Abstract
Purpose
To investigate the efficacy of a poly(ethylene glycol) diacrylate and poly(N-isopropylacrylamide) based thermo-responsive hydrogel drug delivery system (DDS) to deliver prophylactic vancomycin (VAN) following ocular surgery.
Methods
VAN was encapsulated in a hydrogel DDS and characterized in terms of initial burst, release kinetics, bioactivity, and cytotoxicity. Long-Evans rats received an intravitreal injection of Staphylococcus aureus to produce acute endophthalmitis in four experimental groups. One of four treatments were then applied: (1) bolus subconjunctival injection of VAN, (2) blank DDS, (3) saline treatment, and (4) subconjunctival injection of VAN DDS. Animals were scored for infection (0–3) at 12, 24, 48, and 72 hours, and eyes were harvested at 24 and 48 hours for histology.
Results
Following a 36% initial burst, VAN release from the DDS continued at a steady rate for 2 weeks plateauing at 84% after 504 hours. Bioactivity was maintained for all release samples and cytotoxicity analysis for the DDS revealed cell viability >90%. Not until after 12 hours did any of the groups show evidence of infection; however, at 24 hours, animals that received the VAN DDS had significantly lower infection scores (0 ± 0) than those that received a bolus VAN injection, blank DDS, or saline (1.5 ±1.5, 2.3 ± 0.87, and 2.9 ± 0.25; respectively). At 48 and 72 hours, the VAN DDS and bolus VAN treatment groups performed comparably and showed significantly better infection scores than the control groups.
Conclusions
This DDS appears to have promise as a vehicle for short term, prophylactic antibiotic delivery.
Translational Relevance
This DDS may prevent the development of postoperative endophthalmitis.
Keywords: drug delivery, thermoresponsive hydrogel, vancomycin, postoperative acute endophthalmitis
Introduction
During cataract surgery, patients oftentimes receive administration of prophylactic antibiotics via an intracameral injection.1,2 Additionally, at the conclusion of surgery, patients receive a subconjunctival injection of antibiotics, and are then prescribed 1 to 2 weeks of topical antibiotic eye drops (50 mg/cc) two to four times daily. This is done to minimize the potential development of endophthalmitis, an infectious process of the ocular cavity and its adjacent tissues that can lead to severe visual impairment or blindness.3–5 Endophthalmitis most commonly occurs postoperatively due to microflora (usually Staphylococcus aureus) on the ocular surface gaining entry into the eye during surgery and inducing infection.6–8 The reported incidence of endophthalmitis varies and is relatively low (∼0.07%–0.5% after cataract surgery).4,8 Regardless, when it does occur, endophthalmitis is a potentially dangerous condition with potential devastating visual outcomes.3,9–11 Typically, endophthalmitis presents within the first few days following surgery, and 80% of cases present within at least 6 weeks of surgery.4 The first 24 to 48 hours are considered to be the most critical time to prevent endophthalmitis.1 In addition, patient noncompliance with prophylactic topical drops has been shown to be as high or higher than 50% especially among elderly patients, which further complicates preventative care.12,13 These factors warrant the development of new adjunctive prophylactic modalities.3 An ideal drug delivery system (DDS) is one that delivers and maintains a constant drug concentration over time through minimally invasive means without reliance on patient compliance.14 Such a DDS could potentially replace both the initial subconjunctival bolus injection and subsequent topical eye drop treatments.14
To address this need, the goal of this study is to demonstrate the potential of an ocular DDS capable of releasing vancomycin (VAN) for a period of at least 10 days replacing both the bolus subconjunctival injection, and the patient administered topical eye drops. Where hydrogels are already an attractive platform for drug delivery, thermo-responsive hydrogels have shown particular promise as drug delivery vehicles owing to their ability to reversibly change physical state in response to changes in temperature.14,15 Through this property, these hydrogels are liquid-like and injectable through a small gauge needle (27–30 gauge) at room temperature and then gel (collapse) upon reaching body temperature, allowing for the release of encapsulated drug.15,16 Here, we demonstrate the use of a thermo-responsive hydrogel DDS as an effective alternative to a single bolus subconjunctival injection of VAN for the prevention of S. aureus induced endophthalmitis.
Methods
Thermo-Responsive Hydrogel Preparation
Poly(ethylene glycol) diacrylate (PEG-DA) and poly(N-isopropylacrylamide) (NIPAAm) hydrogels were prepared according to a method described and characterized by Kang-Mieler et al.15,17 and Drapala et al.18,19 These hydrogels remain swollen when kept below their transition temperature of 32°C, but collapse and shrink once that temperature is exceeded (i.e., body temperature), allowing for the release of the entrapped drug.15,18 Briefly, hydrogels were synthesized by dissolving PEG-DA (2 mM), N-tert-butylacrylamide (47 mM), and ammonium persulfate (13 mM) in 1 × Dulbecco's phosphate-buffered saline (DPBS) (pH 7.4). NIPAAm (350 mM) was subsequently added to create the hydrogel precursor in a 2-cc microcentrifuge tube and maintained on ice. N,N,N′,N′-Tetramethylethylenediamine (168 mM) (TEMED) was added to initiate hydrogel polymerization. The procedure described uses free radical polymerization that was left to proceed on ice for a duration of 30 minutes. Following polymerization, the newly formed hydrogels were collected and washed five times in double distilled H2O. Hydrogels were prepared in triplicate. While it is well-established that TEMED can cause oxidative damage to cells, studies have shown that washing them at least three times is sufficient to remove residual TEMED and other unreacted monomers.20–23
Encapsulation Efficiency
The encapsulation efficiency (EE) is the initial amount of drug entrapped within the hydrogel. The EE of each DDS was determined indirectly by subtracting the quantity of drug lost during the washing phases from the total drug used for encapsulation.24 Drug quantity in the wash samples was determined using a NanoDrop™ 2000/2000C Spectrophotometer (E1% 40, 280 nm) (Thermo-Fisher Scientific, Grand Island, NY).
Hydrogel Release Profiles
A single (1 mL) hydrogel was placed in 1 mL phosphate-buffered saline (PBS) under static conditions at 37°C. At predetermined intervals, 1 mL aqueous media was removed and replaced with an equal volume of fresh buffer. VAN concentration in the release samples were quantified using a NanoDrop 2000/2000C Spectrophotometer (E1% 40, 280 nm). Cumulative release was calculated relative to EE. The initial burst was defined as the drug released within the first 5, 12, and 24 hours. Release profiles were collected over 3 weeks. All release profiles were performed in triplicate.
In Vitro Bioactivity
To assess drug bioactivity, release samples were assayed by monitoring their efficacies against colonies of S. aureus cells (Carolina Biological Supply Company, Burlington, NC). Cells were cultured in Tryptic Soy Broth (TSB) (Sigma-Aldrich, St. Louis, MO) at 37°C for 24 hours. Filter paper cut into 0.5-inch diameter circular pieces were soaked in the aliquots harvested from the solution released from the hydrogel system at the designated time points. These pieces of filter paper were then placed on a quarter section of a 100 × 15 mm Tryptic Soy Agar (TSA) petri plates swabbed with a 0.1-mL suspension of S. aureus and incubated at 37°C for 24 hours. Following the incubated period, the petri plates were photographed, and the zones of inhibition were measured and compared with the control samples. This experiment was performed in triplicate and all samples at each time point were measured in quadruplicate.
In Vitro Cytotoxicity
The cytotoxicity of drug-free hydrogel release samples was determined using a LIVE/DEAD® assay kit (Invitrogen, Carlsbad, CA). Human Umbilical Vein Endothelial Cells (HUVEC), cells commonly used to asses cytotoxicity for this system, were grown in endothelial growth media (EMG-2, Lonza, Basel, Switzerland), and 23 release samples from a blank hydrogel in a 96-well plate (100 μL media and 30 μL per well) following a growth period of 2 days, 4 μL EthD (red) (sterile), and 1 μL Calcein (green) (sterile) was added to 2 mL PBS.16,18,23 The growth media was then removed from the well plate and replaced with 50 μL/well of the EthD/Calcein solution and incubated for 15 minutes. Each well was imaged (10×, 1.79 microns/pixel resolution) using a confocal microscope (FITc/TRITc; 488 nm/543 nm) (LSM 5 Pascal, Zeiss Microscopy, Thornwood, NY). Cells appearing green (alive) were quantified using the ImageJ cell counting software and compared with the red cells (dead) to determine percent cell viability. Cell viability for each sample was compared with a cell growth media control to assess the risk of cytotoxicity. All experiments were performed in triplicate.
Animal Preparation
Forty Long-Evans male rats (1–3 months, 200–250 g) purchased from Envigo Bioproducts Incorporated (Madison, WI) were used to induce the ocular infection model. The animals were handled and cared for according to the IIT Institutional Animal Care and Use Committee (IACUC) protocol with the principles embodied in the Statement for the Use of Animals in Ophthalmic and Vision Research adopted by the Association for Research in Vision and Ophthalmology. Animals were fed ad libitum. Animals were anesthetized using 0.8 mg/kg body weight of 100 mg/mL ketamine hydrochloride (Fort Dodge Animal Health, Fort Dodge, IA) and 0.1 mg/kg body weight of 100 mg/mL xylazine (AnaSed Injection, Akron, Inc., Decatur, IL) via intraperitoneal (IP) injection. Proparacaine drops (Bausch and Lomb, Rochester, NY) were used to anesthetize the corneas, and pupils were dilated using phenylephrine (Bausch and Lomb) and atropine drops (Bausch and Lomb). Heart rate and blood oxygen saturation were monitored with a Pulse Oximeter (8500AV; Nonin Medical, Inc., Plymouth, MN). Animals were placed on a custom-built heated stage and monitored to maintain a core body temperature of 37°C.
Preparation of S. aureus for Injections
S. aureus cells were maintained in TSB and were incubated on a TSA containing plate at 37°C for 24 hours. Discrete colonies of the bacteria were subcultured into 5 mL sterile TSB. A spectrophotometric optical density of 0.16 to 0.21 at an absorbance of 530 nm corresponded to a viable bacterial count of ∼1.5 × 108 colony forming units (CFU)/mL.25,26 Following 24 hours of dynamic incubation, the suspension was diluted through serial dilution to a concentration of ∼80 CFU/ 5 μL for intravitreal injection.22,25
Sterile Preparation of the DDS
PBS loaded (control) and VAN loaded thermo-responsive hydrogels were prepared as previously described. Under sterile conditions, the hydrogel precursor and initiator were sterile-filtered using 13-mm syringe filters (0.22 μm, Fisherbrand, Thermo Fisher Scientific). Sixty milligrams of VAN was added to 1 mL hydrogel precursor (for drug loaded DDSs), and the tube was vortexed to dissolve the drug. The initiator was then added, and free radical polymerization occurred on ice for 30 minutes. Hydrogels were washed five times in sterile PBS, loaded into 0.5 cc U-100 insulin syringes (28½; Becton Dickinson & Co., Franklin Lakes, NJ), and stored at 4°C.
Experimental Groups
Four groups (20 eyes per group, 16 subgroups) were examined: (1) group 1 (control): bolus saline subconjunctival injection; (2) group 2 (control): blank hydrogel DDS subconjunctival injection; (3) group 3: bolus subconjunctival VAN injection; and (4) group 4: subconjunctival VAN loaded DDS. Before receiving any injections (infection or treatment), eyes were examined using a slit lamp microscope prior to injection to confirm the absence of any abnormalities. Prior to subconjunctival treatment injection, all animals received an intravitreal injection of 5 μL S. aureus cells in suspension (concentration ∼80 CFU per milliliter) through a 0.5-cc U-100 insulin syringe (28G½; Becton Dickinson & Co.).27 The needle was positioned behind the lens in the vitreous body with the bevel directed posteriorly.27 The intraocular pressure (IOP) was measured prior to and following the intravitreal injection using a tonometer (TONO-PEN XL, Medtronic, Minneapolis, MN). The right eye of each experimental animal received a subconjunctival injection of 5 μL sterile PBS or 5 μL blank hydrogel immediately following infection. The left eyes received either an intravitreal injection bolus injection of 0.08 mg/5 μL VAN in PBS (equivalent to a clinical dose) or 5 μL hydrogel DDS (containing 0.06 mg/5 μL VAN). Although the adult rat vitreous volume is ∼14 μL, intravitreal injection volumes of up to 5 μL have been shown to have favorable reproducibility with minimal loss of injected solution.22,28
Tracking of Infection and Scoring Method
At 12, 24, 48, and 72 hours following intravitreal injection, animals were humanely killed after being graded by gross external examination and direct slit lamp ophthalmoscopy. Scoring was based on a clinical scoring method for the severity of endophthalmitis developed by Peyman et al29 and the NIH grading system for vitreous haze in humans.25 The cornea, conjunctiva, and vitreous of each animal received a numerical score between 0 and 3 that each corresponded to a severity of infection.26 Animals were humanely killed using a CO2 chamber, and both eyes were harvested for histopathology.
Histopathology
After gross eye examination, the animals were humanely killed via CO2, and both eyes were harvested for histopathological examination. Three animals were examined at each time point post infection. The eyes were enucleated and placed in 10% buffered formalin phosphate for at least 48 hours. The eyes were processed in graded alcohols and embedded in paraffin. Four-micrometer sections obtained from the center of the vitreous cavities were stained using hematoxylin and eosin. The sections were subsequently examined by light microscopy at using an Olympus DP70 microscope 10×, resolution 4080 × 3072.
Statistical Analysis
All values are reported as sample mean ± standard deviation and in all graphs, error bars represent 1 SD. All statistical differences were determined using one-way analysis of variance testing with Tukey posthoc testing, and unless otherwise noted, significance represents P ≤ 0.05.
Results
DDS Characterization
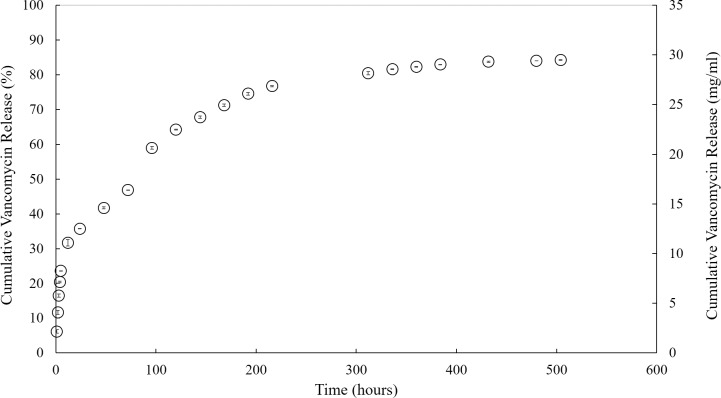
Due to the small fraction of hydrogel accommodated by the rat eye, 60 mg (100%) of VAN was successfully encapsulated into 1 mL PNIPAAm-PEG-DA thermo-responsive hydrogels in order to achieve an equivalent dosage upon injection. The release data from these 1-mL hydrogels is depicted in Figure 1 as a cumulative percent (%) release profile (on the primary Y-axis) and a cumulative release profile in terms of actual amount of VAN released (on the secondary Y-axis). Representing the data in this way gives a complete picture of the release behavior from these hydrogels and allows for an overall picture of VAN release [cumulative percent (%) and amount]. The initial burst VAN released from these 1-mL hydrogels were 23% (8.22 ± 0.10 mg/mL), 31% (11.06 ± 0.10 mg/mL), and 36% (12.47 ± 0.10 mg/mL) at 6, 12, and 24 hours, respectively (Fig. 1). VAN release continued at a steady rate (∼1.5 mg/mL) for 2 weeks until plateauing at 84% (29.4 ± 0.1 mg/mL) cumulative release at 504 hours (21 days) (Fig. 1). By 1 hour, these hydrogels released 2.2 ± 0.6 mg/mL of VAN. During the initial period, the hydrogels released an hourly amount of VAN between 1.1 and 1.9 mg/mL for the first 6 hours, 2.8 ± 0.9 mg/mL between 6 and 12 hours, and 1.4 ± 0.1 mg/mL between 12 and 24 hours. The daily release amount of ∼1.7 mg/mL continued for 13 days (312 hours) and then decreased to 0.2 mg/mL for the remaining duration of the release study (Fig. 1). In all cases, the amount of VAN released was at a detectable level using light spectroscopy (data not shown).
Figure 1.
Average VAN release from a nondegradable PNIPAAm-PEG-DA (MW 575) hydrogel shown as average percent cumulative VAN release from a 1-mL thermo-responsive PNIPAAm-PEG-DA based hydrogel on the primary Y-axis and VAN release in terms of the average cumulative amount (mg/mL) of drug released at each time point on the secondary Y-axis.
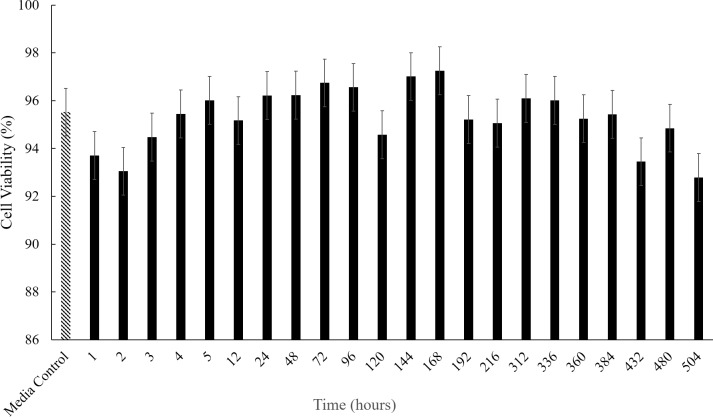
The bioactivity for all release samples was measured. The ZOIs of all release samples collected were found to be at least 10 mm or above, suggesting that throughout the duration of release (504 hours) the antibiotic maintains its antimicrobial bioactivity and is efficacious against S. aureus (Fig. 2). HUVEC cell toxicity was also assessed for the thermo-responsive hydrogel system to determine biocompatibility. For a control hydrogel (no active drug), all release time point samples showed HUVEC cell viability above 90% with no statistical difference between any of the time points and the media control (Fig. 3). These results suggest that the hydrogel delivery system is biocompatible as it does not exhibit any detriment to cell health.
Figure 2.
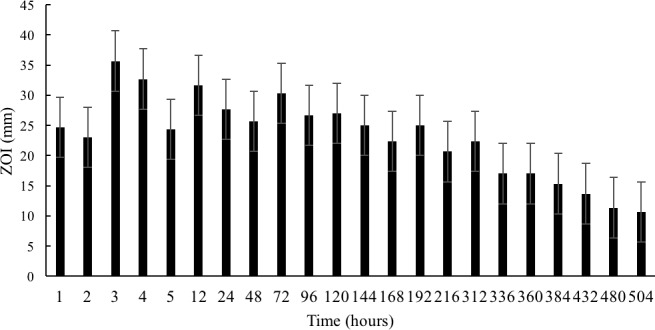
Average bioactivity for VAN released from a PNIPAAm-PEG-DA hydrogel through 504 hours (n = 3).
Figure 3.
Average HUVEC cell toxicity for saline release from a PNIPAAm-PEG-DA hydrogel (n = 3). There was no statistical difference (P > 0.05) between the media control and any of the release time points through 504 hours.
Gross Observations and Clinical Scores After Intravitreal Injection of S. aureus
Figure 4 shows representative images from four experimental groups at each time point. After 12 hours post infection, there was no observable difference between the healthy eyes and any of the treatment groups (Fig. 4, 12 hours). By 24 hours, there were clearly observable signs of infection including clouding of the cornea and redness in the conjunctiva in the eyes of animals that received saline and blank hydrogel injections (Fig. 4, saline and blank DDS, 24 hours). These clinical signs of infection persisted for 72 hours until the animals were humanely killed (Fig. 4, saline and blank DDS, 48 and 72 hours). The conjunctivas and corneas of the animals who received the VAN-loaded DDS remained free of any observable signs of infection throughout the duration of the study.
Figure 4.
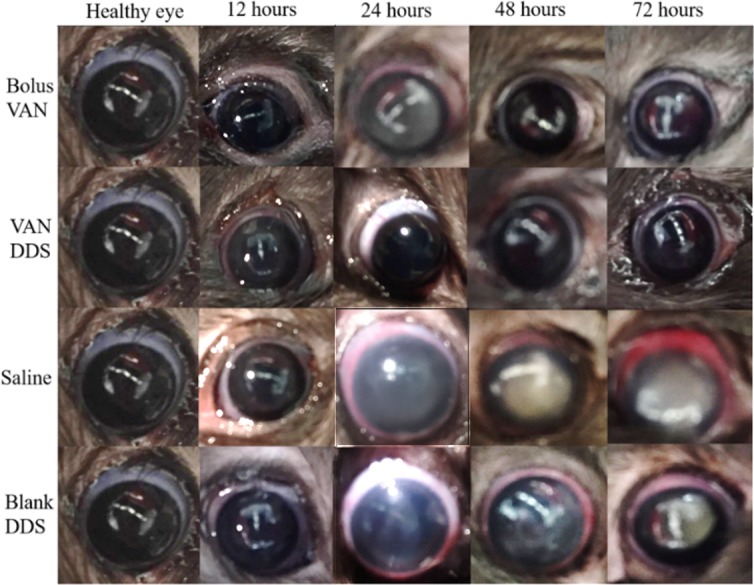
Representative eyes for all four treatment groups at 12, 24, 48, and 72 hours. The bolus VAN row compares eyes that received a subconjunctival bolus injection of VAN after 12, 24, 48, and 72 hours. The VAN DDS row compares eyes that received a subconjunctival injection of the VAN loaded DDS after 12, 24, 48, and 72 hours. The saline row compares eyes that received a subconjunctival injection of the saline (PBS) loaded DDS after 12, 24, 48, and 72 hours. The blank DDS row compares eyes that received a subconjunctival injection of the saline (blank) DDS after 12, 24, 48, and 72 hours.
Table 1 shows the average tissue scores for the cornea, conjunctiva, and vitreous of animals in each treatment group, as well as the significance within a 99% confidence interval between each ocular structure in the VAN DDS group and the bolus VAN group as compared with the corresponding ocular structures in the other groups at the same time point. The first indication of corneal clouding was observed using a slit lamp microscope. Animals with corneas that appeared clear based on gross examination, but were unable to be examined with the slit lamp microscope due to poor light penetration through the cornea, received a corneal infection score of at least 1.0. If further observable clouding was detected, the score was increased. In all cases where the total infection score of higher than 0, the vitreous of the animals received the highest numerical score compared with the cornea and conjunctiva.
Table 1.
Average Tissue Infection Scores for All Treatment Groups (n = 20 Eyes/Experimental Group)
| Time, h |
Infection Score |
|||||||
| Bolus VAN |
VAN DDS |
|||||||
| Conj. |
Cornea |
Vit. |
Total Score |
Conj. |
Cornea |
Vit. |
Total Score |
|
| 12 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 | 1.6 ± 1.3 | 1.6 ± 1.1 | 2.4 ± 0.9 | 1.9 ± 1.0 | 0.0 ± 0.0* | 0.0 ± 0.0* | 0.0 ± 0.0* | 0.0 ± 0.0* |
| 48 | 1.0 ± 1.0x† | 0.4 ± 0.9 | 2.0 ± 1.0 | 1.1 ± 0.8x | 0.2 ± 0.4* | 0.2 ± 0.4 | 0.6 ± 0.5* | 0.3 ± 0.4* |
| 72 | 0.6 ± 0.9 | 0.8 ± 1.1 | 0.8 ± 1.3x† | 0.7 ± 1.1x | 0.0 ± 0.0 | 0.3 ± 0.3 | 0.3 ± 0.3* | 0.1 ± 0.2* |
Numerical score contributions of the conjunctiva (Conj.), cornea, and vitreous (Vit.) to the total infection score for each eye are indicated. An asterisk (*) indicates statistical significance (P < 0.01) between the ocular structure in the VAN DDS group and the corresponding structure in the other groups measured at the same time point. A dagger (†) indicates statistical significance (P < 0.01) between the ocular structure in the bolus VAN group and the corresponding structure in the other groups measured at the same time point. An x indicates no statistical difference (P > 0.05) between the ocular structure marked as significant in the VAN DDS group and the corresponding ocular structure in other groups at that time point.a All statistical significance for this table are within a 99% confidence interval except for where indicated by a b(b), which represents a 95% confidence interval.
For example, the score assigned the cornea at 24 hours in the blank DDS group is not statistically significantly different from the corneal score assigned to the VAN DDS group at 24 hours.
Here, significance is within a 95% confidence interval.
Table 1.
Extended
| Time, h |
Infection Score |
|||||||
| Saline |
Blank DDS |
|||||||
| Conj. |
Cornea |
Vit. |
Total Score |
Conj. |
Cornea |
Vit. |
Total Score |
|
| 12 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 | 2.8 ± 0.5 | 3.0 ± 0.0 | 3.0 ± 0.0 | 2.9 ± 0.2 | 1.8 ± 0.8 | 1.4 ± 1.1x | 2.6 ± 0.9 | 1.9 ± 0.8 |
| 48 | 2.4 ± 0.9 | 1.8 ± 1.3 | 2.8 ± 0.4 | 2.3 ± 0.9 | 2.4 ± 1.1 | 2.0 ± 1.4 | 3.0 ± 0.0 | 2.4 ± 0.8 |
| 72 | 1.2 ± 1.1 | 1.6 ± 0.9 | 2.8 ± 0.4 | 1.9 ± 0.8 | 1.3 ± 1.3 | 1.8 ± 1.0 | 2.8 ± 0.5 | 1.9 ± 0.8b |
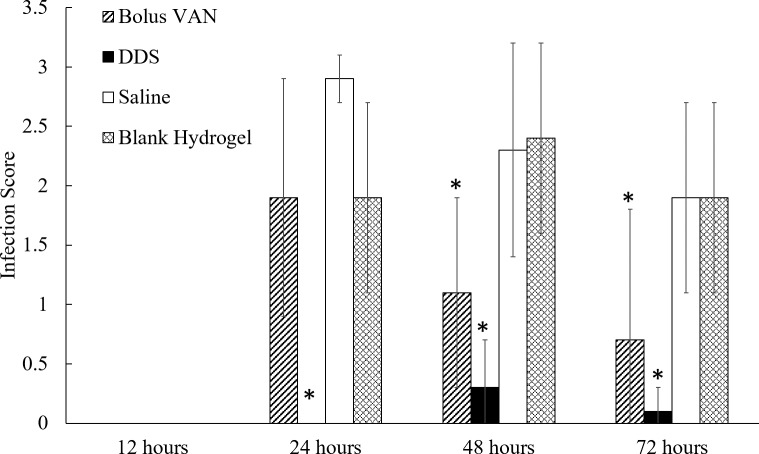
The overall clinical infection scores for each treatment group can be observed graphically in Figure 5. It should also be noted that the representative images in Figure 4 also correspond to the average numerical infection score received by that group. The saline and blank hydrogel groups served as controls to establish a progression of infection. In both of these groups, infection did not present at the 12-hour time point, but by 24 hours, signs of infection were observed in the cornea, conjunctiva, and vitreous of the test animals. The overall infection scores at 24 hours were 2.9 ± 0.2, and 1.9 ± 0.8 for the saline and blank hydrogel groups, respectively. The infection persisted within these groups at 48 and 72 hours with infection scores of 2.3 ± 0.9 and 2.4 ± 0.8 at 48 hours for the saline and blank hydrogel groups, respectively, and 1.9 ± 0.8 for both groups at 72 hours. At every time point, there was no statistical difference (P > 0.05) between the scores assigned to the saline treatment group and the blank hydrogel groups either within time points or between time points. Through these two groups, a pattern of infection was established.
Figure 5.
Average infection scores for all treatment groups (n = 20 eyes/experimental group) at 12, 24, 48, and 72 hours after injection. At 12 hours, none of the groups showed any observable sign of infection. At 24 hours, the DDS treatment group performed statistically better (P < 0.05) than all other groups. At 48 and 72 hours, the bolus VAN and DDS treatment groups performed comparably and received statistically lower overall infection scores than each of the two control groups. *Statistical difference compared with all other treatment groups at each time point (P < 0.05).
Similar to the controls, at 12 hours, clinical signs of infection had not developed in any of the treatment groups (bolus VAN and VAN-loaded DDS), and all animals received an overall infection score of 0. At 24 hours, no signs of infection had developed in the cornea, conjunctiva, or vitreous in any of the animals that received the VAN-loaded DDS treatment (infection score of 0.0 ± 0.0); however, statistically significant (P < 0.01) infection scores (1.9 ± 1.0) were assigned to the animals that received a treatment of a bolus VAN injection. It is worth noting that the infection observed in this group was not statistically (P > 0.05) different from the infection scores received by control groups at 24 hours. By 48 hours, the VAN bolus and VAN-loaded DDS groups received statistically similar scores of 1.1 ± 0.8 and 0.3 ± 0.4, respectively (P > 0.05). In the VAN DDS group, slight clinical infection indicators in a few of the animals resulted in an overall score of greater than zero (0). The 72-hour time point saw the same trend, at which point the bolus VAN and VAN-loaded DDS groups received infection scores of 0.7 ± 1.1 and 0.1 ± 0.2, respectively, which were statistically not different (P > 0.05) from each other, but statistically (P < 0.01) better than the scores received by the saline and blank hydrogel groups (Fig. 5).
Histopathology
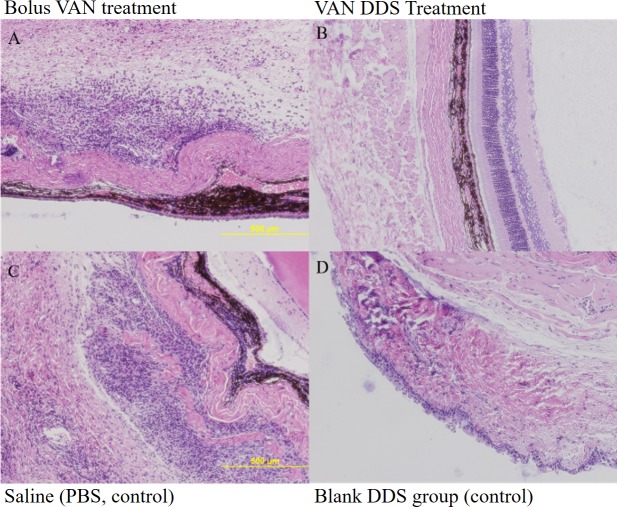
Investigation of the histopathological sections of the vitreous at 48 hours post infection showed infiltration of inflammatory cells in the vitreous and disorganized retinal tissue layers in the eyes that received scores that indicated the presence of infection.30,31 Figure 6A shows that the group that received a bolus VAN injection exhibited cell infiltration and disorganized tissue layers that is a hallmark of infection.32 In the VAN DDS treatment group (Fig. 6B), few inflammatory cells had migrated into the vitreous and the layer structures appeared organized and normal.11 In Figures 6C and 6D, the saline and blank DDS control groups appeared disorganized and infiltrated with inflammatory cells.
Figure 6.
Representative histopathological sections from enucleated specimen stained with hematoxylin-eosin showing vitreous for rats in each experimental group 48 hours after infection (10×). (A) Bolus VAN group showing infiltrated vitreous and disorganized tissue structure. (B) VAN DDS group showing a mostly noninfiltrated vitreous and normal, organized tissue structure. (C) Saline (PBS, control) group showing an infiltrated vitreous disorganized tissue layers. (D) Blank DDS group showing an infiltrated vitreous and disorganized tissue layers.
Intraocular Pressure
IOP measurements were recorded prior to and immediately after intravitreal and subconjunctival injections and at every time point, thereafter. Table 2 shows the average IOP measurements for all treatment groups at each time point. The average control IOP for all animals was 15.4 ± 0.1 mm Hg. As was anticipated, a significant increase of ∼56% in IOP was observed immediately after ocular injections in all treated animals (P < 0.05). Increased IOP remained at 12 hours for all treatment groups. By 24 hours, only the saline and blank hydrogel treated groups had increased IOP (25.0 ± 0.8 mm Hg and 24.4 ± 1.8 mm Hg, respectively) presumably due to the presence of infection. At 48 and 72 hours, the saline and blank hydrogel treated eyes maintained a statistically elevated IOP, while bolus VAN and VAN DDS treated eyes remained statistically unchanged from the control. With the exception of the 12-hour time point and the bolus VAN treated eyes at 24 hours, statistically higher (P < 0.05) IOP measurements correlated with statistically higher infection scores (P < 0.05); however, it should be noted that all IOP values were within the range of what is normal in rats (15–25 mm Hg).33–36
Table 2.
Average IOP Measurements for All Animal Control and Treatment Groups (n = 20 Eyes/Experimental Group) Prior to Injection, Post Injection, and at 12, 24, 48, and 72 Hours After Injection
| Average IOP (mm Hg) |
||||||
| Control |
Postinjection |
12 h |
24 h |
48 h |
72 h |
|
| Bolus VAN | 15.3 ± 0.9 | 23.5 ± 1.6* | 22.0 ± 0.7* | 16.7 ± 0.9 | 16.1 ± 0.6 | 15.9 ± 0.3 |
| DDS | 15.5 ± 0.9 | 24.0 ± 1.4* | 22.9 ± 0.6* | 15.7 ± 2.0 | 15.3 ± 0.6 | 16.0 ± 0.9 |
| Saline | 15.4 ± 0.8 | 23.0 ± 1.4* | 21.2 ± 1.1* | 25.0 ± 0.8* | 24.1 ± 2.4* | 22.5 ± 0.8* |
| Blank hydrogel | 15.5 ± 0.8 | 23.6 ± 1.6* | 22.5 ± 0.7* | 24.4 ± 1.8* | 25.5 ± 2.9* | 23.1 ± 1.1* |
Significant differences in IOP compared with each treatment group's respective control measurement (P < 0.05).
Discussion
In this study, the efficacy of our VAN DDS to prevent S. aureus induced endophthalmitis was evaluated in comparison with a single intravitreal bolus injection of VAN. The VAN-loaded DDS was effective, if not better compared with bolus VAN treatment throughout the duration of the study. At all points where infection was observed (24, 48, and 72 hours), the DDS treatment groups performed significantly (P < 0.05) better than the saline and blank hydrogel groups, demonstrating a clear ability to prevent infection. Our VAN DDS performed significantly better at 24 hours compared with the bolus VAN treated animals. No clinical signs of infection were observed for our VAN DDS, whereas the animals in the bolus group did develop infection. This difference in results is attributed to the steady release of antibiotics provided by our VAN DDS. By 48 and 72 hours, however, these two treatment groups performed comparably, showing low infection scores and in most cases, no signs of infection in the animals.
Table 1 displays the infection scores for the individual ocular structures throughout this study and demonstrates that for all groups, the infection score was highest in the vitreous. Infection scores were significantly higher (P < 0.01) in the saline treatment and the blank hydrogel groups as compared with the VAN DDS groups at 24, 48, and 72 hours. These scores were also significantly higher than the bolus VAN group at 48 and 72 hours (P < 0.01). The significant reduction in vitreal infection scores among the treated eyes suggests that VAN was able to successfully penetrate the vitreous. While beyond 24 hours, our VAN DDS performed comparably with a bolus injection of VAN, the results at 24 hours and overall suggest several potential benefits to a VAN DDS system. Animals in the bolus injection group received a clinical dose of 5 μL VAN (0.08 mg), while the animals that received our VAN DDS were given a DDS that contained a total of 0.06 mg VAN. From Figure 1, we see that only a fraction of the encapsulated drug in the VAN DDS is released over the course of the study, demonstrating that we are able to deliver a lower VAN dose (0.03 mg by 72 hours) with an equivalent result to bolus injection. As such, when treated with the VAN DDS, ocular tissues are exposed to less overall VAN, thereby reducing the potential risk of VAN associated intracameral tissue toxicity.37
The infection scores assigned to the saline treatment group and the blank hydrogel groups were not statistically different (P > 0.05) within or between time points, suggesting that there was no placebo effect encountered from the blank hydrogel system. These results also indicate that the infection did not change over the 72-hour time period that followed injection. Our endophthalmitis model can, therefore, be characterized by signs of clinical infection that presents by 24 hours after exposure to infectious cells and persists for at least 72 hours following exposure. When our VAN DDS was further evaluated for biocompatibility, >90% of HUVEC cells remained viable, a value insignificantly different from a media control (Fig. 3). This finding is consistent with previous published studies that conclude that washing in at least triplicate is sufficient to remove residual chemicals that were not consumed during the polymerization reaction.22,23 From these data, we conclude that our VAN DDS system is biocompatible with endothelial tissue.
The results observed from the histopathological sections are consistent with the inflammatory scores assigned to the corresponding groups of Figures 6B, 6C, and 6D. The VAN DDS treated group received low clinical infection scores (0.3 ± 0.4 at 48 hours), indicating little to no infection. These low clinical infection scores agree with the in vitro data suggesting the biocompatibility and bioactivity of our DDS and are consistent with the histological observations that show an organized tissue that has not experienced any inflammatory cell migration. Conversely, the saline and blank DDS treated control groups received infection scores of 2.3 ± 0.9 and 2.4 ± 0.8 at 48 hours, respectively. These statistically (P < 0.05) higher scores are supported by our histological observations, which show inflammatory cell migration and disorganized tissue due to inflammation.38 The average clinical infection score for the groups that received a bolus VAN injection was 1.1 ± 0.8 at 48 hours and was not statistically different (P > 0.05) from the VAN DDS treated group. However, examination of the histology samples from the bolus VAN group indicates the presence of infection unlike the VAN DDS group, which remained infection-free. It is possible that the presence of VAN prevented S. aureus growth sufficient to minimize clinical symptoms but that some inflammation still occurred.32 This hypothesis is supported by the examination of the clinical infection scores assigned to each individual ocular structure, which shows that the average vitreous score in the bolus VAN group at 48 hours was 2.0 ± 1.0 compared with a significantly lower (P < 0.01) average vitreous score in the VAN DDS group of 0.6 ± 0.5 (Table 1). These scores indicate that the inflammation in the vitreous is higher among the bolus VAN treated group as compared with the VAN DDS treated group and that the VAN DDS is better at preventing the development of infection.
In this study, there was a significant increase in IOP (P < 0.05) immediately following injection of treatment (Table 2). This increased IOP resolved in the group treated with bolus VAN and our VAN DDS after 24 hours but persisted in the two control groups (saline and blank hydrogel treated). This transient increase in IOP may be correlated with the presence of infection due to the influx of inflammatory material that compromise the blood-ocular barrier.39 After 12 hours, all animals that received VAN loaded DDS treatment were measured to have an IOP values that returned to the preinjection values, indicating that subconjunctival injection of our VAN DDS had minimal impact on rat IOP. All IOP values obtained in this study are in agreement with the normal range of IOPs reported for Long-Evans rodents.33–36 Due to the observation that treatment groups with lower infection scores also had lower IOP at 24, 48, and 72 hours, any persistent elevation in IOP was likely due to infection rather than the presence of our DDS.
From the data presented, we believe that our DDS is a promising option for postoperative antibiotic administration to prevent endophthalmitis. Ultimately, for this VAN DDS to be completely developed for clinical use, it needs to be formulated so that it is fully biodegradable. Research conducted by Drapala et al.18 (2014) demonstrated the feasibility of making this system degradable within 12 days; however, it is likely that the degradation time would need to be extended for this application in postoperative antibiotic administration in order to ensure steady drug release for at least 2 weeks.18 Modifications to allow for the complete degradability of our DDS is our next step for this study. VAN is one of several prophylactic antibiotics used for endophthalmitis prevention.10 VAN is increasingly selected for use in this application due to its efficacy against all gram-positive organisms and in an 11-year study, the incidence of endophthalmitis dropped 97% from 0.3% to 0.008% following the prophylactic introduction of VAN intracamerally.40–42 However, while some doctors choose to use VAN alone, others use it in conjunction with an additional antibiotic or elect to use an orthogonal approach using a different agent altogether.13,41,42 Adapting our system for alternative antibiotics could lead to its eventual use in more diverse applications. In applications where VAN is delivered prophylactically, it is often compounded with other antibiotics to provide a more broad-spectrum prophylactic effect.41 Incorporating a complementary antibiotic such as ceftazidime or gentamycin into our DDS for the dual release of the two synergistically effective antibiotics could enable the system to prophylactically protect against both gram-positive and gram-negative bacterial strains to achieve an ideal dual release and is, therefore, recommended.41
To our knowledge, this is the first time a subconjunctival, sustained release DDS to prevent endophthalmitis has been developed and characterized for its release, encapsulation, and biocompatibility. Other studies have explored topical administration of a DDS to improve antibiotic residence time; however, it is generally reported that topical antibiotics do little to prevent infection.43 We believe that the subconjunctival route is viable for antibiotic drug administration based on several reviews and studies; however, some concern exists surrounding the ability of VAN to penetrate the sclera, choroid, and the tight junctions of the retinal pigment epithelium (RPE).44–46 Ambati et al.47 (2000) demonstrated that the sclera is permeable to molecules as large as 70 kDa and thus, owing to the size of VAN (∼1.5 kDa), we believe that scleral permeability is likely47. Similarly, choroidal permeability to even large molecules has been well established.48–50 While the tight junctions of the RPE possibly pose the most restrictive barrier to VAN, we posit that the sustained release model of our system helps to overcome slow penetration and allow enough drug concentration to accumulate such that an efficacious dose is delivered.
Our results suggest VAN penetration into the vitreous. Table 1 displays the infection scores for the individual ocular structures throughout this study and demonstrates that for all groups, the infection score was highest in the vitreous. These numbers were significantly higher in the saline treatment and the blank hydrogel groups as compared with the VAN DDS groups at 24, 48, and 72 hours and significantly higher than the bolus VAN group at 48 and 72 hours. This significant reduction in virtual infection scores among the treated eyes suggests that VAN was able to successfully penetrate the vitreous. Based on the permeability of the ocular tissue and our current data, we believe that VAN can penetrate into the vitreous to have positive effects.
This paper aims to demonstrate the use of a DDS capable of delivering antibiotics, specifically VAN, in a controlled and extended manner and to prevent the development of S. aureus induced endophthalmitis in a rodent model. The findings presented demonstrate that a successful DDS with significant potential for this application has been developed. The efficacy of our system was validated in vivo, and the controlled release of VAN was shown to be comparably efficacious in preventing endophthalmitis to VAN delivered via bolus injection into the subconjunctival space. Additionally, cytotoxic evaluation of all collected samples on HUVEC cells demonstrated that following five washes, the DDS was biocompatible and well-tolerated, with no observable adverse effects. Our DDS remains injectable through small gauge needles (28-30 G needles). Our thermo-responsive DDS may have applications in the prevention of ocular infections and other areas where there is a need to deliver small molecules in a controlled and extended manner.
Acknowledgments
The authors thank Brianna Roux, PhD, for her help with cell culture, Daniel Raba for his help with bacterial cell culture, Kay C. Dee, PhD, for her help with imaging, and the Rose-Hulman Institute of Technology (Terre Haute, IN) for microscope use.
Disclosure: E. Dosmar, None; W. Liu, None; G. Patel, None; A. Rogozinski, None; W.F. Mieler, None; J.J. Kang-Mieler, patent pending on microsphere-hydrogel drug delivery system (P)
References
- 1.Murphy CC, Nicholson S, Quah SA, Batterbury M, Neal T, Kaye SB. Pharmacokinetics of vancomycin following intracameral bolus injection in patients undergoing phacoemulsification cataract surgery. Br J Ophthalmol. 2007;91:1350–1353. doi: 10.1136/bjo.2006.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumi X, Petrovski G, Vasileva B, Thaler A. Endophthalmitis prevention, diagnostic procedures and treatment. Optom Open Access. 2016;1:108. [Google Scholar]
- 3.Kumar A, Singh CN, Glybina IV, Mahmoud TH, Yu FX. Toll-like receptor 2 ligand–induced protection against bacterial endophthalmitis. J Infect Dis. 2010;201:255–263. doi: 10.1086/649589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gower EW, Lindsley K, Tulenko SE, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database Syst Rev. 2017;2:CD006364. doi: 10.1002/14651858.CD006364.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehghani AR, Fazel F, Akhlaghi MR, Ghanbari H, Ilanloo MR, Ahmadi-Azad D. Cefazolin-gentamicin versus vancomycin-ceftazidime eye drops for bacterial corneal ulcers: a randomized clinical trial. J Ophthalmic Vis Res. 2009;4:19–23. [PMC free article] [PubMed] [Google Scholar]
- 6.Souli M, Kopsinis G, Kavouklis E, Gabriel L, Giamarellou H. Vancomycin levels in human aqueous humour after intravenous and subconjunctival administration. Int J Antimicrob Agents. 2001;18:239–243. doi: 10.1016/s0924-8579(01)00375-2. [DOI] [PubMed] [Google Scholar]
- 7.Kessel L, Flesner P, Andresen J, Erngaard D, Tendal B, Hjortdal J. Antibiotic prevention of postcataract endophthalmitis: a systematic review and meta-analysis. Acta Ophthalmol. 2015;9:303–317. doi: 10.1111/aos.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braga-Mele R, Chang DF, Henderson BA, et al. ; ASCRS Clinical Cataract Committee Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40:2134–2142. doi: 10.1016/j.jcrs.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Edelhauser HF, Rowe-Rendleman CL, Robinson MR, et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry P, Cordoves L, Gardner S. ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas, and conclusions. Dublin, Ireland: European Society of Cataract and Refractive Surgeons; 2013. [Google Scholar]
- 11.Sugi N, Whiston EA, Ksander BR, Gregory MS. Increased resistance to staphylococcus aureus endophthalmitis in BALB/c mice: Fas ligand is required for resolution of inflammation but not for bacterial clearance. Infect Immun. 2013;81:2217–2225. doi: 10.1128/IAI.00405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciolino JB, Hoare TR, Iwata NG, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50:3346–3352. doi: 10.1167/iovs.08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alster Y, Herlin L, Lazar M, Loewenstein A. Intraocular penetration of vancomycin eye drops after application to the medial canthus with closed lids. Br J Ophthalmol. 2000;84:300–302. doi: 10.1136/bjo.84.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF. Extended ocular drug delivery systems for the anterior and posterior segments: biomaterial options and applications. Expert Opin Drug Deliv. 2017;14:611–620. doi: 10.1080/17425247.2016.1227785. [DOI] [PubMed] [Google Scholar]
- 15.Kang Derwent JJ, Mieler WF. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc. 2008;106:206–213. discussion 213–214. [PMC free article] [PubMed] [Google Scholar]
- 16.Osswald CR, Kang-Mieler JJ. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr Eye Res. 2016;41:1216–1222. doi: 10.3109/02713683.2015.1101140. [DOI] [PubMed] [Google Scholar]
- 17.Brey E, Kang-Mieler JJ, Perez-Luna V, Jiang B, Drapala P, Rolf Schäfer HH. Thermo-responsive hydrogel compositions. inventors. International patent application PCT/EP2012/053765. March 5, 2012.
- 18.Drapala PW, Jiang B, Chiu YC, et al. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm Res. 2014;31:742–753. doi: 10.1007/s11095-013-1195-0. [DOI] [PubMed] [Google Scholar]
- 19.Drapala PW, Brey EM, Mieler WF, Venerus DC, Kang Derwent JJ, Pérez-Luna VH. Role of thermo-responsiveness and poly(ethylene glycol) diacrylate cross-link density on protein release from poly(N-isopropylacrylamide) hydrogels. J Biomater Sci Polym Ed. 2011;22:59–75. doi: 10.1163/092050609X12578498952315. [DOI] [PubMed] [Google Scholar]
- 20.Moreau MF, Chappard D, Lesourd M, Monthéard JP, Baslé MF. Free radicals and side products released during methylmethacrylate polymerization are cytotoxic for osteoblastic cells. J Biomed Mater Res. 1998;40:124–131. doi: 10.1002/(sici)1097-4636(199804)40:1<124::aid-jbm14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 22.Osswald CR, Guthrie MJ, Avila A, Valio JA, Jr, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res. 2017;42:1293–1301. doi: 10.1080/02713683.2017.1302590. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Lee B-S, Mieler WF, Kang-Mieler JJ. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr Eye Res. 2018;44:264–274. doi: 10.1080/02713683.2018.1533983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honary S, Ebrahimi P, Hadianamrei R. Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm Dev Technol. 2014;19:987–998. doi: 10.3109/10837450.2013.846375. [DOI] [PubMed] [Google Scholar]
- 25.Ravindranath RMH, Mondino BJ, Adamu SA, Pitchekian-Halabi H, Hasan SA, Glasgow BJ. Immunopathologic features of Staphylococcus aureus endophthalmitis in the rat. Invest Ophthalmol Vis Sci. 1995;36:2482–2491. [PubMed] [Google Scholar]
- 26.Engstrom RE, Mondino BJ, Glasgow BJ, Pitchekian-Halabi H, Adamu SA. Immune response to Staphylococcus aureus endophthalmitis in a rabbit model. Invest Ophthalmol Vis Sci. 1991;32:1523–1533. [PubMed] [Google Scholar]
- 27.Giese MJ, Berliner JA, Riesner A, Wagar EA, Mondino BJ. A comparison of the early inflammatory effects of an agr-/sar- versus a wild type strain of Staphylococcus aureus in a rat model of endophthalmitis. Curr Eye Res. 1999;18:177–185. doi: 10.1076/ceyr.18.3.177.5370. [DOI] [PubMed] [Google Scholar]
- 28.Turturro SB, Guthrie MJ, Appel AA, et al. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials. 2011;32:3620–3626. doi: 10.1016/j.biomaterials.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 29.Peyman GA, Paque JT, Meisels HI, Bennett TO. Postoperative endophthalmitis: A comparison of methods for treatment and prophylaxis with gentamicin. Ophthalmic Surgery. (1975);6(1):45–55. [PubMed] [Google Scholar]
- 30.Alfaro DV, Davis J, Kim S, et al. Experimental Bacillus cereus post-traumatic endophthalmitis and treatment with ciprofloxacin. Br J Ophthalmol. 1996;80:755–758. doi: 10.1136/bjo.80.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fortes Filho JB, Maia M, Tartarella MB, Meyer FS, Fortes BG, Kliemann LM. Experimental histopathological study on retinal and renal cellular response to intravitreous antiangiogenic drugs. Int J Ophthalmol. 2014;7:437–440. doi: 10.3980/j.issn.2222-3959.2014.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefèvre S, Saleh M, Marcellin L, et al. Daptomycin versus vancomycin in a methicillin-resistant Staphylococcus aureus endophthalmitis rabbit model: bactericidal effect, safety, and ocular pharmacokinetics. Antimicrob Agents Chemother. 2012;56:2485–2492. doi: 10.1128/AAC.05745-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34:363–369. [PubMed] [Google Scholar]
- 34.Pang IH, Wang WH, Clark AF. Acute effects of glaucoma medications on rat intraocular pressure. Exp Eye Res. 2005;80:207–214. doi: 10.1016/j.exer.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Morrison JC, Johnson E, Cepurna WO. Rat models for glaucoma research. Prog Brain Res. 2008;173:285–301. doi: 10.1016/S0079-6123(08)01121-7. [DOI] [PubMed] [Google Scholar]
- 36.Tummala SR, Benac S, Tran H, et al. Effects of inhibition of neuronal nitric oxide synthase on basal retinal blood flow regulation. Exp Eye Res. 2009;89:801–809. doi: 10.1016/j.exer.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz SG, Flynn HW, Jr, Grzybowski A, Relhan N, Ferris FL., 3rd Intracameral antibiotics and cataract surgery: endophthalmitis rates, costs, and stewardship. Ophthalmology. 2016;123:1411–1413. doi: 10.1016/j.ophtha.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Dureau P, Bonnel S, Menasche M, Dufier J-L, Abitbol M. Quantitative analysis of intravitreal injections in the rat. Curr Eye Res. 2001;22:74–77. doi: 10.1076/ceyr.22.1.74.6974. [DOI] [PubMed] [Google Scholar]
- 39.Kim JM, Park KH, Choi MJ, et al. The effects of Helicobacter pylori infection on intraocular pressure in anterior uveitis. Eye (Lond) 2012;26:1503–1509. doi: 10.1038/eye.2012.206. quiz 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anijeet DR, Palimar P, Peckar CO. Intracameral vancomycin following cataract surgery: An eleven-year study. Clin Ophthalmol. 2010;4:321–326. doi: 10.2147/opth.s9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2009;49:1072–1079. doi: 10.1086/605572. [DOI] [PubMed] [Google Scholar]
- 42.Almeida DRP, Miller D, Alfonso EC. Anterior chamber and vitreous concordance in endophthalmitis: implications for prophylaxis. Arch Ophthalmol. 2010;128:1136–1139. doi: 10.1001/archophthalmol.2010.202. [DOI] [PubMed] [Google Scholar]
- 43.Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther. 2013;29:106–123. doi: 10.1089/jop.2012.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barza M, Kane A, Baum J. The difficulty of determining the route of intraocular penetration of gentamicin after subconjunctival injection in the rabbit. Invest Ophthalmol Vis Sci. 1981;20:509–514. [PubMed] [Google Scholar]
- 45.Cardillo JA, Paganelli F, Melo LA, Jr, et al. Brazilian Ocular Pharmacology and Pharmaceutical Technology Research Group. Subconjunctival delivery of antibiotics in a controlled-release system: a novel anti-infective prophylaxis approach for cataract surgery. Arch Ophthalmol. 2010;128:81–87. doi: 10.1001/archophthalmol.2009.352. [DOI] [PubMed] [Google Scholar]
- 46.Vazirani J, Basu S. Role of topical, subconjunctival, intracameral, and irrigative antibiotics in cataract surgery. Curr Opin Ophthalmol. 2013;24:60–65. doi: 10.1097/ICU.0b013e32835a93be. [DOI] [PubMed] [Google Scholar]
- 47.Ambati J, Canakis CS, Miller JW, et al. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci. 2000;41:1181–1185. [PubMed] [Google Scholar]
- 48.Ambati J, Gragoudas ES, Miller JW, et al. Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci. 2000;41:1186–1191. [PubMed] [Google Scholar]
- 49.Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980;100:332–336. [PubMed] [Google Scholar]
- 50.del Amo EM, Rimpelä AK, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–185. doi: 10.1016/j.preteyeres.2016.12.001. [DOI] [PubMed] [Google Scholar]