Abstract
The perception of pain is strongly influenced by cognitive processes, such as expectations toward the efficacy of pain medication. It is reasonable to assume that such processes, among other sources of fluctuation, are reflected in ongoing brain activity, which in turn influences perceptual processing. To identify specific prestimulus EEG activity, and connectivity patterns related to subsequent pain perception in humans, we contrasted painful with nonpainful sensations delivered at the individual threshold level determined by the psychophysical QUEST estimation method (Watson and Pelli, 1983). The 64-channel EEG was recorded using active electrodes during a constant stimulation procedure. The power contrast between trials sorted by rating revealed a signal decrease of 8% before stimulus onset in theta-band (4–7 Hz) at T7/FT7 as well as increased theta-power by 6% at T8/FT8. Gamma-band power was increased (12%, 28–32 Hz) at frontocentral sites (all p < 0.05). Changes in theta-band power are covarying with subsequent pain perception, as well as lowered frontolateral theta-band connectivity for painful percepts. A decrease in frontoparietal connectivity for painful sensations was also identified in the gamma-band (28–32 Hz). A single-trial logistic regression revealed significant information content in the EEG signal at temporal electrode T7 in theta-band (p < 0.01) and frontal electrode F1 in gamma-band (all p < 0.02). The observed patterns suggest top-down modulation of the theta-band effects by a frontocentral network node. These findings contribute to the understanding of ongoing subjective pain sensitivity, potentially relevant to both clinical diagnostics and pain management.
SIGNIFICANCE STATEMENT The perceived intensity of a constant stimulus is known to vary considerably across multiple presentations. Here, we used state-of-the-art psychophysical methods in an EEG experiment to identify the specific neuronal activity before stimulus onset that reflects the subsequent perception of pain. We found specific oscillatory activity at the bilateral insular cortices as well as connectivity patterns that reflect and correlate with subsequent ratings. These results further the understanding of pain perception and are potentially relevant for the decoding of ongoing pain sensitivity and pain management.
Keywords: EEG, pain, prestimulus, psychophysics, theta-band, threshold
Introduction
The perception of pain is highly subjective and cannot be linked to stimulus intensity alone. Indeed, many cognitive factors, such as attention or expectation, influence whether we perceive a given stimulation as painful (Koyama et al., 2005; Kupers et al., 2005). By now, it has become evident that this perceptual modulation is not only cognitive but also affects signal transduction on the spinal level (Eippert et al., 2009a, b; Sprenger et al., 2012). A more recent study has shown that expectation toward the analgesic potency of opiates interacts with their efficacy, further illustrating the strong impact of the perceptual transformation on nociception (Bingel et al., 2011). In addition to the sensitivity modulation by higher cognitive concepts, there are also accounts of the sensory system using activity fluctuations to enhance its signal detection and discrimination capabilities (Linkenkaer-Hansen et al., 2004). Generally, the subjective perception of constant noxious stimulation exhibits substantial intraindividual variability (Coghill et al., 2003; Boly et al., 2007). This implies that a single stimulus intensity can be experienced as painful, merely aversive, or even nonpainful depending on the current cognitive state (Brown et al., 2008; Wiech et al., 2008). Such a connection between prestimulus state of a neuronal assembly and subsequent stimulus processing has been demonstrated for various cognitive domains, such as attention (Thut et al., 2006), perception (Hesselmann et al., 2008; Salari et al., 2014), and memory (Guderian et al., 2009; Rutishauser et al., 2010). For pain, there is evidence that functional connectivity between the brainstem and the anterior insular cortex determines the susceptibility toward pain (Ploner et al., 2010). It is largely unclear, however, what kind of prestimulus activity is connected to subsequent processing. Likely candidates are activity in the theta-band (4–7 Hz) and gamma-band (>28 Hz), which are evoked during pain processing (Schulz et al., 2012a, b). A correlation between activity after stimulus presentation in both bands and intraindividual variations in the pain experience has previously been demonstrated (Schulz et al., 2011), but prestimulus effects were not yet examined. Furthermore, it is not fully understood how prestimulus activity is related to poststimulus responses and how both interact across the stimulus onset. This also raises the question whether such a relationship occurs spontaneously or is top-down modulated by a larger network. The aim of the current study was to examine whether theta- and/or gamma-band activity in prestimulus time varies systematically with subsequent perceptual decisions about a stimulus of constant intensity being painful or not. We hypothesized that the configuration of ongoing oscillatory theta/gamma activity contains information about the subsequent perceptual processing. This information should result in differential activity between trials rated as painful versus nonpainful under constant stimulation and allow inference about the subsequent ratings. We further aimed to examine whether nociception-related prestimulus patterns in theta- and/or gamma-band coincide with differential states of oscillatory connectivity. Finally, we were interested in localizing the most likely neuronal sources of the identified differential activity. Likely candidates were the areas previously reported for general pain processing, such as the insula or anterior midcingulate cortex (Duerden and Albanese, 2013). Because we contrasted painful and nonpainful conditions under constant stimulation, the corresponding sources can be considered to contribute directly to the transition from innocuous perception to painful sensation (Mouraux et al., 2011).
Materials and Methods
Participants.
A total of 23 healthy right-handed participants were recruited from the institute's participant database. One participant did not complete the experiment due to fatigue and difficulties concentrating. Two more were excluded from the data because both were unable to suppress an involuntary blink response to the painful stimulus. This contaminated poststimulus data and rendered the analysis impossible. The resulting sample thus contains 20 participants (9 female, mean age 26.9 ± 6.9 years). All participants gave written consent and received a compensation of 15 € per hour for their participation. The study was approved by the local ethics committee (PV4509).
Experimental procedure.
Participants were introduced to the study by the experimenter and gave written informed consent.
Noxious stimulation was delivered by applying electrical current to the abductor/flexor pollicis brevis of the left hand using a DS7A Peripheral Stimulator (Digitimer). Stimulation was triggered by the experimental script using MATLAB (R2009b; The MathWorks). The pulse length was fixed at 2 ms, whereas the current was adjusted manually by the experimenter according to the value displayed by the script. Participants were asked to direct their somatosensory attention to a freely chosen location on their body and keep it there constantly throughout the experiment.
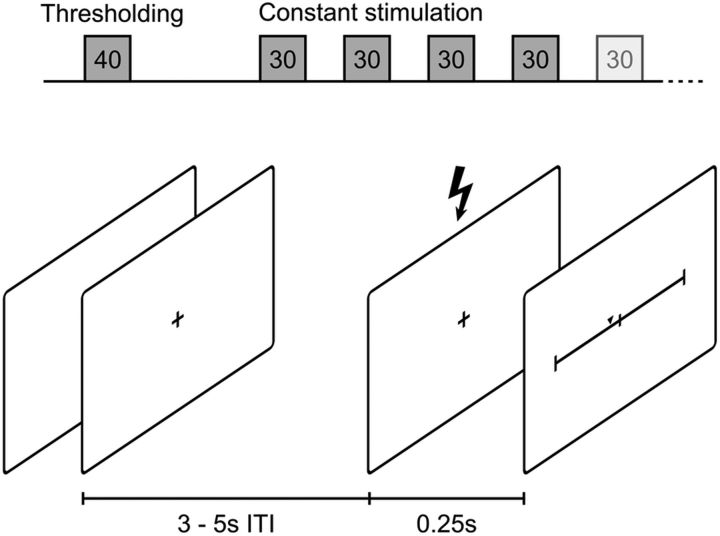
The first block of 40 trials (Fig. 1) was used to determine the 50% pain threshold. Stimulation intensities were chosen according to suggested values from the QUEST algorithm (Watson and Pelli, 1983). This algorithm fits a psychophysical function by probing at stimulus intensities optimized for the maximum likelihood estimation of a threshold. This method is well suited because we wanted to efficiently determine the 50% threshold, located at the midpoint of the psychophysical function (Leek, 2001). Participants were told the stimulus intensity would be chosen at random by the computer. They were asked to rate each stimulation using a mouse on a visual analog scale (VAS) after a 0.25 s delay. The scale ranged from “no sensation” (0) to “most extreme pain” (100), the center of the scale (50) representing the transition point between a strong sensation and painful stimulation. Regarding the classification into “painful” and “not painful,” our experiment resembled a two-alternative forced-choice task because the central point of the scale could not be selected. Two separate thresholding runs of 20 trials each were randomly interleaved to prevent participants from adjusting to the procedure. One thresholding run started from an arbitrarily chosen “high” intensity, whereas the other started out from a “low” intensity, to increase the chance of convergence. The individual threshold intensity for each participant was defined as the mean of the two final QUEST estimates.
Figure 1.
Experimental procedure. Top, Forty trials of thresholding were followed by 4–6 blocks of 30 trials each with constant stimulation, depending on data quality. Bottom, Schematics of a single trial: jittered 3–5 s waiting period (intertrial interval, ISI) followed by stimulation and the visual analog scale for pain rating after 250 ms.
Stimulus intensity was subsequently kept constant at threshold level for the remaining trial blocks. Participants were not made aware of this fact but asked to keep rating the stimuli on the VAS as they had done so far. Dependent on the data quality assessed by online monitoring, participants then completed an additional 4–6 blocks of 30 trials each. This allowed us to directly compare painful and nonpainful sensations within participants at constant stimulus intensity (Oertel et al., 2012).
On each trial, participants were asked to keep their eyes on a fixation cross presented at the center of the screen. After a random waiting period of 3–5 s, the stimulus was delivered. The VAS was presented onscreen 250 ms later. Participants rated the stimulus at their own pace. Following the rating, the fixation cross was shown and the next waiting period began. Visual material (fixation cross, instructions, rating scale) was rendered using the Psychophysics Toolbox version 3 (Brainard, 1997; Pelli, 1997; Kleiner et al., 2007) and displayed on a 23-inch TFT-Screen (SyncMaster P2370; Samsung) positioned centrally at 1.1 m in front of the participant.
The experiment lasted ∼45 min, and participants were offered to take short breaks in between blocks. After finishing the experiment, the true nature of the stimulation was revealed to the participants by the experimenter.
Electroencephalographic (EEG) recordings.
EEG data were acquired using a 64-channel Ag/AgCl active electrode system (ActiCap64; BrainProducts) placed according to the extended 10–20 system (Klem et al., 1999). Sixty electrodes were used on the most central scalp positions. For off-line artifact removal, a bidirectional, bipolar electrooculogram (EOG) was recorded using the remaining four electrodes. The bipolar EOG electrode pairs were placed above and below the left eye as well as on the lateral ends of the bicanthal plane. FCz was used as reference electrode for data recording, the ground electrode was placed at position Iz. The signal was digitized at a sampling rate of 250 Hz and high-pass filtered with a cutoff of 0.5 Hz at recording. All impedances were kept <20 kΩ.
Data analysis.
To test whether the participants habituated to the presented stimuli, we performed a linear regression on the individual rating medians per trial block.
EEG data were analyzed using the FieldTrip toolbox (Oostenveld et al., 2011). For each subject, data were epoched into 2 s trials from −1500 ms to 500 ms around the stimulus onset. For artifact rejection, a 2000 ms padding was added before and after the trials. To assist with visual artifact rejection, an automatic artifact tagging was then performed on the data, calculating trial-based z-scores for EOG artifacts (1–15 Hz), muscle artifacts (100–120 Hz), and peaks in absolute difference between subsequent samples. Trials were then screened for unusual deviations in their maximum z-scores by the experimenter, and an individual threshold for each dataset was chosen. All trials were additionally subjected to a full visual scan of the raw data. Epochs contaminated by artifacts were removed from the dataset in their entirety (24 trials per participant on average). The remaining trials for each subject were split into a “pain” and “no-pain” condition according to the VAS ratings. This was done by sorting the trials by rating in ascending order and selecting a matching number of trials from each end of the list until no more trials were left in either one of the conditions.
Data were transformed into the time-frequency domain using the multitaper method (Thomson, 1982; Mitra and Pesaran, 1999). Slepian sequences were used as tapers; frequency resolution was 1 Hz with a frequency range from 2 to 45 Hz. Time resolution was 50 ms. Length of the time window was varied from 1 to 800 ms; spectral smoothing varied from 2 to 4 Hz across the frequency spectrum. The resulting time-frequency spectra were then averaged for each participant and condition. A grand average across participants was calculated, and power differences were visualized in time-frequency space by calculating the relative signal change between the grand averages of the “no-pain” and “pain” conditions. By subtracting the spectra of both conditions within-participant, the “no-pain” condition served as a baseline for the “pain” condition. This allowed us to analyze the resulting data without resorting to a time-dependent baseline. Due to the time-lock on stimulus onset and averaging, systematic activity was amplified while random activity was attenuated.
To examine functional connectivity, we calculated the trial-based phase locking value (PLV) (Lachaux et al., 1999) for all channel combinations in both conditions. Oscillatory phase synchronization can be interpreted as connectivity measure, which reflects the exchange of information between different neuronal populations (Nunez et al., 1997; Sauseng and Klimesch, 2008). Grand averages were generated as for the time-frequency data. The difference between connectivity in the “pain” and “no-pain” conditions was then calculated.
To test the power and connectivity differences statistically, a Monte Carlo permutation, including 5000 iterations per run, was used. This yielded a permutation distribution of the significance probabilities for dependent-samples t tests between “pain” and “no-pain” conditions for each time-frequency point. Statistics were calculated in the time range of −900 ms to 400 ms, to be able to relate any effects across the stimulus onset. To correct for multiple comparisons in the power analysis, the false discovery rate (FDR) was controlled at q* = 0.05 (Benjamini and Hochberg, 1995; Genovese et al., 2002). In the connectivity analysis, we controlled for multiple comparisons with a nonparametric, cluster-based permutation statistic, as implemented in the FieldTrip toolbox (Maris et al., 2007).
To test our hypothesis on significant information content in the theta-/gamma-bands, we used a logistic regression model predicting pain ratings from single-trial EEG data from all electrodes over frontal and temporal sites that exhibited significant power differences in the group-level analysis, four time windows (−1 s to stimulus onset in 250 ms steps) and theta/gamma frequency bands. The model was fitted on z-transformed data within participants and frequency bands across time and conditions. To make sure the model was not picking up on random noise, we compared it against a random model (predicting rating outcomes at 50% probability) and an arbitrary model, using EEG data from randomly selected frequency bands and time windows, excluding the ones identified by our test model. To assess whether the reliability of our test model was compromised by sampling bias, we estimated the accuracy of the three models using a bootstrapping procedure (Efron, 1979; Chernick, 2011), resampling the trial data 10,000 times. This allowed us to estimate how well the model resisted changes in trial order, the composition of the present sample, and outliers in the data. To test whether the test model reliably outperforms the random and arbitrary models, we calculated CIs around the estimated bootstrap accuracies and computed Cohen's d as effect size measure for the differences (Cohen, 1988).
For connectivity, we restricted our focus on the seeding sites identified in the initial power analysis. We tested the connectivity of each seeding electrode against other local neighbors (local connectivity), and contralateral or frontal electrodes (lateral and frontal connectivity; for details on the areas, see Fig. 4). All connectivity data are reported cluster-corrected with a cluster threshold of p = 0.05, 3 minimum neighbors and 5000 iterations for the permutation statistics.
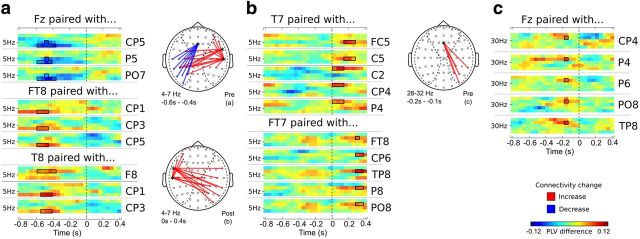
Figure 4.
Connectivity effects. Time-frequency plots of different electrode pairs reflect changes in PLV between pain and no-pain trials. Topo-plots represent the locations of the plotted electrode pairs. Red represents an increase in connectivity. Blue represents a decrease. The matching category is indicated on the bottom right of each topo-plot. Depicted are visualizations of PLV differences from seed electrodes. a, PLV differences in the theta-band (4–7 Hz) and prestimulus time window (−0.8 to 0 s). b, PLV differences in the theta-band (4–7 Hz) and poststimulus time window (0–0.4 s). c, PLV differences in the gamma-band (28–32 Hz) for the prestimulus time window (−0.8 to 0 s). Time-frequency points within the solid lines are significant at p < 0.05 (cluster corrected).
To localize the sources of the observed power differences in 3D head space, we used the exact low resolution brain electromagnetic tomography (eLORETA) method (Pascual-Marqui et al., 2011). To this end, we calculated the average cross-spectrum for each participant in each of the pain and no-pain conditions before passing the data to the LORETA software. We used the eLORETA paired group test to create t-maps of differences between the pain and no-pain conditions in source space. Because we wanted to locate the generators of the power differences, no thresholding was performed, but the source differences with the largest t-values were exported for display.
Results
Behavioral data
The overall median rating was 50.10, suggesting that the QUEST algorithm was successful at identifying the individual threshold intensities. Regression of the median ratings over the blocks did not show a significant change over the time course of the experiment (β = −0.003, p > 0.84). This indicates that no habituation or sensitization toward the presented stimuli has occurred. Although the median rating reflects the 50% threshold, the variability of ratings was smaller for “pain” ratings (SD = 4.63) than for “no-pain” ratings (SD = 14.69).
Oscillatory power
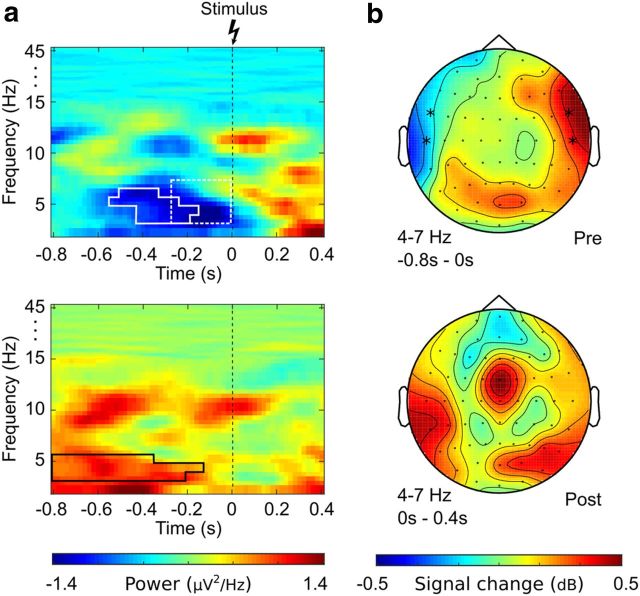
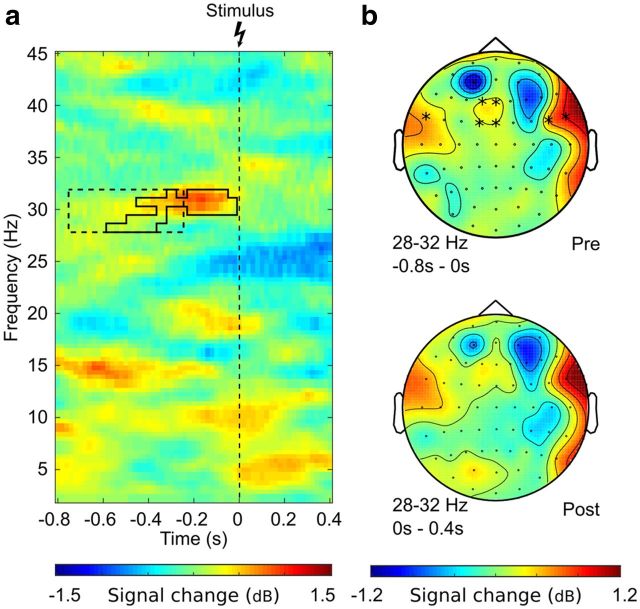
Permutation testing yielded significant clusters in the prestimulus time range in the theta-band (4–7 Hz) as well as gamma-band (cluster at 28–32 Hz). In theta-band, the relative signal change between “no-pain” and “pain” ratings occurred temporally lateralized, with a signal decrease of 8% on the ipsilateral side and a power increase of 6% on the contralateral side. Permutation testing showed a significant power decrease in the −600 ms to 0 ms time range at electrodes FT7 and T7 (tmin = −3.06, p < 0.05, FDR corrected). A significant power increase was detected in the time range of −800 ms to −200 ms at FT8 and T8 (tmax = 2.77, p < 0.05, FDR corrected). For the gamma-band, significant effects were found on frontal as well as temporal electrodes extending from −600 ms up to stimulus onset at Fz, F1, FC1, and FCz (tmax = 3.67, all p < 0.05, peak signal increase 12%, FDR corrected). Detailed displays of the observed effects are given in Figures 2 and 3.
Figure 2.
Power effects in the theta-band (4–7 Hz). a, Time-frequency plot of the difference between pain and no-pain trials averaged across electrodes T7/TF7 (top) and T8/TF8 (bottom). Solid outline indicates the prestimulus effect identified by permutation testing (tmin = −3.06, tmax = 2.77, all p < 0.05, FDR corrected). Dotted outline indicates the data region used as significant regression predictor in single-trial analysis (b = 0.61, p < 0.011). b, The topographies show relative power differences (dB) between pain and no-pain trials in the prestimulus time range (top) and poststimulus time range (bottom). *Electrodes showing significant effects.
Figure 3.
Power effects in the log gamma-band (28–32 Hz). a, Time-frequency plot of the relative power difference (dB) between pain and no-pain trials averaged across electrodes Fz, F1, FC1, and FCz. Solid outline indicates the prestimulus effect identified by permutation testing (tmax = 3.67, p < 0.05, FDR corrected). Dotted outline indicates the data region used as significant regression predictor in single-trial analysis (electrode F1, b = −0.93, p < 0.045). b, Topographies show relative power differences (dB) between pain and no-pain trials in the prestimulus time range (top) and poststimulus time range (bottom). *Electrodes showing significant effects.
The power effects in the poststimulus time range differed from the prestimulus effects. For theta-band, the ipsilateral power decrease disappeared after stimulation, whereas the maximum of the contralateral power increase shifted to parieto-occipital sites. The relative power increase in gamma-band after stimulation was located more laterally.
Connectivity
Trial-based phase-locking indicated systematic functional coupling differences between pain and no-painful sensations in the theta- and gamma-band in the prestimulus as well as in the poststimulus period, as assessed from the seed electrodes Fz and T7/FT7 as well as T8/FT8 (Fig. 4). Prestimulus connectivity in the theta-band (4–7 Hz) was decreased at a cluster between Fz and ipsilateral parietotemporal sites (maximum PLV decrease by 0.12 at T7 and FT7, all p < 0.05, cluster corrected) in trials where the consecutive stimulus was experienced as painful. At the same time, there was increased connectivity between contralateral temporal electrodes T8 and FT8 locally as well as to a cluster on the contralateral side (maximum PLV increase 0.13, all p < 0.05, cluster corrected). During the poststimulus interval, this pattern changed to an increased connectivity between ipsilateral temporal sites T7 and FT7 and an area comprised of contralateral frontotemporal and parietotemporal sites. Another significant difference in connectivity between pain and no-pain trials was found in the gamma-band (28–32 Hz, maximum PLV decrease by 0.12, p < 0.05, cluster corrected). For prestimulus timeframes ∼−0.2 s the connectivity was increased between Fz and a parietotemporal cluster around P6 for painful trials (see Fig. 4c).
Source localization
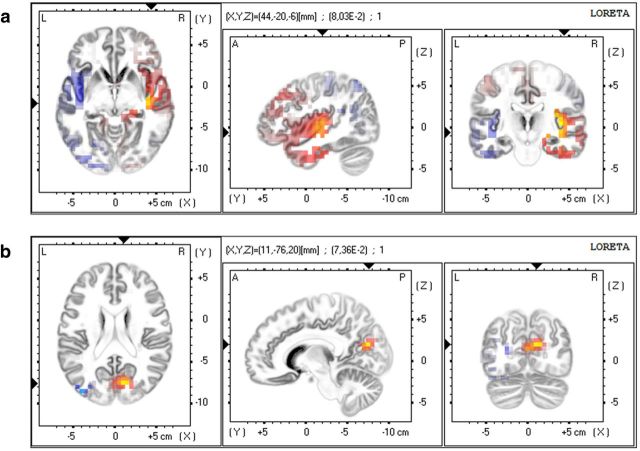
The localization t-maps of generators for the scalp power differences in theta-band revealed two generators at the bilateral insular cortex (IC, Fig. 5a). The ipsilateral IC was less active for subsequent painful ratings (tmin = −0.058), whereas the contralateral IC was more active (tmax = 0.104).
Figure 5.
eLORETA generators for the scalp power differences in the theta-band. a, Differential activity in bilateral insular cortex (tmax = 0.104, tmin = −0.058). b, Differential source activity in the precuneus/posterior cingulate area (tmax = 0.103). Yellow/red: increase, blue: decrease.
eLORETA also revealed a pronounced theta-band difference in the general region of the contralateral precuneus and/or posterior cingulate (tmax = 0.103; Fig. 5b).
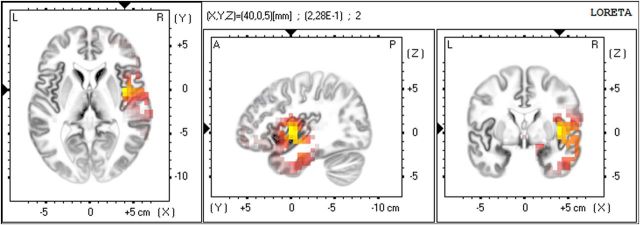
For the low gamma-band, the source localization revealed a single generator in the contralateral IC (tmax = 0.182; Fig. 6).
Figure 6.
eLORETA generator for the scalp power differences in the gamma-band revealing a single effect in the contralateral insular cortex (tmax = 0.182). Yellow/red: increase, blue: decrease.
Information content
The logistic regression model identified three significant predictors from the 32 possible features. One significant predictor was identified in the theta-band at the ipsilateral temporal electrode T7 in the time window between −0.25 s and 0 s (b = −0.48, p < 0.009). Two significant predictors have been identified in the gamma-band at the frontal electrode F1 between −0.75 s and −0.25 s (bmax = 0.61, p < 0.011). The single-trial information from the contralateral temporal electrode FT8 in the time window between −1 s and −0.5 s was not significant but showed a trend in that direction, being the fourth most informative predictor (b = 0.38, p = 0.056). Except for the contralateral-positive difference at T8/FT8 which did not significantly contribute to the model, the identified predictors basically overlap with the power effects on group level (Figs. 2, 3).
In terms of single-trial accuracy in classifying the pain responses, the model is only moderately powerful (acc = 0.565, CI [0.565, 0.566]), but it is significantly better than chance (acc = 0.500, CI [0.499, 0.500]). The model extracts a substantial amount of information in terms of effect size (Cohen's d = 4.50). Also, it significantly outperforms the arbitrary model based on randomly assigned EEG data (acc = 0.506, CI [0.5060, 0.5064]), which cannot extract meaningful single-trial predictions (Cohen's d = 0.45 vs the random model). The small bootstrap CI of model accuracy also indicates that the results are not likely to be a result of sample bias.
Discussion
The present study examined the neuronal activity in the prestimulus time range specific to the subsequent perception of pain. By using constant stimuli at the pain threshold, we were able to observe differences in activity between painful and nonpainful sensation independent of stimulus magnitude. The median of ratings just at the threshold indicates that the psychophysical anchoring of stimulus intensity was successful. The invariance of mean ratings over the course of the experiment rules out effects of habituation or sensitization. It is thus safe to say that the observed systematic effects that distinguish the painful from the nonpainful trials must originate from spontaneous intraindividual fluctuations of neuronal activity and are not caused by external manipulations or linear changes over the course of the experiment, such as gradual weariness.
Power differences and generators
Oscillatory activity in the theta- and gamma-band was enhanced on group level in the prestimulus period in cases where the stimulus was rated as painful, compared with those cases when the stimulus was perceived as nonpainful. This suggests that ongoing activity in these bands is connected to subsequent sensory processing and specific to pain. These findings are also in line with previous research observing a long-lasting increase in theta-band power and a downshift of the cortical frequency spectrum in chronic pain (Sarnthein et al., 2006), and neuropathic disease (Walton et al., 2010), probably due to the inherent sensitization to pain under such conditions.
Prestimulus theta activity on group level showed a topographic pattern indicating the involvement of temporal sites. This result matches the result from the single-trial logistic regression analysis. The topography for gamma also followed this pattern, although significant differential gamma activity in group level occurred at more frontal sites. Source localization, however, revealed generators for both theta and gamma activity in the insular cortices. This is in line with the insula being regarded as an area integrating multiple sensory and affective inputs into a common percept. Previous studies have shown that, in this function, the insular cortex region is contributing to the perception of pain (Starr et al., 2009; Brodersen et al., 2012; Oertel et al., 2012; Segerdahl et al., 2015). Our results add to this interpretation because they demonstrate that the insula is not only involved in sensory processing of noxious stimuli but also involved in the modulation of the ongoing susceptibility to pain.
In addition, source localization also revealed a pronounced pain-specific increase in theta-band activity in the general region of the contralateral precuneus and/or posterior cingulate (PCC). This is in line with recent findings linking baseline functional connectivity between the default mode network and the PCC to pain responses in chronic back pain patients (Loggia et al., 2013). The PCC has also been indicated in self-referential processing (Cavanna and Trimble, 2006; Goffaux et al., 2014). Our results thus add to this interpretation, relating the ongoing state of theta activity in the precuneus/PCC to the formation of a pain percept after stimulus onset.
Network dynamics
Apart from the specific generators and frequencies involved in the prestimulus modulation of pain perception, we were interested in the dynamics behind the occurrence of the patterns we observed. The connectivity analysis reveals that the power difference in the ipsilateral insula coincides with a prestimulus connectivity pattern suggesting a frontocentral involvement. It should be noted that the connectivity changes cannot be explained as a simple reflection of the power effects because the phase locking value estimates connectivity independent of power. Thus, the observed connectivity patterns involving the frontal and temporal sites are specific to establishing the lateralized theta-band power states reflecting the sensitivity setting for the next stimulus. Interestingly, for the gamma-band, we observed a connection between frontal and parietotemporal areas on the contralateral side. This network was more strongly interconnected in painful trials. It seems likely that activity in both bands originates from the same area, possibly the anterior midcingulate cortex or the dorsomedial prefrontal cortex. The anterior midcingulate cortex is part of the cortical network exhibiting pain responses (Peyron et al., 2000; Chapin et al., 2012), whereas the dorsomedial prefrontal cortex is well connected to the bilateral insula and salience network (Eickhoff et al., 2016). Regardless of the exact localization of this frontocentral network node, the observed connectivity pattern indicates that the modulation in pain-specific prestimulus activity is a top-down process within a network, rather than caused by freely fluctuating network nodes.
This interpretation is backed by single-trial results from the logistic regression analysis. The regression model incorporates the information from both frontocentral gamma as well as temporal theta-power. The regression model thus incorporates multivariate single-trial information that might contain information about more complex interactions that are lost in averaging. Indeed, the parallel use of both single-trial and averaged information might help to further disentangle such interactions in future studies.
Interactions across stimulus onset
The effects we observed occurred before stimulus onset during a time range that traditionally constitutes the interstimulus interval. Since we kept experimental conditions constant and only the participants' ratings were used in contrasting pain and nonpain conditions post hoc, the effects must originate from ongoing activity fluctuations during rest. A straightforward interpretation would be that the period before the stimulus can be regarded as a baseline of activity that is carried forward to poststimulus processing. However, a more complicated, nonadditive interaction of prestimulus activity with the later processing stages has previously been established for other perceptual domains (Hesselmann et al., 2008).
Our results show that theta-band effects are specific to the prestimulus time range and end at stimulus onset. This is also reflected in the changing topography and polarity of effects poststimulus. For the gamma-band, the topography of power differences seems to stay similar across the stimulus onset. However, the maxima of the central gamma-band power effects are limited to the prestimulus time range. For connectivity, the theta-band pattern is also specific for prestimulus and poststimulus intervals. The frontocentral sites associated with the gamma-band power effects are interconnected with both temporal areas; however, the pattern observed in theta power is not fully present in theta connectivity. Instead, we found connectivity between frontal and contralateral parietotemporal areas in gamma-band. This pattern is consistent with a cross-frequency interplay between frontal gamma-activity and temporal theta-activity across stimulus onset. The theta-band effects are nonadditive and prestimulus specific, indicating that they interact with poststimulus processing in a more elaborate way. Furthermore, the prestimulus power difference in theta-band was predictive for pain ratings, which indicates a significant role in the formation of the behavioral response. The gamma-band connectivity seems a likely candidate for the transmission of the effect across frequency bands. Together, these findings suggest a top-down moderating role of frontal gamma-band activity in the perceptual nociceptive transformation, such as general perceptual sensitivity. The theta-band activity is specifically related to distinct stages of preparation and processing in the nociceptive system across stimulus onset.
The occurrence of systematic ongoing changes in oscillatory activity as well as connectivity are by now widely accepted (Raichle, 2010; Sadaghiani et al., 2010). It is still an open question, however, whether these fluctuations resemble pure attentional or arousal effects or are intrinsic to the sensory detection system (Linkenkaer-Hansen et al., 2004). To best possible suppress any external effects on attention and expectation, we instructed participants to keep their somatosensory attention constantly focused throughout the experiment. We also led participants to believe that the stimulation was random, so no explicit external modulation of expectation was induced. The fact that the prestimulus effect is most dominant in the theta-band and within the insula underlines the specificity of the observed effects for pain processing and make unspecific fluctuations, such as arousal or attention, unlikely (Dockstader et al., 2010; van Ede et al., 2010).
In conclusion, we were able to show that the activity pattern present at the bilateral insular cortex just before stimulus onset systematically determines the subsequent pain percept under constant stimulation. Herein, the ongoing activity in the theta-band is pain specific and also correlates with behavioral responses. The activity in the gamma-band seems to reflect frontocentral processes related to top-down modulation of the nociceptive system. Our data suggest that these processes might be mediated by gamma-band connectivity between frontocentral and temporal areas. We thus conclude that the resulting ongoing variability in the susceptibility to pain is a systematic effect of complex network dynamics. Our current results as well as future work on this subject might prove beneficial for a deeper understanding of the psychophysical anchoring of pain sensation. Such knowledge could serve as a basis for decoding ongoing pain sensitivity in clinical settings. Also, our results further underline the importance of the insular cortex and potentially the PCC as a potential therapeutic target in pain management.
Footnotes
P.T. was supported by a stipend from the International Graduate Research Group “Cross-Modal Interaction in Natural and Artificial Cognitive Systems” funded by the German Research Foundation (DFG, IGK 1247). The funding organization had no role in the design or conduct of this research.
The authors declare no competing financial interests.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001244. 70ra14-ra70ra14. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci U S A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Brodersen KH, Wiech K, Lomakina EI, Lin CS, Buhmann JM, Bingel U, Ploner M, Stephan KE, Tracey I. Decoding the perception of pain from fMRI using multivariate pattern analysis. Neuroimage. 2012;63:1162–1170. doi: 10.1016/j.neuroimage.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CA, Seymour B, Boyle Y, El-Deredy W, Jones AK. Modulation of pain ratings by expectation and uncertainty: behavioral characteristics and anticipatory neural correlates. Pain. 2008;135:240–250. doi: 10.1016/j.pain.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chapin H, Bagarinao E, Mackey S. Real-time fMRI applied to pain management. Neurosci Lett. 2012;520:174–181. doi: 10.1016/j.neulet.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernick MR. Bootstrap methods: a guide for practitioners and researchers. New York: Wiley; 2011. [Google Scholar]
- Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100:8538–8542. doi: 10.1073/pnas.1430684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- Dockstader C, Cheyne D, Tannock R. Cortical dynamics of selective attention to somatosensory events. Neuroimage. 2010;49:1777–1785. doi: 10.1016/j.neuroimage.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp. 2013;34:109–149. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7:1–26. doi: 10.1214/aos/1176344552. [DOI] [Google Scholar]
- Eickhoff SB, Laird AR, Fox PT, Bzdok D, Hensel L. Functional segregation of the human dorsomedial prefrontal cortex. Cereb Cortex. 2016;26:304–321. doi: 10.1093/cercor/bhu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009a;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009b;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goffaux P, Girard-Tremblay L, Marchand S, Daigle K, Whittingstall K. Individual differences in pain sensitivity vary as a function of precuneus reactivity. Brain Topogr. 2014;27:366–374. doi: 10.1007/s10548-013-0291-0. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson-Klavehn A, Düzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci U S A. 2009;106:5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Eger E, Kleinschmidt A. Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proc Natl Acad Sci U S A. 2008;105:10984–10989. doi: 10.1073/pnas.0712043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard DH, Pelli DG. What's new in Psychtoolbox-3? ECVP '07 Abstracts. Perception. 2007;36:1–235. doi: 10.1177/03010066070360S101. [DOI] [Google Scholar]
- Klem GH, Lüders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation: the International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Faymonville ME, Laureys S. The cognitive modulation of pain: hypnosis- and placebo-induced analgesia. In: Laureys S, editor. The boundaries of consciousness: neurobiology and neuropathology. New York: Elsevier; 2005. pp. 251–600. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63:1279–1292. doi: 10.3758/BF03194543. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Nikulin VV, Palva S, Ilmoniemi RJ, Palva JM. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci. 2004;24:10186–10190. doi: 10.1523/JNEUROSCI.2584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Schoffelen JM, Fries P. Nonparametric statistical testing of coherence differences. J Neurosci Methods. 2007;163:161–175. doi: 10.1016/j.jneumeth.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix.”. Neuroimage. 2011;54:2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ. EEG coherency: I. Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol. 1997;103:499–515. doi: 10.1016/S0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Oertel BG, Preibisch C, Martin T, Walter C, Gamer M, Deichmann R, Lötsch J. Separating brain processing of pain fromthat of stimulus intensity. Hum Brain Mapp. 2012;33:883–894. doi: 10.1002/hbm.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, Tanaka H, Hirata K, John ER, Prichep L, Biscay-Lirio R, Kinoshita T. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci. 2011;369:3768–3784. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. doi: 10.1163/156856897X00366. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain: a review and meta-analysis. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/S0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 2010;107:355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Friston KJ, Kleinschmidt A. The relation of ongoing brain activity, evoked neural responses, and cognition. Front Syst Neurosci. 2010;4:20. doi: 10.3389/fnsys.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari N, Büchel C, Rose M. Neurofeedback training of gamma band oscillations improves perceptual processing. Exp Brain Res. 2014;232:3353–3361. doi: 10.1007/s00221-014-4023-9. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tiemann L, Schuster T, Gross J, Ploner M. Neurophysiological coding of traits and states in the perception of pain. Cereb Cortex. 2011;21:2408–2414. doi: 10.1093/cercor/bhr027. [DOI] [PubMed] [Google Scholar]
- Schulz E, Tiemann L, Witkovsky V, Schmidt P, Ploner M. Gamma oscillations are involved in the sensorimotor transformation of pain. J Neurophysiol. 2012a;108:1025–1031. doi: 10.1152/jn.00186.2012. [DOI] [PubMed] [Google Scholar]
- Schulz E, Zherdin A, Tiemann L, Plant C, Ploner M. Decoding an individual's sensitivity to pain from the multivariate analysis of EEG data. Cereb Cortex. 2012b;22:1118–1123. doi: 10.1093/cercor/bhr186. [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C. Attention modulates spinal cord responses to pain. Curr Biol. 2012;22:1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DJ. Spectrum estimation and harmonic analysis. Proc IEEE. 1982;70:1055–1096. doi: 10.1109/PROC.1982.12433. [DOI] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Jensen O, Maris E. Tactile expectation modulates pre-stimulus β-band oscillations in human sensorimotor cortex. Neuroimage. 2010;51:867–876. doi: 10.1016/j.neuroimage.2010.02.053. [DOI] [PubMed] [Google Scholar]
- Walton KD, Dubois M, Llinás RR. Abnormal thalamocortical activity in patients with Complex Regional Pain Syndrome (CRPS) type I. Pain. 2010;150:41–51. doi: 10.1016/j.pain.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. Quest: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. doi: 10.3758/BF03202828. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]