Abstract
Background:
Magnetic resonance imaging studies of cigarette smoking-related effects on human brain structure primarily focused on cortical volumes. Much less is known about the effects of smoking on cortical thickness. Smokers and Non-smokers were compared on regional cortical thickness. We predicted smokers would demonstrate greater age-related thinning localized to anterior frontal regions that serve as nodes for the executive, salience, and emotional regulation networks (ESER regions) and those demonstrating significant atrophy in early Alzheimer’s Disease (AD regions).
Methods:
Non-smokers (n = 41) and smokers (n = 41), 22–70 years of age, completed a 4T MRI study. Regional cortical thickness was quantitated via FreeSurfer. In smokers, associations between smoking severity, decisionmaking, impulsivity, and regional cortical thickness were examined.
Results:
Smokers demonstrated cortical thinning in the medial and lateral OFC, insula, entorhinal, fusiform, middle temporal, and Composite AD regions. In Smokers, greater pack-years were associated with thinner lateral OFC, middle temporal, inferior parietal, fusiform, precuneus, and Composite AD regions. In Smokers, poorer decision-making/greater risk taking was related to thinner cortices in caudal ACC, rostral middle frontal and superior frontal gyri, and Composite ESER. Higher self-reported impulsivity was associated with thinner rostral and caudal ACC.
Conclusions:
This study provides additional evidence that cigarette smoking is associated with thinner cortices in regions implicated in the development and maintenance of substance use disorders and in regions demonstrating significant atrophy in early AD. The novel structure-function relationships in Smokers further our understanding of the neurobiological substrates potentially underlying the neuropsychological abnormalities documented in smokers.
Keywords: Cigarette smoking, Magnetic resonance imaging, Cortical thickness, Decision-making, Impulsivity, FreeSurfer
1. Introduction
The link between cigarette smoking and markedly increased risk for pulmonary, cardiac, and vascular disease as well as for multiple forms of cancer in humans is essentially indisputable (CDC, 2004). A growing body of evidence also indicates that smoking, in otherwise healthy individuals, is associated with neurobiological and neurocognitive abnormalities that are not directly related to the foregoing diseases (Azizian et al., 2009; Durazzo et al., 2014a, 2010; Durazzo et al., 2017; Sharma and Brody, 2009). Most magnetic resonance (MR) studies investigating the neurobiological consequences of cigarette smoking have focused on volume/density of cortical gray matter (GM) and/or subcortical nuclei (see Durazzo et al., 2010; Pan et al., 2013; Sutherland et al., 2016 for review). Smaller volumes or lower density in dorsal and ventromedial prefrontal cortex, orbitofrontal cortex, insula, thalamus, and cerebellum were the most consistently reported findings (Durazzo et al., 2010; Pan et al., 2013; Sutherland et al., 2016); structural abnormalities in these regions are also apparent in alcohol and substance use disorders (see Buhler and Mann, 2011; Cadet et al., 2014 for review) The anterior frontal, insular, and subcortical regions that show structural abnormalities in smokers are critical nodes in the executive, salience, and emotional regulation networks (Seeley et al., 2007; Williams, 2016). Additionally, in smokers, studies have reported smaller volumes (Almeida et al., 2011, 2008; Peng et al., 2018) in adults and elders, greater longitudinal volume loss over 2 years in elders (Durazzo et al., 2012), and accelerated age-related volume loss in middle-aged adults (Durazzo et al., 2013a, 2017) in cortical and subcortical regions that show atrophic changes in those with mild cognitive impairment and early-stage Alzheimer’s Disease (Vemuri et al., 2010). Correspondingly, chronic smoking during adulthood is associated with significantly elevated risk for Alzheimer’s Disease and related neuro-pathological changes (see Durazzo et al., 2014a for review). The cumulative body of research suggests chronic smoking-related structural abnormalities also show considerable overlap with regions that manifest atrophic changes in alcohol/substance use disorders and Alzheimer’s Disease.
While most MR studies have investigated the consequences of smoking on cerebral volume, fewer studies have examined smoking-related effects on cortical thickness. Cortical thickness is believed to reflect the number and density of cells in a column (Rakic, 1988, 2008) and/or represent neuronal cell body size, the number of spines and synapses, and the degree of myelination (Eickhoff et al., 2005; Fjell and Walhovd, 2010). Cortical thickness is genetically and phenotypically distinct from cortical surface area and volume (Kremen et al., 2010; Panizzon et al., 2009; Winkler et al., 2010), and it is related to neurocognition in healthy adults and adolescents (Choi et al., 2008; Dickerson et al., 2008; Fjell et al., 2006; Schilling et al., 2013; Walhovd et al., 2006) and those with a cocaine use disorder (Makris et al., 2008). Cortical thickness is reputed to be more sensitive to neurodegenerative processes than cortical volumes (Hutton et al., 2009); therefore, this morphometric may increase the ability to detect more subtle structural abnormalities than volumes or density measures and/or be differentially affected by addictive disorders compared to volumes and surface area (Durazzo et al., 2011b; Wang et al., 2016). Taken together, cortical thickness may serve as a proxy for the integrity of cortical cytoarchitecture (Makris et al., 2007). Kuhn and colleagues (Kuhn et al., 2010) reported that adult smokers had thinner left medial orbitofrontal cortex (OFC) than never-smokers. Karama and colleagues (Karama et al., 2015) reported wide-spread reduction of thickness across the cortex, with the greatest thinning in anterior frontal regions in elder active smokers relative to never smokers, after adjusting for vascular risk factors. In a sample of late adolescent/young adults, Li and associates (Li et al., 2015) reported that smokers demonstrated significant cortical thinning in the left caudal anterior cingulate cortex (ACC), right lateral OFC, left insula, left middle temporal gyrus, right inferior parietal lobule, and right parahippocampus. In the above studies, greater pack-years was associated with thinner cortices in the regions that also showed significantly thinner cortices in smokers relative to never-smokers. However, associations between regional cortical thickness and clinically relevant aspects of decision-making and impulsivity, which are key cognitive components in the initiation and maintenance of addictive disorders (Fineberg et al., 2010; Verdejo-Garcia and Bechara, 2009), were not examined in these studies.
The risk for smoking-related diseases increases with years of smoking (CDC, 2004), which is inextricably linked to age. In healthy adults, increasing age is associated with declines in regional cortical thickness (Fjell et al., 2015; Storsve et al., 2014). In the few previous studies investigating smoking-related effects on cortical thickness, mean thickness differences were examined between smokers and never-smokers. While this approach permitted testing for the effect of smoking status (i.e., smoker vs. never-smoker) collapsed across the age range of the participants, no study specifically tested for a smoking status by age interaction. Our previous neuroimaging studies showed that, in healthy participants 25–70 years of age, smokers had both lower mean values and greater age-related declines of anterior frontal brain metabolite concentrations (N-acetylaspartate and glutamate) and hippocampal and subcortical volumes (Durazzo et al., 2016c, a; Durazzo et al., 2017). In these studies, greater cigarette pack-years was related to lower metabolite levels and smaller regional volumes. The greater age-related declines apparent in smokers suggest that chronic smoking amplified the effects of normal aging on multiple aspects of neurobiology. These neurobiological abnormalities may influence reward processing and response to smoking cessation interventions via alterations of the integrity of structural and/or functional connectivity in frontolimbic and/or frontostriatal circuitry (Froeliger et al., 2015; Hong et al., 2009; Li et al., 2017; Sutherland et al., 2013, 2016; Sweitzer et al., 2016). Based on previous neuroimaging findings from our group and others, we predicted that adult smokers would demonstrate greater age-related cortical thinning and lower mean thickness in anterior frontal regions that serve as nodes for the executive, salience, and emotional regulation networks (hereafter referred to as ESER regions) as well as in posterior regions that show significant atrophy in early-stage Alzheimer’s Disease (hereafter referred to as AD regions). In smokers, we hypothesized that higher pack-years are related to thinner cortices in ESER and AD regions. We also predicted that poorer performance on measures of decision-making/risk-taking and impulsivity are associated with thinner cortices in ESER regions of smokers.
2. Methods
2.1. Participants
Eighty-two healthy, community-dwelling participants [41 non-smokers (seven females) and 41 smokers (six females)] were recruited via electronic billboards and word-of-mouth. Participants were between the ages of 22 and 70 (see Table 1 for demographics). Participants gave written informed consent according to the Declaration of Helsinki, and all procedures were approved by the University of California San Francisco and the San Francisco VA Medical Center.
Table 1.
Group Demographics, Alcohol and Cigarette Use Histories, and Self-Report Questionnaires.
| Measure | Non-smokers (n = 41) | Smokers (n = 41) |
|---|---|---|
| Age (years) | 43.2 (12.3) | 46.6 (11.0) |
| Education (years) | 16.1 (2.2) | 14.8 (2.0)* |
| Male (%) | 83 | 85 |
| Caucasian (%) | 56 | 68 |
| Lifetime average drinks/month | 18 (11) | 26 (14)* |
| FTND | NA | 5 (2) |
| Pack-years | NA | 25 (13) |
| BDI | 3 (3) | 6 (4)* |
| STAI | 32 (7) | 34 (9) |
| Intracranial volume (cc) | 1413 (203) | 1425 (249) |
Note. BDI: Beck Depression Inventory. CON: Controls. FTND: Fagerstrom Tolerance Test for Nicotine Dependence. NA: not applicable. STAI: State -trait Anxiety Inventory - Trait.
Smokers > Non-smokers, p < .05. Mean (SD).
Detailed inclusion/exclusion criteria are described elsewhere (Durazzo et al., 2011a). In summary, participants were screened for the following: history of neurologic (e.g., seizure disorder, neurodegenerative disorder, demyelinating disorder, closed head trauma with loss of consciousness), and general medical (e.g., hypertension, myocardial infarction, Type-1 or 2 diabetes, cerebrovascular accident, any form of cancer) and psychiatric (i.e., mood, thought, anxiety, trauma and stressor-related, substance/alcohol use disorders) conditions known or suspected to influence neurocognition and/or brain neurobiology. Non-smoking participants never smoked (n = 36) or smoked less than 40 cigarettes during their lifetime and used no tobacco products for at least 10 years prior to study (n = 5). All smoking participants were actively smoking at the time of assessment, smoked at least 10 cigarettes/day for 5 years or more, and had no periods of smoking cessation greater than 1 month in the 5 years prior to study with no concurrent use of other tobacco products. No smoker was engaged in any pharmacological and/or behavioral smoking cessation program at the time of study.
2.2. Medical, psychiatric, substance, alcohol consumption and behavioral assessment
Participants completed the screening section of the Structured Clinical Interview for DSM-IV Axis I disorders, Patient Edition, Version 2.0 [SCID-I/P; (First et al., 1998)] as well as an in-house questionnaire designed to screen for medical, psychiatric, neurological and developmental conditions that may affect neurocognition or neurobiology (see Durazzo et al., 2004). Participants completed standardized questionnaires assessing substance use [in-house questionnaire assessing substance type, quantity and frequency of use (Abe et al., 2013)] and lifetime alcohol consumption [Lifetime Drinking History, LDH; (Skinner and Sheu, 1982; Sobell et al., 1988)]. From the LDH, average number of drinks per month over lifetime (one drink defined as containing 13.6 g of pure ethanol) was calculated. Participants also completed self-report measures of depressive [(Beck Depression Inventory, BDI; (Beck, 1978)] and anxiety symptomatology [(State-Trait Anxiety Inventory, form Y-2, STAI; (Spielberger et al., 1977)]. Smokers completed a measure of nicotine dependence level [Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991)], self-reported the number of cigarettes currently smoked per day, and number of years of smoking over their lifetime. Pack-years (number of cigarettes per day/20) × total number of years of smoking was calculated for Smokers. An equivalent frequency of Smokers and Non-Smokers (30%) reported intermittent “recreational” use (i.e., ≤ 3 episodes/month) of cannabis or cocaine during late adolescence or early adulthood. Prior to assessment, participants’ urine was tested for five common illicit substances (THC, opiates, PCP, cocaine, amphetamines), and participants were breathalyzed for recent ethanol consumption. No participant was positive for the above common substances or ethanol at the time of assessment. Smokers and Non-Smokers completed the Iowa Gambling Task, [IGT (Bechara, 2007)], a task-based measure of decision-making and risktaking, and the Barrett Impulsivity Scale-11 [BIS-11 (Patton et al., 1995)], a self-report measure of aspects of trait impulsivity.
2.3. Magnetic resonance imaging (MRI) acquisition and processing
Morphological MRI data were acquired on a 4.0 T Bruker MedSpec system using an 8-channel transmit-receive head coil (Siemens, Erlangen, Germany). A Magnetization Prepared Rapid Gradient (TR/TE/TI = 2300/3/950 ms, 7° flip angle, 1.0 × 1.0 × 1.0 mm3 resolution) sequence was used to acquire 3D sagittal T1-weighted images for morphological analyses. The publicly available FreeSurfer (v5.1) segmentation and cortical surface reconstruction methods were used to obtain thickness for 34 bilateral regions (mm) and total intracranial volume (ICV; mm3) (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004, 1999). All segmented subcortical and parcellated cortical T1-weighted images were visually inspected by one of the authors (TCD) for accuracy; any errors in segmentation/parcellation were manually edited, reprocessed, and reinspected as previously described (Durazzo et al., 2014c). The final segmented subcortical and parcellated cortical volumes passed all quality requirements (Durazzo et al., 2014c). Analyses focused on cortical components that serve as nodes for the ESER (Seeley et al., 2007; Williams, 2016) and included the bilateral rostral and caudal ACC, medial and lateral OFC, rostral and caudal middle frontal gyri, and superior frontal gyri. An ESER composite thickness was created by calculating the surface area-weighted average of all ESER ROIs (Durazzo et al., 2014c). AD ROIs included the bilateral entorhinal cortex, fusiform gyri, inferior temporal, inferior parietal lobule, isthmus and posterior cingulate, and precuneus. An AD composite thickness was created by calculating the surface area-weighted average of all AD ROIs (Durazzo et al., 2014c).
2.4. Statistical analyses
2.4.1. Demographic and clinical variables
Demographic and clinical variables were compared between smokers and non-smokers with t-tests and Fisher’s Exact Test where indicated.
2.4.2. Comparisons of smokers and non-smokers on regional ESER and AD thickness
To test our hypothesis of greater age-related regional cortical thinning in smokers, we employed generalized linear modeling (GENLIN) and specifically tested for a smoking status (smoker vs. non-smoker) by age interaction. In preliminary analyses comparing smokers and non-smokers, no consistent pattern emerged for lateralized differences in the ROIs; therefore, results for the average of the left and right hemisphere for each ESER, AD, and Non-ESER/AD ROIs are presented. Dependent measures were ROI thickness, and covariates included age, education, BDI score, ICV, average lifetime drinks/month (smokers and non-smokers were different on education, BDI score, and average life-time drinks/month; see Table 1 and Section 3.1. below). Although cortical thickness is reported to not show the same strong magnitude of relationships with ICV as surface area and volume (e.g., Kremen et al., 2010; Panizzon et al., 2009), cortical thickness was significantly inversely related to ICV in our previous studies of individuals with alcohol use disorder and healthy controls (Durazzo et al., 2013b, b). Main effects and interactions between smoking status and age and follow-up t-tests (two-tailed) were considered significant at p < .05. In all previous neuroimaging-based reports with this cohort (Durazzo et al., 2016c, a; Durazzo et al., 2017), we conservatively corrected t-tests for multiple comparisons. Given our a priori predictions and our consistent findings of neurobiological and neurocognitive abnormalities in smokers in the ROIs investigated in this study, follow-up t-tests were considered statistically significant at p < .05, uncorrected. Effect sizes were calculated with Cohen’s d (Cohen, 1988) for statistically significant differences in ROI thickness between smokers and non-smokers.
2.4.3. Comparisons of smokers and non-smokers on non-ESER/AD ROI thickness
Smokers and Non-Smokers were compared on regions outside the specified ESER and AD ROIs to determine if smoking-related effects on cortical thickness were localized to ESER and AD ROIs or were more widely distributed. GENLIN was used for these comparisons, and, consistent with analyses of Smokers and Non-Smokers on ESER and AD ROIs, we specifically tested for a smoking status (smoker vs. non-smoker) by age interaction and employed the same covariates. Main effects and interactions between smoking status and age and follow-up t-tests (two-tailed) were considered significant at p < .05 in these exploratory analyses. The following cortical regions were compared: banks of superior temporal gyrus, cuneus, frontal pole, lingual, para-central, pars opercularis, pars triangularis, pericalcarine, post central, precentral, superior parietal, superior temporal, and transverse temporal cortices.
2.4.4. Associations between ESER, AD, and non-ESER/AD ROI thickness and smoking severity measures
In Smokers, associations between the ESER, AD, and Non-ESER/AD ROI thickness and cigarette pack-years (measure of exposure magnitude) and FTND score (measure of nicotine dependence level) were examined by linear regression (partial correlations reported) adjusting for age, education, and ICV. A two-tailed p < .05 was considered statistically significant, and only associations of at least moderate magnitude (i.e., r ≥ |0.30|) were reported.
2.4.5. Associations between ESER, AD, and non-ESER/AD ROI thickness and decision-making/risk-taking and impulsivity in smokers and Non-smokers
Associations of ESER and AD ROI thickness and performance on the IGT Total Score (raw score) and BIS-11 total score, attentional impulsivity, motor impulsivity, and non-planning impulsivity were separately examined in Smokers and Non-Smokers with linear regression (partial correlations reported) adjusted for age, education, and ICV. A two-tailed p < .05 was considered statistically significant, and only associations of at least moderate magnitude (i.e., r ≥ |0.30|) were reported. See Durazzo et al. (2016c) for comparisons of Smokers and Non-Smokers from this cohort on the IGT and BIS-11, in which Smokers performed more poorly on the IGT (indicative of poorer decision-making/greater risk taking) and showed higher BIS-11 scores (indicative of greater impulsivity) than Non-Smokers.
3. Results
3.1. Participant demographics and clinical variables
Smokers and Non-Smokers were equivalent on age, sex, percent of Caucasians, and level of anxiety symptomatology. Smokers had significantly lower educational level, higher BDI scores, and consumed more average lifetime alcoholic drinks per month (all p < .05). Although statistically different between groups, the average BDI score for both groups was in the normal range (i.e., < 10) and well below the cutoff for mild depressive symptomatology (Richter et al., 1998). Participant alcohol consumption did not approach a hazardous level [see (McKee et al., 2007; Mertens et al., 2005)] (see Table 1). All females were pre-menopausal, by self-report.
3.2. Comparisons of smokers and non-smokers on ESER and AD ROI cortical thickness
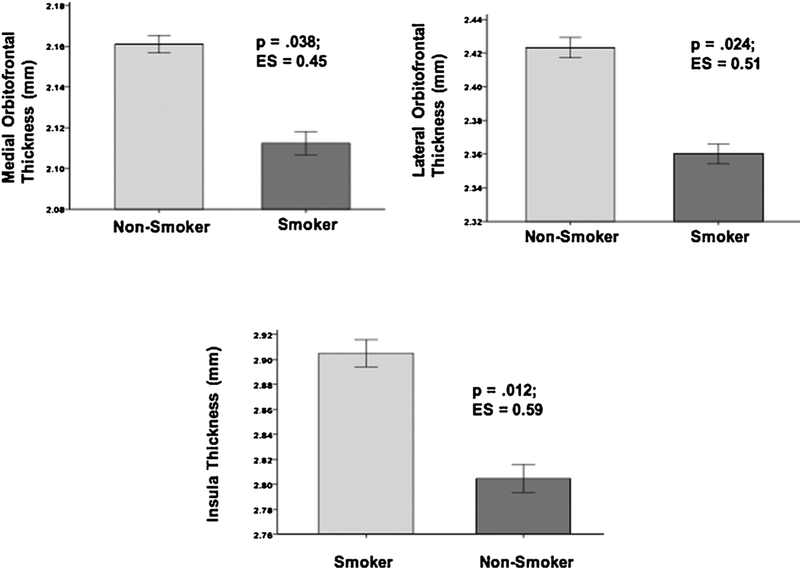
Models containing smoking status, age, and ICV were significant predictors of thickness in the medial OFC [χ2(3) = 9.56, p = .023], lateral OFC [χ2(3) = 13.5, p = .004], entorhinal cortex [χ2(3) = 14.3, p = .003], fusiform gyrus [χ2(3) = 39.4, p < .001], and middle temporal gyrus [χ2(3) = 37.2, p < .001]; greater age was related to thinner cortex, and larger ICV was associated with thicker cortex in these ROIs (all p < .01). The model with smoking status, age, and lifetime average drinks per month showed they were significant predictors of insula thickness [χ2(3) = 35.3, p < .001]; greater age and lifetime average drinks per month were related to thinner insular cortex (all p < .05). The model with smoking status, age, and education showed them to be predictors of Composite AD ROI thickness [χ2(3) = 44.3, p < .001]; greater age was related to thinner Composite AD ROIs and higher education, and ICV was associated with thicker Composite AD ROIs (all p < .05). Smokers demonstrated thinner cortices than Non-Smokers in all above ROIs (see Figs. 1 and 2). BDI score was not a significant predictor of thickness in any region. The smoking status × age interactions were not significant for any ROI examined.
Fig. 1.
ESER Regions significantly different between Non-Smokers and Smokers.
Fig. 2.
AD Regions significantly different between Non-Smokers and Smokers.
3.3. Comparisons of smokers and non-smokers on non-ESER and AD cortical ROI thickness
No significant smoking status × age interactions or main effects for smoking status were observed for any Non-ESER/AD ROI.
3.4. Associations of pack-years and nicotine dependence and regional thickness in smokers
3.4.1. ESER and AD ROIs
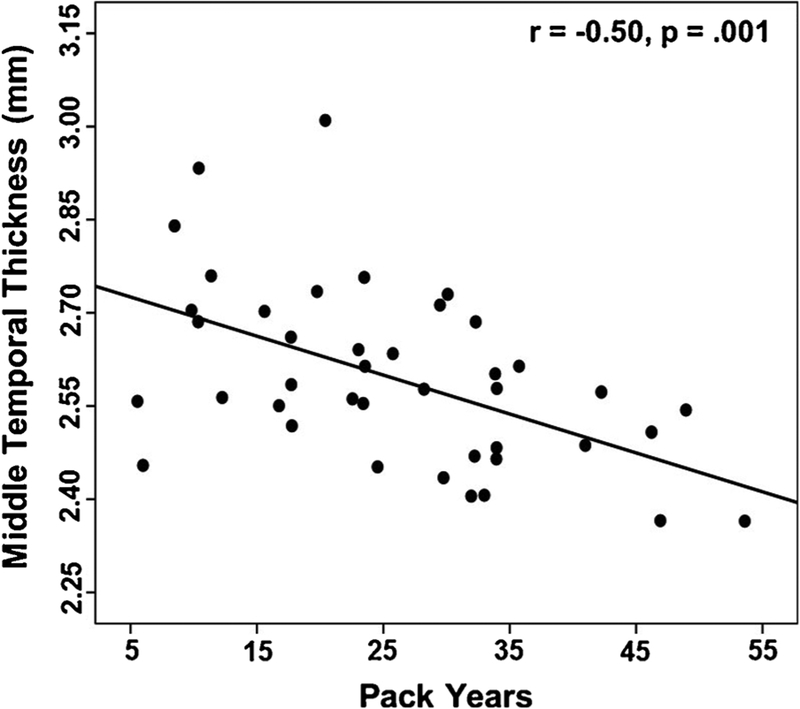
In Smokers, higher pack-years was related to thinner cortices in the middle temporal (r = −0.50, p = .001; see Fig. 3), inferior parietal (r = −0.42, p = .010), fusiform (r = −0.41, p = .013), precuneus (r = −0.37, p = .024), lateral OFC (r = −0.33, p = .047), and composite AD (r = −0.40, p = .013) ROIs. No significant associations were observed between FTND score and ESER or AD ROIs.
Fig. 3.
Association of middle temporal gyri thickness and pack years in Smokers.
3.4.2. Non-ESER/AD ROIs
There were no significant associations between pack-years and FTND score for any region.
3.5. Associations of measures of decision-making and impulsivity with regional thickness in smokers and non-smokers
3.5.1. ESER and AD ROIs
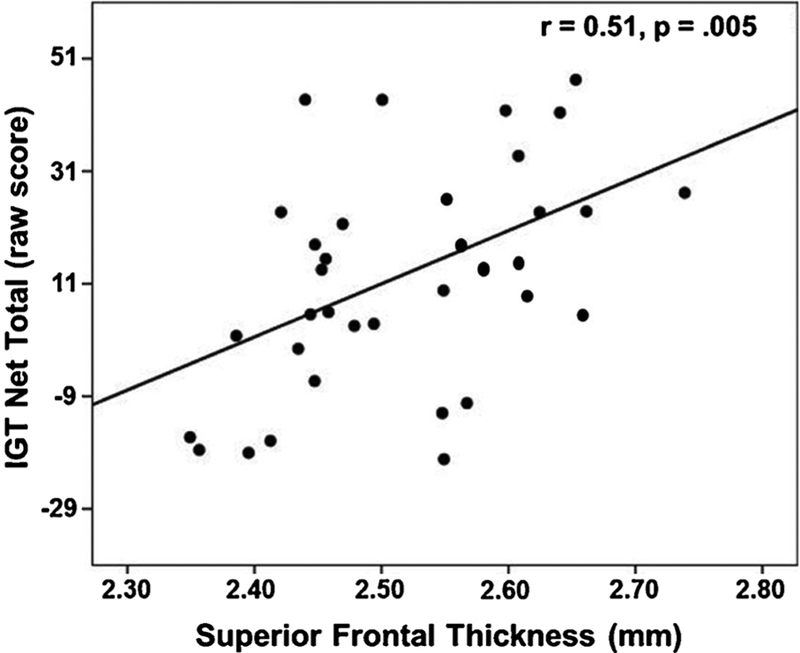
In Smokers, lower IGT Net Total Score (indicative of poorer decision-making/lower risk-taking) was associated with thinner cortices in the superior frontal gyrus (r = 0.51, p = .005; see Fig. 4), caudal ACC (r = 0.44, p = .020), rostral middle frontal gyrus (r = 0.43, p = .022), and composite ESER (r = 0.43, p = .022). On the BIS-11, higher nonplanning impulsivity (r = −0.35, p = .046), motor impulsivity (r = −0.38, p = .028), and BIS-11 total score (r = −0.43, p = .022) were related to thinner rostral ACC, and higher non-planning impulsivity was associated with thinner caudal ACC (r = −0.37, p = .030). The associations between the IGT, BIS-11, and above ROIs largely showed equivalent magnitudes for the left and right hemisphere for both groups (data not shown). There were no significant relationships between IGT, BIS-11, and ESER or AD ROI thickness in Non-Smokers.
Fig. 4.
Association of IGT Net Total Score and superior frontal gyri thickness in Smokers.
3.5.2. Non-ESER/AD
There were no significant associations between IGT Net Total Score, BIS-11, and cortical thickness in the non-ESER/AD ROIs in smokers and Non-Smokers.
4. Discussion
The primary findings of this 4 T quantitative MRI study were: 1) Otherwise healthy adult Smokers demonstrated significant cortical thinning compared to Non-Smokers in the medial and lateral OFC, insula, entorhinal, fusiform, middle temporal, and Composite AD ROIs. Smokers and Non-Smokers did not show statistically significant differences in thickness in areas outside ESER and AD ROIs. 2) In Smokers, greater pack-years were associated with thinner cortices in the lateral OFC, middle temporal, inferior parietal, fusiform, precuneus, and Composite AD ROIs. 3) In Smokers, poorer decision-making/lower risk taking was related to thinner cortices in the caudal ACC, rostral middle frontal gyrus, superior frontal gyrus, and Composite ESER. Higher measures of impulsivity were associated with thinner cortices in the rostral and caudal ACC in Smokers.
The overall pattern of findings indicates the regional cortical thinning and associations with pack-years demonstrated by this group of smokers were limited to ESER and AD ROIs. These results are highly consistent with the few previous published studies of smoking-related effects on regional cortical thickness in non-clinical populations (Karama et al., 2015; Kuhn et al., 2010; Li et al., 2015) and also congruent with studies investigating the effects of smoking on regional brain volumes (see Durazzo et al., 2010; Pan et al., 2013; Sutherland et al., 2016). Contrary to predictions, smokers did not show greater age-related cortical thinning in any ROI, unlike the greater age-related decline in hippocampal and subcortical volumes (Durazzo et al., 2013a, 2017) and brain metabolites (Durazzo et al., 2016c) previously observed in this cohort. As this study excluded individuals with clinically significant smoking-related morbidity, it is possible that the age-related effects on cortical thickness were underestimated in this otherwise healthy cohort (Durazzo et al., 2014a), and/or the age range was not sufficient to yield a smoking status by age interaction.
In ESER regions, Smokers showed thinning of the medial and lateral OFC and insula. Collectively, the OFC is implicated in appraisal of stimulus saliency and representation of reward magnitude, self-monitoring, emotional and impulse regulation, and aspects of decision-making and executive skills (Fettes et al., 2017; Kringelbach and Rolls, 2004; Rolls and Grabenhorst, 2008). The insula is involved in monitoring internal physiological states/sensations (i.e., interoception), adjustment of attentional control based on internal physiological states (Naqvi and Bechara, 2010; Paulus, 2007), and is a major node in the salience network (Williams, 2016). A double blind, placebo-controlled trial of repetitive transcranial magnetic stimulation found that high frequency stimulation (one session/day for 13 days) to the bilateral insula and dorsolateral prefrontal cortex was associated with a smoking cessation rate of 44% immediately after treatment and 33% cessation rate in the 6 months following the treatment (Dinur-Klein et al., 2014). In AD regions, Smokers demonstrated thinner cortices in the entorhinal, fusiform, and middle temporal gyrus as well as across the collective AD ROIs. These regions subserve aspects of face and body recognition, color processing, word recognition (left hemisphere), and declarative and spatial memory (Grill-Spector and Weiner, 2014; Kolb and Whishaw, 2009). Compromised structural integrity of the ESER and AD cortical regions, as indicated by the reduced thickness, may contribute to the abnormalities observed in smokers in mood regulation, cue reactivity, impulse control, decision-making, learning and memory, executive skills (Durazzo et al., 2016c, 2010, 2011a; Fergusson et al., 2003), and to altered functional connectivity (Fedota and Stein, 2015).
Greater pack-years showed moderate magnitude associations with thinner cortices in the lateral OFC, middle temporal, inferior parietal, fusiform, precuneus, and composite AD ROIs. The lateral OFC, middle temporal, fusiform, and Composite AD thicknesses were significantly reduced in Smokers, which suggests a cigarette dose/duration-response relationship. No significant associations were observed between pack-years and thickness in areas outside the ESER and AD ROIs. Increased smoking-related cerebral oxidative stress (see Durazzo et al., 2016a, b; Durazzo et al., 2014a, b) and/or chronic nicotine exposure (see Durazzo et al., 2014a; Sutherland et al., 2016) are hypothesized to be fundamental mechanisms contributing to the structural and other neurobiological abnormalities observed in smokers. Replication in larger samples is required to determine if ESER and AD thickness show selective vulnerability to cigarette smoking and if premorbid or comorbid factors not investigated in this study also contribute to the altered structural integrity observed in these regions.
In Smokers, poorer performance on the IGT (indicative of worse decision-making/greater risk-taking) was related to thinner cortices in the superior frontal gyrus, caudal ACC, rostral middle frontal gyrus, and composite ESER. These results are congruent with findings that tissue integrity of the dorsolateral and ventral medial prefrontal cortex and caudal ACC is related to performance on the IGT and other tasks/situations that require adjustment of goal-directed activity based on current environmental contingencies and anticipated future consequences (Bush et al., 2002; Clark et al., 2003; Fellows and Farah, 2005; Mega and Cummings, 1994). Higher aspects of self-reported impulsivity were related to thinner rostral and caudal ACC, which is consistent with the role of the ACC in monitoring and regulation of behavior and emotions (Bush et al., 2000; Goodkind et al., 2015; Williams, 2016). There were no significant associations between the IGT and BIS-11 and regional cortical thickness in Non-Smokers. The variance on the IGT and BIS-11 in Non-Smokers was relatively low, which appears to have restricted the range on these measures and likely dampened the magnitude of the associations between these measures and regional cortical thickness in this group.
This study has limitations that may affect the generalizability of the findings. Undocumented premorbid/comorbid group differences in lifestyle or subclinical biomedical conditions (e.g., poor diet and nutrition, lack of exercise, subclinical pulmonary or cardiovascular dysfunction) and/or genetic polymorphisms (Mon et al., 2013) may have influenced the results. Additionally, the small number of females precluded assessment of sex effects.
5. Conclusions
This study provides additional evidence that cigarette smoking is associated with cortical thinning in regions implicated in the development and maintenance of substance use disorders as well as those that show significant atrophy in mild cognitive impairment and early AD. The novel structure-function relationships apparent in Smokers offer further insight into the neurobiological substrates related to the multiple neuropsychological abnormalities documented in cigarette smokers across adulthood. Smoking-related diseases kill at least 6 million individuals annually across the globe (WHO, 2015), and smoking is linked to increased risk for the development of Alzheimer’s Diseasedisease and related neuropathology (Durazzo et al., 2016a, b; Durazzo et al., 2014a). Studies with elder adults suggest that at least partial recovery of regional morphological abnormalities is possible with extended smoking cessation (Almeida et al., 2011; Karama et al., 2015). Therefore, longitudinal research on the effects of chronic smoking on brain micro-and-macrostructural integrity, biochemistry, functional and structural connectivity, and their functional correlates is essential to inform the development of new treatment modalities to promote sustained smoking cessation (Ding et al., 2018; Durazzo et al., 2014a; Sweitzer et al., 2016).
Acknowledgements
This material is the result of work supported by the National Institute on Drug Abuse (DA24136 to TCD), the Department of Defense (W81XWH-05-2-0094 to TCD), Department of Veteran Affairs (RX002303 to TCD), the National Institute on Alcohol and Alcoholism (AA10788 to DJM), and by resources and the use of facilities at the Palo Alto Veterans Administration Health Care System, Palo Alto, CA and San Francisco Veterans Administration Medical Center, San Francisco, CA. We thank Drs. Anderson Mon and Donna Murray for assistance in MR data acquisition of study participants. We also wish to extend our gratitude to the study participants, who made this research possible.
Role of the funding source
The study sponsors (Department of Veteran Affairs, National Institutes of Health and Department of Defense) had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Conflict of interest
No conflict declared.
References
- Abe C, Mon A, Hoefer ME, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ, 2013. Metabolic abnormalities in lobar and subcortical brain regions of abstinent polysubstance users: magnetic resonance spectroscopic imaging. Alcohol Alcohol 48, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L, 2008. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am. J. Geriatr. Psychiatry 16, 92–98. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Garrido GJ, Alfonso H, Hulse G, Lautenschlager NT, Hankey GJ, Flicker L, 2011. 24-month effect of smoking cessation on cognitive function and brain structure in later life. Neuroimage 55, 1480–1489. [DOI] [PubMed] [Google Scholar]
- Azizian A, Monterosso J, O’Neill J, London ED, 2009. Magnetic resonance imaging studies of cigarette smoking. Handb. Exp. Pharmacol 192, 113–143. [DOI] [PubMed] [Google Scholar]
- Bechara A, 2007. Iowa Gambling Task, Professional Manual. Psychological Assessment Resources. Inc., Lutz, FL. [Google Scholar]
- Beck AT, 1978. Depression Inventory. Center for Cognitive Therapy, Philadelphia. [Google Scholar]
- Buhler M, Mann K, 2011. Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcohol. Clin. Exp. Res 35, 1771–1793. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR, 2002. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc. Natl. Acad. Sci. U. S. A 99, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM, 2014. Neuropathology of substance use disorders. Acta Neuropathol. 127, 91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Services, U.S.D.o.H.a.H, 2004. The Health Consequences of Smoking: A Report of the Surgeon General. Centers for Disease Control and Prevention; https://www.cdc.gov/tobacco/data_statistics/sgr/2004/. [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, DeYoung CG, Lee MJ, Lee JM, Kim SI, Cho ZH, Kim K, Gray JR, Lee KH, 2008. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J. Neurosci 28, 10323–10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW, 2003. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia 41, 1474–1483. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat DH, Wolk DA, Maguire RP, Desikan R, Pacheco J, Quinn BT, Van der Kouwe A, Greve DN, Blacker D, Albert MS, Killiany RJ, Fischl B, 2008. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage 39, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Salmeron BJ, Wang J, Yang Y, Stein EA, Ross TJ, 2018. Evidence of subgroups in smokers as revealed in clinical measures and evaluated by neuroimaging data. A Preliminary Study. Addict Biol. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A, 2014. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biol. Psychiatry 76, 742–749. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ, 2004. Cigarette smoking exacerbates chronic alcohol-induced brain damage: A preliminary metabolite imaging study. Alcohol. Clin. Exp. Res 28, 1849–1860. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ, 2010. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int. J. Environ. Res. Public Health 7, 3760–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ, 2011a. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 122, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ, 2011b. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol. Clin. Exp. Res 35, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo T, Insel PS, Weiner MW, 2012. Greater regional brain atrophy rate in healthy elders with a history of cigarette smoking. Alzheimers Dement. 8, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ, 2013a. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depend. 133, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ, 2013b. Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict. Biol 18, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, 2014a. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 10, S122–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, Korecka M, Trojanowski JQ, Shaw LM, 2014b. History of cigarette smoking in cognitively-normal elders is associated with elevated cerebrospinal fluid biomarkers of oxidative stress. Drug Alcohol Depend. 142, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abe C, Gazdzinski S, Meyerhoff DJ, 2014c. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict. Biol 19, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Korecka M, Trojanowski JQ, Weiner MW, O’Hara R, Ashford JW, Shaw LM, 2016a. Active cigarette smoking in cognitively-normal elders and probable Alzheimer’s disease is associated with elevated cerebrospinal fluid oxidative stress biomarkers. J. Alzheimers Dis 54, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, 2016b. Interaction of cigarette smoking history with APOE genotype and age on amyloid level, glucose metabolism, and neurocognition in cognitively normal elders. Nicotine Tob. Res 18, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, Abe C, Gazdzinski S, Murray DE, 2016c. Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain N-acetylaspartate and glutamate levels. Biol. Psychiatry 79, 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK, Murray DE, 2017. Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend. 177, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JD, Amunts K, 2005. High-resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum. Brain Mapp 24, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Stein EA, 2015. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann. N.Y. Acad. Sci 1349, 64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ, 2005. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb. Cortex 15, 58–63. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ, 2003. Major depression and cigarette smoking: results of a 21-year longitudinal study. Psychol. Med 33, 1357–1367. [DOI] [PubMed] [Google Scholar]
- Fettes P, Schulze L, Downar J, 2017. Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: promising therapeutic targets in psychiatric illness. Front. Syst. Neurosci 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E, 2010. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 35, 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1998. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 8/98 Revision). Biometrics Research Department, New York, NY. [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM, 1999. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatic parcellation of the human cerebral cortex. Cereb. Cortex 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, 2010. Structural brain changes in aging: courses, causes and cognitive consequences. Rev. Neurosci 21, 187–221. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM, 2006. Selective increase of cortical thickness in high-performing elderly—Structural indices of optimal cognitive aging. Neuroimage 29, 984–994. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, Storsve AB, Tamnes CK, Sala-Llonch R, Due-Tonnessen P, Bjornerud A, Solsnes AE, Haberg AK, Skranes J, Bartsch H, Chen CH, Thompson WK, Panizzon MS, Kremen WS, Dale AM, Walhovd KB, 2015. Development and aging of cortical thickness correspond to genetic organization patterns. Proc. Natl. Acad. Sci. U. S. A 112, 15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM, 2015. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 156, 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A, 2015. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 72, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Weiner KS, 2014. The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci 15, 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br. J. Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA, 2009. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch. Gen. Psychiatry 66, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N, 2009. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 48, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr JM, Wardlaw JM, Bastin ME, Deary IJ, 2015. Cigarette smoking and thinning of the brain’s cortex. Mol. Psychiatry 20, 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ, 2009. Fundamentals of Human Neuropsychology. Worth Publishers, New York, NY. [Google Scholar]
- Kremen WS, Prom-Wormley E, Panizzon MS, Eyler LT, Fischl B, Neale MC, Franz CE, Lyons MJ, Pacheco J, Perry ME, Stevens A, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Dale AM, Fennema-Notestine C, 2010. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. Neuroimage 49, 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET, 2004. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol 72, 341–372. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Schubert F, Gallinat J, 2010. Reduced thickness of medial orbitofrontal cortex in smokers. Biol. Psychiatry 68, 1061–1065. [DOI] [PubMed] [Google Scholar]
- Li Y, Yuan K, Cai C, Feng D, Yin J, Bi Y, Shi S, Yu D, Jin C, von Deneen KM, Qin W, Tian J, 2015. Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend. 151, 211–219. [DOI] [PubMed] [Google Scholar]
- Li S, Yang Y, Hoffmann E, Tyndale RF, Stein EA, 2017. CYP2A6 genetic variation alters striatal-cingulate circuits, network hubs, and executive processing in smokers. Biol. Psychiatry 81, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ, 2007. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb. Cortex 17, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, Kim BW, Blood AJ, Evins AE, Seidman LJ, Iosifescu DV, Lee S, Baxter C, Perlis RH, Smoller JW, Fava M, Breiter HC, 2008. Cortical thickness abnormalities in cocaine addiction-a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron 60, 174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Falba T, O’Malley SS, Sindelar J, O’Connor PG, 2007. Smoking status as a clinical indicator for alcohol misuse in US adults. Arch. Intern. Med 167, 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, 1994. Frontal-subcortical circuits and neuropsychiatric disorders. J. Neuropsychiatry Clin. Neurosci 6, 358–370. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K, 2005. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol. Clin. Exp. Res 29, 989–998. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Hutchison KE, Pennington D, Meyerhoff DJ, 2013. Brain-derived neurotrophic factor (BDNF) genotype is associated with lobar gray and white matter volume recovery in abstinent alcohol dependent individuals. Genes Brain Behav. 12, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A, 2010. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct 214, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Shi H, Zhong J, Xiao P, Shen Y, Wu L, Song Y, He G, 2013. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol. Sci 34, 813–817. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS, 2009. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 19, 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Paulus MP, 2007. Neural basis of reward and craving— A homeostatic point of view. Dialogues Clin. Neurosci 9, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P, Li M, Liu H, Tian YR, Chu SL, Van Halm-Lutterodt N, Jing B, Jiang T, 2018. Brain structure alterations in respect to tobacco consumption and nicotine dependence: A comparative voxel-based morphometry study. Front. Neuroanat 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, 1988. Specification of cerebral cortical areas. Science 241, 170–176. [DOI] [PubMed] [Google Scholar]
- Rakic P, 2008. Confusing cortical columns. Proc. Natl. Acad. Sci. U. S. A 105, 12099–12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Werner J, Heerlein A, Kraus A, Sauer H, 1998. On the validity of the beck depression inventory. A review. Psychopathology 31, 160–168. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, 2008. The orbitofrontal cortex and beyond: from affect to decision-making. Prog. Neurobiol 86, 216–244. [DOI] [PubMed] [Google Scholar]
- Schilling C, Kuhn S, Paus T, Romanowski A, Banaschewski T, Barbot A, Barker GJ, Bruhl R, Buchel C, Conrod PJ, Dalley JW, Flor H, Ittermann B, Ivanov N, Mann K, Martinot JL, Nees F, Rietschel M, Robbins TW, Smolka MN, Strohle A, Kathmann N, Garavan H, Heinz A, Schumann G, Gallinat J, 2013. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol. Psychiatry 18, 624–630. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Brody A, 2009. In vivo brain imaging of human exposure to nicotine and tobacco. Handb. Exp. Pharmacol 192, 145–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ, 1982. Reliability of alcohol use indices. The lifetime drinking history and the MAST. J. Stud. Alcohol 43, 1157–1170. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI, 1988. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. J. Stud. Alcohol 49, 225–232. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA, 1977. Self-Evaluation Questionnaire. Consulting Psychologist Press, Palo Alto, CA. [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB, 2014. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J. Neurosci 34, 8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA, 2013. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol. Psychiatry 74, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA, Laird AR, 2016. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav. Brain Funct 12, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Addicott MA, Denlinger R, Raiff BR, Dallery J, McClernon FJ, Donny EC, 2016. Smoking abstinence-induced changes in resting state functional connectivity with ventral striatum predict lapse during a quit attempt. Neuropsychopharmacology 41, 2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR Jr., 2010. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology 75, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, Salat D, Reinvang I, 2006. Regional cortical thickness matters in recall after months more than minutes. Neuroimage 31, 1343–1351. [DOI] [PubMed] [Google Scholar]
- Wang GY, Demirakca T, van Eijk J, Frischknecht U, Ruf M, Ucar S, Hermann D, Mann K, Kiefer F, Ende G, 2016. Longitudinal mapping of gyral and sulcal patterns of cortical thickness and brain volume regain during early alcohol abstinence. Eur. Addict. Res 22, 80–89. [DOI] [PubMed] [Google Scholar]
- WHO, 2015. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2015. World Health Organization, Geneva. [Google Scholar]
- Williams LM, 2016. Precision psychiatry: A neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 3, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC, 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53, 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]