Abstract
Context
Poor quadriceps force control has been observed after anterior cruciate ligament (ACL) reconstruction but has not been examined after ACL injury. Whether adaptations within the central nervous system are contributing to these impairments is unknown.
Objective
To examine quadriceps force control in individuals who had sustained a recent ACL injury and determine the associations between cortical excitability and quadriceps force control in these individuals.
Design
Cross-sectional study.
Setting
Research laboratory.
Patients or Other Participants
Eighteen individuals with a recent unilateral ACL injury (6 women, 12 men; age = 29.6 ± 8.4 years, height = 1.74 ± 0.07 m, mass = 76.0 ± 10.4 kg, time postinjury = 69.5 ± 42.5 days) and 18 uninjured individuals (6 women, 12 men; age = 29.2 ± 6.8 years, height = 1.79 ± 0.07 m, mass = 79.0 ± 8.4 kg) serving as controls participated.
Main Outcome Measure(s)
Quadriceps force control was quantified as the root mean square error between the quadriceps force and target force during a cyclical force-matching task. Cortical excitability was measured as the active motor threshold and cortical silent period. Outcome measures were determined bilaterally in a single testing session. Group and limb differences in quadriceps force control were assessed using mixed analyses of variance (2 × 2). Pearson product moment correlations were performed between quadriceps force control and cortical excitability in individuals with an ACL injury.
Results
Individuals with an ACL injury exhibited greater total force-matching error with their involved (standardized mean difference [SMD] = 0.8) and uninvolved (SMD = 0.9) limbs than did controls (F1,27 = 11.347, P = .03). During the period of descending force, individuals with an ACL injury demonstrated greater error using their involved (SMD = 0.8) and uninvolved (SMD = 0.8) limbs than uninjured individuals (F1,27 = 4.941, P = .04). Greater force-matching error was not associated with any cortical excitability measures (P > .05).
Conclusions
Quadriceps force control was impaired bilaterally after recent ACL injury but was not associated with selected measures of cortical excitability. The findings highlight a need to incorporate submaximal-force control tasks into rehabilitation and “prehabilitation,” as the deficits were present before surgery.
Keywords: knee injury, motor control, transcranial magnetic stimulation, cortical excitability
Key Points
Individuals with a recent anterior cruciate ligament (ACL) injury exhibited deficits in submaximal quadriceps force control.
Quadriceps force control was compromised in both the involved and uninvolved limbs of individuals after ACL injury compared with the limbs of uninjured individuals.
Quadriceps cortical excitability measured from the rectus femoris was not associated with quadriceps force control.
Further investigation is needed to fully understand the mechanistic underpinnings and functional relevance of force-control deficits after ACL injury.
Quadriceps weakness after anterior cruciate ligament (ACL) injury and ACL reconstruction (ACLR) has important clinical implications, given the associations with patient-reported disability,1 functional deficits,2 and knee osteoarthritis.3,4 For these reasons, numerous authors have assessed quadriceps strength after ACL injury and ACLR.5 However, people do not routinely perform tasks requiring maximal quadriceps effort during activities of daily living (ADLs).6,7 The ability to control submaximal quadriceps forces may be an important determinant of knee function for individuals after ACLR8 and potentially after ACL injury.
Muscle force control is a term used to describe the steadiness or accuracy of the voluntary force produced by skeletal muscles.9–11 The accuracy of quadriceps force production is typically quantified by examining the average force deviation (ie, error) from a submaximal isometric target force.10,11 Deficits in quadriceps force control are apparent in individuals with ACLR compared with uninjured individuals,12 and these deficits are associated with worse knee-joint function in this population.8 Quadriceps force control after ACL injury has been assessed using a constant submaximal isometric target force.13 However, during most functional activities, the quadriceps muscles are required to produce continually fluctuating force levels.12 Therefore, using an isometric testing protocol that involves a fluctuating target force may better approximate quadriceps action during functional activities (eg, walking and running).
Quadriceps dysfunction after ACL injury and ACLR may be caused, in part, by altered mechanoreceptor-mediated feedback from the injured joint to the central nervous system (CNS).14 Mechanistically, diminished quadriceps force control after ACL rupture and ACLR may be attributed to alterations in the primary motor cortex.14–18 Transcranial magnetic stimulation (TMS) can be used to noninvasively examine modulation of intracortical and corticomotor excitability after injury.19 Single-pulse TMS is commonly used to assess corticomotor excitability, which is a measure of the membrane threshold needed to generate and propagate an action potential.19 An increase in the motor threshold suggests reduced excitability and potential difficulty in generating a motor response.19 Authors of TMS studies20,21 have provided mixed evidence on whether primary motor-cortex excitability regarding the motor threshold is altered after ACL injury. In a concurrent study, Ward et al21 demonstrated that rectus femoris active motor threshold (AMT) and measures of intracortical inhibition were not different between individuals with and those without ACL injury. However, the duration of the cortical silent period (cSP), a gross measure of cortical inhibition, was reduced in the involved limb after ACL injury, suggesting a dysfunction in inhibitory rather than excitatory systems; the meaningfulness of a shortened cSP has not been established.21
Assessing quadriceps-muscle force control in people with an ACL injury may highlight important neuromuscular deficits, which may be associated with changes in corticomotor excitability. Therefore, the primary purpose of our study was to examine submaximal quadriceps force control and quadriceps corticomotor excitability in individuals who had recently sustained an ACL injury. We hypothesized that individuals with a recent ACL injury (ie, <8 months before the study) would demonstrate poorer submaximal quadriceps force control than uninjured controls. Our secondary purpose was to examine associations between quadriceps force control and measures of rectus femoris cortical excitability.
METHODS
Participants
Eighteen recreationally active adults (age range = 18–50 years) who had sustained a unilateral ACL injury that was confirmed on magnetic resonance imaging (MRI) within the 8 months before the study were recruited from 2 local orthopaedic surgery clinics using consecutive sampling. Eighteen healthy, recreationally active adults were recruited from local sporting clubs to serve as a control group. Participants were considered recreationally active if they self-reported being active for at least 30 minutes, 2 or more days each week. Participant characteristics are presented in Table 1. Participants with an ACL injury were, on average, 69.5 ± 42.5 days (range = 11–138 days) postinjury at the time of testing. Thirteen individuals (72.2%) had injured their dominant limb and 5 (27.8%) had injured their nondominant limb. Limb dominance was determined as the preferred foot for kicking a ball.22 Volunteers with multi-ligament or meniscal trauma, or both; grade III or IV chondral defects; or previous ACL injury or surgery, or both, of either limb were excluded. Volunteers were excluded from the control group if they had a history of (1) a substantial orthopaedic injury requiring surgery or (2) a lower limb musculoskeletal injury in the year before the study that caused them to seek treatment or affected their function for more than 1 week. Based on the work of Perraton et al,8 who examined quadriceps force control, we determined that a minimum of 12 participants would be needed to find a difference (β = 80%, α = .05) and strong standardized mean differences (SMDs; SMD = 1.673) in root mean square error (RMSE) between limbs and groups. However, we recruited 18 participants to increase the statistical power for the correlation analysis.
Table 1.
Group Demographics
| Variable |
Group, Mean ± SD |
P Value |
|
| Anterior Cruciate Ligament Injury |
Control |
||
| Age, y | 29.6 ± 8.4 | 29.2 ± 6.8 | .87 |
| Height, m | 1.74 ± 0.07 | 1.79 ± 0.07 | .08 |
| Mass, kg | 76.0 ± 10.4 | 79.0 ± 8.4 | .36 |
| Body mass index, kg·m2 | 24.8 ± 2.3 | 24.6 ± 2.3 | .76 |
| Knee Osteoarthritis Outcome Score | |||
| Pain | 73 ± 19a | 99 ± 3 | <.001 |
| Activities of daily living | 78 ± 17a | 100 ± 1 | <.001 |
| Symptoms | 68 ± 19a | 99 ± 2 | <.001 |
| Quality of life | 38 ± 22a | 100 ± 0 | <.001 |
| Visual analog scale, mm | 1.4 ± 1.7a | 0 ± 0 | .005 |
| Dominant limb, No. | |||
| Right | 17 | 17 | NA |
| Left | 1 | 1 | |
| Sex, No. | |||
| Male | 12 | 12 | NA |
| Female | 6 | 6 | |
Abbreviation: NA, not applicable.
Different from control group (P ≤ .05).
Participants completed a 15-item questionnaire to assess contraindications to TMS.23 No participant in either group had neurologic or other medical problems that would contraindicate noninvasive brain stimulation. We instructed participants to abstain from caffeine intake in the 12 hours before testing and, at the start of the testing session, asked them whether they had consumed caffeine (eg, tea, coffee, other beverages) in the previous 12 hours. The Knee Injury and Osteoarthritis Outcome Score (KOOS) was used to evaluate self-reported knee function. Individuals in the ACL-injury group completed the KOOS questionnaire with reference to their injured knee, whereas individuals in the control group considered their knees in general. A visual analog scale (VAS) was used to measure current knee-pain levels during the testing procedures.24 All outcome measures were collected during a single data-collection session. Participants provided written informed consent, and the University of Melbourne Human Ethics Sub-Committee approved the study (ID: 1340551.2).
Quadriceps Force-Matching Task
Participants performed a submaximal isometric open kinetic chain quadriceps force-matching task at a standardized angle of 60° of knee flexion and 90° of hip flexion while seated in a chair. The intensity of the isometric contraction varied constantly between 5% and 25% of the participant's body weight (BW) in a sinusoidal pattern at a frequency of 0.10 Hz. Therefore, participants experienced periods of ascending and descending target force. We used BW to determine force targets rather than percentage of maximal voluntary isometric contraction (MVIC) because we could not accurately assess MVIC in individuals with an ACL injury given the likely presence of joint effusion, arthrogenic muscle inhibition, or both.25
Quadriceps contraction intensity was measured using a force transducer (model 60001 S-Beam; Sensortronics, Covina, CA) attached to a chair via an adjustable arm and to the distal shin via a soft cuff to ensure that the participants' hip joints remained at 90° and knee joints remained at 60° of flexion (Figure 1). This position has been demonstrated to minimize tibiofemoral shear forces26 while maintaining an optimal quadriceps length-tension relationship for force production.6 Four 60-second trials were performed, with a 60-second rest between trials. Participants were instructed to match their quadriceps force with that of the target for the duration of the test. Using a custom LabView (National Instruments, Austin, TX) program, we set the target force for each participant and displayed it on a computer screen directly in front of the participant at a fixed distance of 40 cm (Figure 2). No oral feedback regarding the performance was provided during the trials; however, at the end of the test, visual feedback of the performance was provided using a graph that displayed the participant's force throughout the trial superimposed over the target force.8,12
Figure 1.
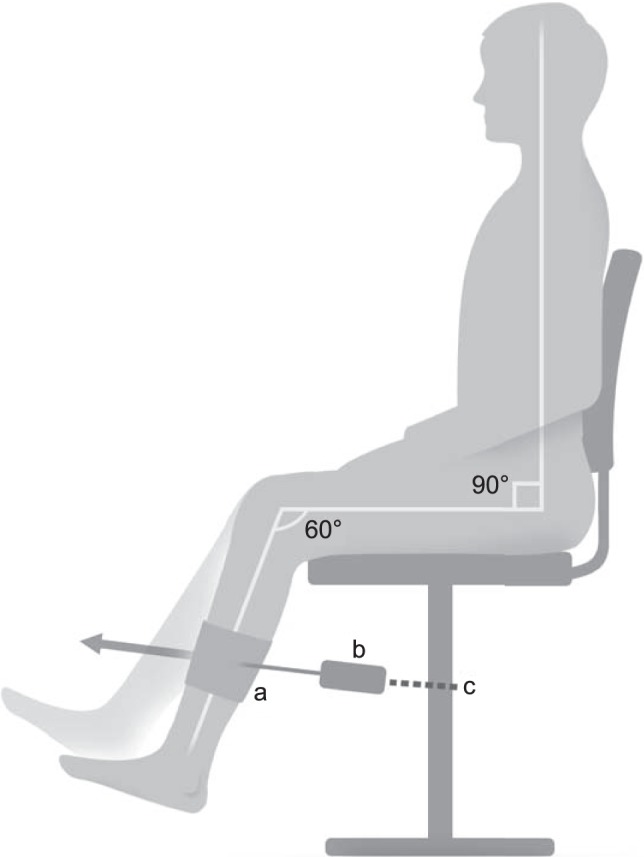
Schematic of experimental setup for the participants. a Hook-and-loop cuff. b Load cell. c Chain.
Figure 2.
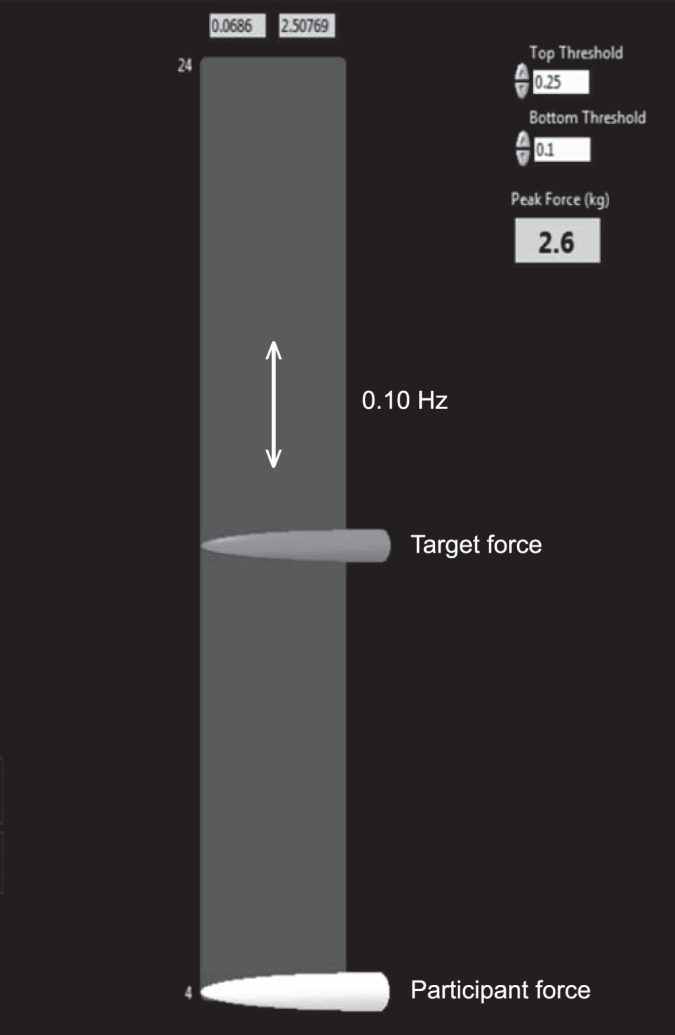
Force-matching task and participant interface. The target force arrow oscillated up and down the screen at a preset frequency. Participants attempted to match the target using their quadriceps force.
Accuracy of quadriceps force control was assessed using the RMSE of quadriceps torque relative to the target torque12 and expressed as a percentage of BW. The first and last repetitions of each 60-second trial were removed from the analysis, and the remaining repetitions were averaged for each trial. In addition to determining total RMSE (RMSEtotal), we performed a subregional analysis to assess the accuracy of the quadriceps force control over the increasing force ramp (ascending [RMSEascending]) and the decreasing force ramp (descending [RMSEdescending]) components of the task. The intersession reliability for this task in our laboratory has been reported previously (intraclass correlation coefficient = 0.91).12
Quadriceps Corticomotor Excitability and Inhibition
A Bi-Stim2 magnetic stimulator (Magstim Inc, Eden Prairie, MN) producing a monophasic pulse shape with a figure-eight 70-mm coil (Magstim Inc) held tangential to the skull was used to examine rectus femoris corticomotor excitability. A custom-designed, form-fitting cap (EasyCap GmbH, Herrsching, Germany) with stimulus sites marked at 1-cm spacing in latitude and longitude was fitted to each participant's head.27–30 The interaural and nasion-inion distances were measured for each participant, and the vertex was aligned with the center of the cap coordinates.31 The belly of the rectus femoris was identified through palpation during manually resisted knee extension. We prepared the skin site by shaving, debriding, and cleaning it with alcohol. A Trigno wireless electromyography (EMG) electrode (Delsys Inc, Natick, MA) was affixed to the skin over the muscle belly halfway between the anterior-superior iliac spine and patella in the direction of muscle-fiber orientation.32 We sampled EMG signals at 2000 Hz for 500 milliseconds and set the EMG amplification at a gain of 1000 (PowerLab 4/35; ADInstruments, Colorado Springs, CO) with a 10-Hz, high-pass filter. The common mode rejection ratio of the EMG amplifier was 100 dB with an input impedance of 1 MΩ. Sites near the estimated center of the rectus femoris area (1–3 cm lateral to the vertex) were explored at 50% maximal stimulator output to determine the site at which the largest motor-evoked potential (MEP) could be obtained for each limb individually.19 This was defined as the optimal stimulation site where the AMT was established for the rectus femoris muscle of each limb. At the optimal site, the stimulator intensity was adjusted until the AMT was identified. We defined the AMT as the minimal stimulus intensity required to elicit an MEP greater than 200 μV while the participant maintained a quadriceps contraction at 10% BW.21,29,33 The specific methods for determining the cSP duration were reported by Ward et al.21 The cSP duration was defined as the onset of the MEP to the return of normal, uninterrupted EMG activity.21,34
Statistical Analysis
We determined the percentage of dominant limbs that were injured in the ACL-injury group and randomly assigned which of the control participants would contribute a dominant limb. Data were assessed for normality using Shapiro-Wilk tests. Independent-samples t tests were used to compare participant demographics. A separate mixed 2 × 2 analysis of variance with repeated measures for limb was used to assess differences in quadriceps RMSE between test limbs and participant groups. We used SMDs to examine the magnitude of any group differences. The SMDs were classified as small (<0.2), moderate (0.2–0.5), or large (>0.5). Separate Pearson product moment correlations (r) were used to examine associations between quadriceps RMSE and cortical excitability in the ACL-injury group. Associations were classified as weak (0.00–0.39), moderate (0.40–0.69), or strong (0.70–1.00).35 Statistical analyses were performed using SPSS (version 21; IBM Corp, Armonk, NY), and the α level was set a priori at .05.
RESULTS
The ACL-injury group self-reported worse knee function (KOOS-Pain, KOOS-ADLs, KOOS-Symptoms, and KOOS-Quality of Life) and greater pain (VAS) than the control group (Table 1).
Submaximal Quadriceps Force Control
The means and standard deviations for RMSEtotal, RMSEascending, and RMSEdescending for the ACL-injury and control groups are provided in Table 2. We observed no limb-by-group interaction for RMSEtotal (F1,27 = 0.071, P = .79) but found a main effect for participant group (F1,27 = 11.347, P = .03). The ACL-injury group had greater RMSEtotal in both the involved (29% difference; SMD = 0.8) and uninvolved (27% difference; SMD = 0.9) limbs than the control group. We observed no main effect of test limb for RMSEtotal (F1,27 = 0.033, P = .86).
Table 2.
Quadriceps Root Mean Square Error for Involved and Uninvolved Limbs (Mean ± SD)
| Root Mean Square Error, % Body Weight |
Group |
|||
| Anterior Cruciate Ligament Injury |
Control |
|||
| Involved Limb |
Uninvolved Limb |
Matched Involved Limb |
Matched Uninvolved Limb |
|
| Total | 3.36 ± 1.81a | 3.28 ± 1.15a | 2.40 ± 0.61 | 2.40 ± 0.58 |
| Ascending | 2.80 ± 1.06 | 3.17 ± 0.97 | 2.42 ± 0.73 | 2.49 ± 0.70 |
| Descending | 3.92 ± 2.89a | 3.39 ± 1.69a | 2.43 ± 0.61 | 2.36 ± 0.52 |
Different from matched control limb (P ≤ .05).
Similarly, we noted no limb-by-group interaction for RMSEdescending (F1,27 = 0.951, P = .34). We demonstrated a main effect for participant group (F 1,27 = 4.941, P = .04), and the ACL-injury group had poorer submaximal force control, as evidenced by higher RMSEdescending, in both the involved (38% difference; SMD = 0.8) and uninvolved (30% difference; SMD = 0.8) limbs than in the control group. No main effect of test limb was present (F1,27 = 1.498, P = .22).
We observed no limb-by-group interaction (F1,27 = 3.759, P = .06), group main effect (F1,27 = 0.727, P = .40), or limb main effect (F1,27 = 1.496, P = .23) for RMSEascending.
Associations Between Quadriceps Force Control and Corticomotor Excitability
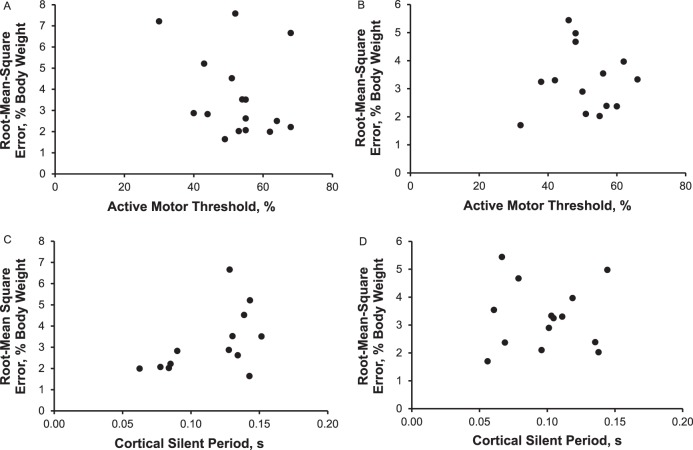
In the ACL-injury group, we found no associations between RMSE and AMT for the involved limb (r = −0.289, P = .28) or uninvolved limb (r = −0.139, P = .64) or between RMSE and cSP for the involved limb (r = 0.509, P = .08) or uninvolved limb (r = −0.192, P = .51; Figure 3).
Figure 3.
Scatterplots of the association between measures of quadriceps force control and cortical excitability in the involved and uninvolved limbs of individuals with anterior cruciate ligament injury. A, Involved-limb active motor threshold. B, Uninvolved-limb active motor threshold. C, Involved-limb cortical silent period. D, Uninvolved-limb cortical silent period.
DISCUSSION
The primary aim of this study was to examine the accuracy of submaximal quadriceps force production in individuals who had recently sustained an ACL injury. In accordance with our hypothesis, quadriceps force control was compromised in both the involved and uninvolved limbs after ACL injury compared with that of healthy, uninjured individuals. An exploratory aim of this study was to examine associations between quadriceps force control and measures of rectus femoris cortical excitability in order to evaluate the mechanisms of quadriceps force-control deficits. We found no associations between measures of quadriceps cortical excitability measured from the rectus femoris and quadriceps force control.
Quadriceps strength-based measures are commonly used to indicate physical function and determine readiness for return to sport after ACL injury and ACLR.5,36 Researchers have used maximal isokinetic tasks to provide insight into force fluctuations during maximal-effort muscle contraction after ACLR37–39 and have demonstrated diminished steadiness of both the quadriceps38 and hamstrings.37 More recently, quadriceps and hamstrings maximal isokinetic steadiness has been examined in individuals after ACL injury, with diminished steadiness evident in both the quadriceps and hamstrings muscle groups.40 Submaximal strength and force control have not been widely examined after ACL injury, but considering that most ADLs are performed at submaximal intensities, these measures may be more appropriate determinants of physical function after ACL injury and ACLR. The findings of our study add to this body of research and demonstrated that individuals with a recent ACL injury had ongoing impairment in their ability to produce an accurate submaximal quadriceps contraction. After ACL injury, individuals had bilateral impairments in quadriceps submaximal force control, as evidenced by greater force-matching error with both limbs than in a control group. The deficits in force control were largest during the RMSEdescending as the contraction intensity was decreasing. These control deficits could be contributing to the differences in the RMSEtotal. It appears that matching an isometric force that was decreasing in intensity was more challenging for the ACL-injury group than the control group. Conversely, the performance of the ACL-injury group during the RMSEascending component of the task using the involved limb was similar to that of the control group. However, the ACL-injury group also performed worse than the control group in the RMSEdescending component of the task using the uninvolved limb. Exploring the reasons for this unexpected finding was beyond the scope of our study, but the finding could be related to cross-adaptation or neuromuscular deficits that existed before ACL injury. Bilateral alterations in quadriceps strength28 and voluntary activation41 have been found after ACL injury. It is possible that a cross-education effect occurs after ACL injury, resulting in neuromuscular alterations, including submaximal force-control deficits, in both the involved and uninvolved limbs.42 The deficits in force control may have implications for knee-joint loading and the development of early-onset knee osteoarthritis after ACL injury. Specifically, an inability to accurately control forces through the knee joint during everyday activities, particularly during activities requiring control of changing intensities of force, could alter joint-contact forces and load areas of the cartilage that are not adapted to regular loading.43 However, this is an area for future research.
Although no comparable studies have included patients with ACL deficiency, our protocol has been used in an ACLR cohort.8,12 In these previous studies, individuals who were, on average, 18 months post-ACLR demonstrated 23% to 48% greater quadriceps force-matching error (RMSE) using their involved limb than did the uninjured control participants.8,12 Furthermore, submaximal force control was related to patient-reported function and functional performance (3 hop tests) in individuals with ACLR; specifically, diminished quadriceps force control was associated with worse knee function.8 The results from participants with ACL injuries demonstrated that these impairments in force control are present postinjury and are more likely to be due to the initial injury than to a longer postinjury or postsurgery time. Longitudinal data are needed to better understand the timescale of such changes.
Quadriceps dysfunction has been well documented after ACL injury44 and ACLR,45–49 and researchers14,18,49 have hypothesized that altered quadriceps corticomotor excitability underlies quadriceps dysfunction after ACL injury and ACLR. Altered quadriceps motor threshold has been demonstrated after ACL injury,20 and authors of a concurrent study21 found a difference in the rectus femoris cSP of individuals with ACL injury.
A secondary aim of our study was to explore associations between quadriceps force control and cortical excitability and the cSP duration in particular; however, no associations were found among any measures of rectus femoris cortical excitability and quadriceps force control. The rectus femoris has an important role in force attenuation and locomotion, but as the biarticular quadriceps muscle, its contribution to knee extension depends on hip and knee position.50–53 Although the rectus femoris demonstrated high EMG activity during pilot testing of the task, the testing position used in our study placed the rectus femoris in a shortened position, which influenced the force-generating capacity of the muscle.50,51 Investigators50,51,54–57 have suggested that, in the shortened position, the contribution of the rectus femoris to knee-extension force is reduced compared with the vasti. The results of the exploratory correlations would support the notion that the rectus femoris is not a major contributor relative to the other quadriceps muscles in this hip and knee position. It is possible that rectus femoris excitability is not contributing to the force-control task when assessed in a seated position, where the contribution of the rectus femoris may be less. In future studies, researchers may want to investigate corticomotor excitability of the vasti muscles to further explore the relationship between corticomotor excitability and quadriceps force control. Although not assessed in our study, one mechanism that may also contribute to quadriceps dysfunction is altered spinal reflex inhibition.44,45,49 Acute-injury models have demonstrated alterations in quadriceps spinal reflex excitability58,59 but not quadriceps corticomotor excitability60 after experimental joint effusion. The ACL injury and associated effusion are thought to alter the ascending signal from the knee joint to the CNS, resulting in inhibitory descending signals to the quadriceps α motor-neuron pool and, thus, a reduced ability to voluntarily activate the muscle.61
Existing TMS paradigms in sports medicine research have generally assessed only the primary motor cortex. Data from studies using functional MRI (fMRI) would suggest that CNS adaptations to ACL injury62 and ACLR63 are not limited to the primary motor cortex. An apparent shift occurs in how the brain generates the motor drive after injury, most likely resulting from changes in sensorimotor input and integration in the cortical regions.17,62,63 The authors of fMRI studies62,63 demonstrated increased activation in motor planning, visual processing, and sensory regions during a simple knee-extension task. Typical peak or maximal force tasks, such as the MVIC, may be less dependent on visual-motor integration and more related to primary motor-cortex excitability. The force-matching task that we used requires a combination of visual and sensory feedback from the knee joint to drive motor output matched to the target force. Given the visual nature of the force-matching task, it is possible that the sensory or visual integration region, rather than primary motor-cortex excitability, is the limiting region. Using advanced paired-pulse TMS techniques to target nonprimary motor-cortex regions or fMRI-compatible force-matching tasks would enable a more in-depth examination of the relationship between quadriceps cortical excitability and force control, providing further insight into the mechanisms that underlie neuromuscular adaptations after ACL injury.6,18
In our study, individuals with an ACL injury reported pain during functional activities (KOOS-Pain subscale) but had minimal pain during force-control testing as assessed on a VAS. Quadriceps strength64 and submaximal force control10 were diminished with experimentally induced pain, indicating that pain may be an independent factor in quadriceps dysfunction after ACL injury.64 Given that pain is an important confounder during strength and force-control tasks, the low levels of pain during testing in our study are important. However, the isometric nature of matching muscle and target forces with visual feedback may not be generalizable to normal functional movements that occur during ADLs or sport activity. Although not representative of a true eccentric muscle contraction, the changing target torque resulted in small increases and decreases in muscle and tendon length that may be more representative of functional movement than a static isometric task.65
Because of the cross-sectional nature of this study, we could not draw causal conclusions regarding altered quadriceps force control in individuals with an ACL injury, as we did not know whether force-control deficits existed before the injury. The force-matching task was novel for all participants in our study and the study of Perraton et al,8 and although we attempted to provide a standardized but limited familiarization process to avoid a large practice effect, it is possible that the differences in RMSE reported in these studies were related to neuromuscular training that patients with ACLR undertook during rehabilitation. Alternatively, given the limitations of our sampling strategy, it is possible that these different groups of individuals were inherently better or worse at performing a novel task. Our findings highlight a potential need to incorporate submaximal force-control–type tasks into rehabilitation protocols and prehabilitation, as deficits were present before surgery in these individuals. However, a cause-and-effect relationship between a force-control intervention and improved quadriceps force control has not been established.
CONCLUSIONS
Individuals who had sustained an ACL injury within the 8 months before the study exhibited bilateral deficits in submaximal quadriceps force control. We found no relationship between diminished force control and rectus femoris cortical excitability measures. Further investigation is needed to fully understand the mechanistic underpinnings and functional relevance of force-control deficits after ACL injury.
ACKNOWLEDGMENTS
This study was supported by an RD Wright Biomedical Fellowship 1053521 (Dr Bryant) and an NHMRC Principal Research Fellowship 1058440 (Dr Bennell) from the National Health and Medical Research Council.
We thank Dr Ross Clark for developing the custom LabVIEW programs used in this study and Mr Vinura Jayaneththi for creating the schematic images.
REFERENCES
- 1.Pietrosimone B, Lepley AS, Harkey MS, et al. Quadriceps strength predicts self-reported function post-ACL reconstruction. Med Sci Sports Exerc. 2016;48(9):1671–1677. doi: 10.1249/MSS.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42(9):750–759. doi: 10.2519/jospt.2012.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Øiestad BE, Juhl CB, Eitzen I, Thorlund JB. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23(2):171–177. doi: 10.1016/j.joca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Segal NA, Glass NA, Felson DT, et al. Effect of quadriceps strength and proprioception on risk for knee osteoarthritis. Med Sci Sports Exerc. 2010;42(11):2081–2088. doi: 10.1249/MSS.0b013e3181dd902e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction: a systematic review. Orthop J Sports Med. 2014;2(1):2325967113518305. doi: 10.1177/2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011;29(5):633–640. doi: 10.1002/jor.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng. 2010;12:401–433. doi: 10.1146/annurev-bioeng-070909-105259. [DOI] [PubMed] [Google Scholar]
- 8.Perraton L, Clark R, Crossley K, et al. Impaired voluntary quadriceps force control following anterior cruciate ligament reconstruction: relationship with knee function. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1424–1431. doi: 10.1007/s00167-015-3937-5. [DOI] [PubMed] [Google Scholar]
- 9.Hortobágyi T, Garry J, Holbert D, Devita P. Aberrations in the control of quadriceps muscle force in patients with knee osteoarthritis. Arthritis Rheum. 2004;51(4):562–569. doi: 10.1002/art.20545. [DOI] [PubMed] [Google Scholar]
- 10.Rice DA, McNair PJ, Lewis GN, Mannion J. Experimental knee pain impairs submaximal force steadiness in isometric, eccentric, and concentric muscle actions. Arthritis Res Ther. 2015;17:259. doi: 10.1186/s13075-015-0768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol (1985) 2002;92(3):1004–1012. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- 12.Telianidis S, Perraton L, Clark RA, Pua YH, Fortin K, Bryant AL. Diminished sub-maximal quadriceps force control in anterior cruciate ligament reconstructed patients is related to quadriceps and hamstring muscle dyskinesia. J Electromyogr Kinesiol. 2014;24(4):513–519. doi: 10.1016/j.jelekin.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Williams GN, Barrance PJ, Snyder-Mackler L, Axe MJ, Buchanan TS. Specificity of muscle action after anterior cruciate ligament injury. J Orthop Res. 2003;21(6):1131–1137. doi: 10.1016/S0736-0266(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 14.Kapreli E, Athanasopoulos S. The anterior cruciate ligament deficiency as a model of brain plasticity. Med Hypotheses. 2006;67(3):645–650. doi: 10.1016/j.mehy.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 15.Baumeister J, Reinecke K, Schubert M, Weiss M. Altered electrocortical brain activity after ACL reconstruction during force control. J Orthop Res. 2011;29(9):1383–1389. doi: 10.1002/jor.21380. [DOI] [PubMed] [Google Scholar]
- 16.Baumeister J, Reinecke K, Weiss M. Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: an EEG study. Scand J Med Sci Sports. 2008;18(4):473–484. doi: 10.1111/j.1600-0838.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 17.Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47(7):1271–1288. doi: 10.1007/s40279-016-0666-y. [DOI] [PubMed] [Google Scholar]
- 18.Ward S, Pearce AJ, Pietrosimone B, Bennell K, Clark R, Bryant AL. Neuromuscular deficits after peripheral joint injury: a neurophysiological hypothesis. Muscle Nerve. 2015;51(3):327–332. doi: 10.1002/mus.24463. [DOI] [PubMed] [Google Scholar]
- 19.Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123(5):858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Héroux ME, Tremblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):823–833. doi: 10.1007/s00167-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 21.Ward SH, Pearce A, Bennell KL, Pietrosimone B, Bryant AL. Quadriceps cortical adaptations in individuals with an anterior cruciate ligament injury. Knee. 2016;23(4):582–587. doi: 10.1016/j.knee.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Pietrosimone BG, Park CM, Gribble PA, Pfile KR, Tevald MA. Inter-limb differences in quadriceps strength and volitional activation. J Sports Sci. 2012;30(5):471–477. doi: 10.1080/02640414.2011.645054. [DOI] [PubMed] [Google Scholar]
- 23.Rossi S, Hallett M, Rossini P, Pascual-Leone A. Screening questionnaire before TMS: an update. Clin Neurophysiol. 2011;122(8):1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson AM. Assessment of chronic pain: I. Aspects of the reliability and validity of the visual analogue scale. Pain. 1983;16(1):87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 25.Palmieri-Smith RM, Villwock M, Downie B, Hecht G, Zernicke R. Pain and effusion and quadriceps activation and strength. J Athl Train. 2013;48(2):186–191. doi: 10.4085/1062-6050-48.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelburne KB, Pandy MG. A musculoskeletal model of the knee for evaluating ligament forces during isometric contractions. J Biomech. 1997;30(2):163–176. doi: 10.1016/s0021-9290(96)00119-4. [DOI] [PubMed] [Google Scholar]
- 27.Al Sawah M, Rimawi M, Concerto C, et al. Symmetric corticospinal excitability and representation of vastus lateralis muscle in right-handed healthy subjects. Clin Anat. 2014;27(7):1053–1057. doi: 10.1002/ca.22438. [DOI] [PubMed] [Google Scholar]
- 28.Lepley AS, Gribble PA, Thomas AC, Tevald MA, Sohn DH, Pietrosimone BG. Quadriceps neural alterations in anterior cruciate ligament reconstructed patients: a 6-month longitudinal investigation. Scand J Med Sci Sports. 2015;25(6):828–836. doi: 10.1111/sms.12435. [DOI] [PubMed] [Google Scholar]
- 29.Ward S, Bryant AL, Pietrosimione B, Bennell KL, Clark R, Pearce AJ. Cortical motor representation of the rectus femoris does not differ between the left and right hemisphere. J Electromyogr Kinesiol. 2016;28:46–52. doi: 10.1016/j.jelekin.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Wilson SA, Thickbroom GW, Mastaglia FL. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects: the representation of two intrinsic hand muscles. J Neurol Sci. 1993;118(2):134–144. doi: 10.1016/0022-510x(93)90102-5. [DOI] [PubMed] [Google Scholar]
- 31.Di Lazzaro V, Restuccia D, Oliviero A, et al. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508(pt 2):625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowling EJ, Steele JR, McNair PJ. Effect of verbal instructions on muscle activity and risk of injury to the anterior cruciate ligament during landing. Br J Sports Med. 2003;37(2):126–130. doi: 10.1136/bjsm.37.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossini PM, Burke D, Chen R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wassermann EM, Epstein CM, Ziemann U, et al. The Oxford Handbook of Transcranial Stimulation. Oxford, United Kingdom: Oxford University Press;; 2008. [Google Scholar]
- 35.Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 36.Undheim MB, Cosgrave C, King E, et al. Isokinetic muscle strength and readiness to return to sport following anterior cruciate ligament reconstruction: is there an association? A systematic review and a protocol recommendation. Br J Sports Med. 2015;49(20):1305–1310. doi: 10.1136/bjsports-2014-093962. [DOI] [PubMed] [Google Scholar]
- 37.Bryant AL, Clark RA, Pua YH. Morphology of hamstring torque-time curves following ACL injury and reconstruction: mechanisms and implications. J Orthop Res. 2011;29(6):907–914. doi: 10.1002/jor.21306. [DOI] [PubMed] [Google Scholar]
- 38.Bryant AL, Pua YH, Clark RA. Morphology of knee extension torque-time curves following anterior cruciate ligament injury and reconstruction. J Bone Joint Surg Am. 2009;91(6):1424–1431. doi: 10.2106/JBJS.H.01335. [DOI] [PubMed] [Google Scholar]
- 39.Tsepis E, Giakas G, Vagenas G, Georgoulis A. Frequency content asymmetry of the isokinetic curve between ACL deficient and healthy knee. J Biomech. 2004;37(6):857–864. doi: 10.1016/j.jbiomech.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Pua YH, Ong PH, Ho JY, Bryant AL, Webster KE, Clark RA. Associations of isokinetic knee steadiness with hop performance in patients with ACL deficiency. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2185–2195. doi: 10.1007/s00167-014-2995-4. [DOI] [PubMed] [Google Scholar]
- 41.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris: a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83(8):1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- 42.Hendy AM, Spittle M, Kidgell DJ. Cross education and immobilisation: mechanisms and implications for injury rehabilitation. J Sci Med Sport. 2012;15(2):94–101. doi: 10.1016/j.jsams.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27(3):383–404. doi: 10.1016/j.csm.2008.03.004. vii. [DOI] [PubMed] [Google Scholar]
- 45.Hart JM, Pietrosimone BG, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45(1):87–97. doi: 10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuenze CM, Hertel J, Weltman A, Diduch D, Saliba SA, Hart JM. Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. J Athl Train. 2015;50(3):303–312. doi: 10.4085/1062-6050-49.5.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Øiestad BE, Holm I, Gunderson R, Myklebust G, Risberg MA. Quadriceps muscle weakness after anterior cruciate ligament reconstruction: a risk factor for knee osteoarthritis? Arthritis Care Res (Hoboken) 2010;62(12):1706–1714. doi: 10.1002/acr.20299. [DOI] [PubMed] [Google Scholar]
- 48.Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43(7):1662–1669. doi: 10.1177/0363546515578252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pietrosimone BG, McLeod MM, Lepley AS. A theoretical framework for understanding neuromuscular response to lower extremity joint injury. Sports Health. 2012;4(1):31–35. doi: 10.1177/1941738111428251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasler EM, Denoth J, Stacoff A, Herzog W. Influence of hip and knee joint angles on excitation of knee extensor muscles. Electromyogr Clin Neurophysiol. 1994;34(6):355–361. [PubMed] [Google Scholar]
- 51.Maffiuletti NA, Lepers R. Quadriceps femoris torque and EMG activity in seated versus supine position. Med Sci Sports Exerc. 2003;35(9):1511–1516. doi: 10.1249/01.MSS.0000084426.03247.93. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery WH, III, Pink M, Perry J. Electromyographic analysis of hip and knee musculature during running. Am J Sports Med. 1994;22(2):272–278. doi: 10.1177/036354659402200220. [DOI] [PubMed] [Google Scholar]
- 53.Salzman A, Torburn L, Perry J. Contribution of rectus femoris and vasti to knee extension: an electromyographic study. Clin Orthop Relat Res. 1993;290:236–243. [PubMed] [Google Scholar]
- 54.Ema R, Wakahara T, Kawakami Y. Effect of hip joint angle on concentric knee extension torque. J Electromyogr Kinesiol. 2017;37:141–146. doi: 10.1016/j.jelekin.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Herzog W, Hasler E, Abrahamse SK. A comparison of knee extensor strength curves obtained theoretically and experimentally. Med Sci Sports Exerc. 1991;23(1):108–114. [PubMed] [Google Scholar]
- 56.Jacobs R, Bobbert MF, van Ingen Schenau GJ. Mechanical output from individual muscles during explosive leg extensions: the role of biarticular muscles. J Biomech. 1996;29(4):513–523. doi: 10.1016/0021-9290(95)00067-4. [DOI] [PubMed] [Google Scholar]
- 57.Pincivero DM, Salfetnikov Y, Campy RM, Coelho AJ. Angle- and gender-specific quadriceps femoris muscle recruitment and knee extensor torque. J Biomech. 2004;37(11):1689–1697. doi: 10.1016/j.jbiomech.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Hopkins JT, Ingersoll CD, Krause BA, Edwards JE, Cordova ML. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33(1):123–126. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 59.Palmieri RM, Tom JA, Edwards JE, et al. Arthrogenic muscle response induced by an experimental knee joint effusion is mediated by pre- and post-synaptic spinal mechanisms. J Electromyogr Kinesiol. 2004;14(6):631–640. doi: 10.1016/j.jelekin.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Lepley AS, Bahhur NO, Murray AM, Pietrosimone BG. Quadriceps corticomotor excitability following an experimental knee joint effusion. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1010–1017. doi: 10.1007/s00167-013-2816-1. [DOI] [PubMed] [Google Scholar]
- 61.Kent Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19(7):861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 62.Kapreli E, Athanasopoulos S, Gliatis J, et al. Anterior cruciate ligament deficiency causes brain plasticity: a functional MRI study. Am J Sports Med. 2009;37(12):2419–2426. doi: 10.1177/0363546509343201. [DOI] [PubMed] [Google Scholar]
- 63.Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2017;47(3):180–189. doi: 10.2519/jospt.2017.7003. [DOI] [PubMed] [Google Scholar]
- 64.Park J, Hopkins JT. Induced anterior knee pain immediately reduces involuntary and voluntary quadriceps activation. Clin J Sport Med. 2013;23(1):19–24. doi: 10.1097/JSM.0b013e3182717b7b. [DOI] [PubMed] [Google Scholar]
- 65.Manini TM, Cook SB, Ordway NR, Ploutz Snyder RJ, Ploutz Snyder LL. Knee extensor isometric unsteadiness does not predict functional limitation in older adults. Am J Phys Med Rehabil. 2005;84(2):112–121. doi: 10.1097/01.phm.0000151940.47912.df. [DOI] [PubMed] [Google Scholar]