Abstract
Objective: To test whether the addition of an insulin pump or continuous glucose monitor (CGM) related to reduced glycated hemoglobin A1c (HbA1c) in large cohort of children, 5–9 years old, and within 1 year of their type 1 diabetes (T1D) diagnosis.
Research Design and Methods: The study uses data from families of children with recent-onset T1D and who were between 5 and 9 years old. Study analyses used children's HbA1c values at baseline and at the 6-month follow-up. Parents reported on family demographics and children's T1D device use in their daily management (e.g., insulin pump or CGM). Children's mean T1D duration was 4.70 ± 3.28 months at baseline, so the 6-month assessment point was ∼12 months postdiagnosis.
Results: One hundred-eleven families participated. At baseline, child mean age was 7.51 ± 1.37 years, and mean child HbA1c was 7.65% ± 1.40%. In addition, 17% of children used an insulin pump, and 17.1% of children used CGM. Six months later, 35.1% of children had started an insulin pump and 25.2% had started CGM. Repeated measures analyses of variance results showed a smaller overall HbA1c between baseline and 6 months for children using an insulin pump versus children not on a pump. For CGM, results showed that children starting a CGM during this window had a significantly lower HbA1c level than children who had not started on CGM.
Conclusions: The study results suggest that early initiation of either an insulin pump or CGM in children newly diagnosed with T1D may help to improve child HbA1c levels within the first 12 months of diabetes.
Keywords: Type 1 diabetes, New onset, HbA1c, Continuous glucose monitoring, Continuous subcutaneous insulin infusion, Pediatrics
Children newly diagnosed with type 1 diabetes (T1D) face many challenges as they learn to adhere to a rigorous and complicated daily medical regimen. As per international guidelines, all children <18 years old should strive to achieve and maintain a hemoglobin A1c (HbA1c) level of ≤7.0% (53 mmol/mol) to reduce their long-term risk for T1D-related vascular complications.1–4 In addition, emerging evidence suggests that early attainment of this goal may offer some future protection against vascular complications (e.g., metabolic memory).5,6
However, in the early months of T1D (aka, honeymoon phase) achieving optimal glycemic control can be difficult.7 Indeed, in the honeymoon phase children can experience rapid changes in their daily glycemic control due to unpredictable effects of their own endogenous insulin levels, the addition of exogenous insulin through an insulin pump or injections, as well as their own emerging knowledge and experience with the disease. In the presence of seemingly unpredictable blood glucose levels and/or rising HbA1c levels, families and T1D providers may seek to add an insulin pump and/or continuous glucose monitor (CGM) early in the management of T1D to help with glycemic management.
While previous research supports the potential efficacy of adding one or more of these devices in lowering HbA1c levels for youth with established T1D,8–12 there is limited research exploring the effect of these devices on HbA1c in youth with recent-onset T1D. Moreover, challenges for existing studies examining the impact of insulin pumps in the recent-onset period are that they are small, underpowered, and/or lack a comparison group (e.g., youth on multiple daily injections [MDI]),13–15 while no previous research has examined HbA1c levels in youth who adopt CGM early in T1D.
This study expands on the literature by (1) testing the impact of adding an insulin pump on child HbA1c in the recent-onset period using a larger sample of youth and including a comparison group of children who do not start an insulin pump and (2) presenting new information on the impact of CGM on child HbA1c in the recent-onset period. Specifically, this study tests the hypothesis that children who start a pump or CGM within the first year after diagnosis will have lower HbA1c or experience a smaller increase in HbA1c at 1 year than children who do not start one of these devices.
Methods
Participants and procedure
Researchers recruited families of children 5–9 years of age and within the first 12 months of their T1D diagnosis to participate in a 30-month longitudinal study from two hospital-based pediatric diabetes clinics in the United States. The Institutional Review Boards at both hospitals approved all study procedures before recruitment. Data for the current study included outcomes collected during the first 6 months of study participation.
Eligible youth were diagnosed with T1D for ≤12 months, were between 5 and 9 years old, used intensive insulin therapy, and were English speaking. Youth ineligible to participate included youth with a diagnosis of developmental delay (i.e., autism, cerebral palsy, or intellectual disability) or severe psychiatric disorder, youth with a comorbid chronic condition (e.g., renal disease), youth with evidence of Type II or monogenic diabetes, and youth with chronic medication use that may impact glycemic control (e.g., systemic steroids).
Parents who agreed to participate provided informed consent. Children >7 years old at the time of study enrollment provided assent. Researchers collected child HbA1c levels at the initial study visit and every 3 months after, and parents completed study questionnaires using a clinic tablet. Researchers completed all study procedures during routine clinic visits and provided monetary compensation each time parents completed questionnaires and gave children a toy valued at 10 U.S. dollars for each HbA1c check.
Measures
Demographics
Parents reported on child and family demographics at baseline. The electronic medical record (EMR) served as the source for collecting children's diagnosis date, pump and CGM start dates, point-of-care (POC) HbA1c values, and body mass index (BMI).
Child HbA1c
This study used HbA1c level as an indicator of average glycemic control. Researchers collected child HbA1c levels at baseline and 6-month follow-up using a finger-stick blood sample and laboratory kit. Researchers sent all samples to a central laboratory for processing using automated high-performance liquid chromatography (reference range 4.0%–6.0% [20–42 mmol/mol]; Tosoh Corporation, San Francisco, CA). This method is traceable to Diabetes Control and Complications Trial standards.16,17 For missing or diluted laboratory samples, researchers used the corresponding POC values from the EMR (baseline n = 16 and 6-month n = 6). The correlations between laboratory and POC HbA1c values for the sample were high (baseline r = 0.98; 6-month follow-up r = 0.97).
Analyses
Researchers used descriptive statistics to examine sample characteristics and to test for assumptions. Then, researchers used correlations to examine associations among study variables and conducted independent sample comparisons to examine differences in HbA1c for children who started a pump between baseline and 6-month follow-up and for children who started a CGM between baseline and 6-month follow-up. To examine change in HbA1c based on device starts between baseline and 6-month follow-up, researchers conducted two repeated measures analyses of variance (ANOVA) (one model for each device: insulin pump and CGM).
For all analyses, researchers treated device starts between baseline and 6-month follow-up as a between-subjects dichotomous variable and child HbA1c at baseline and 6-month follow-up as a within-subjects variable and included duration of T1D diagnosis as a study covariate. Investigators used SPSS (Version 25; SPSS, Inc., Chicago, IL) to conduct all the analyses.
Results
Of the 126 families who enrolled, 112 completed baseline and 6-month study visits (88.9%). One child HbA1c value was >3 standard deviations above the mean and thus removed, resulting in a final sample of 111 families. At baseline, children had a mean age of 7.51 ± 1.37 years and a mean time since diagnosis of 4.70 ± 3.28 months. Forty-seven percent of children were male, and 88.7% identified as non-Hispanic and White. The mean child BMI percentile at baseline was 61.0% and 62.6% at 6-month follow-up and did not differ between baseline and follow-up (t = −1.50, P = 0.14, mean difference = 1.6%). Parents were 88.1% mothers and had a mean age of 36.75 ± 6.27 years. The majority of parents were married or living with their significant other (86.2%), and the median family income was $70,000–$79,999 (range $10,000–19,000 to ≥$100,000).
Mean child HbA1c was 7.65% ± 1.40% (60 mmol/mol; 37.8% of values were ≤7.0%) at baseline and 8.05% ± 1.08% (64 mmol/mol; 13.5% of values were ≤7.0%) at 6-month follow-up. Seventeen percent of children used an insulin pump before completing their baseline visit, and 35.1% started an insulin pump between their baseline and 6-month follow-up. For children who started a pump between baseline and 6-month follow-up, 30.8% showed a decline in HbA1c, 2.5% showed no change, and 66.7% showed an increase. Similarly, among children who did not start a pump during this time, 36.1% showed a decline in HbA1c, 2.8% showed no change, and 61.1% showed an increase.
For CGM, 17.1% of children used a CGM before completing their baseline visit and 25.2% started using a CGM between baseline and 6-month follow-up. For children who started a CGM between baseline and 6-month follow-up, 40.7% showed a decline in HbA1c, 3.7% showed no change, and 55.6% showed an increase, whereas 32.1% of children who did not start a CGM during this time showed a decline in HbA1c, 2.4% showed no change, and 65.5% showed an increase. Table 1 displays the means and correlations among study variables.
Table 1.
Correlations and Descriptives Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Baseline HbA1c | — | |||||||||
| 2. 6-month HbA1c | 0.434*** | — | ||||||||
| 3. Pump start | −0.146 | −0.235** | — | |||||||
| 4. CGM start | −0.020 | −0.267*** | 0.615*** | — | ||||||
| 5. Duration of T1D | 0.086 | 0.262** | −0.199* | −0.081 | — | |||||
| 6. Child age | −0.126 | −0.074 | 0.007 | −0.123 | −0.085 | — | ||||
| 7. Child sex | 0.161 | 0.125 | −0.047 | −0.058 | −0.064 | 0.125 | — | |||
| 8. Child race/ethnicity | −0.078 | −0.057 | −0.058 | −0.012 | 0.136 | 0.027 | 0.034 | — | ||
| 9. Marital status | −0.169 | −0.216* | 0.181 | 0.168 | 0.113 | −0.123 | −0.268*** | 0.026 | — | |
| 10. Family income | −0.275*** | −0.269*** | 0.177 | −0.038 | −0.009 | −0.079 | −0.362*** | 0.047 | 0.446*** | — |
| Mean ± SD | ||||||||||
| Total, n = 111 | 7.65 ± 1.40 | 8.05 ± 1.08 | — | — | 4.70 ± 3.28 | 7.51 ± 1.37 | — | — | — | — |
| Pump start, n = 39 (No Pump start, n = 72) | 7.38 ± 1.17 (7.81 ± 1.50) | 7.71 ± 0.77 (8.24 ± 1.18) | — | — | 3.82 ± 2.92 (5.18 ± 3.38) | 7.71 ± 0.77 (7.51 ± 1.25) | — | — | — | — |
| CGM start, n = 28 (No CGM start, n = 83) | 7.61 ± 1.34 (7.68 ± 1.43) | 7.55 ± 0.78 (8.22 ± 1.12) | — | — | 4.25 ± 3.33 (4.86 ± 3.27) | 7.22 ± 1.42 (7.61 ± 1.34) | — | — | — | — |
| Percentile, 25th; 50th; 75th | ||||||||||
| Total, n = 111 | — | — | — | — | 3.0; 4.0; 7.0 | 6.5; 8.0; 9.0 | — | — | — | — |
| Pump start, n = 39 (No Pump start, n = 72) | — | — | — | — | 2.0; 3.0; 4.0 (3.0; 4.0; 8.5) | 6.0; 7.0; 9.0 (7.0; 8.0; 8.0) | — | — | — | — |
| CGM start, n = 28 (No CGM start, n = 83) | — | — | — | — | 3.0; 3.0; 6.0 (3.0; 4.0; 7.0) | 6.0; 7.0; 8.2 (7.0; 8.0; 9.0) | — | — | — | — |
Duration of T1D = Duration of T1D diagnosis in months; Point-biserial correlations shown for correlations between continuous and dichotomous variables (Pump start: 0 = No pump start between baseline and 6-month follow-up, 1 = Pump start between baseline and 6-month follow-up; CGM start: 0 = No CGM start between baseline and 6-month follow-up, 1 = CGM start between baseline and 6-month follow-up; Child sex: 0 = female, 1 = male; Child race/ethnicity: 0 = Non-White, Hispanic, 1 = White, Non-Hispanic; Marital status: 0 = not married or living together; 1 = married or living together).
P < 0.05; **P < 0.01; ***P < 0.001.
CGM, continuous glucose monitor; HbA1c, hemoglobin A1c; T1D, type 1 diabetes.
In independent sample comparisons, children who started a pump between baseline and 6-month follow-up had a significantly lower HbA1c at 6-month follow-up (t = −2.84, P = 0.01; mean difference = 0.53%) and a shorter duration of T1D diagnosis at baseline (t = −2.22, P = 0.03; mean difference = 1.36 months) compared to children who did not start a pump during this time.
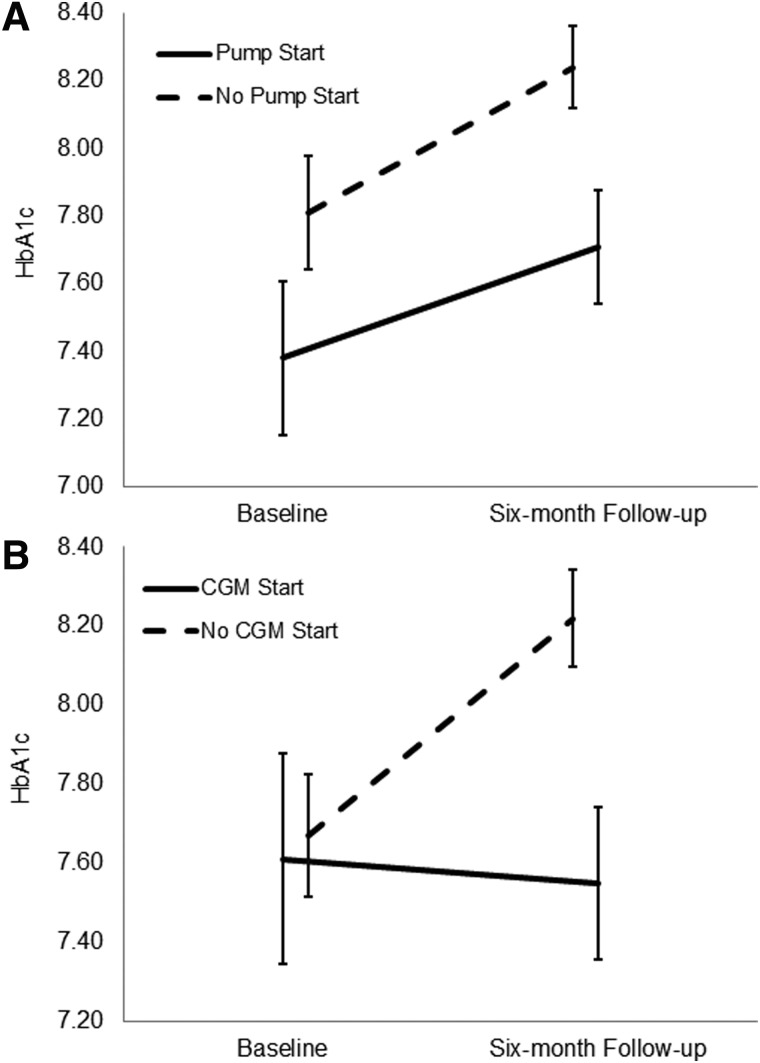
Results of the repeated measures ANOVA showed no significant within-subjects effects for change in HbA1c over time (F = 0.47, P = not significant [ns]) or for the time-by-pump start interaction (F = 0.09, P = not significant; Fig. 1A). However, the results for between-subjects showed a significant group effect (F = 3.90, P = 0.05, ηp2 = 0.04). Specifically, children who started a pump between baseline and 6 months had a significantly lower HbA1c averaged across baseline and 6-month follow-up (mean HbA1c across baseline and 6 months = 7.54 ± 0.97) compared to children who did not start a pump between baseline and 6-month follow-up (mean HbA1c across baseline and 6 months = 8.02 ± 1.34).
FIG. 1.
(A) The change in HbA1c from baseline to 6-month follow-up for children starting a pump between baseline and 6-month follow-up and children not starting a pump between baseline and 6-month follow-up. (B) The change in HbA1c from baseline to 6-month follow-up for children starting a CGM between baseline and 6-month follow-up and children not starting a CGM between baseline and 6-month follow-up. Error bars represent the standard error. CGM, continuous glucose monitor; HbA1c, hemoglobin A1c. Supplemental materials present pump and CGM data that include error bars that represent 95% confidence intervals (Supplementary Fig. S1) and error bars that represent ±1 standard deviation (Supplementary Fig. S2).
For CGM starts between baseline and 6 months, independent sample comparisons showed that children who started a CGM between baseline and 6-month follow-up had a significantly lower HbA1c at 6-month follow-up compared to children who did not start a CGM during this time (t = −2.89, P = 0.01; Mean difference = 0.66%).
Results of the repeated measures ANOVA also showed a significant within-subjects effect for the time-by-CGM start interaction (F = 3.84, P = 0.05, ηp2 = 0.03; Fig. 1B), suggesting that children who started a CGM between baseline and 6 months showed virtually no change in HbA1c, while children who did not start a CGM during this time period showed an increase in HbA1c. There was no significant effect for the within-subjects change in HbA1c over time (F = 0.03, P = ns) or the between-subjects group effect (F = 2.10, P = ns).
Discussion
This study examines the impact of insulin pump and CGM starts on child HbA1c in a sample of 5- to 9-year olds recently diagnosed with T1D. Overall, the study results showed that children's mean HbA1c levels increased 0.4% between the baseline and the 6-month follow-up assessments, which are generally consistent with the findings of Cengiz et al.15 However, novel to the literature, in a large observational design, this study also showed that children who started an insulin pump early in the course of T1D had a lower HbA1c at the 6-month follow-up compared to children who did not start an insulin pump during this time.
Moreover, the results for CGM uptake showed that children who started on CGM had a lower HbA1c at the 6-month follow-up compared to children who had not started CGM during this time, while findings examining change over time found that children who started on CGM showed minimal change in HbA1c from baseline to 6-month follow-up, whereas children who did not start a CGM during this time showed an increase in HbA1c.
In the present sample, approximately one-quarter of children used CGM by the 6-month follow-up, and 35.1% of children used an insulin pump. Overall, this pump rate is comparable to existing multicenter research.13,15 But, unlike the current study, these existing multicenter studies did not relate youth pump use to future HbA1c levels.13–15 There are two existing studies that do relate youth pump use to future HbA1c.18,19 In one study, 28 consecutive youth diagnosed with T1D (M age 12.1 ± 6.2 years) started an insulin pump within 1 day of their diagnosis. All youth saw a decrease in HbA1c from time of diagnosis to 18 months postdiagnosis. However, because all youth started a pump and there was no comparison group, it is impossible to discern the specific impact of an insulin pump versus the addition of exogenous insulin.18
In the second study, researchers randomized 24 youth with new-onset T1D (age range 8–18 years) to either start a pump or use MDI. At 6 months postdiagnosis, there was a statistical trend (P = 0.06) for child HbA1c favoring slightly lower HbA1c for youth on a pump versus MDI. Unfortunately, at 12 months postdiagnosis, there was no difference in HbA1c for youth on a pump versus MDI.19 Therefore, the present study builds on the extant literature by examining the potential benefit of early initiation of an insulin pump on child HbA1c in the recent-onset period in a larger and younger sample of children and by providing a comparison group of children who did not start an insulin pump.
In contrast, there are no previous studies which have examined the impact of starting CGM in the recent-onset period of T1D and children's future HbA1c levels. Thus, this study extends the literature by presenting novel information specific to CGM use in the recent-onset period and suggests that early adoption of CGM may buffer against the increase in HbA1c that has been previously reported in the new-onset period of T1D.15,20
The present study is also notable because of its focus on two potentially modifiable variables (e.g., CGM or pump use) in the recent-onset period of T1D. While large multicenter studies exist examining factors related to child HbA1c in the new-onset period of T1D,15,19 a notable limitation of these studies is that they primarily identified the influence of nonmodifiable factors, such as child race, family socioeconomic status, the presence of a two-parent household, and children's insulin levels13–15 on children's HbA1c levels.
However, by focusing on CGM and pump uptake, this study provides novel data that can directly inform patient care and help families and T1D providers help children to achieve and maintain optimal HbA1c levels earlier in T1D, which could have other important benefits, such as later protection from T1D complications.5,6,21,22 In addition, these results can inform the development and refinement of new behavioral interventions to promote better assimilation of pumps and CGMs within children's daily T1D management, as well as interventions that may promote longer maintenance of use of these devices in children.
Strengths of the present study include its use of a central laboratory for measuring HbA1c, its longitudinal design, its use of a relatively large sample size, its focus on children diagnosed with T1D between 5 and 9 years old, an age group which has previously shown deteriorating HbA1c levels,20 and its observational design, which likely provided a more ecologically-valid examination of the impact of adding either CGM or a pump on children's HbA1c levels.
There are also a few limitations to the present study. First, the present study may be limited by its relatively homogeneous sample. The results of the present study may not generalize to younger children and adolescents with recent-onset T1D, children with comorbid developmental delays, psychiatric disorders, and/or medical conditions, or children from a racial or ethnic background that is considerably different from the present sample. Second, the study used parent report to measure T1D device (e.g., CGM and insulin pump) use at baseline and at the 6-month follow-up. It is possible that parents could have under-reported device use if their child was not using a device on the day of the study visit.
Similarly, based on how these data were collected, it is not possible to conduct sensitivity analyses to determine if time using a T1D device may have influenced children's HbA1c levels at the 6-month assessment point. This question remains for a future study. Third, the researchers acknowledge that due to low power, it was not possible to test for an association between child HbA1c and simultaneous use of a pump and CGM (n = 23). Therefore, the question remains if using both an insulin pump and CGM has an even larger effect on child HbA1c through 12 months. Fourth, this study is limited because it did not consider the impact of CGM or pump starts on child and caregiver quality of life. Finally, there remains a possibility that families who reported starting an insulin pump or CGM displayed better glycemic control for reasons unaccounted for in this study (e.g., medical insurance).
In a sample of 111 children, between the ages of 5–9 years old and within 1 year of their T1D diagnosis, the current data suggest that initiating insulin pump or CGM use close to the time of diagnosis may have beneficial effects on children's HbA1c through 12 months. The clinical implication of these findings is further support for early introduction of T1D devices in children's daily management even within the first few months of T1D. However, future research is needed to explore whether early introduction of T1D devices leads to better assimilation of these devices in children's daily management, better maintenance of these devices in daily care, and better quality of life and glycemic control. In addition, as T1D devices continue to advance, future studies need to explore how starting a hybrid closed loop system in children with recent-onset T1D relates to their glycemic control.
Supplementary Material
Acknowledgments
The authors thank the parents and children who contributed the data for the current analyses. This research was supported by a grant R01-DK100779 (to S.R.P.) from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
Author Disclosure Statement
No competing financial interest exists.
Supplementary Material
References
- 1. Chiang J, Kirkman M, Laffel L, Peters A: Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2034–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Association AD: Children and adolescents: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S126–S136 [DOI] [PubMed] [Google Scholar]
- 3. Rewers MJ, Pillay K, De Beaufort C, et al. : Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes 2014;15:102–114 [DOI] [PubMed] [Google Scholar]
- 4. DiMeglio LA, Acerini CL, Codner E, et al. : ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes 2018;19:105–114 [DOI] [PubMed] [Google Scholar]
- 5. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group: Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group: Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdul-Rasoul M, Habib H, Al-Khouly M: ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes 2006;7:101–107 [DOI] [PubMed] [Google Scholar]
- 8. Clements M, Foster N, Maahs D, et al. : Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes 2016;17:327–336 [DOI] [PubMed] [Google Scholar]
- 9. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) Trial. Diabetes Care 2010;33:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsen B, Johannesen J, Fredheim S, et al. : Insulin pump treatment; increasing prevalence, and predictors for better metabolic outcome in Danish children and adolescents with type 1 diabetes. Pediatr Diabetes 2015;16:256–262 [DOI] [PubMed] [Google Scholar]
- 11. Szypowska A, Schwandt A, Svensson J, et al. : Insulin pump therapy in children with type 1 diabetes: analysis of data from the SWEET registry. Pediatr Diabetes 2016;17:38–45 [DOI] [PubMed] [Google Scholar]
- 12. Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R: Insulin pump therapy. Diabetes Care 2003;28:15–19 [DOI] [PubMed] [Google Scholar]
- 13. Lin MH, Connor CG, Ruedy KJ, et al. : Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technol Ther 2013;15:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redondo M, Connor C, Ruedy K, et al. : Pediatric diabetes consortium type 1 diabetes New Onset (NeOn) study: factors associated with HbA1c levels one year after diagnosis. Pediatr Diabetes 2014;15:294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cengiz E, Connor C, Ruedy K, et al. : Pediatric diabetes consortium T1D New-onset (NeOn) study: clinical outcomes during the first year following diagnosis. Pediatr Diabetes 2014;15:287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Glycohemoglobin Standardization Program (NGSP). (2016, November). List of NGSP certified methods. Retrieved from http://www.ngsp.org/certified.asp
- 17. Lenters-Westra E, Slingerland R: Three of 7 Hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem 2014;60:1062–1072 [DOI] [PubMed] [Google Scholar]
- 18. Ramchandani N, Ten S, Anhalt H, et al. : Insulin pump therapy from the time of diagnosis of type 1 diabetes. Diabetes Technol Ther 2006;8:663–670 [DOI] [PubMed] [Google Scholar]
- 19. Thrailkill KM, Moreau CS, Swearingen C, et al. : Insulin pump therapy started at the time of diagnosis: effects on glycemic control and pancreatic β-cell function in type 1 diabetes. Diabetes Technol Ther 2011;13:1023–1030 [DOI] [PubMed] [Google Scholar]
- 20. Clements MA, Lind M, Raman S, et al. : Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res Care 2014;2:e000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ceriello A, Ihnat MA, Thorpe JE: The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab 2009;94:410–415 [DOI] [PubMed] [Google Scholar]
- 22. Berezin A: Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr 2016;10:S176–S183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.