Abstract
Aging weakens memory functions. Exposing healthy rodents or pathological rodent models to environmental enrichment (EE) housing improves their cognitive functions by changing neuronal levels of excitation, cellular signaling, and plasticity, notably in the hippocampus. At the molecular level, brain derived-neurotrophic factor (BDNF) represents an important player that supports EE-associated changes. EE facilitation of learning was also shown to correlate with chromatin acetylation in the hippocampus. It is not known, however, whether such mechanisms are still into play during aging. In this study, we exposed a cohort of aged rats (18-month-old) to either a 6 month period of EE or standard housing conditions and investigated chromatin acetylation-associated events [histone acetyltranferase activity, gene expression, and histone 3 (H3) acetylation] and epigenetic modulation of the Bdnf gene under rest conditions and during learning. We show that EE leads to upregulation of acetylation-dependent mechanisms in aged rats, whether at rest or following a learning challenge. We found an increased expression of Bdnf through Exon-I-dependent transcription, associated with an enrichment of acetylated H3 at several sites of Bdnf promoter I, more particularly on a proximal nuclear factor κB (NF-κB) site under learning conditions. We further evidenced p65/NF-κB binding to chromatin at promoters of genes important for plasticity and hippocampus-dependent learning (e.g., Bdnf, CamK2D). Altogether, our findings demonstrate that aged rats respond to a belated period of EE by increasing hippocampal plasticity, together with activating sustained acetylation-associated mechanisms recruiting NF-κB and promoting related gene transcription. These responses are likely to trigger beneficial effects associated with EE during aging.
SIGNIFICANCE STATEMENT Aging weakens memory functions. Optimizing the neuronal circuitry required for normal brain function can be achieved by increasing sensory, motor, and cognitive stimuli resulting from interactions with the environment (behavioral therapy). This can be experimentally modeled by exposing rodents to environmental enrichment (EE), as with large cages, numerous and varied toys, and interaction with other rodents. However, EE effects in aged rodents has been poorly studied, and it is not known whether beneficial mechanisms evidenced in the young adults can still be recruited during aging. Our study shows that aged rats respond to a belated period of EE by activating specific epigenetic and transcriptional signaling that promotes gene expression likely to facilitate plasticity and learning behaviors.
Keywords: acetylation, aging, chromatin immunoprecipitation, environmental enrichment, NF-κB, spatial memory
Introduction
Aging is characterized by a gradual cognitive decline often associated with weakened cerebral plasticity (e.g., synaptic plasticity, neurogenesis) in brain regions involved in memory formation. Deregulations of specific transcriptional programs involved in plasticity processes could be one cause of the age-related cognitive decline (Blalock et al., 2003; Burger et al., 2008; Peleg et al., 2010). The regulation of these genetic programs is operated by the coordinated action of transcription factors and coactivators. Importantly, their accessibility and recruitment to chromatin is modulated in part by histone posttranslational modifications, of which acetylation is a well documented trigger of memory formation (Gräff and Tsai, 2013; Peixoto and Abel, 2013; Lopez-Atalaya and Barco, 2014). In the hippocampus, histone tails are actually acetylated following learning experience (Sultan and Day, 2011; Castellano et al., 2012; Bousiges et al., 2013), some of these changes being associated with the transcription of several plasticity genes required for memory formation (Bousiges et al., 2010; Gräff and Tsai, 2013; Peixoto et al., 2015). It is currently thought that aging may alter these mechanisms. For instance, H4K12 acetylation and associated genetic programs become unresponsive to experience in the hippocampal region of aged mice (Peleg et al. 2010). Another study suggests that loss of coordinated control of epigenetic landscape may account for age-associated dysfunctions rather than a specific histone modification (Castellano et al., 2012; Sewals et al., 2015). Such impairments may lead to age-associated memory deficits. Interestingly, some of these deficits could be reversed by treatment with a histone deacetylase (HDAC) inhibitor [suberoylanilide hydroxamic acid (SAHA)], as demonstrated in vivo with the rescue of learning-induced gene expression, enhancement of associative learning (Peleg et al., 2010), and, as described more recently, age-induced aberrant exon usage associated with a neuron-specific decrease in H4K12ac (Benito et al., 2015). Thus, compensating for acetylation deficiencies during aging with such inhibitor molecules could have an important impact on plasticity and memory functions.
Aged-induced learning and memory deficits can also be attenuated by sustained exposure of rodents to environmental enrichment (EE; Harati et al., 2011, 2012; Simpson and Kelly, 2011). EE promotes various plasticity mechanisms in the hippocampus, including long-term potentiation, Bdnf gene upregulation, enhanced dendritic branching, and stimulation of adult neurogenesis (van Praag et al., 2000; Nithianantharajah and Hannan, 2006; O'Callaghan et al., 2009; Simpson and Kelly, 2011; Novkovic et al., 2015). An important step toward the understanding of beneficial effects of EE was to show that, at the molecular level, EE correlated with increased histone-tail acetylation in young rodents (Fischer et al., 2007). Furthermore, mice deficient for an epigenetic enzyme such as the acetyltransferase CBP presented marked deficiencies in EE-induced neurogenesis and were less responsive to EE-mediated enhancement of spatial navigation capabilities (Lopez-Atalaya et al., 2011). Thus, epigenetic regulations, particularly histone acetylation, represent a molecular mean by which EE can translate into long-lasting changes and plasticity. However, molecular effects induced by EE have been poorly studied in aged rodents, and it is not known to which extent beneficial mechanisms evidenced in young adults can still be recruited during aging.
The present study investigated whether EE, when provided to rats at a late stage during their life (i.e., between the age of 18 and 24 months), could impact on acetylation-related regulations relevant to cognitive functions. Our results show that in aged rats, late EE improved plasticity, spatial memory acquisition, activated several acetylation-dependent processes [global histone acetyltranferase (HAT) activity, Pcaf expression, histone H3 acetylation], and fostered the expression of Bdnf Exon-I transcripts in the dorsal hippocampus. We also found that EE could favor p65/nuclear factor κB (NF-κB)-dependent regulations in aged rats. Indeed, EE induced p65/NF-κB enrichment on the proximal region of Bdnf promoter I and CamK2D promoter, both genes showing enhanced expression in response to EE. Thus, our data provide molecular evidence accounting for beneficial cognitive effects in aged rodents exposed to a belated EE.
Materials and Methods
Animals and housing conditions.
Five-week-old female Long–Evans rats (Janvier Labs; n = 107 total) were housed in pairs in transparent cages (46 × 26 × 15 cm) until the age of 18 months [standard conditions (SCs)]. At this age, they were further kept in SCs or randomly assigned to enriched conditions (ECs) for 6 months. Enriched rats were gathered in groups of 10–12 in two contiguous wire mesh cages (112 × 40 × 40 cm) with various objects (tunnels, ramps, shelters, wooden objects, but no running wheel) changed five times a week. Food and water were available ad libitum in a temperature-controlled (22 ± 1°C) and humidity-controlled (55 ± 5%) room under a 12 h light/dark cycle. At the end of differential housing, the now 24-month-old rats were placed in individual transparent cages for 20 d before undergoing spatial training (tr) or not. Subsequently, they were killed for molecular biology experiments. All animals with detectable pathologies (e.g., hydrocephalus, palpable tumors) that might influence the principal outcome measures were killed or excluded a posteriori from the study. Experimental protocols and animal care were in compliance with national (Council Directive 87-848; October 19, 1987; Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale) and international (Directive 86-609; November 24, 1986; European Community and new guidelines of the European Parliament 2010/63/UE of September 22, 2010) laws and policies (Personal Authorization No. 67-167 for A.B., No. 67-289 for M.M., No. 67-215 for J.-C.C.).
Morris water maze training.
The Morris water maze (MWM) consisted of a circular pool (160 cm diameter) filled with opaque water (20°C) placed in an experimental room containing extramaze cues and paintings on the walls (for details, see Lopez et al., 2012). The platform (11 cm diameter) was placed 1 cm underneath the water surface. Rats were first subjected to a 1 d habituation with a visible platform followed by a 3 d training (4 trials/day) with a hidden platform according to our previous study in young adult rats (Bousiges et al., 2010). For each trial, the rat was placed in the pool at a randomly designed starting point and given a maximum of 60 s to reach the submerged platform. When the rat had climbed onto the platform, it was left on it for 10 s, then removed, and then the next trial was started. When the rat failed to find the platform within 60 s, it was gently guided to it by the experimenter and was left there for 10 s. Parameters were recorded using a video-tracking system (Noldus). The distance to the platform, corrected according to the method described by Lindner (1997), and the percentage of thigmotaxis [(time spent in the periphery/total latency to reach the platform) × 100] were calculated.
Tissue collections for biochemical studies.
Rats subjected to learning in the MWM (training groups) were killed 1 h after the last training trial (Bousiges et al., 2010). Freshly dissected tissues were immediately frozen at −80°C until being processed for biochemical studies (RNA, total protein) or were immediately processed (chromatin and nuclear protein extractions).
Real-time reverse transcription-PCR.
Total RNA was extracted from the dorsal hippocampus using TRizol reagent (Invitrogen) as reported previously (Bousiges et al., 2010). cDNA synthesis was performed on 1 μg of total RNA (iScript cDNA synthesis kit; Bio-Rad). Quantitative PCR (qPCR) analysis was performed on a Bio-Rad iCycler System using SsoAdvanced Green Supermix. A specific standard curve was performed in parallel for each gene, and each sample was quantified in duplicate. PCR conditions were 3 min at 94°C, followed by 40 cycles of 45 s at 94°C and 10 s at 58−62°C. Data were analyzed using the iCycler software and normalized to the RNA polymerase II mRNA. CamK2D primers were F_tcagtggtgagaagatgcatg and R_cagattctagcttccctttccag (for all others, see Bousiges et al., 2010).
Protein preparation, Western blot analyses, and HAT activity measurements.
Total protein preparations were analyzed by Western blot as described previously (Bousiges et al., 2010). Primary antibodies were as follows: acetyl-histone H3 (06-599; Millipore), total histone H3 (ab1791, Abcam), p65 (ab31481, Abcam), K310acp65 (ab52175, Abcam), actin (Sigma-Aldrich), BDNF (AB9612, Millipore), and GFAP (MAB360, Millipore). The secondary antibodies were from Jackson Immunoresearch. Blots were revealed with Clarity ECL (Bio-Rad). Results were quantified using ImageLab software. HAT activity was measured on nuclear protein extracts prepared as by Bousiges et al. (2010), with a fluorimetric assay kit (Active Motif). All samples were tested in duplicates. The activity was analyzed in a fluorescent microplate reader at 360–390 and 450–470 nm.
Immunohistochemistry.
Animals were deeply anesthetized with pentobarbital (60 mg/kg) and perfused transcardially with 150 ml ice-cold paraformaldehyde (4% in 0.1 m PB, 4°C). Brains were then rapidly removed from the skulls and postfixed for 4 h in the same fixative at 4°C. Fixed brains were then kept in sucrose at 4°C for 48 h. The freezing of the brains was performed in isopentane for 1 min at −40°C. The brains were stored in a −08°C freezer until being processed further. Coronal sections, 20 μm in thickness, were made through the dorsal hippocampus using a cryostat (Microm HM560). The tissue sections were permeabilized in 1× PBS/2% Triton for 15 min. Nonspecific labeling was blocked by 1× PBS/0.1% Triton/5% horse serum for 30 min, and sections were then incubated overnight with the indicated antibodies [doublecortin (DCX), sc-8066, Santa Cruz Biotechnology; synaptophysin (SYP), S5768, Sigma]. After three washes, they were incubated with the appropriate secondary antibody. For immunofluorescence (SYP), sections were incubated with donkey anti-mouse conjugated with fluorescent far red (FR) dye (1 h, room temperature), followed by three washes with 1× PBS/0.1% Triton; the nuclei were stained with DAPI (1:1000 dilution) for 5 min. After two PBS washes, the sections were mounted. For immunohistochemistry (DCX), sections were incubated with anti rabbit horseradish peroxidase-conjugated antibody for 1 h. After three washes with 1×PBS/0.1% Triton, the revelation was performed with diaminobenzidine (0.05% DAB, 0.04 m Tris, pH 7.5, 0.03% H2O2), and sections were mounted with Roti Histokit II (Roth). Images were acquired with a Zeiss (ApoTome2) imaging system.
Synaptic densities counting.
Images from SYP immunofluorescence were analyzed blind using the spot detector plug-in of ICY software (F. de Chaumont, Institut Pasteur, Paris). To estimate the number of presynaptic terminals in both CA3 and CA1 areas, fluorescence puncta were filtered by size (spots >100 or <5 pixels were rejected), and spots were binarized to get an optimal visual control.
Chromatin immunoprecipitation.
Freshly dissected tissues were chopped with a razor blade and rapidly put in 5 ml PBS containing 1% formaldehyde for 15 min at room temperature followed by the addition of glycine (0.125 m final concentration). Samples were prepared as in the study by Bousiges et al. (2010) and sonicated with a Diagenode Bioruptor (30 cycles of 30 s ON/30 s OFF on high power). Sonicated chromatin was diluted 10 times in chromatin immunoprecipitation (ChIP) dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-Cl, pH 8.1, 167 mm NaCl). A fraction of supernatant (50 μl) from each sample was saved before immunoprecipitation (IP) and served as “total input chromatin.” Supernatants were incubated overnight (4°C) with 2–5 μg of primary antibody (H3K9/K14ac, 06-599, Millipore; p65, ab31481, Abcam; or no antibody as negative control), followed by a mix of protein A/G Dynabeads (Invitrogen) for 3 h. After several washing steps (low salt, high salt, LiCl, and TE buffers), complexes were eluted in 300 μl of Elution buffer (1% SDS, 0.1 m NaHCO3). The cross-linking was reversed (overnight at 65°C), and the DNA was subsequently purified with RNase (30 min, 37°C) and proteinase K (2 h, 45°C) and then subjected to phenol/chloroform extraction and ethanol precipitation. After a last wash with 70% ethanol, pellets were resuspended in 50 μl of nuclease-free milliQ water and analyzed by semiquantitative PCR (MyCycler system, Bio-Rad; 95°C for 4 min, 95°C for 40 s, 58−62°C for 40 s, 72°C for 40 s for 30–36 cycles) or by qPCR (CFX96, Bio-Rad; 98°C for 3 min, and 98°C for 15 s, 63°C for 45 s for 50 cycles, then 98°C for 10 s; melt curve, 65 to 98°C; increment, 0.5°C, 5 s). IP enrichment and specificity were checked with control genes either expressed ubiquitously (Gapdh) or not expressed in the hippocampus (TsH2B).
Primers for Bdnf promoter I are from Tian et al. (2009): neural-restrictive silencer element (NRSE), F_ccaaagcccaccttctggagct R_gccctagatctctgagcgaagaggtata; cAMP response element (CRE), F_aacttttctaagaagtttcctttttacca, R_tgagccagttacgtgaccaact; NF-κB, F_gcagttggacagtcattggtaacc, R_acgcaaacgccctcattctg. The promoter of rat CamK2D was found with the UCSC genome browser and Transcriptional Regulatory Element Database (TRED) and the Fasta sequence entered in Jaspar, computational regulatory genomics software, to identify the predictive recognition sites of p65/NF-kB. Primers were then designed with Primer3Plus bioinformatics software to the proximal region as noted in Figure 4A. CamK2D promoter primers were F_gggtctgggagcactaaaca and R_ggacagagatctcgcagtga.
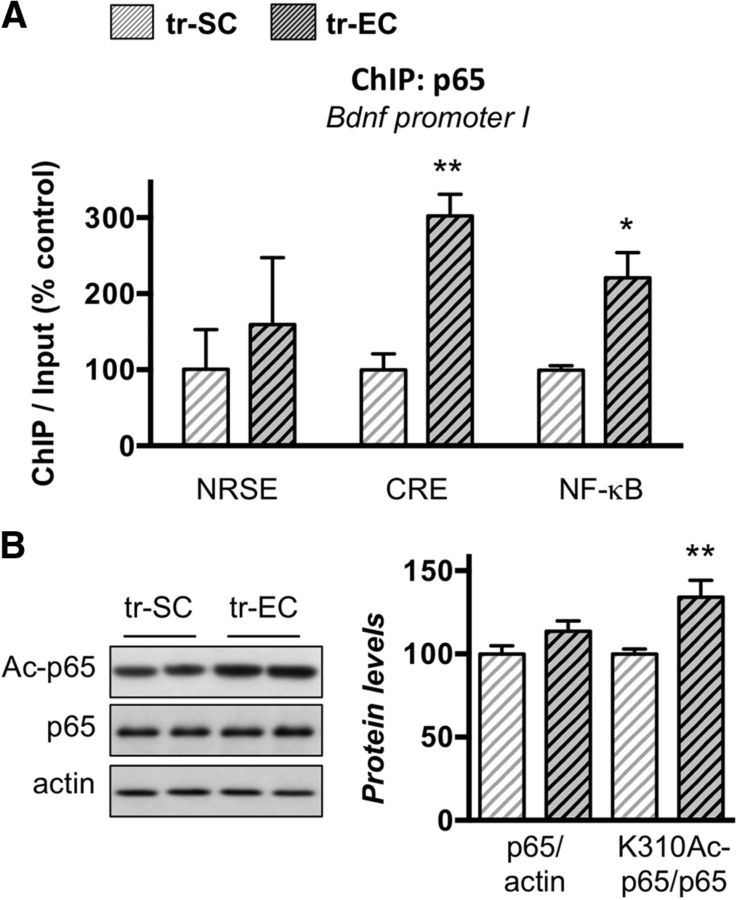
Figure 4.
Late-life enrichment induces p65/NF-κB binding on the Bdnf promoter I and acetylation of p65/NF-κB protein. A, ChIP performed with an antibody against p65/NF-κB on chromatin isolated from dorsal hippocampi of SCs or ECs aged rat groups that were trained in the MWM for 3 d as described. The quantity of immunoprecipitated DNA was assessed by qPCR for the three sites (NRSE, CRE, NF-κB) present in the Bdnf promoter I. The net quantities of enriched DNA were corrected with their corresponding input DNA. Data are expressed as mean ± SEM (n = 3 per group) with percentage relative to the tr-SC control group arbitrarily set at 100%. EE promotes p65/NF-κB binding to the proximal region of the Bdnf promoter I. B, Western blot analyses of p65, acetylated-p65 at K310 (Ac-p65), and actin levels performed on lysates from dorsal hipocamppi of MWM-trained aged SC or EC rats. Representative results are presented as duplicates (left), and their quantification is expressed as mean ± SEM (n = 6 per group) with percentage relative to the tr-SC control group arbitrarily set at 100%. Enrichment housing induces acetylation of p65. *p < 0.05; **p < 0.01 (Student's t test to compare the effect of housing on trained aged rats, tr-EC vs tr-SC; A, B).
Statistical analyses.
Data were analyzed using a two-way ANOVA (“housing” and “training” conditions) followed, where appropriate, by post hoc comparisons using the Newman–Keuls multiple range test or a Student's t test for the data obtained in one condition only (training or housing).
Results
Late-life EE improved adult neurogenesis and synaptic densities in aged rats
A cohort of rats was raised in standard conditions for 18 months, and half of them were then either exposed to enriched conditions for the next 6 months or left in their standard conditions (Fig. 1A). We first asked whether belated EE had an impact on hippocampal plasticity and verified several criteria such as the formation of neuronal progenitors in the dentate gyrus and the synaptic density in two hippocampal areas. We performed immunohistochemistry studies on DCX, a marker of new yet immature neurons in the adult brain. Overall, a significant increase in the number of DCX-positive neurons (almost twofold, p < 0.05) was observed in the subgranular zone of the hippocampus of aged rats raised during 6 months in ECs compared to SCs (Fig. 2B). Another typical hallmarks of aged, memory-impaired animals is the alteration of hippocampal synaptic plasticity at CA3–CA1 synapses (Kumar, 2011). In addition, levels of the synaptic marker synaptophysin were found to be increased by EE in young (Fischer et al., 2007) and aged (Frick and Fernandez, 2003) mice. We thus measured synaptic densities by synaptophysin immunolabeling in the stratum radiatum of the dorsal hippocampal CA1 region (Fig. 2C, inset, DAPI) and in the CA3 region (Fig. 2C). The intrinsic fluorescence of lipofuscin classically observed in aged brain tissues (Fig. 2C, inset, FITC) was circumvented by using a secondary antibody with an emission in the FR light wavelength. EC rats displayed a significantly higher number of synaptic contacts in CA1 as indicated by a higher number of synaptophysin-positive (SYP+) puncta/1000 μm2 (p < 0.01), whereas a tendency to increase was observed in CA3 (p = 0.07). This suggests that a cellular adaptation of the neuronal network is induced by the 6 month period of EE. However, we found that the aged brains displayed a higher level of inflammation compared to young rats, as attested by GFAP measurements, a process that was not counteracted by the enrichment (Fig. 1D,E). This result emphasizes that the cellular content of aged hippocampi may be comparable in the two environmental conditions (aged rat groups), but is different from that found in the young rats. Therefore, biochemical measurements were performed in tissue samples of aged rats only. Overall, late-life EE produced more plasticity in the aged brain without changing the inflammation process.
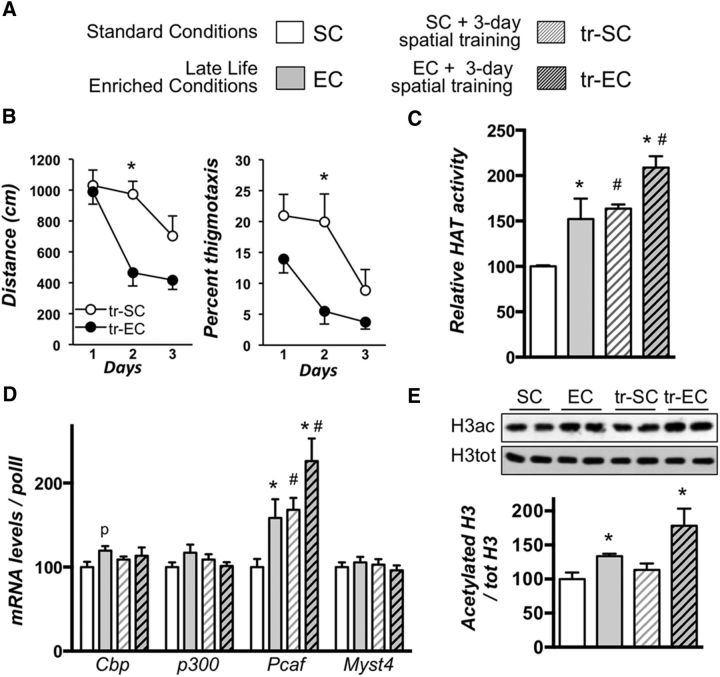
Figure 1.
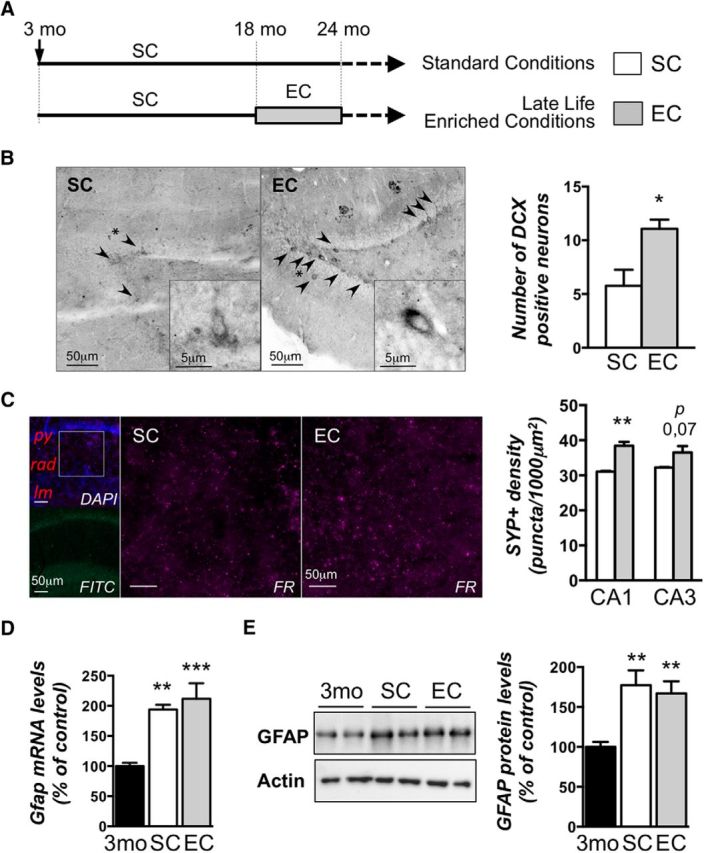
Enriched environment housing conditions applied in late life for aged rats increases plasticity in the hippocampus without impacting inflammation. A, Experimental design. A cohort of 5-week-old rats was housed in pairs over 18 months (standard conditions). Then, rats were randomly kept in standard conditions or assigned to enriched conditions for another 6 month period, after which the 24-month-old rats were placed in individual cages for 20 d. B–E, A subgroup was used for baseline assessments: immunostological (B, C) or biochemical (D, E) analyses. Three-month-old rats (3mo) placed in standard conditions were used as young controls (D, E). B, Hippocampal neurogenesis was evaluated by immunohistochemical detection of the immature neuronal marker DCX in the dentate gyrus subgranular zone from aged rat groups (SC and EE) under basal conditions. Arrowheads indicate DCX positive neurons, and asterisks indicate the neuron focused on in the inset. DCX-positive neurons were counted, and data are expressed as mean ± SEM (n = 4 per group). *p < 0.05 (SC vs EE; Student's t test). A significant increase of the number of DCX-positive cells in the dentate gyrus of aged rats exposed to EE compared to those reared in standard conditions is observed. C, Synapse densities were visualized by synaptophysin immunolabeling (FR) in the CA1 and CA3 regions of the dorsal hippocampus. Typical photographs observed in the CA1 area are shown for SC and EC rats. The inset in the photograph on the left (top, DAPI) represents the region of CA1 that was investigated. py, Pyramidal layer; rad, stratum radiatum; lm, stratum lacunosum moleculare. The FITC photograph below shows the background fluorescence of lipofuscin typically observed in the brains of aged animals. The synapse density is represented as the number of puncta per square 1000 micrometers counted in each photograph (3–5 slices/animal; n = 3–4/group). **p = 0.0025. D, mRNA expression of the glial marker GFAP was evaluated by RT-qPCR in the dorsal hippocampi of young adult rats (3mo) housed in standard conditions and aged rats (24 months of age) housed either in standard conditions or enriched conditions. Values were normalized to the RNA polymerase II expression levels. Data are expressed as mean ± SEM (n = 7–8 per group) with the percentage relative to the young control group arbitrarily set at 100%. **p < 0.01; ***p < 0.001 [NK post hoc comparisons after one-way ANOVA, significantly different from control (3mo) group]. E, GFAP protein levels were assessed by Western blot in nuclear protein extracts from the dorsal hippocampi of the three previously described groups. Typical blots are shown in duplicates. Blots were quantified, and the results are expressed as mean ± SEM (n = 4–5 per group) with the percentage relative to the control group arbitrarily set at 100%. **p < 0.01 [NK post hoc after one-way ANOVA, significantly different from the control (3mo) group].
Figure 2.
Late-life enrichment improves spatial learning and increases acetylation-related events in the dorsal hippocampus. A, Experimental design. A cohort of 5-week-old rats was raised and housed in the same conditions than those of Figure 1. Half of each cohort was then trained (tr-SC, tr-EC) during 3 d for acquisition of reference memory in the MWM (see Material and Methods), the other half being used for baseline assessments. Thereafter, all rats were killed and their brains processed for biochemical analyses within the same range of time. B, Average distance (centimeters) to reach the platform (left) and percentage of thigmotaxis (right) during the 3 d acquisition period (learning based on 4 trials per day) in the water maze. C, HAT activity was measured on nuclear protein extracts from the dorsal hippocampi of the different aged rat groups (n = 4–5 per group) in basal (SC, white bars; EC, gray bars) and trained (tr-SC, hatched white bars; tr-EC, hatched dark bars) conditions. The color code used herein will be the same in the following figures. D, mRNA expression for several HATs (Cbp, p300, Pcaf, and Myst4) was evaluated by RT-qPCR in the dorsal hippocampi of all groups of aged rats (n = 7–10 per group). Values were normalized to RNA polymerase II expression levels. E, Total and acetylated histone H3 protein levels were assessed by Western blot in total protein extracts from the dorsal hippocampi of aged rats (n = 4–5 per group) as noted. Typical blots are shown in duplicates and quantifications of the ratio (acH3/total H3) are shown below (C–E). Data are expressed as mean ± SEM, with the percentage relative to the SC control group set at 100%. *p < 0.05 (for housing, SC vs EC); #p < 0.05 (for training comparison; post hoc comparisons using the Newman–Keuls multiple range test). p denotes p = 0.07 when the EC group was compared to the SC group.
EE improved spatial learning and increased acetylation-related events in aged rats
Another cohort of rats was raised in SCs or ECs as in Figure 1A, among which the half was trained in the MWM for 3 d (tr-SC and tr-EC) to induce learning-related signaling (Fig. 2A). Trained rats were killed 1 h after training, and nontrained rats were killed at the same time. We first monitored the effect of late EE on the spatial training itself. The results obtained are shown in Figure 2B. The average distance traveled to reach the platform decreased in both groups (tr-SC and tr-EC) from day 1 to day 3. The ANOVA showed significant housing and day of training effects for the distance traveled (F(1,11) = 13.13, p < 0.01 and F(2,22) = 11.14, p < 0.001, respectively) and the percentage of thigmotaxis (F(1,11) = 10.45, p < 0.01 and F(2,22) = 6.62, p < 0.01, respectively). However, the overall distance swum was higher in the tr-SC than in the tr-EC rat group. Post hoc comparisons showed that at day 2, the tr-SC rats swam a longer distance to reach the platform than the tr-EC rats (p < 0.01) and stayed closer to the walls (p < 0.05), suggesting that rats raised in standard conditions displayed slower acquisition performance than those raised in EE. It is noteworthy that rats raised in the same EE conditions show a significantly improved retention performance when tested 24 h after 5 d of acquisition (Fuchs et al., 2016). Together, our data show that a belated EE has a beneficial effect on hippocampal plasticity by increasing adult neurogenesis, synaptic densities, and spatial learning abilities in aged rats.
We then evaluated the impact of EE on acetylation regulations in the dorsal hippocampus of these aged rats, measuring different outcomes: global HAT enzyme activity (Fig. 2C), global acetylation of H3 (D), and gene expression of several HAT enzymes (E), in basal and trained conditions. Our results indicated significant housing (F(1,12) = 20.68, p < 0.01) and training (F(1,12) = 13,55, p < 0.001) effects on HAT activity, and no interaction between the two factors (Fig. 2C). Post hoc comparisons indicated that EE led to increased global HAT activity in the dorsal hippocampus of aged rats under basal conditions (SC vs EC, p < 0.05) and under the condition of ongoing learning (tr-SC vs tr-EC, p < 0.05). We showed previously that spatial memory induces the upregulation of Cbp, p300, and Pcaf acetyltransferase transcripts in the dorsal hippocampus of young adult rats (Bousiges et al., 2010). Interestingly, in aged rats, we found that only Pcaf mRNA levels were significantly responsive to training (SC vs tr-SC, p < 0.05; Fig. 2D). Pcaf was also upregulated in response to housing conditions (SC vs EC, p < 0.05; tr-SC vs tr-EC, p < 0.05), while Cbp only tended to increase in response to EE (p = 0.07). The effect of EE was further evaluated on bulk histone H3 acetylation. We detected a significant increase in acetylated H3 levels in aged rats in response to EE in both basal and training conditions (SC vs EC, p < 0.05; tr-SC vs tr-EC, p < 0.05; Fig. 2E). However, contrasting with our previous findings in young adults (Bousiges et al., 2010), spatial training did not activate H3 acetylation in aged rats (Fig. 2E, SC vs tr-SC), suggesting a possible age-related impairment of this regulation.
Altogether, these data point to PCAF as a selective EE-responsive HAT able to contribute to increased HAT activity and histone H3 acetylation levels in the dorsal hippocampus of aged rats. Importantly, EE counteracted the lack of acetylation on H3 observed in tr-SC rats.
EE induced an up-regulation of bdnf expression through specific promoter I-dependent acetylation at different enhancer sites
EE beneficial effects are known to involve Bdnf transcription (Novkovic et al., 2015), even in the aged rat (O'Callaghan et al., 2009). We thus measured the expression of specific bdnf transcripts (total, Exon-I, Exon-IV, and Exon-VI) by RT-qPCR in the dorsal hippocampus of aged rats. As shown in Figure 3A, training was able to induce a moderate but significant increase of total Bdnf transcripts (SC vs tr-SC, p < 0.05). Interestingly, a dramatic upregulation of Bdnf Exon-I mRNA levels (SC vs tr-SC, p < 0.05) was observed in the dorsal hippocampus of aged rats in response to training, but no significant difference was seen for Bdnf Exon-IV and Exon-VI mRNA levels. Whereas EE did not significantly affect total Bdnf mRNA levels in the absence of training, despite a tendency to increase (SC vs EC, p = 0.07), a significant housing effect was observed in the training condition (tr-SC vs tr-EC, p < 0.05), suggesting that housing could enhance dynamic processes. The other Bdnf exons tested (Exon-IV and Exon-VI) were not responsive to EE in aged rats, yet EE induced a specific and marked increase in bdnf Exon-I expression in the dorsal hippocampus, under both basal and training conditions (SC vs EC, p < 0.05; tr-SC vs tr-EE, p < 0.05). Consistently, we found increased production of the 28 KDa pro-BDNF protein in trained rats in response to EE by Western blot analyses (Fig. 3B); however, mature BDNF levels could not be detected in our extracts.
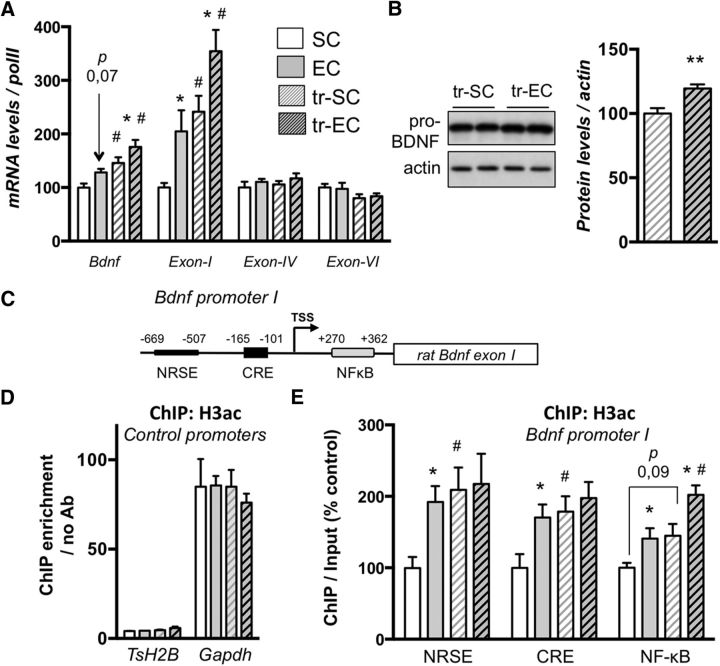
Figure 3.
Late-life enrichment improves learning-induced Bdnf expression from Exon-1-dependent transcription and acetylation of the NF-κB regulatory promoter region. A, mRNA expression for total Bdnf and several specific exons (Exon-I, Exon-IV, and Exon-VI) was evaluated by RT-qPCR in the dorsal hippocampi of all groups of aged rats as noted. Values were normalized to RNA polymerase II expression levels. Data are expressed as mean ± SEM (n = 7–10 per group), with the percentage relative to the SC control group set at 100%. *p < 0.05 (housing, SC vs EC); #p < 0.05 (training; post hoc comparisons using the Newman Keuls multiple range test). p = 0.07 when the EC group was compared to the SC group for total Bdnf transcripts. B, Western blot analyses of pro-BDNF and actin levels performed on lysates from dorsal hipocamppi of MWM-trained aged SC or EC rats. Representative results are presented as duplicates (left), and their quantification is expressed as mean ± SEM (n = 6 per group), with the percentage relative to the tr-SC control group arbitrarily set at 100%. Enrichment housing induces pro-BDNF expression. **p < 0.01 (Student's t test to compare the effect of housing on trained aged rats (tr-EC vs tr-SC). C, Relative location of enhancer elements within the rat Bdnf Exon-I specific promoter (adapted from Tian et al., 2009). The three sites examined (NRSE, CRE, and NF-κB) are located with their respective position in the proximal region of the promoter [transcription start site (TSS)]. D, E, ChIP performed with an antibody against acetylated-histone H3 on chromatin isolated from dorsal hippocampi of aged rat groups. The quantity of immunoprecipitated DNA was assessed by qPCR for control genes (testis H2B histone TsH2B and Gapdh) relative to the no antibody control to establish the enrichment of the IP (D) and Bdnf I promoter regions of interest as noted (E). The net quantities of enriched DNA were corrected with their corresponding input DNA. Data are expressed as mean ± SEM (n = 4 per group), with the percentage relative to the SC control group arbitrarily set at 100%. *p < 0.05 (housing, SC vs EC); #p < 0.05 (training; post hoc comparisons using the Newman–Keuls multiple range test). p denotes p = 0.09 when training was compared to basal in SC for the NF-κB site.
To establish a functional link between acetylation-dependent mechanisms and specific gene transcription, we next evaluated the putative elements responsive to EE of the proximal bdnf promoter I region by ChIP. This promoter was well characterized in previous studies (Lubin et al., 2008; Tian et al., 2009), which indicated a correlation between NMDA receptors stimulation and Bdnf Exon-I transcriptional activation with increased acetylated-H3 levels on specific sites: NRSE, CRE, and NF-κB-responsive element. These sites are depicted in Figure 3C. We thus performed a series of ChIP experiments, targeting the proximal region of bdnf promoter I with acetylated-H3 histone antibodies using chromatin extracts from the dorsal hippocampus of a new group of aged rats (SC vs EC and tr-SC vs tr-EC). Figure 3D shows the specificity and the enrichment of the immunoprecipitation: no enrichment was detected using primers against a gene that is not expressed in the hippocampus, but only in the testis (TsH2B), and an enrichment of ∼80-fold was found when testing binding to Gapdh, a highly expressed gene. On Bdnf promoter I (Fig. 3E), we detected a marked enrichment of acetylated-H3 levels induced by training on the NRSE- and CRE-binding sites in the aged rats reared in standard conditions (SC vs tr-SC, NRSE, p < 0.05; CRE, p < 0.05; NF-κB, p = 0.09), suggesting that Bdnf expression is activated to some extent through these elements in aged rats. Interestingly, EE did not impact the three responsive sites to a comparable extent under basal and trained conditions: a significant increase in acetylated-H3 levels was measured at the three sites (NRSE, CRE, and NF-κB) under basal conditions in EE housed rats (SC vs EC, NRSE, p < 0.05; CRE, p < 0.05; NF-κB, p < 0.05), whereas only the NF-κB binding site displayed an additional enrichment of acetylated-H3 over training (tr-SC vs tr-EC, p < 0.05). Thus, the additional effect found on this NF-κB site suggests that this site could contribute to the Bdnf Exon-I transcriptional upregulation observed in aged enriched rats during the formation of spatial memory.
EE exposure increases the binding of p65/NF-κB to target gene promoters and acetylation of p65/NF-κB during spatial training of aged rats
Having pointed to an EE-induced H3 acetylation at a proximal NF-κB site of Bdnf promoter I during training, our next goal was to assess whether this site was enriched with bound p65/NF-κB. Figure 4A shows that both CRE and NF-κB sites retained more p65/NF-κB protein during training when rats were exposed to EE (tr-SC vs tr-EE, CRE, p < 0.01; NF-κB, p < 0.05), whereas this was not the case on the NRSE site. Increased NF-κB on the CRE site may result from close proximity of the two sites (Fig. 3C). Our results thus indicate that NF-κB is a target for EE that could be relevant for improving hippocampal plasticity.
Interestingly, PCAF, p300, and CBP acetylate the NF-κB subunit p65 (Sheppard et al., 1999; Chen et al., 2001, 2002; Hoberg et al., 2006), and K310 acetylation is required for full transcriptional activity (Chen et al., 2002). We investigated whether EE housing conditions could impact on p65/NF-κB expression and acetylation status on K310 by Western blot analyses. As shown in Figure 4B, p65/NF-κB protein levels were not changed by EE, whereas its acetylation at K310 was significantly induced by EE exposure (p < 0.01).
Last, we further looked at genes implicated in spatial memory and that may be responsive to p65/NF-κB, among which CamK2D is a good candidate (Federman et al., 2013). We checked for p65/NF-κB binding on its proximal promoter in a region enriched on potential p65/NF-κB sites (Fig. 5A, left) and found an increase in p65/NF-κB binding (right). Finally, we found a significant increase of CamK2D gene transcription in EE-exposed rats compared to rats raised in standard conditions (Fig. 5B). Therefore, we propose that EE housing beneficial effects observed on aging are produced in part by boosting acetylation and NF-κB-dependent regulations, by the mean of increased HAT activity and expression (e.g., Pcaf), and increased transcription of genes important for spatial memory and hippocampal plasticity (e.g., Bdnf, CamK2D; Fig. 5C).
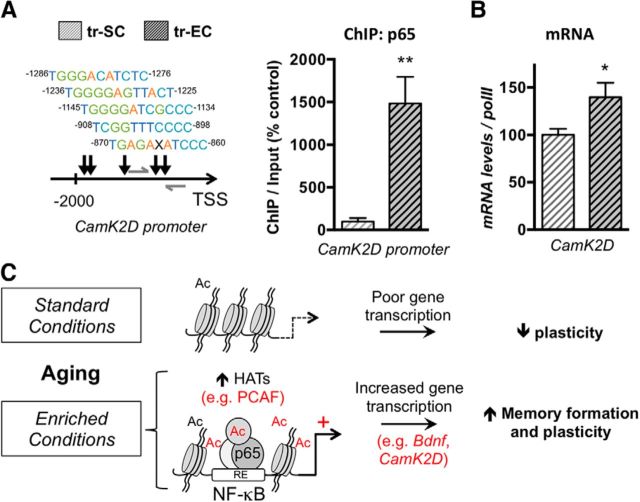
Figure 5.
Late-life enrichment induces p65/NF-κB binding at NF-κB sites of spatial training-induced genes. A, ChIP performed with an antibody against p65/NF-κB on chromatin isolated from dorsal hippocampi of SC or EC aged rat groups that were trained in the MWM for 3 d as described. Left, The scheme depicts the rat CamK2D proximal promoter and the potential p65/NF-κB binding sites (“X” represents any of the 4 bases) with their distance relative to the transcription start site (TSS), as found with TRED software. Right, The quantity of immunoprecipitated DNA was assessed by qPCR with specific primers (gray arrows on the scheme). The net quantities of enriched DNA were corrected with their corresponding input DNA. Data are expressed as mean ± SEM (n = 3 per group), with the percentage relative to the tr-SC control group set at 100%. EE promotes p65/NF-κB binding to the proximal region of the rat CamK2D promoter. B, RT-qPCR evaluation of CamK2D transcripts in the dorsal hippocampi of trained groups of aged rats, enriched (EC) or not (SC). Values were normalized to RNA polymerase II expression levels. Data are expressed as mean ± SEM (n = 6 per group), with the percentage relative to the tr-SC control group set at 100%. EE significantly induced CamK2D expression. *p < 0.05; **p < 0.01 (Student's t test to compare the effect of housing on trained aged rats, tr-EC vs tr-SC). C, Proposed model, Animals raised in standard conditions during their whole life present decreased plasticity with aging. EE induces acetylation-related events (in part through increased PCAF expression) and promotes histone (H3) and non-histone (p65/NF-κB) acetylation that leads to increased NF-κB-dependent gene transcription of specific plasticity and memory related genes, such as Bdnf and CamK2D. Ac, Acetylation.
Discussion
As aging may have detrimental effects on memory function, much effort is made to develop mild therapeutic strategies such as behavioral therapies for preventing/delaying cognitive symptoms. Enrichment of physical and social environments belongs to such strategies. Herein, we present evidence that a belated 6 month EE exposure of rats exerts beneficial effects on plasticity and memory compared to their counterparts raised in standard conditions. These effects are in part transduced through specific cellular signaling regulating chromatin acetylation and NF-κB-dependent transcription and leading to the expression of genes important for memory and plasticity, such as Bdnf and CamK2D.
In our study, EE was found to induce global HAT activity, with a significant increase of Pcaf expression and of bulk histone H3 acetylation, events likely to lead to increased chromatin H3 acetylation at the Bdnf promoter I and to the production of pro-BDNF in the dorsal hippocampus of aged rats. Several studies have reported increased plasticity mechanisms in the hippocampus of rodents exposed to EE, including upregulation of Bdnf expression (van Praag et al., 2000; Novkovic et al., 2015), as well as in mouse and rat models of aging (O'Callaghan et al., 2009; Grinan-Ferre et al., 2015). Only a few previous studies addressed the question of exon-specific expression, and it seems that short-term enrichment can activate Bdnf expression through multiple promoters in young adult animals (Zajac et al., 2010; Branchi et al., 2011; Jha et al., 2011; Kuzumaki et al., 2011). We found that Bdnf Exon-I transcripts were highly upregulated in EE-exposed aged rats. The Bdnf promoter I was found more responsive to histone acetylation changes compared to Bdnf promoter IV in HDAC inhibitor-treated neuronal primary cultures (Hara et al., 2009; Tian et al., 2009). In addition, this particular transcript increases after a single new context exposure (Lubin et al., 2008). As a short EE exposure capable of inducing recovery of hippocampal-dependent memory was associated with increased global histone H3/H4 acetylation levels in the dorsal hippocampus of young adult mice (Fischer et al., 2007), our data also provide evidence that EE is able to increase H3 acetylation in aged rats. Furthermore, sustained acetylated-H3 occupancy at Bdnf promoter I has been shown to lead to a persistent increase of Bdnf Exon-I transcripts over time (Hara et al., 2009). Thus, our data show that EE can still favor a dynamic epigenetic regulation of Bdnf through increased chromatin acetylation at promoter I in the aged rats, despite that mature old rats are known to benefit less than their young counterparts from the social and physical stimulation provided by EE (Mora-Gallegos et al., 2015). Of note, PCAF can acetylate histone H3K9 (Nagy et al., 2010) and is implicated in learning and memory in young and old mice (Maurice et al., 2008). Thus, PCAF could be a molecular link by which EE can modify chromatin structure. As synaptic plasticity and memory deficits that occur during aging are associated with altered histone acetylation mechanisms (Peleg et al., 2010; Benito et al. 2015), our data emphasize that a 6 month period of EE in aged rats could improve hippocampal plasticity, in part through Pcaf- and Bdnf promoter I–mediated regulations.
Our data also point to NF-κB as an important player in EE-induced plasticity-related transcription that is still adjustable in the aged rats. Indeed, we evidenced an increase in p65/NF-κB occupancy at plasticity-related gene promoters, e.g., Bdnf and camK2D. The role of the transcription factor p65/NF-κB in memory is now well documented (Mattson and Meffert, 2006; Alberini, 2009; Gutierrez and Davies, 2011). For instance, it has been associated with the control of excitatory synapse and dendritic spine formation and morphology in hippocampal neurons, a process that requires, in part, NF-κB-dependent transcription of Psd-95, but also the local presence of p65 in the dendritic spines (Boersma et al., 2011). Interestingly, a previous study demonstrated that the target gene Camk2D that bears canonical NF-κB sites displayed increased p65 occupancy in this region during object recognition memory consolidation (Federman et al., 2014). Importantly, NF-κB regulation of memory was associated with increased acetylation at these sites, which occurred in a NF-κB-dependent manner (Federman et al., 2014). Our results also demonstrate an increased p65/NF-κB binding to the canonical NF-κB sites present within the rat promoter after spatial learning, and it is associated with an enhancement of CamK2D transcripts (Fig. 5A,B). However if the acetylation status of these sites was not determined in our study, the Bdnf promoter I presented an additive H3ac enrichment on the NF-κB site after training and EE. Interestingly, such additive effect of housing and training was observed in aged rats for several parameters (Figs. 2D, Pcaf; C, HAT activity; 3A, Bdnf Exon-1), suggesting that both housing and training induced the same outcome may be by different means. Also, the effects of 6 months of EE were most often observed after training as well (e.g., Bdnf Exon-1 induction). It is noteworthy that “housing” reflects a chronic stimulation, whereas “training” reflects an acute one. Thus, it is likely that responses that might appear similar at the molecular level in the first place would actually differ in strength as well as in duration. Nevertheless, regarding bdnf promoter I, the additive effect of housing and training was only observed on the NF-κB site. Accordingly, NF-κB binding was found increased at the Bdnf promoter I (Fig. 4A). This suggests that EE ultimately impacts chromatin acetylation at different sites, and particularly at NF-κB-dependent sites in the hippocampus, to further modulate the response during experience (here, Bdnf expression after spatial training). This more “plastic” genome induced by EE, as also attested by the increased number of doublecortin-positive neurons and synapse densities in the hippocampus (Fig. 1B,C), may be relevant for differences in the strength and duration of gene (Bdnf) expression. In addition, increased acetylation of the p65/NF-κB protein might reinforce this mechanism (Fig. 4B). Indeed, p65 transcriptional activity is regulated by acetylation of lysine 310 and by several HATs, including CBP, P300, and PCAF (Sheppard et al., 1999; Chen et al., 2001, 2002; Schmitz et al., 2004). K310ac-p65 has been implicated in hippocampus-dependent memory formation (Meffert et al., 2003; Yeh et al., 2004). The fact that PCAF is highly dynamic in response to training and EE in aged rats could be associated with increased K310ac-p65. Interestingly, a previous study described a loss of p65/NF-κB occupancy at active genes downregulated in Kat2a/GCN5 cKO mice, in which memory functions are severely impaired (Stilling et al., 2014). Kat2a/GCN5 is an acetyltransferase from the same family as PCAF, also called Kat2b (Allis et al., 2007). Stilling et al. (2014) point to a concomitant action of NF-κB and Kat2a/GCN5 at the promoter region of target genes, which activates gene expression via acetylation of the p65 subunit and nearby histone tails (Stilling et al., 2014). Such a regulation between PCAF and p65/NF-κB could be at play in aged rats that were enriched. Of note, another study demonstrated that cbp+/− mice displayed impaired neuroadaptive transcriptional response to EE in the hippocampus and pointed to NF-κB binding site motifs (among two others) present on EE-inducible promoters in the wild type, but not in the cbp+/− phenotype (Lopez-Atalaya et al., 2011). Thus, HAT activation and NF-κB regulation stand as possible adaptive responses to EE.
EE could also trigger chromatin acetylation through HDAC inhibition. BDNF was shown to increase S-nitrosylation of HDAC2, thereby inhibiting its deacetylase activity (Nott et al., 2008). Interestingly, a genomewide study showed that in vivo HDAC inhibition with molecules such as Trichostatin A preferentially impacted NF-κB-dependent regulations in the hippocampus of treated mice (Lopez-Atalaya et al., 2013). Benito et al. (2015) demonstrated that oral administration of SAHA, another HDAC inhibitor, could ameliorate age-associated memory impairment in spatial reference memory by partly reinstating physiological exon usage through H4K12ac levels in neuronal cells. However, Sewal et al. (2015) showed that acute HDAC inhibition administration in animals having water maze experience could improve memory and extend the transcription profile in the hippocampus of young but not old rats. Thus, it is possible that the beneficial effects provided by EE in the aged rat shown in our study not only occur through NF-κB- and acetylation-related events, but also through other EE-induced pathways activated concomitantly (e.g., Phosphorylation). In addition, it will be important to discriminate the participation of glial (inflammation) versus neuronal cells (plasticity) in these processes.
Altogether, our data support the involvement of the NF-κB complex and acetylation regulations in driving hippocampal plasticity in response to enriched housing conditions in aged rats, in part through PCAF activity and the regulation of memory-dependent genes responsive to NF-κB (e.g., Bdnf, CamK2D; Fig. 5D). Future work investigating the relationship between EE-induced plasticity genes expression (as Bdnf) and particular transcriptional regulators such as NF-κB may provide insights into the development of new therapeutic strategies to prevent/damper age-related cognitive decline. Our results further support cognitive/behavioral therapeutic strategies during aging, as we show they can still be effective through specific pathways even when applied late in lifetime.
Footnotes
This work was supported by the CNRS, Institut National de la Santé et de la Recherche Médicale, University of Strasbourg, and several contracts and associations: the Ligue Européenne Contre la Maladie d'Alzheimer [LECMA Project 10702 (A.L.B.); Project 10702 (O.B., R.N.)], Agence Nationale de la Recherche [ANR-12-MALZ-0002 (A.L.B., A.S.)], Alsace Alzheimer 67 (A.L.B., Dr. F. Blanc (Hôpitaux Universitaires Strasbour, France), J.-C.C.), and the Fondation Unistra-don Pierre Fabre (A.S.). R.N. is the recipient of a doctoral fellowship from the French government. We thank O. Bildstein, O. Egesi, and G. Edomwonyi for their assistance in animal care and B. Cosquer, K. Herbeaux, A. Bombardier, and M. J. Ruivo for their excellent technical assistance. We thank Prof. B. Frenguelli (University of Warwick, Coventry, UK) for his useful comments on an earlier version of this manuscript, and Dr. Karine Merienne (University of Strasbourg, Strasbourg, France) for her critical reading of this manuscript.
The authors declare no competing financial interests.
References
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Benito E, Urbanke H, Ramachandran B, Barth J, Halder R, Awasthi A, Jain G, Capece V, Burkhardt S, Navarro-Sala M, Nagarajan S, Schütz AL, Johnsen SA, Bonn S, Lührmann R, Dean C, Fischer A. HDAC inhibitor-dependent transcriptome and memory reinstatement in cognitive decline models. J Clin Invest. 2015;125:3572–3584. doi: 10.1172/JCI79942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J Neurosci. 2011;31:5414–5425. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousiges O, Vasconcelos AP, Neidl R, Cosquer B, Herbeaux K, Panteleeva I, Loeffler JP, Cassel JC, Boutillier AL. Spatial memory consolidation is associated with induction of several lysine-acetyltransferase (histone acetyltransferase) expression levels and H2B/H4 acetylation-dependent transcriptional events in the rat hippocampus. Neuropsychopharmacology. 2010;35:2521–2537. doi: 10.1038/npp.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousiges O, Neidl R, Majchrzak M, Muller MA, Barbelivien A, Pereira de Vasconcelos A, Schneider A, Loeffler JP, Cassel JC, Boutillier AL. Detection of histone acetylation levels in the dorsal hippocampus reveals early tagging on specific residues of H2B and H4 histones in response to learning. PLoS One. 2013;8:e57816. doi: 10.1371/journal.pone.0057816. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Branchi I, Karpova NN, D'Andrea I, Castrén E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Burger C, Lopez MC, Baker HV, Mandel RJ, Muzyczka N. Genome-wide analysis of aging and learning-related genes in the hippocampal dentate gyrus. Neurobiol Learn Memory. 2008;89:379–396. doi: 10.1016/j.nlm.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JF, Fletcher BR, Kelley-Bell B, Kim DH, Gallagher M, Rapp PR. Age-related memory impairment is associated with disrupted multivariate epigenetic coordination in the hippocampus. PLoS One. 2012;7:e33249. doi: 10.1371/journal.pone.0033249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Lf, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Federman N, de la Fuente V, Zalcman G, Corbi N, Onori A, Passananti C, Romano A. Nuclear factor kappaB-dependent histone acetylation is specifically involved in persistent forms of memory. J Neurosci. 2013;33:7603–7614. doi: 10.1523/JNEUROSCI.4181-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federman N, Zalcman G, de la Fuente V, Fustinana MS, Romano A. Epigenetic mechanisms and memory strength: a comparative study. J of physiology, Paris. 2014;108:278–285. doi: 10.1016/j.jphysparis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447:178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–626. doi: 10.1016/S0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Cosquer B, Penazzi L, Mathis C, Kelche C, Majchrzak M, Barbelivien A. Exposure to an enriched environment up to middle age allows preservation of spatial memory capabilities in old age. Behav Brain Res. 2016;299:1–5. doi: 10.1016/j.bbr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. Histone acetylation: molecular mnemonics on the chromatin. Nature reviews Neuroscience. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Griñan-Ferré C, Pérez-Cáceres D, Gutiérrez-Zetina SM, Camins A, Palomera-Avalos V, Ortuño-Sahagún D, Rodrigo MT, Pallàs M. Environmental enrichment improves behavior, cognition, and brain functional markers in young senescence-accelerated prone mice (SAMP8) Mol Neurobiol. 2015;27:1–16. doi: 10.1007/s12035-015-9210-6. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, Davies AM. Regulation of neural process growth, elaboration and structural plasticity by NF-kappaB. Trends Neurosci. 2011;34:316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara D, Miyashita T, Fukuchi M, Suzuki H, Azuma Y, Tabuchi A, Tsuda M. Persistent BDNF exon I-IX mRNA expression following the withdrawal of neuronal activity in neurons. Biochem Biophys Res Commun. 2009;390:648–653. doi: 10.1016/j.bbrc.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Harati H, Majchrzak M, Cosquer B, Galani R, Kelche C, Cassel JC, Barbelivien A. Attention and memory in aged rats: Impact of lifelong environmental enrichment. Neurobiol Aging. 2011;32:718–736. doi: 10.1016/j.neurobiolaging.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Harati H, Barbelivien A, Herbeaux K, Muller MA, Engeln M, Kelche C, Cassel JC, Majchrzak M. Lifelong environmental enrichment in rats: impact on emotional behavior, spatial memory vividness, and cholinergic neurons over the lifespan. Age (Dordr) 2012;35:1027–1043. doi: 10.1007/s11357-012-9424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoberg JE, Popko AE, Ramsey CS, Mayo MW. IkappaB kinase alpha-mediated derepression of SMRT potentiates acetylation of RelA/p65 by p300. Mol Cell Biol. 2006;26:457–471. doi: 10.1128/MCB.26.2.457-471.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl Psychiatry. 2011;1:e40. doi: 10.1038/tp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. Long-term potentiation at CA3-CA1 hippocampal synapses with special emphasis on aging, disease, and stress. Front Aging Neurosci. 2011;20:3–7. doi: 10.3389/fnagi.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Tamura R, Hareyama N, Imai S, Narita M, Torigoe K, Niikura K, Takeshima H, Ando T, Igarashi K, Kanno J, Ushijima T, Suzuki T. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus. 2011;21:127–132. doi: 10.1002/hipo.20775. [DOI] [PubMed] [Google Scholar]
- Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem. 1997;68:203–220. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- Lopez J, Herbeaux K, Cosquer B, Engeln M, Muller C, Lazarus C, Kelche C, Bontempi B, Cassel JC, de Vasconcelos AP. Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus. 2012;22:827–841. doi: 10.1002/hipo.20943. [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Barco A. Can changes in histone acetylation contribute to memory formation? Trends Genet. 2014;30:529–539. doi: 10.1016/j.tig.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Ciccarelli A, Viosca J, Valor LM, Jimenez-Minchan M, Canals S, Giustetto M, Barco A. CBP is required for environmental enrichment-induced neurogenesis and cognitive enhancement. EMBO J. 2011;30:4287–4298. doi: 10.1038/emboj.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Atalaya JP, Ito S, Valor LM, Benito E, Barco A. Genomic targets, and histone acetylation and gene expression profiling of neural HDAC inhibition. Nucleic Acids Res. 2013;41:8072–8084. doi: 10.1093/nar/gkt590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Maurice T, Duclot F, Meunier J, Naert G, Givalois L, Meffre J, Célérier A, Jacquet C, Copois V, Mechti N, Ozato K, Gongora C. Altered memory capacities and response to stress in p300/CBP-associated factor (PCAF) histone acetylase knockout mice. Neuropsychopharmacology. 2008;33:1584–1602. doi: 10.1038/sj.npp.1301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Mora-Gallegos A, Rojas-Carvajal M, Salas S, Saborio-Arce A, Fornaguera-Trias J, Brenes JC. Age-dependent effects of environmental enrichment on spatial memory and neurochemistry. Neurobiol Learn Memory. 2015;118:96–104. doi: 10.1016/j.nlm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Riss A, Fujiyama S, Krebs A, Orpinell M, Jansen P, Cohen A, Stunnenberg HG, Kato S, Tora L. The metazoan ATAC and SAGA coactivator HAT complexes regulate different sets of inducible target genes. Cell Mol Life Sci. 2010;67:611–628. doi: 10.1007/s00018-009-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- Novkovic T, Mittmann T, Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus. 2015;25:1–15. doi: 10.1002/hipo.22342. [DOI] [PubMed] [Google Scholar]
- O'Callaghan RM, Griffin EW, Kelly AM. Long-term treadmill exposure protects against age-related neurodegenerative change in the rat hippocampus. Hippocampus. 2009;19:1019–1029. doi: 10.1002/hipo.20591. [DOI] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto LL, Wimmer ME, Poplawski SG, Tudor JC, Kenworthy CA, Liu S, Mizuno K, Garcia BA, Zhang NR, Giese K, Abel T. Memory acquisition and retrieval impact different epigenetic processes that regulate gene expression. BMC Genom. 2015;16(Suppl 5):S5. doi: 10.1186/1471-2164-16-S5-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Mattioli I, Buss H, Kracht M. NF-kappaB: a multifaceted transcription factor regulated at several levels. Chembiochem. 2004;5:1348–1358. doi: 10.1002/cbic.200400144. [DOI] [PubMed] [Google Scholar]
- Sewal AS, Patzke H, Perez EJ, Park P, Lehrmann E, Zhang Y, Becker KG, Fletcher BR, Long JM, Rapp PR. Experience modulates the effects of histone deacetylase inhibitors on gene and protein expression in the hippocampus: impaired plasticity in aging. J Neurosci. 2015;35:11729–11742. doi: 10.1523/JNEUROSCI.4339-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/MCB.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats–behavioural and neurochemical aspects. Behav Brain Res. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Rönicke R, Benito E, Urbanke H, Capece V, Burkhardt S, Bahari-Javan S, Barth J, Sananbenesi F, Schütz AL, Dyczkowski J, Martinez-Hernandez A, Kerimoglu C, Dent SY, Bonn S, Reymann KG, Fischer A. K-Lysine acetyltransferase 2a regulates a hippocampal gene expression network linked to memory formation. EMBO J. 2014;33:1912–1927. doi: 10.15252/embj.201487870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan FA, Day JJ. Epigenetic mechanisms in memory and synaptic function. Epigenomics. 2011;3:157–181. doi: 10.2217/epi.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, Hu XZ, Wu X, Jiang H, Pan H, Marini AM, Lipsky RH. Dynamic chromatin remodeling events in hippocampal neurons are associated with NMDA receptor-mediated activation of Bdnf gene promoter 1. J Neurochem. 2009;109:1375–1388. doi: 10.1111/j.1471-4159.2009.06058.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, Craig JM, Saffery R, Hannan AJ. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2010;20:621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]