Abstract
Previous studies have indicated that bone morphogenetic protein (BMP) 6 may play an important role in skeletal system development and progression. However, the mechanism underlying the effects of BMP6 in cartilage cell proliferation and differentiation remains unknown. In this study, cartilage cells were isolated from shanks of chicken embryos and treated with different concentrations of Growth Hormone. Cell proliferation potential was assessed using real-time polymerase chain reaction (RT-PCR), western blotting and CCK-8 assays in vitro. The results showed that at 48 h, the Collagen II and BMP6 expression levels in 50 ng/μl GH-treated cartilage cells were significantly higher than in groups treated with 100 ng/μl or 200 ng/μl GH. We further observed that knockdown of BMP6 in cartilage cells led to significantly decreased expression mRNAs and proteins of Collagen II and Collagen X. Moreover, the suppression of BMP6 expression by a specific siRNA led to significantly decreased expression mRNA levels of IGF1R, JAK2, PKC, PTH, IHH and PTHrP and decreased protein levels of PKC, IHH and PTHrP. Taken together, our data suggest that BMP6 may play a critical role in chicken cartilage cell proliferation and differentiation through the regulation of IGF1, JAK2, PKC, PTH, and IHH-PTHrP signaling pathways.
Introduction
Bone morphogenetic proteins (BMPs) are secreted-type multifunctional proteins belonging to the transforming growth factor (TGF)-β superfamily. Many studies have reported that BMPs play very important roles in bone formation and cartilage induction in both vertebrates and invertebrates [1,2]; moreover, they are also considered crucial molecules involved in cell growth, differentiation, chemotaxis and apoptosis during embryonic development and postnatal tissue remodeling [3]. BMPs stimulate target cells mainly through their specific type I and type II receptors on the cell membrane. When signal transduction occurs, BMPs usually combine with the type II receptor, then activation of receptor type I [4,5]. BMPs first bind to the receptors on the membrane and transmit this signal through the Smads pathway to promote the differentiation of chondrocytes into the osteogenic lineage [6]. In addition to the Smads signaling pathway, other signaling pathways can also be transmitted from BMP family, such as mitogen-activated protein kinase (MAPK) pathways [7,8].
In the BMP family, BMP2, 4 and 6 are all thought to play the most important roles in skeletogenesis. Many studies have suggested that BMP2 is a pivotal signal for the regulation of osteoblastogenesis [9]. Mas et al [10] also showed that BMP2 promotes the expression of IHH in anterior hypertrophic chondrocytes and the proliferation of chondrocytes. BMP6 is mainly expressed in cartilaginous tissue, where it stimulates mesenchymal cell differentiation into chondrocytes and promote the synthesis of chondrocytes and articular cartilage-specific glycoproteins [11]. BMP6 can also induce the differentiation of MSCs into chondrocytes [12]. In BMPs, BMP6 is a strong factor for bone induction [13]. In addition to the differentiation of MSCs, chondrocytes can be derived from BMSCs, ADSCs and other stem cells induced by BMP6 [14–16]. These findings indicate that BMP6 is an important regulator of bone and cartilage cell proliferation and differentiation. However, the biological activity of BMP6 in cartilage cell proliferation and differentiation, as well as related signaling pathways, has remained unclear. Therefore, a further understanding of the molecular mechanism of BMP6 in cartilage is urgently needed.
GH is an important regulatory factor for longitudinal growth of the bone [17]. Local injection of GH can increase the number of cartilage cells in rats [18].In this study, we first extracted and cultured cartilage cells from different breeds of chickens, and we then investigated the expression of BMP6 and the changes in expression of key genes involved in related signaling pathways through GH (Growth hormone)-mediated induction at different concentrations to determine its potential role in cell proliferation and differentiation. Finally, to explore the mechanism of BMP6-mediated effects on the proliferation and differentiation of cartilage cells, we modulated the expression of BMP6 through siRNA and measured its effects by quantitative real-time PCR analysis. Collectively, this study provides evidence that within cartilage cells, BMP signaling regulates genes associated with both cell proliferation and differentiation.
Materials and methods
Animals
Avian broiler and Yellow bantam chickens, which have major differences, were used in this study. Avian broilers were provided by the Zheng Da Company (Chengdu, China). Yellow bantam chickens were provided by the Jin Ling Company (Guangzhou, China). All animal studies were performed in accordance with appropriate guidelines. All experimental protocols were approved by the Committee on Experimental Animal Management of Sichuan Agricultural University, permit number 2014–18.
Cell culture
The eggs were incubated for 15 days after sterilization. Break the egg shell on the end of the air chamber with forceps and place the whole chicken embryo into the sterile culture dishes in PBS solution. Primary cartilage cells were isolated from the shank of 15-day-old chicken embryos with 0.25% trypsin (Gibco, USA), digestion for 0.5 h and then 0.1% collagenase Ⅱ (Gibco, USA) for 1.5 h at 37°C under sterile conditions. The cells were grown in DMEM/F12 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/ml penicillin and 100 U/ml streptomycin (Gibco, USA) in a humidified atmosphere of 5% CO2 at 37°C.
Immunofluorescence
Cartilage cells were fixed in 4% paraformaldehyde for 20 min after adhering for 24 h in the incubator and were then washed three times in PBS, 5 min per wash. The cells were permeabilized with 0.5% Triton-X-100 (Gibco, USA) for 15 min at room temperature and then washed three times in PBS, 5 min per wash. The cells were blocked with Blocking buffer (Bio-Rad, USA) for 2 h at 37°C and then washed as above. The cells were incubated in PBS containing an antibody towards type II collagen (1:1000) (Abcam, USA) overnight at 4°C. The following day, the cells were washed as described above and incubated in PBS containing IgG (1:250) (Abcam, USA) for 2 h at 37°C under dark conditions. After washing three times, the cells were counterstained with DAPI. Photomicrographs were taken using an Olympus digital camera system.
Cell viability assay
Cell proliferation activity was evaluated via Cell Counting Kit-8 assay (CCK8, Bioss, China). At different time points, the medium was replaced with 100 μl of fresh medium containing 10 μl of CCK8. Three hours after the addition of CCK8, cell viability was determined with a microplate reader (Thermo Electron, USA) at a wavelength of 450 nm. All plates had control wells containing medium without cells to obtain a value for background luminescence, which was subtracted from the test sample readings. Each experiment was performed in triplicate. The growth kinetics were performed by the OD values (y axis) and day times (x axis).
Cell treatments
Cells were treated with 0 ng/μl, 50 ng/μl, 100 ng/μl, and 200 ng/μl GH (Biovision, USA) while cells were grown to 80% confluence. Cells were harvested after transfection for 24 h, 48 h, and 72 h. Each experiment was performed in triplicate.
Two siRNAs were designed based on the sequence from NCBI (XM-418956.4) and are listed in Table 1. The cartilage cells were grown to 80% confluence and transfected with 5 μl of Lipofectamine 2000 (Invitrogen, USA) and 5 μl of siRNA (Sangon Biotech, China) according to the manufacturer’s instructions. Cells were harvested after transfection at 24 h, 48 h, and 72 h. Each experiment was performed in triplicate.
Table 1. Sequences of siRNA targeting the BMP6 gene.
| siRNA | Sequences (5’-3’) | |
|---|---|---|
| siBMP6.1 | sense | CCCAATGACACCAAATCAATT |
| antisense | TTGATTTGGTGTCATTGGGTT | |
| siBMP6.2 | sense | GCGAACAGCTCATCTGTGTTT |
| antisense | ACACAGATGAGCTGTTCGCTT |
RNA extraction and qRT-PCR
Total RNA was extracted from cells using the RNAiso plus Reagent (Takara, Japan) according to the manufacturer’s instructions. The first-strand cDNA was synthesized using the PrimeScript RT reagent Kit reverse transcriptase (Takara, Japan) with 1 μg of total RNA according to the manufacturer’s instructions. β-actin was chosen as an internal standard to control for variability in amplification due to differences in the starting mRNA concentrations. The forward and reverse primer sequences used to amplify the genes were designed according to the sequence retrieved from the NCBI and listed in Table 2. Quantitative PCR was performed using SYBR Green PCR technology with a Bio-Rad CFX Connect Real Time System (Bio-Rad, USA). PCR was performed at 98°C for 120 s, followed by 40 amplification cycles (98°C for 2 s, X°C for 15 s, 72°C for 10 s), followed by 65°C to 95°C per second and then 4°C forever. The relative gene expression level was calculated using the 2− ΔΔCT method. All PCR runs were performed in triplicate.
Table 2. Primer sequences for qRT-PCR.
| gene | primer | Sequence (5’-3’) | Temperature |
|---|---|---|---|
| BMP6 | F | CACGCCATCGTCCAAACTCT | 60 |
| R | TGACATCCACAGGCTCTTACTACC | 60 | |
| β-actin | F | TGTGCTGTCCCTGTATGCCTC | 60 |
| R | GGAGGGCGTAGCCTTCATAGA | 60 | |
| BMP2 | F | TGGTGGAGGTGGTTCACTTGGA | 60 |
| R | TGTTTGTGTTTCGCTTGACGCTTT | 60 | |
| Collagen II | F | CCACCCTCAAATCCCTCAACA | 60 |
| R | GTAATCTCCGCTCTTCCACTCG | 60 | |
| Collagen X | F | GCCCAGGGTAACAGGGGTCTTC | 60 |
| R | GCTTGCCGATGCCAACTTCT | 60 | |
| IGF1R | F | CTTTGTCCCCAGACCTGATCG | 60 |
| R | GAACGGGTATTCCACCTCACTC | 60 | |
| IHH | F | CGCTCGCCTACAAGCAGTTC | 60 |
| R | GGTTGTAGTTGGGCGTGAGC | 60 | |
| JAK2 | F | AGTCTTGGGCAGGGCAGATT | 60 |
| R | TTGCTGCCTCAAAGAAGGACTC | 60 | |
| PKC | F | GCTGACGAGGATGGAAATGC | 60 |
| R | TTCACCCTGTCCAGATTGTTG | 60 | |
| PTHrP | F | GTTATTCTGTGCCCTCCTACGG | 60 |
| R | TCTTGGATTGATTTGCCCTTG | 60 | |
| PTH | F | ACAGTGCCCTTGAGGATGCC | 60 |
| R | CAATGGATTTCTTCTGCACTGC | 60 |
Western blotting
Proteins were extracted from cells by total proteins extraction kit (Tiangen, China) according the manuscripture. Total proteins were separated on a 12% SDS-PAGE, and transferred to a polyvinylidene fluoride membrane (Beyotime, China). The membrane was blocked in 5% BSA blocking solution for 1 h at room temperature and incubated overnight at 4°C with primary antibodies (Bioss, China) as follows: BMP6 (bs-10090R, diluted 1:500), Collagen II (bs-10589R, diluted 1:500), Collagen X (bs-0554R, diluted 1:500), BMP2 (bs-10696R, diluted 1:500), JAK2 (bs-0908R, diluted 1:500), PKC (bs-3729R, diluted 1:500), IHH (bs-6624R, diluted 1:500), PTHrP (bs-1107R, diluted 1:500), IGF1R (bs-0680R, diluted 1:500) and beta-actin (bsm-33036M, diluted 1:1,000). After that, the membrane was washed with scrubbing solution (Beyotime, China) and then developed with anti-mouse or rabbit horseradish peroxidase conjugated secondary antibodies (Beyotime, China, diluted 1:1,000). Protein bands were visualized using enhanced chemiluminescence (ECL) system (Beyotime, China) and quantified with an Image Lab system (Bio-Rad, USA).
Statistical analyses
All cell culture experiments were performed a minimum of three times. Statistical analyses were conducted using SAS 8.0 software for Windows. All data are expressed as means ± SEM, and statistical analysis was performed usingone-way ANOVA. P-values < 0.05 were considered statistically significant.
Results
Immunofluorescence of cartilage cells
Collagen II as a special marker for cartilage cells [19–26] was imaged in cartilage cells isolated from avian broiler and yellow bantam chickens, and the cells morphologies of the two breeds was consistent (Fig 1). These results indicate that the cells we cultured were cartilage cells.
Fig 1. Immunofluorescence of markers in cartilage cells.
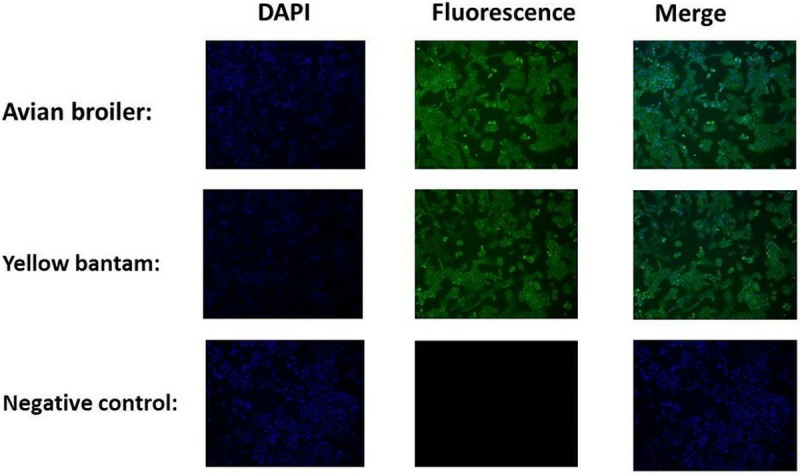
Nuclei stained with DAPI are shown in the left panels. The pictures above indicated that staining of the cells for the marker collagen II was positive. The merged images are shown in the right-most panels. Scale bar = 100 μm.
Growth kinetics of cartilage cells of the two breeds
The growth kinetics of the cartilage cells from the two breeds at different time points are shown by the growth curves. Avian broiler cartilage cells entered the logarithmic phase after approximately 3 days, which ended at the ninth day, whereas yellow bantam cartilage cells entered the logarithmic after approximately 4 days and ended at the tenth day (Fig 2). Avian broiler cartilage cells might have stronger proliferation than yellow bantam cartilage cells, but there were no significant difference between the two breeds according the growth kinetics of cartilage cells.
Fig 2. Growth curves of two breeds cartilage cells had no significant difference.
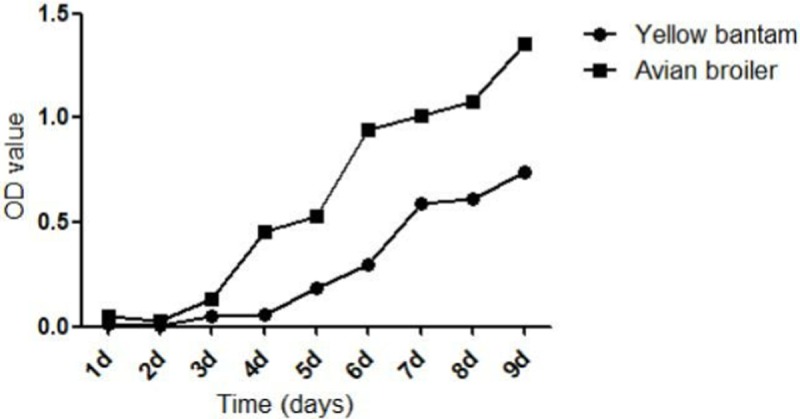
The growth curves of cells were typically sigmoidal, with cell density reflected by the vertical axis. The growth curve consisted of a latent phase, a logarithmic phase, and a plateau phase (n = 3).
Expression of BMP6 in cartilage cells of the two chicken breeds
The relative expression level of BMP6 mRNA was detected in cartilage cells from avian broilers and yellow bantams at day 0, day 1, day 2, day 3, day 4, and day 5 according to the growth curves of cells (Fig 3). Real-time PCR experiments showed that the cellular expression of BMP6 significantly (P < 0.05) increased at the fourth day and fifth day, which was consistent with the growth curves.
Fig 3. The expression of BMP6 gene was consistent with the growth curves at different days.
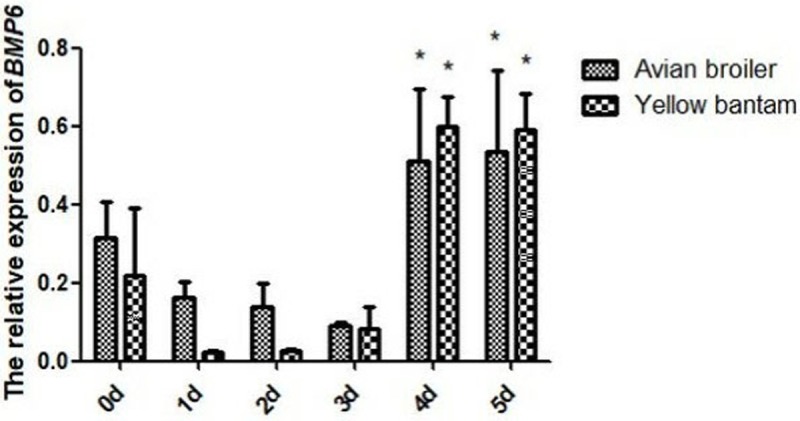
All values are presented as the means ± SEM (n = 3). (*) represents statistical significance (P < 0.05).
Expression of BMP6 mRNA after GH induction
Based on the growth curves and the relative expression of BMP6 mRNA in cartilage cells of avian broilers and yellow bantams, we selected cartilage cells of avian broilers for induction by GH and interference by siRNA targeting BMP6. Collagen II, a special marker for cartilage cells, was detected in cartilage cells after GH-induction. Relative to the β-actin gene, the expression levels of BMP6 mRNA varied considerably at different times (Fig 4). The expression levels of Collagen II and BMP6 in the three treatment groups at 24 h and 72 h was not significantly (P > 0.05) higher than that in the blank treatment group. However, at 48 h, the proliferation of cartilage cells was significantly (P < 0.05) elevated in the treatment group 50 ng/ul compared to that of the blank group, 100 ng/μl and 200 ng/μl groups (Fig 4). Considering the proliferation of cartilage cells of 200 ng/ul treatment group wasn’t significantly (P > 0.05) changed at 24 h, 48 h and 72 h, we detected the proteins of Collagen II and BMP6 in the blank group, 50 ng/ul and 100ng/ul treatment groups. The protein of Collagen II at 48 h in the 50 ng/ul treatment groups was significantly (P<0.05) higher than the blank group and 100 ng/μl groups (Fig 5). These results indicated that the cells were most sensitive to the concentration of 50 ng/μl GH. The relative expression levels of BMP6 mRNA in cartilage cells after induction are shown in Fig 4 (B). In the 50 ng/μl and 100 ng/μl treatment groups, the expression of BMP6 mRNA was significantly (P < 0.05) increased relative to the blank treatment group and 200 ng/μl at 48 h, which was consistent with the expression of Collagen II mRNA. However, the protein of BMP6 showed no significantly changes between treatment groups and blank group (Fig 5). This showed that there might be post-transcriptional modification to regulate the expression of BMP6 in the process of proliferation of cartilage cells.
Fig 4. The relative expression of Collagen II and BMP6 mRNA in cartilage cells after GH induction was significantly increased.
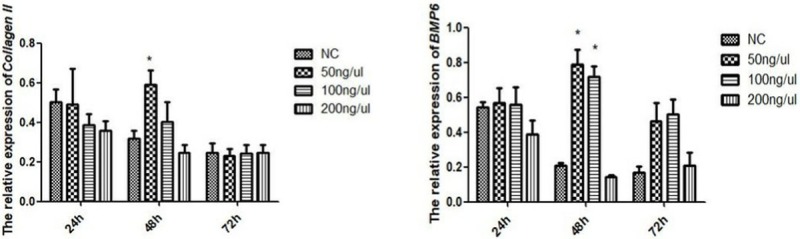
All values are represented as the means ± SEM (n = 3). (*) represent statistical significance (P < 0.05).
Fig 5.
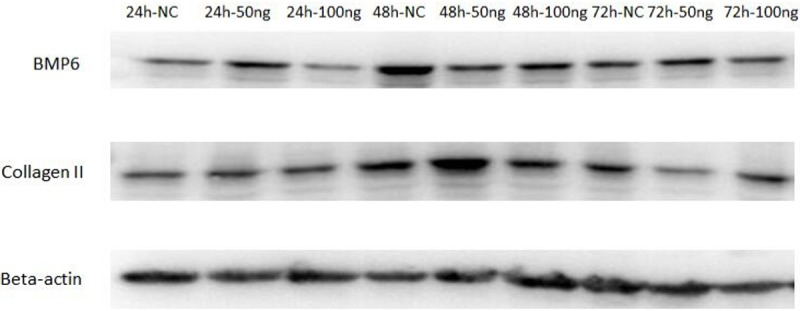
The protein levels of Collagen II (A) and BMP6 (B) by Western blot in cartilage cells after GH induction was significantly increased. The protein beta-actin was used as reference gene.
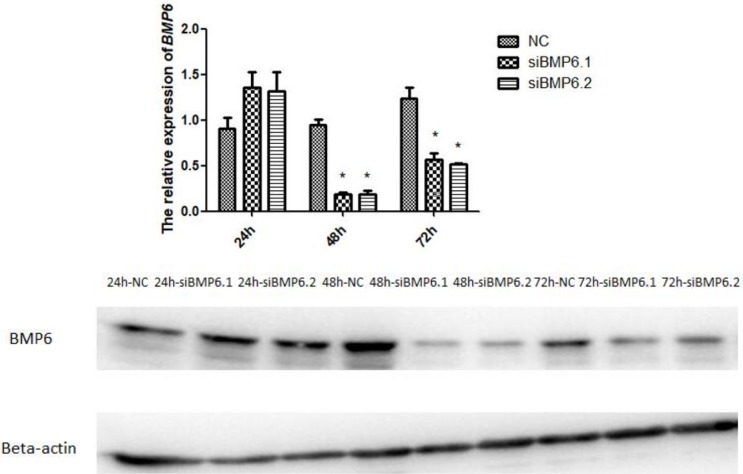
The interference efficiency of the two siRNAs
It can be seen from Fig 6 that the relative expression of BMP6 mRNA and protein levels of cartilage cells after interference with siBMP6.1 and siBMP6.2 at 24 h was not significantly (P > 0.05) different from the control group, indicating that the efficiency of siRNA interference was not reflected at this time. At 48 h and 72 h, the mRNA and protein levels of BMP6 in the treatment groups were significantly (P < 0.05) decreased compared with the control group. The interference efficiency of the two siRNAs at 48 h and 72 h were marked and stable, indicating their utility for subsequent experiments.
Fig 6. The interference efficiency of the two siRNAs were marked and stable.
(A) The expression levels of BMP mRNA by qRT-PCR. All values represent means ± SEM (n = 3). (*) represents statistical significance (P < 0.05). (B) The expression levels of BMP6 protein by western blot. The protein beta-actin was used as reference gene.
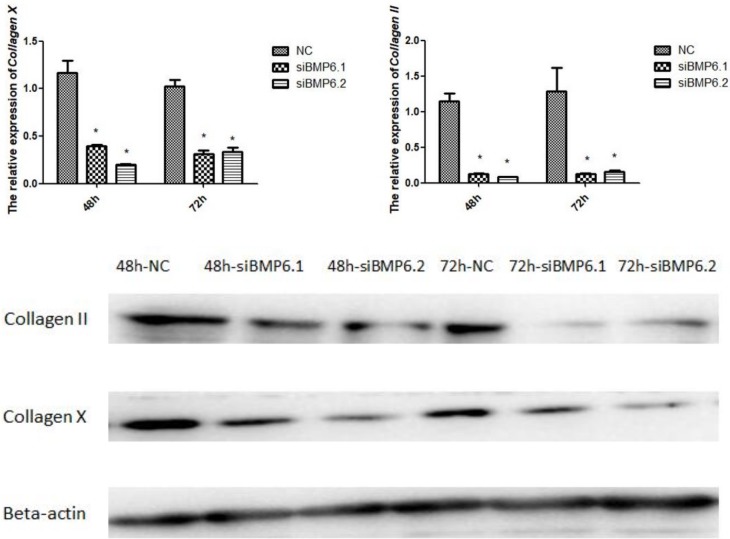
The expression levels of Collagen II and Collagen X after interference with BMP6
Collagen II and Collagen X have been suggested to be important markers of chondrocyte proliferation and differentiation [19–26]. We therefore further measured their gene expression levels and protein levels after interfering with BMP6 expression. As shown in Fig 7, we found that at 48 h and 72 h after interference with BMP6, the Collagen Ⅱ and Collagen X mRNA expression and protein expression levels in chondrocytes were significantly (P < 0.05) lower than in the control group.
Fig 7. The expression of Collagen II and Collagen X in cartilage cells were significantly decreased after siRNA treatment.
(A) The expression levels of Collagen II and Collagen X mRNA by qRT-PCR. All values are represented as the means ± SEM (n = 3). (*) represents statistical significance (P < 0.05). (B) The expression levels of Collagen II and Collagen X protein by western blot. The protein beta-actin was used as reference gene.
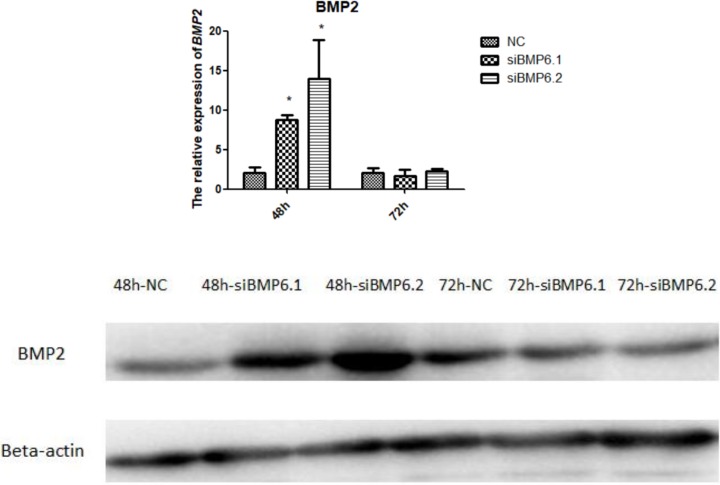
The expression levels of BMP2 after interference with BMP6
We measured the BMP2 expression levels after interfering with BMP6 expression. As shown in Fig 8, we found that at 48 h after interference with BMP6, the BMP2 mRNA expression and protein expression levels in chondrocytes were significantly higher than in the control group (P < 0.05), but there was no significant (P > 0.05)difference between treatment group and control group at 72 h.
Fig 8. The expression of BMP2 in cartilage cells were significantly increased at 48 hafter siRNA treatment.
(A) The expression levels of BMP2 mRNA by qRT-PCR. All values are represented as the means ± SEM (n = 3). (*) represents statistical significance (P < 0.05). (B) The expression levels of BMP2 protein by western blot. The protein beta-actin was used as reference gene.
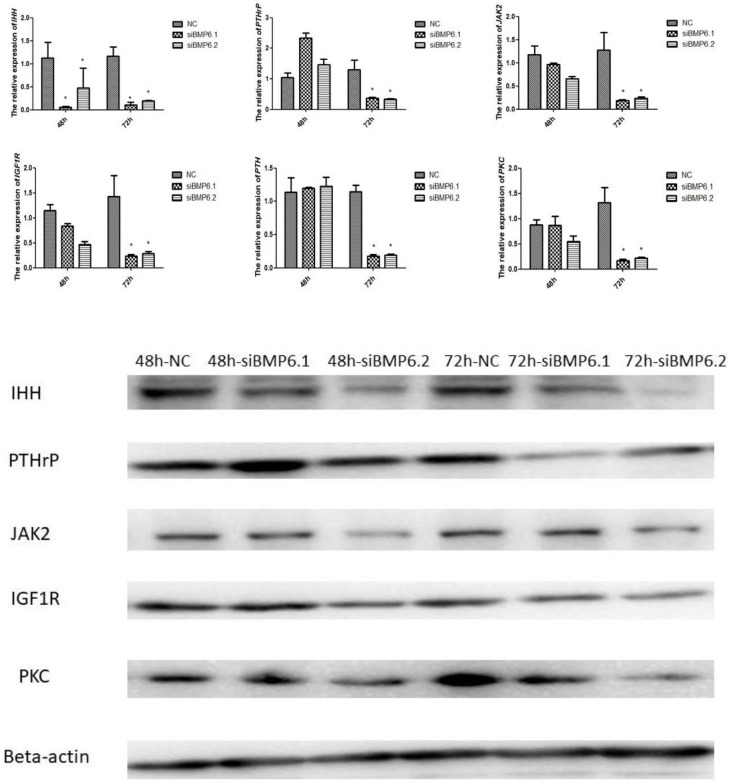
The expression levels of IGF1R, JAK, PKC, PTH and PTHrP after interference with BMP6
Since IGF1, PKC, JAK2/STAT, IHH/PTHrP and PTH signaling have been reported to affect the proliferation and differentiation of chondrocytes, we further investigated whether BMP6 was also involved in these processes. We used siRNA to knock down BMP6 gene expression in plasmid-transfected cells. Real-time PCR analysis showed that transfecting chondrocytes with BMP6-targeting siRNA resulted in a significant inhibition of expression of IHH, IGF1R, JAK2, PKC, PTH and PTHrP at 72 h (Fig 9). However, at 48 h, the relative expression of IGF1R, JAK2, PKC, PTH and PTHrP mRNAs had no significant (P > 0.05) differences between the treatment and control cartilage cells, whereas the relative expression of IHH in the treatment groups was significantly (P < 0.05) lower than in the control group.
Fig 9. The mRNA expression of selected genes in cartilage cells after siRNA treatment were significantly decreased.
(A) The expression levels of genes mRNA by qRT-PCR. All values represent means ± SEM (n = 3). (*) represents statistical significance (P < 0.05). (B) The expression levels of proteins by western blot. The protein beta-actin was used as reference gene.
The proteins of IHH, PTHrP and PKC at 72 h in the treatment showed significantly (P < 0.05) lower than in the control group. The proteins of JAK2 and IGF1R had no significant (P > 0.05) differences between the treatment and control cartilage cells. As there wasn’t appropriate primary antibody for PTH, we didn’t detect the protein of PTH.
Discussion
Currently, studies regarding the BMP6 gene in chondrocytes have primarily focused on humans, mice, rabbits and other mammals, with few studies focused on birds. Previous study demonstrated that BMP6 function in the appendicular bone development was dispensable but was required for the progression of ossification of the sternal elements [27]. Considering the biological difference between mammals and birds, there may be some different functions of BMP6. As members of BMPs, BMP2 and BMP4 induce an increase in the volume of skeletal elements by recruiting non-chondrogenic precursors to form cartilage [28,29]. But BMP4 is more effective than BMP2 at promoting differentiation [28]. BMP6 stimulates the phenotypic changes associated with chondrocyte differentiation such as increased alkaline phosphatase activity and decreased proliferation [29]. BMP6 has high levels of expression in the developing growth plate, which is on the contrary with BMP2, BMP4 and BMP7 that are expressed at lower levels or are absent in the growth plate[30,31]. In our previous studies we found that the polymorphism in BMP6 gene in chicken had significant correlation with femur perimeter [32], but the expression of BMP6 in cartilage plate of 15 E, 1 day avian broiler and yellow bantam chickens detected by RT-PCR was high (not published). BMP6 gene plays an important role in the chondrocytes in the chicken embryonic development. But the research about the signaling pathway of BMP6 is few except the SMADS signaling pathway. To figure out the signal pathways related to BMP6, we used immunofluorescence to identify and observe the cell morphology, used CCK8 assays to detect the proliferation of cartilage cells and real-time PCR to detect the BMP6 mRNA expression in cartilage cells cultured from avian broiler and yellow bantam chickens. Collagen II is a representative protein indicating the proliferation of chondrocytes, and thus can be used as a cell marker; many studies have examined the expression of collagen II in the mandibular condyle, using both immunohistochemistry and in situ hybridization techniques [19–26]. Our results showed that the avian and yellow bantam cartilage cells had no significant difference. According to these results, we selected cartilage cells cultured from avian chicken growth plates to research the function of BMP6 for regulating the proliferation and differentiation of cartilage cells.
The expression of GH can promote the expression of Collagen II mRNA in cartilage cells and maintain a chondrocyte phenotype. We used GH to induce the cartilage cells in order to detect the expression of BMP6 mRNA in proliferative cells. The results showed that the sensitivity of cartilage cells to GH varied with GH concentration. When cartilage cells proliferated, the expression of BMP6 mRNA was significantly increased but the protein levels of BMP6 wasn’t significantly changed. Therefore, we speculated that BMP6 participates in the proliferation of cartilage cells or promote cells differentiation to relieve the competitive pressure brought by cell proliferation. But the proliferation of cartilage cells controls the expression of BMP6 to maintain the proliferation state.
RNA interference uses homologous double-stranded RNA (dsRNA) to induce the silencing of specific target genes and block gene activity. SiRNA (small interfering RNA) is the intermediate of RNA interference, which is necessary for the RNA interference and can stimulate the complementary target mRNA silencing. Collagen X is specifically expressed in hypertrophic chondrocytes [33]. In this experiment, two siRNAs were designed and synthesized according to the BMP6 gene CDS (NCBI, XM-418956.4) region, and the expression of BMP6 mRNA was inhibited. BMP6 was involved in the proliferation of cartilage cells, and, when the expression of BMP6 mRNA was suppressed in cartilage cells, the expression of Collagen II and Collagen X mRNA—two marker genes representing the proliferation and differentiation of cartilage cells—decreased in cartilage cells. These results showed that the proliferation and differentiation of chondrocytes were both blocked following the disruption of the expression of BMP6. Together with the previous results, we speculate that BMP6 is involved in the proliferation and differentiation of cartilage cells, which is consistent with the existing research findings. IGF1 plays an important role in the growth and development of bone cells; it can regulate the function of osteoblasts in various forms and participates in bone reconstruction. IGF1 can significantly promote the proliferation of bone progenitor cells both in vivo and in vitro [34]. The signal transmission mediated by IGF1 is specifically induced by the IGF1R on the cell surface, thus promoting the growth, differentiation and apoptosis of tissue cells. To explore whether IGF1R is involved in the regulation of chondrocyte proliferation and differentiation of BMP6, the expression of IGF1R mRNA and protein levels were detected while using RNAi technology to interfere with the expression of BMP6. When the expression of BMP6 was inhibited, the expression of IGF1R mRNA was also inhibited, otherwise the protein of IGF1R wasn’t significantly varied. Therefore, we speculate that BMP6 has a regulatory effect on IGF1R but there may be some post-transcriptional modification to regulate the protein level of IGF1R.
JAK2 is a common signaling pathway induced by multiple cytokine- and growth factor-mediated signaling within cells [35]. When JAK activity is inhibited, osteoarthritis articular chondrocytes can reproduce and differentiate normally [36]. Interleukin-6 and interleukin-7 can induce cartilage cell activity through the JAK2/STAT signaling pathway [37,38]. It has been found that BMP7 can promote osteogenic differentiation of osteoblasts through JAK2/STAT5B signaling [39], and we hypothesized that BMP6 may regulate the proliferation and differentiation of chondrocytes through the JAK2/STAT signaling pathway. To explore whether JAK2 is involved in the regulatory effects of BMP6 on the proliferation and differentiation of cartilage cells, we used RNAi technology to interfere with the expression of BMP6 and subsequently detect the expression of JAK2 in the JAK2/STAT signaling pathway. Our results showed that the expression of JAK2 was also inhibited when the expression of BMP6 was decreased, otherwise the protein of JAK2 wasn’t significantly varied. We hypothesize that BMP6 has regulatory effects on JAK2 expression but there may be some post-transcriptional modification to regulate the protein level of JAK2.
PKC is an important substance in cell signal transduction pathways and participates in the process of proliferation and differentiation of chondrocytes; it is one of the important signal transducers affecting the growth and development of cartilage and its eventual degeneration [40]. PKC signaling and multiple other signaling pathways participate in IGF1 induction of chondrocyte proliferation and differentiation [41]. Estrogen and vitamin D-mediated signaling depends on the PKCα pathway to regulate the activity of cartilage cells [42]. To explore whether PKC is involved in the BMP6-mediated regulation of the proliferation and differentiation of cartilage cells, we used RNAi technology to interfere with the expression of BMP6 and detect the subsequent expression of PKC. Our results showed that PKC expression was inhibited both mRNA and protein levels when the expression of BMP6 was inhibited. We hypothesize that PKC is involved in the regulation of chondrocyte proliferation and differentiation mediated by BMP6. But the work to study the regulation is according phosphorylation or other modifications still needed more.
The main function of PTH is to regulate Ca2+ and Phosphorous metabolism and promote bone absorption. PTH stimulates the expression of Collagen II in cartilage cells [43]. A previous study found that PTH interacts with the TGF-beta signaling pathway at the receptor level [44]. BMP6 belongs to the TGF-beta family, therefore we speculated that PTH may participate in the regulation of chondrocytes by BMP6. We used RNAi technology to interfere with the expression of BMP6 and detected subsequent PTH expression. Our results showed that PTH mRNA expression was also inhibited when the expression of BMP6 was inhibited. We hypothesize that PTH participates in the regulation of chondrocyte proliferation and differentiation by BMP6.
IHH/PTHrP signaling is important in bone development regulation, modulating the differentiation of chondrocytes and osteoblasts, maintaining the cartilage cell proliferation state [45] and determining the length of the growth plate cartilage by regulating the bone longitudinal growth rate [46,47]. PTHrP can inhibit osteoblast differentiation by down-regulating BMP2 expression [47]. We hypothesized that the IHH/PTHrP signaling pathway may be involved in the regulation by BMP6 of chondrocytes. We used RNAi technology to interfere with the expression of BMP6 and detect the subsequent expression of IHH and PTHrP. Our results showed that the expression of IHH and PTHrP mRNA and protein levels were also inhibited when the expression of BMP6 was decreased. The IHH/PTHrP signaling pathway participates in the regulation by BMP6 of the proliferation and differentiation of cartilage cells. But the work to study the regulation is according phosphorylation or other modifications still needed more.
While the expression of BMP6 was decreased, the expression of BMP2 was increased, the BMP2 may brevity replace the function of BMP6. That may be the reason the expression of IGF1/JAK/PKC/PTH/IHH-PTHrP weren’t decreased at 48 h but significantly decreased at 72 h. BMP6 belongs to the TGFβ family, and current research indicates that the regulation of chondrocytes is mainly achieved through the Smad signaling pathway of TGFβ [48]. Smads may be required, but not sufficient for the differentiation of chondrogenic differentiation of ATDC5 cells [49]. Further studies are needed to identity the signaling pathways responsible for biological effects. BMP2, BMP9 and BMP6 belong to the BMP family. BMP2 can increase the proliferation of chondrocytes and the elongation of chondrocytes by inducing the expression of IHH [10]. BMP9 and GH can synergize the osteogenic differentiation of mesenchymal stem cells through the JAK/STAT/IGF1 signaling pathway. As one of the strongest members of the BMPs family, BMP6 may regulate the proliferation and differentiation of chondrocytes through other signaling pathways in addition to the Smad signaling pathway. Therefore, we analyzed the IGF1/JAK/PKC/PTH/IHH-PTHrP signaling pathways, which play important roles in the process of proliferation and differentiation of cartilage cells, and detected the expression changes (mRNA and protein levels) of several key genes (IGF1R, JAK2, PKC, PTH, IHH, PTHrP) in these signaling pathways when the expression of BMP6was inhibited. The results collectively showed that the expression of IHH, PTHrP and PKC genes decreased significantly on mRNA and protein levels and the expression of IGF1R and JAK2 mRNA were significantly decreased while the protein levels of IGF1R and JAK2 had no significantly varied which might because there were less p+ signal transmit to the intracellular by BMP6 or because the cell activity was affected caused by the decreased expression of BMP6. IGF1/JAK/PKC/PTH/IHH-PTHrP are involved in the regulation by BMP6 of the proliferation and differentiation of cartilage cells, but there should be more work to verify how the signal pathway and its phosphorylation transmit. The work to study the regulation is according phosphorylation or other modifications still needed more.
Conclusion
There was no significant difference in the shape, proliferation and differentiation of cartilage cells cultured from different chicken breeds. BMP6 was highly expressed when cartilage cells proliferated. When the expression of BMP6 was decreased, the proliferation and differentiation of chondrocytes was blocked, indicating that BMP6 is involved in the proliferation and differentiation of cartilage cells. The expression levels of key genes involved in the IGF1R, JAK2, PKC, PTH, IHH and PTHrP signaling pathways were significantly lower in cartilage cells when the expression of BMP6 was decreased. These key genes involved in signaling pathways may related to the regulation by BMP6 of the proliferation and differentiation of cartilage cells, but how the signal such as phosphorylation or other protein modification transmit still need more work to verify.
Acknowledgments
We would like to thank AMERICAN JOURNAL EXPERTS (https://secure.aje.com) for providing linguistic assistance during the preparation of this manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was financially supported by the project of Sichuan Science and Technology Department (2019YJ0513), the China Agricultural Research System (CARS-41) and the 13th Five year Plan for Breeding Program in Sichuan (2016NYZ0050).
References
- 1.Karsenty G, Wagner EF (2002) Reaching a genetic and molecular understanding of skeletal development. Developmental cell 2: 389–406. [DOI] [PubMed] [Google Scholar]
- 2.Zuscik MJ, Hilton MJ, Zhang X, Chen D, O’Keefe RJ (2008) Regulation of chondrogenesis and chondrocyte differentiation by stress. The Journal of clinical investigation 118: 429–438. 10.1172/JCI34174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandell LJ, Nalin AM, Reife RA (1994) Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non‐cartilaginous tissues during early mouse development. Developmental dynamics 199: 129–140. 10.1002/aja.1001990206 [DOI] [PubMed] [Google Scholar]
- 4.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, et al. (2000) Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proceedings of the National Academy of Sciences 97: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazono K, Miyazawa K (2002) Id: a target of BMP signaling. Science signaling 2002: pe40–pe40. [DOI] [PubMed] [Google Scholar]
- 6.Vukicevic S, Grgurevic L (2009) BMP-6 and mesenchymal stem cell differentiation. Cytokine & growth factor reviews 20: 441–448. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Yang X, He Y, Ye G, Li X, et al. (2012) Bone morphogenetic protein-9 induces osteogenic differentiation of rat dental follicle stem cells in P38 and ERK1/2 MAPK dependent manner. International journal of medical sciences 9: 862–871. 10.7150/ijms.5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheu S-Y, Tsai C, Sun J, Chen M, Liu M, et al. (2012) Stimulatory effect of puerarin on bone formation through co-activation of nitric oxide and bone morphogenetic protein-2/mitogen-activated protein kinases pathways in mice. Chinese medical journal 125: 3646–3653. [PubMed] [Google Scholar]
- 9.Farnum CE, Wilsman NJ (1989) Cellular turnover at the chondro‐osseous junction of growth plate cartilage: Analysis by serial sections at the light microscopical level. Journal of orthopaedic research 7: 654–666. 10.1002/jor.1100070505 [DOI] [PubMed] [Google Scholar]
- 10.Uyama Y, Yagami K, Hatori M, Kakuta S, Nagumo M (2004) Recombinant human bone morphogenetic protein‐2 promotes Indian hedgehog‐mediated osteo‐chondrogenic differentiation of a human chondrocytic cell line in vivo and in vitro. Differentiation 72: 32–40. 10.1111/j.1432-0436.2004.07201001.x [DOI] [PubMed] [Google Scholar]
- 11.Cook SD, Rueger DC (1996) Osteogenic protein-1: biology and applications. Clinical orthopaedics and related research 324: 29–38. [PubMed] [Google Scholar]
- 12.de Mara CS, Duarte A, Sartori-Cintra A, Luzo A, Saad ST, et al. (2013) Chondrogenesis from umbilical cord blood cells stimulated with BMP-2 and BMP-6. Rheumatology international 33: 121–128. 10.1007/s00296-011-2328-6 [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Cho T-J, Kwon S-K, Lee G, Cho J (2013) Chondrogenesis of periodontal ligament stem cells by transforming growth factor-&bgr; 3 and bone morphogenetic protein-6 in a normal healthy impacted third molar. International journal of oral science 5: 7–13. 10.1038/ijos.2013.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Buckley CT, Mulhall KJ, Kelly DJ (2013) Combining BMP-6, TGF-β3 and hydrostatic pressure stimulation enhances the functional development of cartilage tissues engineered using human infrapatellar fat pad derived stem cells. Biomaterials Science 1: 745–752. [DOI] [PubMed] [Google Scholar]
- 15.Estes BT, Wu AW, Guilak F (2006) Potent induction of chondrocytic differentiation of human adipose‐derived adult stem cells by bone morphogenetic protein 6. Arthritis & Rheumatism 54: 1222–1232. [DOI] [PubMed] [Google Scholar]
- 16.Kayabaşi GK, Tiğli Aydin RS, Gümüşderelioğlu M (2013) In vitro chondrogenesis by BMP6 gene therapy. Journal of Biomedical Materials Research Part A 101: 1353–1361. 10.1002/jbm.a.34430 [DOI] [PubMed] [Google Scholar]
- 17.Gevers EF, Van Der Eerden BC, Karperien M, Raap AK, Robinson IC, et al. (2002) Localization and regulation of the growth hormone receptor and growth hormone‐binding protein in the rat growth plate. Journal of Bone and Mineral Research 17: 1408–1419. 10.1359/jbmr.2002.17.8.1408 [DOI] [PubMed] [Google Scholar]
- 18.Dos Santos C, Essioux L, Teinturier C, Tauber M, Goffin V, et al. (2004) A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nature genetics 36: 720–724. 10.1038/ng1379 [DOI] [PubMed] [Google Scholar]
- 19.Silbermann M, von der Mark K (1990) An immunohistochemical study of the distribution of matrical proteins in the mandibular condyle of neonatal mice. I. Collagens. Journal of anatomy 170: 11 [PMC free article] [PubMed] [Google Scholar]
- 20.Mizoguchi I, Nakamura M, Takahashi I, Kagayama M, Mitani H (1992) A comparison of the immunohistochemical localization of type I and type II collagens in craniofacial cartilages of the rat. Cells Tissues Organs 144: 59–64. [DOI] [PubMed] [Google Scholar]
- 21.SHIBATA S, FUKADA K, SUZUKI S, YAMASHITA Y (1997) Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. The Journal of Anatomy 191: 561–570. 10.1046/j.1469-7580.1997.19140561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luder HU, Leblond C, Von Der Mark K (1988) Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Developmental Dynamics 182: 197–214. [DOI] [PubMed] [Google Scholar]
- 23.Visnapuu V, Peltomäki T, Säämänen A, Rönning O (2000) Collagen I and II mRNA distribution in the rat temporomandibular joint region during growth. Journal of craniofacial genetics and developmental biology 20: 144–149. [PubMed] [Google Scholar]
- 24.Ohashi N, Ejiri S, Hanada K, Ozawa H (1997) Changes in type I, II, and X collagen immunoreactivity of the mandibular condylar cartilage in a naturally aging rat model. Journal of bone and mineral metabolism 15: 77–83. [Google Scholar]
- 25.FUKADA K, SHIBATA S, SUZUKI S, OHYA K, KURODA T (1999) In situ hybridisation study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. The Journal of Anatomy 195: 321–329. 10.1046/j.1469-7580.1999.19530321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizoguchi I, Nakamura M, Takahashi I, Kagayama M, Mitani H (1990) An immunohistochemical study of localization of type I and type II collagens in mandibular condylar cartilage compared with tibial growth plate. Histochemistry 93: 593–599. [DOI] [PubMed] [Google Scholar]
- 27.Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, et al. (1998) Mice lacking Bmp6 function. Developmental genetics 22: 321–339. [DOI] [PubMed] [Google Scholar]
- 28.Volk SW, Luvalle P, Leask T, Leboy PS (1998) A BMP responsive transcriptional region in the chicken type X collagen gene. Journal of Bone and Mineral Research 13: 1521–1529. 10.1359/jbmr.1998.13.10.1521 [DOI] [PubMed] [Google Scholar]
- 29.Duprez D, Bell EJdH, Richardson MK, Archer CW, Wolpert L, et al. (1996) Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mechanisms of development 57: 145–157. [DOI] [PubMed] [Google Scholar]
- 30.Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, et al. (1996) Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273: 613–622. 10.1126/science.273.5275.613 [DOI] [PubMed] [Google Scholar]
- 31.Lyons KM, Pelton R, Hogan B (1989) Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes & Development 3: 1657–1668. [DOI] [PubMed] [Google Scholar]
- 32.Cui C, Ye F, Li Y, Yin H, Ye M, et al. (2017) Detection of SNPs in the BMP6 Gene and Their Association with Carcass and Bone Traits in Chicken. Revista Brasileira de Ciência Avícola 19: 673–682. [Google Scholar]
- 33.Aigner T, Greskötter K-R, Fairbank J, Von Der Mark K, Urban J (1998) Variation with age in the pattern of type X collagen expression in normal and scoliotic human intervertebral discs. Calcified tissue international 63: 263–268. [DOI] [PubMed] [Google Scholar]
- 34.Kasukawa Y, Stabnov L, Miyakoshi N, Baylink DJ, Mohan S (2002) Insulin‐Like Growth Factor I Effect on the Number of Osteoblast Progenitors Is Impaired in Ovariectomized Mice. Journal of Bone and Mineral Research 17: 1579–1587. 10.1359/jbmr.2002.17.9.1579 [DOI] [PubMed] [Google Scholar]
- 35.Ghoreschi K, Laurence A, O’Shea JJ (2009) Janus kinases in immune cell signaling. Immunological reviews 228: 273–287. 10.1111/j.1600-065X.2008.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Beuningen HM, de Vries-van Melle ML, Vitters EL, Schreurs W, van den Berg WB, et al. (2014) Inhibition of TAK1 and/or JAK can rescue impaired chondrogenic differentiation of human mesenchymal stem cells in osteoarthritis-like conditions. Tissue Engineering Part A 20: 2243–2252. 10.1089/ten.TEA.2013.0553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yammani RR, Long D, Loeser RF (2009) Interleukin‐7 stimulates secretion of S100A4 by activating the JAK/STAT signaling pathway in human articular chondrocytes. Arthritis & Rheumatism 60: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aida Y, Honda K, Tanigawa S, Nakayama G, Matsumura H, et al. (2012) IL‐6 and soluble IL‐6 receptor stimulate the production of MMPs and their inhibitors via JAK—STAT and ERK—MAPK signalling in human chondrocytes. Cell biology international 36: 367–376. 10.1042/CBI20110150 [DOI] [PubMed] [Google Scholar]
- 39.Joung YH, Lim EJ, Darvin P, Jang JW, Park KD, et al. (2013) Hwanggeumchal sorghum extract enhances BMP7 and GH signaling through the activation of Jak2/STAT5B in MC3T3‑E1 osteoblastic cells. Molecular medicine reports 8: 891–896. 10.3892/mmr.2013.1593 [DOI] [PubMed] [Google Scholar]
- 40.Nakajima T (2008) Positive and negative regulation of radiation-induced apoptosis by protein kinase C. Journal of radiation research 49: 1–8. 10.1269/jrr.07053 [DOI] [PubMed] [Google Scholar]
- 41.Ciarmatori S, Kiepe D, Haarmann A, Huegel U, Tönshoff B (2007) Signaling mechanisms leading to regulation of proliferation and differentiation of the mesenchymal chondrogenic cell line RCJ3. 1C5. 18 in response to IGF-I. Journal of molecular endocrinology 38: 493–508. 10.1677/jme.1.02179 [DOI] [PubMed] [Google Scholar]
- 42.Boyan B, Schwartz Z (2004) Rapid vitamin D-dependent PKC signaling shares features with estrogen-dependent PKC signaling in cartilage and bone. Steroids 69: 591–597. 10.1016/j.steroids.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 43.Siebuhr A, Gudmann N, Musa K, Kehlet S, Hansen G, et al. (2015) AB0086 PTH Stimulates Cartilage Formation in Low Turnover Patients–a Possible Systemic Anabolic Treatment for OA? Annals of the Rheumatic Diseases 74: 919–919. [Google Scholar]
- 44.Qiu T, Xian L, Crane J, Wen C, Hilton M, et al. (2015) PTH receptor signaling in osteoblasts regulates endochondral vascularization in maintenance of postnatal growth plate. Journal of Bone and Mineral Research 30: 309–317. 10.1002/jbmr.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Litherland GJ, Elias MS, Hui W, Macdonald CD, Catterall JB, et al. (2010) Protein kinase C isoforms ζ and ι mediate collagenase expression and cartilage destruction via STAT3-and ERK-dependent c-fos induction. Journal of Biological Chemistry 285: 22414–22425. 10.1074/jbc.M110.120121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, et al. (1996) Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proceedings of the National Academy of Sciences 93: 10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, et al. (1996) PTH/PTHrP receptor in early development and Indian hedgehog—regulated bone growth. Science 273: 663–666. 10.1126/science.273.5275.663 [DOI] [PubMed] [Google Scholar]
- 48.Aref-Eshghi E, Liu M, Harper PE, Doré J, Martin G, et al. (2015) Overexpression of MMP13 in human osteoarthritic cartilage is associated with the SMAD-independent TGF-β signalling pathway. Arthritis research & therapy 17: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujii M, Takeda K, Imamura T, Aoki H, Sampath TK, et al. (1999) Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Molecular biology of the cell 10: 3801–3813. 10.1091/mbc.10.11.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.