Abstract
Given the prevalence of lateral ankle sprains during physical activity and the high rate of reinjury and chronic ankle instability, clinicians should be cognizant of the need to expand the evaluation of ankle instability beyond the acute time point. Physical assessments of the injured ankle should be similar, regardless of whether this is the initial lateral ankle sprain or the patient has experienced multiple sprains. To this point, a thorough injury history of the affected ankle provides important information during the clinical examination. The physical examination should assess the talocrural and subtalar joints, and clinicians should be aware of efficacious diagnostic tools that provide information about the status of injured structures. As patients progress into the subacute and return-to-activity phases after injury, comprehensive assessments of lateral ankle-complex instability will identify any disease and patient-oriented outcome deficits that resemble chronic ankle instability, which should be addressed with appropriate interventions to minimize the risk of developing long-term, recurrent ankle instability.
Lateral ankle sprains (LASs), the most prevalent neuromusculoskeletal injury in general and among physically active populations, are associated with long-term pain, disability, and a substantial financial burden.1,2 Between 50% and 70% of the people who sustain an LAS will develop chronic ankle instability (CAI), a condition characterized by lingering pain, instability, injury recurrence, and persistent functional disability.3 Chronic symptoms and reinjury have been shown to occur rapidly, with recent authors4–8 demonstrating the onset of CAI symptoms as early as 6 to 12 months after the initial acute LAS. The steps for determining the status of an LAS through clinical examination are well established and can be supplemented with advanced imaging. However, given the high rates of reinjury and CAI, clinicians should recognize the need to expand the evaluation of ankle instability beyond the acute time point. As patients progress through rehabilitation, lateral ankle complex instability should be assessed to identify any deficits characteristic of CAI, so that appropriate interventions can be implemented.
The ligamentous support of the ankle complex can be considered in terms of the talocrural, distal tibiofibular, and subtalar articulations. With lateral instability being the most common source of ankle instability, I will focus on the assessment of lateral talocrural instability and consider the contributions of the subtalar joint to LAS. The mechanism of injury for LAS has been described consistently in the literature1 as an acute episode of excessive supination of the rearfoot in relation to an excessively externally rotated lower limb during a closed kinetic chain position, typically excessive inversion with accompanying plantar flexion. This injury mechanism is associated with an index LAS as well as repetitive injuries that are experienced by patients with CAI. Therefore, physical assessments of the injured ankle should be similar, regardless of whether this is the patient's first LAS or one in a series of recurrent sprains. To this point, a thorough injury history of the affected ankle offers important value during the clinical examination.
The focal point of the examination of a suspected LAS often becomes the status of the critical lateral ligamentous structures. After completing a history, identifying symptoms, and ruling out a potential fracture, the clinician should test the integrity of the potentially affected soft tissues, specifically the lateral ligaments: anterior talofibular (ATF), calcaneofibular (CF), and posterior talofibular (PTF).9 Athletic trainers are well educated in procedures for examining overall ankle motion and performing stress tests to isolate these and other ligaments. However, advances in technology provide resources for obtaining additional objective information via stress testing and imaging. Although these resources offer confirmatory utility, the clinician must determine if they are available and necessary to supplement the physical examination.
Subtalar joint disruption and resultant instability have been suggested to accompany lateral talocrural joint instability. Symptoms of subtalar joint instability are not as apparent as those of lateral ligamentous instability during clinical examination, but this does not diminish the importance of assessing the subtalar joint when evaluating a suspected LAS, which may provide critical insight into the progression of CAI.
A thorough physical examination of the affected articulations and associated structures during the acute and subacute stages of recovery is vital for determining the injury classification of an LAS. Yet because of the high risk for reinjury and lingering symptoms, it is also important to comprehensively evaluate patient-reported and neuromuscular-control outcomes as the patient progresses toward a return to activity.9 Treating clinicians should be aware of the most common CAI deficiencies in order to identify any lingering deficiencies that need continued intervention. Recognizing and addressing the deficiencies during these early stages are likely to emerge as critical to reducing the rate of CAI.
The intention of this review article is to provide evidence-informed guidelines for comprehensively assessing patients with acute and subacute lateral ankle instability. The paper is organized in 2 main sections: (1) overview of the physical examination of the lateral ankle complex and (2) examination of the patient with subacute LAS for characteristics of CAI before the return to activity.
REVIEW OF COMMON EVIDENCE-BASED PRINCIPLES FOR DIAGNOSTIC OUTCOMES
To emphasize the influence of the literature summarized in this review, I present several evidence-based statistical outcomes to support recommendations for best-practice evaluation approaches and techniques. Selected outcomes from the reviewed studies are provided in Table 1. For example, diagnostic statistics such as sensitivity and specificity can be helpful in determining the strength of an evaluation tool in making the correct assessment.23 Sensitivity indicates how well a diagnostic tool rules out a condition, or in more practical terms, the rate of true positive case identification by the diagnostic tool (ie, a positive anterior drawer test equates with an actual case of ankle instability). Similarly, specificity helps to rule in, or in practical terms, highlights the rate of true negative identification using that diagnostic tool. Both sensitivity and specificity are represented as percentage scores, with values closer to 100% indicating higher rates of true positives and true negatives, respectively. Sensitivity or specificity >70% commonly implies a strong outcome.
Table 1.
Efficacy of Diagnostic Tests for Evaluating Instability of the Anterior Talofibular (ATF) and Calcaneofibular (CF) Ligamentsa
| Diagnostic Test |
Reported Scores |
Calculated Scores |
||
| Sensitivity |
Specificity |
+ Likelihood Ratio |
− Likelihood Ratio |
|
| Anterior drawer | ||||
| In vivo10,11 | 73–96 | 84–97 | 6.00–24.33 | 0.048–0.278 |
| Cadaver12,13 | 75–100 | 50–67 | 1.5–3.02 | 0.001–0.500 |
| Anterior lateral drawer (cadaver)12,13 | 100 | 67–100 | 3.02–999.0 | 0.001–0.002 |
| Stress radiographs14 | 31–74 | 92–100 | 1.92–710.0 | 0.290–0.693 |
| Diagnostic ultrasound | ||||
| ATF ligament15–18 | 94–100 | 50–100 | 12.25–940.0 | 0.010–0.060 |
| CF ligament17 | 94 | 91 | 10.44 | 0.066 |
| Magnetic resonance imaging | ||||
| ATF ligament19–22 | 67–87 | 53–100 | 1.766–4.47 | 0.001–0.321 |
| CF ligament20,21 | 40–47 | 83–100 | 1.66–2.76 | 0.002–0.638 |
Sensitivity and specificity scores, reported as %, are represented from the published studies. Positive and negative likelihood ratios were calculated from these published scores. Diagnostic tests are grouped with bolded and lightface rows. When a sensitivity or specificity was reported as 100%, a value of 99.9% was substituted in the formula to calculate the likelihood ratios.
From sensitivity and specificity, positive and negative likelihood ratios can be calculated. These ratios indicate how well a diagnostic tool validates a prediction that a patient does or does not have the injury or condition.23 For example, if a patient reports that an injury mechanism (inversion) occurred with painful symptoms over the affected body part (lateral ankle), the clinician may or may not suspect the presence of a fracture. The clinician may apply the Ottawa Ankle Rules (OAR), which have diagnostic capabilities.24–26 If the clinician determines the probability of a fracture in the ankle complex is high, then a positive outcome on the OAR would validate the suspicion of a fracture, as this diagnostic tool has a strong positive likelihood ratio. In contrast, if the clinician suspects that the patient did not sustain a fracture, then a negative outcome on the OAR would validate the clinician's suspicion, as this diagnostic tool has a strong negative likelihood ratio. The larger the positive likelihood ratio, the greater the strength of the positive test validation.23 In contrast, negative likelihood values that approach zero provide stronger support that the diagnostic tool can validate a clinician-suspected negative case.
Identifying diagnostic tools and techniques that possess good sensitivity and specificity and can provide strong positive and negative likelihood ratios supplies important categorical information for interpreting the presence or absence of a symptom or injury (eg, yes or no, positive or negative). Clinicians may also use diagnostic tools that provide a range or scale of information that could indicate if the patient presents with a deficiency (eg, range of motion, perceived disability) that helps to define a condition. These noncategorical outcomes help clinicians determine if the patient's presentation differs from that of an uninjured “normal” patient, such as a healthy individual or the unaffected contralateral side. Additionally, the clinician may want to know how much change in a diagnostic test indicates that the patient's previous deficiency has diminished enough to be considered inconsequential during a follow-up appointment. These statistical outcomes are based on a concept of minimally important change, or how much of a difference between 2 scores (eg, bilateral comparison, acute versus subacute assessment) indicates a meaningful difference.23 Two examples that are common in the athletic training-related literature and will be highlighted in this review are the minimally important change or minimal detectable change (MDC) and minimal clinically important difference (MCID). The MDC is the amount of change needed to exceed a natural variation in a measurement, which would represent a true change, whereas the MCID is the smallest amount of difference recorded on an instrument that a patient would perceive as beneficial.23 Both may or may not be reported in a published study, but typically the MDC for a diagnostic tool is lower than the MCID.23
A final aspect of the available evidence-based assessments of diagnostic tools is the importance of consistent findings in the literature. A clinician must decide if conclusions from published studies are homogeneous or heterogeneous. Obviously, if the findings related to the utility of a diagnostic tool are more homogeneous, then the clinician should be more willing to implement the tool if the findings suggest consistent positive utility; in contrast, if multiple studies show the tool cannot provide good diagnostic discrimination, the clinician should not spend time and resources using the tool. If the literature is more divided, or heterogeneous, then this suggests to the clinician that the literature cannot yet support use or non-use of that diagnostic tool in clinical practice.
OVERVIEW OF PHYSICAL EXAMINATION OF THE LATERAL ANKLE COMPLEX
Clinical Examination
Any evaluation of a patient with a suspected neuromusculoskeletal injury should begin with thorough observation to determine any obvious signs of trauma or deformity and a historical interview to determine the mechanism of injury, as well as sensations and audible clues, such as potential popping or snapping, and pain over the joint complex. Identifying a history of LAS is also necessary. This information should be noted for important follow-ups after the extent of the current injury is established. Additionally, the clinician should inquire about any injury history in the contralateral ankle before performing bilateral comparisons to judge the status of the currently injured ankle.
The OAR have been widely accepted as a valid and reliable set of palpation sites used to determine the risk of a potential fracture and whether radiographic imaging is warranted or unnecessary. The OAR recommend radiographs for a patient who (1) is unable to bear weight or walk 4 steps, (2) has palpable tenderness at the posterior edge or tip of the medial or lateral malleolus, or (3) has pain at the base of the fifth metatarsal.24–26 Systematic reviews and meta-analyses consistently demonstrate high and homogeneous sensitivities (>97%), indicating a robust rate of identifying true positives, supporting the use of the OAR to rule out the risk of an ankle fracture.24–26 However, specificities in these reviews and analyses are heterogeneous and low, raising concern about the ability to identify true negative cases. Nonetheless, the OAR have the potential to reduce unnecessary ankle radiographs by up to 40%.24,26 The clinician's level of experience, patient's age, cultural perceptions and communication of pain, and time since the acute insult all can influence the successful application of the OAR. A reasonable approach to clinical decision making is that if the patient does not present with OAR signs and symptoms, then radiographs are not immediately warranted, but regular follow-ups are good practice; any positive OAR criteria necessitate strong consideration for further consultation.
Assuming that the OAR indicate a low risk of ankle fracture, the physical evaluation may continue with assessments of swelling, palpable tenderness, and range of motion. Palpable pain is expected over and around the sinus tarsi in the area of the ATF and possibly under the lateral malleolus in the area of the CF. With involvement of the dynamic restraints from muscle contractions, soreness may occur along muscles such as the fibularis longus or brevis. A thorough palpation of the ankle complex and identification of areas of tenderness should be completed. Palpable swelling may be present in these and other areas around the lateral ankle complex. However, accumulated edema and effusion may not be evident at the time of the acute injury assessment. Bilateral comparisons of active, passive, and resistive motion to determine deficiencies should be conducted and any pain during these motions noted. Clinicians must consider the injury history of the affected ankle as they assess range of motion because persistent arthrokinematic restrictions from a previous LAS could result in motion limitations. It is important to compare motion restrictions observed during the acute stage with a reassessment after pain and swelling have subsided to ascertain the source of any motion limitations. Often, clinicians will compare the joints bilaterally to determine the status and progression of the recently injured ankle, but as stated earlier, the clinician must inquire about the injury history of the contralateral ankle. Overlooking a previous injury to the contralateral ankle could introduce error when determining if the currently injured ankle has any measurable restrictions or limitations.
The final component of the physical assessment for acute lateral ankle instability should focus on ligamentous integrity using mechanical stress tests. The most commonly implicated lateral ankle ligament is the ATF, followed in order of injury prevalence by the CF and PTF. Therefore, the anterior drawer test (ADT) is typically the first test performed. The clinician usually places the lower leg on the edge of a table, preferably in slight knee flexion to relax the gastrocnemius. However, Kovaleski et al27 reported that the greatest amount of anterior translation and isolation of the ATF ligament occurred with the knee flexed to 90° and the ankle in 10° of plantar flexion. With the lower limb stabilized using 1 hand and the heel firmly gripped using the other hand, the calcaneus is translated anteriorly (Figure 1). A positive test is associated with excessive anterior translation and the lack of a solid end feel or perhaps a clunking sensation. The amount of linear excursion for a positive test is debated, and hence, bilateral comparison is recommended.28 An accompanying sulcus sign of the sinus tarsi is likely to emerge, and the patient may or may not describe pain. In isolation, a positive ADT had good to excellent sensitivity (73%–96%) and specificity (84%–97%) for identifying a compromised ATF.10,11 Palpable pain and a hematoma with a positive anterior drawer improve the sensitivity (100%) but provide only good specificity (77%), thereby offering perfect true positive identification but only moderate true negative conclusions.10
Figure 1.
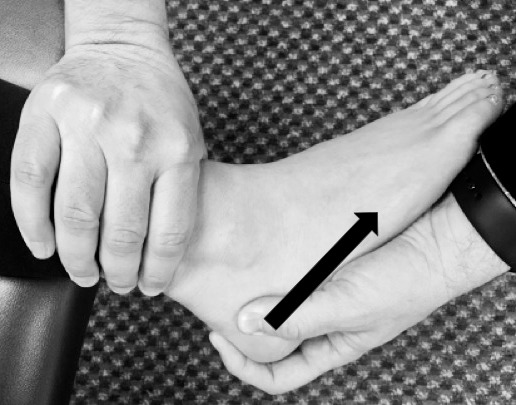
Anterior drawer test.
A variation of the ADT, the anterolateral drawer test (ALDT), has been suggested to minimize potential movement of the subtalar joint with the goal of reducing potentially false findings of isolated talocrural instability.12,13,29 The ALDT stabilizes the lower limb as in the ADT but the thumb of the translating hand is placed over the sinus tarsi, with the tip touching the anterolateral surface of the lateral malleolus (Figure 2). As anterior translation is applied to the calcaneus, the clinician can appreciate talar movement and the associated sulcus created in the sinus tarsi when ATF instability is present.12,13,29 Miller et al29 observed 3 to 4 mm of increased anterior talar translation on the ALDT compared with the ADT. With a cutoff score of 3 mm of anterior talar translation, Phisitkul et al13 found perfect sensitivity (100%) and specificity (100%) with the ALDT but only good sensitivity (75%) and poor specificity (50%) with the ADT. When the diagnostic cutoff was increased to 4 mm of translation, Vaseenon et al12 noted that both the ALDT and ADT had the same perfect sensitivity scores (100%) and moderate specificity scores (67%). However, the ALDT yielded stronger validity (intraclass correlation coefficient [ICC] = 0.73) with a criterion standard of direct anatomical measures than the ADT (ICC = 0.57).12 The major limitation to these 3 studies was that the assessments were performed on cadaveric specimens.12,13,29 Clinical studies of patients with or without ankle instability are needed to strengthen the case for using the ALDT.
Figure 2.
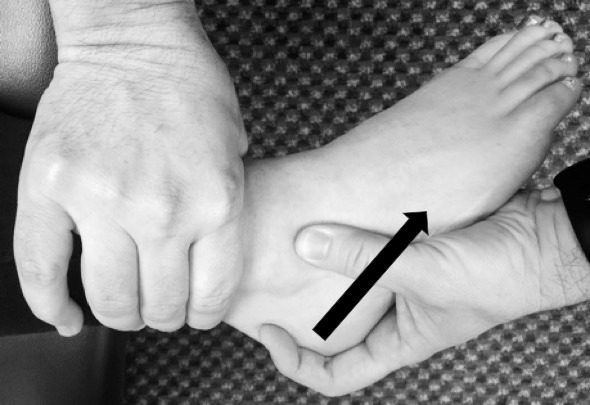
Anterolateral drawer test.
During an LAS, the CF ligament is typically implicated secondary to an ATF ligament injury. Therefore, CF ligament integrity is commonly assessed after the ATF ligament is evaluated. Excessive rearfoot adduction, or talocrural inversion, is checked by the CF ligament. Subsequently, a rotational stress test to the talocrural joint is applied in the form of a talar tilt test. With the ankle in neutral position, the distal tibia and fibula are stabilized while the opposite hand provides a medial rotational force to stress the lateral portion of the joint (Figure 3). The mobilizing hand grasps the calcaneus, but care should be taken to move the talus with the calcaneus so as to isolate the talocrural joint from the subtalar joint. The range of positive findings has been debated, but in general, more than 10° of movement is believed to indicate instability.28 As with the ADT, the best comparison is with the opposite ankle, yet as mentioned previously, the clinician should be aware of a patient's history of ankle sprains, which could have resulted in preexisting instability.
Figure 3.

Talar tilt test.
Subtalar instability has been suggested as accompanying talocrural instability when individuals sustain an LAS. However, the subtalar contribution to overall ankle instability is often overlooked because the symptoms and ligamentous integrity are more challenging to quantify than those in the talocrural joint. The subtalar joint may be considered as 2 primary articulations: the anterior (talonavicular) and the posterior (talocalcaneal).30,31 The important ligamentous contributions to these articulations can be grouped into intrinsic (interosseus talocalcaneal and cervical) and extrinsic (calcaneofibular and tibiocalcaneal fascicle of the deltoid) ligaments.30,31 Injury to the subtalar joint typically accompanies suspected injury to the CF ligament during an LAS when excessive supination of the rearfoot, or overall inversion, is experienced. Excessive palpable pain in the sinus tarsi along with laxity during an inversion stress test may provide insight regarding potential subtalar instability, but it is challenging to differentiate subtalar joint instability from talocrural instability during the evaluation of a patient with an acute LAS.30–33
Subsequently, follow-up assessment of the subtalar joint after the initial pain and swelling from the LAS have subsided is warranted, and specific tests have been suggested. Thermann et al34 developed a stress test that involves internal rotation with varus stress to the calcaneus to elicit medial shifting of the calcaneus; however, this test has not been well validated. In a small sample of CAI patients, Hertel et al35 found that the medial subtalar glide test elicited excessive medial calcaneal excursion in 78% of the patients who also had a positive talar tilt test. Positive results on these stress tests may provide insight to the clinician. However, more evidence is needed about the use of these and other clinical stress tests during the examination of a patient with an acute LAS. Therefore, advanced imaging for more definitive documentation is currently recommended. For example, using stress radiography, separation of the posterior talocalcaneal facet of more than 7 mm36 and a 5-mm anterior displacement of the calcaneus37 have been used to define subtalar instability. More recent validation of these recommendations is needed. Other imaging techniques such as fluoroscopy, computed tomography, and magnetic resonance imaging (MRI) may also prove useful, but additional evidence is needed to determine the best-practice recommendations.
Grading of Severity
Clinicians commonly rate the severity of ligamentous injury associated with LAS. The typical grading scale of a ligament injury consists of grade I, which describes minor elongation with microdamage; grade II, more involved stretching and insult but without compromised structural integrity; and grade III, complete rupture. Often with an LAS, the focus is also on which ligaments are involved.28,38–41 With a mild or grade I LAS, minor ligamentous involvement may or may not be specific to the ATF. A moderate or grade II LAS is associated with a significant loss of ligamentous integrity of the ATF and possible mild CF involvement. Finally, a severe or grade III LAS indicates significant structural compromise of the ATF and CF and likely some threat to the PTF or capsular integrity (or both). A summary of frequently used criteria for grading the severity of grade I to III LASs is provided in Table 2.
Table 2.
Summary of Common Lateral Ankle Sprain Severity Grading Scalesa
| Grade I | |
| Little to no pain and swelling | |
| Minimal loss of weight-bearing ability | |
| Minimal loss of mechanical stability: | |
| Partial tear of the lateral ligament complex; typically isolated to the anterior talofibular ligament | |
| Minimal loss of motion | |
| Short-term loss of function | |
| Grade II | |
| Moderate pain and swelling | |
| Moderate loss of weight-bearing ability | |
| Moderate loss of mechanical stability | |
| Significant compromise to the anterior talofibular ligament | |
| Intact but partially disrupted calcaneofibular ligament | |
| Moderate loss of joint motion | |
| Significant loss of function | |
| Grade III | |
| Severe pain and swelling | |
| Diffuse swelling | |
| Extreme point tenderness | |
| Severe loss of weight-bearing ability | |
| Severe loss of mechanical stability | |
| Complete tear of lateral ankle complex | |
| Disrupted ankle capsule | |
| Significant loss of joint motion | |
| Severe loss of function | |
After a detailed history, a thorough physical examination should supply the clinician with a strong suspicion of the status of a suspected acute LAS and an indication of its severity. Damage to the bony, vascular, or neural structures is important to recognize at the time of acute injury, followed by appropriate referral for more complex assessment and management. If compromise to these structures is ruled out, follow-up assessment is recommended after initial management (protection, rest, ice, compression, elevation), with the best results emerging at 5 to 6 days after injury.11,42,43 Additionally, advanced diagnostic imaging may prove useful for verifying the clinical evaluation and follow-up assessments.
Instrumented Assessment and Imaging of Lateral Ankle Ligaments
As discussed earlier, athletic trainers and other health care providers are well prepared to assess a patient with a possible acute LAS. These examination procedures are appropriate for determining the risk of a fracture and arriving at a confident decision about the presence of an LAS. However, clinicians should be aware of various diagnostic options for verifying ligamentous deficiencies and other sources of ankle-joint instability after the traditional clinical physical examination. Subsequently, if verification of the physical examination is warranted, clinicians should determine the availability of resources such as arthrometry, stress radiography, diagnostic ultrasound, and MRI. These imaging options can be helpful in making clinical decisions, but the cost-to-benefit ratio must be considered. Furthermore, the efficacy of the imaging options must be considered and is summarized in the next sections.
Stress Radiography
When an ankle fracture is suspected after application of the OAR, standard radiography is appropriate and is typically accessible for most patients with sport-related ankle injuries. However, standard radiography is not the ideal imaging choice for assessing integrity of the ankle soft tissues. Stress radiography can quantify the extent of ankle-joint laxity by demonstrating the separation of the bony joint structures while a force is applied, revealing potentially excessive strain within the ligamentous structures. Anteroposterior and lateral views are usually obtained while the ankle is manually translated in the direction of suspected ligamentous laxity. Traditionally, total anterior translation >9 mm or translation >5 mm (or both) compared with the contralateral side is thought to indicate significant laxity of the ATF, whereas a talar tilt angle >10° in total or more than 5° greater than the contralateral limb indicates pathologic laxity of the CF ligament.44,45 However, other authors19,45,46 have suggested slight variations to these ranges, as well as concerns about high levels of variability and inconsistency.
Using a standardized device to apply the force reduces the potential limitation of an inconsistently applied stress, which may improve the value of the diagnostic information. The sensitivity of stress radiography applied to patients with LAS is concerning (31%–74%), whereas the specificity is much stronger (92%–100%).14 These results suggest that this resource should be considered for identifying true negative cases, but identifying true positive cases may be challenging. Stress radiography is relatively low in cost, but the patient is exposed to radiation; additional potential limitations include inconsistent orientations and views of the ankle and patient cooperation during the examination while the ankle is stressed, especially if the injury is acute.47
Arthrometry
Similar to stress radiography, arthrometers can provide a controlled stress to the ankle joint during anterior and inversion translation, demonstrating associated linear (mm) and angular (degrees) displacement for an objective quantification of laxity. Thus, they offer a cost-effective, dynamic assessment of ligamentous integrity as an alternative to stress radiography, while avoiding the radiation exposure that may concern some patients. Also similar to stress radiography, arthrometry may not be ideal during the acute assessment of a suspected LAS due to pain and apprehension.
Hubbard and Cordova48 compared arthrometry measures bilaterally between a group with acute LAS and an uninjured control group. Significant laxity was noted on the injured side compared with the uninjured side and for anterior displacement and inversion rotation in the control group, supported by strong effect sizes. Interestingly, those same relationships persisted at the 8-week postinjury follow-up. Hubbard et al49 used ankle arthrometry to quantify bilateral differences in patients with unilateral functional ankle instability; greater anterior linear displacement was present in the affected limb compared with the uninjured limb. However, the effect size was weak with a 95% confidence interval that crossed zero (d = 0.32 [−0.08, 0.71]). Additionally, the investigators did not find limb differences for inversion angular displacement. In a follow-up comparison with an uninjured group, Hubbard50 reported greater anterior displacement and inversion rotation on the CAI group's injured side, this time supported by strong effect sizes (0.85–1.36) with 95% confidence intervals that did not cross zero. Thus, instrumented ankle arthrometry may provide objective laxity data for the assessment of acute injury and CAI. Other devices have been examined51 but have not yielded equivalent reliability or pathologic differentiation.
Ankle arthrometry is likely to provide a useful objective diagnostic outcome in ankle-ligament evaluation. Yet further validation of the technique against other diagnostic tools is needed. Strong agreement was present between arthrometry measures and stress radiography in cadaveric samples,52 but to date, no authors have studied patient samples. Continued work is required to fully quantify the utility of ankle arthrometry in comparison with other diagnostic techniques when assessing patients with LAS.
Diagnostic Ultrasound
The use of diagnostic ultrasound for detecting ligamentous lesions of the ankle is growing as the technology has become more affordable and available. It may be used for static assessment of ligamentous integrity or cross-sectional area, as well as dynamic assessment of the articulating surfaces as the joint is stressed (similar to stress radiography). Diagnostic ultrasound provides an inexpensive imaging option that is versatile due to its portability and appealing to many patients because it does not expose them to radiation or enclosures. Also, it can be used during the acute phase of an LAS with little patient setup and without stressing the joint.
The evidence suggests that diagnostic ultrasound offers moderate to strong confirmation of lateral ligamentous injury in patients with suspected LAS. Reported sensitivities for identifying ATF injuries in patients with acute LAS using static ultrasound ranged from 94% to 100% and specificities ranged from 50% to 100%.15–18 Cheng et al17 observed strong sensitivity (94%) and specificity (91%) for identifying CF injury. Croy et al,53 using a stress ultrasound assessment, demonstrated increased lateral ligament lengths during the anterior drawer and talar tilt tests on the injured side compared with the noninjured side, as well as among individuals with a history of CAI compared with healthy controls.54 The literature indicates that diagnostic ultrasound may be a viable diagnostic tool for identifying true positive cases of ATF injury, but the evidence is inconsistent for identifying true negative cases. Until more evidence becomes available, it is difficult to conclude whether diagnostic ultrasound can identify CF injury.
Magnetic Resonance Imaging
Noninvasive MRI is becoming increasingly available in health care systems that treat injuries in the physically active and can provide detailed visualization of the structures implicated in an LAS. A static measurement like ultrasound, MRI can illustrate disruption of stabilizing soft tissue structures and may be more tolerable for patients, especially during the acute phase when dynamic assessment may be challenging. Although MRI as a medium has advantages in displaying orientation and resolution, debate persists as to its utility for identifying compromised ankle ligamentous structures. An ankle-specific coil is likely to improve imaging consistency but is not always available, and surface or extremity coils can be used with relative confidence. A routine oblique axial view is typically adequate to observe the lateral ligaments, but Kim et al55 suggested that a full-length view of the ATF ligament may prove more useful, especially in patients with a CAI history.
Magnetic resonance imaging may be a surrogate measure for identifying true negative cases, yet it should be used with caution for true positive identification. Joshy et al20 examined the integrity of the ATF and CF ligaments documented by MRI in 24 patients scheduled for ankle reconstruction. Identification of true negative cases (specificity) was perfect (ATF = 100%, CF = 100%), but the determination of true positives (sensitivity) was only fair to good (ATF = 67%, CF = 40%). Kumar et al21 reported that for the CF, sensitivity (47%) was worse than specificity (83%), but for the ATF, sensitivity was better (87%) than specificity (60%). More recently, Kim et al19 found stronger sensitivity (76%–84%) and specificity (83%–92%) in identifying ATF injuries using MRI. Finally, Jolman et al22 found that MRI assessment of patients with CAI had strong sensitivity (83%) but weak specificity (53%). Thus, for CF injuries, MRI is likely to be useful for identifying true negatives while providing poor true positive identification. However, the conclusions regarding the use of MRI for identifying true positive versus true negative ATF injuries are not definitive. The other caveat to consider is that these authors included patients with acute and chronic instability who were scheduled for arthroscopic ankle reconstructions, meaning that the level of suspected ligamentous instability was quite severe. To date, no researchers have examined the use of MRI for verifying less severe suspected ankle instability (eg, grade I or II). Therefore, although MRI offers the potential for advanced diagnostic imaging, the inconsistencies noted in the literature may not support its selection as the primary choice for all patients with suspected ankle ligamentous damage. Furthermore, it is likely to provide a more definitive diagnosis of a CF injury than an ATF injury.45,47
Comparison of Resources
Clinical assessment of patients with ankle injuries should include physical examination tests to verify the suspected ligamentous insult. The ADT and talar tilt test are used most commonly, though even in the hands of experienced clinicians, the outcomes could be improved with further diagnostic testing. For example, Croy et al56 found the ADT had good sensitivity (74%–83%) but weak specificity (38%–40%) when compared with stress ultrasound. Cho et al57 demonstrated approximately 79% accuracy in grading the severity of LAS using the ADT versus arthroscopy; however, stress radiography, diagnostic ultrasound, and MRI were comparable in defining the injury. In contrast, Jolman et al22 stated that MRI offered better true positive identification (sensitivity = 83%, specificity = 53%), whereas stress radiography had better true negative identification (sensitivity = 66%, specificity = 97%) of ankle instability among patients with CAI. Finally, Sisson et al58 showed that stress ultrasound and ankle arthrometry measures of ankle anterior displacement and inversion rotation were not strongly correlated, suggesting they may be assessing different aspects of ankle-joint laxity. Considering which diagnostic tools provide better options for ruling in or ruling out a suspected diagnosis is important. Yet the evidence has been dominated by studies involving small samples of patients, many with severe or grade III injuries who were candidates for surgical procedures, which typically allows arthroscopic confirmation. Additional evidence is needed to determine which diagnostic options may be best for confirming grade I and II injuries. In the meantime, factors such as availability, cost, and patient comfort should be considered when advanced confirmatory assessment is desired.
SUBACUTE AND RETURN-TO-PLAY ASSESSMENTS OF THE PATIENT WITH ANKLE INSTABILITY
As described earlier, repeating the clinical assessment of an ankle sprain several days after the acute injury to improve the diagnostic accuracy of the physical examination tests is recommended. Along with evaluating the mechanical instability of the affected joint, the clinician should take into account other characteristics that may indicate progress toward return to play and any lingering deficiencies that could signal the development of CAI. Hertel59 first proposed a paradigm outlining the contributions of mechanical and functional insufficiencies that combine to create CAI. Later, Hiller et al60 and Delahunt et al61 built on the possible characteristics of CAI, leading to an understanding that many functional insufficiencies linger in patients with a history of LAS; the contributions from mechanical instability were not as strong. Most recently, the International Ankle Consortium (IAC)62 provided a succinct definition of CAI that focuses on repetitive episodes of giving way and self-reported dysfunction. This literature has deemphasized the importance of lingering mechanical instability from a previous LAS, yet that is often the focal point in evaluating and diagnosing an LAS in a patient. In a review article, Pourkazemi et al63 suggested that patients with a more severe index LAS were more likely to sustain a recurrent LAS, although the evidence was limited and not in total agreement. The literature reflects a shift in the focus from an exclusively structural assessment to include subjective and perceptual findings from patient-reported outcome instruments. Despite the importance of sequential assessments of ligamentous instability among patients with LAS, clinicians should concentrate on the physical and patient-reported functional deficiencies to improve the chances of a successful return to activity and reduction in reinjury risk.
Range of Motion
Full and pain-free range of motion is 1 of the first milestones that should be achieved and maintained during recovery from an LAS. A dorsiflexion deficit often persists and is attributed to soft tissue restriction. However, patients with CAI typically present with hypomobility that can be linked to arthrokinematic limitations and positional faults in the ankle complex.64–66 For example, the talus may be anteriorly displaced in the ankle mortise, creating a bony block to full dorsiflexion.67,68 Fibular positional faults have been associated with CAI: the distal end is anteriorly translated, which may contribute to the difficulty the talus has in gliding posteriorly during dorsiflexion.69,70 A clinical examination based on arthrokinematic principles and including manual-gliding tests is effective in identifying these bony restrictions to global joint motion and should help in determining the corrective course.67–70
Strength
Another tenet of clinical examination and a common focus of rehabilitation is strength surrounding a joint. Specific deficiencies in ankle strength have been identified as risk factors for LAS,71 as well as a characteristic of CAI.72 Ankle strength is easily assessed using manual muscle testing, which may be supplemented with measures of handheld or instrumented dynamometry. In addition to ankle strength, knee and hip strength should be evaluated because proximal joints in the lower extremity may demonstrate strength deficits in patients with CAI (eg, quadriceps, hamstrings, gluteal muscles).73,74
Postural Control
Postural-control alterations are 1 of the critical physical outcomes associated with a risk of LAS71 and a frequent characteristic of CAI.75,76 Balance can be assessed using instrumented force platforms, but these devices may not be available in most clinical settings. Fortunately, many valid and reliable noninstrumented balance measures can aid in detecting ankle instability.77,78 Assessment of balance while making return-to-activity decisions should follow a progression of static to more dynamic and functional challenges. Two tests that are useful at the beginning of this protocol are the Balance Error Scoring System and the Star Excursion Balance Test (SEBT). Both are easily implemented in clinical settings with little to no financial cost and can indicate deficits in those with a history of LAS.79,80 An MDC cutoff of 7 on the Balance Error Scoring System has been suggested for young adults81; MDC score cutoffs from 1.56% to 4.64%, depending on the reach direction, were cited for the SEBT.82,83 At this point, studies of larger samples are needed to more definitively establish cutoff scores and MDCs for these tests. Progressions to controlled hopping assessments challenge patients to create a new base of support as they move in multiple directions. These assessments can be performed with confidence using cost-effective testing procedures in most clinical settings.77
Patient-Reported Outcomes
Physical testing of disease outcomes is the primary component of the initial and follow-up assessments of LAS patients. However, a critical component that is often downplayed or even omitted is patient-reported outcomes. Clinicians may document outcomes such as self-reported pain and establish functional perceptions through oral interaction with patients, but it is important to quantify their self-perceived deficiencies to determine how they are progressing postinjury. Additionally, this is a way to recognize lingering complaints that may not have been addressed fully and could contribute to a poor result on return to activity. Along with assessments of pain, general function, and activity restrictions, clinicians should consider using ankle-specific questionnaires to ascertain limitations associated with LAS and resulting instability. For example, the IAC62 has endorsed instruments including the Ankle Instability Instrument,84 the Identification of Foot and Ankle Instability,85 and the Cumberland Ankle Instability Tool86 with cutoff scores indicating the presence of CAI among patients with a history of LAS. However, these instruments are not ideal for quantifying changes in patient status during the subacute and return-to-activity phases after an LAS. To track progress in meeting goals, the Foot and Ankle Ability Measure (FAAM)87 is an option and has been endorsed by the IAC.62 The scores on the FAAM should move in a positive direction as disability in a variety of physical tasks diminishes, giving the clinician an objective measure of which tasks (activities of daily living and sport) are presenting the greatest limitations as the patient recovers and progresses toward a return to activity. Martin et al88 reported MCIDs of 8 for the Activities of Daily Living scale and 9 for the Sports scale.
Prediction Models and Additional Considerations
In recent prospective studies and systematic reviews, a few groups have provided models for understanding the deficiencies associated with reinjury and the development of lingering instability from LAS during the 12 months after an acute injury. Pourkazemi et al63 performed a systematic review of prospective studies that focused on perceived ankle instability, postural control, and initial LAS severity to predict resprain rates. Only injury severity predicted LAS reinjury; however, as discussed previously, limitations of the severity categories should be considered. Measures such as the Cumberland Ankle Instability Tool, which was used in the reviewed studies, may not provide optimal estimates of changes in status. The balance task used in the reviewed studies was a foot lift, which did not yield predictive information. Other balance assessments may provide better utility for illustrating injury deficiencies and the risk of reinjury. The authors concluded that this important clinical question posed a gap in the literature that needed to be addressed.63
More recently, Doherty et al89 published multiple articles from a large prospective study that assessed numerous disease and patient outcomes among individuals at the time of LAS and tracked them up to 12 months postinjury. Within 2 weeks of the LAS, patients presented with lingering balance deficits compared with uninjured controls.89 Those who had characteristics of CAI still had deficiencies in balance and altered jump-landing patterns at 6 months4,8,90 and 12 months5,7 after the LAS. Two findings may be most important from this group's work: (1) an inability to perform a jump-landing task during the first 2 weeks after acute LAS predicted 68% of the patients who developed CAI within 1 year and (2) a deficit on the SEBT and poor score on the FAAM-Activities of Daily Living 6 months after the initial LAS identified 85% of the patients who developed CAI within 1 year.6 In a separate analysis,91 the investigators determined that a battery of basic clinical outcomes (talar glide, talar tilt, ADT, and plantar-flexion ROM) performed within 2 weeks of the LAS predicted only 69% of those who developed CAI within 1 year. This highlights the need to include comprehensive follow-up assessments of patients with acute LAS that include more than basic clinical outcomes before return to activity. Clinicians should consider using disease and patient-reported outcomes of function and likely should implement follow-ups several months after patients return to activity to identify any lingering deficiencies that have not been addressed. The researchers provided specific suggestions, but more prospective studies are needed to elucidate the ideal constituents of follow-up assessments.
As prospective information grows, clinicians should be aware of the emerging evidence about alterations to the central nervous system and resulting adaptations in motor strategies associated with CAI. Multiple sensorimotor deficiencies have been observed in patients with a history of LAS,92 and changes in cortical and spinal pathways among populations with CAI have been documented.93–99 These alterations may help to explain many of the disease and patient-reported deficiencies observed during clinical examination but are currently limited to laboratory techniques. It is possible that in the coming years, this emerging body of information may influence the comprehensive assessments of patients with LAS.
SUMMARY
A clinical determination of an LAS should follow a prescribed assessment of disease and patient-reported outcomes and should include comprehensive evaluations at the time of injury with follow-ups at critical time points. Evaluations at all critical time points (acute, subacute, return-to-activity, and postreturn-to-activity follow-ups) should include a thorough history, along with other patient outcomes using subjective interviews and objective scores from validated outcome instruments. Physical examination should assess potential tissue damage and the need for advanced imaging to confirm the findings. These steps should be repeated a few days after the initial insult to maximize the accuracy of the examination. The clinician should supplement the assessment with appropriate clinical and functional outcomes that indicate progress toward a confident return to activity. The clinician should be aware of the propensity of patients with LAS to develop CAI and the complex symptoms and deficiencies that may predispose a patient to the condition. By adopting a thorough strategy for the evaluation of LAS from acute insult to return to activity, the clinician can identify deficient areas that need further intervention, with the goal of positioning the patient for an optimal return to activity that minimizes the risk for reinjury and long-term complications.
REFERENCES
- 1.Gribble PA, Bleakley CM, Caulfield BM, et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;50(24):1496–1505. doi: 10.1136/bjsports-2016-096189. [DOI] [PubMed] [Google Scholar]
- 2.Gribble PA, Bleakley CM, Caulfield BM, et al. 2016 consensus statement of the International Ankle Consortium: prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;40(24):1493–1495. doi: 10.1136/bjsports-2016-096188. [DOI] [PubMed] [Google Scholar]
- 3.Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. Br J Sports Med. 2014;48(13):1014–1018. doi: 10.1136/bjsports-2013-093175. [DOI] [PubMed] [Google Scholar]
- 4.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Dynamic balance deficits 6 months following first-time acute lateral ankle sprain: a laboratory analysis. J Orthop Sports Phys Ther. 2015;45(8):626–633. doi: 10.2519/jospt.2015.5653. [DOI] [PubMed] [Google Scholar]
- 5.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Dynamic balance deficits in individuals with chronic ankle instability compared to ankle sprain copers 1 year after a first-time lateral ankle sprain injury. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1086–1095. doi: 10.1007/s00167-015-3744-z. [DOI] [PubMed] [Google Scholar]
- 6.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: a prospective cohort analysis. Am J Sports Med. 2016;44(4):995–1003. doi: 10.1177/0363546516628870. [DOI] [PubMed] [Google Scholar]
- 7.Doherty C, Bleakley C, Hertel J, et al. Lower limb interjoint postural coordination one year after first-time lateral ankle sprain. Med Sci Sports Exerc. 2015;47(11):2398–2405. doi: 10.1249/MSS.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 8.Doherty C, Bleakley C, Hertel J, et al. Coordination and symmetry patterns during the drop vertical jump, 6-months after first-time lateral ankle sprain. J Orthop Res. 2015;33(10):1537–1544. doi: 10.1002/jor.22915. [DOI] [PubMed] [Google Scholar]
- 9.Delahunt E, Bleakley CM, Bossard DS, et al. Clinical assessment of acute lateral ankle sprain injuries (ROAST): 2019 consensus statement and recommendations of the International Ankle Consortium. Br J Sports Med. 2018;52(20):1304–1310. doi: 10.1136/bjsports-2017-098885. [DOI] [PubMed] [Google Scholar]
- 10.Krips R, de Vries J, van Dijk CN. Ankle instability. Foot Ankle Clin. 2006;11(2):311–329. doi: 10.1016/j.fcl.2006.02.003. vi. [DOI] [PubMed] [Google Scholar]
- 11.van Dijk CN, Lim LS, Bossuyt PM, Marti RK. Physical examination is sufficient for the diagnosis of sprained ankles. J Bone Joint Surg Br. 1996;78(6):958–962. doi: 10.1302/0301-620x78b6.1283. [DOI] [PubMed] [Google Scholar]
- 12.Vaseenon T, Gao Y, Phisitkul P. Comparison of two manual tests for ankle laxity due to rupture of the lateral ankle ligaments. Iowa Orthop J. 2012;32:9–16. [PMC free article] [PubMed] [Google Scholar]
- 13.Phisitkul P, Chaichankul C, Sripongsai R, Prasitdamrong I, Tengtrakulcharoen P, Suarchawaratana S. Accuracy of anterolateral drawer test in lateral ankle instability: a cadaveric study. Foot Ankle Int. 2009;30(7):690–695. doi: 10.3113/FAI.2009.0690. [DOI] [PubMed] [Google Scholar]
- 14.Frost SC, Amendola A. Is stress radiography necessary in the diagnosis of acute or chronic ankle instability? Clin J Sport Med. 1999;9(1):40–45. doi: 10.1097/00042752-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Oae K, Takao M, Uchio Y, Ochi M. Evaluation of anterior talofibular ligament injury with stress radiography, ultrasonography and MR imaging. Skelet Radiol. 2010;39(1):41–47. doi: 10.1007/s00256-009-0767-x. [DOI] [PubMed] [Google Scholar]
- 16.Gun C, Unluer EE, Vandenberk N, Karagoz A, Senturk GO, Oyar O. Bedside ultrasonography by emergency physicians for anterior talofibular ligament injury. J Emerg Trauma Shock. 2013;6(3):195–198. doi: 10.4103/0974-2700.115340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Cai Y, Wang Y. Value of ultrasonography for detecting chronic injury of the lateral ligaments of the ankle joint compared with ultrasonography findings. Br J Radiol. 2014;87(1033):20130406. doi: 10.1259/bjr.20130406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hua Y, Yang Y, Chen S, Cai Y. Ultrasound examination for the diagnosis of chronic anterior talofibular ligament injury. Acta Radiol. 2012;53(10):1142–1145. doi: 10.1258/ar.2012.120171. [DOI] [PubMed] [Google Scholar]
- 19.Kim YS, Kim YB, Kim TG, et al. Reliability and validity of magnetic resonance imaging for the evaluation of the anterior talofibular ligament in patients undergoing ankle arthroscopy. Arthroscopy. 2015;31(8):1540–1547. doi: 10.1016/j.arthro.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Joshy S, Abdulkadir U, Chaganti S, Sullivan B, Hariharan K. Accuracy of MRI scan in the diagnosis of ligamentous and chondral pathology in the ankle. Foot Ankle Surg. 2010;16(2):78–80. doi: 10.1016/j.fas.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Triantafyllopoulos I, Panagopoulos A, Fitzgerald S, van Niekerk L. Deficiencies of MRI in the diagnosis of chronic symptomatic lateral ankle ligament injuries. Foot Ankle Surg. 2007;13(4):171–176. [Google Scholar]
- 22.Jolman S, Robbins J, Lewis L, Wilkes M, Ryan P. Comparison of magnetic resonance imaging and stress radiographs in the evaluation of chronic lateral ankle instability. Foot Ankle Int. 2017;38(4):397–404. doi: 10.1177/1071100716685526. [DOI] [PubMed] [Google Scholar]
- 23.Raab S, Craig D. Evidence-Based Practice in Athletic Training. Champaign, IL: Human Kinetics; 2016. [Google Scholar]
- 24.Bachmann LM, Kolb E, Koller MT, Steurer J, ter Riet G. Accuracy of Ottawa ankle rules to exclude fractures of the ankle and mid-foot: systematic review. BMJ. 2003;326(7386):417. doi: 10.1136/bmj.326.7386.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckenkamp PR, Lin CC, Macaskill P, Michaleff ZA, Maher CG, Moseley AM. Diagnostic accuracy of the Ottawa Ankle and Midfoot Rules: a systematic review with meta-analysis. Br J Sports Med. 2017;51(6):504–510. doi: 10.1136/bjsports-2016-096858. [DOI] [PubMed] [Google Scholar]
- 26.Dowling S, Spooner CH, Liang Y, et al. Accuracy of Ottawa Ankle Rules to exclude fractures of the ankle and midfoot in children: a meta-analysis. Acad Emerg Med. 2009;16(4):277–287. doi: 10.1111/j.1553-2712.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- 27.Kovaleski JE, Norrell PM, Heitman RJ, Hollis JM, Pearsall AW. Knee and ankle position, anterior drawer laxity, and stiffness of the ankle complex. J Athl Train. 2008;43(3):242–248. doi: 10.4085/1062-6050-43.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch SA. Assessment of the injured ankle in the athlete. J Athl Train. 2002;37(4):406–412. [PMC free article] [PubMed] [Google Scholar]
- 29.Miller AG, Myers SH, Parks BG, Guyton GP. Anterolateral drawer versus anterior drawer test for ankle instability: a biomechanical model. Foot Ankle Int. 2016;37(4):407–410. doi: 10.1177/1071100715620854. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson J, Eriksson BI, Renstrom PA. Subtalar ankle instability: a review. Sports Med. 1997;24(5):337–346. doi: 10.2165/00007256-199724050-00005. [DOI] [PubMed] [Google Scholar]
- 31.Barg A, Tochigi Y, Amendola A, Phisitkul P, Hintermann B, Saltzman CL. Subtalar instability: diagnosis and treatment. Foot Ankle Int. 2012;33(2):151–160. doi: 10.3113/FAI.2012.0151. [DOI] [PubMed] [Google Scholar]
- 32.Aynardi M, Pedowitz DI, Raikin SM. Subtalar instability. Foot Ankle Clin. 2015;20(2):243–252. doi: 10.1016/j.fcl.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Keefe DT, Haddad SL. Subtalar instability. Etiology, diagnosis, and management. Foot Ankle Clin. 2002;7(3):577–609. doi: 10.1016/s1083-7515(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 34.Thermann H, Zwipp H, Tscherne H. Treatment algorithm of chronic ankle and subtalar instability. Foot Ankle Int. 1997;18(3):163–169. doi: 10.1177/107110079701800309. [DOI] [PubMed] [Google Scholar]
- 35.Hertel J, Denegar CR, Monroe MM, Stokes WL. Talocrural and subtalar joint instability after lateral ankle sprain. Med Sci Sports Exerc. 1999;31(11):1501–1508. doi: 10.1097/00005768-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Heilman AE, Braly WG, Bishop JO, Noble PC, Tullos HS. An anatomic study of subtalar instability. Foot Ankle. 1990;10(4):224–228. doi: 10.1177/107110079001000407. [DOI] [PubMed] [Google Scholar]
- 37.Kato T. The diagnosis and treatment of instability of the subtalar joint. J Bone Joint Surg Br. 1995;77(3):400–406. [PubMed] [Google Scholar]
- 38.DiGiovanni BF, Partal G, Baumhauer JF. Acute ankle injury and chronic lateral instability in the athlete. Clin Sports Med. 2004;23(1):1–19. doi: 10.1016/S0278-5919(03)00095-4. v. [DOI] [PubMed] [Google Scholar]
- 39.Ferran NA, Oliva F, Maffulli N. Ankle instability. Sports Med Arthrosc Rev. 2009;17(2):139–145. doi: 10.1097/JSA.0b013e3181a3d790. [DOI] [PubMed] [Google Scholar]
- 40.Usuelli FG, Mason L, Grassi M, Maccario C, Ballal M, Molloy A. Lateral ankle and hindfoot instability: a new clinical based classification. Foot Ankle Surg. 2014;20(4):231–236. doi: 10.1016/j.fas.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Bergfeld J, Cox J, Drez D, Raemy H, Weiker G. Symposium: management of acute ankle sprains. Contemp Ortho. 1986;13(3):83–116. [Google Scholar]
- 42.Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers' Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48(4):528–545. doi: 10.4085/1062-6050-48.4.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerkhoffs GM, van den Bekerom M, Elders LA, et al. Diagnosis, treatment and prevention of ankle sprains: an evidence-based clinical guideline. Br J Sports Med. 2012;46(12):854–860. doi: 10.1136/bjsports-2011-090490. [DOI] [PubMed] [Google Scholar]
- 44.Alparslan L, Chiodo CP. Lateral ankle instability: MR imaging of associated injuries and surgical treatment procedures. Semin Musculoskelet Radiol. 2008;12(4):346–358. doi: 10.1055/s-0028-1100641. [DOI] [PubMed] [Google Scholar]
- 45.Shakked R, Sheskier S. Acute and chronic lateral ankle instability diagnosis, management, and new concepts. Bull Hosp Jt Dis (2013) 2017;75(1):71–80. [PubMed] [Google Scholar]
- 46.Hashimoto T, Inokuchi S, Kokubo T. Clinical study of chronic lateral ankle instability: injured ligaments compared with stress X-ray examination. J Orthop Sci. 2009;14(6):699–703. doi: 10.1007/s00776-009-1386-z. [DOI] [PubMed] [Google Scholar]
- 47.Meehan TM, Martinez-Salazar EL, Torriani M. Aftermath of ankle inversion injuries: spectrum of MR imaging findings. Mag Reson Imaging Clin N Am. 2017;25(1):45–61. doi: 10.1016/j.mric.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Hubbard TJ, Cordova M. Mechanical instability after an acute lateral ankle sprain. Arch Phys Med Rehabil. 2009;90(7):1142–1146. doi: 10.1016/j.apmr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Hubbard TJ, Kaminski TW, Vander Griend RA, Kovaleski JE. Quantitative assessment of mechanical laxity in the functionally unstable ankle. Med Sci Sports Exerc. 2004;36(5):760–766. doi: 10.1249/01.mss.0000126604.85429.29. [DOI] [PubMed] [Google Scholar]
- 50.Hubbard TJ. Ligament laxity following inversion injury with and without chronic ankle instability. Foot Ankle Int. 2008;29(3):305–311. doi: 10.3113/FAI.2008.0305. [DOI] [PubMed] [Google Scholar]
- 51.de Vries JS, Kerkhoffs GM, Blankevoort L, van Dijk CN. Clinical evaluation of a dynamic test for lateral ankle ligament laxity. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):628–633. doi: 10.1007/s00167-009-0978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovaleski JE, Hollis J, Heitman RJ, Gurchiek LR, Pearsall AW IV. Assessment of ankle-subtalar-joint-complex laxity using an instrumented ankle arthrometer: an experimental cadaveric investigation. J Athl Train. 2002;37(4):467–474. [PMC free article] [PubMed] [Google Scholar]
- 53.Croy T, Saliba S, Saliba E, Anderson MW, Hertel J. Talofibular interval changes after acute ankle sprain: a stress ultrasonography study of ankle laxity. J Sport Rehabil. 2013;22(4):257–263. doi: 10.1123/jsr.22.4.257. [DOI] [PubMed] [Google Scholar]
- 54.Croy T, Saliba SA, Saliba E, Anderson MW, Hertel J. Differences in lateral ankle laxity measured via stress ultrasonography in individuals with chronic ankle instability, ankle sprain copers, and healthy individuals. J Orthop Sports Phys Ther. 2012;42(7):593–600. doi: 10.2519/jospt.2012.3923. [DOI] [PubMed] [Google Scholar]
- 55.Kim JS, Moon YJ, Choi YS, Park YU, Park SM, Lee KT. Usefulness of oblique axial scan in magnetic resonance imaging evaluation of anterior talofibular ligament in ankle sprain. J Foot Ankle Surg. 2012;51(3):288–292. doi: 10.1053/j.jfas.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 56.Croy T, Koppenhaver S, Saliba S, Hertel J. Anterior talocrural joint laxity: diagnostic accuracy of the anterior drawer test of the ankle. J Orthop Sports Phys Ther. 2013;43(12):911–919. doi: 10.2519/jospt.2013.4679. [DOI] [PubMed] [Google Scholar]
- 57.Cho JH, Lee DH, Song HK, Bang JY, Lee KT, Park YU. Value of stress ultrasound for the diagnosis of chronic ankle instability compared to manual anterior drawer test, stress radiography, magnetic resonance imaging, and arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1022–1028. doi: 10.1007/s00167-015-3828-9. [DOI] [PubMed] [Google Scholar]
- 58.Sisson L, Croy T, Saliba S, Hertel J. Comparison of ankle arthrometry to stress ultrasound imaging in the assessment of ankle laxity in healthy adults. Int J Sports Physl Ther. 2011;6(4):297–305. [PMC free article] [PubMed] [Google Scholar]
- 59.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 60.Hiller CE, Kilbreath SL, Refshauge KM. Chronic ankle instability: evolution of the model. J Athl Train. 2011;46(2):133–141. doi: 10.4085/1062-6050-46.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delahunt E, Coughlan GF, Caulfield B, Nightingale EJ, Lin CW, Hiller CE. Inclusion criteria when investigating insufficiencies in chronic ankle instability. Med Sci Sports Exerc. 2010;42(11):2106–2121. doi: 10.1249/MSS.0b013e3181de7a8a. [DOI] [PubMed] [Google Scholar]
- 62.Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J Orthop Sports Phys Ther. 2013;43(8):585–591. doi: 10.2519/jospt.2013.0303. [DOI] [PubMed] [Google Scholar]
- 63.Pourkazemi F, Hiller CE, Raymond J, Nightingale EJ, Refshauge KM. Predictors of chronic ankle instability after an index lateral ankle sprain: a systematic review. J Sci Med Sport. 2014;17(6):568–573. doi: 10.1016/j.jsams.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Hoch MC, Staton GS, Medina McKeon JM, Mattacola CG, McKeon PO. Dorsiflexion and dynamic postural control deficits are present in those with chronic ankle instability. J Sci Med Sport. 2012;15(6):574–579. doi: 10.1016/j.jsams.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 65.Terada M, Pietrosimone BG, Gribble PA. Therapeutic interventions for increasing ankle dorsiflexion after ankle sprain: a systematic review. J Athl Train. 2013;48(5):696–709. doi: 10.4085/1062-6050-48.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wikstrom EA, Hubbard TJ. Talar positional fault in persons with chronic ankle instability. Arch Phys Med Rehabil. 2010;91(8):1267–1271. doi: 10.1016/j.apmr.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 67.Hubbard TJ, Hertel J. Mechanical contributions to chronic lateral ankle instability. Sports Med. 2006;36(3):263–277. doi: 10.2165/00007256-200636030-00006. [DOI] [PubMed] [Google Scholar]
- 68.Denegar CR, Hertel J, Fonseca J. The effect of lateral ankle sprain on dorsiflexion range of motion, posterior talar glide, and joint laxity. J Orthop Sports Phys Ther. 2002;32(4):166–173. doi: 10.2519/jospt.2002.32.4.166. [DOI] [PubMed] [Google Scholar]
- 69.Hubbard TJ, Hertel J, Sherbondy P. Fibular position in individuals with self-reported chronic ankle instability. J Orthop Sports Phys Ther. 2006;36(1):3–9. doi: 10.2519/jospt.2006.36.1.3. [DOI] [PubMed] [Google Scholar]
- 70.Hubbard TJ, Hertel J. Anterior positional fault of the fibula after sub-acute lateral ankle sprains. Man Ther. 2008;13(1):63–67. doi: 10.1016/j.math.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Witchalls J, Blanch P, Waddington G, Adams R. Intrinsic functional deficits associated with increased risk of ankle injuries: a systematic review with meta-analysis. Br J Sports Med. 2012;46(7):515–523. doi: 10.1136/bjsports-2011-090137. [DOI] [PubMed] [Google Scholar]
- 72.Arnold BL, Linens SW, de la Motte SJ, Ross SE. Concentric evertor strength differences and functional ankle instability: a meta-analysis. J Athl Train. 2009;44(6):653–662. doi: 10.4085/1062-6050-44.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gribble PA, Robinson RH. An examination of ankle, knee, and hip torque production in individuals with chronic ankle instability. J Strength Cond Res. 2009;23(2):395–400. doi: 10.1519/JSC.0b013e31818efbb2. [DOI] [PubMed] [Google Scholar]
- 74.Negahban H, Moradi-Bousari A, Naghibi S, et al. The eccentric torque production capacity of the ankle, knee, and hip muscle groups in patients with unilateral chronic ankle instability. Asian J Sports Med. 2013;4(2):144–152. doi: 10.5812/asjsm.34515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKeon PO, Hertel J. Systematic review of postural control and lateral ankle instability, part I: can deficits be detected with instrumented testing? J Athl Train. 2008;43(3):293–304. doi: 10.4085/1062-6050-43.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnold BL, De La Motte S, Linens S, Ross SE. Ankle instability is associated with balance impairments: a meta-analysis. Med Sci Sports Exerc. 2009;41(5):1048–1062. doi: 10.1249/MSS.0b013e318192d044. [DOI] [PubMed] [Google Scholar]
- 77.Linens SW, Ross SE, Arnold BL, Gayle R, Pidcoe P. Postural-stability tests that identify individuals with chronic ankle instability. J Athl Train. 2014;49(1):15–23. doi: 10.4085/1062-6050-48.6.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pourkazemi F, Hiller C, Raymond J, Black D, Nightingale E, Refshauge K. Using balance tests to discriminate between participants with a recent index lateral ankle sprain and healthy control participants: a cross-sectional study. J Athl Train. 2016;51(3):213–222. doi: 10.4085/1062-6050-51.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Docherty CL. Valovich McLeod TC, Shultz SJ. Postural control deficits in participants with functional ankle instability as measured by the Balance Error Scoring System. Clin J Sport Med. 2006;16(3):203–208. doi: 10.1097/00042752-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Gribble PA, Hertel J, Plisky P. Using the Star Excursion Balance Test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train. 2012;47(3):339–357. doi: 10.4085/1062-6050-47.3.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mulligan IJ, Boland MA, McIlhenny CV. The Balance Error Scoring System learned response among young adults. Sports Health. 2013;5(1):22–26. doi: 10.1177/1941738112467755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sandrey MA, Mitzel JG. Improvement in dynamic balance and core endurance after a 6-week core-stability-training program in high school track and field athletes. J Sport Rehabil. 2013;22(4):264–271. doi: 10.1123/jsr.22.4.264. [DOI] [PubMed] [Google Scholar]
- 83.Hoch MC, Andreatta RD, Mullineaux DR, et al. Two-week joint mobilization intervention improves self-reported function, range of motion, and dynamic balance in those with chronic ankle instability. J Orthop Res. 2012;30(11):1798–1804. doi: 10.1002/jor.22150. [DOI] [PubMed] [Google Scholar]
- 84.Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and reliability of the Ankle Instability Instrument. J Athl Train. 2006;41(2):154–158. [PMC free article] [PubMed] [Google Scholar]
- 85.Simon J, Donahue M, Docherty C. Development of the Identification of Functional Ankle Instability (IdFAI) Foot Ankle Int. 2012;33(9):755–763. doi: 10.3113/FAI.2012.0755. [DOI] [PubMed] [Google Scholar]
- 86.Hiller CE, Refshauge KM, Bundy AC, Herbert RD, Kilbreath SL. The Cumberland Ankle Instability Tool: a report of validity and reliability testing. Arch Phys Med Rehabil. 2006;87(9):1235–1241. doi: 10.1016/j.apmr.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 87.Carcia CR, Martin RL, Drouin JM. Validity of the Foot and Ankle Ability Measure in athletes with chronic ankle instability. J Athl Train. 2008;43(2):179–183. doi: 10.4085/1062-6050-43.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM) Foot Ankle Int. 2005;26(11):968–983. doi: 10.1177/107110070502601113. [DOI] [PubMed] [Google Scholar]
- 89.Doherty C, Bleakley CM, Hertel J, Caulfield B, Ryan J, Delahunt E. Laboratory measures of postural control during the star excursion balance test after acute first-time lateral ankle sprain. J Athl Train. 2015;50(6):651–664. doi: 10.4085/1062-6050-50.1.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Single-leg drop landing movement strategies 6 months following first-time acute lateral ankle sprain injury. Scand J Med Sci Sports. 2015;25(6):806–817. doi: 10.1111/sms.12390. [DOI] [PubMed] [Google Scholar]
- 91.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Clinical tests have limited predictive value for chronic ankle instability when conducted in the acute phase of a first-time lateral ankle sprain injury. Arch Phys Med Rehabil. 2018;99(4):720–725. doi: 10.1016/j.apmr.2017.11.008. e721. [DOI] [PubMed] [Google Scholar]
- 92.Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008;27(3):353–370. doi: 10.1016/j.csm.2008.03.006. vii. [DOI] [PubMed] [Google Scholar]
- 93.Bowker S, Terada M, Thomas AC, Pietrosimone BG, Hiller CE, Gribble PA. Neural excitability and joint laxity in chronic ankle instability, coper, and control groups. J Athl Train. 2016;51(4):336–343. doi: 10.4085/1062-6050-51.5.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harkey M, McLeod MM, Terada M, Gribble PA, Pietrosimone BG. Quadratic association between corticomotor and spinal-reflexive excitability and self-reported disability in participants with chronic ankle instability. J Sport Rehabil. 2016;25(2):137–145. doi: 10.1123/jsr.2014-0282. [DOI] [PubMed] [Google Scholar]
- 95.Kim KM, Hart JM, Saliba SA, Hertel J. Modulation of the fibularis longus hoffmann reflex and postural instability associated with chronic ankle instability. J Athl Train. 2016;51(8):637–643. doi: 10.4085/1062-6050-51.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kosik KB, Terada M, Drinkard CP, McCann RS, Gribble PA. Potential corticomotor plasticity in those with and without chronic ankle instability. Med Sci Sports Exerc. 2017;49(1):141–149. doi: 10.1249/MSS.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 97.McLeod MM, Gribble PA, Pietrosimone BG. Chronic ankle instability and neural excitability of the lower extremity. J Athl Train. 2015;50(8):847–853. doi: 10.4085/1062-6050-50.4.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pietrosimone BG, Gribble PA. Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train. 2012;47(6):621–626. doi: 10.4085/1062-6050-47.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terada M, Bowker S, Thomas AC, Pietrosimone B, Hiller CE, Gribble PA. Corticospinal excitability and inhibition of the soleus in individuals with chronic ankle instability. PM R. 2016;8(11):1090–1096. doi: 10.1016/j.pmrj.2016.04.006. [DOI] [PubMed] [Google Scholar]


