Abstract
The coordinated mechanisms balancing promotion and suppression of dendritic morphogenesis are crucial for the development of the cerebral cortex. Although previous studies have revealed important transcription factors that promote dendritic morphogenesis during development, those that suppress dendritic morphogenesis are still largely unknown. Here we found that the expression levels of the transcription factor Sox11 decreased dramatically during dendritic morphogenesis. Our loss- and gain-of-function studies using postnatal electroporation and in utero electroporation indicate that Sox11 is necessary and sufficient for inhibiting dendritic morphogenesis of excitatory neurons in the mouse cerebral cortex during development. Interestingly, we found that precocious suppression of Sox11 expression caused precocious branching of neurites and a neuronal migration defect. We also found that the end of radial migration induced the reduction of Sox11 expression. These findings indicate that suppression of dendritic morphogenesis by Sox11 during radial migration is crucial for the formation of the cerebral cortex.
SIGNIFICANCE STATEMENT Because dendritic morphology has profound impacts on neuronal information processing, the mechanisms underlying dendritic morphogenesis during development are of great interest. Our loss- and gain-of-function studies indicate that Sox11 is necessary and sufficient for inhibiting dendritic morphogenesis of excitatory neurons in the mouse cerebral cortex during development. Interestingly, we found that precocious suppression of Sox11 expression caused a neuronal migration defect. These findings indicate that suppression of dendritic morphogenesis by Sox11 during radial migration is crucial for the formation of the cerebral cortex.
Keywords: cerebral cortex, dendritic development, radial migration, Sox11, transcription factor
Introduction
Dendritic morphology has profound impacts on neuronal information processing. Appropriate dendritic morphology is essential for receiving inputs from other neurons, and branching patterns of dendrites affect the efficiency in transmitting information from the synapse to the soma (Häusser et al., 2000). Because abnormal development of dendrites has been associated with several neurological disorders (Chahrour and Zoghbi, 2007; Jan and Jan, 2010), it is important to understand the molecular mechanisms regulating dendritic morphogenesis during development.
Previous studies have shown that transcription factors play important roles in dendritic morphogenesis during development (Scott and Luo, 2001; Gao, 2007; Jan and Jan, 2010; Puram and Bonni, 2013). For example, the transcription factor CREST is necessary for promoting activity-dependent dendritic morphogenesis in the developing brain (Aizawa et al., 2004). CREST was reported to contribute to the dendritic complexity of neurons in the cerebral cortex, including dendritic extension and branching in pyramidal neurons (Aizawa et al., 2004). Another transcription factor involved in dendritic morphogenesis is Zfp312, which is expressed in deep layers of the cerebral cortex and is necessary for dendrite elongation and branching (Chen et al., 2005). In contrast to transcription factors promoting dendritic morphogenesis, those suppressing dendritic morphogenesis are still largely unknown (Santiago and Bashaw, 2014). Because it has been demonstrated that the balance between transcription factors promoting and suppressing developmental processes is crucial for appropriate brain development in a various regions (Kageyama et al., 2005), we decided to search for transcription factors suppressing dendritic morphogenesis.
Recent pioneering studies proposed that inhibition of dendritic morphogenesis is crucial for the formation of the cerebral cortex during development (Gupta et al., 2003; Ohshima et al., 2007; Guerrier et al., 2009; Bando et al., 2016). p35 and Cdk5 are required for maintaining the simple morphology of migrating neurons, and the loss of p35 and Cdk5 results in abnormally branched leading processes and severe migration defects (Gupta et al., 2003; Ohshima et al., 2007). In our search for transcription factors suppressing dendritic morphogenesis, Sry-related high-mobility group (HMG) box-containing (Sox) transcription factors seemed like good candidates because they play crucial roles in a variety of developmental processes in the brain (Prior and Walter, 1996; Wegner and Stolt, 2005; Kiefer, 2007; Chew and Gallo, 2009; Bergsland et al., 2011; She and Yang, 2015). Here, we focused on Sox11 because its expression level was high before dendritic morphogenesis at embryonic day (E)18 and rapidly decreased by postnatal day (P)14 when dendritic morphogenesis was initiated (Allen Brain Atlas, http://www.brain-map.org/). We found that Sox11 was necessary and sufficient for inhibiting dendritic morphogenesis of excitatory neurons in the mouse cerebral cortex during development. Interestingly, we also found that precocious suppression of Sox11 expression caused a neuronal migration defect. These findings suggest that the timing of dendritic morphogenesis is genetically fine-tuned at appropriate time points by positive and negative transcriptional factors.
Materials and Methods
Animals.
All procedures were performed in accordance with protocols approved by the University of Tokyo Animal Care Committee and the Kanazawa University Animal Care Committee. Either male or female ICR mice (SLC) and Sox11 knock-out mice (kind gifts from Drs Elisabeth Sock and Michael Wegner, Universität Erlangen-Nürnberg; Sock et al., 2004) were reared on a normal 12 h light/dark schedule. The day of conception and that of birth were counted as E0 and P0, respectively. Experiments were repeated at least three times and gave consistent results.
Plasmids.
The pCAG plasmid vector, pCAG-EGFP, pCAG-mCherry, pThy1S-EGFP, pCAG-FloxedSTOP-EGFP, and pCAG-DN-Ncad were described previously (Sehara et al., 2010; Ako et al., 2011; Wakimoto et al., 2015). pCAG-NLS-Cre was a kind gift from Dr Mikio Hoshino (National Institute of Neuroscience). pCAG-Sox11 was constructed by inserting mouse Sox11 (NM_009234.6) into the pCAG plasmid vector.
The Sox11-shRNA-expression vector (shSox11) was constructed using a pSUPER.basic vector (Oligoengine). The sequences used for shRNA experiments were designed using the web-based software siDIRECT (http://sidirect2.rnai.jp/) and are as follows: 5′-GAGAAGATCCCGTTCATCA-3′ against Sox11 (shSox11 #1), 5′-GGAAATAAGGCAAATAAGT-3′ against Sox11 (shSox11 #2), and 5′-CAACAAGATGAAGAGCACC-3′ as a nontargeting negative control.
Plasmids were purified using an Endofree plasmid Maxi kit (Qiagen). Before electroporation experiments, plasmid DNA was diluted to <3 mg/ml in PBS, and Fast Green solution was added at a final concentration of 0.1% to monitor the injection.
In utero electroporation.
In utero electroporation was performed as described previously with slight modifications (Fukuchi-Shimogori and Grove, 2001; Saito and Nakatsuji, 2001; Tabata and Nakajima, 2001; Sehara et al., 2010; Ako et al., 2011; Wakimoto et al., 2015). Pregnant ICR mice (E15.5) were deeply anesthetized, and the uterine horns were exposed. Approximately 1–3 μl of DNA solution was injected into the lateral ventricle of the embryo using a pulled glass micropipette. Each embryo within the uterus was placed between tweezer-type electrodes with a diameter of 5 mm (NEPA Gene, CUY650P5). Square electric pulses (45 V, 50 ms) were passed five times at 1 Hz using an electroporator (BTX). Care was taken to quickly place embryos back into the abdominal cavity to avoid excessive temperature loss. The wall and skin of the abdominal cavity were sutured.
Postnatal electroporation.
Postnatal electroporation was performed as previously described with modifications (Mizuno et al., 2010). Neonatal ICR mouse pups at P1.5 were anesthetized with hypothermia, and craniotomy was performed. The capillary tube (Drammond, 3-000-203-G/X) was pulled using a two-stage vertical puller (Narishige, PC-10) and was inserted into the primary somatosensory cortex at 700 μm in depth from the surface of the cranial bone. After DNA solution was injected, square electric pulses (80 V, 50 ms) were passed five times at 1 Hz using an electroporator (BTX). The scalp was bonded with Vetbond (3M), and the pups were warmed for recovery from anesthesia.
Immunohistochemistry.
Immunohistochemistry was performed as described previously with modifications (Kawasaki et al., 2000; Toda et al., 2013). Mice were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in PBS. To make coronal sections, the brains were dissected, postfixed by overnight immersion in the same fixative, cryoprotected by 2 d immersion in 30% sucrose in PBS, and embedded in OCT compound. Sections of 50 μm thickness were made using a cryostat, permeabilized with 0.1–0.5% Triton X-100 in PBS and incubated overnight with primary antibodies. After being incubated with AlexaFluor 488-, Cy3-, and/or AlexaFluor 647-conjugated secondary antibodies and 1 μg/ml Hoechst 33342, the sections were washed and mounted. The primary antibodies used here include goat anti-Sox11 (Santa Cruz Biotechnology), rabbit anti-GFP (Medical & Biological Laboratories), goat anti-Brn2 (Santa Cruz Biotechnology), rat anti-Ctip2 (Abcam), and rabbit anti-FOXP2 (Atlas) antibodies.
Imaging and morphometric measures.
Epifluorescence microscopy was performed with an AxioImager A1 microscope (Carl Zeiss), a BIOREVO BZ-9000 (Keyence), and a fluorescence stereomicroscope (MZ16 F, Leica). Confocal microscopy was performed with an LSM510 microscope (Carl Zeiss), an LSM700 microscope (Carl Zeiss), and an FV10i FLUOVIEW microscope (Olympus). For morphometric measures, the Sholl analysis and quantifications of the total length of the dendrites of each neuron, the number of the dendritic branches, the number of the dendritic ends, and the number of primary dendrites per cell were performed using either the Simple Neurite Tracer plug-in of ImageJ software (Longair et al., 2011) or Neurolucida360 (MBF Bioscience). Statistical comparisons were performed using Student's t test, except for the Sholl analysis, which was assessed by multivariate ANOVA.
Quantification of Sox11 expression levels in EGFP-positive nuclei.
Confocal microscopic images were analyzed using ImageJ software. To select the areas of EGFP-positive transfected cell nuclei in the images, Hoechst and EGFP double-positive areas were extracted using the Image Calculator tool of ImageJ software. EGFP-positive cell nuclei were registered to ROI manager using the Analyze particle tool. Sox11 signal intensities in the area of EGFP-positive nuclei were measured. After background signals were subtracted from the Sox11 signal intensities, statistical comparisons were performed using either Student's or Welch's t test.
Quantification of the position of electroporated cells in the cerebral cortex.
Epifluorescence images of coronal sections of the electroporated cortex were taken and analyzed using ImageJ software. The numbers of mCherry-positive electroporated cells in the cortical plate and those in the white matter of electroporated regions were manually and blindly counted by using the Cell Count plug-in. Statistical comparisons were performed using Welch's t test.
Results
The expression levels of Sox11 in the mouse cerebral cortex during development
We focused on Sox11 as a candidate for a transcription factor suppressing dendritic morphogenesis. To test the expression levels of Sox11 using immunohistochemistry, we first tested the specificity of the anti-Sox11 antibody used in this study using coronal sections of the mouse cerebral cortex. Consistent with previous reports (Kuhlbrodt et al., 1998; Dy et al., 2008), immunohistochemistry showed that this antibody widely recognized many cells in the cerebral cortex at E18.5 (Fig. 1A). Importantly, Sox11 immunoreactivity was totally lost in the cerebral cortex of Sox11 knock-out mice (Fig. 1A). Consistent with the fact that Sox11 is a transcription factor, higher-magnification of Hoechst-stained cells revealed that Sox11 immunoreactivity was found in the nucleus (Fig. 1B). Sox11 immunoreactivity was also lost in higher-magnification images of Sox11 knock-out mice (Fig. 1B). These results clearly indicate that the anti-Sox11 antibody used here specifically recognizes Sox11 protein.
Figure 1.
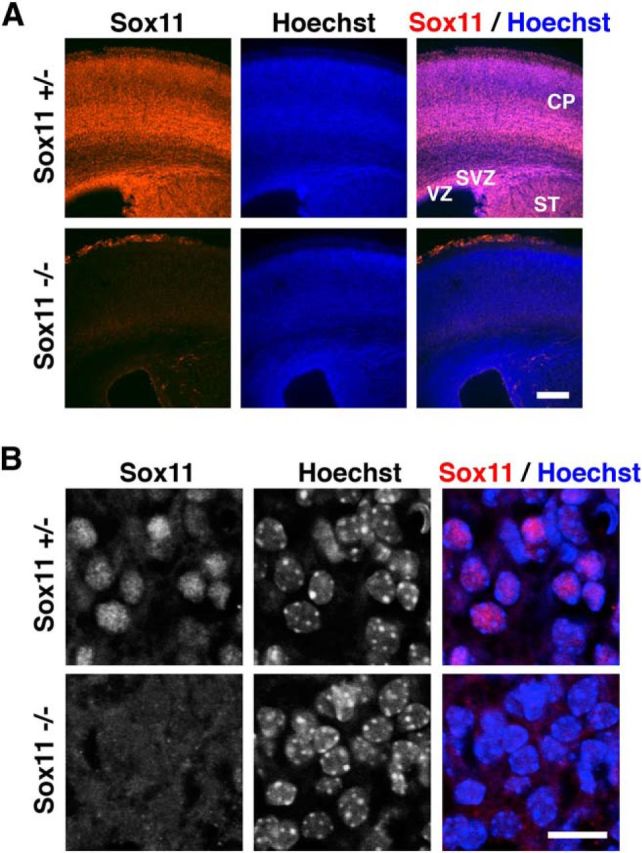
Characterization of anti-Sox11 antibody. A, Coronal sections of the mouse cerebral cortex were prepared from Sox11-deficient (−/−, bottom) and heterozygous (+/−, top) embryos at E18.5. The sections were stained with anti-Sox11 antibody (red) and Hoechst 33342 (blue). CP, Cortical plate; SVZ, subventricular zone; VZ, ventricular zone; ST, striatum. Scale bar, 200 μm. B, High-magnification confocal microscopic images of layer 6. Note that Sox11 immunoreactivity was lost in the sections derived from Sox11-deficient mice. Scale bar, 10 μm.
We next examined Sox11 expression in the mouse cerebral cortex during development. We found that the expression levels of Sox11 decreased dramatically soon after birth (Fig. 2A,B). Although cortical neurons expressed high levels of Sox11 at P0, Sox11 expression rapidly decreased by P3 (Fig. 2A,B). At P3 and P5, Sox11 expression was still detected weakly in layer 2/3, where newly generated young neurons were located, but the expression of Sox11 was lost in all cortical layers at P11 (Fig. 2A,B). These results suggest that Sox11 expression in the cortical neurons decreases soon after cortical neurons arrive at the cortical plate.
Figure 2.
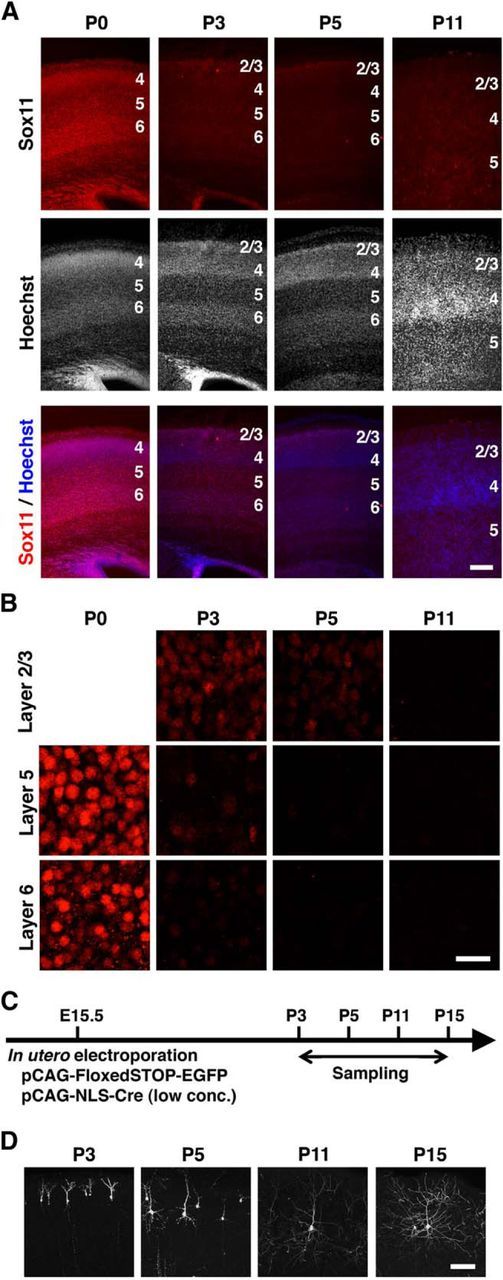
Expression levels of Sox11 and dendritic morphogenesis in the mouse cerebral cortex during development. A, Sox11 immunohistochemistry and Hoechst 33342 staining of coronal sections of the mouse cerebral cortex at P0, P3, P5, and P11. Numbers indicate the corresponding layers in the cerebral cortex. Scale bar, 200 μm. B, Magnified confocal microscopic images of Sox11 immunohistochemistry. Layer 2/3 has not been formed at P0. Scale bar, 20 μm. C, Experimental procedure of in utero electroporation to reveal the dendritic morphology of small numbers of layer 2/3 neurons. pCAG-FloxedSTOP-EGFP (1 mg/ml) and a very low concentration of pCAG-NLS-Cre (1.5 μg/ml) were cotransfected into layer 2/3 neurons using in utero electroporation at E15.5, and coronal sections were prepared at the indicated time points. EGFP signals were enhanced by immunostaining. D, Confocal microscopic images of EGFP-positive layer 2/3 neurons in coronal sections. Scale bar, 100 μm.
We hypothesized that Sox11 regulates dendritic morphogenesis of cortical neurons because Sox11 reduction and dendritic morphogenesis proceed simultaneously soon after birth of mouse pups (Barnes and Polleux, 2009). To visualize the morphology of single layer 2/3 neurons in the mouse cerebral cortex, we expressed EGFP in a small number of cortical neurons by introducing pCAG-FloxedSTOP-EGFP plus a very low concentration of pCAG-NLS-Cre (1.5 μg/ml) using in utero electroporation at E15.5 (Fig. 2C; Okamoto et al., 2013; Kitazawa et al., 2014). Our observation of sparsely labeled neurons confirmed that the dendritic complexity of layer 2/3 neurons increased during the 2 weeks after birth (Fig. 2D). Whereas the dendrites of layer 2/3 neurons were relatively simple at P3, apical and basal dendrites of labeled neurons became longer, and the number of primary basal dendrites was increased at P5. Apical and basal dendrites became much longer and more complex by P15. Because dendritic morphogenesis of layer 2/3 neurons proceeds soon after the reduction of Sox11 expression, we hypothesized that Sox11 suppresses dendritic morphogenesis, and that the reduction of Sox11 expression leads to dendritic morphogenesis.
Precocious Sox11 knockdown is sufficient to promote dendritic morphogenesis of layer 2/3 neurons
To examine the role of Sox11 in dendritic morphogenesis, we tested whether precocious reduction of Sox11 expression promotes dendritic morphogenesis in layer 2/3 neurons. Because Sox11 knock-out mice die soon after birth, it was impossible to use them to examine dendritic morphogenesis. Instead, to suppress Sox11 expression, we designed a Sox11-shRNA-expression vector (shSox11), and confirmed that our shSox11 construct effectively reduced Sox11 expression (Fig. 3). To introduce shSox11 and EGFP in layer 2/3 neurons, we performed postnatal electroporation at P1.5 (Fig. 4A,B; Mizuno et al., 2010). The results showed that Sox11 knockdown resulted in complex dendritic morphology at P5 (Fig. 4C). Quantitative analyses revealed that Sox11-downregulated neurons had longer dendrites (n = 3 animals per each experimental group; 9–10 transfected cells per each experimental group were analyzed; Student's t test, p = 0.035; Fig. 4D), larger numbers of dendritic ends (p = 0.041; Fig. 4F) and larger numbers of primary dendrites per cell (p = 0.008; Fig. 4G) than those of control neurons. Our result also showed that Sox11-downregulated neurons tended to have larger numbers of dendritic branches (p = 0.075; Fig. 4E). Sholl analysis revealed that Sox11-downregulated neurons had more complex morphology than control neurons (multivariate ANOVA, p = 0.012; Fig. 4H). These results suggest that the precocious reduction of Sox11 expression is sufficient for promoting dendritic morphogenesis of layer 2/3 neurons.
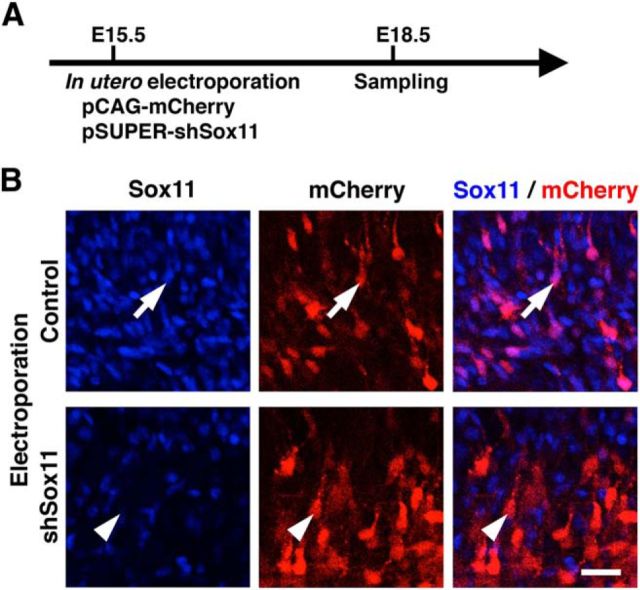
Figure 3.
Characterization of the Sox11-shRNA-expression vector. A, Experimental procedure. pCAG-mCherry (0.3 mg/ml) and pSUPER-shSox11 (1.65 mg/ml) were cotransfected into layer 2/3 neurons using in utero electroporation at E15.5, and coronal sections were prepared at E18.5 and stained with anti-Sox11 antibody and Hoechst 33342. B, Confocal microscopic images of cells transfected with either control (arrow) or shSox11 (arrowhead) vectors. mCherry-positive transfected neurons in the intermediate zone are shown. Scale bar, 25 μm.
Figure 4.
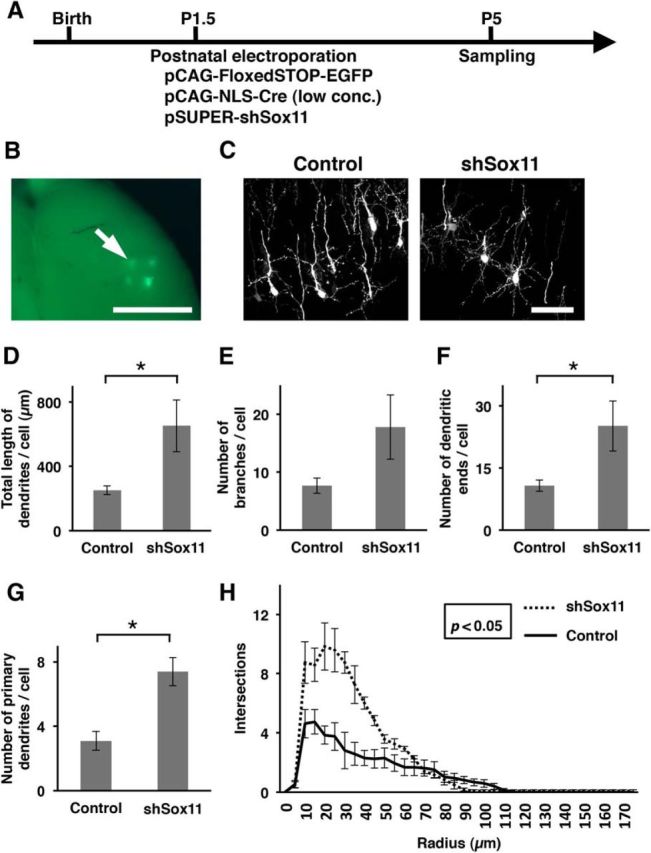
Precocious Sox11 knockdown promotes dendritic morphogenesis in layer 2/3 neurons. A, Experimental procedure of postnatal electroporation to investigate the dendritic morphology of Sox11-downregulated layer 2/3 neurons. pCAG-FloxedSTOP-EGFP (0.75 mg/ml), a very low concentration of pCAG-NLS-Cre (2.0 μg/ml) and pSUPER-shSox11 (1.25 mg/ml) were cotransfected into layer 2/3 neurons using postnatal electroporation at P1.5, and coronal sections were prepared at P5. EGFP signals were enhanced by immunostaining. B, A low-magnification image of the cerebral cortex in which postnatal electroporation was performed. EGFP signals (arrow) were observed at four plasmid injection sites. Scale bar, 2.0 mm. C, Confocal microscopic images of layer 2/3 neurons transfected with either control (left) or shSox11 (right) vectors. Coronal sections are shown. Scale bar, 50 μm. D–G, Quantification of the total length of dendrites per cell (D), the number of dendritic branches per cell (E), the number of dendritic ends per cell (F), and the number of primary dendrites per cell (G). Data are presented as mean ± SEM; n = 3 animals per each experimental group; 9–10 transfected cells per each experimental group were analyzed. *p < 0.05. H, Sholl analysis of the dendritic complexity of the transfected neurons. Data are presented as mean ± SEM; n = 4 animals per each experimental group; 12–14 transfected cells per each experimental group were analyzed.
The reduction of Sox11 is required for the formation of dendrites of layer 2/3 neurons
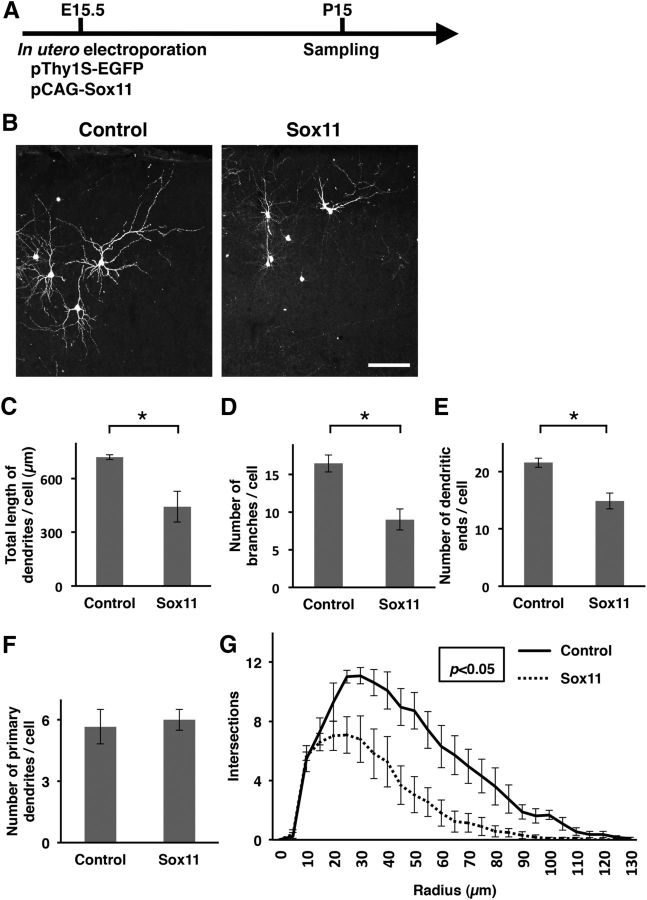
We next tested the necessity of the reduction of Sox11 expression for dendritic morphogenesis. We coexpressed Sox11 and EGFP in layer 2/3 neurons using in utero electroporation at E15.5, and brain samples were obtained at P15 (Fig. 5A). Our immunohistochemical analysis showed that ectopically expressed Sox11 was indeed detected in EGFP-positive transfected layer 2/3 neurons at P15, when endogenous Sox11 was undetectable (Fig. 5B,C). To examine the dendritic morphology of Sox11-overexpressing neurons, we sparsely labeled layer 2/3 neurons with EGFP under the control of the Thy1S promoter (Fig. 6A,B; Ako et al., 2011) and found that dendrites of Sox11-overexpressing neurons were much simpler than those of control neurons (Fig. 6B). To quantitatively analyze the dendritic morphology, we traced the basal dendrites of layer 2/3 neurons, because it was difficult to trace entire apical dendrites of layer 2/3 neurons at P15. Quantitative analyses of the basal dendrites showed that Sox11-overexpressing neurons had shorter dendrites (n = 3 animals per each experimental group; 9 transfected cells per each experimental group were analyzed; Welch's t test, p = 0.040; Fig. 6C), and smaller numbers of branches (Student's t test, p = 0.007; Fig. 6D) and dendritic ends (p = 0.007; Fig. 6E) than those of control neurons. We did not detect any differences in the number of primary dendrites per cell (p = 0.376; Fig. 6F). Consistently, Sholl analysis revealed that Sox11-overexpressing neurons had simpler morphology than control neurons (multivariate ANOVA, p = 0.014; Fig. 6G). These results suggest that the reduction of Sox11 expression is necessary for promoting dendritic morphogenesis of layer 2/3 neurons.
Figure 5.
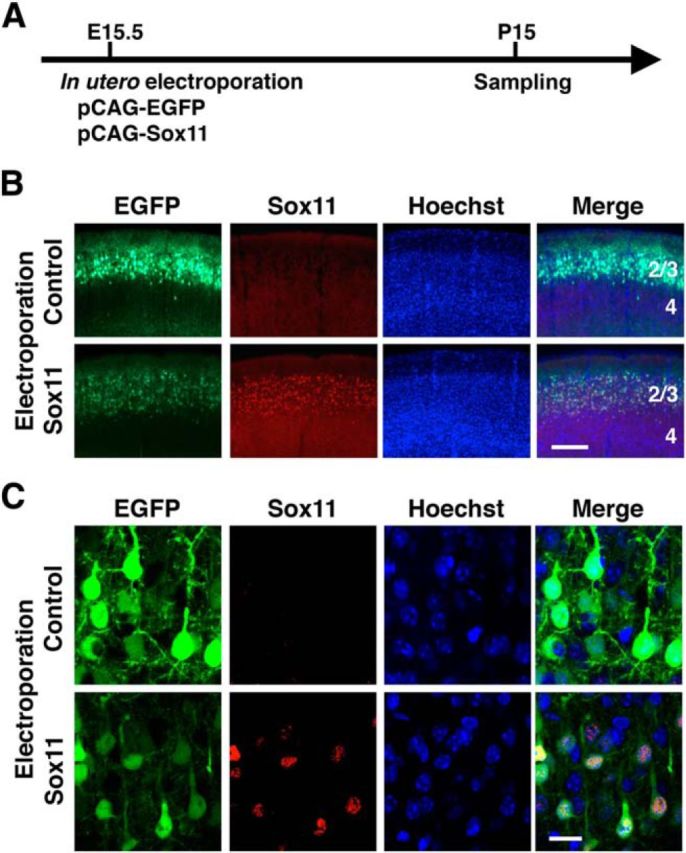
Characterization of the Sox11 expression vector. A, Experimental procedure. pCAG-EGFP (0.5 mg/ml) and pCAG-Sox11 (1.0 mg/ml) were cotransfected into layer 2/3 neurons using in utero electroporation at E15.5, and coronal sections were prepared at P15 and immunostained with anti-Sox11 antibody and Hoechst 33342. B, Sox11 immunoreactivity was detected in the cerebral cortex of the pCAG-Sox11-electroporated brain, whereas no signal was detected in that of the control brain. Numbers indicate the corresponding layers in the cerebral cortex. Scale bar, 200 μm. C, High-magnification confocal microscopic images of layer 2/3 neurons in B. Scale bar, 10 μm.
Figure 6.
The reduction of Sox11 expression is required for dendritic morphogenesis of layer 2/3 neurons. A, Experimental procedure of in utero electroporation to investigate the dendritic morphology of Sox11-overexpressing layer 2/3 neurons. pThy1S-EGFP (1.75 mg/ml) and pCAG-Sox11 (1.0 mg/ml) were cotransfected into layer 2/3 neurons using in utero electroporation at E15.5, and coronal sections were prepared at P15. EGFP signals were enhanced by immunostaining. B, Confocal microscopic images of layer 2/3 neurons transfected with either control (left) or Sox11 (right) vectors. Scale bar, 100 μm. C–F, Quantification of the total length of basal dendrites per cell (C), the number of branches of basal dendrites per cell (D), the number of dendritic ends of basal dendrites per cell (E) and the number of primary basal dendrites per cell (F). Data are presented as mean ± SEM; n = 3 animals per each experimental group; 9 transfected cells per each experimental group were analyzed. *p < 0.05. G, Sholl analysis of the dendritic complexity of the transfected neurons. Data are presented as mean ± SEM; n = 4 animals per each experimental group; 13 transfected cells per each experimental group were analyzed.
Although our results showed that Sox11 overexpression suppressed dendritic morphogenesis of layer 2/3 neurons, it remained possible that Sox11-overexpressing neurons had become neurons other than layer 2/3 neurons, and as a result, their dendritic morphology was changed by Sox11 overexpression. To exclude this possibility, we examined layer markers of the cerebral cortex, such as Brn2, Ctip2, and Foxp2 (Molyneaux et al., 2007; Fig. 7). Brn2 is expressed in layers 2/3 and 5 (Fig. 7A), and Ctip2 and Foxp2 are expressed in layers 5 and 6 (Fig. 7B,C). The expression patterns of Brn2, Ctip2, and Foxp2 were not affected by expressing Sox11 (Fig. 7A–C), suggesting that the identity of Sox11-overexpressing layer 2/3 neurons is not affected by Sox11.
Figure 7.
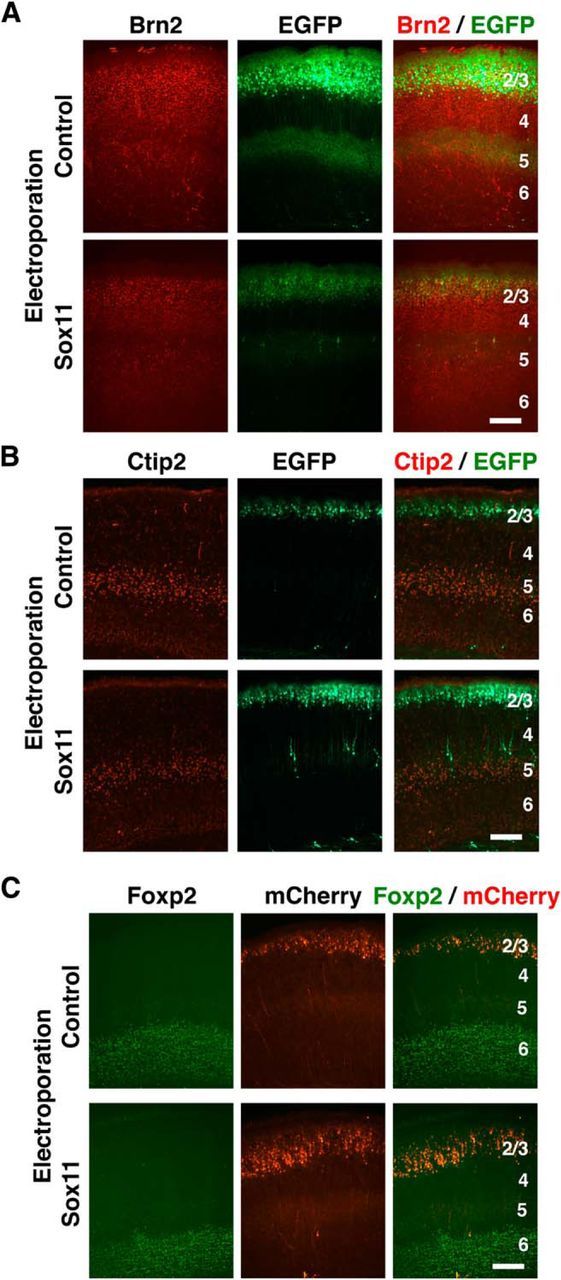
Sox11 overexpression in layer 2/3 neurons does not affect the identities of cortical neurons. Sox11 plus either EGFP or mCherry were cotransfected by using in utero electroporation at E15.5, and sections were prepared at P5 (Ctip2), P6 (Foxp2), or P15 (Brn2). The sections were stained with anti-Brn2, Ctip2, and FOXP2 antibodies. A, Brn2 immunostaining at P15. B, Ctip2 immunostaining at P5. C, Foxp2 immunostaining at P6. Numbers indicate the corresponding layers in the cerebral cortex. Scale bars, 250 μm.
Embryonic knockdown of Sox11 induces precocious neurite elongation and a migration defect
We have shown that Sox11 knockdown after neurons arrived at the cortical plate resulted in precocious dendritic morphogenesis (Fig. 4). We next examined what would happen if Sox11 expression were suppressed before neuronal radial migration. We introduced pSUPER-shSox11, pCAG-mCherry, and pCAG-FloxedSTOP-EGFP with a very low concentration of pCAG-NLS-Cre (1.5 μg/ml) into layer 2/3 neurons using in utero electroporation at E15.5 and prepared coronal sections at E18.5 and P5 (Fig. 8A). This mixture of plasmids enabled us to suppress Sox11 expression and visualize the morphology of single neurons. Interestingly, we found that radial migration of mCherry-positive transfected neurons was markedly inhibited by Sox11 knockdown at E18.5 (Fig. 8B,C). Consistent results were obtained using a different shSox11 construct (Fig. 8C). Our quantification showed that 65.2% of shSox11-transfected neurons were distributed in the white matter at P5, when most control neurons were found in layer 2/3 of the cerebral cortex (Fig. 8B,D). To address the reason of this migration defect, we examined the morphology of each transfected cell (Fig. 8E,F). In control conditions, EGFP-positive cells were still migrating and had a simple morphology with leading processes and trailing processes at E18.5. In contrast, when shSox11 was expressed, EGFP-positive cells had intricately branched processes, suggesting that branching of processes proceeded precociously. Our quantification showed that the morphology of processes of shSox11-transfected migrating neurons was significantly more complex than that of control neurons (Fig. 8G–K). Sox11-downregulated neurons had longer processes (p = 0.000096; Fig. 8G), larger numbers of branches (p = 0.0011; Fig. 8H), larger numbers of terminals (p = 0.00016; Fig. 8I) and larger numbers of primary processes (p = 0.0045; Fig. 8J) than control neurons (n = 4–5 animals per each experimental group; 12–15 transfected cells per each experimental group were analyzed. Welch's t test). Sholl analysis revealed that Sox11-downregulated neurons had more complex morphology than control neurons (multivariate ANOVA, p = 0.00053; Fig. 8K). It seemed plausible that this complex branching resulted in difficulty in migration. This is consistent with previous reports associating precocious branching with radial migration defects (Gupta et al., 2003; Ohshima et al., 2007; Guerrier et al., 2009; Bando et al., 2016). Thus, our findings suggest a biological importance of the suppression of dendritic morphogenesis during migration by Sox11-mediated mechanisms.
Figure 8.
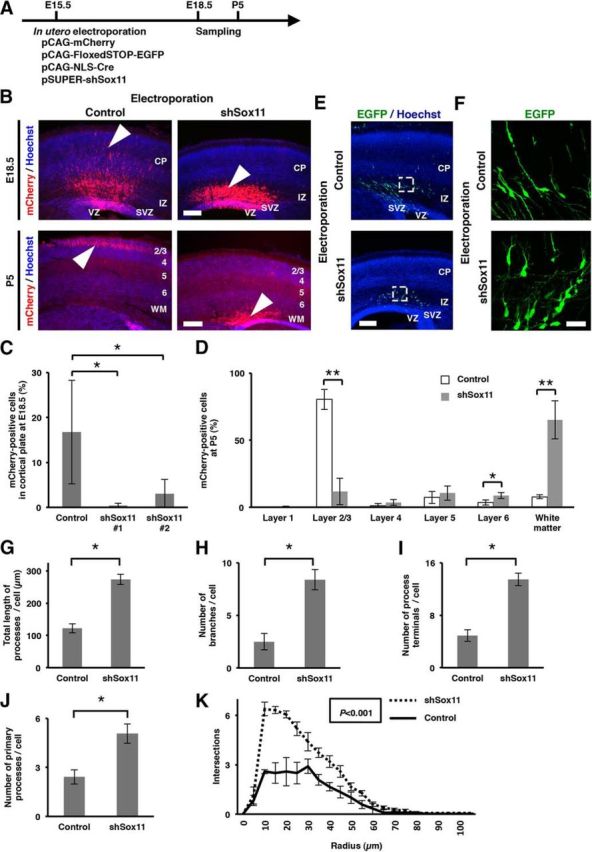
Embryonic knockdown of Sox11 induces precocious dendrite branching and a radial migration defect. A, Experimental procedure. pCAG-mCherry (0.3 mg/ml), pSUPER-shSox11 (1.65 mg/ml), pCAG-FloxedSTOP-EGFP (1.0 mg/ml), and a very low concentration of pCAG-NLS-Cre (1.5 μg/ml) were cotransfected into layer 2/3 neurons using in utero electroporation at E15.5, and coronal sections were prepared at E18.5. EGFP signals were enhanced by immunostaining. B, The distribution of mCherry-positive transfected neurons at E18.5 (top) and at P5 (bottom). Many mCherry-positive control neurons had reached the cortical plate (CP) at E18.5 (arrow), whereas shSox11-transfected neurons were unable to reach cortical plate (arrowhead). At P5, whereas most control neurons were located in the cerebral cortex (arrow), shSox11-transfected neurons were preferentially distributed in the white matter (WM; arrowhead). Cortical layers are indicated with numbers. Scale bars: top, 200 μm; bottom, 300 μm. C, The effects of two independent shSox11 constructs on radial migration. The numbers of mCherry-positive cells in the cortical plate were divided by those of all mCherry-positive cells at E18.5. Data are presented as mean ± SD; n = 3 animals per each experimental group. *p < 0.05. D, The distribution of mCherry-positive cells at P5. The numbers of mCherry-positive cells in the indicated layers were divided by those of all mCherry-positive cells. Data are presented as mean ± SD; n = 3 animals per each experimental group. *p < 0.05; **p < 0.01. E, F, The morphology of neurons transfected with either control (top) or shSox11 (bottom) vectors (green). E, Low-magnification images of the cerebral cortex. Scale bar, 400 μm. F, High-magnification confocal microscopic images of the boxed areas in E. Scale bar, 25 μm. CP, Cortical plate; IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone. G–K, Quantification of the morphology of processes of shSox11-transfected migrating neurons at E18.5. Quantification of the total length of processes per cell (G), the number of branches per cell (H), the number of process ends per cell (I), and the number of primary processes per cell (J). Data are presented as mean ± SEM; n = 4 animals per each experimental group; 12 transfected cells per each experimental group were analyzed. *p < 0.01. K, Sholl analysis of the complexity of the processes of transfected neurons. Data are presented as mean ± SEM; n = 4 animals per each experimental group; 12 transfected cells per each experimental group were analyzed.
The reduction of Sox11 expression is triggered by the end of radial migration
We next explored the mechanism controlling the reduction of Sox11. Because the reduction of Sox11 expression occurs soon after the end of radial migration (Fig. 2), we hypothesized that the end of radial migration triggers the reduction of Sox11 expression. To test this hypothesis, we terminated radial migration precociously by introducing human dominant-negative N-cadherin (DN-Ncad) into layer 2/3 neurons using in utero electroporation (Fig. 9A; Wakimoto et al., 2015). It was reported that loss-of-function of N-cadherin weakened the attachment between migrating neurons and radial glial fibers (Shikanai et al., 2011), and as a result, neurons stopped radial migration precociously. Consistent with previous reports, radial migration of DN-Ncad-transfected neurons was inhibited (Fig. 9B). We found that DN-Ncad-transfected neurons located deep in the cortex had lower levels of Sox11 protein compared with control neurons located in layer 2/3 (n = 3 animals per each experimental group; Student's t test, p = 0.010; Fig. 9C,D). Because migration of DN-Ncad-transfected neurons in the deep cerebral cortex was stopped earlier than neurons in the superficial cortex, this result suggests that precocious termination of radial migration results in earlier reduction of Sox11 protein. Together, our findings are consistent with the idea that the expression level of Sox11 remains high during radial migration, and the end of radial migration triggers the reduction of the Sox11 level. Our findings also support the idea that high expression levels of Sox11 are required for radial migration because Sox11 downregulation resulted in precocious branching and impaired radial migration.
Figure 9.
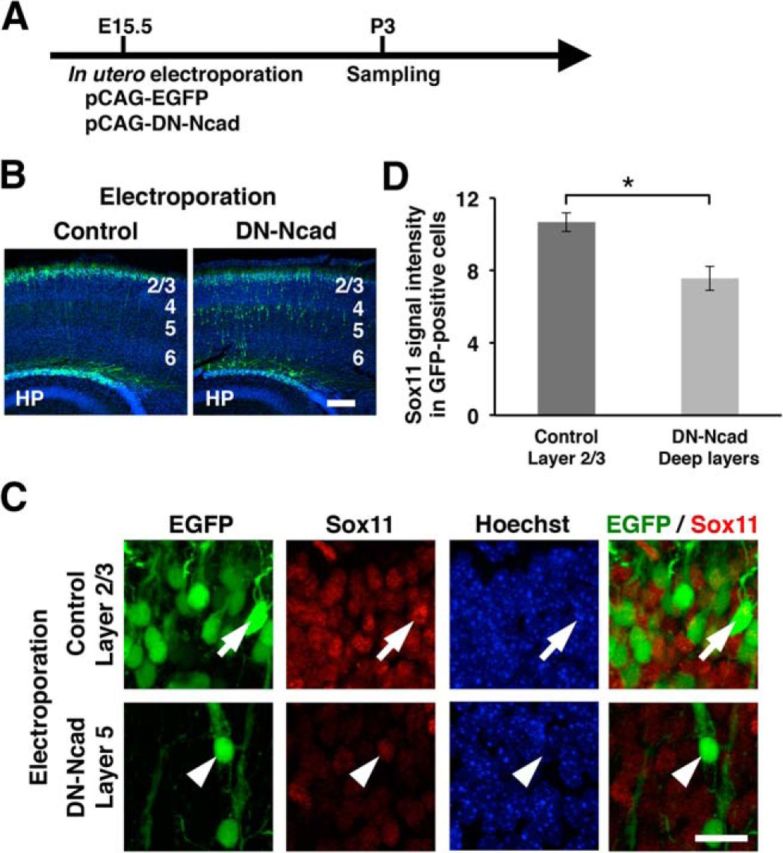
The end of radial migration triggers the reduction of Sox11 expression. A, Experimental procedure. pCAG-EGFP (0.5 mg/ml) and pCAG-DN-Ncad (0.5 mg/ml) were cotransfected into layer 2/3 neurons using in utero electroporation at E15.5. Coronal sections were prepared at P3, and Sox11 immunostaining and Hoechst 33342 staining were performed. B, Layer 2/3 neurons transfected with either control (left) or DN-Ncad (right) vectors (green). Note that DN-Ncad suppresses radial migration of transfected neurons. Numbers indicate the corresponding layers in the cerebral cortex. HP, Hippocampus. Scale bar, 200 μm. C, The expression levels of Sox11 in EGFP-positive transfected neurons. Sections were stained with anti-Sox11 antibody and Hoechst 33342. High-magnification confocal microscopic images of neurons transfected with either control (top) or DN-Ncad (bottom) vectors are shown. Note that the expression levels of Sox11 were lower in DN-Ncad-expressing neurons located in layer 5 (arrowhead) compared with control neurons located in layer 2/3 (arrow). Scale bar, 10 μm. D, Quantification of the expression levels of Sox11 in EGFP-positive neurons. Data are presented as mean ± SEM; n = 3 animals per each experimental group. *p < 0.05.
Discussion
In this study, we have shown that the reduction of Sox11 expression is required and sufficient for dendritic morphogenesis during development. We also showed that precocious inhibition of Sox11 expression resulted in a radial migration defect, presumably due to precocious branching of neurites. Our findings underline a biological importance of the Sox11-mediated mechanisms suppressing dendritic morphogenesis during radial migration.
Sox11 is a transcription factor suppressing dendritic morphogenesis during development
Previous studies have identified transcription factors promoting dendritic morphogenesis in the mammalian cerebral cortex (Puram and Bonni, 2013; Santiago and Bashaw, 2014). Neurogenin2 and Otx1 regulate cell-type-specific morphology by preferentially promoting development of either apical or basal dendrites (Hand et al., 2005; Zhang et al., 2015), and CREST and CREB contribute to regulating the timing of dendritic morphogenesis by responding to neural activity or other extrinsic signals (Redmond et al., 2002; Aizawa et al., 2004; Gaudillière et al., 2004; Wayman et al., 2006).
Although previous findings have been primarily related to the roles of transcription factors in promoting dendritic morphogenesis, our findings indicate that the transcription factor Sox11 plays a crucial role in suppressing dendritic morphogenesis, especially during radial migration. It has already been established that transcription factors are important for promoting and suppressing neurogenesis in neural stem cells (Kageyama et al., 2005). Our results indicate that, as in the case of neurogenesis, transcription factors play important roles in balancing the suppression and promotion of dendritic morphogenesis during development.
Sox11 maintains simple morphology of migrating neurons
Recent pioneering studies proposed that precocious branching of neurites leads to radial migration defects in postmitotic neurons. For example, when the GTPase activating protein srGAP2 is overexpressed in migrating neurons, branching of the leading process occurs, and radial migration is inhibited (Guerrier et al., 2009). p35 and Cdk5 are required for maintaining the simple morphology of migrating neurons, and loss of p35 and Cdk5 results in abnormally branched leading processes and severe migration defects (Gupta et al., 2003; Ohshima et al., 2007). Apart from these molecules regulating the cytoskeleton, transcription factors that ensure radial migration by suppressing branch formation had been unclear. Our study showed that Sox11 is a transcription factor that negatively regulates dendritic morphogenesis and ensures radial migration. Although the mechanisms downstream of Sox11 are unclear, it seems possible that srGAP2, p35, and Cdk5 are involved in the effect of Sox11. It was also reported that an increase in spontaneous neural activity resulted in premature branching of the leading process (Bando et al., 2016). It would be intriguing to investigate whether Sox11 suppresses dendritic morphogenesis by regulating spontaneous neural activity.
Roles of Sox11 in the CNS
Previous studies have shown that Sox11 is required for promoting early stages of neurogenesis. Sox11 is required for neuronal differentiation of neural precursors, and cell-fate specification, differentiation and survival in the spinal cord and the retina (Bergsland et al., 2006; Jiang et al., 2013; Usui et al., 2013b). In the peripheral nervous system, Sox11 promotes cell proliferation (Potzner et al., 2010). In addition to the previously identified roles of Sox11 in early stages of neurogenesis, our findings revealed a novel role of Sox11 in suppressing dendritic morphogenesis in postmitotic neurons. The reduction of Sox11 expression was observed not only in the cortical neurons, but also in other tissues including sympathetic ganglia and retina (Potzner et al., 2010; Usui et al., 2013a,b). Thus, it seems reasonable that Sox11 also regulates dendritic morphology in these tissues. Interestingly, Sox11 is also expressed in the adult brain regions where neurogenesis is observed, such as the dentate gyrus and the subventricular zone (Haslinger et al., 2009; Mu et al., 2012; Wang et al., 2013). Therefore, Sox11 may also play similar roles in adult neurogenesis.
Footnotes
This work was supported by the Global Centers of Excellence program and the Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology-Japan, and Takeda Science Foundation, and by the Iwadare Scholarship Foundation (Y. H.). We thank Drs S. Tsuji, T. Kadowaki, H. Bito (The University of Tokyo), H. Tanaka (Tokyo University of Pharmacy and Life Sciences), E. Nishida (Kyoto University), the late Y. Sasai, and S. Nakanishi for their continuous support and warm encouragement; Drs Y. Nakai (Hirosaki University), H. Mizuno (National Institute of Genetics), E. Sock, M. Wegner (Universität Erlangen-Nürnberg), S. Watanabe (The University of Tokyo), K. Yoshimura, T. Sakurai, and M. Sato (Kanazawa University) for their technical support; and the Kawasaki laboratory members and Z. Blalock for critical discussions and comments on this paper.
The authors declare no competing financial interests.
References
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- Ako R, Wakimoto M, Ebisu H, Tanno K, Hira R, Kasai H, Matsuzaki M, Kawasaki H. Simultaneous visualization of multiple neuronal properties with single-cell resolution in the living rodent brain. Mol Cell Neurosci. 2011;48:246–257. doi: 10.1016/j.mcn.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bando Y, Irie K, Shimomura T, Umeshima H, Kushida Y, Kengaku M, Fujiyoshi Y, Hirano T, Tagawa Y. Control of spontaneous Ca2+ transients is critical for neuronal maturation in the developing neocortex. Cereb Cortex. 2016;26:106–117. doi: 10.1093/cercor/bhu180. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Genes Dev. 2006;20:3475–3486. doi: 10.1101/gad.403406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland M, Ramsköld D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew LJ, Gallo V. The Yin and Yang of sox proteins: activation and repression in development and disease. J Neurosci Res. 2009;87:3277–3287. doi: 10.1002/jnr.22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dy P, Penzo-Méndez A, Wang H, Pedraza CE, Macklin WB, Lefebvre V. The three SoxC proteins—Sox4, Sox11 and Sox12—exhibit overlapping expression patterns and molecular properties. Nucleic Acids Res. 2008;36:3101–3117. doi: 10.1093/nar/gkn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Gao FB. Molecular and cellular mechanisms of dendritic morphogenesis. Curr Opin Neurobiol. 2007;17:525–532. doi: 10.1016/j.conb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudillière B, Konishi Y, de la Iglesia N, Yao GL, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/S0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Guerrier S, Coutinho-Budd J, Sassa T, Gresset A, Jordan NV, Chen K, Jin WL, Frost A, Polleux F. The F-BAR domain of srGAP2 induces membrane protrusions required for neuronal migration and morphogenesis. Cell. 2009;138:990–1004. doi: 10.1016/j.cell.2009.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, Pearlman AL, Brunstrom J, Tsai LH. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003;6:1284–1291. doi: 10.1038/nn1151. [DOI] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, Schuurmans C, Guillemot F, Polleux F. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Haslinger A, Schwarz TJ, Covic M, Chichung Lie DC. Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci. 2009;29:2103–2114. doi: 10.1111/j.1460-9568.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- Häusser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ding Q, Xie X, Libby RT, Lefebvre V, Gan L. Transcription factors SOX4 and SOX11 function redundantly to regulate the development of mouse retinal ganglion cells. J Biol Chem. 2013;288:18429–18438. doi: 10.1074/jbc.M113.478503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/S0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kiefer JC. Back to basics: Sox genes. Dev Dyn. 2007;236:2356–2366. doi: 10.1002/dvdy.21218. [DOI] [PubMed] [Google Scholar]
- Kitazawa A, Kubo K, Hayashi K, Matsunaga Y, Ishii K, Nakajima K. Hippocampal pyramidal neurons switch from a multipolar migration mode to a novel “climbing” migration mode during development. J Neurosci. 2014;34:1115–1126. doi: 10.1523/JNEUROSCI.2254-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Enderich J, Hermans-Borgmeyer I, Wegner M. Cooperative function of POU proteins and SOX proteins in glial cells. J Biol Chem. 1998;273:16050–16057. doi: 10.1074/jbc.273.26.16050. [DOI] [PubMed] [Google Scholar]
- Longair MH, Baker DA, Armstrong JD. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics. 2011;27:2453–2454. doi: 10.1093/bioinformatics/btr390. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, Tagawa Y. Pre-synaptic and post-synaptic neuronal activity supports the axon development of callosal projection neurons during different post-natal periods in the mouse cerebral cortex. Eur J Neurosci. 2010;31:410–424. doi: 10.1111/j.1460-9568.2009.07070.x. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Mu L, Berti L, Masserdotti G, Covic M, Michaelidis TM, Doberauer K, Merz K, Rehfeld F, Haslinger A, Wegner M, Sock E, Lefebvre V, Couillard-Despres S, Aigner L, Berninger B, Lie DC. SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J Neurosci. 2012;32:3067–3080. doi: 10.1523/JNEUROSCI.4679-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Hirasawa M, Tabata H, Mutoh T, Adachi T, Suzuki H, Saruta K, Iwasato T, Itohara S, Hashimoto M, Nakajima K, Ogawa M, Kulkarni AB, Mikoshiba K. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134:2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Namba T, Shinoda T, Kondo T, Watanabe T, Inoue Y, Takeuchi K, Enomoto Y, Ota K, Oda K, Wada Y, Sagou K, Saito K, Sakakibara A, Kawaguchi A, Nakajima K, Adachi T, Fujimori T, Ueda M, Hayashi S, et al. TAG-1-assisted progenitor elongation streamlines nuclear migration to optimize subapical crowding. Nat Neurosci. 2013;16:1556–1566. doi: 10.1038/nn.3525. [DOI] [PubMed] [Google Scholar]
- Potzner MR, Tsarovina K, Binder E, Penzo-Méndez A, Lefebvre V, Rohrer H, Wegner M, Sock E. Sequential requirement of Sox4 and Sox11 during development of the sympathetic nervous system. Development. 2010;137:775–784. doi: 10.1242/dev.042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior HM, Walter MA. SOX genes: architects of development. Mol Med. 1996;2:405–412. [PMC free article] [PubMed] [Google Scholar]
- Puram SV, Bonni A. Cell-intrinsic drivers of dendrite morphogenesis. Development. 2013;140:4657–4671. doi: 10.1242/dev.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/S0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240:237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Santiago C, Bashaw GJ. Transcription factors and effectors that regulate neuronal morphology. Development. 2014;141:4667–4680. doi: 10.1242/dev.110817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Luo L. How do dendrites take their shape? Nat Neurosci. 2001;4:359–365. doi: 10.1038/86006. [DOI] [PubMed] [Google Scholar]
- Sehara K, Toda T, Iwai L, Wakimoto M, Tanno K, Matsubayashi Y, Kawasaki H. Whisker-related axonal patterns and plasticity of layer 2/3 neurons in the mouse barrel cortex. J Neurosci. 2010;30:3082–3092. doi: 10.1523/JNEUROSCI.6096-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She ZY, Yang WX. SOX family transcription factors involved in diverse cellular events during development. Eur J Cell Biol. 2015;94:547–563. doi: 10.1016/j.ejcb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Shikanai M, Nakajima K, Kawauchi T. N-cadherin regulates radial glial fiber-dependent migration of cortical locomoting neurons. Commun Integr Biol. 2011;4:326–330. doi: 10.4161/cib.4.3.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sock E, Rettig SD, Enderich J, Bösl MR, Tamm ER, Wegner M. Gene targeting reveals a widespread role for the high-mobility-group transcription factor Sox11 in tissue remodeling. Mol Cell Biol. 2004;24:6635–6644. doi: 10.1128/MCB.24.15.6635-6644.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/S0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Toda T, Homma D, Tokuoka H, Hayakawa I, Sugimoto Y, Ichinose H, Kawasaki H. Birth regulates the initiation of sensory map formation through serotonin signaling. Dev Cell. 2013;27:32–46. doi: 10.1016/j.devcel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Usui A, Iwagawa T, Mochizuki Y, Iida A, Wegner M, Murakami A, Watanabe S. Expression of Sox4 and Sox11 is regulated by multiple mechanisms during retinal development. FEBS Lett. 2013a;587:358–363. doi: 10.1016/j.febslet.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Usui A, Mochizuki Y, Iida A, Miyauchi E, Satoh S, Sock E, Nakauchi H, Aburatani H, Murakami A, Wegner M, Watanabe S. The early retinal progenitor-expressed gene Sox11 regulates the timing of the differentiation of retinal cells. Development. 2013b;140:740–750. doi: 10.1242/dev.090274. [DOI] [PubMed] [Google Scholar]
- Wakimoto M, Sehara K, Ebisu H, Hoshiba Y, Tsunoda S, Ichikawa Y, Kawasaki H. Classic cadherins mediate selective intracortical circuit formation in the mouse neocortex. Cereb Cortex. 2015;25:3535–3546. doi: 10.1093/cercor/bhu197. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lin L, Lai H, Parada LF, Lei L. Transcription factor Sox11 is essential for both embryonic and adult neurogenesis. Dev Dyn. 2013;242:638–653. doi: 10.1002/dvdy.23962. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wegner M, Stolt CC. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 2005;28:583–588. doi: 10.1016/j.tins.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Liu LX, Cao HT, Ou L, Qu J, Wang Y, Chen JG. Otx1 promotes basal dendritic growth and regulates intrinsic electrophysiological and synaptic properties of layer V pyramidal neurons in mouse motor cortex. Neuroscience. 2015;285:139–154. doi: 10.1016/j.neuroscience.2014.11.019. [DOI] [PubMed] [Google Scholar]