Abstract
Fumonisins are mycotoxins that cause diseases of plants and, when consumed by animals, can damage liver, kidney, lung, brain, and other organs, alter immune function, and cause developmental defects and cancer. They structurally resemble sphingolipids (SLs), and studies nearly 30 years ago discovered that the most prevalent fumonisin [fumonisin B1 (FB1)] potently inhibits ceramide synthases (CerSs), enzymes that use fatty acyl-CoAs to N-acylate sphinganine (Sa), sphingosine (So), and other sphingoid bases. CerS inhibition by FB1 triggers a “perfect storm” of perturbations in structural and signaling SLs that include: reduced formation of dihydroceramides, ceramides, and complex SLs; elevated Sa and So and their 1-phosphates, novel 1-deoxy-sphingoid bases; and alteration of additional lipid metabolites from interrelated pathways. Moreover, because the initial enzyme of sphingoid base biosynthesis remains active (sometimes with increased activity), the impact is multiplied by the continued production of damaging metabolites. Evidence from many studies, including characterization of knockout mice for specific CerSs and analyses of human blood (which found that FB1 intake is associated with elevated Sa 1-phosphate), has consistently pointed to CerS as the proximate target of FB1. It is also apparent that the changes in multiple bioactive lipids and related biologic processes account for the ensuing spectrum of animal and plant disease. Thus, the diseases caused by fumonisins can be categorized as “sphingolipidoses” (in these cases, due to defective SL biosynthesis), and the lessons learned about the consequences of CerS inhibition should be borne in mind when contemplating other naturally occurring and synthetic compounds (and genetic manipulations) that interfere with SL metabolism.
Keywords: cancer, glycolipids, lipid signaling, liver, nutrition/lipids, sphingosine 1-phosphate, toxicology
Graphical Abstract
Fumonisins and related analogs (Fig. 1) were discovered ∼30 years ago (1) during characterization of diseases associated with Fusarium verticillioides (formerly Fusarium moniliforme) and categorized as mycotoxins because they recapitulated the toxicity of culture material for horses and pigs and the carcinogenicity for rats (2). They are produced by over a dozen Fusarium species (3) and serve as phytotoxic virulence factors (4) for maize (corn) and a few other host plants by activation of the pathogen infection-induced hypersensitive response, a form of programmed cell death (5). When consumed by animals, they cause a wide spectrum of diseases that involve multiple organs (6): liver and kidney toxicity in many species, carcinogenicity for rodents, vasculature and brain in equine leukoencephalomalacia, and lung in porcine pulmonary edema syndrome. Health concerns in humans (6) include esophageal cancer, birth defects, growth impairment (7), and perhaps others.
Fig. 1.
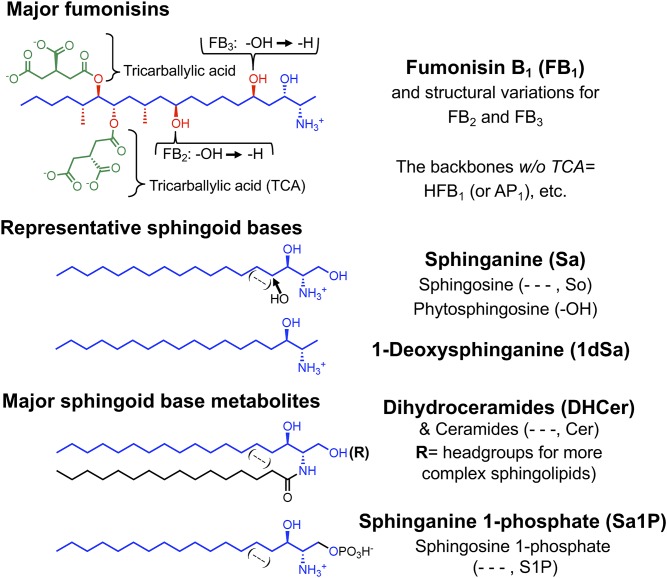
Structures of B family fumonisins and representative SLs. The major fumonisin, FB1, is shown with highlighting of the sphingoid base-like portion in blue, additional functional groups in red, and the tricarballylic acids (TCA) in green. Hydrolyzed fumonisins (such as HFB1) lack the TCA groups, and are sometimes referred to as “AP”. Components that differ for B2 (HFB2) and B3 (HFB3) are shown in black. Four representative mammalian sphingoid bases are displayed by the Sa backbone (in blue), So (with a 4,5-trans-double bond), and phytosphingosine (with a 4-hydroxyl-group) indicated in black, and 1dSa. The lower structures represent N-acyl derivatives of sphingoid bases and the 1-phosphates.
FUMONISINS INHIBIT CERAMIDE SYNTHASE(S)
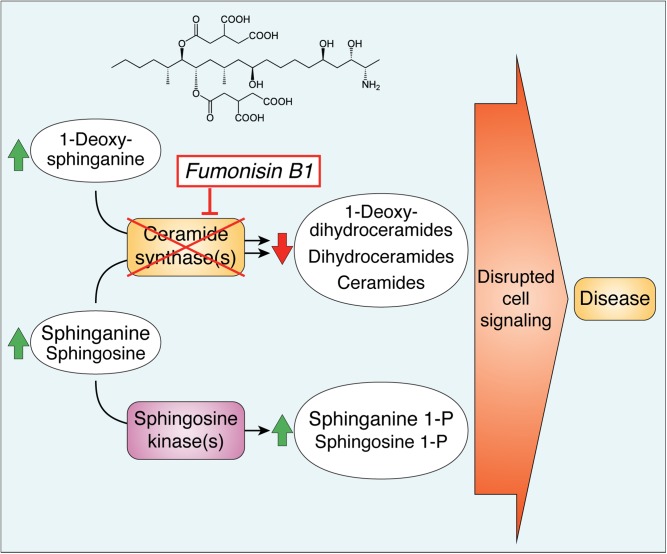
The most prevalent fumonisin, fumonisin B1 (FB1), and its hydrolyzed FB1 [HFB1 or FB1 aminopentol (AP1)] backbone are remarkably similar to the sphingoid bases [sphinganine (Sa) and sphingosine (So)] of sphingolipids (SLs) (Fig. 1). Thus, might fumonisins mimic or interfere with SLs? A connection was indeed found through a collaboration of our laboratories (8): FB1 inhibits ceramide synthases (CerSs), the enzymes that transfer fatty acids from fatty acyl-CoAs to Sa and So.2 In these studies with rat hepatocytes (8), FB1 not only eliminated production of N-acyl-[14C]So [ceramide (Cer)] from [14C]serine but also caused [14C]Sa to accumulate, as expected for CerS inhibition (Fig. 2) (9). Inhibition was confirmed by in vitro assays (8) as well as by finding elevated Sa in the affected tissues, blood, and urine of animals exposed to fumonisins at toxic and carcinogenic levels (10, 11). Therefore, these studies had identified a biochemically and physiologically relevant target of FB1 and, additionally, substantiated the pathway Sa → dihydroceramide (DHCer) → Cer (9) over other hypothesized routes (12) [and was subsequently established by in vitro assay (13) and identification of the desaturase genes (14)]. This was also the first discovery of a sphingolipidosis due to defective SL biosynthesis instead of degradation (15).
Fig. 2.
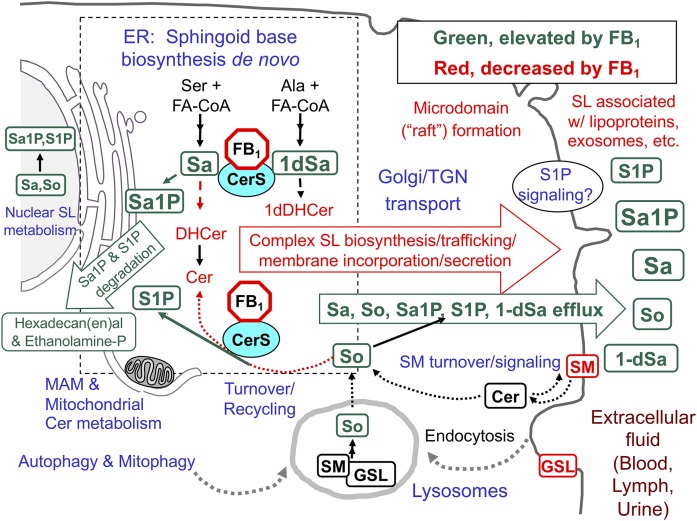
An overview of SL biosynthesis and turnover in mammalian cells with highlighted metabolites that are affected by inhibition of CerSs by fumonisins (FB1). The metabolites in green are generally elevated when CerSs are inhibited by FB1 and metabolites in red eventually decrease. The perinuclear dashed box represents the ER, where de novo sphingoid base biosynthesis occurs, with CerSs in the ER, mitochondria, and associated membranes (MAMs). In these reactions, Ser + a fatty acyl-CoA (usually palmitoyl-CoA) are incorporated into 3-ketosphinganine (not shown) and then Sa followed by N-acylation to DHCers by CerS (likewise 1dSa is made from Ala then acylated to 1dDHCer). DHCer is mostly desaturated to Cer and (DH)Cers are incorporated into more complex SLs (mainly SM and GSLs) beginning in the ER lumen (for galactosylCer), cis-Golgi (for glucosylCer, with more complex GSLs made throughout the Golgi apparatus) and trans-Golgi, and plasma membrane (for SM). The SMs and GSLs arrive at their destinations (plasma membrane, exosomes, and other locations) via vesicular transport, transport proteins, and sorting mechanisms at the trans-Golgi network (TGN). SL turnover occurs in multiple locations in the cell (hydrolases in lysosomes, autophagosomes, and other organelles) to release the sphingoid base (mainly So) that is recycled (salvaged) via CerSs or phosphorylated by So kinases to 1-phosphates in multiple locations in the cell. Sphingoid bases (and the 1-phosphates) can function in cell signaling, undergo degradation (to hexadecenal, hexadecanal, and ethanolamine phosphate), or efflux from the cell. Metabolism of Cer from SM in plasma membrane signaling might not be affected by fumonisins until SM is depleted. For more information about these processes, see the references cited in the text and Supplement A.
FB1 inhibits the six known mammalian CerSs, which differ in tissue distribution and fatty acyl-CoA specificity (16). FB1 inhibition appears to be competitive versus Sa and fatty acyl-CoAs (17). HFB1 is a weaker inhibitor (thus, the side-chains play a role), and both HFB1 and FB1 are N-acylated, placing them in the active site (18, 19). The N-acyl derivatives also inhibit CerSs in vitro, perhaps as product analogs (18, 19). CerS does not appear to be inhibited significantly by the other common amine adducts (N-acetylated fumonisin B1, also known as FA1; amine adducts formed with cyclic 3-hydroxpridinium, also known as the FP series; and nonenzymatic N-glycosylation) (6).
CerS INHIBITION AFFECTS MULTIPLE SL METABOLITES AND LIPIDS OF INTERRELATED PATHWAYS
Figure 2 illustrates key events of SL and glycosphingolipid (GSL) metabolism, trafficking, efflux, and uptake that are altered by inhibition of CerS by FB1 (15, 20). For simplicity, the phytoCer branch of SL metabolism is not shown. More details are provided in the Supplements3 and citations therein.
Elevation of sphingoid bases
Sa is biosynthesized de novo in the ER and usually N-acylated rapidly by CerS there and, to some extent, in mitochondria and mitochondria-associated membranes (MAMs) (21); whereas, Sos (and some Sas) are produced by SL turnover and “salvaged” by CerS or phosphorylated by So kinases for signaling or degradation (Fig. 2). Thus, the elevation of Sa and So by FB1 reflects inhibition of the de novo and “salvage” pathways, respectively (Fig. 2). Sa is usually elevated early and with a large fold change, and So is elevated later and to a lesser extent (22).4 Because both are membrane permeable, they appear in blood and urine as well as target tissues of animals in a manner that correlates with exposure and the progression of the toxic, pathophysiologic, and histopathologic effects (6).
Reduction of DHCer, Cer, and complex SL
Reduced production of DHCer and Cer [(DH)Cer] affects the biosynthesis of SMs and GSLs (Fig. 2) (15, 20). FB1 affects all of these metabolites except, presumably, when Cer is recycled directly to SM by the plasma membrane signaling cycle SM → Cer → SM. Nonetheless, the extent of inhibition is variable; for example, SM mass usually changes slowly because most cells have large amounts; whereas, the effect on SM biosynthesized de novo has been reported to be 10 times higher than for GSL (17). The latter probably reflects the close proximity of Cer biosynthesis to production of galactosylCer (ER lumen) and glucosylCer (cis-Golgi) (so they are made first with whatever Cer is produced) versus the more distant trans-Golgi and plasma membrane for SM (23).
It is undoubtedly important that instead of being shut off by Sa accumulation, serine palmitoyltransferase (SPT) continues to make Sa, even exhibiting higher expression and activity in some cells (24). Some of the elevated SPT activity may be due to release of inhibition by Orm/ORMDL proteins, which are regulated by Cer (25).
Elevation of sphingoid base 1-phosphates
Elevated Sa and So cause both Sa 1-phosphate (Sa1P) and So 1-phosphate (S1P) to increase in fumonisin-exposed cells in culture [including in the nucleus (26)] and in animal tissues and blood (11, 27, 28). Blood spot Sa1P and the Sa1P/S1P ratio are positively correlated with urinary FB1 (28) and have allowed estimation of the dietary exposure that results in detectable perturbation of SL metabolism in humans (29).
1-Deoxysphingoid bases
We were surprised to find that mammalian cells treated with FB1 produce substantial amounts of 1-deoxysphinganine (1dSa; Fig. 1), which was identified by mass spectrometry (30), because wild-type SPT can utilize alanine (30, 31). 1-Deoxysphingoid bases are usually N-acylated by CerS, but the absence of the 1-hydroxyl group precludes their metabolism to more complex SLs or phosphorylation for degradation. They are more cytotoxic than Sa or So (30), and the N-acyl derivatives are membrane disruptive (32). These properties might also be important in the inherited neuropathies where mutations in SPT increase production of 1dSa (33).
Impact on other lipids
Fumonisin exposure affects multiple lipid classes [see (34), for instance, and Supplement B], with the changes appearing to be consequences of altered SL metabolism; for example: elevated fatty alcohols and ethanolamine-phosphate produced by Sa1P and S1P degradation → elevation of 1-acyl- and 1 alkyl-phosphatidylethanolamines; reduced SM biosynthesis (which occurs via phosphatidylcholine + Cer ↔ DAG + SM) → alteration of the composition of phosphatidylcholines; reduced SM and GSL (which alters “raft” composition) → altered cholesterol metabolism and trafficking; and alteration of fatty acid composition via effects on elongases, desaturases, and peroxidation status [via reactive oxygen species (ROS)].
COMPARISON OF THE PERTURBATIONS IN SL METABOLITES AND CELLULAR EFFECTS OF FUMONISINS ASSOCIATED WITH DISEASE
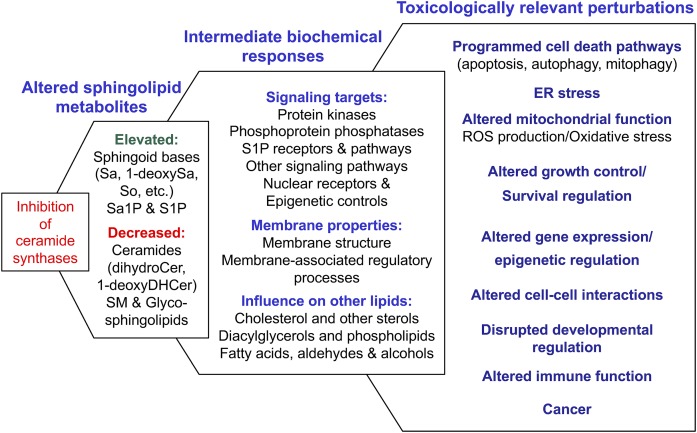
The cytotoxicity of fumonisins was shown to be due to CerS inhibition per se (rather than reduced SL biosynthesis overall) because the SPT inhibitor, myriocin, was not cytotoxic for HT29 cells and prevented the Sa elevation and cytotoxicity caused by FB1 (35). The consequences of CerS inhibition were expanded by the finding that Sa1P and S1P are also elevated (11) because they are involved in growth and anti-apoptotic signaling via S1P receptors and intracellular targets (36) and play important roles in embryonic development (6) and oncogenesis (37). And now we know that the number of bioactive metabolites that change due to CerS inhibition is mind-boggling (Sa, 1dSa, So, Sa1P, S1P, DHCer, Cer, complex SL, and signaling lipids from multiple interrelated pathways), and the number of biochemical processes regulated by these metabolites is also enormous (Fig. 3) (also see Supplement B) (38). Therefore, it is very difficult to pin down a specific mechanism for the pathologic effects.
Fig. 3.
A scheme summarizing how inhibition of CerS(s) alters multiple SLs to affect many biochemical processes and the toxicologically relevant perturbations found in mammalian cells in culture and/or animals exposed to fumonisins. All of these relationships have been experimentally linked (for example, that sphingoid bases can induce ROS and that fumonisins elevate sphingoid bases and ROS), but most are sufficiently complex (i.e., have multiple causes) that they have not been definitively connected across all four columns of this scheme. Each of the components of this figure is described in greater detail with relevant bibliographic citations (247 references) in Supplement B.
What is clear is that the cellular processes affected by CerS inhibition and SLs are highly correlated with the toxicologically relevant biological processes that are altered by fumonisins (Fig. 3, Supplement B). Such findings and others from in vivo toxicologic studies and epidemiologic investigations (6) led to the conclusion at the eighty-third meeting of the Joint FAO/WHO Expert Committee on Food Additives that “the weight of the evidence indicates that the proximate cause for fumonisin-induced toxicity and animal diseases is inhibition of CerS resulting in a global disruption of lipid metabolism” (Ref. 6; p. 426).
RELATED FINDINGS FROM CerS KNOCKDOWN AND CerS GENE KNOCKOUT
Studies of CerS knockdown in cells and knockout mice have provided additional information about the consequences of loss of CerS activity. Knockout of CerS2 is particularly relevant because it is a major CerS isoform in most tissues affected by fumonisins (liver, kidney, and lung). The C22-24 (DH)Cers that are made by CerS2 (15) are nearly eliminated in the knockout mice, with substantial compensation by increases in shorter chain Cers (39). Sa is elevated up to 50-fold in liver (39), resembling the effect of FB1, and CerS2 knockout mice display progressive hepatomegaly and hepatocellular carcinoma (40). Other behaviors of CerS2 knockout mice that resemble fumonisin exposure include changes in biophysical properties of membranes (fluidity, phase separation, curvature, and morphology), multiple membrane functions (receptor internalization, clathrin-mediated endocytosis, and gap junction activity), ROS generation (and related lipid peroxidation and protein nitrosylation), and other pathologies, such as increased susceptibility to diethylnitrosamine-induced liver tumorigenesis, altered renal architecture, and development of pheochromocytoma (see Supplement C).
The limitation of the knockout studies is that they have not recapitulated the inhibition of all of the CerS isozymes by FB1, but one study (41) used siRNA against the major CerSs of the cell line MCF-7 (CerS2, -5, and -6). The results included elevation of So and S1P, but not Sa or Sa1P, and total Cer and SL were not decreased by much, although shifts in Cer subspecies were noted. The authors commented that the lack of pronounced effects might be due to shifts in expression of key enzymes of the pathway (for example, CerS4, -5, and -6 were noted to increase) and/or augmented utilization of SL from the culture medium via the salvage pathway. The latter is an important consideration for studies using cells in culture because media are usually rich in SLs (provided by the serum). Another finding of this study was upregulation of the transcription factor, C/EBP homologous protein (CHOP), an indicator of ER stress. This might provide a link between CerS inhibition by FB1 and the reduced activity of Δ6-fatty acid desaturase (FADS2/D6D) (42) because FADS2 activity is affected by ER stress (43).
SOME OF THE UNKNOWNS OF FUMONISIN ACTION (AND PERSPECTIVES FOR FUTURE RESEARCH)
There are still many questions about the biochemistry, cell biology, and toxicology of fumonisins. Some of these are: Does their interaction with CerS alter the behavior of associated enzymes, such as the fatty acyl-CoA elongases and acyl-CoA-binding protein(s)? Do fumonisins (and/or their N-acyl metabolites) associate with other binding partners, and, if so, do these interactions contribute to the pathobiology? How does subcellular localization affect the metabolic and signaling outcomes? Are there metabolic processes that have not yet been fully explored for a possible connection with fumonisins (for example, does the elevation of Sa, So and 1dSa sometimes drive their N-acylation by reversal of ceramidases) (44, 45)? How many regulatory processes are altered by fumonisins, and which play the most important roles in disease? In this regard, one must be mindful that new connections between SL and cell regulation are still being discovered and some might be pertinent to fumonisin action, such as: the activation of atypical protein kinase Cs (aPKCs) (PKCζ and PKCι/λ, which are involved in diverse cellular functions, and can serve as oncogenes or tumor suppressors) by S1P and Sa1P (46); the activation of SNAI2 (a transcriptional regulator of the epithelial to mesenchymal transition) by S1P (47) and its suppression by Cer (48); and others (see Supplement B).
For all of the targets of the perturbed SLs, it is important to know how fumonisin dosage and length of exposure affect the outcome and what happens after exposure has ended. This latter knowledge gap is important because once CerS activity is restored (by cessation of exposure in the diet, for example), the metabolism of accumulated Sa, So, and 1dSa will elevate (DH)Cer, 1-deoxy(DH)Cer, and other bioactive metabolites. This is one of the “Whack-a-mole” effects of fumonisins: when some bioactive metabolites are knocked down, others pop up, and this can occur in many cycles.
The ultimate questions, of course, are: to what extent does fumonisin intake and CerS inhibition contribute to human disease when contaminated maize is a dietary staple and, how can this knowledge facilitate elimination of these diseases?
SUMMARY
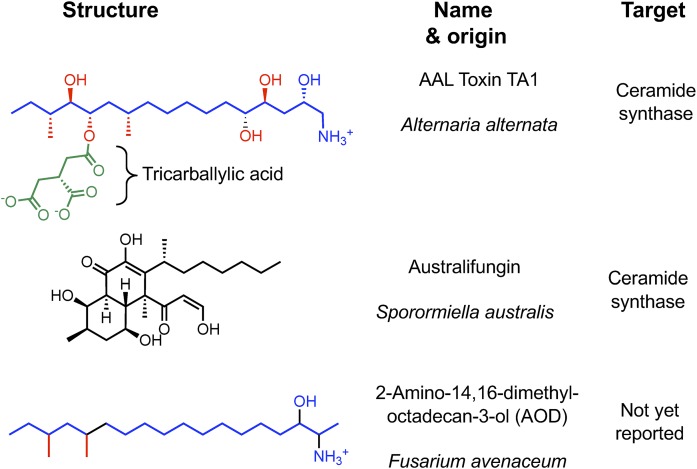
By producing fumonisins, certain Fusarium species have capitalized on CerS inhibition to perturb SL metabolism in host plants and trigger cell death signaling pathways (5, 49). Other fungi are known to produce CerS inhibitors (50), such as the Alternaria species that produce structurally similar AAL toxins and Sporormiella australis that makes the structurally unrelated astralifungins (Fig. 4). This targeting of CerS by independent paths can be viewed as “convergent evolution.”
Fig. 4.
Additional fungal secondary metabolites known to inhibit SL metabolism and/or with close structural similarity to known substrates/inhibitors. The sphingoid base-like structural features of AAL toxin TA1 and AOD are colored blue with additional functional groups in red and green, as in Fig. 1. Australifungin shares some of these features (as an alkyl polyol), but lacks the characteristic amino-group of sphingoid bases. There are also several fungal metabolites, such as myriocin (not shown), that are potent inhibitors of SPT. References for these compounds are given in the text.
All of the evidence to date is consistent with CerS being the primary target of fumonisins for animals, resulting in a perfect storm of perturbed SL metabolism, signaling, and disease. Because sphingoid base-like compounds are widespread in nature (50) and contaminate food (such as 2-amino-14,16-dimethyloctadecan-3-ol, which is made by a common fungus on grain) (Fig. 4), do they impact health? This question should also be borne in mind for synthetic compounds, genetic manipulations, and dietary components that interfere with SL metabolism.
Supplementary Material
Acknowledgments
The authors thank their collaborators, in particular, Elaine Wang and Jency Showker, as well as the many other scientists who have contributed to the understanding of SLs, the mycology and toxicology of fumonisins, and the convergence of these subjects.
Footnotes
Abbreviations:
- AP1
- fumonisin B1 aminopentol or hydrolyzed fumonisin B1
- Cer
- ceramide
- CerS
- ceramide synthase
- DHCer
- dihydroceramide
- (DH)Cer
- dihydroceramide and ceramide
- 1dSa
- 1-deoxysphinganine
- FB1
- fumonisin B1
- GSL
- glycosphingolipid
- HFB1
- hydrolyzed fumonisin B1
- MAM
- mitochondria-associated membrane
- PKC
- protein kinase C
- ROS
- reactive oxygen species
- Sa
- sphinganine
- Sa1P
- sphinganine 1-phosphate
- SL
- sphingolipid
- So
- sphingosine
- S1P
- sphingosine 1-phosphate
- SPT
- serine palmitoyltransferase
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gelderblom W. C., Jaskiewicz K., Marasas W. F., Thiel P. G., Horak R. M., Vleggaar R., and Kriek N. P.. 1988. Fumonisins–novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54: 1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marasas W. F. 2001. Discovery and occurrence of the fumonisins: a historical perspective. Environ. Health Perspect. 109(Suppl 2): 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rheeder J. P., Marasas W. F., and Vismer H. F.. 2002. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 68: 2101–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glenn A. E., Zitomer N. C., Zimeri A. M., Williams L. D., Riley R. T., and Proctor R. H.. 2008. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant Microbe Interact. 21: 87–97. [DOI] [PubMed] [Google Scholar]
- 5.Saucedo-García M., González-Solís A., Rodríguez-Mejía P., Olivera-Flores Tde J., Vázquez-Santana S., Cahoon E. B., and Gavilanes-Ruiz M.. 2011. Reactive oxygen species as transducers of sphinganine-mediated cell death pathway. Plant Signal. Behav. 6: 1616–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley R. T., Edwards S. G., Aidoo K., Alexander J., Bolger M., Boon P. E., Cressey P., Doerge D. R., Edler L., Miller J. D., et al. 2018. Fumonisins (addendum). In WHO Food Additive Series 74: Safety Evaluation of Certain Contaminants in Food. World Health Organization, Geneva, Switzerland. 415–573.
- 7.Chen C., Riley R. T., and Wu R.. 2018. Dietary fumonisin and growth impairment in children and animals: a review. Compr. Rev. Food Sci. Food Saf. 17: 1448–1464. [DOI] [PubMed] [Google Scholar]
- 8.Wang E., Norred W. P., Bacon C. W., Riley R. T., and Merrill A. H. Jr.. 1991. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 266: 14486–14490. [PubMed] [Google Scholar]
- 9.Merrill A. H. Jr., and Wang E.. 1986. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J. Biol. Chem. 261: 3764–3769. [PubMed] [Google Scholar]
- 10.Merrill A. H. Jr., Sullards M. C., Wang E., Voss K. A., and Riley R. T.. 2001. Sphingolipid metabolism: roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 109(Suppl 2): 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley R. T., Enongene E., Voss K. A., Norred W. P., Meredith F. I., Sharma R. P., Spitsbergen J., Williams D. E., Carlson D. B., and Merrill A. H. Jr.. 2001. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 109 (Suppl. 2): 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radin N. S. 1984. Biosynthesis of the sphingoid bases: a provocation. J. Lipid Res. 25: 1536–1540. [PubMed] [Google Scholar]
- 13.Michel C., van Echten-Deckert G., Rother J., Sandhoff K., Wang E., and Merrill A. H. Jr.. 1997. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 272: 22432–22437. [DOI] [PubMed] [Google Scholar]
- 14.Ternes P., Franke S., Zahringer U., Sperling P., and Heinz E.. 2002. Identification and characterization of a sphingolipid delta 4-desaturase family. J. Biol. Chem. 277: 25512–25518. [DOI] [PubMed] [Google Scholar]
- 15.Merrill A. H., Jr. 2011. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111: 6387–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cingolani F., Futerman A. H., and Casas J.. 2016. Ceramide synthases in biomedical research. Chem. Phys. Lipids. 197: 25–32. [DOI] [PubMed] [Google Scholar]
- 17.Merrill A. H. Jr., van Echten G., Wang E., and Sandhoff K.. 1993. Fumonisin B1 inhibits sphingosine (sphinganine) N-acyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J. Biol. Chem. 268: 27299–27306. [PubMed] [Google Scholar]
- 18.Humpf H. U., Schmelz E. M., Meredith F. I., Vesper H., Vales T. R., Wang E., Menaldino D. S., Liotta D. C., and Merrill A. H. Jr.. 1998. Acylation of naturally occurring and synthetic 1-deoxysphinganines by ceramide synthase. Formation of N-palmitoyl-aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. J. Biol. Chem. 273: 19060–19064. [DOI] [PubMed] [Google Scholar]
- 19.Harrer H., Laviad E. L., Humpf H. U., and Futerman A. H.. 2013. Identification of N-acyl-fumonisin B1 as new cytotoxic metabolites of fumonisin mycotoxins. Mol. Nutr. Food Res. 57: 516–522. [DOI] [PubMed] [Google Scholar]
- 20.Harrison P. J., Dunn T. M., and Campopiano D. J.. 2018. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep. 35: 921–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernández-Corbacho M. J., Salama M. F., Canals D., Senkal C. E., and Obeid L. M.. 2017. Sphingolipids in mitochondria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 1862: 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voss K. A., and Riley R. T.. 2013. Fumonisin toxicity and mechanism of action: Overview and current perspectives. Food Safety. 1: 2013006. [Google Scholar]
- 23.Hanada K. 2010. Intracellular trafficking of ceramide by ceramide transfer protein. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 86: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Q., Suzuki H., Sharma N., and Sharma R. P.. 2006. Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse liver. Toxicol. Sci. 94: 388–397. [DOI] [PubMed] [Google Scholar]
- 25.Davis D. L., Gable K., Suemitsu J., Dunn T. M., and Wattenberg B. W.. 2019. The ORMDL/Orm-serine palmitoyltransferase (SPT) complex is directly regulated by ceramide: reconstitution of SPT regulation in isolated membranes. J. Biol. Chem. 294: 5146–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner N. M., Riley R. T., Showker J. L., Voss K. A., Sachs A. J., Maddox J. R., and Gelineau-van Waes J. B.. 2016. Elevated nuclear sphingoid base-1-phosphates and decreased histone deacetylase activity after fumonisin B1 treatment in mouse embryonic fibroblasts. Toxicol. Appl. Pharmacol. 298: 56–65. [DOI] [PubMed] [Google Scholar]
- 27.Gelineau-van Waes J., Rainey M. A., Maddox J. R., Voss K. A., Sachs A. J., Gardner N. M., Wilberding J. D., and Riley R. T.. 2012. Increased sphingoid base-1-phosphates and failure of neural tube closure after exposure to fumonisin or FTY720. Birth Defects Res. A Clin. Mol. Teratol. 94: 790–803. [DOI] [PubMed] [Google Scholar]
- 28.Riley R. T., Showker J. L., Lee C. M., Zipperer C. E., Mitchell T. R., Voss K. A., Zitomer N. C., Torres O., Matute J., Gregory S. G., et al. 2015. A blood spot method for detecting fumonisin-induced changes in putative sphingolipid biomarkers in LM/Bc mice and humans. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 32: 934–949. [DOI] [PubMed] [Google Scholar]
- 29.Riley R. T., Torres O., Matute J., Gregory S. G., Ashley-Koch A. E., Showker J. L., Mitchell T., Voss K. A., Maddox J. R., and Gelineau-van Waes J. B.. 2015. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize-based foods and living in high exposure communities in Guatemala. Mol. Nutr. Food Res. 59: 2209–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitomer N. C., Mitchell T., Voss K. A., Bondy G. S., Pruett S. T., Garnier-Amblard E. C., Liebeskind L. S., Park H., Wang E., Sullards M. C., et al. 2009. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 284: 4786–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan J., and Merrill A. H. Jr.. 2015. 1-Deoxysphingolipids encountered exogenously and made de novo: dangerous mysteries inside an enigma. J. Biol. Chem. 290: 15380–15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiménez-Rojo N., Sot J., Busto J. V., Shaw W. A., Duan J., Merrill A. H. Jr., Alonso A., and Goñi F. M.. 2014. Biophysical properties of novel 1-deoxy-(dihydro)ceramides occurring in mammalian cells. Biophys. J. 107: 2850–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penno A., Reilly M. M., Houlden H., Laura M., Rentsch K., Niederkofler V., Stoeckli E. T., Nicholson G., Eichler F., Brown R. H. Jr., et al. 2010. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285: 11178–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raa H., Grimmer S., Schwudke D., Bergan J., Walchli S., Skotland T., Shevchenko A., and Sandvig K.. 2009. Glycosphingolipid requirements for endosome-to-Golgi transport of Shiga toxin. Traffic. 10: 868–882. [DOI] [PubMed] [Google Scholar]
- 35.Schmelz E. M., Dombrink-Kurtzman M. A., Roberts P. C., Kozutsumi Y., Kawasaki T., and Merrill A. H. Jr.. 1998. Induction of apoptosis by fumonisin B1 in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol. Appl. Pharmacol. 148: 252–260. [DOI] [PubMed] [Google Scholar]
- 36.Pyne S., Adams D. R., and Pyne N. J.. 2016. Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Prog. Lipid Res. 62: 93–106. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel S., Maczis M. A., Maceyka M., and Milstien S.. 2019. New insights into functions of the sphingosine-1-phosphate transporter SPNS2. J. Lipid Res. 60: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannun Y. A., and Obeid L. M.. 2018. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 19: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brugger B., Sachsenheimer T., Wieland F., et al. 2010. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J. Biol. Chem. 285: 10902–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pewzner-Jung Y., Brenner O., Braun S., Laviad E. L., Ben-Dor S., Feldmesser E., Horn-Saban S., Amann-Zalcenstein D., Raanan C., Berkutzki T., et al. 2010. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J. Biol. Chem. 285: 10911–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullen T. D., Spassieva S., Jenkins R. W., Kitatani K., Bielawski J., Hannun Y. A., and Obeid L. M.. 2011. Selective knockdown of ceramide synthases reveals complex interregulation of sphingolipid metabolism. J. Lipid Res. 52: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelderblom W. C., Moritz W., Swanevelder S., Smuts C. M., and Abel S.. 2002. Lipids and delta 6-desaturase activity alterations in rat liver microsomal membranes induced by fumonisin B1. Lipids. 37: 869–877. [DOI] [PubMed] [Google Scholar]
- 43.Teng C. F., Wu H. C., Hsieh W. C., Tsai H. W., and Su I. J.. 2015. Activation of ATP citrate lyase by mTOR signal induces disturbed lipid metabolism in hepatitis B virus pre-S2 mutant tumorigenesis. J. Virol. 89: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okino N., He X., Gatt S., Sandhoff K., Ito M., and Schuchman E. H.. 2003. The reverse activity of human acid ceramidase. J. Biol. Chem. 278: 29948–29953. [DOI] [PubMed] [Google Scholar]
- 45.Novgorodov S. A., Wu B. X., Gudz T. I., Bielawski J., Ovchinnikova T. V., Hannun Y. A., and Obeid L. M.. 2011. Novel pathway of ceramide production in mitochondria: thioesterase and neutral ceramidase produce ceramide from sphingosine and acyl-CoA. J. Biol. Chem. 286: 25352–25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kajimoto T., Caliman A. D., Tobias I. S., Okada T., Pilo C. A., Van A. N., Andrew McCammon J., Nakamura S. I., and Newton A. C.. 2019. Activation of atypical protein kinase C by sphingosine 1-phosphate revealed by an aPKC-specific activity reporter. Sci. Signal. 12: eaat6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W., Hind T., Lam B. W. S., and Herr D. R.. Sphingosine 1-phosphate signaling induces SNAI2 expression to promote cell invasion in breast cancer cells. FASEB J. Epub ahead of print. March 7, 2019; doi:10.1096/fj.201801635R. [DOI] [PubMed] [Google Scholar]
- 48.Lu P., White-Gilbertson S., Nganga R., Kester M., and Voelkel-Johnson C.. 2019. Expression of the SNAI2 transcriptional repressor is regulated by C16-ceramide. Cancer Biol. Ther. 20: 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saucedo-García M., Gavilanes-Ruiz M., and Arce-Cervantes O.. 2015. Long-chain bases, phosphatidic acid, MAPKs, and reactive oxygen species as nodal signal transducers in stress responses in Arabidopsis. Front. Plant Sci. 6: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pruett S. T., Bushnev A., Hagedorn K., Adiga M., Haynes C. A., Sullards M. C., Liotta D. C., and Merrill A. H. Jr.. 2008. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 49: 1621–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.