Abstract
Background:
The shape of the human lens changes from almost spherical at birth to ellipsoid due to a decrease in sagittal thickness and an increase in equatorial diameter during the first two decades of life. Both dimensions increase thereafter. This study was undertaken to determine the reason for the change.
Methods:
Published refractive index gradients, from 20 lenses aged from seven to 82 years, were used to calculate the protein contents of concentric shells of fibre cells in human lenses. The boundaries of nuclear cores containing from 2.5 to 45 mg, in 2.5 mg increments, were determined from the isoindicial shells. Cortex thickness was determined from the distance between the 30 mg nuclear boundary and the capsule.
Results:
The sagittal thickness of every nuclear core decreased until age 40 years and remained constant thereafter. Over the same time frame, the equatorial diameter of the cores containing up to 30 mg of protein increased, while those of cores larger than 30 mg decreased. The volumes of the cores decreased and their shapes changed from near spherical to spheroidal. Equatorial and sagittal cortex thickness increased linearly with age at 0.0082 mm per year. The anterior sagittal cortex was 0.23 mm larger than the posterior and the equatorial cortex was 0.62 mm greater.
Conclusions:
Changes in lens shape observed during the first two decades of life are due to remodelling and compaction of the 30 mg nuclear core. Cortex growth is linear throughout life.
Keywords: ageing, compaction, cortex, dimensions, growth, human lens, nucleus, remodeling, shape
Lens growth commences early in gestation and continues throughout life. In most species, this takes place through a single selflimiting (asymptotic) process, in which the accumulation of mass gradually slows with increasing age.1,2 By contrast, human lens growth takes place through a unique biphasic process, not observed in any other species. This entails an asymptotic mode during gestation and early infancy followed by linear growth for the rest of post-natal life.3,4 Logistic analysis suggests that the human prenatal growth mode is complete not long after birth when cells, already committed to this growth mode at birth, reach maturity. This self-limiting growth mode generates a lens nuclear core containing around 30 mg of protein.4 The core encompasses the so-called foetal, embryonic and juvenile nuclei which, contrary to the implications of their nomenclature, are all laid down during prenatal life and the adult nucleus which is completed within three months after birth.4
The two growth modes produce two distinct tissue compartments in the adult human lens, the fixed-size nucleus and the continuously growing cortex.4 It would appear that these two compartments have different functions, as only the nucleus changes shape during accommodation.5–9
While post-natal lens weight increases linearly with age,3,4 the situation is more complex for lens shape which undergoes substantial changes due to differences in the growth of the dimensions. The equatorial diameter increases monotonically and continuously from the time of vesicle closure during gestation but it is not certain whether this is logarithmic10,11 or linear12,13 in adulthood. Sagittal thickness also increases during gestation and the first year after birth; however, it then starts to decrease, reaching an in vitro minimum around age 18 to 20 years before increasing again for the rest of life.4 Similar changes have been observed in vivo, except that the minimum thickness appears to be reached at around age 10 years.14 What drives these complex changes is not known.
Any change in the shape of the lens must involve alterations in the thickness and length of the fibre cells. These would require relocation of the cytoplasm and its constituent proteins. Thus, determining the location of fibre cell proteins as a function of age could reveal where and when the shape changes originate. The present study was undertaken to explore this possibility by using protein concentrations calculated from the sagittal and equatorial refractive index gradients determined by Jones and colleagues15 and Pierscionek and colleagues.16
METHODS
Refractive index gradients for 20 lenses, ranging in age from seven to 82 years, were obtained from magnetic resonance imaging (MRI) studies by Jones and colleagues,15 who generously made the complete data set available to the author. The sagittal and equatorial gradients were aligned to generate a series of isoindicial contours, which were used to divide each lens into concentric shells of 0.05 mm width. The volumes of the shells, anterior and posterior to the equatorial axis, were calculated from the contour dimensions, assuming these represented the boundaries of oblate ellipsoids of revolution with equators coincident with the lens equator. Although the lens shape is not strictly spheroidal, the calculations will be valid for all shells except the outermost.
Protein concentrations were calculated from the gradients using a refractive index of 1.340 for the aqueous phase and a composite refractive increment of 0.198/mg calculated from the different refractive increments of the three crystallin classes, determined by Pierscionek, Smith and Augusteyn17 and the nuclear crystallin proportions described by Thomson and Augusteyn.18 This yielded concentrations ranging from 150 to 170 mg/ml in the outer 0.05 mm of the cortex to 370 to 410 mg/ml in the centre of most adult lenses. The average value agrees with the 385 mg/ml determined by Heys, Cram and Truscott.19 The concentrations were used to estimate the protein content of concentric shells. The validity of this approach was tested by comparing the calculated protein content for the whole lenses with published dry weights.4, 10 The calculated and experimental data were indistinguishable for most lenses (multiple regression analysis, p = 0.853).
Starting from the centre of the lens, the protein contents of successive concentric shells were added and the outermost dimensions of the combined shells were recorded every 2.5 mg, between 2.5 and 45 mg of accumulated protein. These combined shells were taken to be nuclear cores.
RESULTS
Internal lens changes were examined by determining the effect of age on the dimensions of central cores containing from 2.5 to 42.5 mg protein. The upper limit corresponds to the total protein content of the seven-year-old lens. Representative profiles showing the equatorial and sagittal dimensions of the cores as a function of age are shown in Figure 1.
Figure 1.
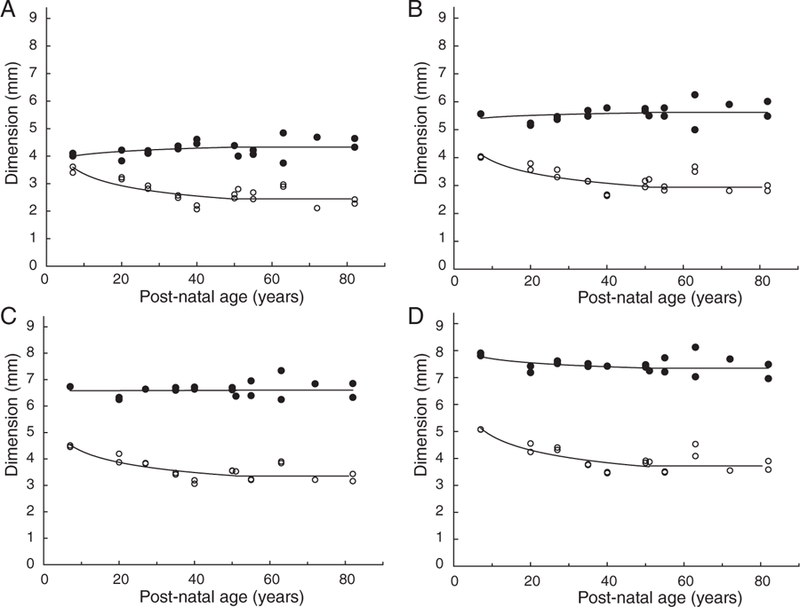
Equatorial diameter (●) and sagittal thickness (○) of the nuclear cores containing 10 (A), 20 (B), 30 (C) and 40 (D) mg protein, as a function of age
In all cases, the sagittal dimension was found to decrease with increasing age until around ages 40 to 50, after which it appears to be constant. For example, the 30 mg core thickness decreases from 4.5 mm at age seven to 3.2 mm at age 40 and above; however, the changes in equatorial diameter are complex. For the more central cores containing up to 25 mg, the diameter appears to increase up to age 40 to 50 years and remain constant thereafter (Figures 1A and 1B); for the 27.5 and 30 mg cores, it appears to be constant at all ages (Figure 1C), while for the larger cores the equatorial diameter appears to decrease until ages 40 to 50 years, after which it also remains constant (Figure 1D).
These changes can be better appreciated by comparison of the dimensions of the cores in seven- and 82-year-old lenses (Figure 2).
Figure 2.
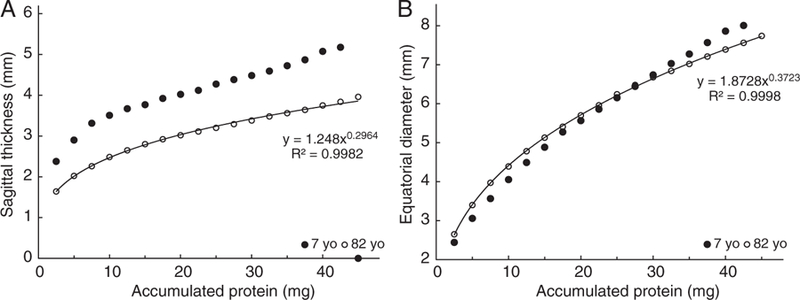
Equatorial diameter (A) and sagittal thickness (B) of nuclear cores in seven- (●) and 82-year-old (○) human lenses as a function of core protein content
As concluded from Figure 1, the sagittal thickness of the various cores decreases substantially with age, by 36 per cent for the smallest (2.5 mg) in the centre, gradually decreasing to 26 per cent for the largest (42.5 mg) examined (Figure 2A). By contrast, the equatorial diameters of the central cores actually increase with age so that in the 82-year-old lens the 2.5 mg core diameter is 0.4 mm (16 per cent) larger than that in the seven-year-old lens. The difference becomes less with increasing core size until 30 mg where the seven- and 82-year-old diameters are the same (Figure 2B). Thereafter, the diameters decrease so that at 42.5 mg, the 82-year-old core is 0.6 mm (8.0 per cent) smaller than that at age seven (Figures 1C and 2B). These observations suggest that there is a change in the growth mode when approximately 30 mg of protein have accumulated.
To examine this more closely, the volumes and aspect ratios were calculated for the various cores at ages seven and 82 years. The results are summarised in Figure 3.
Figure 3.
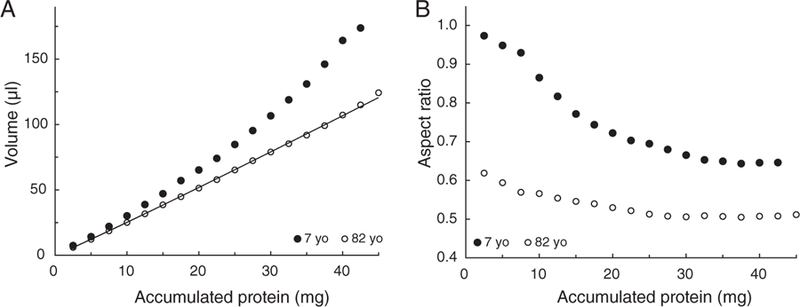
The volumes (A) and aspect ratios (B) of the nuclear cores in seven- (●) and 82-year-old (○) human lenses as a function of core protein content
A substantial decrease is seen in the volumes between ages seven and 82 (Figure 3A). This is consistent with compaction of the cells. For example, the volume occupied by the 30 mg core decreases from around 107 μl at age seven years to 79 μl after age 40 (Figure 3A). The maximum concentration of protein reached in any core was in the range 370 to 410 mg/μl, indicative of a limit in the extent of compaction in the regions examined, as previously noted.15,19,20
The aspect ratios (T/D, Figure 3B) reflect the complex changes in the equatorial diameter. In the seven-year-old lens the two central cores are close to spherical with aspect ratios of greater than 0.96. The ratio decreases outward from the centre, with increasing accumulated protein, to around 0.65 and appears to be constant for cores containing more than 30 mg. As might be expected from the overall decrease in the sagittal dimensions shown in Figures 2 and 3, the aspect ratios in the 82-year-old lens are substantially lower, ranging from 0.63 for the central 2.5 mg to 0.51 for the outer cores. The levelling off in the aspect ratio in the outer layers coincides with the age-related decrease in equatorial diameter. The outer ratio in the older lens is the same as that observed with the whole lens.10,13
The changes in nuclear core thickness appear to resemble those of the whole lens during the remodelling phase which takes place in the first two decades of post-natal life.4,14 To explore this further, the dimensions of the cortex were obtained from the distance between the outer boundaries of the 30 mg core and the capsule. This core was selected because remodelling appeared to involve only the central 30 mg and was complete as judged from the trends in the diameter (Figures 1C and 2B) and the constant aspect ratio in the larger cores (Figure 3B). It appears to correspond to the 30 mg nuclear core identified previously from a variety of observations.4
The dimensions of the cortex around the 30 mg nucleus in both equatorial and sagittal directions, as a function of age, are shown in Figure 4.
Figure 4.
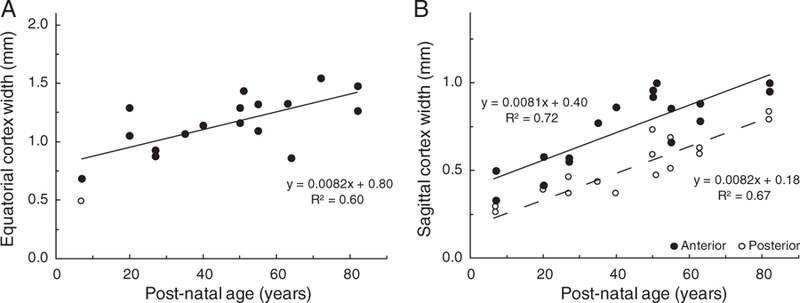
Human lens cortical thickness as a function of age. Thickness was determined from the distance between the outer boundary of the 30 mg nucleus and the lens capsule for (A) the equatorial cortex and (B) the anterior (●) and the posterior (○) sagittal cortex. The open symbol in Figure 4A is an outlier, which differed from the mean for that age by more than 2.5 times the standard deviation. It was not used in the regression analysis.
No significant differences were observed between the widths of the cortex on either side in the equatorial plane so the data from each lens were averaged for regression analysis (Figure 4A). By contrast, the anterior sagittal cortex appears to be 0.23 mm thicker than the posterior (Figure 4B). Some of the extra width probably corresponds to the C1 cortical zone, which in vivo, is approximately 0.1 mm wider in the anterior than in the posterior, plus the thicker anterior capsule and epithelium.6 The equatorial cortex is 0.64 mm greater than the posterior sagittal cortex. Some of this (0.12 to 0.14 mm)21 can be attributed to the germinative and proliferative zones but it would appear that cell thickness is greater in the equatorial plane. Regression analyses indicated that all cortical widths increase linearly with age and at the same rate of 0.0082 mm/year (R2 = 0.60–0.72) (Figures 4A and 4B). A linear increase of around 0.008 mm/year can also be seen with the dimensions of whole lenses over 50 years old in which the nuclear remodelling is essentially finished.10 Identical increases in the equatorial cortex and in the sagittal cortex is what would be expected from the way lens growth takes place, through the deposition of layers of cells. In the absence of uneven compaction, each complete layer of cells increases the diameter and thickness of the lens by the same two cell thicknesses.
Similar calculations were performed using the interferometric data extracted from the published figures of Pierscionek and colleagues.16 Because of their high X-ray refractive index, protein concentrations, calculated using the visible light refractive increments, were unrealistically high (greater than 500 mg/ml in the centre). Consequently, the estimated dimensions of the nucleus were smaller and those of the cortex were larger than the values obtained from the MRI data. Nevertheless, the overall conclusions were similar in that the nuclear equatorial diameter increases while sagittal thickness decreases until sometime after age 40; the anterior sagittal cortex is approximately 0.3 mm wider than the posterior and all cortical dimensions increase linearly at around 0.008 mm/year.
DISCUSSION
Determination of nuclear core dimensions from the published refractive index gradient data of Jones and colleagues15 and Pierscionek and colleagues16 has provided an explanation for the change in lens shape observed during the first two decades of life.
The observations presented here indicate that alterations in the central core containing 30 mg of protein are mostly responsible for the transformation of lens shape. This core contains the cells and proteins produced during the prenatal asymptotic growth mode and will eventually become the distinct human adult lens nucleus.4 The neonatal lens is close to spherical but the shape change starts within a year,4 suggesting that the remodelling probably commences as soon as the prenatal growth mode is complete.
The shape change is not simply due to a rearrangement of the nuclear fibre cells but also involves compaction. Remodelling, through stretching in the equatorial plane converts the core from near spherical to the final spheroidal shape. This increases the equatorial diameter, while decreasing the sagittal thickness of the core. Compaction would decrease both. Thus, with both processes occurring, the equatorial diameter of the core would be expected to increase by a little, while sagittal thickness would substantially decrease. This is what was observed in the present study for the central (less than 30 mg) cores. However, the 30 to 42.5 mg cores are differently affected. Both sagittal and equatorial dimensions decrease with age and the aspect ratios are very similar. This indicates that no further remodelling is taking place and any decrease with age is due to compaction alone.
There appear to be differences between the in vivo and in vitro ages at which the lens thickness minimum is observed. In vivo the minimum is seen around ages 10 to 12 years,14 whereas in vitro it is around 18 to 20.4,11 The minimum would be expected at the time when the amount of cortical thickness increase equals the decrease in nuclear thickness. In vivo, this would be dependent on the accommodative state. In the relaxed or cycloplegic eye, the thickness of the lens, and hence the decrease due to compaction, are reduced by stretching, so an apparent minimum is observed at an earlier age.
Van Alphen suggested that remodelling of the lens takes place as a result of forces exerted by the growing eye;22 however, external human ocular growth ceases around one year after birth,23 while remodelling of the lens and nuclear core is substantial in the first two decades and appears to continue until at least age 40. More likely, the remodelling is due to forces generated by the accommodative system, that is, the zonules and ciliary body muscle while the eye is in the relaxed state. With increasing age, these forces may lessen as the equatorial diameter increases and circumlental space decreases. Alterations in capsular tension with the advent of the post-natal growth mode may also contribute.
After taking into account the nuclear changes, it is clear that growth of the cortex is linear. Linear increases in cortical thickness with little or no change in nuclear thickness are also evident from in vivo Scheimpflug observations. Huggert24 noted that the anterior cortex increases linearly at 0.007 mm/year. Smith and colleagues25 found 0.008 mm/year. Combining the C1, C2 and C3 data of Dubbelman and colleagues6 yielded anterior and posterior sagittal cortex increases of 0.012 and 0.0068 mm/year, respectively. Cook and colleagues26 found 0.012 and 0.005 mm/year. Dubbelman and colleagues6 reported that the nuclear thickness increased by 0.003 mm/year in adults, while Cook and colleagues26 concluded it decreased at the same rate. As noted previously,4 these data were highly scattered and could be interpreted as showing no change in both cases.
It is interesting to note that the nuclear remodelling and compaction parallel the increase in lens nuclear stiffness and the loss of accommodative amplitude.19 Given that only the nucleus is involved in accommodation,5–9 it seems likely that these processes are connected. How remains to be established.
ACKNOWLEDGEMENTS
The author is grateful to Catherine Jones and James Pope for allowing him to use their refractive index data. This work was supported in part by the National Institutes of Health grants R01EY014225, R01EY021834 and P30EY14801 and by the Australian Federal Government CRC Scheme through the Vision Cooperative Research Centre.
REFERENCES
- 1.Augusteyn RC. Growth of the eye lens; I. Weight accumulation in multiple species. Mol Vis 2014; 20: 410–426. [PMC free article] [PubMed] [Google Scholar]
- 2.Augusteyn RC. Growth of the eye lens; II Allometric studies. Mol Vis 2014; 20: 427–440. [PMC free article] [PubMed] [Google Scholar]
- 3.Augusteyn RC. Growth of the human eye lens. Mol Vis 2007; 13: 252–257. [PMC free article] [PubMed] [Google Scholar]
- 4.Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res 2010; 90: 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown N. The change in shape and internal form of the lens of the eye on accommodation. Exp Eye Res 1973; 15: 445–459. [DOI] [PubMed] [Google Scholar]
- 6.Dubbelman M, van der Heijde GL, Weeber H et al. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res 2003; 43: 2363–2375. [DOI] [PubMed] [Google Scholar]
- 7.Hermans E, Dubbelman M, van der Heijde R et al. The shape of the human lens nucleus with accommodation. J Vision 2007; 7: 1–10. [DOI] [PubMed] [Google Scholar]
- 8.Koretz JF, Cook CA, Kaufman PL. Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Invest Ophthalmol Vis Sci 1997; 38: 569–578. [PubMed] [Google Scholar]
- 9.Patnaik B. A photographic study of accommodative mechanisms: Changes in the lens nucleus during accommodation. Invest Ophthalmol Vis Sci 1967; 6: 601–612. [PubMed] [Google Scholar]
- 10.Mohamed A, Sangwan VS, Augusteyn RC. Growth of the human lens in the Indian adult population: preliminary observations. Indian J Ophthalmol 2012; 60: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachar RA. Growth patterns of fresh human crystalline lenses measured by in vitro photographic biometry. J Anat 2005; 206: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith P. Diseases of crystalline lens and capsule. On the growth of the crystalline lens. Trans Ophthalmol Soc UK 1883; 3: 79–99. [Google Scholar]
- 13.Rosen AM, Denham DB, Fernandez V et al. In vitro dimensions and curvatures of human lenses. Vision Res 2006; 46: 1002–1009. [DOI] [PubMed] [Google Scholar]
- 14.Mutti DO, Zadnik K, Fusari RE et al. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci 1998; 39: 120–133. [PubMed] [Google Scholar]
- 15.Jones C, Atchison DA, Meder R et al. Refractive index distribution and optical properties of the isolated human lens measured using magnetic resonance imaging (MRI). Vision Res 2005; 45: 2352–2366. [DOI] [PubMed] [Google Scholar]
- 16.Pierscionek B, Bahrami M, Hoshino M et al. The eye lens: age-related trends and individual variations in refractive index and shape parameters. Oncotarget 2015; 6; 30532–30544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierscionek B, Smith G, Augusteyn RC. The refractive increments of bovine α-, β- and γ-crystallins. Vision Res 1987; 27: 1539–1541. [DOI] [PubMed] [Google Scholar]
- 18.Thomson JA, Augusteyn RC. Ontogeny of human lens crystallins. Exp Eye Res 1985; 40: 393–410. [DOI] [PubMed] [Google Scholar]
- 19.Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the lens nucleus with age: the basis for presbyopia? Mol Vis 2005; 10: 256–263. [PubMed] [Google Scholar]
- 20.Augusteyn RC, Jones C, Pope J. Age-related development of a RI plateau in the human lens: evidence for a distinct nucleus. Clin Exp Optom 2008; 91: 296–30. [DOI] [PubMed] [Google Scholar]
- 21.Lim JC, Walker KL, Sherwin T et al. Confocal microscopy reveals zones of membrane remodeling in the outer cortex of the human lens. Invest Ophthalmol Vis Sci 2009; 50: 4304–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Alphen GW. On emmetropia and ametropia. Optica Acta (Lond) 1961; 142 (Suppl): 1–92. [PubMed] [Google Scholar]
- 23.Augusteyn RC, Mohamed A, Nankivil D et al. Human ocular biometry. Exp Eye Res 2012; 102: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huggert A. The thickness of the cortex of the crystalline lens in different ages. Acta Ophthalmol 1946; 24: 43–62. [Google Scholar]
- 25.Smith GTH, Smith RC, Brown NAP et al. Changes in light scatter and width measurements from the human lens cortex with age. Eye 1992; 6: 55–59. [DOI] [PubMed] [Google Scholar]
- 26.Cook CA, Koretz JF, Pfahnl A et al. Aging of the human crystalline lens and anterior segment. Vision Res 1994; 34: 2945–2954. [DOI] [PubMed] [Google Scholar]


