Abstract
In the majority of cases, the cognitive and behavioral impairments resulting from a mild traumatic brain injury (TBI) (also referred to as concussion) wane within days to weeks. In contrast, these impairments can persist for months to years after repetitive mild TBI (rmTBI). The cellular and molecular mechanisms underlying these impairments are not well understood. In the present study, we examined the consequences of rmTBI (three weight drops each separated by 72 h) on brain tissue respiration, pathology, and cognitive performance in mice. The transcription factor nuclear factor-erythroid 2-realted factor 2 (Nrf2) has been demonstrated to enhance the expression of numerous cytoprotective genes. Carnosic acid (CA) has been shown to activate Nrf2 and suppress the proinflammatory transcription factor nuclear factor kappa B (NF-κB). Because contemporaneous activation of cytoprotective genes and inhibition of proinflammatory genes can be beneficial, we questioned whether CA can be used to mitigate the pathobiology of rmTBI. The rmTBI increased hippocampal adenosine triphosphate-linked tissue respiration and proton leak that were unaffected by CA treatment. The rmTBI also caused significant motor and cognitive dysfunction, as tested using the foot fault, Barnes maze, and novel object recognition tasks. These impairments occurred in the absence of visible neuronal or dendritic loss. Post-rmTBI administration of CA significantly improved motor and cognitive function, and decreased Gfap and Iba1 immunoreactivities within white matter tracks. Taken together, these results show that rmTBI can cause cognitive impairments in the absence of overt neuronal pathologies, and post-injury treatment with CA can lessen some of these impairments.
Keywords: brain tissue respiration, NF-κB, Nrf2 activator, repeat concussive injury, tissue respiration, white matter inflammation
Introduction
Athletes who sustain repetitive mild traumatic brain injury (rmTBI) can have prolonged cognitive and behavioral problems that, in some cases, can last months to years.1–3 The underlying mechanisms by which rmTBI causes long-lasting symptomatologies are not well understood, and no effective treatments have been identified. Activators of the transcription factor nuclear factor-erythroid 2-realted factor 2 (Nrf2) have been demonstrated to enhance the expression of a large group of cytoprotective genes4 and have been shown to be protective in animal models of Alzheimer disease, stroke, and TBI.5–8
Carnosic acid (CA), a naturally occurring proelectrophilic chemical present in sage and rosemary, has been shown to increase the expression of Nrf2-regulated genes.9 The CA also has been shown to inhibit the transcription factor nuclear factor kappa B (NF-κB) and decrease the expression of proinflammatory molecules.10,11 After moderate-severe TBI, intraperitoneal CA (1 mg/kg IP) has been shown to reduce 4-hydroxy-2-nonenal (4-HNE) and 3-nitrotyrosine (3-NT) immunoreactivity in the injured cortex.12 Using a repeat weight drop model of closed head injury, we tested the efficacy of post-injury administration of CA in altering symptophenotypes such as motor and cognitive function.
Methods
C57BL/6 male mice (12–16 weeks old) were housed singly on a 12-h light/dark cycle with ad libitum access to food and water. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee. The rmTBI was performed as described previously with some modifications.13,14 Mild closed head injury (mCHI) was delivered by a free-falling weight onto the head of a mouse positioned on a compressible material (a 17.0 cm × 12.5 cm × 4.0 cm [L × W × H] piece of 1.0 pound per cubic foot [pcf] expanded polystyrene). A total of three hits were delivered, each separated by 72 h. This separation was based on studies that have demonstrated that markers of neuronal health (e.g., N-acetylaspartic acid, NAA) in the brain are depleted maximally three days after concussion in athletes, an effect also observed in rats after mTBI.15,16
Male mice were anesthetized with 5% isoflurane in a 1:1 O2/air mixture, then maintained with a 2.5% isoflurane and 1:1 O2/air mixture. At 40 sec after discontinuation of anesthesia, a single impact was applied to the skull by dropping a 9.8 gram weight through a 4-foot Plexiglas tube. To the tip of the weight, a 1.5 mm thick, 5 mm in diameter piece of butyl rubber (16” bicycle inner tube) was attached (using cyanoacrylic) to reduce the possibility of skull fractures. Sham animals were exposed to identical periods of anesthesia but not injured.
The CA was purchased from Cayman Chemical (cat# 89820). A stock CA solution (7.5 mg/mL in ethanol) was diluted in sterile saline and administered IP (1 mg/kg; 30 min after each injury) as described previously.12 Acute neurological assessments were performed immediately after each injury. Drug administration, motor skills, and cognitive testing were performed as outlined in Figure 1A.
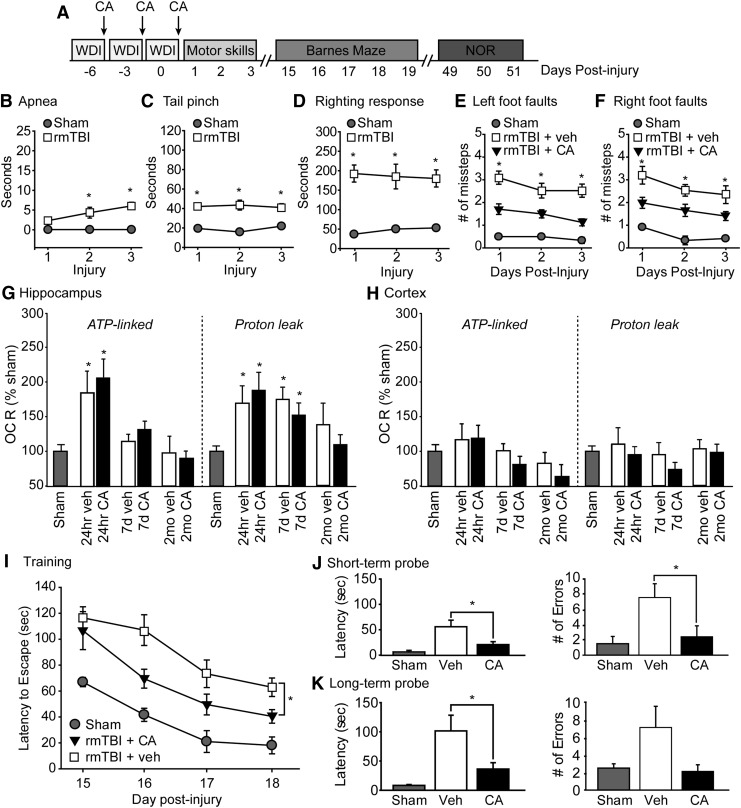
FIG. 1.
Post-injury carnosic acid (CA) treatment improves motor and cognitive performance after repeat mild traumatic brain injury (mTBI). (A) Timeline for injury, CA administration, motor and cognitive assessments. (B) Length of apnea, (C) period of suppression of pain reflex, and D) the duration of suppression of righting reflex are increased after repetitive mTBI (rmTBI) rmTBI (n = 10 sham, n = 16 rmTBI) *significant difference compared with sham. Mice sustaining rmTBI made significantly more (E) left and (F) right foot fault errors (assessed using a 2 cm × 2 cm wire grid) that were reduced by CA treatment. *significant difference between vehicle and CA treated animals. The rmTBI acutely increased adenosine triphosphate (ATP)-linked oxygen consumption rate (OCR) and proton leak in the (G) hippocampus, but not the (H) cortex. These changes were unaffected by CA treatment. *significant differences compared with sham. (I) The rmTBI mice treated with CA had improved learning in the Barnes maze tested 15 days after the last injury and CA treatment as indicated by significantly reduced latencies to enter the escape hole during training. *significant difference between vehicle and CA treated mice. The rmTBI mice treated with CA had improved (J) short-term and (K) long-term memory as indicated by reduced latency to find the target hole and fewer errors when tested 30 min and 24 h after training. *significant difference between vehicle and CA treated mice. Data are presented as mean ± standard error of the mean. *p < 0.05.
Results
Figure 1B shows that the duration of apnea between sham (n = 10) and rmTBI (n = 16) progressively became longer, resulting in a statistically significant increase compared with sham controls that only received anesthesia (F = 23.303, p < 0.001). The duration of suppression of pain reflex (F = 25.165, p < 0.001; Fig. 1C) and latency to recover the righting response (F = 21.770, p < 0.001; Fig. 1D) were found to be significantly different between rmTBI mice and sham controls after each injury. Acute CA treatment (30 min after each injury, for a total of four IP injections) had no significant effect on acute apnea, pain reflex, or righting response measures compared with rmTBI mice receiving vehicle injections.
When motor coordination was tested using the foot fault task after completion of treatments,17 however, CA treated animals had improved motor function as indicated by significantly fewer left (F = 32.33, p < 0.001) and right (F = 14.427, p = 0.002) foot faults recorded one to three days after the last injury (Fig. 1E, 1F).
To examine the effect of rmTBI on mitochondrial respiration, hippocampal and cortical tissue punches (0.5 mm in diameter) were prepared 24 h, seven days, and two months after the last injury from sham, rmTBI mice treated with vehicle, and rmTBI mice treated with CA (four punches from three to four sections per structure from n = 4/group). Oxygen consumption rate (OCR) was measured using a Seahorse XF96 analyzer. The summary data shown in Figure 1G indicate that rmTBI acutely increased adenosine triphosphate (ATP)-linked respiration (as assessed using oligomycin, an F1-Fo ATP synthase/respiratory complex V inhibitor; F = 8.31, p < 0.001) in hippocampal tissue punches. This increase in ATP-linked respiration was accompanied by an increase in proton leak (F = 5.23, p = 0.003 for 24 h, p = 0.001 for seven days).
Acute, post-injury CA treatment did not influence these measures significantly. No significant improvements in either ATP-linked respiration or proton leak were observed in cortical tissue punches from rmTBI mice (Fig. 1H), suggesting that hippocampal mitochondria may be vulnerable particularly to rmTBI.
Spatial learning and memory were tested beginning 15 days after the last injury using the Barnes maze. Training consisted of four trials per day for thee consecutive days followed by a short-term memory test performed 30 min later. The acquisition curve of a group of sham animals (n = 4) is demonstrated to show normal learning in the task (Fig. 1I). When the learning curves of rmTBI animals treated with vehicle (n = 7) and rmTBI animals treated with CA (n = 8) were compared, a group main effect of treatment was observed (F = 5.304, p = 0.040; Fig. 1I). When short-term memory was assessed, rmTBI animals treated with CA took less time to find the target hole (t = 2.472, p = 0.029) and made fewer errors (t = 2.230, p = 0.046; Fig. 1J) than their vehicle-treated counterparts.
To test long-term spatial memory, mice were given four reminder trials, and their memory tested 24 h later. The rmTBI mice treated with CA had reduced latency to find the target hole (t = 2.243, p = 0.045) and trended toward making fewer errors (t = 1.980, p = 0.071; Fig. 1K) than rmTBI mice treated with vehicle.
The novel object recognition (NOR) task was performed 49 days after the last injury as described previously.18,19 Figure 2 shows that while sham animals (n = 4) have intact recognition memory as indicated by significantly more time spent exploring the novel versus familiar object, rmTBI mice treated with vehicle (n = 7) displayed persistent short- (t = -2.130, p = 0.055; Fig. 2A) and long- (t = 0.736, p = 0.476; Fig. 2B) term recognition memory impairments. In contrast, acute post-injury treatment (administered 30 min after each injury) with CA (n = 8) caused an improvement in short- (t = -6.373, p < 0.001; Fig. 2A) and long- (t = -10.552, p < 0.001; Fig. 2B) term recognition memory tested at 30 min and 24 h, respectively.
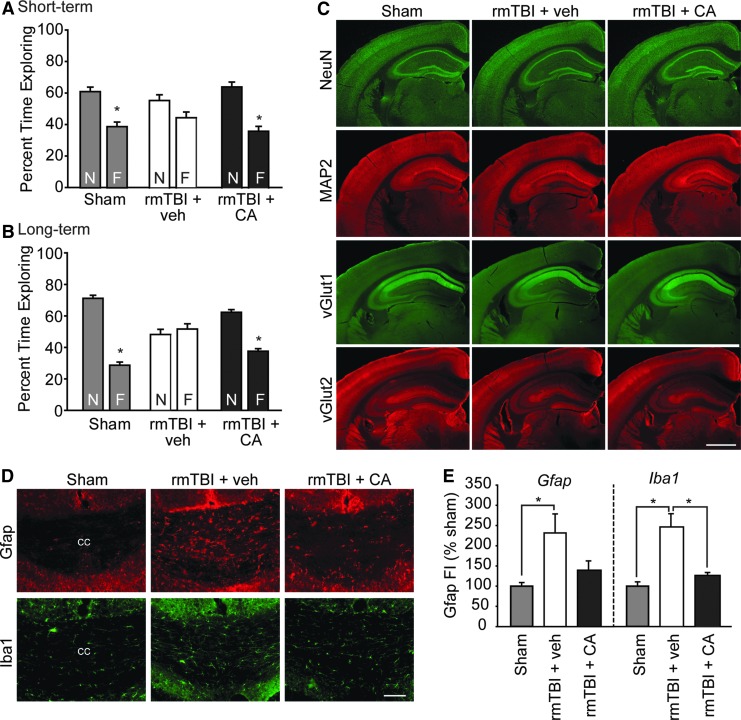
FIG. 2.
Mice with repetitive mild traumatic brain injury (rmTBI) present impaired recognition memory in the chronic stage of injury that was associated with white matter inflammation. (A) Short- and (B) long-term recognition memory was tested beginning 49 days after injury and CA treatment. Sham animals explore the novel object (N) significantly longer than a familiar object (F), indicating intact recognition memory. The rmTBI mice had impaired recognition memory as indicated by no preference for the novel object. The rmTBI mice acutely treated with CA perform similar to sham controls. *significant difference between novel and familiar object. (C) The rmTBI did not cause visible neuronal or dendritic loss as assessed by NeuN and MAP2 immunoreactivities, respectively. No overt changes in synaptic proteins vGlut1 or vGlut2 were observed. Scale bar = 1 mm. (D) Representative photomicrographs showing that rmTBI increased astrocyte (indicated by Gfap immunoreactivity) and microglia (indicated by Iba1 immunoreactivity) activation in the corpus callosum. These increased immunoreactivities were visibly reduced by acute CA treatment. Scale bar = 100 μm. (E) Summary data showing that acute CA treatment significantly reduced Iba1 immunoreactivity in white matter tracts after rmTBI (n = 4/group). Data are presented as mean ± standard error of the mean. *p < 0.05.
After the completion of the behavioral testing (∼2 months post-injury), mice (n = 4/group) were euthanized and brains processed for histological examination. Immunostaining of brain sections using the neuronal marker NeuN (Millipore, cat# MAB377), dendritic marker MAP2 (Millipore, cat# AB5622), and the synaptic vesicle proteins vGlut1 (Synaptic Systems, cat# 135303) and vGlut2 (Synaptic Systems, cat# 135411) did not reveal any overt differences between the treatment groups (Fig. 2C).
We next examined whether this rmTBI increases markers of neuroinflammation (i.e., Gfap and Iba120,21), and whether post-injury CA influences these changes. Although we did not observe any changes in Gfap (a marker of astrocytes) or Iba1 (a marker of microglial activation20) immunoreactivity in either the injured cortex or hippocampus, an increase in Gfap immunoreactivity in the corpus callosum (Fig. 2D), the cingulum, and the external capsule could be seen. These increases appeared to be reduced in rmTBI mice treated with CA. The Iba1 immunoreactivity was also found to be elevated in white matter tracts after rmTBI, an effect that was reduced by acute post-injury administration of CA.
Quantification of these immunoreactivities (by measuring fluorescence intensity) revealed that both Gfap (F = 5.926, p = 0.026) and Iba1 (F = 19.194, p < 0.001) immunoreactivities are significantly increased as a result of rmTBI (Fig. 2E). Post hoc analysis revealed that white matter Iba1 immunoreactivity was significantly reduced by CA treatment (p = 0.004). Gfap was reduced as a result of CA treatment, although this did not reach statistical significance.
Discussion
Our results revealed the following key findings: (1) rmTBI causes significant learning and memory dysfunction in the absence of overt neuronal loss or damage, (2) CA, when administered 30 min after each injury, markedly improves motor and cognitive function after rmTBI, and (3) CA reduced markers of neuroinflammation in white matter tracts. At present, we are uncertain whether the modest inflammation we observed in white matter tracts is the underlying mechanism for the cognitive dysfunction seen after rmTBI.
Mitochondrial damage has been linked to both tissue loss as well as cognitive dysfunction after TBI.22–26 For example, a recent study by Lyons and associates showed that a single mild TBI can cause mitochondrial dysfunction evident at 28 days post-injury.27 Although the rmTBI model employed in our study acutely increased tissue respiration and proton leak, we did not observe any significant changes in mitochondrial respiration at two months post-injury.
Carnosic acid has been reported to protect mitochondria in cultured cells exposed to toxic agents.28 A previous study by Miller and colleagues29 has reported that isolated mitochondria from CA-treated animals (1 mg/kg) display reduced inhibition of respiratory complex I when exposed to 4-HNE.12 Contrary to our expectations, CA did not affect the changes in mitochondrial respiration we observed after rmTBI. The reason for these discrepancies is unclear but may be because of differences in injury magnitude (moderate-severe CCI vs. mild closed head injury) and/or mode for assessments for mitochondrial function (isolated mitochondria vs. tissue respiration) could be contributing factors.
In addition to activating cytoprotective genes, CA has been reported to inhibit NF-κB and reduce prostaglandin E2 production in lipopolysaccharide (LPS)-stimulated macrophages.11 Moreover, CA has been shown to decrease LPS-induced activation of microglia and reduce cytokine (e.g.. interleukin (IL)-1α, IL-6) and chemokine (e.g.. CCL5, CXCL10) production.11,30 Consistent with the anti-inflammatory action of CA, we observed that acute CA treatment decreased Gfap and Iba-1 immunoreactivities in white matter tracts after rmTBI.
Conclusion
Our results show that repeat concussive injury markedly impairs cognitive function that can persist for more than a month after the injuries. These deficits occurred in the absence of overt brain or neuronal pathologies, with only a modest increase in astrocyte and microglial activation observed in white matter tracts. Acute treatment with CA reduced these markers of neuroinflammation and significantly improved cognitive function. One limitation of this study is that only male mice were used. According to the Centers for Disease Control and Prevention, females accounted for approximately 45% of TBI-related emergency room visits, and 25% of TBI-related deaths in 2010.
Recently, it has been found that the male and female brain may respond differently to injury, including the magnitude of inflammation, the types of symptomology, and the degree of brain damage,31,32 suggesting that mechanism-based treatments may have different effects in males and females. The ability of CA to treat TBI in females remains to be determined.
Acknowledgements
This research was supported in part by grants from the National Institutes of Health (NS087149, NS086301), and funds made available by the Gillson Longenbaugh Foundation/Mission Connect.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Fidan E., Lewis J., Kline A.E., Garman R.H., Alexander H., Cheng J.P., Bondi C.O., Clark R.S., Dezfulian C., Kochanek P.M., Kagan V.E., and Bayir H. (2016). Repetitive mild traumatic brain injury in the developing brain: effects on long-term functional outcome and neuropathology. J. Neurotrauma 33, 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mouzon B.C., Bachmeier C., Ojo J.O., Acker C.M., Ferguson S., Paris D., Ait-Ghezala G., Crynen G., Davies P., Mullan M., Stewart W., and Crawford F. (2018). Lifelong behavioral and neuropathological consequences of repetitive mild traumatic brain injury. Ann. Clin. Transl. Neurol. 5, 64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manley G., Gardner A.J., Schneider K.J., Guskiewicz K.M., Bailes J., Cantu R.C., Castellani R.J., Turner M., Jordan B.D., Randolph C., Dvorak J., Hayden K.A., Tator C.H., McCrory P., and Iverson G.L. (2017). A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 51, 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayes J.D. and Dinkova-Kostova A.T. (2014). The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218 [DOI] [PubMed] [Google Scholar]
- 5. Zhao J., Kobori N., Aronowski J., and Dash P.K. (2006). Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci. Lett. 393, 108–112 [DOI] [PubMed] [Google Scholar]
- 6. Zhao J., Moore A.N., Redell J.B., and Dash P.K. (2007). Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J. Neurosci. 27, 10240–10248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lipton S.A., Rezaie T., Nutter A., Lopez K.M., Parker J., Kosaka K., Satoh T., McKercher S.R., Masliah E., and Nakanishi N. (2016). Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer's disease models. Cell Death Dis. 7, e2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma Q. (2013). Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mimura J., Kosaka K., Maruyama A., Satoh T., Harada N., Yoshida H., Satoh K., Yamamoto M., and Itoh K. (2011). Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J. Biochem. 150, 209–217 [DOI] [PubMed] [Google Scholar]
- 10. Aruoma O.I., Spencer J.P., Rossi R., Aeschbach R., Khan A., Mahmood N., Munoz A., Murcia A., Butler J., and Halliwell B. (1996). An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs. Food Chem. Toxicol. 34, 449–456 [DOI] [PubMed] [Google Scholar]
- 11. Schwager J., Richard N., Fowler A., Seifert N., and Raederstorff D. (2016). Carnosol and related substances modulate chemokine and cytokine production in macrophages and chondrocytes. Molecules 21, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller D.M., Singh I.N., Wang J.A., and Hall E.D. (2015). Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 264, 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marmarou A., Foda M.A., van den Brink W., Campbell J., Kita H., and Demetriadou K. (1994). A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 80, 291–300 [DOI] [PubMed] [Google Scholar]
- 14. Kalish B.T. and Whalen M.J. (2016). Weight drop models in traumatic brain injury. Methods Mol. Biol. 1462, 193–209 [DOI] [PubMed] [Google Scholar]
- 15. Vagnozzi R., Tavazzi B., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro V., Finocchiaro A., and Lazzarino G. (2007). Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment—part I. Neurosurgery 61, 379–388 [DOI] [PubMed] [Google Scholar]
- 16. Tavazzi B., Vagnozzi R., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro V., Finocchiaro A., and Lazzarino G. (2007). Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses—part II. Neurosurgery 61, 390–395 [DOI] [PubMed] [Google Scholar]
- 17. Dash P.K., Hylin M.J., Hood K.N., Orsi S.A., Zhao J., Redell J.B., Tsvetkov A.S., and Moore A.N. (2015). Inhibition of eukaryotic initiation factor 2 alpha phosphatase reduces tissue damage and improves learning and memory after experimental traumatic brain injury. J. Neurotrauma 32, 1608–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prins M.L., Hales A., Reger M., Giza C.C., and Hovda D.A. (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 32, 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis A.R., Shear D.A., Chen Z., Lu X.C., and Tortella F.C. (2010). A comparison of two cognitive test paradigms in a penetrating brain injury model. J. Neurosci. Methods 189, 84–87 [DOI] [PubMed] [Google Scholar]
- 20. Lafrenaye A.D., Todani M., Walker S.A., and Povlishock J.T. (2015). Microglia processes associate with diffusely injured axons following mild traumatic brain injury in the micro pig. J. Neuroinflammation 12, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Floyd C.L. and Lyeth B.G. (2007). Astroglia: important mediators of traumatic brain injury. Prog. Brain Res. 161, 61–79 [DOI] [PubMed] [Google Scholar]
- 22. Hubbard W.B., Harwood C.L., Geisler J.G., Vekaria H.J., and Sullivan P.G. (2018). Mitochondrial uncoupling prodrug improves tissue sparing, cognitive outcome, and mitochondrial bioenergetics after traumatic brain injury in male mice. J. Neurosci. Res. 96, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bains M. and Hall E.D. (2012). Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta 1822, 675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fischer T.D., Hylin M.J., Zhao J., Moore A.N., Waxham M.N., and Dash P.K. (2016). Altered mitochondrial dynamics and TBI pathophysiology. Front. Syst. Neurosci. 10, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kochanek P.M., Jackson T.C., Ferguson N.M., Carlson S.W., Simon D.W., Brockman E.C., Ji J., Bayir H., Poloyac S.M., Wagner A.K., Kline A.E., Empey P.E., Clark R.S., Jackson E.K., and Dixon C.E. (2015). Emerging therapies in traumatic brain injury. Semin. Neurol. 35, 83–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiong Y., Gu Q., Peterson P.L., Muizelaar J.P., and Lee C.P. (1997). Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14, 23–34 [DOI] [PubMed] [Google Scholar]
- 27. Lyons D.N., Vekaria H., Macheda T., Bakshi V., Powell D.K., Gold B.T., Lin A.L., Sullivan P.G., and Bachstetter A.D. (2018). A mild traumatic brain injury in mice produces lasting deficits in brain metabolism. J. Neurotrauma 20, 2435–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Oliveira M.R., Peres A., Ferreira G.C., Schuck P.F., Gama C.S., and Bosco S.M. (2017). Carnosic acid protects mitochondria of human neuroblastoma SH-SY5Y cells exposed to paraquat through activation of the Nrf2/HO-1 axis. Mol. Neurobiol. 54, 5961–5972 [DOI] [PubMed] [Google Scholar]
- 29. Miller D.M., Singh I.N., Wang J.A., and Hall E.D. (2013). Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic. Biol. Med. 57, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yanagitai M., Itoh S., Kitagawa T., Takenouchi T., Kitani H., and Satoh T. (2012). Carnosic acid, a pro-electrophilic compound, inhibits LPS-induced activation of microglia. Biochem. Biophys. Res. Commun. 418, 22–26 [DOI] [PubMed] [Google Scholar]
- 31. Villapol S., Loane D.J., and Burns M.P. (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia 65, 1423–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caplan H.W., Cox C.S., and Bedi S.S. (2017). Do microglia play a role in sex differences in TBI? J. Neurosci. Res. 95, 509–517 [DOI] [PubMed] [Google Scholar]