Abstract
Lateral ankle sprains (LASs) are among the most common injuries incurred during participation in sport and physical activity, and it is estimated that up to 40% of individuals who experience a first-time LAS will develop chronic ankle instability (CAI). Chronic ankle instability is characterized by a patient's being more than 12 months removed from the initial LAS and exhibiting a propensity for recurrent ankle sprains, frequent episodes or perceptions of the ankle giving way, and persistent symptoms such as pain, swelling, limited motion, weakness, and diminished self-reported function. We present an updated model of CAI that aims to synthesize the current understanding of its causes and serves as a framework for the clinical assessment and rehabilitation of patients with LASs or CAI. Our goal was to describe how primary injury to the lateral ankle ligaments from an acute LAS may lead to a collection of interrelated pathomechanical, sensory-perceptual, and motor-behavioral impairments that influence a patient's clinical outcome. With an underpinning of the biopsychosocial model, the concepts of self-organization and perception-action cycles derived from dynamic systems theory and a patient-specific neurosignature, stemming from the Melzack neuromatrix of pain theory, are used to describe these interrelationships.
Keywords: ankle sprains, anterior talofibular ligament, calcaneofibular ligament, diagnosis, rehabilitation
Ankle sprains are among the most common injuries in the general population, and the injury reported most frequently by competitive athletes.1–3 Damage to the lateral ligaments of the ankle accounts for the majority of ankle sprains, regardless of patient demographics.4,5 The prevalence of lateral ankle sprains (LASs), coupled with high rates of reinjury, persistent symptoms, and reduced self-reported ankle function, makes LASs and their sequelae a public health concern.6,7 Chronic ankle instability (CAI) is a condition characterized by repetitive episodes or perceptions of the ankle giving way; ongoing symptoms such as pain, weakness, or reduced ankle range of motion (ROM); diminished self-reported function; and recurrent ankle sprains that persist for more than 1 year after the initial injury. Specific diagnostic criteria for CAI have been recommended by the International Ankle Consortium.8 Doherty et al9 performed a prospective study of patients with first-time ankle sprains who sought treatment in a hospital emergency department and found that 40% had developed CAI, as defined by these criteria, at 12-month follow-up.
Our understanding of the factors contributing to CAI has evolved over the past 6 decades. Freeman et al10–12 presented the first comprehensive theory of ankle instability in 1965. They coined the term functional instability, which they operationally defined as “the disability to which patients refer when they say that their foot tends to ‘give way' in the months and years after initial ankle sprain.”12(p678) It must also be noted that the ankle giving way was not the patients' only complaint:
Every patient whose foot gave way stated that such incidents occasionally caused the ankle to be painful or swollen, sometimes to such an extent that the ankle could be said to have been sprained. . . . For this reason no patient complained only of a tendency for the foot to “give way.”10(p666)
Freeman11 was adamant that mechanical instability due to pathologic laxity of the ankle was only rarely the initial cause of the functional instability of the foot. Mechanical instability was specifically defined as increased varus tilt of the talus under inversion stress. Instead, Freeman et al12 asserted that
(1) the afferent nerve fibres in the capsule and ligaments of the foot and ankle subserve reflexes which help to stabilise the foot during locomotion, and (2) when the foot or ankle is “sprained” partial deafferentiation of the injured joints occurs, so that (3) reflex stabilisation of the foot is impaired and the foot tends to “give way.”12(p678)
Additionally, Freeman et al12 provided evidence that patients who performed coordination exercises during their recovery from ankle sprains demonstrated a lower incidence of functional instability.
Tropp et al13–17 conducted a series of studies in the 1980s that aimed to further the understanding of the causes of CAI. Using the mechanical instability–functional instability dichotomy as a starting point,15 they concluded that functional instability could not be due to proprioceptive (ie, sensory) deficits alone, as originally hypothesized by Freeman et al,10–12 but was also due to changes in the motor component of sensorimotor control, particularly impaired postural control,13–15 diminished ankle-eversion strength,16 and alterations in motor control of the muscles proximal to the injured ankle.17 This led to a shift in the literature from describing functional instability as a persistent symptom after LAS, as originally described by Freeman et al,10–12 to the idea that functional instability represented the sensorimotor cause of persistent injury. Functional instability was viewed as contrasting with mechanical instability, due to pathologic ankle-joint laxity, as the cause of recurrent and persistent instability after LAS.15
Hertel18 published a comprehensive literature review on functional ankle instability in 2000 that summarized the evidence of sensorimotor deficits related to ankle instability, including impairments in balance, joint position sense, peroneal muscle reaction time to inversion perturbation, peripheral nerve-conduction properties, muscle strength, and ROM. Two years later, Hertel19 presented an expanded model consisting of a Venn diagram with 2 overlapping circles representing the potential mechanical and functional (sensorimotor) contributions to CAI. In this model, the condition was explicitly labeled CAI in an effort to avoid the confusion over whether functional instability was the involved deficit or a potential cause of the involved deficit.19 Additionally, the terms mechanical instability and functional instability were not used in the model; instead, mechanical insufficiencies and functional insufficiencies were described as specific contributors to the development of CAI.19 Mechanical insufficiencies in the model included pathologic laxity, arthrokinematic restrictions, degenerative changes, and synovial changes, whereas functional insufficiencies included impairments in proprioception, neuromuscular control, strength, and postural control.19 The components of both mechanical and functional instability could now be named, described, and studied to show the relationships within and between the interrelated causes. This model suggested that when insufficiencies were identified clinically in individual patients, treatments to address the specific insufficiencies could be developed in an effort to improve patient outcomes.19
In 2011, Hiller et al20 proposed an extension of the Hertel19 model in the form of multiple clinical subgroups for classifying patients with CAI: mechanical instability, perceived instability, and recurrent sprains, or combinations of these 3 conditions.20 Importantly, the authors validated their model by fitting patients with CAI into the predetermined subgroups. Evaluating 108 ankles with CAI, they found that 56% fit into 1 of the 3 primary categories and 44% did not.20 However, all of the ankles did fit into 1 of the 7 subgroupings when the primary categories were combined.20 Although the Hiller et al model20 evolved the understanding of CAI, the advent of new evidence in areas such as self-reported function, health-related quality of life, kinesiophobia, altered movement patterns, and physical activity levels, as well as contemporary injury paradigms such as the biopsychosocial model,21,22 dynamic systems theory,23–26 and neuromatrix of pain theory27,28 emphasizes the need for an updated model. The purpose of our article was to describe an updated model that provides a theoretical framework for the contemporary understanding of the causes of CAI while simultaneously offering a framework for clinicians evaluating and treating patients with LASs and CAI.
UPDATED MODEL OF CAI
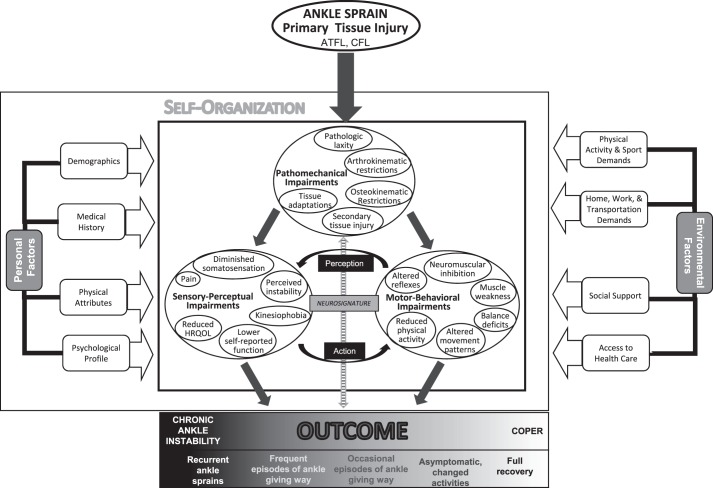
The updated model of CAI has 8 primary components: (1) primary tissue injury, (2) pathomechanical impairments, (3) sensory-perceptual impairments, (4) motor-behavioral impairments, (5) personal factors, (6) environmental factors, (7) component interactions, and (8) the spectrum of clinical outcomes (Figure 1). All patients with CAI will have had a primary injury to the anterior talofibular ligament (ATFL) and possibly the calcaneofibular ligament (CFL) at the time of their index LAS. Each specific impairment listed under the categories of pathomechanical, sensory-perceptual, and motor-behavioral impairments is a factor that has been identified in the literature as being different between patients with CAI and healthy participants without a history of LAS. The list of many specific impairments in the model is not meant to imply that every patient with CAI will present with each individual impairment; instead, these are characteristics that the patients as a group are likely to demonstrate. Patient-specific personal and environmental factors play critical roles in how an individual responds to injury and its consequences.21,22 The component interactions are drawn from dynamic systems theory23–26 and the Melzack neuromatrix theory of pain27,28 and used to hypothesize how the primary tissue injury, the 3 categories of impairments, and personal and environmental factors may interrelate to produce a patient's clinical outcome. Lastly, the spectrum of outcomes ranges from a fully successful recovery (coper) to an indisputably unsatisfactory outcome (CAI).
Figure 1.
The updated model of chronic ankle instability (CAI). The outcome is determined at least 12 months after the initial ankle sprain. Abbreviations: ATFL, anterior talofibular ligament; CFL, calcaneofibular ligament; HRQOL, health-related quality of life.
Primary Tissue Injury
For CAI to develop, a patient must first sustain an index LAS. Lateral ankle sprains are typically caused by excessive supination of the rearfoot on an externally rotated tibia. These injuries are often referred to as inversion ankle sprains, but this term represents a reductionist approach to describing the mechanism of injury and ignores the oblique axes of rotation of the talocrural and subtalar joints.19 Through robust analysis of several LASs that occurred in athletes and were captured on video, the kinematics of the injury mechanism were shown to consist of both excessive inversion and internal rotation of the rearfoot on the tibia.29,30 Interestingly, this work has also challenged the dogma of LASs as plantar flexion-inversion injuries by demonstrating that in some athletes, the peak angles and angular velocities of inversion and internal rotation occurred not while the ankle was in plantar flexion but when it was in sagittal-plane neutral or dorsiflexed.30 Perhaps the term inversion–internal-rotation sprain would be a more apt kinematic description of the mechanism of injury for LAS.
The ATFL is the ligament injured most commonly during an LAS.1 Concurrent injury of the CFL is present in many more severe ankle sprains.1 Clinicians must be cognizant of other potential injuries when evaluating patients who have experienced an inversion–internal-rotation mechanism of injury, including but not limited to fibular fracture, fifth metatarsal fracture, osteochondral lesion of the talus, high ankle sprain (injury to the anterior inferior tibiofibular ligament and tibiofibular syndesmosis), subtalar-joint sprain, bifurcate ligament sprain, fibularis tendon and retinacular lesions, and injury to the superficial fibular, tibial, or sural nerve.
An initial LAS results in stretching or disruption of the collagen fibers of the lateral ligaments, causing structural tissue damage. After an LAS, patients quickly develop the clinical signs and symptoms of pain, swelling, and inflammation. Simultaneously, but often less obviously, alterations in sensorimotor function also occur. Together, the injured tissues, accompanying inflammatory responses, and the patient's psychological and emotional responses to the injury (eg, pain and mechanical and sensorimotor alterations in response to ligamentous injury) drive the specific impairments that can cause an individual to deviate from successful healing toward CAI.
Pathomechanical Impairments
Pathomechanical impairments are operationally defined as structural abnormalities to the ankle joint and surrounding tissues, secondary to an index LAS, that contribute to ankle dysfunction and CAI. The impairments in this category represent the biological component of the biopsychosocial model.
Pathologic Laxity
Loss of the structural integrity of the lateral ankle ligaments results in pathologic laxity of the talocrural joint and possibly the subtalar joint. This laxity represents the mechanical instability described in earlier models of CAI. Disruption of the ATFL is associated with increased anterior drawer, or translation, of the talus within the tibiofibular mortise. Although most often evaluated with a common physical examination test, increased anterior translation of the talus has also been consistently demonstrated among patients with CAI using objective measurements such as instrumented arthrometry31–33 and stress radiographic34 and ultrasound imaging.35,36 Excessive internal rotation of the talus on the tibia has also been described in relation to lateral ankle instability.37–39 The anterolateral drawer test is performed by passively internally rotating the rearfoot while stabilizing the tibia.37–39 The absence of a firm end feel at maximal internal rotation indicates a rupture of the ATFL. In some patients with extensive laxity, a “clunk” of the talus may be felt, similar to that found with a positive pivot shift test in a patient with an anterior cruciate ligament-deficient knee. Although this test is popular in some orthopaedic circles,37–39 further research is needed to validate the diagnostic properties of the anterolateral drawer test.
Integrity of the CFL is most often assessed using the inversion stress test. This test is performed by passively inverting the rearfoot to its end ROM. Similar to the anterior drawer test, the inversion stress test has also been quantified using arthrometry.31–33,40 The CFL may be better isolated by conducting the inversion stress test in a dorsiflexed position, whereas the integrity of both the CFL and ATFL can be evaluated by performing the test in a plantar-flexed position.41 Clinicians must also be cognizant of the potential for increased laxity in adjacent joints, including the distal tibiofibular and subtalar joints, as a subset of patients with LAS and CAI presents with instability of these joints.1
Evidence of an initial increase in laxity after acute LAS and a subsequent return toward preinjury laxity in the weeks and months afterward has been reported in a few prospective studies42,43; some residual laxity is likely to remain in most patients who incur an LAS.
Arthrokinematic Restrictions
In contrast to pathologic laxity, particular accessory joint motions may be limited after LAS or with CAI. Over the past 2 decades, substantial advances in our understanding of arthrokinematic restrictions in the ankle and foot complex have emerged in the manual therapy literature.44–47 Restrictions in anterior-to-posterior glide of the talus on the tibia have been well documented as being associated with limited osteokinematic dorsiflexion of the talocrural joint in patients with lateral ankle instability.44,45,48 Also, small amounts of anterior displacement of the talus on the distal tibia may be associated with restricted glide of the talus.49 Furthermore, many patients have demonstrated anterior displacement of the distal fibula relative to the tibia and associated restriction of anterior-to-posterior glide of the distal fibula.50,51 Lastly, the potential for arthrokinematic restrictions at the subtalar, midtarsal, and tarsometatarsal joints has also been described.46,47
Osteokinematic Restrictions
Patients recovering from LAS or with CAI often demonstrate restricted dorsiflexion ROM. Possible causes of this deficit include the previously mentioned restriction of anterior-to-posterior talar glide and soft tissue restrictions in the triceps surae. These soft tissue restrictions may be due to inflexibility of the musculotendinous structures, neuromuscular spasm mediated by the γ motor-neuron system, myofascial constraints, or a combination of these.45 Patients with longstanding CAI may also exhibit limitations in foot and ankle motion in multiple planes as a consequence of osteoarthritis in the ankle complex.52,53
Secondary Tissue Injury
As mentioned earlier, clinicians must be vigilant in assessing concomitant injuries to structures other than the lateral ligaments in patients who have sustained LASs. Similarly, repetitive bouts of excessive inversion-internal rotation, which may result in recurrent ankle sprains or less severe giving-way episodes, can result in further insult to the ATFL and CFL as well as secondary tissue damage about the ankle complex. Of particular concern are lesions of the fibularis longus and brevis tendons, the osteochondral surfaces of the talus and tibia, the synovial membrane of the talocrural and subtalar joints, and the ligaments of adjacent joints on the medial side of the ankle.54 Ultimately, ankle osteoarthritis can be a serious sequela of CAI.55
Tissue Adaptations
Injured tissues will adapt to the demands placed on them over time and may develop alterations that are not identifiable on routine physical examination. For example, the involved ATFL of both CAI and coper groups has been demonstrated to be substantially thicker than in healthy controls who have never incurred an LAS.56 Additionally, subclinical alterations in the osteochondral surface of the talus as identified by higher T1ρ57 and T258 relaxation times during advanced magnetic resonance imaging have been identified in patients with CAI compared with controls. Also, volume alterations have been seen in the intrinsic and extrinsic foot muscles of patients with CAI.59 Clinicians should be mindful that such “hidden” structural changes may be contributing to specific impairments identified during the physical examination and functional testing of these patients.
Sensory-Perceptual Impairments
Sensory-perceptual impairments are operationally defined as conditions that the patient senses or feels about the body, the injury, or the self. These impairments represent physiological constructs such as somatosensation (bio in the biopsychosocial model), psychophysiological constructs such as pain (biopsycho), and psychosocial constructs such as kinesiophobia. These latter 2 constructs represent the patient's perceptions of the injury and the effects they have on his or her well-being. This grouping purposely includes impairments that involve both conscious and unconscious sensation and perception.
Diminished Somatosensation
Several domains of somatosensation have been noted to be impaired in patients with CAI. These impairments are hypothesized to occur because of damage to the ligamentous and articular proprioceptors during injury and possible nerve injury secondary to ligament injury. Deficits have been reported in both the active and passive joint position sense of frontal- and sagittal-plane ankle motion, with CAI groups demonstrating more proprioceptive errors.60,61 The inability of patients with CAI to accurately sense the position of their ankle joint before initial contact during gait or landing has been theorized to increase the risk of recurrent ankle sprain because the foot is likely to contact the ground in a position that predisposes the ankle to move into supination rather than pronation during the loading response.62
Measures of force sense in all directions of ankle motion among patients with CAI have indicated that the ability to sense and regulate muscle-contraction output is impaired after joint injury, even in the absence of musculotendinous injury.63–68 Interestingly, weak and nonsignificant correlations were found between measures of active position sense and force sense in patients with CAI, suggesting that these measures assess different constructs of somatosensation.69
Differences in cutaneous sensation have also been demonstrated between CAI and control groups. The CAI groups have displayed poorer plantar sensation as evaluated with both vibrotactile stimuli70 and Semmes-Weinstein monofilaments71,72 at the heel, base of the fifth metatarsal, and head of the first metatarsal. Burcal and Wikstrom72 observed impaired sensation over the sinus tarsi in both CAI and coper groups versus a healthy control group. Interestingly, the sinus tarsi was the only site at which the coper group exhibited sensory deficits, whereas the CAI group had deficits in plantar sensation in addition.72
The ability to integrate different sensory inputs appears to be compromised in CAI. Song et al73 performed a meta-analysis to investigate postural control in eyes-open and eyes-closed positions. Compared with healthy controls, patients with CAI relied more heavily on visual information than somatosensory information during unipedal-stance balance tasks. Additionally, those with CAI appeared to be unable to dynamically reweight sensory inputs to the same extent as healthy controls.74 The physiological mechanism of these differences is currently unknown. To date, only 1 group75 has evaluated somatosensory cortex activity in patients with ankle instability; they found no differences in electroencephalography-derived somatosensory cortex activity during a controlled ankle-joint–loading task among CAI, coper, and healthy groups.
Pain
Pain is a hallmark of most chronic musculoskeletal conditions. Surprisingly, quantification of pain has received relatively little attention in the CAI literature,76 although clinical experience tells us that persistent pain is a common reason for patients with CAI to seek health care. The Melzack neuromatrix theory of pain27,28 indicates that in chronic pain conditions, the pain is generated not exclusively from the sensory input evoked by injury, inflammation, or other damage at the site of symptoms but is instead produced by the output of the neuromatrix, a widely distributed neural network in the brain. Chronic psychological and physical stress associated with chronic pain can further diminish a patient's ability and willingness to participate in functional activities.27,28 The influence of pain on other impairments commonly seen among patients with CAI is likely to be clinically important, but currently these relationships are poorly understood.
Perceived Instability
A common complaint of those with CAI is the perception that the ankle is unstable or that it is at risk of giving way during functional activities. Patients reporting perceived instability may or may not actually experience episodes of excessive ankle inversion; however, the perception of instability represents a clinically important impairment.20 The Cumberland Ankle Instability Tool (CAIT)77 and Identification of Functional Ankle Instability (IdFAI)78 questionnaire have both been widely used in the CAI literature as screening tools. Both survey instruments ask individuals to self-report the frequency and circumstances of the perceived instability episodes. The CAIT consists of 9 questions, 1 about pain and 8 about perceived instability. A score of <27 points out of a possible 30 points was originally considered the threshold for identifying functional ankle instability.77 However, a CAIT ≤24 is now considered a diagnostic criterion of CAI.79 The IdFAI consists of 10 questions about ankle-sprain history and perceived instability. A score of ≥11 out of a possible 37 points is necessary for a diagnosis of CAI.78
Kinesiophobia
Fears of movement and reinjury during functional activities have been reported in patients with CAI.80 Kinesiophobia is most often assessed with the Fear-Avoidance Beliefs Questionnaire81 and the Tampa Scale for Kinesiophobia (TSK-11).82 The Fear Avoidance Beliefs Questionnaire is a 16-item survey that addresses the fear of movement during physical activity and work.81 The TSK is an 11-item questionnaire that assesses fears of movement and reinjury.82 The perception that movement of the involved ankle will be harmful runs counter to the emphasis on therapeutic exercise as a primary treatment for CAI and represents an important obstacle to be managed when treating this condition.
Self-Reported Function
Reduced self-reported function has been consistently demonstrated in patients with CAI.80,83–85 These deficits have most often been identified using a region-specific questionnaire such as the Foot and Ankle Ability Measure (FAAM).86 The FAAM consists of a 21-item Activities of Daily Living (ADL) scale and an 8-item Sports scale; it requires patients to rate their difficulty when performing specific ADL or sport activities due to their involved ankle.86 Measures of self-reported function provide insight into the types of actions and activities these patients are able to perform.
Health-Related Quality of Life
Measures of health-related quality of life (HRQOL) were diminished in patients with CAI.80,85,87 Global, or generic, HRQOL focuses on broader concerns, such as mood, vitality, and social interactions, that are not as directly linked to ankle function as are the items on region-specific function scales. The most commonly used HRQOL scales in medicine are the Short Form-36 and Short Form-12 questionnaires. These scales are particularly adept at tracking HRQOL in patients with chronic conditions. Both have physical health and mental health subscales. Patients with CAI have displayed deficits in physical HRQOL but not in mental HRQOL.87 A criticism of the Short Form scales is that they may not be appropriate for athletic or otherwise highly physically active populations because of a ceiling effect in their psychometric properties.88 In response to this weakness, the Disability in the Physically Active Scale was developed to more accurately assess HRQOL in this population.89,90 Using the Disability in the Physically Active Scale, Houston et al80 demonstrated a large deficit in HRQOL among patients with CAI.
Motor-Behavioral Impairments
Motor-behavioral impairments among patients with CAI constitute deficiencies and alterations in muscle contractility, motion patterns, and physical activities that they choose to partake in or avoid. These factors constitute the motor aspect of sensorimotor function. All impairments in this category fall into the bio construct of the biopsychosocial model except for the reduced physical activity impairment, which includes both a bio component related to the physiological costs and benefits related to exercise and physical activity and a psychosocial component representing intentional behavior.
Altered Reflexes
A large body of literature has examined muscle-contraction timing and amplitude in response to inversion perturbations of the ankle.91 The most common measures were the electromyographic, force, and kinematic responses to inversion of a platform with a trapdoor mechanism that caused the ankle to be suddenly inverted or, in some designs, concomitantly plantar flexed and inverted. Participants were typically in bipedal stance when 1 foot was perturbed, although some researchers92–94 have tested participants during walking. In a meta-analysis, Hoch and McKeon91 found delayed reaction time of the fibularis longus and brevis muscles in response to sudden-inversion perturbations in patients with CAI. The delayed motor response may be due to alterations in somatosensation, nerve conduction velocity, or central processing of the monosynaptic stretch reflex. Regardless of the physiological source, delayed contraction of the fibularis muscles results in an electromechanical delay in the ability to create an eversion force to counteract the ankle moving quickly into inversion.91
Neuromuscular Inhibition
Arthrogenic muscle inhibition has been well documented in chronically unstable ankles,95 most often by assessing the H-reflex response in the fibularis longus muscle. The H-reflex is an electrically induced surrogate of the monosynaptic stretch reflex and represents spinal-level motor control. Participants receive transdermal electrical stimulation of a motor nerve, and the H-reflex output is measured via surface electromyography of the muscle of interest. Several groups have reported diminished H-reflex amplitude in the fibularis longus92,96 and soleus.97 Kim et al98 also found that the constrained ability to modulate the H-reflex in the fibularis longus and soleus muscles across different postural positions (ie, moving from lying prone to bipedal stance or from bipedal stance to unipedal stance) was impaired in patients with CAI. Additionally, they were unable to modulate paired reflex depression of the soleus during positional changes similarly to healthy participants and demonstrated greater levels of recurrent inhibition of the soleus.99
The inhibition of muscles proximal to the ankle has also been reported in patients with CAI. Using measures of central activation, investigators100 observed that patients with unilateral CAI had bilateral inhibition of the hamstrings muscles and ipsilateral facilitation of the quadriceps muscles compared with healthy controls. Remarkably, impaired contractility of the diaphragm muscle has also been reported in patients with CAI, indicating that proximal muscle function was affected not only in the lower extremity musculature but also in the trunk.101
In recent years, the influence of supraspinal motor control in patients with CAI has been studied using measures of motor-cortex excitability and inhibition. Electromyographic measures are taken from peripheral muscles immediately after transcranial magnetic stimulation of the motor cortex in areas of the homunculus specific to the muscles of interest. Higher resting102 and lower active103 motor thresholds of the fibularis longus were present bilaterally in patients with unilateral CAI. Kosik et al104 identified less fibularis longus recruitment map volume and area in the motor cortex among patients with CAI than healthy individuals, suggesting that the former had a more concentrated and restricted area of neurons able to recruit the fibularis longus muscle. Altered balance between corticospinal inhibition and excitability of the soleus among patients with CAI compared with healthy controls has also been suggested.105 Correlations between measures of cortical excitability and ankle laxity106 and self-reported function107 have been reported among patients with CAI.
Muscle Weakness
The clinical assessment of muscle function among patients with CAI most often relies on measures of strength using manual muscle tests. Using a handheld dynamometer, Fraser et al108 recently reported that patients with CAI were weaker than healthy controls in isometric eversion, inversion, and plantar flexion but not in dorsiflexion.
Donnelly et al109 also demonstrated deficits in isometric eversion strength but no differences in corresponding surface electromyography amplitude of the fibularis longus and brevis muscles. Interestingly, eversion force and electromyographic amplitude were significantly correlated in the healthy group but not the CAI group, indicating an uncoupling of muscle contractility and force production among patients. Additionally, Terrier et al110 described a weight-bearing test of eversion strength that discriminated between CAI and healthy groups.
Ankle strength among patients with CAI has been studied extensively using isokinetic dynamometry. Meta-analyses111,112 have shown consistent eversion concentric-strength deficits in patients with CAI. Deficits have also been reported in concentric inversion113,114 and plantar-flexion115,116 strength and eccentric eversion,117,118 inversion,118,119 plantar-flexion,120 and dorsiflexion121 strength.
Weakness of the muscles proximal to the unstable ankle, including deficits in concentric knee flexion and extension,116 isometric hip abduction,115,121 extension,115,121 external rotation,121,122 and eccentric hip flexion,123 has also been identified among patients with CAI. Distally, weakness in hallux and lesser toe-flexion strength108 and diminished volume of the flexor hallucis brevis and adductor hallucis oblique muscles59 have been reported in patients.
Balance Deficits
The relationship between ankle instability and balance deficits was first noted by Freeman et al10–12 more than 50 years ago. In the ensuing decades, dozens of researchers have described balance, or postural-control, deficits in patients with CAI. The most common balance tasks reported in the literature were maintenance of quiet unipedal stance124 and the Star Excursion Balance Test (SEBT).125 The former represents static balance, or the ability to remain as still as possible while standing on 1 leg, whereas the latter represents dynamic balance, which requires the participant to reach as far as possible in a prescribed direction with 1 leg while maintaining balance on the other limb. Balance deficits among patients with CAI may be due to somatosensory impairments, motor impairments, or both.
Static balance is typically assessed with a participant performing trials in eyes-open and then in eyes-closed conditions. Assessment of static balance may consist of no-, low-, or high-technology methods. No-technology assessment relies on patient or clinician judgment to subjectively identify impairment while the patient with unilateral CAI balances on the involved limb compared with the uninvolved limb.12 A low-technology approach to measuring static balance assesses the amount of time a patient can maintain unipedal stance.126 Performing the unipedal components of the Balance Error Scoring System on firm and foam surfaces by counting the number of predefined errors during a 20-second trial is another low-technology approach that has been used to quantify balance deficits among patients with CAI.126,127
The most common high-technology approach to measuring balance is to have a participant maintain single-limb stance while standing on a force plate that measures 3-dimensional forces and moments.124 Although dozens of force-plate measures have been reported in the CAI literature,60,124,128 the key conclusion is that balance deficits have been consistently demonstrated in these patients. Generally, the measures evaluate the magnitude, velocity, or variability of postural sway. Song et al73 postulated that patients with CAI did not use somatosensory information to the same extent as healthy controls but instead relied more heavily on visual input to maintain unipedal stance.
The SEBT, originally conceived as a “no-tech” measure of dynamic balance, has been used extensively to identify deficits in patients with CAI, who are unable to reach as far as healthy controls.125 Surprisingly, the reach deficits have been shown to be more strongly related to diminished knee and hip flexion than to limited ankle dorsiflexion.129 Similarly, diminished hip-abduction and external-rotation strength has also been correlated with reduced reach distances in patients with CAI.121 In addition, patients exhibited more trunk and pelvis rotation when executing select SEBT reach tasks.130
Altered Movement Patterns
Individuals with CAI displayed altered movement patterns in a spectrum of functional activities, including walking, running, cutting, and landing, compared with control participants. Such alterations have been demonstrated using biomechanical measures of kinematics, kinetics, plantar pressure, and electromyography.
During walking, patients with CAI tend to exhibit greater inversion and plantar flexion of the foot relative to the tibia, a more laterally deviated center of pressure throughout stance, and alterations in fibularis muscle activation.131 Biomechanical alterations during jogging and running tend to mimic those seen during walking.131 The kinematic changes were amplified using a dual-task paradigm in which participants performed a cognitive task while ambulating.132 A more inverted foot is likely to lead to an LAS. Investigators133 have speculated that because the foot tends to be more inverted during midswing in patients with CAI, the fibularis muscles must activate during late swing to actively move the foot into eversion in preparation for initial contact. This is in contrast to healthy individuals, who typically contract the fibularis longus muscle after initial contact, as part of the loading response in which the fibularis longus muscle contracts to plantar flex the first ray.133 This contraction would be associated with a medial displacement of the center of pressure as the foot also everts. If the fibularis longus is already contracted before initial contact, as it is in these patients, it cannot be contracted again to plantar flex the first ray during the loading response. Thus, this is a likely reason why the foot remains more inverted and the center of pressure stays more lateral throughout the stance phase among patients with CAI.133 Although gait alterations in CAI are often described in terms of greater inversion, an alternative view associates CAI with less eversion, and hence, less pronation during the stance phase. This may be why patients with CAI produce greater impact force and a faster loading rate of the vertical ground reaction force during the loading response.134
Alterations in the stride-to-stride variability of various gait factors have also been reported in patients with CAI. However, increases and decreases in variability have both been seen. These discrepancies likely depend on gait speed (walking, running), task constraints (fatigue, dual tasking), the specific biomechanical measure being analyzed, and the method used to calculate variability (linear, nonlinear). Increased variability in frontal-plane ankle kinematics during running among patients with CAI has been reported using linear variability calculations based on intraindividual standard deviations across multiple steps.135,136 During walking, ankle frontal-plane kinematic variability was amplified in patients with CAI during a dual-task paradigm.132 Conversely, Terada et al137 reported less stride-to-stride variability in frontal-plane ankle kinematics among patients using measures of sample entropy, a nonlinear variability estimate during single-task walking.
Another approach to analyzing stride-to-stride variability is to assess the kinematic coupling behavior, or coordinated movement, of different segments of the lower extremity using vector-coding techniques. Patients with CAI have less variability in coupling between transverse-plane shank and frontal-plane rearfoot motion during walking and jogging.138,139 Differences in coupling variability have also been examined between ankle motion and more proximal joints. During walking, patients with CAI have demonstrated less variability in frontal-plane ankle-hip coupling140 and greater variability in ankle frontal-knee sagittal-plane motions.141 During jogging, patients have exhibited less coupling variability between the ankle-hip and the ankle-knee in both frontal- and sagittal-plane motions.141
Patients with CAI have also been reported to require a higher level of gait disturbance, defined by alterations in walking speed and dual tasks, to reduce stride-time variability, a spatiotemporal gait measure, compared with healthy controls.142 This change was hypothesized to be due to less adaptability of the sensorimotor system in response to task constraints.142 Koldenhoven et al143 observed that patients with CAI had greater stride-to-stride variability in the location of their center of pressure during the first 10% of stance phase during walking compared with controls but no differences later in the stance phase, despite their center of pressure staying more lateral.144 Interestingly, the patients with CAI also exhibited less variability in electromyographic amplitude of the fibularis longus muscle throughout the swing phase and the beginning of the stance phase.143
During cutting tasks requiring rapid lateral movement, patients with CAI activated the fibularis longus earlier than healthy controls, in a manner similar to that seen while walking.145 Reduced amplitude of fibularis longus surface electromyographic activity both before and after initial contact has been noted,145,146 as has activation of other ankle and hip muscles.146 Patients have exhibited greater ankle-inversion147 and less dorsiflexion146 motion, as well as pronounced changes in knee and hip motion,146,147 during cutting tasks. In terms of kinetics, patients with CAI have shown greater peak vertical ground reaction force, less time to peak force,148 and increased external knee- and hip-extensor moments149 during cutting tasks.
Alterations in single-limb landing tasks have also occurred in patients with CAI. In a recent systematic review, Simpson et al150 concluded that patients with CAI tended to display altered kinematic, kinetic, and muscle-activation patterns during single-limb landings. They consistently landed in a more dorsiflexed position and underwent less sagittal-plane motion during the absorption phase of landing.150 Higher peak vertical ground reaction forces and faster loading rates have also been reported in patients with CAI, indicating a stiffer landing strategy.150 These landing strategies were associated with proximal kinematic and kinetic changes at the knee and hip.149,151,152 Reduced fibularis longus muscle activation among patients with CAI has been seen in some studies,150 but conflicting results152 showed increased fibularis activation. Increased activation of the gluteus maximus muscle before initial contact has also been demonstrated in patients with CAI.153
Reduced Physical Activity
Patients with CAI may avoid physical activity because of their ankle instability. College students with CAI took more than 2100 fewer steps per day than healthy counterparts with no history of ankle injury.154 The long-term health consequences of reduced physical activity in patients with CAI are a concern that requires further study. It is also possible that these patients change the type of physical activities in which they choose to participate; however, this area has not been widely studied. Toward this purpose, Halasi et al155 modified the Tegner Activity Scale,156 a survey instrument that assesses changes in physical activity of patients with knee injuries, to be appropriate for patients with ankle injuries; however, this instrument has not been used widely in the CAI literature. We chose to include reduced physical activity in the category of motor-behavioral impairments because participating or not participating in specific physical activities is a motor behavior that is distinct from the sensory and perceptual impairments described elsewhere in the model. Yet the specific impairments clearly interact, as described in the model.
Personal Factors
Individual patients will respond to injury in unique ways based on their own distinctive characteristics. Such characteristics are referred to as personal factors in the International Classification of Functioning model.157 In our CAI model, we identify the personal factors of patient demographics, medical history, physical attributes, and psychological profile. Still, additional personal factors may influence a patient's response to injury. Demographic factors such as age, body mass index, and sex may have important biological influences on healing and other physiological processes after injury. A patient's medical history, including the presence of comorbidities, structural deficits due to past injury, and how an individual has recovered from previous injuries and illnesses, can affect the response to a new or recurrent injury. A patient's physical attributes, such as the level of strength and conditioning (ie, strength and flexibility) or skeletal alignment (ie, foot morphotype), can influence the response to and recovery from injury. Finally, an individual's psychological profile, including characteristics such as self-efficacy and anxiety, can play important roles in the response to injury. Our decision to exclude other potential personal factors is not meant to deny the importance of those factors but was an effort to simplify the presentation of the CAI model. Clinicians should be cognizant of how patient-specific personal factors may influence an individual's response to and recovery from acute and chronic ankle injury.158
Environmental Factors
Factors outside of a patient's organism that may affect the response to injury are termed environmental factors in the International Classification of Functioning model157 and are included in our CAI model. These factors include societal expectations the individual perceives regarding physical activity and sports participation as well as expectations for his or her role in home, family, work, and transportation activities. Social support networks can also play an important role in the response to and recovery from injury. Finally, a patient's access to health care facilities and providers can have a large influence on the type and frequency of health care received. Similar to how personal factors are portrayed in the CAI model, other environmental factors may be important to an individual patient; excluding any of these potential factors from our model is not meant to imply that such factors do not exist. In an effort to provide holistic care to each patient we evaluate and treat, clinicians should seek to identify and address any environmental factors that may influence a patient's recovery from injury.158
From Impairments to CAI Manifestation
Chronic ankle instability is a heterogeneous injury in which individual patients present with unique combinations of pathomechanical, sensory-perceptual, and motor-behavioral impairments. Rather than positing multiple subgroups of patients in an effort to identify homogeneity among CAI patients, the updated model accounts for the heterogeneity of impairment presentation through the interactions of 3 conjectural constructs: self-organization, perception-action cycles, and neurosignature. The first 2 constructs are derived from the dynamic systems theory of motor control,23–26 and the third stems from the Melzack neuromatrix theory of pain.27,28
Self-Organization
Dynamic systems theory is a universal theory of science used to describe complex phenomena in a diverse array of disciplines.26 Multilevel components influence human movement, including but not limited to cells, tissues, systems, organisms, and social constructs. At the crux of dynamic systems theory are principles indicating that the component levels are not equivalent to each other because the influence of 1 level on another level is typically nonlinear (eg, a small change at the tissue level can cause a large effect at the systems level); circular causality exists among levels, indicating the role of both feedback and feedforward relationships; relationships among levels change over time; and no predefined motor programs are directing system interactions.26
The generation and control of specific movements are dictated by a process of self-organization that weighs the potential movement strategies available, given the relevant constraints, to achieve the desired movement goal.23–25 Types of constraints include task, environmental, and organismic. Task constraints represent the limitations that govern how a movement may occur (eg, track athletes always race in counterclockwise direction). Environmental constraints are external to the organism and are due to the surroundings in which movement is being executed (eg, uneven grass surface or a flat, paved surface). The primary tissue injury and accompanying pathomechanical, sensory-perceptual, and motor-behavioral impairments in the model represent organismic constraints, which can influence how a patient with CAI moves and engages in physical activity.25 The unique organismic constraints in an individual patient, coupled with the task and environmental constraints specific to a given situation, influence how a patient behaves and moves. During rehabilitation, a clinician may manipulate task and environmental constraints in an effort to generate a specific motor output aimed at addressing a specific impairment (eg, designing a balance exercise that specifically requires a large amount of fibularis muscle activation).25
Perception-Action Cycles
A cyclical relationship exists between perception and action, meaning that perception (sensory input) influences action (motor output), and action affects perception, and the cycle repeats in perpetuity.159 In the CAI model, the perception-action cycle represents the circular causality between sensory-perceptual impairments and motor-behavioral impairments. Understanding the inherent linkage between the sensory and motor contributions to CAI is essential to successful assessment and treatment of patients with this complex condition. An intervention that addresses a sensory-perceptual impairment alters motor behavior and vice versa.
Neurosignature
In his neuromatrix of pain theory,27,28 Melzack proposed 4 core components that contribute to chronic pain conditions: (1) the body-self neuromatrix; (2) cyclical processing and synthesis producing a continuous neurosignature outflow; (3) sentient neural hubs in the brain where the flow of neurosignature is integrated into the flow of sensory inputs; and (4) an action neuromatrix, also influenced by the neurosignature, that produces movements aimed at achieving a desired goal. The neuromatrix comprises a series of neural networks throughout the brain that process sensory information and generate a stream of neurosignature output that contributes to both the body possessing a sense of itself in perceptual and emotional terms and production of movement.27,28 The neuromatrix and thus the neurosignature are influenced by genetics and modified by lived experiences.27,28 These lived experiences are incorporated as personal and environmental factors in our proposed model. Persistent pain and stress are posited to substantially alter the neurosignature in a negative manner,27,28 whereas targeted therapies such as manual therapy and therapeutic exercise can alter it in a positive manner. In the CAI model, the neurosignature represents the neural patterns unique to the individual patient that influence sensory and emotional perception and motor function. A patient's neurosignature acts as a continuous modifier of the perception-action cycle.
How Do the Component Interactions Work Together in Patients With Ankle Sprain or CAI?
Acute injury to the lateral ankle ligaments produces specific pathomechanical impairments related to ligamentous and, potentially, other tissue damage around the ankle. The injury also initially triggers sensorimotor changes via inflammatory and pain mediators that result in specific sensory-perceptual and motor-behavioral impairments. How a patient responds to these impairments influences his or her perception of the injury and behavior, including motor output, in the presence and aftermath of the injury. An individual's personal factors, such as a history of musculoskeletal injury and level of self-efficacy, will affect perceptions and behaviors. Environmental factors, such as social support and expectations for the patient to fulfill defined roles relative to home, family, work, or sport, further influence the individual's perceptions and behaviors in response to injury. Physiological responses to injury mediated by inflammatory, neurologic, and hormonal processes produce local changes at the site of injury, such as edema, and in the central nervous system, such as neuromuscular inhibition in the injured limb. Neuroendocrine responses to injury, including the release of stress hormones, further influence the patient's perception of injury and movement. Together, these factors and processes affect the flow of afferent and efferent neural signals that constitute the patient's neurosignature.
Before injury, a person's neurosignature is in a state of homeostasis. Injury, such as an acute ankle sprain, leads to an immediate change in the neurosignature in response to tissue damage, inflammation, and stress. This initial change in the neurosignature is protective in nature. Patients who recover quickly after an acute ankle sprain are able to restore their neurosignature to preinjury homeostasis as injury symptoms are eliminated and sensorimotor function is restored. In contrast, patients who are unable to reset their neurosignature soon after injury may develop chronic symptoms and altered movement patterns.
Relative to the neuromusculoskeletal system, perception-action cycles are at the crux of an individual's neurosignature. Action in the form of motor output is a product of self-organization. Acute injury and subsequent manifestations of that injury create impairments that impose organismic constraints on movement strategies. Movement, however, is endemic to the human condition, and the body will self-organize to find a motor strategy that circumvents organismic constraints to accomplish the tasks that one deems necessary. For example, a patient who lacks 10° of ankle dorsiflexion is still able to walk but must use motor strategies that bypass the organismic constraint of restricted ankle dorsiflexion. This movement solution introduces unfamiliar signals into the nervous system, thereby producing unaccustomed perception-action cycles. Left unabated, this movement strategy can become the preferred motor output. Failure to address specific impairments postinjury can lead to longstanding constraints that normalize altered movement patterns, resulting in chronically altered perception-action cycles and a neurosignature that predisposes an individual to recurrent episodes of the ankle giving way and ankle sprains. Clinicians are thus encouraged to not only address patient-specific impairments during rehabilitation but to also emphasize perception-action processes in an effort to return the patient's neurosignature to a condition of healthy homeostasis.
Spectrum of Clinical Outcomes
We propose a spectrum of clinical outcomes that ranges from copers on the positive end to CAI on the negative end. (Figure 1 displays negative outcomes to the left and a positive outcome to the right of the clinical outcome spectrum.) The outcome is meant to be determined more than 12 months after the initial ankle sprain, as deficits during the first year would not be deemed chronic.
A coper is defined as an individual who is more than 12 months removed from the index ankle sprain, has incurred no recurrent ankle sprains, reports no or very minimal symptoms or deficits in self-reported function, and perceives a full recovery.160 The goal of clinicians in treating a patient with a first-time ankle sprain should be to produce an outcome in which the patient becomes a coper. We assert that empirical measures to define a coper should include no ankle pain at rest or during physical activity; self-reported function scores greater than 95% on both the FAAM–ADL and –Sports subscales; a CAIT score of 28 or higher; an IdFAI score of 10 or lower; and no recurrent ankle sprains or perceptions of the ankle giving way. It should be noted that copers may have some identifiable residual impairments, such as increased laxity35; however, these impairments do not adversely affect the function or perception of the patient's ankle. The long-term consequences of these residual impairments are unknown at this time.
Ideally, an ankle-sprain patient becomes a coper without changing the type or volume of physical activities that he or she participated in preinjury. If a patient is asymptomatic but has altered physical activities because of the ankle, that cannot be considered a full recovery and the patient is not a true coper. Some patients choose to alter their physical activity to avoid symptoms or recurrent sprains. Although the outcomes of these patients are on the more positive side of the spectrum, a full recovery has not occurred because of the patient's failure to return to the preinjury level of physical activity. Moving in a negative direction on the outcome spectrum, the increasing frequency of ankle giving-way episodes and the frequency and severity of symptoms such as pain, swelling, and weakness are associated with poorer outcomes, as are recurrent ankle sprains. Repeated episodes of giving way and recurrent ankle sprains are likely to produce further secondary tissue damage, thus resulting in additional pathomechanical impairment. This is represented on the model by the dashed arrow between the outcome and the pathomechanical impairment circle. This “new” secondary tissue damage can then further exacerbate sensory-perceptual and motor-behavioral impairments, creating a cyclical condition associated with a poorer outcome.
On the most negative end of the outcome spectrum is the clinical designation of CAI, which is characterized by a patient who is more than 12 months removed from the initial ankle sprain; has a propensity for recurrent ankle sprains; and experiences frequent episodes or perception of the ankle giving way, as well as persistent symptoms such as pain, swelling, diminished ROM, weakness, and reduced self-reported function. We recommend that empirical measures to define CAI should include a CAIT score of 24 or lower, an IdFAI score of 11 or higher, and self-reported function scores of less than 90% on the FAAM–ADL and less than 80% on the FAAM–Sport. At present, we are unable to recommend specific diagnostic thresholds for other impairment categories.
APPLYING THE MODEL TO RESEARCH AND CLINICAL PRACTICE
The aims of the updated CAI model are to serve as (1) a paradigm for the current state of the science regarding the causes of CAI and (2) a framework to aid clinicians in managing patients with LASs or CAI. With respect to the first aim, we acknowledge that the updated model of CAI, while based on our synthesis of the current research, is theoretical. Like previous models of CAI, this model needs validation and refinement through continued research. In particular, little is known about the relationships between specific impairments and how these relationships affect clinical outcomes.
To accomplish the second aim, we recommend the application of the Donovan and Hertel assess-treat-reassess paradigm161 and the International Ankle Consortium rehabilitation-oriented–assessment approach,162 which explicitly link the identification of specific impairments during clinical assessment with corresponding treatment goals for rehabilitation. During the assessment of patients with ankle injuries, clinicians should routinely try to identify the source of the primary tissue injury and evaluate specific pathomechanical, sensory-perpetual, and motor-behavioral impairments by taking a thorough injury history and performing a comprehensive physical examination. Clinicians are also encouraged to look not just at the composite scores of questionnaires used to assess perceived ankle instability, pain, kinesiophobia, self-reported function, and HRQOL but also at the individual item responses on these survey instruments to identify patient-specific complaints and impairments. These findings should then be used to guide the development of rehabilitation goals and treatment decisions.
Not all patients will exhibit evidence of each specific impairment in the model. Each patient will present with a unique combination of impairments. As such, rather than applying a uniform rehabilitation protocol to all patients with LASs or CAI, clinicians should tailor a specific rehabilitation plan for each person based on the unique set of impairments identified during assessment. The targeted rehabilitation plan should address the patient's unique collection of impairments in an effort to modify the neurosignature that is driving the cyclical nature of the condition and shift the patient's outcome toward the positive (coper) side of the outcome spectrum. As illustrations, we have created 3 hypothetical patients, each representing a unique collection of impairments within the CAI model and requiring a uniquely targeted rehabilitation approach.
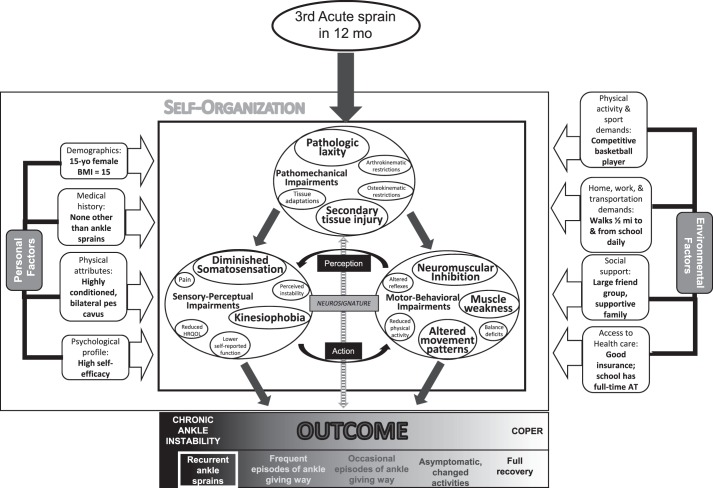
Patient 1 is a 15-year-old female high school basketball player who has sustained 3 LASs in the past 12 months (Figure 2). Her outcome is CAI, as evidenced by multiple recurrent ankle sprains. Her specific impairments, as identified on clinical examination and represented by enlarged circles and text in the figure, include the pathomechanical impairments of secondary tissue damage and pathologic laxity; the sensory-perceptual impairments of diminished somatosensation and heightened kinesiophobia; and the motor-behavioral impairments of neuromuscular inhibition, muscle weakness, and altered movement patterns. The repetitive ankle sprains and subsequent impairments have negatively affected her neurosignature, resulting in substantial neuromuscular dysfunction. This patient is likely to respond favorably to a rehabilitation approach that includes ankle taping or bracing during physical activity to address her ankle laxity and a therapeutic exercise program aimed at improving somatosensation, muscle activation, and strength; restoring functional movement patterns; and reducing her kinesiophobia.
Figure 2.
Adaptation of the model to illustrate the specific impairments of a 15-year-old female high school basketball player who has chronic ankle instability (CAI). The enlarged circles and text indicate specific impairments that are contributing to her condition and health status. Abbreviations: AT, athletic trainer; BMI, body mass index; HRQOL, health-related quality of life.
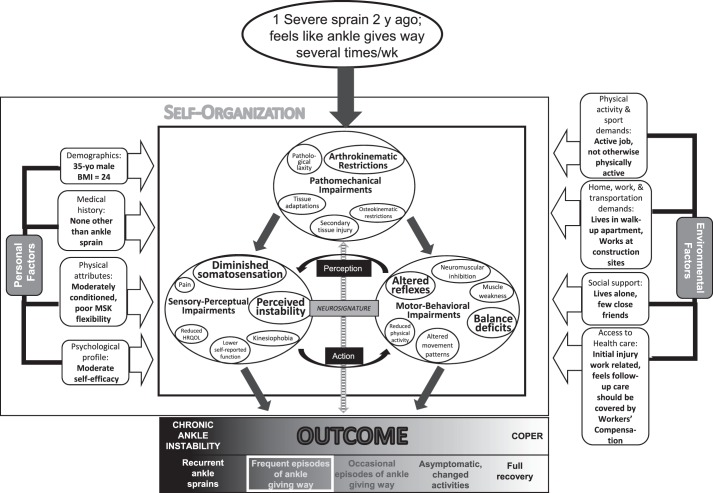
Patient 2 is a 35-year-old male construction worker who incurred a severe ankle sprain 2 years ago and now has a primary complaint of his ankle giving way several times per week (Figure 3). His outcome is CAI as characterized by repeated episodes of giving way and considerable perceived instability. Upon examination, his specific impairments include arthrokinematic restrictions and perceived instability and deficits in somatosensation, reflex responses to unexpected inversion, and static and dynamic balance. The repeated episodes of the ankle giving way and subsequent impairments have negatively affected his neurosignature, resulting in neuromuscular dysfunction. An important environmental factor that may influence the patient's perception of the injury is that it was work related and subject to Workers' Compensation. This patient is likely to respond favorably to a rehabilitation program that includes manual therapy focused on passive accessory joint mobilizations to address specific arthrokinematic restrictions and a therapeutic exercise program aimed at improving somatosensation, reflexive control of the ankle, and postural control in an effort to lessen his perceived ankle instability.
Figure 3.
Adaptation of the model to illustrate the specific impairments of a 35-year-old male construction worker who has chronic ankle instability (CAI). The enlarged circles and text indicate specific impairments that are contributing to his condition and health status. Abbreviations: BMI, body mass index; HRQOL, health-related quality of life.
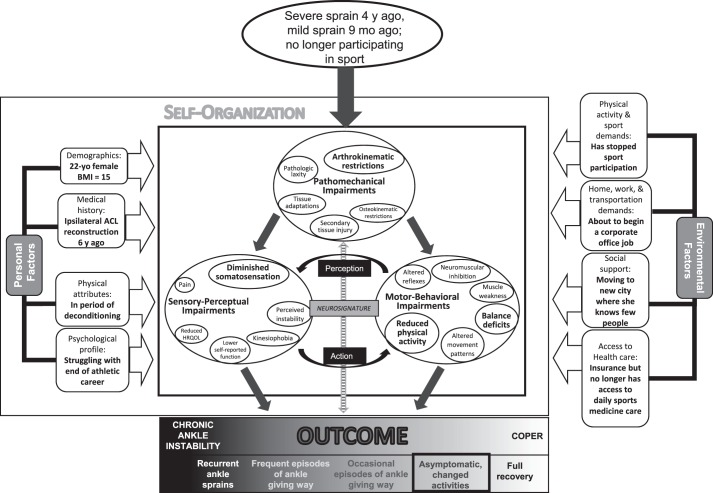
Patient 3 is a 22-year-old graduating collegiate student-athlete who had a severe ankle sprain 4 years ago and a mild recurrent ankle sprain 9 months ago (Figure 4). She is no longer playing competitive sports and has no plans to do so in the future, partly because of her history of ankle and knee injuries. Because her ankle is not symptomatic when she does not participate in sport, she has dramatically reduced the amount of physical activity in which she participates. Although her symptoms do not warrant a diagnosis of CAI, she clearly has not had a full recovery and cannot be classified as a coper. As such, her outcome has moved away from the most positive end of the outcome spectrum to indicate that she is asymptomatic because she has substantially altered her physical activity level. The figure depicts a few specific impairments identified by larger circles and text, but these are not of the same magnitude as those seen in patients 1 and 2. Patient 3's outcome could be characterized as a subclinical condition, and she would benefit from addressing her specific impairments to increase her overall level of physical activity.
Figure 4.
Adaptation of the model to illustrate the specific impairments of a 22-year-old graduating female collegiate athlete who has a history of ankle sprains and has changed her level of physical activity to cope with her ankle injury. The enlarged circles and text indicate specific impairments that are contributing to her condition and health status. Abbreviations: ACL, anterior cruciate ligament; HRQOL, health-related quality of life.
These 3 examples are presented for illustrative purposes only. Clinicians must be vigilant in assessing each individual patient and developing a holistic plan of care that addresses the primary condition and identified impairments along with the relevant component interactions, personal factors, and environmental factors. Validation of the clinical application of the CAI model is also needed. At this time, an understanding of the interrelationships among specific impairment categories is lacking. Lastly, we assert that the model could serve as the framework for developing a clinical predictor rule to aid clinicians by identifying the characteristics of patients who are most likely to respond favorably (or unfavorably) to specific treatment approaches.
CONCLUSIONS
We have presented an updated model of CAI that aims to both synthesize the current understanding of the causes of CAI and serve as a framework for the clinical assessment and rehabilitation of patients with LASs or CAI. The model describes how primary tissue injury to the lateral ankle ligaments after an acute ankle sprain may lead to a collection of interrelated pathomechanical, sensory-perceptual, and motor-behavioral impairments that influence a patient's clinical outcome. Using the biopsychosocial model of health care as a foundation, the concepts of self-organization and perception-action cycles, derived from dynamic systems theory, and a patient-specific neurosignature, stemming from the Melzack neuromatrix of pain theory, are incorporated to describe these interrelationships.
ACKNOWLEDGMENTS
We acknowledge Patrick McKeon, PhD, ATC, CSCS, of Ithaca College and the members of the Exercise and Sport Injury Laboratory at the University of Virginia for their feedback during the development of the conceptual model presented in this paper.
REFERENCES
- 1.Swenson DM, Collins CL, Fields SK, Comstock RD. Epidemiology of US high school sports-related ligamentous ankle injuries, 2005/06 to 2010/11. Clin J Sport Med. 2013;23(3):190–196. doi: 10.1097/JSM.0b013e31827d21fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roos KG, Kerr ZY, Mauntel TC, Djoko A, Dompier TP, Wikstrom EA. The epidemiology of lateral ligament complex ankle sprains in National Collegiate Athletic Association sports. Am J Sports Med. 2017;45(1):201–209. doi: 10.1177/0363546516660980. [DOI] [PubMed] [Google Scholar]
- 3.Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont PJ., Jr The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92(13):2279–2284. doi: 10.2106/JBJS.I.01537. [DOI] [PubMed] [Google Scholar]
- 4.Doherty C, Delahunt E, Caulfield B, Hertel J, Ryan J, Bleakley C. The incidence and prevalence of ankle sprain injury: a systematic review and meta-analysis of prospective epidemiological studies. Sports Med. 2014;44(1):123–140. doi: 10.1007/s40279-013-0102-5. [DOI] [PubMed] [Google Scholar]
- 5.Bridgman SA, Clement D, Downing A, Walley G, Phair I, Maffulli N. Population based epidemiology of ankle sprains attending accident and emergency units in the West Midlands of England, and a survey of UK practice for severe ankle sprains. Emerg Med J. 2003;20(6):508–510. doi: 10.1136/emj.20.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gribble PA, Bleakley CM, Caulfield BM, et al. 2016 consensus statement of the International Ankle Consortium: prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;50(24):1493–1495. doi: 10.1136/bjsports-2016-096188. [DOI] [PubMed] [Google Scholar]
- 7.Gribble PA, Bleakley CM, Caulfield BM, et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;50(24):1496–1505. doi: 10.1136/bjsports-2016-096189. [DOI] [PubMed] [Google Scholar]
- 8.Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J Orthop Sports Phys Ther. 2013;43(8):585–591. doi: 10.2519/jospt.2013.0303. [DOI] [PubMed] [Google Scholar]
- 9.Doherty C, Bleakley C, Hertel J, Caulfield B, Ryan J, Delahunt E. Recovery from a first-time lateral ankle sprain and the predictors of chronic ankle instability: a prospective cohort analysis. Am J Sports Med. 2016;44(4):995–1003. doi: 10.1177/0363546516628870. [DOI] [PubMed] [Google Scholar]
- 10.Freeman MA. Treatment of ruptures of the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47(4):661–668. [PubMed] [Google Scholar]
- 11.Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47(4):669–677. [PubMed] [Google Scholar]
- 12.Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47(4):678–685. [PubMed] [Google Scholar]
- 13.Tropp H, Ekstrand J, Gillquist J. Stabilometry in functional instability of the ankle and its value in predicting injury. Med Sci Sports Exerc. 1984;16(1):64–66. [PubMed] [Google Scholar]
- 14.Tropp H, Ekstrand J, Gillquist J. Factors affecting stabilometry recordings of single limb stance. Am J Sports Med. 1984;12(3):185–188. doi: 10.1177/036354658401200302. [DOI] [PubMed] [Google Scholar]
- 15.Tropp H, Odenrick P, Gillquist J. Stabilometry recordings in functional and mechanical instability of the ankle joint. Int J Sports Med. 1985;6(3):180–182. doi: 10.1055/s-2008-1025836. [DOI] [PubMed] [Google Scholar]
- 16.Tropp H. Pronator muscle weakness in functional instability of the ankle joint. Int J Sports Med. 1986;7(5):291–294. doi: 10.1055/s-2008-1025777. [DOI] [PubMed] [Google Scholar]
- 17.Tropp H, Odenrick P. Postural control in single-limb stance. J Orthop Res. 1988;6(6):833–839. doi: 10.1002/jor.1100060607. [DOI] [PubMed] [Google Scholar]
- 18.Hertel J. Functional instability following lateral ankle sprain. Sports Med. 2000;29(5):361–371. doi: 10.2165/00007256-200029050-00005. [DOI] [PubMed] [Google Scholar]
- 19.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 20.Hiller CE, Kilbreath SL, Refshauge KM. Chronic ankle instability: evolution of the model. J Athl Train. 2011;46(2):133–141. doi: 10.4085/1062-6050-46.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 22.Jull G. Biopsychosocial model of disease: 40 years on. Which way is the pendulum swinging? Br J Sports Med. 2017;51(16):1187–1188. doi: 10.1136/bjsports-2016-097362. [DOI] [PubMed] [Google Scholar]
- 23.Davids K, Glazier P, Araújo D, Bartlett R. Movement systems as dynamical systems: the functional role of variability and its implications for sports medicine. Sports Med. 2003;33(4):245–260. doi: 10.2165/00007256-200333040-00001. [DOI] [PubMed] [Google Scholar]
- 24.Glazier PS, Davids K. Constraints on the complete optimization of human motion. Sports Med. 2009;39(1):15–28. doi: 10.2165/00007256-200939010-00002. [DOI] [PubMed] [Google Scholar]
- 25.Wikstrom EA, Hubbard-Turner T, McKeon PO. Understanding and treating lateral ankle sprains and their consequences: a constraints-based approach. Sports Med. 2013;43(6):385–393. doi: 10.1007/s40279-013-0043-z. [DOI] [PubMed] [Google Scholar]
- 26.Balagué N, Torrents C, Hristovski R, Kelso JA. Sport science integration: an evolutionary synthesis. Eur J Sport Sci. 2017;17(1):51–62. doi: 10.1080/17461391.2016.1198422. [DOI] [PubMed] [Google Scholar]
- 27.Melzack R. Pain-an overview. Acta Anaesthesiol Scand. 1999;43(9):880–884. doi: 10.1034/j.1399-6576.1999.430903.x. [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001;65(12):1378–1382. [PubMed] [Google Scholar]
- 29.Mok KM, Fong DT, Krosshaug T, et al. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: 2 cases during the 2008 Beijing Olympics. Am J Sports Med. 2011;39(7):1548–1552. doi: 10.1177/0363546511399384. [DOI] [PubMed] [Google Scholar]
- 30.Fong DT, Ha SC, Mok KM, Chan CW, Chan KM. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: five cases from televised tennis competitions. Am J Sports Med. 2012;40(11):2627–2632. doi: 10.1177/0363546512458259. [DOI] [PubMed] [Google Scholar]
- 31.Hubbard TJ, Kaminski TW, Vander Griend RA, Kovaleski JE. Quantitative assessment of mechanical laxity in the functionally unstable ankle. Med Sci Sports Exerc. 2004;36(5):760–766. doi: 10.1249/01.mss.0000126604.85429.29. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard TJ. Ligament laxity following inversion injury with and without chronic ankle instability. Foot Ankle Int. 2008;29(3):305–311. doi: 10.3113/FAI.2008.0305. [DOI] [PubMed] [Google Scholar]
- 33.Hirai D, Docherty CL, Schrader J. Severity of functional and mechanical ankle instability in an active population. Foot Ankle Int. 2009;30(11):1071–1077. doi: 10.3113/FAI.2009.1071. [DOI] [PubMed] [Google Scholar]
- 34.Jung HG, Kim NR, Kim TH, Eom JS, Lee DO. Magnetic resonance imaging and stress radiography in chronic lateral ankle instability. Foot Ankle Int. 2017;38(6):621–626. doi: 10.1177/1071100717693207. [DOI] [PubMed] [Google Scholar]
- 35.Croy T, Saliba SA, Saliba E, Anderson MW, Hertel J. Differences in lateral ankle laxity measured via stress ultrasonography in individuals with chronic ankle instability, ankle sprain copers, and healthy individuals. J Orthop Sports Phys Ther. 2012;42(7):593–600. doi: 10.2519/jospt.2012.3923. [DOI] [PubMed] [Google Scholar]
- 36.Mizrahi DJ, Nazarian LN, Parker L. Evaluation of the anterior talofibular ligament via stress sonography in asymptomatic and symptomatic populations. J Ultrasound Med. 2018;37(8):1957–1963. doi: 10.1002/jum.14542. [DOI] [PubMed] [Google Scholar]
- 37.Phisitkul P, Chaichankul C, Sripongsai R, Prasitdamrong I, Tengtrakulcharoen P, Suarchawaratana S. Accuracy of anterolateral drawer test in lateral ankle instability: a cadaveric study. Foot Ankle Int. 2009;30(7):690–695. doi: 10.3113/FAI.2009.0690. [DOI] [PubMed] [Google Scholar]
- 38.Vaseenon T, Gao Y, Phisitkul P. Comparison of two manual tests for ankle laxity due to rupture of the lateral ankle ligaments. Iowa Orthop J. 2012;32:9–16. [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AG, Myers SH, Parks BG, Guyton GP. Anterolateral drawer versus anterior drawer test for ankle instability: a biomechanical model. Foot Ankle Int. 2016;37(4):407–410. doi: 10.1177/1071100715620854. [DOI] [PubMed] [Google Scholar]
- 40.Brown CN, Rosen AB, Ko J. Ankle ligament laxity and stiffness in chronic ankle instability. Foot Ankle Int. 2015;36(5):565–572. doi: 10.1177/1071100714561057. [DOI] [PubMed] [Google Scholar]
- 41.Bahr R, Pena F, Shine J, Lew WD, Engebretsen L. Ligament force and joint motion in the intact ankle: a cadaveric study. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):115–121. doi: 10.1007/s001670050083. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard TJ, Cordova M. Mechanical instability after an acute lateral ankle sprain. Arch Phys Med Rehabil. 2009;90(7):1142–1146. doi: 10.1016/j.apmr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Croy T, Saliba S, Saliba E, Anderson MW, Hertel J. Talofibular interval changes after acute ankle sprain: a stress ultrasonography study of ankle laxity. J Sport Rehabil. 2013;22(4):257–263. doi: 10.1123/jsr.22.4.257. [DOI] [PubMed] [Google Scholar]
- 44.van der Wees PJ, Lenssen AF, Hendriks EJ, Stomp DJ, Dekker J, de Bie RA. Effectiveness of exercise therapy and manual mobilisation in ankle sprain and functional instability: a systematic review. Aust J Physiother. 2006;52(1):27–37. doi: 10.1016/s0004-9514(06)70059-9. [DOI] [PubMed] [Google Scholar]
- 45.Terada M, Pietrosimone BG, Gribble PA. Therapeutic interventions for increasing ankle dorsiflexion after ankle sprain: a systematic review. J Athl Train. 2013;48(5):696–709. doi: 10.4085/1062-6050-48.4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser JJ, Feger MA, Hertel J. Midfoot and forefoot involvement in lateral ankle sprains and chronic ankle instability. Part 1: anatomy and biomechanics. Int J Sports Phys Ther. 2016;11(6):992–1005. [PMC free article] [PubMed] [Google Scholar]
- 47.Fraser JJ, Feger MA, Hertel J. Clinical commentary on midfoot and forefoot involvement in lateral ankle sprains and chronic ankle instability. Part 2: clinical considerations. Int J Sports Phys Ther. 2016;11(7):1191–1203. [PMC free article] [PubMed] [Google Scholar]
- 48.Green T, Refshauge K, Crosbie J, Adams R. A randomized controlled trial of a passive accessory joint mobilization on acute ankle inversion sprains. Phys Ther. 2001;81(4):984–994. [PubMed] [Google Scholar]
- 49.Wikstrom EA, Hubbard TJ. Talar positional fault in persons with chronic ankle instability. Arch Phys Med Rehabil. 2010;91(8):1267–1271. doi: 10.1016/j.apmr.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 50.Hubbard TJ, Hertel J, Sherbondy P. Fibular position in individuals with self-reported chronic ankle instability. J Orthop Sports Phys Ther. 2006;36(1):3–9. doi: 10.2519/jospt.2006.36.1.3. [DOI] [PubMed] [Google Scholar]
- 51.Hubbard TJ, Hertel J. Anterior positional fault of the fibula after sub-acute lateral ankle sprains. Man Ther. 2008;13(1):63–67. doi: 10.1016/j.math.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Valderrabano V, von Tscharner V, Nigg BM, et al. Lower leg muscle atrophy in ankle osteoarthritis. J Orthop Res. 2006;24(12):2159–2169. doi: 10.1002/jor.20261. [DOI] [PubMed] [Google Scholar]
- 53.Nüesch C, Valderrabano V, Huber C, von Tscharner V, Pagenstert G. Gait patterns of asymmetric ankle osteoarthritis patients. Clin Biomech (Bristol, Avon) 2012;27(6):613–618. doi: 10.1016/j.clinbiomech.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 54.Vertullo C. Unresolved lateral ankle pain. It's not always “just a sprain”. Aust Fam Physician. 2002;31(3):247–253. [PubMed] [Google Scholar]
- 55.Carbone A, Rodeo S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res. 2017;35(3):397–405. doi: 10.1002/jor.23341. [DOI] [PubMed] [Google Scholar]
- 56.Liu K, Gustavsen G, Royer T, Wikstrom EA, Glutting J, Kaminski TW. Increased ligament thickness in previously sprained ankles as measured by musculoskeletal ultrasound. J Athl Train. 2015;50(2):193–198. doi: 10.4085/1062-6050-49.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wikstrom EA, Song K, Tennant JN, Dederer KM, Paranjape C, Pietrosimone B. T1ρ MRI of the talar articular cartilage is increased in those with chronic ankle instability. Osteoarthritis Cartilage. 2019;27(4):646–649. doi: 10.1016/j.joca.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Golditz T, Steib S, Pfeifer K, et al. Functional ankle instability as a risk factor for osteoarthritis: using T2-mapping to analyze early cartilage degeneration in the ankle joint of young athletes. Osteoarthritis Cartilage. 2014;22(10):1377–1385. doi: 10.1016/j.joca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 59.Feger MA, Snell S, Handsfield GG, et al. Diminished foot and ankle muscle volumes in young adults with chronic ankle instability. Orthop J Sports Med. 2016;4(6):2325967116653719. doi: 10.1177/2325967116653719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munn J, Sullivan SJ, Schneiders AG. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Med Sport. 2010;13(1):2–12. doi: 10.1016/j.jsams.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 61.McKeon JM, McKeon PO. Evaluation of joint position recognition measurement variables associated with chronic ankle instability: a meta-analysis. J Athl Train. 2012;47(4):444–456. doi: 10.4085/1062-6050-47.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konradsen L, Voigt M. Inversion injury biomechanics in functional ankle instability: a cadaver study of simulated gait. Scand J Med Sci Sports. 2002;12(6):329–336. doi: 10.1034/j.1600-0838.2002.00108.x. [DOI] [PubMed] [Google Scholar]
- 63.Docherty CL, Arnold BL, Hurwitz S. Contralateral force sense deficits are related to the presence of functional ankle instability. J Orthop Res. 2006;24(7):1412–1419. doi: 10.1002/jor.20195. [DOI] [PubMed] [Google Scholar]
- 64.Arnold BL, Docherty CL. Low-load eversion force sense, self-reported ankle instability, and frequency of giving way. J Athl Train. 2006;41(3):233–238. [PMC free article] [PubMed] [Google Scholar]
- 65.Docherty CL, Arnold BL. Force sense deficits in functionally unstable ankles. J Orthop Res. 2008;26(11):1489–1493. doi: 10.1002/jor.20682. [DOI] [PubMed] [Google Scholar]
- 66.Wright CJ, Arnold BL. Fatigue's effect on eversion force sense in individuals with and without functional ankle instability. J Sport Rehabil. 2012;21(2):127–136. doi: 10.1123/jsr.21.2.127. [DOI] [PubMed] [Google Scholar]
- 67.Sousa ASP, Leite J, Costa B, Santos R. Bilateral proprioceptive evaluation in individuals with unilateral chronic ankle instability. J Athl Train. 2017;52(4):360–367. doi: 10.4085/1062-6050-52.2.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hagen M, Lemke M, Lahner M. Deficits in subtalar pronation and supination proprioception in subjects with chronic ankle instability. Hum Mov Sci. 2018;57:324–331. doi: 10.1016/j.humov.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Kim CY, Choi JD, Kim HD. No correlation between joint position sense and force sense for measuring ankle proprioception in subjects with healthy and functional ankle instability. Clin Biomech (Bristol, Avon) 2014;29(9):977–983. doi: 10.1016/j.clinbiomech.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Hoch MC, McKeon PO, Andreatta RD. Plantar vibrotactile detection deficits in adults with chronic ankle instability. Med Sci Sports Exerc. 2012;44(4):666–672. doi: 10.1249/MSS.0b013e3182390212. [DOI] [PubMed] [Google Scholar]
- 71.Powell MR, Powden CJ, Houston MN, Hoch MC. Plantar cutaneous sensitivity and balance in individuals with and without chronic ankle instability. Clin J Sport Med. 2014;24(6):490–496. doi: 10.1097/JSM.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 72.Burcal CJ, Wikstrom EA. Plantar cutaneous sensitivity with and without cognitive loading in people with chronic ankle instability, copers, and uninjured controls. J Orthop Sports Phys Ther. 2016;46(4):270–276. doi: 10.2519/jospt.2016.6351. [DOI] [PubMed] [Google Scholar]
- 73.Song K, Burcal CJ, Hertel J, Wikstrom EA. Increased visual use in chronic ankle instability: a meta-analysis. Med Sci Sports Exerc. 2016;48(10):2046–2056. doi: 10.1249/MSS.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 74.Song K, Kang TK, Wikstrom EA, Jun HP, Lee SY. Effects of reduced plantar cutaneous sensation on static postural control in individuals with and without chronic ankle instability. J Sci Med Sport. 2017;20(10):910–914. doi: 10.1016/j.jsams.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 75.Needle AR, Swanik CB, Schubert M, et al. Decoupling of laxity and cortical activation in functionally unstable ankles during joint loading. Eur J Appl Physiol. 2014;114(10):2129–2138. doi: 10.1007/s00421-014-2929-3. [DOI] [PubMed] [Google Scholar]
- 76.Doherty C, Bleakley C, Delahunt E, Holden S. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med. 2017;51(12):113–125. doi: 10.1136/bjsports-2016-096178. [DOI] [PubMed] [Google Scholar]
- 77.Hiller CE, Refshauge KM, Bundy AC, Herbert RD, Kilbreath SL. The Cumberland Ankle Instability Tool: a report of validity and reliability testing. Arch Phys Med Rehabil. 2006;87(9):1235–1241. doi: 10.1016/j.apmr.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 78.Simon J, Donahue M, Docherty C. Development of the Identification of Functional Ankle Instability (IdFAI) Foot Ankle Int. 2012;33(9):755–763. doi: 10.3113/FAI.2012.0755. [DOI] [PubMed] [Google Scholar]
- 79.Wright CJ, Arnold BL, Ross SE, Linens SW. Recalibration and validation of the Cumberland Ankle Instability Tool cutoff score for individuals with chronic ankle instability. Arch Phys Med Rehabil. 2014;95(10):1853–1859. doi: 10.1016/j.apmr.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 80.Houston MN, Van Lunen BL, Hoch MC. Health-related quality of life in individuals with chronic ankle instability. J Athl Train. 2014;49(6):758–763. doi: 10.4085/1062-6050-49.3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A. Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 82.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117(1–2):137–144. doi: 10.1016/j.pain.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 83.Hale SA, Hertel J. Reliability and sensitivity of the Foot and Ankle Disability Index in subjects with chronic ankle instability. J Athl Train. 2005;40(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 84.Carcia CR, Martin RL, Drouin JM. Validity of the Foot and Ankle Ability Measure in athletes with chronic ankle instability. J Athl Train. 2008;43(2):179–183. doi: 10.4085/1062-6050-43.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Houston MN, Hoch JM, Hoch MC. Patient-reported outcome measures in individuals with chronic ankle instability: a systematic review. J Athl Train. 2015;50(10):1019–1033. doi: 10.4085/1062-6050-50.9.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM) Foot Ankle Int. 2005;26(11):968–983. doi: 10.1177/107110070502601113. [DOI] [PubMed] [Google Scholar]
- 87.Arnold BL, Wright CJ, Ross SE. Functional ankle instability and health-related quality of life. J Athl Train. 2011;46(6):634–641. doi: 10.4085/1062-6050-46.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huffman GR, Park J, Roser-Jones C, Sennett BJ, Yagnik G, Webner D. Normative SF-36 values in competing NCAA intercollegiate athletes differ from values in the general population. J Bone Joint Surg Am. 2008;90(3):471–476. doi: 10.2106/JBJS.G.00325. [DOI] [PubMed] [Google Scholar]
- 89.Vela LI, Denegar C. Transient disablement in the physically active with musculoskeletal injuries, part I: a descriptive model. J Athl Train. 2010;45(6):615–629. doi: 10.4085/1062-6050-45.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vela LI, Denegar CR. The Disablement in the Physically Active Scale, part II: the psychometric properties of an outcomes scale for musculoskeletal injuries. J Athl Train. 2010;45(6):630–641. doi: 10.4085/1062-6050-45.6.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoch MC, McKeon PO. Peroneal reaction time after ankle sprain: a systematic review and meta-analysis. Med Sci Sports Exerc. 2014;46(3):546–556. doi: 10.1249/MSS.0b013e3182a6a93b. [DOI] [PubMed] [Google Scholar]
- 92.Palmieri-Smith RM, Hopkins JT, Brown TN. Peroneal activation deficits in persons with functional ankle instability. Am J Sports Med. 2009;37(5):982–988. doi: 10.1177/0363546508330147. [DOI] [PubMed] [Google Scholar]
- 93.Hopkins JT, Brown TN, Christensen L, Palmieri-Smith RM. Deficits in peroneal latency and electromechanical delay in patients with functional ankle instability. J Orthop Res. 2009;27(12):1541–1546. doi: 10.1002/jor.20934. [DOI] [PubMed] [Google Scholar]
- 94.Donahue MS, Docherty CL, Riley ZA. Decreased fibularis reflex response during inversion perturbations in FAI subjects. J Electromyogr Kinesiol. 2014;24(1):84–89. doi: 10.1016/j.jelekin.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 95.Hertel J. Sensorimotor deficits with ankle sprains and chronic instability. Clin Sports Med. 2008;27(3):353–370. doi: 10.1016/j.csm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 96.McVey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005;26(12):1055–1061. doi: 10.1177/107110070502601210. [DOI] [PubMed] [Google Scholar]
- 97.Bowker S, Terada M, Thomas AC, Pietrosimone BG, Hiller CE, Gribble PA. Neural excitability and joint laxity in chronic ankle instability, coper, and control groups. J Athl Train. 2016;51(4):336–343. doi: 10.4085/1062-6050-51.5.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim KM, Ingersoll CD, Hertel J. Altered postural modulation of Hoffmann reflex in the soleus and fibularis longus associated with chronic ankle instability. J Electromyogr Kinesiol. 2012;22(6):997–1002. doi: 10.1016/j.jelekin.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Sefton JM, Hicks-Little CA, Hubbard TJ, et al. Segmental spinal reflex adaptations associated with chronic ankle instability. Arch Phys Med Rehabil. 2008;89(10):1991–1995. doi: 10.1016/j.apmr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 100.Sedory EJ, McVey ED, Cross KM, Ingersoll CD, Hertel J. Arthrogenic muscle response of the quadriceps and hamstrings with chronic ankle instability. J Athl Train. 2007;42(3):355–360. [PMC free article] [PubMed] [Google Scholar]
- 101.Terada M, Kosik KB, McCann RS, Gribble PA. Diaphragm contractility in individuals with chronic ankle instability. Med Sci Sports Exerc. 2016;48(10):2040–2045. doi: 10.1249/MSS.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 102.Pietrosimone BG, Gribble PA. Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train. 2012;47(6):621–626. doi: 10.4085/1062-6050-47.6.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McLeod MM, Gribble PA, Pietrosimone BG. Chronic ankle instability and neural excitability of the lower extremity. J Athl Train. 2015;50(8):847–853. doi: 10.4085/1062-6050-50.4.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kosik KB, Terada M, Drinkard CP, McCann RS, Gribble PA. Potential corticomotor plasticity in those with and without chronic ankle instability. Med Sci Sports Exerc. 2017;49(1):141–149. doi: 10.1249/MSS.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 105.Terada M, Bowker S, Thomas AC, Pietrosimone B, Hiller CE, Gribble PA. Corticospinal excitability and inhibition of the soleus in individuals with chronic ankle instability. PM R. 2016;8(11):1090–1096. doi: 10.1016/j.pmrj.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 106.Needle AR, Palmer JA, Kesar TM, Binder-Macleod SA, Swanik CB. Brain regulation of muscle tone in healthy and functionally unstable ankles. J Sport Rehabil. 2013;22(3):202–211. doi: 10.1123/jsr.22.3.202. [DOI] [PubMed] [Google Scholar]
- 107.Harkey M, McLeod MM, Terada M, Gribble PA, Pietrosimone BG. Quadratic association between corticomotor and spinal-reflexive excitability and self-reported disability in participants with chronic ankle instability. J Sport Rehabil. 2016;25(2):137–145. doi: 10.1123/jsr.2014-0282. [DOI] [PubMed] [Google Scholar]
- 108.Fraser JJ, Koldenhoven RM, Jaffri AH, et al. Foot impairments contribute to functional limitation in individuals with ankle sprain and chronic ankle instability. Knee Surg Sports Traumatol Arthrosc. 2018 doi: 10.1007/s00167-018-5028-x. published online ahead of print July 6. [DOI] [PubMed]
- 109.Donnelly L, Donovan L, Hart JM, Hertel J. Eversion strength and surface electromyography measures with and without chronic ankle instability measured in 2 positions. Foot Ankle Int. 2017;38(7):769–778. doi: 10.1177/1071100717701231. [DOI] [PubMed] [Google Scholar]
- 110.Terrier R, Degache F, Fourchet F, Gojanovic B, Forestier N. Assessment of evertor weakness in patients with chronic ankle instability: functional versus isokinetic testing. Clin Biomech (Bristol, Avon) 2017;41:54–59. doi: 10.1016/j.clinbiomech.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Arnold BL, Linens SW, de la Motte SJ, Ross SE. Concentric evertor strength differences and functional ankle instability: a meta-analysis. J Athl Train. 2009;44(6):653–662. doi: 10.4085/1062-6050-44.6.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson C, Schabrun S, Romero R, Bialocerkowski A, van Dieen J, Marshall P. Factors contributing to chronic ankle instability: a systematic review and meta-analysis of systematic reviews. Sports Med. 2018;48(1):189–205. doi: 10.1007/s40279-017-0781-4. [DOI] [PubMed] [Google Scholar]
- 113.Wilkerson GB, Pinerola JJ, Caturano RW. Invertor vs. evertor peak torque and power deficiencies associated with lateral ankle ligament injury. J Orthop Sports Phys Ther. 1997;26(2):78–86. doi: 10.2519/jospt.1997.26.2.78. [DOI] [PubMed] [Google Scholar]
- 114.Cho BK, Park JK, Choi SM, Kang SW, SooHoo NF. The peroneal strength deficits in patients with chronic ankle instability compared to ankle sprain copers and normal individuals. Foot Ankle Surg. 2019;25(2):231–236. doi: 10.1016/j.fas.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 115.Hubbard TJ, Kramer LC, Denegar CR, Hertel J. Contributing factors to chronic ankle instability. Foot Ankle Int. 2007;28(3):343–354. doi: 10.3113/FAI.2007.0343. [DOI] [PubMed] [Google Scholar]
- 116.Gribble PA, Robinson RH. An examination of ankle, knee, and hip torque production in individuals with chronic ankle instability. J Strength Cond Res. 2009;23(2):395–400. doi: 10.1519/JSC.0b013e31818efbb2. [DOI] [PubMed] [Google Scholar]
- 117.Yildiz Y, Aydin T, Sekir U, Hazneci B, Komurcu M, Kalyon TA. Peak and end range eccentric evertor/concentric invertor muscle strength ratios in chronically unstable ankles: comparison with healthy individuals. J Sports Sci Med. 2003;2(3):70–76. [PMC free article] [PubMed] [Google Scholar]
- 118.David P, Halimi M, Mora I, Doutrellot PL, Petitjean M. Isokinetic testing of evertor and invertor muscles in patients with chronic ankle instability. J Appl Biomech. 2013;29(6):696–704. doi: 10.1123/jab.29.6.696. [DOI] [PubMed] [Google Scholar]
- 119.Munn J, Beard DJ, Refshauge KM, Lee RY. Eccentric muscle strength in functional ankle instability. Med Sci Sports Exerc. 2003;35(2):245–250. doi: 10.1249/01.MSS.0000048724.74659.9F. [DOI] [PubMed] [Google Scholar]
- 120.Fox J, Docherty CL, Schrader J, Applegate T. Eccentric plantar-flexor torque deficits in participants with functional ankle instability. J Athl Train. 2008;43(1):51–54. doi: 10.4085/1062-6050-43.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCann RS, Crossett ID, Terada M, Kosik KB, Bolding BA, Gribble PA. Hip strength and Star Excursion Balance Test deficits of patients with chronic ankle instability. J Sci Med Sport. 2017;20(11):992–996. doi: 10.1016/j.jsams.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 122.McCann RS, Bolding BA, Terada M, Kosik KB, Crossett ID, Gribble PA. Isometric hip strength and dynamic stability of individuals with chronic ankle instability. J Athl Train. 2018;53(7):672–678. doi: 10.4085/1062-6050-238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Negahban H, Moradi-Bousari A, Naghibi S, et al. The eccentric torque production capacity of the ankle, knee, and hip muscle groups in patients with unilateral chronic ankle instability. Asian J Sports Med. 2013;4(2):144–152. doi: 10.5812/asjsm.34515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKeon PO, Hertel J. Systematic review of postural control and lateral ankle instability, part I: can deficits be detected with instrumented testing? J Athl Train. 2008;43(3):293–304. doi: 10.4085/1062-6050-43.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gribble PA, Hertel J, Plisky P. Using the Star Excursion Balance Test to assess dynamic postural-control deficits and outcomes in lower extremity injury: a literature and systematic review. J Athl Train. 2012;47(3):339–357. doi: 10.4085/1062-6050-47.3.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Linens SW, Ross SE, Arnold BL, Gayle R, Pidcoe P. Postural-stability tests that identify individuals with chronic ankle instability. J Athl Train. 2014;49(1):15–23. doi: 10.4085/1062-6050-48.6.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Docherty CL, Valovich McLeod TC, Shultz SJ. Postural control deficits in participants with functional ankle instability as measured by the balance error scoring system. Clin J Sport Med. 2006;16(3):203–208. doi: 10.1097/00042752-200605000-00003. [DOI] [PubMed] [Google Scholar]
- 128.Arnold BL, De La Motte S, Linens S, Ross SE. Ankle instability is associated with balance impairments: a meta-analysis. Med Sci Sports Exerc. 2009;41(5):1048–1062. doi: 10.1249/MSS.0b013e318192d044. [DOI] [PubMed] [Google Scholar]
- 129.Gribble PA, Hertel J, Denegar CR. Chronic ankle instability and fatigue create proximal joint alterations during performance of the Star Excursion Balance Test. Int J Sports Med. 2007;28(3):236–242. doi: 10.1055/s-2006-924289. [DOI] [PubMed] [Google Scholar]
- 130.de la Motte S, Arnold BL, Ross SE. Trunk-rotation differences at maximal reach of the Star Excursion Balance Test in participants with chronic ankle instability. J Athl Train. 2015;50(4):358–365. doi: 10.4085/1062-6050-49.3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moisan G, Descarreaux M, Cantin V. Effects of chronic ankle instability on kinetics, kinematics and muscle activity during walking and running: a systematic review. Gait Posture. 2017;52:381–399. doi: 10.1016/j.gaitpost.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 132.Tavakoli S, Forghany S, Nester C. The effect of dual tasking on foot kinematics in people with functional ankle instability. Gait Posture. 2016;49:364–370. doi: 10.1016/j.gaitpost.2016.07.302. [DOI] [PubMed] [Google Scholar]
- 133.Feger MA, Donovan L, Hart JM, Hertel J. Lower extremity muscle activation in patients with or without chronic ankle instability during walking. J Athl Train. 2015;50(4):350–357. doi: 10.4085/1062-6050-50.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bigouette J, Simon J, Liu K, Docherty CL. Altered vertical ground reaction forces in participants with chronic ankle instability while running. J Athl Train. 2016;51(9):682–687. doi: 10.4085/1062-6050-51.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hamacher D, Hollander K, Zech A. Effects of ankle instability on running gait ankle angles and its variability in young adults. Clin Biomech (Bristol, Avon) 2016;33:73–78. doi: 10.1016/j.clinbiomech.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 136.McGrath D, Patterson M, Persson UM, Caulfield B. Frontal-plane variability in foot orientation during fatiguing running exercise in individuals with chronic ankle instability. J Athl Train. 2017;52(11):1019–1027. doi: 10.4085/1062-6050-52.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Terada M, Bowker S, Thomas AC, et al. Alterations in stride-to-stride variability during walking in individuals with chronic ankle instability. Hum Mov Sci. 2015;40:154–162. doi: 10.1016/j.humov.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 138.Herb CC, Chinn L, Dicharry J, McKeon PO, Hart JM, Hertel J. Shank-rearfoot joint coupling with chronic ankle instability. J Appl Biomech. 2014;30(3):366–372. doi: 10.1123/jab.2013-0085. [DOI] [PubMed] [Google Scholar]
- 139.Herb CC, Hertel J. Shank-rearfoot joint coupling in young adults with chronic ankle instability: a cross-correlation analysis. J Sports Med Phys Fitness. 2015;55(6):639–646. [PubMed] [Google Scholar]
- 140.Yen SC, Chui KK, Corkery MB, Allen EA, Cloonan CM. Hip-ankle coordination during gait in individuals with chronic ankle instability. Gait Posture. 2017;53:193–200. doi: 10.1016/j.gaitpost.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Lilley T, Herb CC, Hart J, Hertel J. Lower extremity joint coupling variability during gait in young adults with and without chronic ankle instability. Sports Biomech. 2018;17(2):261–272. doi: 10.1080/14763141.2017.1287215. [DOI] [PubMed] [Google Scholar]
- 142.Springer S, Gottlieb U. Effects of dual-task and walking speed on gait variability in people with chronic ankle instability: a cross-sectional study. BMC Musculoskelet Disord. 2017;18(1):316. doi: 10.1186/s12891-017-1675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koldenhoven RM, Feger MA, Fraser JJ, Hertel J. Variability in center of pressure position and muscle activation during walking with chronic ankle instability. J Electromyogr Kinesiol. 2018;38:155–161. doi: 10.1016/j.jelekin.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 144.Koldenhoven RM, Feger MA, Fraser JJ, Saliba S, Hertel J. Surface electromyography and plantar pressure during walking in young adults with chronic ankle instability. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1060–1070. doi: 10.1007/s00167-016-4015-3. [DOI] [PubMed] [Google Scholar]
- 145.Suda EY, Sacco IC. Altered leg muscle activity in volleyball players with functional ankle instability during a sideward lateral cutting movement. Phys Ther Sport. 2011;12(4):164–170. doi: 10.1016/j.ptsp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 146.Son SJ, Kim H, Seeley MK, Hopkins JT. Movement strategies among groups of chronic ankle instability, coper, and control. Med Sci Sports Exerc. 2017;49(8):1649–1661. doi: 10.1249/MSS.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 147.Koshino Y, Ishida T, Yamanaka M, et al. Kinematics and muscle activities of the lower limb during a side-cutting task in subjects with chronic ankle instability. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1071–1080. doi: 10.1007/s00167-015-3745-y. [DOI] [PubMed] [Google Scholar]
- 148.Dayakidis MK, Boudolos K. Ground reaction force data in functional ankle instability during two cutting movements. Clin Biomech (Bristol, Avon) 2006;21(4):405–411. doi: 10.1016/j.clinbiomech.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 149.Kim H, Son SJ, Seeley MK, Hopkins JT. Kinetic compensations due to chronic ankle instability during landing and jumping. Med Sci Sports Exerc. 2018;50(2):308–317. doi: 10.1249/MSS.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 150.Simpson JD, Stewart EM, Macias DM, Chander H, Knight AC. Individuals with chronic ankle instability exhibit dynamic postural stability deficits and altered unilateral landing biomechanics: a systematic review. Phys Ther Sport. 2018 doi: 10.1016/j.ptsp.2018.06.003. published online ahead of print June 13. [DOI] [PubMed]
- 151.Terada M, Pietrosimone B, Gribble PA. Individuals with chronic ankle instability exhibit altered landing knee kinematics: potential link with the mechanism of loading for the anterior cruciate ligament. Clin Biomech (Bristol, Avon) 2014;29(10):1125–1130. doi: 10.1016/j.clinbiomech.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 152.Herb CC, Grossman K, Feger MA, Donovan L, Hertel J. Lower extremity biomechanics during a drop-vertical jump in participants with or without chronic ankle instability. J Athl Train. 2018;53(4):364–371. doi: 10.4085/1062-6050-481-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Webster KA, Pietrosimone BG, Gribble PA. Muscle activation during landing before and after fatigue in individuals with or without chronic ankle instability. J Athl Train. 2016;51(8):629–636. doi: 10.4085/1062-6050-51.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hubbard-Turner T, Turner MJ. Physical activity levels in college students with chronic ankle instability. J Athl Train. 2015;50(7):742–747. doi: 10.4085/1062-6050-50.3.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Halasi T, Kynsburg A, Tállay A, Berkes I. Development of a new activity score for the evaluation of ankle instability. Am J Sports Med. 2004;32(4):899–908. doi: 10.1177/0363546503262181. [DOI] [PubMed] [Google Scholar]
- 156.Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43–49. [PubMed] [Google Scholar]
- 157.International Classification of Functioning, Disability and Health (ICF) World Health Organization Web site. 2019 www.who.int/classifications/icf/en/ Accessed January 17.
- 158.Snyder AR, Parsons JT, Valovich McLeod TC, Curtis Bay R, Michener LA, Sauers EL. Using disablement models and clinical outcomes assessment to enable evidence-based athletic training practice, part I: disablement models. J Athl Train. 2008;43(4):428–436. doi: 10.4085/1062-6050-43.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fuster JM. Prefrontal cortex and the bridging of temporal gaps in the perception-action cycle. Ann N Y Acad Sci. 1990;608:318–329. doi: 10.1111/j.1749-6632.1990.tb48901.x. [DOI] [PubMed] [Google Scholar]
- 160.Wikstrom EA, Brown CN. Minimum reporting standards for copers in chronic ankle instability research. Sports Med. 2014;44(2):251–268. doi: 10.1007/s40279-013-0111-4. [DOI] [PubMed] [Google Scholar]
- 161.Donovan L, Hertel J. A new paradigm for rehabilitation of patients with chronic ankle instability. Phys Sportsmed. 2012;40(4):41–51. doi: 10.3810/psm.2012.11.1987. [DOI] [PubMed] [Google Scholar]
- 162.Delahunt E, Bleakley CM, Bossard DS, et al. Clinical assessment of acute lateral ankle sprain injuries (ROAST): 2019 consensus statement and recommendations of the International Ankle Consortium. Br J Sports Med. 2018;52(20):1304–1310. doi: 10.1136/bjsports-2017-098885. [DOI] [PubMed] [Google Scholar]