Abstract
Background
Experiments in vitro have shown that the drug amodiaquine may inhibit Ebola virus activity. During the Ebola virus disease (EVD) epidemic in West Africa in 2014–2016, 2 mass drug administrations (MDAs) of artesunate-amodiaquine (ASAQ) were implemented to decrease the burden of malaria. The objective of this study was to assess the effect of the ASAQ MDAs on the mortality of patients with EVD.
Methods
A retrospective cohort design was used to analyze mortality data for patients with EVD admitted to 5 Ebola treatment units in Liberia and Sierra Leone. Patients admitted to the ETUs during the time period of ASAQ’s therapeutic effect from areas where the MDA was implemented were matched to controls not exposed to ASAQ, using a range of covariates, including malaria co-infection status, and a logistic regression analysis was performed. The primary outcome was Ebola treatment unit mortality.
Results
A total of 424 patients with EVD had sufficient data for analysis. Overall, the mortality of EVD patients was 57.5%. A total of 22 EVD patients were exposed to ASAQ during the MDAs and were found to have decreased risk of death compared with those not exposed in a matched analysis, but this did not reach statistical significance (relative risk, 0.63; 95% confidence interval, 0.37–1.07; P = .086).
Conclusions
There was a non–statistically significantly decreased risk of mortality in EVD patients exposed to ASAQ during the 2 MDAs as compared with EVD patients not exposed to ASAQ. Further prospective trials are needed to determine the direct effect of ASAQ on EVD mortality.
Keywords: amodiaquine, Ebola virus disease, epidemic, mass drug administration, mortality
Experiments in vitro have shown that amodiaquine may inhibit Ebola virus activity. This study found a non-statistically significant decreased risk of death for patients with Ebola Virus Disease exposed to amodiaquine during mass drug administration of artesunate-amodiaquine in the 2014-15 West African Ebola outbreak suggesting possible therapeutic effect of amodiaquine and need for further trials of this drug in patients with Ebola Virus Disease.
The Ebola virus disease (EVD) epidemic in West Africa in 2014–2016 was the largest EVD outbreak ever recorded, infecting >28 000 people, and causing >11 000 deaths [1]. Despite ongoing research efforts, there remain no approved treatments for EVD. Given that human communities continue to migrate to forested regions harboring animal reservoirs of Ebola virus (EBOV), future epidemics are likely to occur [2, 3]. The recent declarations of 2 EVD outbreaks in the Democratic Republic of Congo (DRC) in 2018 underscore the relevance and need to improve clinical management and treatment for patients with EVD [4]. Although efforts to create Ebola vaccines and novel therapeutics are promising, effective and readily available treatments are still urgently needed, as large-scale vaccination campaigns will take significant time and resources to implement and investigational drugs may not be obtainable in quantities needed to halt another outbreak [3, 5, 6].
Although there are no definitive treatments for EVD, several candidate treatments have been proposed. One of these proposed drugs, amodiaquine, has shown inhibition of EBOV activity in vitro by preventing the entry of EBOV into host cells [7–9]. Computational methods have shown amodiaquine docking to viral protein 35, a common target for other potential Ebola virus inhibitors, as a possible mechanism of action [7, 8]. Additionally, a 2016 retrospective study showed a 31% decreased mortality risk in EVD patients treated with artesunate-amodiaquine (ASAQ) during a brief time period when the standard antimalarial drug artemether-lumefantrine was unavailable at an Ebola treatment center supported by Médicins Sans Frontières (MSF) in Foya, Liberia [10]. Despite these promising findings, no further studies using human clinical data have evaluated the effects of amodiaquine on mortality in patients with EVD. Furthermore, the authors of the MSF study also speculated that their findings could potentially be explained by artemether-lumefantrine causing increased risk of death, rather than a protective effect of ASAQ [10].
During the West African Ebola outbreak, the government of Sierra Leone implemented 2 mass drug administrations (MDAs) of ASAQ to decrease the burden of malaria on the severely strained health system and reduce the number of febrile cases that could be considered as suspected EVD cases, in accordance with World Health Organization (WHO) recommendations [11]. The 2 MDAs were implemented in 8 of Sierra Leone’s 14 districts, which are highly endemic to malaria and had been affected by the EVD epidemic. In 6 districts, the MDA was conducted only in chiefdoms (administrative units below the district level) with previously confirmed EVD cases; all zones (equivalent to chiefdoms) in the 2 districts of the Western Area (1 of the 4 principal divisions of Sierra Leone, and where the capital Freetown is located) were covered with the MDA [11]. Each MDA was distributed over a 4-day period by 8830 health workers who provided directly observed treatment (DOT) for the first dose and gave recipients instructions to complete the remaining 2 days of treatment unsupervised [11]. Population coverage was high, with 87% coverage of the targeted population (the total population of all targeted chiefdoms) of 3 million during the first MDA round (implemented December 5–8, 2014) and 96% coverage during the second MDA round (implemented January 16–19, 2015) [11]. Persons residing in quarantined houses (with confirmed or suspected EVD cases) received ASAQ by Ebola surveillance teams with personal protective equipment [11].
Throughout the course of the 2014–2016 epidemic, the international humanitarian organization International Medical Corps (IMC) developed a database utilizing data collected during clinical care provision at 5 Ebola treatment units (ETUs) in cooperation with local authorities in Liberia and Sierra Leone, as previously described in the literature [12]. Using this robust database of clinical information, this study aims to assess the effect of ASAQ distribution on the mortality of patients admitted to the 5 ETUs with final diagnosis of EVD during the West African outbreak.
METHODS
Study Design and Participants
This retrospective cohort study utilized data collected from 5 ETUs operated by IMC between September 2014 and September 2015. The 5 ETUs were located in Bong and Margibi counties in Liberia and in Port Loko District, Bombali District, and Kambia District in Sierra Leone. All patients presenting to the 5 IMC ETUs who met the case definition for suspected EVD (which was based on WHO and MSF guidelines in consultation with local health authorities) at triage were admitted to the ETU suspect ward and had a blood sample drawn for EBOV and malaria testing [13, 14]. EBOV testing was performed using real-time polymerase chain reaction, with a cycle threshold (CT) <40 cycles considered positive for EBOV. Malaria testing was performed using BinaxNow (Alere, Waltham, MA) rapid diagnostic testing (RDT), which identifies 4 plasmodium species: Plasmodium falciparum, P. malariae, P. vivax, and P. ovale. All patients admitted to the ETUs received antimalarial treatment empirically (regardless of whether a malaria RDT was performed) with oral artemether-lumefantrine (89.6% of patients) or parenteral artemether (4.5% of patients) or artesunate (5.7% of patients) if unable to take oral medications, in accordance with recommended guidelines. ASAQ was not used in any of the 5 ETUs’ clinical protocols, and no patients received amodiaquine-containing medications while admitted to the ETUs. All patients with a final diagnosis of EVD who had data available on chiefdom/zone of home residence, clinical predictors of mortality, and final mortality outcome data were included in this study and analyzed to evaluate the effect of ASAQ exposure from the MDAs on mortality.
The Sierra Leone Ethics and Scientific Review Committee, the University of Liberia Pacific Institute for Research & Evaluation Institutional Review Board, and the Lifespan (Rhode Island Hospital) Institutional Review Board provided ethical approval and exemption from informed consent for this study.
Data Collection
Patient demographic data, including the chiefdom/zone of the patient’s home residence, were collected at admission. Trained providers recorded baseline clinical signs and symptoms on standardized forms, as previously described [12]. Patients with positive EBOV test results were moved to the ETU-confirmed ward. Patients with initially negative EBOV tests were kept as inpatients for repeated testing after 2 days. Patients with a second negative EBOV result were discharged if clinically stable or transferred to another health facility if available. Mortality outcomes were collected at patient discharge. All data were later combined into a single research database, which was audited for quality, as described previously [12].
Data Analysis
Descriptive Statistics
Descriptive analyses were performed, with results presented as frequencies and percentages for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences in characteristics between patients presumed treated with ASAQ vs those not treated were assessed using independent-sample t tests or Mann-Whitney tests for continuous variables and Fisher exact tests for categorical variables.
Determination of ASAQ Exposure
As no individual-level information was collected regarding patient treatment with ASAQ from the MDA, a time period for ASAQ’s therapeutic effect was estimated based on the known pharmacodynamic half-life of 10 days of ASAQ [11, 13, 15]. This time period was set as the day following the first day of each MDA round until 10 days after the last day of the MDA distribution for that round (December 6–December 19, 2014, or January 17–January 30, 2015). Patients admitted on the first day of each MDA round (December 5, 2014, or January 16, 2015) were excluded as persons with fever or who were feeling unwell were excluded from the MDA and therefore would not have received ASAQ [11]. Patients who were admitted to the ETUs during the time periods of ASAQ’s therapeutic effect and whose reported home residence was in a chiefdom that received the MDA were considered exposed to ASAQ. All other patients were presumed not exposed to ASAQ.
Matching Method
Univariate analysis was used to assess the association of a broad range of covariates (such as time from ETU opening, age, sex, CT value, malaria RDT results, abnormal bleeding, dyspnea, fever, coma, confusion, anorexia, vomiting, diarrhea, dysphagia, abdominal pain) with the treatment (ASAQ exposure) as well as outcome (mortality). A 1:1 matching method using nearest-neighbor matching without replacement was used to match patients exposed to ASAQ during the MDA to patients not exposed. The covariates found to be associated with ASAQ exposure and mortality in our univariate analysis were included in the matching criteria in order to reduce the impact of potential confounding factors. Matching between the exposed and unexposed cohorts for variables associated with ASAQ exposure and mortality was assessed using the standardized bias (difference in the exposed and unexposed mean for each covariate divided by the standard deviation) before and after matching. A predefined threshold of <0.25 was used to ensure balance between treatment groups, as recommended in the literature [16].
Patients were matched exactly on malaria infection status (positive for malaria, negative for malaria, or missing result), as ASAQ has known antimalarial activity and patients with malaria and EBOV co-infection have been shown to have higher mortality rates in previous studies [17]. A covariate coding the days from the opening of the ETU was also added to control for secular trends in epidemic mortality outcomes [18, 19]. Patients were matched nearest-neighbor on the days from the opening of the ETU, as this was a continuous variable. CT values were matched exactly on categorization as “high,” “low,” or “missing”; CT values (a surrogate for EBOV viral load) were categorized as low if ≤22 cycles and high if they were >22 cycles. Finally, patients were matched on the presence of abnormal bleeding. Abnormal bleeding included unexplained bleeding of any cause (including but not limited to mucosal bleeding, epistaxis, gastrointestinal bleeding, and metrorrhagia). After treated patients were matched to a control, a logistic regression model was constructed to assess the effect of exposure to ASAQ on the primary outcome of mortality in EVD patients. The logistic model was fitted using the generalized estimating equation (GEE) method to account for the correlation among matched pairs and to obtain a robust statistical inference for estimating the relative risk (RR) of mortality. RRs, estimated here as odds ratios, may be biased in matched study designs [20]. Statistical significance was predefined using a P value <.05. All data preparation and statistical analyses were performed using R, version 3.5.0 (http://cran.r-project.org), and STATA 14 (StataCorp, College Station, TX).
RESULTS
Study Population Characteristics
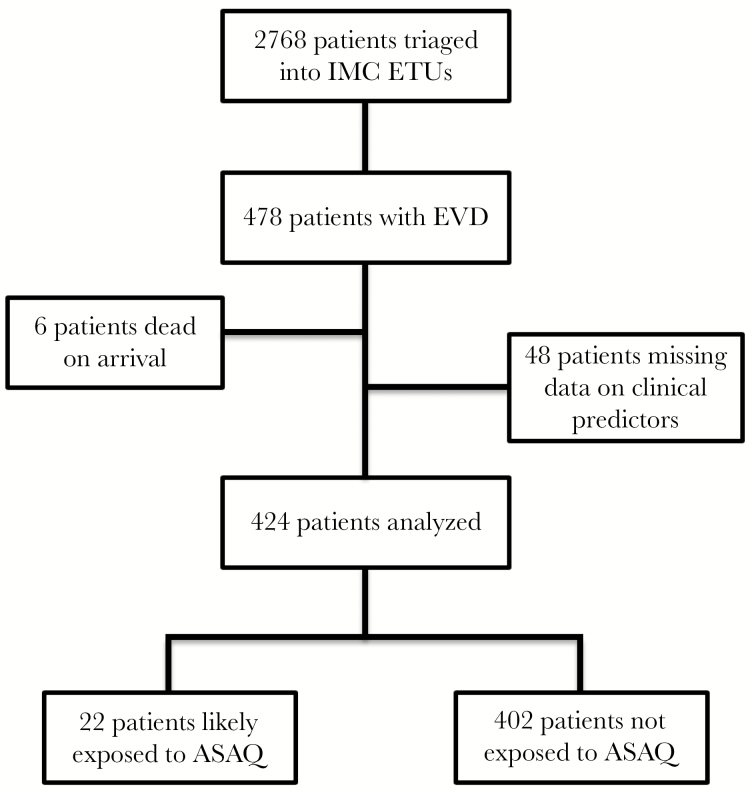
A total of 478 patients treated at the 5 ETUs had a final diagnosis of EVD, and 424 had sufficient available data on clinical condition, mortality outcome, and home residence to be included in this study (Figure 1). The median age (IQR) was 30 (16–44) years, and females accounted for 59.4% of all patients (Table 1). Overall mortality for all EVD patients included in the study was 57.5%. Patients with a home residence in a chiefdom/zone where the MDA was implemented made up 39.6% of the study population. Results of malaria RDT testing were available in 243 patients (57%), with 11.3% of all EVD patients having malaria co-infection. CT values were available for 281 patients, of whom 129 (56.6%) had a CT value ≤22 cycles, indicating a high EBOV viral load.
Figure 1.
Study profile. Abbreviations: ASAQ, artesunate-amodiaquine; ETU, Ebola treatment unit; IMC, International Medical Corps; EVD, Ebola virus disease.
Table 1.
Cohort Characteristics
| Patients (n = 424) | |
|---|---|
| Sex | |
| Male | 172 (40.6) |
| Female | 252 (59.4) |
| Age, y | 30 (16–44) |
| ETU | |
| Bong | 129 (30.4) |
| Margibi | 5 (1.2) |
| Port Loko | 148 (34.9) |
| Bombali | 109 (25.7) |
| Kambia | 33 (7.8) |
| Mortality outcome | |
| Survived | 180 (42.5) |
| Died | 244 (57.5) |
| Malaria co-infection | |
| Malaria positive | 48 (11.3) |
| Malaria negative | 195 (46.0) |
| Missing malaria result | 181 (42.7) |
| EBOV cycle threshold value | |
| Low (≤22) | 159 (37.5) |
| High (>22) | 122 (28.8) |
| Missing | 143 (33.7) |
Data are No. (%) or median (interquartile range).
Abbreviations: EBOV, Ebola virus; ETU, Ebola treatment unit.
Univariate Analyses and Matching
A total of 22 patients were in the cohort exposed to ASAQ, and 402 patients were in the unexposed cohort. Covariates significantly associated with ASAQ exposure included time to ETU opening, malaria co-infection status, CT value, abnormal bleeding, vomiting, and confusion (Table 2). Covariates significantly associated with mortality outcome included time to ETU opening, CT value, malaria co-infection status, abnormal bleeding, diarrhea, dysphagia, and dyspnea (Table 3).
Table 2.
Characteristics of Cases by ASAQ Exposure Status
| ASAQ Exposed (n = 22) | ASAQ Unexposed (n = 402) | P Value | |
|---|---|---|---|
| Sex | |||
| Male | 5 (22.7) | 166 (41.3) | .062 |
| Female | 17 (77.3) | 236 (58.7) | |
| Age, y | 34.5 (20–44) | 30 (15–44) | .446 |
| Malaria co-infection | |||
| Malaria positive | 4 (18.2) | 44 (11.0) | <.001 |
| Malaria negative | 18 (81.8) | 177 (44.0) | |
| Missing malaria result | 0 (0) | 181 (45.0) | |
| EBOV cycle threshold value | |||
| Low (≤22) | 17 (77.3) | 185 (46.0) | .011 |
| High (>22) | 5 (22.7) | 156 (38.8) | |
| Missing | 0 (0.0) | 61 (15.2) | |
| Presenting symptoms | |||
| Anorexia | 10 (45.5) | 195 (48.5) | .787 |
| Abnormal bleeding | 2 (9.1) | 92 (22.9) | .047 |
| Abdominal pain | 12 (54.5) | 214 (53.2) | .907 |
| Coma | 0 (0) | 2 (0.5) | .158 |
| Confusion | 0 (0) | 20 (5.0) | .000 |
| Diarrhea | 16 (72.7) | 229 (57.0) | .129 |
| Dysphagia | 5 (22.7) | 129 (32.1) | .331 |
| Dyspnea | 7 (31.8) | 112 (27.9) | .707 |
| Fever | 15 (68.2) | 302 (75.1) | .511 |
| Vomiting | 16 (72.7) | 187 (46.5) | .015 |
Data are No. (%) or median (interquartile range).
Abbreviations: ASAQ, artesunate-amodiaquine; EBOV, Ebola virus.
Table 3.
Characteristics of Cases by Mortality Outcome
| Survived (n = 180) | Died (n = 244) | P Value | |
|---|---|---|---|
| Sex | |||
| Male | 72 (40) | 99 (40.6) | .906 |
| Female | 108 (60) | 145 (59.4) | |
| Age, y | 28 (17–40) | 32 (14–45) | .080 |
| Malaria co-infectiona | |||
| Malaria positive | 17 (9.4) | 31 (12.7) | .082 |
| Malaria negative | 94 (52.2) | 101 (41.4) | |
| Missing malaria result | 69 (38.3) | 112 (45.9) | |
| EBOV cycle threshold valuea | |||
| Low (≤22) | 59 (32.8) | 143 (58.6) | <.001 |
| High (>22) | 100 (55.6) | 61 (25.0) | |
| Missing | 21 (11.7) | 40 (16.4) | |
| Presenting symptoms | |||
| Anorexia | 86 (47.8) | 119 (48.8) | .840 |
| Abnormal bleeding | 32 (17.8) | 62 (25.4) | .057 |
| Abdominal pain | 91 (50.6) | 135 (55.3) | .332 |
| Coma | 0 (0.0) | 2 (0.8) | .158 |
| Confusion | 6 (3.3) | 14 (5.7) | .231 |
| Diarrhea | 93 (51.7) | 152 (62.3) | .029 |
| Dysphagia | 44 (24.4) | 90 (36.9) | .006 |
| Dyspnea | 39 (21.7) | 80 (32.8) | .010 |
| Fever | 133(73.9) | 184 (75.4) | .723 |
| Vomiting | 87 (48.3) | 116 (47.5) | .872 |
Data are No. (%) or median (interquartile range).
Abbreviation: EBOV, Ebola virus.
aPercentages may not equal 100% due to rounding.
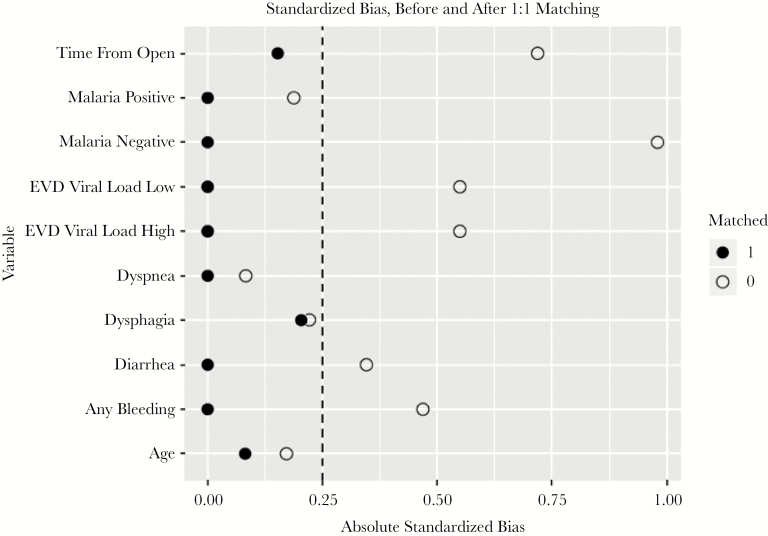
Based on the univariate analyses, covariates of malaria co-infection status, CT value, abnormal bleeding, and time from opening of ETU were included in the matching criteria. The results of matching on covariate balance showed that balance was achieved for malaria co-infection status, CT value, age, abnormal bleeding, dyspnea, diarrhea, dysphagia, and time from opening of ETU in the matched cohort, and the results were visually represented using a Love plot (Figure 2).
Figure 2.
Absolute standardized biases before and after matching. Abbreviation: EVD, Ebola virus disease.
Mortality
Overall mortality rates were similar across the study population, regardless of whether patients came from chiefdoms/zones where the MDA was implemented; a total of 96/168 patients died (57.1%; 95% confidence interval [CI], 49.5%–64.5%) who were from chiefdoms/zones where the MDA was implemented, as compared with 148/256 patients (57.8%; 95% CI, 51.6%–63.8%) from chiefdoms/zones that did not receive the MDA. However, the overall mortality for patients from the MDA chiefdoms/zones presenting during the time period of ASAQ distribution was lower, at 45.5% (95% CI, 25.1%–67.5%), with a total of 10/22 patients who died. In the matched cohort analysis, the mortality for patients likely to be exposed to ASAQ during the MDAs was 45.5% compared with 72.7% in patients not exposed to ASAQ (risk difference, 27.2%). The results of the GEE regression analysis showed that EVD patients likely exposed to ASAQ during the MDAs had a decreased risk of death (RR, 0.63; 95% CI, 0.37–1.07; P = .086) compared those who were not exposed, but their difference was marginally insignificant.
DISCUSSION
The results of this study showed a nonsignificant trend toward decreased mortality in patients with EVD with likely exposure to ASAQ during the MDAs as compared with patients with EVD not exposed to ASAQ. Given the large risk difference in mortality of 27.2% between patients likely exposed and not exposed to ASAQ, the lack of statistical significance is most likely due to the small number of patients presenting to the study ETUs with EVD from the MDA chiefdoms/zones during the periods of the therapeutic effect of ASAQ. The current findings are quite congruent, however, with a 2016 MSF study that showed a 31% decreased mortality risk in EVD patients treated with ASAQ at a single ETU in Foya, Liberia, although this study was also limited by its retrospective design and multiple potential confounding effects, as its authors have stated [10]. Additionally, the findings from the present study lend support to the hypothesis that ASAQ has a protective effect against EVD mortality rather than artemether-lumefantrine causing increased mortality. Together, these findings indicate that more rigorous clinical evaluation of amodiaquine treatment for patients with EVD is warranted. Given that current guidelines for the management of EVD recommend empiric treatment for malaria in malaria-endemic regions, the results of this study suggest that ASAQ may be the agent of choice in ETUs in populations for whom no other contraindications to using ASAQ exist. The widespread availability of ASAQ and its low cost also make it particularly appealing for use in resource-limited settings [21].
Although the study uses a robust set of data collected under difficult conditions at 5 separate ETUs, this study had several limitations. One important limitation was that as this was a retrospective study, there was no way to determine from this data set whether individual patients had received ASAQ during the MDA. Given the high reported coverage of 87%–96% with ASAQ during the 2 MDAs, an assumption that all patients presenting from MDA chiefdoms/zones during or immediately after the MDAs had been treated with ASAQ seems reasonable. Although a total of 18.2% of patients exposed to ASAQ had a positive malaria RDT result, which may suggest that some of these patients did not actually receive ASAQ as presumed, an alternate explanation is that malaria RDTs based on detection of histidine-rich protein 2 (HRP-2) have been shown to remain positive for up to 2 weeks after parasite clearance [22, 23]. Further speculation based on the limited available data are unfortunately impossible. The effective time period of therapeutic effect of ASAQ used in this analysis was selected using the half-life of the drug amodiaquine; however, there is no clinical evidence to date on the true therapeutic level above which amodiaquine may reasonably have an effect on EBOV activity. Amodiaquine is rapidly converted by cytochrome P450 enzyme CYP2C8 into its active metabolite destheylamodiaquine, which is eliminated much more slowly, having an estimated terminal half-life (range) of 10 (7–12) days [15]. Using the assumption that amodiaquine pharmacokinetics in EVD patients are similar to that seen when used for antimalarial treatment, using a half-life of 10 days appeared appropriate, although it is possible that ASAQ may lose effectiveness against EVD earlier than this or persist for longer time periods [11]. As ASAQ is also the firstline antimalarial treatment in Sierra Leone and antimalarial medications are often obtained over the counter without prescription in many malaria-endemic regions, it is also possible that patients in the group unexposed to ASAQ during the MDA may have taken ASAQ at some point in the past few weeks, although in this case, it would be expected that the results would be skewed more toward the null hypothesis.
Prior studies have noted that the overall mortality rate in ETUs decreased during the later periods of the outbreak [18, 19]. If the decreased mortality of patients exposed to ASAQ was simply due to variations in mortality over time as the outbreak persisted, a higher mortality in the cohort of patients exposed to ASAQ would have been expected, as the MDAs occurred during the earlier months of the operation of IMC’s ETUs, which was not found in this study [24]. It is also possible that there were unmeasured clinical differences between the patients exposed to ASAQ compared with the cohort that was unexposed. However, covariate balance between cohorts was achieved in CT value (a surrogate for EBOV viral load), malaria co-infection status, time since opening of the ETUs, and other major potential clinical confounders—indicating that patients were comparable between the 2 cohorts and supporting a potential causal effect of ASAQ on mortality outcomes.
Another limitation was the small sample size of the cohort exposed to ASAQ. This not only limited the power of the analysis to identify all potential confounders and adjust for them, but also limited the power to statistically test the effect of ASAQ. Therefore, although our findings were not statistically significant, the reduction in mortality was clinically meaningful and deserves further investigation.
CONCLUSIONS
There was a nonsignificant trend toward decreased mortality in EVD patients likely exposed to ASAQ during 2 MDAs in Sierra Leone as compared with EVD patients unexposed to ASAQ. Further prospective trials of amodiaquine in patients with EVD are needed to determine whether amodiaquine can decrease mortality and the mechanism by which this may occur.
Acknowledgments
The authors thank International Medical Corps and the Governments of Liberia, Sierra Leone, and Guinea for contributing data for this research. The authors also thank the generous institutional, corporate, foundation, and individual donors who placed their confidence and trust in International Medical Corps and made its work during the Ebola epidemic possible. The authors thank the United States Naval Medical Research Center, Public Health England, and the Nigerian/European Union Mobile Laboratory for providing laboratory support to the International Medical Corps Ebola treatment units in Liberia and Sierra Leone and making their data available for this research. Finally, the authors thank all the International Medical Corps clinical and nonclinical staff in Liberia and Sierra Leone, including the data collection officers at each Ebola treatment unit, without whom these data would not be available for analysis.
Author contributions.S.G., A.A., A.C., and T.L. conceived the study idea, developed the research design, and wrote the manuscript. A.C. and T.L. supervised the project. D.Y., T.L., and S.G. performed the statistical analysis. D.C. performed the literature review and contributed to writing the manuscript. S.K., M.M., F.S., and S.P. participated in data collection, provided technical guidance, and offered critical comments in reviewing the manuscript. All authors have read and approved the final manuscript.
Availability of data and materials. The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclaimer.The funding sources had no involvement in the design or conduct of the study or the decision to submit for publication.
Financial support.This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (R03AI132801). T.L. and D.Y. were partially supported by Institutional Development Award Number U54GM115677 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds Advance Clinical and Translational Research (Advance-CTR).
Potential conflicts of interest.All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Ebola situation report - 30 March 2016.2016. Available at: http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016. Accessed September 1, 2018.
- 2. Diallo B, Sissoko D, Loman NJ, et al. Resurgence of Ebola virus disease in guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis 2016; 63:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. WHO supports Ebola vaccination of high risk populations in the Democratic Republic of the Congo 2018. Available at: http://www.who.int/news-room/detail/21-05-2018-who-supports-ebola-vaccination-of-high-risk-populations-in-the-democratic-republic-of-the-congo. Accessed September 3, 2018.
- 4. World Health Organization. Ebola situation reports: Democratic Republic of the Congo. 2018. Available at: http://www.who.int/ebola/situation-reports/drc-2018/en/. Accessed September 1, 2018.
- 5. PREVAIL II Writing Group. A randomized, controlled trial of ZMapp for Ebola virus infection. New England J Med 2016; 375:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennedy SB, Bolay F, Kieh M, et al. ; PREVAIL I Study Group Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zilbermintz L, Leonardi W, Jeong SY, et al. Identification of agents effective against multiple toxins and viruses by host-oriented cell targeting. Sci Rep 2015; 5:13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ekins S, Freundlich JS, Coffee M. A common feature pharmacophore for FDA-approved drugs inhibiting the Ebola virus. F1000Res 2014; 3:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakurai Y, Sakakibara N, Toyama M, et al. Novel amodiaquine derivatives potently inhibit Ebola virus infection. Antiviral Res 2018; 160:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gignoux E, Azman AS, de Smet M, et al. Effect of artesunate-amodiaquine on mortality related to Ebola virus disease. N Engl J Med 2016; 374:23–32. [DOI] [PubMed] [Google Scholar]
- 11. Aregawi M, Smith SJ, Sillah-Kanu M, et al. Impact of the mass drug administration for malaria in response to the Ebola outbreak in Sierra Leone. Malar J 2016; 15:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roshania R, Mallow M, Dunbar N, et al. Successful implementation of a multicountry clinical surveillance and data collection system for Ebola virus disease in West Africa: findings and lessons learned. Glob Health Sci Pract 2016; 4:394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Clinical management of patients with viral haemorrhagic fever: a pocket guide for front-line health workers: interim emergency guidance for country adaptation. 2016. https://apps.who.int/iris/handle/10665/205570. Accessed October 1, 2018. [Google Scholar]
- 14. Medicins Sans Frontieres. Filovirus haemorrhagic fever guidelines. 2008. https://www.ghdonline.org/uploads/MSF_Ebola_2008.pdf. Accessed October 15, 2018. [Google Scholar]
- 15. Stepniewska K, Taylor W, Sirima SB, et al. Population pharmacokinetics of artesunate and amodiaquine in African children. Malar J 2009; 8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waxman M, Aluisio AR, Rege S, Levine AC. Characteristics and survival of patients with Ebola virus infection, malaria, or both in Sierra Leone: a retrospective cohort study. Lancet Infect Dis 2017; 17:654–60. [DOI] [PubMed] [Google Scholar]
- 18. WHO Ebola Response Team. After Ebola in West Africa—unpredictable risks, preventable epidemics. New England J Med 2016; 375:587–96. [DOI] [PubMed] [Google Scholar]
- 19. Garske T, Cori A, Ariyarajah A, et al. Heterogeneities in the case fatality ratio in the West African Ebola outbreak 2013–2016. Phil Trans R Soc B 2017; 372:20160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med 2011; 30:1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Checchi F, Roddy P, Kamara S, et al. ; Sierra Leone Antimalarial Efficacy Study Collaboration Evidence basis for antimalarial policy change in Sierra Leone: five in vivo efficacy studies of chloroquine, sulphadoxine-pyrimethamine and amodiaquine. Trop Med Int Health 2005; 10:146–53. [DOI] [PubMed] [Google Scholar]
- 22. Dalrymple U, Arambepola R, Gething PW, Cameron E. How long do rapid diagnostic tests remain positive after anti-malarial treatment? Malar J 2018; 17:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grandesso F, Nabasumba C, Nyehangane D, et al. Performance and time to become negative after treatment of three malaria rapid diagnostic tests in low and high malaria transmission settings. Malar J 2016; 15:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weppelmann TA, Donewell B, Haque U, et al. Determinants of patient survival during the 2014 Ebola virus disease outbreak in Bong County, Liberia. Glob Health Res Policy 2016; 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]