-concentration behavior of mAbs can also be predicted by determining the interaction parameter (KD) derived from concentration dependence of the protein diffusivity obtained by dynamic light scattering (DLS).11-16 Prior studies have suggested that KD could serve as a qualitative screening tool to assess protein colloidal stability, particularly with regard to viscosity and phase separation.17-19 This method requires measurement of diffusion coefficient within a protein concentration range, typically from 1 mg/mL to 20 mg/mL. Even with high-throughput adaptation, KD determination still consumes ≥20 mg of protein to obtain reliable results for multiple formulation conditions. Thus, this method is better suited for formulation screening for a low number of molecules, such as in later development stages. Recent studies have also reported conflicting observations regarding the correlation between KD and mAb viscosity, especially at low ionic strength conditions,20,21 raising concerns about utilizing KD alone for prediction of high protein concentration behavior. Orthogonal methods for predicting and screening mAb solubility are still desired.
In order to address these deficiencies, we explored an approach where solubility or insolubility is directly assessed with the use of solution additives (precipitants) that shift the intrinsic solubility maxima to a much lower mAb concentration. There is a long history in the biochemical sciences of using protein precipitants to explore protein solution behaviors such as solubility and phase separation. These practices have also been applied to protein purification, protein crystallization, and liquid-liquid phase separation.22-32 Particularly relevant to this work, it has been demonstrated that the extrapolated protein solubility by polyethylene glycol (PEG) precipitation has a strong correlation to experimentally measured solubility, including antibodies.26,27,29,33 Gibson et al.34 utilized the PEGmidpt values (the weight percent PEG in solution required to decrease the protein concentration by 50%) to compare relative protein solubility of IgG1 mAbs under various pH and buffer conditions for formulation development. Similarly, Li et al.35 used a simplified PEG precipitation method for studying high concentration formulation of mAbs. Using 10 mAbs as model proteins, the authors demonstrated that the minimum PEG concentration required to cause precipitation correlated with both the ability to concentrate various antibodies and “opalescence” of high-concentration (100 mg/mL) solutions. These studies focused on fewer candidate molecules and are formulation-centric. Both methods consume at least 20 mg of material for evaluating one formulation condition, with a starting mAb concentration of ≈10–20 mg/mL.
Based on these prior studies, we aimed to develop an assay that can screen a large panel of mAbs during early discovery, aiming for greater throughput with lower material consumption. Herein, we describe the establishment of two assay formats designed for miniaturization and automation that can be used to qualitatively characterize the solubility properties of mAbs and to provide a ranking of mAbs based on their solubility. The screening assays require small quantities of mAb (up to ≈3 mg) and are capable of assessing solubility in a variety of buffer conditions with relatively high throughput. These assays were successfully applied to the selection of mAbs with superior solubility properties, either among diverse discovery hits or related mAb engineering variants.
Results
Developing a minimum solubility “phase diagram” using precipitants
We initially explored whether a protein precipitant, specifically ammonium sulfate (AS, (NH4)2SO4), could be used to rank the solubility of different antibodies. Rather than determining mAb solubility at varying precipitant concentrations, we characterized the minimum concentration of AS required to induce insolubility under various buffer conditions at a constant mAb concentration. This was accomplished by employing the traditional “sitting drop” protein crystallization technique to drive mAb precipitation under incrementally higher AS concentrations, followed by visual inspection under a microscope to record the droplet phenomenon. This approach relies on vapor diffusion to drive the protein concentration to a super-saturated state, by using the buffer and AS concentrations that are twofold greater in the well relative to the small drop containing antibody. Preliminary experiments with a variety of in-house antibodies suggested that an AS concentration range of 0.9 M to 1.9 M worked best for this purpose. Within this AS range, at a starting protein concentration of 4 mg/mL, we observed that most antibodies exhibit various levels of amorphous precipitation under a variety of pH conditions. To achieve a broader understanding of pH-dependent solubility with minimal material usage, we designed a 4 × 6 pH and AS concentration matrix (24 conditions), where the pH conditions reflect typical manufacturing, formulation, and physiological conditions. Thus, one 96-well crystallization tray contains 4 of the 4 × 6 matrix (24 conditions each) for characterizing 4 mAbs.
To further explore the translatability of AS-induced insolubility to mAb solubility behavior, we examined four model mAbs with known solubility properties (Table 1) in this assay format. Examples of the image panel of droplet behavior for mAb1 and mAb2 are shown in Figure 1(a). Visually, it is evident that for mAb1, nearly all of the droplets appear clear, with only light precipitation at the highest AS concentration, whereas visible to heavy precipitation is observed for many of the droplets of mAb2. To simplify the visualization of imaging results, and to aid in ranking mAbs by their solubility properties, we graded the extent of precipitation based on visual observation of each droplet (Figure 1(b)). The grading result yielded a graphical display of a solubility “phase diagram” for each antibody (Figure 1(c)) across the pH and AS concentration matrix. Overall, the patterns in the diagrams for these four mAbs were consistent with known solubility properties (Table 1), and suggest that this approach has the potential to predict the solubility behavior of a variety mAbs with significantly reduced material requirements (<1 mg protein).
Table 1.
Summary of solubility behavior of the 4 model mAbs used for developing AS induced solubility screening assay.
| Solubility behavior | |
|---|---|
| mAb1 | Good solubility: >150 mg/mL (C5, C6, and PBS) |
| mAb2 | Higher levels of aggregate and insoluble particles; protein precipitation at pH 5 |
| mAb3 | Good solubility: ≥195 mg/mL (C6.5); ≥ 168 mg/mL (PBS) |
| mAb4 | Poor solubility with strong pH dependency: 7.4 mg/mL (C6.5); ≥80 mg/mL (PBS) |
Figure 1.

Use of ammonium sulfate (AS) or 4 kDa polyethylene glycol (PEG 4K) as a precipitant to evaluate and rank order mAbs solubility. (a) Images of the droplet in the 4 × 6 matrix for mAb1 and mAb2. Horizontally, there is an increase of AS concentration applied (0.9 M to 1.9 M) and each row represents one pH condition as labeled on the right. (b) Legend of grading system utilized for recording the solubility behavior of each droplet. (c) Solubility phase diagram of mAb 1, 2, 3 and 4 graded by the legend shown in (b). (d) Images of droplet behavior of mAb B2 by PEG 4K induced precipitation (8% to 25% PEG 4K; with and without salt).
We also used PEG as the protein precipitant in order to interrogate the impact of salt on protein solubility. Prior investigations have demonstrated that PEG of different sizes possesses different protein precipitation efficiency.23,36,37 Based on the reported studies27,38 and our empirical experience, we evaluated three types of PEG (PEG 400, PEG 4000, and PEG 8000) with several model mAbs (data not shown). PEG 4000 (PEG 4K) was chosen because it demonstrated optimal efficiency in mAb precipitation at the desired mAb concentration (1 mg/mL), and possessed manageable solution viscosity at the required concentrations to yield accurate dispensing and mixing. Example droplet images for a mAb with known salt-dependent solubility are shown in Figure 1(d); the antibody used, mAb B2, is described in more detail below (Table 3). Using PEG 4K with a concentration range from 8% to 25% (by weight percent), in the absence or presence of added sodium chloride (NaCl, 0.1 M), we were able to induce observable precipitation and reveal expected improvement (i.e., increased resistance to precipitation) upon the inclusion of NaCl.
Table 3.
A panel of 12 mAbs (B1-B12) that were selected for validation of PEG 4K induced precipitation assay. Their solubility related properties and sequence-based properties are summarized. The reported pI values were calculated using MOE2017 (Chemical computing group).
| mAb | Isotype | pI (calculated) | Solubility properties |
|---|---|---|---|
| B1 | IgG4 | 7.1 | Poor solubility; insoluble aggregates in high concentration solution, precipitation without salt |
| B2 | IgG4 | 8.3 | Salt-dependent solubility: concentrations > 60 mg/ml in PBS; < 10 mg/ml at C6 (salt free buffer) |
| B3 | IgG4 | 8.2 | Phase separation in the absence of salt or low salt |
| B4 | IgG4 | 7.6 | No solubility Issues observed |
| B5 | IgG4 | 8.2 | No solubility Issues observed |
| B6 | IgG4 | 7.1 | No solubility Issues observed |
| B7 | IgG4 | 8.4 | High molecular weight increase and particle formation at concentrations > 25 mg/mL of citrate condition |
| B8 | IgG4 | 4.9 | No solubility Issues observed |
| B9 | IgG4 | 7.6 | Insoluble aggregate, particle and cryoglobulin formation at concentrations > 50 mg/mL |
| B10 | IgG1 | 8 | No solubility Issues observed |
| B11 | IgG1 | 8.6 | No solubility Issues observed |
| B12 | IgG4 | 7.8 | Phase separation observed in the absence of salt |
Encouraged by these initial results, we further explored assay miniaturization and automation by utilizing robotics often used for protein crystallization practices. In our experiments, a Crystal Phoenix Robot (Art Robbins Instruments) was used for setting up trays to achieve smaller-volume drops with precision (0.5 µL). This was followed by automated image collection with a Rock Imager 1000 (Formulatrix, Inc.), scheduled at 24 and 48 h after tray setup. All imaging data and grading results were captured and stored in a database. These adaptations further reduced material consumption (requiring 0.5 mg protein per molecule) and provided a substantial time saving by eliminating the manual inspection procedure previously employed. For example, we characterized 12 related mAbs, which bear identical complementarity-determining regions and only differ in the variable domain framework regions. The resulting phase diagrams (Figure 2(a)) suggested that these mAbs exhibit a wide range of solubility behavior in spite of their sequence similarity and comparable binding activity. Based on the solubility screening profile, V3 was one of the superior molecules in this panel as it exhibited the most resistance to AS-induced precipitation at all four pH conditions evaluated (Figures S1 and S2). Subsequent in-depth characterization of V3 confirmed that it was easily concentrated to over 100 mg/mL under a variety of conditions (citrate buffer at pH 6 with and without 150 mM NaCl, and in phosphate-buffered saline (PBS) at pH 7.4; Table 2). Interestingly, variant V4 at pH 5.5 grew protein crystals (marked as X in Figure 2(a), shown in Figure 2(b)) in multiple AS concentrations (Figure S3), suggesting lower solubility at this pH condition as it reached saturation even at the lowest AS concentration of 0.9 M. Importantly, because of the reduced material requirements and automated process, solubility ranking of this series of mAbs was achieved by using ~0.5 mg of each antibody and was completed within three days. This demonstrated the effective use of the assay for the rapid selection of highly soluble antibodies during mAb discovery.
Figure 2.

Solubility screening of 12 mAbs (V1-V12) by AS-induced precipitation. (a) Based on the screening profile, V3 demonstrated the least amount of precipitation under AS-induced precipitation at all four pH conditions. (b) Protein crystals (marked as “X”) observed for V4 at pH 5.5 in multiple wells from low to high AS concentration (0.9 M to 1.9 M).
Table 2.
Solubility determination of mAb variant V3, a molecule selected based on its superior solubility phase diagram.
| Formulation | Solubility (mg/mL) | %HMW |
|---|---|---|
| C6 | ≥138 | 1.7 |
| C6N | ≥132 | 1.4 |
| PBS (pH 7.4) | ≥125 | 1.8 |
Development of a homogenous, high-throughput assay format
We next explored whether turbidity measurement was a more effective way of determining insolubility than visual inspection and grading. We observed that the absorbance at 350 nm (A350) was sensitive to mAb precipitation, but required larger solution volume for reading (when using a standard, 96-well microtiter plate). In order to minimize material requirements, we examined parameters such as initial protein concentration, minimal solution volume and incubation time. We chose a starting protein concentration of 1 mg/mL with 25 μL protein solution in each well to mix with the corresponding PEG 4K reagent. To determine the optimal time window for turbidity measurement, we performed a time–course study, which indicated that relative protein precipitation onset conditions become stable after ≈2-h incubation at 25°C (Figure S4). Extended incubation time (>6 h) leads to heavy precipitation settling to the bottom of the well, which results in inconsistent A350 measurements. Therefore, 2-h incubation was selected, which also allows the entire assay process to be completed within one day. Combining these modifications allowed for a homogenous assay that required only appropriate mixing of solutions (mAb solution mixed with increasing concentrations of PEG 4K) into individual wells of a 96-well plate by automated liquid handling, followed by A350 reading using a standard plate-reader after a 2-h incubation.
To extract a broader range of data from this screening format, we designed an expanded set of conditions, including various buffers, pHs and salt, for solubility screening. We settled on eight conditions, which represent manufacturing purification process-related and formulation-related conditions that a typical antibody might experience. The conditions used are acetate buffer pH 5 (A5), acetate buffer pH 5 with sodium sulfate (A5 + SO4), acetate buffer pH 5 with NaCl (A5 + NaCl), bicine buffer pH 8.1 (B8.1), citrate buffer pH 6 (C6), citrate buffer pH 6 with NaCl (C6N), histidine buffer (H6), and PBS (P7.4). The final design is a matrix of 8 buffer/pH conditions by 12 PEG 4K concentrations (ranging from 4%-26% with 2% incremental increases), with one 96-well assay plate per mAb. Compared with the previous design of four conditions by six PEG 4K points (range 8%-25%), this format offers more defined resolution and evaluates broader formulation conditions. The increased material demand was balanced by further miniaturization, using a 384-well plate format. The resulting solubility profile provides an assessment of eight buffer conditions simultaneously and consumes only ≈3 mg of mAb. This amount is substantially less than what would be required using traditional solubility assessment methods.
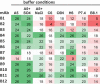
Using this assay format, we performed an in-depth evaluation of 12 mAbs chosen from a variety of in-house mAb programs, with each mAb recognizing different therapeutic targets. Their sequence-based properties and qualitative solubility properties are summarized in Table 3. The PEG 4K induced precipitation results, grouped by mAb, are shown in Figure 3. Qualitatively, it is easy to rank-order mAbs on their solubility properties by visual inspection of the precipitation profile. For example, mAbs with poor solubility start to show protein precipitation earlier in the PEG 4K gradient, and higher overall turbidity (A350), as exhibited by mAbs B1 and B2. The A350 baseline reading of buffers is consistently 0.2 ± 0.01 for the specified assay plate we used. For data aggregation purposes, we chose to extract the onset of precipitation (PEGonset) for easy comparison and ranking of solubility across mAbs and conditions. PEGonset was defined as the minimal PEG 4K concentration (weight percent) that induces protein precipitation, with A350 reading ≥0.4 AU (≈twofold increase over the baseline) for each precipitation curve. The corresponding PEGonset values of the 12 mAbs are reported in Table 4. Conditional formatting was applied to help visualization, green for higher PEGonset value and red for lower PEGonset value. It becomes easy to determine which mAbs show superior solubility profiles for all of the conditions evaluated (e.g., mAbs B4 and B11) or which mAbs exhibit poor overall solubility profiles (e.g., mAbs B1 and B2). Collectively, these observations are consistent with the known solubility behavior for these mAbs. Among the eight buffer conditions, A5 appears to be a favorable buffer for most of the mAbs, except for mAb B8. This could be attributed to the isoelectric point (pI) of mAb B8 (pI = 4.9) being close to pH 5 of A5.
Figure 3.

Solubility screening profile of 12 mAbs (B1-B12) by PEG 4K induced precipitation method. The turbidity (absorbance 350 nm, AU) for the various PEG 4K concentrations (%) are shown, with each curve representing one formulation condition (indicated in the figure legend).
Table 4.
The panel of 12 mAbs (B1-B12) were assessed in the PEG-induced precipitation assay at eight buffer conditions and the corresponding PEGonset values (%) are reported. Conditional formatting is applied to guide visualization. Red: lower PEGonset, lower solubility; Green: higher PEGonset, higher solubility.
 |
In order to assess the predictive value of the assay more rigorously, we carried out a conventional solubility assessment by ultracentrifugation for some of the same mAbs in select buffer conditions. The determined solubilities are summarized in Table 5. The solubility values (mg/mL) obtained by ultracentrifugation were plotted against the PEGonset derived from the above screening assay (Figure 4). Visually, there appears to be a correlation between measured solubility and the corresponding PEGonset values, suggesting that this assay is capable of differentiating molecules based on their solubility properties, as well as identifying buffer conditions that yield improved solubility for a particular molecule.
Table 5.
Solubility assessment by ultracentrifugation of mAbs with selected formulation conditions. For those with repeated solubility experiments (marked by *), both results are listed in the table (slash separated). PEG-induced precipitation screening were performed twice, and both PEGonset values are reported (PEGonset,1 PEGonset2).
| mAb | buffer condition | solubility (mg/mL) | PEGonset1 (%) | PEGonset2 (%) |
|---|---|---|---|---|
| B1* | C6* | 4.9/4.2 | 10 | 10 |
| B1 | H6 | 47 | 12 | 12 |
| B1 | PBS | 156.3 | 16 | 18 |
| B2* | C6* | 4.6/3.4 | 4 | 4 |
| B2 | H6 | 176.5 | 18 | 20 |
| B2 | PBS | 84.6 | 10 | 12 |
| B4 | C6 | 143.8 | 18 | 20 |
| B5* | C6* | 112.6/101.7 | 18 | 16 |
| B5 | H6 | 170.3 | 20 | 20 |
| B5 | PBS | 175.5 | 20 | 18 |
| B6 | C6 | 148.8 | 16 | 16 |
| B7 | C6 | 149.9 | 14 | 14 |
| B8* | C6* | 151.2/165.5 | 18 | 20 |
| B8 | H6 | 1.3 | 8 | 6 |
| B8 | PBS | 180.8 | 20 | 22 |
| B9 | C6 | 124.6 | 16 | 18 |
| B10 | C6 | 139.6 | 16 | 16 |
| B11 | C6 | 164.5 | 20 | 20 |
| B11 | H6 | 173.7 | 26 | 26 |
| B11 | PBS | 175.8 | 24 | 24 |
| B11 | A5 | 156.6 | 26 | 26 |
Figure 4.

Assessing the predictive value of PEG 4K induced precipitation method by plotting solubility (mg/mL) value obtained through ultracentrifugation against PEGonset (%) obtained by the screening method. Among the 12 model mAbs tested, mAb B1 (orange dots), B2 (purple dots), B5 (gray triangles), B8 (green dots), and B11 (black-white squares) were assessed at multiple formulation conditions. The rest of the other mAbs (assessed at single condition, i.e., C6) are shown as blue dots.
Once established, this assay has been used for numerous mAb discovery projects. A total of 107 data points (where both the PEGonset (%) determined by precipitation assay and solubility (mg/mL) determined by ultracentrifugation were available), derived from 21 independent mAb discovery programs, were curated for retrospective analysis. As can be seen in the scatterplot between PEGonset (x-axis) and solubility (y-axis), mAbs cluster into two groups (Figure 5). The first group includes molecules that exhibit poor solubility (<50 mg/mL) and lower PEGonset (<10% PEG 4K), boxed in the lower left corner (n = 17). The second group covers mAbs exhibiting better solubility and higher PEGonset values. These results have led us to apply an empirical cutoff of PEGonset ≤10% to flag or eliminate molecules during solubility screening.
Figure 5.

Scatterplot between PEGonset (x-axis) and solubility (y-axis) of historical data on mAbs, where both solubility value (obtained through ultracentrifugation) and its corresponding PEGonset (obtained using the screening assay) are available. For mAbs whose concentration reached the pre-set solubility threshold value (such as ≥100) and therefore stopped further concentration, the threshold values were plotted (pink circles). The Spearman correlation between solubility and PEGonset is ρ = 0.524 (p < 0.0001) (including all data points) and ρ of 0.69 (p < 0.0001) (excluding data points in pink). Data points can be seen clustered in two groups, boxed by the gray dashed lines. In general, PEGonset <10% suggests solubility lower than 50 mg/mL, which was used as the guideline for flagging molecules with poor solubility in the screening assay.
Discussion
The principle of using precipitants (either AS or PEG) for relative protein solubility has been elaborated elsewhere,35,39,40 and numerous methods for determining the relative or apparent solubility of proteins have been described.34,35,41,42 In this work, we explored two uniquely adapted approaches for screening the relative solubility of a larger number of mAbs, in relevant formulation conditions, that can be used during early antibody discovery. These assay formats enabled rapid assessment of solubility properties, using high-throughput screening with minimal material usage, thus providing valuable information critical for molecule selection and design at an early stage. These approaches aim to mimic a protein high-concentration environment by applying a crowding reagent (AS or PEG) and assessing protein solubility directly in solution in a miniaturized setting. Both reagents are well established, inert protein precipitants, exhibiting minimal perturbation of tested proteins.43,44 Compared with the other high-throughput screening methods (CIC or AC-SINS), our approaches avoid the complications caused by unusual surfaces (beads or resin) or unrelated antibodies (polyclonal IgGs).
Our first approach used a standard protein crystallization technique of sitting drop with vapor diffusion to characterize mAb solubility under various pH, buffer, and salt conditions. The resultant phase diagram matrix derived from imaging and grading of protein droplet behavior can be used to qualitatively rank the solubility for screening mAbs. Subsequent optimization of this approach, including the use of automated liquid handling and image collection, significantly enhanced the efficiency such that ≈1 mg of mAb was sufficient for both AS- and PEG-based experiments, compatible with the low material availability during early antibody discovery. In addition to solubility screening, some unexpected benefits also became apparent. First, the observed droplet phenomenon provided relevant phase behavior of mAbs, including phase separation or “oil droplets”, skin formation, or gelation. Such observations could indicate undesirable protein behavior at high concentration, such as phase separation or high viscosity.29,33 The additional, qualitative information can aid in molecule selection or in identifying whether follow-up studies are warranted for molecules of interest. Second, protein crystals sometimes grew in the assay trays and occasionally yielded protein structures when used for X-ray diffraction experiments.
The second approach, based on PEG-induced protein precipitation, is similar to other recently published methodologies34,35,41,42 except that we utilize turbidity (i.e., light scattering due to protein precipitation) as the primary readout. The rank-order of mAb solubility references the value of PEGonset, slope, and magnitude of the precipitation curve, collectively. We chose PEGonset over the more commonly reported PEGmidpt approach34,41 for three practical reasons. First, we assay our plate directly after 2 h of incubation time and avoid overnight incubation as used by other methods. Second, the simplified method enables higher efficiency and reduced protein material consumption by skipping two extra steps: centrifugation to remove protein precipitate, and transfer of the supernatant to another assay plate for determination of remaining soluble protein concentration by A280 measurement. Hence, 3 mg of protein is adequate to simultaneously obtain the solubility profile for eight different buffer conditions. Third, the use of PEGonset is better suited for turbidity determination (i.e., A350).
Turbidity is affected by many parameters including particle number, particle size, and particle shape.45 Usually, when heavy protein precipitation occurs, heterogeneous aggregates co-exist as a mixture of different particle sizes. Therefore, it is likely that the A350 signal is not linear when higher levels of aggregate formation occur (as seen in Figure 3). The concept of PEGonset is similar to the term ‘discontinuity point’ (m*), which describes the PEG concentration at which protein solubility starts to decrease under the corresponding formulation condition tested.46 Based on the notion that a more soluble mAb requires higher % PEG 4k to precipitate, and thus a later PEGonset, we believe that PEGonset is a reliable way for rank-ordering solubility of mAbs, when the other assay parameters are kept constant (i.e., initial protein concentration, protein shape and size, temperature).
The robustness of using PEGonset values to rank-order mAbs by their solubility is also confirmed by a more comprehensive correlation analysis. We chose Spearman’s rank correlation (ρ, determined using JMP, version 12, SAS Institute), as there is no expectation that the solubility and PEGonset values should be linearly related. For the data plotted in Figure 4 (21 measurements), ρ = 0.72 (p < 0.0001), suggesting a statistically significant correlated ranking between the two measurements. With the larger dataset (Figure 5, n = 107 measurements), there is still modest Spearman correlation between PEGonset and the solubility determined by ultracentrifugation, but the correlation appears to be weaker (ρ = 0.52, p < 0.0001). The weaker correlation is likely due to two reasons: 1), a fraction of solubility data were only minimum solubility measurements (colored in pink), meaning the protein solubility reached the pre-set solubility threshold value (such as 50 or 100 mg/mL) and therefore stopped further concentrating; and 2) some mAbs exhibited no precipitation even at the highest PEG 4K concentration, thus marked as ≥26% PEGonset, suggesting a potentially higher PEGonset than reported. When the minimum solubility data points (pink) are excluded (resulting in n = 63 measurements), a correlation close to the initial data set is obtained (ρ = 0.69, p < 0.0001), demonstrating the robustness in using PEGonset values to rank mAbs by their solubility. As the focus of our approach is to screen mAbs for ranking, rather than to ascribe absolute protein solubility, we routinely include two control mAbs (B11 – good solubility; B2 – poor solubility) in each experiment to serve as internal references for comparison.
Similar methods reported previously focused on formulation development and consumed large amounts of protein, and are better suited for downstream, late-stage candidate molecules.34,35 Our optimized approach can be used to screen diverse discovery hits or related engineering variants using several milligrams of material, thus moving solubility assessment to the earlier stage of antibody discovery. For the selected molecules, the solubility profile obtained from the panel of formulation conditions also readily inform and guide the study design of extended high-concentration characterizations. Depending on the particular program needs, abridged versions of the assay could also be devised, e.g., using 0.3 mg protein for evaluating one formulation condition. Using these high-throughput methods, we were able to identify the improved engineering variants to achieve the desired pH- or salt-dependent solubility behavior. Thus, the assay is a well-suited companion tool when coupled with protein engineering efforts.
The methods we have described are tailored specifically for mAbs and have been exclusively applied for this purpose. Recent publications suggest that PEG-induced precipitation may not apply to entirely different moieties other than mAbs,47 such as single-domain antibodies or other protein therapeutics. Protein behavior and precipitation efficiency in the presence of PEG are influenced by many factors, including protein size, protein shape, and PEG properties.48 Therefore, the extrapolated apparent solubility could be molecule-type dependent. We recommend exercising caution when comparing proteins of different modalities (e.g., mAb vs fusion protein) because the solubility rank-order derived by such a screening assay may not be relevant.
In summary, we developed two high-throughput screening assays that are capable of predicting the solubility of mAbs without the requirement of high concentration studies or the need for large quantities of material. The described assay formats were used to screen a large number of mAbs for solubility, and to recommend formulation conditions for optimal solubility. The value of early developability assessment for therapeutic mAbs, especially characterizing high-concentration behavior, is widely recognized for reasons such as cost and attrition rate reduction. Our screening assay enables the assessment of this critical property early-on and facilitates the discovery, selection, and optimization of the most promising mAb molecules.
Materials and methods
Materials
All mAb samples tested were produced internally at Eli Lilly & Co. They were expressed in either HEK293 or Chinese hamster ovary cells. mAbs were purified through typical antibody purification procedures (protein A capture followed by polishing steps). All reagents and excipients were commercially available from Hampton Research, EM Chemicals, JT Baker, Sigma-Aldrich, and Mallinckrodt, and were of high grade (>98% purity).
mAb precipitation by ammonium sulfate
Stock solutions of 1 M sodium citrate at pH 3.5, 5.5, 6.5 and 0.5 M phosphate at pH 7.4 containing 3.5 M ammonium sulfate were mixed to prepare solutions of varying final ammonium sulfate levels (from 0.9 M to 1.9 M) in a deep-well Master Block plate. These solutions were added (70 μL) to the reservoir well of each quadrant of a CrystalQuick 96-well plate (Greiner 609171). mAb samples were dialyzed into 20 mM sodium citrate, pH 6.5. Final mAb concentrations were adjusted to 4.0 mg/mL. A volume of 0.5 μL of mAb sample was dispensed into the center of the stage of each well and 0.5 μL of the Master Block solution was also added onto the center of the stage. The 96-well plate(s) were incubated for 48 h at 25°C. Each well of the plate was viewed by stereomicroscope with zoom (8 to 12.5:1) and 10 × to 100 × magnification. The wells were assessed for a clear drop, precipitation, phase-separation, and other phenomena in accordance with a standard crystallization scoring procedure (described in Figure 1(b)). For miniaturization and optimization, Crystal Phoenix Robot (Art Robbins Instruments) was used to set up trays to achieve smaller volume drops with precision (0.5 µL). This was followed by automated image collection of the trays on Rock Imager 1000 (Formulatrix, Inc.) scheduled at 24 and 48 h after tray setup. The Rock Imager 1000 uses a 5-megapixel, 2/3” color charge-coupled device camera that captures high-quality images, with a 12 x motorized zoom function. All imaging data and grading results are captured and stored in a database.
PEG-induced precipitation with buffer screens & automation
Stock solutions of 1 M sodium acetate, pH 5.0, 1 M sodium citrate, pH 6.0, 0.5 M L-histidine, pH 6.0, 0.5 M bicine, pH 8.1, 1 M sodium sulfate, 5 M NaCl and 10X PBS were mixed to prepare solutions of varying final PEG 4000 (PEG 4K) levels (from 4% to 26%) in a deep well Master Block plate using a Formulator (Formulatrix, Bedford, MA). A Biomek FXp (Beckman Coulter, Indianapolis, IN) liquid handler was used to add 25 μL/well of the stock solutions from the Master Block plate into 96-well polystyrene, V-bottomed assay plates (Greiner Cat # REF651101). mAb samples were normalized at 1 mg/mL in water and plated (25 μL/well) into all wells of the 96-well PS plate (containing the Master Block solutions) using a Biomek FXp. The final assay composition on the PS plate (in a total volume of 50 µL) contains 25 μg of sample mAb per well, plus buffers (in appropriate rows) of 10 mM sodium acetate, 10 mM sodium citrate, 10 mM L-histidine, 10 mM bicine, with or without 300 mM sodium sulfate, with or without 150 mM NaCl, and 1x PBS of the corresponding PEG 4K concentration. The assay plates were sealed with clear sealing film (Cat# HR3-609, Hampton Research, Aliso Viejo, CA) and then incubated at 25°C on a titer plate shaker (Lab-Line Instruments, Melrose Park, IL) for 2 h with gentle shaking (150 rpm). After incubation, the contents of the 96-well plates were transferred to a 384-well optical-bottom plate (Thermo Scientific, Cat# 242764) using a Biomek FXp. The optical-bottom plate was read on a SpectraMax Pro plate reader (Molecular Devices, San Jose, CA) at 350 nm. The absorbance data were de-convoluted and plotted in Excel (Microsoft, Redmond, WA), where the onset of precipitation (abrupt increase in A350, ≥0.4 AU) was determined visually. The nearest PEG 4K concentration (%) that corresponds to the onset of precipitation is defined as PEGonset and refers to the PEG concentration (%) of the original stock solution prepared before plating.
Solubility assessment by ultracentrifugation
mAb samples were dialyzed against buffers of interest overnight using 30 kDa MWCO Slide-A-Lyzer G2 dialysis cassettes overnight at 4°C (a minimum of 1:200 level of exchange). After dialysis, 3–5 mg of each sample was placed in a Vivaspin-2 centrifugal concentrator (Sartorius Stedim Biotech, 30 kDa MWCO) and centrifuged at 3,100 × g for at least 3 h or until volume ceased to decrease. The concentrated sample was recovered following the manufacturer’s instructions. Concentration was determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and volume determined via Rainin pipettor. SEC-UPLC analysis was performed on both pre-and post-concentration samples at 1 mg/mL with 4 μg per injection (Waters Acquity H-Class Bio UPLC system with Waters BEH-200 SEC column, 4.6 × 150 mm, 1.7 μm, Milford, MA). Visual inspections were made pre- and post-dialysis and post-concentration for turbidity. After overnight incubation at 4°C, samples were examined visually for signs of phase separation.
Acknowledgments
All the antibodies characterized in this work were identified, produced, purified and confirmed through the combined efforts from the antibody engineering group, automation group and protein bioscience group at the Lilly Biotechnology Center and Lilly Bioprocess Research & Development. Specifically, we want to thank Gerard Kelly, Shane Atwell, Stephanie Truhlar, Karen Hill, and William Weiss IV for their support. We also appreciate the critical review and discussion provided by Tao Wei and Suntara Cahya.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Abbreviations
- AC-SINS
affinity-capture self-interaction nanoparticle spectroscopy
- AS
ammonium sulfate
- CIC
cross-interaction chromatography
- CSI-BLI
clone self-interaction by bio-layer interferometry
- DLS
dynamic light scattering
- KD
interaction parameter
- mAb
monoclonal antibody
- PEG
polyethylene glycol
- pI
isoelectric point
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Shire SJ, Shahrokh Z, Liu J.. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93:1390–402. doi: 10.1002/jps.20079. [DOI] [PubMed] [Google Scholar]
- 2.Shire SJ. Formulation and manufacturability of biologics. Curr Opin Biotechnol. 2009;20:708–14. doi: 10.1016/j.copbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Lowe D, Dudgeon K, Rouet R, Schofield P, Jermutus L, Christ D. Aggregation, stability, and formulation of human antibody therapeutics. Adv Protein Chem Struct Biol. 2011;84:41–61. doi: 10.1016/B978-0-12-386483-3.00004-5. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs SA, Wu SJ, Feng Y, Bethea D, O’Neil KT. Cross-interaction chromatography: a rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res. 2010;27:65–71. doi: 10.1007/s11095-009-0007-z. [DOI] [PubMed] [Google Scholar]
- 5.Spencer S, Bethea D, Raju TS, Giles-Komar J, Feng Y. Solubility evaluation of murine hybridoma antibodies. MAbs. 2012;4:319–25. doi: 10.4161/mabs.19869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sule SV, Dickinson CD, Lu J, Chow CK, Tessier PM. Rapid analysis of antibody self-association in complex mixtures using immunogold conjugates. Mol Pharm. 2013;10:1322–31. doi: 10.1021/mp300524x. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Schultz JS, Weldon CL, Sule SV, Chai Q, Geng SB, Dickinson CD, Tessier PM. Discovery of highly soluble antibodies prior to purification using affinity-capture self-interaction nanoparticle spectroscopy. Protein Eng Des Sel. 2015;28:403–14. doi: 10.1093/protein/gzv045. [DOI] [PubMed] [Google Scholar]
- 8.Geng SB, Wittekind M, Vigil A, Tessier PM. Measurements of monoclonal antibody self-association are correlated with complex biophysical properties. Mol Pharm. 2016;13:1636–45. doi: 10.1021/acs.molpharmaceut.6b00071. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Caffry I, Wu J, Geng SB, Jain T, Sun T, Reid F, Cao Y, Estep P, Yu Y, et al. High-throughput screening for developability during early-stage antibody discovery using self-interaction nanoparticle spectroscopy. MAbs. 2014;6:483–92. doi: 10.4161/mabs.27431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun T, Reid F, Liu Y, Cao Y, Estep P, Nauman C, Xu Y. High throughput detection of antibody self-interaction by bio-layer interferometry. MAbs. 2013;5:838–41. doi: 10.4161/mabs.26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He F, Becker GW, Litowski JR, Narhi LO, Brems DN, Razinkov VI. High-throughput dynamic light scattering method for measuring viscosity of concentrated protein solutions. Anal Biochem. 2010;399:141–43. doi: 10.1016/j.ab.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99:1152–68. doi: 10.1002/jps.21898. [DOI] [PubMed] [Google Scholar]
- 13.Yadav S, Scherer TM, Shire SJ, Kalonia DS. Use of dynamic light scattering to determine second virial coefficient in a semidilute concentration regime. Anal Biochem. 2011;411:292–96. doi: 10.1016/j.ab.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, Shire S, Gokarn Y. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78. doi: 10.1016/j.bpj.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi S, Uchida M, Cheung J, Antochshuk V, Shameem M. Method qualification and application of diffusion interaction parameter and virial coefficient. Int J Biol Macromol. 2013;62:487–93. doi: 10.1016/j.ijbiomac.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Garidel P, Blume A, Wagner M. Prediction of colloidal stability of high concentration protein formulations. Pharm Dev Technol. 2015;20:367–74. doi: 10.3109/10837450.2013.871032. [DOI] [PubMed] [Google Scholar]
- 17.Neergaard MS, Kalonia DS, Parshad H, Nielsen AD, Moller EH, van de Weert M. Viscosity of high concentration protein formulations of monoclonal antibodies of the IgG1 and IgG4 subclass - prediction of viscosity through protein-protein interaction measurements. Eur J Pharm Sci. 2013;49:400–10. doi: 10.1016/j.ejps.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Nichols P, Li L, Kumar S, Buck PM, Singh SK, Goswami S, Balthazor B, Conley TR, Sek D, Allen MJ. Rational design of viscosity reducing mutants of a monoclonal antibody: hydrophobic versus electrostatic inter-molecular interactions. MAbs. 2015;7:212–30. doi: 10.4161/19420862.2014.985504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow CK, Allan BW, Chai Q, Atwell S, Lu J. Therapeutic antibody engineering to improve viscosity and phase separation guided by crystal structure. Mol Pharm. 2016;13:915–23. doi: 10.1021/acs.molpharmaceut.5b00817. [DOI] [PubMed] [Google Scholar]
- 20.Pindrus MA, Shire SJ, Yadav S, Kalonia DS. The effect of low ionic strength on diffusion and viscosity of monoclonal antibodies. Mol Pharm. 2018;15:3133–42. doi: 10.1021/acs.molpharmaceut.8b00210. [DOI] [PubMed] [Google Scholar]
- 21.Woldeyes MA, Qi W, Razinkov VI, Furst EM, Roberts CJ. How well do low- and high-concentration protein interactions predict solution viscosities of monoclonal antibodies? J Pharm Sci. 2019;108:142–54. doi: 10.1016/j.xphs.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Foster PR, Dunnill P, Lilly MD. The precipitation of enzymes from cell extracts of saccharomyces cerevisiae by polyethyleneglycol. Biochim Biophys Acta. 1973;317:505–16. [DOI] [PubMed] [Google Scholar]
- 23.Ingham KC. Protein precipitation with polyethylene glycol. Methods Enzymol. 1984;104:351–56. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson CL, Hageman MJ. Estimation of recombinant bovine somatotropin solubility by excluded-volume interaction with polyethylene glycols. Pharm Res. 1995;12:1671–76. [DOI] [PubMed] [Google Scholar]
- 25.Vivares D, Kaler EW, Lenhoff AM. Quantitative imaging by confocal scanning fluorescence microscopy of protein crystallization via liquid-liquid phase separation. Acta Crystallogr D Biol Crystallogr. 2005;61:819–25. doi: 10.1107/S090744490402949X. [DOI] [PubMed] [Google Scholar]
- 26.Jion AI, Goh LT, Oh SK. Crystallization of IgG1 by mapping its liquid-liquid phase separation curves. Biotechnol Bioeng. 2006;95:911–18. doi: 10.1002/bit.21054. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Annunziata O. Comparison between protein-polyethylene glycol (PEG) interactions and the effect of PEG on protein-protein interactions using the liquid-liquid phase transition. J Phys Chem B. 2007;111:1222–30. doi: 10.1021/jp065608u. [DOI] [PubMed] [Google Scholar]
- 28.Boncina M, Rescic J, Vlachy V. Solubility of lysozyme in polyethylene glycol-electrolyte mixtures: the depletion interaction and ion-specific effects. Biophys J. 2008;95:1285–94. doi: 10.1529/biophysj.108.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumetz AC, Lewus RA, Lenhoff AM, Kaler EW. Effects of ammonium sulfate and sodium chloride concentration on PEG/protein liquid-liquid phase separation. Langmuir. 2008;24:10345–51. doi: 10.1021/la801180n. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Rajagopalan R, Jiang J. Polymer-induced phase separation and crystallization in immunoglobulin G solutions. J Chem Phys. 2008;128:205105. doi: 10.1063/1.2919565. [DOI] [PubMed] [Google Scholar]
- 31.Banks DD, Latypov RF, Ketchem RR, Woodard J, Scavezze JL, Siska CC, Razinkov VI. Native-state solubility and transfer free energy as predictive tools for selecting excipients to include in protein formulation development studies. J Pharm Sci. 2012;101:2720–32. doi: 10.1002/jps.23219. [DOI] [PubMed] [Google Scholar]
- 32.Yamniuk AP, Ditto N, Patel M, Dai J, Sejwal P, Stetsko P, Doyle ML. Application of a kosmotrope-based solubility assay to multiple protein therapeutic classes indicates broad use as a high-throughput screen for protein therapeutic aggregation propensity. J Pharm Sci. 2013;102:2424–39. doi: 10.1002/jps.23618. [DOI] [PubMed] [Google Scholar]
- 33.Ahamed T, Esteban BN, Ottens M, van Dedem GW, van der Wielen LA, Bisschops MA, Lee A, Pham C, Thömmes J. Phase behavior of an intact monoclonal antibody. Biophys J. 2007;93:610–19. doi: 10.1529/biophysj.106.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson TJ, McCarty K, McFadyen IJ, Cash E, Dalmonte P, Hinds KD, Dinerman AA, Alvarez JC, Volkin DB. Application of a high-throughput screening procedure with PEG-induced precipitation to compare relative protein solubility during formulation development with IgG1 monoclonal antibodies. J Pharm Sci. 2011;100:1009–21. doi: 10.1002/jps.22350. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Kantor A, Warne N. Application of a PEG precipitation method for solubility screening: a tool for developing high protein concentration formulations. Protein Sci. 2013;22:1118–23. doi: 10.1002/pro.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atha DH, Ingham KC. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981;256:12108–17. [PubMed] [Google Scholar]
- 37.Sim SL, He T, Tscheliessnig A, Mueller M, Tan RB, Jungbauer A. Protein precipitation by polyethylene glycol: a generalized model based on hydrodynamic radius. J Biotechnol. 2012;157:315–19. doi: 10.1016/j.jbiotec.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Sharma VK, Kalonia DS. Effect of polyols on polyethylene glycol (PEG)-induced precipitation of proteins: impact on solubility, stability and conformation. Int J Pharm. 2009;366:38–43. doi: 10.1016/j.ijpharm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Latypov RF, Lomakin A, Meyer JA, Kerwin BA, Vunnum S, Benedek GB. Quantitative evaluation of colloidal stability of antibody solutions using PEG-induced liquid-liquid phase separation. Mol Pharm. 2014;11:1391–402. doi: 10.1021/mp400521b. [DOI] [PubMed] [Google Scholar]
- 40.Loschen C, Klamt A. Solubility prediction, solvate and cocrystal screening as tools for rational crystal engineering. J Pharm Pharmacol. 2015;67:803–11. doi: 10.1111/jphp.12376. [DOI] [PubMed] [Google Scholar]
- 41.Toprani VM, Joshi SB, Kueltzo LA, Schwartz RM, Middaugh CR, Volkin DB. A micro-polyethylene glycol precipitation assay as a relative solubility screening tool for monoclonal antibody design and formulation development. J Pharm Sci. 2016;105:2319–27. doi: 10.1016/j.xphs.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Sormanni P, Amery L, Ekizoglou S, Vendruscolo M, Popovic B. Rapid and accurate in silico solubility screening of a monoclonal antibody library. Sci Rep. 2017;7:8200. doi: 10.1038/s41598-017-07800-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pullara F, Emanuele A, Palma-Vittorelli MB, Palma MU. Protein crystallization: universal thermodynamic vs. specific effects of PEG. Faraday Discuss. 2008;139:299–308. discussion 9–25, 419–20. [DOI] [PubMed] [Google Scholar]
- 44.Burgess RR. Protein precipitation techniques. Methods Enzymol. 2009;463:331–42. doi: 10.1016/S0076-6879(09)63020-2. [DOI] [PubMed] [Google Scholar]
- 45.Kleizen HH, de Putter AB, van der Beek M, Huynink SJ. Particle concentration, size and turbidity. Filtrat Separat. 1995;32:897–901. [Google Scholar]
- 46.Hammerling F, Ladd Effio C, Andris S, Kittelmann J, Hubbuch J. Investigation and prediction of protein precipitation by polyethylene glycol using quantitative structure-activity relationship models. J Biotechnol. 2017;241:87–97. doi: 10.1016/j.jbiotec.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann M, Winzer M, Weber C, Gieseler H. Limitations of polyethylene glycol-induced precipitation as predictive tool for protein solubility during formulation development. J Pharm Pharmacol. 2018;70:648–54. doi: 10.1111/jphp.12699. [DOI] [PubMed] [Google Scholar]
- 48.Sim SL, He T, Tscheliessnig A, Mueller M, Tan RB, Jungbauer A. Branched polyethylene glycol for protein precipitation. Biotechnol Bioeng. 2012;109:736–46. doi: 10.1002/bit.24343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


