Abstract
Harnessing the endogenous immune system to eliminate malignant cells has long been an intriguing approach. After considerable success in the treatment of B-cell acute lymphoblastic leukemia, chimeric antigen receptor (CAR)-modified T cells have entered early clinical evaluation in the field of multiple myeloma (MM). The choice of suitable non-CD19 target antigens is challenging and a variety of myeloma-associated surface molecules have been under preclinical investigation. Most recent clinical protocols have focused on targeting B-cell maturation antigen (BCMA), and early results are promising. The trials differ in receptor constructs, patient selection, dosing strategies and conditioning chemotherapy and will thus pave the way to eventually define the optimal parameters. Other sources for autologous T-cell therapy of MM include affinity-enhanced T-cell receptor-modified cells and marrow infiltrating lymphocytes. In summary, adoptive T-cell transfer for the treatment of MM is still in its infancy, but if early response rates indicate durability, will be a paradigm changing therapeutic modality for the treatment of MM.
Keywords: multiple myeloma, immunotherapy, T-lymphocytes, chimeric antigen receptor, B-cell maturation antigen, BCMA, adoptive transfer
Introduction
Multiple myeloma (MM) is the second most commonly diagnosed hematologic malignancy. Despite considerable improvement in therapeutic approaches, it still remains an incurable disease in most cases [1, 2]. Recent treatment strategies are increasingly focusing on a redirection of the immune system to harness the intrinsic immune defense against the disease [3]. The prime example of immunotherapy is allogeneic hematopoietic stem cell transplantation (alloHSCT) with its desired graft-versus-myeloma effect. While alloHSCT and donor lymphocyte infusions have demonstrated the curative potential that can be mediated by T-cell anti-myeloma immunity [4], transplant related mortality and graft-versus-host disease limit their clinical application for MM [5, 6]. Therefore, other concepts to exploit the anti-myeloma capacities of autologous lymphocytes are desirable. Here, we discuss the three major concepts for adoptive transfer of T cells with reactivity against MM: chimeric antigen receptors, affinity-enhanced T-cell receptors, and marrow infiltrating lymphocytes.
Chimeric antigen receptors
In order to recognize a specific tumor-associated surface antigen, T cells can be redirected by genetic modification to express a chimeric antigen receptor (CAR). In 2017, the FDA granted approval of CD19-directed CAR-T cells for young patients with relapsed/refractory B-cell acute lymphoblastic leukemia and for adults with certain types of relapsed/refractory aggressive non-Hodgkin lymphomas. The success stories from CAR T cell therapy for CD19-positive B-cell malignancies [7–10] have attracted enormous attention to the field and currently, a large variety of alternative target antigens for various types of cancer are under investigation.
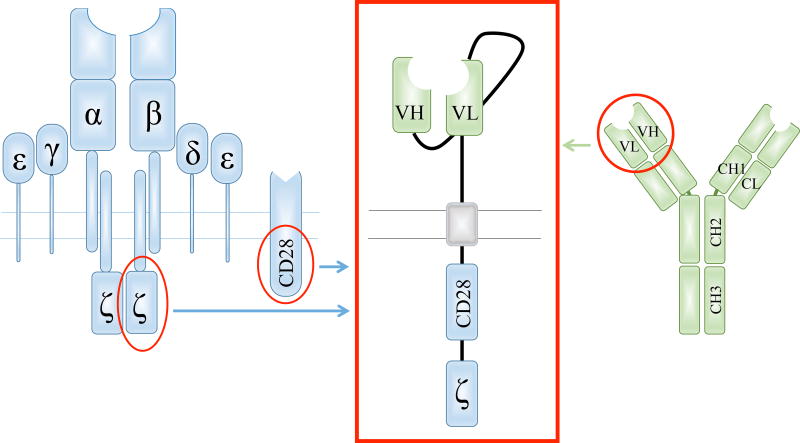
The basic property of the CAR construct is to establish cell surface antigen-specific binding to a target cell and thereon initiate supra-physiologic T-cell activation in an HLA-independent manner. For this purpose, the CAR consists of an extracellular targeting moiety and intracellular signaling domains [11, 12]. For antigen binding, most commonly, single-chain variable fragments (scFv) derived from murine immunoglobulins or human antibody libraries are used (Figure 1). The scFv is connected to the intracellular moiety by an extracellular spacer and a transmembrane domain, often derived from IgG4 and CD8, respectively. Historically, the intracellular component of the CAR consisted solely of the CD3ζ signaling domain of the T-cell receptor [11]. The failure of the CAR-T cells to proliferate led to the development of dual-signaling receptors to provide the cells with a co-stimulatory signal [13]. These second generation CARs most commonly contain co-stimulatory domains derived from either CD28 or 4-1BB in addition to CD3ζ (Figure 1). Whether combination of multiple co-stimulatory domains in so-called third generation CARs can convey improved antitumor efficacy in a clinical context is yet to be determined.
Figure 1. Schematic composition of a second generation chimeric antigen receptor.
The extracellular domain of the chimeric antigen receptor (CAR, red box) contains a single-chain variable fragment (scFv), most commonly derived from the heavy (VH) and light (VL) chain variable fragments of a monoclonal antibody (green). The components of the CAR are connected by an extracellular spacer and a transmembrane domain. The intracellular moiety comprises the CD3ζ signaling domain of the T-cell receptor (blue) and, in a second generation CAR, a co-stimulatory domain derived from a co-receptor like CD28 (blue).
CAR-T cell associated toxicities
Apart from the toxicities of pre-conditioning regimens for lymphodepletion prior to adoptive transfer, a new complex of side effects has been observed in CAR-T cell trials. This includes cytokine release syndrome (CRS), resulting from the excessive release of cytokines after T-cell activation, neurologic toxicities, or “on-target, off-tumor” targeted destruction of healthy tissue [12].
CRS can range from flu-like symptoms to hypotension, capillary leak, coagulopathy and organ dysfunction [14]. Besides supportive measures like fluid replacement and vasopressor support, the specific IL-6 receptor blockade by tocilizumab has proven an extremely effective treatment strategy at rapidly reversing toxicity; potentially without compromising CAR-T cell efficiency. Approaches to prevent the development of CRS in the first place by modification of T-cell composition and dose reduction have also been successful in some trials [10].
Most neurologic adverse events are observed during or after severe CRS. Clinical presentations range from mild confusion to seizures or coma requiring ventilatory support [15]. Pathophysiological correlates of severe neurotoxicity appear to be high cytokine levels in the cerebrospinal fluid and increased permeability of the blood-brain barrier [16]. Unlike for CRS, the effect of tocilizumab on neurologic sequelae is so far unclear and alternative treatment strategies are under development.
“On-target, off-tumor” toxicity is highly dependent on the chosen antigen. One of the best examples is the B-cell aplasia associated with CD19 CAR-T cell treatment [14], but while this side effect can be addressed by immunoglobulin replacement, damage to more essential tissue types can be fatal. Reportedly, targeting her2 in a patient with advanced colonic cancer resulted in severe pulmonary edema and cardiac arrest due to low-level antigen expression in the heart and lung vasculature [17]. The fact that CAR-T cells require extremely low antigen densities for activation limits the transferability of safety observations from monoclonal antibody therapies.
CAR targets
For safe and efficient integration of CAR-T cell therapy into clinical practice, the choice of a suitable target antigen is crucial [18]. As the antigen recognition of the CAR is independent from HLA-molecules, the antigen must be expressed on the surface of the malignant cells. Homogeneous expression in the tumor cell population is required to prevent the outgrowth of antigen deficient clones (antigen escape). To reduce the risk of antigen downregulation, the surface molecule ideally should play a relevant role in the survival of the malignant cells. Most importantly, the target antigen must not be expressed on essential healthy tissues to avoid the development of potentially fatal on-target, off-tumor toxicities [17]. As MM does not belong to the group of B-cell malignancies with uniform CD19 surface expression, a variety of alternative targets for CAR T-cell therapy are currently under pre-clinical (Table 1) and clinical investigation (Table 1, Table 2).
Table 1.
Expression of potential target antigens for CAR-T cell therapy of multiple myeloma
| Surface antigen |
Expression in multiple myeloma (% of cases) |
Expression in normal hematopoietic cells |
Known expression in normal solid organ tissues |
References |
|---|---|---|---|---|
| CD70 | 0,2 – 42% | activated T and B cells, dendritic cells, plasma cells | [17, 18, 20] | |
| CD56 | 78%, reduced in extramedullary disease | T and NK cells | neuronal cells | [21, 22] |
| CD44v6 | 43% in advanced stage | activated T cells, monocytes | keratinocytes | [27] |
| SLAMF7 | high and uniform expression | T, B and NK cells, macrophages, dendritic cells, plasma cells | [30, 32] | |
| CD38 | high and uniform expression | activated T cells, NK cells, plasma cells, early B cells, immature myeloid and erythroid cells, thymocytes | prostatic epithelium, pancreatic islet cells, cerebellar Purkinje cells | [35–37] |
| CD138 | high expression | plasma cells | liver, skin and glandular epithelial cells | [45, 47] |
| κ light chain | expression on κ-restricted disease propagating cells | mature B cells | [77] | |
| CD19 | low expression in most cases; potentially mostly on disease propagating cells | B cells | [51] | |
| BCMA | 60 – 100% | mature B cells, plasma cells | [53, 78] |
Abbreviations: NK – natural killer; SLAMF7 - signaling lymphocytic activation molecule family member 7; BCMA – B cell maturation antigen
Table 2.
Selection of ongoing and completed clinical trials with CAR-T cells for the treatment of multiple myeloma
| antigen | trial site | scFv | signaling domains |
safety gene |
gene transfer |
conditioning therapy |
T-cell dose | patients treated |
reported responses |
clinical trial identifier, ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| CD138 | PLA General Hospital Beijing | NK-92 | 4-1BB/CD3ζ | - | lentiviral | various | 0.44×106-3.78×106 CAR+ T cells/kg | 5 | SD (4 pts) | NCT01886976 [44] |
| κ light chain | Baylor College of Medicine | CRL-1758 | CD28/ CD3ζ | - | gamma-retroviral | Cy 12.5 mg/kg | 2×107; 1×108; 2×108 CAR+ T cells/m2 | 7 | SD (4 pts) | NCT00881920 [49] |
| CD19 | University of Pennsylvania | FMC63 | 4-1BB/CD3ζ | - | lentiviral | Mel 140–200 mg/m2 +HSCT, AT d12-14 | 1–5×107 CAR+ T cells | 10 | longer PFS than after 1st ASCT in 2 pts | NCT02135406 [50,79] |
| BCMA | National Cancer Institute | 11D5-3 | CD28/ CD3ζ | - | gamma-retroviral | Cy 300 mg/m2 ×3 + Flu 30 mg/m2 ×3 | 0.3–9.0×106 CAR+ T cells/kg | 26 | sCR (2 pts), VGPR (9), PR (4), SD (10), PD (1) | NCT02215967 [55] |
| BCMA | Bluebird Bio, multicenter | NR, murine | 4-1BB/CD3ζ | - | lentiviral | Cy 300 mg/m2 ×3 + Flu 30 mg/m2 ×3 | 5, 15, 45 and 80×107 CAR+ T cells | 33 | 21 pts evaluable (escalation phase): CR (10 pts), VGPR (6), PR (2), SD (1), PD (2) | NCT02658929 [56] |
| BCMA | University of Pennsylvania | NR, human | 4-1BB/CD3ζ | - | lentiviral | none or Cy 1500 mg/m2 | 1–5×107 or 1–5×108 CAR+ T cells | 24 | CR (2 pts), VGPR (3), PR (6), MR (5), SD (5), PD (3) | NCT02546167 [57] |
| BCMA | Nanjing Legend Biotech | NR | NR | - | lentiviral | Cy 300 mg/m2 ×3 | 1.5–7.0×106 CAR+ T cells/kg | 35 | CR (15 pts), VGPR (13), PR (7) | NCT03090659 [59] |
| BCMA | Memorial Sloan Kettering Cancer Center | NR, human | 4-1BB/CD3ζ | EGFRt | gamma-retroviral | Cy 3000 mg/m2 or Cy 300 mg/m2 ×3 + Flu 30 mg/m2 ×3 | 1×106 CAR+ T cells/kg; 15, 45 and 80×107 CAR+ T cells | 6 | 4 pts evaluable: VGPR (2), PR (1), SD (1) | NCT03070327 [58] |
| BCMA/ CD19 | First Affiliated Hospital, Soochow Univ. | NR | CD28/OX40 /CD3ζ | - | lentiviral | Cy 300 mg/m2 ×3 + Flu 30 mg/m2 ×3 | 5–50×106 CAR+ T cells/kg | 10 | CR (2), VGPR (1), PR (6), MR (1) | NCT03196414 [81] |
Abbreviations: SD - stable disease; Cy - cyclophosphamide; Mel - melphalan; HSCT - hematopoietic stem cell transplantation; AT - adoptive transfer; PFS - progression free survival; Flu - fludarabine; VGPR - very good partial response; PR - partial response; NR - not reported; F/U -follow-up; MR - minor response, EGFRt – truncated epidermal growth factor receptor.
CAR targets in pre-clinical evaluation for multiple myeloma
CD70
One of the first CARs engineered to target MM was directed against CD70, a ligand of the co-stimulatory receptor CD27 that is transiently expressed on T, B, and dendritic cells and regulates proliferation and maturation [19]. It also plays a role in the induction of plasma cell differentiation and aberrant expression was found in carcinomas and hematologic malignancies including MM [20]. Shaffer et al engineered CD70-directed CAR-T cells with an antigen-binging domain derived from CD27 [21]. Therewith, CAR-recognition of the target antigen not only resulted in specific lysis of CD70-positive tumor cell lines, but also conveyed CD27 co-stimulation resulting in improved T-cell survival. However, data on elimination of primary myeloma cells have not been presented so far. As more recent flow cytometry analyses demonstrated low and infrequent CD70 expression in a cohort of 101 myeloma patients [22], the suitability of CD70 for targeted therapy of MM remains questionable.
CD56
The neural cell adhesion molecule CD56 (NCAM1, Leu-19) is expressed on a variety of neuronal and immune cells with expression on natural killer cells [23]. Albeit absent on normal plasma cells, CD56 is frequently expressed in MM [24]. Five years ago, Benjamin et al showed potent anti-myeloma function for CD56-directed CAR-T cells in vitro and in a systemic xenograft model and demonstrated considerable T-cell persistence in the animals [25]. Given that CD56 expression is potentially located on neuronal, as well as myocardial tissue [26], particular caution is required for a potential clinical translation of CD56-directed CAR-T cells.
CD44v6
CD44 glycoproteins were first identified on the surface of human leukocytes and are now known to be encoded by a highly conserved gene which is expressed in most vertebrate cells [27]. Due to alternative splicing and post-transcriptional modifications, the heterogeneity of the proteins is considerable. The CD44 isoform variant 6 (CD44v6) is expressed on monocytes and epithelia including keratinocytes [28]. Its expression on MM was found to be associated with adverse prognosis [29]. Casucci et al generated a CD44v6-directed CAR derived from the monoclonal antibody bivatuzumab and demonstrated elimination of myeloma cells and monocytes, while normal CD44v6-low expressing keratinocytes were spared [30]. Conversely, the clinical development of a bivatuzumab based radio-immunoconjugate was discontinued due to excess skin-related adverse events [31]. Therefore, Casucci et al integrated a suicide gene to allow for pharmacological ablation of the CAR-T cells to reverse possible toxicities such as skin damage. In aggregate, these data provide the basis for careful clinical evaluation of CD44v6-directed CAR-T cells [30].
SLAMF7
With the successful clinical introduction of elotuzumab and daratumumab, two other potential target antigens for CARs against MM have moved to the focus of interest. The elotuzumab target SLAMF7 (CD319, CS1, CRACC), a member of the signaling lymphocytic activation molecule (SLAM) family of transmembrane receptors, has first been described in natural killer cells [32]. SLAMF7 expression has also been documented in a proportion of T cells, B cells, macrophages and dendritic cells [33], where it mediates activating or suppressive functions. High SLAMF7 expression was found on normal and malignant plasma cells [34] which led to development and clinical introduction [35] of elotuzumab. In-depth immunohistochemistry-based evaluation has not revealed SLAMF7 expression on hematopoietic stem cells and solid organ tissues. Development of an elotuzumab-derived scFv resulted in successful generation of SLAMF7-directed CAR-T cells with considerable antimyeloma function [36]. Fratricide of other SLAMF7 expressing lymphocytes did not prevent the outgrowth of SLAMF7 negative virus specific T cells with preserved in vitro functionality [36]. Provided clinical confirmation of safety, SLAMF7 CAR-T cells have the potential to improve myeloma treatment options significantly.
CD38
CD38 is a type II transmembrane glycoprotein that was first identified on the surface of T cells as “intermediate” to “late” activation marker [37]. Moreover, CD38 is expressed on thymocytes [37], natural killer cells and monocytes, immature myeloid and erythroid bone marrow (BM) cells and plasma cells [38]. CD38 is further expressed on prostatic epithelium, pancreatic islet cells and cerebellar Purkinje cells [39]. Plasma cell dyscrasias demonstrate strong CD38 expression [38]. However, the expression levels seem to decrease during the evolution of the disease and complete antigen loss has been observed in extramedullary myeloma [40]. By contrast, possible disease propagating myeloma cells might be CD38 positive [41]. Daratumumab, the first approved anti-CD38 monoclonal antibody has demonstrated single-agent antimyeloma reactivity and a favorable safety profile [42]. Mihara et al were the first to generate CD38-directed CAR-T cells with potent anti-myeloma function in preclinical models [43]. Drent et al confirmed antimyeloma reactivity of CD38-directed CAR-T cells that developed a CD38 negative phenotype during cell culture but preserved their T-cell effector functions [44]. To reduce potential on-target, off-tumor toxicity, Drent et al performed modifications of the antigen-binding domain to lower its affinity to the target antigen such that low CD38 positive (normal) cells were spared whereas high CD38 antigen density myeloma cells were eliminated [45].These data indicate considerable potential for CD38 as target antigen in myeloma therapy.
CARs in clinical trials for multiple myeloma
CD138
The first clinical data of CAR-T cells for the treatment of MM were reported by Guo et al from the PLA General Hospital in Beijing [46], after 5 individuals had received 4-1BB co-stimulated CD138-directed CAR-T cells (NCT01886976). CD138 (Syndecan1) is an adhesion molecule expressed on the majority of normal and malignant plasma cells [47] and has been associated with negative prognosis [48]. However, CD138 is also expressed on a variety of epithelial cells [49]. In their first-in-human CD138 CAR-T cell trial, Guo et al observed post-infusional fever in four out of five patients and moderate elevation of liver enzymes in one patient, but no other non-hematologic toxicities [44]. CAR transgenes were detectable for more than 80 days, four out of five patients achieved stable disease for up to 7 months and one patient with plasma cell leukemia showed a reduction in circulating plasma cells to less than 30% from baseline persisting for 12 weeks. With the limited data reported, conclusions from this study should be drawn carefully. It is noteworthy, however, that the CAR-T cells used in the study displayed relatively low capacity of homing to myeloma lesions, despite anti-myeloma reactivity in vitro. Whether this was due to intrinsic functional defects of the CAR-T cells or increased susceptibility to inhibitory signals remains unclear. It yet resulted in limited therapeutic efficacy in the BM niche and absent epithelial toxicity. Due to the potential on-target, off-tumor effects of CD138-directed treatment approaches, it would be advisable to include suicide genes as safety switch.
κ light chain
Inspired by the effects of CD19-directed CAR-T cells, the Baylor group engineered an anti-κ light chain (LC) CAR to prevent complete B-cell depletion [50]. Preclinical evidence for the surface expression of immunoglobulins in disease-propagating myeloma cells encouraged the investigators to open their phase I basket trial for myeloma patients with κ-restricted disease (NCT00881920) [51]. Seven myeloma patients thus received κ LC-directed CAR-T cells comprising a CD28 co-stimulatory domain. Good tolerability was reported despite CAR-T cell doses exceeding 108 cells in all individuals. No CRS occurred, despite the potential of soluble immunoglobulin to induce cytokine production in vitro [50]. CAR transgenes in the peripheral blood were detectable at 6 weeks after adoptive transfer, but lost at 6 months, while BM analyses were not routinely performed. The anti-myeloma effect was moderate with stable disease in 4 out of 7 patients for up to 24 months. Conceptually, one restriction to targeting the involved LC by CAR-T cells in MM is that LC is generally secreted, and not retained as CAR target on the surface of myeloma cells, thus, this approach might be more suitable for the treatment of lymphoma [51].
CD19
Another pioneering CAR-T cell study in MM influenced from the success in B-cell malignancies was conducted at the University of Pennsylvania to evaluate 4-1BB co-stimulated CD19-directed CAR-T cells in the post-transplant setting (NCT02135406) [52]. Despite the absence of CD19 on the majority of plasma cells, extensive research on putative “myeloma stem cells” identified CD19 as potentially expressed by disease-propagating clones [53]. To reduce the burden of predominantly CD19-negative myeloma cells, ten patients with relapsed disease post-prior autologous hematopoietic stem cell transplantation (autoHSCT) received a second autoHSCT and subsequent CD19-targeted CAR-T cells. No higher grade CRS was apparent despite treatment with the target dose of 5*107 CAR-positive cells in all cases. Transgene-positive T cells persisted for a median of 6 weeks and were detectable in the BM in 9 out of 10 patients. Most patients had progressed within one year after their initial transplant. Progression free survival resulting from the salvage treatment exceeded that of the first transplant, a so-called “remission inversion”, in two of ten patients, although all went on to ultimately relapse.
BCMA
Over the last 5 years, our understanding of B-cell maturation antigen (BCMA, CD296) has evolved to place it in the spotlight for targeted cell therapy of MM. BCMA belongs to the tumor necrosis factor receptor superfamily and is expressed on the surface of mature B cells and plasma cells including MM [54] but absent from other normal tissues [55]. The first BCMA-directed CAR was generated by Carpenter et al [55] and consisted of a scFv derived from a murine anti-human BCMA antibody and a cytoplasmic CD28 co-stimulatory domain. After demonstration of potent anti-myeloma efficacy in preclinical models [55], Ali et al initiated a first-in-human dose-escalation trial at the National Cancer Institute in 2014 (NCT02215967) [56]. The investigators recently [57] reported on 26 myeloma patients who received treatment with cyclophosphamide/fludarabine for lymphodepletion before BCMA-directed CAR-T cells. Noteworthy, CAR-T cell production was successful in these patients despite a median of 10 prior lines of therapy. Dose escalation to 9×106 CAR-T cells/kg led to objective responses in 13 out of 16 patients including an ongoing stringent complete remission (CR) for 37 weeks. Response to the treatment was associated with the highest numbers of CAR-T cells in the peripheral blood, but also grade 3/4 toxicities including CRS and delirium. As high disease burden in the BM seemed to correlate with grade 3/4 CRS, enrollment of the last 14 individuals was restricted to patients with BM infiltration of 30% or less which led to reduced incidence of severe CRS.
Currently, further BCMA CAR-T cell studies are ongoing. At the last interim analysis, Bluebird Bio reported data (NCT02658929) [58] on a cohort of 21 heavily pre-treated myeloma patients who received a 4-1BB co-stimulated BCMA-directed CAR-T cell product (bb2121) across nine sites in the US. While the dose of 50×106 CAR-T cells did not produce significant efficacy in 3/3 patients, dose escalation to 150–800×106 CAR-T resulted in an overall response rate of 94% including 10/18 patients in CR. Within a median follow-up period of 40 weeks, four patients have progressed so far. Grade 3/4 adverse events included CRS in two patients, tocilizumab use in 4, but no dose-limiting toxicities. A separate dose expansion trial investigating doses between 150–300×106 CAR-T cells is ongoing. Cohen et al presented updated interim data from a BCMA CAR-T cell trial conducted at the University of Pennsylvania (NCT02546167) [59]. The first cohort (nine patients) received split-dose infusions of 100–500×106 CAR-T cells over three days without prior preconditioning, whereas cohort 2 (five patients) and cohort 3 (ten patients) had been treated with 1.5g/m² cyclophosphamide three days prior to 10–50×106 and 100–500×106 CAR T cells, respectively. Four patients did not receive the target dose, as their third infusion was held for fever or CRS. Despite this dose reduction and lack of conditioning, massive CAR-T cell expansion correlated with robust anti-myeloma efficacy in one patient who achieved a stringent complete response which has been ongoing for 24 months. Overall, 11 patients out of 24 achieved a partial response (PR) or better including six out of ten patients in cohort 3. Patients achieving an objective response demonstrated higher peak CAR-T cell levels and pre-conditioning cyclophosphamide boosted peak CAR-T cell expansion. Notably, in five of six patients with better or equal to a PR, where residual MM cells were available for evaluation, a reduction of BCMA antigen density was discovered, pointing to antigen down-regulation or the outgrowth of an antigen low clone as potential sources of relapse. Data on the first two dose level cohorts were reported for a phase I trial using BCMA-targeted CAR-T cell therapy at Memorial Sloan Kettering (MSK). In view of potential CAR-T cell associated toxicities, the integration of a truncated epidermal growth factor receptor (EGFR) surface marker that can possibly be used as an elimination strategy was incorporated into the MSK vector, which also includes a novel human scFv (Smith et al, NCT03070327). They note an overall response rate of three (2 very good partial responses and 1 PR) of their first four evaluable patients treated [60].
Zhang et al from Nanjing Legend Biotech in China generated an extracellular domain comprising two heavy-chain variable fragments to target distinct epitopes of BCMA (NCT03090659)[61]. The group reported on the treatment of 35 patients who received preconditioning with cyclophosphamide and split-dose infusions of up to 7×106 T cells/kg within a week. Apart from grade 3/4 CRS in three patients, toxicities were minimal. An overall response rate of 100% was reported, including CR in 14 of the first 20 patients treated. The interpretation of these data is challenging due to missing information on the patient population and the design of the CAR. One notable difference between this trial and the US trials is that patients treated had an average prior lines of therapy of 3, compared to 7–10 in US trials.
In aggregate, the ongoing studies demonstrate that targeting BCMA with CAR-T cells does not cause unmanageable CRS or elicit unexpected on-target, off-tumor toxicities, but has the potential to induce considerable anti-myeloma efficacy. However, further data will be required to determine the durability of these initial responses, as well as the optimal clinical setting, CAR construct and composition of the cell product to maximize the benefit for MM patients.
Affinity-enhanced T-cell receptors
Another approach to genetically engineer tumor-reactive T cells is to modify physiologically occurring T-cell receptors (TCRs) in a way to increase the receptor affinity to selected tumor-associated antigens. A group of potential target antigens are the cancer-testis antigens that are absent in non-gametogenic tissue, but present in a variety of malignancies [62]. However, possibly due to thymic selection, T cells targeting cancer-testis antigens such as MAGE-A3 have shown low functional avidity [63]. Adoptive transfer with affinity-enhanced TCRs therefore appears attractive to establish increased anti-tumor reactivity.
Enhanced-affinity TCRs differ from CARs in various aspects, especially the MCH-dependent binding of the TCR and its potential to recognize intracellular peptides with extremely high sensitivity. Furthermore, due to the plasticity of the TCR, the recognition of alternative targets with similarity in the MHC-presented peptide sequence can result in unpredictable off-target toxicity [64]. This became apparent when one patient with advanced melanoma and a second patient with relapsed MM were treated with T cells expressing an affinity-enhanced TCR against HLA-A*01-restricted MAGE-A3 [65]. Both patients developed fever, progressive hypoxia and cardiogenic shock and suffered from fatal complications 4–5 days after the treatment. Post hoc analyses ruled out mispairing of introduced and endogenous TCR α/β chains, alloreactivity and MAGE-A3 expression of normal tissue. Elaborate in vitro and in silico investigations identified a cross-reactive epitope derived from the human protein, titin, expressed in beating cardiomyocytes [64]. The exuberant T-cell activation had resulted in severe, eventually fatal, myocarditis unresponsive to treatment with corticosteroids.
A phase I/II trial to evaluate affinity-enhanced TCRs recognizing NY-ESO/LAGE-1 demonstrated safety in 20 patients with advanced MM; specifically off-target toxicities or severe CRS did not occur [66]. After high-dose chemotherapy and autoHSCT, patients underwent adoptive transfer on day 2 and the investigators noted persistence of the T cells for over 2 years in 9 out of 10 evaluable patients. 14 out of 20 patients obtained CR or near CR on day 100 post-autoHSCT and the estimated median progression free survival was 19.1 months. Relapses correlated with loss of gene-modified cells in eight patients and outgrowth of NY-ESO-1-negative subclones in two patients. Interpretation of the clinical benefit of these results is limited by the fact that in most of these patients this was their first autoHSCT, so while the immune correlatives are impressive, it is impossible to know to what extent the TCR modified cells contributed to the depth or durability of the clinical responses. In summary, affinity-modification of TCRs bear great potential for targeted cell therapy, and we eagerly await a trial in MM in a similar relapsed/refractory setting with minimal concomitant anti-MM therapy to judge their ability to eradicate high MM burden. However, the example of MAGE-A3 demonstrates the enormous deleterious potential of the T cells and extreme caution is advisable for any first-in-human studies.
Marrow-infiltrating lymphocytes
Another approach to achieve enhanced antitumor efficacy of autologous T cells is to make use of the distinctive characteristics of marrow-infiltrating lymphocytes (MILs). The observation that tumor-specific T cells are more likely to be found in close proximity to the tumor had led to clinical trials for solid tumors [67], with the disadvantages that tumor-infiltrating lymphocytes are not present in every patient and harvesting requires a surgical intervention. In MM, MILs are present in every patient and can routinely be obtained by BM aspiration. The immunologic character of the BM imparts essential properties for the cytotoxic effect of the MILs and their broad endogenous antigen specificity has exerted significant antitumor activity in preclinical models. On this rationale, Noonan et al treated 22 myeloma patients with high-dose melphalan and autoHSCT followed by an infusion of ex vivo expanded MILs on day 3 [68]. Isolation and over five-fold ex vivo expansion of MILs was feasible in all patients. The infusion of activated MILs did not result in CRS. In depth immunologic analyses demonstrated that enhanced central memory phenotype and increased capacity of IFN-γ and IL-2 secretion in the MIL product was associated with higher likelihood of achieving a CR. Accordingly, patients in CR demonstrated increased CD8 cytotoxicity and better immune responses to a pneumococcal vaccine after one year. As the efficacy of the adoptive T-cell transfer can hardly be distinguished from the previously administered high-dose melphalan, a randomized clinical trial is under way to evaluate the additional benefit of MILs post-autoHSCT (NCT01858558). Due to their broad endogenous antigen specificity, MILs might be a more favorable source than peripheral blood lymphocytes for genetically engineered T cells.
Future directions
Several strategies to further improve anti-tumor activity of T cells are currently under pre-clinical or clinical investigation.
As discussed in detail, the choice of a suitable target antigen is essential, with uniform, but exclusive expression on the malignant cells and high relevance for the maintenance of the disease. In reality, these antigens are rare. Simultaneous targeting of two tumor-associated antigens can reduce the risk of antigen escape, and in fact, a bispecific CAR that conveyed T-cell activation upon binding of either CD19 or CD20 demonstrated improved anti-tumor efficacy in preclinical B-cell lymphoma models [69]. In MM, bispecific CARs targeting BCMA and a second antigen such as CD38 (or even potentially CD19) have the potential to minimize the risk of BCMA-negative relapse and first clinical trials are ongoing (NCT03196414). However, the danger of on-target, off-tumor toxicity increases likewise. Overcoming limited expansion and/or persistence of the T cells is likely another essential issue that genetic engineering and culture methods must convey on adoptively transferred T cells. This is addressed through tailored construction of the CAR to incorporate optimized co-stimulation and avoid immunogenicity of the extracellular moiety, as well as, by controlling the composition of the T-cell subsets after ex vivo activation and expansion. In preclinical models, cell products derived from CD8+ central memory and CD4+ naïve T-cells demonstrated improved persistence [70], and the approach to administer T-cell products at a defined CD4/CD8 T-cell ratio has been incorporated successfully into several clinical trials for CD19 CAR-T cells [10, 71]. In addition, pharmacological inhibition of the serine/threonine kinase AKT has promoted memory features of therapeutic T cells, resulting in improved antitumor efficacy and persistence in preclinical models [72], and is now entering clinical evaluation (NCT 03139370).
Another major reason for reduced T-cell efficiency is the ability of myeloma cells and the immune-suppressive microenvironment to hamper T-cell activation by the expression of inhibitory molecules like programmed death-ligand-1 (PD-L1) [73, 74]. PD-L1 binding to the activation-induced T-cell inhibitory receptor programmed death-1 (PD1) results in T-cell anergy or apoptosis, but can be abrogated by checkpoint inhibition; conceptually, a co-administration with T-cell based therapies appears promising. Other approaches to reduce the immune-suppressive effects of the microenvironment include the systemic administration of lenalidomide (NCT03070327) or phosphodiesterase-5 inhibitors like tadalafil (NCT01858558) [75]. To further improve CAR T-cell function the cells can be modified to co-express selected cytokines or receptors to generate a pro-inflammatory microenvironment by autocrine or paracrine stimulation. Examples for these so called “armored” CAR T-cells include CD40L or 4-1BBL co-expressing CD19 CAR-T cells that have demonstrated improved proliferation and efficacy in preclinical models [76] and IL-12 secreting CAR-T cells directed against the ectoplasmic domain of MUC16 for the treatment of ovarian cancer [77]. In summary, these modifications of the T cells appear a promising approach to increase therapeutic efficiency to overcome the local constraints of the microenvironment while minimizing the systemic side effects. With further improvements of the efficacy to toxicity ratio [78] CAR-T cells have the potential to play a major role in the treatment of MM.
Summary
After proof-of-concept in the treatment of B-ALL, adoptive T-cell therapy has more recently also been explored for other malignancies. Similar to B-ALL, multiple myeloma is predominantly a disease of the bone marrow. However, CD19 is not expressed in most myeloma cases and other target antigens appear more promising. Based on preclinical investigations of a variety of myeloma antigens, including CD38, SLAMF7, CD138, and κ light chains, the B-cell maturation antigen (BCMA) has been identified as the most promising target for treatment with chimeric antigen receptor (CAR)-modified T cells. In at least four independent clinical trials, encouraging results have been documented to date, but also limitations have emerged. A major challenge is the reduction of antigen expression on the malignant cells under selection pressure of the CAR-T cells. This can be addressed by targeting two tumor-associated antigens simultaneously which, on the other hand, can increase the risk of on-target, off-tumor toxicities. Furthermore, the persistence of functional CAR-T cells is still limited; and loss of the genetically modified T cells has been associated with relapse. Other approaches to exploit the anti-myeloma efficacy of T cells is the modification of the cells with affinity-enhanced T-cell receptors (TCRs) targeting myeloma-associated antigens or the enrichment of marrow-infiltrating lymphocytes (MILs) with intrinsic anti-myeloma reactivity. Common efforts to improve efficacy and safety of adoptive T-cell transfer include the refinement of ex vivo activation and expansion methods, the development of strategies to overcome the immune-suppressive myeloma microenvironment and the definition of suitable combination therapies.
Practice Points.
-
-
Autologous T-cell therapy for treatment of multiple myeloma has entered phase I/II clinical testing
-
-
A number of myeloma antigens qualify as potential targets for receptor-modified T cells
-
-
Clinical evaluation has focused on chimeric antigen receptor-modified (CAR)-T cells targeting the B-cell maturation antigen (BCMA); so far, the treatment of more than 100 myeloma patients across the world have been treated; results demonstrate considerable anti-myeloma efficacy
-
-
Side effects of adoptive T-cell therapy include cytokine release syndrome (CRS) and on-target, off-tumor toxicities
-
-
Resistance or relapse has frequently been associated with insufficient T-cell functionality or reduced target antigen expression
Research Agenda.
-
-
For potential integration of adoptive T-cell therapy into standard of care in multiple myeloma, more data from clinical trials are required to define optimal strategies for patient selection, conditioning therapy and generation of the cell product
-
-
Clonal tiding in myeloma implicates the risk of target antigen reduction, therefore concepts such as dual targeting or combination therapy should be evaluated
-
-
Limited reliability for the prediction of clinical side effects in preclinical models calls for the incorporation of strategies to specifically eliminate therapeutic T cells to manage potential severe toxicities
Acknowledgments
SD is supported by the IZKF Wuerzburg (Interdisziplinäres Zentrum für Klinische Forschung) and is a fellow of the Clinician Scientist Program of the Else-Kröner Forschungskolleg. MH is supported by the Young Scholar Program of the Bavarian Academy of Sciences and the Myeloma Crowd Research Initiative. ELS is supported by LLS, ASH, SITC, MSK Cancer Center Support
Core Grant (P30 CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
SD has no conflict of interest.
MH is coinventor on a patent application (PCT/US2013/055862) related to CAR technologies that has been filed by the Fred Hutchinson Cancer Research Center (Seattle, WA) and licensed by JUNO Therapeutics, Inc. In addition, MH is coinventor on patent applications related to CAR technologies that have been filed by the University of W¨urzburg.
ELS is an inventor of BCMA and other targeted CAR T cell vectors for the treatment of MM that have been licensed to Juno Therapeutics and is a consultant for Juno Therapeutics. He is an inventor of BCMA and other targeted bispecific antibodies for the treatment of MM that are unlicensed.
Contributor Information
Sophia Danhof, Department of Hematology and Medical Oncology, University Hospital Wuerzburg, Versbacherstrasse 5, 97078 Wuerzburg, Germany, Tel. +49(0)931/201-71091, Fax. +49(0)931/201-671091, danhof_s@ukw.de.
Michael Hudecek, Department of Hematology and Medical Oncology, University Hospital Wuerzburg, Versbacherstrasse 5, 97078 Wuerzburg, Germany, Tel. +49(0)931/201-71091, Fax. +49(0)931/201-671091, hudecek_m@ukw.de.
Eric L. Smith, Myeloma Service, Director of Clinical Translation - Cellular Therapeutics Center, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, NY 10065, New York, USA, Tel: 212-639-2157, smithe3@mskcc.org.
References
- 1.Alexanian R, Delasalle K, Wang M, Thomas S, Weber D. Curability of multiple myeloma. Bone Marrow Res. 2012;2012:916479. doi: 10.1155/2012/916479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kazandjian D, Landgren O. A look backward and forward in the regulatory and treatment history of multiple myeloma: Approval of novel-novel agents, new drug development, and longer patient survival. Semin Oncol. 2016;43:682–9. doi: 10.1053/j.seminoncol.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasche L, Weinhold N, Morgan GJ, van Rhee F, Davies FE. Immunologic approaches for the treatment of multiple myeloma. Cancer Treat Rev. 2017;55:190–9. doi: 10.1016/j.ctrv.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith E, Devlin SM, Kosuri S, Orlando E, Landau H, Lesokhin AM, et al. CD34-Selected Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Relapsed, High-Risk Multiple Myeloma. Biol Blood Marrow Transplant. 2016;22:258–67. doi: 10.1016/j.bbmt.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokhorst HM, van der Holt B, Cornelissen JJ, Kersten M-J, van Oers M, Raymakers R, et al. Donor versus no donor comparison of newly diagnosed myeloma patients included in the HOVON 50 multiple myeloma study. Blood. 2012;119:6219–25. doi: 10.1182/blood-2011-11-393801. [DOI] [PubMed] [Google Scholar]
- 6.Gahrton G, Iacobelli S, Björkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous / reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–64. doi: 10.1182/blood-2012-11-469452. [DOI] [PubMed] [Google Scholar]
- 7.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtle CJ, Hanafi L, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR- T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadelain M, Brentjens R, Rivière I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor. Nat Biotechnol. 2002;20:70–5. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med. 2014;6:224ra25–224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7:1404–19. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maus MV, June CH. Zoom Zoom: Racing CARs for Multiple Myeloma. Clin Cancer Res. 2013;19:1917–9. doi: 10.1158/1078-0432.CCR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendriks J, Gravestein La, Tesselaar K, van Lier RAW, Schumacher TNM, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–40. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 20.McEarchern JA, Oflazoglu E, Francisco L, McDonagh CF, Gordon KA, Stone I, et al. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood. 2006;109:1185–92. doi: 10.1182/blood-2006-07-034017. [DOI] [PubMed] [Google Scholar]
- 21.Shaffer DR, Savoldo B, Yi Z, Chow KKH, Kakarla S, Spencer DM, et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011:117. doi: 10.1182/blood-2010-04-278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng W, Liu D, Fan X, Powers L, Goswami M, Hu Y, et al. Potential therapeutic biomarkers in plasma cell myeloma: A flow cytometry study. Cytom Part B Clin Cytom. 2013;84B:222–8. doi: 10.1002/cyto.b.21083. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–8. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahara N, Takeshita A, Shigeno K, Fujisawa S, Takeshita K, Naito K, et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. Br J Haematol. 2002;117:882–5. doi: 10.1046/j.1365-2141.2002.03513.x. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin R, Condomines M, Gertrude Gunset A, Sadelain M. CD56 targeted chimeric antigen receptors for immunotherapy of multiple myeloma. Cancer Res; Proc 103rd Annu Meet Am Assoc Cancer Res; 2012 Mar 31-Apr 4; Chicago, IL Philadelphia AACR. 2012. p. 3499. [Google Scholar]
- 26.Al-Mahdawi S, Shallal A, Wyse RKH. Neural cell adhesion molecule (N-CAM) in fetal and mature human heart. Fed Eur Biochem Soc Lett. 1990;267:183–5. doi: 10.1016/0014-5793(90)80920-e. [DOI] [PubMed] [Google Scholar]
- 27.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 28.Heider K-H, Kuthan H, Stehle G, Munzert G. CD44v6: a target for antibody-based cancer therapy. Cancer Immunol Immunother. 2004;53:567–79. doi: 10.1007/s00262-003-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebisch P, Eppinger S, Schöpflin C, Stehle G, Munzert G, Döhner H, et al. CD44v6, a target for novel antibody treatment approaches, is frequently expressed in multiple myeloma and associated with deletion of chromosome arm 13q. Haematologica. 2005;90:489–93. [PubMed] [Google Scholar]
- 30.Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–72. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 31.Tijink BM, Buter J, de Bree R, Giaccone G, Lang MS, Staab A, et al. A Phase I Dose Escalation Study with Anti-CD44v6 Bivatuzumab Mertansine in Patients with Incurable Squamous Cell Carcinoma of the Head and Neck or Esophagus. Clin Cancer Res. 2006;12:6064–72. doi: 10.1158/1078-0432.CCR-06-0910. [DOI] [PubMed] [Google Scholar]
- 32.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–49. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Zhong M-C, Guo H, Davidson D, Mishel S, Lu Y, et al. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493–7. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 36.Gogishvili T, Danhof S, Prommersberger S, Rydzek J, Schreder M, Brede C, et al. SLAMF7-CAR T-cells eliminate myeloma and confer selective fratricide of SLAMF7 + normal lymphocytes. Blood. 2017 doi: 10.1182/blood-2017-04-778423. blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 37.Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF. Discrete stages of human intrathymic differentiation: Analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci. 1980;77:1588–92. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada H, Kawano MM, Huang N, Harada Y, Iwato K, Tanabe O, et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 1993;81:2658–63. [PubMed] [Google Scholar]
- 39.Mizuguchi M, Otsuka N, Sato M, Ishii Y, Kon S, Yamada M, et al. Neuronal localization of CD38 antigen in the human brain. Brain Res. 1995;697:235–40. doi: 10.1016/0006-8993(95)00885-t. [DOI] [PubMed] [Google Scholar]
- 40.Tembhare P, Yuan C, Korde N, Maric I, Landgren O. Antigenic drift in relapsed extramedullary multiple myeloma: plasma cells without CD38 expression. Leuk Lymphoma. 2012;53:721–4. doi: 10.3109/10428194.2011.623257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Park CY, Medeiros BC, Weissman IL. CD19-CD45low/−CD38high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia. 2012;26:2530–7. doi: 10.1038/leu.2012.140. [DOI] [PubMed] [Google Scholar]
- 42.Touzeau C, Moreau P. Daratumumab for the treatment of multiple myeloma. Expert Opin Biol Ther. 2017;17:887–93. doi: 10.1080/14712598.2017.1322578. [DOI] [PubMed] [Google Scholar]
- 43.Mihara K, Bhattacharyya J, Kitanaka A, Yanagihara K, Kubo T, Takei Y, et al. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia. 2012;26:365–7. doi: 10.1038/leu.2011.205. [DOI] [PubMed] [Google Scholar]
- 44.Drent E, Groen RWJ, Noort WA, Themeli M, Lammerts van Bueren JJ, Parren PWHI, et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. 2016;101:616–25. doi: 10.3324/haematol.2015.137620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drent E, Themeli M, Poels R, de Jong-Korlaar R, Yuan H, de Bruijn J, et al. A Rational Strategy for Reducing On-Target Off-Tumor Effects of CD38-Chimeric Antigen Receptors by Affinity Optimization. Mol Ther. 2017;25:1946–58. doi: 10.1016/j.ymthe.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo B, Chen M, Han Q, Hui F, Dai H, Zhang W, et al. CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma. J Cell Immunother. 2015 doi: 10.1016/j.jocit.2014.11.001. [DOI] [Google Scholar]
- 47.Wijdenes J, Vooijs WC, Clément C, Post J, Morard F, Vita N, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996;94:318–23. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- 48.Seidel C, Sundan A, Hjorth M, Turesson I, Dahl IM, Abildgaard N, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000;95:388–92. [PubMed] [Google Scholar]
- 49.Leonova EI, Galzitskaya OV. Structure and functions of syndecans in vertebrates. Biochem. 2013;78:1071–85. doi: 10.1134/S0006297913100015. [DOI] [PubMed] [Google Scholar]
- 50.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramos CA, Savoldo B, Torrano V, Ballard B, Zhang H, Dakhova O, et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J Clin Invest. 2016;126:2588–96. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garfall AL, Maus MV, Hwang W-T, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373:1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajek R, Okubote SA, Svachova H. Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol. 2013;163:551–64. doi: 10.1111/bjh.12563. [DOI] [PubMed] [Google Scholar]
- 54.Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brudno J, Lam N, Wang M, Stroncek D, Maric I, Stetler-Stevenson M, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor with a CD28 Costimulatory Moiety Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. Blood. 2017:130. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berdeja JG, Lin Y, Raje N, Munshi N, Siegel D, Liedtke M, et al. Durable Clinical Responses in Heavily Pretreated Patients with Relapsed/Refractory Multiple Myeloma: Updated Results from a Multicenter Study of bb2121 Anti-Bcma CAR T Cell Therapy. Blood. 2017;130:740. [Google Scholar]
- 59.Cohen AD, Garfall AL, Stadtmauer EA, Lacey SF, Lancaster E, Vogl DT, et al. Safety and Efficacy of B-Cell Maturation Antigen (BCMA)-Specific Chimeric Antigen Receptor T Cells (CART-BCMA) with Cyclophosphamide Conditioning for Refractory Multiple Myeloma (MM) Blood. 2017;130:505. [Google Scholar]
- 60.Smith EL, Mailankody S, Ghosh A, Masakayan R, Staehr M, Purdon TJ, et al. Development and Evaluation of a Human Single Chain Variable Fragment (scFv) Derived Bcma Targeted CAR T Cell Vector Leads to a High Objective Response Rate in Patients with Advanced MM. Blood. 2017;130:742. [Google Scholar]
- 61.Zhang W, Zhao W, Liu J, He A, Chen Y, Cao X, et al. Phase I, open-label trial of anti- BCMA chimeric antigen receptor T cells in patients with relapsed/refractory multiple myeloma. Haematologica. 2017;102(suppl) Abstract no S103. [Google Scholar]
- 62.Condomines M, Hose D, Raynaud P, Hundemer M, De Vos J, Baudard M, et al. Cancer/Testis Genes in Multiple Myeloma: Expression Patterns and Prognosis Value Determined by Microarray Analysis. J Immunol. 2007;178:3307–15. doi: 10.4049/jimmunol.178.5.3307. [DOI] [PubMed] [Google Scholar]
- 63.Connerotte T, Van Pel A, Godelaine D, Tartour E, Schuler-Thurner B, Lucas S, et al. Functions of Anti-MAGE T-Cells Induced in Melanoma Patients under Different Vaccination Modalities. Cancer Res. 2008;68:3931–40. doi: 10.1158/0008-5472.CAN-07-5898. [DOI] [PubMed] [Google Scholar]
- 64.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, et al. Identification of a Titin-Derived HLA-A1-Presented Peptide as a Cross-Reactive Target for Engineered MAGE A3-Directed T Cells. Sci Transl Med. 2013;5:197ra103–197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–71. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914–21. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Noonan KA, Huff CA, Davis J, Lemas MV, Fiorino S, Bitzan J, et al. Adoptive transfer of activated marrow-infiltrating lymphocytes induces measurable antitumor immunity in the bone marrow in multiple myeloma. Sci Transl Med. 2015;7:288ra78–288ra78. doi: 10.1126/scitranslmed.aaa7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zah E, Lin M-Y, Silva-Benedict A, Jensen MC, Chen YY. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2015;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turtle CJ, Hanafi L-A, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkins lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8:355ra116–355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 74.Görgün G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. 2015;21:4617–8. doi: 10.1158/1078-0432.CCR-15-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting Immune Suppression with PDE5 Inhibition in End-Stage Multiple Myeloma. Cancer Immunol Res. 2014;2:725–31. doi: 10.1158/2326-6066.CIR-13-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curran KJ, Seinstra BA, Nikhamin Y, Yeh R, Usachenko Y, van Leeuwen DG, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther. 2015;23:769–78. doi: 10.1038/mt.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koneru M, O’Cearbhaill R, Pendharkar S, Spriggs DR, Brentjens RJ. A phase I clinical trial of adoptive T cell therapy using IL-12 secreting MUC-16ecto directed chimeric antigen receptors for recurrent ovarian cancer. J Transl Med. 2015;13:102. doi: 10.1186/s12967-015-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mikkilineni L, Kochenderfer JN. Chimeric Antigen Receptor T-cell Therapies for Multiple Myeloma. Blood. 2017 doi: 10.1182/blood-2017-06-793869. blood-2017-06-793869. [DOI] [PMC free article] [PubMed] [Google Scholar]