Abstract
Lateral ankle sprains are the most common injuries sustained during physical activity. The epidemiologic trends associated with chronic ankle instability (CAI) suggest that current rehabilitation approaches may be inadequate. We sought to synthesize best-practices evidence for the rehabilitation of patients with acute ankle sprains and CAI through the integration of emerging paradigms in perception, the dynamics of skill acquisition, and the biopsychosocial model of function, disability, and health. From the best available evidence, 4 key factors emerged for effective treatment and rehabilitation strategies: pain reduction, external ankle support for up to 1 year, progressive return to motion, and coordination training. We combined these factors into a meta-theoretical framework that centers on the perceptual interdependence of the cellular, local, and global functioning levels by linking insights from the body-self neuromatrix, the dynamics of skill acquisition, and the biopsychosocial model. Based on the best-practice recommendations from systematic reviews, ankle-sprain rehabilitation represents a multidimensional phenomenon governed by perception. The impairments, activity limitations, and participation restrictions associated with CAI may be linked to perceptual-interdependence alterations. Pain and edema reduction, the use of external ankle support for up to 1 year, progressive return to motion, and coordination training foster enhanced perceptual interdependence from cells to society. Using the perceptual-interdependence framework for ankle-sprain rehabilitation, we offer new insights for charting the course of effective strategies for enhancing function, reducing disability, and preventing the long-term sequelae associated with CAI.
Keywords: body-self neuromatrix, biopsychosocial model, skill acquisition
Ankle sprains are ubiquitous in physical activity.1,2 These injuries represent the most common lower extremity injuries associated with sport participation and have the highest recurrence rates.2 At least 1 in 3 patients will develop recurrent problems after an index sprain.1,3 Chronic ankle instability (CAI) is characterized by repeated giving-way episodes, functional performance deficits, decreased physical activity, recurrent ankle sprains, and the early onset of ankle osteoarthritis.4,5 Numerous clinical features and contributing factors have been identified for those at risk of developing CAI.6 The most consistent evidence is that ankle sprains continue to be the most frequent injuries seen in sports, and a history of ankle sprains is the strongest predictor for sustaining one.
In line with the literature addressing factors that contribute to the development of CAI, numerous studies and systematic reviews have been conducted to identify the most effective treatment strategies for resolving symptoms, reducing functional deficits, and decreasing the risk of patients sustaining another ankle sprain. As with the predictors, the literature has now reached the point that systematic reviews are being conducted of systematic reviews of the evidence.7 Although the recurrence rates of these injuries remain very high, the evidence from systematic reviews, best-practice recommendations, and clinical commentaries identified 4 factors that appear to be most advantageous in enhancing patient function and reducing the risk of recurrent ankle sprains7–10:
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) to reduce pain and enhance self-reported function in conjunction with ice, compression, and elevation early in the rehabilitation process.
Early return to motion rather than prolonged immobilization after an ankle sprain.
The use of external ankle support, either a functional ankle brace or tape, for up to 1 year after an ankle sprain.
The incorporation of balance, exercise, and coordination training in the rehabilitation plan as soon as weight bearing can be tolerated.
Given the overwhelming evidence to support the use of these 4 components, it would be easy to end this clinical commentary here. However, a trend in the evidence points to an underlying phenomenon that drives both the predictors of injury and the preventive strategies to reduce risk. The purpose of this clinical commentary is to provide a theoretical framework for interpreting the evidence associated with treatment and rehabilitation strategies through the integration of emerging paradigms in perception, the dynamics of skill acquisition, and the biopsychosocial model of function, disability, and health. The key concept discussed throughout is the interdependence of the perceptions of cells and tissues, the body, the perception of self, and the perception of self in the context of society. Ankle sprains can alter this perceptual interdependence, resulting in a continuum of disability.5 Rehabilitation strategies that use the 4 key components highlighted earlier provide a framework for breaking this continuum and restoring health.
Before delving into the perceptual-interdependence framework for ankle-sprain rehabilitation, it is important to revisit the seminal works that govern the integrated paradigm proposed. The collective works that have inspired much of the evidence associated with ankle-sprain treatment and rehabilitation emerged in 1965 and continue to influence clinical decisions for patients with ankle sprains today.
The Recognition of CAI
In 1965, Freeman et al published their seminal companion papers11,12 on the nature and treatment of functional instability of the foot with a line that would continue to echo through history:
Functional instability of the foot [a term used in this paper to designate the disability to which patients refer when they say that their foot tends to “give way”] follows about 40% of injuries to the lateral ligament of the ankle.12
The evidence from these 2 studies is best captured in 3 key findings:
Strapping and early mobilization of the ankle after an acute ankle sprain yielded faster resolution of symptoms than immobilization but did not reduce the risk of developing functional instability of the foot.11
Conventional treatment (ice, compression, resisted movements, stabilization exercises, and walking reeducation) combined with coordination training improved self-reported function and episodes of giving way more than conventional treatment alone.12
Functional instability of the foot was attributed to a deafferentation of articular structures in the ankle due to injury. Motor incoordination was a consequence of this deafferentation, but when treated appropriately (with coordination training), this residual phenomenon could be alleviated.12
These initial authors charted the course of ankle-instability rehabilitation research, first by defining the condition (functional instability of the foot) and second by detailing a method for preventing it. Freeman et al11,12 provided a framework derived from the work of Sir Charles Sherrington13 on the role of sensory information in motor coordination: that motor control is subservient to the available relevant sources of sensory information. Freeman et al11,12 did not start coordination training with their patients until pain had decreased sufficiently. Pain was recognized as a limiting factor in the ability to effectively coordinate movements. Patients performed coordination exercises over an average of 5 sessions, progressing from a single-axis tilt board to a semispherical wobble board with the clear movement goal to “maintain balance with neither end of the board touching the floor.”12 Once patients mastered performance on the single-axis board, they progressed to the semispherical wobble board.
Coordination training would spark a paradigm shift in the treatment of ankle sprains. When comparative outcomes were examined, 7% of patients treated with coordination training reported episodes of giving way after treatment compared with 46% who received immobilization or conventional therapy alone. This would indicate a successful treatment strategy, with an 85% relative risk reduction in the coordination group versus all other treatment groups. However, follow-up in this study was poor (<80%), so the point measure (85% relative risk reduction) should be interpreted with caution. Based on the results from the coordination training study, Freeman et al12 concluded:
Treatment by coordination exercises was based upon the hope that some central process might compensate for articular deafferentation and its consequent proprioceptive deficit, and that such a process might be made more effective by deliberate “training.” What neurophysiological events might underlie this “training” is hard to say, but presumably a similar process occurs when a subject learns to ride a bicycle or walk a tight-rope.12
Freeman et al12 set out to help their patients learn the skill dynamics for balancing on 1 leg. They observed lasting training effects after coordination training, including improved balance and reduced giving-way episodes. The critical take-home message was that an early return to motion combined with progressive coordination training reduced the chances of developing CAI.
Since its inception as a paradigm, CAI has undergone several evolutions beyond the simple deafferentation of ankle articular structures causing alterations in motor coordination.14,15 In its current state, the CAI model highlights contributing factors from the pathomechanical, sensory, and behavioral domains, resulting in neurologic consequences that alter both perception and action. However, contemporary rehabilitation for ankle sprains does not reflect the evolution of CAI. The complexity of the clinical phenomenon may require a rehabilitation paradigm that mirrors this complexity. Based on its epidemiologic persistence, we suggest that a new rehabilitation paradigm needs to be developed in the context of emerging trends in CAI etiology. To better develop this paradigm, we must revisit the emergence of the biopsychosocial model of disability.
The Advent of the Biopsychosocial Model of Disability
In the same year that Freeman et al11,12 charted the course for effective ankle-sprain rehabilitation strategies, Nagi16 proposed that disability was the culmination of impairments, functional limitations, and the corresponding individual and societal perceptions of the health condition. Before 1965, injuries and illnesses were viewed mainly as their anatomic and physiologic manifestations. From this point of view, resolving these 2 concerns should logically result in the alleviation of the condition. However, many patients continued to suffer long after the markers of injury had resolved.
Nagi16 characterized disability as a perceptual framework for interpreting the dynamic nature of function. He described “active pathology” (ie, the body's defensive and coping mechanisms) as producing impairments (anatomical or physiological abnormalities or losses or both). Impairments might still be present even after the active condition resolved. Functional limitations were the perceived and actual consequences of impairments in the ability to perform tasks and obligations associated with one's usual roles and normal daily activities. These functional limitations were highly influenced by the patient's perceptions of the injury and the impairments. The patient's perception was highly influenced by the reactions and expectations of significant others (stakeholders) around them. Disability then emerged as a biopsychosocial pattern of behavior of long-term impairments and functional limitations in the context of the patient's perception and the perception of the society to which he or she belongs. The biopsychosocial model introduced an entirely new way of viewing disability. Rather than requiring an active condition to be present, disability was the culmination of actual and perceived impairments and functional limitations due to the perceptions of the patient and society.
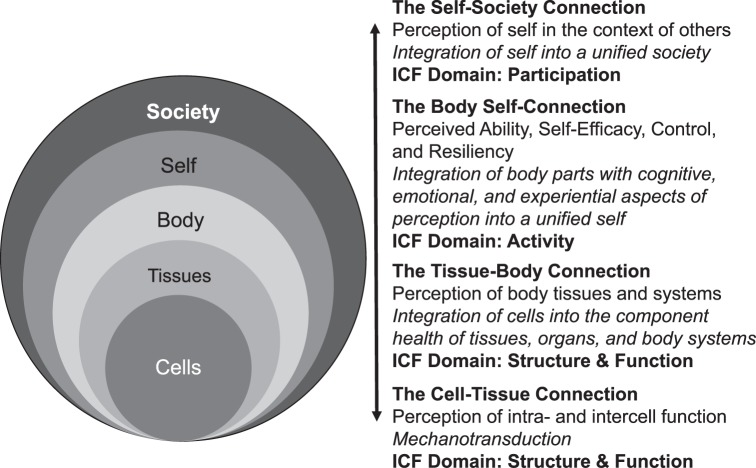
Key topics have arisen out of the biopsychosocial model, specifically in the context of perception. Based on the biopsychosocial model, the term perception not only refers to the structure and function of body systems (eg, the perception of pain in an injured ankle) but also the whole person as he or she is able to accomplish activities and participate in meaningful life events (eg, perceived ability, self-efficacy, and resilience in coping with change).17,18 Furthermore, the perception of society strongly influences the ability of the person to participate meaningfully in desired life experiences. These concerns are critical to understanding the multidimensional nature of perception as a complex biopsychosocial phenomenon. In this way, strong connections exist among the cells and tissues of the body, the self (whole person), and society.
Similar to CAI, the biopsychosocial model has also undergone several evolutions.19 The World Health Organization's International Classification of Functioning, Disability, and Health (ICF) is the currently accepted model across many health care professions.19,20 The ICF defines function as the dynamic interplay among 3 domains: structure and function (the body and its component parts), activity (the function of the whole person), and participation (the whole person in the context of his or her complete environment). Disability is characterized by structural and functional impairments, activity limitations, and participation restrictions. These 3 domains are highly influenced by environmental factors, including the physical, social, and attitudinal environment in which a person lives. In addition, personal factors such as coping mechanisms, habits, lifestyle choices, previous experiences with disease, and other psychological characteristics influence the perception of disability. Most importantly, the ICF captures the essence of the dynamic nature of function and its perceptual manifestation.
The Advent of the Body-Self Neuromatrix
In 1965, Melzack and Wall21 introduced the gate control theory of pain modulation that would revolutionize pain management in health care. They proposed that pain could be modulated through the manipulation of non-nocioceptive information from the periphery. In their theory, pain was the product of central processing factors rather than peripheral damage. However, sensory information from the periphery played a large role in triggering the perception of pain. In the wake of advances in central sensitization, higher-order modulation of perception, neuroplasticity, and the roles that previous experience, cognition, and emotion play in pain perception,22–24 the gate control theory fell short in explaining and predicting the modulation of pain as a complex biopsychosocial phenomenon. Accordingly, it has since evolved into a more complex paradigm for explaining phenomena such as chronic pain and phantom limb pain.25 This evolution, known as the body-self neuromatrix paradigm, centers on parallel and hierarchical processing loops of sensory, cognitive, and motivational domains within the central nervous system that govern both perception and action.26,27 Through the integration of wide-ranging areas within the central nervous system, the body-self neuromatrix produces a perception of unity across the body's parts into a unified self. Behavioral patterns, known as neurosignatures, develop dynamically based on the interplay of the perceptual and action systems within the body-self neuromatrix. Sensory sources from the periphery tune neurosignatures, but once formed, the patterns and resulting actions can continue without the need for sensory input.28
In this context, ankle sprains and the subsequent changes in relevant sensory information from the periphery can influence the perceptions of body parts and to a broader extent, the self and the behavioral patterns that result.27 The initial injury can change the neurosignature patterns related to the perception of body parts, specifically the foot and ankle, integrated within the unified self. Whereas the initial sensory alterations due to tissue damage in the periphery may resolve over time, the altered neurosignature patterns may remain. This may explain the continuum of disability experienced by those with CAI long after the injury has resolved.5
Despite resolution of the active condition, substantial self-reported deficits persist in the perceived ability to perform activities of daily living and sport, increased fear of movement, a tendency to be less physically active, and increased perceptions of ankle instability.29 Perhaps disability in CAI is highly influenced by the development of inappropriate neurosignatures within the body-self neuromatrix, representing a disunity of the foot and ankle within the context of body-self perception and action. Given the biopsychosocial nature of perception, this may be an important link among the affected cells and tissues, the body-self connection, and the self-society connection. Rehabilitation goals should no longer focus on regaining appropriate action (increasing strength, balance, power) but rather on enhancing the patient's perception and influencing advantageous neurosignatures that promote a sense of unity and health within the body-self neuromatrix.
RECOMMENDATIONS FOR ANKLE-SPRAIN REHABILITATION THROUGH THE PERCEPTUAL-INTERDEPENDENCE FRAMEWORK
The factors shown to be most predictive of functional status after an ankle sprain were the severity of the sprain, the location and provocation of pain, and weight-bearing status.30,31 Medial joint-line pain, pain with weight-bearing dorsiflexion, and unwillingness to perform a drop jump are potential predictors of lower functional status for patients with residual problems.3,30,31 Across these studies, increased pain was a critical feature of those who went on to develop poorer functional status.
The Importance of Pain Reduction
The greatest amount of pain reduction after acute ankle sprains takes place within the first 2 weeks postinjury.30 After this time, pain reduction occurs at a much slower pace, with up to 33% of patients still reporting pain after 1 year.30 In addition, the persistent pain experienced was not proportional to the severity of the sprain. This supports the perceptual framework derived from the biopsychosocial model and the body-self neuromatrix that an active injury produces impairments, but impairments are not always linked to an active injury. Along with this trend is the tendency to report subjective instability, less dorsiflexion, decreased physical activity, and recurrent sprains.
Controlling pain is widely accepted as a standard treatment goal for any acute injury. It is also important to view pain as a multidimensional product of the sensory, cognitive, and motivational factors that influence the perception of a unified self.28 Pain influences volitional and reflexive action patterns, stress responses, and even social interactions.28 Reducing pain is not only important from the perspective of tissue healing but also for preventing adverse alterations in the neurosignature patterns within the body-self neuromatrix.28,32 Residual pain and the subsequent alterations in neurosignature patterns may explain the increased fear and avoidance, diminished self-efficacy and self-reported function, and lower quality of life in those with CAI.17,29
The NSAIDs, when combined with cryotherapy, compression, and elevation (ICE), have produced superior pain reduction and improvements in self-reported function for patients with acute ankle sprains compared with ICE alone.33,34 The combination of NSAIDs and ICE not only modulates the perception of pain but also enhances the clearance of chemical and biological mediators (referred to as inflammatory soup32) that increase the transmission of nocioceptive signals related to pain perception. Psychosocial factors related to the perception of pain reduction may be more critical than the underlying physiological processes associated with mitigating the inflammatory response to an active injury.35 Therefore, reducing pain in those with acute ankle sprains should involve a combination of ICE and NSAIDs, with attentive reflection to reducing pain. Helping patients perceive reduced pain is a critical step in enhancing a return to homeostasis within the body-self neuromatrix.28 A word of caution: The evidence to support NSAIDs and ICE (NICE) is based on group trends rather than individual patient responses. Whereas NICE appears to be a logical framework for controlling pain at the cellular and tissue levels, it is also important to consider pain reduction in the context of appropriate healing based on physiological markers of tissue-integrity restoration in individual patients. Although the literature points toward enhanced functional outcomes in those who use NSAIDs, evidence36 suggests that NSAIDs early in the healing phase may increase swelling, reduce the integrity of ligamentous healing, and heighten the risk of gastrointestinal complaints. If one is concerned about the potentially negative consequences of NSAIDs early in the healing phase, then acetaminophen for pain control appears to be an equally effective alternative.37 The long-term effects of NSAIDs and a connection to CAI have not been established. More prognostic investigation and evidence are needed in this area.
Progressive Return to Motion: Restoring Ankle Perception Within the Body-Self Neuromatrix
Coupled with pain reduction is the goal of restoring motion to the ankle early and progressively during the rehabilitation process. Rest (complete elimination of loading) is no longer considered the most appropriate intervention strategy for musculoskeletal injuries and has been replaced by the concept of optimal loading, based on evidence suggesting that early motion promotes better recovery from ankle injury.7,38 When combined with protection (functional external support via bracing or taping) during functional activities,9,10,39 an early return to motion results in a quicker return to work and sport, reduced edema in the short term, and enhanced treatment satisfaction among patients upon discharge from rehabilitation compared with immobilization.
Early, protected return to motion stimulates the somatosensory afferents of the foot and ankle in the articular, cutaneous, and musculotendinous receptors. Within this framework, sensory information from the foot and ankle can shape the perception within the body-self neuromatrix. Including other lower extremity joints in motions such as triple flexion and extension in proprioceptive neuromuscular-facilitation patterns is also beneficial38 and may promote the restoration of healthy neurosignature patterns. Through this lens, joint mobilizations,40–43 plantar massage,44 stretching,43 and strengthening45,46 techniques serve to hone the inputs related to the local perception of the ankle and foot through articular, cutaneous, and musculotendinous stimulation. Controlled progressive motion passively, actively, and with resistance reduces the local structural and functional impairments that may continue long after the active ankle sprain has subsided.
Return to motion in a controlled fashion with protection is also beneficial in triggering cellular mechanisms for maintaining tissue health. Mechanotransduction is the process by which mechanical stimuli trigger cell-to-cell communication that results in changes in the mechanical and chemical properties of tissues.47 The controlled- and progressive-loading demands produce physiological mechanisms to promote cellular health and tissue repair and healing. Whereas progressive motion reduces edema and pain in the short term,48 it also promotes appropriate perception and action at the cellular level. Mechanotherapy is the purposeful manipulation of loading to stimulate cells in the tissue to promote repair and restore the compromised biomechanical properties.47 This treatment paradigm has led to optimal loading as an update for the early phases of healing, now referred to as POLICE (protection, optimal loading, and integrated control exercises).49 However, the ICE of POLICE may be better considered in this perceptual context as Integrated Control Exercises that promote the restoration of control in both the sensory and motor pathways associated with the foot and ankle. In this way, POLICE can be used to enhance the cell-tissue-body perceptual connection during the return to motion.
The severity of the sprain is a key factor to consider, and in this context, for some patients with severe sprains, optimal loading may initially consist of complete non–weight bearing due to pain and tissue integrity disruption.8 Immobilization for no more than 10 days followed by a progressive return to motion is recommended for these severe injuries.8 The POLICE treatment paradigm is a viable strategy for encouraging appropriate signaling of the tissue at the cellular level and promoting the maintenance of healthy neurosignatures within the body-self neuromatrix. Careful consideration should be given to recalcitrant pain and swelling because these can have deleterious effects on behavior and performance.30,31 As Freeman11 pointed out, this strategy may not be effective in preventing subsequent perceived disability. Reintroducing the healing ankle into the unified body-self neuromatrix becomes the next logical goal. This process may best be captured with coordination training.
Coordination Training: Restoring Global Body-Self Perception
Strong evidence has associated balance and coordination training with improving self-reported function and reducing the recurrence of ankle sprains after an acute injury.7,50 Since Freeman et al12 introduced the concept, numerous exercise protocols have been developed to challenge patients in single-limb stance, ranging from the use of wobble boards to more complex hopping and landing tasks.7 Most of these programs incorporate a time-based progression in which patients move from less difficult to more difficult exercises over 4 to 12 weeks. The underlying mechanisms for improvements in self-reported function and the risk of recurrent ankle sprains after coordination training remain unclear. The central mechanism may relate to enhanced global perception-action and the ability to reincorporate the ankle and foot into effective movement strategies.5,51
The theory that coordination training enhances proprioception may simply be scratching the tip of the perceptual iceberg. An evaluation of many of the coordination-training programs indicated that all progress occurred by increasing demands from simple to more complex tasks or from predictable to unpredictable environments (or both).5,7,10,52 The Freeman et al12 original proposal for coordination training was that patients progressed based on their mastery of the movement goal. Once learned, the improved performance was retained. The underlying mechanisms for these improvements may be best explained through the theoretical framework for the dynamics of skill acquisition.
Coordination Training and the Dynamics of Skill Acquisition
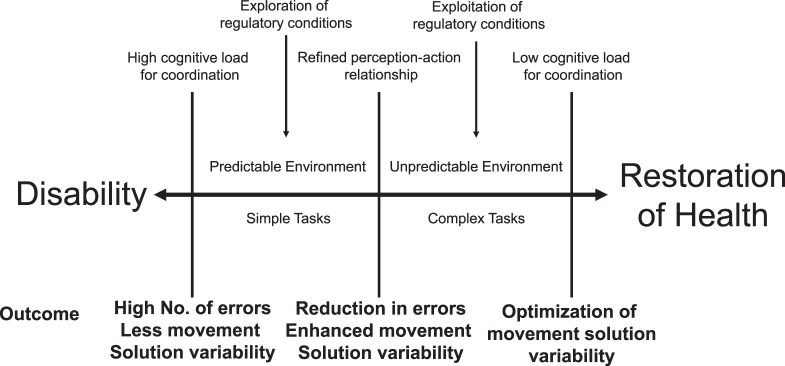
Learning a particular skill is a progressive and multidimensional process that involves refining the relationship between perception and action.53–55 Coordination is the mark of a goal-oriented dynamic system governed by internal and external constraints.51,54 These constraints include the health of the individual, the complexity of the task being performed, and the predictability of relevant sensory cues from the environment.51,56 Coordination of the body changes according to the demands of the movement goal in the context of task complexity and environmental predictability. Skill acquisition then becomes the process of exploring the regulatory conditions57,58 of the skill (the task and environmental constraints) and learning to exploit them.54,55,57,58 This process is marked by the ability to discriminate between relevant and irrelevant sources of sensory information. Exploratory behavior surrounding simple tasks in predictable environments with high cognitive load represents the early stages of learning a new skill. A great deal of effort is put into exploring how to consistently accomplish a movement goal. Therefore, it is critically important that the movement goal be perceived clearly by the learner to ensure that he or she can recognize success versus failure in attaining it.5,51 The ability to reduce errors enhances the probability of success and is an important factor in promoting the perception of skill mastery.17,18 As performance errors are reduced, the task and environmental constraints can be progressed in complexity and unpredictability, respectively, to increase the difficulty in perceiving the regulatory conditions governing coordination of the movement goal.5,51
As deliberate practice continues, the cognitive load concurrently decreases because changes in perception are more strongly associated with anticipated changes in action. The resulting actions are then associated with anticipated changes in perception and reduced cognitive demand.55,58 Appropriate behavioral patterns retained through the tuning of perception and action reduce the perceived effort to maximize the outcome.51,54,55,57 This represents the underlying process that Freeman et al12 originally pointed out: once the ability to balance was learned, it appeared to be retained and the patients were able to progress to more difficult balance challenges. In this way, the error-based progression with the purposeful manipulation of task and environmental constraints in the context of sport-specific activities enhances the perception of mastery of sport-specific demands.
The functional redundancy and overlap of degrees of freedom (eg, bones, joints, muscles) within the body's movement system offers great flexibility in developing effective movement solutions to meet the demands of the task and environmental constraints.5,51,54,55 Rather than having preprogrammed responses, the sensorimotor system is capable of self-organization based on the level of constraints acting on it. These constraints regulate the configurations of degrees of freedom to allow the sensorimotor system to form patterns. In the context of the neuromatrix, self-organized coordination is the result of encapsulated cognitive, affective, and sensory patterns linked to functionally effective movement solutions.27 Coordination training via purposeful and progressive manipulation of task and environmental constraints affords perceptual tuning of the body-self connection. Skill reacquisition, then, is the optimization of the relationship between perception and action in a system that has been constrained by injury or illness. A patient should be progressed to more complex tasks and unpredictable environments when there is evidence of a shift from a high to a low cognitive load while also maximizing the outcome of the movement goal.5,51 In this way, coordination training promotes an enhanced sense of mastery for the unified self (“I can do it better now”), which encourages enhanced self-efficacy and resilience (the ability to cope with change).
Patient-oriented evidence from those with CAI indicates heightened levels of disability, fear and avoidance behaviors, and decreased engagement in physical activity.29 The driving force for these perceptions of function may be impaired skill-reacquisition dynamics. Coordination training appears to be effective for reducing the residual disability after an ankle sprain, but based on the low compliance rates reported in the literature for home-based coordination-training programs, supervised training sessions may be required to optimize the therapeutic benefits.59 Emerging evidence17,60,61 also suggests coupling home-based interventions with educational materials about the exercises; providing the framework of relearning how to master movement may be critical for enhancing compliance, fidelity, and adherence to these types of intervention strategies. It is important to consider these factors on a patient-by-patient basis given that each individual's unique experiences and perceptions can shape his or her compliance, fidelity, and adherence.62,63
The remaining questions about coordination training focus on the dosage. How long do patients need to perform the programs? How often? How many different exercises should be performed? Although the literature provides no clear answers as to which programs appear to be most beneficial, it seems that the longer the patient participates in progressive coordination training, the more robust the responses.7 Our recommendation is to talk with patients about their perceptions of self-efficacy17 and their specific activity and participation goals. Be sure to reflect on their level of perceived disability and how it changes through the intervention process. If their confidence or perceived ability seems to be diminishing, then it might be time to revisit the coordination exercises. By being mindful of the movement goal, the errors associated with it, and the perceived change in function through progression, the clinician can establish a maintenance program for the patient in the context of this perceptual framework. A framework (Figure 1) is supplied for discussing with patients where they are in their own skill-reacquisition process and how they may need to ramp up or down in the manipulation of task and environmental constraints to tune their body-self connection. When patients have a perceptual guide through the rehabilitation process, the continuum of disability that affects many of them can be broken.
Figure 1.
The dynamics of skill acquisition for ankle-sprain rehabilitation. Based on the level of self-reported function during a particular activity (eg, running, cutting, balancing), a clinician and patient can gauge the appropriate demands from the task and environmental constraints. Discussing that the initial phases of learning are marked by high cognitive load is important when helping patients to understand that errors in the movement goal are expected initially but should reduce with deliberate practice.
PERCEPTUAL INTERDEPENDENCE IN THE CONTEXT OF CLINICAL PRACTICE
As discussed earlier, a new rehabilitation paradigm centers on a framework of perceptual interdependence from cells to society in the context of the biopsychosocial model (Figure 2). Although the cells and society are furthest from each other, the levels are still interdependent. For example, chemical mediators released during the inflammatory process that generate pain at the cellular level can ultimately influence the patient's perception at the society level. As well, society may perceive an ankle sprain as a benign injury that should be “ready to go” within a couple of days, thereby increasing perceived pressure and resulting in an inappropriately early return to play and stress on tissues. Coupling the lack of compliance with the rapid return to play that most athletes experience with ankle sprains,64 we recognize the growing need to educate stakeholders who influence societal decisions and perceptions.
Figure 2.
The perceptual-interdependence framework from cell to society. This figure depicts the interdependent perceptual relationships among the cells and tissues of the body, the body-self connection, and the self-society connection. On the right side, the domains of the International Classification of Function, Disability, and Health (ICF) are used to contextualize these relationships.
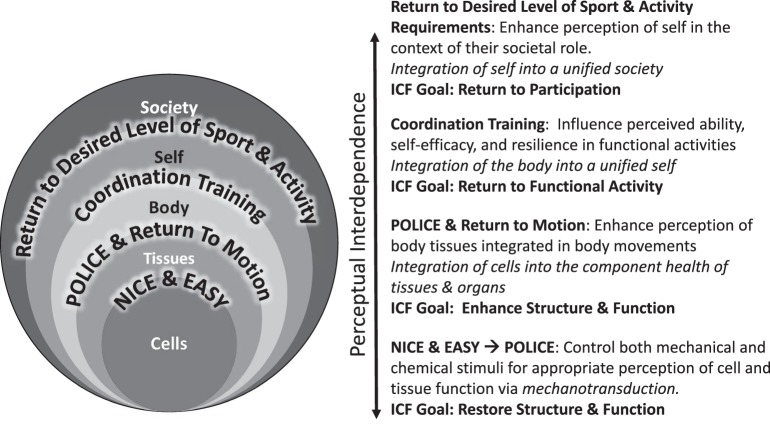
As a guide for clinicians using this framework, the evidence-based recommendations stated earlier transform through the lens of the perceptual-interdependence framework (Figure 3):
Figure 3.
Evidence-based rehabilitation recommendations from the perceptual-interdependence framework. The 4 evidence-based treatment recommendations are overlaid on the perceptual-interdependence framework. On the right side, the evidence-based recommendations are contextualized through the International Classification of Function, Disability, and Health (ICF). Abbreviations: EASY, external ankle support for up to 1 year; NICE, nonsteroidal anti-inflammatory drugs, ice, compression, elevation; POLICE, protection, optimal loading, and integrated control exercises.
In the acute phase of ankle-sprain rehabilitation, clinicians should talk with patients about taking it NICE and EASY (external ankle support for up to 1 year). This encapsulates 2 of the evidence-based recommendations: control pain and edema via NICE and use EASY. However, this recommendation has 2 caveats. As stated earlier, the evidence for NSAIDs for early pain relief and anti-inflammatory benefit is strong, but prognostic investigations are needed to determine the long-term effects of their use during the early stages of recovery and rehabilitation. Acetaminophen appears to be a viable alternative to NSAIDs for pain relief early in the recovery process.37 It is also important to note that the role and implementation of EASY changes over the course of the year. During the acute phase of rehabilitation, an external ankle support should be used during activities of daily living; this practice has been shown to improve self-reported function and reduce the risk of sustaining a recurrent sprain.7
Regaining motion is critical to restoring tissue integrity, promoting the optimal health of cells and tissues, and preventing the formation of dysfunctional neurosignature patterns within the body-self neuromatrix. When regaining motion, use POLICE as a guide. Protection continues to focus on EASY during activities of daily living. Optimal loading centers on the appropriate stimulation of cells and tissues via mechanotransduction and the stimulation of relevant sources of sensory information within the skin, articular structures, and surrounding musculature to prevent perceptual smudging of the foot and ankle. We recommend incorporating Integrated Control Exercises that target the relevant sources of both sensory and motor control of the ankle and surrounding structures. These include the use of sensory-targeted strategies such as joint mobilization, plantar massage, stretching to target relevant sensory information as well as progressive resistive exercises to promote volitional muscle control, strength, endurance, and reflex patterns. In this capacity, POLICE and the restoration of motion encapsulate the perceptual interdependence of the cell-tissue-body connection.
Coordination training should start early in the rehabilitation process and focus on the body-self connection throughout the progression. The purposeful and progressive manipulation of task and environmental constraints using coordination exercises should afford the patient the opportunity to experience mastery of these activities. Progression occurs from simple to complex tasks and predictable to more unpredictable environmental constraints. More advanced progression should include relevant activities in the context of the patient's sport and activity demands. No high-level evidence supports or refutes the use of an external ankle support during coordination training in the context of rehabilitation. Evidence from crossover studies65,66 suggested that ankle braces reduced lower extremity muscle activity during walking and functional exercises in patients with CAI, but only immediate effects were examined. Given the lack of evidence and considering that the goals of coordination exercises are to optimize functional variability and cultivate a perception of mastery, the use of an external support is not currently recommended during coordination training. However, using EASY during unsupervised daily and high-risk activities (eg, walking on uneven surfaces, running, cutting, jumping) is recommended.
When returning a patient to his or her desired level of sport and physical activity, it is important to consider the perceptual interdependence from the cell to society. Educating stakeholders close to the patient on the appropriate prognostic timelines, expectations, and progressions in return to play may be just as important as educating the patient. As patients return to participation in high-risk activities and sport, EASY should continue to be implemented based on the overwhelming evidence that it substantially decreases the risk of recurrent ankle sprains.7 The low compliance rates for using external ankle supports63,67 suggest that evidence-informed education of and behavioral change among all stakeholders are needed. All patients and stakeholders should be informed that the risk of sustaining a recurrent ankle sprain after 1 year of being injury free decreases to the level of a person who has never had a sprain.68 The combination of NICE and EASY, POLICE, coordination training, and return to participation using the perceptual-interdependence paradigm provides a logical framework for reducing the risk of injury during this critical time frame.
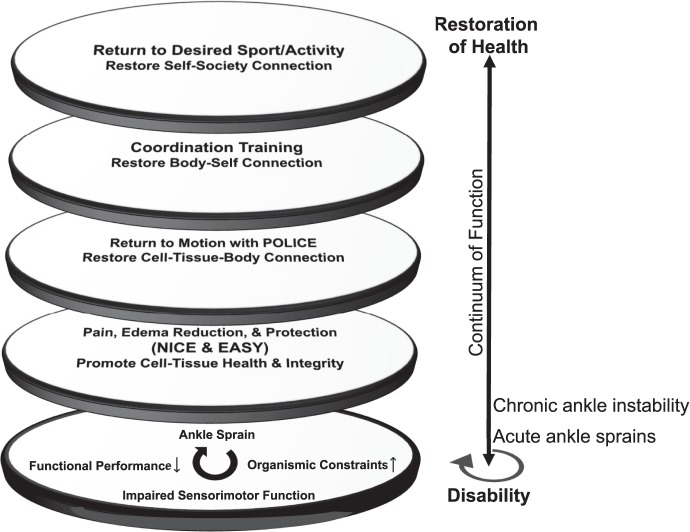
The Table offers examples of goals and interventions that can be used to promote the restoration and enhancement of the perceptual-interdependence framework in the context of these recommendations. Using the lenses of perceptual interdependence, practitioners can break the continuum of disability in patients with CAI. However, inadequate reduction of pain and edema and restoration of motion and coordination may cloud the clinician's ability to break this continuum in patients (See Figure 4).
Table.
Intervention Strategies for Promoting and Enhancing the Perceptual Interdependence From Cell to Society for Ankle-Sprain Rehabilitationa
| Level |
Goal(s) of Rehabilitation |
Impairment-Based Interventions |
Proposed Interventions to Target Perception |
Clinician-Oriented Consequences |
Patient-Oriented Consequences |
| Cell ↕ Tissue | Reduce pain; restore integrity of damaged structures; control inflammation | NICE and EASY joint mobilization mechanotherapy electrotherapy | Pain-management education; injury-management education | Reduced integrity of tissue; prolonged inflammation | Perception of pain without necessarily stimulating pain nerve fibers; chronic pain |
| Tissue ↕ Body | Return to motion (increase range of motion, strength, neuromuscular control, and improve gait mechanics) | POLICE STARS resistive training | Supervised rehabilitation; interventions that target both the sensory and motor aspects of foot and ankle control | Reduced range of motion, strength, neuromuscular control, and altered gait mechanics | Feeling of instability during motion; pain during motion |
| Body ↕ Self | Return to sport-specific activity | Coordination training; task and environment manipulation for sport-specific context; gait retraining; functional movement exercises | Error-based progressions of sport-specific skills; virtual reality and/or imagery of skill in a sport-specific environment; positive self-talk; realistic expectations of skill performance and enhancement | Inability to perform sport-specific activities | Diminished self-efficacy; feeling of pain during sport-specific activity; fear and avoidance of sport-specific activity |
| Self ↕ Society | Return to sport participation | Practice with the team | Virtual reality/imagery of sport participation; education on coping and stress management; education of stakeholders about appropriate prognostic timelines and expectations | Inability to play the sport; decrease in physical activity | Feeling of disability during sport; feeling of pain during sport; feeling of lack of contribution to sport |
Abbreviations: Mechanotherapy indicates instrumented soft-tissue mobilization, local and whole-body vibration, massage, and ultrasound; NICE and EASY, nonsteroidal anti-inflammatory drugs, ice, compression, and elevation and external ankle support for up to 1 year; POLICE, protection, optimal loading, and integrated control exercises; STARS, striated muscle activator of Rho signaling.
From left to right, the columns identify the level of relationship, the evidence-based intervention strategies, the proposed effects of these interventions on perception, and the consequences of not achieving these goals on clinician- and patient-oriented outcomes. Bold = substantial evidence to support the use of the intervention for treating ankle sprains; italics = limited or inconclusive evidence to support the use of the intervention for treating ankle sprains.
Figure 4.
The lenses of perceptual-interdependence intervention recommendations on the continuum of disability. The multiple lenses of the perceptual framework for ankle-sprain rehabilitation. The continuum of disability (bottom lens) is marked by increased organismic constraints, impaired sensorimotor function, and decreased functional performance. The progression from disability to health should focus on pain and edema reduction, early return to motion, and coordination training. Through these lenses, the patients' needs become apparent based on the level of disability experienced. Abbreviations: EASY, external ankle support for up to 1 year; NICE, nonsteroidal anti-inflammatory drugs, ice, compression, elevation; POLICE, protection, optimal loading, and integrated control exercises.
CONCLUSIONS
As with the evolution of CAI, the biopsychosocial model, and the perceptual framework of pain, it is time for rehabilitation strategies for ankle sprains to evolve. The major evidence-based goals in the conservative care of patients with ankle sprains center on perceptual interdependence of our cells to society. Perhaps the concerns related to CAI disability stem from the perception that “it's just an ankle sprain.” As we move forward in charting the course of ankle rehabilitation over the next 50 years, the personal and societal perceptions of ankle sprains are most likely where intervention is needed. By integrating the dynamics of skill acquisition, the biopsychosocial model, and the body-self neuromatrix, the perceptual-interdependence framework has emerged as a new rehabilitation paradigm. This framework allows us to capitalize on the essential elements of effective rehabilitation strategies for patients with ankle sprains and their negative sequelae from the cell to society.
REFERENCES
- 1.Attenborough AS, Hiller CE, Smith RM, Stuelcken M, Greene A, Sinclair PJ. Chronic ankle instability in sporting populations. Sports Med. 2014;44(11):1545–1556. doi: 10.1007/s40279-014-0218-2. [DOI] [PubMed] [Google Scholar]
- 2.Gribble PA, Bleakley CM, Caulfield BM, et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;50(24):1496–1505. doi: 10.1136/bjsports-2016-096189. [DOI] [PubMed] [Google Scholar]
- 3.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: a prospective cohort analysis. Am J Sports Med. 2016;44(4):995–1003. doi: 10.1177/0363546516628870. [DOI] [PubMed] [Google Scholar]
- 4.Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. Br J Sports Med. 2014;48(13):1014–1018. doi: 10.1136/bjsports-2013-093175. [DOI] [PubMed] [Google Scholar]
- 5.Wikstrom EA, Hubbard-Turner T, McKeon PO. Understanding and treating lateral ankle sprains and their consequences: a constraints-based approach. Sports Med. 2013;43(6):385–393. doi: 10.1007/s40279-013-0043-z. [DOI] [PubMed] [Google Scholar]
- 6.Thompson C, Schabrun S, Romero R, Bialocerkowski A, van Dieen J, Marshall P. Factors contributing to chronic ankle instability: a systematic review and meta-analysis of systematic reviews. Sports Med. 2018;48(1):189–205. doi: 10.1007/s40279-017-0781-4. [DOI] [PubMed] [Google Scholar]
- 7.Doherty C, Bleakley C, Delahunt E, Holden S. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51(2):113–125. doi: 10.1136/bjsports-2016-096178. [DOI] [PubMed] [Google Scholar]
- 8.Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers' Association position statement: conservative management and prevention of ankle sprains in athletes. J Athl Train. 2013;48(4):528–545. doi: 10.4085/1062-6050-48.4.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerkhoffs GM, van den Bekerom M, Elders LA, et al. Diagnosis, treatment, and prevention of ankle sprains: an evidence-based clinical guideline. Br J Sports Med. 2012;46(12):854–860. doi: 10.1136/bjsports-2011-090490. [DOI] [PubMed] [Google Scholar]
- 10.Verhagen EA, Bay K. Optimising ankle sprain prevention: a critical review and practical appraisal of the literature. Br J Sports Med. 2010;44(15):1082–1088. doi: 10.1136/bjsm.2010.076406. [DOI] [PubMed] [Google Scholar]
- 11.Freeman MA. Treatment of ruptures of the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47(4):661–668. [PubMed] [Google Scholar]
- 12.Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47(4):678–685. [PubMed] [Google Scholar]
- 13.Burke RE. Sir Charles Sherrington's the integrative action of the nervous system: a centenary appreciation. Brain. 2007;130(pt 4):887–894. doi: 10.1093/brain/awm022. [DOI] [PubMed] [Google Scholar]
- 14.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 15.Hiller CE, Kilbreath SL, Refshauge KM. Chronic ankle instability: evolution of the model. J Athl Train. 2011;46(2):133–141. doi: 10.4085/1062-6050-46.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagi SZ. Some conceptual issues in disability and rehabilitation. In: Sussman M, editor. Sociology and Rehabilitation. Washington, DC: American Sociological Society; 1965. p. 100. [Google Scholar]
- 17.McCann RS, Gribble PA. Resilience and self-efficacy: a theory-based model of chronic ankle instability. Int J Athl Ther Train. 2016;21(3):32–37. [Google Scholar]
- 18.Connolly FR, Aitken LM, Tower M. An integrative review of self-efficacy and patient recovery post acute injury. J Adv Nurs. 2014;70(4):714–728. doi: 10.1111/jan.12237. [DOI] [PubMed] [Google Scholar]
- 19.Snyder AR, Parsons JT. Valovich McLeod TC, Bay RC, Michener LA, Sauers EL. Using disablement models and clinical outcomes assessment to enable evidence-based athletic training practice, part I: disablement models. J Athl Train. 2008;43(4):428–436. doi: 10.4085/1062-6050-43.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86(5):726–734. [PubMed] [Google Scholar]
- 21.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 22.Price DD, Verne GN, Schwartz JM. Plasticity in brain processing and modulation of pain. Prog Brain Res. 2006;157:333–352. doi: 10.1016/s0079-6123(06)57020-7. [DOI] [PubMed] [Google Scholar]
- 23.Villemure C, Schweinhardt P. Supraspinal pain processing: distinct roles of emotion and attention. Neuroscientist. 2010;16(3):276–284. doi: 10.1177/1073858409359200. [DOI] [PubMed] [Google Scholar]
- 24.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 25.Melzack R. Phantom limb pain. Patol Fiziol Eksp Ter. 1992;(4):52–54. [PubMed] [Google Scholar]
- 26.Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001;65(12):1378–1382. [PubMed] [Google Scholar]
- 27.Melzack R, Katz J. Pain. Wiley Interdiscip Rev Cogn Sci. 2013;4(1):1–15. doi: 10.1002/wcs.1201. [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. Evolution of the neuromatrix theory of pain. The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract. 2005;5(2):85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- 29.Houston MN, Hoch JM, Hoch MC. Patient-reported outcome measures in individuals with chronic ankle instability: a systematic review. J Athl Train. 2015;50(10):1019–1033. doi: 10.4085/1062-6050-50.9.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Rijn RM, van Os AG, Bernsen RM, Luijsterburg PA, Koes BW, Bierma-Zeinstra SM. What is the clinical course of acute ankle sprains? A systematic literature review. Am J Med. 2008;121(4):324–331. doi: 10.1016/j.amjmed.2007.11.018. e326. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor SR, Bleakley CM, Tully MA, McDonough SM. Predicting functional recovery after acute ankle sprain. PLoS One. 2013;8(8):e72124. doi: 10.1371/journal.pone.0072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler DS, Moseley GL. Explain Pain. Adelaide, Australia: Noigroup Publications; 2003. [Google Scholar]
- 33.van den Bekerom MPJ. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating acute ankle sprains in adults: benefits outweigh adverse events. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2390–2399. doi: 10.1007/s00167-014-2851-6. [DOI] [PubMed] [Google Scholar]
- 34.van den Bekerom MP, Struijs PA, Blankevoort L, Welling L, van Dijk CN, Kerkhoffs GM. What is the evidence for rest, ice, compression, and elevation therapy in the treatment of ankle sprains in adults? J Athl Train. 2012;47(4):435–443. doi: 10.4085/1062-6050-47.4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moseley GL, Butler DS. Fifteen years of explaining pain: the past, present, and future. J Pain. 2015;16(9):807–813. doi: 10.1016/j.jpain.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Slatyer MA, Hensley MJ, Lopert R. A randomized controlled trial of piroxicam in the management of acute ankle sprain in Australian Regular Army recruits. The Kapooka Ankle Sprain Study. Am J Sports Med. 1997;25(4):544–553. doi: 10.1177/036354659702500419. [DOI] [PubMed] [Google Scholar]
- 37.Jones P, Dalziel SR, Lamdin R, Miles-Chan JL, Frampton C. Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database Syst Rev. 2015;(7) doi: 10.1002/14651858.CD007789.pub2. :CD007789. [DOI] [PubMed] [Google Scholar]
- 38.Bleakley CM, O'Connor SR, Tully MA, et al. Effect of accelerated rehabilitation on function after ankle sprain: randomised controlled trial. BMJ. 2010. 340:c1964. [DOI] [PubMed]
- 39.Kerkhoffs GM, Struijs PA, Marti RK, Blankevoort L, Assendelft WJ, van Dijk CN. Functional treatments for acute ruptures of the lateral ankle ligament: a systematic review. Acta Orthop Scand. 2003;74(1):69–77. doi: 10.1080/00016470310013699. [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Diaz D. Lomas Vega R, Osuna-Perez MC, Hita-Contreras F, Martinez-Amat A. Effects of joint mobilization on chronic ankle instability: a randomized controlled trial. Disabil Rehabil. 2015;37(7):601–610. doi: 10.3109/09638288.2014.935877. [DOI] [PubMed] [Google Scholar]
- 41.Loudon JK, Reiman MP, Sylvain J. The efficacy of manual joint mobilisation/manipulation in treatment of lateral ankle sprains: a systematic review. Br J Sports Med. 2014;48(5):365–370. doi: 10.1136/bjsports-2013-092763. [DOI] [PubMed] [Google Scholar]
- 42.Wikstrom EA, McKeon PO. Manipulative therapy effectiveness following acute lateral ankle sprains: a systematic review. Athl Train Sports Health Care. 2011;3(6):271–279. [Google Scholar]
- 43.Terada M, Pietrosimone BG, Gribble PA. Therapeutic interventions for increasing ankle dorsiflexion after ankle sprain: a systematic review. J Athl Train. 2013;48(5):696–709. doi: 10.4085/1062-6050-48.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKeon PO, Wikstrom EA. Sensory-targeted ankle rehabilitation strategies for chronic ankle instability. Med Sci Sports Exerc. 2016;48(5):776–784. doi: 10.1249/MSS.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall EA, Docherty CL, Simon J, Kingma JJ, Klossner JC. Strength-training protocols to improve deficits in participants with chronic ankle instability: a randomized controlled trial. J Athl Train. 2015;50(1):36–44. doi: 10.4085/1062-6050-49.3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright CJ, Linens SW, Cain MS. A randomized controlled trial comparing rehabilitation efficacy in chronic ankle instability. Sport Rehabil. 2017;26(4):238–249. doi: 10.1123/jsr.2015-0189. [DOI] [PubMed] [Google Scholar]
- 47.Khan KM, Scott A. Mechanotherapy: how physical therapists' prescription of exercise promotes tissue repair. Br J Sports Med. 2009;43(4):247–252. doi: 10.1136/bjsm.2008.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerkhoffs GM, Struijs PA, Marti RK, Assendelft WJ, Blankevoort L, van Dijk CN. Different functional treatment strategies for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002;(3) doi: 10.1002/14651858.CD002938. :CD002938. [DOI] [PubMed] [Google Scholar]
- 49.Bleakley CM, Glasgow P, MacAuley DC. PRICE needs updating, should we call the POLICE? Br J Sports Med. 2012;46(4):220–221. doi: 10.1136/bjsports-2011-090297. [DOI] [PubMed] [Google Scholar]
- 50.Kosik KB, McCann RS, Terada M, Gribble PA. Therapeutic interventions for improving self-reported function in patients with chronic ankle instability: a systematic review. Br J Sports Med. 2017;51(2):105–112. doi: 10.1136/bjsports-2016-096534. [DOI] [PubMed] [Google Scholar]
- 51.McKeon PO. Dynamic systems theory as a guide to balance training development for chronic ankle instability: a review of the literature. Athl Train Sports Health Care. 2012;4(5):230–236. [Google Scholar]
- 52.Webster KA, Gribble PA. Functional rehabilitation interventions for chronic ankle instability: a systematic review. Sport Rehabil. 2010;19(1):98–114. doi: 10.1123/jsr.19.1.98. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein NA. The Co-ordination and Regulation of Movements. Oxford, UK: Pergamon Press; 1967. [Google Scholar]
- 54.Davids K, Button C, Bennett S. Dynamics of Skill Acquisition: A Constraints-Led Approach. Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 55.Newell KM. Motor skill acquisition. Annu Rev Psychol. 1991;42:213–237. doi: 10.1146/annurev.ps.42.020191.001241. [DOI] [PubMed] [Google Scholar]
- 56.Hoch MC, McKeon PO. Integrating contemporary models of motor control and health in chronic ankle instability. Athl Train Sports Health Care. 2010;2(2):82–88. [Google Scholar]
- 57.Gentile AM. A working model of skill acquisition with application to teaching. Quest. 1972;17(1):3–23. [Google Scholar]
- 58.Williams AM, Grant A. Training perceptual skill in sport. Int J Sport Psychol. 1999;30(2):194–220. [Google Scholar]
- 59.Feger MA, Herb CC, Fraser JJ, Glaviano N, Hertel J. Supervised rehabilitation versus home exercise in the treatment of acute ankle sprains: a systematic review. Clinics in Sports Med. 2015;34(2):329–346. doi: 10.1016/j.csm.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 60.Van Reijen M, Vriend I, van Mechelen W, Verhagen EA. Preventing recurrent ankle sprains: is the use of an app more cost-effective than a printed booklet? Results of a RCT. Scand J Med Sci Sports. 2018;28(2):641–648. doi: 10.1111/sms.12915. [DOI] [PubMed] [Google Scholar]
- 61.Van Reijen M, Vriend I, Zuidema V, van Mechelen W, Verhagen EA. The “strengthen your ankle” program to prevent recurrent injuries: a randomized controlled trial aimed at long-term effectiveness. J Sci Med Sport. 2017;20(6):549–554. doi: 10.1016/j.jsams.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009. 339:b2684. [DOI] [PMC free article] [PubMed]
- 63.Janssen KW, van Mechelen W, Verhagen EA. Bracing superior to neuromuscular training for the prevention of self-reported recurrent ankle sprains: a three-arm randomised controlled trial. Br J Sports Med. 2014;48(16):1235–1239. doi: 10.1136/bjsports-2013-092947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medina McKeon JM, Bush HM, Reed A, Whittington A, Uhl TL, McKeon PO. Return-to-play probabilities following new versus recurrent ankle sprains in high school athletes. J Sci Med Sport. 2014;17(1):23–28. doi: 10.1016/j.jsams.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Barlow G, Donovan L, Hart JM, Hertel J. Effect of lace-up ankle braces on electromyography measures during walking in adults with chronic ankle instability. Phys Ther Sport. 2015;16(1):16–21. doi: 10.1016/j.ptsp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Feger MA, Donovan L, Hart JM, Hertel J. Effect of ankle braces on lower extremity muscle activation during functional exercises in participants with chronic ankle instability. Int J Sports Phys Ther. 2014;9(4):476–487. [PMC free article] [PubMed] [Google Scholar]
- 67.Janssen KW, van der Zwaard BC, Finch CF, van Mechelen W, Verhagen EA. Interventions preventing ankle sprains; previous injury and high-risk sport participation as predictors of compliance. J Sci Med Sport. 2016;19(6):465–469. doi: 10.1016/j.jsams.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Verhagen EA, Van der Beek AJ, Bouter LM, Bahr RM, Van Mechelen W. A one season prospective cohort study of volleyball injuries. Br J Sports Med. 2004;38(4):477–481. doi: 10.1136/bjsm.2003.005785. [DOI] [PMC free article] [PubMed] [Google Scholar]