Abstract
Introduction:
Routine immunization of pregnant women with seasonal inactivated influenza vaccines (IIV) is recommended in all trimesters of pregnancy. A review of the Vaccine Adverse Event Reporting System (VAERS) during 1990–2009 did not find any unexpected patterns of pregnancy complications or fetal outcomes after administration of IIV or live attenuated influenza vaccines (LAIV). During 2009–2010 pandemic H1N1 vaccination campaign, a study noted that the numbers of VAERS reports from pregnant women who received the influenza A (H1N1) 2009 inactivated monovalent vaccine (N=288) increased compared to 1990–2009 seasonal IIV pregnancy reports (N=148).
Objectives:
To assess the safety of seasonal influenza vaccines in pregnant women and their infants whose reports were submitted to VAERS during 2010–2016.
Methods:
We searched VAERS for US reports of adverse events (AEs) in pregnant women who received IIV or LAIV from 7/1/2010–5/06/2016. Clinicians reviewed reports and available medical records and assigned a primary clinical category for each report. Reports were coded as serious, based on the Code of Federal Regulations.
Results:
We identified 671 reports after seasonal influenza vaccines administered to pregnant women: 544 after IIV and 127 after LAIV. Serious events occurred among 61 (11.2%) reports following IIV and 1 (0.8%) report following LAIV. No deaths were reported. Among reports with trimester information (n=296), IIV was administered during the first trimester in 116 (39.2%). Among IIV reports, the most frequent pregnancy-specific AE was spontaneous abortion in 62 (11.4%) reports, followed by stillbirth in 10 (1.8%) and preterm delivery in 6 (1.1%). The most common non-pregnancy specific AEs were injection site reactions (55, 10.1%). Neonatal or infant outcomes were reported in 22 (4.0%) reports, 7 of which had major birth defects of different types and no neonatal deaths.
Conclusion:
As in 2009–2010, no new or unexpected patterns in maternal or fetal outcomes were observed during 2010–2016.
Keywords: adverse events, epidemiology, inactivated influenza vaccines, live attenuated influenza vaccines, pregnancy, surveillance, vaccine safety
1. Introduction
During the 2009 H1N1 pandemic, pregnant women were found to have a higher risk for serious complications and death from influenza than non-pregnant women of reproductive age [1,2]. Since 1997 the Advisory Committee on Immunization Practices (ACIP) recommended routine immunization of pregnant women with trivalent inactivated influenza vaccine (IIV3) after the first trimester; in 2004 the recommendation was expanded to all trimesters [3]. Live attenuated influenza vaccine (LAIV) is not recommended for use in pregnant women. Maternal influenza vaccination is an important strategy for preventing severe influenza infections in pregnant women and newborns. Some studies suggest that inactivated influenza vaccine (IIV) may protect against certain outcomes such as preterm delivery or small for gestational age [4] and it may also confer protection to infants who cannot be vaccinated until 6 months [5]. Despite improvements in influenza vaccination coverage among pregnant women in the post-H1N1 pandemic period, only 41% were vaccinated during 2013–2014 in the US [6].
Patients’ concerns regarding the safety of influenza vaccines during pregnancy continue to be a barrier to vaccine uptake [7] despite several studies showing the safety of influenza vaccine in pregnancy [8,9]. A review of Vaccine Adverse Event Reporting System (VAERS) during 1990–2009 found no unusual patterns of pregnancy complications or fetal outcomes after administration of IIV3 or trivalent live attenuated influenza vaccine (LAIV3) [10]. During the 2009–2010 H1N1 influenza pandemic, an expected increase in reported adverse events (AEs) in pregnant women was observed following vaccination with the inactivated 2009 H1N1 vaccines (N=288) [11] compared to seasonal influenza vaccines given to pregnant women during 1990–2009 (N=148) [10]. This increase, may be due in part to enhanced reporting and increased media coverage and heightened awareness, and was noted for most pregnancy outcomes, including reports of spontaneous abortion (SAB), and stillbirth. The objective of our current study was to assess the safety of IIV and LAIV in pregnant women and their infants whose reports were submitted to VAERS during 2010–2016, after the increased reporting observed during the 2009–2010 pandemic H1N1 vaccination campaign. Although LAIV is not recommended in pregnant women, inadvertent exposure to this vaccine may occur [10].
2. Materials and Methods
2.1. Vaccine Adverse Events Reporting System (VAERS)
VAERS is a national vaccine safety surveillance system, co-administered by the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), which receives spontaneous (passive) reports of AEs following immunization [12]. Reports to VAERS can be submitted by healthcare providers, vaccine recipients, vaccine manufacturers, and other reporters. The VAERS report form collects information on the age, sex, vaccines administered, AE experienced, medical conditions at the time of vaccination and medical history of the vaccinee. The Medical Dictionary for Regulatory Activities (MedDRA®), a clinically validated, internationally standardized medical terminology [13], is used to code signs and symptoms of AEs which are entered into a database by trained personnel. One or more MedDRA® preferred terms (PT) may be used to characterize the AEs in a VAERS report. A PT is a distinct descriptor for a symptom, sign, disease, diagnosis, therapeutic indication, investigation, surgical, or medical procedure, or medical, social, or family history characteristic [14]. The definition of serious reports, based on the Code of Federal Regulations, is if one of the following is reported: death, life-threatening illness, hospitalization or prolongation of hospitalization, or permanent disability [15]. It is based on the reporter’s assessment of the condition and, for pregnancy reports, it often is based on the effects on the mother and not necessarily the fetus (e.g., SAB may not always be defined as serious). Medical records are routinely requested for non-manufacturer reports. Reports with no AE, those describing only a vaccination error, (e.g., drug administered to patient of inappropriate age), may also be reported and are assigned MedDRA® PTs. Monitoring the safety of influenza vaccines administered during pregnancy is a priority activity at CDC; therefore medical records were also requested for reports if SAB, stillbirth, or birth defect occurred.
We searched the VAERS database for reports of pregnant women vaccinated in the United States with seasonal influenza vaccines with or without other vaccines from July 1, 2010 through May 6, 2016 and received by May 27, 2016. Although live attenuated influenza vaccines (LAIV) are not recommended for use in pregnant women, these vaccines may be given inadvertently to pregnant women; thus we also reviewed pregnancy reports after LAIV. To search for pregnancy reports, we conducted an automated search using the following three approaches: i) MedDRA® terms in two system organ classes (SOC), “Pregnancy, Puerperium, and Perinatal Conditions” and “Congenital, Familial, and Genetic Disorders”; SOC is the highest level of the MedDRA® hierarchy that provides the broadest classification for AEs [10]; ii) MedDRA® terms “Drug exposure during pregnancy”, “Maternal exposure during pregnancy”, and “Exposure during pregnancy”; and iii) a text string search for the term “preg” in the report. Reports that had at least one of these criteria were included in the data set for further evaluation.
2.2. Clinical reviews
All US reports identified through the automated search of the VAERS database were reviewed by medical officers from the FDA and CDC to ascertain pregnancy status at time of vaccination, calculate gestational age, and characterize AEs. We included reports of infants born to women vaccinated with IIV or LAIV during pregnancy. For each report, we assigned a primary diagnosis. If more than one AE was reported for the same individual, we assigned the diagnosis based on what we believed was the primary clinical event of concern, and assumed that the primary event was the pregnancy-specific event unless information suggested otherwise. Nonpregnancy-specific medical conditions were categorized into SOC. We excluded reports that indicated the reported subject was not pregnant or that IIV or LAIV was administered prior to the last menstrual period. Gestational ages at the time of vaccination and at the time of the AE were calculated based on: (1) clinical determination by the health care provider (method not specified), (2) earliest ultrasound assessment (if the former was not available), or (3) last menstrual period, estimated delivery date, or estimated date of conception (if the first two options were unavailable) in the VAERS report and/or medical records. We used the following definition for trimesters: first (0–13 weeks), second (14–27 weeks), and third (≥28 weeks). SAB was defined as fetal demise <20 weeks’ gestation; stillbirth was defined as fetal demise ≥ 20 weeks’ gestation, and preterm delivery was defined as a live birth <37 weeks’ gestation [16].
2.3. Data mining
We used empirical Bayesian (EB) data mining [17] to identify AEs reported more frequently than expected following IIV by brand of influenza vaccine in the VAERS database. EB data mining can address the inherent limitation of absent denominator data (e.g., number of overall relevant doses administered) in VAERS by screening for vaccine-event pairs that are reported more frequently than expected. EB05 is defined as the lower 90% confidence intervals (CI) limit of the adjusted ratios of the observed counts over expected counts [18]. Through this data mining analysis, IIV reports were compared with all other vaccines in the VAERS database. We used published criteria [18,19] to identify, with a high degree of confidence, IIV pregnancy-specific event pairs reported at least twice as frequently as would be expected (i.e., lower bound of the 90% CI surrounding the EB geometric mean [EB05] >2). We clinically reviewed those IIV reports containing PTs for pregnancy-specific conditions which exceeded an EB05 of 2 to characterize and verify the signal.
3. Results
During July 1, 2010 through May 6, 2016, VAERS received a total of 671 US reports after seasonal influenza vaccines in pregnant women or their infants; 544 reports were after IIV and 127 were after LAIV. The average annual number of IIV pregnancy reports received was 112 per influenza season.
Serious events occurred among 61 (11.2%) reports after IIV and 1 (0.8%) report after LAIV. Figure 1 shows annual pregnancy reports after IIV in VAERS since 1991. Following the 2009–2010 influenza season peak of H1N1 vaccine pregnancy reports, there was a decline in the number of pregnancy reports after IIV submitted to VAERS. Characteristics of reports after IIV are shown in Table 1. In most reports, IIV was given during the first or second trimester (76.7%). In over half of the reports (51.8%), women were 30 or more years of age; in 21.3% (116/544) women were aged ≥35 years.
Figure 1.
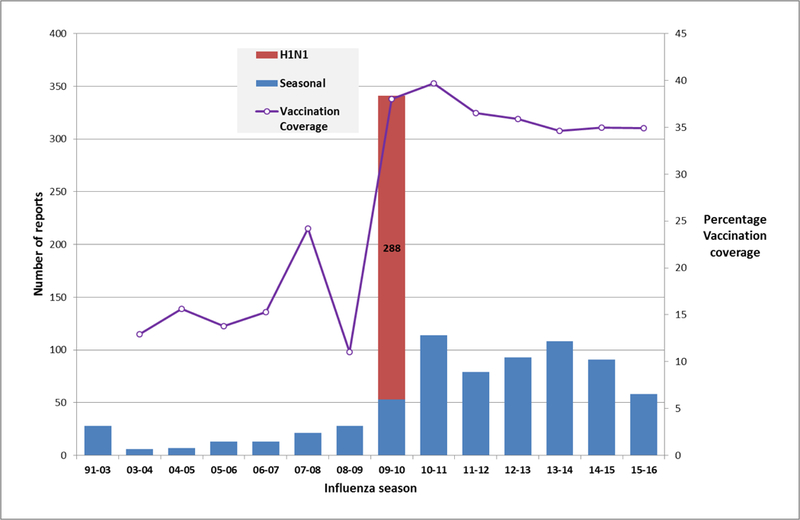
Reports of inactivated influenza vaccines given during pregnancy, Vaccine Adverse Event Reporting System (VAERS), 1991–2016a
a Season unknown for one report Legend: H1N1: H1N1pdm09 monovalent vaccine; Seasonal influenza vaccines may include trivalent or quadrivalent inactivated influenza vaccines Vaccination coverage source: Internet panel surveys; http://www.cdc.gov/flu/fluvaxview/pregnant-women-nov2015.htm
Table 1.
Characteristics of VAERS reports received following inactivated influenza vaccines in pregnant women, United States, 2010–2016
| Characteristic | |
|---|---|
| Total reports | 544 |
| Reports with no adverse events, n (%) | 168 (30.9) |
| Maternal age in years, median (range) | 31 (12–48) |
| Interval from vaccination to adverse event in days, median (range)a | 1 (0–1098) |
| Gestational age in weeks at time of vaccination, median (range)b | 18.0 (1–38) |
| Reports of serious adverse events, n (%)c | 61 (11.2) |
| Type of reporter, N (%) | |
| Manufacturer | 186 (34.2) |
| Provider | 156 (28.7) |
| Patient/parent | 130 (23.9) |
| Other | 72 (13.2) |
| Maternal age groups, years | |
| 10 – 17 | 25 (4.6) |
| 18 – 29 | 198 (36.4) |
| 30 – 39 | 262 (48.2) |
| ≥ 40 | 21 (3.9) |
| Unknown | 38 (6.9) |
| Vaccines administered | |
| IIV3 alone | 306 (56.3) |
| IIV4 | 28 (5.1) |
| IIV3, Tdap | 26 (4.8) |
| Other combinations | 184 (33.8) |
| Trimester of pregnancy at time of vaccination (N=296)b, N (%) | |
| First (0 – 13 weeks) | 116 (39.2) |
| Second (14 – 27 weeks) | 111 (37.5) |
| Third (28 + weeks) | 69 (23.3) |
Interval unknown for 44 reports with adverse events
Gestational age at time of vaccination is unknown for 248 reports.
IIV3: trivalent inactivated influenza vaccines; IIV4 quadrivalent inactivated influenza vaccines; Tdap: Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed
A report is defined as serious when one of the following is reported: death, life-threatening illness, hospitalization or prolongation of hospitalization, or permanent disability [12]
Table 2 shows AEs following IIV in pregnant women during 2010–2016. One hundred sixty-eight (30.9%) reports did not describe an AE: 157 were reports submitted by the vaccine manufacturer corresponding to women enrolled in pregnancy registries and 11 described vaccination errors (e.g. administration of expired vaccine). The most frequent pregnancy-specific condition reported was SAB, in 11.4% (62/544) reports. The median interval from vaccination to occurrence of symptoms or signs associated with SAB was 5.5 days (0–58 days). During the 2010–2011 influenza season, 26 of 62 (41.9%) SABs were reported, the most for any season. Most SAB reports (69%) were in women aged ≥30 years. There were ten reports of stillbirth and six reports of preterm delivery. The most commonly reported nonpregnancy-specific condition was injection site reactions, in 10.1% (55/544) of IIV reports. Immune system disorders, which included mostly non-anaphylaxis allergic reactions, were the second most commonly reported condition in 7.2% (39/544) followed by respiratory events in 6.9% (38/544). Eleven of these were reports of vaccination failure (infection with influenza A or B) which were submitted by the same reporter during the 2010–2011 influenza season. Twenty-two reports involved infant conditions and seven of these were major birth defects. Vaccination occurred during the first trimester of pregnancy in five of these reports. However, in one report of ectopic kidney, vaccination occurred during the second trimester and in one of two reports of polydactyly, vaccination occurred during the third trimester.
Table 2.
Reported adverse events (AEs)† in pregnant women following receipt of inactivated influenza vaccines in pregnant women, VAERS, 2010 – 2016 (N=544)
| Adverse Events ǂ | N (%) |
|---|---|
| Pregnancy-specific AEs | 102 (18.8) |
| Spontaneous abortion (< 20 weeks gestation) | 62 (11.4) |
| Stillbirth (≥ 20 weeks gestation) | 10 (1.8) |
| Fetal death (gestational age unknown) | 2 (0.4) |
| Preterm delivery (< 37 weeks) | 6 (1.1) |
| Preeclampsia/pregnancy induced hypertension | 6 (1.1) |
| Vaginal bleeding | 5 (0.9) |
| Other a | 11 (2.0) |
| Non-pregnancy specific AEsb | 252 (46.3) |
| General disorders and administration site conditions | 106 (19.5) |
| Injection site reactions | 55 |
| Immune system disorders | 39 (7.2) |
| Anaphylaxis | 5 |
| Non-anaphylaxis allergic reactions | 34 |
| Respiratory, thoracic and mediastinal disorders | 38 (6.9) |
| Nervous system disorders | 24 (4.4) |
| Bell’s palsy | 8 |
| Guillain-Barré syndrome | 8 |
| Musculoskeletal and connective tissue disorders | 20 (3.7) |
| Infections and infestations | 8 (1.5) |
| Gastrointestinal disorders | 8 (1.5) |
| Other c | 9 (1.7) |
| Infant outcomes | 22 (4.0) |
| Intrauterine growth restriction | 1 |
| Neonatal hypoxia | 1 |
| Low birth weight | 1 |
| Laryngomalacia | 1 |
| Ventricular septal defect | 1 |
| Patent foramen ovale | 1 |
| Vascular malformation (Port wine stain) | 1 |
| Jaundice | 1 |
| Lack of respiratory effort | 1 |
| Left foot ligament laxity | 1 |
| Hydronephrosis | 1 |
| Aplasia cutis congenital | 1 |
| Hooded foreskin/hemophilia A | 1 |
| Autism spectrum disorder | 1 |
| Fetal pyelectasis | 1 |
| Major birth defects d | 7 |
| Polydactyly | 2 |
| Ectopic kidney in newborn | 1 |
| Cleft lip and palate | 1 |
| Cleft palate (incomplete) | 1 |
| Trisomy 18 | 1 |
| Multiple birth defects (complex congenital heart disease, microtia, cleft lip) | 1 |
| No adverse event reported e | 168 (30.9) |
Adverse events are based on primary reported diagnoses identified during clinical review. One diagnosis assigned to one report. Proportions calculated using all IIV reports as denominator (N=544)
Sixty-one (11.2%)were serious reports
Other pregnancy specific adverse events included two reports of gestational diabetes, and one report each of placental abruption, threatened abortion, gestational trophoblastic disease, preterm labor, placenta previa, excessive labor bleeding and injection site reaction, chorioamnionitis, increased fetal movement, and aborted pregnancy due to Guillain-Barré syndrome
Selected adverse event shown under each system organ class
Other non-pregnancy specific adverse events included one report each of diabetes type 1 uncontrolled, increased blood pressure/dizziness/flushed skin, acute myeloblastic leukemia, anemia, thrombocytopenia, pyelonephritis, tachycardia, and two reports of an unspecified adverse event
Gestational age in weeks (in parentheses) at the time of vaccination for birth defects were: polydactyly (8, 29), ectopic kidney (18), cleft lip and palate (9), cleft palate/incomplete (10), trisomy 18 (2), and multiple birth defects (8)
Reports with no AE comprised vaccination errors and/or reports submitted to the manufacturer pregnancy registry
The 61 serious reports after IIV included 21 pregnancy-specific conditions (SAB [7], stillbirth [5], preterm delivery [3], preeclampsia [3], preterm labor [1], abruptio placentae [1] fetal death [1]); 36 nonpregnancy-specific conditions (respiratory disorders [11], nervous system disorders [11], general administration disorders [5], immune system disorders [5], infections and infestations [2], neoplasia [1], and genitourinary system disorders [1]); and four neonatal conditions (one report each of laryngomalacia, cleft palate incomplete, multiple birth defects, and neonatal hypoxia).
Data mining analysis found no disproportionate reporting for any pregnancy-specific MedDRA® PT.
3.1. Reports after LAIV
One hundred-twenty seven reports after LAIV in pregnant women or their infants were submitted to VAERS during 2010–2016. One serious report involved an infant hospitalized in the neonatal intensive care unit for persistent pulmonary hypertension. The infant recovered and was discharged. Among 47 reports with information on gestational age at time of vaccination, LAIV was administered during the first trimester in 24 (51.1%) reports, second trimester in 15 (31.9%) reports, and third trimester in 8 (17.0%) reports. One hundred-twelve (88.2%) of 127 reports did not report an AE and the AEs in the other 15 reports included the following: two reports each of SAB, elective terminations, nasal congestion and one report each of transverse myelitis, abdominal pain, preterm delivery, chest pain with dyspnea due to trauma, pure cell aplasia, headache, common cold, pulmonary hypertension in a newborn infant and an unspecified pregnancy complication.
4. Discussion
During the period from July 1, 2010 to May 6, 2016 following the 2009 H1N1 influenza pandemic, 671 reports after influenza vaccines in pregnant women were submitted to VAERS; 544 were after IIV. This represents a decrease in the annual number of pregnancy reports following the 2009 H1N1 pandemic (Figure 1) despite similar vaccination coverage as observed during the pandemic year, but an increase over the period 1990–2009 before the H1N1 pandemic. Prior to the 2009 H1N1 pandemic, pregnancy reports after IIV had been sparsely reported to VAERS. The peak in the number of pregnancy reports observed during 2009–2010 followed by a decrease in reporting suggests that the 2009 spike in pregnancy reports after 2009 H1N1 inactivated vaccines may have been due to stimulated reporting. During the current review, we did not observe any new AE or condition of concern. Moreover, data mining analysis did not reveal any disproportionate reporting for any pregnancy-specific MedDRA® PT.
Among pregnancy-specific conditions, SAB was the most commonly reported event in 11.4% of reports; similar to a previous review of IIV in pregnancy during 1990–2009 in VAERS which found that SAB was reported in 11% of reports [10]. SAB is a relatively common condition during pregnancy occurring in up to 22% of pregnancies among women 34 years of age or older [20]. During the 2009 H1N1 pandemic, SAB accounted for 41% of all reported events in pregnant women who received the H1N1 inactivated monovalent influenza vaccine. Stillbirths were also more frequently reported during the pandemic accounting for 6.5% of reports in contrast to 1.8% of reports in the current review [11]. At the time of the pandemic, these events of fetal demise were expected to be reported more frequently to VAERS due to its nature as a spontaneous system, which is subject to overreporting bias surrounding publicity around certain events [21]. The decrease in the number of pregnancy reports following the 2009 H1N1 pandemic, including reports of SAB and stillbirth, further suggests that there was stimulated reporting to the VAERS system during 2009–2010. Following the 2009 H1N1 pandemic, several cohort studies have demonstrated the safety of the 2009 H1N1 monovalent and seasonal trivalent and quadrivalent inactivated influenza vaccines [22]. Many of these studies showed that administration of these vaccines was not associated with an increased risk of SAB, stillbirth or fetal demise [12,23,24].
Similar to the period 1990–2009, major birth defects were infrequently reported [10] to VAERS during 2010–2016 accounting for only seven reports or less than 2% of reports. Since the population prevalence of major birth defects is approximately 3% [25] a similar frequency of major birth defects is expected to occur among all women who receive influenza vaccines. The substantial level of underreporting may in part be due to lack of reporting of many birth defects that are not recognized immediately after birth. Underreporting might also result from lack of recognition of a possible link between an exposure to vaccine earlier in gestation and a defect noted at birth. Also similar to the period 1990–2009, injection site reactions were the most common type of event reported among nonpregnancy-specific conditions, [10].
In our analysis of pregnancy reports after IIV, we used EB data mining to detect disproportionate reporting of any MedDRA® code within the two SOC’ i) pregnancy, puerperium and perinatal conditions, and ii) congenital, familial and genetic disorders. It is reassuring that no MedDRA® PT was disproportionally reported.
Although VAERS may be useful to study rare AEs and detect potential safety signals, there are important inherent limitations in this system that call for caution in the interpretation of its findings. VAERS is a passive surveillance system that may be prone to biased reporting (over-or underreporting) and inconsistency in the quality and completeness of reports. Because VAERS accepts reports from any reporter, the information provided by individuals with little or no medical training may adversely affect the quality of the report. Events that occur close to the time of vaccination are more likely to be reported to VAERS. Recall bias may occur as pregnancies that experience adverse outcomes may be more likely to recall/report vaccination during pregnancy. VAERS also generally cannot determine whether a vaccine caused an AE [11]. VAERS does not collect data on the number of individuals vaccinated therefore it is not possible to calculate the incidence or prevalence of AEs. The regulatory definition of a serious report in VAERS as it applies to pregnancy conditions can have limitations as it often is applied to how conditions affect the mother and not necessarily the fetus. For example, in our review, seven of 62 SAB reported were coded as serious because the mothers were hospitalized, whereas 55 were coded as non-serious because the report did not indicate that the mother had been hospitalized.
5. Conclusions
During the study period 2010–2016, the average annual number of VAERS reports following IIV in pregnancy decreased compared to the pandemic 2009–2010 pandemic period but remained higher than prior to the pandemic. Similar to the 2009–2010 period, no new or unexpected patterns in maternal or fetal outcomes were observed during 2010–2016. Maternal influenza vaccination benefits both the mother and the infant protecting both from influenza disease [26]. Given that the antigenic composition of these vaccines usually changes each season, CDC and FDA will continue to monitor the safety of influenza vaccines in pregnant women.
Acknowledegements
We thank CDC’s Immunization Safety Office staff whose work allowed this activity to be conducted. We also thank staff from cSRA, Inc. for their work on this project.
Funding:
no external or internal sources of funding were used for this study
Footnotes
Conflict of interest:
None of the authors have a conflict of interest. Part of this research was presented during the August 22–26, 2015 31st International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE).
Ethics: Because VAERS is a routine surveillance program that does not meet the definition of research, it is not subject to Institutional Review Board review and informed consent requirements.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Food and Drug Administration
References
- 1.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ; Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009;374:451–8. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-Related Mortality Resulting From Influenza in the United States During the 2009–2010 Pandemic. Obstet Gynecol. 2015. Sep;126(3):486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skoronski DM, De Serres G. Is routine influenza immunization warranted in early pregnancy? Vaccine 2009;27:4754–70. [DOI] [PubMed] [Google Scholar]
- 4.Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye-Gbewonyo D, Ault K, Gallagher M, Orenstein W, Davis RL, Omer SB. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013. May;56(9):1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shakib JH, Korgenski K, Presson AP, Sheng X, Varner MW, Pavia AT, Byington CL. Influenza in Infants Born to Women Vaccinated During Pregnancy. Pediatrics. 2016. June;137(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding H, Santibanez TA, Jamieson DJ, Weinbaum CM, Euler GL, Grohskopf LA, Lu PJ, Singleton JA. Influenza vaccination coverage among pregnant women--National 2009 H1N1 Flu Survey (NHFS). Am J Obstet Gynecol. 2011. June;204(6 Suppl 1):S96–106 [DOI] [PubMed] [Google Scholar]
- 7.Kharbanda EO, Vargas CY, Castaño PM, Lara M, Andres R, Stockwell MS. Exploring pregnant women’s views on influenza vaccination and educational text messages. Prev Med 2011;52: 75–7. [DOI] [PubMed] [Google Scholar]
- 8.Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines. 2012. August;11(8):911–21. [DOI] [PubMed] [Google Scholar]
- 9.Fell DB, Dodds L, MacDonald NE, Allen VM, McNeil S. Influenza vaccination and fetal and neonatal outcomes. Expert Rev Vaccines. 2013. December;12(12):1417–30. [DOI] [PubMed] [Google Scholar]
- 10.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C.. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol 2011;204:146.e1–7. [DOI] [PubMed] [Google Scholar]
- 11.Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, Barash F, Arana J, Brantley MD, Ding H, Singleton JA, Walton K, Haber P, Lewis P, Yue X, Destefano F, Vellozzi C. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011. November;205(5):473.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015. August 26;33(36):4398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medical Dictionary for Regulatory Activities. Available at: http://www.meddramsso.com/. Accessed April 28, 2011.
- 14.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals. http://www.ich.org/products/meddra.html. [DOI] [PMC free article] [PubMed]
- 15.Cunningham G, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams obstetrics. New York (NY): McGraw-Hill Companies, Inc; 2010. [Google Scholar]
- 16.Food and Drug Administration. 21 CFR Part 600.80. Postmarketing reporting of adverse experiences. Vol 62: Federal Register, 1997:52252–3. [Google Scholar]
- 17.DuMouchel W Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am Stat 1999;53:177–90. [Google Scholar]
- 18.Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf 2002;25:381–92. [DOI] [PubMed] [Google Scholar]
- 19.Martin D, Menschik D, Bryant-Genevier M, Ball R. Data Mining for Prospective Early Detection of Safety Signals in the Vaccine Adverse Event Reporting System (VAERS): A Case Study of Febrile Seizures after a 2010–2011 Seasonal Influenza Virus Vaccine. Drug Saf 2013. May 9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, Miller E, Andrews N, Stowe J, Salmon D, Vannice K, Izurieta HS, Akhtar A, Gold M, Oselka G, Zuber P, Pfeifer D, Vellozzi C. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009. December 19;374(9707):2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman KB1, Demakas AR, Dimbil M, Tatonetti NP, Erdman CB. Stimulated reporting: the impact of US food and drug administration-issued alerts on the adverse event reporting system (FAERS). Drug Saf. 2014. Nov;37(11):971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis. 2015. March 1;60(5):e11. [DOI] [PubMed] [Google Scholar]
- 23.Regan AK, Moore HC, de Klerk N, Omer SB, Shellam G, Mak DB, Effler PV. Seasonal Trivalent Influenza Vaccination During Pregnancy and the Incidence of Stillbirth: Population-Based Retrospective Cohort Study. Clin Infect Dis. 2016. May 15;62(10):1221–7 [DOI] [PubMed] [Google Scholar]
- 24.Wortman AC, Casey BM, McIntire DD, Sheffield JS. Association of influenza vaccination on decreased stillbirth rate. Am J Perinatol. 2015. May;32(6):571–6 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC). Update on overall prevalence of major birth defects--Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008. January 11;57(1):1–5. Available at https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5701a2.htm Accessed August 7, 2016 [PubMed] [Google Scholar]
- 26.Phadke VK, Omer SB. Maternal vaccination for the prevention of influenza: current status and hopes for the future. Expert Rev Vaccines. 2016. Apr 22:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]


