Abstract
Cavitation-Based ultrasound histotripsy has shown potential for treating infections on surgical mesh. The goal of this study was to explore a new scan strategy while assessing the impact of scan speed, scan step size, and number of cycles in the tone burst on the destruction of S. aureus biofilms grown on surgical mesh samples using ultrasound histotripsy pulses (150 MPa/–17 MPa). For each exposure, the number of colony forming units (CFUs) on the mesh and released onto the surrounding gel was quantified. Most of the exposed mesh samples had no CFUs, and there was a statistically significant reduction in CFUs on the mesh for each of the exposures, with an average reduction of 3.8-log10 relative to the sham. Compared with the sham, there was also a statistically significant reduction in CFUs on the gel with the highest exposures.
Index Terms: Ultrasound Therapy, Histotripsy, biofilm infection
I. INTRODUCTION
WHILE sterile surgical techniques and perioperative care have made a significant impact on minimizing the risk of surgical site infection (SSI), these infections still occur. While many SSIs can be effectively treated by local care with or without antibiotics, bacteria biofilms are highly resistant to antibiotics [1]. When associated with prosthetic material, the effect is deleterious requiring removal of the prosthesis in most instances. This increased biofilm resistance is likely due to the reduced growth rate of the bacteria in the biofilm and decreased penetration of antibacterial agents in the biofilm. Bacteria biofilms consist of structured bacteria communities encased in a protective matrix. The biofilm also offers protection from antibodies [2, 3] and phagocytes [4, 5]. Therefore, while antibiotics can treat the planktonic bacteria that are periodically released by the biofilm, the bacteria under the protection of the biofilm remain viable and are a source of continuing infection [6].
One common site for bacteria biofilms in vivo is on medical implants. For example, 95% of urinary tract infections are associated with catheters, 87% of bloodstream infections are attributed to indwelling vascular catheters, and 86% of pneumonias originate from mechanical ventilation [7]. In order to treat these infections, the catheter needs to be removed and a new catheter installed. However, other implants are not so easy to replace. For example, mesh infections are some of the most severe complications following hernia repair [8–12]. Infections on the mesh require removal ~70% of the time with a high risk of hernia re-occurrence following removal of the mesh [8, 12, 13].
Cavitation-based ultrasound histotripsy has already been shown to have the potential to destroy bacteria biofilms non-invasively. In our first study, Escherichia coli (E. coli) was grown as a biofilm and exposed at different pressure levels using a 1.1 MHz spherically focused source [14]. E. coli was selected due to its prevalence in catheter-related infections [15]. The study found a threshold for the rarefaction pressure between 10 and 12 MPa for biofilm destruction with a typical reduction of 4-log10 colony forming units (CFUs). In our second study, the effectiveness of the therapy for destroying Pseudomonas aeruginosa (P. aeruginosa) biofilms was demonstrated [16]. P. aeruginosa was selected for the difficulty when treating this biofilm with ultrasound using a different treatment strategy [17], for its general resistance to antibiotic treatment, and for its major contribution to nosocomial infections.
In addition, the potential for using ultrasound histotripsy to treat Staphylococcus aureus (S. aureus) biofilms has also been demonstrated [18]. S. aureus was selected for its prevalence in mesh infections following hernia repair [8, 10, 12]. The S. aureus study varied the number of cycles in the tone burst used to generate and excite the histotripsy bubble cloud. The histotripsy exposures resulted in a reduction of 2.9-log10, 2.6-log10, and 3.8-log10 CFUs for the 3-cycle, 5-cycle, and 10 cycle tone bursts respectively with no statistical difference between the different tone bursts. However, the 3-cycle exposures had the least amount of collateral tissue damage. As a comparison, the FDA defines high-level disinfection as a reduction of 6-log10 (i.e., 6 orders of magnitude) in the number of CFUs. However, 4-log10 is typical for many ex vivo sterilization methods such as the initial cleaning of endoscopes and other directed energy methods such as microwaves [19–21].
The goal of this study is to vary the scan parameters to see if higher levels of biofilm destruction can be achieved. Specifically, our prior study moved the focus of our therapy transducer over the surface of the mesh in a raster pattern with a step-size of 750 μm (~0.6 of the beam width) in both directions of the treatment plane. A smaller step size may result in better biofilm destruction as the focus may not be directly targeted on the fibers of the mesh. However, a smaller step size for a raster scan is not practical as it would result in unreasonably long treatment times in the clinic. Therefore, for this study, the focus was continuously scanned along one axis in the treatment plane while only stepping in one direction. We then varied the scan speed and the step size and quantified the impact of each on CFUs. Lastly, we did exposures with both 3-cycle and 10-cycle tone bursts to confirm our results from our earlier study under these new scan conditions.
II. Methods
A. Biofilm Preparation
Staphylococcus aureus subsp. Aureus (ATCC®25923™) biofilms were grown on Polypropylene mesh samples (PPKM301, Surgical Mesh Division, Textile Development Associates Inc., Brookfield, CT) as described in [18]. Briefly, the 10 mm × 10 mm mesh samples were individually placed in 20 ml of tryptic soy broth (TSB) that had been inoculated with 20 μl of stationary phase bacteria. The samples and TSB were then incubated for three days at 37°C. However, every 24 hours, the mesh samples were placed in a fresh solution of TSB so that the bacteria could continue to have an ample supply of nutrients. As was stated in our earlier paper, PPKM301 was selected as it is a small-diameter monofilament mesh with relatively large pores which seems to correlate with better clinical outcomes when conducting hernia repair [9, 22].
B. Ultrasound Alignment and Exposure of Infected Mesh
After growing the biofilms on the mesh samples, the biofilms were inserted into Aquaflex Ultrasound Gel Pad Standoffs (Parker Laboratories Inc., Fairfield, NJ) as shown in Figure 1.
Fig. 1.
An example image showing the mesh sample inside the gel pad that is then placed in the holder utilized for the experiments. The mesh sample is inside the red rectangle in the image.
Prior to insertion, the mesh samples were rinsed with 10 ml of sterile phosphate buffered saline (PBS) to remove any planktonic bacteria. To insert the mesh into the gel pads, the gel pads were first cut in half. Then, a slit was cut in the center of the gel pad allowing the mesh to be placed inside the pad. After insertion, 100 μl of sterile PBS was also injected into the slit with the mesh to minimize the chance of trapped air in the slit. The gel pads were used as an easily reproducible sterile tissue mimic as they have approximately the same elastic modulus as abdominal muscle [23–25]. The elastic modulus of the medium has a significant impact on the level of damage observed following ultrasound histotripsy experiments, and therefore must be matched when utilizing a material as a tissue mimic [26–28]. The gel pad with the inserted mesh was then placed inside an aluminum holder, and the position of the mesh relative to the edges of the holder was measured. The holder with the gel pad and mesh was then placed in a water bath as shown in Figure 2.
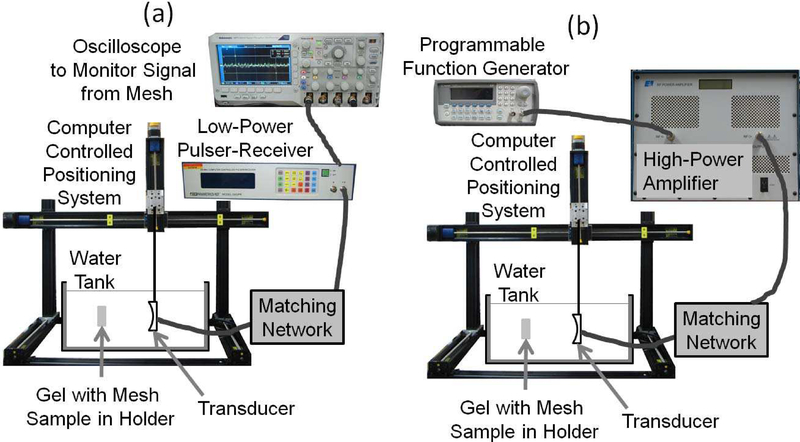
Fig. 2.
The set-up utilized for the experiments when (a) aligning the focus of the ultrasound transducer on the mesh sample prior to the histotripsy exposures, and (b) exposing the mesh samples to the high-intensity tone bursts for the histotripsy treatment.
After being placed in the water bath, the focus of the ultrasound transducer was first aligned with the left and bottom internal edges of the holder as viewed when looking along the beam-axis in the direction of ultrasound propagation (left and bottom sides of the holder in Figure 1) as illustrated in Figure 2a. The alignment was done by using a low-power signal from a pulser-receiver (Panametrics 5900, Olympus Corporation, Tokyo, Japan) and noting when the signal from the edges began to drop off significantly. The transducer was then moved until the focus was approximately in the middle of the mesh based on the measurements taken of the mesh position prior to insertion into the water tank. The transducer was then moved along the beam axis in steps of 1 mm and transverse to the beam axis in steps of 350 μm until a maximum echo was received from one of the mesh fibers. This was done to ensure that the mesh was precisely positioned in the focal plane. Once aligned, the edges of the mesh were found by moving the transducer parallel to the plane of the mesh until the echo from the mesh could no longer be observed. For the alignment, the Panametrics 5900 was set to excite the transducer with a voltage spike at a pulse repetition frequency of 200 Hz. A lower voltage spike corresponding to a focal pressure of 0.076 MPa was used when finding the edges of the holder while a higher voltage spike corresponding to a focal pressure of 0.15 MPa was used when aligning on the mesh. These focal pressures were determined using our capsule hydrophone (ONDA HGL-0200, Onda Corporation, Sunnyvale, CA).
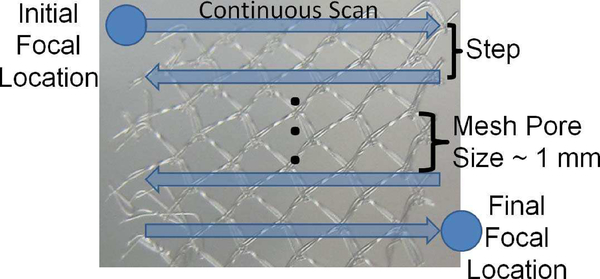
After alignment on the mesh, the mesh was randomly exposed to either a sham exposure or one of the histotripsy exposures as illustrated in Figure 2b. All the exposures were done with a single-element spherically focused transducer with an operating frequency of 1.1 MHz, a focal length of 12.5 cm, and a diameter of 12.5 cm (H-161C, Sonic Concepts Inc., Bothell, WA). The transducer was excited to transmit tone bursts at a pulse repetition frequency (PRF) of 1 kHz with a peak-compressional pressure of approximately 150 MPa and a peak rarefactional pressure of approximately 17 MPa. The therapy exposures were calibrated via a combination of hydrophone measurements and computer modelling as was explained in detail in our earlier publications [16, 26, 29, 30]. The 3 dB beam width for the transducer as measured by reflection from a wire target was 1.2 mm. For the exposures, we continuously scanned the transducer in the horizontal direction and stepped the transducer in the vertical direction once we reached the edge of the mesh treatment zone as illustrated in Figure 3. Also, rather than performing the treatment in a single pass, the treatment scan was performed 5 times for each mesh sample. Therefore, once the focal spot reached the end of the mesh, denoted Final Focal Location in Figure 3, it was returned to the Initial Focal Location, and the treatment was repeated 4 more times. To ensure that the entire mesh was exposed, the treatment dimensions varied from 12 to 16 mm on a side with 12 mm × 12 mm being the most common. The treatment dimensions for each mesh were based on the mesh edges identified from the alignment exposures.
Fig. 3.
Illustration of ultrasound exposure parameters where the focal spot was continuously scanned in the horizontal direction and stepped in the vertical direction when treating the mesh samples.
For the exposures, the speed for the continuous portion of the scan was varied as 0.4 mm/sec, 0.8 mm/sec, 1.2 mm/sec, and 1.6 mm/sec corresponding to 0.33 beam widths/sec, 0.67 beam widths/sec, 1 beam width/sec, and 1.33 beam widths/sec respectively. In addition, the step size for the vertical portion was varied as 350 μm (0.3 beam widths) and 700 μm (0.6 beam widths). Lastly, the number of cycles in the tone burst was either 3 cycles or 10 cycles. Since the speed and step size varied for the different exposures, the total treatment time also varied from about 20 minutes for the fastest exposures to about 90 minutes for the longest exposures. For the sham exposures, ultrasound was off, the scan speed was 0.4 mm/sec, and the step size was 350 μm corresponding to the longest scan times. All of the different exposure conditions are summarized in Table I. Each exposure was repeated 7 times except for the sham exposure which was repeated 9 times. The extra repetitions for the sham exposure were due to requiring at least one sham exposure every week to help ensure the bacteria biofilms were growing as expected.
TABLE I.
Summary of exposure conditions used in the experiment.
| Exposure Condition | Scan Speed (mm/sec) | Scan Step Size (pm) | # of Cycles |
|---|---|---|---|
| Sham (9 repetitions) | 0.4 | 350 | Off |
| A (7 repetitions) | 0.4 | 350 | 10 |
| B (7 repetitions) | 0.8 | 350 | 10 |
| C (7 repetitions) | 1.2 | 350 | 10 |
| D (7 repetitions) | 1.6 | 350 | 10 |
| E (7 repetitions) | 0.8 | 700 | 10 |
| F (7 repetitions) | 0.8 | 350 | 3 |
C. Quantification of Colony Forming Units (CFUs)
After each exposure was completed, the gel pad with the inserted mesh was removed from the holder. The mesh was then removed from the gel pad by trimming the gel pad with a sterile blade so that it could be separated by the slit that was originally cut in the gel pad when the mesh was inserted. The mesh was then lifted off the gel pad using sterile forceps and dipped into a tube of sterile PBS to remove any loose gel fragments. The mesh was then placed in a 1.5 ml microcentrifuge tube containing 0.5 ml of sterile PBS. The tube was then vortexed for 60 seconds and placed in a Symphony Ultrasonic Cleaner (1.9 L, VWR, Radnor, PA) and sonicated for two minutes. It was then vortexed again for 60 seconds, sonicated again for two minutes, and vortexed again for 60 seconds. The vortexing/sonication cycle would release and disperse the bacteria from the mesh without killing them. After the vortexing/sonication, 0.5 ml was removed and serially diluted in sterile PBS at dilutions of 10−1, 10−2, 10−3, 10−4, and 10−5. 100 μl of each dilution was then plated on tryptic soy agar and incubated overnight at 37°C.
In addition to processing the mesh, the gel in the vicinity of the mesh was also scraped by a sterile blade. The scrapped gel and fragments were then placed in the same container in which the mesh had been dipped (tube with 4.5 ml of sterile PBS). The tube of gel fragments was then vortexed for 3 minutes. After vortexing, 0.5 ml was removed and serially diluted in sterile PBS at the same dilutions of 10−1, 10−2, 10−3, 10−4, and 10−5. Following dilution, 100 μl was once again plated on tryptic soy agar and incubated overnight at 37°C.
The number of CFUs on each plate for both the mesh and gel dilutions were counted following the overnight incubation. The dilution that had counts between 30 and 300 was then used to back calculate the total number of CFUs remaining on the mesh and gel following the exposure. If two different dilutions had counts in the 30–300 range, the numbers were averaged after accounting for the dilution process. If all the counts were outside the 30 to 300 range, then dilution that resulted in a count that was closest to 300 was used. Lastly, if no CFUs were observed on the lowest dilution plate, then the count was set to zero for that exposure. When counting CFUs, numbers in the range of 30 to 300 are the most reliable as plates with fewer than 30 colonies may not give statistically reliable results and plates with more than 300 colonies can be too crowded to allow the bacteria to form distinct colonies. Therefore, focusing on counts in this range has become standard practice in microbiology.
After determining the number of CFUs for each exposure, we calculated log10(count+1) to translate the count into log number for comparison. This transformation facilitates the calculation of the log10 reduction in the number of CFUs. In addition, the transformed data for the gel for each of the exposures (including the sham exposure) follows an approximately normal distribution whereas the original count data does not. It is important for the data to follow a normal distribution if traditional statistical analysis approaches are to be used [31].
The transformed data for the gel was analyzed using a one-way analysis of variance (ANOVA) and the JMP software (Pro 12, The SAS Institute). The data included 51 observations, 9 for the sham and 7 for each of the six histotripsy exposures. Dunnett’s method [32] was used for simultaneously comparing the six histotripsy exposures with the sham, with an overall type I error rate of no more than 5%. The plot of residuals versus predicted values and the normal probability plot of the residuals indicate that the model was adequate.
The transformed data for the mesh also included 51 observations, 9 for the sham and 7 for each of the six histotripsy exposures. Because most of the exposed mesh samples had no CFUs, most of the transformed values are zero, invalidating the normality and equal-variance assumptions for the standard one-way ANOVA. Thus a more robust procedure was used [33] for pairwise comparisons between the seven exposures, with an overall type I error rate of no more than 5%. The procedure also gave 95% simultaneous confidence intervals for all pairwise differences. We used the R software (version 3.1.0, The R Foundation for Statistical Computing) to analyze the transformed data for the mesh because the robust procedure was implemented in the R software [33].
III. Results
A. Mesh Results
Figures 4, 5, and 6 show the numbers of CFUs remaining on the mesh for the different treatments as a function of scan speed, step size, and the number of cycles in the tone burst respectively.
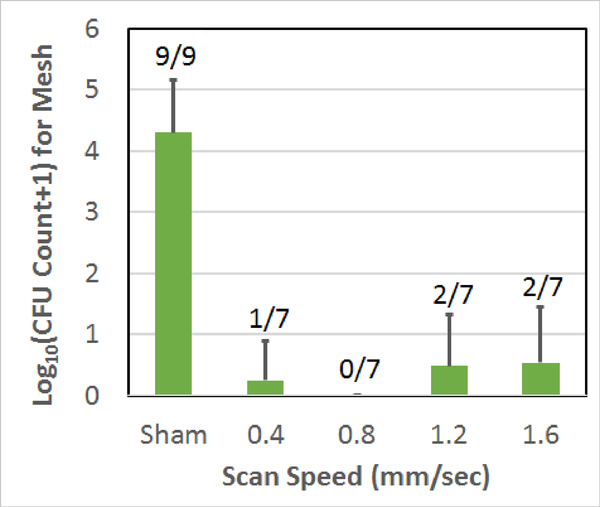
Fig. 4.
Count of CFUs surviving on the mesh sample following the treatment as a function of scan speed used to treat the biofilm on the mesh. The error bars correspond to one standard deviation. The numbers above each bar correspond to the number of repetitions for which CFUs were found on the mesh.
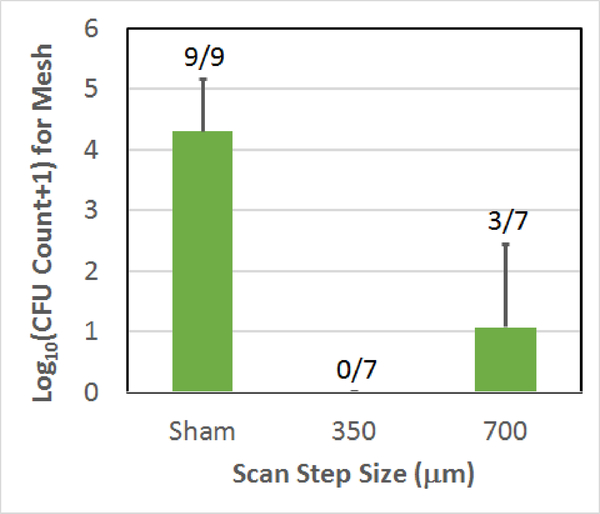
Fig. 5.
Count of CFUs surviving on the mesh sample following the treatment as a function of scan step size used to treat the biofilm on the mesh. The error bars correspond to one standard deviation. The numbers above each bar correspond to the number of repetitions for which CFUs were found on the mesh.
Fig. 6.
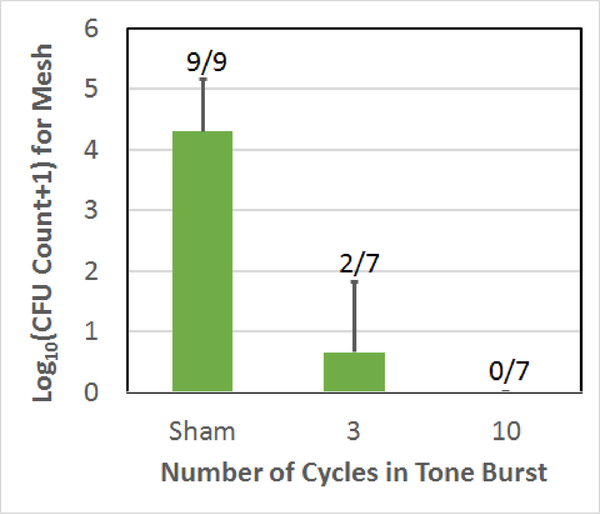
Count of CFUs surviving on the mesh sample following the treatment as a function of the number of cycles in the tone burst used to treat the biofilm on the mesh. The error bars correspond to one standard deviation. The numbers above each bar correspond to the number of repetitions for which CFUs were found on the mesh.
As was given in Table I, for the scan speed comparison, all of the speeds used a step size of 350 μm and 10-cycle tone bursts. Also, when comparing step size, the scan speed was maintained at 0.8 mm/sec with 10-cycle tone bursts. Likewise, the tone burst comparison used a 0.8 mm/sec scan speed and a step size of 350 μm. The sham exposure, while duplicated in each plot, was the same data set corresponding to a 350 μm step size and a scan speed of 0.4 mm/sec. The 0.8 mm/sec scan speed, 350 μm step size, 10-cycle exposure (the exposure condition B in Table I) in each plot was also the same data (with no CFUs on all the seven mesh samples). The error bars in each figure correspond to one standard deviation. The numbers above each bar in each plot give the number of repetitions for which CFUs were found on the mesh. Therefore, a 0/7 means that of the 7 repetitions, none of the meshes had any surviving CFUs for that exposure.
From these results, it is clear that the vast majority of the exposures had no CFUs remaining on the mesh following the treatment and all of the exposed meshes were statistically different from the sham exposures. The 0.8 mm/sec scan speed, 700 μm step size, 10-cycle exposure had the most meshes with CFUs (3 out of 7) and the highest average CFU count at 1.1±1.4-log10. However, none of the average CFU counts for the six histotripsy exposures were statistically different from each other. Table II summarizes the average reduction in CFUs for each of the exposures relative to the sham exposure.
TABLE II.
Summary of reduction in CFUs on the mesh for each exposure RELATIVE TO THE SHAM EXPOSURE.
| Exposure Condition (Speed/Step/# of Cycles) | CFU reduction (log10) |
|---|---|
| A (0.4/350/10) | 4.1 |
| B (0.8/350/10) | 4.3 |
| C (1.2/350/10) | 3.8 |
| D (1.6/350/10) | 3.8 |
| E (0.8/700/10) | 3.2 |
| F (0.8/350/3) | 3.7 |
| Average | 3.8 |
The reduction in CFUs ranged from 3.2-log10 for the 700 μm step size to 4.3-log10 with an average reduction of 3.8-log10.
B. Gel Results
In addition to quantifying the number of CFUs surviving on the mesh samples, the surviving bacteria released onto the gel surrounding the mesh was also quantified. As a reminder, biofilms continuously release planktonic bacteria. Therefore, CFUs on the gel would include both spontaneously released bacteria and bacteria released from the biofilm due to the ultrasound exposure.
Figures 7, 8, and 9 show the numbers of CFUs remaining on the gel for the different treatments as a function of scan speed, step size, and the number of cycles in the tone burst respectively.
Fig. 7.
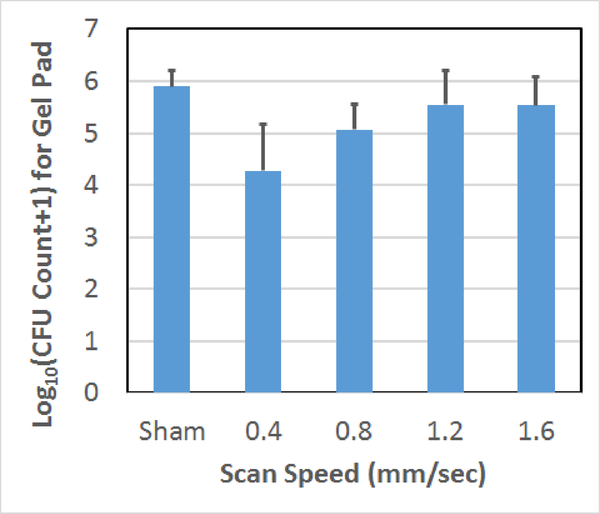
Count of CFUs surviving on the gel following the treatment as a function of scan speed used to treat the biofilm on the mesh. The error bars correspond to one standard deviation.
Fig. 8.
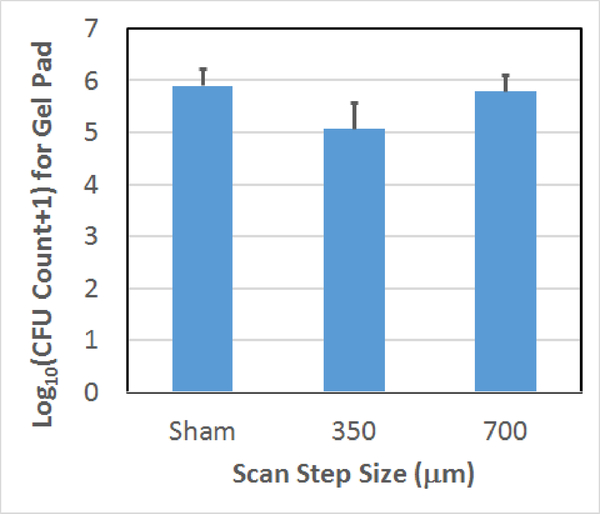
Count of CFUs surviving on the gel following the treatment as a function of scan step size used to treat the biofilm on the mesh. The error bars correspond to one standard deviation.
Fig. 9.
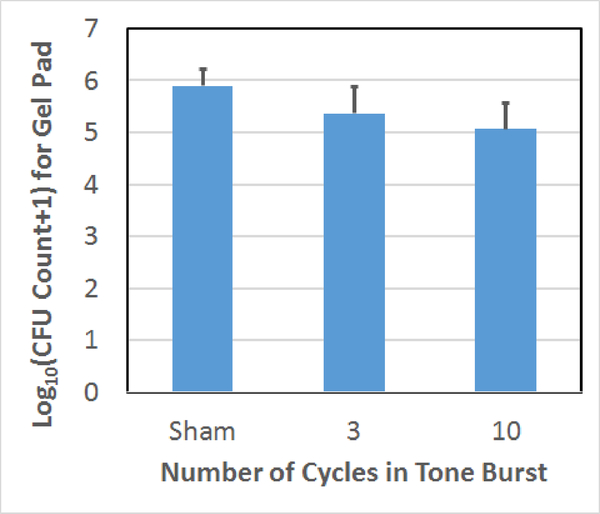
Count of CFUs surviving on the gel following the treatment as a function of the number of cycles in the tone burst used to treat the biofilm on the mesh. The error bars correspond to one standard deviation.
For the scan speed results, 0.4 and 0.8 mm/sec scan speeds had statistically fewer average surviving CFUs than the sham exposure. The 1.2 and 1.6 mm/sec exposures were not statistically different from the sham exposure. For the step size comparison, the average numbers of CFUs on the gel are not statistically different for the 700 μm step and the sham. However, there is a statistically significant difference between the 350 μm step and the sham as was already stated. Likewise, for the cycle number comparison, the 3-cycle exposure was not statistically different from the sham; however, the 10-cycle and sham exposures were statistically different from each other. In addition, we qualitatively observed less damage to the gel with the 3-cycle exposures which was consistent with our earlier observations regarding the dependence of collateral damage on pulse duration [18].
IV. Discussion
In this study, the average reduction in CFUs on the mesh was 3.8-log10. As a comparison, our average reduction in our earlier study was 3.1-log10 [18]. Also, in this study, 76% of the exposed meshes had no CFUs while in the previous study, only 38% of the meshes had no surviving CFUs. Hence, our new continuously scanning method is more effective at destroying the biofilm on the mesh samples. In addition, our CFU reduction for this study is likely higher than measured as most of the meshes had no surviving CFU. Since it is impossible to have a count less than zero, this introduces a bias in the estimated CFU reductions. While having no surviving CFUs would be extremely advantageous from a clinical perspective, it made comparing the different exposure conditions difficult.
In the future, it would be best to have more bacteria on the mesh prior to the exposure, so that the actual reduction in CFUs could be more accurately quantified. one method would be to increase the growth time for the biofilm. our present study used a growth time of 3 days based on a relatively quick exploratory study. A three-day growth interval also worked well for some of our earlier studies (Xu, et al. 2012). However, if we increase the growth time to 6 days, as was done after completing the study described in this paper, the number of CFUs on the mesh increases to 7.4-log10. This is 3 orders of magnitude more CFUs than the 4.3±0.9-log10 CFUs on the mesh in the sham exposures used in this study. Therefore, in the future, longer biofilm growth times should be used when quantifying the destruction of the biofilm on the mesh.
In addition to our mesh results, there is clearly a trend of reduced CFUs on the gel as the scan speed and step size decrease. The clear reduction in CFUs relative to the sham at these higher exposure levels shows that the bacteria in the vicinity of the mesh are being killed by the ultrasound histotripsy at least at the higher levels. This is in agreement with our earlier studies which showed a fragmentation of microalgae following histotripsy level exposures [34, 35]. The fate of the bacteria on the mesh at the lower exposure levels is still not certain due to the enormous number of bacteria spontaneously released by the biofilm as is evidenced by the large number of CFUs on the gel for the sham exposures.
In addition to the decrease in bacteria on the gel with decreasing scan speed and step size, there is also a decrease in CFUs with increasing tone burst duration. This decrease may just be due to larger bubble clouds more effectively treating bacteria in the pre-focal region. However, exploratory studies have shown that diffusion of the bacteria into the gel is minimal. Therefore, bacteria should not be able to diffuse axially. Hence, expanding the treatment zone axially should have only a minimal impact on treating the bacteria. Another possibility is that at larger tone burst durations, a larger volume of the gel in the pre-focal region is liquefied by the cavitation activity. This liquefied chamber could potentially allow for the formation of strong streaming currents and associated shearing forces on the bacteria when the focal zone passes through the treatment zone on subsequent passes as the focal zone was passed over the mesh 5 times. In our earlier studies with microalgae [34, 35], it is clear that the fragmentation of micro-organisms his greatly enhanced when fluid streaming is present. However, the creation of these liquefied pockets is likely not desirable in general as they would greatly increase the collateral damage to healthy surrounding tissue.
Another compounding factor when assessing the gel data is that when scraping the gel, we collected gel fragments from beyond the boundary of the exposed region. This was done as the boundary of the exposed region was very difficult to visualize for most of the exposures after the mesh was removed as the gel damage tended to be only on the side closest to the transducer. Therefore, we purposely scraped a larger region to ensure that we did not miss the mesh location. Hence, depending on the diffusion of the bacteria upon release, many of the bacteria collected from the gel may never have been exposed to any of the ultrasound exposures. Regardless, methods for controlling the released planktonic bacteria, such as combining our treatment with antibiotics, should also be explored. Also, it may be possible to reduce the number of CFUs on the gel by increasing the number of passes of the focal region over the treated region. In the current treatment, the focus was scanned over the mesh 5 times, and increasing this number to improve the destruction of CFUs on the gel should be explored. These additional passes could also be implemented at a higher speed and/or cover a larger area to treat the bacteria more effectively as they spread away from the mesh.
V. CONCLUSION
In this study, we explored the impact of scan speed and scan step size on the destruction of S. aureus bacteria biofilms grown on surgical mesh samples using ultrasound cavitation-based histotripsy exposures. We also re-evaluated the difference between using 3-cycle tone bursts and 10-cycle tone bursts in our exposures. For the mesh results, none of the ultrasound histotripsy exposures were statistically different and the average reduction in CFUs was 3.8-log10 relative to the sham. It is likely that the reduction was even greater than this with more differences between the exposure conditions, but these differences were masked by the large number of meshes that had no surviving CFUs.
In addition to quantifying the CFUs on the mesh, we also quantified the CFUs released onto the surrounding gel. The highest exposures (0.4 mm/sec, 350 μm step, 10-cycle exposure and 0.8 mm/sec, 350 μm step, 10-cycle exposure) resulted in a clear reduction in the average number of CFUs near the mesh relative to the sham exposure. The fate of the bacteria for the other exposures remains uncertain due to the large number of bacteria spontaneously released by the biofilm.
Acknowledgments
This research was supported by National Institutes of Health (NIH) grant number R21EB020722.
Contributor Information
Timothy A. Bigelow, Center for Nondestructive Evaluation, Iowa State University, Ames, IA 50011.
Clayton L. Thomas, Center for Nondestructive Evaluation, Iowa State University, Ames, IA 50011
Huaiqing Wu, Department of Statistics, Iowa State University, Ames, IA 50011.
Kamal MF. Itani, Itani is with the VA Boston Healthcare System, Boston University and Harvard Medical School, West Roxbury, MA 02132.
References
- [1].Olson ME, Ceri H, Morck DW, Buret AG, and Read RR, “Biofilm bacteria: formation and comparative susceptibility to antibiotics,”Can J Vet Res, vol. 66, pp. 86–92, 2002. [PMC free article] [PubMed] [Google Scholar]
- [2].Cochrane DM, Brown MR, Anwar H, Weller PH, Lam K, and Costerton JW, “Antibody response to Pseudomonas aeruginosa surface protein antigens in a rat model of chronic lung infection,” J Med Microbiol, vol. 27, pp. 255–261, December 1, 1988 1988. [DOI] [PubMed] [Google Scholar]
- [3].De Beer D, Stoodley P, and Lewandowski Z, “Measurement of local diffusion coefficients in biofilms by microinjection and confocal microscopy,” Biotechnology and Bioengineering, vol. 53, pp. 151–158, 1997. [DOI] [PubMed] [Google Scholar]
- [4].Rodgers J, Phillips F, and Olliff C, “The effects of extracellular slime from Staphylococcus epidermidis on phagocytic ingestion and killing,” FEMS Immunology and Medical Microbiology, vol. 9, pp. 109–115, 1994. [DOI] [PubMed] [Google Scholar]
- [5].Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, et al. , “Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system,” Cellular Microbiology,vol. 6, pp. 269–275, 2004. [DOI] [PubMed] [Google Scholar]
- [6].Trautner BW and Darouiche RO, “Catheter-Associated Infections: Pathogenesis Affects Prevention,” Arch Intern Med, vol. 164, pp. 842–850, April 26, 2004 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Richards MJ, Edwards JR, Culver DH, and Gaynes RP, “Nosocomial infections in medical intensive care units in the United States,” Critical Care Medicine, vol. 27, pp. 887–892, May 1999. [DOI] [PubMed] [Google Scholar]
- [8].Mavros M, Athanasiou S, Alexiou V, Mitsikostas P, Peppas G, and Falagas M, “Risk Factors for Mesh-related Infections After Hernia Repair Surgery: A Meta-analysis of Cohort Studies,” World Journal of Surgery, vol. 35, pp. 2389–2398, 2011. [DOI] [PubMed] [Google Scholar]
- [9].Amid P, “Classification of biomaterials and their related complications in abdominal wall hernia surgery,” Hernia, vol. 1, pp. 15–21, 1997. [Google Scholar]
- [10].Falagas ME and Kasiakou SK, “Mesh-related infections after hernia repair surgery,” Clinical Microbiology and Infection, vol. 11, pp. 3–8, 2005. [DOI] [PubMed] [Google Scholar]
- [11].Lüning T and Spillenaar-Bilgen EJ, “Parastomal hernia: complications of extra-peritoneal onlay mesh placement,” Hernia,vol. 13, pp. 487–490, 2009. [DOI] [PubMed] [Google Scholar]
- [12].Sanchez VM, Abi-Haidar YE, and Itani KMF, “Mesh infection in ventral incisional hernia repair: Incidence, contributing factors, and treatment,” Surgical Infections, vol. 12, pp. 205–210, 2011. [DOI] [PubMed] [Google Scholar]
- [13].LeBlanc KA, “Laparoscopic incisional and ventral hernia repair: Complications—how to avoid and handle,” Hernia,vol. 8, pp. 323–331, 2004. [DOI] [PubMed] [Google Scholar]
- [14].Bigelow TA, Northagen T, Hill TM, and Sailer FC, “The Destruction of Escherichia Coli Biofilms Using High-Intensity Focused Ultrasound,” Ultrasound in Medicine & Biology,vol. 35, pp. 1026–1031, 2009. [DOI] [PubMed] [Google Scholar]
- [15].Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, and Hultgren SJ, “Intracellular Bacterial Biofilm-Like Pods in Urinary Tract Infections,” Science, vol. 301, pp. 105–107, July 4, 2003 2003. [DOI] [PubMed] [Google Scholar]
- [16].Xu J, Bigelow TA, Halverson LJ, Middendorf JM, and Rusk B, “Minimization of treatment time for in vitro 1.1 MHz destruction of Pseudomonas aeruginosa biofilms by high-intensity focused ultrasound,” Ultrasonics, vol. 52, pp. 668–675, 2012. [DOI] [PubMed] [Google Scholar]
- [17].Carmen JC, Roeder BL, Nelson JL, Robison Ogilvie RL, Robison RA, Schaalje GB, et al. , “Treatment of biofilm infections on implants with low-frequency ultrasound and antibiotics,” American Journal of Infection Control, vol. 33, pp. 78–82, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bigelow TA, Thomas CL, Wu H, and Itani KMF, “Histotripsy Treatment of S. Aureus Biofilms on Surgical Mesh Samples under Varying Pulse Durations,” IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, vol. 64, pp. 1420–1428, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rutala W and Weber D, “Reprocessing endoscopes: United States perspective,” J Hosp Infect, vol. 56, pp. S27–39, 2004. [DOI] [PubMed] [Google Scholar]
- [20].Rutala WA and Weber DJ, “FDA Labeling Requirements for Disinfection of Endoscopes: A Counterpoint,” Infection Control and Hospital Epidemiologyvol. 16, pp. 231–235, 1995. [DOI] [PubMed] [Google Scholar]
- [21].Liu Q, Zhang M, Fang Z.-x., and Rong X.-h., “Effects of ZnO nanoparticles and microwave heating on the sterilization and product quality of vacuum-packaged Caixin,” Journal of the Science of Food and Agriculture,pp. 2547–2554, 2014. [DOI] [PubMed] [Google Scholar]
- [22].Klosterhalfen B and Klinge U, “Retrieval study at 623 human mesh explants made of polypropylene - impact of mesh class and indication for mesh removal on tissue reaction,” Journal of Biomedical Materials Research Part B: Applied Biomaterials,pp. n/a–n/a, 2013. [DOI] [PubMed] [Google Scholar]
- [23].Brown SHM, Carr JA, Ward SR, and Lieber RL, “Passive Mechanical Properties of Rat Abdominal Wall Muscles Suggest an Important Role of the Extracellular Connective Tissue Matrix,” Journal of Orthopaedic Research,vol. 30, pp. 1321–1326, 01/20 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ogneva IV, Lebedev DV, and Shenkman BS, “Transversal Stiffness and Young’s Modulus of Single Fibers from Rat Soleus Muscle Probed by Atomic Force Microscopy,” Biophysical Journal,vol. 98, pp. 418–424, 2010 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dogan F and Celebi MS, “Quasi-non-linear deformation modeling of a human liver based on artificial and experimental data,” The International Journal of Medical Robotics and Computer Assisted Surgery, vol. 12, pp. 410–420, 2016. [DOI] [PubMed] [Google Scholar]
- [26].Xu J and Bigelow TA, “Experimental Investigation of the Effect of Stiffness, Exposure Time and Scan Direction on the Dimension of Ultrasound Histotripsy Lesions,” Ultrasound in Medicine & Biology,vol. 37, pp. 1865–1873, 2011. [DOI] [PubMed] [Google Scholar]
- [27].Xu J, Bigelow TA, Davis G, Avendano A, Shrotriya P, Bergler K, et al. , “Dependence of ablative ability of high-intensity focused ultrasound cavitation-based histotripsy on mechanical properties of agar,” The Journal of the Acoustical Society of America, vol. 136, pp. 3018–3027, 2014. [DOI] [PubMed] [Google Scholar]
- [28].Xu J, Bigelow TA, and Nagaraju R, “Precision control of lesions by high-intensity focused ultrasound cavitation-based histotripsy through varying pulse duration,” Ultrasonics, Ferroelectrics and Frequency Control, IEEE Transactions on, vol. 60, 2013. [DOI] [PubMed] [Google Scholar]
- [29].Xu J, Bigelow TA, and Riesberg GM, “Impact of Preconditioning Pulse on Lesion Formation During High-Intensity Focused Ultrasound Histotripsy,” Ultrasound in Medicine & Biology,vol. 38, pp. 1918–1929, 2012. [DOI] [PubMed] [Google Scholar]
- [30].Xu J, Bigelow TA, and Whitley EM, “Assessment of Ultrasound Histotripsy-Induced Damage to Ex Vivo Porcine Muscle,” Journal of Ultrasound in Medicine, vol. 32, pp. 69–82, January 1, 2013 2013. [DOI] [PubMed] [Google Scholar]
- [31].Olsen CH, “Review of the Use of Statistics in Infection and Immunity,” Infection and Immunity, vol. 71, pp. 6689–6692, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dunnett CW, “A Multiple Comparison Procedure for Comparing Several Treatments with a Control,” Journal of the American Statistical Association, vol. 50, pp. 1096–1121, 1955/12/01 1955. [Google Scholar]
- [33].Herberich E, Sikorski J, and Hothorn T, “A Robust Procedure for Comparing Multiple Means under Heteroscedasticity in Unbalanced Designs,” PLoS ONE,vol. 5, p. e9788, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bigelow TA, Xu J, Stessman DJ, Yao L, Spalding MH, and Wang T, “Lysis of Chlamydomonas reinhardtii by high-intensity focused ultrasound as a function of exposure time,” Ultrasonics Sonochemistry,vol. 21, pp. 1258–1264, 2014. [DOI] [PubMed] [Google Scholar]
- [35].Riesberg G, Bigelow TA, Stessman DJ, Spalding MH, Yao L, Wang T, et al. , “Flow rate and duty cycle effects in lysis of Chlamydomonas reinhardtii using high-energy pulsed focused ultrasound,” The Journal of the Acoustical Society of America,vol. 135, pp. [DOI] [PubMed] [Google Scholar]