Advances in understanding the neural basis of mental processes have long been hampered by the mere extent and complexity of the underlying neuronal networks. Recent attempts to overcome this challenge include rendering brain networks in the form of simple and abstract graphs (Bullmore and Sporns, 2009). The nodes of a graph can represent anatomical brain areas, while the edges can represent either physical connections (axons) or functional interactions between nodes. Such an abstract representation allows researchers to study the higher-order organization of brain networks by using the powerful analytic tools of network science and graph theory. Brain networks indeed exhibit a range of complex properties optimized for efficient information processing. These include the presence of hubs (structurally central and highly interconnected nodes), small-world topology (dense local clustering combined with sparse long-range connections), and modular organization (groups of strongly interconnected nodes with few between-groups connections; Bullmore and Sporns, 2009). Importantly, these organizational features are found regardless of the neuroimaging method used and are associated with the cognitive abilities and state of the subjects (Fornito et al., 2015).
Although the emerging field of network neuroscience holds great potential, several challenges remain to be addressed (Fornito et al., 2013; Papo et al., 2014). First, in the great majority of studies functional networks are estimated based on the resting-state activity of the brain. Consequently, interpretation of the findings is limited by the fact that the subject's internal states during data acquisition cannot be controlled. Second, while neuronal activity and information routing are dynamic and flexible, analyses of brain functional networks typically reflect only a static and time-invariant approximation of the underlying dynamics. Third, with the current state of knowledge, graph measures are difficult to interpret in physiological terms and, accordingly, the majority of network neuroscience studies are rather exploratory. These caveats, if left unresolved, might prevent network neuroscience from maturing into a coherent theory accounting for the functional role of the complex organization features of the brain network.
A recent publication by Vatansever et al. (2015) provides a compelling demonstration of how the network neuroscience approach can advance our understanding of brain processes. A novelty of this study is that the authors investigated the dynamic reorganization of brain functional networks during performance of the classic n-back working-memory task. The cognitive effort was manipulated by changing the difficulty level of the task (from 0-back to 3-back), and whole-brain functional networks were created based on fMRI recordings. The nodes of the networks represented brain anatomical areas, and the edges represented functional interactions between areas estimated by correlation coefficient between BOLD signals. Vatansever et al. (2015) hypothesized that, with the increasing cognitive effort, (1) the whole-brain functional network would reorganize toward a more globally integrated and interconnected pattern and (2) that within the whole-brain network the default mode network (DMN) areas will play the role of a “global integrator of information.”
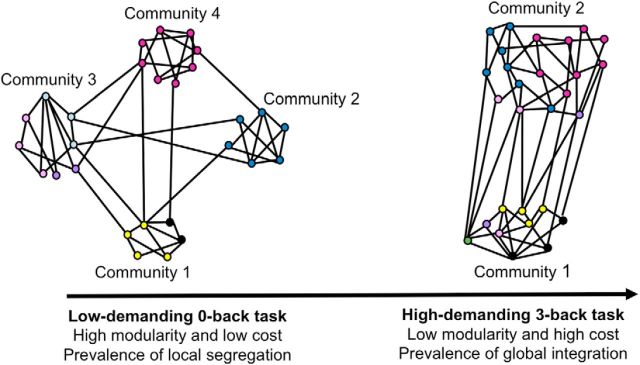
To test these hypotheses, Vatansever et al. (2015) investigated the community structure (modularity) of the network. A community was defined as a set of strongly interconnected nodes exhibiting only sparse interactions with nodes in other communities. The analysis revealed that the modularity index (Q) of the network and the number of modules decreased with increasing cognitive effort. Specifically, the Louvain algorithm divided the whole-brain network into four modules in the least demanding 0-back condition, but only two modules were detected in the most demanding 3-back condition (Fig. 1). The second part of the analysis concerned specifically the brain regions constituting DMN and revealed that in the 3-back condition the DMN nodes increased their nodal connectivity strength. Based on these findings, the authors conclude that cognitive processing is related to more extensive integration of information among brain subnetworks. In other words, during task performance transient interactions occur among brain regions that do not interact at rest. Because the DMN showed high flexibility in community membership and extensive interactions with other brain subnetworks during task performance, it has been assigned an active role in functional integration of task-related information.
Figure 1.
Schematic representation of the main findings of Vatansever et al. (2015). Community representation and colors are in the style of Figures 1 and 3 in the article by Vatansever et al. (2015), and the DMN is represented by Community 4. In the low-demanding 0-back condition, the network was highly modular (high Q index) and was divided into four distinct modules. With the increasing cognitive load, the modularity of the network decreased, and three communities merged into one. Thus, while local segregation was prevalent in the low-demanding task, increasing cognitive effort was associated with more pronounced global integration.
The major advantage of the work by Vatansever et al. (2015) is that the network neuroscience approach was used to test predictions of a specific cognitive theory. The global workspace theory (GWT), originally proposed in 1988 by Baars (2005), assumes that conscious processing requires the integration of information among sensory subsystems. Although the GWT was put forward long before graph theory was introduced to neuroscience, the recent developments of graph analytic tools provide an ideal opportunity to test its main assumptions. Indeed, in agreement with Vatansever et al. (2015), several recent studies also confirmed the predictions of the GWT. First, using fMRI, Godwin et al. (2015) demonstrated that the act of conscious perception, in contrast to subthreshold processing, is related to the decreasing modularity of functional networks. Second, using EEG and source-modeling algorithms Bola and Sabel (2015) revealed that during the processing of target stimuli in a visual discrimination task the functional network transiently shifts toward less modular and more globally integrated arrangements. Third, decreases in the modularity of the whole-brain network during the performance of a demanding perceptual task were observed in an fMRI study by Elton and Gao (2015). Finally, these recent results corroborate earlier findings, for instance from an MEG study of working memory (Kitzbichler et al., 2011), and from fMRI studies of recollection (Fornito et al., 2012) and emotional stimulus processing (Kinnison et al. 2012). Therefore, converging evidence indicates that the prevalence of global integration among brain subnetworks might be a genuine correlate of cognitive processing.
Of note, the network reorganization observed in the aforementioned studies, including the study by Vatansever et al. (2015), was assumed to reflect dynamic rerouting of task-related information for cognitive processing. But several modulatory factors, like arousal, fatigue, emotions, and mood, also dynamically affect brain functional networks. Indeed, performing a cognitively demanding task produces carryover effects, which can be detected in an fMRI BOLD signal even several minutes after task completion (Barnes et al., 2009; Grigg and Grady, 2010). This finding supports the hypothesis that the amplitude coupling, which is estimated by fMRI-derived functional connectivity, reflects a modulatory mechanism regulating the activation level of neuronal populations (Engel et al., 2013). Therefore, an important goal for future studies is to identify the aspects of network reorganization related to the genuine transfer of task-related information. This might require focusing on the networks underlying neurophysiological phase coupling, which is a supposed mechanism of effective communication between neural assemblies (Engel et al., 2013; Fries, 2015).
The brain has long been hypothesized to exhibit modular organization across spatial scales—from cortical microcolumns to functional subsystems. Thus, in contrast to other graph measures, changes in the community structure of the network might be easier to interpret in physiological terms. Importantly, perturbing the baseline (i.e., the resting state) community structure with a demanding task, as was done by Vatansever et al. (2015), allows new insights into the functional roles of brain modules. For instance, it is hypothesized that complex networks are organized to satisfy two conflicting demands lowering the wiring cost of the network (i.e., the length of connections) and increasing the efficiency of information processing (Bullmore and Sporns, 2012). The modular organization with sparse but strategically placed intermodular connections seems to fulfill both, and the balance between cost and efficiency can be easily adjusted by adding more intermodular connections. Indeed, the results of the study by Vatansever et al. (2015) indicate that during cognition the whole-brain functional network transiently reorganizes toward more efficient, but also more costly, arrangements.
An important goal of the study by Vatansever et al. (2015) was to pinpoint the anatomical substrate of the global workspace. Whereas the global workspace is typically identified with the broadly defined frontoparietal system (Dehaene and Changeux, 2011), Vatansever et al. (2015) argue that the DMN is also crucial for the global integration of information during cognition. However, we think their claim asserting the “central role for the DMN in higher cognitive processing” (Vatansever et al., 2015, p. 15254) might require stronger support. Although the authors had a strong a priori hypothesis that the DMN will integrate information during task performance, they did not show the specificity of this effect. The presented data indicate that several other subnetworks also change their modular allegiance (Vatansever et al., 2015, their Fig. 3) and exhibit high values of the global variable connectivity measure (Vatansever et al., 2015, their Fig. 4). Therefore, it seems likely that the reported increase in connectivity strength, on which the discussed claim is based, is not specific to the DMN. We believe that supporting a statement that the DMN is crucial for task-related integration requires examining a statistical interaction (task difficulty × subnetwork), and not a simple effect of the task difficulty within the DMN (Nieuwenhuis et al., 2011).
Finally, to further test the hypothesis that the DMN is “actively contributing to greater functional integration” (Vatansever et al., 2015, p. 15259), future studies might use “effective connectivity” methods, which provide information on the direction of information transfer (Friston et al., 2013). If DMN indeed integrates information during cognitive processing, then the effective connectivity analysis should reveal DMN as an important functional “sink” (i.e., there should be a substantial flow of information into the DMN during cognitive processing).
In conclusion, by studying whole-brain functional network reorganization during cognition, Vatansever et al. (2015) contribute to two research fields. From the cognitive neuroscience perspective, tracking the dynamic reorganization of large-scale brain networks might reveal new mechanisms supporting cognitive processing. At the same time, revealing the genuine role of the complex organization patterns of the brain networks, which is the ultimate goal of network neuroscience, might be advanced by observing how these patterns change in response to external demands rather than by studying the resting-state networks only.
Footnotes
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
We thank Łukasz Bola, Artur Marchewka, and Monika Riegel for their comments on an earlier version of the manuscript.
The authors declare no competing financial interests.
References
- Baars BJ. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog Brain Res. 2005;150:45–53. doi: 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4:e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bola M, Sabel BA. Dynamic reorganization of brain functional networks during cognition. Neuroimage. 2015;114:398–413. doi: 10.1016/j.neuroimage.2015.03.057. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Elton A, Gao W. Task-related modulation of functional connectivity variability and its behavioral correlations. Hum Brain Mapp. 2015;36:3260–3272. doi: 10.1002/hbm.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Gerloff C, Hilgetag CC, Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron. 2013;80:867–886. doi: 10.1016/j.neuron.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci U S A. 2012;109:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage. 2013;80:426–444. doi: 10.1016/j.neuroimage.2013.04.087. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88:220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Moran R, Seth AK. Analysing connectivity with Granger causality and dynamic causal modelling. Curr Opin Neurobiol. 2013;23:172–178. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin D, Barry RL, Marois R. Breakdown of the brain's functional network modularity with awareness. Proc Natl Acad Sci U S A. 2015;112:3799–3804. doi: 10.1073/pnas.1414466112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O, Grady CL. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS ONE. 2010;5:e13311. doi: 10.1371/journal.pone.0013311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnison J, Padmala S, Choi JM, Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. J Neurosci. 2012;32:8361–8372. doi: 10.1523/JNEUROSCI.0821-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler MG, Henson RN, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci. 2011;31:8259–8270. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Papo D, Zanin M, Pineda-Pardo JA, Boccaletti S, Buldú JM. Functional brain networks: great expectations, hard times and the big leap forward. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0525. 20130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D, Menon XDK, Manktelow AE, Sahakian BJ, Stamatakis EA. Default mode dynamics for global functional integration. J Neurosci. 2015;35:15254–15262. doi: 10.1523/JNEUROSCI.2135-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]