Abstract
Early molecular and developmental events impacting many incurable mitochondrial disorders are not fully understood and require generation of relevant patient- and disease-specific stem cell models. In this study, we focus on the ability of a nonviral and integration-free reprogramming method for deriving clinical-grade induced pluripotent stem cells (iPSCs) specific to Leigh's syndrome (LS), a fatal neurodegenerative mitochondrial disorder of infants. The cause of fatality could be due to the presence of high abundance of mutant mitochondrial DNA (mtDNA) or decline in respiration levels, thus affecting early molecular and developmental events in energy-intensive tissues. LS patient fibroblasts (designated LS1 in this study), carrying a high percentage of mutant T8993G mtDNA, were reprogrammed using a combined mRNA–miRNA nonviral approach to generate human iPSCs (hiPSCs). The LS1-hiPSCs were evaluated for their self-renewal, embryoid body (EB) formation, and differentiation potential, using immunocytochemistry and gene expression profiling methods. Sanger sequencing and next-generation sequencing approaches were used to detect the mutation and quantify the percentage of mutant mtDNA in the LS1-hiPSCs and differentiated derivatives. Reprogrammed LS-hiPSCs expressed pluripotent stem cell markers including transcription factors OCT4, NANOG, and SOX2 and cell surface markers SSEA4, TRA-1-60, and TRA-1-81 at the RNA and protein level. LS1-hiPSCs also demonstrated the capacity for self-renewal and multilineage differentiation into all three embryonic germ layers. EB analysis demonstrated impaired differentiation potential in cells carrying high percentage of mutant mtDNA. Next-generation sequencing analysis confirmed the presence of high abundance of T8993G mutant mtDNA in the patient fibroblasts and their reprogrammed and differentiated derivatives. These results represent for the first time the derivation and characterization of a stable nonviral hiPSC line reprogrammed from a LS patient fibroblast carrying a high abundance of mutant mtDNA. These outcomes are important steps toward understanding disease origins and developing personalized therapies for patients suffering from mitochondrial diseases.
Keywords: mitochondrial disease, Leigh's syndrome, hiPSC, pluripotent stem cell, heteroplasmy, differentiation, reprogramming
Introduction
Human mitochondrial DNA (mtDNA) disorders affect energy-intensive tissues such as the brain, heart, and muscle, and are clinically complex and often fatal [1,2]. These disorders represent a large group of diseases with heterogeneous clinical and pathological expressions characterized by improper functions of and sometimes irreversible damage to specialized neuronal or cardiac cells or populations. The causes and mechanisms of cell death and related defects in many of these disorders, although not fully understood, derive from a high abundance of mutations in mtDNA or decline in energy levels. Mutant mtDNA not only influences clinical severity in rare childhood mitochondrial disorders, but also affects common disorders in adults, such as Parkinson's and Alzheimer's disorders, diabetes, cardiovascular disorders, and infertility [3,4].
Energy-intensive tissues are particularly vulnerable to insults (mutation and deletions) to mtDNA or respiration defects in the electron transport chain, thus contributing to heterogeneity seen in various clinical phenotypes [1,5,6]. Clinical severity can also be influenced by the percentage of mutant mtDNA genome versus normal mtDNA genome (heteroplasmy) in affected tissues or cells leading to impaired respiration.
mtDNA mutations are often heteroplasmic, where both wild type and mutant mtDNA coexist at an organismal, tissue, or cellular level. Mitochondrial disorders such as Leigh's syndrome (LS) arise due to a high abundance of mutant mtDNA, leading to respiratory defects [7,8]. A high abundance of mutant mtDNA influences the severity of the symptoms in LS patients causing developmental delays, respiratory and muscle impairments, cardiac defects, and chronic energy loss in cells of various affected tissues [8]. The classic example used in this study is the T8993G mutation present in the mtDNA-encoded ATP synthase 6 gene. When the mutation is present in low abundance, it results in NARP (neurogenic, muscle weakness, ataxia, and retinitis pigmentosa) disease, and the individuals survive to adulthood.
However, when the mutation is present in high abundances, it results in LS disease, leading to rapid lethality in infants [9–11]. Currently there is no specific treatment available for any of these disorders. Therefore, these diseases that are unique in their polyploidy of mitochondrial genetics are in dire need of patient-specific models to develop therapeutics.
The percentage of mutation load correlates with disease severity, so understanding the mechanism of heteroplasmy inheritance is of major medical importance. However, the precise mechanism of heteroplasmy is still unknown and remains controversial. One school of thought explained the rapid shift in heteroplasmy between generations, by the mitochondrial genetic “bottleneck hypothesis,” whereby only a small number of the mtDNA molecules rapidly multiplied to populate the entire cell or organism during a single cell division [12–14]. Other studies showed a wide range of heteroplasmy values in primary human cells and proposed “random genetic drift” as a possible mechanism [15,16]. A major confounding factor that must be taken into consideration when studying heteroplasmy variance is that determining mechanism of inheritance is critically dependent on the initial heteroplasmy level.
Initial efforts carried out by our research team and others have focused on mtDNA manipulations in either the oocyte, embryonic stem cell, or progenitor cell stage in mammalian development to predict disease severity and ultimately design therapeutic strategies that eliminate and/or reduce developmental delays caused due to the high percentage of mutant mtDNA [17–19]. Taking advantage of viral-based reprogramming methodologies pioneered by Yamanaka, two groups have successfully created human-induced pluripotent stem cells (hiPSCs) for two mitochondrial diseases (mitochondrial encephalomyopathy [MELAS], lactic acidosis, and stroke-like episodes; Pearson's syndrome [PS]) [20,21]. Their studies showed that isogenic hiPSCs exhibited variable mutant mtDNA levels, which influenced the cell fate and mitochondrial function.
Although the mechanism of why the reprogrammed hiPSC clones retained varying levels of mutant mtDNA levels was unknown, these studies are particularly attractive for understanding and treating mitochondrial disorders because cytoplasmic genetic material is retained during direct reprogramming. However, use of the integrating viral-based reprogramming methodology raises significant concerns with respect to random insertional mutagenesis, leading to unknown off target gene expression and commonly observed spontaneous reactivation of integrated reprogramming genes causing abnormal gene expression and differentiation of derived cells [22]. Both of these factors could significantly affect how these mtDNA-derived induced pluripotent stem cell (iPSC) lines differentiate and respond to potential therapeutics.
The use of virus-derived cells also raises concerns of tumorigenicity, eliminating the potential for these cells for transplant therapy upon correction of mtDNA defects [22]. An improvement to these strategies is the use of modified mRNAs encoding the reprogramming factors OCT4, SOX2, c-MYC, KLF4, and LIN28A [23]. In addition to eliminating concerns of insertional mutagenesis and spontaneous reactivation of reprogramming genes, mRNA reprogramming is typically faster and generates higher yields [24]. mRNA reprogramming also allows for dosage control of multiple proteins simultaneously, thus permitting much more control of the reprogramming process. It is also reasonable to conclude that upon further investigation, direct differentiation procedures can be developed using appropriate mRNAs [23].
In this study, we have used a combined mRNA–miRNA nonviral approach to generate hiPSCs from a LS patient-fibroblast cells containing T8993G mutation in high abundance. We optimized the reprogramming conditions to ensure that mitochondrial genome integrity is maintained, fully characterized the generated LS1-hiPSC lines, demonstrated differentiation potential, and used next-generation sequencing methods to quantitate the heteroplasmy burden in patient fibroblasts, LS1-hiPSCs, and differentiated cell populations.
Materials and Methods
Cell culture
Primary culture of healthy control BJ (ATCC® CRL-2522™) fibroblasts (ATCC, Manassas, VA) and LS LS1 fibroblasts (female, 3 years at diagnosis of LS) was maintained in fibroblast medium (minimal essential medium [MEM] w/10% fetal bovine serum [FBS] and 2 mM glutamine). Fibroblasts were briefly expanded through 0.05% trypsin enzymatic passaging. hiPSCs generated from these fibroblasts as well as H9 healthy control human embryonic stem cell (hESC) lines were maintained and expanded on feeder-free defined extracellular matrix, Growth Factor Reduced Matrigel™ (BD Biosciences, San Jose, CA), and coated cell culture dishes in mTeSR1 medium (Stem Cell Technologies, Vancouver, Canada). Stem cell colonies were enzymatically passaged at high density (1:3–1:6) using Dispase 1 mg/mL (Stem Cell Technologies). All cell cultures were handled in Biosafety Type II sterile hoods regularly cleaned with UV irradiation and 70% ethanol, and were grown in 37°C incubators at 5% CO2 and 95% humidity.
Somatic cell reprogramming to hiPSC cultures
Rapid nonintegrating mRNA reprogramming of the BJ control and LS1 patient fibroblasts was performed using the mRNA reprogramming factor set and microRNA Booster Kit (both from Reprocell, Beltsville, MD). In brief, fibroblast cultures were transfected daily for 12 days with modified mRNAs OCT4, SOX2, c-MYC, KLF4, and LIN28A, along with an epidermal growth factor receptor (eGFR) transfection-reporter mRNA [24]. In addition, a cocktail of proprietary microRNAs available as part of the booster kit was added to the transfection mixture on days 0 and 4 to accelerate the reprogramming process. The LS disease cell lines were recalcitrant to initial reprogramming attempts.
Successful reprogramming to stable pluripotent hiPSC colonies was achieved using NuFF irradiated human fibroblasts (GSC-3002G, GlobalStem, Rockville, MD) conditioned antibiotic-free medium, feeder-free conditions using Matrigel-coated cell dishes, and pretreatment with the type-1 interferon inhibitor B18R (Thermo Fisher Scientific, Waltham, MA). Initial fibroblast plating density of 5–7.0 × 104 cells per well of the six-well plate was found to be optimal. After 12 days of transfections, well-circumscribed stem-cell-like colonies began to arise. hiPSC colonies were confirmed by live cell microscopy staining with Alexa 488-fluorophore-conjugated TRA-1-81 StainAlive™ antibody and manually harvested on days 14–15 using pulled glass pipette tip hooks.
Pluripotency and differentiation analysis
For confirmation of pluripotency, hiPSC cultures and hiPSC-differentiated cultures were tested for expression of pluripotency markers and for tri-lineage differentiation potential by immunofluorescence (IF) staining and quantitative real-time polymerase chain reaction (RT-PCR). Stem cells were nonspecifically differentiated through adherent culture protocol [25]. Stem cells were grown to confluence in mTeSR medium on Matrigel-coated plates or chamber slides, washed with Dulbecco's modified Eagle's medium (DMEM), and then cultured for an additional 21 days in defined differentiation medium: DMEM/F-12 with 20% KnockOut Serum Replacement, β-mercaptoethanol, 2 mM Glutamax, and 0.1 mM nonessential amino acids (all from Invitrogen/Gibco, Grand Island, NY). Embryoid body (EB) differentiation was carried out using Aggrewell plates (Stem Cell Technologies) according to established protocols that allow for creating uniform cellular aggregates of equal starting number [26].
In brief, stem cells grown to 70%–80% confluence were dissociated to single cells using Accutase (Stem Cell Technologies), washed, single cell strained through a 37 μm filter, and resuspended in EB formation medium supplemented with 10 μM ROCK inhibitor Y-27632 (Stem Cell Technologies) to improve dissociated stem cell survival [27]. Resuspended single cells were counted for number and concentration of live cells and plated 1.2 × 106 cells per well to generate EBs of 1,000 cells each. Cell solutions were gently pipetted to ensure even distribution of cells within the wells and centrifuged at 100 g for 3 min. Aggrewell plates were cultured in incubators at 37°C with 5% CO2 and 95% humidity for 24 h before collection of spherical EBs for culture in ultra-low adherence plates in EB medium.
Immunocytochemical analysis
For IF cell marker detection, cells were cultured on Matrigel-coated glass chamber slides (Nunc Lab-Tek II Chamber Slide System; Thermo Fisher Scientific). Cultures were fixed in 4% paraformaldehyde solution for 15 min, followed by washes in phosphate-buffered saline (PBS) with Ca and Mg. For intracellular epitope antibody staining, fixed cells were permeabilized with 0.1% Triton X-100 and 1% polyvinylpyrrolidone in a 4% normal goat serum PBS blocking solution. For extracellular epitopes, cells were blocked in 4% normal goat serum containing PBS. Primary antibodies were diluted in the respective blocking solutions, with concentrations listed hereunder, and incubated for 1 h at room temperature.
The primary antibodies used for hiPSC characterization are POU5F1/OCT4 (cat. no. AF1759; 1:200, R&D Systems), NANOG (cat. no. AB9220, 1:200; Millipore), SOX2 (cat. no. MAB2018, 1:200; R&D Systems), SSEA-4 (cat. no. MC-813-70; DSHB 1:200), TRA-1-60 (cat. no. MAB4360; 1:200, Millipore-Sigma), and TRA-1-81 (cat. no. MAB4381; 1:200, Millipore-Sigma). After washes, fluorophore-labeled secondary antibodies Alexa Fluor 488 and Alexa Fluor 594 were used to detect the primary antibodies. Immunofluorescently labeled cells were washed, cell nuclei costained with 4′,6-diamidino-2-phenylindole (DAPI) (1:1,000), and slides sealed with Prolong Gold (Invitrogen). BJ and LS1-hiPSC cultures at passage #9 were stained for intracellular (OCT4, NANOG, and SOX2) and extracellular (SSEA4, TRA-1-60, and TRA-1-81) markers of pluripotency. Parental fibroblasts at passage #5 and H9 hESCs at passage #55 were also stained as negative and positive controls, respectively.
Differentiated cultures generated from hiPSCs and hESCs were stained for intracellular cytoskeletal markers of each of the three embryonic germ layers: βIIITub and MAP2 for neural ectoderm, desmin (DES) and alpha smooth muscle actin (αSMA) for muscle mesoderm, and vimentin (VIM) for mesendodermal endoderm. The primary antibodies used for characterization of hiPSC differentiation are βIIITub (1:1,000 dilution, Novus Bio NB100-1612), MAP2 (1:500 dilution, Millipore AB5622), DES (1:100 dilution, Thermo RB-9014-P1), αSMA (1:800 dilution, Thermo MS-113-P1), and VIM (1:200 dilution, BD Bioscience 550513). Undifferentiated hiPSCs were stained as negative controls.
Gene expression measurements
For pluripotency and germ layer differentiation analysis, total mRNA was extracted from cell samples using RNeasy Plus Mini kits with gDNA eliminator column (Qiagen, CA), and quantified using NanoDrop 8000 spectrometer (Thermo Scientific, MA). Reverse transcription cDNA synthesis was performed on 1 μg total mRNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative RT-PCR analysis of 94 pluripotency and differentiation lineage gene primers was conducted on the Applied Biosystems TaqMan human pluripotent stem cell (hPSC) Scorecard panel (Thermo Scientific) using the 7900HT Real-Time PCR system with 384w block (Thermo Scientific), in accordance with the instructions of the manufacturer. The TaqMan hPSC Scorecard Kit is a predesigned gene expression quantitative PCR (qPCR) assay consisting of the TaqMan probes specific for reference markers.
The contents of the scorecard panel are well established and have been validated against multiple hESC and hiPSC lines [28]. Reference standards include 94 validated controls, housekeeping, self-renewal, and lineage-specific genes. The resulting expression data set was analyzed by using TaqMan hPSC scorecard software (Thermo Scientific) to compare acquired gene expression patterns with assay-included reference standards.
mtDNA isolation and purification
Frozen cell pellets from different samples containing ∼500,000 cells were thawed and processed. The QIAamp DNA mini kit (Qiagen, CA) manufacturer protocol was followed to extract total DNA, which resulted in an elution of 80 μL of distilled water (dH2O) and total DNA from all cells. To obtain the circular mtDNA, all other DNA was degraded using the Plasmid-Safe ATP-Dependent DNase (Epicentre, WI). This product selectively degrades all noncircular DNA molecules. The manufacturer-recommended minipreparation protocol was followed. The 50 μL solutions containing the mtDNA were further treated with 1 μL of RNaseA for 1 h at 37°C to further avoid any non-mtDNA nucleic acids. It is expected that at this point, the mtDNA should be intact, and all other forms of DNA should be degraded. The samples were further processed before use in sequencing protocols.
To rid the mtDNA samples of previously used enzymes, salts, the samples were cleaned using the UltraClean 15 DNA Purification Kit (MoBio, CA). To ensure the mtDNA was maintained in a 16 kb circle, the samples were run on a 0.8% agarose gel, and the 16 kb band that appeared for each sample was extracted from the gel. Because the samples were extracted from an agarose gel, they needed to be cleaned again using the UltraClean 15 DNA Purification Kit. With the mtDNA isolated and purified, the samples were prepared for sequencing.
Sample preparation for sequencing
The previous procedures resulted in a 30 μL solution of dH2O with an unknown quantity of mtDNA. It was necessary to quantify and dilute the amount of mtDNA to meet the specifications for Sanger sequencing and whole exome next-generation sequencing. The mtDNA was quantified using a NanoDrop One UV/Vis Spectrophotometer (Thermo Scientific). A blank of 1.5 μL of dH2O was used to establish a zero, and 1.5 μL of each sample was used to determine the concentration. Exome sequencing requires ∼25 ng of DNA, and the samples were diluted accordingly. Sanger sequencing, however, requires the area of interest (region of ATP6 gene containing 8993) to be PCR amplified, purified, and diluted to a concentration of 10 ng/μL.
Primers for use in PCR were generated using the human mtDNA sequence provided by mitomap.org [3] and IDT's PrimerQuest tool (IDT, Coralville, Iowa). Forward primer: TATCGAAACCATCAGCCTACTC; reverse primer: GCTTCCAATTAGGTGCATGAG generates a 100 bp amplified sequence that contains the 8993 site. A standard PCR was carried out using the Takara Taq PCR Amplification Kit (Clontech, Mountain View, CA). Ten microliters of the PCR product for each sample was run on a 2% agarose gel to confirm proper amplification of the region of interest. After gel confirmation, the PCR products were cleaned again using the UltraClean 15 DNA Purification Kit (Mo Bio, Carlsbad, CA). The cleaned PCR products were quantified using a NanoDrop One UV/Vis Spectrophotometer (Thermo Scientific), and diluted to 10 ng/μL with dH2O for Sanger sequencing. The primers were also diluted to 20 ng/μL for use in Sanger sequencing.
Sanger sequencing analysis
Sequencing reactions were carried out with the forward and reverse primers as designed to amplify the specific region of mtDNA. The cycle sequencing reaction was carried out on a 96-capillary 3730XL (Applied Biosystems) with BigDye Taq FS Terminator V 3.1. The genotyping and fragment sizing were done on a 3130XL (Applied Biosystems). The files generated from the sequencing were observed using CodonCode Aligner (CodonCode Corporation, Centerville, MA).
Next-generation sequencing for whole exome and heteroplasmy analysis
The DNA concentration was verified using a Qubit fluorometer (Thermo Scientific). Instead of the standard DNA fragmentation, an enzymatic fragmentation was performed using the KAPA Frag Enzyme from the KAPA HyperPlus Library Preparation Kit (KAPA Biosystems, Wilmington, MA). This alternative was performed to increase yield during the fragmentation step. Fragmented DNA was purified using Ampure beads (Beckman Coulter, Brea, CA). DNA libraries were prepared using the Accel-NGS 2S Plus DNA Library Kit (Swift Biosciences, Ann Arbor, MI). Ten PCR cycles were carried out during the Library Amplification step. The final libraries were analyzed with a 2100 Bioanalyzer to assess library size distribution (Agilent Technologies, Santa Clara, CA).
DNA libraries were quantified with the KAPA Library Quantification Kit to ensure accuracy (KAPA Biosystems). Based on the qPCR results, the DNA libraries were compiled in equimolar amounts and sequenced with the HiSeq 2500 using TruSeq v3 reagents according to the 2 × 100 bp protocol (Illumina, San Diego, CA).
The file generated from sequencing was separated out into FASTQ files using bcl2fastq software version 2.17 (Illumina). These files were aligned to the mtDNA reference human genome 19 with Burrows–Wheeler Alignment Tool [29]. The alignment tool generates sequence alignment map (SAM) files, which are sorted based on the coordinates of the sequence. The SAM files are then converted to a compressed form, BAM format, using SAMTools. PCR duplicates were removed from the alignment using the Picard toolkit (Broad Institutes, Cambridge, MA). Finally, the BAM format files were sent to Atlas2 to generate variant calling files (VCFs) (Human Genome Sequencing Center, Houston, TX). Integrative Genomics Viewer was used to view the sequences [30], whereas the VCFs were viewed with a word processor.
Results
Reprogramming of LS fibroblasts generated iPSCs
To investigate the ability of the mRNA–miRNA approach to generate hiPSCs from patient LS1 fibroblasts carrying the T8993G mutation (Fig. 1), we conducted a side-by-side comparison of reprogramming with healthy control BJ fibroblasts. Previous studies have generated iPSCs from mitochondrial disorders such as Pearson syndrome [20] and MELAS syndrome [21,31] using integrating retroviral transfection of OS(K)M reprogramming factors. Our approach utilized feeder-free culture and transient transfection of mRNA reprogramming factors OCT4, SOX2, KLF4, and c-MYC to generate hiPSCs free of feeder-cell line contamination, genomic integration of transgenes, or residual reprogramming vectors [24,32]. Additional transfection of selected micro-RNAs increased the speed and success of the reprogramming process by enhancing the MET, or mesenchymal-to-epidermal transition, a rate-limiting step in the conversion of fibroblasts to stem cells [33].
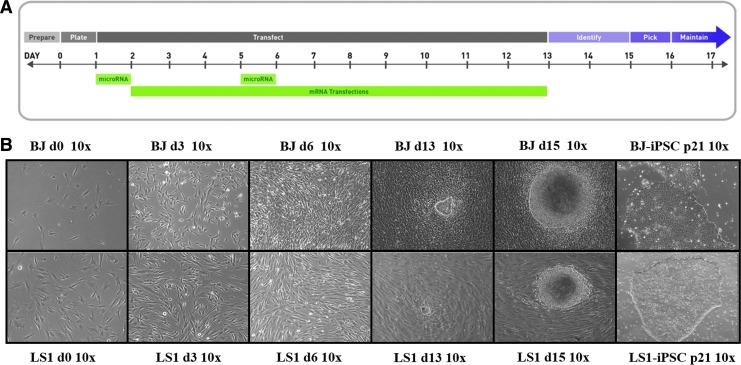
FIG. 1.
Human patient LS dermal fibroblasts reprogrammed into LS-iPSCs using mRNA–miRNA transfection. (A) Reprogramming schematic for mRNA and microRNA transfections. (B) Bright field images (10 × ) of healthy control BJ fibroblasts and human disease LS1 fibroblasts undergoing reprogramming. Multipolar spindle-shaped fibroblast cells transitioning to a more compact cobblestone appearance from days 6 to 12 as cells undergo MET, with early stem cell colonies arising by day 13 and expanding into well-circumscribed pluripotent stem cell colonies at day 15. Isolated stem cells maintain characteristic well-circumscribed morphology with minimal spontaneous differentiations through 20+ passages. iPSC, induced pluripotent stem cell; LS, Leigh's syndrome; MET, mesenchymal-to-epidermal transition.
Thus, following the mRNA–miRNA transfection protocol (Fig. 1A), LS1 fibroblasts were reprogrammed into LS1-hiPSCs in 13–15 days. Clonal stem cell colonies began to arise on day 13 of the protocol, formed well-circumscribed compact colonies by day 15 (Fig. 1B), and were picked for expansion on day 16. The reprogramming efficiency of LS1 cultures was lower than that of the BJ controls. LS1 fibroblasts generated 5 colonies whereas BJ fibroblasts generated 15 colonies. Initial growth was slower in early LS1-hiPSC cultures, cell doubling and passaging rate equaled that of the control BJ-hiPSC cultures in extended feeder-free passage on Matrigel in mTeSR stem cell maintenance medium. LS1-hiPSC cultures continued to demonstrate the characteristic stem cell appearance of compact and well-circumscribed colonies through 20+ passages (Fig. 1B). After stable proliferation and expansion, these stem cell lines were assessed for pluripotency characteristics of self-renewal and multipotent differentiation.
LS-hiPSCs express markers characteristic of pluripotent stem cells
To confirm stem cell reprogramming, LS1-hiPSCs were assessed for expression of pluripotency transcription factors OCT4, SOX2, and NANOG. Establishing the endogenous expression of these transcription factors is a necessary step in the induction of pluripotency, as they stabilize the genetic circuits of pluripotent cell fate and self-renewal [34,35]. Immunocytochemical staining of LS1-hiPSCs cultures demonstrates positive expression of OCT4, SOX2, and NANOG, comparable with control BJ-hiPSCs and H9 hESCs (Fig. 2A). As the mRNA-based reprogramming method utilized transient expression of reprogramming factors, this result indicates activation of the endogenous pluripotent stem cell network and stable expression of the stem cell identity.
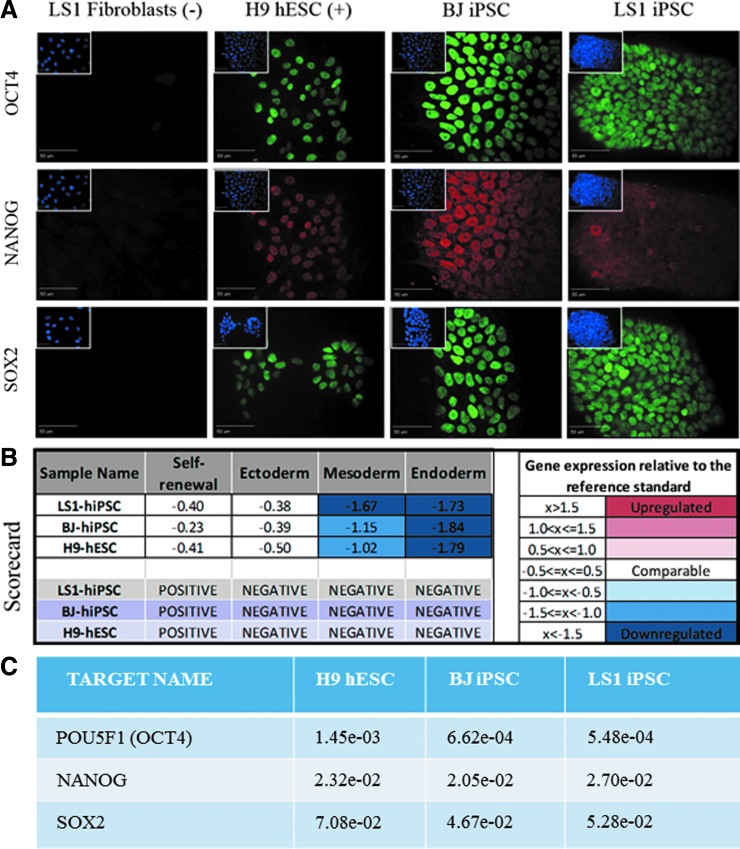
FIG. 2.
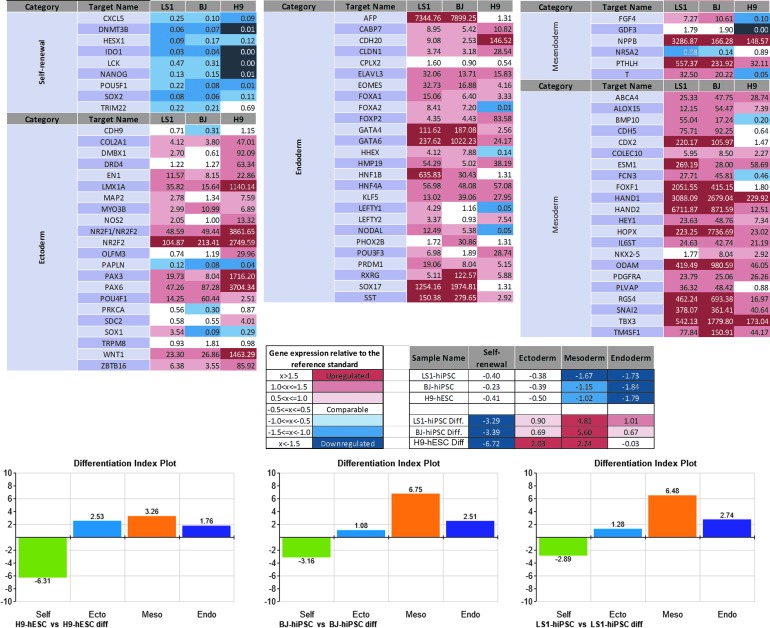
Putative LS-patient iPSCs express the core pluripotency transcription factors. (A) LS1 fibroblasts are negative for pluripotency markers OCT4, SOX2, and NANOG, whereas positive control H9 ESCs, BJ-hiPSCs, and LS1-hiPSCs are positive for pluripotent markers. Scale bar = 50 μm. (B) hPSC Scorecard qPCR analysis compares gene expression signatures for self-renewal and multilineage differentiation to standard reference stem cell cultures. Relative to hPSC standards, LS1-iPSCs have comparable expression of self-renewal genes and downregulated ectoderm, mesoderm, and endoderm gene expression. Healthy control BJ-hiPSC and H9-hESC lines also show similar expression. Overall LS1-iPSC gene expression signatures, as well as those of BJ-hiPSC and H9-hESC, rate as positive for pluripotent self-renewal and negative for all ectoderm-, mesoderm-, and endoderm-differentiated lineages. (C) qPCR gene expression values from the Scorecard Array for OCT4, SOX2, and NANOG. Individual gene expression values from the Scorecard qPCR array were calculated using the 2−ΔCT and normalized against β-actin expression levels (Supplementary Table S1). hESC, human embryonic stem cell; hiPSC, human-induced pluripotent stem cell; hPSC, human pluripotent stem cell; qPCR, quantitative PCR.
Expression of pluripotency factors and self-renewal potential was additionally assessed by gene expression using the qPCR array hPSC Scorecard™ system. Two hallmarks of pluripotent stem cells are self-renewal and the ability to generate somatic cell types from the three embryonic germ layer lineages ectoderm, endoderm, and mesoderm [36,37]. The “Pluripotency Scorecard” system characterizes the self-renewal and tri-lineage differentiation potential of sample cultures by comparing gene expression profiles with a predictive databank of pluripotent stem cell standards [28], generating composite gene expression values for each category and a quality control “score” for the tested cell lines. LS-hiPSCs were analyzed with this system and found to have characteristic expression of undifferentiated pluripotent stem cells, scoring positive for markers of self-renewal and negative for all three germ layer differentiation lineages (Fig. 2B). Included in the 84 qPCR probes for genes involved in pluripotency and differentiation, OCT4, SOX2, and NANOG were found to be upregulated in LS1-hiPSCs comparable with control stem cells (Supplementary Table S1), corroborating the immunocytochemistry results.
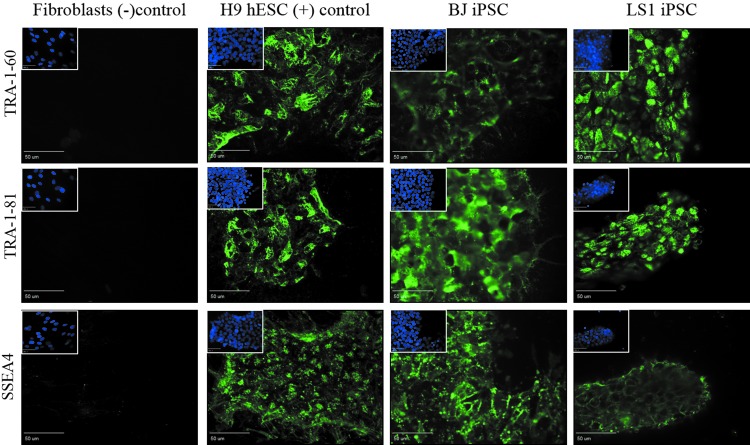
Subsequent immunocytochemical examination of extracellular markers of stem cell identity further supported successful reprogramming. The cell surface glycoprotein and glycolipid epitopes SSEA4, TRA1-60, and TRA1-81, found specifically on pluripotent cells, are routinely used as stem cell markers [38–40]. LS patient fibroblast cells were negative for these markers, whereas reprogrammed LS-hiPSCs expressed SSEA4, TRA1-60, and TRA1-81 (Fig. 3).
FIG. 3.
LS1-iPSCs express the cell surface pluripotency markers SSEA4, TRA-1-60, and TRA-1-81. LS fibroblasts are negative for cell surface pluripotency markers, whereas LS1-hiPSCs reprogrammed from these cells become positive for SSEA4, TRA-1-60, and TRA-1-81 stem cell markers. LS1-hiPSCs expression is comparable with healthy control H9-hESCs and BJ-hiPSCs stem cells. Scale bar = 50 μm. Image inserts contain corresponding DAPI nuclear counterstain. DAPI, 4′,6-diamidino-2-phenylindole.
LS-hiPSCs are capable of multilineage differentiation
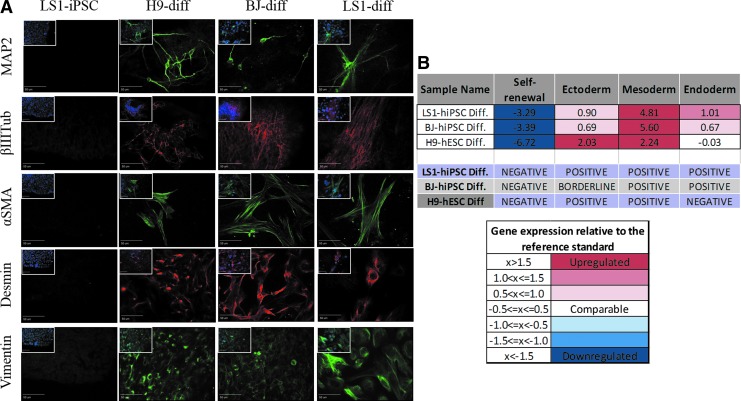
To assess the ability of LS1-hiPSCs to differentiate into ectoderm, mesoderm, and endoderm, LS1-hiPSC cultures underwent unguided spontaneous differentiation utilizing a previously established approach [25]. Adherent hiPSC cultures were grown to confluence followed by replacement of stem cell maintenance medium with growth factor free, defined differentiation medium. After 21 days of differentiation, cultures were fixed and stained for markers of cell types representative of the three embryonic germ layer lineages. LS1-hiPSC unguided spontaneously differentiated cultures (LS1-Diff) produced cells positive for neuroectodermal markers MAP2 and βIII-Tubulin, mesoderm markers αSMA and DES, and endodermal marker VIM (Fig. 4A).
FIG. 4.
Differentiation of LS-iPSCs cultures generates cells representative of ectoderm, mesoderm, and endoderm embryonic germ layer lineages. (A) LS1-iPSCs cultures do not express markers for differentiated cell types. Upon differentiation, LS1-iPSC derived cultures contain cells that are positive for markers of ectodermal (MAP2, βIIITub), mesodermal (αSMA, DES), and endodermal (VIM) lineages, indicating pluripotent differentiation potential. Scale bar = 50 μm. Image inserts show DAPI nuclear counterstain overlay. (B) Analysis of differentiated cultures by the Scorecard qPCR array demonstrates downregulation of stem cell self-renewal genes, with upregulation of genes involved in germ layer specification and differentiated cell identity. Comparison of gene expression signatures with standard control databases allows for the analysis of lineage bias of each lines differentiation potential. Differentiated LS1-iPSCs result in gene expression signatures positive for all three germ layers, exhibiting full differentiation potential. αSMA, alpha smooth muscle actin; DES, desmin; VIM, vimentin.
Using mRNA isolated from these same differentiation cultures, Taqman Scorecard™ qPCR further evaluated the differentiation potential of the LS1-hiPSCs. Compared with undifferentiated LS1-hiPSCs and Scorecard reference standards, LS1-Diff cultures downregulated expression of stem cell self-renewal genes, while upregulating lineage-specific differentiated cell type genes (Fig. 5). Scorecard analysis scored LS1-Diff cultures as positive for ectoderm, mesoderm, and endoderm lineage differentiation (Fig. 4B).
FIG. 5.
Expression profiles of pluripotent- and trilineage-specific genes in LS1-hiPSCs. Pluripotency and trilineage differentiation potential were assessed with the TaqMan Scorecard qPCR assay and readouts were normalized against the pluripotency and germline-specific controls. Fold changes in individual gene expression in the sample cell lines were obtained by comparing differentiated versus undifferentiated sample of the same cell line. Data analysis and normalization were performed using the cloud-based TaqMan hPSC scorecard analysis software. LS1, differentiated sample obtained from LS1-hiPSC; BJ, differentiated sample obtained from BJ-hiPSC; H9, differentiated sample obtained from H9-hESC.
When LS1-hiPSCs were differentiated using another common method for generating multilineage cultures, EB suspension culture [25], they demonstrated slower growth rates and higher cell death than cultures from control stem cells. Using the Aggrewell™ generation method, EBs of consistent size and number were generated from LS1-hiPSCs, BJ-hiPSCs, and H9-hESCs. Although initial growth of LS1-EBs mirrored that of the control stem cell lines, by day 7 of suspension culture, the LS1-EBs began to rapidly regress and die out (Supplementary Fig. S1). As LS1-hiPSCs did not show obvious deficits in cell growth, possessing doubling and passaging rates equal to healthy control BJ-iPSC and other control stem cell lines, this deficit appears to be specific to the differentiation process.
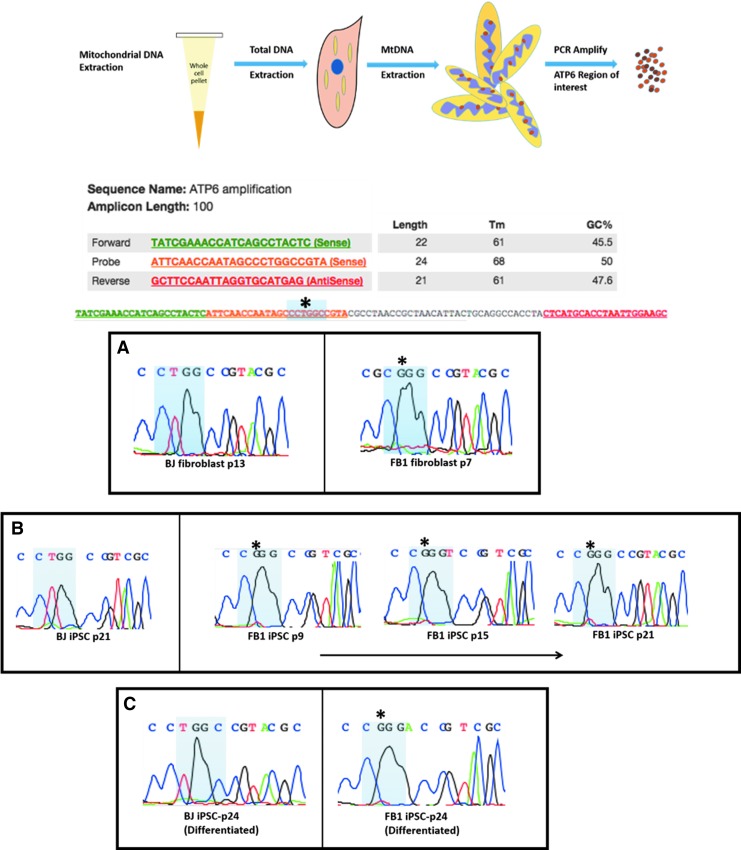
Sanger sequencing demonstrates presence of 8993T>G mutation in patient fibroblast, hiPSCs, and differentiated cells
To confirm the presence of the mutation, the mtDNA was extracted from whole cell pellets, and specific region of interest within the ATP6 gene was PCR amplified. The PCR product was purified and quantified to the appropriate specification. This allowed for Sanger sequencing of the specific ATP6 region containing the 8993 site. Sanger sequencing of our samples was necessary to confirm the presence of the disease-causing mutation after reprogramming and spontaneous differentiation. The results demonstrate that the 8993 T > G mutation is present in all LS1 samples (LS1 fib p7, LS1-hiPSC p9, LS1-hiPSC p15, LS1-hiPSC p21, and LS1 differentiated p24) (Fig. 6). Verifying the presence of the mutation was a necessary step before the quantification of mutation burden using next-generation sequencing for whole exome.
FIG. 6.
Detection of mutation through PCR amplification and Sanger sequencing of MtDNA: LS (8993 T>G). Following extraction of mtDNA, detection of mutation through PCR amplification and Sanger sequencing of MtDNA: LS (8993 T>G) was conducted in (A) Parental LS1 (BJ fibroblast-control); (B) LS1 iPSC across multiple passages (BJ-iPSC-control); (C) differentiated LS1-iPSC (differentiated BJ-iPSC-control). mtDNA, mitochondrial DNA.
Next-generation sequencing demonstrates presence of heteroplasmy burden
With the presence of the mutation confirmed, the percentage of mutation burden in each sample was measured using high-throughput next-generation sequencing for whole exome. This strategy has previously been utilized to effectively measure heteroplasmies in the mitochondrial genome [41–43]. The mtDNA was extracted from whole cell pellets, and then purified before sequencing. The sequencing results yielded a range of total sequence reads between 388 and 2031 at the 8993 position in the different cell samples. This large of a sample size allows us to be confident about the percentages measured. As expected, BJ-fib, BJ-hiPSC, and BJ-Diff (unguided spontaneously differentiated) mostly possessed the wild type thymine with only a few sequences containing the mutated guanine. The Leigh's patient sample LS1 fibroblast exhibited the mutation in 85% of the sequences read. The LS1-hiPSCs of various passage numbers (p9, p15, p21) and the differentiated cell sample (from LS1-hiPSC p24) displayed similar mutation levels with a range of 80%–88% (Table 1).
Table 1.
Quantification of Heteroplasmy by next-Generation Sequencing
| Sample name | Total number sequenced | 8993 T | 8993 G | Percentage of mutation |
|---|---|---|---|---|
| BJ fibroblast | 689 | 689 | 0 | 0 |
| BJ iPSC | 1,812 | 1,801 | 11 | 1 |
| BJ differentiated | 388 | 377 | 9 | 3 |
| LS1 fibroblast | 548 | 84 | 464 | 85 |
| LS1 iPSC p9 | 1,987 | 234 | 1,753 | 88 |
| LS1 iPSC p15 | 2,031 | 305 | 1,726 | 85 |
| LS1 iPSC p21 | 589 | 92 | 497 | 84 |
| LS1 iPSC differentiated | 1,000 | 203 | 797 | 80 |
The extracted mtDNA was next-generation sequenced for whole exome. The sequencing results were compiled, and the results were analyzed with the Integrative Genomics Viewer. This allows us to view heteroplasmic sequences with an exact measure of the variants. Results demonstrate the presence of pathogenic mtDNA burden (8993T>G) in parent LS1 fibroblast, LS1-hiPSCs across multiple passages, and in differentiated samples obtained from LS1-hiPSCs. hiPSC, human-induced pluripotent stem cell; iPSC, induced pluripotent stem cell; LS, Leigh's syndrome.
Discussion
Despite the mitochondrial genome being rather small, effectively researching the pathogenesis of mitochondrial diseases caused by mtDNA mutations has been difficult. LS has the added complexity of being dependent upon a high threshold effect of mutation burden to manifest a clinical phenotype in various tissues. Previous studies of detecting mtDNA mutation heteroplasmy have shown conflicting results when addressing the question of mutation load through reprogramming. Using viral transfection of c-Myc, Oct4, Sox2, and Klf4 to generate Pearson syndrome hiPSCs, researchers found that mtDNA heteroplasmy persisted through reprogramming but mutation copy number began to drop after passage #10 [20]. In another study, using viral transfection of c-Myc, Oct4, and Sox2 to generate MELAS syndrome hiPSCs, similar selective loss of mtDNA heteroplasmy was seen [21].
Alternatively, another study that used viral transfection of c-Myc, Oct4, and Sox2 to generate MELAS syndrome hiPSCs found that consistent levels of heteroplasmy were seen through extended passaging of hiPSCs and through multilineage and neural-specific differentiation [31]. Our results agree with the latter study, as LS-hiPSCs maintained heteroplasmy levels equally in the initial patient fibroblasts, over 20+ passages of hiPSCs culture, and through differentiation (Table 1).
In this study, we were able to successfully reprogram the LS1 fibroblast cells into LS1-iPSCs despite generating fewer initial colonies relative to control cells. The LS1-iPSCs showed reduced growth and survival upon differentiation. The observed LS cell growth defect in LS1 EB differentiated cells is similar to findings in previous studies of mitochondrial disorders, where reprogramming progressed normally, but growth and survival defects were apparent upon differentiation [20,44]. Our results were in concordance with findings from a mouse iPSC model of mtDNA disease [45], where mutant iPSCs behaved similar to WT iPSCs in terms of expression of pluripotency markers and proliferation rate, but when differentiated by EB formation, the mtDNA mutants with higher mutation loads showed decreased proliferation of EB aggregates, with growth arrest and regression after day 5.
This suggests that our LS1-hiPSCs carried significantly high percentage of T8993G mutant mtDNA, as demonstrated by our next-generation sequencing analysis. These and other studies [46,47] show the crucial role of mitochondria in the metabolic shift from stem cell glycolysis to somatic cell oxidative phosphorylation, suggesting the importance of understanding the role of such deleterious effects of pathogenic mtDNA mutations during early cellular differentiation events.
Our methods included the use of Sanger sequencing, next-generation sequencing, and subsequent analysis of the results confirmed the presence of the 8993 T > G mutation in all cells derived from the LS1 patient fibroblasts. In addition, the mutation burden was maintained at a consistently elevated percentage through reprogramming, several passages, and spontaneous differentiation. Thus, our experiments have resulted in the creation of a hiPSC line with a mutation burden comparable with that of the LS patient fibroblast from which it was derived. The continued stability of this mutation load through extended passaging and nonspecific differentiation demonstrates these LS1-hiPSCs to be a valid model for the study of the ATP6 8993 T > G mutation in vitro. Generating this cell line with the disease-causing mutation intact is a very important step in confirming nonviral nonintegrating reprogramming technologies as a viable option of creating a cell-based model for LS disease.
Clinically, LS displays variability among patients and variability among different tissues within individual patients, which can complicate translational research. Our cell-based model addresses both of these issues, by providing a stable and renewable source for the generation of various differentiated cells, all carrying the same pathogenic mtDNA load from a severely affected LS patient. These stem cells could then be directly differentiated into specific cell types of interest such as neurons or myocytes. Further experiments can be carried out to determine how the disease affects specific tissues on a molecular basis. This is a promising approach for the investigation of patient- and tissue-specific pathophysiology, and for generating possible therapeutics for individual patients affected by LS disease and other mitochondrial diseases. The methods used to successfully create this patient-specific and disease-specific clinical-grade model in this study could also be adapted to investigate other devastating mitochondrial diseases such as MELAS, PS (Leber's hereditary optic neuropathy), and LHON [1,19,48,49].
In addition to confirming the consistent mutation burden of 8993 T>G, the next-generation sequencing results also identified a number of other heteroplasmic variants (Supplementary Table S2). Owing to the highly polymorphic nature of the mitochondrial genome, a number of these variants are characterized as normal sequence variants that are not likely to contribute to LS. Some of the variants displayed in the LS patient sample were also present in the BJ control samples in similar quantities (Supplementary Table S2). Thus, it is unlikely that these sequence variants contribute to LS. Two other variants in the LS patient samples were also present in the BJ control samples, but in lower quantities (Supplementary Table S2). When cross examined with the MitoMap database [3], both of these sites were determined to be normal polymorphisms.
Also, a number of sequence variants were associated with previously defined mitochondrial haplogroups. It is widely known that haplogroup designations are used in population genetics to denote people with a common ancestry. The haplogroups connected to the LS1 cell line are indicative of European ancestry, which correlates with the origin of the cell line used in this study (Supplementary Table S3). It would be interesting to see whether other patients with LS could be connected to similar haplogroups, information that could be beneficial in identifying at risk populations for LS.
With the normal variants identified, there were a few remaining sites of interest for possible future studies (Supplementary Table S2). The 310 T > C mutation is in the hypervariable II region of the mitochondrial genome. This control region of the genome is heavily concentrated with polymorphisms that have inconclusive effects, but the 310 T > C mutation has been implicated in malignant melanoma, colorectal cancer, and breast cancer [50–52]. The presence of this mutation is detected in these types of cancer patients at a statistically higher amount when compared with control populations. This mutation is expected to hinder replication or transcription of the mitochondrial genome, which could elevate reactive oxygen species (ROS) production [52]. Another possible variant of interest is 12372 A>G, which is in the ND5 gene coding for subunit five of NADH dehydrogenase. This mutation is present in a statistically significant higher number of sporadic Creutzfeldt–Jakob disease cases compared with controls, and is reported to be correlated with altered brain pH values [53]. It is hypothesized that this could be caused by a lack of coupling in complex I of the electron transport chain. Based on current knowledge, it is unclear how these mutations might affect or correlate with LS.
Our research was focused on the 8993 T > G mutation in the ATP6 gene, however, many LS disease cases are caused by mutations in genes that code for subunits of complex I. Next-generation sequencing of the LS1 patient cell line revealed 10 sequence variants in genes that code for subunits of complex I. None of these variants has previously been mentioned as causes of LS, but it is not inconceivable that they could in some way contribute to the disease state. Cross-referencing these variants with other LS patient sequencing results could allow for the determination of their importance to LS disease.
Our results have opened up numerous avenues for future areas of research. The generation of the LS1-hiPSC provides a unique window into studying the effects of the mutation during early embryonic development. An important area of research is elucidating interactions between the mitochondrial and nuclear genomes and stem cell metabolism. This will be critical for better understanding the origins of the disease. The clinical manifestation of the LS phenotype due to the 8993T>G mutation is a result of energy deprivation and cell death in high-energy-dependent tissues such as the brain, muscles, and the heart. Because LS disease has more drastic effects on neural cells and myocytes, the next logical step would be to directly differentiate the LS1-hiPSC line into these cell types.
It is useful to note that specific transcription factors have been used to direct differentiation into neural cell types [54]. Direct differentiation to cardiomyocytes has been difficult in humans, but mouse embryonic fibroblasts have been directed into cardiomyocytes [55]. Furthermore, the developmental impact and timing of the LS pathology could be examined with step-wise differentiation of neural progenitors [56–59] or self-organizing organoid structures [60]. In addition to sequencing for heteroplasmies, another interesting area of research could be connecting the level of mutation burden to the degree of mitochondrial dysfunction. Select biomarkers for mitochondrial dysfunction such as carnitine, pyruvic acid, and lactic acid have been used in the past to assess mitochondrial respiration.
More recently, use of Seahorse XF(e)24 Extracellular Flux Analyzer technology allows mitochondrial respiration and glycolysis to be measured simultaneously in live cells. Preliminary bioenergetic characterization of the LS1 fibroblasts indicates a compromise in ATP synthesis (unpublished data). We, therefore, expect that LS1-hiPSCs and their differentiated derivatives will exhibit bioenergetics defects due to increased mutation burden. It is widely acknowledged that determining oxygen consumption rate and lactic acid production rate is accurate measures of mitochondrial function and has been extensively used in reprogrammed stem cells, progenitors, and differentiated cells [61–64]. Our future studies will focus on quantitatively determining correlations between level of mutation burden and alteration in mitochondrial function in the context of LS.
In summary, we have demonstrated the derivation and characterization of hiPSCs from a patient fibroblast corresponding to a classic mitochondrial disease, LS, using a combined mRNA–miRNA nonviral approach. Comparative analyses with hiPSCs derived from BJ-control fibroblasts and H9-hESCs demonstrate the ability of the LS1-hiPSCs to maintain self-renewal capabilities and exhibit multilineage differentiation potential. We also demonstrated presence of consistent mutation burden in the LS1-hiPSCs and differentiated derivatives. The possible role of the specific mutations in the context of LS during early development and differentiation into specialized cell types and its effect on mitochondrial function and bioenergetics can now be elucidated in detail using the patient-specific and disease-specific hiPSC model. This now allows us to (1) study mtDNA transmission and its role in self-renewal and differentiation, (2) study how mtDNA transmission affects disease severity, (3) study how mtDNA transmission affects stem cell energetics, and (4) to apply cell-based and tissue-on-chip models for drug discovery, development, and toxicity testing.
Supplementary Material
Acknowledgments
We thank Dr. Myrna Serrano and Dr. Gregory Buck at Virginia Commonwealth University for assistance with Sanger sequencing and next-generation sequencing resources and analysis. We also thank Dr. Johannes A. Mayr and Dr. Wolfgang Sperl from the Medical University of Salzburg Austria, and Dr. Daniela Karall from the Medical University of Innsbruck, Austria, for kindly providing us fibroblasts used in the study. We also thank Dr. Raj Rao at the University of Arkansas for kind assistance with analysis and discussions. This study was supported, in part, by NIH-R15R15NS080157-01A1 (S.I.) and NIH CTSA UL1TR000058 (E.J.L. and S.I.) by VCU's Center for Clinical and Translational Research and DoD W81XWH-16-1-0181 (F.D.W., E.J.L., and S.I.). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the article. This study is being conducted in the spirit of “Gentle Science” and the authors wish to thank all hands and minds involved in this study.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Iyer S, Alsayegh K, Abraham S. and Rao RR. (2009). Stem cell-based models and therapies for neurodegenerative diseases. Crit Rev Biomed Eng 37:321–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Picard M, Wallace DC. and Burelle Y. (2016). The rise of mitochondria in medicine. Mitochondrion 30:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, Procaccio V. and Wallace DC. (2018). MITOMAP: a human mitochondrial genome database. https://mitomap.org/foswiki/bin/view/MITOMAP/CitingMitomap
- 4. Schapira AH. (2012). Mitochondrial diseases. Lancet 379:1825–1834 [DOI] [PubMed] [Google Scholar]
- 5. Dimauro S. (2011). A history of mitochondrial diseases. J Inherit Metab Dis 34:261–276 [DOI] [PubMed] [Google Scholar]
- 6. Schon EA, DiMauro S, Hirano M. and Gilkerson RW. (2010). Therapeutic prospects for mitochondrial disease. Trends Mol Med 16:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leigh D. (1951). Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry 14:216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loeffen JL, Smeitink JA, Trijbels JM, Janssen AJ, Triepels RH, Sengers RC. and van den Heuvel LP. (2000). Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum Mutat 15:123–134 [DOI] [PubMed] [Google Scholar]
- 9. Holt IJ, Harding AE, Petty RK. and Morgan-Hughes JA. (1990). A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 46:428–433 [PMC free article] [PubMed] [Google Scholar]
- 10. Shoffner JM, Fernhoff PM, Krawiecki NS, Caplan DB, Holt PJ, Koontz DA, Takei Y, Newman NJ, Ortiz RG, et al. (1992). Subacute necrotizing encephalopathy: oxidative phosphorylation defects and the ATPase 6 point mutation. Neurology 42:2168–2174 [DOI] [PubMed] [Google Scholar]
- 11. Tatuch Y, Christodoulou J, Feigenbaum A, Clarke JT, Wherret J, Smith C, Rudd N, Petrova-Benedict R. and Robinson BH. (1992). Heteroplasmic mtDNA mutation (T—-G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high. Am J Hum Genet 50:852–858 [PMC free article] [PubMed] [Google Scholar]
- 12. Olivo PD, Van de Walle MJ, Laipis PJ. and Hauswirth WW. (1983). Nucleotide sequence evidence for rapid genotypic shifts in the bovine mitochondrial DNA D-loop. Nature 306:400–402 [DOI] [PubMed] [Google Scholar]
- 13. Payne BA, Wilson IJ, Yu-Wai-Man P, Coxhead J, Deehan D, Horvath R, Taylor RW, Samuels DC, Santibanez-Koref M. and Chinnery PF. (2013). Universal heteroplasmy of human mitochondrial DNA. Hum Mol Genet 22:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson IJ, Carling PJ, Alston CL, Floros VI, Pyle A, Hudson G, Sallevelt SC, Lamperti C, Carelli V, et al. (2016). Mitochondrial DNA sequence characteristics modulate the size of the genetic bottleneck. Hum Mol Genet 25:1031–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wai T, Teoli D. and Shoubridge EA. (2008). The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 40:1484–1488 [DOI] [PubMed] [Google Scholar]
- 16. Samuels DC, Wonnapinij P, Cree LM. and Chinnery PF. (2010). Reassessing evidence for a postnatal mitochondrial genetic bottleneck. Nat Genet 42:471–472; author reply 472–473 [DOI] [PubMed] [Google Scholar]
- 17. Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O. and Mitalipov S. (2009). Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature 461:367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, et al. (2010). Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465:82–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iyer S, Xiao E, Alsayegh K, Eroshenko N, Riggs MJ, Bennett JP., Jr. and Rao RR. (2012). Mitochondrial gene replacement in human pluripotent stem cell-derived neural progenitors. Gene Ther 19:469–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cherry ABC, Gagne KE, McLoughlin EM, Baccei A, Gorman B, Hartung O, Miller JD, Zhang J, Zon RL, et al. (2013). Induced pluripotent stem cells with a mitochondrial DNA deletion. Stem Cells 31:1287–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folmes CDL, Martinez-Fernandez A, Perales-Clemente E, Li X, McDonald A, Oglesbee D, Hrstka SC, Perez-Terzic C, Terzic A. and Nelson TJ. (2013). Disease-causing mitochondrial heteroplasmy segregated within induced pluripotent stem cell clones derived from a patient with MELAS. Stem Cells 31:1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okita K, Ichisaka T. and Yamanaka S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317 [DOI] [PubMed] [Google Scholar]
- 23. Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7:618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mandal PK. and Rossi DJ. (2013). Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc 8:568. [DOI] [PubMed] [Google Scholar]
- 25. Sheridan SD, Surampudi V. and Rao RR. (2012). Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem Cells Int 2012:738910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abraham S, Sheridan SD, Miller B. and Rao RR. (2010). Stable propagation of human embryonic and induced pluripotent stem cells on decellularized human substrates. Biotechnol Prog 26:1126–1134 [DOI] [PubMed] [Google Scholar]
- 27. Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S-I, Muguruma K. and Sasai Y. (2007). A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681–686 [DOI] [PubMed] [Google Scholar]
- 28. Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, et al. (2011). Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H. (2012). Exploring single-sample SNP and INDEL calling with whole-genome de novo assembly. Bioinformatics 28:1838–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thorvaldsdottir H, Robinson JT. and Mesirov JP. (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hämäläinen RH, Manninen T, Koivumäki H, Kislin M, Otonkoski T. and Suomalainen A. (2013). Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc Natl Acad Sci 110:E3622–E3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinton DA. and Daley GQ. (2012). The promise of induced pluripotent stem cells in research and therapy. Nature 481:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao B, Bao X, Liu L, Feng S, Zovoilis A, Liu W, Xue Y, Cai J, Guo X, et al. (2011). MicroRNA cluster 302–367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem 286:17359–17364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buganim Y, Faddah DA. and Jaenisch R. (2013). Mechanisms and models of somatic cell reprogramming. Nat Rev Genet 14:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao RR. and Stice SL. (2004). Gene expression profiling of embryonic stem cells leads to greater understanding of pluripotency and early developmental events. Biol Reprod 71:1772–1778 [DOI] [PubMed] [Google Scholar]
- 36. Cai J, Chen J, Liu Y, Miura T, Luo Y, Loring JF, Freed WJ, Rao MS. and Zeng X. (2006). Assessing self-renewal and differentiation in human embryonic stem cell lines. Stem Cells 24:516–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loring JF. and Rao MS. (2006). Establishing standards for the characterization of human embryonic stem cell lines. Stem Cells 24:145–150 [DOI] [PubMed] [Google Scholar]
- 38. Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H. and Andrews PW. (2002). Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells 20:329–337 [DOI] [PubMed] [Google Scholar]
- 39. Natunen S, Satomaa T, Pitkänen V, Salo H, Mikkola M, Natunen J, Otonkoski T. and Valmu L. (2011). The binding specificity of the marker antibodies Tra-1-60 and Tra-1-81 reveals a novel pluripotency-associated type 1 lactosamine epitope. Glycobiology 21:1125–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rao RR, Johnson AV. and Stice SL. (2007). Cell surface markers in human embryonic stem cells. Methods Mol Biol 407:51–61 [DOI] [PubMed] [Google Scholar]
- 41. Carroll CJ, Brilhante V. and Suomalainen A. (2014). Next-generation sequencing for mitochondrial disorders. Br J Pharmacol 171:1837–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li M, Schonberg A, Schaefer M, Schroeder R, Nasidze I. and Stoneking M. (2010). Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. Am J Hum Genet 87:237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang S. and Huang T. (2010). Characterization of mitochondrial DNA heteroplasmy using a parallel sequencing system. Biotechniques 48:287–296 [DOI] [PubMed] [Google Scholar]
- 44. Wahlestedt M, Ameur A, Moraghebi R, Norddahl GL, Sten G, Woods NB. and Bryder D. (2014). Somatic cells with a heavy mitochondrial DNA mutational load render induced pluripotent stem cells with distinct differentiation defects. Stem Cells 32:1173–1182 [DOI] [PubMed] [Google Scholar]
- 45. Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C. and Terzic A. (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bukowiecki R, Adjaye J. and Prigione A. (2014). Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology 60:174–182 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, Nuebel E, Daley GQ, Koehler CM. and Teitell MA. (2012). Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 11:589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iyer S, Bergquist K, Young K, Gnaiger E, Rao RR. and Bennett JP., Jr. (2012). Mitochondrial gene therapy improves respiration, biogenesis, and transcription in G11778A Leber's hereditary optic neuropathy and T8993G Leigh's syndrome cells. Hum Gene Ther 23:647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iyer S. (2013). Novel therapeutic approaches for Leber's hereditary optic neuropathy. Discov Med 15:141–149 [PMC free article] [PubMed] [Google Scholar]
- 50. Ebner S, Lang R, Mueller EE, Eder W, Oeller M, Moser A, Koller J, Paulweber B, Mayr JA, Sperl W. and Kofler B. (2011). Mitochondrial haplogroups, control region polymorphisms and malignant melanoma: a study in middle European Caucasians. PLoS One 6:e27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Govatati S, Saradamma B, Malempati S, Dasi D, Thupurani MK, Nagesh N, Shivaji S, Bhanoori M, Tamanam RR, Nallanchakravarthula V. and Pasupuleti SR. (2017). Association of mitochondrial displacement loop polymorphisms with risk of colorectal cancer in south Indian population. Mitochondrial DNA A DNA Mapp Seq Anal 28:632–637 [DOI] [PubMed] [Google Scholar]
- 52. Tipirisetti NR, Govatati S, Pullari P, Malempati S, Thupurani MK, Perugu S, Guruvaiah P, Rao KL, Digumarti RR, et al. (2014). Mitochondrial control region alterations and breast cancer risk: a study in South Indian population. PLoS One 9:e85363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang J, Zhang ZX, Du PC, Zhou W, Wu SD, Wang QL, Chen C, Shi Q, Chen C, et al. (2015). Analyses of the mitochondrial mutations in the Chinese patients with sporadic Creutzfeldt-Jakob disease. Eur J Hum Genet 23:86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim J, Ambasudhan R. and Ding S. (2012). Direct lineage reprogramming to neural cells. Curr Opin Neurobiol 22:778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J. and Ding S. (2011). Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol 13:215–222 [DOI] [PubMed] [Google Scholar]
- 56. Shi Y, Kirwan P. and Livesey FJ. (2012). Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7:1836–1846 [DOI] [PubMed] [Google Scholar]
- 57. Kirwan P, Turner-Bridger B, Peter M, Momoh A, Arambepola D, Robinson HP. and Livesey FJ. (2015). Development and function of human cerebral cortex neural networks from pluripotent stem cells in vitro. Development 142:3178–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dhara SK, Hasneen K, Machacek DW, Boyd NL, Rao RR. and Stice SL. (2008). Human neural progenitor cells derived from embryonic stem cells in feeder-free cultures. Differentiation 76:454–464 [DOI] [PubMed] [Google Scholar]
- 59. Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R. and Stice SL. (2006). Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells 24:125–138 [DOI] [PubMed] [Google Scholar]
- 60. Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL. and Vaccarino FM. (2012). Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci U S A 109:12770–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aldana BI, Zhang Y, Lihme MF, Bak LK, Nielsen JE, Holst B, Hyttel P, Freude KK. and Waagepetersen HS. (2017). Characterization of energy and neurotransmitter metabolism in cortical glutamatergic neurons derived from human induced pluripotent stem cells: a novel approach to study metabolism in human neurons. Neurochem Int 106:48–61 [DOI] [PubMed] [Google Scholar]
- 62. Zhou Y, Al-Saaidi RA, Fernandez-Guerra P, Freude KK, Olsen RK, Jensen UB, Gregersen N, Hyttel P, Bolund L, et al. (2017). Mitochondrial spare respiratory capacity is negatively correlated with nuclear reprogramming efficiency. Stem Cells Dev 26:166–176 [DOI] [PubMed] [Google Scholar]
- 63. Lorenz C. and Prigione A. (2017). Mitochondrial metabolism in early neural fate and its relevance for neuronal disease modeling. Curr Opin Cell Biol 49:71–76 [DOI] [PubMed] [Google Scholar]
- 64. Cliff TS. and Dalton S. (2017). Metabolic switching and cell fate decisions: implications for pluripotency, reprogramming and development. Curr Opin Genet Dev 46:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.