Abstract
Background:
The incidence of permanent pacemaker (PPM) implantation is higher following mitral valve surgery (MVS) with ablation for atrial fibrillation (AF) compared to MVS alone.
Objectives:
We identified risk factors and outcomes associated with PPM implantation in a randomized trial evaluating ablation for AF in recipients of MVS.
Methods:
Two hundred forty-three patients with AF and without prior PPM were randomly assigned to MVS alone (n=117) or MVS+ablation (n=126). Patients in the ablation group were further randomized to pulmonary vein isolation (PVI; n=62) or biatrial maze (n=64). Using competing risk models, we examined the association between PPM and baseline and operative risk factors, and the effect of PPM on time to discharge, readmissions and 1-year mortality.
Results:
Thirty-five patients received a PPM within the first year (14.4%), 29 (83%) were implanted during the index hospitalization. The frequency of PPM implantation was 7.7% in patients randomized to MVS alone, 16.1% in MVS+PVI, and 25% in MVS+biatrial maze. The indications for PPM were similar among patients having MVS with and without ablation. Ablation, multi-valve surgery, and NYHA Class III/IV were independent risk factors for PPM implantation. Length of stay post-surgery was longer in patients receiving a PPM, but it was not significant when adjusted for randomization assignment (MVS vs. ablation) and age (HR 0.81;95%CI 0.61–1.08; p=0.14). PPM implantation did not increase 30-day readmission rate (HR 1.43;95%CI 0.50–4.05; p=0.50). The need for PPM was associated with a higher risk of 1- year mortality (HR 3.21;95%CI 1.01–10.17; p=0.05) after adjustment for randomization assignment, age and NYHA Class.
Conclusions:
AF ablation, multi-valve surgery and NYHA Class III/IV are associated with an increased risk for permanent pacing. PPM implantation following MVS is associated with a significant increase in 1-year mortality.
Keywords: permanent pacemaker, valvular heart disease, cardiac surgery, biatrial maze, pulmonary vein isolation
Condensed Abstract:
Permanent pacemaker (PPM) implantation is higher following mitral valve surgery (MVS) with ablation for atrial fibrillation (AF) compared to MVS alone. Risk factors and outcomes associated with PPM implantation were examined in a randomized trial evaluating ablation for AF in 243 recipients of MVS. Thirty-five patients received a PPM within the first year (14.4%). The frequency was 7.7% in MVS alone, 16.1% in MVS + pulmonary vein isolation and 25% in MVS+biatrial maze. Ablation, multi-valve surgery, and NYHA Class III/IV were independent risk factors for permanent pacing. PPM implantation was associated with a higher risk of 1-year mortality.
Clinical Trial: ClinicalTrials.gov: NCT00903370.
Introduction
Patients undergoing mitral-valve surgery (MVS) frequently present with atrial fibrillation (AF), which is associated with reduced survival and increased stroke risk (1–3). In a recent randomized trial conducted within the Cardiothoracic Surgical Trials Network (CTSN) (4), 260 patients with persistent or long-standing persistent AF who required MVS underwent either MVS alone or MVS with surgical ablation by pulmonary-vein isolation (PVI) or a biatrial maze procedure. Although significantly more patients were free from AF on 72-hour continuous Holter monitoring at 6 and 12 months in the ablation group than in the control group, ablation was associated with more permanent pacemaker (PPM) implantations (21.5 vs. 8.1 per 100 ptyrs; p=0.01).
A variety of factors may contribute to this observation (5–8). Differences in incidence may be attributed to choice of surgical approach. Almost all current surgical ablation procedures include PVI. But the biatrial maze approach is more complex and requires right and left atriotomies, more time on cardiopulmonary bypass, and the creation of endocardial ablation lesions extending to the mitral and tricuspid annuli. The type of ablation device (i.e., radiofrequency versus cryoablation) may contribute to differences in PPM risk. Additionally, the complexity of the surgical procedure (i.e., MVS alone versus multivalve procedures), as well as patient characteristics (i.e. age, presence of left ventricular dysfunction and other comorbidities), can affect need for PPM implantation. Pacing following cardiac surgery is undertaken for symptomatic bradycardia due to sinus node (SN) dysfunction and/or high grade atrioventricular (AV) block. The simultaneous need for rate control of intermittent atrial tachyarrhythmias in many post-operative patients is an additional consideration.
The goal of this secondary analysis was to identify risk factors leading to placement of a PPM and the association of PPM placement with post-operative length of hospital stay, readmissions, and mortality in patients undergoing MVS with and without concomitant ablation procedure.
Methods
Trial Design
The trial evaluating ablation for AF during mitral valve surgery, sponsored by the National Institutes of Health (NIH) and the Canadian Institute of Health Research, was conducted at 20 centers within the CTSN, and was previously published (4). Briefly, between 2010 and 2013, 260 patients with persistent or long-standing persistent AF with mitral-valve disease requiring surgical intervention were randomized in a 1:1 ratio to surgical ablation (n=133) or to no ablation (n=127) during the mitral-valve operation. Patients in the ablation group were further randomized to PVI (n=67) or biatrial maze (n=66). All patients underwent left atrial appendage management. The primary endpoint was freedom from AF at both 6 and 12 months post-surgery assessed by 72-hour Holter monitor. Based on sample size calculations, a total of 260 patients provided 90% power to detect an absolute increase of 20% (25%−45%) in the proportion of patients free of AF in the ablation arm compared to MVS alone. Additionally, weekly transtelephonic monitoring was collected. Secondary endpoints included death, stroke, heart failure hospitalization, mitral valve re-intervention, need for rhythm-related interventions, quality of life and re-hospitalization.
The study received institutional review board approval at each participating center and all patients gave written informed consent. A data coordinating center, an independent event adjudication committee, and a data and safety monitoring board appointed by the NIH oversaw trial progress. The data coordinating center had full and independent access to all the data and was responsible for analyzing the data. The writing committee wrote the manuscript and vouched for the integrity, accuracy, and completeness of the analysis. The trial was conducted under an investigational device exemption because devices were not all approved for treatment of AF.
Endpoint
The primary outcome for this analysis was the placement of a PPM due to SN dysfunction and/or cardiac conduction abnormalities within 1 year of surgery. Secondary outcomes included post-operative length of index hospital stay, readmissions, and all-cause mortality associated with PPM implantation.
Data Analysis
The data set for the current analysis consisted of 243 of the 260 (93.5%) randomized patients who did not have a PPM at baseline. Fine and Gray’s proportional sub-distribution hazards model (9) with death as a competing risk was used to examine the association between PPM implantation and selected risk factors (Supplemental Table 1). Candidate variables included demographics, comorbidities, baseline medications, ejection fraction, CHA2DS2-VASc score (Congestive heart failure, Hypertension, Age (≥75=2 points), Diabetes mellitus, prior Stroke or transient ischemic attack (2 points), Vascular disease (peripheral arterial disease, previous myocardial infarction, aortic atheroma), Age (65–74=1 point) and Sex category (female gender)), electrocardiogram (EKG) parameters, operative factors, randomization assignment to MVS alone, PVI, or biatrial maze, and whether patients underwent multi-valve surgery. Variables that were significant at the 0.20 level in the univariate regression analysis were considered for the multivariable model. A stepwise backward selection approach was used to identify significant risk factors at the 0.05 level. All models were tested for the proportional hazards assumption. Additionally we ran a separate model that included type of MV surgery (repair or replacement) and whether or not concomitant surgery was performed (aortic valve and/or tricuspid regurgitation valve surgery).
To evaluate the association of PPM with post-operative length of stay of the index hospitalization, the cardiac impulse formation or conduction abnormality adverse event that led to the placement of PPM was treated as a time-dependent variable in a Fine and Gray’s model that had time to hospital discharge from surgery as the endpoint of interest and in-hospital death as a competing risk. Hazard ratios (HR) <1 corresponded to longer length of stay (i.e., there is a lower probability of being discharged early).
Readmissions were analyzed at 30 days and 1 year. Since no patient experienced multiple re-hospitalizations within 30 days of surgery, Fine and Gray’s model was used to assess the effect of time-varying PPM implantation on time to first readmission by 30 days with death as a competing risk. For readmissions that occurred within 1 year of surgery, the Andersen and Gill’s counting processes method (10) was applied to compare readmission rates between patients with and without a PPM. The Andersen-Gill model was chosen to account for the repeated hospitalizations in a patient and the time-varying nature of PPM placement. If a PPM was inserted on the day of admission, the patient was not considered as having a PPM at the time of the admission except in cases where the reason for hospitalization was PPM implantation.
One-year mortality differences between patients with and without a PPM were compared using Cox proportional hazards model with PPM implantation as a time-dependent variable. Models for post-operative length of stay, readmissions, and survival were adjusted for randomization assignment (MVS vs ablation) regardless of its significance. Analyses were performed using SAS version 9.4 (SAS, Cary, North Carolina) and followed the intent-to-treat principle.
Results
Patients
Table 1 depicts the baseline and operative characteristics of patients with and without a PPM. For the entire group, the mean age was 69 years and CHA2DS2-VASc score 3.4 (±1.6); 68% were using beta-blockers or amiodarone at enrollment. Mitral-valve surgery consisted of replacement in 45% of patients and repair in 55% of patients. Concomitant surgeries included coronary artery bypass grafting in 19% of patients, tricuspid valve procedure in 38%, and aortic valve surgery in 13%.
Table 1.
Patient and Operative Characteristics*
| PPM (N = 35) |
No PPM (N = 208) |
Total (N = 243) |
P Value** | |
|---|---|---|---|---|
| Age, years | 70 ± 9.2 | 68.9 ± 10.5 | 69.1 ± 10.3 | 0.56 |
| Female sex | 18 (51.4) | 96 (46.2) | 114 (46.9) | 0.56 |
| Congestive Heart Failure | 16 (45.7) | 97 (46.6) | 113 (46.5) | 0.92 |
| Hypertension | 28 (80.0) | 164 (78.8) | 192 (79.0) | 0.88 |
| Diabetes | 9 (25.7) | 47 (22.6) | 56 (23.0) | 0.69 |
| Previous stroke or TIA | 5 (14.3) | 20 (9.6) | 25 (10.3) | 0.38 |
| Vascular disease† | 11(31.4) | 29 (13.9) | 40 (16.5) | 0.01 |
| CHA2DS2-VASc score | 3.7 ± 1.6 | 3.3 ± 1.6 | 3.4 ± 1.6 | 0.16 |
| Previous cardiac surgery | 5 (14.3) | 21 (10.1) | 26 (10.7) | 0.55 |
| Beta blockers at baseline | 23 (65.7) | 137 (65.9) | 160 (65.8) | 0.99 |
| Amiodarone at baseline | 1 (2.9) | 15 (7.2) | 16 (6.6) | 0.48 |
| NYHA Class III/IV | 23/35 (65.7) | 85/207(41.1) | 108/242 (44.6) | 0.01 |
| Atrial Fibrillation | 0.92 | |||
| Long-standing persistent | 19 (54.3) | 111 (53.4) | 130 (53.5) | |
| Persistent | 16 (45.7) | 97 (46.6) | 113 (46.5) | |
| Cause of mitral-valve disease | 0.24 | |||
| Organic primary MR | 17 (48.6) | 123 (59.1) | 140 (57.6) | |
| Functional, non-ischemic MR | 13 (37.1) | 71 (34.1) | 84 (34.6) | |
| Functional, ischemic MR | 5 (14.3) | 14 (6.7) | 19 (7.8) | |
| Left ventricular ejection fraction, % | 54.7 ± 7.2 | 56.3 ± 7.5 | 56.0 ± 7.5 | 0.30 |
| Preoperative AF (assessed by EKG)‡ | 31/34 (91.2) | 189/208 (90.9) | 220/242 (90.9) | >0.99 |
| Randomization Assignment | 0.01 | |||
| MVS alone | 9 (25.7) | 108 (51.9) | 117(48.1) | |
| MVS + PVI | 10 (28.6) | 52 (25.0) | 62 (25.5) | |
| MVS + Biatrial maze | 16 (45.7) | 48 (23.1) | 64 (26.3) | |
| Mitral-valve surgery§ | 0.05 | |||
| Valve replacement | 21/35 (60.0) | 87/207 (42.0) | 108/242 (44.6) | |
| Valve repair | 14/35 (40.0) | 120/207 (58.0) | 134/242 (55.4) | |
| Concomitant procedure§ | ||||
| Tricuspid valve surgery | 21/35 (60.0) | 71/207 (34.3) | 92/242 (38.0) | 0.004 |
| Aortic valve surgery | 4/35 (11.4) | 27/207 (13.0) | 31/242 (12.8) | >0.99 |
| CABG | 9/35 (25.7) | 38/207 (18.4) | 47/242 (19.4) | 0.31 |
| Other | 7/35 (20.0) | 18/207(8.7) | 25/242 (10.3) | 0.07 |
| Cardiopulmonary bypass time, min | 142.6 ± 56.5 | 140.4 ± 58.8 | 140.7 ± 58.4 | 0.84 |
| Aortic cross-clamp time, min | 99.9 ± 36.8 | 95.2 ± 43.5 | 95.8 ± 42.6 | 0.55 |
Abbreviations: AF, Atrial fibrillation; CABG, coronary artery bypass graft; CHA2DS2-VASc score, Congestive heart failure, Hypertension, Age (≥75=2 points), Diabetes mellitus, prior Stroke or transient ischemic attack (2 points), Vascular disease (peripheral arterial disease, previous myocardial infarction, aortic atheroma), Age (65–74=1 point) and Sex category (female gender); EKG, electrocardiogram; MR, mitral regurgitation; MVS, mitral-valve surgery, NYHA, New York Heart Association; PPM, permanent pacemaker; PVI, pulmonary-vein isolation; TIA, transient ischemic attack
Plus-minus values are means ± SD, categorical values are n (%)
P values based on two independent sample t-test for continuous variables and chi-squared test or Fisher’s exact test for categorical variables
Vascular disease defined as presence of peripheral vascular disease or history of myocardial infarction
To be eligible for study, must have AF documented by direct EKG upon arrival in OR or within 6 months prior to randomization
One patient withdrew consent before index surgery
Distribution, Timing, and Indications for PPM Placement
Of the 243 patients available for analysis, a total of 35 (14.4%) patients had PPM insertion within 1 year of surgery, including 9 (7.7%) patients receiving MVS alone and 26 (20.6%) patients who underwent MVS + ablation. In all, 19 patients received dual chamber pacemakers, 12 patients had single lead pacemakers and 4 patients biventricular devices. Further stratification of the ablation procedure revealed that 10 patients (16.1%) with PVI and 16 patients (25%) with biatrial maze underwent PPM placement. Similar proportions of patients in the PVI and biatrial groups received cryoablation alone (PVI: 33.9%; biatrial maze: 36.5%) and radiofrequency with or without additional cryoablation (PVI: 66.1%; biatrial maze: 63.5%).
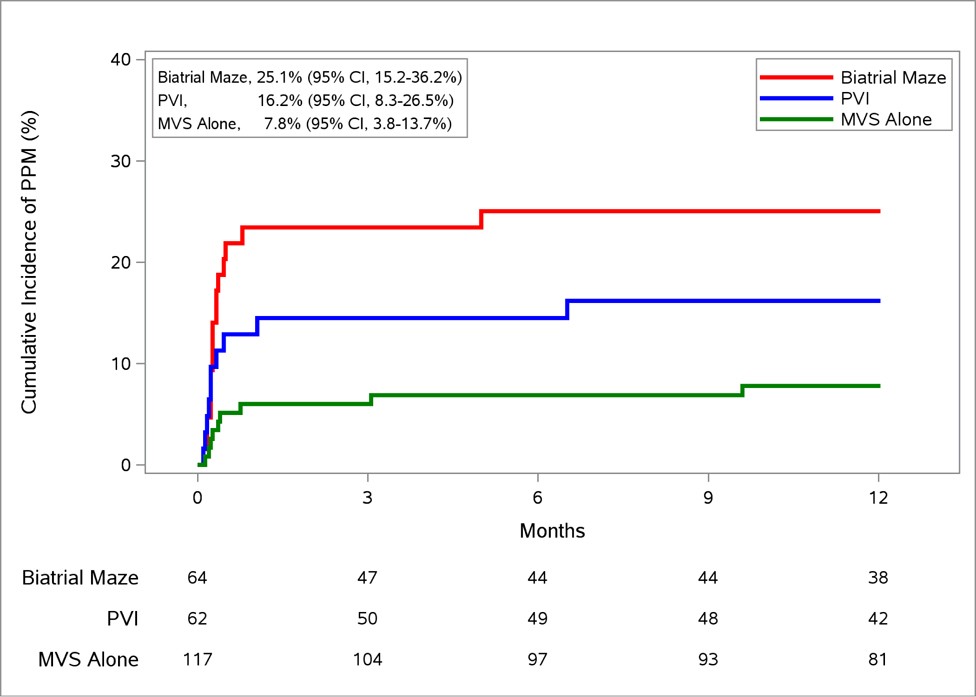
Among the 35 patients who received a PPM, the vast majority (82.9%) underwent implantation during the index hospitalization. Median time to PPM implant was 11 days postoperatively in MVS alone, 7 days in PVI, and 8 days in biatrial maze. The crude cumulative incidence of PPM implantation at 12 months (accounting for competing risk of death) for all patients was 14.5% (95% CI, 10.4–19.3 %). The crude cumulative incidence for the 3 groups was 7.8% (MVS alone), 16.2% (PVI), and 25.1 % (biatrial maze) respectively (Figure 1). Patients were followed for a median duration of 11.5 (interquartile range, 5.7–12.3) months after PPM implantation.
Figure 1. Cumulative Incidence of PPM Placement by Randomization Assignment.
Nonparametric estimates of the cumulative incidence functions for permanent pacemaker implantation with death as a competing risk over 12 months after mitral-valve surgery ± concomitant ablation procedure. Abbreviations: CI, confidence interval; MVS, mitral-valve surgery; PPM, permanent pacemaker; PVI, pulmonary-vein isolation.
The main indications for PPM insertion were high grade AV block in 51% of patients and SN dysfunction in 43%. The distribution in the indication for PPM was similar between the MVS + ablation and MVS alone groups.
Risk Factors for PPM Placement
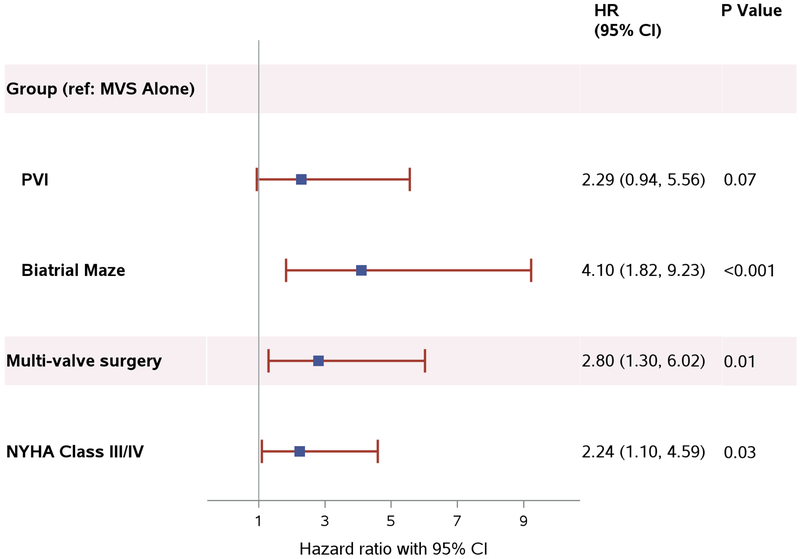
Patient characteristics and operative factors associated with PPM placement were ablation, multi-valve surgery, and New York Heart Association (NYHA) Class III/IV (Figure 2). Both PVI and biatrial maze were associated with an increased incidence of PPM compared to MVS alone, with a hazard ratio of 2.29 (95% CI 0.94–5.56; p=0.07) in the PVI group and 4.10 (95% CI 1.82–9.23; p < 0.001) in the biatrial maze group. There was no significant difference in the incidence of PPM implantation between the two ablation lesion sets (biatrial maze vs. PVI: HR 1.79; 95% CI 0.82–3.92; p=0.15). In a separate analysis, radiofrequency ablation had a higher incidence of PPM than cryoablation alone adjusting for multi-valve surgery and NYHA class but was not statistically significant (HR 1.90; 95% CI 0.78–4.61; p=0.16). Multi-valve surgery involving the aortic and/or tricuspid valve (HR 2.80; 95% CI 1.30–6.02; p=0.01) and severity of heart failure as measured by NYHA Class (HR 2.24; 95% CI 1.10–4.59; p=0.03) were also independent risk factors for PPM implantation. Age and left ventricular ejection fraction were not associated with need for PPM. In a separate model looking at MV replacement versus MV repair (adjusted for concomitant aortic and/or tricuspid valve surgery, randomization assignment, and NYHA Class III/IV), there was no significant difference in the need for PPM with either type of MV procedure.
Figure 2. Patient and Procedure Characteristics Associated with PPM Placement.
Patient and operative risk factors associated with permanent pacemaker placement. The blue square represents the estimated hazard ratio and the red lines extend from the lower limit to the upper limit of the estimated 95% confidence interval. Abbreviations: CI, confidence interval; HR, hazard ratio; MVS, mitral-valve surgery; NYHA, New York Heart Association; PPM, permanent pacemaker; PVI, pulmonary-vein isolation.
Length of Stay and Readmissions
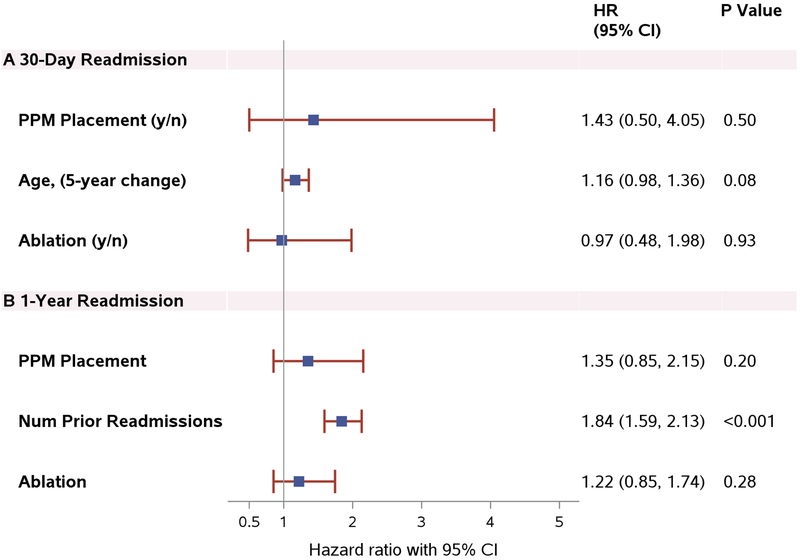
The length of stay during the index hospitalization was slightly longer in patients who underwent PPM placement than in those who did not (HR 0.78; 95% CI 0.59–1.03; p=0.08). When adjusted for age and randomization assignment (MVS vs. ablation), this difference was not statistically significant (HR 0.81; 95% CI 0.61–1.08; p=0.14). A total of 34 patients were readmitted within 30 days of the index surgery. PPM placement was not associated with an increased incidence of 30-day readmission (HR 1.43; 95% CI 0.50–4.05; p=0.50) (Figure 3A). Over 12 months, 79 patients were readmitted for a total of 120 re-hospitalizations. Of the 120 re-admissions, 66 (55%) were cardiovascular in nature. About 40% (n=27) were arrhythmia-related: 17 atrial fibrillation, 2 atrial tachycardia, 2 atrial flutter, 1 sinus tachycardia, and 5 non-specific. Heart failure was the second most frequent reason (27.3%; 18). There were 54 non-cardiovascular re-admissions. The primary reasons were infection (18.5%; 10) and pleural effusion (13%; 7). Causes of cardiovascular-related readmissions in the PPM group included heart failure, atrial fibrillation, and cardiac conduction abnormalities that led to PPM implantation. One patient was admitted for pacemaker reprogramming due to muscle fasciculations. The number of previous readmissions, treated as time-varying, was a strong predictor of subsequent readmissions (HR 1.84; 95% CI 1.59–2.13, p<0.001) (Figure 3B) but no difference in readmission rates was observed between patients with and without a PPM (HR 1.35; 95% CI 0.85–2.15; p=0.20). Gender, NYHA Class, and multi-valve surgery were not associated with 30-day and 1-year readmission. Older age was marginally associated with short-term readmission only (5-year change, HR 1.16; 95% CI 0.98–1.36; p=0.08).
Figure 3. PPM Placement and Readmission.
3A, Impact of PPM Placement, Age and Ablation on 30-Day Readmission. 3B, Impact of PPM Placement, Number of Previous Readmissions and Ablation on 1-Year Readmission. Abbreviations: CI, confidence interval; HR, hazard ratio; PPM, permanent pacemaker.
Mortality
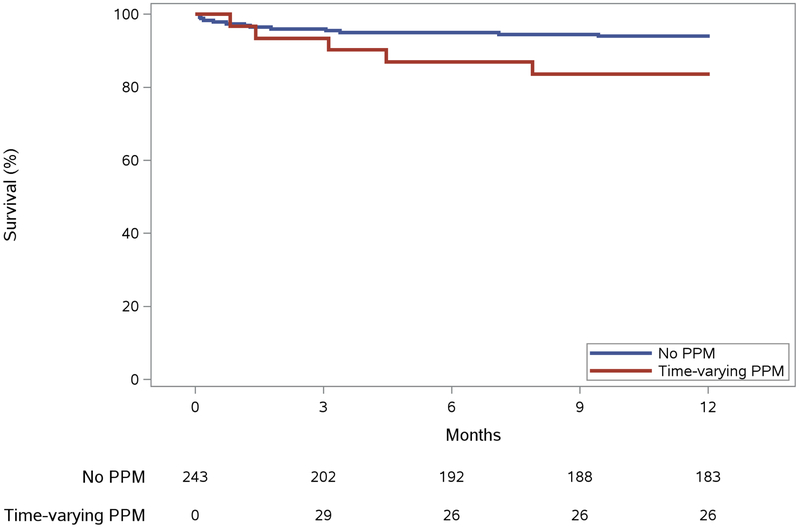
Five (14.3%) deaths were reported in patients with a PPM compared to 13 (6.3%) without a device. The underlying causes of mortality in the PPM group included sepsis (1 patient, related to chemotherapy treatment for a brain tumor); respiratory failure (2), heart failure (1) and unknown causes (1). In patients without PPM, deaths were related to heart failure (3 patients), sepsis (3), bleeding (2), myocardial infarct (1), aspiration pneumonitis (1) and to unknown causes (3). The mean baseline LVEF for patients with PPM who expired was 57.6 ± 3.8 % and 60.8 ± 9.6 % for patients without the device who died. PPM implantation was associated with a deleterious effect on 1-year survival (Central Illustration) (HR 3.21; 95% CI 1.01–10.17; p=0.05) (Table 2) as was NYHA Class III/IV (HR 3.40; 95% CI 1.09–10.56; p=0.03). An increased risk of mortality was observed in older patients (1-year change, HR 1.10; 95% CI 1.03–1.17, p=0.01) but did not differ by gender nor for those receiving multi-valve surgery.
Central Illustration. Permanent Pacemaker (PPM) Placement and Survival.
Extended Kaplan-Meier curve assessing the effect of PPM on survival probability using method by Snappin et al. (12). Median observation time was 1.3 (IQR, 0.4, 3.4) months for patients who died. Abbreviations: PPM, permanent pacemaker.
Table 2.
Impact of PPM Placement, Age, NYHA Class III/IV and Ablation on 1-Year Mortality
| Variable | HR (95% CI) | P Value |
|---|---|---|
| PPM Placement | 3.21 (1.01, 10.17) | 0.05 |
| Age, years | 1.10 (1.03, 1.17) | 0.01 |
| NYHA Class III/IV | 3.40 (1.09, 10.56) | 0.03 |
| Ablation (yes/no) | 0.66 (0.25, 1.77) | 0.41 |
Abbreviations: CI, confidence interval; HR, hazard ratio; NYHA, New York Heart Association; PPM, permanent pacemaker
Late Heart Rhythm among Patients Receiving PPM
Continuous 72-hour Holter monitoring assessment at 12 months was available for 25 (83%) of the 30 living patients who underwent PPM implantation. All patients had appropriate intermittent pacing with 10 (40%) in atrial fibrillation, 12 (48%) in sinus rhythm (2 with first degree AV block and 1 with episodic junctional rhythm) and 3 (12%) in atrial flutter. Twenty nine of the 35 pacemaker patients had weekly transtelephonic monitoring which demonstrated pacing episodes in 76 % (n=22) of the patients.
Discussion
The prevalence of AF in patients referred for MVS is high (5). The effects of strategies to achieve restoration of sinus rhythm on long term morbidity and mortality, including the development of the need for PPM following surgical ablation remain poorly understood because of the paucity of data from adequately-powered prospective trials which include uniform enrollment, lesion sets and rigorous rhythm follow-up.
The trial conducted by the CTSN comparing MVS alone to MVS plus ablation employed standard lesion sets and strict rhythm monitoring procedures, including weekly trans-telephonic monitoring and 6 and 12 month Holter monitoring. Only patients with persistent/long-standing persistent AF were enrolled in this study; paroxysmal AF was an exclusion criterion for randomization. The one-year freedom from AF (the primary endpoint) was 63.2% in the ablation group compared to 29.4% in the MVS only group (p<0.001) (2). However, the one-year rate of PPM implantation was significantly higher in the ablation group than in the MVS group (21.5 vs. 8.1 per 100 pt-yrs; p=0.01).
The incidence of PPM implantation in this study was significantly higher than the 5–10% rate seen in most observational series (5). The relatively older age of our cohort, the large number of multi-valve operations (>45%), the inclusion of patients with only persistent/longstanding persistent atrial fibrillation in our study and rigorous prospective data collection within the context of a randomized trial are all potential causes for the higher PPM rate relative to that seen in other studies. Age was not a predictor of the need for PPM. However, multivalve surgery, biatrial maze and NYHA Class were strong predictors of PPM implantation. In a retrospective review of 305 patients undergoing ablation during MVS, Soni and colleagues reported a 16.5% incidence of PPM in patients undergoing biatrial maze compared to 7.5% in patients undergoing left sided ablation only (11). The strongest predictors for PPM in their study were biatrial maze and multi-valve surgery. Churyla and colleagues reported a retrospective series of 724 patients from Northwestern undergoing either biatrial ablation (n=257) or left atrial ablation only (n=359) during MVS (6). In the unmatched groups, there was a significantly higher incidence of persistent versus paroxysmal AF. In these unmatched cohorts, biatrial ablation resulted in a statistically significant difference in post-operative pacemaker rate of 13% compared to 7% for left atrial ablation only. However, when the groups were propensity matched across 17 variables (n=147), the rates of post-operative PPM were similar between the groups (biatrial 10% versus left atrial 12%). In another retrospective series of 340 patients from Washington University undergoing ablation for either lone atrial fibrillation (n=112) or during other cardiac operations (n=228), age was the only variable associated with an increased risk for PPM (7). However, the combination of paroxysmal and persistent AF patients together with the heterogeneity of the other cardiac operations performed makes it difficult to compare results with the current study.
The relatively higher rate of PPM in this trial as compared to previous studies may raise the question of whether permanent pacing was necessary in all patients. We found evidence of intermittent pacing on all 12-month Holter tracings. Of the surviving patients with a PPM who remained in AF or atrial flutter at 12 months (52%) all exhibited pauses requiring intermittent pacing. Similarly, the patients in sinus rhythm with a PPM exhibited both SN and AV nodal dysfunction requiring pacing as detected on 12-month Holter examination. It can be assumed that the initial indications for PPM placement after surgery were accurate and that most, if not all, patients warranted long-term pacing.
The indication for PPM was similar among MVS alone and MVS + ablation patients in this study. AV block or a combination of AV block and SN dysfunction was present in 51% of patients; isolated SN dysfunction was observed in 43% of patients. The high rate of multivalve surgeries and the relative older age of our cohort are likely causes of the relative incidence of post-operative AV block as the cause of PPM and are consistent with rates in similar recent retrospective cohorts (11). In the Washington University series, SN dysfunction was the predominant indication for PPM insertion (68%). Only 31% of their patients had either isolated AV node dysfunction (20%) or combined sino-atrial (SA) + AV node dysfunction (11%) as the indication for PPM. The large numbers of patients undergoing ablation for lone atrial fibrillation and the heterogeneity of combined operation and underlying type of AF may again explain these results.
Our study revealed that the need for a post-operative PPM resulted in no untoward consequences on either readmission rate or post-operative length of stay. Prior readmission was the most powerful predictor of subsequent readmissions through 1 year and this observation is consistent with several previous surgical studies across different specialties. Although the postoperative length of stay of patients without PPM was shorter than those with a PPM, when adjusted for age and randomization this was not statistically significant. It is difficult to make conclusions about this finding as post-operative length of stay may have numerous subjective cofounders that are not accounted for in our analysis and that the number of patients with a PPM inserted was small.
PPM insertion has consequences. In other cardiac pathologies, chronic right ventricular pacing is associated with increased mortality. The specific situation of PPM placement after ablation during MVS has also been implicated as an independent predictor of short-term mortality (8). In our study, PPM and NYHA Class were strong predictors of 1-year mortality. However, the number of deaths was small so findings should be interpreted cautiously. Most of our patients who received VVI pacemakers had a high burden of RV pacing but it remains unclear if chronic right ventricular pacing over the ensuing post-operative year is the etiology of increased mortality or if PPM is merely a marker of a sicker patient cohort. Specifically if these patients required more extensive surgery, were higher NYHA Class, or had greater use of antiarrhythmic drugs.
Limitations of study
Although our analysis of the risks of PPM is based on a rigorous randomized trial of the effectiveness of AF ablation during MVS, there are some limitations to the current study. First, our patient population included only patients with persistent or long-standing persistent AF and the results may not be generalizable to patients with paroxysmal AF. Second, the patient cohort included primarily older patients, with a large proportion of patients undergoing mitral valve replacement. The results may not be generalizable to younger patient cohorts undergoing mitral valve repair.
Conclusions
In summary, in this prospective randomized trial a more extensive ablation lesion set, multi-valve surgery and NYHA Class III/IV all increased the risk of the need for PPM implantation following MVS. The impact of PPM on short-term mortality makes it important to weigh the risk of requiring a PPM after MVS + ablation as part of pre-procedural planning and to tailor the ablation strategy to the individual patient.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care and Procedural Skills:
Patients with long-standing, persistent atrial fibrillation undergoing ablation in conjunction with mitral valve surgery are more likely to require pacemaker implantation, particularly when patients have heart failure or the procedures involve bi-atrial maze or multivalve surgery.
Translational Outlook:
Additional research is needed to determine whether modifications of surgical or ablation techniques might reduce the need for pacemaker implantation and improve long-term outcomes in patients with atrial fibrillation undergoing mitral valve surgery.
Funding:
The AF ablation trial was supported by a cooperative agreement (U01 HL088942) funded by the National Heart Lung and Blood Institute, the National Institutes of Neurological Disorders and Stroke of the National Institutes of Health (NIH), Bethesda, MD, and the Canadian Institutes for Health Research (CIHR), Ottawa, ON, Canada. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services. Note: This trial was designed to evaluate ablation as a therapeutic approach, not to support FDA-approval for any individual device.
Abbreviations
- AF
Atrial fibrillation
- EKG
Electrocardiogram
- MVS
Mitral-valve surgery
- NIH
National Institutes of Health
- NYHA
New York Heart Association
- PPM
Permanent pacemaker
- PVI
Pulmonary vein isolation
- SN
Sinus node
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Gillinov reports: consulting for Edwards Lifesciences, Medtronic, AtriCure, Abbott, CryolLife, ClearFlow; royalties and equity rights for ClearFlow; institutional royalties for AtriCure. Dr. Mack reports: Co-PI relationship with Edwards Lifesciences and Abbott Vascular; Executive Board member for Medtronic, all uncompensated. All other authors have nothing to disclose.
REFERENCES
- 1.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018–22. [DOI] [PubMed] [Google Scholar]
- 2.Gillinov AM, Saltman AE. Ablation of atrial fibrillation with concomitant cardiac surgery. Semin Thorac Cardiovasc Surg. 2007;19:25–32. [DOI] [PubMed] [Google Scholar]
- 3.Lee R, McCarthy PM, Wang EC, et al. Midterm survival in patients treated for atrial fibrillation: a propensity-matched comparison to patients without a history of atrial fibrillation. J Thorac Cardiovasc Surg. 2012;143:1341–51. [DOI] [PubMed] [Google Scholar]
- 4.Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015;372:1399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2008;85:909–14. [DOI] [PubMed] [Google Scholar]
- 6.Churyla A, Iddriss A, Andrei AC, et al. Biatrial or left atrial lesion set for ablation during mitral valve surgery: Risks and benefits. Ann Thorac Surg. 2017;103:1858–65. [DOI] [PubMed] [Google Scholar]
- 7.Robertson JO, Cuculich PS, Saint LL, et al. Predictors and risk of pacemaker implantation after the Cox-maze IV procedure. Ann Thorac Surg. 2013;95:2015–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worku B, Pak SW, Cheema F, et al. Incidence and predictors of pacemaker placement after surgical ablation for atrial fibrillation. Ann Thorac Surg. 2011;92:2085–89. [DOI] [PubMed] [Google Scholar]
- 9.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496–509. [Google Scholar]
- 10.Andersen PK, Gill RD. Cox’s regression model for counting processes: A large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 11.Soni LK, Cedola SR, Cogan J, et al. Right atrial lesions do not improve the efficacy of a complete left atrial lesion set in the surgical treatment of atrial fibrillation, but they do increase procedural morbidity. J Thorac Cardiovasc Surg. 2013;145:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snappin SM, Jiang Q, Iglewitz B. Illustrating the impact of time varying covariate with an extended Kaplan-Meier estimator. The American Statistician. 2005;59:301–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.