Abstract
Aims: Human embryonic stem cell derived-cardiovascular progenitor cells (hESC-CVPCs) are a promising cell source for cardiac repair, while the underlying mechanisms need to be elucidated. We recently observed cardioprotective effects of human pluripotent stem cell (hPSC)-CVPCs in infarcted nonhuman primates, but their effects on inflammation during early phase of myocardial infarction (MI) and the contribution of such effect to the cardioprotection are unclear.
Results: Injection of hESC-CVPCs into acutely infarcted myocardium significantly ameliorated the functional worsening and scar formation, concomitantly with reduced inflammatory reactions and cardiomyocyte apoptosis as well as increased vascularization. Moreover, hESC-CVPCs modulated cardiac macrophages toward a reparative phenotype in the infarcted hearts, and such modulation was further confirmed in vitro using human cardiovascular progenitor cell (hCVPC)-conditioned medium (hCVPC-CdM) and highly contained interleukin (IL)-4/IL-13. Furthermore, signal transducer and activator of transcription 6 (STAT6) was activated in hCVPC-CdM- and IL-4/IL-13-treated macrophages in vitro and in hESC-CVPC-implanted MI hearts, resulting in the polarization of macrophages toward a reparative phenotype in the post-MI hearts. However, hESC-CVPC-mediated modulation on macrophages and cardioprotection were abolished in STAT6-deficient MI mice.
Innovation: This is the first report about the immunoregulatory role played by hESC-CVPCs in the macrophage polarization in the infarcted hearts, its importance for the infarct repair, and the underlying signaling pathway. The findings provide new insight into the mechanism of microenvironmental regulation of stem cell-based therapy during acute MI.
Conclusions: Implantion of hESC-CVPCs during the early phase of MI promotes infarct repair via the modulation of macrophage polarization through secreted cytokine-mediated STAT6 activation. The findings suggest a therapeutic potential by modulating macrophage polarization during acute phase of MI.
Keywords: human cardiovascular progenitor cells, myocardial infarction, inflammation, macrophages, STAT6
Introduction
Myocardial infarction (MI) and the resulting heart failure (HF) are major causes of morbidity and mortality worldwide. During cardiac remodeling after acute MI (AMI), an ischemia-induced inflammatory cascade occurs via recruitment of a mixture of both protective and cytotoxic cell types as a driver for healing and exacerbating scar formation (19, 48). Cumulated evidence suggests that modulation of the inflammatory response during the early phase of MI improves infarct repair (13, 15, 40); however, clinically useful approaches targeting the modulation of inflammation toward the healing process are lacking (13).
Innovation
Human pluripotent stem cell (hPSC)-derived cardiovascular progenitor cells (hPSC-CVPCs) are a promising cell source for ischemic cardiac repair, but their roles and mechanisms in acute myocardial infarction remain largely unknown. Here, we demonstrate that human embryonic stem cell-derived cardiovascular progenitor cells (hESC-CVPCs) repair infarcted hearts. Such effect is at least achieved by the hESC-CVPC-polarized macrophages to a reparative phenotype through hESC-CVPC-secreted cytokines via activation of signal transducer and activator of transcription 6. These findings extend the knowledge of the benefits and mechanisms of hPSC-CVPCs in the infarct repair, and provide further evidence to support the view that priming macrophages toward a reparative phenotype might be a potential therapeutic approach to facilitate infarct healing.
Macrophages function as primary responder cells in modulating the inflammatory response to AMI (4, 34). After AMI, monocytes/macrophages are activated by chemotactic factors, and polarized into either classically activated inflammatory macrophages (M1-like) or alternatively activated reparative macrophages (M2-like) (4, 34). M1-like macrophages are characterized by secretion of interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) that amplify the inflammation cascade, while M2-like macrophages predominantly secrete anti-inflammatory factors IL-10, IL-13, transforming growth factor-β, and proangiogenic factors that can improve cell survival and promote fibroblast activation necessary for scar formation and angiogenesis (18, 42, 53). Recent studies suggest that shifting macrophages away from a M1-like phenotype and/or polarizing macrophages into a M2-like phenotype improve infarct repair (4, 7, 13, 34, 53), while the direct impact of modulating macrophage subpopulations on infarct repair remains to be fully investigated; and effective regulators and approaches targeting macrophage plasticity need to be further developed for priming macrophages toward a reparative phenotype during the early phase of AMI to improve cardiac remodeling and promote the infarct healing.
Human embryonic stem cell-derived cardiovascular progenitor cells (hESC-CVPCs) improve cardiac function of rodent infarcted hearts when implanted during the subacute stage of ischemia/reperfusion (I/R) (17). Besides, nonhuman primate ESC-derived SSEA1+ CVPCs can differentiate into ventricular myocytes and reconstituted part of the scar when transplanted into nonhuman primate hearts at 2-week post-MI (5). However, the precise mechanisms and time windows for the treatment remain largely unknown, and whether hESC-CVPCs have beneficial effects when administered during the early phase of MI remains elusive. We recently demonstrated that transplantation of SSEA1+ hESC-CVPCs into the AMI hearts of nonhuman primates improves recovery of left ventricular (LV) function, but the engraftment rate declines to ∼0.4% on day 3 after delivery (64), suggesting the involvement of paracrine action in their cardioprotection. However, it is unknown how these cells protect damaged hearts through paracrine action when administered during acute phase of MI, whether and how the inflammation, especially macrophage plasticity, is regulated by these cells, and whether such regulation contributes critically to the infarct repair of these cells.
Accordingly, in this study, using a murine MI model involving implantation of SSEA1+ hESC-CVPCs during AMI, combining in vivo and in vitro studies of macrophage polarization and signal transducer and activator of transcription 6 (STAT6) knockout (STAT6KO), we investigated (i) whether hESC-CVPCs delivered during AMI improve the infarct healing, (ii) the effects and regulatory mechanisms of hESC-CVPCs on macrophage plasticity, and (iii) the contribution of such modulation to infarct repair. Our data reveal previously unrecognized important roles and regulatory mechanisms of hESC-CVPCs in priming macrophage polarization toward a reparative phenotype during the early phase of MI and their impact on the infarct healing.
Results
Characteristics of SSEA1+ hESC-CVPCs (hCVPCs) and human cardiac fibroblasts
SSEA1+ hCVPCs were generated from H9 ESCs and displayed CVPC markers mesoderm posterior BHLH transcription factor 1 (MESP1), insulin gene enhancer protein (ISL1), GATA binding protein 4 (GATA4), NK2 homeobox 5 (NKX2.5), and myocyte enhancer factor 2 (MEF2C) (Supplementary Fig. S1A) as previously reported (8, 9, 64). Fluorescence-activated cell sorting (FACS) analysis showed that the purity of human cardiovascular progenitor cells (hCVPCs) was between 96.1% and 97.6% when evaluated for stage-specific embryonic antigen 1 (SSEA1) expression, a surface marker of hCVPCs (5, 36), and ∼98% of hCVPCs were enhanced green fluorescent protein (EGFP) positive (Supplementary Fig. S1B). Human cardiac fibroblasts (hcFbs) expressed fibroblast markers Vimentin and discoidin domain receptor-2 (DDR2) (Supplementary Fig. S1C) as reported (1).
hCVPCs improve contractile function and reduce scar size
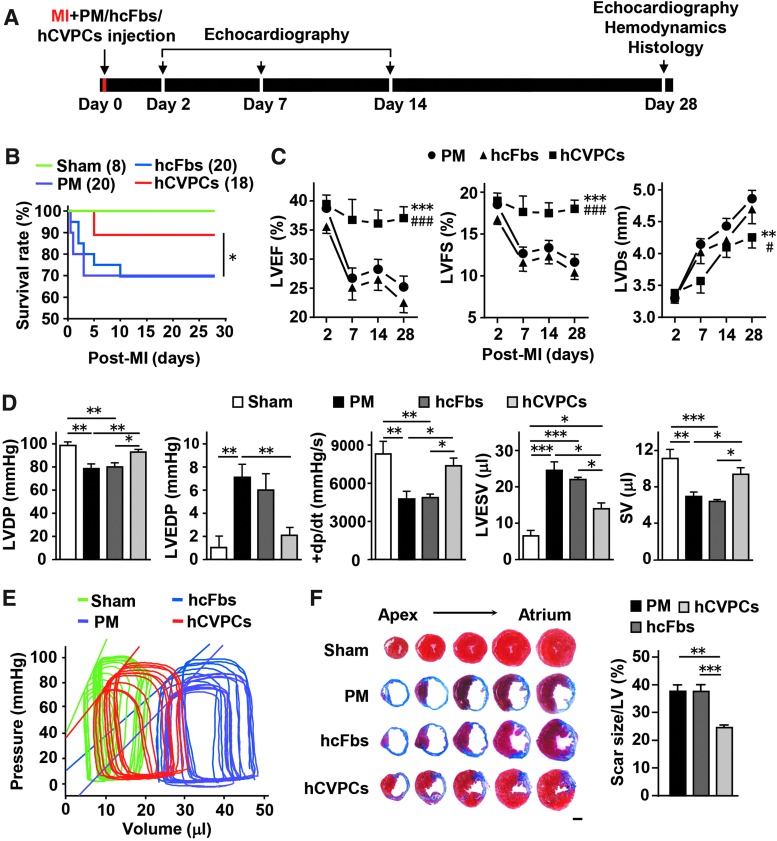
To determine whether hESC-CVPCs improve cardiac function after implantation during AMI phase, we compared the functional outcome at days 2, 7, 14, and 28 post-MI as indicated in Figure 1A. The death rate of MI mice did not significantly differ between the PuramatrixTM (PM) control and hcFbs groups (6 deaths of 20 each) during the 4-week follow-up period, but it was significantly reduced by 43% in the hCVPCs group (3 deaths of 18) (Fig. 1B). The MI was confirmed by echocardiography, and all MI groups showed comparably reduced LV ejection fraction (LVEF), LV fractional shortening (LVFS), and LV systolic dimension (LVDs) at day 2 post-MI (Fig. 1C). These parameters worsened in the PM and hcFbs groups up to day 28 post-MI; however, they were significantly improved during day 7 through day 28 post-MI in the hCVPCs group (Fig. 1C). Hemodynamic changes were further analyzed in a terminal study at day 28 post-MI using a pressure–volume loop. The MI-induced deterioration in LV developed pressure (LVDP), LV end-diastolic pressure (LVEDP), LV maximum ascending rates of pressure (+dp/dt max), and stroke volume (SV) was significantly improved by hCVPCs but not hcFbs (Fig. 1D). MI induced a rightward shift of the LV end-systolic pressure–volume relationship (ESPVR) with a shallow slope, while these changes were partially reversed by hCVPCs but not by PM and hcFbs (Fig. 1E). Consistently, all MI animals showed scar formation at day 28 post-MI as analyzed by Masson's trichrome staining, while the scar size in hCVPC-treated hearts was smaller than that in the PM and hcFbs groups (Fig. 1F). These data further indicate that hCVPCs not only improve LV function but also prevent the progressive deterioration of LV remodeling.
FIG. 1.
Cardiac repair of hCVPCs delivered to acutely infarcted mice hearts. (A) Schematic of cell transplantation and analysis. (B) Kaplan–Meier survival curves for MI mice. (C) LVEF, LVFS, and LVD measured by echocardiography. n = 7 (Sham), 13 (PM and hCVPCs), and 11 (hcFbs). (D) LVDP, LVEDP, LV maximum ascending rates of pressure (+dp/dt max) and SV measured by pressure–volume loop at day 28 post-MI. n = 5–8. (E) Representative LV ESPVR. (F) Representative cross-sectional images and quantitative data of hearts stained with Masson's trichrome at day 28 post-MI. n = 6–8. Scale bars, 1 mm. *p < 0.05, **p < 0.01, ***p < 0.001 versus the PM or as indicated; #p < 0.05, ###p < 0.001 versus hcFbs. ESPVR, end-systolic pressure–volume relationship; hcFb, human cardiac fibroblast; hCVPC, human cardiovascular progenitor cell; LV, left ventricle; LVD, left ventricular systolic dimension; LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; MI, myocardial infarction; PM, Puramatrix™; SV, stroke volume.
hCVPCs improve cardiomyocyte survival and promote angiogenesis
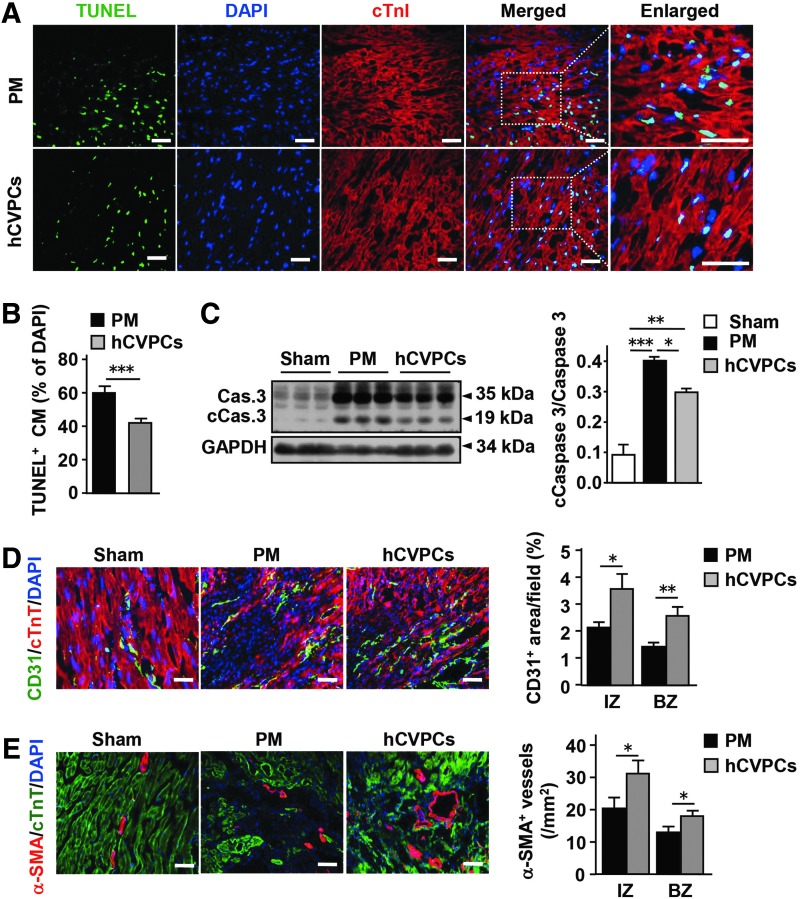
The reduction of scar size may be at least partially due to a reduction of cardiomyocyte death and/or an increase of angiogenesis. We thus examined the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL+) cardiomyocytes, CD31+ endothelial cells, and alpha-smooth muscle actin (α-SMA+) vessels in the infarcted hearts. At day 3 post-MI, TUNEL+ cardiomyocytes within the infarct and border zones in the hCVPCs group were significantly reduced compared with the PM group (Fig. 2A, B). Consistently, the MI-increased caspase-3 cleavage in the infarct zones was reduced by the hCVPCs but not the PM (Fig. 2C and Supplementary Fig. S2). At day 28 post-MI, immunohistochemistry (IHC) showed an increased number of CD31+ endothelial cells (Fig. 2D) and α-SMA+ vessels (Fig. 2E) in the infarct and border zones of hCVPC-treated MI hearts compared with the PM.
FIG. 2.
hCVPCs increase angiogenesis and reduce cardiomyocyte apoptosis. (A, B) Representative and quantification of IHC staining for TUNEL+ cardiomyocytes in the border zone of infarcted hearts at day 3 post-MI. n = 5 hearts each. Scale bar, 100 μm. (C) Representative and averaged Western blot analysis for caspase-3 (Cas. 3), cleaved caspase-3 (cCas.3), and GAPDH in the infarct zone of day 3 post-MI hearts. n = 3 hearts each. (D) Immunochemistry staining for CD31+ endothelial cells at day 28 post-MI. n = 4 hearts each. Scale bar, 100 μm. (E) Immunochemistry staining for α-SMA+ blood vessels at day 28 post-MI. n = 4 hearts each. Scale bar, 100 μm. *p < 0.05, **p < 0.01, ***p < 0.001. α-SMA, alpha-smooth muscle actin; BZ, border zone; cTnI, cardiac troponin I; cTnT, cardiac troponin T; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IHC, immunohistochemistry; IZ, infarct zone; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Myocardial sections were then stained for mouse cardiac troponin T (cTnT) and GFP antibody to distinguish whether these improvements are related to the engraftment of implanted cells. Transplanted hCVPCs were easily detected in the border zone at day 1, significantly decreased at day 3, and hardly detected at day 7 after cell delivery (Supplementary Fig. S3A). Analysis of human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA levels also showed that the engraftment rate of hCVPCs declined quickly to a level of 0.05% at day 7 after cell delivery (Supplementary Fig. S3B, C). Such a rapid decline in the number of transplanted cells in the host heart hardly allows differentiation; instead, the beneficial effects of hCVPCs on myocardial repair may result from paracrine action in the regulation of inflammatory responses during early phase of MI.
hCVPCs attenuate inflammatory responses and shift macrophages to a reparative phenotype
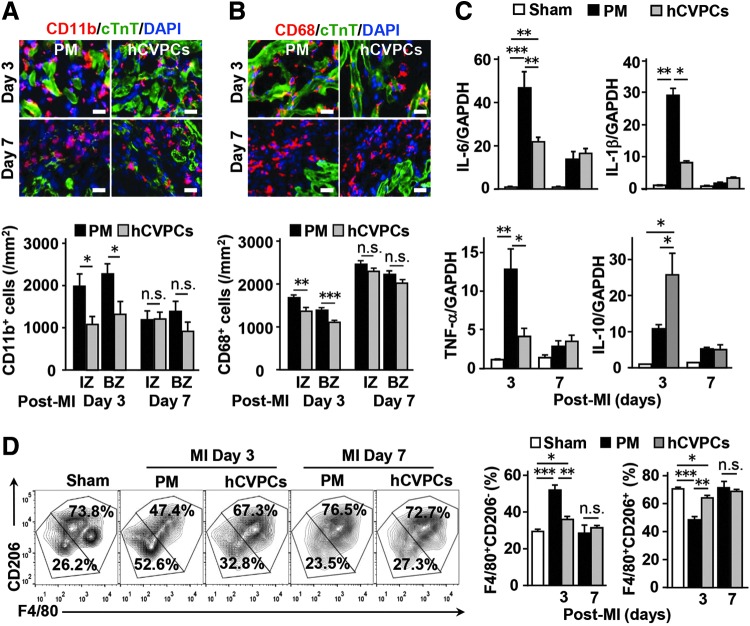
To test the above hypothesis, we then analyzed the infiltration of inflammatory cells and expression of inflammatory factors in the infarcted LV. Hematoxylin-eosin (HE) staining demonstrated that AMI-induced infiltration of inflammatory cells was reduced by hCVPCs on day 3 but not day 7 post-MI (Supplementary Fig. S4). This was confirmed by IHC staining, showing that hCVPCs significantly reduced infiltration of CD11b+ cells (neutrophils and monocytes/macrophages) and CD68+ cells (monocytes/macrophages) in the infarct and border zones on day 3 but not day 7 post-MI (Fig. 3A, B). Consistently, on day 3 post-MI, AMI-enhanced gene expression of proinflammatory cytokines IL-6, TNF-α, and IL-1β [products of inflammatory macrophages (42)] in the infarct and border zones was markedly reduced by the hCVPCs, whereas on day 7 post-MI these levels markedly declined and did not differ between the PM and hCVPC groups (Fig. 3C). Interestingly, the anti-inflammatory factor IL-10 commonly secreted from reparative macrophages (4, 16, 17, 63) was significantly enhanced by the hCVPCs on day 3 post-MI (Fig. 3C). These results suggest that hCVPCs attenuate inflammatory responses in the infarcted myocardium and might polarize macrophages toward a reparative phenotype.
FIG. 3.
hCVPCs attenuate inflammatory response and increase F4/80+CD206+ M2-like macrophages. (A, B) IHC for CD11b+ (A) and CD68+ (B) inflammatory cells at the border zone. n = 5 hearts each. (C) Quantitative reverse transcription-polymerase chain reaction analysis of IL-6, TNF-α, IL-1β, and IL-10 in the infarcted area. n = 6 hearts each. (D) Fluorescence-activated cell sorting analysis of M2 proportion (F4/80+CD206+) and M1 proportion (F4/80+CD206-). n = 4 hearts each. Scale bar, 50 μm. *p < 0.05, **p < 0.01, ***p < 0.001, and n.s., no statistical significance. IL, interleukin; TNF-α, tumor necrosis factor alpha.
To determine whether the hCVPCs affect the polarization of macrophages after MI, the proportion of inflammatory macrophages and reparative macrophages in the infarcted hearts were examined by FACS (Supplementary Fig. S5A, B). The treated hearts were digested and stained simultaneously with antibodies of CD11b, CD45, Ly6G, F4/80, and CD206 (Supplementary Fig. S5A). CD45+CD11b+Ly-6G−F4/80+ macrophages were divided based on CD206- (M1-like) and CD206+ (M2-like) (Supplementary Fig. S5B). The proportion of CD45+CD11b+Ly-6G−F4/80+CD206− M1-like macrophages in the infarcted LV was significantly higher than that in the Sham group, but the CD45+CD11b+Ly-6G−F4/80+CD206+ M2-like macrophages were decreased at day 3 post-MI, and both of them returned to the level of the Sham group at day 7 post-MI (Fig. 3D). Interestingly, at day 3 post-MI, the MI-induced opposite changes in the proportions of CD45+CD11b+Ly-6G−F4/80+CD206− M1-like macrophages and CD45+CD11b+Ly-6G−F4/80+CD206+ M2-like macrophages in the hCVPC groups were both returned to a level similar to that in the Sham, while no difference was detected at day 7 post-MI between the PM and hCVPC groups (Fig. 3D). These data indicate that the hCVPCs promote the transition of macrophages toward a reparative phenotype but shift from an inflammatory phenotype during the early phase of MI.
hCVPCs modulate macrophage polarization through the paracrine effect
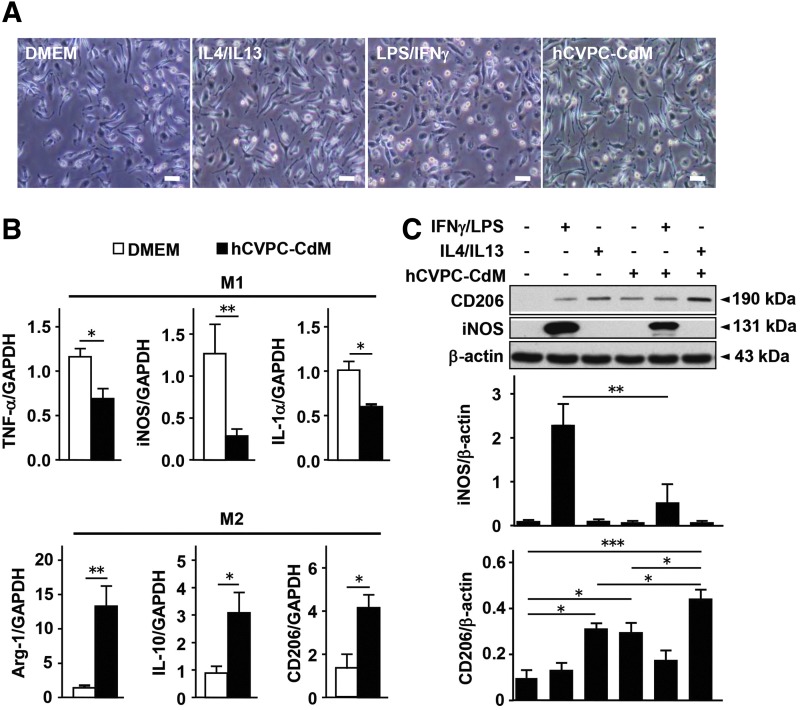
Next, we examined whether the hCVPC-modulated macrophage polarization is mediated by the paracrine effect. Isolated murine peritoneal macrophages were differentiated into M1-like (interferon gamma [IFNγ] and lipopolysaccharide [LPS]) or M2-like (IL-4 and IL-13) population with or without treatment of hCVPC-conditioned medium (hCVPC-CdM) for 24 h. Morphologically, hCVPC-CdM-treated macrophages were similar to those treated with IL-4/IL-13, while they differed from those treated with IFNγ/LPS or Dulbecco's Modified Eagle Medium (DMEM) (Fig. 4A). Quantitative reverse transcription–polymerase chain reaction (RT-qPCR) analysis showed that the hCVPC-CdM significantly reduced the expression of M1 markers TNFα, inducible nitric oxide synthase (iNOS), and IL-1α but increased the expression of M2 markers Arg1, IL-10, and CD206 (Fig. 4B). Western blot analysis further confirmed that IFNγ/LPS-activated macrophages expressed a higher level of M1 marker iNOS that was largely suppressed by the hCVPC-CdM, while IL-4/IL-13-stimulated macrophages expressed the M2 marker CD206 (Fig. 4C and Supplementary Fig. S6). Interestingly, the latter was mimicked by the hCVPC-CdM and was further elevated when treated with both (Fig. 4C and Supplementary Fig. S6). However, FACS analysis of the macrophages uptaking FITC-beads did not detect the effect of hCVPCs-CdM on macrophage phagocytosis (Supplementary Fig. S7A–C). These data suggest that hCVPCs may secrete certain stimulators to activate macrophages toward a M2-like status. The human antibody array revealed abundant proteins (Supplementary Fig. S8A) and the large amount of IL-4 and IL-13 contained in the hCVPC-CdM (Supplementary Fig. S8B). RT-qPCR analysis further detected the higher expression of M2 stimulators IL-4 and IL-13 in the hCVPCs than in the hESCs (Supplementary Fig. S8C).
FIG. 4.
hCVPC-CdM increases reparative macrophages but decreases M1-like macrophages. (A) Morphology of murine peritoneal macrophages treated with DMEM, IL-4/IL-13 (10 ng/mL), LPS (50 ng/ml)/IFNγ (100 ng/mL), or hCVPC-CdM for 24 h. Scale bar, 100 μm. (B) RT-qPCR analysis of M1 markers (TNF-α, iNOS, and IL-1α) and M2 markers (Arg-1, IL-10, and CD206) of macrophages treated with DMEM and hCVPC-CdM for 24 h. n = 4 each. (C) Western blot analysis of CD206 and iNOS levels in the hCVPC-CdM and DMEM groups with and without LPS (100 ng/mL)/IFNγ (50 ng/mL) or IL-4 (10 ng/mL)/IL-13 (10 ng/mL) in isolated peritoneal macrophages. n = 3 each. *p < 0.05, **p < 0.01, ***p < 0.001. DMEM, Dulbecco's Modified Eagle Medium; hCVPC-CdM, human cardiovascular progenitor cell-conditioned medium; IFN, interferon; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; RT-qPCR, quantitative reverse transcription polymerase chain reaction.
hCVPC-polarized M2-like macrophages via the cytokine-activated STAT6
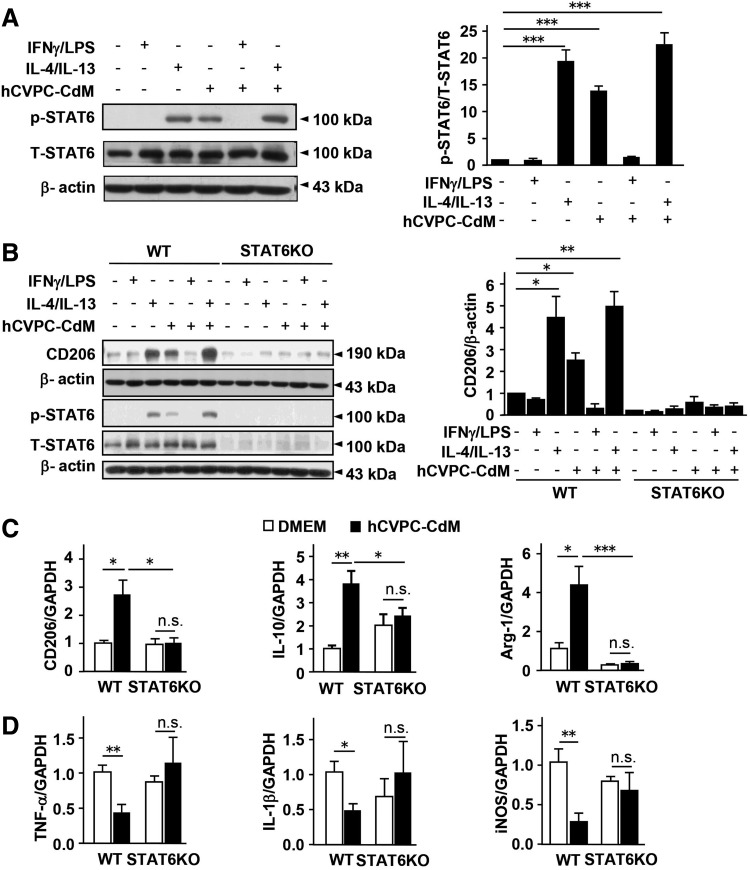
We next sought to identify signaling pathways involved in the regulation of hCVPC-CdM on the macrophage polarization with or without IFNγ/LPS or IL-4/IL-13 in the presence or absence of hCVPC-CdM on cultured macrophages. The signaling pathways protein kinase B (Akt) (47), extracellular signal-regulated kinases 1/2 (Erk1/2) (57), STAT3 (21, 54), and STAT6 (25, 44, 55) related to the macrophage regulation were examined. The treatments did not affect total protein levels of Akt, Erk1/2, STAT3, and STAT6, while IFNγ/LPS upregulated phosphorylation of Erk1/2 and STAT3 but not Akt and STAT6, and these upregulations were not affected by hCVPC-CdM (Supplementary Figs. S9A, B, S10, and S11 and Fig. 5A). In contrast, IL-4/IL-13 and hCVPC-CdM alone did not affect the phosphorylation of Akt, Erk1/2, and STAT3 but increased STAT6 phosphorylation, and the latter was further enhanced when given together for 3 h (Supplementary Figs. S9A, B, S10 and S11 and Fig. 5A). This was further confirmed by Western blot analysis, showing that IL-4/IL-13 and hCVPC-CdM upregulated the level of M2 marker CD206, and STAT6 phosphorylation after 24 h of treatment in the cultured peritoneal macrophages isolated from wild type (WT) mice was abolished in the cells isolated from STAT6KO mice (Fig. 5B and Supplementary Fig. S12). RT-qPCR analysis showed that the STAT6KO suppressed hCVPC-CdM-increased expression of M2 markers CD206, IL-10, and Arg-1 (Fig. 5C), and hCVPC-CdM-reduced the expression of M1 markers TNF-α, IL-1β and iNOS was not observed in STAT6KO macrophages (Fig. 5D). These data indicate that the hCVPCs shift the macrophages toward a M2-like phenotype via the paracrine effect-activated STAT6 signaling pathway.
FIG. 5.
hCVPC-CdM polarizes macrophages into a M2-like phenotype via the activation of STAT6. (A) Effects of LPS (100 ng/mL)/IFNγ (50 ng/mL), IL-4 (10 ng/mL)/IL-13 (10 ng/mL), or hCVPC-CdM treated for 3 h in the level of total (T) and phosphorylation (p) of STAT6 in mice peritoneal macrophages analyzed by Western blot. n = 3 each. (B) The level of CD206, total, and phosphorylation of STAT6 after 24 h of treatment with LPS (100 ng/mL)/IFNγ (50 ng/mL), IL-4 (10 ng/mL)/IL-13 (10 ng/mL), or hCVPC-CdM in the peritoneal macrophages isolated from WT and STAT6KO mice. n = 3 each. RT-qPCR analysis of M2 (CD206, IL-10, Arg-1) (C) and M1 markers (TNF-α, IL-1β, iNOS) (D) in the macrophages isolated from WT and STAT6KO mice and cultivated with DMEM and hCVPC-CdM for 24 h. n = 4 each. *p < 0.05, **p < 0.01, ***p < 0.001, and n.s., no statistical significance. STAT6, signal transducer and activator of transcription 6; STAT6KO, STAT6 knockout; WT, wild type.
STAT6 mediates myocardial wound repair of hCVPCs
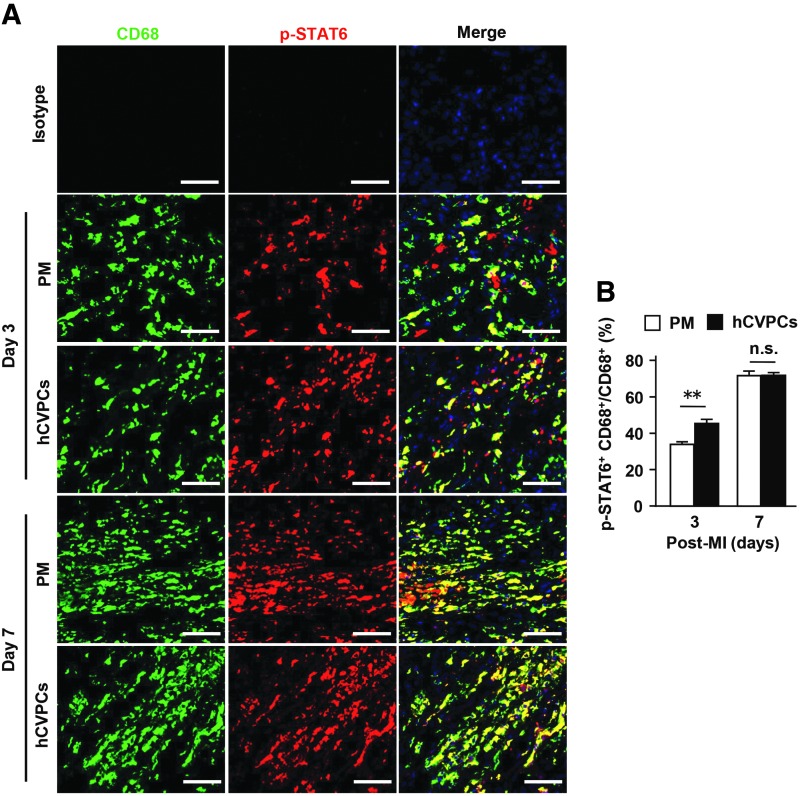
Next, we sought to determine whether hCVPCs activate STAT6 in the macrophages of infarcted hearts. The STAT6 phosphorylation in the border zone of infarcted hearts was enhanced by hCVPCs when compared with that of the PM group at day 3 post-MI (Fig. 6A, B).
FIG. 6.
hCVPCs activate macrophage STAT6 in border zone of day 3 post-MI hearts. (A) IHC for p-STAT6 and CD68 at day 3 and 7 post-MI. Scale bar, 50 μm. (B) Quantification of ratio of p-STAT6+CD68+ macrophages to CD68+ macrophages. n = 6 hearts each. **p < 0.01, and n.s., no statistical significance.
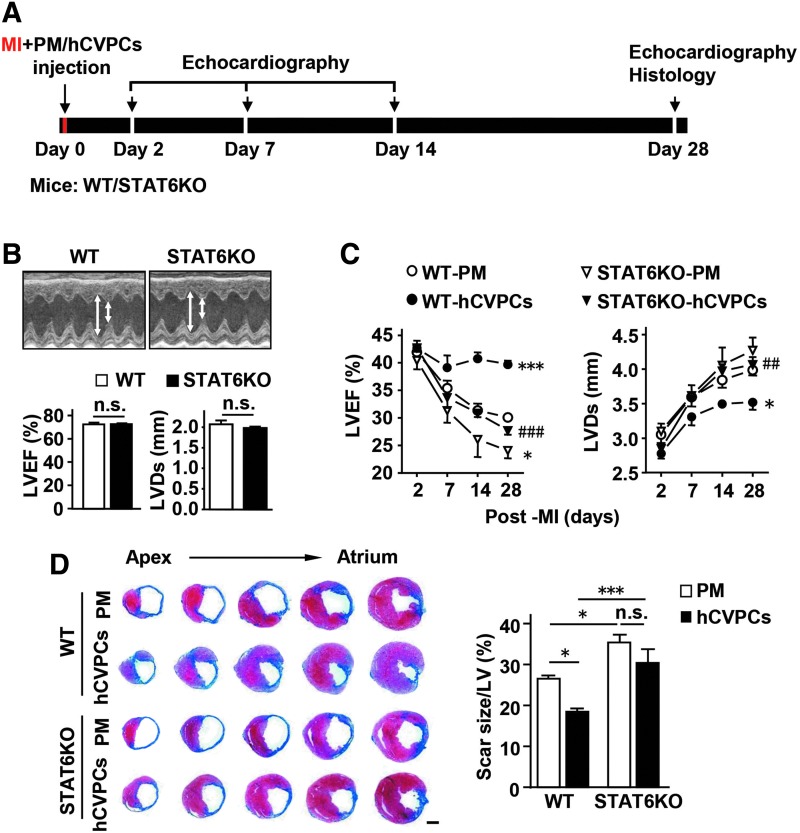
We then verified whether the activation of STAT6 contributes to the hCVPC-mediated benefits in the infarcted hearts in the WT and STAT6KO mice (Fig. 7A). The efficiency of STAT6KO in the heart was confirmed by RT-PCR (Supplementary Fig. S13A) and Western blot (Supplementary Fig. S13B). The LVEF and LVDs were similar between the WT and STAT6KO mice (Fig. 7B) as well as between the infarcted WT and STAT6KO mice at day 2 post-MI (p > 0.05) (Fig. 7C), but the STAT6KO MI mice exhibited worsened LVEF and LVDs (Fig. 7C) and larger scar size (Fig. 7D) than those observed in the WT MI mice at day 28 post-MI. Moreover, the functional improvement (Fig. 7C) and the limitation in scar size (Fig. 7D) by the hCVPCs were abolished in STAT6KO mice at day 28 post-MI, though these parameters were comparable at day 2 post-MI among the groups.
FIG. 7.
Cardioprotective effects of hCVPCs are dependent on the activation of STAT6. (A) Schematic of cell transplantation and analysis in WT and STAT6KO mice. (B) Echocardiographic analysis of LVEF and LVDs in the WT and STAT6KO mice. n = 6 each. (C) Echocardiographic analysis of LVEF and LVDs in the MI WT and STAT6KO mice with PM or hCVPCs treatment. n = 5–8 each. (D) Representative cross-sectional images and quantitative data of hearts stained with Masson's trichrome at day 28 post-MI. n = 5–8 hearts each. Scale bar, 1 mm. *p < 0.05, ***p < 0.001 versus the WT-PM or as indicated; ##p < 0.01, ###p < 0.001 versus the WT-hCVPCs, n.s., no statistical significance. LVEF, left ventricular ejection fraction.
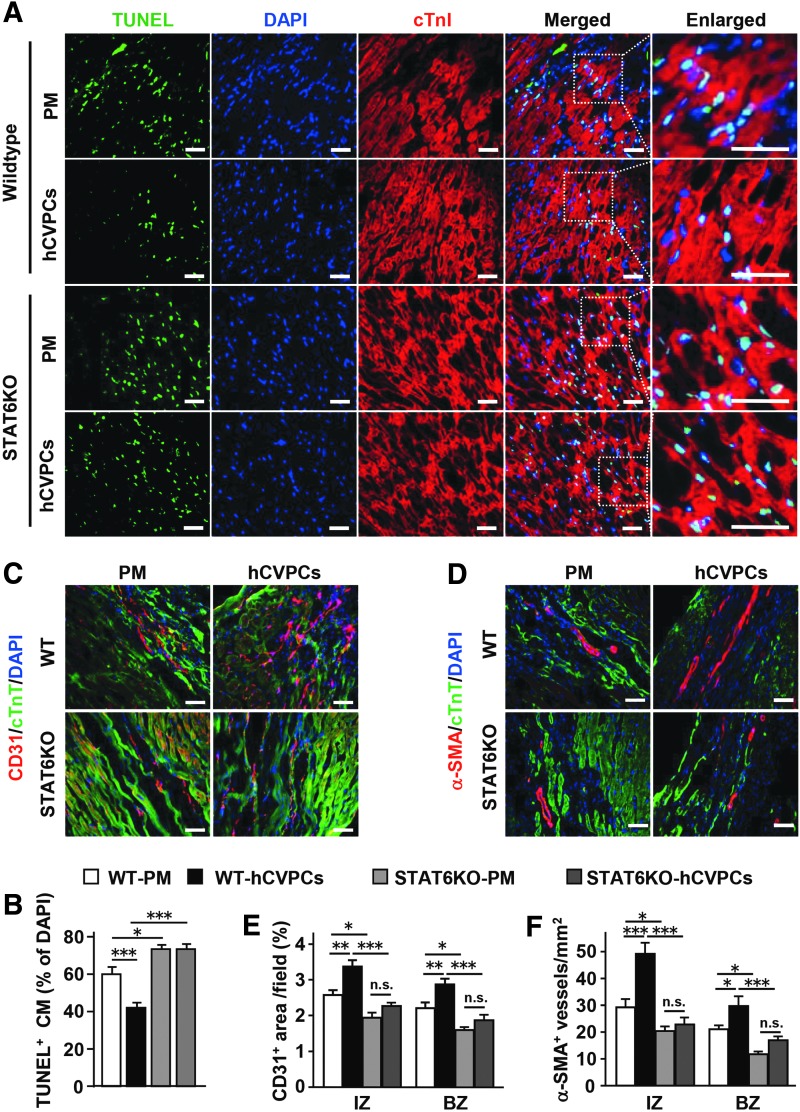
We then investigated whether the STAT6KO affects the benefits of hCVPCs in promoting angiogenesis and antiapoptosis of cardiomyocytes. The apoptotic responses induced by 0.5 and 1 h of oxygen glucose deprivation (OGD) treatment in cardiomyocytes isolated from adult WT and STAT6KO mice were comparable, but the majority of cardiomyocytes lost their myofilament phenotype and died after a 3-h OGD treatment in both WT and KO cardiomyocytes (Supplementary Fig. S14A, B). However, STAT6KO increased MI-induced apoptosis and abolished hCVPC-reduced apoptotic cardiomyocytes in the border zone detected with TUNEL staining at day 3 post-MI (Fig. 8A, B, left panel). Moreover, STAT6KO reduced the number of CD31+ endothelial cells (Fig. 8C, E, middle panel) and α-SMA+ vessels (Fig. 8D, F, right panel) in the infarct and border zones of the PM hearts, and blocked hCVPC-promoted angiogenesis (Fig. 8C–F).
FIG. 8.
hCVPC-promoted angiogenesis and antiapoptosis of cardiomyocytes are blunted by STAT6KO. (A) Representative TUNEL-stained images of the border zone in MI mouse heart. Scale bar, 100 μm. (B) Quantification of TUNEL+ cardiomyocytes. n = 3 hearts each. Representative of immunochemistry staining for CD31+ endothelial cells (C) and α-SMA+ blood vessels (D) at day 28 post-MI. Scale bar, 100 μm. Quantification of CD31 (E) and α-SMA for blood vessels (F) at day 28 post-MI. n = 4 hearts each. *p < 0.05, **p < 0.01, ***p < 0.001, and n.s., no statistical significance.
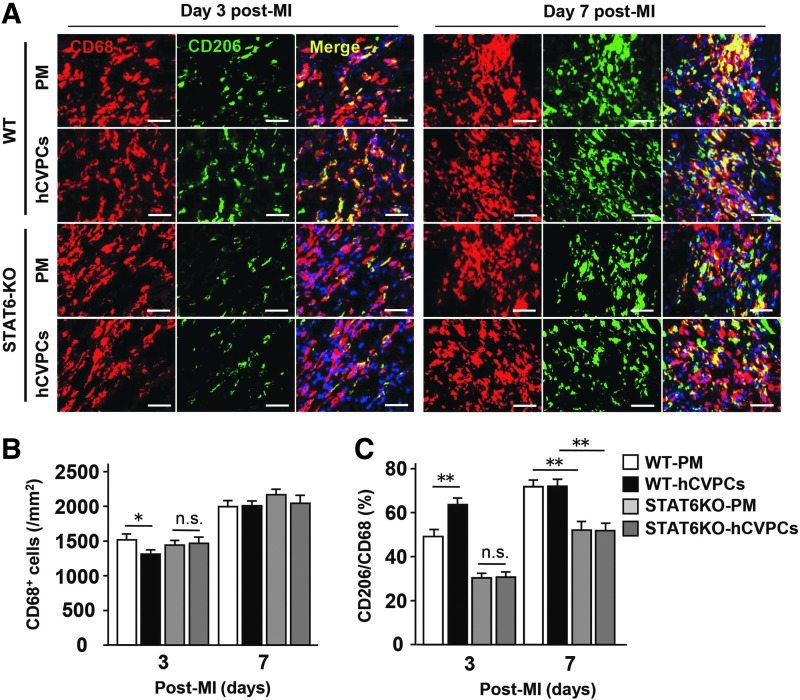
Next, to confirm whether lack of STAT6 interferes with the polarization of macrophages after the MI and the hCVPC treatment, we analyzed CD206+ and CD68+ macrophages at the border zone of infarcted hearts with and without treatment of hCVPCs by IHC staining (Fig. 9A–C). In the infarcted hearts, STAT6KO did not affect the number of CD68+ macrophages at day 3 and 7 post-MI (Fig. 9B), while it decreased the ratio of CD206+/CD68+ at day 3 as well as day 7 post-MI compared with those observed in the WT mice (Fig. 9C). Moreover, on day 3 post-MI, the hCVPC-reduced CD68+ macrophage population and increased CD206+/CD68+ ratio were canceled in STAT6KO mice (Fig. 9C).
FIG. 9.
STAT6KO abolishes hCVPC treatment-induced polarization of reparative macrophages after MI. (A) IHC for CD206+ and CD68+ macrophages at the border zone of hearts treated with PM or hCVPCs at day 3 and day 7 post-MI of WT and STAT6KO mice. Scale bar, 50 μm. (B) Quantification of total CD68+ cells at the border zone of hearts. n = 3 hearts each. (C) Quantification of CD206+/CD68+ ratio at the border zone of hearts. n = 3 hearts each. *p < 0.05, **p < 0.01, n.s., no statistical significance.
Discussion
hESC-CVPCs represent an attractive cell type for cardiac repair, but their roles in AMI and mechanisms of action remain largely unknown. This is the first report showing that the infarct repair of SSEA1+ hESC-CVPCs implanted during the acute phase of MI is concomitantly decreasing the number of inflammatory macrophages and increasing reparative macrophages in the infarcted hearts. These effects are achieved by hESC-CVPC-secreted cytokines via activation of STAT6, resulting in the improvement of existing cardiomyocyte survival and vascularization in the infarcted hearts. The findings extend the benefits of human pluripotent stem cell (hPSC)-CVPCs to ischemic myocardial damage, and provide new knowledge into the mechanisms mediating infarct repair via modulating inflammatory responses and the involved signaling. The findings also provide further evidence to support the view that the priming macrophages toward to a reparative phenotype might be a potential therapeutic approach to facilitate infarct healing (4, 7, 13, 34, 53).
Transplantation of hESC-CVPCs during the subacute stage of I/R or chronic stage of MI has been shown to improve recovery of cardiac function from I/R in rodent (17) and nonhuman primate models (5). Here, we demonstrate that SSEA1+ hESC-CVPC delivered at the acute phase of MI confers cardioprotection characterized by improving cardiac performance, survival of existing cardiomyocytes, promoting angiogenesis, and limiting scar size. These observations are consistent with the findings in nonhuman primates, in which the transplantation of SSEA1+ hESC-CVPCs significantly reduced apoptotic cardiomyocytes in the infarcted hearts at day 3 post-MI and improved LV function at day 28 post-MI (64). These findings indicate that the acute stage of MI is an important window for hESC-CVPCs therapy targeting at saving cardiomyocyte and promoting vascularization in addition to their benefits at the subacute and chronic stages of MI (5, 17). Supportively, implantation of ESC-derived ISL1+-CVPCs (3) and cardiomyocytes (56), mesenchymal stromal cells (MSCs) (4), or cardiosphere-derived cells (CDCs) during early reperfusion (13, 30) or AMI significantly improves healing process of infarct hearts. Thus, the acute phase of MI is a critical window for cell therapy targeting outcome of cardiac repair.
The critical role of inflammatory modulation in the hESC-CVPC-mediated myocardial repair after ischemic injury is revealed in this study. Adequate evidence suggests that the activation of reparative macrophages during acute phase of MI is critical for the healing process. It seems that modulation of macrophage polarization to a reparative phenotype is an important mechanism for stem cell/cytokine therapy. This is supported by the reports showing that transforming macrophages away from inflammatory macrophages to reparative macrophages by delivery of CDCs (6, 13), MSCs (4), IL-4 (51, 53), IL-10 (29), or inactivation of cyclic GMP–AMP synthase (7) improves the infarct repair after MI. Interestingly, hESC-CVPCs not only increase the population of reparative macrophages but also reduce the population of inflammatory macrophages, while MSCs seem to mainly activate reparative macrophages in the infarcted hearts (4). These findings extend previous observations (4, 13, 20, 24, 34, 35, 43, 58), indicating that the hESC-CVPCs may serve as a modulator of the innate immune responses to favor the infarct repair, and the transition of inflammatory macrophages to a reparative phenotype might be an effective target for infarct repair.
The polarization of macrophages to a reparative phenotype during the acute phase of MI may decrease the MI scar via the following mechanisms: (i) improving the survival of cardiomyocytes via the downregulation of proinflammatory cytokines inducing apoptosis of cardiomyocytes, such as IL-1β and TNF-α (49, 61), when suppressing M1 macrophage polarization (27) and the enhancement of anti-inflammatory cytokines attenuating apoptosis of cardiomyocytes, such as IL-10 (14). Similar changes were observed here in the hCVPC-implanted MI hearts; (ii) promoting angiogenesis through macrophage-secreted proangiogenic factors such as fibroblast growth factor-2, vascular endothelial growth factor alpha, insulin-like growth factor, and platelet-derived growth factor (28, 32, 33, 37). We observed that STAT6KO-abolished hCVPC-reduced number of CD68+ macrophages and increased the ratio of CD206+/CD68+ in the infarcted hearts (Fig. 9C), which are accompanied by the blockage of hCVPC-promoted angiogenesis by STAT6KO (Fig. 8C–F), suggesting that the proangiogenic effect induced by SSEA1+ hCVPC-modulated macrophages may contribute to the healing process of the infarcted heart. The precise mechanisms behind the hCVPC-educated macrophage-stimulated angiogenesis and antiapoptosis of cardiomyocytes need to be further elucidated in future. Besides, the hCVPCs might stimulate proangiogenic effect and exert antiapoptotic effect via a macrophage-independent way, and this possibility needs to be explored.
Our results also indicate that benefits associated with the hESC-CVPCs in cardiac repair are mediated by paracrine effects due to the change of microenvironment. A rapid decline in the engraftment rate of the transplanted hESC-CVPCs (Supplementary Fig. S3) as seen in our recent study using the infarcted primates (64) suggesting the paracrine-related anti-inflammatory effects and modulation of macrophage phenotypes may play as a major mechanism for the cardioprotection of hPSC-CVPCs when administered during early phase of MI. This is supported by the experimental data, showing that (i) hESC-CVPCs secrete abundant proteins, including cytokines IL-4 and IL-13 (Supplementary Fig. S8), to promote polarization of macrophages into a reparative phenotype via activating STAT6 (Fig. 5A, B); (ii) the hCVPC-CdM modulates the macrophage phenotype and activates STAT6 in the macrophages (Figs. 4 and 5); (iii) at day 3 post-MI, the macrophages are polarized into a reparative phenotype via activation of STAT6 in the infarcted hearts by implanted hCVPCs (Figs. 3C and 6), while at that time majority implanted cells are gone (Supplementary Fig. S3); and (iv) these processes are associated with the hCVPC-promoted healing process. Thus, hESC-CVPCs provide an approach to modulate macrophage phenotypes and functions by changing the microenvironment in the infarcted hearts. In addition, SSEA1+ hPSC-CVPC-secreted vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic postinfarct HF murine model (31), and paracrine effects play major roles in the salvage of infarcted myocardium by hPSC-derived cardiac lineage cells implanted during acute phase of MI (56). Therefore, further studies are required to dissect their secretome to explore the potential use of a cell-derived but cell-free therapy for cardiac repair. Besides, upon the development of approaches to improve the survival of implanted hPSC-CVPCs, the contribution of differentiated cardiovascular cells to the infarct repair needs to be evaluated.
Another interesting observation is STAT6, serving as a key signal mediating the therapeutic effects of hESC-CVPCs in the infarcted heart. Although STAT6KO mice exhibit normal cardiac function as previously reported (26), the LV function and fibrosis has shown to be worse, and the benefits of hESC-CVPCs in cell survival, angiogenesis, functional recovery, and scar formation are cancelled by STAT6KO. This indicates the critical role of STAT6 in the myocardial repair, especially with respect to the hESC-CVPC-promoted healing process. This protection can be interpreted by the following hypothetical mechanisms: STAT6 may act as a key signal priming macrophages to a reparative phenotype in response to IL-4/IL-13 and hESC-CVPCs in the MI hearts, resulting in cardiac repair (Figs. 5–9 and Supplementary Fig. S8). Supportively, the STAT6 in macrophages is activated by IL-4/IL-13 as reported (25) but not Akt, Erk1/2, and STAT3 (Supplementary Fig. S9), by hCVPC-CdM in vitro (Fig. 5), and by hESC-CVPCs in the infarcted hearts (Fig. 6), while IL-4/IL-13- or hCVPC-CdM-activated reparative macrophages are suppressed by STAT6KO (Fig. 5) and hESC-CVPC-promoted reparative macrophage polarization in MI mice is cancelled by STAT6KO (Fig. 9). Moreover, isolated STAT6KO cardiomyocytes show similar OGD injury to that in the WT cells, supporting the indirect protection of STAT6 activation on cardiomyocyte survival in the infarcted hearts. These observations provide further evidence, showing the essential role of STAT6 in the activation of reparative macrophages (11, 44) and promoting the infarct healing. Thus, activation of STAT6 might be a potential target for the treatment of AMI via specific polarization of monocytes/macrophages into a reparative phenotype, though the regulatory effects of STAT6 on other cell types in cardiac repair need to be elucidated. Besides STAT6, other proteins could be involved in the modulation of macrophage phenotypes, resulting in the promotion of infarct heart healing. It is thus worthy to explore whether other proteins secreted by hESC-CVPCs would contribute to the cardioprotection via polarization of macrophages into a reparative phenotype in the MI hearts.
There are limitations in this study. Transplantation of the hESC-derived CVPCs in a mouse model could lead to confusing rejection activity. It would be helpful to control these studies with experiments where mouse ESC-derived CVPCs are used, and verify that the cardioprotection of these cells is also dependent on their paracrine effect and related to modulation of macrophage phenotypes. In addition, as the xenograft model and consequent rejection of hESC-CVPCs in this study prevent us from assessing engraftment and subsequent cellular contribution to cardiac recovery, our experiments do not at all exclude other mechanisms by which hESC-CVPCs could help the repair process of the injured heart, for example by engrafting and becoming functional cardiac cells as seen in hESC-derived cardiomyocytes (10, 41) and MSCs (2, 45).
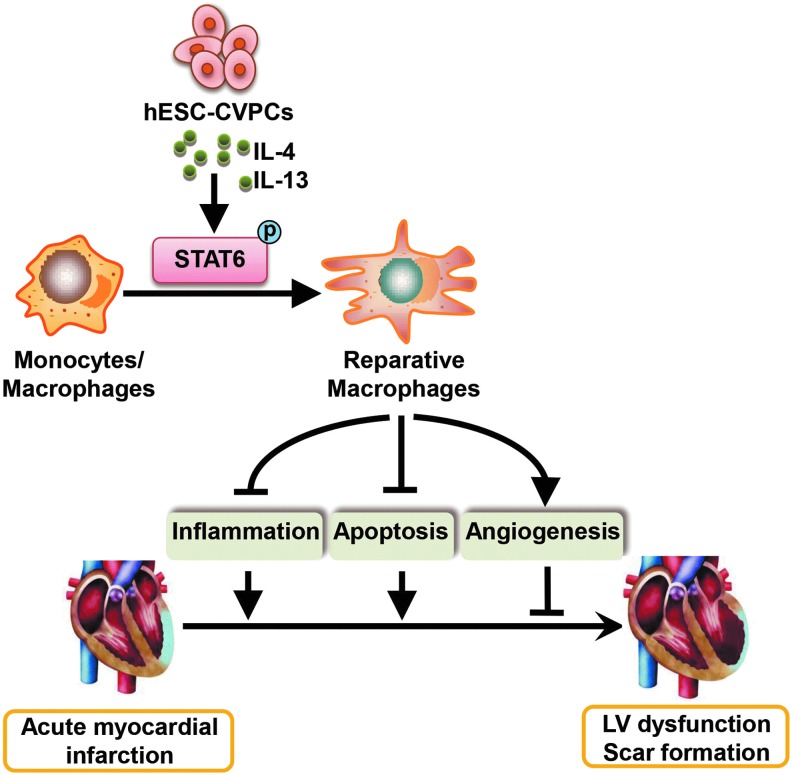
In conclusion, we demonstrate that modulation of macrophage polarization by delivery of hESC-CVPCs into acutely infarcted mice hearts plays a significant role in blunting the worsening of heart function and fibrosis via their paracrine effect-mediated activation of STAT6. The benefits are associated with a reduced inflammatory response, improved survival of existing cardiomyocytes, and promoted angiogenesis (Fig. 10). These findings not only indicate that hESC-CVPCs may be a therapeutic option for infarct repair, but also suggest that modulation of macrophage polarization to a reparative phenotype and activation of STAT6 in the infarcted heart might be a promising therapeutic approach for ischemic heart disease.
FIG. 10.
A proposed model for the cadioprotection of hESC-CVPCs in the mice model of acute MI. hESC-CVPCs implanted at acute phase of MI regulate immune regulatory activity, including the modulation of reparative macrophage polarization by activating STAT6 through at least secreted IL-4 and IL-13, which dominantly contributes to the cardioprotective effect of hESC-CVPCs in the infarcted hearts. hESC-CVPC, human embryonic stem cell-derived cardiovascular progenitor cell.
Materials and Methods
Cell culture, differentiation, and characterization
H9 hESCs (WiCell) were routinely maintained in commercially available mTeSR1 medium (Stem Cell Technologies) on Matrigel-coated plates (hESC qualified; BD Biosciences) according to manufacturer's instruction. The induction of hESC-CVPCs followed the protocol reported previously (8, 9, 64). In brief, undifferentiated hESCs were dissociated with Accutase (Stem Cell Technologies) and then plated onto Matrigel-coated culture dishes at a density of 5 × 104 cells/cm2 in hESC-CVPC induction medium (DMEM/F12, 1 × B27 supplement without vitamin A, 1% l-Glutamine, 1% penicillin/streptomycin, 400 μM 1-thioglycerol, 50 μg/mL ascorbic acid, 25 ng/mL bone morphogenetic protein 4, and 3 μM CHIR99021). hESC-CVPCs were harvested after 3 days of differentiation for analysis and implantation. hcFbs were purchased from Sciencell (Catalog No. 6330; Shanghai, China) and cultured on DMEM/F12 with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Immunostaining assays for characterization of hESC-CVPCs and hcFbs were performed as described previously (1, 8, 22). In brief, cells were fixed with 4% paraformaldehyde (PFA), permeabilized in 0.3% Triton X-100, blocked in 10% normal goat serum (Vector Laboratories), and then incubated with primary antibodies against MESP1, MEF2C, ISL1, GATA4, NKX2.5, Vimentin, and DDR2 at 4°C overnight and detected by DyLight 488- or DyLight 549-conjugated secondary antibodies. Nuclei were stained with Hoechest33342 or 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). The SSEA1-positive and GFP-positive proportion of hESC-CVPCs were analyzed and quantified by flow cytometry (FACStar Plus Flow Cytometer; BD Bioscience) as reported previously. The antibodies used are listed in Supplementary Table S1.
Plasmid and cell line construction
EGFP-positive hESCs were generated by using the pCDH cDNA cloning and expression lentivector system (System Biosciences) as described previously (64). In brief, the EGFP cDNA was subcloned into a pCDH-EF1-MCS-T2A-Puro plasmid (System Biosciences) between EcoRI and NotI restriction endonuclease sites. Then, the EGFP-containing lentivirus was packaged into 293FT cells (Invitrogen), and lentivirus particles were collected via centrifugation at 130,000 g for 2 h. hESCs (H9-WA09 hESCs, provided by WiCell Research Institute, Wisconsin) were transfected with the particles and cultured with puromycine (1 mg/mL; Life Technologies) for 7 days. Then, the puromycine-resistant (i.e., EGFP-expressing) cells were collected for use in subsequent experiments.
MI surgical procedure and study design
All procedures involving animals were performed in accordance with the Guidelines for Care and Use of Laboratory Animals published by the United States National Institutes of Health (43a), and were approved by the Institutional Animal Care and Use Committee of Shanghai Institutes for Biological Sciences. Adult male C57BL/6 and BALB/c (WT) mice (The Shanghai Slac Laboratory Animal Co. Ltd., Shanghai, China) and BALB/c background STAT6 knockout (STAT6KO) mice (The Jackson Laboratory, Bar Harbor, ME) were used in this study.
Sex- and age-matched animals were used for all experiments. MI was produced as previously described (12, 40). In brief, male mice aged 10–12 weeks were anesthetized via intraperitoneal injection of 50 mg/kg sodium pentobarbital and ventilated with a volume-regulated respirator (SAR830; Cwe Incorporated) at a frequency of 90 bpm, and the tidal volume ranged 200–300 cc/min. The body temperature was maintained at 37°C throughout the surgical procedure. The mice were randomly selected for the Sham group and the induction of MI. The left chest of mice was opened between the second and the third ribs, and the hearts were exposed. Then, Sham-operated mice had a loose suture placed around the left anterior descending (LAD) coronary artery, and the MI was produced through ligation of the LAD coronary artery with 8-0 Prolene suture at the 3–4 mm on the lower edge of the left auricle. Of 10 μL 10% sucrose solution containing 5 × 105 hCVPCs or hcFbs cells were mixed with 10 μL 0.4% self-assembling nanopeptide (PM; 3-D Matrix, Ltd.) to generate 20 μL of 0.2% PM cell mixture, and then they were directly injected into two sites in the border zone of infarcted myocardium (10 μL per site) just after the ligation of LAD coronary artery as previously described (40). The muscle and skin of the chest were then sutured with 6-0 and 5-0 Prolene suture, respectively. The disposition of animals is listed in Supplementary Table S2.
Heart function measurements
Transthoracic echocardiography (Vevo 2100; Visual Sonics) with a 25-MHz imaging transducer was performed on anesthetized animals as previously described (12) with measurement of LVEF, LVFS, and LVDs. The average values were collected from three consecutive cardiac cycles.
Hemodynamic analysis was performed using a pressure–volume loop as previously described (52, 59). In brief, at 28 days post-MI after echocardiography, a polyethylene pressure transducer catheter (Millar Spr-839 1.4F; Millar Instruments, Houston, TX) was inserted from the right carotid artery into LV for hemodynamic study as previously described (52, 59). LVDP, LVEDP, LV maximum ascending rates of LV pressure (+dp/dt max), and SV were recorded simultaneously. Inferior vena cava occlusion was performed with external compression to produce variably loaded beats to determine the LV ESPVR. Chart software (AD Instrument Ltd., Australia) was used for data processing.
Immunohistochemical staining
Hearts were quickly removed from anesthetized mice and washed with 5 mL cold cardiac arresting buffer (10% KCl), phosphate buffered saline (PBS), and 4% PFA solution. Ventricular tissues were embedded in optimal cutting temperature (SAKURA) compound for histological analysis. Transversal sections (5 μm) were prepared at 400-μm intervals.
For fluorescent IHC, fresh frozen sections were fixed with 4% PFA, permeabilized in 0.4% Triton X-100 (Sigma), and stained with anti-CD31, α-SMA, CD11b, CD68, CD206, cardiac troponin I, cTnT, and GFP antibody. Antibodies were detected by fluorescent-conjugated secondary antibodies. Nuclei were stained with DAPI (Sigma-Aldrich). Histological images were blindly measured using a Zeiss inverted microscope and processed using ZEN software. Three microscopic fields were quantified for each slice by two independent observers. The image-processing software (ImageJ) was used as the image quantification software. The antibodies used are listed in Supplementary Table S1.
Apoptosis was evaluated with an In situ Cell Death Detection Kit (Roche Applied Science, Germany) for TUNEL staining as directed by the manufacturer's instructions. The percentage of TUNEL+ cardiomyocytes was quantified as the ratio of TUNEL+ cardiomyocytes to the DAPI-positive cells. The ImageJ is used to quantificate the TUNEL+ nucleus and total nucleus.
Masson's trichrome staining and HE staining
From Masson's trichrome-stained images, morphometric parameters in each heart including total LV area and scar area were analyzed on five slices (from the point of ligation to the apex of the heart) with ImageJ, as described previously (13, 23, 46). Infarcted scar size was calculated as total scar area divided by LV area. The HE staining analysis was performed as described previously (60).
Isolation of cardiac immune cells and FACS analysis
The procedure and analysis were performed as previously described (62). In brief, the hearts were rapidly removed after being anesthetized, and then minced, digested for 80 min with prewarmed buffer containing 2% collagenase type 2 (Worthington), 0.25% elastase (Worthington), and 0.05% DNase I. After the enzymatic reaction was stopped, the cells were filtered through a 70-μm cell strainer. The filtered cells were subjected to density gradient centrifugation with Percoll to obtain inflammatory cells as described previously (62). Isolated cells were stained at 4°C for 40 min simultaneously with the following antibodies: FITC-labeled rat antimouse CD45, PE-labeled rat antimouse Ly-6G, PE-cy7-labeled rat antimouse CD11b, Bv421-labeled rat antimouse F4/80, Alexa Fluor 647-labeled rat antimouse CD206 to analyze the polarization of macrophages. Cell fluorescence was measured with EPICS ALTRA flow cytometer using EXPO32 software, version 1.2 (Beckman Coulter, Inc.). All antibodies have been listed in Supplementary Table S1.
Isolation and culture of abdominal macrophage
Peritoneal macrophages from C57BL/6 or BALB/c or STAT6KO mice were harvested under aseptic conditions at 3 days after intraperitoneal injection with 3% thioglycollate (Sigma-Aldrich) as previously described (39, 50). Red blood cells were lysed in erythrocyte lysis buffer (10 mmol/L Tris-HCl, pH 7.2), containing 150 mmol/L NH4Cl. The resultant peritoneal macrophages were seeded onto six-well tissue culture plates at 1 × 106 cells/well in 1640 medium containing 10% FBS, and 4 h later the nonadherent cells were removed. The macrophages were then cultured for 24 h in DMEM medium containing 1% FBS before treatment with hCVPC-CdM, LPS (100 ng/mL) + IFNγ (50 ng/mL), or IL-4 (10 ng/mL) + IL-13 (10 ng/mL) in serum-free DMEM. After 24-h treatment, the macrophages were collected for further analysis and dissociated with Trizol to isolate total RNA. After the reverse transcription reaction, M1 (TNF-α, iNOS, and IL-1α) and M2 markers (Arg-1, IL-10, and CD206) were measured by RT-qPCR. Proteins were collected after treatment for 3 or 24 h, dissociated with RIPA buffer, and Western blotted with anti-iNOS, anti-CD206, STAT6, p-STAT6, STAT3, p-STAT3, Akt, p-Akt, Erk1/2, p-Erk1/2, or β-actin. Detailed antibody information is listed in Supplementary Table S1.
Preparation of hCVPC-CdM
hESCs were seeded onto 10-cm dishes at a field density of 3 × 104 cells/cm2. After culture for 72 h in hCVPC-induced medium, cells were washed with PBS thoroughly three times, and medium was changed to serum-free DMEM. After incubation for 48 h in serum-free medium, the supernatant was collected as hCVPC-CdM and cells were removed by centrifugation at 2000 g for 10 min.
Protein analysis
The heart was harvested and rinsed in precold PBS. The infarct with border zone was dissected and stored at −80°C until use. Tissues were minced, homogenized, and digested in tissue lysis buffer (with protease and phosphatase inhibitors; Thermo Scientific). The cells were scraped off culture plates on ice and lysed with RIPA (with protease and phosphatase inhibitors; Thermo Scientific). The resulting suspensions were centrifuged at 10,000 g for 30 min at 4°C, and the protein supernatant was collected. Protein concentrations were measured using a BCA assay (Thermo Scientific). Protein samples were prepared for polyacrylamide gel electrophoresis. Proteins were then transferred to a polyvinylidene difluoride membrane (BioRad) for immunoblotting with relevant antibodies. The antibodies and usage dilution are listed in Supplementary Table S1. Bands were visualized after activation with ECL (Thermo Scientific) and exposure on film (Kodak Carestream Biomax; Sigma-Aldrich). Cytokine release was measured in culture supernatant by cytokine antibody array (Raybiotech L-Series Human Antibody Array L-507), according to the manufacturer's instructions (RayBiotech, Inc.).
Reverse transcription–polymerase chain reaction and RT-qPCR
Reverse transcription–polymerase chain reaction (RT-PCR) and RT-qPCR were performed as described previously (8). In brief, total RNA was extracted from the LV tissue using a RNeasy Plus Mini Kit (QIAGEN) following the manufacturer's instructions and treated with DNase I (Promega) for 15 min to eliminate the potential contamination of genomic DNA. cDNA was generated by reverse-transcribed total RNA (1 μg) using oligo (dT) primer and ReverTra Ace reverse transcriptase (Toyobo). PCR was carried out using Taq DNA Polymerase (Takara). qPCR was performed using the ABI PRISM 7900 system (Applied Biosystems) with the SYBR Green Realtime PCR Master Mix plus (TOYOBO) for relative quantification of the indicated genes. The transcript of GAPDH was used for internal normalization. The RT-PCR and RT-qPCR primers are listed in Supplementary Table S3.
Phagocytosis assay
The analysis was performed as previously described (13). To examine the phagocytic capacity of unstimulated and stimulated macrophage with hCVPC-CdM, LPS (100 ng/mL) + IFNγ (50 ng/mL), or IL-4 (10 ng/mL) + IL-13 (10 ng/mL), we performed a fluorescent latex bead assay (Cayman Chemical) according to the manufacturer's protocol. After prestimulation, macrophages were treated overnight with FITC fluorescently labeled microspheres (0.1-μm diameter). Cells were collected and then stained with F4/80-PE (eBioscience) to examine all macrophages. We then performed FACS analysis for quantitation assessment of microsphere uptake in each group.
Isolation and culture of adult mouse cardiomyocytes and OGD treatment
The adult mouse cardiomyocytes (AMCMs) were isolated from adult WT and STAT6KO mice hearts, and cultured as previously described (38). AMCMs were subjected to hypoxia in vitro in an oxygen control cabinet (Ruskinn, England) mounted within an incubator, and equipped with oxygen controller and sensor for continuous oxygen-level monitoring. A mixture of 85% nitrogen, 10% hydrogen, and 5% CO2 was utilized to create hypoxia, and the O2 in the chamber was monitored and maintained at a level <0.1%. The AMCMs were treated with OGD injury by cultured medium without serum and glucose and in hypoxic condition (<0.1% O2) for 0.5, 1, and 3 h. Then, the cardiomyocytes were stained by TUNEL, cTnT, and DAPI to evaluate the level of apoptosis in the AMCMs. The ImageJ is used to quantify the TUNEL+ nucleus and total nucleus.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Statistical significance was analyzed by using unpaired Student's t test or one-way analysis of variance (ANOVA), followed by Bonferroni's multiple as appropriate. Two-way ANOVA was applied with Tukey's multiple comparison for analysis of echocardiographic data. Statistical analyses were performed with Graphpad Prism software (version 6.1). A p-value <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by grants from National Key R&D Program of China (2017YFA 0103700 to H.-T.Y. and Z.C., 2016YFC1301204 to H.-T.Y.), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA16010201 to H.-T.Y.), National Natural Science Foundation of China (81520108004, 81470422 to H.-T.Y.), and the National Institute of Health grant HL136025-01A1 (for Y.W.). The authors thank WiCell Research Institute for providing the H9 hESCs, Chunxing Zheng from Key Laboratory of Stem Cell Biology, SIBS for the assistance in mice peritoneal macrophage isolation and cultivation and in the hESC-CVPCs preparation, and Jizhen Lu and Qiao Liu in the Laboratory of Molecular Cardiology.
Abbreviations Used
- α-SMA
alpha-smooth muscle actin
- Akt
protein kinase B
- AMCMs
adult mice cardiomyocytes
- AMI
acute myocardial infarction
- ANOVA
analysis of variance
- CDCs
cardiosphere-derived cells
- cTnT
cardiac troponin T
- DAPI
4′,6-diamidino-2-phenylindole
- DDR2
discoidin domain receptor-2
- DMEM
Dulbecco's Modified Eagle's Medium
- EGFP
enhanced green fluorescent protein
- Erk1/2
extracellular signal-regulated kinases 1/2
- ESPVR
end-systolic pressure–volume relationship
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GATA4
GATA binding protein 4
- hcFb
human cardiac fibroblast
- hCVPC-CdM
human cardiovascular progenitor cell-conditioned medium
- HE
hematoxylin-eosin
- hESC-CVPC
human embryonic stem cell-derived cardiovascular progenitor cell
- HF
heart failure
- hPSC
human pluripotent stem cell
- I/R
ischemia/reperfusion
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- ISL1
insulin gene enhancer protein
- LAD
left anterior descending
- LPS
lipopolysaccharide
- LV
left ventricle or ventricular
- LVD
left ventricular systolic dimension
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end-diastolic pressure
- LVEF
left ventricular ejection fraction
- LVFS
left ventricular fractional shortening
- MEF2C
myocyte enhancer factor 2c
- MESP1
mesoderm posterior BHLH transcription factor 1
- MI
myocardial infarction
- MSC
mesenchymal stromal cell
- NKX2.5
NK2 homeobox 5
- OGD
oxygen glucose deprivation
- PBS
phosphate buffered saline
- PFA
paraformaldehyde
- PM
Puramatrix™
- RT-PCR
reverse transcription–polymerase chain reaction
- RT-qPCR
quantitative reverse transcription-polymerase chain reaction
- SSEA1
stage-specific embryonic antigen 1
- STAT6
signal transducer and activator of transcription 6
- STAT6KO
signal transducer and activator of transcription 6 knockout
- SV
stroke volume
- TNF-α
tumor necrosis factor alpha
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild type
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AM, Volz KS, Tang Z, Red-Horse K, and Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115: 625–635, 2014 [DOI] [PubMed] [Google Scholar]
- 2. Amado LC, Saliaris AP, Schuleri KH., , St. John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, and Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A 102: 11474–11479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartulos O, Zhuang ZW, Huang Y, Mikush N, Suh C, Bregasi A, Wang L, Chang W, Krause DS, Young LH, Pober JS, and Qyang Y. ISL1 cardiovascular progenitor cells for cardiac repair after myocardial infarction. JCI Insight 1: pii:, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH, Silman Z, Cohen S, and Leor J. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol 62: 1890–1901, 2013 [DOI] [PubMed] [Google Scholar]
- 5. Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rucker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagege A, Desnos M, Renaud JF, Menasche P, and Puceat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest 120: 1125–1139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith R R, Marban L, and Marban E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 9: 337–352, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao DJ, Schiattarella GG, Villalobos E, Jiang N, May H I, Li T, Chen ZJ, Gillette TG, and Hill JA. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation 137: 2613–2634, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao N, Liang H, Huang J, Wang J, Chen Y, Chen Z, and Yang HT. Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Res 23: 1119–1132, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao N, Liang H, and Yang HT. Generation, expansion, and differentiation of cardiovascular progenitor cells from human pluripotent stem cells. Methods Mol Biol 1212: 113–125, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, and Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510: 273–277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Czimmerer Z, Daniel B, Horvath A, Ruckerl D, Nagy G, Kiss M, Peloquin M, Budai MM, Cuaranta-Monroy I, Simandi Z, Steiner L, Nagy B, , Jr., Poliska S, Banko C, Bacso Z, Schulman IG, Sauer S, Deleuze JF, Allen JE, Benko S, and Nagy L. The transcription factor stat6 mediates direct repression of inflammatory enhancers and limits activation of alternatively polarized macrophages. Immunity 48: 75–90.e76, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai B, Huang W, Xu M, Millard RW, Gao MH, Hammond HK, Menick DR, Ashraf M, and Wang Y. Reduced collagen deposition in infarcted myocardium facilitates induced pluripotent stem cell engraftment and angiomyogenesis for improvement of left ventricular function. J Am Coll Cardiol 58: 2118–2127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, and Marban E. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 125: 3147–3162, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhingra S, Sharma AK, Arora RC, Slezak J, and Singal PK. IL-10 attenuates TNF-alpha-induced NF kappaB pathway activation and cardiomyocyte apoptosis. Cardiovasc Res 82: 59–66, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Epstein SE, Luger D, and Lipinski MJ. Paracrine-mediated systemic anti-inflammatory activity of intravenously administered mesenchymal stem cells: a transformative strategy for cardiac stem cell therapeutics. Circ Res 121: 1044–1046, 2017 [DOI] [PubMed] [Google Scholar]
- 16. Faiella W. and Atoui R. Therapeutic use of stem cells for cardiovascular disease. Clin Transl Med 5: 34, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandes S, Chong JJ, Paige SL, Iwata M, Torok-Storb B, Keller G, Reinecke H, and Murry CE. Comparison of human embryonic stem cell-derived cardiomyocytes, cardiovascular progenitors, and bone marrow mononuclear cells for cardiac repair. Stem Cell Rep 5: 753–762, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 110: 159–173, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol 30: 240–245, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frantz S, Hofmann U, Fraccarollo D, Schafer A, Kranepuhl S, Hagedorn I, Nieswandt B, Nahrendorf M, Wagner H, Bayer B, Pachel C, Schon MP, Kneitz S, Bobinger T, Weidemann F, Ertl G, and Bauersachs J. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 27: 871–881, 2013 [DOI] [PubMed] [Google Scholar]
- 21. Fujiwara Y, Takeya M, and Komohara Y. A novel strategy for inducing the antitumor effects of triterpenoid compounds: blocking the protumoral functions of tumor-associated macrophages via STAT3 inhibition. Biomed Res Int 2014: 348539, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furtado MB, Costa MW, Pranoto EA, Salimova E, Pinto AR, Lam NT, Park A, Snider P, Chandran A, Harvey RP, Boyd R, Conway SJ, Pearson J, Kaye DM, and Rosenthal NA. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res 114: 1422–1434, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galvez-Monton C, Fernandez-Figueras MT, Marti M, Soler-Botija C, Roura S, Perea-Gil I, Prat-Vidal C, Llucia-Valldeperas A, Raya A, and Bayes-Genis A. Neoinnervation and neovascularization of acellular pericardial-derived scaffolds in myocardial infarcts. Stem Cell Res Ther 6: 108, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gombozhapova A, Rogovskaya Y, Shurupov V, Rebenkova M, Kzhyshkowska J, Popov SV, Karpov RS, and Ryabov V. Macrophage activation and polarization in post-infarction cardiac remodeling. J Biomed Sci 24: 13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gordon S. and Martinez F O. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Hikoso S, Yamaguchi O, Higuchi Y, Hirotani S, Takeda T, Kashiwase K, Watanabe T, Taniike M, Tsujimoto I, Asahi M, Matsumura Y, Nishida K, Nakajima H, Akira S, Hori M, and Otsu K. Pressure overload induces cardiac dysfunction and dilation in signal transducer and activator of transcription 6-deficient mice. Circulation 110: 2631–2637, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Hu Y, Zhang H, Lu Y, Bai H, Xu Y, Zhu X, Zhou R, Ben J, Xu Y, and Chen Q. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res Cardiol 106: 1311–1328, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, and Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17: 109–118, 2014 [DOI] [PubMed] [Google Scholar]
- 29. Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, and Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marban L, and Marban E. Cellular postconditioning: allogeneic cardiosphere-derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail 8: 322–332, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kervadec A, Bellamy V, El Harane N, Arakelian L, Vanneaux V, Cacciapuoti I, Nemetalla H, Perier MC, Toeg HD, Richart A, Lemitre M, Yin M, Loyer X, Larghero J, Hagege A, Ruel M, Boulanger CM, Silvestre JS, Menasche P, and Renault NK. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant 35: 795–807, 2016 [DOI] [PubMed] [Google Scholar]
- 32. Kluge A, Zimmermann R, Munkel B, Mohri M, Sack S, Schaper J, and Schaper W. Insulin-like growth factor I is involved in inflammation linked angiogenic processes after microembolisation in porcine heart. Cardiovasc Res 29: 407–415, 1995 [PubMed] [Google Scholar]
- 33. Lamagna C, Aurrand-Lions M, and Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 80: 705–713, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Lambert JM, Lopez EF, and Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol 130: 147–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, and Caplice NM. Systemic and cardiac depletion of M2 macrophage through CSF-1R signaling inhibition alters cardiac function post myocardial infarction. PLoS One 10: e0137515, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leschik J, Stefanovic S, Brinon B, and Puceat M. Cardiac commitment of primate embryonic stem cells. Nat Protoc 3: 1381–1387, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Lewis C. and Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol 167: 627–635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liao R. and Jain M. Isolation, culture, and functional analysis of adult mouse cardiomyocytes. Methods Mol Med 139: 251–262, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clement K, and Jain MK. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest 121: 2736–2749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu ML, Nagai T, Tokunaga M, Iwanaga K, Matsuura K, Takahashi T, Kanda M, Kondo N, Naito AT, Komuro I, and Kobayashi Y. Anti-inflammatory peptides from cardiac progenitors ameliorate dysfunction after myocardial infarction. J Am Heart Assoc 3: e001101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, and Murry CE. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 36: 597–605, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, and Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, and Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43a. National Research Council. Guide for the Care and Use of Laboratory Animals, 8th edition. Washington, DC: The National Academic Press, 2011 [Google Scholar]
- 44. Ohmori Y. and Hamilton TA. STAT6 is required for the anti-inflammatory activity of interleukin-4 in mouse peritoneal macrophages. J Biol Chem 273: 29202–29209, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, and Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A 106: 14022–14027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quijada P, Salunga HT, Hariharan N, Cubillo JD, El-Sayed FG, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R, Jr., Gude NA, and Sussman MA. Cardiac stem cell hybrids enhance myocardial repair. Circ Res 117: 695–706, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rocher C. and Singla DK. SMAD-PI3K-AKT-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One 8: e84009, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruparelia N, Godec J, Lee R, Chai JT, Dall'Armellina E, McAndrew D, Digby JE, Forfar JC, Prendergast BD, Kharbanda RK, Banning AP, Neubauer S, Lygate CA, Channon KM, Haining NW, and Choudhury RP. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur Heart J 36: 1923–1934, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shen Y, Qin J, and Bu P. Pathways involved in interleukin-1beta-mediated murine cardiomyocyte apoptosis. Tex Heart Inst J 42: 109–116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, Sharma A, Kaplan D, Greaves DR, Wang Y, and Simon DI. Down-regulation of the forkhead transcription factor FOXP1 is required for monocyte differentiation and macrophage function. Blood 112: 4699–4711, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shintani Y, Ito T, Fields L, Shiraishi M, Ichihara Y, Sato N, Podaru M, Kainuma S, Tanaka H, and Suzuki K. IL-4 as a repurposed biological drug for myocardial infarction through augmentation of reparative cardiac macrophages: proof-of-concept data in mice. Sci Rep 7: 6877, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shioura KM, Geenen DL, and Goldspink PH. Assessment of cardiac function with the pressure-volume conductance system following myocardial infarction in mice. Am J Physiol Heart Circ Physiol 293: H2870–H2877, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, and Suzuki K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 126: 2151–2166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shirakawa K, Endo J, Kataoka M, Katsumata Y, Yoshida N, Yamamoto T, Isobe S, Moriyama H, Goto S, Kitakata H, Hiraide T, Fukuda K, and Sano M. IL-10-STAT3-galectin-3 axis is essential for osteopontin-producing reparative macrophage polarization after myocardial infarction. Circulation 138: 20212035, 2018 [DOI] [PubMed] [Google Scholar]
- 55. Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM, Barak Y, Schwabe J, and Nagy L. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity 33: 699–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, and Yang PC. Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ Res 121: e22–e36, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tian L, Li W, Yang L, Chang N, Fan X, Ji X, Xie J, Yang L, and Li L. Cannabinoid receptor 1 participates in liver inflammation by promoting m1 macrophage polarization via Rhoa/NF-kappaB p65 and Erk1/2 pathways, respectively, in mouse liver fibrogenesis. Front Immunol 8: 1214, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, and van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170: 818–829, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Zhang D, Ashraf M, Zhao T, Huang W, Ashraf A, and Balasubramaniam A. Combining neuropeptide Y and mesenchymal stem cells reverses remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 298: H275–H286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xie Y, Zhu WZ, Zhu Y, Chen L, Zhou ZN, and Yang HT. Intermittent high altitude hypoxia protects the heart against lethal Ca2+ overload injury. Life Sci 76: 559–572, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Xu H, Li J, Zhao Y, and Liu D. TNFalpha-induced downregulation of microRNA-186 contributes to apoptosis in rat primary cardiomyocytes. Immunobiology 222: 778–784, 2017 [DOI] [PubMed] [Google Scholar]
- 62. Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, and Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62: 24–35, 2013 [DOI] [PubMed] [Google Scholar]
- 63. Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, and Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 15: 750–761, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu K, Wu Q, Ni C, Zhang P, Zhong Z, Wu Y, Wang Y, Xu Y, Kong M, Cheng H, Tao Z, Yang Q, Liang H, Jiang Y, Li Q, Zhao J, Huang J, Zhang F, Chen Q, Li Y, Chen J, Zhu W, Yu H, Zhang J, Yang HT, Hu X, and Wang J. Lack of remuscularization following transplantation of human embryonic stem cell-derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ Res 122: 958–969, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.