Abstract
The purpose of this work was to investigate whether changes in oxysterol and apolipoprotein levels over 5 years are associated with disease course and disability progression in multiple sclerosis (MS). This study included 139 subjects [39 healthy controls (HCs), 61 relapsing-remitting MS (RR-MS) patients, and 39 progressive MS (P-MS) patients]. Oxysterols [24-hydroxycholesterol (24HC), 25-hydroxycholesterol (25HC), 27-hydroxycholesterol (27HC), 7α-hydroxycholesterol (7αHC), and 7-ketocholesterol (7KC)] were measured at baseline and 5 years using a novel mass spectrometric method, and apolipoproteins were measured using immunoturbidometric diagnostic kits. Levels of 24HC (P = 0.004), 25HC (P = 0.029), and 27HC (P = 0.026) increased in P-MS patients. 7KC (P = 0.047) and 7αHC (P = 0.001) levels decreased in RR-MS patients, and there were no changes in any oxysterols in HCs. In MS patients, ApoC-II (all P ≤ 0.01) and ApoE (all P ≤ 0.01) changes were positively associated with all oxysterol levels. Increases in 24HC (P = 0.038) and ApoB (P = 0.038) and decreases in 7KC (P = 0.020) were observed in RR-MS patients who converted to secondary P-MS (SP-MS) at follow-up and in SP-MS patients compared with RR-MS patients. Oxysterols and their associations with apolipoproteins differed between MS patients and HCs over 5 years. Oxysterol and apolipoprotein changes were associated with conversion to SP-MS.
Keywords: cholesterol, disease progression, metabolism
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the CNS that results in blood-brain barrier (BBB) breakdown, inflammation, and neurodegeneration, and causes physical and cognitive disability.
There is extensive evidence suggesting that higher levels of total cholesterol (TC) and LDL cholesterol are associated with increasing disability in MS (1–4). Cholesterol is required for myelin structure and proper functioning of neuronal, vascular, and immune cells in the CNS. Cholesterol and lipoproteins do not cross the BBB, so the brain is dependent on de novo cholesterol synthesis.
Oxysterols are oxygenated cholesterol metabolites that traverse the BBB rapidly (5) and act as signaling mediators between the periphery and the CNS (6) to enable the maintenance of cholesterol homeostasis in the brain. Oxysterols are ligands for LXR (7, 8) and are involved in regulating the biosynthesis, cellular efflux, and elimination of cholesterol (9–13). We investigated three side-chain oxysterols [24-hydroxycholesterol (24HC), 25-hydroxycholesterol (25HC), and 27-hydroxycholesterol (27HC)] and two B-ring oxysterols [7α-hydroxycholesterol (7αHC) and 7-ketocholesterol (7KC)]. 24HC is produced exclusively in the brain (11) and is the primary regulator of cholesterol synthesis and the principal mechanism for cholesterol elimination in the brain (14–16). 25HC is produced in activated macrophages and mediates inflammatory signaling. 7αHC is the product of the rate-limiting step in the bile acid pathway of cholesterol elimination (17), and it is a surrogate marker for bile acid synthesis and cholesterol excretion (17). 27HC is produced by CYP27A1 in the acidic pathway (18). 7KC results from oxidative stress and can induce apoptosis (19), inflammation in endothelial cells, and neuronal injury in the brain (20). van de Kraats et al. (21) found that MS patients had lower levels of serum 24HC and 27HC compared with healthy controls (HCs), and higher 24HC levels were associated with decreased normalized brain volume measures. Our group also found that 24HC, 27HC, and 7αHC (all P < 0.015) were lower in MS patients compared with HCs, and 7KC was higher in progressive MS (P-MS) compared with relapsing-remitting MS (RR-MS) (P < 0.001) (22).
Apolipoproteins contribute to maintaining peripheral cholesterol homeostasis and are surrogates for oxysterol signaling. ApoA-I acts as an acceptor of cholesterol following efflux from cells (23). Serum ApoB is strongly associated with multiple oxysterols, including 24HC, 25HC, 27HC, 7αHC, and 7KC (22, 24), and is associated with inflammatory markers (25). In this study, we will focus on ApoE and ApoC-II as the primary biomarkers for LXR signaling. ApoC-II shuttles between lipoproteins and is important for the removal of cholesterol from tissues, and its levels are unaffected by inflammation (26). ApoE, which is modulated by 24HC, plays an important role in maintaining cholesterol homeostasis in the CNS by accepting cholesterol from astrocytes and shuttling it to neurons (7).
The associations of oxysterol changes with MS disease progression have not been extensively investigated in longitudinal studies. The goals of this study were to investigate oxysterol and apolipoprotein levels in 5 year follow-up samples and assess whether changes in these lipid mediators were associated with MS disease course conversion and disability progression.
METHODS
Study population
Study setting and design.
This study included samples and clinical data from a single-center longitudinal prospective study of clinical, genetic, and environmental risk factors in MS at the MS Center of the State University of New York at Buffalo. The study includes patients with MS and clinically isolated syndrome, HCs, and controls with other neurological diseases.
Patients and controls provided blood samples, underwent neurological examination, and responded to a structured questionnaire administered in-person at baseline and at 5 year follow-up visits.
Informed consent.
The University at Buffalo Human Subjects Institutional Review Board approved the study protocol, and all participants provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Inclusion and exclusion criteria.
This substudy was limited to HCs and MS patients 18–65 years of age with oxysterol and apolipoprotein measures available at baseline and 5 years. MS disease course at baseline and follow-up were reported by MS specialists based on patients’ clinical characteristics and published disease course classification (27).
HCs needed to meet the health-screen requirements and had to have a normal physical and neurological examination. They were recruited from respondents to a local advertisement.
Children younger than 18 years of age, adults over 65 years of age, clinically isolated syndrome, neuromyelitis optica, or other neurological diseases were excluded from this substudy. P-MS consisted of patients with secondary P-MS (SP-MS) or primary P-MS.
A conversion to SP-MS categorical variable was defined to classify MS patients into the three groups: i) RR-MS at baseline and remained RR-MS at follow-up (RR-MS); ii) RR-MS at baseline and converted to SP-MS by the 5 year follow-up visit (RR-MS to SP-MS); and iii) SP-MS at baseline and follow-up (SP-MS).
Oxysterol assays
The plasma total plasma oxysterol assay (inclusive of free and esterified oxysterols) included room temperature saponification, solid phase extraction, and a modification of the LC-mass spectrometry analysis conditions previously described (28).
Sample preparation.
Plasma samples were stored at −80°C until use without prior freeze thaw cycles. Baseline and 5 year follow-up samples were grouped together by a technician separate from the analytical laboratory and analysts were blind to case/control and baseline/follow-up status.
In-house quality control materials consisting of −80°C frozen aliquots of pooled human plasma and lipid-stripped human blood plasma spiked with authentic oxysterol standards demonstrated −80°C stability (<15% deviation from nominal) for 4.5 years.
Samples, matrix calibrators, and quality control materials were prepared as previously described (28). In brief, 200 μl of sample were vortex-mixed with 100 μl of deuterated internal standard mix [150 ng/ml each of vitamin D3 (d3), 22HC (d7), 7αHC (d7), 7KC (d7)] and 10 μl of 50 mg/dl ethanolic butylated hydroxytoluene and saponified using 875 μl of 0.5 M ethanolic KOH for 3 h at room temperature under argon. The pH was neutralized using 20 μl of 85% phosphoric acid; 1 ml of water was added, the vial centrifuged, and the supernatant was loaded under gravity onto a HyperSep C18 solid phase extraction cartridge (200 mg, 3 ml) that had been conditioned with 1 ml of hexane:isopropanol (50:50 v/v), 1 ml of methanol, and 2 ml of water, sequentially. Polar lipids were washed off the SPE column using 4 ml of methanol:water (75:25 v/v). Nonpolar sterols (including cholesterol and oxysterols) were eluted using 2 ml of hexane:isopropanol (50:50 v/v). The eluate was evaporated under nitrogen, reconstituted in 300 μl of methanol:water (90:10, v/v), and 75 μl injected for LC-mass spectrometry analysis.
LC-mass spectrometry analysis.
Oxysterols were analyzed on a Shimadzu Scientific (Columbia, MD) LCMS-2010A mass spectrometer system with atmospheric pressure chemical ionization interface in positive ion mode. Mobile phase composition was 100% methanol to pump A and methanol:water (50:50 v/v) to pump B, both containing 0.1% formic acid. The chromatographic conditions were optimized from our originally reported method (28). Oxysterols were separated on a Supelcosil LC-18-S, 10 cm × 3.0 mm, 3 μm column (Sigma-Aldrich, St. Louis, MO). Column oven temperature was set at 10°C and flow rate was at 0.75 ml/min. The mobile phase gradient was as follows: 80% A for 10 min, linear increase to 100% A over 5 min and held for 13 min followed by re-equilibration at 80% A for 6 min; total run time was 34 min. Temperature settings for the mass spectrometer were: interface at 400°C, CDL at 230°C, and heat block at 200°C. Nitrogen was used as a nebulizing gas for the ion source at a flow rate of 2.5 l/min. Data were acquired in a time segmented-single ion monitoring manner to achieve maximum sensitivity. The m/z ratios used for quantifying oxysterols were: m/z 367.30 for 24HC, 25HC, and 7αHC; m/z 385.30 for 27-OHC; m/z 401.40 for 7KC; m/z 374.30 for 22-OHC(d7); m/z 374.30 for 7αHC(d7); and m/z 408.40 for 7KC(d7). Calibrator standards were included in every run and the oxysterol concentrations (in picograms per milliliter) were calculated using calibrator standards.
Lipids and apolipoproteins
EDTA plasma samples for lipid and apolipoprotein analyses were obtained in the nonfasted state. The methods used for lipid and apolipoprotein analyses were previously published (29). Analysts were blinded to the clinical status of samples.
Immunoturbidometric diagnostic reagent kits, calibrators, and controls (Kamiya Biomedical, Thousand Oaks, CA) were used for the apolipoprotein (ApoA-I, ApoA-II, ApoB, ApoC-II, and ApoE) assays. The coefficient of variation of these assays is <5%.
Data analysis
The SPSS (IBM Inc., Armonk, NY, version 24.0) statistical program was used.
Oxysterol concentrations were log-transformed to reduce skew. Apolipoprotein concentrations were normally distributed and not log-transformed.
Paired t-tests were used to investigate changes in 24HC, 25HC, 27HC, 7αHC, 7KC, ApoA-I, ApoA-II, ApoB, ApoC-II, and ApoE levels from baseline to follow-up in RR-MS, P-MS, and HC groups alone. Additionally, we used repeated measures analyses to investigate associations between change in oxysterol or apolipoprotein level over 5 years with disease course at baseline (either HC vs. MS or RR-MS vs. P-MS) following adjustment for age, gender, and BMI.
The independent samples t-test was used to assess differences in baseline and follow-up levels of 24HC, 25HC, 27HC, 7αHC, 7KC, ApoA-I, ApoA-II, ApoB, ApoC-II , and ApoE between HCs and MS patients and between RR-MS and P-MS patients.
Linear regression was used to investigate the associations between change in TC and change in each of the apolipoproteins (ApoA-I, ApoA-II, ApoB, ApoC-II, or ApoE) with the change in each of the oxysterol levels (24HC, 25HC, 27HC, 7αHC, or 7KC) over 5 years. The change in TC or change in the apolipoprotein level of interest was the dependent variable; and age, gender, BMI, and the change in the oxysterol level of interest were treated as covariates.
Linear regression was used to investigate associations between changes in oxysterol and apolipoprotein levels from baseline to 5 years with the conversion to SP-MS variable and SP-MS subgroups variable. In these analyses, the change in oxysterol or apolipoprotein variable of interest from baseline to 5 years was the dependent variable; and the predictor variables included age, gender, baseline BMI, baseline oxysterol, or apolipoprotein of interest and either the conversion to SP-MS variable or the SP-MS subgroups variable.
RESULTS
Demographic and clinical characteristics
Baseline and follow-up clinical and demographic characteristics of the entire study sample and for HC, RR-MS, and P-MS groups are summarized in Table 1. The higher average age and median Expanded Disability Status Scale (EDSS) scores observed in P-MS patients are representative of the progressive disease course. The majority of subjects in the HC (55%), RR-MS (53%), and P-MS (54%) groups had a BMI of 25 kg/m3 or more at baseline. At baseline, the percentages of RR and P-MS patients on disease-modifying therapies (DMTs) were 80% and 77%, respectively. At baseline, the percentages of HC, RR-MS, and P-MS patients on statins were 8, 12, and 13%, respectively.
TABLE 1.
Demographic and clinical characteristics by disease course at baseline visit
| HC | RR-MS | P-MS | |
| Sample size, number | 39 | 61 | 39 |
| Percent female | 28 (71.8%) | 43 (70.5%) | 29 (74.4%) |
| Age, years | |||
| Baseline | 46.4 ± 12.7 | 44.7 ± 11.1 | 56.0 ± 6.1 |
| Follow-up | 52.2 ± 12.7 | 50.7 ± 11.2 | 62.2 ± 6.0 |
| Disease duration, years | |||
| Baseline | — | 13.2 ± 8.7 | 21.3 ± 10.4 |
| Follow-up | — | 19.2 ± 8.9 | 27.3 ± 10.2 |
| BMI, kg/m2a | |||
| Baseline BMI | 27.0 ± 6.1 | 28.1 ± 6.3 | 26.0 ± 3.9 |
| Normal/overweight/obese | 45%/31%/24% | 47%/25%/28% | 46%/44%/10% |
| Follow-up BMI | 26.6 ± 6.7 | 28.8 ± 7.9 | 26.5 ± 4.7 |
| Normal/overweight/obese | 55%/24%/21% | 33%/35%/32% | 39%/41%/21% |
| EDSS, median (IQR)b | |||
| Baseline | — | 2.5 (2.0) | 5.0 (3.0) |
| Follow-up | — | 2.5 (1.5) | 6.0 (2.8) |
| TC, mg/dl | |||
| Baseline | 207 ± 42 | 210 ± 39 | 214 ± 35 |
| Follow-up | 235 ± 43 | 227 ± 38 | 244 ± 41 |
| DMT at baselinec | |||
| Interferon | — | 19 (32%) | 14 (36%) |
| Glatiramer acetate | — | 13 (22%) | 10 (26%) |
| Natalizumab | — | 14 (23%) | 2 (5%) |
| Other | — | 1 (2%) | 3 (8%) |
| None | — | 12 (20%) | 9 (23%) |
| DMT at follow-up | |||
| Interferon | — | 16 (27%) | 13 (33%) |
| Glatiramer acetate | — | 18 (30%) | 13 (33%) |
| Natalizumab | — | 2 (3%) | 1 (3%) |
| Orals | — | 10 (17%) | 2 (5%) |
| Other | — | 7 (12%) | 2 (5%) |
| None | — | 6 (10%) | 8 (21%) |
| Statins | |||
| Baseline | 8% | 12% | 13% |
| Follow-up | 10% | 20% | 13% |
All continuous variables (age, BMI, disease duration) are mean ± standard deviation.
EDSS in median [interquartile range (IQR)].
The BMI category variable was defined according to National Institutes of Health guidelines (https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm): normal weight (BMI <25 kg/m2), overweight (BMI ≥25 to <30 kg/m2), and obese (BMI ≥30 kg/m2). BMI is missing for one HC and one RR-MS subject at baseline.
DMT data for two RR-MS subjects are missing at baseline and follow-up. DMT data for one P-MS subject is missing at baseline. The interferon category includes the products AVONEX®, REBIF®, BETASERON®, EXTAVIA®, and PLEGRIDY™, whereas orals include fingolimod, dimethyl fumarate, and teriflunomide.
Oxysterol changes at 5 year follow-up
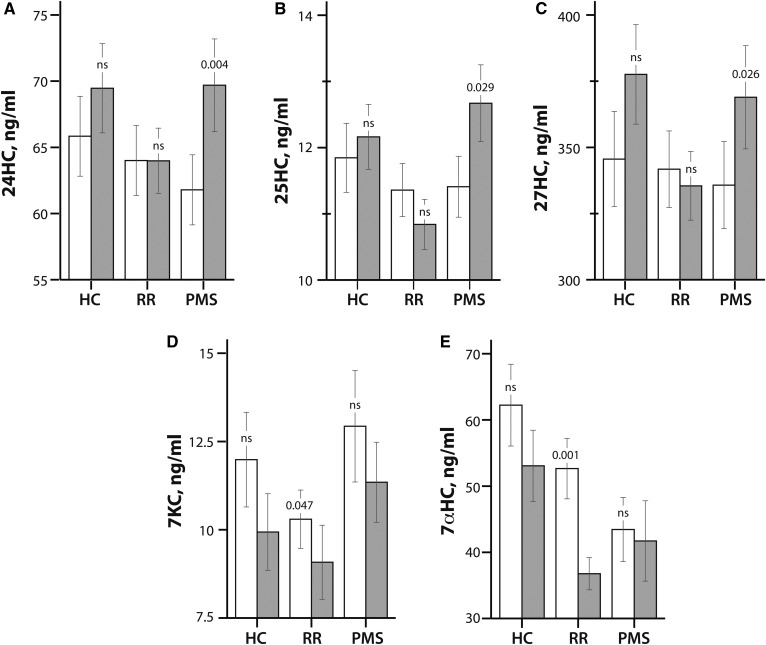
Figure 1 summarizes the changes in oxysterol (24HC, 25HC, 27HC, 7KC, and 7αHC) levels from baseline to 5 years in HC, RR-MS, and P-MS patients.
Fig. 1.
Oxysterol levels in HCs, RR-MS patients (RR), and P-MS patients (PMS) at baseline (white bars) and 5 years (gray bars). The values of 24HC (A), 25HC (B), 27HC (C), 7KC (D), and 7αHC (E) are shown in nanograms per milliliter by disease course (HC, RR-MS, and P-MS). The bars compare mean oxysterol values shown on the y axis by disease course shown on the x axis. The error bars indicate the standard error of the mean. The P-values from paired t-tests are provided for the changes in oxysterol levels from baseline to 5 years within each disease course. ns, not significant.
There were no significant changes in any oxysterol levels between baseline and 5 years in HCs. We compared oxysterol levels in the entire MS patient group to the HC group and found that follow-up 7αHC levels were 14.5 ng/ml lower in MS patients compared with HCs (P = 0.012). No other differences were observed between baseline or follow-up levels of oxysterols in the MS patient group compared with HCs.
In RR-MS patients, 7KC levels decreased by 1.2 ng/ml (P = 0.047), and 7αHC levels decreased by 15.8 ng/ml (P = 0.001) from baseline to 5 years. No significant changes in 24HC, 25HC, or 27HC were observed in the RR-MS patients.
In P-MS patients, 24HC levels increased by 7.9 ng/ml (P = 0.004), 25HC levels increased by 1.3 ng/ml (P = 0.029), and 27HC levels increased by 33.2 ng/ml (P = 0.026) from baseline to 5 years. There were no significant changes in 7KC or 7αHC from baseline to 5 years in the P-MS group.
Follow-up levels of 25HC were 1.4 ng/ml higher in P-MS patients compared with RR-MS patients (P = 0.008). Follow-up levels of 7KC were 2.6 ng/ml higher in P-MS patients compared with RR-MS patients (P = 0.023). There were no other differences in baseline or follow-up oxysterols between RR-MS and P-MS patients.
In repeated measures analyses that adjusted for age, gender, BMI, and RR-MS versus P-MS diagnosis at baseline, the greater decreases in 7KC and 7αHC over time in RR-MS compared with P-MS patients, and the greater increases in 25HC over time in P-MS patients compared with RR-MS patients remained significant (data not shown).
These results suggest that MS disease course at baseline (RR-MS or P-MS) is associated with changes in oxysterol levels over 5 years.
Apolipoprotein changes at 5-year follow-up
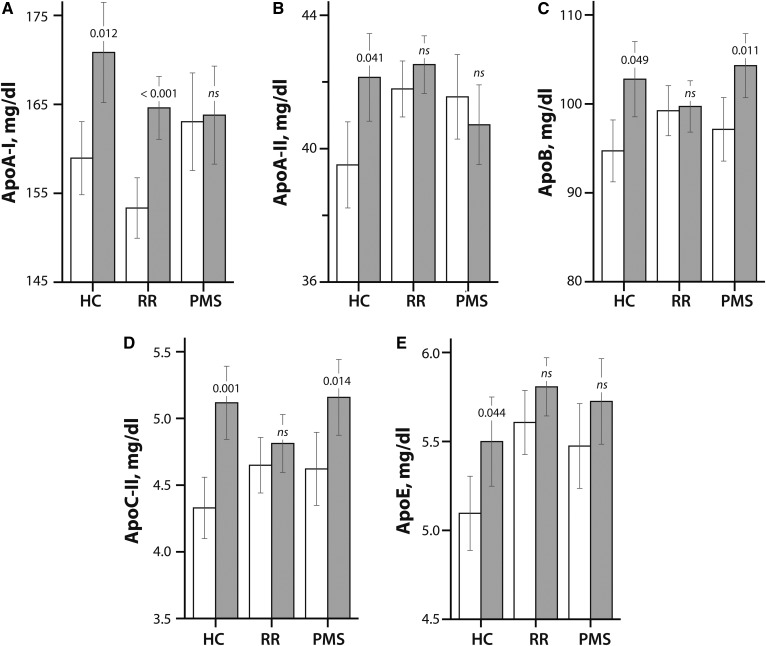
Figure 2 summarizes the changes in apolipoproteins (ApoA-I, ApoA-II, ApoB, ApoC-II, and ApoE) levels from baseline to 5 years in the HCs, RR-MS patients, and P-MS patients.
Fig. 2.
Apolipoprotein levels in HCs, RR-MS patients (RR), and P-MS patients (PMS) at baseline (white bars) and at 5 year follow-up (gray bars). The values for ApoA-I (A), ApoA-II (B), ApoB (C), ApoC-II (D), and ApoE (E) are shown in millligrams per deciliter. The bars compare mean apolipoprotein values on the y axis by disease course shown on the x axis. The error bars indicate the standard error of the mean. The P-values are provided from paired t-tests assessing the differences between baseline and 5 year apolipoprotein levels within each disease course.
In HCs, ApoA-I levels increased by 10.9 mg/ml (P = 0.012), ApoA-II levels increased by 2.0 mg/ml (P = 0.041), ApoB levels increased by 7.1 mg/ml (P = 0.049), ApoC-II levels increased by 0.55 mg/ml (P = 0.001), and ApoE levels increased by 0.37 mg/ml (P = 0.044) over 5 years.
In the RR-MS group, only ApoA-I levels increased by 12.5 mg/ml (P < 0.001) from baseline to 5 years. No significant changes from baseline to follow-up were observed for ApoA-II, ApoB, ApoC-II, or ApoE levels in the RR-MS group.
In the P-MS group, ApoC-II levels increased by 0.6 mg/ml (P = 0.014), and ApoB levels increased by 6.2 mg/ml (P = 0.011) from baseline to 5 years. No significant changes were observed in ApoA-I, ApoA-II, and ApoE levels in the P-MS group.
In repeated measures analyses that corrected for age, gender, BMI, and HC versus MS diagnosis at baseline, we found that the increases in ApoC-II levels from baseline to 5 years in HCs compared with MS patients remained significant (data not shown).
Interdependence of apolipoproteins, cholesterol, and oxysterol changes over 5 years
We investigated the associations, if any, between apolipoprotein, cholesterol, and oxysterol changes, because oxysterols are known to induce ApoE and ApoC-II biosynthesis via transcriptional activation of LXR (30).
In the HC group, greater increases in 24HC (partial correlation rp = 0.66, P < 0.001) and 27HC (rp = 0.60, P = 0.001) were associated with greater increases in TC, whereas in the MS group, greater increases in 24HC (rp = 0.40) and 27HC (rp = 0.52) and also 25HC (rp = 0.51), 7αHC (rp = 0.29), and 7KC (rp = 0.31) levels from baseline to follow-up were associated with increases in TC (all P ≤ 0.011).
In HCs, changes in 25HC were positively associated with changes in ApoA-I (P = 0.041). In MS patients, changes in 7αHC levels were positively associated with changes in ApoA-I over 5 years (P = 0.043). There were no significant associations between changes in any of the oxysterol levels and the changes in ApoA-II in HCs. In MS patients, changes in 24HC (P = 0.008), 27HC (P = 0.005), and 7αHC (P = 0.018) were positively associated with changes in ApoA-II over 5 years. None of the other oxysterol changes were associated with ApoA-I changes in HCs or with ApoA-I and ApoA-II changes in MS patients.
In HCs, changes in 24HC (P < 0.001), 25HC (P = 0.002), and 27HC (P < 0.001) levels were positively associated with increases in ApoB over 5 years. ApoB changes were not associated with 7αHC or 7KC changes in HCs. In MS patients, changes in 24HC, 25HC, 27HC, 7αHC, and 7KC levels were positively associated with ApoB (all P ≤ 0.008).
In HCs, only changes in 27HC levels were positively associated with changes in ApoC-II (P = 0.009). Changes in the other oxysterols were not associated with ApoC-II changes over time in HCs. There were no significant associations between changes in any oxysterol levels and the changes in ApoE in HCs. In MS patients, changes in 24HC, 25HC, 27HC, 7αHC, and 7KC levels were all positively associated with ApoC-II (all P ≤ 0.01) and ApoE (all P ≤ 0.01) changes over the 5 year study period.
These results indicate that increases in ApoC-II and ApoE are associated with increases in oxysterols in MS patients, but not in HCs.
Oxysterol and apolipoprotein changes in RR-MS patients converting to SP-MS
Next, we investigated whether changes in oxysterol and apolipoprotein levels from baseline to 5 years differed in the subset of patients who converted from RR-MS at baseline to SP-MS at 5 years. There were 52 patients with RR-MS at baseline and 5 years, 31 patients with SP-MS at baseline and 5 years, and 9 patients with RR-MS at baseline that progressed to SP-MS at 5 years.
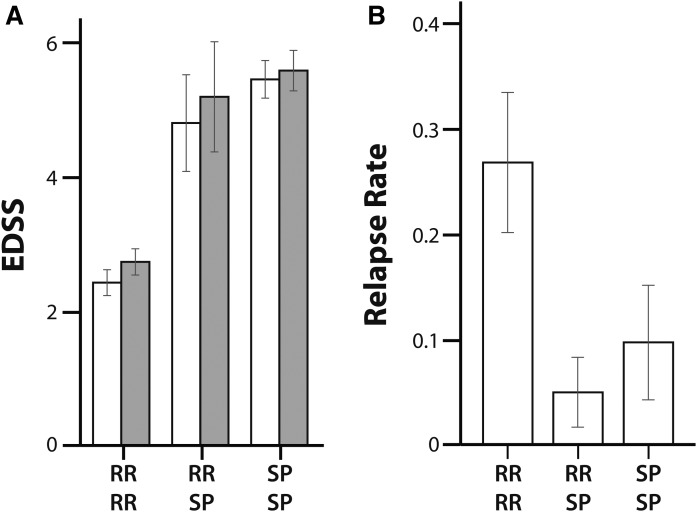
The RR-MS patients who converted to SP-MS had a higher average EDSS score at both baseline and follow-up visits and a lower relapse rate compared with RR-MS patients (Fig. 3), which is a clinical profile consistent with the conversion of the MS disease course from RR-MS to SP-MS.
Fig. 3.
EDSS score at baseline (white bars) and 5 years (gray bars) (A) and relapse rate over 5 years (B) in patients who were diagnosed with RR-MS at baseline and remained diagnosed with RR-MS at 5 years (RR RR), patients who switched from a RR-MS diagnosis at baseline to SP-MS at 5 years (RR SP), and patients who were diagnosed with SP-MS at baseline and remained SP-MS at 5 years (SP SP). The error bars indicate the standard error of the mean.
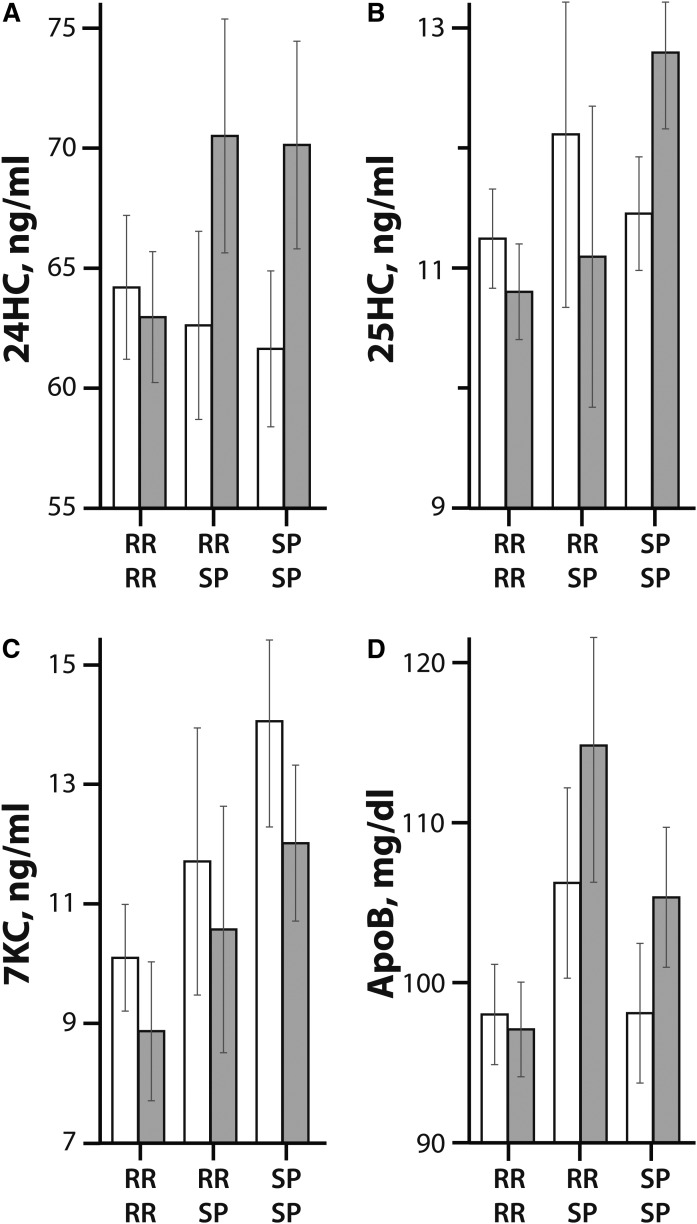
Greater increases in 24HC (P = 0.038, Fig. 4A) and ApoB (P = 0.038, Fig. 4D) and greater decreases in 7KC (P = 0.020, Fig. 4C) were observed in the RR-MS patients who converted to SP-MS and SP-MS patients compared with the RR-MS patients. However, greater decreases in 25HC (P = 0.020, Fig. 4B) were seen in the RR-MS patients, and the RR-MS patients who converted to SP-MS compared with the SP-MS patients. These results suggest that 24HC, 7KC, and ApoB are associated with progression from RR-MS to SP-MS.
Fig. 4.
Oxysterol and apolipoprotein levels at baseline (white bars) and 5 years (gray bars) by the disease course conversion status (RR RR, RR SP, and SP SP; as described in the legend to Fig. 3). The mean values for the oxysterols, 24HC (A), 25HC (B), 7KC (C), and, ApoB (D) are shown in milligrams per deciliter by disease course conversion status. The error bars indicate standard error of the mean.
DISCUSSION
We measured oxysterol and apolipoprotein levels from baseline to 5 years in MS patients and HCs, and investigated whether these changes were associated with disease course conversion and disability progression. 24HC, 25HC, and 27HC levels increased in P-MS patients, whereas 7KC and 7αHC decreased in RR-MS patients over 5 years. However, no changes in oxysterol levels were observed in HCs. Interestingly, all of the apolipoprotein levels investigated increased in HCs, while ApoB increased and ApoC-II decreased in P-MS patients and only ApoA-I increased in RR-MS patients. Increases in many of the oxysterols measured were associated with increases in ApoB over 5 years in both MS patients and HCs. However, increasing levels of almost every oxysterol were associated with increases in ApoA-II, ApoC-II, and ApoE levels in MS patients but not HCs. Changes in oxysterol and apolipoprotein levels were observed with conversion of RR-MS patients to SP-MS at follow-up.
We compared the oxysterol and apolipoprotein profiles in the patients who progressed from RR-MS at baseline to SP-MS at 5 years with those of the patients who remained RR-MS at 5 years and the patients who remained SP-MS at 5 years. There were nine patients who converted from RR-MS at baseline to SP-MS at follow-up. These patients exhibited EDSS scores and relapse rates qualitatively similar to those of the patients who were diagnosed with SP-MS at baseline and follow-up. Additionally, the changes in 24HC, 7KC, and ApoA-I over 5 years in the nine RR-MS patients who converted to SP-MS by follow-up are similar to the changes seen in the SP-MS patients rather than the RR-MS patients.
A strength of this study was its longitudinal follow-up analysis that included an extensive oxysterol and apolipoprotein panel assayed in paired baseline and follow-up samples. However, the limitations of this study warrant discussion. There were a relatively small number of patients who converted from RR-MS at baseline to SP-MS at follow-up, which limits the statistical power in this study. Furthermore, the diagnosis of SP-MS is subjective and is arrived at retrospectively (31). Our study would also be strengthened with the inclusion of CSF measures of oxysterols and apolipoproteins, which would provide additional information on the cross-talk between cholesterol in the CNS and periphery via the oxysterol network and apolipoprotein signaling.
In our previous study (22), we showed how baseline oxysterol levels differed by disease course. This study expands on our previous work by including 5 year follow-up data, which allowed us to investigate long-term changes in oxysterols and apolipoproteins. The results from this study, which are concordant with our previous results, indicate that the RR-MS and P-MS disease courses are associated with changes in different oxysterol levels. RR-MS patients exhibited decreases in sterol B-ring oxysterols (7αHC and 7KC) in contrast to P-MS patients who exhibited increases in side-chain oxysterols (24HC, 25HC, and 27HC), all ligands of the LXR receptor.
From the analytical standpoint, 7αHC and 7βHC are generally considered difficult to resolve chromatographically. We included a deuterated 7αHC internal standard and further optimized the chromatographic conditions for our previously published assay method (28) to ensure separation of 7αHC from 7βHC. While we used −80°C frozen samples that had not been previously thawed, we did not include an antioxidant, such as dibutylhydroxytoluene, to minimize autoxidation during −80°C storage. While BHT addition did not improve bioanalytical stability of oxysterols in a 2 year standardization study (32), it should be considered for future studies.
Oxysterols exert their immunomodulatory effects on immune cells via LXR and LXR-independent EBI2-mediated signaling pathways (33). LXR signaling results in polarization of CD4 helper T cells into Th17 cells in humans (34) and suppresses T cell proliferation (35–37). LXR activation downregulates IL-17 production and Th17 cells, resulting in amelioration of experimental allergic encephalomyelitis, while LXR deficiency leads to exacerbation (34). Activation of LXR also inhibits production of pro-inflammatory cytokines. Finally, LXR agonists can regulate macrophage phagocytosis. Macrophages have been shown to accumulate cholesterol from myelin debris, and these myelin-laden macrophages express LXR-induced genes and an increase in the efflux of cholesterol (38).
In conclusion, our results indicate differences in baseline and 5 year oxysterol levels between RR-MS patients, P-MS patients, and HCs. The direction and the extent of change in oxysterol and apolipoprotein levels also differ between the MS disease courses and HCs. The changes in oxysterols are associated with changes in apolipoprotein levels to a greater extent in MS patients than in HCs. Furthermore, there are distinct differences in oxysterol and apolipoprotein profiles in patients progressing to SP-MS. These findings may be useful for developing future studies that aim to delineate the mechanisms by which oxysterols, as well as other cholesterol mediators, may contribute to MS pathogenesis.
Acknowledgments
The authors thank the patients who participated in this study.
Footnotes
Abbreviations:
- BBB
- blood-brain barrier
- DMT
- disease-modifying therapy
- EDSS
- Expanded Disability Status Scale
- HC
- healthy control
- 24HC
- 24-hydroxycholesterol
- 25HC
- 25-hydroxycholesterol
- 27HC
- 27-hydroxycholesterol
- 7αHC
- 7α-hydroxycholesterol
- 7KC
- 7-ketocholesterol
- MS
- multiple sclerosis
- P-MS
- progressive multiple sclerosis
- RR-MS
- relapsing-remitting multiple sclerosis
- SP-MS
- secondary progressive multiple sclerosis
- TC
- total cholesterol
This work was supported by National Institute of Neurological Disorders and Stroke Grant 1R21NS098169 to the Ramanathan laboratory and National Center for Advancing Translational Sciences of the National Institutes of Health Grant UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. K.F.M., S. B., M.L.B., D.J., J.H., and R.W.B. have nothing to disclose. R.Z. has received speaker honoraria and consultant fees from Genzyme-Sanofi, Novartis, Celgene, Alexion,and EMD Serono. He has received research support from the Genzyme-Sanofi, Mapi-Pharma, PRAH, Protembis, Celgene, Novartis, and V-WAVE Medical. B.W-G. received honoraria for serving in advisory boards and educational programs from Teva Pharmaceuticals, Biogen Idec, Novartis, Acorda, EMD Serono, Pfizer, Novartis, Genzyme, and Sanofi. She also received support for research activities from the National Institutes of Health, National Multiple Sclerosis Society, National Science Foundation, Department of Defense, EMD Serono, Biogen Idec, Teva Neuroscience, Cyberonics, Novartis, Acorda, and the Jog for the Jake Foundation. M.R. received research funding from the National Multiple Sclerosis Society, Department of Defense, and National Institute of Neurological Diseases and Stroke. He received funding for unrelated research from Otsuka Pharmaceutical and Development, and receives royalty from a self-published textbook.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Tettey P., Simpson S. Jr., Taylor B. V., and van der Mei I. A.. 2014. Vascular comorbidities in the onset and progression of multiple sclerosis. J. Neurol. Sci. 347: 23–33. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock-Guttman B., Zivadinov R., Mahfooz N., Carl E., Drake A., Schneider J., Teter B., Hussein S., Mehta B., Weiskopf M., et al. . 2011. Serum lipid profiles are associated with disability and MRI outcomes in multiple sclerosis. J. Neuroinflammation. 8: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandoj C., Renna R., Plantone D., Sperduti I., Cigliana G., Conti L., and Koudriavtseva T.. 2015. Anti-annexin antibodies, cholesterol levels and disability in multiple sclerosis. Neurosci. Lett. 606: 156–160. [DOI] [PubMed] [Google Scholar]
- 4.Tettey P., Simpson S. Jr., Taylor B., Blizzard L., Ponsonby A. L., Dwyer T., Kostner K., and van der Mei I.. 2014. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult. Scler. 20: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 5.Heverin M., Meaney S., Lutjohann D., Diczfalusy U., Wahren J., and Bjorkhem I.. 2005. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 46: 1047–1052. [DOI] [PubMed] [Google Scholar]
- 6.Jeitner T. M., Voloshyna I., and Reiss A. B.. 2011. Oxysterol derivatives of cholesterol in neurodegenerative disorders. Curr. Med. Chem. 18: 1515–1525. [DOI] [PubMed] [Google Scholar]
- 7.Abildayeva K., Jansen P. J., Hirsch-Reinshagen V., Bloks V. W., Bakker A. H., Ramaekers F. C., de Vente J., Groen A. K., Wellington C. L., Kuipers F., et al. . 2006. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281: 12799–12808. [DOI] [PubMed] [Google Scholar]
- 8.Leoni V., and Caccia C.. 2013. 24S-hydroxycholesterol in plasma: a marker of cholesterol turnover in neurodegenerative diseases. Biochimie. 95: 595–612. [DOI] [PubMed] [Google Scholar]
- 9.Björkhem I., and Meaney S.. 2004. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24: 806–815. [DOI] [PubMed] [Google Scholar]
- 10.Diczfalusy U., Lund E., Lutjohann D., and Bjorkhem I.. 1996. Novel pathways for elimination of cholesterol by extrahepatic formation of side-chain oxidized oxysterols. Scand. J. Clin. Lab. Invest. Suppl. 226: 9–17. [PubMed] [Google Scholar]
- 11.Dietschy J. M., and Turley S. D.. 2004;Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 45: 1375–1397. [DOI] [PubMed] [Google Scholar]
- 12.Meaney S., Heverin M., Panzenboeck U., Ekstrom L., Axelsson M., Andersson U., Diczfalusy U., Pikuleva I., Wahren J., Sattler W., et al. . 2007. Novel route for elimination of brain oxysterols across the blood-brain barrier: conversion into 7alpha-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 48: 944–951. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz M., Lund E. G., Setchell K. D., Kayden H. J., Zerwekh J. E., Bjorkhem I., Herz J., and Russell D. W.. 1996. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J. Biol. Chem. 271: 18024–18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lund E. G., Xie C., Kotti T., Turley S. D., Dietschy J. M., and Russell D. W.. 2003. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 278: 22980–22988. [DOI] [PubMed] [Google Scholar]
- 15.Björkhem I., Lütjohann D., Breuer O., Sakinis A., and Wennmalm A.. 1997. Importance of a novel oxidative mechanism for elimination of brain cholesterol. Turnover of cholesterol and 24(S)-hydroxycholesterol in rat brain as measured with 18O2 techniques in vivo and in vitro. J. Biol. Chem. 272: 30178–30184. [DOI] [PubMed] [Google Scholar]
- 16.Lütjohann D., Breuer O., Ahlborg G., Nennesmo I., Sidén A., Diczfalusy U., and Björkhem I.. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc. Natl. Acad. Sci. USA. 93: 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosignani A., Zuin M., Allocca M., and Del Puppo M.. 2011. Oxysterols in bile acid metabolism. Clin. Chim. Acta. 412: 2037–2045. [DOI] [PubMed] [Google Scholar]
- 18.Chawla A., Saez E., and Evans R. M.. 2000. Don’t know much bile-ology. Cell. 103: 1–4. [DOI] [PubMed] [Google Scholar]
- 19.Terasaka N., Wang N., Yvan-Charvet L., and Tall A. R.. 2007. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA. 104: 15093–15098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diestel A., Aktas O., Hackel D., Hake I., Meier S., Raine C. S., Nitsch R., Zipp F., and Ullrich O.. 2003. Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: a link between demyelination and neuronal damage. J. Exp. Med. 198: 1729–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Kraats C., Killestein J., Popescu V., Rijkers E., Vrenken H., Lutjohann D., Barkhof F., Polman C. H., and Teunissen C. E.. 2014. Oxysterols and cholesterol precursors correlate to magnetic resonance imaging measures of neurodegeneration in multiple sclerosis. Mult. Scler. 20: 412–417. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S., Fellows K., Browne R. W., Khare P., Krishnan Radhakrishnan S., Hagemeier J., Weinstock-Guttman B., Zivadinov R., and Ramanathan M.. 2017. Interdependence of oxysterols with cholesterol profiles in multiple sclerosis. Mult. Scler. 23: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz K., Lawn R. M., and Wade D. P.. 2000. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun. 274: 794–802. [DOI] [PubMed] [Google Scholar]
- 24.Alkazemi D., Egeland G., Vaya J., Meltzer S., and Kubow S.. 2008. Oxysterol as a marker of atherogenic dyslipidemia in adolescence. J. Clin. Endocrinol. Metab. 93: 4282–4289. [DOI] [PubMed] [Google Scholar]
- 25.Sniderman A. D., and Faraj M.. 2007. Apolipoprotein B, apolipoprotein A-I, insulin resistance and the metabolic syndrome. Curr. Opin. Lipidol. 18: 633–637. [DOI] [PubMed] [Google Scholar]
- 26.Isshiki M., Hirayama S., Ueno T., Ito M., Furuta A., Yano K., Yamatani K., Sugihara M., Idei M., and Miida T.. 2018. Apolipoproteins C-II and C-III as nutritional markers unaffected by inflammation. Clin. Chim. Acta. 481: 225–230. [DOI] [PubMed] [Google Scholar]
- 27.Lublin F. D., Reingold S. C., Cohen J. A., Cutter G. R., Sorensen P. S., Thompson A. J., Wolinsky J. S., Balcer L. J., Banwell B., Barkhof F., et al. . 2014. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanaswamy R., Iyer V., Khare P., Bodziak M. L., Badgett D., Zivadinov R., Weinstock-Guttman B., Rideout T. C., Ramanathan M., and Browne R. W.. 2015. Simultaneous determination of oxysterols, cholesterol and 25-hydroxy-vitamin D3 in human plasma by LC-UV-MS. PLoS One. 10: e0123771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browne R. W., Weinstock-Guttman B., Horakova D., Zivadinov R., Bodziak M. L., Tamano-Blanco M., Badgett D., Tyblova M., Vaneckova M., Seidl Z., et al. . 2014. Apolipoproteins are associated with new MRI lesions and deep grey matter atrophy in clinically isolated syndromes. J. Neurol. Neurosurg. Psychiatry. 85: 859–864. [DOI] [PubMed] [Google Scholar]
- 30.Mak P. A., Laffitte B. A., Desrumaux C., Joseph S. B., Curtiss L. K., Mangelsdorf D. J., Tontonoz P., and Edwards P. A.. 2002. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J. Biol. Chem. 277: 31900–31908. [DOI] [PubMed] [Google Scholar]
- 31.Lorscheider J., Buzzard K., Jokubaitis V., Spelman T., Havrdova E., Horakova D., Trojano M., Izquierdo G., Girard M., Duquette P., et al. ; MSBase Study Group . 2016. Defining secondary progressive multiple sclerosis. Brain. 139: 2395–2405. [DOI] [PubMed] [Google Scholar]
- 32.Helmschrodt C., Becker S., Thiery J., and Ceglarek U.. 2014. Preanalytical standardization for reactive oxygen species derived oxysterol analysis in human plasma by liquid chromatography-tandem mass spectrometry. Biochem. Biophys. Res. Commun. 446: 726–730. [DOI] [PubMed] [Google Scholar]
- 33.Poli G., Biasi F., and Leonarduzzi G.. 2013. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 1: 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui G., Qin X., Wu L., Zhang Y., Sheng X., Yu Q., Sheng H., Xi B., Zhang J. Z., and Zang Y. Q.. 2011. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J. Clin. Invest. 121: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glass C. K., and Saijo K.. 2008. Immunology: oxysterols hold T cells in check. Nature. 455: 40–41. [DOI] [PubMed] [Google Scholar]
- 36.Bensinger S. J., Bradley M. N., Joseph S. B., Zelcer N., Janssen E. M., Hausner M. A., Shih R., Parks J. S., Edwards P. A., Jamieson B. D., et al. . 2008. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 134: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensinger S. J., and Tontonoz P.. 2008. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 454: 470–477. [DOI] [PubMed] [Google Scholar]
- 38.Bogie J. F., Timmermans S., Huynh-Thu V. A., Irrthum A., Smeets H. J., Gustafsson J. A., Steffensen K. R., Mulder M., Stinissen P., Hellings N., et al. . 2012. Myelin-derived lipids modulate macrophage activity by liver X receptor activation. PLoS One. 7: e44998. [DOI] [PMC free article] [PubMed] [Google Scholar]