Abstract
A newly developed microscale protocol for profiling serum O-glycans has been validated here with multiple serum samples obtained from different cohorts of colorectal cancer patients. The simultaneous cleavage and permethylation steps in this procedure preserve the integrity of released minor O-glycans, so that 39 O-linked oligosaccharides could be reliably recorded in a profile. This is far more detected components than shown in any previous studies. The analytical results were further subjected to a battery of statistical tests. Our O-glycan compositions compare favorably with the previous results obtained with solid tumors and cancer cell lines, suggesting that smaller circulatory mucins protruding into the blood circulation may be one source of O-glycans that we observe in the serum samples. While the control vs. cancer statistical comparisons generally agree with the expected glycosylation trends, the comparisons of male vs. female subjects have led to some surprising results for which we do not have a ready explanation due to lack of any literature describing hormonal control of O-glycosylation. Our results thus underscore the necessity of applying new analytical technologies to clinically interesting sample sets.
Graphical Abstract

Many clinically relevant analyses now utilize human blood serum or plasma for both the routine clinical measurements and various efforts to look for potential disease biomarkers. Glycoproteins are among the most targeted biomarker molecules. Aberrant glycosylation of proteins in different types of cancer has provided stimulus for numerous studies for already several decades 1-3, but detection of definitive glycoconjugate cancer biomarkers in blood still remains somewhat elusive. At present, there are many efforts to utilize relatively new technologies, such as glycan and lectin arrays, and mass spectrometry (MS)-based analytical techniques for glycomic profiling 4-7. MS and its ancillary techniques have now been recognized to have the unparalleled capabilities and potential in providing definitive structural information on glycoconjugate attributes of malignant cellular transformation.
The proteins in blood span a very large range of concentrations 8, while more than 50 % of these proteins appear glycosylated at asparagine or serine/threonine residues and rarely at other sites. At this time, the glycan profiling techniques have been far more successfully developed for asparagine (N-linked) oligosaccharides than the serine/threonine (O-linked) glycans, as exemplified by the recent applications to cancer research 9-13. This situation is primarily due to the availability of a reliable enzymatic cleavage for the former glycan type, which cannot be readily achieved for O-glycans. With O-glycans, many investigators still rely on the classical beta-elimination procedure 14, facilitated through alkaline chemical cleavage, or its more recent modifications toward the glycan analysis. The main drawbacks of this approach are: (a) the need to use relatively large amounts of biological material; and (b) perhaps most importantly, a danger of degrading O-glycan structures during the so-called “peeling reactions”. Consequently, most attempts at profiling report only a limited number of major O-glycans rather than their more complete profiles15. This situation has called for substantial procedural improvements for some time, as underscored by the obvious functional importance of large mucinous proteins in relation to cancer 1,16-18
As demonstrated in this communication, we can now successfully detect a wide range of O-glycan structures through a modified and miniaturized version of our previously reported procedure19, where the enzymatically degraded proteins were subjected to a cleavage during the following permethylation step. As shown below, only small volumes of blood serum (low microliters) are needed in these determinations. In order to enhance profiling selectivity for O-glycans, we first remove potential interferences from N-linked oligosaccharides by their prior enzymatic cleavage with N-glycanase. The simultaneous O-glycan cleavage and permethylation is an inherently advantageous feature of this procedure, in which we protect the minor glycans that could otherwise be lost due to the peeling reactions and formation of other procedural artifacts. This has allowed us to record fairly comprehensive profiles of serum O-glycans during this study.
For the initial validation of our O-glycan profiling procedure using MALDI-MS, we have chosen the set of samples from colorectal cancer patients. These very same samples were initially collected for the benefits of N-glycan profiling, as previously described in this journal 20.
Colorectal cancer (CRC) is one of the most common oncological diseases with sporadic causes, high tendency toward metastasis and relatively high mortality21,22. The glycosylation events in colon cancer were recently reviewed extensively by Holst et al.23. Several hallmark aberrations due to this disease are quite similar to what has been observed in several other cancers, including but not limited to, an increased β1-6 branching and poly-N-acetyl-lactosamine extension in N-glycan structures, increase in truncated paucimannosidic structures and a decrease of bisecting N-acetyl-lactosamine (LacNAc) structures. The changes in O-glycosylation can include decrease in Core 3 and Core 4 structures and increased levels of structures such as (sialyl) Tn and (sialyl) T antigen. Increases in sialylation, fucosylation and occurrence of the Lewis-type antigens and Type 2 chains have been indicated in tissue biopsies and cancer cell lines. Considerable evidence points to modifications of short-chain O-glycans (known as “T”, “Tn” and sialylated Tn antigens) on the serial sections of mucin-based serine and threonine residues24, but only a limited number of actual glycans have been structurally indicated. In contrast, in our report, around 40 oligosaccharides can now be routinely assessed quantitatively, providing the basis for statistically evaluating different cohort groups of colorectal cancer patients.
The O-glycan profiles from 62 patients’ serum samples (both males and females at different cancer stages, (referred here Cycle 1 and 2) and 20 healthy control individuals provided the basis for statistical evaluations: one-way ANOVA analysis; principal component analysis (PCA); and Receiver Operating Characteristics (ROC) evaluation plots for selected O-glycans. Five O-glycans were significantly altered in cancer (Cycle 1 and 2 combined) at the 0.05 level. The changes in glycan profiles correlated with cancer progression, but also importantly and unexpectedly, we observed gender differences in these O-glycan profiles. To our knowledge, this has not been reported previously. Glycosylation changes due to cancer could be expected in different forms (either lower or higher expression of certain structures, incomplete glycosylation, or even a possibility of previously unobserved structures), however, it is commonly believed that these changes do not occur at random 1. With the possibility of reproducibly profiling a large number of glycans, seemingly complex process of disordered glycosylation in cancer can now be increasingly approached. This study further underscores the great untapped information on O-glycosylation and its role in cancer as indicated by the close correlation between altered glycan profiles and cancer progression.
EXPERIMENTAL SECTION
Chemicals and Reagents
Beta-mercaptoethanol, sodium hydroxide beads, methyl iodide, trypsin (EC 3.4.21.4, TPCK-treated from bovine pancreas) were purchased from Sigma-Aldrich (St. Louis, MO). 2,5-Dihydroxybenzoic acid (2,5-DHB) was received from Alfa Aesar (Ward Hill, MA). N-Glycanase (PNGase F) was purchased from Prozyme (Hayward, CA), while Pronase (from Streptomyces griseus) was a product of Roche Diagnostics (Indianapolis, IN), as was Nonidet P-40. LC-MS grade water and acetonitrile were purchased from EMD Chemicals (Gibbstown, NJ). Trifluoroacetic acid (TFA), glacial acetic acid, formic acid (FA), chloroform, and N,N’-dimethylformamide (DMF) were products of Mallinckrodt Baker (Phillipsburg, NJ). Micro SpinColumn Empty and Ultra-Micro SpinColumn amino columns were purchased from Harvard Apparatus (Hayward, CA), while Amicon Ultra-0.5 Centrifugal Filter Unit with Ultracel-10 membrane was from EMD Millipore (a part of Millipore Sigma). Sodium dodecylsulfate (SDS) was acquired from Biorad (Hercules, CA).
Clinical Specimens
The human blood serum samples investigated in this study were obtained from Hoosier Oncology Group, Inc. (Indianapolis, IN) as a part of sample collection targeted to provide specimens to investigators pursuing different biomarker research objectives. The Walther Cancer Institute associated with the Indiana University School of Medicine facilitated coordination of the sample collection effort. The study was approved by the institutional ethics committee of Indiana University and performed in accordance with the ethical standards of the Helsinki Declaration. Blood samples were collected from the various patients diagnosed with various stages and types of colorectal cancer (n=62) as well as healthy control individuals (n=20). Blood was collected into sterile vacutainer tubes and allowed to clot for 30 min at ambient temperature, then subjected to centrifuging, aliquoting and freeze and storage at −80 °C. All samples were deidentified to protect the sample donors’ privacy.
All colorectal cancer patients were subjected to blood collection after their primary cancer treatment was finished (further referred to as “Cycle 1” in our text) and at the time of patients’ relapse (Cycle 2). There were 20 male patients and 18 female patients in Cycle 1 and 15 males and 9 females in Cycle 2. The colorectal cancer patients under this study underwent a variety of individualized treatments, including different types of chemotherapy with single or multiple agents, radiation therapy and surgery. For statistical comparison, all samples were divided according to their gender and “cycles”. Although there were some differences in race, ethnicity and smoking status, the number of cases were insufficient for statistical evaluations.
Serum O-Glycan Measurements
O-Glycans were extracted from the human blood serum (HBS) of colorectal cancer patients and healthy individuals as based on our previously reported method 19 with minor modifications regarding the proteolysis since the first publication. Briefly, HBS (2.5 μL) was denatured by 25 μL of 0.1% SDS and 0.1% β-mercaptoethanol in 10 mM sodium phosphate (pH 7.5) at 60°C for 1 h. After the samples were cooled for 10 min at room temperature, 2.5 μL of 10% Nonidet P-40 (NP-40) was added and allowed to equilibrate for 10 min. Subsequently, 1 μL of 0.25 mU of PNGase F in 10 mM sodium phosphate (pH 7.5) was added to each sample and incubated at 37°C for 21 h. N-glycans were removed by size exclusion through Amicon® Ultra-0.5 (10 KDa) (flow through). To separate the proteins from N-glycans, 10,000 MWCO filters (Amicon Ultra Centrifugal filters 0.5 mL, Millipore, Billerica, MA) were prepared by adding 400-μL water and centrifuging for 10 min at 9300 ×g, followed by 400-μL 10-mM sodium phosphate buffer (pH 7.5) and centrifuging for 10 min at 9,300 ×g. Subsequently, 300-μL 10-mM sodium phosphate buffer (pH 7.5) was first added to the centrifugal filters and then the PGNase F-digested sample (50 μL) was added and mixed by pipetting. The centrifugal filters were centrifuged for 15 min at 14,000 ×g, followed by addition of 2× 400-μL 10-mM sodium phosphate buffer (pH 7.5), and centrifuged at 14,000 ×g for 15 min. The first flow-through and sodium phosphate buffer flow-through containing the N-glycans were pooled and dried with a CentriVap concentrator. The centrifugal filters were then turned upside down and centrifuged for 15 min at 14,000 ×g to elute the proteins. The protein fractions were continued in the O-glycan preparation as follows.
The O-glycosylated peptides were purified by amino Ultra SpinColumns (Harvard Apparatus). The columns were washed 3× with buffer B (10%/90%/0.1% acetonitrile, water, trifluoroacetic acid) and 3× with buffer A (90%/10%/0.1% acetonitrile, water, trifluoroacetic acid) and centrifuged for 1 min at 200 ×g. Two hundred fifty-microliter volumes of buffer A were added to the digested protein samples and vortexed before applying samples to the columns. The columns were centrifuged for 1 min at 200 ×g; the flow-throughs were collected and reapplied to the column. The samples were reapplied and centrifuged two more times to ensure retention on the column. Following that, the columns were washed with 2× 300-μL buffer A and centrifuged for 1 min at 200 ×g. The O-glycopeptide fractions were eluted by adding 300-μL buffer B, centrifuged at 200 ×g for ~ 10 s, left equilibrating for 10 min, then centrifuged for 1 min at 200 ×g. Another 300-μL buffer B was added and centrifuged for 1.5 min at 800 ×g. The two buffer B eluate fractions were pooled and dried by a CentriVap concentrator. The O-glycopeptides were permethylated, in which the permethylation was also used here to release the O-glycans from the peptides and the glycans were thus not reduced first.
Permethylation
Permethylation was carried out as follows. First, the permethylation reactors were created by taking a 20-mL glass vial, filled with roughly one fourth with NaOH beads, and followed with acetonitrile. By using a cut 1-mL pipette tip, the NaOH beads were transferred to empty Micro SpinColumns (Harvard Apparatus). Approximately three fourths of the column volume was filled up with the NaOH beads. The columns were centrifuged at 200 ×g for 1 min to remove acetonitrile and then washed with 3× ~ 300-μL DMF (dimethylformamide) and centrifuged for 1 min at 200 ×g. The not-reduced O-glycans from the peptides were supplemented with 5-μL water, vortexed, and centrifuged for 30 s at 600 ×g. Next, 65-μL DMF and 35-μL methyl iodide were added to the samples and mixed. The samples were applied onto the permethylation reactors, and the reactors were put in the original sample centrifugation tubes, which were sealed by putting a cap on them. The reactors were set horizontally, slightly elevated, and left to react for 20 min. Afterwards, the caps were removed and the permethylation reactors were centrifuged for 1.5 min at 200 ×g. The flow-throughs were reapplied to the reactors after 35-μL methyl iodide was added and mixed. Caps were used to seal the reactors and they were set horizontally, slightly elevated, and left to react for another 20 min. The caps were removed and reactors were centrifuged for 1.5 min at 2,300 ×g. Then, 400-μL chloroform and 1-mL 0.5-M NaCl were added to the flow-throughs and shaken gently by hand for 2 min before centrifuging the samples at 600 ×g for 30 s. The upper aqueous phases were discarded and 1-mL water was added to the lower chloroform phases. The mixtures were gently shaken by hand for 2 min and centrifuged for 30 s at 600 ×g. The upper aqueous phases were discarded again, while 1-mL water was added and gently shaken by hand for 2 min. The mixtures were centrifuged for 30 s at 600 ×g and the upper aqueous phases were discarded. The chloroform phases were dried in a CentriVap concentrator and stored in a freezer until analysis.
Data Collection and Analysis
All the O-glycan profiles were recorded using MALDI-TOF/TOF mass spectrometry using an Applied Biosystems 4800 TOF-TOF instrument. The laser intensity was set at 4100, light intensity of 20, DIE 500, and mass range (500-3000). Briefly, the permethylated samples (dissolved in 1:1 methanol: water) were spotted and dried in room temperature (RT) on the plates for 10 min and DHB matrix (10 mg/mL in 1:1 methanol: water), while a final concentration of 1-mM sodium acetate was added to each spot and dried again under vacuum for 2 min. For each sample, three spectra were recorded. The spectra were baseline-corrected and noise-filtered (0.7) in the Applied Biosystems Data Explorer software (4.9) before export to text file format. The text files were uploaded to the ExPASy online tool GlycoMod that can predict the possible oligosaccharide structures from their experimentally determined masses. In GlycoMod, the UniCarbKB database was used for peak identification (+/− 0.2 Dalton filter was used for peak identification) and the structure/composition with a traceable UniCarbKB link were selected for constructing the O-glycan library of the study. A list of 39 oligosaccharide monoisotopic masses was made for the in-house developed program PeakCalc. The relative abundance and associated standard deviation of each peak was calculated with PeakCalc (+/− 0.5 Dalton filter was used for peak identification). Relative abundance (normalization) was calculated by dividing the intensity of each peak with the total intensity of all 39 O-glycans. Triplicate sample files exported from PeakCalc in CSV data format were merged and imported into Microsoft Excel for rendering of plots and sorting of data.
Statistical Tests
Advanced statistical tests such as one-way ANOVA, Principal Component Analysis (PCA) and Receiver Operating Characteristics (ROC) Area Under the Curve (AUC) plots were performed with OriginPro2018 (9.5.1.195) on the normalized MS intensity data imported from Microsoft Excel sheets. Briefly, the one-way ANOVA was performed pairwise with control vs Cycle 1 or Cycle 2 with 0.05 significance level. The means comparisons were performed with Tukey test at the 0.05 significance level, while box plots were chosen for the output format. The p-values of the one-way ANOVA reported in this communication refer to the means comparison with the Tukey test, not the F-value of the ANOVA.
RESULTS AND DISCUSSION
Aberrant glycosylation of cellular surfaces in tumorigenesis and metastatic promotion has been a subject of numerous studies for quite some time1-3. New analytical techniques that improve our structural knowledge of glycan antigens are becoming increasingly important for diagnostic, prognostic and even therapeutic purposes25,26. Most contemporary knowledge of O-glycosylation in cancer has been primarily obtained from cancer cell lines and tumor tissue analyses 23,27, and much less frequently from the analyses of body fluids such as blood serum or plasma23 Moreover, structural considerations of O-glycans have often been deduced from transcriptomic data31 rather than direct measurements. Whereas N-glycan profiling in serum samples, for the sake of biomarker discovery, has seen some progress during the recent years9-13, sufficiently sensitive analytical techniques for serum O-glycans have been rare and limited to a few isolated structures,15,28,30,32-34 some of which may even be degradation products of uncontrolled deglycosylation. This is, to a large extent, due to unavailability of the “cleaner” glycan release protocols, which caused various investigators to resort to a chemical cleavage from serine and threonine residues in the protein backbone with its earlier mentioned methodological disadvantages.
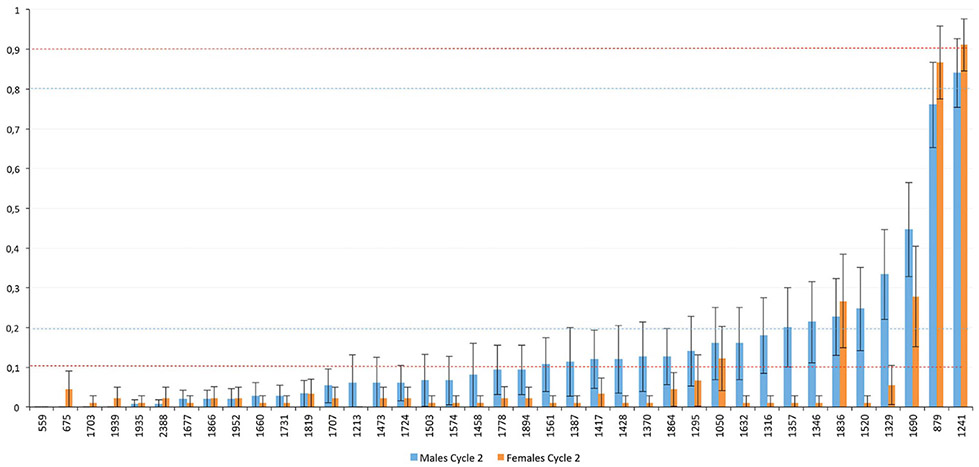
The modification and miniaturization of the previously reported procedure 19, as shown in this work, leads us to a substantially enhanced profiling capability, now including numerous O-glycans from low-microliter volumes of serum samples. After enzymatic removal of the N-glycans and complete proteolysis, the O-glycans were released and permethylated in one step. This allowed us to record about 40 O-glycan components within a substantial concentration range, as seen in the Excel plot of MALDI-MS data shown in Figure 1. With the expected repeatability of the MALDI-MS-based measurements (approximately ±15 % variation), the large standard deviation error bars very likely reflect substantial variations due to a disease state and possibly gender. A dot plot of the same data set is shown in Figure S1 (supporting information). A typical raw spectrum of the serum-originated components (Figure S2) and the O-glycan annotated spectral profile (Figure S3) are also shown in the Supporting Information. Table 1 further specifies the individual m/z-ratios together with the glycan compositions, which are very likely due to a close correspondence between the measured and expected masses. Besides the few main O-glycans that are usually seen in biological specimens (that is m/z 879, 1241), minority peaks (<10 %) have now been reproducibly recorded here as well, primarily due to the fact that their structures are preserved with minimal degradation, as the cleavage and permethylation occur rapidly at the same time. To validate our protocol, we have chosen the sets of samples from colorectal cancer patients at two different times in disease progression and compared them with appropriately documented controls. As described below, this conceptually important application of a new profiling technique has led to both expected (cancer vs. control) results, but also an unprecedented finding of the gender (male vs. female) and cancer treatment (Cycle 1 vs Cycle 2) differences in the extent of glycosylation during cancer progression. The large standard deviations seen in Figure 1 represent inclusion of all data, presumably due to “biochemical individuality” of different patients.
Figure 1.
A comprehensive serum O-glycan profile derived from control and colorectal cancer Cycle 1 and Cycle 2 samples. The relative abundance of 39 permethylatedO-glycanswas determined using MALDI-MS and the in-house developed software PeakCalc. With the expected repeatability of the MALDI-MS-based measurements (approximately ±15% variation), the large standard deviation error bars very likely reflect substantial variations due to a disease state and possibly gender.
Table 1.
Compositions of 39 serum O-glycans. The O-glycans were released and permethylated prior to MALDI-MS detection. Theoretical monoisotopic masses are listed side-by-side with the observed values.
| Peak ID | Presumptive composition | Observed mass [M +Na]+ |
GlycoMod expected mass [M +Na]+ |
|---|---|---|---|
| 1 | (HexNAc)2 | 559.3 | 559.25 |
| 2 | (HexNAc)1(NeuAc)1 | 675.4 | 675.27 |
| 3 | (Hex)1(HexNAc)1(NeuAc)1 | 879.4 | 879.38 |
| 4 | (HexNAc)4 | 1049.6 | 1049.62 |
| 5 | (Hex)2(HexNAc)3 | 1212.6 | 1212.64 |
| 6 | (Hex)1(HexNAc)2(NeuAc)2 | 1240.6 | 1240.56 |
| 7 | (HexNAc)5 | 1294.6 | 1294.79 |
| 8 | (Hex)2(HexNAc)2(dHex)2 | 1315.6 | 1315.80 |
| 9 | (Hex)2(HexNAc)2(NeuAc)1 | 1328.6 | 1328.81 |
| 10 | (Hex)3(HexNAc)2(dHex)1 | 1345.7 | 1345.87 |
| 11 | (Hex)1(HexNAc)3(dHex)2 | 1356.7 | 1356.83 |
| 12 | (Hex)1(HexNAc)3(NeuAc)1 | 1369.7 | 1369.76 |
| 13 | (Hex)2(HexNAc)3(dHex)1 | 1386.7 | 1386.80 |
| 14 | (Hex)3(HexNAc)3 | 1416.7 | 1416.68 |
| 15 | (Hex)1(HexNAc)4(dHex)1 | 1427.7 | 1427.82 |
| 16 | (Hex)2(HexNAc)4 | 1457.7 | 1457.86 |
| 17 | (Hex)1(HexNAc)2(dHex)1(NeuAc)1 | 1472.7 | 1472.92 |
| 18 | (Hex)2(HexNAc)2(dHex)1(NeuAc)1 | 1502.7 | 1502.86 |
| 19 | (Hex)3(HexNAc)2(dHex)2 | 1519.8 | 1519.90 |
| 20 | (Hex)2(HexNAc)3(dHex)2 | 1560.8 | 1560.89 |
| 21 | (Hex)2(HexNAc)3(NeuAc)1 | 1573.8 | 1573.89 |
| 22 | (Hex)2(HexNAc)4(dHex)1 | 1631.8 | 1631.94 |
| 23 | (Hex)1(HexNAc)2(dHex)1(NeuAc)2 | 1659.9 | 1659.78 |
| 24 | (Hex)2(HexNAc)2(dHex)2(NeuAc)1 | 1676.8 | 1678.87 |
| 25 | (Hex)2(HexNAc)2(NeuAc)2 | 1689.8 | 1689.81 |
| 26 | (Hex)2(HexNAc)5 | 1702.8 | 1702.91 |
| 27 | (Hex)3(HexNAc)2(dHex)1(NeuAc)1 | 1706.8 | 1706.94 |
| 28 | (Hex)4(HexNAc)2(dHex)2 | 1723.9 | 1723.94 |
| 29 | (Hex)1(HexNAc)3(NeuAc)2 | 1730.9 | 1730.93 |
| 30 | (Hex)3(HexNAc)3(NeuAc)1 | 1777.8 | 1777.84 |
| 31 | (Hex)2(HexNAc)4(NeuAc)1 | 1818.9 | 1819.03 |
| 32 | (Hex)3(HexNAc)4(dHex)1 | 1835.9 | 1835.86 |
| 33 | (Hex)2(HexNAc)2(dHex)1(NeuAc)2 | 1863.9 | 1863.93 |
| 34 | (Hex)4(HexNAc)4 | 1865.9 | 1865.90 |
| 35 | (Hex)3(HexNAc)2(NeuAc)2 | 1893.9 | 1893.92 |
| 36 | (Hex)2(HexNAc)3(NeuAc)2 | 1935.0 | 1934.98 |
| 37 | (Hex)3(HexNAc)3(dHex)3 | 1938.9 | 1939.03 |
| 38 | (Hex)3(HexNAc)3(dHex)1(NeuAc)1 | 1952.0 | 1951.89 |
| 39 | (Hex)4(HexNAc)4(dHex)3 | 2388.1 | 2388.06 |
In assessing the profiling power of our procedure, we have included the sera of colorectal cancer patients from two different progressions of the disease (referred to as Cycle 1, n=38, and Cycle 2, n=24), and age-matched control individuals (n=20) for comparative analyses. Both male and female patients have been included. From the selected 39 O-glycans (Table 1), all but two major components (m/z 879 and m/z 1241) are less than 3 % in abundance (Figure 1). These main m/z values were structurally confirmed with MS/MS to represent sialyl-T (Neu-Gal-GalNAc) and disialyl T (Neu-Gal-(Neu)GalNAc) antigen, respectively (Figure S4, Supporting Information). Disialylated extended Core 2 (m/z 1690) was also confirmed with MS/MS (Figure S4, supporting information). This indicates that peaks observed in the O-glycan MALDI-MS spectrum (Figure S3) may represent extended Core 1 and Core 2 O-glycans in addition to extended Core 3. Most minor glycans were detectable at below 0.5% abundance. The T antigen (m/z 518, Figure S3) was excluded from the calculations due to its unpredictable detection levels in both controls and cancer samples, a phenomenon that was not observed for any of the other 39 glycans that we describe in this communication. This was presumably due to interference from the lower molecular weight components of the MALDI matrix. We have previously observed that N-glycans were not detected from fetuin using the same O-glycan release protocol (unpublished data). In the unlikely event that any remaining N-glycans were to remain after PNGase F treatment and they were released together with the O-glycans during the permethylation treatment, they would be observed as high molecular weight peaks in the MALDI-MS spectrum. Of the 39 O-glycan peaks that we report in this study there is only one potential N-glycan overlap with the peak m/z 1836 which could potentially represent a small N-glycan (Table 1, peak 32). The glycan was not significantly altered in any of the statistical tests, however, and did not alter the conclusions of this study.
It appears that sialyl-T antigen and disialyl-T antigen are increasing in relative abundance as cancer progresses (i.e., comparing Cycle 2 to Cycle 1), while the reverse is true for the larger extended O-glycans (Figure 1). The decrease in the lower- abundance glycans is not due to the normalization of the data since the same trend was seen in the original spectra (a plot of non-normalized raw data is shown in Figure S5).
While there is a plausible agreement between the measured and computed m/z values, the more definitive structures will need to be assigned by additional MS/MS fragmentation measurements, which, alas, could not be accomplished due to the very low signals for the minor profile components. We, however, feel encouraged that their detection became feasible as the result of the simultaneous cleavage/immediate permethylation in our protocol, which largely rules out a formation of artifacts. Sample enrichment procedures still need to be developed in future studies to secure MS/MS recordings for the minor glycans. However, our results are in fair agreement with the previous results from transcriptomic data31. While transcriptomic data may be considered less than definitive by today’s technology standards, the general agreement with our suggested structures appears plausible. A selective advantage of these truncated structures seem to preclude other core structures and extended O-glycans to reach significant abundance numbers. The early sialylation precludes further extensions of the core structures. For readers’ orientation with the structural relations of Core 1-4, see Supplementary Figure S6, Supporting Information. However, the relative depression of these structures is quite dramatic, correlating the disease in a statistically significant way as demonstrated in a battery of statistical tests indicated below.
For an initial statistical evaluation of the different cohort groups, we chose Principal Component Analysis (PCA). The PCA plots shown in Figure 2 indicate some basic trends: (A) different cohorts are not easily segregated when both males and females are included; (B) for females alone, distinction is readily seen for both cancer cycles against controls; and (C) for males alone, a distinction is observed for Cycle 2 only when compared to the control and Cycle 1. Females and males thus appear to have distinct differences in how the glycan profiles correlate with disease progression.
Figure 2.
PCA plot of principal component 1 and 2 of 39 O-glycansfrom three different cancer conditions: control (blue), Cycle 1 (orange), and Cycle 2 (gray). The groups are not easily segregated when both males and females are included (A). For females the PCA segregates both cancer cycles from the control (B), while the PCA of males segregate Cycle 2 from the control and Cycle 1(C).
Another means to determine if and how the data differ between the controls and cancer patients has been a one-way ANOVA test (at the p=0.05 level), with Tukey means comparison test. While comparing the data obtained from 39 individual glycans as control vs. cancer (Cycle 1 and Cycle 2 combined), five glycan entries were deemed significant (Figure S7A, Supporting Information), but when judging the O-glycan level in different cycles, most entries were altered in Cycle 2 and only few in Cycle 1 (Figure S7B, Supporting Information). The ANOVA p-values and boxplots of cancer and control samples of the five significant glycans m/z 559 (HexNAc2), 1212 (Hex3HexNAc2), 1294 (HexNAc5), 1703 (Hex2HexNAc5) and 1865 (Hex4HexNAc4) are shown in Figure 3.
Figure 3.
Boxplots of O-glycanssignificantly altered in cancer as determined with one-way ANOVA (at appropriate p = 0.05 values) and ROC curves with AUC values and associated standard errors. The low AUC values represent strong negative correlation. Carbohydrate representations are drawn according to symbol nomenclature as suggested by Varki et al.36
A diagnostic value of individual glycans in the entire O-glycan profile was further assessed through the Receiver Operating Characteristic (ROC) evaluation, which is a widely recognized criterion to validate biomedical investigations35. In this test, the area-under-the-curve (AUC) values range from 0 to 1, while AUC=0.5 represents totally insignificant results; values between 0.7 to 0.8, or 0.2 and 0.3 are viewed as “moderately accurate”, values between 0.8 and 0.9 or 0.1 to 0.2 are viewed as “accurate” and, finally, values below 0.1 or above 0.9 are viewed as highly accurate. Values above and below 0.5 represent positive and negative correlation, respectively. The ROC curve AUC values for all 39 glycans (control vs. cancer (both cycles combined)) are shown in Figure S8 (Supporting Information). Four O-glycans display accurate AUC values below 0.2 (m/z 559, 1212, 1703, and 1865), 31 glycans are moderately accurate and four glycans (m/z 1050, 1241, 1690, and 1836) have ROC values that are not accurate (Figure S8, Supporting Information). Four of the five glycans plotted in Figure 3 are both significant in the one-way ANOVA test and deemed accurate by ROC under the curve test (m/z 559, 1212, 1703, and 1865), Figure 3.
All five significant O-glycans progressively decrease with the successive cancer cycles, with less relative abundance overlap with controls, lower p-values and better AUC values to support this notion. The PCA clusters (Figure 2) already indicated that there are gender-specific differences in the colorectal cancer disease progression. To investigate this further, we performed gender- and cycle-specific ROC analysis for all 39 glycans. In Cycle 1, the O-glycan serum profiles contain no accurate predictors of cancer in males, while the female profiles contain 10 highly accurate ROC AUC scores, 23 accurate and 2 moderately accurate scores (Figure S9, Supporting Information). In summary, the female O-glycan profiles change significantly already at Cycle 1, while the male O-glycan profiles were not changed to the same extent.
In Cycle 2, the ROC AUC predictive values of the O-glycans had increased dramatically in males (Figure 4). Twenty-one O-glycans were now highly accurate, 11 were accurate and 3 were moderately accurate. For females, the accuracy levels were now even higher in Cycle 2: 35 highly accurate ROC AUC values and four accurate ROC values (Figure 4). Our statistical tests indicate that the O-glycosylation clearly correlates with disease progression. Some O-glycans even display AUC values of zero, indicating complete segregation between Cycle 2 and controls. For comparative purposes, the five O-glycans that were significant in Figure 3 (control vs cancer) are again plotted from the female Cycle 2 subgroup with boxplots and ROC curves now shown in Figure 5.
Figure 4.
ROC AUC values of controls versus Cycle 2 of 39 serum O-glycans. Female (orange) and male (blue) data bars are plotted separately. The error bars denote standard error of the AUC plots. The glycans are listed in increasing ROC area order. Bars below 0.2 orabove 0.8 are considered accurate, while values below 0.1 or above 0.9 are highly accurate. The lower values indicate decreased expression in cancer, while the positive values represent higher expression in cancer.
Figure 5.
Female Cycle 2 O-glycansdisplay strong statistical change compared to controls as demonstrated by one-way ANOVA and ROC AUC plots with associated standard error values.
The value of our sample treatment and profiling protocol for the measurement and monitoring of serum O-glycans seems evident from the presented data and their statistical analyses: 39 glycans have been measurable and quantifiable in human serum, which is significantly more components than shown in any previous communication using MS-based techniques. Moreover, it is somewhat unclear as to how many glycans previously shown by others were actually procedural artifacts of beta-elimination.
Our MALDI-MS profiles indicate that increase of m/z 879 (sialyl-T antigen) and m/z 1241 (disialyl-T antigen) in blood serum is accompanied by a depression of numerous other O-glycans in the glycan profile. This is in contrast to what is observed in healthy human colon, where Core 1-4 are all expressed with Core 3 being the most abundant16. Up-regulation of both the sialyl T and disialyl T antigens is compliant with a strong expression of T-synthase (C1GalT1), ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3Gal-1) and ST6 N-acetylgalactosaminide alpha-2,6-sialyltransferase 1 (ST6GalNAc-1). Increase in disialyl-T antigen on serum hemopexin peptides was previously reported to be associated with colorectal cancer37. Low levels of Core 3 (GlcNAcβ3GalNAc) and sialyl-Tn (Siaα6GalNAc) indicate that T-synthase competes effectively with Core 3 β3GNT and ST6GalNAc I glycosyltransferases. The reciprocal relationship between these enzymes has also been shown in vitro38. Low or non-existent expression of Core 3 enzyme has previously been reported in colon cancers39.
Recently, it has also been shown that reduced Core 3 correlates with metastasis and poor prognosis of colorectal cancer patients by affecting MUC-1, P53 and miR-200c 40. In addition, MUC-1 and MUC-2 expression seems to be dependent on Core 3 expression 40,41. Down-regulation of MUC-1 and MUC-2 and other mucins has been reported to correlate with more aggressive cancers and poor prognosis42. Due to the small signals, we did not observe the Tn antigen (GalNAcα, theoretical m/z 314) in the MALDI-MS spectra. This is to be expected due to strong matrix interference in the low m/z range. Tn expression is strongly correlated with various cancers and not expressed in healthy tissues or, if so, at very low levels16. In our study, we observed sialyl Tn, STn (m/z 675, Siaα6GalNAc) that, interestingly, decreased with the disease progression. This is somewhat in contrast to previous reports from solid tumors, with the ratio of Tn to T antigen increased during cancer progression16. Strong expression of ST3Gal-1 blocks the production of Core 2 (GaLβ3(GlcNAcβ6)GalNAc), since Core 2 GNT1-2 competes with ST3Gal-1 over the Core 1 substrate and the two glycosyltransferases are known to co-localize43. In breast cancer, the dominant up-regulation of ST3Gal-1 is observed43. With low levels of Core 2, extended structures, such as polyLacNAc, are also notably down-regulated. This observation in serum is in contrast to what has been reported in colon cancer cell lines, where up-regulation of C2GnT1 and down-regulation of C2GnT2 were observed, resulting in up-regulation of Core 2 and Core 2-extended structures at the expense of Core 4 structures16.
The source of O-glycans that we detect in serum has not been determined as yet in the necessary detail. The O-glycans may be derived from abundant proteins in serum, such as some O-glycosylated immunoglobulins or acute-phase proteins as well. Some O-glycans could also originate from inflammatory cells that are extensively coated with sialic acids. Moreover, it is also possible that our observed signals may originate from glycoproteins that have been released from the primary tumor. Only a limited number of glycoproteins have been reported to carry sialylated- or unsialylated T- and Tn-antigens in malignant tissues including MUC-1 and CD44V6 and osteopontin44. Several glycoprotein-based serum biomarkers have been used to differentiate cancer types. They are believed to be shedded from the tumor and are detected by monoclonal antibodies that recognize O-glycoproteins or O-glycans such as MUC-1 (CA15-3, breast cancer), MUC-16 (CA125, ovarian cancer), SLea (CA19-9, pancreatic cancer), and STn (evaluated as biomarker for ovarian cancer)45. Transformed colon cancer tissue excrete glycoproteins, including mucins, to the circulation in contrast to healthy differentiated tissue 45. Thus, the glycans that we detect could originate from the solid tumor. In all regards, the total glycan profile of the serum should not be expected to be entirely the same as the profile of the solid tumor, even though some overlap is theoretically possible.
The altered “glycan coat” on tumor cells may be immunomodulatory, which is something that has raised interest recently46. Sialic acid-binding immunoglobulin-like lectins (SIGLECs), macrophage galactose-specific lectin (MGL) and dendritic cell (DC)-specific ICAM-3-grabbing non-integrin 1 (DC-SIGN/CD209) are expressed on immune cells and can mediate immune suppression by distinct pathways through binding to specific glycans covering tumor cells46. It has been suggested that the altered glycosylation in cancer may be a novel immune checkpoint, suitable as a therapeutic target46.
While differences in the onset of O-glycosylation between males and females as observed in our study are striking, our exhaustive literature search did not reveal any studies in connection with hormonal regulation of O-glycosylation effects that would justify the reason behind this observation. Besides the well-known studies on pregnancy-associated changes in glycodelin47 and uromodulin48 conducted in the laboratories of Howard Morris and Ann Dell two decades ago, the literature on hormonally-dependent N-glycosylation is not particularly abundant either. The relatively recent reports include post-translationally-induced functional targeting of the hormone thyrotropin49, N-glycome changes in plasma during pregnancy50, and the sex-related differences in N-glycosylation of IgG in both humans and mice51-53. We believe the knowledge discrepancies between N-glycosylation and O-glycosylation is largely attributable to different methodological difficulties in the sample treatment for N-versus O-glycans, respectively. Our observations show that the serum O-glycan profiles correlate strongly with cancer progression and display marked gender differences. Even if the observation of aberrant O-glycosylation in the serum profile of a late stage cancer is of limited prognostic value, these observations should encourage the research community to investigate more extensively the biological roles of O-glycosylation in cancer.
CONCLUSIONS
A new analytical protocol for the recovery of O-glycans from minute volumes of blood serum has been established and validated through MALDI-MS profiling of 39 glycans in 20 control and 62 colorectal cancer serum samples. The nearly simultaneous alkaline-based O-glycan cleavage from serum glycoproteins and a quantitative permethylation is the key to preserving the integrity of minor glycans and their quantitative profiling. The following statistical evaluation of the acquired O-glycan profiles from different colorectal cancer patient cohorts shows substantial agreement with the previously reported or suspected glycan structures extracted from either tissue biopsies or cancer cell lines. While our data obtained from the already advanced cancer cases do not yield any potential diagnostic biomarkers, they still indicate some promise for prognostic measurements using relatively non-invasive blood samples. A most remarkable outcome of our profiling measurements has been the finding of gender-dependent O-glycosylation during cancer progression. Whereas no explanations of this can be provided at this time, this finding, together with the availability of the described protocol and methodology, should stimulate further research in this area.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the grants from the National Institute of General Medical Sciences, U.S. Department of Health (NIH R21GM118340 and NIH R01GM106084). We thank Hoosier Oncology Group for providing the serum samples and clinical information on all cancer patients. These activities were, in turn, supported under Grant U01 CA128535 from the National Cancer Institute. Reporting on experimental details has been prepared in accordance with MIRAGE (The Minimum Information Required for a Glycomics Experiment) sample preparation (dx.doi.org/10.3762/mirage.1) and MS guidelines (dx.doi.org/10.3762/mirage.2).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Varki A, Kannagi R, Toole B, et al. In Essentials of Glycobiology; Varki A, Cummings RD, Esko JD, et al. , Ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor (NY), 2017; p Chapter 47. [Google Scholar]
- (2).Hakomori S-I Cancer Res. 1985, 45 (6), 2405–2414. [PubMed] [Google Scholar]
- (3).Hakomori S Proc. Natl. Acad. Sci. U. S. A 2002, 99 (16), 10231–10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ribeiro JP; Mahal LK Curr. Opin. Chem. Biol 2013, 17 (5), 827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Novotny MV; Alley WR Curr. Opin. Chem. Biol 2013, 17 (5), 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Alley WR; Mann BF; Novotny MV Chem. Rev 2013, 113 (4), 2668–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Xia L; Gildersleeve JC Methods Mol. Biol 2015, 1331, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Surinova S; Schiess R; Hüttenhain R; Cerciello F; Wollscheid B; Aebersold R J. Proteome Res 2011, 10 (1), 5–16. [DOI] [PubMed] [Google Scholar]
- (9).Alley WR Jr.; Vasseur JA; Goetz JA; Svoboda M; Mann BF; Matei DE; Menning N; Hussein A; Mechref Y; Novotny MV J Proteome Res 2012, 11 (4), 2282–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Vasseur JA; Goetz JA; Alley WR; Novotny MV Glycobiology 2012, 22 (12), 1684–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Mann BF; Goetz JA; House MG; Schmidt CM; Novotny MV Mol. Cell. Proteomics 2012, 11 (7), M111.015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Holst S; Deuss AJM; van Pelt GW; van Vliet SJ; Garcia-Vallejo JJ; Koeleman CAM; Deelder AM; Mesker WE; Tollenaar RA; Rombouts Y; Wuhrer M Mol. Cell. Proteomics 2016, 15 (1), 124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Biskup K; Braicu EI; Sehouli J; Fotopoulou C; Tauber R; Berger M; Blanchard VJ Proteome Res. 2013, 12 (9), 4056–4063. [DOI] [PubMed] [Google Scholar]
- (14).Carlson DM J. Biol. Chem. 1968, 243 (3), 616–626. [PubMed] [Google Scholar]
- (15).Maho Amano MT J. Glycomics Lipidomics 2013, 03 (01), 1–8. [Google Scholar]
- (16).Brockhausen I EMBO Rep. 2006, 7 (6), 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Tarp MA; Clausen H Biochim. Biophys. Acta 2008, 1780 (3), 546–563. [DOI] [PubMed] [Google Scholar]
- (18).Hollingsworth MA; Swanson BJ Nat. Rev. Cancer 2004, 4 (1), 45–60. [DOI] [PubMed] [Google Scholar]
- (19).Goetz JA; Novotny MV; Mechref Y Anal. Chem 2009, 81 (23), 9546–9552. [DOI] [PubMed] [Google Scholar]
- (20).Snyder CM; Alley WR; Campos MI; Svoboda M; Goetz JA; Vasseur JA; Jacobson SC; Novotny MV Anal. Chem 2016, 88 (19), 9597–9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ferlay J; Steliarova-Foucher E; Lortet-Tieulent J; Rosso S; Coebergh JWW; Comber H; Forman D; Bray F Eur. J. Cancer 2013, 49 (6), 1374–1403. [DOI] [PubMed] [Google Scholar]
- (22).Schmoll HJ; Van Cutsem E; Stein A; Valentini V; Glimelius B; Haustermans K; Nordlinger B; van de Velde CJ; Balmana J; Regula J; Nagtegaal ID; Beets-Tan RG; Arnold D; Ciardiello F; Hoff P; Kerr D; Köhne CH; Labianca R; Price T; Scheithauer W; Sobrero A; Tabernero J; Aderka D; Barroso S; Bodoky G; Douillard JY; El Ghazaly H; Gallardo J; Garin A; Glynne-Jones R; Jordan K; Meshcheryakov A; Papamichail D; Pfeiffer P; Souglakos I; Turhal S; Cervantes A Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 2012, 23 (10), 2479–2516. [DOI] [PubMed] [Google Scholar]
- (23).Holst S; Wuhrer M; Rombouts Y Adv. Cancer Res 2015, 126, 203–256. [DOI] [PubMed] [Google Scholar]
- (24).Pinho SS; Reis CA Nat. Rev. Cancer 2015, 15 (9), 540–555. [DOI] [PubMed] [Google Scholar]
- (25).Dalziel M; Crispin M; Scanlan CN; Zitzmann N; Dwek RA Science 2014, 343 (6166), 1235681. [DOI] [PubMed] [Google Scholar]
- (26).Dube DH; Bertozzi CR Nat. Rev. Drug Discov. 2005, 4 (6), 477–488. [DOI] [PubMed] [Google Scholar]
- (27).Christiansen MN; Chik J; Lee L; Anugraham M; Abrahams JL; Packer NH Proteomics 2014, 14 (4–5), 525–546. [DOI] [PubMed] [Google Scholar]
- (28).Williams TI; Saggese DA; Muddiman DC J. Proteome Res 2008, 7 (6), 2562–2568. [DOI] [PubMed] [Google Scholar]
- (29).Kirmiz C; Li B; An HJ; Clowers BH; Chew HK; Lam KS; Ferrige A; Alecio R; Borowsky AD; Sulaimon S; Lebrilla CB; Miyamoto S Mol. Cell. Proteomics 2007, 6 (1) 43–55. [DOI] [PubMed] [Google Scholar]
- (30).An HJ; Miyamoto S; Lancaster KS; Kirmiz C; Li B; Lam KS; Leiserowitz GS; Lebrilla CB J. Proteome Res 2006, 5 (7), 1626–1635. [DOI] [PubMed] [Google Scholar]
- (31).Vavasseur F; Dole K; Yang J; Matta KL; Myerscough N; Corfield A; Paraskeva C; Brockhausen I Eur. J. Biochem 1994, 222 (2), 415–424. [DOI] [PubMed] [Google Scholar]
- (32).Miura Y; Kato K; Takegawa Y; Kurogochi M; Furukawa J-I; Shinohara Y; Nagahori N; Amano M; Hinou H; Nishimura S-I Anal. Chem 2010, 82 (24), 10021–10029. [DOI] [PubMed] [Google Scholar]
- (33).Yoshida Y; Furukawa J-I; Naito S; Higashino K; Numata Y; Shinohara Y Proteomics 2016, 16 (21), 2747–2758. [DOI] [PubMed] [Google Scholar]
- (34).Furukawa J; Piao J; Yoshida Y; Okada K; Yokota I; Higashino K; Sakairi N; Shinohara Y Anal Chem 2015, 87 (15), 7524–7528. [DOI] [PubMed] [Google Scholar]
- (35).Zweig MH; Campbell G Clin. Chem. 1993, 39 (4), 561–577. [PubMed] [Google Scholar]
- (36).Varki A; Cummings RD; Esko JD; Freeze HH; Stanley P; Marth JD; Bertozzi CR; Hart GW; Etzler ME Proteomics 2009, 9 (24), 5398–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Darebna P; Novak P; Kucera R; Topolcan O; Sanda M; Goldman R; Pompach P J. Proteomics 2016, 153, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Barrow H; Tam B; Duckworth CA; Rhodes JM; Yu LG PLoS One 2013, 8 (3), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Brockhausen I Biochim. Biophys. Acta - Gen. Subj. 1999, 1473 (1), 67–95. [DOI] [PubMed] [Google Scholar]
- (40).Ye J; Wei X; Shang Y; Pan Q; Yang M; Tian Y; He Y; Peng Z; Chen L; Chen W; Wang R Oncogene 2017, 36 (46), 6391–6407. [DOI] [PubMed] [Google Scholar]
- (41).Xia L Methods Enzymol. 2010, 479 (10), 123–141. [DOI] [PubMed] [Google Scholar]
- (42).Behera SK; Praharaj AB; Dehury B; Negi S Glycoconj. J 2015, 32 (8), 575–613. [DOI] [PubMed] [Google Scholar]
- (43).Dalziel M; Whitehouse C; McFarlane I; Brockhausen I; Gschmeissner S; Schwientek T; Clausen H; Burchell JM; Taylor-Papadimitriou J J. Biol. Chem 2001, 276 (14), 11007–11015. [DOI] [PubMed] [Google Scholar]
- (44).Häuselmann I; Borsig L Front. Oncol 2014, 4 (February), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kudelka MR; Ju T; Heimburg-Molinaro J; Cummings RD Adv. Cancer Res. 2015, 126, 53–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).RodrÍguez E; Schetters STT; van Kooyk Y Nat. Rev. Immunol 2018, 18 (3), 204–211. [DOI] [PubMed] [Google Scholar]
- (47).Morris HR; Dell A; Easton RL; Panico M; Koistinen H; Koistinen R; Oehninger S; Patankar MS; Seppala M; Clark GF J. Biol. Chem 1996, 271 (50), 32159–32167. [DOI] [PubMed] [Google Scholar]
- (48).Easton RL; Patankar MS; Clark GF; Morris HR; Dell A J. Biol. Chem 2000, 275 (29), 21928–21938. [DOI] [PubMed] [Google Scholar]
- (49).Ikegami K; Liao XH; Hoshino Y; Ono H; Ota W; Ito Y; Nishiwaki-Ohkawa T; Sato C; Kitajima K; Iigo M; Shigeyoshi Y; Yamada M; Murata Y; Refetoff S; Yoshimura T Cell Rep. 2014, 9 (3), 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Ruhaak LR; Uh HW; Deelder AM; Dolhain RE; Wuhrer M J. Proteome Res 2014, 13 (3),1657–1668. [DOI] [PubMed] [Google Scholar]
- (51).Chen G; Wang Y; Qiu L; Qin X; Liu H; Wang X; Wang Y; Song G; Li F; Guo Y; Li F; Guo S; Li Z J. Proteomics 2012, 75 (10), 2824–2834. [DOI] [PubMed] [Google Scholar]
- (52).Melanson E; Bombardier C; Saraux A; Combe B; Dougados M; Bengtsson C; Hausmann JS; Campbell H; Kaiser UB; Rudd PM; Lauc G; Wilson JF; Finkelstein JS; Nigrovic PA J Appl Physiol 2017, 119 (9), 975–981. [Google Scholar]
- (53).Krištić J; Zaytseva OO; Ram R; Nguyen Q; Novokmet M; Vučković F; Vilaj M; Trbojević-Akmačić I; Pezer M; Davern KM; Morahan G; Lauc G Nat. Chem. Biol. 2018, 14 (5), 516–524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.