Abstract
Background
Type 1, type 17, and other proinflammatory cytokines are important in host immunity to tuberculosis (TB) in animal models. However, their role in human immunity to TB is not completely understood.
Methods
To examine the association of proinflammatory cytokines with pulmonary TB (PTB), we examined the plasma levels of type 1 (interferon [IFN]γ and tumor necrosis factor [TNF]α), type 17 (interleukin [IL]-17A and IL-17F), and other proinflammatory (IL-6, IL-12, and IL-1β) cytokines in individuals with PTB, latent TB (LTB), or healthy controls (HC).
Results
Individuals with PTB exhibited significantly higher plasma levels of most of the above cytokines compared with LTB or HC individuals. Principal component analysis based on these cytokines could clearly distinguish PTB from both LTB or HC individuals. Pulmonary TB individuals with bilateral or cavitary disease exhibited significantly higher levels of IFNγ, TNFα, IL-17A, and IL-1β compared with those with unilateral or noncavitary disease. Pulmonary TB individuals also exhibited a significant positive relationship between IFNγ, TNFα, and IL-17A levels and bacterial burdens. In addition, PTB individuals with delayed culture conversion exhibited significantly higher levels of IFNγ, TNFα, IL-17A, and IL-1β at baseline. Finally, the plasma levels of all the cytokines examined were significantly reduced after successful chemotherapy.
Conclusions
Therefore, our data demonstrate that PTB is associated with heightened levels of plasma proinflammatory cytokines, which are reversed after chemotherapy. Our data also reveal that proinflammatory cytokines are markers of disease severity, bacterial burden, and delayed culture conversion in PTB.
Keywords: biomarkers, cytokines, tuberculosis
Mycobacterium tuberculosis (Mtb) is one of the most common infections worldwide and causes a spectrum of infection and disease manifestations [1]. Thus, approximately 90% of infected individuals remain asymptomatic throughout their lifetime and are thought to resist the infection, whereas 5%–10% of infected individuals progress to active tuberculosis (TB) [1]. However, progression to disease is not thought to be a linear process, but, instead, there are individuals in the spectrum who have subclinical, asymptomatic disease and individuals who have incipient disease [2]. The immunological pathways that underlie these different disease phenotypes remain unclear. It is known that the interaction between Mtb and the host evokes innate and adaptive immune responses. However, the immune responses that drive the transition from latent to active disease are not completely understood [3].
Proinflammatory cytokines are signaling molecules that are produced by T cells, macrophages, and other immune cells and promote inflammation and immunity. They are predominantly involved in the upregulation of immune responses, including activation of macrophages, induction of apoptosis, and recruitment of additional immune cells. These cytokines are thought to play a major role in host resistance to TB based mostly on studies in animal models [3, 4]. Thus, gene knockout mice lacking type 1 (interferon [IFN]γ, tumor necrosis factor [TNF]α), type 17 (interleukin [IL]-17), and other proinflammatory cytokines (IL-12, IL-1α, IL-1β) are known to be more susceptible to TB infection [3, 4]. However, this mechanism is not as well defined in humans. The only 2 major causative links in humans are the increased susceptibility to mycobacterial infection in humans with mutations in the IL-12 and IFNγ pathway [5] and the increased susceptibility to TB disease in individuals treated with TNFα blockade [6]. However, these cytokines also appear to exhibit duality in their functions [7]. Although important for host resistance, they are not sufficient, and in the setting of chronic infection, they can also induce harmful effects, including delayed resolution of inflammation, delayed tissue repair, and excessive tissue damage [8, 9]. Indeed, persistently elevated levels of these cytokines could mediate or prolong the underlying immune pathology in TB disease [9, 10].
To understand the role of proinflammatory cytokines in TB infection and disease, we sought to measure the levels of a panel of cytokines in pulmonary TB (PTB), latent TB (LTB), and healthy control (HC) individuals, correlate the levels of cytokines with disease severity/extent and bacterial burden/time to culture conversion, and examine the changes after anti-TB treatment. Our data reveal that proinflammatory cytokines are indeed associated with most of the above processes in PTB.
MATERIALS AND METHODS
Ethics Statement
All individuals were examined as part of a clinical research protocol (NCT01154959) approved by the Institutional Review Board of the National Institute for Research in Tuberculosis, and informed written consent was obtained from all participants.
Study Population and Procedures
Plasma samples were collected from 88 individuals with active PTB, 74 individuals with LTB, and 74 individuals with no TB infection or disease (HC), recruited in Chennai, India. Individuals with PTB were diagnosed by positive solid cultures in Lowenstein–Jensen medium. Chest x-rays were used to determine cavitary disease as well as unilateral versus bilateral involvement. Smear grade was used to determine bacterial burdens and classified as 1+, 2+, and 3+. Culture conversion (from positive to negative) at 2 months was defined as the cutoff for fast responders ([FR] who culture converted) versus slow responders ([SR] who were still culture positive). At the time of enrollment, all active TB cases had no record of prior TB disease or anti-TB treatment (ATT). Latent TB diagnosis was based on tuberculin skin test (TST) and QuantiFERON TB-Gold In-Tube positivity (double positive for both tests to reduce false-positive results), absence of chest radiograph abnormalities or pulmonary symptoms, and negative sputum smears. A positive TST result was defined as an induration at the site of tuberculin inoculation of at least 12 mm in diameter to minimize false positivity due to exposure to environmental mycobacteria [11]. The HC individuals were asymptomatic with normal chest x-rays and negative TST (indurations <5 mm in diameter) and QuantiFERON results. All participants were Bacillus Calmette-Guérin (BCG) vaccinated (identified by scar status), human immunodeficiency virus negative (using rapid test), nondiabetic (HbA1c levels <5.7), and had normal body mass index (>18.5 and <24.9 kg/m2). Participants were excluded if they exhibited signs or symptoms of any associated lung or systemic disease. The study groups were similar with regard to age and gender, and the baseline characteristics of the study participants are shown in Table 1. Anti-TB treatment (2 months of rifampicin, isoniazid, ethambutol, and pyrazinamide plus 4 months of rifampicin and isoniazid) was administered to PTB individuals using the directly observed treatment, short course (DOTS) strategy. At 6 months after ATT initiation, fresh plasma samples were obtained. All PTB individuals were culture negative at the end of ATT.
Table 1.
Demographics of the Study Groups
| Study Demographics | PTB | LTB | HC |
|---|---|---|---|
| Number of subjects recruited | 88 | 74 | 74 |
| Male | 61 | 46 | 42 |
| Female | 27 | 28 | 32 |
| Median age (range) | 48 (25–70) | 40 (25–67) | 32 (23–55) |
| Smear grade: 0/1+/2+/3+ | 0/32/27/29 | - | - |
| Lung lesions (bilateral) | 56 | - | - |
| Lung lesions (unilateral) | 32 | - | - |
| Cavitary disease | 26 | - | - |
| No cavitary disease | 62 | - | - |
Abbreviations: HC, healthy controls; LTB, latent tuberculosis; PTB, pulmonary tuberculosis.
Enzyme-Linked Immunosorbent Assay
Circulating levels of IFNγ, TNFα, IL-17A, IL-17F, IL-6, IL-12, and IL-1β were measured using the Bio-Plex multiplex cytokine assay system (Bio-Rad, Hercules, CA). The lowest detection limits were as follows: IFNγ, 4.39 pg/mL; TNFα, 3.24 pg/mL; IL-17A, 1.69 pg/mL; IL-17F, 46.8 pg/mL; IL-6, 7.7 pg/mL; IL-12, 4.66 pg/mL; and IL-1β, 3.96 pg/mL.
Statistical Analysis
Geometric means (GM) were used for measurements of central tendency. Statistically significant differences between PTB and LTB as well as PTB and HC were analyzed using the Kruskal-Wallis test with Dunn’s multiple comparisons. Principal component analysis (PCA) was done using statistical software JMP 13.0 (SAS, Cary, NC). Mann-Whitney test was used to compare cytokines concentrations between the individuals with PTB with unilateral or bilateral lung lesions or cavitary or noncavitary disease and SR or FR with Holm’s correction for multiple comparisons. Linear trend posttest was used to compare cytokine concentrations with smear grades (reflecting bacterial burdens). Wilcoxon signed-rank test was used to compare cytokines concentrations before and after ATT. Analyses were performed using GraphPad Prism, version 7.0.
RESULTS
Plasma Levels of Proinflammatory Cytokines Are Elevated in Pulmonary Tuberculosis
To determine the systemic levels of proinflammatory cytokines in TB infection and disease, we measured the circulating levels of IFNγ, TNFα, IL-17A, IL-17F, IL-6, IL-12, and IL-1β in PTB (n = 88), LTB (n = 74), and HC individuals (n = 74) (Figure 1). As shown in Figure 1A, the systemic levels of IFNγ (GM of 407.2 pg/mL in PTB vs 178.2 pg/mL in LTB and 90.63 pg/mL in HC; PTB vs LTB, P < .0001 and PTB vs HC, P < .0001), TNFα (GM of 260.2 pg/mL in PTB vs 133.9 pg/mL in LTB and 50.6 pg/mL in HC; PTB vs LTB, P < .0001 and PTB vs HC, P < .0001), IL-17A (GM of 101.9 pg/mL in PTB vs 52.1 pg/mL in LTB and 36.2 pg/mL in HC; PTB vs LTB, P < .0001 and PTB vs HC, P < .0001), and IL-17F (GM of 385.8 pg/mL in PTB vs 92.8 pg/mL in LTB and 59.8 pg/mL in HC; PTB vs LTB, P < .0001 and PTB vs HC, P < .0001) were significantly higher in PTB compared with both LTB and HC individuals. Likewise, as shown in Figure 1B, the systemic levels of IL-6 (GM of 148.3 pg/mL in PTB vs 141.8 pg/mL in LTB and 98.2 pg/mL in HC; PTB vs LTB, P = .9991 and PTB vs HC, P = .0003), IL-12 (GM of 60.5 pg/mL in PTB vs 41.9 pg/mL in LTB and 34.6 pg/mL in HC; PTB vs LTB, P = .2713 and PTB vs HC, P = .0012), and IL-1β (GM of 68 pg/mL in PTB vs 32.5 pg/mL in LTB and 18.4 pg/mL in HC; PTB vs LTB, P < .0001 and PTB vs HC, P < .0001) were significantly higher in PTB compared with LTB and/or HC individuals. A PCA model using data on all the cytokines was performed to compare and visualize the grouping between PTB, LTB, and HC individuals. This enables the detection of variation and set patterns in our dataset. Our PCA analysis clearly demonstrates the ability of these cytokines to discriminate PTB from LTB or HC individuals. Thus, PTB is associated with elevated systemic levels of proinflammatory cytokines.
Figure 1.

Elevated plasma levels of type 1, type 17, and proinflammatory cytokines in pulmonary tuberculosis (PTB) individuals. (A) The plasma levels of type 1 and type 17 cytokines were measured in individuals with PTB (n = 88) and latent TB ([LTB] n = 74) and healthy controls ([HC] n = 74). The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Kruskal-Wallis test with Dunn’s post hoc comparison. (B) The plasma levels of proinflammatory cytokines were measured in PTB (n = 88), LTB (n = 74), and HC (n = 74) individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Kruskal-Wallis test with Dunn’s post hoc comparison. (C) Principle component analysis (PCA) plot computing normalized enzyme-linked immunosorbent assay data from baseline plasma levels of cytokines in combination of 2 different experimental groups: first, PTB (blue) vs LTB (red) - the PCA shows the 2 principal components of variation, accounting for 41.8% (x-axis) and 15.7% (y-axis); and second, PTB (blue) vs HC (red) - the PCA shows the 2 principal components of variation, accounting for 48.3% (x-axis) and 15.2% (y-axis). IFN, interferon; IL, interleukin, TNF, tumor necrosis factor.
Plasma Proinflammatory Cytokines Are Markers of Disease Severity in Pulmonary Tuberculosis
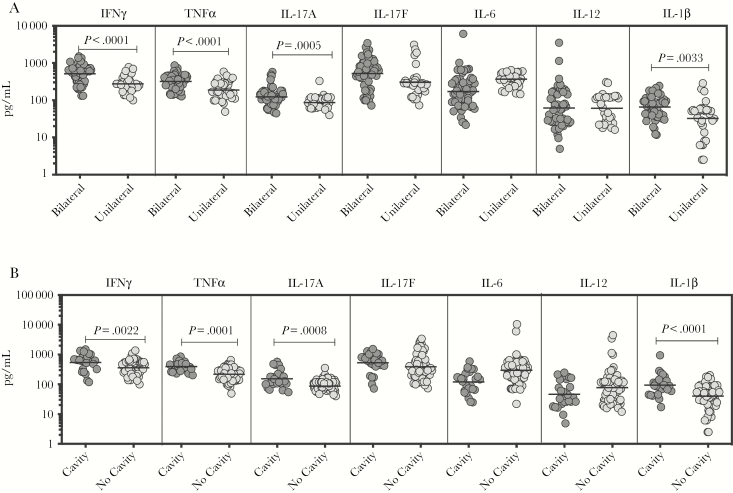
To determine the association between the systemic levels of proinflammatory cytokines and disease severity in PTB, we measured the circulating levels of IFNγ, TNFα, IL-17A, IL-17F, IL-6, IL-12, and IL-1β in PTB individuals with unilateral versus bilateral disease and with cavitary versus noncavitary disease (Figure 2). As shown in Figure 2A, the systemic levels of IFNγ (GM of 509 pg/mL in bilateral vs 275.6 pg/mL in unilateral disease, P < .0001), TNFα (GM of 316.4 pg/mL in bilateral vs 187.7 pg/mL in unilateral disease, P < .0001), IL-17A (GM of 123.4 pg/mL in bilateral vs 86.7 pg/mL in unilateral disease, P = .0005), and IL-1β (GM of 65.7 pg/mL in bilateral vs 32.5 pg/mL in unilateral disease, P = .0033) were significantly higher in PTB individuals with bilateral disease compared with unilateral disease. No significant differences were seen in the levels of IL-17F (GM of 502 pg/mL in bilateral vs 390 pg/mL in unilateral disease, P = .0601), IL-6 (GM of 204 pg/mL in bilateral vs 302 pg/mL in unilateral disease, P = .0533), and IL-12 (GM of 60 pg/mL in bilateral vs 62 pg/mL in unilateral disease, P = .9878) in PTB individuals with bilateral disease compared with unilateral disease. Likewise, as shown in Figure 2B, the systemic levels of IFNγ (GM of 544.7 pg/mL in cavitary disease vs 360.4 pg/mL in noncavitary disease, P = .0022), TNFα (GM of 397.4 pg/mL in cavitary disease vs 217.8 pg/mL in noncavitary disease, P = .0001), IL-17A (GM of 153.4 pg/mL in cavitary disease vs 87.2 pg/mL in noncavitary disease, P = .0008), and IL-1β (GM of 94 pg/mL in cavitary disease vs 39.9 pg/mL in noncavitary disease, P < .0001) were significantly higher in PTB individuals with cavitary disease compared with those without cavitary disease. No significant differences were seen in the levels of IL-17F (GM of 501 pg/mL in cavitary disease vs 412 pg/mL in noncavitary disease, P = .0634), IL-6 (GM of 190 pg/mL in cavitary disease vs 270 pg/mL in noncavitary disease, P = .0501), and IL-12 (GM of 46 pg/mL in cavitary disease vs 70 pg/mL in noncavitary disease, P = .3761) in PTB individuals with cavitary disease compared with those without cavitary disease. Thus, disease severity in PTB is associated with elevated systemic levels of proinflammatory cytokines.
Figure 2.
Plasma type 1 and type 17 cytokines are associated with (1) extent of disease or (2) disease severity in pulmonary tuberculosis (PTB) individuals. (A) The plasma levels of type 1, type 17, and proinflammatory cytokines were measured in in PTB individuals with bilateral versus unilateral disease, reflecting the extent of disease. (B) The plasma levels of type 1, type 17, and proinflammatory cytokines were measured in PTB individuals with cavitary versus noncavitary disease, reflecting the disease severity. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. IFN, interferon; IL, interleukin, TNF, tumor necrosis factor.
Plasma Proinflammatory Cytokines Are Markers of Bacterial Burdens in Pulmonary Tuberculosis and Are Elevated in Slow Responders
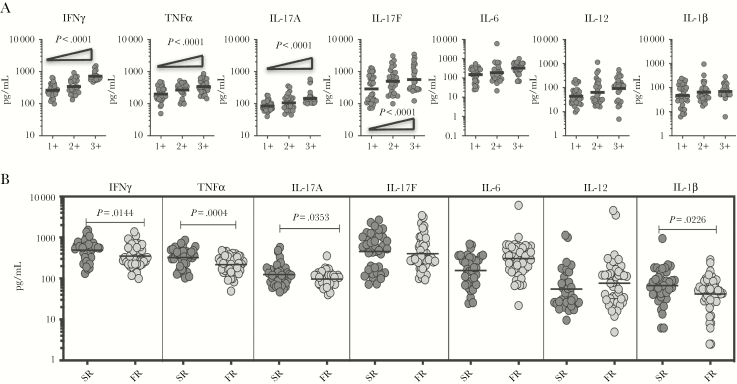
To determine the association between the systemic levels of proinflammatory cytokines and bacterial burden in PTB, we correlated the plasma levels of IFNγ, TNFα, IL-17A, IL-17F, IL-6, IL-12, and IL-1β in PTB individuals with smear grade classified as 1+, 2+, and 3+ (Figure 3). As shown in Figure 3A, the systemic levels of IFNγ, TNFα, IL-17A, and IL-17F exhibited a significant positive correlation with smear grades in PTB individuals, indicating a positive association of these factors with bacterial burdens. To determine the association between the systemic levels of proinflammatory cytokines and bacterial culture conversion in PTB, we correlated the plasma levels of IFNγ, TNFα, IL-17A, IL-17F, IL-6, IL-12, and IL-1β in PTB individuals with SR versus FR. As shown in Figure 3B, the systemic levels of IFNγ (GM of 493.2 pg/mL in SR vs 349.6 pg/mL in FR, P = .1433), TNFα (GM of 325.5 pg/mL in SR vs 217.7 pg/mL in FR, P = .0004), IL-17A (GM of 124.7 pg/mL in SR vs 96.7 pg/mL in FR, P = .0353), and IL-1β (GM of 66.7 pg/mL in SR vs 41.7 pg/mL in FR, P = .0226) were significantly higher in SR compared with FR. No significant differences were seen in the levels of IL-17F (GM of 457 pg/mL in SR vs 402 pg/mL in FR, P = .3899), IL-6 (GM of 188 pg/mL in SR vs 283 pg/mL in FR, P = .0511), and IL-12 (GM of 56 pg/mL in SR vs 75 pg/mL in FR, P = .2414) in SR versus FR. Thus, plasma proinflammatory cytokines are also markers of slow culture conversion in PTB.
Figure 3.
Plasma type 1 and type 17 cytokines are associated with bacterial burdens in pulmonary tuberculosis (PTB) individuals. (A) The relationship between the plasma levels of type 1, type 17, and proinflammatory cytokines and smear grades as estimated by sputum smears was examined in PTB individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Linear trend posttest. (B) The plasma levels of type 1, type 17, and proinflammatory cytokines were measured in PTB individuals, who were slow responders (sputum positive during second month of anti-TB treatment [ATT], SR) versus fast responders (sputum negative during second month of ATT, FR). The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons. IFN, interferon; IL, interleukin, TNF, tumor necrosis factor.
Plasma Proinflammatory Cytokine Levels Are Significantly Diminished After Antituberculosis Treatment
To determine whether the elevated levels of proinflammatory cytokines are directly associated with TB disease, we determined the levels of these cytokines in PTB individuals before and after a standard course of ATT (pre-T versus post-T). As shown in Figure 4, at the end of ATT, the plasma levels of IFNγ (GM of 407.2 pg/mL pre-T vs 222.2 pg/mL post-T, P < .0001), TNFα (GM of 260.2 pg/mL pre-T vs 154.5 pg/mL post-T, P < .0001), IL-17A (GM of 101.9 pg/mL pre-T vs 71.7 pg/mL post-T, P < .0001), IL-17F (GM of 385.8 pg/mL pre-T vs 126 pg/mL post-T, P < .0001), IL-6 (GM of 148.3 pg/mL pre-T vs 120.6 pg/mL post-T, P < .0001), IL-12 (GM of 60.5 pg/mL pre-T vs 25.1 pg/mL post-T, P < .0001), and IL-1β (GM of 68 pg/mL pre-T vs 35.4 pg/mL post-T, P < .0001) were all significantly lower compared with pretreatment levels. Thus, successful treatment of active TB results in significantly diminished levels of proinflammatory cytokines in PTB.
Figure 4.
Diminished plasma levels of type 1, type 17, and proinflammatory cytokines at the end of standard anti-tuberculosis (TB) therapy in pulmonary TB (PTB) individuals. The plasma levels of type 1, type 17, and proinflammatory cytokines PTB at baseline (pre-T) and at 6 months of anti-TB treatment (post-T). The data are presented as line graphs with each line representing a single individual. P values were calculated using the Wilcoxon signed-rank test. IFN, interferon; IL, interleukin, TNF, tumor necrosis factor.
DISCUSSION
Cytokines are typically thought of as mediators of resistance in TB, mainly from data from animal models but also due to human studies implicating IL-12/IFNγ and TNFα as primary effectors [3]. However, data from both animal models and human studies also indicate that cytokines can serve as a double-edged weapon with the levels and timing of cytokine production acting as determinants of protection versus pathology [7]. After infection, the interplay of proinflammatory and anti-inflammatory signals is vital in establishing the granuloma and in influencing its eventual trajectory [2, 12]. Skewing towards a robust proinflammatory cytokine milieu can promote bacterial dissemination, remodeling of the granuloma, and destruction of lung parenchyma [2, 13]. These processes typically underlie active TB and aid transmission. In our study, we demonstrate that heightened levels of proinflammatory cytokines are a hallmark of PTB disease, and these serve as markers of disease severity and bacterial burden and can be altered by ATT.
Type 1 cytokines, specifically IFNγ and TNFα, are crucial for protection against mycobacteria [3, 4]. The induction of IFNγ is mainly regulated by IL-12, which is produced by antigen-presenting cells. Genetic mutations affecting the IL-12/IFNγ pathway enhance the susceptibility to severe infections due to mycobacteria [5]. However, IFNγ alone is not sufficient to confer immunity because IFNγ levels in PTB are not usually compromised. It has also been reported that plasma IFNγ levels are highest in patients with advanced TB and decrease after ATT [14–16]. Tumor necrosis factor-α is also essential for control of Mtb infection [17]. In addition, TNFα is important in containment of persistent infection and prevention of reactivation in animal models [3]. Finally, there is a 5-fold increase in the rates of TB in individuals with LTB who have received anti-TNF therapy for rheumatoid arthritis or Crohn’s disease [6]. However, it has also been demonstrated that TNFα was a main determinant of pathogenesis and disease progression in a rabbit model of TB [18] and that high levels of TNFα could promote granuloma dissemination, progressive infection, and pathology in a zebra fish model of Mycobacterium marinum [19]. In addition, incomplete anti-inflammatory treatment regulating TNF improved TB outcomes [20]. Our data clearly imply that the presence of elevated levels of type 1 cytokines is a feature secondary to disease severity because heightened levels of both IFNγ and TNFα are observed in PTB individuals. More interestingly, our data also suggest an important association of IFNγ and TNFα with both the degree of pathology in TB as well as the extent of disease. Finally, our data reveal a direct correlation of IFNγ and TNFα levels with bacterial burdens and a negative correlation with time to culture conversion, suggesting that type 1 cytokines in PTB are mainly influenced by the density of infection. This is further corroborated by the fact that type 1 cytokine levels are significantly reduced after successful chemotherapy.
Type 17 cytokines are proinflammatory cytokines that primarily mediate resistance to extracellular bacteria and fungi but have been implicated in host immunity to TB. Interleukin-17A has been shown to be essential for the formation of mature granulomas in BCG and Mtb infection [21]. However, overproduction of IL-17A could also lead to increased immunopathology, associated with influx of neutrophils and onset of tissue destruction [8]. The role of IL-17F in TB is poorly understood. Our data clearly reveal that type 17 cytokines, especially IL-17A, is a marker of disease severity, bacterial burden, and time to culture conversion. The induction of protective type 1 immune responses to TB are dependent on IL-12 [4, 22]. Mice lacking IL-12 have been shown to be susceptible to TB disease [23]. Interleukin-6 is critical to resistance against TB after infection with high doses of intravenously delivered Mtb, but it is dispensable for the control of mycobacterial growth after low-dose aerosol-delivered infection [24, 25]. However, neither IL-12 nor IL-6 exhibited any major association with disease severity or bacterial burdens. The IL-1 family of cytokines have been shown to play an important role in resistance to murine infection with TB [12]; however, elevated levels of IL-1β in humans was associated with the development of active TB, the severity of pulmonary disease, and poor treatment outcome in TB patients [26]. Thus, our findings confirm and extend on this observation and suggest that IL-1β is a marker of disease severity and time to culture conversion in active TB. In addition, proinflammatory cytokine responses appear to be correlated to each other such that they are all linked to different measures of disease severity.
CONCLUSIONS
A recent systematic review of TB antigen-stimulated cytokines for monitoring anti-TB therapy reported that TNFα, IL-2, IL-6, IL-10, and IL-12 were the most promising biomarkers but that there was a great deal of variation between the studies [27]. In contrast, a targeted screen of 70 host biomarkers revealed IL-6 and IL-1β as the only proinflammatory cytokines that reflect treatment response and correlate with smear grade [28]. Although various studies have assessed the association between cytokines and chest x-ray abnormalities in TB, only TNFα and IL-1β have been shown to exhibit a positive correlation with the presence or size of cavities and to also exhibit reduction in plasma levels after treatment [9]. Another recent systematic review on TB biomarkers concluded that most of the biomarker studies suffer from poor design and that findings are rarely validated [29]. Our data expand on these findings and suggest that a larger panel of cytokines are associated with disease pathology and severity. A variety of studies have examined the cytokine response in antigen-stimulated samples from PTB individuals [30–33]. However, the disadvantage of using stimulated samples is the difficulty in translating the assays to a point-of-care mode. Therefore, our study fills in the knowledge gap by performing longitudinal profiling of plasma cytokines and describing the association with disease severity and bacterial burdens. Our study also delineates cytokine predictors of time to culture conversion by demonstrating the association of certain cytokines with FR versus SR in terms of time to culture conversion. In addition, our PCA analysis clearly demonstrate the importance of these cytokines in demarcating TB disease from TB infection and HC. The limitations of our study include the lack of direct cause and effect linkage and the absence of a validation cohort, but this is countered by the relatively good sample size. There is also a significant overlap in the distribution of cytokines between the different groups signifying the need for the results to be tested in future studies. In conclusion, the findings of this study highlight the importance of cytokines in pathology of TB disease and can be validated in multiple settings in the future.
Acknowledgments
We thank the staff of Department of Clinical Research and Department of Social Work, National Institute for Research in Tuberculosis, for valuable assistance in recruiting the patients for this study, the staff of Department of Bacteriology for cultures, and Prabbu Balakrishnan and Yukthi Bhootra of the National Institutes of Health-International Center for Excellence in Research for technical assistance.
Financial support. This work was funded by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers 2016; 2:16076. [DOI] [PubMed] [Google Scholar]
- 2. Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol 2017; 17:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Garra A, Redford PS, McNab FW, et al. The immune response in tuberculosis. Annu Rev Immunol 2013; 31:475–527. [DOI] [PubMed] [Google Scholar]
- 4. Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 2009; 27:393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 2002; 20:581–620. [DOI] [PubMed] [Google Scholar]
- 6. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–104. [DOI] [PubMed] [Google Scholar]
- 7. Dorhoi A, Kaufmann SH. Pathology and immune reactivity: understanding multidimensionality in pulmonary tuberculosis. Semin Immunopathol 2016; 38:153–66. [DOI] [PubMed] [Google Scholar]
- 8. Torrado E, Robinson RT, Cooper AM. Cellular response to mycobacteria: balancing protection and pathology. Trends Immunol 2011; 32:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stek C, Allwood B, Walker NF, et al. The immune mechanisms of lung parenchymal damage in tuberculosis and the role of host-directed therapy. Front Microbiol 2018; 9:2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cicchese JM, Evans S, Hult C, et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev 2018; 285:147–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radhakrishna S, Frieden TR, Subramani R; Tuberculosis Research Centre (ICMR) Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in South India: a 15-year follow-up. Int J Tuberc Lung Dis 2003; 7:1083–91. [PubMed] [Google Scholar]
- 12. Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol 2011; 4:252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coleman MT, Maiello P, Tomko J, et al. Early changes by (18)Fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun 2014; 82:2400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsao TC, Huang CC, Chiou WK, et al. Levels of interferon-gamma and interleukin-2 receptor-alpha for bronchoalveolar lavage fluid and serum were correlated with clinical grade and treatment of pulmonary tuberculosis. Int J Tuberc Lung Dis 2002; 6:720–7. [PubMed] [Google Scholar]
- 15. Vankayalapati R, Wizel B, Weis SE, et al. Serum cytokine concentrations do not parallel Mycobacterium tuberculosis-induced cytokine production in patients with tuberculosis. Clin Infect Dis 2003; 36:24–8. [DOI] [PubMed] [Google Scholar]
- 16. Juffermans NP, Verbon A, van Deventer SJ, et al. Elevated chemokine concentrations in sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with tuberculosis: a possible role for mycobacterial lipoarabinomannan. Infect Immun 1999; 67:4295–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995; 2:561–72. [DOI] [PubMed] [Google Scholar]
- 18. Tsenova L, Bergtold A, Freedman VH, et al. Tumor necrosis factor alpha is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc Natl Acad Sci U S A 1999; 96:5657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tobin DM, Vary JC Jr, Ray JP, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 2010; 140:717–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsenova L, Sokol K, Freedman VH, Kaplan G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J Infect Dis 1998; 177:1563–72. [DOI] [PubMed] [Google Scholar]
- 21. Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8:369–77. [DOI] [PubMed] [Google Scholar]
- 22. Cooper AM, Khader SA. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunol Rev 2008; 226:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 1997; 186:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saunders BM, Frank AA, Orme IM, Cooper AM. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun 2000; 68:3322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ladel CH, Blum C, Dreher A, et al. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun 1997; 65:4843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang G, Zhou B, Li S, et al. Allele-specific induction of IL-1β expression by C/EBPβ and PU.1 contributes to increased tuberculosis susceptibility. PLoS Pathog 2014; 10:e1004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clifford V, Zufferey C, Street A, et al. Cytokines for monitoring anti-tuberculous therapy: a systematic review. Tuberculosis (Edinb) 2015; 95:217–28. [DOI] [PubMed] [Google Scholar]
- 28. Sigal GB, Segal MR, Mathew A, et al. Biomarkers of tuberculosis severity and treatment effect: a directed screen of 70 host markers in a randomized clinical trial. EBioMedicine 2017; 25:112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacLean E, Broger T, Yerlikaya S, et al. A systematic review of biomarkers to detect active tuberculosis. Nat Microbiol 2019; 4:748–58. [DOI] [PubMed] [Google Scholar]
- 30. Chegou NN, Sutherland JS, Namuganga AR, et al. Africa-wide evaluation of host biomarkers in QuantiFERON supernatants for the diagnosis of pulmonary tuberculosis. Sci Rep 2018; 8:2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sutherland JS, Adetifa IM, Hill PC, et al. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol 2009; 39:723–9. [DOI] [PubMed] [Google Scholar]
- 32. Awoniyi DO, Teuchert A, Sutherland JS, et al. Evaluation of cytokine responses against novel Mtb antigens as diagnostic markers for TB disease. J Infect 2016; 73:219–30. [DOI] [PubMed] [Google Scholar]
- 33. Clifford V, Tebruegge M, Zufferey C, et al. Cytokine biomarkers for the diagnosis of tuberculosis infection and disease in adults in a low prevalence setting. Tuberculosis (Edinb) 2019; 114:91–102. [DOI] [PubMed] [Google Scholar]