Abstract
Background:
Organochlorine pesticides are associated with an increased risk of Parkinson’s disease. A preliminary analysis from the Honolulu-Asia Aging Study suggested that heptachlor epoxide, a metabolite from an organochlorine pesticide extensively used in Hawaii, may be especially important. This was a cross sectional analysis to evaluate the association of heptachlor epoxide and other organochlorine compounds with Lewy pathology in an expanded survey of brain organochlorine residues from the longitudinal Honolulu-Asia Aging Study.
Methods:
Organochlorines were measured in frozen occipital or temporal lobes in 705 brains using gas chromatography with mass spectrometry. Lewy pathology was identified using hematoxylin and eosin- and α-synuclein immunochemistry-stained sections from multiple brain regions.
Results:
The prevalence of Lewy pathology was nearly doubled in the presence versus the absence of heptachlor epoxide (30.1% versus 16.3%, P < 0.001). Although associations with other compounds were weaker, hexachlorobenzene (P = 0.003) and α-chlordane (P = 0.007) were also related to Lewy pathology. Most of the latter associations, however, were a result of confounding from heptachlor epoxide. Neither compound was significantly related to Lewy pathology after adjustment for heptachlor epoxide. In contrast, the association of heptachlor epoxide with Lewy pathology remained significant after adjustments for hexachlorobenzene (P = 0.013) or α-chlordane (P = 0.005). Findings were unchanged after removal of cases of PD and adjustment for age and other characteristics.
Conclusions:
Organochlorine pesticides are associated with the presence of Lewy pathology in the brain, even after exclusion of PD cases. Although most of the association is through heptachlor epoxide, the role of other organochlorine compounds is in need of clarification.
Keywords: epidemiology, heptachlor epoxide, Lewy pathology, organochlorine pesticide, Parkinson’s disease
Idiopathic Parkinson’s disease (PD) is pathologically characterized by neuronal loss in the substantia nigra and by the formation of Lewy bodies and Lewy neurites through deposition of α-synuclein in degenerating neurons. The presence of Lewy bodies in asymptomatic individuals, commonly referred to as incidental Lewy bodies, has long been considered a hallmark of pre-symptomatic PD. The frequency of incidental Lewy bodies is 5 to 20 times higher than overt clinical PD.1 Although the underlying mechanism leading to nigral neuronal loss and Lewy pathology is unknown, a number of pesticides including organochlorines have been implicated.2 Heptachlor epoxide, a metabolite of the organochlorine pesticide heptachlor, is one such compound that is of special interest in Hawaii. As a pesticide, heptachlor was heavily used by pineapple growers in Hawaii after World War II, with its epoxide metabolite finding its way into the dairy herds and milk supply at levels that were reported to be “extraordinarily high.”3
In a previous report from the Honolulu-Asia Aging Study (HAAS), combinations of heptachlor epoxide, methoxychlor, and hexachlorobenzene were associated with the presence of Lewy pathology; however, the association was not entirely convincing because of a limited sample size and statistical issues related to multiple testing involving several organochlorine pesticides. Given that dairy products have been linked to clinical PD,4,5 documentation of the contamination of the milk supply in Hawaii with heptachlor epoxide is both unique and important. Recent findings from the HAAS also suggest that milk consumption is associated with early nigral neurodegeneration in nonsmoking men without PD and that the relationship is partly attributed to the coexistence of brain residues of heptachlor epoxide.6 Based on an expanded survey of brain organochlorine residues in the HAAS, using larger amounts of brain tissue and improved analytical procedures, the purpose of this study was to better characterize the association of heptachlor epoxide with Lewy pathology.
Methods
Background and Study Sample
The HAAS was initiated in 1991 as a continuation of the Honolulu Heart Program (HHP), a longitudinal population-based cohort study of cardiovascular disease that began in 1965. At that time, the HHP enrolled a cohort of 8006 Japanese-American men aged 45–68 years living on the island of Oahu.7,8 The HAAS was developed at a time of increased focus on neurodegeneration, cognitive decline, and other conditions affecting the elderly.9 There were follow-up examinations performed approximately every 2 years as well as surveillance of hospital and death records. Standardized methods were used to identify all PD cases in the cohort beginning in 1991.10,11 The Institutional Review Board of Kuakini Medical Center, Honolulu, Hawaii, reviewed and approved the study, and written informed consent was obtained from all participants.
Coinciding with initiation of the HAAS was the launch of an autopsy study. Autopsy was discussed with HAAS participants at the time of each examination. Participants received counseling and were encouraged to discuss their wishes to take part in the autopsy program at the time of their deaths with their family members. For those who had expressed willingness, autopsy consent was sought from the closest living relative according to Hawaii state law.
Measurement of Heptachlor Epoxide and Other Organochlorine Residues
Brain organochlorine pesticides were measured in frozen occipital or temporal lobes in 2 overlapping sample sets. Preliminary feasibility work using 15 brains from highly exposed individuals demonstrated that organochlorine values from occipital and frontal lobes were nearly identical, suggesting that over time, levels of these compounds are distributed evenly throughout cortical tissues. Measurements included residues of heptachlor epoxide and other organochlorine compounds. The sample set from the initial study comprised frozen occipital lobes from 431 brains.12 A second sample set consisted of frozen temporal lobes from 677 brains, including 392 brains from the original study and 285 that were newly collected. After removing 11 decedents with missing data on Lewy pathology, the combined sample sets yielded 705 brains for which survey results were available on organochlorine pesticides. An organochlorine pesticide was considered present if found in either sample.
Increased aliquot size as well as some modification of the original analytic method12 resulted in improved sensitivity in the second sample set. An aliquot (0.1 to 0.2 g) of frozen temporal lobe tissue was weighed and transferred to a conical vial. Sodium sulfate and an internal standard solution containing 13C-labeled analogues of all the target analytes were added, and the sample was homogenized 3 times with hexane. The combined extracts were brought to 10 mL with hexane, and 1.0 mL was removed for lipid determination. The concentrated extracts were cleaned and fractionated using partially deactivated Florisil as described previously, using an automated solid-phase extraction system (Gilson GX-271, Middleton, WI). Collected fractions were concentrated to 0.2 mL and placed in autosampler vials. Samples were analyzed by gas chromatography with mass selective detection (Agilent 7890–5977, Santa Clara, CA) using large-volume injection to increase sensitivity. Lipids were determined gravimetrically following evaporation of solvent from the lipid aliquot. The lipid concentration was reported as percentage of sample mass. Organochlorine concentrations were reported in parts per billion (ng/g). An organochlorine was considered not present if reported to be “not detected” or “detected at less than the level of calibration.”12
Determination of Lewy Pathology
Standardized gross and microscopic assessments were performed as described previously.13–15 This included examination of all brains for Lewy bodies within hematoxylin and eosin-stained single sections of the substantia nigra and locus coeruleus. Immunohistochemical staining for α-synuclein of multiple limbic and neocortical regions was performed on those with Lewy bodies present in the substantia nigra or locus coeruleus according to published criteria.16 For a subset of 228 brains, modified Braak staging was performed with α-synuclein-stained sections to assess the distribution of pathology within the anatomic loci, which included the olfactory bulb, medulla, pons, midbrain, hippocampus, amygdala, striatum at the level of the nucleus accumbens, basal forebrain, and 9 cortical regions (anterior cingulate gyrus, anterior temporal mesocortex, entorhinal, insula, mid frontal, anterior superior, and mid temporal, inferior parietal, calcarine, and superior pre- and postcentral gyri).13 From each brain region, at least 2 foci from the original Braak staging description were evaluated: olfactory bulb and dorsal motor nucleus of the vagus (stage 1); pontine raphe nucleus and locus coeruleus (stage 2); substantia nigra pars compacta and nucleus basalis of Meynert (stage 3); basolateral nuclear complex of the amygdala, CA2/3 region of Ammon’s horn, anterotemporal mesocortex, and transentorhinal cortex (stage 4); insula, anterior cingulate (stage 5); and motor, primary sensory, and middle temporal cortices (stage 6).17 Semiquantitative pathology density assessment was carried out for each focus examined, with a score of 0–3 assigned, regardless of Lewy pathology morphology, with a score of 0 representing no pathological aggregates, 1 some, 2 moderate, and 3 numerous pathological aggregates, as suggested previously.18 For this analysis, the presence of Lewy bodies or Lewy neurites in any region was considered positive for the presence of Lewy pathology. Diagnosis of PD was based on clinical criteria as described elsewhere11 and pathologically confirmed by the presence of Lewy pathology.
Other Characteristics Measured During Life
To help to identify an independent association between heptachlor epoxide and other organochlorine residues with prevalent Lewy pathology, adjustments were made for characteristics measured during life that are commonly correlated with PD. Characteristics included midlife cigarette smoking in pack-years and daily coffee intake. Data on smoking and coffee intake were collected at the baseline examination of the Honolulu Heart Program (1965–1968) as markers of a life-time of exposure to these factors.11 Late-life coffee intake was not collected at initiation of the HAAS (1991–1993) and late-life cigarette smoking was too uncommon to be a reliable measure of a lifetime of exposure. Coffee consumption was measured by a dietitian using 24-hour dietary recall methods with validation based on 7-day food records in a subset of the cohort.19 As a measure of cognitive function, adjustments were made for performance on the Cognitive Abilities Screening Instrument (CASI). Scores range from 0 to 100, with high scores indicating better cognitive function than low scores.20 As a possible depot for lipid-soluble neurotoxins, adjustments for body fat were based on the body mass index (kg/m2).21 Adjustments were also made for age at death.
Statistical Methods
To identify features that might indicate that the autopsied sample was less than typical, study characteristics were compared between decedents with and without an autopsy using 2-sample t tests. Similar methods were used to compare average characteristics between decedents with and without Lewy pathology. Frequency of Lewy pathology was compared between those with and without measurable levels of an organochlorine compound using standard chi-square tests of association. When samples were small, comparisons were based on Fisher’s exact test.
To help to determine if an association between heptachlor epoxide and Lewy pathology was real or could be explained by associations with other organochlorine compounds, adjustments were made for the other compounds (singly or in combination) using logistic regression models. Here, Lewy pathology was modeled as a dependent variable (presence versus absence), whereas heptachlor epoxide and the other compounds were modeled as predictor variables (measurable versus non-measurable). In the event that combinations of Lewy pathology and measurable levels of an organochlorine compound resulted in a small sample size, logistic regression estimation and inference were based on exact testing methods.22 Further adjustments were made for age at death, pack-years of cigarette smoking, coffee intake, body mass index, and performance on the CASI. Analyses were repeated after removing cases of PD. All reported P values were based on 2-sided tests of significance.
Results
There were 705 available brains screened for Lewy pathology and heptachlor epoxide and other organochlorine compounds. Among the sample, 35 decedents had a diagnosis of PD with pathological confirmation by the presence of Lewy pathology. In those without PD (670), incidental Lewy pathology was present in 135 decedent brains (20.2%). Table 1 compares the average characteristics for the autopsied and nonautopsied deceased participants. Other than age, there were no significant differences between the groups. On average, autopsied men were slightly more than 6 months older than nonautopsied men (P < 0.001).
TABLE 1.
Average characteristics in decedents with and without an autopsy
| Characteristic | Autopsied (n = 705) |
Not autopsied (n = 2981) |
|---|---|---|
| Age at death (years)b | 88.2 ± 5.3a | 87.5 ± 5.4 |
| Cigarette smoking (pack-years) | 26.8 ± 28.4 | 27.9 ± 27.9 |
| Coffee intake (oz/day) | 13.7 ± 13.2 | 13.6 ± 12.6 |
| Body mass index (kg/m2) | 24.1 ± 2.9 | 23.9 ± 3.0 |
| Cognitive Abilities Screening Instrument |
82.1 ± 16.3 | 82.6 ± 14.8 |
Mean ± standard deviation
Autopsied decedents are significantly older than decedents without an autopsy (P < 0.001).
Note: Cigarette smoking data are missing for 2 decedents with an autopsy and 26 decedents without an autopsy. Data on body mass index are missing for 2 decedents without an autopsy. Data on the Cognitive Abilities Screening Instrument are missing for 44 decedents with an autopsy and 707 decedents without an autopsy.
Table 2 provides further comparisons of the study characteristics in Table 1 between the autopsied decedents with and without Lewy pathology. Among the characteristics, only CASI was significantly different between the groups. Here, CASI score was lower in the presence versus the absence of Lewy pathology (79.6 vs 82.9; P = 0.027).
TABLE 2.
Average characteristics in autopsied decedents with and without Lewy pathology
| Lewy pathology | ||
|---|---|---|
| Characteristic | Absent (n = 535) |
Present (n = 170) |
| Age at death (years) | 88.4 ± 5.4a | 87.6 ± 5.0 |
| Cigarette smoking (pack-years) | 26.9 ± 27.9 | 26.6 ± 29.9 |
| Coffee intake (oz/day) | 14.0 ± 13.4 | 12.7 ± 12.6 |
| Body mass index (kg/m2) | 24.0 ± 3.0 | 24.3 ± 2.6 |
| Cognitive Abilities Screening Instrumentb |
82.9 ± 15.8 | 79.6 ± 17.6 |
Mean ± standard deviation.
Performance on the Cognitive Abilities Screening Instrument is significantly lower in the presence versus the absence of Lewy pathology (P = 0.027).
Note: Cigarette smoking data are missing for 2 decedents without Lewy pathology. Data on the Cognitive Abilities Screening Instrument are missing for 35 decedents without Lewy pathology and 9 decedents with Lewy pathology.
The percentage of brains with Lewy pathology by the presence versus absence of measurable levels of heptachlor epoxide and 15 other organochlorine compounds is shown in Table 3. Among the compounds, only 3 had a significant relationship with Lewy pathology. The percent of brains with Lewy pathology was significantly higher in the presence versus absence of heptachlor epoxide (30.1% vs 16.3%; P < 0.001), hexachlorobenzene (27.7 vs 17.8%, P = 0.003), and a-chlordane (33.3% vs 22.1%, P = 0.007). Relationships with other compounds were less clear and not significant.
TABLE 3.
Percentage of decedents with Lewy pathology by the presence and absence of measurable levels of an organochlorine compound
| Measurable levels of anorganochlorine compound |
||||
|---|---|---|---|---|
| Organochlorine | Absent | Present | Relative odds | P |
| Heptachlor epoxide | 16.3 (50/306)a | 30.1 (120/399) | 2.2 (1.5–3.2)b | < 0.001 |
| Hexachlorobenzene | 17.8 (45/253) | 27.7 (125/452) | 1.8 (1.2–2.6) | 0.003 |
| α-Chlordane | 22.1 (127/576) | 33.3 (43/129) | 1.8 (1.2–2.7) | 0.007 |
| t-Nonachlor | 11.8 (4/34) | 24.7 (166/671) | 2.5 (0.9–7.1) | 0.085 |
| 4,4′-DDD | 23.6 (161/681) | 37.5 (9/24) | 1.9 (0.8–4.5) | 0.119 |
| Endrin | 24.6 (165/670) | 14.3 (5/35) | 0.5 (0.2–1.3) | 0.163 |
| Lindane | 23.7 (139/586) | 29.6 (24/81) | 1.4 (0.8–2.3) | 0.246 |
| γ-Chlordane | 23.0 (97/422) | 26.1 (69/264) | 1.2 (0.8–1.7) | 0.349 |
| Methoxychlor | 23.8 (161/678) | 33.3 (5/15) | 1.6 (0.4–5.2) | 0.370 |
| Dieldrin | 23.1 (72/312) | 24.9 (98/393) | 1.1 (0.8–1.6) | 0.567 |
| β-BHC | 23.8 (149/625) | 26.3 (21/80) | 1.1 (0.7–1.9) | 0.635 |
| Oxychlordane | 24.4 (142/581) | 22.6 (28/124) | 0.9 (0.6–1.4) | 0.660 |
| 4,4′-DDE | 22.5 (9/40) | 24.2 (161/665) | 1.1 (0.5–2.4) | 0.806 |
| Endosulfan 1 | 24.1 (161/667) | 23.7 (9/38) | 1.0 (0.5–2.1) | 0.943 |
| Mirex | 24.1 (114/473) | 24.1 (56/232) | 1.0 (0.7–1.4) | 0.992 |
| α-BHC | 24.1 (163/676) | 24.1 (7/29) | 1.0 (0.4–2.4) | 0.998 |
Number with Lewy pathology/sample size.
95% confidence interval.
Note: Data on methoxychlor are missing for 8 decedents without Lewy pathology and 4 decedents with Lewy pathology. Data on γ-chlordane are missing for 15 decedents without Lewy pathology and 4 decedents with Lewy pathology. Data on lindane are missing for 31 decedents without Lewy pathology and 7 decedents with Lewy pathology.
Among the organochlorine compounds in Table 3, only the association between heptachlor epoxide and Lewy pathology is left unexplained by covariates measured during life or the presence of any of the other compounds. Excluding PD cases did not change the results. As an example (Table 4), after adjusting for hexachlorobenzene and α-chlordane, the relative odds of Lewy pathology (ranging from 1.7 to 2.0) remained significant in the presence versus the absence of heptachlor epoxide. Without adjustment (Table 3), the corresponding relative odds was 2.2. In contrast, associations involving hexachlorobenzene and α-chlordane were no longer significant after adjusting for heptachlor epoxide. Although not significant, the elevated odds of Lewy pathology (ranging from a 1.2- to 1.6-fold excess) in the presence versus the absence hexachlorobenzene or a-chlordane makes it difficult to rule out an association with these latter compounds.
TABLE 4.
Relative odds of Lewy pathology in the presence versus the absence of heptachlor epoxide, hexachlorobenzene, and α-chlordane with adjustment for the presence of each of the other organochlorine compounds
| Compound adjustment | Without other adjustments | P | Covariate adjusteda | P |
|---|---|---|---|---|
| Heptachlor epoxide | ||||
| Hexachlorobenzene | 2.0 (1.3–3.0)b | 0.001 | 1.8 (1.1–2.8) | 0.013 |
| α-Chlordane | 2.0 (1.4–3.0) | <0.001 | 1.8 (1.2–2.8) | 0.005 |
| Hexachlorobenzene | ||||
| Heptachlor epoxide | 1.3 (0.9–2.0) | 0.225 | 1.2 (0.8–2.0) | 0.421 |
| α-Chlordane | 1.6 (1.1–2.4) | 0.016 | 1.5 (1.0–2.3) | 0.076 |
| α-Chlordane | ||||
| Heptachlor epoxide | 1.3 (0.8–2.0) | 0.224 | 1.2 (0.8–2.0) | 0.380 |
| Hexachlorobenzene | 1.6 (1.0–2.4) | 0.041 | 1.4 (0.9–2.2) | 0.180 |
| Excluding cases of PD | ||||
| Heptachlor epoxide | ||||
| Hexachlorobenzene | 1.9 (1.2–2.9) | 0.006 | 1.7 (1.1–2.8) | 0.031 |
| α-Chlordane | 1.9 (1.3–3.0) | 0.003 | 1.8 (1.1–2.9) | 0.013 |
| Hexachlorobenzene | ||||
| Heptachlor epoxide | 1.3 (0.8–2.1) | 0.247 | 1.3 (0.7–2.1) | 0.398 |
| α-Chlordane | 1.6 (1.0–2.5) | 0.029 | 1.5 (0.9–2.5) | 0.087 |
| α-Chlordane | ||||
| Heptachlor epoxide | 1.3 (0.8–2.1) | 0.250 | 1.2 (0.7–2.1) | 0.433 |
| Hexachlorobenzene | 1.6 (1.0–2.5) | 0.060 | 1.4 (0.8–2.3) | 0.229 |
Covariates include age at death, pack-years of cigarette smoking, coffee intake, body mass index, and performance on the Cognitive Abilities Screening Instrument.
95% Confidence interval.
The importance of heptachlor epoxide is further illustrated in Figure 1, especially after adjusting for covariates measured during life. The right side of the left panel shows that in the absence of brain residues of heptachlor epoxide, hexachlorobenzene, and α-chlordane, the prevalence of Lewy pathology was 15.9%. In the presence of either hexachlorobenzene or α-chlordane (without brain residues of heptachlor epoxide), the prevalence was 20.3%. In decedents with brain residues of heptachlor epoxide alone, Lewy pathology prevalence increased to 29.2%. With the addition of either hexa-chlorobenzene or α-chlordane to brains already containing residues of heptachlor epoxide, the increase in Lewy pathology prevalence was negligible (from 29.2% to 29.5%). Removing the 35 cases of PD only had a modest effect on this pattern.
FIG. 1.
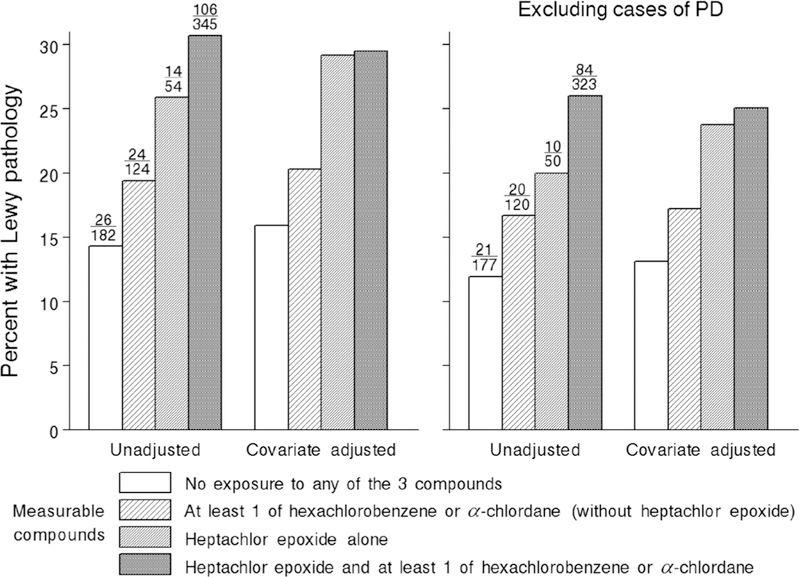
Percentage of decedents with Lewy pathology by detectable levels of heptachlor epoxide, hexachlorobenzene, and α-chlordane before and after removing cases of PD. Numbers above the unadjusted bars are the decedents with Lewy pathology/sample size. Adjusted percentages are adjusted for age at death, pack-years of cigarette smoking, coffee intake, body mass index, and performance on the Cognitive Abilities Screening Instrument.
Discussion
Findings confirmed that heptachlor epoxie is significantly associated with Lewy pathology. The association remained strong after adjusting for other organochlorine compounds and characteristics that exist during life and after removing cases of PD. Hexachlorobenzene and α-chlordane were also associated with Lewy pathology, but their relationships were largely explained by co-occurrence with heptachlor epoxide. Regardless, in the presence of a positive but weaker relationship with Lewy pathology, associations with hexachlorobenzene and α-chlordane cannot be excluded. Indeed, the co-occurrence of organochlorine pesticides in the environment was pervasive. In the current sample of 705 brains, 108 contained residues of all 3 compounds, 241 contained residues of 2 of the compounds, 54 contained residues of heptachlor epoxide alone, 117 contained residues of hexachlorobenzene alone, and 3 contained residues of α-chlordane alone. In an effort to determine the relative importance of each compound individually, analyses were conducted to examine independent relationships with Lewy pathology. With only 3 brains containing α-chlordane alone, an independent relationship with Lewy pathology could not be assessed. Nevertheless, its coexistence with other organochlorine residues could be important, although its presence was not necessary to observe an independent effect involving heptachlor epoxide. Figure 1 illustrates this further, as the percentage of decedents with Lewy pathology was nearly maximized in the brains containing heptachlor epoxide alone.
The evidence in support of a distinct association between heptachlor epoxide and Lewy pathology in Hawaii is especially noteworthy. Heptachlor epoxide is an oxidative metabolite of heptachlor, an insecticide that was used extensively by the Hawaiian pineapple industry and as a termiticide until it was banned in 1988. Hawaii is especially sensitive to groundwater contamination from agricultural pesticides because of the high permeability of the volcanic soil.23 An additional source of potential exposure on the island of Oahu occurred in 1982, when the commercial milk supply on the island of Oahu was found to be contaminated with excessively high levels of heptachlor epoxide. The contamination was traced to the use of chopped pineapple leaves from the plantations where heptachlor was in use to create a “wholesome” diet for the Hawaiian dairy herd.3,24 Earlier reports from the HAAS found a higher risk of clinical PD and lower substantia nigra neuron density at death in nonsmokers who were heavy consumers of milk.5,6 Heptachlor epoxide was also more commonly found in the brains of heavy milk consumers than in nondrinkers.6
In addition to heptachlor epoxide, α-chlordane, and hexachlorobenzene were also important in Hawaiian agriculture. α-Chlordane (cis-chlordane) is a major component of technical chlordane, a chlorinated cyclo-diene termiticide commonly used in Hawaii between 1948 and 1983. Chlordane contains lesser amounts of other distinct compounds as by-products, including heptachlor and trans-nonachlor, so that chlordane and heptachlor have properties that are similar.25 Hexa- chlorobenzene, a fungicide, was widely used to coat seeds. Both heptachlor and chlordane and their metabolites have been associated with cognitive decline and certain cancers including breast, prostate, and testicular cancer.26–28
Although the associations of hexachlorobenzene and α-chlordane with Lewy pathology were weaker than they were for heptachlor epoxide, a relationship with early PD neuropathology remains a possibility. This is also true for other organochlorine compounds considered in this report. Organochlorine compounds are measurable in blood and tissue and persist for many years owing to very long half-lives and lipophilic properties.23 Earlier studies with small numbers of cases found that PD brains were more likely to have detectable levels of dieldrin29 and higher mean concentrations of lindane and dieldrin30 than normal brains or brains with Alzheimer’s disease (AD) pathology. In a recent small case-control study of serum organochlorines and PD, β-hexachlorocyclohexane was more often detected in PD cases versus normal and AD controls.31 In a larger follow-up study, the odds of PD was significantly elevated in subjects with β-hexachlorocyclohexane levels above the detectable interquartile range.32 In another case-control study, using prospectively collected serum, a higher mean concentration of only dieldrin of 5 analyzed organochlorine pesticides was associated with a higher odds of PD.33
Epidemiological studies have also reported associations between self-reported pesticide exposures and clinical PD.34–39 Professional use of organochlorines was associated with more than twice the odds of PD compared with those not exposed.36 Use of 2,4-dichlorophenoxyacetic acid, a constituent of the herbicide agent orange, increased the odds of PD in a large case-control study.39 Exposure to pesticides through work on a pineapple or sugarcane plantation has also been shown to increase the risk of PD in the HAAS.40
Heptachlor belongs to the cyclodiene class of organo-chlorine compounds that includes dieldrin. Much of the work examining the neurotoxicity of these compounds has been performed in cell cultures and animal studies.41,42 Studies have documented selective dopaminergic neuron toxicity and diminished brain dopamine levels in animals exposed to dieldrin. Selective vulnerability of nigral neurons to these compounds may be explained by studies documenting disruption of dopamine transport by heptachlor epoxide and dieldrin. In addition, these compounds have been shown to enhance processes implicated in the mechanisms of nigral cell death in PD, namely, oxidative stress, apoptosis, and mitochondrial dysfunction. Especially relevant to this report is a single study demonstrating that dieldrin can promote formation of α-synuclein intracellular inclusions in dopamine neurons in rat mesencephalic cells overexpressing α-synuclein.43
Generalization of HAAS findings to the larger US population is limited by the all-male Japanese American cohort. However, rates and risk factors for PD in the HAAS are similar to those in studies of other ethnic groups that also include women. An association of organochlorine exposure and PD risk has been reported in numerous other populations as well.36,37,39 An alternative explanation of the association of organochlorines and Lewy pathology is the possibility that processes associated with α-synuclein deposition could also impede the clearance of organochlorine compounds from the brain. This is deemed less likely because exposure to these compounds occurred approximately 30 years prior to death.
Altogether, the evidence is persuasive that certain organochlorine pesticides may contribute to the cause of PD. In particular, heptachlor epoxide may be especially important in increasing the accumulation of α-synuclein deposits in the brain in the form of Lewy pathology and in lowering substantia nigra neuron density.6 Although further validation is needed, it is important to avoid exposure to suspected compounds, especially heptachlor epoxide in the instances when it continues to be used, most commonly in developing countries.44 Unfortunately, because of their environmental persistence, it may take years before existing legislative actions banning or regulating these highly pervasive neurotoxic substances will have an effect on reducing the frequency and burden of PD.
Acknowledgments:
We thank the many volunteers and families who have participated in the Honolulu Heart Program and Honolulu-Asia Aging Study. The information here does not necessarily reflect the position or the policy of the US government, and no official endorsement should be inferred.
Funding agencies: This work was supported by the United States Department of the Army, USAMRMC, grant WX81XWH-13–1-0085; United States Department of the Army, grant DAMD17–98-1–8621; National Institutes of Health: National Institute of Neurological Disorders and Stroke grant 5 R01 NS041265; National Institute on Aging grants 1 U01 AG19349 and 5 R01 AG017155; and the Office of Research and Development, Department of Veterans Affairs. This research was also supported in part by the Intramural Research Program of the NIH, National Institute on Aging, National Institutes of Health. Brain Pool Program (grant 2018HID3A2065629), National Research Foundation of Korea.
Footnotes
Relevant conflicts of interest/financial disclosures: None.
References
- 1.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 2008; 115(4):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman SM. Environmental toxins and Parkinson’s disease. Annu Rev Pharmacol Toxicol 2014;54:141–164. [DOI] [PubMed] [Google Scholar]
- 3.Smith RJ. Hawaiian milk contamination creates alarm. A sour response by state regulators. Science 1982;217(4555):137–140. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Diet and Parkinson’s disease: a potential role of dairy products in men. Ann Neurol 2002;52(6):793–801. [DOI] [PubMed] [Google Scholar]
- 5.Park M, Ross GW, Petrovitch H, et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology 2005;64(6):1047–1051. [DOI] [PubMed] [Google Scholar]
- 6.Abbott RD, Ross GW, Petrovitch H, et al. Midlife milk consumption and substantia nigra neuron density at death. Neurology 2016; 86(6):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: introduction. Am J Epidemiol 1975;102(6):477–480. [DOI] [PubMed] [Google Scholar]
- 8.Worth RM, Kagan A. Acertainment of men of Japanese ancestry in Hawaii through World War II selective service registration. J Chron Dis 1970;23:389–397. [DOI] [PubMed] [Google Scholar]
- 9.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia Aging Study. JAMA 1996;276(12):955–960. [PubMed] [Google Scholar]
- 10.Morens DM, Davis JW, Grandinetti A, Ross GW, Popper JS, White LR. Epidemiologic observations on Parkinson’s disease: Incidence and mortality in a prospective study of middle-aged men. Neurology 1996;46:1044–1050. [DOI] [PubMed] [Google Scholar]
- 11.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 2000; 283(20):2674–2679. [DOI] [PubMed] [Google Scholar]
- 12.Ross GW, Duda JE, Abbott RD, et al. Brain organochlorines and Lewy pathology: the Honolulu-Asia Aging Study. Mov Disord 2012;27(11):1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milber JM, Noorigian JV, Morley JF, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology 2012;79(24):2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrovitch H, White LR, Ross GW, et al. Accuracy of clinical criteria for AD in the Honolulu-Asia Aging Study, a population-based study. Neurology 2001;57(2):226–234. [DOI] [PubMed] [Google Scholar]
- 15.Ross GW, Petrovitch H, Abbott RD, et al. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol 2004;56(4):532–539. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 2004;318(1):121–134. [DOI] [PubMed] [Google Scholar]
- 18.Alafuzoff I IP, Arzberger T, Al-Sarraj S, et al. Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol 2009;117(6):635–652. [DOI] [PubMed] [Google Scholar]
- 19.McGee D, Rhoads G, Hankin J, Yano K, Tillotson J. Within-person variability of nutrient intake in a group of Hawaiian men of Japanese ancestry. Am J Clin Nutr 1982;36:657–663. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 1994;6(1):45–58. [DOI] [PubMed] [Google Scholar]
- 21.Abbott RD, Ross GW, White LR, et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology 2002;59(7):1051–1057. [DOI] [PubMed] [Google Scholar]
- 22.Mehta CR, Patel NR. Exact logistic regression: theory and examples. Stat Med 1995;14(19):2143–2160. [DOI] [PubMed] [Google Scholar]
- 23.Allen RH, Gottlieb M, Clute E, Pongsiri MJ, Sherman J, Obrams GI. Breast cancer and pesticides in Hawaii: the need for further study. Environ.Health Perspect 1997;105(Suppl 3):679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker DB, Loo S, Barker J. Evaluation of human exposure to the heptachlor epoxide contamination of milk in Hawaii. Hawaii Med J 1991;50(3):108–112, 118. [PubMed] [Google Scholar]
- 25.Registry AfTSaD May, 1994;Pages. Accessed at U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/toxprofiles/tp31.pdf. [Google Scholar]
- 26.Kim SA, Lee YM, Lee HW, Jacobs DR Jr, Lee DH. Greater cognitive decline with aging among elders with high serum concentrations of organochlorine pesticides. PLoS.One 2015;10(6):e0130623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst 2008; 100(9):663–671. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal NR. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults. Environ Health Perspect 2010;118(1):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol 1994;36(1):100–103. [DOI] [PubMed] [Google Scholar]
- 30.Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organ-ochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A 2000;59(4):229–234. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JR, Shalat SL, Buckley B, et al. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol 2009;66(7): 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson JR, Roy A, Shalat SL, et al. β-Hexachlorocyclohexane levels in serum and risk of Parkinson’s disease. Neurotoxicology 2011;32(5):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisskopf MG, Knekt P, O’Reilly EJ, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology 2010;74(13):1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol 2006;60(2):197–203. [DOI] [PubMed] [Google Scholar]
- 35.Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol 2009;169(8):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elbaz A, Clavel J, Rathouz PJ, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol 2009;66(4):494–504. [DOI] [PubMed] [Google Scholar]
- 37.Kamel F, Tanner C, Umbach D, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol 2007;165(4):364–374. [DOI] [PubMed] [Google Scholar]
- 38.Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 2011;119(6):866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanner CM, Ross GW, Jewell SA, et al. Occupation and risk of parkinsonism: a multicenter case-control study. Arch Neurol 2009; 66(9):1106–1113. [DOI] [PubMed] [Google Scholar]
- 40.Petrovitch H, Ross GW, Abbott RD, et al. Plantation work and risk of Parkinson disease in a population-based longitudinal study. Arch Neurol 2002;59(11):1787–1792. [DOI] [PubMed] [Google Scholar]
- 41.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends Pharmacol Sci 2008;29(6): 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson’s disease pathogenesis. Neurotoxicology 2005;26(4):701–719. [DOI] [PubMed] [Google Scholar]
- 43.Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther 2005;315(1):69–79. [DOI] [PubMed] [Google Scholar]
- 44.Furlong M, Tanner CM, Goldman SM, et al. Protective glove use and hygiene habits modify the associations of specific pesticides with Parkinson’s disease. Environ.Int 2015;75:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]


