Abstract
Objective:
To evaluate and summarize reports to the Vaccine Adverse Event Reporting System (VAERS), a spontaneous reporting system, in pregnant women who received Influenza A (H1N1) 2009 Monovalent Vaccine to assess for potential vaccine safety problems.
Methods:
We reviewed reports of adverse events (AEs) in pregnant women who received 2009-H1N1 vaccines from 10/0½009–02/28/2010.
Results:
VAERS received 294 reports of AEs in pregnant women who received 2009-H1N1 vaccine: 288 after inactivated and 6 after the live attenuated vaccines. Two maternal deaths were reported. Fifty-nine (20.1%) women were hospitalized. We verified 131 pregnancy-specific outcomes: 95 spontaneous abortions (<20 weeks), 18 stillbirths (≥20 weeks), 7 preterm deliveries (<37 weeks), 3 threatened abortions, 2 preterm labor, 2 preeclampsia, and 1 each of fetal hydronephrosis, fetal tachycardia, intrauterine growth retardation, and cleft lip.
Conclusions:
Review of reports to VAERS following H1N1 vaccination in pregnant women did not identify any concerning patterns of maternal or fetal outcomes.
Introduction
Pregnant women are at higher risk for complications of influenza than non-pregnant women. 1 Since 1997 the Advisory Committee on Immunization Practices (ACIP) has recommended trivalent inactivated influenza vaccine (TIV) for pregnant women. 2 In 2004 the recommendation was expanded to cover all trimesters of pregnancy. 2 In April 2009, pandemic H1N1 2009 influenza A (H1N1) virus infections (2009 H1N1) were identified in the United States. 3 The second documented death associated with 2009 H1N1 virus was in a previously healthy pregnant woman, and enhanced surveillance for 2009 H1N1 infection in pregnant women in the United States was initiated. 4 Analyses of these cases showed that pregnant women had a higher rate of complications from 2009 H1N1 infection than the general population. 4 ACIP recommended that pregnant women (in any trimester) be included as an initial target group for 2009 H1N1 vaccination. 1,5 On September 15, 2009, the Food and Drug Administration (FDA) licensed the first 2009 influenza A (H1N1) vaccines which were initially available as live, attenuated vaccine (live H1N1) for intranasal administration (although live vaccines are not recommended for pregnant women 5) and as inactivated, split-virus or subunit vaccines for injection (inactivated H1N1). 6 Since the licensure and manufacturing process for the 2009 H1N1 vaccines was the same as that used for seasonal influenza vaccines, 7 the safety profile of licensed 2009 H1N1 vaccine was anticipated to be similar to that of seasonal influenza vaccine, which has an excellent safety record. 8
Monitoring adverse events (AEs) following 2009 H1N1 vaccine administration in pregnant women was a priority for the Centers for Disease Control and Prevention (CDC) in collaboration with FDA. The Vaccine Adverse Event Reporting System (VAERS) is the largest United States postlicensure surveillance system and has provided useful information on the safety profile of TIV in the general population of adults 9 and children. 10 Because of its size and national scope, VAERS can provide information on rare AEs that may not be detected in pre-licensure clinical trials.
As part of national 2009 H1N1 vaccine safety monitoring, we conducted rapid surveillance for adverse events reported to VAERS in pregnant women who received H1N1 vaccines to assess for potential vaccine safety problems.
Material and Methods
Vaccine Adverse Events Reporting System (VAERS)
VAERS is a spontaneous reporting system used to monitor vaccine safety. It was established in 1990 and is jointly administered by CDC and FDA. 11 VAERS accepts reports of AEs after vaccination from vaccine manufacturers, healthcare providers, vaccine recipients, and others. VAERS generally cannot assess whether a vaccination caused an AE, but can identify possible vaccine safety problems for further investigation. 11 Reports are classified as serious, based on the Code of Federal Regulations, if they result in death, life-threatening illness, hospitalization or prolongation of hospitalization, permanent disability or congenital anomaly. 12
This regulatory definition of a serious report does not always reflect the severity of an outcome. Thus some reports describing severe clinical conditions could be classified as non-serious if none of the above mentioned situations are not met.
As part of the federal response to monitor the safety of 2009 H1N1 vaccine, VAERS was enhanced in several ways: providing VAERS contact information on influenza vaccination cards given to vaccinees at the time of vaccination; advertising in medical journals and on the internet; communicating with professional associations (such as the American College of Obstetricians and Gynecologists, American Academy of Pediatrics, American Medical Association) and state vaccine safety coordinators; and, increasing the capacity to process reports and obtain and review medical records.
Review of reports
We analyzed VAERS reports received through March 1, 2010 on pregnant women who were vaccinated with 2009 H1N1 vaccine during October 1, 2009 through February 28, 2010. Non-US reports were excluded. We included reports of AEs in neonates if the report indicated that their mothers had received 2009 H1N1 vaccine during pregnancy. The Medical Dictionary for Regulatory Activities (MedDRA) 13 is an internationally standardized terminology used for coding clinical signs, symptoms and diagnoses. An individual VAERS report may be coded with one or more MedDRA terms. We manually reviewed all VAERS reports for pregnant cases. Additionally, we identified reports by searching for MedDRA terms in two System Organ Classes (SOC): ‘Pregnancy, Puerperium, and Perinatal Conditions’ and ‘Congenital, Familial and Genetic Disorders’, and a text string search for the term ‘PREG’ in the report.
Medical records were requested for all reports that 1) suggested the vaccinee was pregnant at the time of receipt of the 2009 H1N1 vaccine or 2) were from infants born to mothers who received 2009 H1N1 vaccine during pregnancy. One medical officer (PM) conducted a clinical review of all VAERS reports retrieved using both search strategies described above to distinguish between pregnancy and non-pregnancy related reports. Medical officers with obstetrical expertise reviewed medical records of case-reports presenting more specialized pregnancy information (DK,FB, NR, NT, YZ). Reports indicating that influenza vaccine was administered prior to last menstrual period were excluded. Pre-specified pregnancy outcomes under surveillance included: spontaneous abortion (SAB) defined as a fetal death occurring < 20 weeks gestation, stillbirth defined as a fetal death occurring ≥ 20 weeks gestation, preterm delivery occurring < 37 weeks delivery, preeclampsia, and eclampsia. We reviewed the medical records of all reports to verify the diagnosis and to obtain additional data, including state of residence, maternal date of birth, maternal age, gestational age at the time of vaccination and at the time of event, time between vaccination and onset of AE (onset interval), major symptoms, laboratory data, concomitant vaccinations, illness(es) at the time of vaccination, and pre-existing medical conditions. We also reviewed medical records of women who reported a spontaneous abortion or a stillbirth to identify risk factors (as reported in literature or identified by expert opinion) 14, for these conditions. Gestational age at the time of vaccination and at the time of AE was calculated based on 1) clinical determination of healthcare provider; or 2) earliest ultrasound assessment (if the former was not available); or 3) last menstrual period, estimated delivery date, or estimated date of conception (if the first 2 options were not available). If applicable, the sex of the infant or fetus and the type of congenital anomaly were collected. We defined trimesters by gestational age as: first (0–13 weeks), second (14–27 weeks), and third (28 + weeks). Reports of anaphylaxis or Guillain-Barré Syndrome were verified based on the attending physician’s assessment or criteria established by the Brighton Collaboration definition, an international collaboration for establishing standardized case definitions for AEs following vaccination. 15–17 When more than one AE were reported for the same person, we selected what we believed to be the primary clinical event of concern determined after medical record review. We did not assess causality of AEs. Because VAERS is a routine surveillance program that does not meet the definition of research, it is not subject to Institutional Review Board review and informed consent requirements.
Reporting rates
We estimated the number of pregnant women during the period of our surveillance by adapting previously published methods. 4 To estimate the number of pregnant women vaccinated with inactivated 2009 H1N1 vaccine, we used influenza vaccination coverage data from October 1, 2009 through February 28, 2010 from the National 2009 H1N1 Flu Survey (NHFS) 18 [CDC unpublished data]. To estimate the number of pregnant women during the period of this review we used the most recent vital statistics data available on fertility, fetal loss, and induced abortions.19–21 The 2009 population of women of reproductive age (61,948,144) was determined from US Census estimates.22 We calculated reporting rates of AEs in pregnant women by dividing the number of AEs by the estimated number of inactivated 2009 H1N1 vaccine doses administered to pregnant women during the time period covered in this review. A supplementary technical appendix shows in detail the calculations used to determine the number of pregnant women vaccinated with inactivated 2009 H1N1 vaccine.
Analysis
We calculated frequencies of the most common MedDRA coding terms, demographic and medical history information, and selected pregnancy and fetal outcomes and reporting rates for selected outcomes using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
From October 1, 2009 through February 28, 2010, VAERS received a total of 10,186 reports after H1N1 vaccination; 422 of these reports involved pregnant women. We excluded 128 pregnancy reports where no AE was reported after administration of inactivated or live 2009 H1N1 vaccine. These pregnant women are being followed prospectively as part of a separate CDC study that seeks to assess delivery and infant outcomes. Of the remaining 294 reports, 288 followed inactivated and six followed live vaccines. Medical records were obtained for 240 reports (81.6%). Sixty (20.4%) reports were coded as serious, including two maternal deaths. Serious reports corresponded to 59 pregnant women who were hospitalized for any reason and one to an infant born with a congenital anomaly. The 2009 H1N1 vaccine was reported as administered mostly during the first (133; 53.9%) and second trimester of pregnancy (67; 27.1%). In 31 (10.5%) reports, 2009 H1N1 vaccine was administered on the same date as seasonal influenza vaccine (Table 1). The most frequent pregnancy-specific AE reported following 2009 H1N1 vaccine administration was SAB in 121 women (41.2% of reports), followed by stillbirth in 19 women (6.5% of reports) (Table 2). Most (62) SAB reports were received in November 2009 but reporting rapidly declined thereafter (Figure 1). All stillbirths were reported during October and November 2009 with 10 and 9 reports, respectively (Figure 1). In 42.4% of SAB reports the onset interval from the day of vaccination to onset of AE was 0–3 days (61 reports with onset 0–6 days). In 47.4% of stillbirth reports the onset interval was 0–6 days. We did not observe any clustering of SAB or stillbirth cases by geographic location or lot number. Ninety-five (78.5%) SAB and 18 (94.7%) stillbirths were verified by review of medical records. Five fetal adverse outcomes were reported, 2 cases of intrauterine growth restriction and one case each of hydronephrosis, tachycardia, and cleft lip. The maternal deaths included one patient who experienced hemorrhagic shock due to uterine atony following a cesarean section and one patient who experienced a ruptured aortic aneurysm which resulted in cardiac tamponade and subsequent death. The most common pregnancy-specific AEs were SAB (121), stillbirth (19), and preterm delivery (7). The most common non-pregnancy specific AEs were non-anaphylactic allergic reactions (36), constitutional symptoms (28), and local reactions (10). Two-hundred thirty-five (79.9%) of total reports did not require hospitalization.
Table 1.
Characteristics of the Vaccine Adverse Event Reporting System (VAERS) reports following receipt of 2009 H1N1 vaccine in pregnant women October 1, 2009 through February 28, 2010 United States (n=294).
| Characteristic | |
|---|---|
| Serious reports | 20.4% (60/294) |
| Deaths | 0.7% (2/294) |
| Maternal age in years (median [range]) a | 30.0 (15.0 – 46.0) |
| Onset interval in daysb (median [range]) | 2.0 (0 – 84.0) |
| Gestational age in weeks at time of vaccination, median (range) c | 13.0 (3.0 –39.0) |
| Gestational age at time of vaccination (n=247) | |
| First trimester (0 – 13 weeks) | 53.9% (133/247) |
| Second trimester (14 – 27 weeks) | 27.1% (67/247) |
| Third trimester (28 + weeks) | 19.0% (47/247) |
| Type of reporter | |
| Patient | 45.6% (134/294) |
| Vaccine provider | 39.1% (115/294) |
| Other | 11.6% (34/294) |
| Healthcare provider | 2.4% (7/294) |
| Manufacturer | 0.6% (2/294) |
| 2009 H1N1 vaccine given on the same day as seasonal influenza vaccine (%) | 10.5% (31/294) |
| No. MedDRAd terms coded for all reports | 2182 |
| Median No. MedDRA terms per report | 6.0 |
Age missing for one pregnant woman. One neonate report not included for age calculation.
Onset unknown for 19 reports. Onset is the difference between date of onset of adverse event and vaccination date.
Gestational age determined at time of vaccinationGestational age determined at time of vaccination. Gestational age unknown for 49 reports.
The Medical Dictionary for Regulatory Activities (MedDRA) was used to code symptoms and/or conditions described in each report.
Table 2.
Adverse events a in pregnant women following 2009 H1N1 influenza vaccine, VAERS, October 1, 2009 through February 28, 2010 (n=294).
| N | % | |
|---|---|---|
| Pregnancy-specific outcomes | ||
| Spontaneous Abortion (< 20 weeks gestation) † | 121 | 41.2 |
| Stillbirth (≥ 20 weeks gestation) | 19 | 6.5 |
| Preterm deliveryb | 7 | 2.4 |
| Threatened abortion | 4 | 1.4 |
| Preterm labor | 3 | 1.0 |
| Maternal death c | 2 | 0.7 |
| Preeclampsia | 2 | 0.7 |
| Intrauterine growth restriction | 2 | 0.7 |
| Fetal hydronephrosis | 1 | 0.3 |
| Fetal tachycardia | 1 | 0.3 |
| Cleft lip d | 1 | 0.3 |
| Neonatal intraparenchymal hemorrhage | 1 | 0.3 |
| Intrauterine fetal death e | 1 | 0.3 |
| Subtotal | 165 | 56.1 |
| Non-pregnancy specific outcomes | ||
| Allergic reactions other than anaphylaxis | 36 | 12.2 |
| General or constitutional symptoms f† | 28 | 9.5 |
| Local reactions | 10 | 3.4 |
| Paresthesias | 8 | 2.7 |
| Bell’s palsy | 5 | 1.7 |
| Syncope | 5 | 1.7 |
| Dizziness† | 5 | 1.7 |
| Hypoesthesia | 3 | 1.0 |
| Headache† | 3 | 1.0 |
| Anaphylaxis | 1 | 0.3 |
| Other g† | 25 | 8.5 |
| Subtotal | 129 | 43.9 |
| Total | 294 | 100.0 |
Adverse events based on main diagnosis following medical chart review
Infant of one mother died approximately one month after delivery.
Maternal deaths included a case of ruptured aortic aneurysm and a case of hemorrhagic shock. In both cases the neonates were born alive.
19 weeks gestational age when vaccinated
Could not be categorized as spontaneous abortion or stillbirth because the gestational age was unknown
Does not include paresthesias, or headache.
Includes: anxiety, arthralgia, asthenia, asthma, arthritis, blurred vision, chest pain, chronic bronchitis, cough, diarrhea, dyspnea, eye swelling, influenza, influenza like illness, lip injury, nail bleeding, panic attack, shingles, sneezing, tachycardia, tremors, unusual taste, upper respiratory infection (2), wheezing
Live H1N1 vaccine cases: two constitutional reactions and one of each
Figure 1.
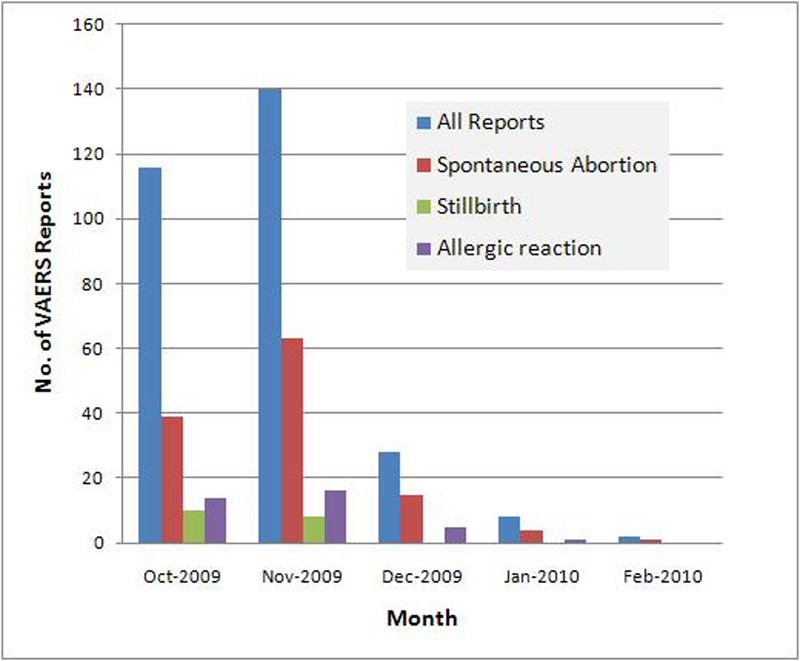
Monthly Reports of spontaneous abortions, stillbirth and allergic reactions among pregnant women who received 2009 H1N1 vaccine and who reported to VAERS
Only one congenital anomaly was reported to VAERS, a cleft lip in an infant born to a 23 year-old woman who was vaccinated at 19 weeks gestation. The cleft lip was first detected during a prenatal ultrasound exam at 22 weeks gestation. Because cleft lip is embryologically determined during the first trimester of pregnancy, it is implausible to consider vaccination at 19 weeks gestation as a possible causative agent. In another report, mild fetal hydronephrosis was detected in a pregnant woman during an ultrasound exam at 36 weeks gestation. However, a postnatal ultrasound exam of the infant performed on day 2 of life failed to confirm the presence of hydronephrosis; thus we did not consider this infant to have a congenital anomaly.
Five cases of Bell’s palsy and one case of anaphylaxis (meeting Brighton Collaboration case definition) 17 were reported. No cases of Guillain-Barré Syndrome were identified.
Medical record review of spontaneous abortions and stillbirths reported to VAERS
One-hundred twenty-one cases of SAB after 2009 H1N1 vaccination were reported to VAERS (120 after inactivated, and 1 after live vaccine) of which 95 were verified. Medical records were not received for 16 reports, and in 10 reports the available records were not sufficient to confirm the initial diagnosis of SAB. Risk factors for SAB were identified in 47 (49.5%) of the 95 verified reports (Table 3). Thirty-six women had only one risk factor for SAB. The most common risk factor was advanced maternal age (defined as age ≥ 35 years) in 25 (26.3%) reports.
Table 3.
Risk Factors for Spontaneous Abortions (SAB) and Stillbirths in Verified Reports Received Following 2009 H1N1Influenza Vaccination, VAERS, October 14, 2009 through February 28, 2010.
| SAB (N=95) | Stillbirths (N=18) | |
|---|---|---|
| Median age years | 32.0 | 29.0 |
| Median onset interval in days a | 7.0 | 7.0 |
| Median duration of pregnancy in weeks b | 8.0 | 30.0 |
| Risk factors c: | N (%) | |
| Advanced maternal age (≥ 35 years of age) | 25 (26.3) | 5 (27.8) |
| Smoking | 7 (7.4) | 3 (16.7) |
| History of intrauterine fetal death | 4 (4.2) | 2 (11.1) |
| Hypothyroidism | 4 (4.2) | 0 |
| Diabetes mellitus d | 3 (3.2) | 2 (11.1) |
| Urinary tract infection | 0 | 5 (27.8) |
| Alcohol or drug use | 2 (2.1) | 4 (22.2) |
| Obesity | 1 (1.1) | 3 (16.7) |
| Other e | 2 (2.1) | 7 (38.9) |
| Pathological findings/observations at delivery: | ||
| Chorioamnionitis | 1 (1.1) | 7 (38.9) |
| Tight nuchal cord | NA | 2 (11.1) |
| Fetal-maternal hemorrhage | NA | 2 (11.1) |
| Fetal growth restriction | NA | 2 (11.1) |
Onset interval unknown for 2 SAB reports. Onset interval is the difference between onset of adverse event and vaccination date.
Duration of pregnancy unknown for 10 SAB reports.
Risk factors are not mutually exclusive; 47 (49.5%) of the 95 had at least one risk factor for a SAB; Among stillbirths, 13 (72.2%) of 18 reports had ≥ 1 maternal risk factor for stillbirth
For SAB reports it includes one case of type II diabetes, type I and history of gestational diabetes; For stillbirth reports it includes on case of type I and one of gestational diabetes
Other risk factors for spontaneous abortion included: chlamydia infection and complicated pregnancy; Other risk factors for stillbirths included: congenital malformations in past pregnancies, uncontrolled diabetes, chronic hypertension, positive Down syndrome screening, chronic hypertension, and macrocytic hyperchromic anemia
NA: not applicable
Nineteen stillbirths were reported to VAERS. Of 18 with verified diagnosis, 13 (72.2%) had at least one maternal risk factor for stillbirth (e.g., obesity) or a pathological finding that had at least one maternal risk factor for SB or a finding on pathological evulation of the fetus or placenta that may have contributed to the fetal demisemay have contributed to the fetal demise (Table 3). Fourteen of 18 reports had pathology reports. Pathological/placental or other findings were present in 13 of the 14 reports and included: chorioamnionitis in 7 reports, and 2 reports each of tight nuchal cord, fetal-maternal hemorrhage, and fetal growth restriction. or a finding on pathological evulation of the fetus or placenta that may have contributed to the fetal demise
Adverse event reporting rates
We estimated that during October 2009 through February 2010, 2,437,113 (95% CI, 1,865,373 – 3,008,853) pregnant women were vaccinated with inactivated 2009 H1N1 vaccine in the United States. The overall reporting rate of AEs to VAERS in pregnant women after inactivated 2009 H1N1 vaccine was 118.2 reports per one million pregnant women vaccinated. The reporting rates for SABs and stillbirths were 49.2 and 7.8 reports per one million pregnant women vaccinated, respectively.
Comment
During October 2009 through February 2010, approximately 3% of reports to VAERS after 2009 H1N1 vaccine were of pregnant women who experienced at least one adverse event after vaccination. Among 294 VAERS reports of AEs in pregnant women after 2009 H1N1 vaccination with inactivated and live vaccines, we did not observe any unusual patterns of adverse maternal or fetal outcomes. Reporting for all AEs was highest during the month of November 2009 and markedly declined in subsequent months which may be due to higher H1N1 vaccination coverage during the earlier part of the 2009 H1N1 vaccination program (Dr. Helen Ding, CDC, unpublished observations). The observed greater number of reports may also reflect a Weber-like effect, 23 likely due to enhancements made to VAERS and to publicity surrounding the H1N1 vaccination program. As described by Hartnell et al 23 the Weber effect is an epidemiologic phenomenon whereby new products or products perceived to be new have higher reporting rates initially, which then decline despite steadily increasing prescribing rates. Similar phenomena have been observed with other vaccines. 24, 25
The most common pregnancy-specific AE reported was SAB. In our review of medical records, we found that more than a third of women who experienced a SAB had at least one risk factor, with the most common being advanced maternal age. Pregnant women 35 years of age or older constitute approximately 16% of the pregnant population 26; however, among women of reproductive age (pregnant and non-pregnant) who received 2009 H1N1 vaccine and reported to VAERS, 43% were within this age group. This suggests that women with a higher risk for SAB are overrepresented in the VAERS database. Reasons for increased representation for older pregnant women in the VAERS database are not clear. Although there are no published reports that would suggest so, it is possible that coverage for 2009 H1N1 vaccine or adverse event reporting practices varied by age group for pregnant women.
The second most common pregnancy-specific AE was stillbirth. Although stillbirths occur less frequently than preterm delivery (third most frequent pregnancy-specific outcome reported to VAERS) or preeclampsia in the general population,27,28 the complex interaction of factors such as voluntary reporting to VAERS, the reporter’s perception of the AE 29 and other causes of biased reporting, may have contributed to the preferential reporting of the more serious events, such as stillbirths. Careful review of medical records of stillbirth reports received by VAERS following 2009 H1N1 vaccine revealed that 72% of these women had at least one risk factor or had a pathological finding in the fetus or placenta suggesting factors other than vaccination that may have contributed to the fetal demise.
We observed that the onset interval for approximately half of SAB and stillbirth reports clustered during the first week following vaccination. This is a typical reporting pattern in VAERS and other spontaneous systems whereby a larger number of reports are reported closer to the vaccination date.11
SAB is a relatively frequent event in pregnancy, with a rate as high as 22.4% in women aged ≥34 years and 10.4% in women younger than age 25 years.27 Stillbirths occur at a background rate of 0.4% of all pregnancies or 6.22 per 1,000 live births and fetal deaths. 30 There is under-reporting to VAERS in general and the proportion of AEs following immunization among pregnant women that are reported to VAERS is unknown. Nonetheless, the reporting rates to VAERS for SABs and stillbirths after H1N1 vaccine was several orders of magnitude lower than the expected rates of fetal losses in the general population of pregnant women 27,30 during a time of heightened awareness about vaccine safety. The VAERS data provide no indication that the occurrence of SAB and stillbirths following influenza vaccination is higher than in the general population. The reporting rate for SAB in the current review of H1N1 vaccine reports was 25-times higher than the reporting rate to VAERS for SAB after seasonal influenza vaccine during the period 1990–2009. 31 This difference between 2009 H1N1 and 1990–2009 seasonal influenza vaccines is likely the result of enhanced and stimulated reporting. As part of the H1N1 response, CDC partnered with several state and local health departments and private and academic institutions throughout the United States to increase communication about pandemic (H1N1) 2009 influenza and the 2009 H1N1 vaccine program, particularly targeting pregnant women and those who care for them. Special attention was placed on the safety of the H1N1 vaccines through enhanced monitoring and surveillance. 32
Only one congenital anomaly was reported. Although this probable represents underreporting of these AEs, no unusual clustering of birth defects were reported. Passive surveillance systems tend to receive AE reports closer to the exposure due to recall bias and since delivery and awareness of a congenital anomaly likely occur many weeks after receipt of vaccination they are less likely to be reported.11
Other AEs observed in pregnant women were mostly non-serious and were similar to those reported in a preliminary review of the safety of 2009 H1N1 vaccines among the general population. 33 For example, non-anaphylactic allergic type reactions were observed in 12% of pregnancy reports following 2009 H1N1 vaccination, compared with 9% of non-pregnancy reports. 33 Bell’s palsy, a mononeuropathy of the VII (facial) cranial nerve, has been reported to occur 3 times more frequently in pregnant women than in non-pregnant women of child-bearing age. 34 However, in our review of VAERS the reporting rate of Bell’s palsy after 2009 H1N1 vaccination among pregnant women was only 0.2 cases per 100,000 in comparison to the incidence rate of 25 cases per 100,000 in the general population. 35 Other than paresthesias, no other neurological outcomes, including Guillain-Barré syndrome, were reported. 2009 H1N1 vaccines were anticipated to have a similar safety profile as seasonal influenza vaccines. Initial data during the first 2 months of the 2009 H1N1 vaccination program and an end-of-season analysis supported this assumption. 32,33 Several studies suggest that seasonal influenza vaccine is safe in pregnancy. A large prospective study with seven years of follow-up of 2,291 pregnant women who received seasonal inactivated vaccine did not find any congenital malformations, malignancies, or increased childhood mortality that could be associated with exposure to the vaccine in pregnancy. 36 A retrospective study of 252 pregnant women vaccinated with inactivated seasonal influenza vaccine matched to a control group of 826 unvaccinated healthy pregnant women found no serious adverse events within 42 days of vaccination; no differences in pregnancy outcomes and infant conditions up to 6 months after birth were found. 37 Additional studies in over 7,000 pregnant women vaccinated with inactivated seasonal influenza vaccine have failed to demonstrate increased risk of maternal, fetal or infant complications. 8 However, relatively few of these studies have included first trimester pregnant women, demonstrating a gap that should be addressed in future vaccine safety research.
VAERS is a national surveillance system used to detect signals of potential adverse events following vaccination. For example, VAERS reports of intussusception following receipt of Rotashield®, a tetravalent rotavirus vaccine no longer in use 38, prompted studies that confirmed this finding. 39,40 Any finding in VAERS needs to be interpreted with caution given its inherent limitations. VAERS is a passive surveillance system that may be prone to biased reporting (over- or underreporting) and inconsistency in the quality and completeness of reports. VAERS also generally cannot determine whether a vaccine caused an AE. 11 Stimulated reporting can occur following publicity around a potential AE. For example, number of reports of intussusception after Rotashield® vaccination received by VAERS increased almost 12-fold after a report in CDC’s Morbidity and Mortality Weekly Report (MMWR) described the association between Rotavirus vaccine and intussusceptions. 41
To assess the clinical circumstances of reported cases of fetal demise we reviewed available medical records to identify potential risk factors for SAB or stillbirths. However, it is important to point out that this assessment may be incomplete since medical records may not provide complete information on the number and nature of these risk factors. The regulatory definition of a serious report in VAERS can have limitations as it may not reflect the true severity of an outcome. For example, in VAERS any hospitalization of a woman for dilation and curettage following a spontaneous abortion (even a brief one) would be coded as serious. One important limitation of VAERS is that it only collects data on vaccinated individuals who have experienced an AE; the total vaccinated population is not known with certainty and therefore incidence rates of AE cannot be directly calculated. The number of individuals vaccinated is often estimated using the number of doses distributed or vaccine coverage data, derived from special surveys. Both of these approaches have limitations. We used preliminary coverage data from the National 2009 H1N1 Flu survey (NHFS) which shows that 43% of pregnant women received the 2009 H1N1 vaccine [CDC unpublished data]. This reported 2009 H1N1 vaccination coverage was higher than reported for pregnant women in previous seasons. 1
NHFS did not collect data on the vaccination coverage by trimester of pregnancy. We did not identify other data sources that provided vaccination coverage data by trimester of pregnancy. While it may be of interest to calculate reporting rates for SAB using only women during the first 20 weeks of pregnancy, reported SAB background rates generally have been calculated using all pregnant women. In this study, SAB reporting rates were compared to SAB background rates from vaccine safety studies where all trimester pregnant women were used as the denominator for calculating rates of SAB.27
Our estimates of the population of pregnant women may not reflect the true population of pregnant women during the study period. For example, to estimate the population of pregnant women in 2009 we used the most recent fertility, induced abortion, and fetal loss rates; 2009 rates are not yet available. In addition we used the 10-year average of monthly proportions for live births and applied this to our pregnant population estimate with the assumption that induced abortions and fetal losses would vary in a similar manner. We used vaccination coverage for women that reported they were pregnant from October 2009 through February 28, 2010. This estimate of the vaccination coverage may be subject to limitations such as small sample size, self-reported pregnancy and vaccination status, or non-response bias. Our estimates of the number of vaccinated pregnant women may also be imprecise. Indeed the 95% confidence intervals of our estimates were wide which indicate imprecision. We found that a little more than half of the pregnant women who reported to VAERS received the vaccine during the first trimester of pregnancy. This is likely an artifact due to the predominance of reports of SAB which tend to occur mostly (80%) during the first trimester of pregnancy 14 (temporal bias) and may also be a reflection of biased reporting of more serious events to VAERS.
Although VAERS provided rapid initial assessment of reports of AEs among pregnant women, it is only one system that served to monitor the safety of 2009 H1N1 vaccines. Several other federally supported systems have been part of the vaccine safety monitoring effort, including the Vaccine Safety Datalink (VSD) and the Vaccines and Medications in Pregnancy Surveillance System (VAMPSS). 42,43 Unlike VAERS, these systems are designed to assess for potential associations between vaccines and adverse events using comparisons groups. Data from these and other systems are anticipated to provide further information about the safety of 2009 H1N1 vaccines among pregnant women.
On February 22, 2010. the FDA’s Vaccines and Related Biological Products Advisory Committee voted to include the 2009 monovalent H1N1 vaccine strain (an A/California7/2009-like strain) in the 2010–11 “seasonal” trivalent influenza vaccine (http://www.cdc.gov/vaccines/recs/ACIP/livemeeting-feb10.htm#inf). Pregnant women will continue to be recommended for vaccination, along with all persons 6 months and older. Our review of VAERS reports following 2009 H1N1 vaccination has provided a comprehensive and rapid assessment of the safety of inactivated 2009 H1N1 vaccine among pregnant women during a period when influenza vaccine coverage during pregnancy was at its highest recorded levels ever. 1,18 We will continue to use VAERS to monitor AEs in pregnant women following future seasonal influenza vaccines.
Condensation.
We characterized reports to the Vaccine Adverse Event Reporting System in pregnant women who received Influenza A (H1N1) 2009 Monovalent Vaccine to assess for potential vaccine safety concerns.
Acknowledgements
We would like to thank Drs. Janet Cragan, Dixie Snider, and Sonja Rasmussen, Ms. Nadine Shehab, and Mr. Thomas H. Taylor for their valuable comments and advice. We thank CDC’s Immunization Safety Office staff whose indefatigable labor allowed this activity to be conducted. Disclosures: No authors have a conflict of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Part of this research was presented at the Thirteenth Annual Conference on Vaccine Research held in Bethesda, Maryland during April 26-April 28, 2010, and during the October 28, 2010 meeting of the Advisory Committee on Immunization Practices in Atlanta, Georgia.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or Food and Drug Administration.
References
- 1.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009; 58:1–52. [PubMed] [Google Scholar]
- 2.Skowronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine 2009; 27:4754–70. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Swine Influenza A (H1N1) Infection in Two Children --- Southern California, March--April 2009. MMWR. 2009; 58:400–2. [PubMed] [Google Scholar]
- 4.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451–8. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Use of Influenza A (H1N1) 2009 Monavalent Vaccine Recommendations of the Advisory Committee on Immunization Practices. MMWR; 2009; 58:1–8. [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Influenza A (H1N1) 2009 Monovalent, available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm181950.htm Retrieved January 4, 2010.
- 7.Food and Drug Administration. Vaccines Licensed for Immunization and Distribution in the US with Supporting Documents (Influenza vaccine), available at http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093830.htm, Retrieved January 4, 2010.
- 8.Tamma PD, Ault KA, Del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009; 201:547–52. [DOI] [PubMed] [Google Scholar]
- 9.Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine. 2009; 27:2114–20. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg M, Sparks R, McMahon A, Iskander J, Campbell JD, Edwards KM. Serious adverse events rarely reported after trivalent inactivated influenza vaccine (TIV) in children 6–23 months of age. Vaccine. 2009; 27:4278–83. [DOI] [PubMed] [Google Scholar]
- 11.Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J 2004; 23:287–94. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. 21 CFR Part 600.80 Postmarketing reporting of adverse experiences. Vol 62: Federal Register; 1997:52252–52253. [Google Scholar]
- 13.Web site: Medical Dictionary for Regulatory Activities. http://www.meddramsso.com/MSSOWeb/index.htm. Retrieved August 24, 2009.
- 14.Cunningham G, Leveno KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstrom KD. (Editors). Williams Obstetrics; McGraw-Hill Companies, Inc; 2001. [Google Scholar]
- 15.Kohl KS, Gidudu J, Bonhoeffer J, et al. The development of standardized case definitions and guidelines for adverse events following immunization. Vaccine 2007; 25:5671–4. [DOI] [PubMed] [Google Scholar]
- 16.Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011; 29:599–612 [DOI] [PubMed] [Google Scholar]
- 17.Ruggeberg JU, Gold MS, Bayas JM, et al. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2007; 25:5675–84. [DOI] [PubMed] [Google Scholar]
- 18.Interim Results: State-Specific Influenza A (H1N1) 2009 Monovalent Vaccination Coverage --- United States, October 2009 -- January 2010. Morbidity and Mortality Weekly Report (MMWR). 2010;59(12):363–638. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5912a2.htm. Retrieved July 27, 2010. [PubMed] [Google Scholar]
- 19.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2008. National vital statistics reports; vol 58 no 16. Hyattsville, MD: National Center for Health Statistics; Released April 6, 2010. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_16.pdf [Google Scholar]
- 20.Centers for Disease Control and Prevention. Abortion Surveillance — United States, 2006. Surveillance Summaries, November 27, 2009. MMWR 2009; 58(No. SS-8). Available at http://www.cdc.gov/mmwr/pdf/ss/ss5808.pdf [Google Scholar]
- 21.Ventura S, Abma JC, Mosher WD. Estimated Pregnancy rates for the United States, 1990–2005: An Update. National Vital Statistics Reports Volume 58, Number 4 (October 14, 2009). Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_04.pdf Retrieved May 4, 2010. [PubMed] [Google Scholar]
- 22.US Census Bureau, Population Estimates Program. Available at http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=PEP&_submenuId=datasets_3&_lang=en
- 23.Hartnell NR, Wilson JP. Replication of the Weber Effect using Postmarketing adverse event Reports Voluntarily Submitted to the United States Food and Drug Administration. Pharmacotherapy 2004; 24:743–49. [DOI] [PubMed] [Google Scholar]
- 24.Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005; 294:2720–5. [DOI] [PubMed] [Google Scholar]
- 25.Chaves SS, Haber P, Walton K, et al. Safety of Varicella Vaccine after Licensure in the United States: Experience from Reports to the Vaccine Adverse Event Reporting System, 1995–2005. J Infect Dis. 2008; 197 Suppl 2:S170–7. [DOI] [PubMed] [Google Scholar]
- 26.Ventura SJ, Abma JC, Mosher WD. Estimated Pregnancy Rates by Outcome for the United States, 1990–2004. National Vital Statistics Reports. 2008; 56:1–28. http://www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_15.pdf. Retrieved August 24, 2009. [PubMed] [Google Scholar]
- 27.Black S, Eskola J, Siegrist CA, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunization with pandemic H1N1 influenza vaccines. Lancet 2009; 374:2115–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008; 21:521–26. [DOI] [PubMed] [Google Scholar]
- 29.Woo EJ, Ball R, Bostrom A, et al. Vaccine risk perception among reporters of autism after vaccination: vaccine adverse event reporting system 1990–2001. Am J Public Health 2004; 94:990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDorman MF, Kirmeyer S. Fetal and Perinatal Mortality, United States, 2005. National Vital Statistics Reports. 2009; 57(8):1–20. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_08.pdf. Retrieved May 6, 2010. [PubMed] [Google Scholar]
- 31.Moro PL, Broder K, Zheteyeva Y, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. A J Obstet Gynecol 2011. 204:146.e1–7 [DOI] [PubMed] [Google Scholar]
- 32.Vellozzi C, Broder KR, Haber P, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine. 2010; 28:7248–55 [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC). Safety of influenza A (H1N1) 2009 monovalent vaccines - United States, October 1-November 24, 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:1351–6. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58e1204a1.htm, Retrieved February 2, 2010. [PubMed] [Google Scholar]
- 34.Shapiro JL, Yudin MH, Ray JG. Bell’s Palsy and Tinnitus during Pregnancy: Predictors of Preeclampsia: Three Cases and a Detailed Review of the Literature. Acta Oto-Laryngologica 1999; 119:647–51. [DOI] [PubMed] [Google Scholar]
- 35.Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell’s palsy in the UK. Eur J Neurol 2002; 9:63–67 [DOI] [PubMed] [Google Scholar]
- 36.Heinonen OP, Shapiro S, Monson RR, Hartz SC, Rosenber L, Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. Int J Epidemiol 1973; 2:229–35. [DOI] [PubMed] [Google Scholar]
- 37.Munoz FM, Greisinger AJ, Wehmanen OA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2005; 192:1098–106. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC). Intussusception among recipients of rotavirus vaccine--United States, 1998–1999. MMWR Morb Mortal Wkly Rep 1999; 48:577–81. [PubMed] [Google Scholar]
- 39.Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J 2001; 20:410–6. [DOI] [PubMed] [Google Scholar]
- 40.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344:564–72. [DOI] [PubMed] [Google Scholar]
- 41.Haber P, Chen RT, Zanardi LR, Mootrey GT, English R, Braun MM. VAERS Working Group. An analysis of rotavirus vaccine reports to the vaccine adverse event reporting system: more than intussusception alone? Pediatrics 2004; 113:e353–9. [DOI] [PubMed] [Google Scholar]
- 42.Federal Immunization Safety Task Force. Federal Plans to Monitor Immunization Safety for the Pandemic 2009 H1N1 Influenza Vaccination Program. http://www.flu.gov/professional/federal/monitor_immunization_safety.html. Retrieved June 4, 2010.
- 43.The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS). http://www.otispregnancy.org/vaccines-and-medications-in-pregnancy-surveillance-system-vampss-s13053. Retrieved June 4, 2010.


