Abstract
Alpha-5 gamma-aminobutyric acid type A receptors (α5-GABAARs) are located extrasynaptically, regulate neuronal excitability through tonic inhibition, and are fundamentally important for processes such as plasticity and learning. For example, pharmacological blockade of α5-GABAAR in mice with ischemic stroke improved recovery of function by normalizing exaggerated perilesional α5-GABAAR-dependent tonic inhibition. S44819 is a novel competitive selective antagonist of the α5-GABAAR at the GABA-binding site. Pharmacological modulation of α5-GABAAR-mediated tonic inhibition has never been investigated in the human brain. Here, we used transcranial magnetic stimulation (TMS) to test the effects of a single oral dose of 50 and 100 mg of S44819 on electromyographic (EMG) and electroencephalographic (EEG) measures of cortical excitability in 18 healthy young adults in a randomized, double-blinded, placebo-controlled, crossover phase I study. A dose of 100 mg, but not 50 mg, of S44819 decreased active motor threshold, the intensity needed to produce a motor evoked potential of 0.5 mV, and the amplitude of the N45, a GABAAergic component of the TMS-evoked EEG response. The peak serum concentration of 100 mg S44819 correlated directly with the decrease in N45 amplitude. Short-interval intracortical inhibition, a TMS–EMG measure of synaptic GABAAergic inhibition, and other components of the TMS-evoked EEG response remained unaffected. These findings provide first time evidence that the specific α5-GABAAR antagonist S44819 reached human cortex to impose an increase in cortical excitability. These data warrant further development of S44819 in a human clinical trial to test its efficacy in enhancing recovery of function after ischemic stroke.
SIGNIFICANCE STATEMENT The extrasynaptic α-5 gamma-aminobutyric acid type A receptor (α5-GABAAR) regulates neuronal excitability through tonic inhibition in the mammalian brain. Tonic inhibition is important for many fundamental processes such as plasticity and learning. Pharmacological modulation of α5-GABAAR-mediated tonic inhibition has never been investigated in the human brain. This study demonstrates that S44819, a selective α5-GABAAR antagonist, increases cortical excitability in healthy human subjects, as indicated by specific markers of transcranial magnetic stimulation-induced muscle and brain responses measured by electromyography and electroencephalography. Our findings imply that tonic inhibition in human cortex can be modified effectively and that this modification can be quantified with noninvasive brain stimulation methods. The actions of S44819 may be suitable to improve plasticity and learning.
Keywords: α5-GABAAR, excitability, human cortex, motor evoked potential, TMS–EEG, tonic inhibition
Introduction
The α5-subunit containing gamma-aminobutyric acid type A receptors (α5-GABAARs) predominate in the hippocampus, but are also expressed in the neocortex (Quirk et al., 1996; Möhler et al., 2002). They are located extrasynaptically at the base of the spines and on the adjacent shafts of pyramidal cell dendrites and are therefore in a privileged position to modulate excitatory input to pyramidal cells through tonic inhibition (Brünig et al., 2002; Farrant and Nusser, 2005; Möhler, 2006). Accordingly, increasing tonic inhibition shifts the input–output relationship of single cells to the right; that is, the probability of action potential generation to a given excitatory input decreases (Mitchell and Silver, 2003). Animal models demonstrated that specific pharmacological blockade, point mutations, or null mutants of α5-GABAARs enhance learning processes (Crestani et al., 2002; Maubach, 2003; Martin et al., 2010), whereas activation of α5-GABAARs reduces synaptic plasticity (Martin et al., 2010).
Acute ischemic stroke in mice and rats causes hypoexcitability in the peri-infarct cortex through increased tonic inhibition by overexpression and overactivation of α5-GABAARs (Clarkson et al., 2010; Schmidt et al., 2012). Pharmacological blockade or genetic lowering of the expression of α5-GABAARs enhances functional recovery after stroke in mice (Clarkson et al., 2010). Although increased tonic inhibition may be neuroprotective in the acute phase after ischemic stroke, counteracting excessive α5-GABAAR-mediated tonic inhibition in the subacute phase of stroke may allow a greater and/or more rapid recovery in stroke patients (Carmichael, 2012).
S44819 is a novel potent, competitive,and selective antagonist at the GABA-binding site of the α5-GABAAR tested in vitro (Etherington et al., 2016). It is as of yet unclear to what extent a single oral dose of S44819 is capable of reducing inhibition mediated by α5-GABAARs and thus increasing excitability in the human brain. Here, transcranial magnetic stimulation (TMS) was used in young healthy adults to obtain electromyographic (EMG) and electroencephalographic (EEG) markers of cortical excitability. Motor threshold, intensity needed to elicit a motor evoked potential (MEP) of a given amplitude, and short-interval intracortical inhibition (SICI) were obtained as classical TMS–EMG markers. Motor threshold represents axon membrane excitability, while SICI reflects synaptic GABAAergic inhibition of corticospinal neurons (for review (Ziemann et al., 2015)). The N45 component of the TMS-evoked EEG potential (TEP) involves GABAAergic activity, as benzodiazepines, i.e., allosteric positive modulators at GABAARs increase this potential (Premoli et al., 2014a; Premoli et al., 2014b). We hypothesized that S44819 would decrease the motor threshold and the intensity needed to elicit an MEP of a given amplitude, signifying a leftward shift in the input-output relationship of corticospinal neurons to TMS excitation, but would not affect SICI, a paired-pulse TMS measure of synaptic (phasic) rather than extrasynaptic (tonic) inhibition. Furthermore, we expected that S44819 would decrease the N45 amplitude given its previously established GABAAergic nature.
Materials and Methods
Participants
Eighteen healthy male volunteers (mean age ± SD: 27.5 ± 6.0 years; range 21–43) participated in this study after having provided written informed consent. All subjects were strongly right-handed according to the Edinburgh handedness inventory (mean laterality index of handedness ± SD: 87 ± 9; range 75–100; Oldfield, 1971), free of medication and any drug abuse (including alcohol and nicotine), and without any history of neurological or psychiatric diseases. All subjects underwent the Transcranial Magnetic Stimulation Adult Safety Screen (TASS; Keel et al., 2001), followed by a physical examination and a diagnostic EEG to rule out any contraindications against the TMS procedures of this study. At screening, participants were included if the resting motor threshold (RMT) was ≤50% of maximum stimulator output (MSO) and the stimulus intensity (SI) needed to elicit MEPs of, on average, 0.5 mV in peak-to-peak amplitude was ≤70% MSO. Blood alcohol test, drug screening, and urinary cotinine test were performed on the day before each experiment and participants had to have negative results in all tests to be allowed to take part in the study. Experimental procedures conformed to the Declaration of Helsinki and the Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte) and the local ethics committee of the Medical Faculty of Eberhard-Karls-University Tübingen approved the study (EudraCT #2014-004681-13).
Experimental design
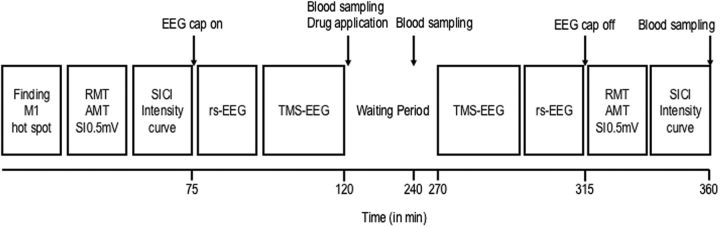
To evaluate whether the antagonistic action of S44819 on α5-GABAARs detected in preclinical studies in vitro is relevant for the modulation of the human primary motor cortex (M1) excitability, a randomized, double-blinded, placebo-controlled, crossover study investigated the effects of a single oral dose of 50 and 100 mg of S44819 on TMS–EMG and TMS–EEG measures of corticospinal and cortical excitability. Participants were assigned to one of the six possible sequences of treatment (three subjects were allocated in each of the six sequences of treatment), which all included three successive treatment periods during which placebo, 50 mg S44819 or 100 mg S44819 was administered. One week separated each of the treatment periods to exclude the possibility of carryover effects between treatment periods according to a S44819 serum half-life of ∼7 h in human (Institut de Recherches Internationales Servier, 2014). Drug dosages were chosen based on extrapolation of pharmacokinetic (PK) results in animal models and doses presenting satisfactory clinical safety in a first human phase I study of S44819 (Institut de Recherches Internationales Servier, 2016). To ensure controlled conditions for food and fluids for all participants, they were admitted to our phase I unit on the evening of the day before each treatment period. TMS measurements were performed on next morning starting either at 8:00 A.M. or 10:00 A.M. (always at the same time for a given participant). TMS sessions always followed the same sequence and timing of investigations (Fig. 1): baseline TMS–EMG and TMS–EEG measurements; first PK blood sampling, oral study drug intake; waiting period of 150 min; second PK blood sampling (at +2 h after study drug intake); TMS–EEG and TMS–EMG postdrug measurements; and further PK blood samplings (at +4 h, +6 h, +8 h after study drug intake). The timing of TMS postdrug measurements was based on the PK data from a first human phase I study estimating the maximum systemic levels of S44819 between ∼2.5 and 4 h after oral intake (Institut de Recherches Internationales Servier, 2014).
Figure 1.
Timeline of experiments. TMS–EMG and TMS–EEG measures were obtained immediately before and 150 min after study drug intake (100 mg S44819, 50 mg S44819, or placebo).
Data recording
TMS.
Participants were asked to sit on a comfortable reclining chair and stay awake with eyes open. Monophasic TMS pulses were applied over the hand area of the dominant (left) M1 using two Magstim 2002 magnetic stimulators connected to a figure-eight coil (outer diameter of each wing, 70 mm) through a BiStim Module (Magstim). The coil was placed tangentially on the scalp with the handle pointing backwards and 45° away from the midline. This way, the direction of the TMS-induced current in the brain was from lateral–posterior to medial–anterior, leading to largely transsynaptic excitation of corticospinal cells through horizontal corticocortical connections (Di Lazzaro et al., 2008). The hand representation of the left M1 was determined and marked with a pen on the scalp as the coil position, where TMS at a marginally suprathreshold stimulus intensity consistently resulted in largest MEPs in the right first dorsal interosseus (FDI) muscle. MEPs were recorded by using surface EMG with Ag-AgCl cup electrodes in a belly–tendon arrangement. EMG data were recorded by spike2 software (Cambridge Electronic Design), the raw signal was amplified (Digitimer D360 8-channel amplifier), band-pass filtered (20 Hz to 2 kHz), and digitized at an A/D rate of 5 kHz (CED Micro 1401; Cambridge Electronic Design). Single-pulse TMS was used to determine RMT, active motor threshold (AMT), and stimulus intensity needed to elicit a motor evoked potential of 0.5 mV amplitude (SI0.5mV). RMT was defined as the lowest stimulus intensity eliciting a MEP of ≥50 μV in peak-to-peak amplitude in at least five of 10 successive trials (Groppa et al., 2012). AMT was determined in the slightly voluntarily contracting FDI muscle (∼10% of maximum voluntary contraction monitored by audiovisual feedback of the EMG signal) as the lowest stimulus intensity resulting in a MEP of >100 μV in peak-to-peak amplitude in at least five of 10 successive trials. SI0.5mV was determined as the stimulus intensity required for MEPs of, on average, 0.5 mV in peak-to peak amplitude in the voluntarily relaxed FDI. SICI was tested by paired-pulse TMS. The SICI paradigm involved pairing of a conditioning stimulus (CS) followed by a test stimulus (TS) at a short interstimulus interval of 2.0 ms to avoid possible contamination by short-interval intracortical facilitation (Peurala et al., 2008). A SICI intensity curve was obtained with CS intensities ranging from 50% AMT to 120% AMT in steps of 10% AMT (i.e., 8 different CS intensities) and TS intensity of SI0.5mV. TS intensity was adjusted to maintain a test MEP amplitude of, on average, 0.5 mV in the postdrug SICI measurements. CS/TS and TS alone conditions were repeated 10 times each in randomized order in a block of 90 trials. The intertrial interval varied randomly between 4 and 8 s to limit anticipation of the next trial. SICI datasets from 5/18 subjects had to be discarded from analysis because of incomplete voluntary relaxation of the FDI or too small test MEP amplitudes (<200 μV). Both factors can lead to a nonspecific (drug-unrelated) reduction of SICI (Ridding et al., 1995; Sanger et al., 2001).
EEG recordings.
To evaluate TEPs, EEG was recorded parallel with the EMG recordings. EEG signals were acquired through TMS-compatible EEG equipment (BrainAmp DC; Brain Products) using a 64-channel EEG cap (BrainCap-Fast'n Easy; Brain Products). FCz and AFz served as the active reference and ground electrodes, respectively. To monitor eye movement artifacts and blinks, two more electrodes were placed outside of the outer canthus and over the right eye. Electrode impedances were maintained at <5kΩ throughout the experiment. EEG signals were recorded via BrainVision Recorder software version 1.20 (BrainProducts) with a resolution of 0.5 μV/bit, a low-pass filter of 1 kHz, and a sampling rate of 5 kHz. During the TMS–EEG recordings, white noise was applied through ear phones to mask the TMS click and to avoid TMS-evoked auditory potentials (Massimini et al., 2005; Casarotto et al., 2010). A total of 130 TMS pulses were applied at baseline and 150 min after drug intake over the FDI hotspot of the left M1 at 100% RMT as determined at baseline; that is, no adjustment of stimulus intensity was made in the postdrug TMS–EEG measurements. Because there were no drug effects on RMT (cf. Fig. 3), the observed drug effects on TEPs (see below) cannot be accounted for by changes motor threshold. MEPs were monitored visually during TMS–EEG recording. No or only miniature MEPs were elicited. This ensured that the somatosensory afferent signals caused by muscle twitches were absent and therefore did not contaminate the TEPs. The intertrial interval varied randomly between 4 and 8 s to limit anticipation of the next trial.
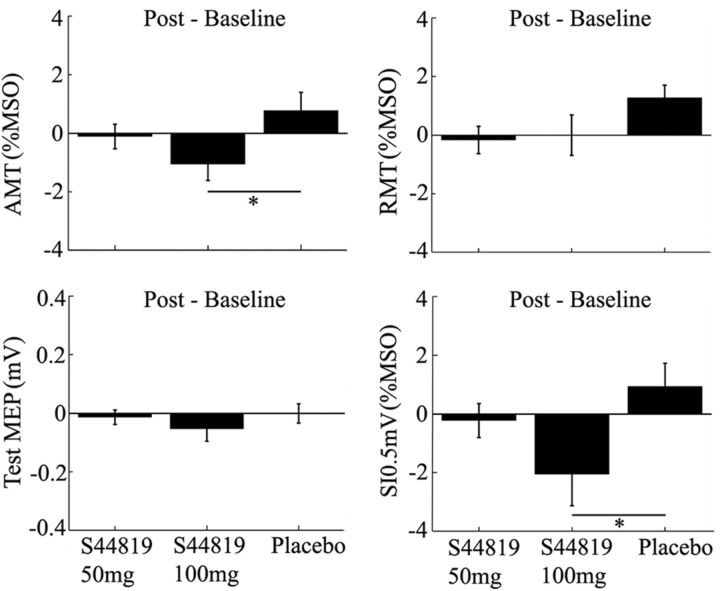
Figure 3.
Mean changes (±1 SEM) in RMT, AMT, SI0.5mV, and test-MEP (postdrug − baseline) in the three drug conditions (50 mg S44819, 100 mg S44819, and placebo). The 100 mg S44819 dose decreased AMT and SI0.5mV compared with placebo (*p < 0.05).
Data analyses
TMS–EMG analysis.
EMG data were analyzed via Spike2 software (Cambridge Electronic Design) and MATLAB (R2015a, RRID:SCR_000903; The MathWorks). Peak-to-peak MEP amplitudes were calculated for each trial and averaged per each condition. The SICI intensity curve was obtained by calculating the ratio of mean conditioned MEP (eight different CS intensities: 50%, 60%, 70%, 80%, 90%, 100%, 110%, and 120% AMT) over mean test MEP (SI0.5mV). For assessment of possible drug-induced changes in RMT, AMT, SI0.5mV, and test-MEP, two-way repeated measures ANOVA (rmANOVA) was used, with the main within-subject effects of time (two levels: baseline and postdrug) and drug condition (three levels: placebo, 50 mg S44819, and 100 mg S44819). For SICI, a three-way rmANOVA with the main within-subject effects of time, drug condition, and CS intensity (8 levels: 50–120% AMT) was run. Order effects were assessed by substituting the main effect of drug condition by period (three levels: period 1, period 2, and period 3). In case of significant interactions between time and drug condition (or period), post hoc tests were applied to compare effects between the single drug conditions. Differences were considered significant whenever p < 0.05.
TMS–EEG analysis.
EEG data were processed offline using BrainVision Analyzer software (version 2.0, RRID:SCR_002356; BrainProducts) and the Fieldtrip open source toolbox (www.ru.nl/fcdonders/fieldtrip/, RRID:SCR_004849) running in MATLAB (R2015a; The MathWorks). The EEG raw data were re-referenced to linked mastoid electrodes and downsampled to 1 kHz. All trials were inspected visually to remove artifact-contaminated trials caused by movements, blinks, or TMS-related muscle artifacts. Artifact-free trials were segmented from −500 ms to 500 ms relative to the TMS pulse and then, to remove the electromagnetic TMS artifact, a linear interpolation was applied from −10 ms to 10 ms around each TMS pulse (Thut et al., 2011; Premoli et al., 2014b). Next, epochs were baseline corrected by subtracting the mean amplitude of the channel signal during an interval between −500 ms and −100 ms before the TMS pulse. A digital band-pass filter was then applied (2–80 Hz). Further, a notch filter with a stop band centered at 50 Hz was applied for noise-line correction. Independent component analysis was then used to remove components reflecting TMS-induced muscle activity and TMS artifacts (within the first 50 ms after the TMS pulse) from TEPs based on each participant's data (Rogasch and Fitzgerald, 2013; Rogasch et al., 2014). Averages at each recording channel were calculated across the retained trials (mean ± SEM, 98 ± 4, range 74–118), and finally grand averaged TEPs were computed by averaging per condition (2 levels of time, 3 drug conditions) across all participants. A 45 Hz low-pass filter was applied to smooth the TEP components with latency <200 ms. Five TEP components were considered (P25, N45, P70, N100, and P180) due to their consistent reproducibility upon M1 stimulation as reported in several other studies (Bonato et al., 2006; Lioumis et al., 2009; Ferreri et al., 2011; Premoli et al., 2014b). For their quantitative analysis, five time windows of interest (TOIs) were defined, based on the grand average TEP components: P25 (15–35 ms), N45 (38–60 ms), P70 (63–82 ms), N100 (85–119 ms), and P180 (156–230 ms; Fig. 2; Premoli et al., 2014a; Premoli et al., 2014b). These TOIs were adjusted individually to take into account interindividual variability of TEP peak latencies and peak amplitudes of the TEP components were determined for each participant, time point, and drug condition. To correct for multiple comparisons (i.e., electrodes, time points within TOIs), a cluster-based permutation analysis (Maris and Oostenveld, 2007) was conducted as implemented in FieldTrip (http://fieldtrip.fcdonders.nl/; Litvak et al., 2007; Premoli et al., 2014a; Premoli et al., 2014b). A paired t test was applied to compare the postdrug versus baseline data within the same drug condition or postdrug data between different drug conditions for each electrode at each data point within the five different TOIs. t-values exceeding an a priori threshold of p < 0.05 were clustered based on adjacent data points and neighboring electrodes. Cluster-level statistics were calculated by taking the sum of the t-values within every cluster. The statistical comparisons were done with respect to the maximum values of summed t-values. By means of a permutation test (i.e., randomizing data across postdrug and baseline conditions and rerunning the statistical test 1000 times), a reference distribution of the maximum of summed cluster t-values was obtained for evaluating the statistics of the actual data. Clusters in the original dataset were considered to be significant at an α level of 5% if <5% of the permutations used to construct the reference distribution yielded a maximum cluster-level statistic larger than the cluster-level value observed in the actual data.
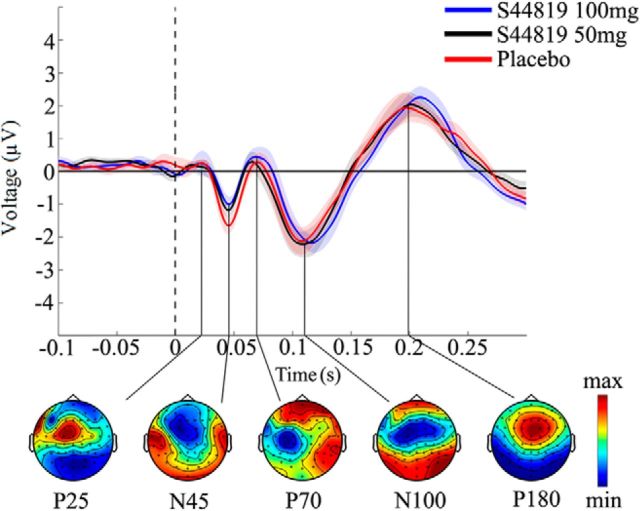
Figure 2.
Grand average TEPs before drug intake. TEPs were averaged over all channels and artifact-free trials at baseline in the three different drug conditions (100 mg S44819, 50 mg S44819, and placebo) and labeled based on their polarity (P: positive; N: negative) and approximate latency relative to the time of applying TMS (time 0, vertical dashed line) over the left M1 (P25, N45, P70, N100, and P180). Topographical distributions of surface voltages illustrated in the bottom were grand averaged over the three drug conditions in nonoverlapping TOIs after TMS (P25: 15–35 ms; N45: 38–60 ms; P70: 63–82 ms; N100: 85–119 ms; P180: 156–230 ms). Color coding of each map was calibrated according to the maximum positivity (red) and negativity (blue) of the separate grand-averaged TEPs.
The data from three participants had to be discarded from analysis due to large artifacts in at least one recording session. Therefore, the presented TEP data and analyses are based on 15 participants.
Analysis of resting-state EEG data postdrug versus predrug.
To investigate drug-induced changes of spontaneous oscillations, 3 min periods of eyes-closed resting-state EEG (rs-EEG) data recorded postdrug versus predrug (cf. Fig. 1) were analyzed. Data were preprocessed consistently with the TEP analysis (see above) and then divided into nonoverlapping 2 s time windows. The power spectra of the rs-EEG signal postdrug and predrug were analyzed in the theta-frequency (4–7 Hz), alpha-frequency (8–12 Hz), and beta-frequency (13–30 Hz) bands by means of Hanning taper with frequency-dependent window length (frequency steps: 1 Hz in the range 4–50 Hz). Then, the same cluster statistical test described previously for TEPs was applied to the topoplots of the rs-EEG power in the three bands (postdrug vs predrug; Maris and Oostenveld, 2007).
Results
S44819 and all study procedures were generally well tolerated. Serious adverse events did not occur.
PK
The mean (±SD) peak serum concentrations were 9.09 ± 1.94 ng/ml (range 6.0–12.6 ng/ml) for 50 mg S44819 and 16.47 ± 4.70 ng/ml (range 10.0–28.9 ng/ml) for 100 mg S44819. A model-based PK approach was used to assess individual and mean PK parameters. Mean time to peak concentration (Fig. 1) was 198 min for 50 mg S44819 and 184 min for 100 mg S44819. These times correspond to 318 min and 314 min, respectively, on the time axis in Figure 1. Therefore, the time to peak concentration was reached, on average, at approximately the end of the postdrug TMS–EEG recordings and immediately before the postdrug TMS–EMG recordings.
Drug effects on RMT, AMT, SI0.5mV, and unconditioned test-MEP amplitude
RMT, AMT, SI0.5mV, and unconditioned test-MEP amplitude in the SICI measurements were not different at baseline between drug conditions (Table 1, all p > 0.05). This ensured that baseline differences could not account for any of the drug effects (see below). Drug effects on RMT, AMT, SI0.5mV, and unconditioned test-MEP amplitude (postdrug − baseline) are summarized in Figure 3. Two-way rmANOVA with the main effects of time (two levels: baseline and postdrug) and drug condition (three levels: placebo, 50 mg S44819, and 100 mg S44819) did not reveal any significant main effects or an interaction of time and drug condition for RMT and test-MEP. In contrast, AMT and SI0.5mV showed a significant interaction between time and drug condition (AMT: F(2,34) = 3.361, p = 0.047; SI0.5mV: F(2,34) = 3.526, p = 0.041). Post hoc testing revealed that these effects were explained by a significant decrease in AMT and SI0.5mV in the 100 mg S44819 condition compared with the placebo condition (AMT: p = 0.004; SI0.5mV: p = 0.022; Fig. 3). None of the other post hoc comparisons between drug conditions was significant. Two-way rmANOVAs with the main effects of time (two levels: baseline and post drug) and period (period 1, period 2, and period 3) did not reveal any significant main effect or interaction of time and period for RMT, AMT, SI0.5mV, or unconditioned test-MEP, signifying that there were no order effects.
Table 1.
TMS–EMG measures at baseline in the different drug conditions
Drug effects on SICI
The three-way rmANOVA with main effects of time (two levels: baseline and postdrug), CS intensity (eight levels: 50% to 120% AMT), and drug condition (three levels: placebo, 50 mg S44819, and 100 mg S44819) did not reveal any significant interaction between time and drug conditions (F(2,24) = 1.538, p = 0.235), or time, drug condition, and CS intensity (F(5.48,65.73) = 0.698, p = 0.640; Fig. 4). Similarly, the three-way rmANOVA with the main effects of time, CS intensity, and period (three levels: period 1, period 2, and period 3) did not show significant interactions of time with period, or time, period, and CS intensity, excluding an order effect.
Figure 4.
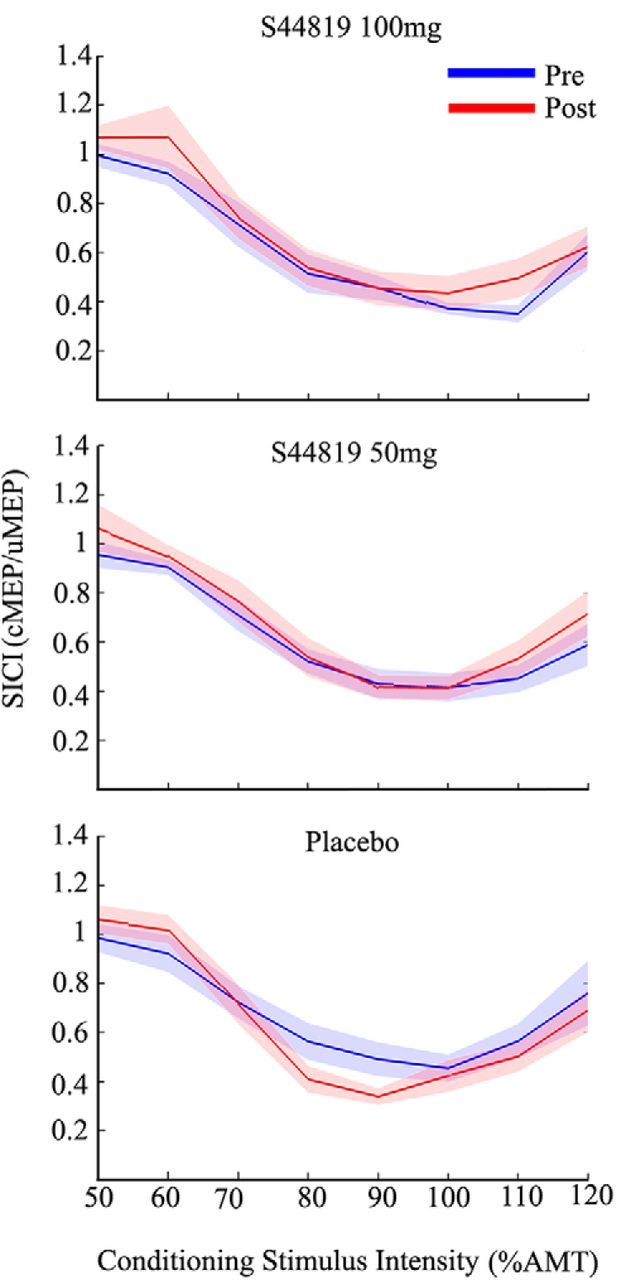
Mean SICI intensity curves (shadings: ±1 SEM) plotted against eight different conditioning stimulus intensities before (blue) and after (red) intake of S44819 (100 mg; top), S44819 (50 mg; middle), and placebo (bottom).
Drug effects on TEPs
Figure 2 illustrates the grand average TEPs of 15 participants at baseline in each of the three drug conditions (placebo, 50 mg S44819, and 100 mg S44819). Five typical TEP components were identified (P25, N45, P70, N100, and P180) and are shown with their topographical surface voltages. TEPs were highly reproducible and there were no differences at baseline between the three drug conditions.
The 100 mg S44819 dose suppressed the N45 over the period of 43–61 ms after stimulus (postdrug: −0.52 ± 0.27 μV; baseline: −1.34 ± 0.27 μV; p < 0.05; Fig. 5A). There was also a significant reduction in the N45 in the postdrug 100 mg S44819 condition versus postdrug placebo condition over the period 33–55 ms after stimulus (postdrug 100 mg S44819: −0.64 ± 0.27 μV; postdrug placebo: −2.17 ± 0.38 μV; p = 0.01; Fig. 5B). In contrast, there were no significant differences in postdrug versus baseline in the placebo (Fig. 5E) or 50 mg S44819 conditions (Fig. 5C) or the postdrug 50 mg S44819 versus postdrug placebo conditions (Fig. 5D) in any of the TOIs (all p > 0.05).
Figure 5.
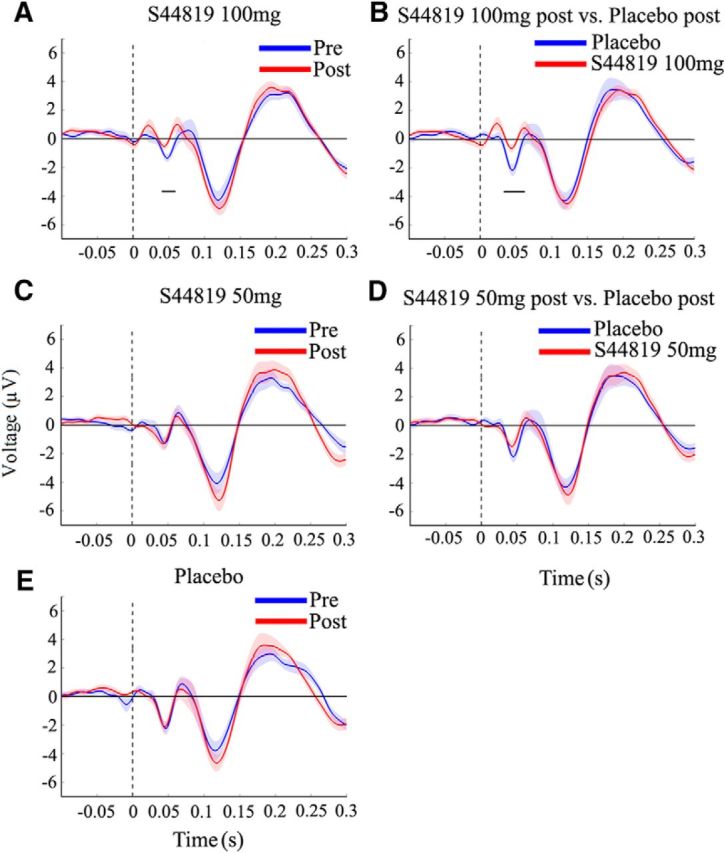
Grand-averaged TEPs (shadings: ±1 SEM) elicited by TMS of left M1 before (blue) versus after (red) intake of S44819 (100 mg; A), S44819 (50 mg; C), and placebo (E) or after drug intake comparing S44819 (100 mg; red) versus placebo (blue; B) and S44819 (50 mg; red) versus placebo (blue; bottom; D). The 100 mg S44819 dose decreased specifically the N45 TEP component compared with the baseline measurement (A) and compared with postdrug placebo (B), whereas there were no changes in other drug conditions and/or TEP components. Horizontal black bars underneath the N45 denote the significant periods of drug-induced changes. Data are grand averages of those channels that showed a significant difference in the N45 TEP component in the 100 mg S44819 postdrug versus baseline (A) or between the postdrug 100 mg S44819 and placebo conditions (B). These channels are indicated in Figure 6, A and B, respectively.
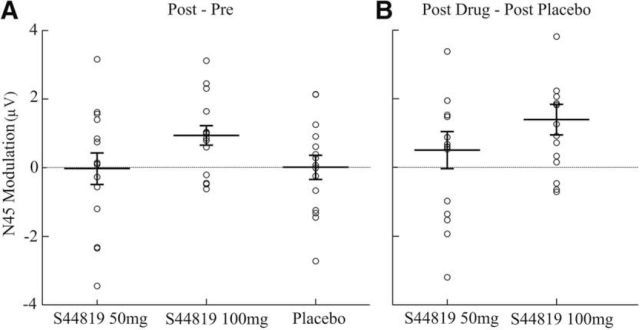
Figure 6 shows the topological distribution of changes in EEG surface voltage in the N45 component caused by the study drug (Fig. 6A: postdrug vs baseline 100 mg S44819; Fig. 6B: postdrug 100 mg S44819 vs postdrug placebo). Cluster-based analyses revealed that this significant depression of N45 amplitude occurred largely in the nonstimulated right frontal and central cortex (postdrug vs baseline 100 mg S44819: p < 0.05, significant channels: FC2, FC6, F2, F4, F6, C6, and AF8; postdrug 100 mg S44819 vs postdrug placebo: p = 0.01, significant channels: FC2, FC6, F2, F4, F6, F8, Cz, C2, C4, C6, CP2, CP6, and AF8); that is, in a region where the N45 is typically expressed (cf. Fig. 2 and left column of Fig. 6). Finally, the individual N45 data are displayed in Figure 7 to demonstrate the consistency of the suppressive effect by 100 mg S44819 across subjects. In summary, results suggest a highly specific effect of S44819; that is, a selective depression of the N45 TEP component at the dose of 100 mg S44819.
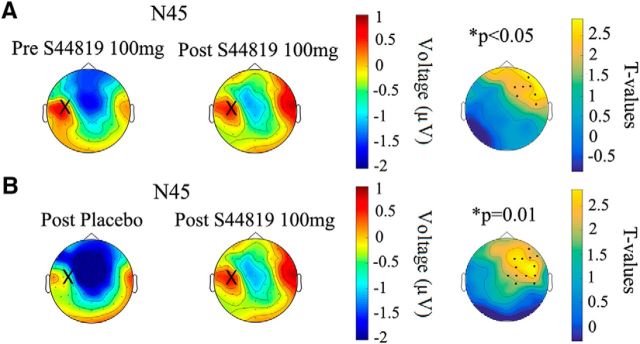
Figure 6.
Topographical surface voltage plots of the N45 TEP component. A, Topoplots before (left column) and after (middle column) intake of 100 mg S44819 and t-statistic map (right column) of the postdrug versus baseline differences (postdrug − baseline 100 mg S44819). B, Topoplots of the N45 TEP component after intake of placebo (right column) and S44819 (100 mg; middle column), and t-statistic map (right column) of the postdrug 100 mg S44819 versus post placebo differences (postdrug 100 mg S44819 − post placebo). Large crosses on the left hemispheres indicate the site of TMS over left M1. Yellow color on the t-statistic maps represents a decrease of N45 negativity. Black dots on the t-statistics maps represent the channels showing a significant difference. Note that these channels are located in the nonstimulated right hemisphere in the region where the N45 is predominantly expressed.
Figure 7.
Drug-induced modulation of the N45 TEP component (single-subject data). Scatter plots of individual amplitude modulations (A: postdrug − baseline; B: postdrug − postplacebo) of the N45 for all conditions are illustrated. In all conditions, data were extracted from EEG channels showing a significant difference for postdrug 100 mg S44819 − baseline S44819 (100 mg; A), or postdrug 100 mg S44819 − postplacebo (B). Error bars indicate the group mean ±1 SEM.
Linear regression analyses were performed to determine whether the depression in N45 amplitude (postdrug − baseline 100 mg S44819) correlated with the TMS–EMG changes observed for AMT and SI0.5mV. None of these correlations showed significance (all p > 0.05).
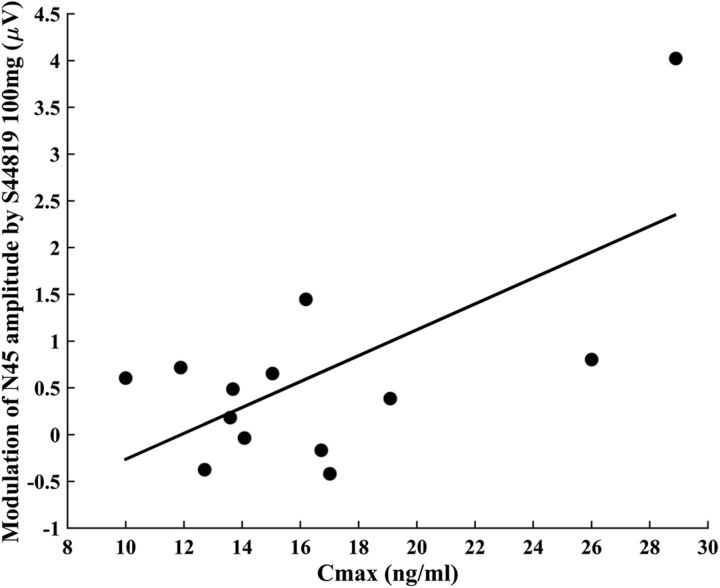
Finally, peak 100 mg S44819 serum concentration correlated with the change in N45 amplitude (postdrug − baseline 100 mg S44819; Fig. 8, Pearson correlation coefficient r = 0.066, p = 0.015), but not with the changes in AMT or SI0.5mV (all p > 0.05).
Figure 8.
Correlation between the peak 100 mg S44819 serum concentration and the change in N45 amplitude (postdrug 100 mg S44819 − baseline). Pearson correlation coefficient r = 0.66, p = 0.015. The data from only 13/15 subjects were subjected to this correlation analysis because two subjects had no identifiable N45 potential.
Drug effects on rs-EEG power
The cluster-based analysis did not reveal any significant effect of any of the drugs on rs-EEG power in any of the frequency bands (theta-, alpha-, and beta-frequency bands; all p > 0.05).
Discussion
Significant effects of the specific α5-GABAAR antagonist S44819 on excitability of the human cortex could be demonstrated by TMS–EMG and TMS–EEG assessments. Main findings were decreases in AMT and SI0.5mV and a reduction specifically of the N45 TEP component of the TMS-induced EEG response, whereas SICI and other TEP components remained unaffected. In the following paragraphs, these findings will be discussed and interpreted.
Dose effects
All significant effects of S44819 on TMS–EMG and TMS–EEG measures of cortical excitability were observed only with the 100 mg dose, but not with the 50 mg dose. The presence of significant effects strongly suggests that a single oral dose of 100 mg S44819 reaches the human brain to a relevant extent. All observed effects are consistent with the notion that 100 mg S44819 increased cortical excitability via reduction of tonic inhibition. Importantly, the decrease in N45 amplitude correlated directly with the peak serum concentration of 100 mg S44819, suggesting that the modulation of N45 can be used as a direct marker of S44819 action, most likely on α5-GABAARs, in the human brain.
SICI
S44819 did not change SICI. Several factors likely account for this nil finding. SICI has been established as a marker for GABAAR-mediated inhibition (for review, see Ziemann et al., 2015). Benzodiazepines, allosteric positive modulators of GABAARs containing α1-, α2-, α3-, or α5-subunits, consistently increased SICI (Ziemann et al., 1996a; Di Lazzaro et al., 2000; Ilić et al., 2002; Di Lazzaro et al., 2005; Di Lazzaro et al., 2006; Di Lazzaro et al., 2007; Müller-Dahlhaus et al., 2008; Teo et al., 2009). There have been two important specifications to these findings. First, zolpidem, a benzodiazepine-like hypnotic with largely specific positive modulation of the α1-GABAAR, had no effect on SICI (Di Lazzaro et al., 2007; Teo et al., 2009). This suggested that SICI represents inhibition mediated by GABAARs other than the α1-GABAAR (i.e., the α2-, α3-, and/or α5-subtypes of the GABAAR). Second, the benzodiazepine antagonist flumazenil did not change SICI (Jung et al., 2004). This provided evidence that there is normally no significant endogenous activity at the benzodiazepine GABAAR-binding site in the human M1.
Furthermore, cortical polarization by anodal or cathodal transcranial direct current stimulation (tDCS) did not affect SICI (Nitsche et al., 2005). This is a very important nil finding because pharmacological blockade of the α5-GABAAR would be expected to shift the resting membrane potential toward depolarization (similar to anodal tDCS; and see below on the effects of S44819 on AMT and SI0.5mV). In contrast to the synaptic α1-, α2-, and α3-GABAARs, the α5-GABAAR is localized extrasynaptically (Brünig et al., 2002; Farrant and Nusser, 2005) and is involved in tonic inhibition that decreases pyramidal neuron excitability (Mitchell and Silver, 2003; Caraiscos et al., 2004). Because neither intervention (tDCS or S44819) affected SICI, the most straightforward conclusion is that SICI measures specifically synaptic, but not extrasynaptic, GABAAergic inhibition in human M1. This conclusion is in full accordance with the currently accepted notion that the subthreshold CS of the SICI paired-pulse protocol excites low-threshold inhibitory interneurons that synapse via GABAARs onto pyramidal neurons that are then less excitable to the succeeding suprathreshold test stimulus (Ilić et al., 2002; Di Lazzaro and Ziemann, 2013).
Single pulse TMS–EMG measures (RMT, AMT, SI0.5mV, test MEP)
The 100 mg S44819 dose reduced AMT and SI0.5mV. This suggests that blockade of extrasynaptic α5-GABAARs increases the excitability of corticospinal neurons to single-pulse TMS. This is plausible because the blockade of extrasynaptic α5-GABAARs by S44819 will result in a less hyperpolarized (i.e., more depolarized) state of pyramidal neurons in M1. Basic experiments showed that an increase of tonic inhibition shifts the input–output relationship of single cells to the right; that is, the probability of action potential generation to a given excitatory input is decreased (Mitchell and Silver, 2003). At the systems level, depolarization, probably of the somatic region of corticospinal cells, by anodal tDCS over M1 increases MEP amplitude (Nitsche and Paulus, 2000, 2001; Nitsche et al., 2005). However, RMT and AMT remain unchanged after anodal tDCS (Nitsche et al., 2005). In addition, an increase of ambient GABA in the extracellular space by vigabatrin, an irreversible inhibitor of the GABA transaminase, or by tiagabine, a GABA-transporter-blocking GABA reuptake inhibitor, had no effect on motor thresholds or MEP amplitude (Ziemann et al., 1996b; Werhahn et al., 1999). However, the change of GABA concentration at extrasynaptic sites by these drugs may be too small to drive changes in excitability of corticospinal neurons to single-pulse TMS. Consistent with the current findings, some, but not all, of the previous pharmacological TMS–EMG studies that tested the effects of benzodiazepines reported an increase in motor threshold (Ilić et al., 2002) or a decrease in MEP amplitude (Di Lazzaro et al., 2000; Boroojerdi et al., 2001). These effects may be explained by the positive modulation of benzodiazepines at the extrasynaptic α5-GABAAR. S44819 did not affect RMT, but there was a nonsignificant trend for 100 mg S44819 to reduce RMT compared with placebo (p = 0.074, cf. Fig. 3). There were no significant drug effects on the unconditioned test-MEP amplitude because test stimulus intensity was adjusted, whenever necessary, to maintain a test MEP amplitude of, on average 0.5 mV, in the postdrug paired-pulse SICI measurements.
TMS–EEG measures of TEPs
The 100 mg S44819 dose reduced the N45 amplitude and the amount of N45 amplitude reduction was correlated directly with the peak serum concentration of 100 mg S44819. The effect was expressed in the frontal and central region of the nonstimulated right hemisphere, where the N45 potential is predominantly localized (Komssi et al., 2004; Bonato et al., 2006; Litvak et al., 2007; Rogasch et al., 2013; Premoli et al., 2014a; Premoli et al., 2014b). In addition, the effect was specific because none of the other TEP amplitudes (P25, P70, N100, or P180) was altered by S44819. Furthermore, the absence of drug effects on rs-EEG power suggests that the observed reduction of N45 amplitude by 100 mg S44819 cannot be explained by concomitant alterations in rs-EEG power. The reduction of the N45 amplitude by S44819 is opposite to the significant increase observed in our previous studies by single doses of the benzodiazepines alprazolam and diazepam and the hypnotic zolpidem, a largely selective positive modulator at the α1-GABAAR (Premoli et al., 2014a; Premoli et al., 2014b). Because alprazolam, diazepam, and zolpidem exhibit a common receptor affinity profile targeting the α1-subunit of the GABAAR, these data strongly suggested that activation of α1-subunit-containing GABAARs contributes to the generation of the N45 potential. The current findings extend this notion by providing evidence that the α5-GABAAR also contributes to the generation of the N45 potential.
The lack of an effect of S44819 on the N100 TEP component may be explained by the fact that activation of GABABRs, but not GABAARs, contributes to its generation at the site of its predominant expression in the stimulated M1, as indicated by the significant increase of the N100 amplitude by baclofen, a selective GABABR agonist (Premoli et al., 2014b). Furthermore, we had observed a decrease of the N100 amplitude in our previous studies by alprazolam and diazepam, but not zolpidem (Premoli et al., 2014b). The current findings suggest that the transcallosal and/or corticothalamo-cortical interactions, which are probably responsible for the modulation of the N100 amplitude in the nonstimulated hemisphere by diazepam and alprazolam, depend on activation of α2- and/or α3-GABAARs, but not α1- (zolpidem) or α5-GABAARs (S44819).
Two limitations of this study should be noted: behavioral data have not been obtained because this study was designed to test primarily the effects of S44819 on cortical and corticospinal excitability as measured with TMS–EEG and TMS–EMG. However, investigation of behavioral measures, in particular on memory and learning processes that are expected to be influenced by S44819, and the relation of the currently obtained electrophysiological measures with behavior would be of interest in future studies. Furthermore, implementation of navigated TMS would be advantageous to optimize test–retest reliability in future pharmaco-TMS–EEG studies (Casarotto et al., 2010).
Conclusions
The present data provide evidence that the selective α5-GABAAR antagonist S44819 reached human cortex at a sufficient concentration to impose an increase in corticospinal and cortical excitability, as indexed by a decrease in motor threshold measured by single-pulse TMS–EMG and a decrease of the amplitude of the N45 component of the TMS–EEG response. These data warrant the further development of S44819 in a human clinical trial to test its efficacy in enhancing recovery of function after ischemic stroke, in which tonic inhibition mediated by α5-GABAARs is abnormally increased in the peri-infarct zone.
Footnotes
This work was supported by the Institut de Recherches Internationales Servier. M.S. was supported by Robert Bosch Stiftung, Stuttgart, Germany. We thank Drs. Moerike, Gleiter, and Igel for local study support.
U.Z. received personal fees from Biogen Idec GmbH, Bayer Vital GmbH, Bristol Myers Squibb GmbH, CorTec GmbH, Medtronic GmbH and grants from Biogen Idec GmbH and Janssen Pharmaceuticals NV outside of the submitted work. The remaining authors declare no competing financial interests.
References
- Bonato C, Miniussi C, Rossini PM. Transcranial magnetic stimulation and cortical evoked potentials: a TMS/EEG co-registration study. Clin Neurophysiol. 2006;117:1699–1707. doi: 10.1016/j.clinph.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellbacher W, Cohen LG. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin Neurophysiol. 2001;112:931–937. doi: 10.1016/S1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- Brünig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012;69:161–167. doi: 10.1001/archneurol.2011.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, Romero Lauro LJ, Bellina V, Casali AG, Rosanova M, Pigorini A, Defendi S, Mariotti M, Massimini M. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS One. 2010;5:e10281. doi: 10.1371/journal.pone.0010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front Neural Circuits. 2013;7:18. doi: 10.3389/fncir.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/S1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol. 2007;118:2207–2214. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Pasqualetti P, Määttä S, Ponzo D, Ferrarelli F, Tononi G, Mervaala E, Miniussi C, Rossini PM. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. Neuroimage. 2011;54:90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Solé J, Siebner HR. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut de Recherches Internationales Servier. S44819. Investigator's brochure. 2014 [Google Scholar]
- Jung HY, Sohn YH, Mason A, Considine E, Hallett M. Flumazenil does not affect intracortical motor excitability in humans: a transcranial magnetic stimulation study. Clin Neurophysiol. 2004;115:325–329. doi: 10.1016/S1388-2457(03)00335-3. [DOI] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation [letter] Clin Neurophysiol. 2001;112:720. doi: 10.1016/S1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kähkönen S, Ilmoniemi RJ. The effect of stimulus intensity on brain responses evoked by transcranial magnetic stimulation. Hum Brain Mapp. 2004;21:154–164. doi: 10.1002/hbm.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioumis P, Kicić D, Savolainen P, Mäkelä JP, Kähkönen S. Reproducibility of TMS-Evoked EEG responses. Hum Brain Mapp. 2009;30:1387–1396. doi: 10.1002/hbm.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Komssi S, Scherg M, Hoechstetter K, Classen J, Zaaroor M, Pratt H, Kahkonen S. Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage. 2007;37:56–70. doi: 10.1016/j.neuroimage.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA. Alpha5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci. 2010;30:5269–5282. doi: 10.1523/JNEUROSCI.4209-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Maubach K. GABA(A) receptor subtype selective cognition enhancers. Curr Drug Targets CNS Neurol Disord. 2003;2:233–239. doi: 10.2174/1568007033482779. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/S0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Möhler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Müller-Dahlhaus JF, Liu Y, Ziemann U. Inhibitory circuits and the nature of their interactions in the human motor cortex: a pharmacological TMS study. J Physiol. 2008;586:495–514. doi: 10.1113/jphysiol.2007.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/WNL.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005;568:291–303. doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pálvölgyi A, Etherington LA, Mihalik B, Ling I, Pallagi K, Kertész S, Gunn BG, Brown AR, Livesey MR, Belelli D, Barkóczy J, Varga P, Spedding M, Gacsályi I, Lambert JJ, Antoni FA. Selective targeting of extra-synaptic α5-GABAA receptors by S44819 (Egis-13529), a novel competitive GABAA receptor inhibitor compound. Soc Neurosci Abstr. 2016 124.16. [Google Scholar]
- Peurala SH, Müller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Premoli I, Rivolta D, Espenhahn S, Castellanos N, Belardinelli P, Ziemann U, Müller-Dahlhaus F. Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS–EEG. Neuroimage. 2014a;103:152–162. doi: 10.1016/j.neuroimage.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Premoli I, Castellanos N, Rivolta D, Belardinelli P, Bajo R, Zipser C, Espenhahn S, Heidegger T, Müller-Dahlhaus F, Ziemann U. TMS–EEG signatures of GABAergic neurotransmission in the human cortex. J Neurosci. 2014b;34:5603–5612. doi: 10.1523/JNEUROSCI.5089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/S0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Fitzgerald PB. Assessing cortical network properties using TMS–EEG. Hum Brain Mapp. 2013;34:1652–1669. doi: 10.1002/hbm.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Thomson RH, Daskalakis ZJ, Fitzgerald PB. Short-latency artifacts associated with concurrent TMS–EEG. Brain Stimul. 2013;6:868–876. doi: 10.1016/j.brs.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Thomson RH, Farzan F, Fitzgibbon BM, Bailey NW, Hernandez-Pavon JC, Daskalakis ZJ, Fitzgerald PB. Removing artefacts from TMS–EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. Neuroimage. 2014;101:425–439. doi: 10.1016/j.neuroimage.2014.07.037. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Bruehl C, Frahm C, Redecker C, Witte OW. Age dependence of excitatory-inhibitory balance following stroke. Neurobiol Aging. 2012;33:1356–1363. doi: 10.1016/j.neurobiolaging.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ, Rothwell JC. Differing effects of intracortical circuits on plasticity. Exp Brain Res. 2009;193:555–563. doi: 10.1007/s00221-008-1658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996b;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Müller-Dahlhaus F. TMS and drugs revisited 2014. Clin Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]