Abstract
Context
Motor planning, a prerequisite for goal-driven movement, is a complex process that occurs in the cortex. Evidence has suggested that motor planning is altered in patients with chronic ankle instability (CAI). We know balance training can improve balance, but we do not know if it also improves motor planning. Such changes in cortical activity can be assessed using electroencephalography.
Objective
To evaluate changes in cortical measures of motor planning after balance training in patients with CAI.
Design
Controlled laboratory study.
Setting
Research laboratory.
Patients or Other Participants
Fifteen patients with CAI (age = 20.80 ± 2.37 years, height = 169.47 ± 7.95 cm, mass = 70.45 ± 19.25 kg).
Intervention(s)
A 4-week progression-based balance-training program.
Main Outcome Measure(s)
Motor planning was assessed via electroencephalography before a lateral-stepping task. We calculated event-related spectral perturbations in the θ (4–8 Hz), α (8–12 Hz), β (14–25 Hz), and γ (30–50 Hz) bands. The change in power (in decibels) was calculated in each band for the 500 milliseconds before the onset of the lateral-stepping movement. Additional outcomes were the Foot and Ankle Ability Measure (FAAM)–Activities of Daily Living and Sport subscales; the anterior-, posteromedial-, and posterolateral-reach directions of the Star Excursion Balance Test; and static balance. Patients completed 3 test sessions: baseline, 24- to 48-hour posttest, and 1-week posttest. Repeated-measures analyses of variance were used to assess changes over time. The α level was set at .05.
Results
The FAAM–Activities of Daily Living subscale score was improved at both posttests (P < .05), and the FAAM-Sport subscale score was improved at the 1-week posttest (P = .008). Balance was better in all 3 directions of the Star Excursion Balance Test at both posttest sessions (P < .001). After balance training, no differences were identified in cortical activity at either posttest session (P > .05).
Conclusions
No improvements were identified in electroencephalography measures of motor planning during lateral stepping in patients with CAI. Improved balance suggested that sensorimotor adaptations occurred, but they may not have transferred to the lateral-stepping task or they may have been mediated via other processes in patients with CAI.
Keywords: coordination, electroencephalography, sensorimotor control, treatment mechanisms
Key Points
Sensorimotor control and patient-reported outcomes of perceived function and health-related quality of life improved after a 4-week balance-training program in patients with chronic ankle instability.
Improvements in patient-reported outcomes, dynamic balance, and static balance were observed at the 1-week posttest.
No changes were evident in premovement cortical activity, as measured by event-related spectral perturbations, after the balance-training program.
A long-term consequence of lateral ankle sprains is chronic ankle instability (CAI), which is estimated to occur in about 40% of individuals who sustained a lateral ankle sprain.1 The hallmark symptoms of CAI are recurrent ankle sprains and reports of instability or giving way,2 which may considerably limit an individual's ability to remain physically active.3 Repetitive damage to the ankle-joint complex results in a variety of impairments, including altered sensorimotor function.4–6 Researchers have suggested that these patients may adopt a conservative approach to motor planning, as evidenced by decreased displacement of the center of pressure (COP) during gait initiation4 and altered neuromuscular activation during planned gait termination.7 Furthermore, Van Deun et al5 reported that individuals without CAI activated their muscles before transitioning from double- to single-limb support, but patients with CAI activated the shank muscles, such as the fibularis longus, tibialis anterior, and gastrocnemius, after movement began. Changes in corticomotor excitability of muscles, such as the fibularis longus8 and soleus,9 indicate that long-term changes in cortical function may occur in patients with CAI. The excitability of the motor cortex appears to be related to the sensorimotor activity of the cortex, even in individuals without CAI. Needle et al10 demonstrated that participants without CAI and with decreased corticomotor excitability of the soleus displayed greater somatosensory cortical activity during early joint loading. Among patients with CAI, these cortical adaptations may result from ligamentous trauma, as investigators have also identified decreased corticomotor excitability11 and somatosensory activity12 in patients with anterior cruciate ligament deficiency (for a review, see Needle et al13). Taken together, altered motor planning, execution, and sensory processing may represent the underlying mechanisms for one of the most commonly described impairments in the population with CAI: balance.6 Understanding the mechanisms behind balance impairments is important because poor balance, which is a modifiable risk factor, places one at up to 4 times greater risk of ankle injury.14
Impaired balance in patients with CAI can be improved after balance training.6,15–17 Whereas multiple balance-training programs have been developed, researchers have recommended that the sensorimotor system be progressively challenged to develop motor “solutions” to new motor “problems” by increasing the difficulty of motor tasks after movement proficiency is demonstrated.15,18 Progressively increasing the difficulty of hopping and balance exercises may be beneficial, as it may allow the sensorimotor system time to adapt to more challenging tasks.18 Dynamic exercises included in balance-training programs, such as hopping, require effective motor-planning strategies; when these exercises are incorporated into balance-training programs, progression to higher levels of difficulty may reflect improvements in motor planning. In other words, this represents a greater ability to plan for and achieve a movement goal without errors when individuals encounter increased constraints, such as increased hopping distance or an unstable landing surface. Some transfer of these benefits to common tasks, such as gait, also occurs. McKeon et al19 reported improvements in shank and rearfoot coupling (ie, coordination) during walking after participants underwent 4 weeks of balance training. This improvement may represent better motor planning and execution. Similarly, increases in reach distance during the Star Excursion Balance Test (SEBT)15–17 may be due, in part, to improved motor planning. Whereas direct evidence is lacking among patients with CAI, these results suggest that motor-planning processes can be modified using common therapeutic interventions.
Over the past decade, investigators have studied the neural mechanisms of balance training using a variety of laboratory techniques. Taube et al20 hypothesized that, after balance training, the cerebral cortex has a decreased role and greater emphasis is placed on activity in the subcortical structures. Researchers have used electroencephalography (EEG) to investigate adaptations in cortical activity after skill and balance training. The EEG measures the electrical fields generated by the coordinated activity of large populations of neurons and can be used to infer motor-planning processes using time-frequency analysis. A popular time-frequency analysis is the calculation of an event-related spectral perturbation (ERSP), which is a normalized change of signal power within a given bandwidth of activity (eg, θ, α, β, γ). For instance, Schattin et al21 found that θ-band activity (4–8 Hz) was decreased after balance training in older adults. This cortical activity is thought to reflect executive functions, as well as movement selection and initiation.22 Activity in the α (8–12 Hz) and β (14–25 Hz) bands has long been accepted as sensorimotor rhythms representing movement preparation and planning.23,24 Activity in these 2 bands was depressed (ie, smaller decrease in spectral power) after task training, which may indicate more automaticity in motor-planning processes.25,26 High-frequency γ-band activity (>30 Hz), which is thought to represent cortical arousal (ie, processing) during motor tasks,24 has also been shown to decrease after training.25
Together, the evidence suggests that beneficial neuroplasticity may be present after a common intervention used for CAI rehabilitation based on the changes in spectral power that have been observed. The neuroplasticity observed in older adults21 and individuals without CAI25,26 may represent a less-constrained movement pattern and a more-automated motor-planning process because the influence of the premotor cortex or supplementary motor area, or both, which are thought to constrain movement patterns, has been reduced (ie, decreased cortical activity).13 Patients with CAI have constrained sensorimotor systems (eg, poor balance) that appear to respond well (ie, balance improves) to common therapeutic interventions, such as balance training.6,15 However, we do not have evidence to support these proposed adaptations of cortical activity in patients with CAI because the neural mechanisms of therapeutic interventions have not been investigated. Therefore, the purpose of our study was to evaluate measures of motor-planning change in patients with CAI who completed a progressive15 balance-training program. Specifically, we assessed the response in cortical activity, measured by EEG, before a lateral-stepping task previously described in CAI research.5,27 We calculated ERSPs in the θ (4–8 Hz), α (8–12 Hz), β (14–25 Hz), and γ (30–50 Hz) bands to measure the change in power before movement initiation. Based on the literature,20,21,25,26,28 we hypothesized that the amount of cortical activity would be decreased before lateral stepping after the intervention. Specifically, we hypothesized that α- and β-band activity would increase and θ- and γ-band activity would decrease after patients completed 4 weeks of balance training. We also wanted to examine the retention of balance improvements after balance training. To accomplish this, we assessed both EEG and balance outcomes within 24 to 48 hours of completing the balance-training program and again 1 week later. Delayed improvements in balance and patient-reported function (ie, delayed acquisition) have been reported after therapeutic interventions in patients with CAI.16,29 Given these results, we anticipated that we would observe a greater amount of measurable improvement in balance at 1 week after patients completed the intervention than at 24 to 48 hours postintervention.
METHODS
Design
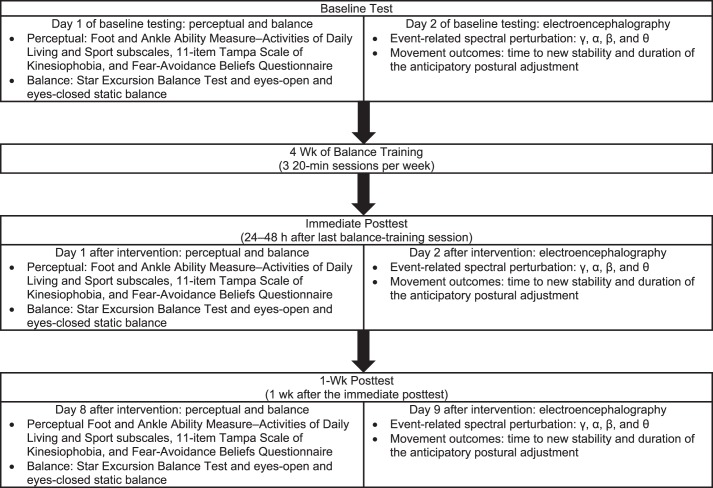
This report is part of a larger investigation of cortical activity and treatment responses among patients with CAI. We used a pretest-posttest with repeated-measures design. All patients were tested 3 times: at baseline (before the intervention), immediately posttest (24–48 hours postintervention), and at 1-week posttest (1 week after the immediate posttest). The immediate and 1-week posttests were conducted after patients had completed the 4-week balance-training program (Figure 1).
Figure 1.
Description of test sessions and study design. Perceptual and balance testing were always performed on day 1 and electroencephalography testing was performed on day 2 to minimize fatigue.
Participants
A total of 15 physically active patients with self-reported CAI participated in this investigation (Table 1). This study was powered to detect improvements in balance after training, with a minimum recommendation of 13 participants (effect size = 0.33, β = .8, α = .05). Consistent with the recommendations of the International Ankle Consortium,30 we defined a patient with CAI as having sustained at least 1 lateral ankle sprain, experienced at least 2 episodes of the ankle giving way in the 6 months before the investigation, and scored at least 11 on the Identification of Functional Ankle Instability instrument. Individuals with both unilateral and bilateral CAI were eligible for this investigation. In patients with bilateral CAI, the limb with the lower scores on the Foot and Ankle Ability Measure (FAAM)–Activities of Daily Living (FAAM-ADL) and Sports (FAAM-Sports) subscales was used as the training limb.15 Scores on the FAAM-ADL and FAAM-Sports were not used as inclusion criteria. Exclusion criteria were known balance and vision problems, acute lower extremity or head injury within 3 months of study enrollment, a history of concussion, any chronic musculoskeletal condition known to affect balance (eg, anterior cruciate ligament deficiency), a history of lower extremity musculoskeletal surgery, or any other neurologic condition that might affect postural control (eg, diabetes) or EEG signal analysis (eg, epilepsy). All participants provided written informed consent, and the study was approved by the University of North Carolina–Charlotte Institutional Review Board.
Table 1.
Participant Demographics
| Measure |
No. (%) |
| Sex | |
| Male | 7 (46.67) |
| Female | 8 (53.33) |
| Mean ± SD |
|
| Age, y | 20.80 ± 2.37 |
| Height, cm | 169.47 ± 7.95 |
| Mass, kg | 70.45 ± 19.25 |
| No. |
|
| Lateral ankle sprains | 2.67 ± 2.02 |
| Ankle rolls in the 6 mo before the study | 4.93 ± 3.31 |
| Score |
|
| Identification of Functional Ankle Instability | 18.33 ± 4.47 |
| Tampa Scale for Kinesiophobia—11 items | 19.60 ± 4.34 |
| Fear-Avoidance Beliefs Questionnaire | 10.00 ± 3.91 |
| Percentage |
|
| Foot and Ankle Ability Measure | |
| Activities of Daily Living subscale | 88.11 ± 6.04 |
| Sports subscale | 76.88 ± 14.11 |
Test Protocol
At the baseline session, participants were provided with a description of the investigation. They completed the same battery of tests at each of the 3 time points to assess perceptual, balance, and cortical outcome measures (Figure 1). The assessments took place over 2 days, with perceptual and balance testing (30 minutes) on day 1 and EEG testing (75–90 minutes) on day 2. Two days were allocated to reduce the potential that fatigue from the balance testing would affect the EEG signal during testing.
Perceptual and Balance Testing
Perceptual testing comprised 4 patient-oriented outcomes (PROs): the FAAM-ADL and FAAM-Sports to assess region-specific perceived function and the 11-item Tampa Scale for Kinesiophobia (TSK-11) and Fear-Avoidance Beliefs Questionnaire physical activity subscale (FABQ) to assess psychosocial aspects of the patients' health-related quality of life. The FAAM-ADL and FAAM-Sports were graded using a 4-point scale of Likert-style responses, with scores of 100% representing no perceived disability and 0% representing complete disability.31 The FAAM is a valid PRO for testing perceived function over the past week among patients with CAI.31 Whereas responsiveness has not been specifically defined for these individuals, minimal clinically important differences (MCIDs) of 8 and 9 points on the FAAM-ADL and FAAM-Sports, respectively, have been established.32 The TSK-11 was graded based on the shortened 11-item list of questions. Scores range from 11 to 44, with higher scores indicating a greater fear of reinjury or movement or both.33 Although this tool has not been validated for use among patients with CAI, the suggested MCID is 4 points.33 The FABQ physical activity score was the sum of the answers to the first 5 items on the full FABQ. Scores range from 0 to 30, with higher scores indicating worse perceived function due to pain.
Balance was assessed using the SEBT and instrumented force-platform trials of single-limb balance. The SEBT was performed in the anterior (SEBT-A), posteromedial (SEBT-PM), and posterolateral (SEBT-PL) directions. Participants completed 4 practice trials before the average of 3 test trials was used for analysis.34 Reach distance was normalized to limb length, which was measured as the distance between the anterior-superior iliac spine and the ipsilateral medial malleolus.34 The MCID has not been established in patients with CAI; however, the minimal detectable change scores have been reported as 6.86% for SEBT-A, 8.15% for SEBT-PM, and 7.11% for SEBT-PL.35 Single-limb balance was measured on a force platform during three 10-second trials of eyes-open and eyes-closed balance with the hands on the hips, the hip flexed to 45°, and the knee flexed to 90°. All test trials were performed on the training limb.
Electroencephalography Testing
Cortical activity was measured while patients completed a lateral-stepping task. We chose this task because it has been associated with impairments in patients with CAI5,27 and evaluated using EEG in adults without CAI.36 Patients stood within a shoulder-width box drawn on a force platform with their hands relaxed and hanging by their sides. On oral cue, they transitioned from double- to single-limb support and maintained single-limb balance for approximately 6 seconds. Given that this study was part of a larger investigation, both limbs were tested in a computer-generated random order; therefore, the oral cue that the patient received was “right” or “left.” An individual trial lasted 8 seconds, and the oral cue was delivered between 1 and 2 seconds after the trial began. Patients were instructed to distribute weight equally between their limbs and not move until the oral cue. A total of 4 testing blocks with a 4-minute seated break between blocks was completed. Each block consisted of 60 trials for a total of 240 trials, with 120 trials completed on each limb. Only trials for the trained limb were included in this analysis.
Force-platform data were collected using an Accusway force platform (AMTI Inc, Watertown, MA) connected to a laptop computer using a PJB-101 interface (AMTI Inc). Data for the static-balance trials were recorded with BalanceClinic software (version 1.4.2; AMTI Inc) at 50 Hz, and data for EEG testing were sampled at 200 Hz using NetForce software (version 3.5.3; AMTI Inc). NetForce was used for the lateral-stepping task because it allows external triggering for synchronizing EEG and force-platform data.
We collected the EEG data using a 32-channel Quick-Cap (Compumedics Neuroscan, Charlotte, NC) connected to a 40-channel NuAmps (Compumedics Neuroscan) digital EEG amplifier. A custom montage was used to collect data from 14 EEG channels (FP1, FP2, F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, CP4), 4 electro-oculographic channels placed above and below the left eye and lateral to each eye, and 2 earlobe clip electrodes (A1, A2). The linked A1/A2 earlobe clip electrodes served as references for all EEG recordings and analyses. Electrode impedance was maintained at less than 5 kΩ throughout testing. The EEG signals were amplified at a gain of 19, filtered at DC 400 Hz, sampled at 1000 Hz in Curry 7 software (version 7.0.9; Compumedics Neuroscan) on a dedicated computer, and saved for offline analysis. Force-platform and EEG data were synchronized using a custom-built trigger device that delivered a 4.8-V transistor-transistor–logic pulse simultaneously to the NuAmps amplifier and the PJB-101 force-platform interface system. The transistor-transistor–logic pulse created an event code in the continuous EEG file and triggered the beginning of the trial in NetForce, resulting in a single file for each lateral-stepping trial.
Balance-Training Intervention
All patients completed the 4-week balance-training program developed by McKeon et al.15 This program consists of twelve 20-minute sessions comprising 5 exercises over a 4-week period. These exercises were 2 hopping exercises completed in 4 directions: anterior-posterior, medial-lateral, anterolateral-posteromedial, and anteromedial-posterolateral.15 The specific exercises were the hop to stabilization (10 repetitions per direction), during which patients hopped to a target and stabilized before hopping back, and the hop to stabilization and reach (5 repetitions per direction), which was the same as the hop to stabilization but with a dynamic-reaching component for the nonstance limb.15 Patients also completed an unanticipated hop-to-stabilization exercise (3 repetitions), during which a randomly generated digit (range, 1–9; dial-pad layout) appeared on a screen and patients hopped to the target and stabilized.15 The program includes static single-limb eyes-open and eyes-closed balance exercises of varying difficulty (3 repetitions each).15 This program is designed to continually challenge the sensorimotor system by manipulating both task (eg, hop distance) and environmental (eg, support-surface) constraints. Progressions occurred only after patients displayed proficient (ie, error-free) movement for every repetition of an individual direction or exercise, or both, during a training session. Progressions in exercise difficulty were exercise specific but included increases in hop distance, hopping with the hands on the hips, increasing the duration of the balance activities, the addition of unstable surfaces, or all of these.15 Progression was determined independently for each exercise and direction within an activity by the same certified athletic trainer (C.J.B.). Complete descriptions of the exercises and progression have been presented in a previous study.15
Data Analysis and Outcome Measures
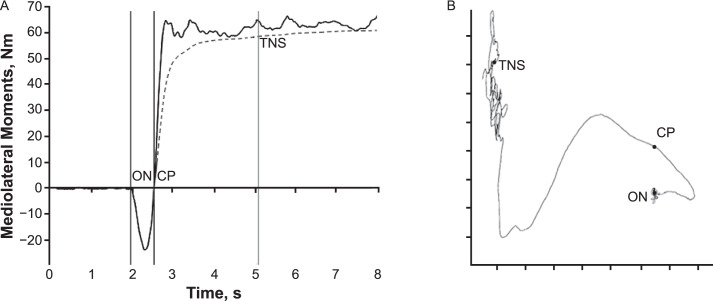
Static-balance trials were analyzed using the BalanceClinic software, and outcomes included the path length of the COP (in centimeters), peak velocity (in cm/s) in the anteroposterior and mediolateral (ML) planes, and the 95% confidence ellipse of the COP area (in cm2). For the lateral-stepping trials, we used a modified version of the time to new stability (TNS) that was originally described by Dingenen et al27 (Figure 2A). Given the software restrictions for external triggers, the force platform went to zero before each trial, making it impossible to capture accurate vertical force values and subsequently calculate COP during these trials. However, analysis of our data and subsequent follow-up testing in our laboratory revealed that the data from the force-moment channels (Mx and My) during the zeroed-out trials were very strongly correlated with the COP locations during the stepping task (Figure 2B). Therefore, we used the Mx and My channels to calculate the TNS outcome.27 The duration of the anticipatory postural adjustment was also calculated (Figure 2A).
Figure 2.
A, The onset of the lateral step (ON) is identified when the mediolateral (My) moments begin shifting toward the nonstance limb. The crossing point (CP), which is the point where the coordinates of the moment cross ON, is also present. The cumulative displacement of each successive point from the average position of the anteroposterior (Mx) and My signal before ON is then calculated. The time to new stability (TNS) is identified after the cumulative displacement becomes and stays less than 0.25 SDs of the cumulative displacement at the end of the trial. The full calculations can be seen in the original report.4 The duration of the anticipatory postural adjustment outcome was calculated as the time (in milliseconds) between ON and CP, which represents the duration of the initial anticipatory postural adjustment. B, The trace of the graphed Mx and My moments can be seen. The similarity to the trace observed in center-of-pressure data meant that the TNS outcome as described by Dingenen et al27 could be calculated, which is why no units are given.
We processed the EEG data using Curry 7 and MATLAB (version R2016a; The MathWorks, Inc, Natick, MA). We used Curry 7 for baseline and ocular artifact correction and exported the data into MATLAB for further processing using scripts in EEGLAB (version 13.6.5b; The MathWorks, Inc).37 The data were low-pass filtered at 100 Hz to remove trigger artifact and segmented into 3-second epochs around the initiation of the double- to single-limb transition (−1500 to 1500 milliseconds). Baseline activity (−1200 to −1000 milliseconds) was subtracted from each epoch,36 and each epoch was visually inspected by a single, nonblinded investigator (C.J.B.). Trials with excessive noise or substantial movement artifact in any channel were rejected. To attain a signal-to-noise ratio similar to that of Varghese et al,36 a minimum of 40 trials per participant was required for analysis. All participants met this criterion (minimum = 42, median = 52, maximum = 69 trials).
An EEG signal comprises multiple frequency components. An ERSP analysis is a time-frequency transform that computes the change in signal power at each frequency from baseline for an event to represent event-related change in activity. The power of the EEG signal can change when an increase or decrease occurs in the synchronized activity of large populations of neurons.24 In this investigation, the ERSP was calculated for the 500-millisecond window just before the initiation of the lateral step (ie, the event) with respect to the spectral power that occurred 1200 to 1000 milliseconds before initiating the lateral step (ie, the baseline activity).36 These values, log transformed to decibels, were grand averaged across the 500-millisecond window before the lateral step in 4 bands of activity: θ (4–8 Hz), α (8–12 Hz), β (14–25 Hz), and γ (30–50 Hz; Figure 3). These frequency bands were selected because of their roles in sensorimotor processing and movement preparation.22,24,36 A decrease in upper α and β power represents an increase in cortical excitability, whereas an increase in γ power represents rapid sensorimotor processing.24 Increased θ oscillations are commonly associated with executive functions and decision making21; however, recent evidence22 has suggested that they may reflect movement selection and initiation. The ERSP was calculated separately for all 4 bands at the Cz electrode.36 The central electrodes are most commonly evaluated during sensorimotor tasks,24,36 and the Cz is located closest to the premotor and supplementary motor cortex. Therefore, activity from this electrode best represents motor-planning processes and minimizes the influence of other ongoing brain processes that may be detected because of volume conduction of the EEG signal.
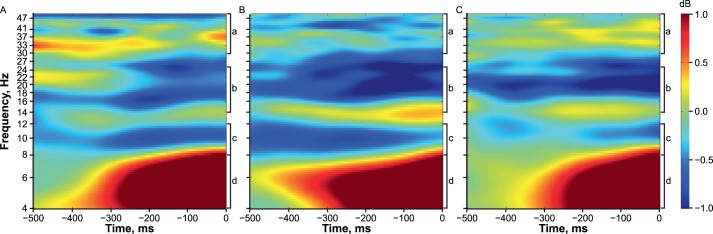
Figure 3.
The grand averaged (n = 15) event-related spectral perturbations output at each time can be seen. A, Baseline. B, Immediate posttest. C, 1-week posttest. a γ Band (30–50 Hz). b β Band (14–25 Hz). c α Band (8–12 Hz). d θ Band (4–8 Hz). More blue colors indicate a decrease in signal power, and therefore an increase in activity in frequencies less than 30 Hz. Yellow to red colors indicate a decrease in activity in frequencies less than 30 Hz. Increased activity in the γ band (30–50 Hz) is indicated by more yellow and red colors, whereas decreased activity in the γ band is indicated by more green and blue colors.
Statistical Analysis
All outcome measures were analyzed with 1-way (baseline, immediate posttest, 1-week posttest) repeated-measures analyses of variance. The Tukey honestly significant difference was used for post hoc testing when indicated. Change scores were calculated for the immediate and delayed response to treatment as immediate posttest minus baseline and 1-week posttest minus baseline. Hedges g effect sizes and 95% confidence intervals (CIs) were also calculated for the immediate and delayed treatment responses as immediate posttest minus baseline and 1-week posttest minus baseline. Effect sizes were interpreted as weak (≤0.40), moderate (0.41–0.69), or strong (≥0.70).38 We set the α level at .05, and all statistical tests were performed in SPSS (version 23; IBM Corp, Armonk, NY).
RESULTS
Perceptual Outcomes
Means and standard deviations of all outcome measures and the change scores and Hedges g effect sizes with 95% CIs are presented in Table 2. We observed an effect of time in FAAM-ADL (F2,28 = 6.903, P = .005), FAAM-Sports (F2,28 = 6.456, P = .02), and FABQ (F2,28 = 5.615, P = .009) scores. Relative to baseline, FAAM-ADL scores were improved at the immediate (P = .047) and 1-week (P = .006) posttests. A delayed improvement in the FAAM-Sports score was identified at 1-week posttest relative to baseline (P = .008). The FAAM-Sports score also showed improvement between the immediate and 1-week (P = .004) posttests, but it was not clinically important.29 Improvements were observed in FABQ scores at the immediate (P = .01) and 1-week (P = .009) posttests compared with baseline. No changes were identified for TSK-11 scores (P > .05).
Table 2.
Patient-Reported Outcomes, Balance, and Cortical Activity at Each Timepoint
| Measure |
Baseline |
Immediate Posttest |
Initial Treatment |
1-wk Posttest, Mean ± SD |
Delayed Treatment |
||
| Mean ± SD |
Change Score, Mean ± SD |
Hedges g Effect Size (95% CI) |
Change Score, Mean ± SD |
Hedges g Effect Size (95% CI) |
|||
| Perceptual outcomes | |||||||
| Foot and Ankle Ability Measure, % | |||||||
| Activities of Daily Living subscalea | 88.11 ± 6.04 | 91.50 ± 5.78b | 3.39 ± 6.04 | 0.79 (0.05, 1.54)c | 93.29 ± 6.75b | 5.18 ± 6.24 | 0.75 (0.01, 1.49)c |
| Sports subscalea | 76.88 ± 14.11 | 83.96 ± 11.74 | 7.08 ± 15.47 | 0.67 (−0.07, 1.40) | 88.33 ± 8.95b,d | 11.46 ± 14.26 | 0.74 (0.00, 1.48)c |
| Tampa Scale for Kinesiophobia—11-item | 19.60 ± 4.34 | 17.73 ± 2.71 | 1.87 ± 3.93 | 0.48 (−0.25, 1.21) | 18.13 ± 4.93 | 1.47 ± 2.90 | 0.46 (−0.26, 1.19) |
| Fear-Avoidance Beliefs Questionnairea | 10.00 ± 3.91 | 6.80 ± 4.06b | 3.20 ± 4.33 | 0.61 (−0.12, 1.35) | 6.73 ± 4.40b | 3.27 ± 4.17 | 0.71 (−0.03, 1.45) |
| Balance outcomes | |||||||
| Star Excursion Balance Test, % | |||||||
| Anterior directiona | 74.71 ± 5.37 | 80.38 ± 4.03b | 5.66 ± 3.41 | 1.47 (0.66, 2.27)c | 79.70 ± 4.19b | 4.99 ± 3.32 | 1.43 (0.63, 2.23)c |
| Posteromedial directiona | 84.56 ± 9.05 | 92.50 ± 7.37b | 7.94 ± 6.47 | 1.41 (0.61, 2.21)c | 92.59 ± 7.83b | 8.04 ± 6.31 | 1.16 (0.39, 1.94)c |
| Posterolateral directiona | 80.98 ± 12.16 | 90.27 ± 7.60b | 9.29 ± 7.96 | 0.83 (0.08, 1.58)c | 91.51 ± 7.81b | 10.52 ± 8.37 | 1.27 (0.48. 2.05)c |
| Eyes open | |||||||
| Center-of-pressure path length, cma | 42.33 ± 10.89 | 37.56 ± 9.36 | 4.76 ± 8.69 | 0.50 (−0.22, 1.23) | 34.74 ± 10.10b | 7.59 ± 8.84 | 0.78 (0.03, 1.52)c |
| Anteroposterior maximal velocity, cm/s | 11.77 ± 2.66 | 12.75 ± 3.34 | −0.99 ± 2.17 | −0.24 (−0.95, 0.48) | 11.22 ± 3.12 | −0.54 ± 2.75 | −0.18 (−0.90, 0.54) |
| Mediolateral maximal velocity, cm/sa | 14.31 ± 4.36 | 12.96 ± 3.50 | 1.35 ± 3.63 | 0.33 (−0.39, 1.06) | 10.83 ± 3.70b | 3.48 ± 3.77 | 0.84 (0.09, 1.59)c |
| Center-of-pressure area, cm2 | 8.10 ± 4.51 | 5.80 ± 2.06 | 2.31 ± 4.03 | 0.50 (−0.23, 1.22) | 6.89 ± 3.17 | 1.22 ± 4.70 | 0.24 (−0.48, 0.96) |
| Eyes closed | |||||||
| Center-of-pressure path length, cma | 92.31 ± 21.68 | 83.73 ± 20.71 | 8.58 ± 22.63 | 0.39 (−0.33, 1.11) | 78.26 ± 23.49b | 14.05 ± 21.23 | 0.60 (−0.13, 1.33) |
| Anteroposterior maximal velocity, cm/s | 27.26 ± 6.02 | 26.54 ± 5.42 | 0.72 ± 5.38 | 0.08 (−0.63, 0.80) | 24.93 ± 7.45 | −2.33 ± 9.09 | −0.23 (−0.95, 0.49) |
| Mediolateral maximal velocity, cm/s | 31.30 ± 8.36 | 28.08 ± 7.08 | 3.23 ± 8.62 | 0.34 (−0.39, 1.06) | 27.27 ± 9.50 | 4.03 ± 10.05 | 0.36 (−0.36, 1.08) |
| Center-of-pressure area, cm2 | 27.25 ± 12.09 | 21.90 ± 6.51 | 5.35 ± 10.98 | 0.47 (−0.25, 1.20) | 21.04 ± 8.13 | 6.21 ± 11.12 | 0.52 (−0.21, 1.25) |
| Time to new stability, s | 2.23 ± 0.22 | 2.22 ± 0.17 | 0.01 ± 0.24 | 0.03 (−0.69, 0.74) | 2.18 ± 0.13 | 0.04 ± 0.25 | 0.17 (−0.55, 0.88) |
| Duration of the anticipatory postural adjustment, ms | 545.23 ± 76.19 | 554.26 ± 77.88 | −9.04 ± 50.54 | −0.16 (−0.87, 0.55) | 553.55 ± 59.94 | −6.32 ± 54.47 | −0.14 (−0.86, 0.57) |
| Electroencephalography event-related spectral perturbation, dBe | |||||||
| Θ | 0.86 ± 0.97 | 0.80 ± 1.31 | 0.00 ± 1.08 | −0.05 (−0.76, 0.67) | 0.81 ± 0.86 | 0.01 ± 0.89 | −0.05 (−0.76, 0.67) |
| α | −0.35 ± 1.47 | −0.48 ± 1.30 | −0.11 ± 1.33 | −0.08 (−0.80, 0.63) | −0.25 ± 0.88 | 0.17 ± 1.11 | 0.09 (−0.62, 0.81) |
| β | −0.38 ± 0.80 | −0.62 ± 0.83 | −0.24 ± 1.04 | −0.21 (−0.92, 0.51) | −0.52 ± 0.54 | −0.17 ± 0.99 | −0.13 (−0.85, 0.59) |
| γ | −0.09 ± 0.47 | −0.28 ± 0.54 | −0.20 ± 0.50 | 0.35 (−0.37, 1.08) | 0.01 ± 0.57 | 0.05 ± 0.52 | −0.17 (−0.89, 0.55) |
Abbreviation: CI, confidence interval.
Indicates a main effect of time (P < .05).
Difference from baseline (P < .05).
The 95% CI did not cross zero.
Difference from the immediate posttest (P < .05).
For event-related spectral perturbation outcomes, decibel values closer to zero indicate no change in signal power in a given frequency band. Negative values indicate a widespread increase in activity for the θ, α, and β. bands; positive values indicate more focal activity and increased activity for the γ band. Signs have been changed for outcome measures so a positive change score or Hedges g effect size indicates an improvement in the outcome measure. For ERSP effect sizes, a positive point estimate represents a decrease in cortical activity; this means a less negative decibel value for the θ, α, and β bands and a more negative decibel value for the γ band.
Balance Outcomes
Effects of time were identified in SEBT-A (F2,28 = 29.40, P < .001), SEBT-PM (F2,28 = 20.76, P < .001), SEBT-PL (F2,28 = 21.21, P < .001), eyes-open COP path length (F2,28 = 5.81, P = .008), eyes-open ML peak velocity (F2,28 = 6.70, P = .004), and eyes-closed COP path length (F2,28 = 3.53, P = .043). The SEBT reach distance was improved at both posttest sessions compared with baseline (P < .05). Compared with baseline, eyes-open COP path length (P = .02) and ML peak velocity (P = .009) and eyes-closed COP path length (P = .02) were improved at 1-week posttest but were not different at immediate posttest. Therefore, we considered the improvements to be delayed. No differences were identified in either the TNS or duration of anticipatory postural adjustment outcome among any test sessions (P > .05).
Electroencephalography Outcomes
No differences in premovement cortical activity were noted at either time after balance training (P > .05). Hedges g effect sizes ranged from −0.21 to 0.35, indicating a weak effect. The 95% CIs included zero for all EEG effect sizes (Table 2).
DISCUSSION
Contrary to our hypothesis, we did not observe any changes in premovement cortical activity after patients with CAI completed 4 weeks of balance training. We identified improvements in PROs, SEBT performance, and instrumented balance outcomes, which are consistent with results reported for this balance-training program in the literature.15–17 Our findings suggested that perceptual and balance improvements among patients with CAI were not mediated by changes in premovement cortical activity during a lateral-stepping task.
Our ERSP measure of motor planning during a lateral-stepping task did not change after balance training. We expected to observe differences in the ERSPs based on reports in the balance-training21 and skill-training25,26 literature. Our findings indicated that balance training did not produce changes in cortical activity. However, the differential responsiveness of our dependent variables suggested that balance training may improve dynamic balance. No improvements were identified in ERSP or static-balance measures, but performance in all 3 directions of the SEBT was improved. Whereas these balance improvements were likely due to changes in central nervous system (CNS) function, our ERSP analysis before a lateral-stepping task did not reveal any changes in premovement cortical activity. It is likely that changes to cortical activity occurred but may not have transferred to the lateral-stepping task. In other words, beneficial adaptations to motor preparation or execution, or both, likely occurred for tasks similar to those being trained but not for the lateral-stepping task that we used to assess cortical changes. For example, the hop-to-stabilization-and-reach task and the SEBT share a similar reaching component. It is also possible that our methods of grand averaging ERSPs across the entire band may not be the ideal approach for tracking neurophysiological changes after interventions. Cortical oscillations (eg, α and β bands) are known to have large interindividual differences in peak activity within a given band.24 For example, patient A may have had peak α activity around 11 Hz, and patient B may have had peak α activity around 9 Hz. Researchers39 have suggested that these α peaks may shift according to the demand or difficulty of a balance task. Therefore, identifying the peak frequency for each patient and the response to balance training may be a more sensitive approach than averaging the activity across an entire band.
Another possible reason why we observed changes in balance but not cortical activity is that these changes may be mediated via other mechanisms. This may include an upregulation in sensory processing and muscular coordination in subcortical structures, such as the basal ganglia or cerebellum,20 which is not easily measured with EEG. Another option is that balance improvements occur due to adaptations at the spinal level. Both gait19,21 and balance6 improved after balance training, and these tasks rely on spinal-level activation of muscle synergies through either afferent or efferent input. Intricate networks of interneurons help to regulate the activation of the muscle synergies, and it is likely they are susceptible to neuroplastic changes after balance training. Researchers should explore alternative approaches to evaluating EEG activity or combining EEG and other measurement modalities, such as Hoffman-reflex testing, transcranial magnetic stimulation, magnetic resonance imaging, or all of these, to improve our understanding of the CNS adaptations that result from balance training.
The research in which investigators have used EEG to examine how balance training affects the CNS is limited. Schattin et al21 noted that exergaming decreased prefrontal θ activity more than standard balance training in older adults; however, the magnitude of this decrease was not different in the balance-training group. The results of Schattin et al21 and our study may indicate that balance training is not a robust enough intervention to cause large-scale changes to cortical activity. We identified improvements in balance (Table 2), and Schattin et al21 reported greater improvement in gait measures in the balance-training group, yet these were paired with minor changes in θ activity. The remaining frequency bands analyzed (Δ, lower α, upper α, and β) did not change in either group.21 Whereas θ activity has been implicated in movement selection and execution, it has also traditionally been accepted as a rhythm representing executive function in the frontocentral regions of the brain.22 Therefore, the findings reported in the literature may represent an improvement in the age-related decline in executive functioning rather than adaptations to motor functions. Another possible explanation for why we did not observe changes in our ERSP measures is that patients with CAI may not have impairments in premovement cortical activity measured with EEG. Although researchers using peripheral outcomes (eg, electromyographic and kinetic) have suggested that alterations to or impaired motor planning were present in patients with CAI,5,7 these may not be drastic enough to be detected by EEG measures or the gains may not have transferred from balance-training exercise to the lateral-stepping task used in this investigation. Therefore, as a field, we are limited by not knowing whether differences in cortical activity exist between uninjured controls and patients with CAI before or during a lateral-stepping task.
We observed improvements in PROs, SEBT, and static balance more frequently at the 1-week posttest (Table 2). This supports the idea that the benefits of balance training take time to mature after treatment.16,40 We identified improvements in PROs (FAAM-Sports) and instrumented balance measures (eyes-open COP path length and ML peak velocity and eyes-closed COP path length) at 1-week posttest that were not present at the immediate posttest, which was consistent with previous findings.16 Whereas delayed improvements have been identified in participants without CAI,40 these studies are often designed to evaluate motor learning, as opposed to studies of patients with CAI that are more focused on improving clinically important outcomes (eg, balance). Thus, it is not possible to determine whether delayed improvements in balance are a natural response to balance training or attributed to impairments in patients with CAI.
The mechanisms of these delayed improvements are not well understood; however, they may represent the time the sensorimotor system needs to fully develop new movement strategies based on the feedback obtained during the training period. Regardless of the underlying mechanism, this pattern of delayed improvements may have clinical implications. For example, the FAAM assesses the perception of function over the past week,31 so immediate postintervention assessments would not capture all benefits of the entire intervention. Although it is impractical to wait a week before assessing the outcome of an intervention in clinical practice, our results and the existing literature could support the concept of serial testing to quantify continued improvements, retention, or the need for a training bolus because of performance declines.
Several additional limitations should be considered when interpreting our results. An unintended consequence of the data-collection protocol was that we were unable to include certain outcome measures. The motor-related cortical potential (MRCP) is an event-related potential that precedes movement, and it has been measured during lateral stepping.36 In our study design, a patient had to respond to an oral command of “right” or “left” and then execute the lateral step. Under this protocol, the patient could have prepared for lateral stepping to either limb. This choice-response study design results in an event-related potential called contingent negative variation that is different from the MRCP, which is a measure of motor planning that is observed during self-initiated movements.36 The electrode montage that we used made it impractical to perform an independent-components analysis on the data because of the small number of channels. This limited our ability to isolate the movement-related activity from movement artifacts. The independent-components analysis is a computational method that can be used to clean data by isolating and correcting movement artifacts, but it can also be used to analyze specific components, such as the isolated MRCP reported during lateral stepping.36 However, we believe that minimal movement artifact was present, as we analyzed only activity before movement and discarded contaminated trials. Lastly, this report is part of a larger investigation that was powered a priori to detect balance improvements among 15 patients with CAI. It is possible that this small sample size and limited variability among patients may have masked cortical differences that could be identified with larger sample sizes; therefore, given our largest effect size (Hedges g = 0.35), we suggest investigators include at least 25 patients with CAI when analyzing cortical adaptations to balance training.
CONCLUSIONS
A 4-week balance-training program improved sensorimotor control and PROs of perceived function and health-related quality of life in patients with CAI. Improvements were more pronounced 1 week after completing the intervention, possibly indicating the presence of a buffering period during which patients fully integrated their newly acquired skills into their full repertoire of movement patterns to maximize motor control. However, a 4-week balance-training program did not alter motor planning as measured by ERSP in patients with CAI. Given this lack of responsiveness, ERSP may not be the best outcome for assessing treatment efficacy in patients with CAI. Based on our findings of delayed improvements in balance, a follow-up may be necessary to assess the full treatment effect of the intervention(s) used when determining return to participation for these patients.
ACKNOWLEDGMENTS
Funding for this study was provided by a research grant from the Mid-Atlantic Athletic Trainers' Association (Dr Burcal). We thank Casey Bruce and Brandon Glover for their assistance with data collection and Sharnali Ghoshdastidar and Avanish Madhavaram for their assistance with data processing. We also thank Bart Dingenen, PhD; Kevin Deschamps, PhD; Filip Staes, PhD; and Dirk Desmet for their invaluable assistance with refining the code to analyze our postural data.
REFERENCES
- 1.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: a prospective cohort analysis. Am J Sports Med. 2016;44(4):995–1003. doi: 10.1177/0363546516628870. [DOI] [PubMed] [Google Scholar]
- 2.Delahunt E, Coughlan GF, Caulfield B, Nightingale EJ, Lin CW, Hiller CE. Inclusion criteria when investigating insufficiencies in chronic ankle instability. Med Sci Sports Exerc. 2010;42(11):2106–2121. doi: 10.1249/MSS.0b013e3181de7a8a. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard-Turner T, Turner MJ. Physical activity levels in college students with chronic ankle instability. J Athl Train. 2015;50(7):742–747. doi: 10.4085/1062-6050-50.3.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med. 2010;38(4):829–834. doi: 10.1177/0363546509351562. [DOI] [PubMed] [Google Scholar]
- 5.Van Deun S, Staes FF, Stappaerts KH, Janssens L, Levin O, Peers KK. Relationship of chronic ankle instability to muscle activation patterns during the transition from double-leg to single-leg stance. Am J Sports Med. 2007;35(2):274–281. doi: 10.1177/0363546506294470. [DOI] [PubMed] [Google Scholar]
- 6.Wikstrom EA, Naik S, Lodha N, Cauraugh JH. Balance capabilities after lateral ankle trauma and intervention: a meta-analysis. Med Sci Sports Exerc. 2009;41(6):1287–1295. doi: 10.1249/MSS.0b013e318196cbc6. [DOI] [PubMed] [Google Scholar]
- 7.Wikstrom EA, Bishop MD, Inamdar AD, Hass CJ. Gait termination control strategies are altered in chronic ankle instability subjects. Med Sci Sports Exerc. 2010;42(1):197–205. doi: 10.1249/MSS.0b013e3181ad1e2f. [DOI] [PubMed] [Google Scholar]
- 8.Pietrosimone BG, Gribble PA. Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train. 2012;47(6):621–626. doi: 10.4085/1062-6050-47.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terada M, Bowker S, Thomas AC, Pietrosimone B, Hiller CE, Gribble PA. Corticospinal excitability and inhibition of the soleus in individuals with chronic ankle instability. PM R. 2016;8(11):1090–1096. doi: 10.1016/j.pmrj.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Needle AR, Baumeister J, Farquhar WB, et al. The relationship between the sensory responses to ankle-joint loading and corticomotor excitability. Int J Neurosci. 2018;128(5):435–441. doi: 10.1080/00207454.2017.1396219. [DOI] [PubMed] [Google Scholar]
- 11.Heroux ME, Tremblay F. Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):823–833. doi: 10.1007/s00167-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 12.Courtney C, Rine RM, Kroll P. Central somatosensory changes and altered muscle synergies in subjects with anterior cruciate ligament deficiency. Gait Posture. 2005;22(1):69–74. doi: 10.1016/j.gaitpost.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Needle AR, Lepley AS, Grooms DR. Central nervous system adaptation after ligamentous injury: a summary of theories, evidence, and clinical interpretation. Sports Med. 2017;47(7):1271–1288. doi: 10.1007/s40279-016-0666-y. [DOI] [PubMed] [Google Scholar]
- 14.Attenborough AS, Sinclair PJ, Sharp T, et al. The identification of risk factors for ankle sprains sustained during netball participation. Phys Ther Sport. 2017;23:31–36. doi: 10.1016/j.ptsp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 15.McKeon PO, Ingersoll CD, Kerrigan DC, Saliba E, Bennett BC, Hertel J. Balance training improves function and postural control in those with chronic ankle instability. Med Sci Sports Exerc. 2008;40(10):1810–1819. doi: 10.1249/MSS.0b013e31817e0f92. [DOI] [PubMed] [Google Scholar]
- 16.Burcal CJ, Trier AY, Wikstrom EA. Balance training versus balance training with STARS in patients with chronic ankle instability: a randomized controlled trial. J Sport Rehabil. 2017;26(5):347–357. doi: 10.1123/jsr.2016-0018. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer JL, Sandrey MA. Effects of a 4-week dynamic-balance-training program supplemented with Graston instrument-assisted soft-tissue mobilization for chronic ankle instability. J Sport Rehabil. 2012;21(4):313–326. doi: 10.1123/jsr.21.4.313. [DOI] [PubMed] [Google Scholar]
- 18.McKeon PO. Dynamic systems theory as a guide to balance training development for chronic ankle instability: a review of the literature. Athl Train Sports Health Care. 2012;4(5):230–236. [Google Scholar]
- 19.McKeon PO, Paolini G, Ingersoll CD, et al. Effects of balance training on gait parameters in patients with chronic ankle instability: a randomized controlled trial. Clin Rehabil. 2009;23(7):609–621. doi: 10.1177/0269215509102954. [DOI] [PubMed] [Google Scholar]
- 20.Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf) 2008;193(2):101–116. doi: 10.1111/j.1748-1716.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- 21.Schattin A, Arner R, Gennaro F, de Bruin ED. Adaptations of prefrontal brain activity, executive functions, and gait in healthy elderly following exergame and balance training: a randomized-controlled study. Front Aging Neurosci. 2016;8:278. doi: 10.3389/fnagi.2016.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan R, Doeller CF, Barnes GR, et al. Movement-related theta rhythm in humans: coordinating self-directed hippocampal learning. PLoS Biol. 2012;10(2):e1001267. doi: 10.1371/journal.pbio.1001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfurtscheller G, Berghold A. Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol. 1989;72(3):250–258. doi: 10.1016/0013-4694(89)90250-2. [DOI] [PubMed] [Google Scholar]
- 24.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 25.Houweling S, Daffertshofer A, van Dijk BW, Beek PJ. Neural changes induced by learning a challenging perceptual-motor task. Neuroimage. 2008;41(4):1395–1407. doi: 10.1016/j.neuroimage.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Pollok B, Latz D, Krause V, Butz M, Schnitzler A. Changes of motor-cortical oscillations associated with motor learning. Neuroscience. 2014;275:47–53. doi: 10.1016/j.neuroscience.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Dingenen B, Staes FF, Janssens L. A new method to analyze postural stability during a transition task from double-leg stance to single-leg stance. J Biomech. 2013;46(13):2213–2219. doi: 10.1016/j.jbiomech.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Taube W, Gruber M, Beck S, Faist M, Gollhofer A, Schubert M. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol (Oxf) 2007;189(4):347–358. doi: 10.1111/j.1748-1716.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 29.McKeon PO, Wikstrom EA. Sensory-targeted ankle rehabilitation strategies for chronic ankle instability. Med Sci Sports Exerc. 2016;48(5):776–784. doi: 10.1249/MSS.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J Orthop Sports Phys Ther. 2013;43(8):585–591. doi: 10.2519/jospt.2013.0303. [DOI] [PubMed] [Google Scholar]
- 31.Carcia CR, Martin RL, Drouin JM. Validity of the Foot and Ankle Ability Measure in athletes with chronic ankle instability. J Athl Train. 2008;43(2):179–183. doi: 10.4085/1062-6050-43.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM) Foot Ankle Int. 2005;26(11):968–983. doi: 10.1177/107110070502601113. [DOI] [PubMed] [Google Scholar]
- 33.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1–2):137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Gribble PA, Hertel J, Plisky P. Using the Star Excursion Balance Test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train. 2012;47(3):339–357. doi: 10.4085/1062-6050-47.3.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munro AG, Herrington LC. Between-session reliability of the Star Excursion Balance Test. Phys Ther Sport. 2010;11(4):128–132. doi: 10.1016/j.ptsp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Varghese JP, Merino DM, Beyer KB, McIlroy WE. Cortical control of anticipatory postural adjustments prior to stepping. Neuroscience. 2016;313:99–109. doi: 10.1016/j.neuroscience.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 37.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- 39.Hulsdunker T, Mierau A, Struder HK. Higher balance task demands are associated with an increase in individual alpha peak frequency. Front Hum Neurosci. 2015;9:695. doi: 10.3389/fnhum.2015.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elion O, Sela I, Bahat Y, Siev-Ner I, Weiss PL, Karni A. Balance maintenance as an acquired motor skill: delayed gains and robust retention after a single session of training in a virtual environment. Brain Res. 2015;1609:54–62. doi: 10.1016/j.brainres.2015.03.020. [DOI] [PubMed] [Google Scholar]