Abstract
Rapidly growing mycobacteria (RGM) are environmental bacteria found worldwide with a propensity to produce skin and soft-tissue infections. Among them, the most clinically relevant species is Mycobacterium abscessus. Multiple resistance to antibiotics and the ability to form biofilm contributes considerably to the treatment failure. The search of novel anti-mycobacterial agents for the control of biofilm growth mode is crucial. The aim of the present study was to evaluate the activity of carvacrol (CAR) against planktonic and biofilm cells of resistant RGM strains. The susceptibility of RGM strains (n = 11) to antibiotics and CAR was assessed by MIC/MBC evaluation. The CAR activity was estimated by also vapour contact assay. The effect on biofilm formation and preformed biofilm was measured by evaluation of bacterial growth, biofilm biomass and biofilm metabolic activity. MIC values were equal to 64 μg/mL for most of RGM isolates (32–512 μg/mL), MBCs were 2–4 times higher than MICs, and MICs of vapours were lower (16 μg/mL for most RGM isolates) than MICs in liquid phase. Regarding the biofilm, CAR at concentrations of 1/2 × MIC and 1/4 × MIC showed a strong inhibition of biofilm formation (61–77%) and at concentration above the MIC (2–8 × MIC) produced significant inhibition of 4- and 8-day preformed biofilms. In conclusion, CAR could have a potential use, also in vapour phase, for the control of RGM.
Introduction
Rapidly growing mycobacteria (RGM) are environmental bacteria capable of causing a wide spectrum of infections [1]. Among them, Mycobacterium abscessus is an emerging human pathogen causing lung infection but also responsible for wound, catheter and eye infections and also tattooing [2]. Mycobacterium chelonae is commonly associated with skin and soft tissue infections and also causes catheter-related and post-surgical infections; invasive infections are common in immunosuppressed patients but pulmonary infections are rare when compared to M. abscessus [3]. Mycobacterium fortuitum is responsible for the most of post-surgical wound and catheter infections produced by the RGM [4]. Mycobacterium smegmatis, previously considered an environmental saprophyte without any clinical significance, is accountable for community-acquired disease including cellulitis, localized abscesses and osteomyelitis [5]. Mycobacterium mucogenicum causes osteomyelitis and respiratory tract, bloodstream and disseminated infections in both immunocompetent and immunocompromised hosts [4].
Most of the M. chelonae, M. abscessus and M. mucogenicum isolates are widely resistant to antibiotics and disinfectants [6,7]. In particular, M. abscessus is resistant to the common and currently existing antibiotics [5]. An important virulence factor for RGM is the production of biofilms, both in medical and in environmental settings [8], that contribute to the therapy failure and relapses [9]. Notably, smooth M. abscessus strains are major biofilm-producers respect to rough M. abscessus strains [10].
The volatile monoterpene carvacrol (CAR) [2-Methyl-5-(1-methylethyl) phenol], a major constituent of many essential oils of the Labiatae family, is classified among the substances generally recognized as safe (GRAS) and approved for use in food [11]. Several studies have demonstrated its biological properties such as antioxidant, anti-inflammatory, antitumor, analgesic, anti-hepatotoxic and insecticidal activities [12–15]. Carvacrol has been known for its wide antimicrobial activity against food or pathogenic microorganisms, including drug-resistant bacteria [8,16,17]. CAR is also efficacious against organisms in the biofilm growth mode [18]. Specifically, it is able to interfere with biofilm growth of clinically relevant Staphylococcus aureus and S. epidermidis [19–21], Salmonella tiphymurium [22], Listeria monocytogenes [23] titanium-adherent oral microrganisms [24] and carbapenemase-producing Gram negative bacilli. The efficacy of CAR against microbial fungal biofilms has also been investigated [25]. Despite extensive research on the monoterpenic phenol CAR, there is little information on its efficacy against RGM. To our knowledge, the activity of CAR has been documented against fast-growing M. phlei ATCC 11758, M. smegmatis ATCC 19420 and M. fortuitum ATCC 6841 (MICs of 80–100 μg/mL) [26].
The goal of the present study was to extend the research and to evaluate the antimicrobial activity of CAR, either in liquid and vapour phase, against different species of resistant RGM. Moreover, the antibiofilm activity of CAR, in liquid phase, was also evaluated against RGM species capable to form biofilm.
Materials and methods
RGM strains and growth media
Eleven anonymized clinical strains of RGM were used for this study. Strains, were stored in the private collection of Unit of Microbiology, Department of Biomedical Sciences and Public Health, Polytechnic University of Marche, Ancona, Italy. Rapidly growing mycobacteria strains were identified as smooth morphotypes M. abscessus #09716, #29904, #30235, #70513, #73596, #90459, #74600; M. chelonae #74471; M. fortuitum #26647; M. mucogenicum #45646 and M. smegmatis #44041 by line-probe reverse hybridization assay (GenoType CM, Hain Lifescience, Nehren, Germany) and conventional biochemical and cultural methods, as suggested by Clinical and Laboratory Standard Institute (CLSI) [27]. Blood agar base (BAB, Oxoid, Basingstoke, UK) and Müller-Hinton cation-adjusted agar (CAMHA, Oxoid), both supplemented with 5% sheep blood; Müller-Hinton cation-adjusted broth (CAMHB, Oxoid); Middlebrook broth (MBB, Oxoid) and agar (MBA, Oxoid), both supplemented with 10% oleic albumin dextrose catalase (OADC, Oxoid) and 0.5% glycerol, were used for the experiments. Bacterial isolates were stored in cryovials with glycerol (20% v/v glycerol).
Susceptibility of RGM strains to antibiotics and CAR
All antibiotics: amikacin (AMK), cefoxitin (FOX), ciprofloxacin (CIP), clarithromycin (CLR), linezolid (LZD), meropenem (MEM), sulfamethoxazole (SX) and tigecycline (TGC) as well as carvacrol (CAR; W224502, purity ≥98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibiotics stock solutions (10 mg/mL) were stored in absolute ethanol at -20°C. Doubling broth dilutions of antibiotics and CAR were prepared in 96-well microtitre plates. In order to categorize RGM strains as susceptible, intermediate or resistant, antibiotic concentrations two-fold dilutions away from their breakpoints (Table 1) [28] were used in susceptibility testing. Currently there are no interpretative breakpoints available for meropenem, ertapenem and doripenem antibiotics for which those related to imipenem are considered [29]. Breakpoints for tigecycline were based on those used by Ananta [30].
Table 1. Antibiotic breakpoints used for interpretation of RGM susceptibility.
| Antibiotics | MIC (μg/ml) | ||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| AMK | ≤16 | 32 | ≥64 |
| FOX | ≤16 | 32–64 | ≥128 |
| CIP | ≤1 | 2 | ≥4 |
| CLR | ≤2 | 4 | ≥8 |
| LZD | ≤8 | 16 | ≥32 |
| MEM | ≤4 | 8 | ≥16 |
| SX | ≤32 | - | ≥64 |
| TGC | ≤1 | 2 | ≥4 |
The final concentration of CAR ranging from 4096 to 8 μg/mL. Ethanol maximum concentration was 1.25% (v/v). Minimum inhibitory concentration (MIC) was determined by microdilution methods and interpreted following the Clinical and Laboratory Standards Institute guidelines [28]. Growth controls consisting of medium and medium with ethanol were included. Inoculated 96-well microtiter plates were covered with adhesive seals and incubated at 30°C for 4/5 days. Bacterial growth was controlled by visual inspection. The minimum bactericidal concentration (MBC) of CAR was determined by sub-culturing 10 μL of each microdilution on MHA plates followed by incubation at 30°C for 4–5 days. All experiments were performed in triplicate.
Vapour contact assay
The effect of CAR vapours was evaluated with an invert Petri dishes method as previously described [31,32]. Briefly, CAMHA was inoculated with 5 μL of a suspension of the strain (104 CFU/mL). Different volumes of CAR were added in a glass slide placed in the upper lid of each Petri dish. The final concentration ranging from 4096 to 8 μg/mL of the air space. The plates were sealed with adhesive seals and incubated at 30°C for 4–5 days. Each assay was made in triplicate. The lowest concentration of CAR vapours preventing visible growth was recorded as MIC.
Effect of CAR on biofilm
The biofilm-forming ability of Mycobacterium strains was tested on 96-well polystyrene flat-bottomed microtitre plates as previously described [19]. Then, the effect of exposure of biofilm to CAR was determined either on biofilm formation and preformed biofilm.
Effect on biofilm formation
The effect of sub-inhibitory concentrations (sub-MICs) of CAR (ranging from 1/2 × MIC to 1/16 × MIC) on biofilm-forming ability was studied [33]. Bacterial cultures, were grown for 3–4 days in MBB + 0.5% glycerol + 10% OADC, standardized to 1 × 108 CFU/mL by optical density (OD492) measurements and diluted 1:100 was inoculated (100 μL) into each well of microtiter plates in the presence of sub-MICs of CAR (100 μL) or medium (control). The correlation between CFU/mL and optical density (OD492) was obtained through the use of a standard curve and the following equation which was developed by plotting the OD values as a function of the log CFU/mL:
| (1) |
After incubation for 3–4 days at 37°C, the effect on planktonic bacterial growth i) and biofilm biomass ii) was estimated as follows:
the medium (planktonic bacterial growth) was removed from the each well and transferred to wells of a new 96-well polystyrene flat-bottomed microtitre plates in order to evaluate the total mass amount by measuring the OD492 using a spectrophotometer EIA reader (Bio-Rad Model 2550, Richmond, CA, USA);
the biofilm biomass was evaluated as follows: the wells of microtitre plates were washed twice with sterile PBS, dried, stained with 0.1% safranin and washed with water. The biofilm biomass eluted in acetic acid 30% (v/v) was evaluated by OD492 measurements. The biofilm reduction was estimated by the following equation:
| (2) |
All experiments were made in triplicate.
Effect on preformed 4 and 8 day-biofilms
Strains grown for 3–4 days in MBB + 0.5% glycerol + 10% OADC were standardized to 1 × 106 CFU/mL and were inoculated (200 μL) in 96-well polystyrene flat-bottomed microtitre plates. After incubation for 4 and 8 days at 37°C, the planktonic bacterial growth was dislodged and the wells were washed with sterile PBS and filled with twofold dilutions of CAR, ranging from the MIC to a 16-fold the MIC (referred to the values reported in Table 2). After incubation for 4 days at 37°C, the effects on i) biofilm supenatant growth ii) biofilm biomass and iii) biofilm metabolic activity were estimated as follows.
Table 2. Antibacterial activity of CAR, in liquid and vapour phase, against RGM strains.
| Strain # | Mycobacterium spp. | MIC BMa |
MBC BMa |
MIC VCb |
|---|---|---|---|---|
| 09716 | M. abscessus | 128 | 256 | 64 |
| 29904 | M. abscessus | 256 | 512 | 16 |
| 30235 | M. abscessus | 512 | 2048 | 16 |
| 70513 | M. abscessus | 64 | 128 | 16 |
| 73596 | M. abscessus | 64 | 128 | 16 |
| 90459 | M. abscessus | 64 | 128 | 16 |
| 74600 | M. abscessus | 64 | 128 | 16 |
| 74471 | M. chelonae | 64 | 128 | 16 |
| 26647 | M. fortuitum | 64 | 256 | 16 |
| 45646 | M. mucogenicum | 64 | 64 | 16 |
| 44041 | M. smegmatis | 32 | 128 | 16 |
a BM, broth microdilution method: values are given as μg/mL.
bVC, vapour contact method: values are given as μg/mL air space.
Biofilm supernatant growth. To determine whether CAR treatment prevented the growth in the biofilm supernatant, the medium was removed from the each well and transferred to wells of a new 96-well polystyrene flat-bottomed microtitre plates in order to evaluate the total mass amount by measuring the OD492;
Biofilm biomass. The planktonic growth was dislodged and each well was washed with PBS, dried, stained with 0.1% safranin and then washed with water. The biofilm biomass was eluted in acetic acid 30% (v/v) and the OD492 was quantified.
Biofilm metabolic activity. The planktonic growth was dislodged and, after washing, each well was treated with the Cell Proliferation Kit II XTT (Roche Diagnosis, Mannheim, Germany) as previously reported [34]. This assay is based on the metabolic reduction of a tetrazolium salt [2,3-bis[methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5carboxanilide carboxanilide (XTT)] to a colored water-soluble formazan derivative. Briefly, each well was filled with the XTT solution (final concentration 0.3 mg/mL) for 5 h at 37°C and the formazan derivative was measured spectrophotometrically at 492 nm.
All experiments were made in triplicate.
The reduction of biofilm supernatant growth/biofilm biomass/biofilm metabolic activity was estimated using Eq 1. The action of CAR was classified according to Lemos et al. [35] classification, as follows: < 25%—low efficacy; 60%—moderate efficacy; 60 ≤—< 90%—high efficacy; 90 ≤—≤ 100%—excellent efficacy.
Statistical analysis
ANOVA was used to evaluate the significant differences between the samples treated with CAR and the samples without CAR. A p value <0.05 was assumed as significant.
Results
Susceptibility of RGM strains to antibiotics and CAR
The results of susceptibility tests showed that M. abscessus strains were resistant to AMK (#90459, MIC 64 μg/mL), CIP (#09716, #29904, #30235, #70513, #73596, #90459, #74600, MIC ≥ 4 μg/mL), CLR after 72 h incubation (#29904, #70513, #73596, MIC 8 μg/mL), CLR after 14 incubation days (#09716, #29904, #30235, #70513, #73596, #74600, MIC > 8 μg/mL), LZD (#09716, #29904, #70513, #73596, #90459, MIC 32 μg/mL), MEM (#29904, #73596, #74600, MIC > 16 μg/mL and #09716, #70513, MIC 16 μg/mL), SX (#09716, #29904, #70513, #73596, #90459, #74600, MIC > 64 μg/mL), TGC (#29904, MIC 8 μg/mL) and intermediate to AMK (#09716, #29904, #70513, #73596, #74600, MIC 32 μg/mL), FOX (#29904, #70513, #73596, MIC 32 μg/mL), LZD (#74600, MIC 16 μg/mL); M. chelonae #74471 was resistant to AMK (MIC 64 μg/mL), FOX (MIC > 128 μg/mL), CIP (MIC > 4 μg/mL), MEM (MIC >16 μg/mL), SX (MIC > 64 μg/mL) and intermediate to LZD (MIC 16 μg/mL); M. fortuitum #26647 was resistant to CIP (MIC 4 μg/mL), CLR after 14 incubation days (MIC > 8 μg/mL), SX (MIC: > 64 μg/mL) and intermediate to CLR after 72 h of incubation (MIC 4 μg/mL), FOX (MIC 32 μg/mL); M. mucogenicum #45646 was resistant to CIP (MIC > 4 μg/mL), SX (MIC > 64 μg/mL); M. smegmatis #44041 was resistant to SX (MIC > 64 μg/mL).
The susceptibility patterns of RGM strains to CAR, in liquid and vapour phase are shown in Table 2. The MIC values obtained using microdilution technique were equal to 64 μg/mL for 7 out of 11 RGM isolates; the MICs of the remaining four M. abscessus isolates were 32 μg/mL (#44041), 128 μg/mL (#09716), 256 μg/mL (#29904) and 512 μg/mL (#30235). The MBC values were 2–4 times higher than MICs (from 64 μg/mL to 2048 μg/mL) in all RGM strains, except for one isolate (# 45646) for which the MIC was equal to the MBC value. The MIC values obtained using vapour contact assays were lower than those in liquid medium for all the RGM isolates. Specifically, MICs were equal to 16 μg/mL for all strains except for one of them (#09716), for which the MIC was 64 μg/mL.
Efficacy of CAR on biofilm formation
Biofilm biomass measurements showed the efficacy of CAR against biofilm formation of randomly selected Mycobacterium strains, i.e. M. abscessus (n = 4;) and M. fortuitum (Table 3). Although concentrations of 1/2 × MIC and 1/4 × MIC produced a relative inhibition of planktonic growth (28–46%), these concentrations showed a strong inhibition of biofilm (61–77%) in respect to 1/8 × MIC and 1/16 × MIC (Table 3); notably, CAR at 1/2 × MIC caused reductions of biofilm biomass accumulation ≥ 70% for all tested strains. According to Lemos et al. [35] classification, CAR at 1/2 × MIC and 1/4 × MIC was classified as highly effective (60% ≤ inhibition value < 90%).
Table 3. Effect of CAR at sub-MICs on planktonic growth and biofilm formation.
| Strain | CAR sub-MIC | Planktonic Growth | Biofilm Biomass | ||
|---|---|---|---|---|---|
| OD492 | Reduction (%) |
OD492 | Reduction (%) |
||
|
M. abscessus #09716 |
1/2 | 0.382±0.022 | 35 | 0.365±0.010 | 71 |
| 1/4 | 0.410±0.012 | 31 | 0.464±0.018 | 63 | |
| 1/8 | 0.432±0.025 | 27 | 0.715±0.027 | 43 | |
| 1/16 | 0.482±0.031 | 18 | 0.863±0.045 | 31 | |
| Control | 0.590±0.034 | 1.256±0.038 | |||
|
M. abscessus #29904 |
1/2 | 0.343±0.031 | 40 | 0.351±0.041 | 70 |
| 1/4 | 0.410±0.022 | 28 | 0.440±0.030 | 63 | |
| 1/8 | 0.450±0.041 | 21 | 0.720±0.052 | 39 | |
| 1/16 | 0.482±0.012 | 15 | 0.854±0.043 | 28 | |
| Control | 0.568±0.033 | 1.180±0.180 | |||
|
M. abscessus #70513 |
1/2 | 0.358±0.009 | 47 | 0.312±0.008 | 72 |
| 1/4 | 0.425±0.011 | 38 | 0.425±0.012 | 61 | |
| 1/8 | 0.532±0.015 | 22 | 0.625±0.024 | 42 | |
| 1/16 | 0.653±0.024 | 4 | 0.958±0.028 | 11 | |
| Control | 0.682±0.022 | 1.083±0.026 | |||
|
M. abscessus #73596 |
1/2 | 0.343±0.012 | 45 | 0.384±0.010 | 73 |
| 1/4 | 0.405±0.014 | 35 | 0.425±0.025 | 70 | |
| 1/8 | 0.442±0.024 | 29 | 0.742±0.042 | 48 | |
| 1/16 | 0.486±0.033 | 22 | 0.825±0.038 | 42 | |
| Control | 0.625±0.041 | 1.425±0.034 | |||
|
M. fortuitum #26647 |
1/2 | 0.405±0.025 | 46 | 0.372±0.032 | 77 |
| 1/4 | 0.532±0.022 | 29 | 0.427±0.041 | 73 | |
| 1/8 | 0.736±0.033 | 2 | 0.537±0.028 | 66 | |
| 1/16 | 0.743±0.028 | 1 | 0.693±0.052 | 56 | |
| Control | 0.752±0.052 | 1.592±0.043 | |||
Data are presented as the mean ± standard deviation of three independent experiments.
Efficacy of CAR on preformed 4 and 8 day-biofilms
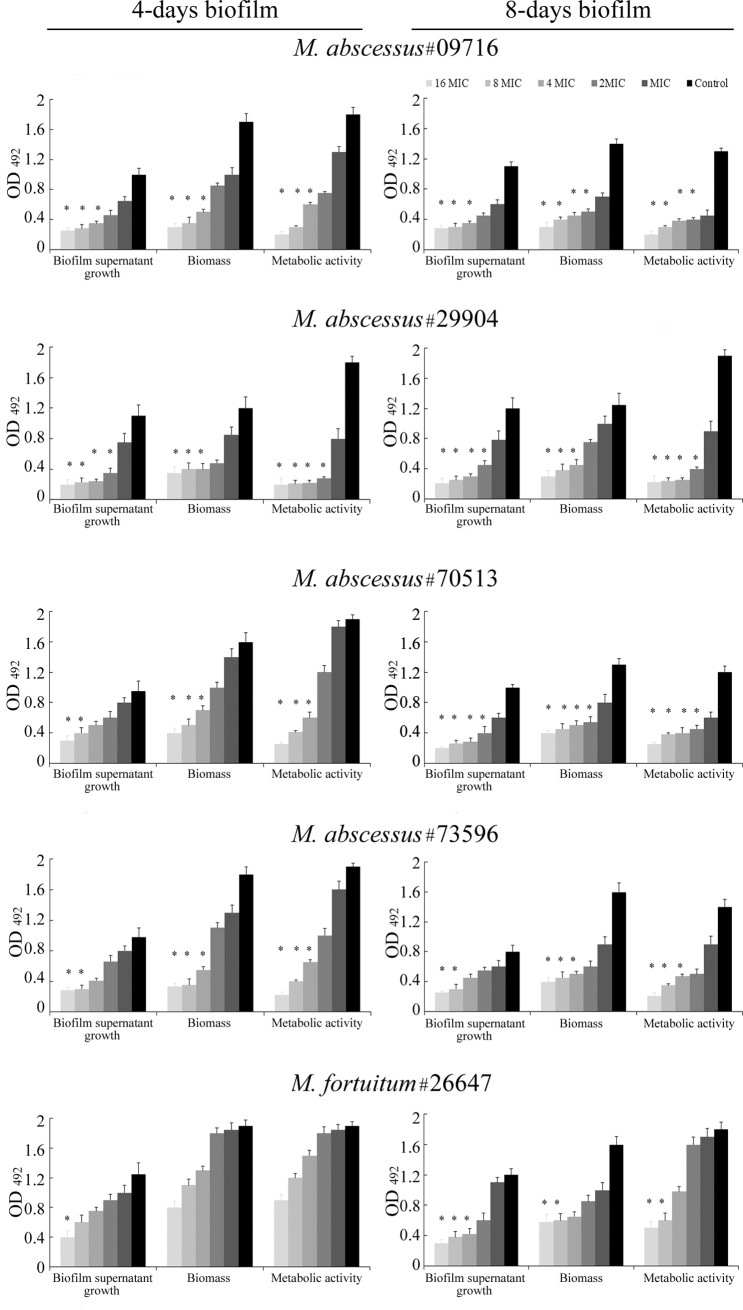
The effect of CAR on preformed biofilms at two different maturation stages (4- and 8-days old) was evaluated in terms of influence on biofilm supernatant growth, biofilm biomass and biofilm metabolic activity (Fig 1). CAR showed a significant high efficacy (60 ≤–< 90%) against preformed biofilm at concentrations slightly higher respect to those on the planktonic phase (from 2 × MIC to 8 × MIC). Furthermore, 4 d- and 8 d-preformed biofilm showed a similar susceptibility profile for M. abscessus #29904 and M. abscessus #73596 whereas showed a susceptibility related to the maturation stage for M. abscessus #09716 and M. abscessus #70513. On the contrary, for M. fortuitum a minor effect of CAR on both 4 and 8 days old biofilms was observed.
Fig 1. Activity of CAR against 4- and 8-days old rapidly growing mycobacteria biofilms.
Effects of different concentrations, ranging from MIC to 16 x MIC, on biofilm supernatant growth, biofilm biomass and biofilm metabolic activity. * indicate significant differences between the samples treated with CAR and the samples without CAR (ANOVA, p < 0.05).
Specifically for M. abscessus #29904, the effective percentage reductions of biofilm supernatant growth (68% - 62%, for 4 d- and 8 d-biofilm), biofilm biomass (67% - 64%, for 4 d- and 8 d-biofilm) and biofilm metabolic activity (84% - 79%, for 4 d- and 8 d-biofilm) were detected with 2 × MIC, 4 × MIC and 2 × MIC, respectively. Moreover, even using increasing CAR concentrations (from 2 x MIC to 16 x MIC), similar OD results for 4 d-biofilm were detected. This evidence is probably related to the higher MIC value of CAR (256 μg/&mL) respect to most RGM (64 μg/mL).
For M. abscessus #73596, the effective percentage reductions of biofilm supernatant growth (69% - 62%, for 4- and 8 d-biofilm), biofilm biomass (70% - 69%, for 4- and 8 d-biofilm) and biofilm metabolic activity (66%, for 4- and 8 d-biofilm) were detected with 8 × MIC, 4 × MIC and 4 × MIC, respectively.
Differently, a more susceptibility of 8 d- respect to 4 days old biofilm was detected for M. abscessus #09716 and M. abscessus #70513. Despite effective percentage reductions (65% - 68%, for 4 d- and 8 d-biofilm) of biofilm supernatant growth of M. abscessus #09716 were achieved with the same concentration of CAR (4 × MIC), the significant biofilm biomass and biofilm metabolic activity decrease (> 65%) was detected with 4 × MIC for 4 d-biofilm and 2 × MIC for 8 d-biofilm. Equally, significant reduction of biofilm supernatant growth (> 60%) of 4 d- and 8 d-biofilm M. abscessus #70513 was achieved with 4–8 × MIC and 2 × MIC, respectively.
Discussion
The search of novel anti-mycobacterial agents for the control of biofilm growth mode is crucial. At this regard, we have recently reported the antimicrobial activity of curcumin, a phenolic compound extracted from the Curcuma longa, against a lung isolate of M. abscessus [33]. CAR has been identified as a novel small molecule with activity against M. avium subsp. paratuberculosis and as a monoterpenic inhibitor of the chorismate mutase enzyme of M. tuberculosis [36, 37]. Moreover, CAR showed to be selective for M. tuberculosis, have efflux pumps activity and induce rough bacillary and agglomerates [38]. However, few data demonstrated the efficacy of CAR against RGM [26]. The present study demonstrated a good activity of CAR against resistant RGM isolates with MICs equal to 64 μg/mL for most strains and MBC values from 2 to 4 times higher. This suggests interesting applicative prospects as new anti-RGM agent. CAR could be considered a valuable support in the therapeutic treatment of RGM also for topical treatment of mucous, skin and wounds microbial infections [39]. In addition, CAR is a promising molecule with a potential in disinfection pratice e.g. in drinking water distribution systems. Furthermore, the activity of CAR vapours against RGM strains were first shown: CAR in vapour phase has shown higher antimicrobial activity (MICs equal to 16 μg/mL for most strains) than that revealed in liquid state. Some evidences proved that the effect of vapours is due to the combined action of deposition of CAR on bacteria together with adsorption through the agar medium [40,41]. Carvacrol is a volatile molecule and the effectiveness of its vapours without requiring direct contact could have additional applicative advantages. In this context, the administration of carvacrol by inhalation could implement the strategies in the treatment of RGM. With regard to the development of new preparations for inhalation therapy, Houdkova et al. [42] described the antibacterial potential of CAR in the liquid and vapour phase and its low cytotoxicity in lung fibroblast cells MRC-5. The easy penetration of vapours into inaccessible areas could be exploited for the treatment of extrapulmonary RGM diseases such as otomastoiditis or chronic otitis media [43,44]. Specifically, the potential role of CAR in the inhalation therapy or in the treatment of acute otitis media against Hemophilus infuenzae, Streptococcus pyogenes, Streptococcus pneumoniae, and Staphylococcus aureus pathogens [40,45] has been studied. Finally, a further use of CAR vapours by airing could concern the environmental remediation in order to reduce the persistence of RGM, e.g. in hospital sinks, showerheads and homes and therefore the risk of infection.
Rapidly growing mycobacteria have been studied for their ability to form biofilm [9]. CAR emerges as promising antimicrobial molecule with high potential for the control of microorganisms that are currently difficult to treat either as free- and sessile-growth lifestyle organisms [12,17–19,40,46,47]. In this context, the obtained results on the biofilm taking into account the limit of the study related to the use of only one species of M. fortuitum represent another important finding. Concentrations below the MIC (1/2–1/4 × MIC) impaired biofilm formation whereas concentration above the MIC (2–8 × MIC) caused significant disaggregation effect on the biofilm biomass and metabolic viability of cells embedded in a biofilm matrix at two maturation state (4-day and 8-day biofilms). According to Lemos et al. [35] classification, this action of CAR was classified as highly effective because the percentage of reduction ranged from 60% to 90% (60 ≤—< 90%). Regarding the influence of biofilm maturity on CAR activity, we observed that the 8-day biofilm of M. abscessus was more sensitive than the 4-day biofim. The effect could be due to a poorer 8-day biofilm in terms of biomass and metabolic activity. In contrast, the 4- and 8-day biofilm of M. fortuitum showed a similar susceptibility trend. About CAR toxicity, Cacciatore et al. [48] demonstrated that the toxic concentration of CAR (hemolytic activity on human red cells) was more than 6200 μg/mL. In the light of this, except for the highest value of 16 x MIC, corresponding to 8192 μg/mL when MIC value was equal to 512 μg/mL, the other concentrations were under 6200 μg/mL.
Conclusion
In conclusion, within the limits of the present study CAR could have the potential for implementation of strategies for treating Mycobacterium strains also in a sessile lifestyle and offers interesting applicative prospects related to its volatility such as diffusion and penetration into inaccessible areas.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Brown-Elliott BA, Philley JV. Rapidly growing mycobacteria. Microbiol Spectr. 2017; 5(1) 10.1128/microbiolspec.TNMI7-0027-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee MR, Sheng WH, Hung CC, Yu CJ, Lee LN, Hsueh PR. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015; 21(9):1638–1646. 10.3201/2109.141634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akram SM, Bhimji SS. Mycobacterium chelonae. Treasure Island (FL): StatPearls Publishing; 2018; October 27. [Google Scholar]
- 4.De Groote MA, Huitt G. Infections due to rapidly growing mycobacteria. Clin Infect Dis. 2006; 42(12):1756–1763. 10.1086/504381 [DOI] [PubMed] [Google Scholar]
- 5.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother. 2012; 67(4):810–818. 10.1093/jac/dkr578 [DOI] [PubMed] [Google Scholar]
- 6.van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 2012; 5(3):149–161. 10.1016/j.drup.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 7.Burgess W, Margolis A, Gibbs S, Duarte RS, Jackson M. Disinfectant susceptibility profiling of glutaraldehyde-resistant nontuberculous mycobacteria. Infect Control Hosp Epidemiol. 2017; 38(7):784–791. 10.1017/ice.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteban J, García-Coca M. Mycobacterium Biofilms. Front Microbiol. 2017; 8:2651 10.3389/fmicb.2017.02651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteban J, Martín-de-Hijas NZ, Kinnari TJ, Ayala G, Fernández-Roblas R, Gadea I. Biofilm development by potentially pathogenic non-pigmented rapidly growing mycobacteria. BMC Microbiol. 2008; 8:184 10.1186/1471-2180-8-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MM, Yakrusn MA, Arduino MJ, Cooksey RC, Crane CB, Banerjee SN, et al. Structural analysis of biofilm formation by rapidly and slowly growing nontuberculous mycobacteria. Appl Environ Microbiol. 2009; 75(7):2091–2098. 10.1128/AEM.00166-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Parliament and Council. (1996). Regulation (EC) No 2232/96 the European Parliament, and of the Council on 28 October 1996, Commission Decision of 23 February 1999 adopting a register of flavouring substances used in or on foodstuffs. Off J Eur Commun. L84:1999/217/EC 1–37.
- 12.Nostro A, Papalia T. Antimicrobial activity of carvacrol: current progress and future prospectives. Recent Pat Anti Infect Drug Discov. 2012; 7(1):28–35. [DOI] [PubMed] [Google Scholar]
- 13.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012; 3:12 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magi G, Marini E, Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A streptococci. Front Microbiol. 2015; 6:165 10.3389/fmicb.2015.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langeveld WT, Veldhuizen EJA, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014; 40(1):76–94. 10.3109/1040841X.2013.763219 [DOI] [PubMed] [Google Scholar]
- 16.Burt S. Essential oils: their antibacterial properties and potential applications in foods. Int J Food Microbiol. 2004; 94(3):223–253. 10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 17.Nostro A, Blanco AR, Cannatelli MA, Enea V, Flamini G, Morelli I, et al. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol Lett. 2004; 230(2):191–195. 10.1016/S0378-1097(03)00890-5 [DOI] [PubMed] [Google Scholar]
- 18.Marchese A, Arciola CR, Coppo E, Barbieri R, Barreca D, Chebaibi S, et al. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: mechanisms, synergies and bio-inspired anti-infective materials. Biofouling. 2018; 34(6):630–656. 10.1080/08927014.2018.1480756 [DOI] [PubMed] [Google Scholar]
- 19.Nostro A, Sudano Roccaro A, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilm. J Med Microbiol. 2007; 56(4):519–523. [DOI] [PubMed] [Google Scholar]
- 20.Nostro A, Marino A, Blanco AR, Cellini L, Di Giulio M, Pizzimenti F, et al. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J Med Microbiol. 2009; 58(6):791–797. 10.1099/jmm.0.009274-0 [DOI] [PubMed] [Google Scholar]
- 21.Espina L, Pagán R, López D, García-Gonzalo D. Individual Constituents from essential oils inhibit biofilm mass production by multi-drug resistant Staphylococcus aureus. Molecules. 2015; 20(6):11357–11372. 10.3390/molecules200611357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soni KA, Oladunjoye A, Nannapaneni R, Schilling MW, Silva JL, Mikel B, et al. Inhibition and inactivation of Salmonella typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J Food Protect. 2013; 76(2):205–212. 10.4315/0362-028X.JFP-12-196 [DOI] [PubMed] [Google Scholar]
- 23.Upadhyay A, Upadhyaya I, Kollanoor-Johny A, Venkitanarayanan K. Antibiofilm effect of plant derived antimicrobials on Listeria monocytogenes. Food Microbiol. 2013; 36(1):79–89. 10.1016/j.fm.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 24.Ciandrini E, Campana R, Federici S, Manti A, Battistelli M, Falcieri E, et al. In vitro activity of carvacrol against titanium-adherent oral biofilms and planktonic cultures. Clin Oral Investig. 2014; 18(8):2001–2013, 10.1007/s00784-013-1179-9 [DOI] [PubMed] [Google Scholar]
- 25.Doke SK, Raut JS, Dhawale S, Karuppayil SM. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J Gen Appl Microbiol. 2014; 60(5):163–168. [DOI] [PubMed] [Google Scholar]
- 26.Al-Ani I, Zimmermann S, Reichling J, Wink M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine. 2015; 22(2):245–255. 10.1016/j.phymed.2014.11.019 [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. Laboratory detection and identification of mycobacteria; proposed guideline M48. P. Forbes, BA, USA: CLSI; 2007. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes: approved standard. In M24–A2 (2nd ed). Wayne, PA, USA: CLSI; 2011. [PubMed] [Google Scholar]
- 29.Chihara S., Smith G., Petti CA. Carbapenem susceptibility patterns for clinical isolates of Mycobacterium abscessus determined by the Etest method. J Clin Microbiol. 2010; 48(2): 579–580. 10.1128/JCM.01930-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ananta P, Kham-Ngam I, Chetchotisakd P, Chaimanee P, Reechaipichitkul W, Namwat W, Lulitanond V, Faksri K. Analysis of drug-susceptibility patterns and gene sequences associated with clarithromycin and amikacin resistance in serial Mycobacterium abscessus isolates from clinical specimens from Northeast Thailand. PLoS One. 2018; 13(11):e0208053 10.1371/journal.pone.0208053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tullio V, Nostro A, Mandras, Dugo P, Banche G, Cannatelli MA, et al. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J Appl Microbiol. 2007; 102(6):1544–1550. 10.1111/j.1365-2672.2006.03191.x [DOI] [PubMed] [Google Scholar]
- 32.Mandras N, Nostro A, Roana J, Scalas D, Banche G, Ghisetti V, et al. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement Alter Med. 2016; 16(1):330 10.1186/s12906-016-1316-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marini E, Di Giulio M, Magi G, Di Lodovico S, Cimarelli ME, Brenciani A, et al. Curcumin, an antibiotic resistance breaker against a multiresistant clinical isolate of Mycobacterium abscessus. Phytother Res. 2018; 32(3):488–495. 10.1002/ptr.5994 [DOI] [PubMed] [Google Scholar]
- 34.Nostro A, Scaffaro R, D’Arrigo M, Botta L, Filocamo A, Marino A, et al. Development and characterization of essential oil component-based polymer films: a potential approach to reduce bacterial biofilm. Appl Microbiol Biotechnol. 2013; 97(21):9515–9523. 10.1007/s00253-013-5196-z [DOI] [PubMed] [Google Scholar]
- 35.Lemos M, Borges A, Teodósio J, Araújo P, Mergulhão F, Melo L, et al. The effects of ferulic and salicylic acids on Bacillus cereus and Pseudomonas fluorescens single and dual-species biofilms. Int Biodeter Biodegrad. 2014; 86(Part A):42–51. [Google Scholar]
- 36.Alokam R, Jeankumar VU, Sridevi JP, Matikonda SS, Peddi S, Alvala M, et al. Identification and structure–activity relationship study of carvacrol derivatives as Mycobacterium tuberculosis chorismate mutase inhibitors. J Enzyme Inhib Med Chem. 2014; 29(4):547–554. 10.3109/14756366.2013.823958 [DOI] [PubMed] [Google Scholar]
- 37.Wong SYY, Grant IR, Friedman M, Elliott CT, Situ C. Antibacterial activities of naturally occurring compounds against Mycobacterium avium subsp. paratuberculosis. Appl Environ Microbiol. 2008; 74(19):5986–5990. 10.1128/AEM.00981-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura de Vasconcelos SS, Caleffi-Ferracioli KR, Hegeto LA, Baldin VP, Nakamura CV, Stefanello TF, et al. Carvacrol activity & morphological changes in Mycobacterium tuberculosis. Fut Microbiol. 2018; 13:877–888. 10.2217/fmb-2017-0232 [DOI] [PubMed] [Google Scholar]
- 39.Scaffaro R, Lopresti F, D’Arrigo M, Marino A, Nostro A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Appl Microbiol Biotechnol. 2018; 102:4171–4181. 10.1007/s00253-018-8879-7 [DOI] [PubMed] [Google Scholar]
- 40.Inouye S, Takizawa T, Yamaguchi H. Antibacterial activity of essential oils and their major constituents against respiratory tract pathogens by gaseous contact. J Antimicrob Chemother. 2001; 47(5):565–573. 10.1093/jac/47.5.565 [DOI] [PubMed] [Google Scholar]
- 41.Inouye S, Tsuruoka T, Watanabe M, Takeo K, Akao M, Nishiyama Y, et al. Inhibitory effect of essential oils on apical growth of Aspergillus fumigatus by vapour contact. Mycoses. 2000; 43(1–2):17–23. [DOI] [PubMed] [Google Scholar]
- 42.Houdkova M, Rondevaldova J, Doskocil I, Kokoska L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia. 2017;118:56–62. 10.1016/j.fitote.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 43.Sugimoto H, Ito M, Hatano M, Nakanishi Y, Maruyama Y, Yoshizaki T. A case of chronic otitis media caused by Mycobacterium abscessus. Auris Nasus Larynx. 2010; 37(5):636–639. 10.1016/j.anl.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 44.Tang IP, Singh S, Rajagopalan R. Bilateral nontuberculous mycobacterial middle ear infection: a rare case. Ear Nose Throat J. 2014; 93(9):390–394. [PubMed] [Google Scholar]
- 45.Kristinsson KG, Magnusdottir AB, Peterson H, Hermansson A. Effective treatment of experimental acute otitis media by application of volatile fluids into the ear canal. J Infect Dis. 2005; 191(11):1876–1880. 10.1086/430003 [DOI] [PubMed] [Google Scholar]
- 46.Nostro A, Marino A, Blanco AR, Cellini L, Di Giulio M, Pizzimenti F, et al. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J Med Microbiol. 2009; 58(6):791–797. 10.1099/jmm.0.009274-0 [DOI] [PubMed] [Google Scholar]
- 47.Gaio V, Lima CA, Oliveira F, França Â, Cerca N. Carvacrol is highly disruptive against coagulase-negative staphylococci in in vitro biofilms. Fut Microbiol. 2017; 12(16):1487–1496. 10.2217/fmb-2017-0122 [DOI] [PubMed] [Google Scholar]
- 48.Cacciatore I, Di Giulio M, Fornasari E, Di Stefano A, Cerasa LS, Marinelli L, et al. Carvacrol codrugs: a new approach in the antimicrobial plan. PLoS One. 2015; 10(4):e0120937 10.1371/journal.pone.0120937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.