Abstract
Context
Constant-tension (CT) stretching has been used to reduce hamstrings passive stiffness; however, the time course of hamstrings stiffness responses during a short bout of this type of stretching and the effects on maximal and explosive strength remain unclear.
Objective
To examine the time course of hamstrings passive-stiffness responses during a short, practical bout of manual straight-legged–raise (SLR) CT passive stretches and their effects on maximal and explosive strength in healthy young women.
Design
Descriptive laboratory study.
Setting
Research laboratory.
Patients or Other Participants
Eleven healthy women (age = 24 ± 4 years, height = 167 ± 4 cm, mass = 65 ± 8 kg) participated.
Intervention(s)
Participants underwent four 15-second SLR CT passive stretches of the hamstrings.
Main Outcome Measurement(s)
Hamstrings passive stiffness was calculated from the slopes of the initial (phase 1) and final (phase 2) portions of the angle-torque curves generated before and after the stretching intervention and at the beginning of each 15-second stretch. Hamstrings peak torque and rate of torque development were derived from maximal voluntary isometric contractions performed before and after the stretching intervention.
Results
The slope coefficients (collapsed across phase) for the third and fourth stretches and the poststretching assessment were lower than the prestretching assessment (P range = .004–.04), but they were not different from each other (P > .99). In addition, no differences in peak torque (t10 = −0.375, P = .72) or rate of torque development (t10 = −0.423, P = .68) were observed between prestretching and poststretching.
Conclusions
A short bout of SLR CT passive stretching may effectively reduce hamstrings stiffness without negatively influencing maximal and explosive strength.
Keywords: hamstrings, range of motion, straight-legged raise, peak torque, rate of torque development
Key Points
Two 15-second constant-tension passive stretches reduced phase 1 and 2 slope coefficients.
Two subsequent 15-second stretches did not further decrease the slope coefficients.
No changes in isometric peak torque and rate of torque development were observed from prestretching to poststretching.
Preactivity passive stretching is commonly performed in clinical and athletic settings to increase range of motion (ROM) and decrease passive stiffness of the muscle-tendon unit (MTU).1 Reduced hamstrings ROM has been identified as a substantial risk factor for hamstrings injuries in elite-level athletes.2 Researchers3 examining the flexibility effects of hamstrings passive stretching have reported that 5 stretches held for 15 seconds each are needed to successfully increase hamstrings ROM in younger adults. Whereas these data have important clinical implications for improving hamstrings flexibility, ROM, which is a single measurement in time in a static system, provides limited information regarding the viscoelastic behavior of the MTU.4 Alternatively, investigators have suggested that musculotendinous stiffness, which is typically calculated as the slope of the angle-torque curve recorded during a passive stretch,5 may provide more valid evidence concerning the effectiveness of stretching in decreasing the likelihood of muscle strains and other hamstrings-related injuries.6 McHugh et al7 reported that individuals with stiffer hamstrings were more susceptible to exercise-induced muscle injury than individuals with compliant hamstrings. Consequently, knowledge about the effect of preactivity passive stretching on hamstrings stiffness may be important. Such information could help improve our understanding of stretch-induced changes in the passive mechanical properties of the MTU, as well as guide the development of stretching interventions aimed at reducing the risk of hamstrings injuries in individuals with shorter and stiffer muscles.
Recently, a number of authors8–11 have compared the effects of constant-angle and -torque stretching maneuvers on the passive stiffness characteristics of the hamstrings. Specifically, they found that stretches held at a constant tension (CT) more effectively decreased hamstrings stiffness than stretches held at a constant joint angle. Stretching at CT causes an increase in ROM referred to as muscle creep.9 Researchers9,12 have hypothesized that, given the muscle-creep–induced increase in ROM, CT stretching applies more work on the MTU, which may explain its greater effects on the passive stiffness properties of the muscle when compared with constant-angle stretching. Given that clinicians and other practitioners often see patients for a limited time,1 previous investigators have been heavily focused on determining the minimum number of CT stretches required for eliciting changes in stiffness. Ryan et al1 found that two 30-second CT passive stretches were needed to effectively reduce passive stiffness in the plantar flexors. Moreover, Herda et al9 reported a decrease in hamstrings passive stiffness after a single 30-second CT passive stretch and subsequent decreases after additional 30-second stretches. Whereas these findings provide important information regarding the time course of the stiffness effects of multiple 30-second CT passive stretches, Costa et al13 suggested that 15-second stretches may better reflect the stretching durations performed in a clinical setting. Therefore, examining the time course of the effects of a shorter, more practical bout of 15-second CT passive stretches on hamstrings stiffness may be of great clinical interest. Furthermore, numerous researchers have reported transient decreases in muscle power14 and maximal15 and explosive14,16 strength after various acute stretching interventions. Given that authors14,16 of 2 of these studies evaluated strength before and after prolonged stretching bouts (>4 minutes), it remains unclear whether strength was influenced by a shorter stretching duration. Therefore, to fully understand the application of a short bout of CT passive stretching, it may be important to examine how this duration and type of stretching affects maximal and explosive strength, as well as the passive mechanical properties of the MTU.10
Whereas CT stretching may effectively decrease hamstrings passive stiffness, such techniques require relatively large and expensive isokinetic devices,8–11 rendering the use of such stretching as a stiffness-reducing modality unfeasible in most clinical settings. Our laboratory has developed a relatively simple yet cost-effective manual straight-legged–raise (SLR) technique for assessing hamstrings passive stiffness that consists of the investigator applying force against a load cell attached to the heel of the participant while the limb is moved toward the head.17,18 We have recently modified this technique by adding another load cell to the system and connecting it to a strain-gauge meter, which provides real-time feedback of the tension applied during a passive stretch. With this modification, our manual SLR technique can now be used as a portable CT stretching tool, which may be an attractive alternative to isokinetic devices for performing CT stretching in the field. We are aware of no authors who have examined the stiffness and strength effects of a CT passive-stretching protocol using a manual SLR technique. Moreover, given that the effects of CT stretching on stiffness and strength have been examined almost exclusively in young males,9–11 examining the effects of CT stretching in young females may help shed light on the stretch-induced stiffness and strength changes in this population. Therefore, the purpose of our study was to examine the time course of hamstrings stiffness responses during a short, practical bout of four 15-second manual SLR CT passive stretches and their effects on maximal and explosive strength in young, healthy women.
METHODS
Participants
Eleven young healthy women (age = 24 ± 4 years, height = 167 ± 4 cm, mass = 65 ± 8 kg, body mass index = 23 ± 3 kg/m2) volunteered to participate. Before testing, participants completed a self-administered questionnaire that assessed their health history and volume of physical activity. Volunteers were excluded if they had sustained an injury in the 6 months before the study or had undergone surgery involving the ankle, knee, or hip joint in the year before the study. No participant reported any current or ongoing neuromuscular disease of or musculoskeletal injury to these areas. All participants were considered recreationally active based on their self-reported levels of exercise activity (5.7 ± 3.2 h/wk).8 All participants provided written informed consent, and the study was approved by the Kansas State University Institutional Review Board.
Experimental Design
We used a repeated-measures design to investigate the acute effects of manual SLR CT stretching on passive stiffness and isometric maximal and rapid torque production of the hamstrings muscles in healthy young women. Participants visited the laboratory on 2 occasions separated by 2 to 3 days and at approximately the same time of day (±2 h). During the first visit, they practiced the passive SLR and isometric strength assessments and experienced several SLR CT passive stretches. During the second visit, participants underwent the prestretching assessments (1 manual SLR assessment and 2 isometric strength tests), four 15-second SLR CT passive stretches, and the poststretching assessments that occurred immediately after the stretching intervention.
Passive Stiffness
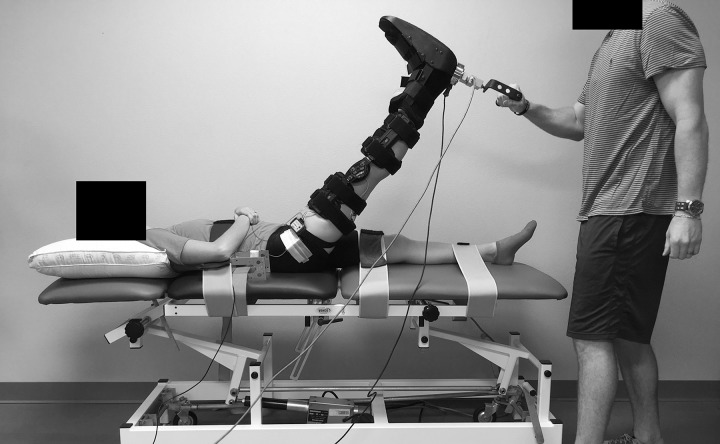
Passive stiffness of the hamstrings was examined from the angle-torque curves generated before and after the stretching intervention and at the beginning of each 15-second stretch using a manual SLR technique as previously described,17 consisting of the primary investigator (T.B.P.) applying force against a load cell (model LCHD-250; Omega Engineering Inc, Bridgeport, NJ) positioned immediately posterior to the heel while the limb was moved slowly toward the head (Figure 1). For each SLR, the knee was braced in full extension, and the ankle was immobilized in a neutral 90° position (between the foot and leg) with a custom-made cast that was fixed around the foot and held with straps above the ankle and over the toes and metatarsals. We used an electrogoniometer (model TSD130B; BIOPAC Systems Inc, Santa Barbara, CA) to measure the hip-joint angle. The distal and proximal endblocks of the electrogoniometer were attached using methods similar to those described previously.17 During the SLR, participants lay supine with restraining straps placed over the waist, left thigh, and ankle. All SLRs were performed on the right limb to the point of discomfort but not pain as indicated by the participant, which was regarded as the maximal ROM. After maximal ROM was reached, the limb was either returned immediately (for the prestretching and poststretching SLR assessments) or was stretched and returned (for each 15-second stretch) to the baseline position, which was a hip-joint angle of 20° above the horizontal plane.
Figure 1.
The manual straight-legged–raise technique. The primary investigator applied force against a load cell attached to the heel of the participant while the limb was slowly moved toward the head.
Surface Electromyography
To ensure that all SLRs were passive, we placed bipolar pregelled disposable electrodes (model EL502; BIOPAC Systems Inc) with an interelectrode distance of 25 mm over the biceps femoris muscle of the right limb at 50% of the distance between the ischial tuberosity and the lateral epicondyle of the tibia.5,9,19 To decrease interelectrode impedance, the skin was cleansed with isopropyl alcohol before electrode placement. A single, pregelled disposable electrode was placed on the palmar side of the right wrist to serve as a reference electrode. Electromyographic (EMG) amplitude was calculated using a root mean square function for 200-millisecond epochs corresponding to each whole-number degree during the ROM and normalized to the corresponding prestretch maximal voluntary isometric contraction (MVIC) peak EMG amplitude.10,17
Isometric Peak Torque and Rate of Torque Development
To determine the stretch-induced changes in isometric peak torque (PT) and rate of torque development (RTD) and to normalize the EMG amplitude values, participants performed 2 MVICs of the hamstrings before and after the stretching intervention. All MVICs were performed using a manual isometric-strength–assessment technique on the right limb at a hip-joint angle of 20° above the horizontal plane,20 which was the same hip-joint angle as the starting point of the passive SLR assessments. Participants lay supine during each MVIC, and we placed restraining straps over the waist, left thigh, and ankle. One-minute rests were allotted between MVIC assessments. Of the 2 MVICs performed before and after stretching, the MVIC with the higher PT was selected for further analysis. For all MVICs, participants were instructed to extend the thigh “as hard and fast as possible” for a total of 3 to 4 seconds, and strong oral encouragement was given throughout the duration of the contraction.5
Signal Processing
During each MVIC and SLR, force (in newtons), joint-angle position (in degrees), and EMG signals (in microvolts) were sampled simultaneously at 1 kHz (model MP150WSW; BIOPAC Systems Inc), stored on a personal computer (model Dell Inspiron 8200; Dell Inc, Round Rock, TX), and processed offline using custom-written software (LabVIEW version 11.0; National Instruments, Austin, TX). Force and position signals were low-pass filtered using a zero-phase lag, fourth-order Butterworth filter with a 10-Hz cutoff. A torque signal (in newton-meters) was subsequently derived offline by multiplying the force signal (in newtons) from the load cell by the limb length (in meters) for each participant.17 The EMG signal was scaled and bandpass filtered with a zero-phase lag, fourth-order Butterworth filter from 20 to 400 Hz. All subsequent analyses were conducted on the scaled and filtered signals.
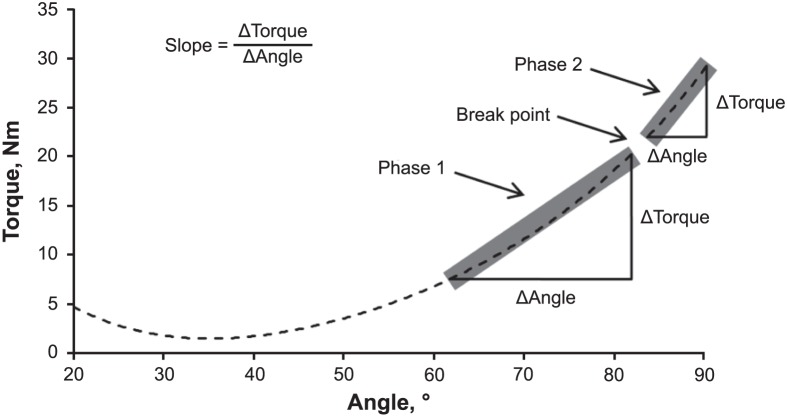
The MVIC PT (in newton-meters) was calculated as the highest mean 500-millisecond epoch during the entire 3- to 4-second MVIC plateau. We calculated the RTD (in newton-meters per second) as the peak of the first derivative of the torque signal. For passive stiffness, gravity correction was performed during each SLR using a cosine function in which the limb mass was subtracted from the torque signal across the ROM. The scaled and gravity-corrected torque and joint-angle signals were plotted as passive angle-torque curves and fitted with a fourth-order polynomial regression model based on the equation of Nordez et al.21 Passive stiffness was calculated as the slopes of the initial linear portion (phase 1) and the second steeper portion (phase 2) of the angle-torque curve using a previously described procedure (Figure 2).5 The slope of phase 1 was defined as the change in passive tension from 60% to 88% of the total ROM. The slope of phase 2 was defined as the change in passive tension from 90% to the end ROM. The break point (in degrees) between phases 1 and 2 was defined as 89% of each participant's total ROM, which was selected based on the mean break point relative to the total ROM reported by Chleboun et al.22 Passive tension at the beginning and end of phases 1 and 2 was quantified at common joint angles for all SLRs performed on each participant. Consequently, the same absolute joint angles could be used to calculate the absolute slope coefficients (in newton-meters per degree) and mean EMG amplitudes of phases 1 and 2 for each SLR.
Figure 2.
An example of an angle-torque curve during a single, passive, straight-legged–raise assessment of the hamstrings muscles. The slope coefficients (Δtorque/Δangle in newton-meters per degree) of the initial linear portion (phase 1) and the second steeper portion (phase 2) of the angle-torque curve were defined based on percentages relative to the total range of motion (ROM). The break point denoted the angle (in degrees) where phase 1 ends and phase 2 begins and was calculated as 89% of the total ROM. The slope of phase 1 represented the change in passive tension from 60% to 88% of the total ROM. The slope of phase 2 represented the change in passive tension from 90% to the end ROM. In this example, the phase 1 (63°–82°) slope is 0.56, the phase 2 (84°–91°) slope is 1.09, and the break point is 83°.
Reliability
Based on the procedures described by Weir,23 test-retest reliability in our laboratory was examined during the manual SLR and MVIC assessments from a subset of the participants measured 48 to 72 hours apart. The intraclass correlation coefficient and standard error of measurement expressed as a percentage of the mean were 0.70 and 19.54% for the phase 1 slope, 0.89 and 15.89% for the phase 2 slope, 0.99 and 4.60% for PT, and 0.95 and 13.34% for the RTD, respectively. In addition, we observed no systematic differences among testing sessions for any variable (P > .05).
Passive Stretching
Repeated passive stretching of the right hamstrings muscles was performed by the primary investigator using the manual SLR technique. He passively moved the limb toward the head at a slow, controlled speed until the participant orally acknowledged discomfort but not pain by saying “Stop.” The tension measured at this position was kept constant by the primary investigator, who stretched the limb for 15 seconds. An additional load cell (model LC105-500; Omega Engineering Inc, Bridgeport, NJ) attached to the participant's heel was connected to a high-performance strain-gauge meter (model DP41-S; Omega Engineering Inc), which provided the primary investigator with real-time feedback of the tension applied during the passive stretch. The investigator maintained CT by increasing the position of the participant's limb when he observed reduced tension on the strain gauge. A 15-second rest, in which the limb was returned to the baseline position, was allotted between stretches. Each participant underwent four 15-second bouts of stretching, totaling 1 minute under stretch and lasting approximately 2 minutes total.24
Statistical Analyses
Dependent-samples t tests were used to examine the differences in PT and RTD from prestretching to poststretching. Two separate 2-way repeated-measures analyses of variance (time [prestretching versus stretch 1 versus stretch 2 versus stretch 3 versus stretch 4 versus poststretching] × phase [phase 1 versus phase 2]) were used to analyze the slope coefficients and EMG amplitude data. A separate 1-way repeated-measures analysis of variance (stretch 1 versus stretch 2 versus stretch 3 versus stretch 4) was used to analyze the torque maintained by the primary investigator during each stretch. We reported t statistics and type 1 error rates (P) for the t tests and F ratios and P values for the interaction and main effects. Cohen d effect sizes were calculated for each pairwise comparison, in which values of 0.20, 0.50, and 0.80 corresponded to small, medium, and large effect sizes, respectively.25 Statistical analyses were performed using SPSS (version 22; IBM Corp, Armonk, NY), and the α level was set at ≤.05.
RESULTS
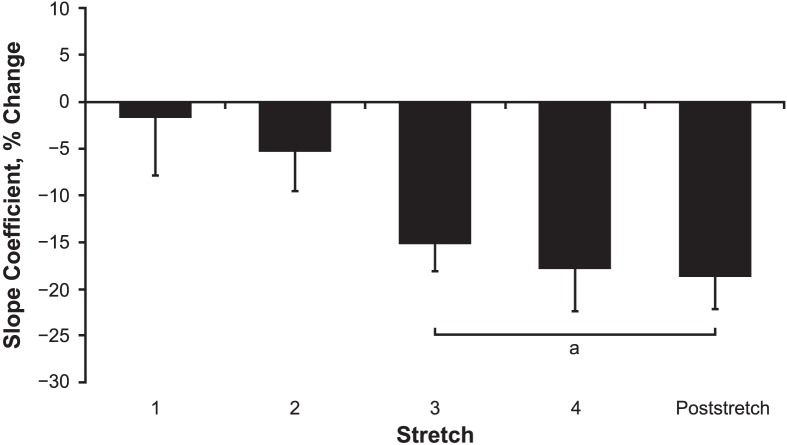
For maximal and rapid strength, no differences in PT (t10 = −0.375, P = .72, d = 0.03) and RTD (t10 = −0.423, P = .68 d = 0.18) were found from prestretching to poststretching (Table 1). The means and standard deviations for the slope coefficients of phases 1 and 2 for each SLR are shown in Table 2. For the slope coefficients, we observed no interaction (F5,50 = 0.152, P = .98) but noted main effects for time (F5,50 = 4.920, P = .001) and phase (F1,10 = 11.998, P = .006). The marginal means collapsed across phase for stretches 3 and 4 and the poststretching assessment were lower than the prestretching assessment (P range = .004–.04, d range = 0.71–0.83), but they were not different from each other (P > .99, d ≤ 0.17; Figure 3). In addition, the marginal mean for the slope coefficients collapsed across time increased from phase 1 to 2 (P = .006, d = 0.86). For EMG amplitude, we observed no interaction (F5,50 = 0.016, P > .99) and no main effects for time (F5,50 = 0.027, P > .99) or phase (F1,10 = 0.042, P = .84). The EMG amplitude values were 0.39% and 0.37% of the MVIC for phases 1 and 2, respectively. The torque maintained by the primary investigator did not change among stretches (stretch 1 = 36.82 ± 7.00 Nm; stretch 2 = 37.89 ± 5.21 Nm; stretch 3 = 35.78 ± 6.58 Nm; stretch 4 = 38.58 ± 6.28 Nm; F3,30 = 1.269, P = .30).
Table 1.
Prestretching and Poststretching Isometric Peak Torque and Rate of Torque Development, Mean ± SD
| Variable |
Prestretching |
Poststretching |
| Isometric peak torque, Nm | 140.35 ± 36.11 | 141.31 ± 33.46 |
| Rate of torque development, Nm/s | 996.82 ± 286.26 | 1044.71 ± 259.96 |
Table 2.
Slope Coefficients of Phases 1 and 2 for Each Straight-Legged Raise, Mean ± SD
| Time |
Slope, Nm/° |
||
| Phase 1 |
Phase 2 |
Meana |
|
| Prestretching | 0.62 ± 0.13 | 0.77 ± 0.17 | 0.69 ± 0.14 |
| Stretch 1 | 0.61 ± 0.18 | 0.74 ± 0.24 | 0.67 ± 0.16 |
| Stretch 2 | 0.57 ± 0.15 | 0.72 ± 0.14 | 0.65 ± 0.09 |
| Stretch 3 | 0.53 ± 0.11 | 0.65 ± 0.18 | 0.59 ± 0.14b |
| Stretch 4 | 0.52 ± 0.14 | 0.62 ± 0.19 | 0.57 ± 0.14b |
| Poststretching | 0.52 ± 0.15 | 0.62 ± 0.21 | 0.57 ± 0.16b |
Main effect for time (collapsed across phase).
Lower than the prestretching assessment (P ≤ .05).
Figure 3.
Percentage change in the slope coefficients (collapsed across phase) from the prestretching assessment for the angle-torque curves generated at the beginning of stretches 1, 2, 3, and 4, as well as the poststretching assessment. Values are mean ± SEM. a Decrease from the prestretching assessment.
DISCUSSION
Our primary findings were that hamstrings passive stiffness, as indicated by the slope coefficients, decreased after two 15-second SLR passive stretches, with no additional decreases after stretches 3 and 4 when compared with the prestretching assessment (Table 2). We also observed no changes in PT and RTD from prestretching to poststretching (Table 1). Our findings indicated that the cumulative effects of 2 repeated bouts of 15-second SLR passive stretching could reduce passive stiffness in the hamstrings. Researchers have shown decreases in passive stiffness of the plantar flexors26 and hamstrings9 after a single 30-second passive stretch. However, other authors27,28 examining similar stretching durations (20–60 seconds) have reported no changes in passive stiffness for these muscles. The discrepancies between these findings and ours may be due to differences in the types of passive stretching that were performed. We performed a CT passive-stretching protocol (4 × 15-second passive stretches), whereas similar bouts of constant-angle stretching (1 × 20-second, 1 × 60-second, 2 × 30-second, or 4 × 15-second passive stretches) were performed in previous studies.27,28 Greater decreases in passive stiffness have been reported after holding stretches at a constant torque (or tension) compared with a constant position.8–11,29 Ryan et al12 suggested that the muscle-creep–induced increase in ROM during CT stretching applies more work on the MTU, which could result in greater changes in the stiffness properties of the muscle than constant-angle stretching. Whereas the slope coefficients in this study decreased after the second SLR stretch, we demonstrated no changes in these variables after the first stretch. These findings suggest that one 15-second SLR CT passive stretch is insufficient for reducing stiffness and a second stretch (for a total time of 30 seconds) may be needed before reductions in stiffness can occur.
The reason for the stretch-induced declines in passive stiffness is unclear24 but could be due to several factors, including increases in tendon compliance,30 muscle-fascicle length,15 and deformation of the noncontractile proteins of the endosarcomeric and exosarcomeric cytoskeletons (ie, titin, desmin).31 Moreover, given that connective tissue has been reported to be a major contributor to passive stiffness,32 increases in the length of these structures may also play a role in the stiffness decreases observed after stretching.8,24 Future investigations involving more sophisticated methods (ie, ultrasound imaging combined with passive torque and ROM data) are needed to further examine these findings.
The lack of differences in slope coefficients among the third, fourth, and poststretching assessments in this study indicated that 15-second CT stretches beyond 2 repetitions may not elicit further decreases in passive stiffness of the hamstrings. In contrast, Herda et al9 found a decrease in hamstrings passive stiffness after 30 seconds of CT passive stretching and subsequent decreases after longer stretching durations. However, these additional decreases in stiffness were not observed until after 4 minutes of stretching.9 Therefore, based on these findings and ours, 30 seconds may be as effective as 45 or 60 seconds of stretching in reducing stiffness in the hamstrings. However, if stretching is performed for longer periods (>4 minutes), subsequent declines in passive tissue resistance may occur and could result in greater reductions in stiffness.9 Whereas these findings provide support for prolonged passive-stretching protocols greater than 4 minutes, stretching for such a duration has been shown to elicit reductions in numerous performance-based outcomes, including muscle power14 and maximal and rapid force production.14,16 Given that lower stiffness values have been associated with a decreased ability to generate force rapidly in younger adults,5 the aforementioned declines in performance may be partially due to the large decreases in muscle stiffness that are typically observed after long durations of passive stretching. A key finding of our study was that maximal and explosive strength, as indicated by PT and RTD, were not affected by the stretching intervention. Researchers13,33 have suggested that, given its smaller effects on stiffness, shorter-term stretching may cause a smaller change in MTU compliance that is not detrimental to the force-producing capabilities of the muscle. In our study, four 15-second passive stretches elicited a 17% decrease in hamstrings muscle stiffness (Figure 3), which was substantially smaller than the stiffness reductions (approximately 31%–38%) reported previously after longer-term passive stretching (≥4 minutes).9 Therefore, the possibility of smaller reductions in the passive-stiffness properties of the muscle may explain why we observed no stretch-induced changes in maximal and explosive strength. Whereas further research is still needed to test these hypotheses regarding the influence of stretching duration on stiffness and active force production, our findings of declines in the slope coefficients but no changes in PT and RTD provide support that a short bout of SLR CT passive stretching may effectively reduce stiffness without negatively influencing maximal and explosive strength capacities of the hamstrings.
To our knowledge, we are the first to examine the stiffness and strength effects of a CT passive-stretching protocol using a manual SLR technique. Whereas previous investigators have reported beneficial effects on stiffness from CT stretching using isokinetic dynamometers,1,8–10 these devices are relatively expensive, immobile, and inaccessible to many researchers and clinicians.17 The manual SLR technique that we proposed uses equipment (load cells, strain gauge meter, electrogoniometer, etc) that is portable and less costly than isokinetic devices. Moreover, the ability of this technique to provide visual feedback of the passive tension applied to the muscles being stretched may make it an attractive alternative for performing CT stretching in both laboratory and clinical settings. Researchers have suggested that a decrease in hamstrings-stiffness characteristics may alleviate tightness of the lower extremity musculature,8 which in turn, could help reduce the potential risk of muscle strains and other injuries to the MTU.34 Given our findings of a stretch-induced reduction in the slope coefficients, the stretching protocol used in our study may be beneficial for mitigating subsequent injuries in younger adults. Future studies of the effects of CT passive stretching as part of a warmup routine on the incidence of muscle injuries to the hamstrings are needed to test this hypothesis.
A potential limitation of this study was the absence of a control treatment (ie, no stretch). However, given that the prestretching SLR assessment and stretch 1 produced angle-torque curves before CT stretching was initiated, this period without intervention may serve as an alternative control condition. No reductions in the slope coefficients were observed between the prestretching SLR assessment and stretch 1. Therefore, based on these findings, we can conclude that the prestretching testing by itself did not influence passive stiffness. Furthermore, the torque values maintained by the primary investigator were not different among stretches, indicating that the SLRs performed in this study were of a similar intensity and, thus, comparable with the CT movements performed previously using isokinetic dynamometers.8–10 Finally, we acknowledge that the generalizability of our findings is limited to a population of healthy young females. Therefore, further research is warranted to extend these research objectives to other populations, including healthy young males, athletes, and older individuals.
CONCLUSIONS
Two 15-second SLR CT passive stretches reduced phase 1 and 2 slope coefficients; however, 2 subsequent 15-second stretches did not further decrease the slope coefficients. In addition, no changes in isometric PT and RTD were observed from prestretching to poststretching. Taken together, these findings provide support that a short bout of SLR CT passive stretching may be an effective modality for reducing passive stiffness without decreasing maximal and explosive strength in the hamstrings. Moreover, given that the CT passive-stretching protocol in our study was performed manually without using an isokinetic dynamometer, these findings also perhaps demonstrate the practicality and utility of the manual SLR technique as an attractive alternative to isokinetic devices for performing CT stretching and stiffness-based assessments in healthy populations. Whereas large decreases in hamstrings stiffness can occur after 4 minutes of CT passive stretching,9 such stretching durations have been shown to elicit declines in maximal and explosive strength of the lower body musculature.14,16 Therefore, if athletic trainers and other practitioners want to prescribe a pre-exercise stretching intervention to reduce stiffness while minimizing any potential deficits in maximal and explosive strength of the hamstrings, our findings suggest that 2 repeated bouts of 15-second SLR CT passive stretching may be an effective strategy for achieving these desired outcomes.
ACKNOWLEDGMENTS
Funding for this study was provided by the Mid-America Athletic Trainers' Association Research and Education Committee (Drs Palmer and Thiele).
REFERENCES
- 1.Ryan ED, Herda TJ, Costa PB, et al. Determining the minimum number of passive stretches necessary to alter musculotendinous stiffness. J Sports Sci. 2009;27(9):957–961. doi: 10.1080/02640410902998254. [DOI] [PubMed] [Google Scholar]
- 2.Witvrouw E, Danneels L, Asselman P, D'Have T, Cambier D. Muscle flexibility as a risk factor for developing muscle injuries in male professional soccer players: a prospective study. Am J Sports Med. 2003;31(1):41–46. doi: 10.1177/03635465030310011801. [DOI] [PubMed] [Google Scholar]
- 3.Boyce D, Brosky JA., Jr Determining the minimal number of cyclic passive stretch repetitions recommended for an acute increase in an indirect measure of hamstring length. Physiother Theory Pract. 2008;24(2):113–120. doi: 10.1080/09593980701378298. [DOI] [PubMed] [Google Scholar]
- 4.Magnusson SP, Simonsen EB, Aagaard P, Kjaer M. Biomechanical responses to repeated stretches in human hamstring muscle in vivo. Am J Sports Med. 1996;24(5):622–628. doi: 10.1177/036354659602400510. [DOI] [PubMed] [Google Scholar]
- 5.Palmer TB, Thompson BJ. Influence of age on passive stiffness and size, quality, and strength characteristics. Muscle Nerve. 2017;55(3):305–315. doi: 10.1002/mus.25231. [DOI] [PubMed] [Google Scholar]
- 6.Ryan ED, Beck TW, Herda TJ, et al. The time course of musculotendinous stiffness responses following different durations of passive stretching. J Orthop Sports Phys Ther. 2008;38(10):632–639. doi: 10.2519/jospt.2008.2843. [DOI] [PubMed] [Google Scholar]
- 7.McHugh MP, Connolly DA, Eston RG, Kremenic IJ, Nicholas SJ, Gleim GW. The role of passive muscle stiffness in symptoms of exercise-induced muscle damage. Am J Sports Med. 1999;27(5):594–599. doi: 10.1177/03635465990270050801. [DOI] [PubMed] [Google Scholar]
- 8.Palmer TB. Acute effects of constant-angle and constant-torque static stretching on passive stiffness of the posterior hip and thigh muscles in healthy, young and old men. J Strength Cond Res. 2017] doi: 10.1519/JSC.0000000000002157. [published online ahead of print July 24, [DOI] [PubMed]
- 9.Herda TJ, Costa PB, Walter AA, Ryan ED, Cramer JT. The time course of the effects of constant-angle and constant-torque stretching on the muscle-tendon unit. Scand J Med Sci Sports. 2014;24(1):62–67. doi: 10.1111/j.1600-0838.2012.01492.x. [DOI] [PubMed] [Google Scholar]
- 10.Herda TJ, Costa PB, Walter AA, et al. Effects of two modes of static stretching on muscle strength and stiffness. Med Sci Sports Exerc. 2011;43(9):1777–1784. doi: 10.1249/MSS.0b013e318215cda9. [DOI] [PubMed] [Google Scholar]
- 11.Cabido CE, Bergamini JC, Andrade AG, Lima FV, Menzei HJ, Chagas MH. Acute effect of constant torque and angle stretching on range of motion, muscle passive properties, and stretch discomfort perception. J Strength Cond Res. 2014;28(4):1050–1057. doi: 10.1519/JSC.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 12.Ryan ED, Herda TJ, Costa PB, et al. Viscoelastic creep in the human skeletal muscle-tendon unit. Eur J Appl Physiol (1985) 2010;108(1):207–211. doi: 10.1007/s00421-009-1284-2. [DOI] [PubMed] [Google Scholar]
- 13.Costa PB, Graves BS, Whitehurst M, Jacobs PL. The acute effects of different durations of static stretching on dynamic balance performance. J Strength Cond Res. 2009;23(1):141–147. doi: 10.1519/JSC.0b013e31818eb052. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Ishii K, Yamanaka M, Yasuda K. Acute effect of static stretching on power output during concentric dynamic constant external resistance leg extension. J Strength Cond Res. 2006;20(4):804–810. doi: 10.1519/R-18715.1. [DOI] [PubMed] [Google Scholar]
- 15.Fowles JR, Sale DG, MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol (1985) 2000;89(3):1179–1188. doi: 10.1152/jappl.2000.89.3.1179. [DOI] [PubMed] [Google Scholar]
- 16.Allison SJ, Bailey DM, Folland JP. Prolonged static stretching does not influence running economy despite changes in neuromuscular function. J Sports Sci. 2008;26(14):1489–1495. doi: 10.1080/02640410802392715. [DOI] [PubMed] [Google Scholar]
- 17.Palmer TB, Jenkins NDM, Cramer JT. Reliability of manual versus automated techniques for assessing passive stiffness of the posterior muscles of the hip and thigh. J Sports Sci. 2013;31(8):867–877. doi: 10.1080/02640414.2012.753159. [DOI] [PubMed] [Google Scholar]
- 18.Palmer TB, Jenkins NDM, Thompson BJ, Cramer JT. Influence of stretching velocity on musculotendinous stiffness of the hamstrings during passive straight-leg raise assessments. Musculoskelet Sci Pract. 2017;30:80–85. doi: 10.1016/j.msksp.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 20.Palmer TB, Thiele RM, Thompson BJ. Age-related differences in maximal and rapid torque characteristics of the hip extensors and dynamic postural balance in healthy, young and old females. J Strength Cond Res. 2017;31(2):480–488. doi: 10.1519/JSC.0000000000001503. [DOI] [PubMed] [Google Scholar]
- 21.Nordez A, Cornu C, McNair P. Acute effects of static stretching on passive stiffness of the hamstring muscles calculated using different mathematical models. Clin Biomech (Bristol, Avon) 2006;21(7):755–760. doi: 10.1016/j.clinbiomech.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Chleboun GS, Howell JN, Conatser RR, Giesey JJ. The relationship between elbow flexor volume and angular stiffness at the elbow. Clin Biomech (Bristol, Avon) 1997;12(6):383–392. doi: 10.1016/s0268-0033(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 23.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 24.Palmer TB, Agu-Udemba CC, Palmer BM. Acute effects of static stretching on passive stiffness and postural balance in healthy, elderly men. Phys Sportsmed. 2018;46(1):78–86. doi: 10.1080/00913847.2018.1421396. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates;; 1988. [Google Scholar]
- 26.Opplert J, Genty JB, Babault N. Do stretch durations affect muscle mechanical and neurophysiological properties? Int J Sports Med. 2016;37(9):673–679. doi: 10.1055/s-0042-104934. [DOI] [PubMed] [Google Scholar]
- 27.McNair P, Dombroski EW, Hewson DJ, Stanley SN. Stretching at the ankle joint: viscoelastic responses to holds and continuous passive motion. Med Sci Sports Exerc. 2001;33(3):354–358. doi: 10.1097/00005768-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo S, Suzuki S, Iwata M, et al. Acute effects of different stretching durations on passive torque, mobility, and isometric muscle force. J Strength Cond Res. 2013;27(12):3367–3376. doi: 10.1519/JSC.0b013e318290c26f. [DOI] [PubMed] [Google Scholar]
- 29.Yeh CY, Chen JJ, Tsai KH. Quantifying the effectiveness of the sustained muscle stretching treatments in stroke patients with ankle hypertonia. J Electromyogr Kinesiol. 2007;17(4):453–461. doi: 10.1016/j.jelekin.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Kubo K, Kanehisa H, Fukunaga T. Effects of transient muscle contractions and stretching on the tendon structures in vivo. Acta Physiol Scand. 2002;175(2):157–164. doi: 10.1046/j.1365-201X.2002.00976.x. [DOI] [PubMed] [Google Scholar]
- 31.Gajdosik RL. Passive extensibility of skeletal muscle: review of the literature with clinical implications. Clin Biomech (Bristol, Avon) 2001;16(2):87–101. doi: 10.1016/s0268-0033(00)00061-9. [DOI] [PubMed] [Google Scholar]
- 32.Purslow PP. Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. J Biomech. 1989;22(1):21–31. doi: 10.1016/0021-9290(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 33.Reis Eda F, Pereira GB, de Sousa NM, et al. Acute effects of proprioceptive neuromuscular facilitation and static stretching on maximal voluntary contraction and muscle electromyographical activity in indoor soccer players. Clin Physiol Funct Imaging. 2013;33(6):418–422. doi: 10.1111/cpf.12047. [DOI] [PubMed] [Google Scholar]
- 34.Cross KM, Worrell TW. Effects of a static stretching program on the incidence of lower extremity musculotendinous strains. J Athl Train. 1999;34(1):11–14. [PMC free article] [PubMed] [Google Scholar]