OBJECTIVES:
Telomeres and telomerase play important roles in maintaining chromosome integrity and genomic stability. To address a lack of consensus about the association between leukocyte telomere length and colorectal cancer, we investigated this association in the Singapore Chinese Health Study.
METHODS:
Relative telomere length in white blood cells was quantified using a validated quantitative polymerase chain reaction method in 26,761 participants, including 776 incident colorectal cancer cases. The Cox proportional hazard regression method was used to calculate the hazard ratio and the corresponding 95% confidence interval (CI) for colorectal cancer associated with longer telomeres.
RESULTS:
Longer telomeres were significantly associated with a higher risk of colorectal cancer (Ptrend = 0.02). Compared with the lowest quartile, subjects with the highest quartile of telomere length had a hazard ratio of 1.32 (95% CI: 1.08–1.62) for developing colorectal cancer. The corresponding elevation in rectal cancer risk for the highest quartile of telomere length was 71% (95% CI: 22–140, Ptrend = 0.02). There was no statistically significant association between telomere length and risk of colon cancer.
DISCUSSION:
This large cohort study of Singapore Chinese, the first study using a cohort study design with more than 26,000 participants that yielded 776 incidence colorectal cancer cases during 12 years of follow-up, provides evidence in support of longer telomeres being associated with a higher risk of colorectal cancer, particularly rectal cancer.
INTRODUCTION
Colorectal cancer is the second and third most common cancer among men and women worldwide, respectively (age-standardized incidence rates per 100,000: 20.6 in men and 14.3 in women) (1), with approximately 1,360,000 new cases and 690,000 deaths per year (2). Colorectal cancer is also ranked among the top 20 causes of death globally, and this rank is expected to rise in the coming decades (3). Known environmental risk factors for colorectal cancer include consumption of red meat and processed meat, smoking, alcohol consumption, low physical activity, obesity, and type 2 diabetes (review by Kuipers et al. (1)). Genetic factors have also been found to play an important role in the development of colorectal cancer (4). Different susceptibility genes for colorectal cancer have been identified, including Kirsten rat sarcoma viral oncogene homolog, B-Raf murine sarcoma viral oncogene homolog, p53, and in microsatellite-containing genes vulnerable to mismatch repair defects (5). It is also commonly believed that many common but low penetrance genetic variants collectively explain a substantial proportion of colorectal cancer burden in general populations (6).
Telomeres are repeated TTAGGG sequences at the ends of chromosomes and are coated by shelterin proteins, involved in the process of telomere length homeostasis to maintain chromosomal integrity and stability (7). Telomerase is the enzyme that lengthens telomeres and therefore is the primary positive regulator of telomere length. In human telomerase, the telomerase RNA component serves as a template for the catalytic component, and telomerase reverse transcriptase adds telomeric repeats (8). Both telomeres and telomerase play important roles in genomic stability because they protect chromosome ends from degradation, fusion, and abnormal recombination (9,10). Although the human telomeres are approximately 10–15 kb long, they shorten by approximately 30–200 bp after each cycle of cell division (11). In addition to age being a strong predictor of telomere length, different genetic and environmental factors (i.e., smoking, diabetes, obesity, or physical activity) are also predictors of telomere length (12,13). In normal circumstances, the incomplete replication of linear DNA molecules at the end of each chromosome results in telomere shortening, leading to cell senescence or apoptosis (9). On the other hand, if the cells bypass senescence due to dysfunctional checkpoint pathways, the telomeres will continue to shorten, driving chromosome fusions and genomic instability. Survivors of this telomere crisis then maintain telomeres, a cancer hallmark, by upregulating telomerase in most cases (14). Thus, these surviving cells with longer telomeres have a replicative advantage (15) and consequently undergo more cell divisions before telomere crisis, resulting in the increase of likelihood for acquiring mutations that drive malignant transformation (16).
Previous epidemiologic studies (16–21) produced inconsistent results on the associations between leukocyte telomere length and risk of colorectal cancer. Some studies found that shorter, while others reported longer, leukocyte telomeres were significantly associated with higher risks of colorectal cancer (16,17,20,21). In addition, other studies (18,19) reported a null association. This inconsistency prompted us to investigate the association between telomere length and colorectal cancer risk in the Singapore Chinese Health Study, a prospective study of more than 60,000 middle-aged or older Chinese men and women in Singapore.
METHODS
Study population
The current analysis was based on the data from the Singapore Chinese Health Study, a population-based prospective cohort study of Hokkien and Cantonese, the 2 major dialect groups of Chinese in Singapore, who originated from Fujian and Guangdong provinces in southern China, respectively. Detailed information on designs and methods has been described elsewhere (22). Briefly, from April 1993 through December 1998, 63,257 Chinese men and women were enrolled into the study at age 45–74 years who resided in government-built housing estates. At baseline, a trained interviewer conducted an in-person interview at home. The structured questionnaire asked for information on demographics, body weight and height, current physical activity, menstrual and reproductive history (for women only), lifetime use of tobacco (cigarettes and waterpipe), occupational exposure, medical history, and family history of cancer. Body mass index (BMI) was calculated as the current weight in kilograms divided by height in meters squared. A 165-item food frequency questionnaire was used to obtain information on current diet and consumption of beverages. This food frequency questionnaire was validated against a series of 24-hour dietary recall interviews and selected biomarker studies conducted on random subsets of cohort participants (23–25). All study participants provided written informed consent. The Singapore Chinese Health Study was approved by the Institutional Review Boards of the National University of Singapore and the University of Pittsburgh.
Initially, 3% random sample of cohort participants were selected for blood and urine sample collection during 1994–1999. At the beginning of year 2000, the collection of blood and urine samples was extended to all surviving members of the entire cohort. The first follow-up telephone interview was conducted during July 1999–December 2003. The follow-up I questionnaire updated information on cigarette smoking, alcohol consumption, medical history, and current body weight. After the telephone interviews, participants were asked for donation of blood and urine samples for research use, if declined, mouthwash was collected.
We consented and collected blood samples from 28,346 (57%) of all eligible subjects. The study participants who provided blood samples were more educated (33.6% vs 25.1% having secondary or higher education) than those who did not, younger (mean age ± SD: 60.9 ± 7.7 vs 62.4 ± 8.2), more likely to be men (45.5% vs 39.1%), and were otherwise similar in the smoking rate (ever smokers: 32% vs 30.2%) and alcohol consumption (weekly consumers of alcohol: 18.3% vs 14.8%).
Assessment of colorectal cancer cases
The Nationwide Singapore Cancer Registry and the Birth and Death Registry were used to identify incident colorectal cancer cases and all-cause deaths via annual record linkage analysis for all surviving cohort participants. Cases of colorectal cancer were determined by the International Classification of Diseases–Oncology Second Edition Codes—C18 for colon cancer and C19-20 for rectal cancer (26). Both cancer and death registries have recorded all incident cancer cases and deaths with a high completion rate in Singapore. The identification of both incident cancer and death cases among all study participants was virtually complete, as to date, there were only 56 participants (<0.1%) of the whole cohort lost to follow-up due to migration out of Singapore.
In the current analysis, the total follow-up time as of December 2015 after blood donation was 11.8 years. After excluding 1,585 subjects with a history of cancer, the present analysis included 26,761 participants who were free of cancer at the time of blood collection, with 776 incident colorectal cancer cases as of December 31, 2015.
Measurements of leukocyte telomere length
Telomere length was measured in genomic DNA. The quantitative polymerase chain reaction (qPCR) method for quantification of relative telomere length was described previously (27). Briefly, relative telomere length in genomic DNA was determined by the ratio of telomere repeat copy number (T) to single (albumin) gene copy number (S) in experimental samples relative to a reference sample. We used 77 samples from a previous study in the Singapore Chinese Health Study to construct the standard curve. Individual telomere length measurements of these samples were within 10% of the cohort mean. The pooled DNA samples of these 77 participants were then run on all qPCR plates, of which 8 replications were performed for each of 4 concentrations: 4, 0.8, 0.16, and 0.032 ng/μL. Thermal cycling was conducted on an Applied Biosystem 7900 HT machine according to the conditions described previously (28). Telomere length was calculated using real-time PCR cycle thresholds with normalization of telomere length for each 384-well plate. The reproducibility rate of telomere length was excellent, at 3.5%, for all technical sample duplicates. Each experimental DNA sample was assayed twice, and the mean of the 2 test values was used for statistical analysis.
Statistical analysis
The t and χ2 tests were used to compare the distributions of continuous and categorical variables, respectively, between cases and noncases, as well as across quartiles of telomere length. The analysis of covariance method was used to examine the difference in geometric mean and 95% confidence intervals (CIs) of telomere length by selected characteristics after adjustment for age and sex.
In the current analysis, we further excluded 221 participants with missing data on telomere length measurement due to failed assays, leaving the current analysis of 26,540 total participants, including 776 incident cases of colorectal cancer. For each participant, we calculated person-years at risk from the date of blood draw to earliest date of colorectal cancer diagnosis, death, migration out of Singapore, or December 31, 2015.
The Cox proportional hazard regression method was used to estimate hazard ratios (HRs) and their corresponding 95% CIs for colorectal cancer associated with higher quartiles of telomere lengths comparing with the lowest quartile. Telomere length was grouped into 4 quartiles: Q1 (relative length: 0.19–0.87), Q2 (relative length: 0.87–1.00), Q3 (relative length: 1.00–1.15), and Q4 (relative length: 1.15–3.24). The linear trend was done by treating the telomere length quartiles as an ordinal variable. Proportional hazard assumption was tested using the Schoenfeld residuals test, and no violation was observed for telomere length. All multivariable Cox proportional hazards models included multiple potential confounders—age, sex, dialect group (Hokkien or Cantonese), level of education (no formal education, primary school, secondary, or higher education), BMI (<18.5, 18.5 to 23.0, 23.0 to <27.0, or ≥27 kg/m2), smoking, alcohol consumption (nondrinkers, 1 to <7, or ≥7 drinks per week), weekly physical activity (no/yes), history of diabetes (no/yes), and family history of colorectal cancer (no/yes). The BMI categories were based on the recommendation for Asians by the World Health Organization (29). The status of lifetime light or heavy smoker was defined previously (30); heavy smokers were those who started to smoke before age 15 years and smoked 13 or more cigarettes per day, whereas all remaining ever smokers were light smokers. The weekly physical activity was defined as any moderate or vigorous activity or strenuous sports lasting at least 30 minutes per week.
We performed both stratified and sensitivity analyses to examine the robustness of the association between telomere length and risk of colorectal cancer. The analyses in subgroups stratified by major risk factors for colorectal cancer such as the mean age (≤62 vs >62 years), sex, smoking status (ever vs never smoked), and history of diabetes (yes vs no). The likelihood ratio test was used to evaluate interactions between telomere length in quartile and these main risk factors by comparing nested models with and without the corresponding product term besides their main variables. For sensitivity analysis, we divided colorectal cancer cases with various number of years from blood draw to cancer diagnosis to evaluate whether the underlying subclinical disease progression had any impact on telomere length, and so on the association between telomere length and risk of colorectal cancer. P for heterogeneity in the sensitivity analysis was performed based on the comparison of 2 HRs (31).
We performed all statistical analyses using the computing software SAS version 9.4 (SAS Institute, Cary, NC). All P values presented are 2 sided. P values below 0.05 were considered being statistically significant.
RESULTS
The mean age at diagnosis for colorectal cancer cases was 72.5 (SD 8.2) years. The median time from blood collection to diagnosis of colorectal cancer was 7.04 years (range: <1 month to 16.6 years).
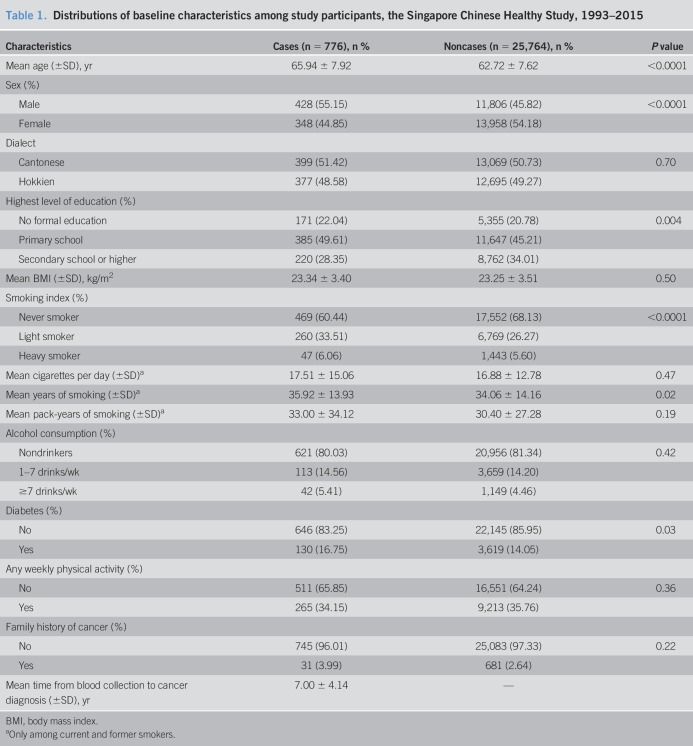
The distribution of baseline demographics and selected characteristics of cancer cases and noncancer cases are shown in Table 1. Patients with blood donation who later developed incident colorectal cancer were older, had lower level of education, ever smoked cigarettes, or more likely to be male or have a history of diabetes.
Table 1.
Distributions of baseline characteristics among study participants, the Singapore Chinese Healthy Study, 1993–2015
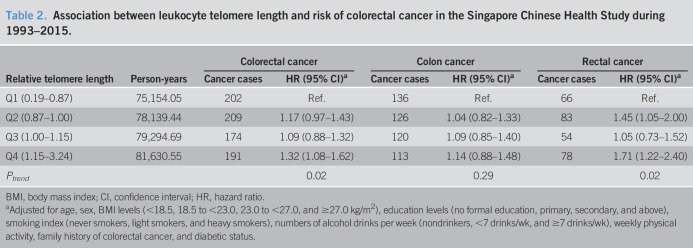
In our previous report (27), we found that shorter telomeres were associated with older age, male sex, lower level of education, ever cigarette smoking, daily drinkers of alcoholic beverages, and less physical activity. We also published previously the associations of cigarette smoking and alcohol drinking (30), diabetes (32), and physical activity (33) with the risk of colorectal cancer within this cohort. After adjustment for these potential confounding factors, telomere length was positively associated with the risk of colorectal cancer combined and rectal cancer alone (Table 2). Compared with the lowest quartile of telomere length, participants with the highest quartile of telomere length had a statistically significant 32% higher risk of developing colorectal cancer (95% CI: 8%–62%, Ptrend = 0.02). The corresponding elevation in rectal cancer risk associated with the highest quartile of telomere length was 71% (95% CI: 22%–140%, Ptrend = 0.02). Although longer telomere length was associated with a higher risk of colon cancer, the result did not reach statistical significance (HR = 1.14, 95% CI: 0.88–1.48, Ptrend = 0.29, compared highest to the lowest quartile). However, we did not detect a statistically significant heterogeneity in the association for telomere length with the risk of colon cancer in comparison with the risk of rectal cancer (Pheterogeneity = 0.22).
Table 2.
Association between leukocyte telomere length and risk of colorectal cancer in the Singapore Chinese Health Study during 1993–2015.
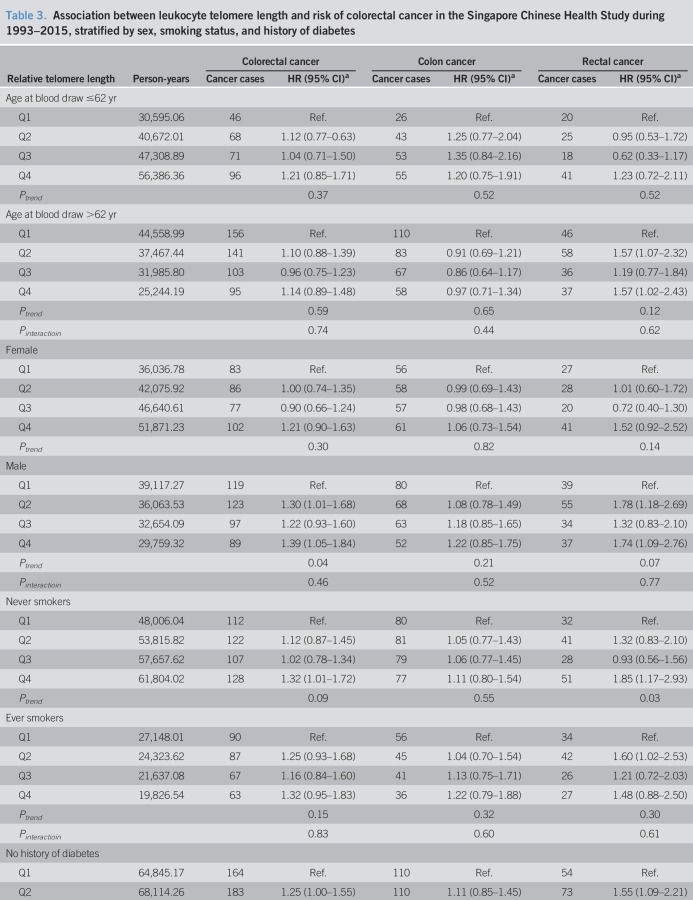
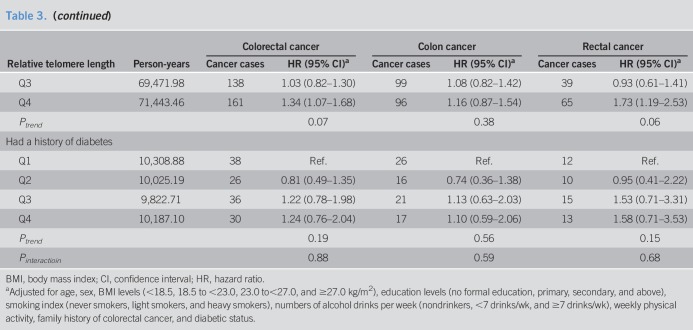
In a stratified analysis by selected risk factors including age at blood draw, sex, smoking status, and history of diabetes, the highest quartile of telomere length was significantly associated with an elevated HR of colorectal cancer combined and rectal cancer alone for men, never smokers, and subjects without a history of diabetes (Table 3). In addition, the quartile of telomere length was significantly associated with the risk of rectal cancer for older subjects (above the median age of 62 years) (Table 3). However, their interaction terms with telomere length on the risk of colorectal cancer combined or rectal cancer alone were not statistically significant (Pinteraction > 0.30). No significant association between telomere length and colon cancer risk was found in all the subgroups stratified by these factors. Further analysis did not reveal any difference in the association for telomere length with rectal cancer risk as compared to that with colon cancer risk in these subgroups (Pheterogeneity > 0.14).
Table 3.
Association between leukocyte telomere length and risk of colorectal cancer in the Singapore Chinese Health Study during 1993–2015, stratified by sex, smoking status, and history of diabetes
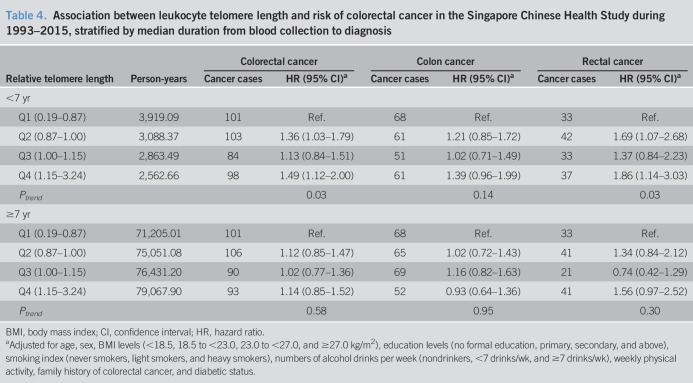
We conducted a sensitivity analysis by various time intervals from blood draw to cancer diagnosis. Table 4 shows the results derived from the sensitivity analysis for cases stratified by the median time interval (i.e., 7 years). The highest quartile of telomere length was significantly associated with an elevated risk of colorectal cancer combined and rectal cancer alone within the first 7 years of follow-up after blood draw. There was also a marginally elevated risk of colon cancer in the highest quartile of telomere length (HR = 1.39, 95% CI: 0.96–1.99). There was, however, no significant association in subjects with longer duration (≥7 years) of follow-up (Pheterogeneity = 0.164, 0.256, and 0.428 for colorectal cancer, colon cancer, and rectal cancer, respectively).
Table 4.
Association between leukocyte telomere length and risk of colorectal cancer in the Singapore Chinese Health Study during 1993–2015, stratified by median duration from blood collection to diagnosis
DISCUSSION
Our prospective analysis of 26,540 participants revealed that participants with longer telomeres at baseline had a higher risk of developing colorectal cancer, specifically rectal cancer. In subgroup analysis, longer telomeres were associated with an increased risk of colorectal cancer for men, never smokers, or those without a history of diabetes diagnosis, although the differences in the telomere length–colorectal cancer risk association among subgroups were not statistically significant.
Aviv et al. (15) recently proposed a 2-stage clonal expansion model to explain the observed differential association between telomere length and cancer risk in healthy individuals from that in cancer patients. The first hits of mutation at the stem-cell level are independent of telomere length and generate a clone with replicative advantage. The second hits of mutation occur in somatic cells where those with longer telomeres have greater potential for cell division before telomere exhaustion and increase the likelihood of acquiring mutations or have higher chance of malignant transformation. This model illustrates a possible biological mechanism by which under normal circumstance, individuals with longer telomere lengths in circulating leukocytes, representing for other tissues in the body, are at a higher risk of developing different cancers (i.e., lung, stomach, ovary, breast, or melanoma), as observed in our current and previous studies (27,34,35) and in studies by others (36–39).
Previous retrospective case-control studies reported inconsistent association between leukocyte telomere length and colorectal cancer risk (16,17,21). The earliest retrospective case-control study in China reported that shorter telomeres were associated with significantly elevated risk of colorectal cancer (21), primarily in younger persons, women, nonsmokers, or nondrinkers of alcohol. A retrospective case-control study in the United States reported that longer telomeres were significantly associated with a lower risk of colon cancer, but not rectal cancer (17). A third retrospective case-control study in the United States found that overall, patients with colorectal cancer had significantly longer leukocyte telomeres than controls (16) and that longer telomeres were associated with a higher risk of colorectal cancer in younger persons (aged <50 years), but lower risk in older persons (aged ≥ 50 years). Because blood samples for telomere measurement in retrospective case-control studies were collected from patients after their cancer diagnosis and potentially cancer treatment, the observed association between telomere length and risk of colorectal cancer could be confounded if the underlying disease progression and/or treatment had any impact on leukocyte telomere length.
Prospectively designed studies, in which blood samples were collected from subjects many years before their cancer diagnosis and treatment, avoid such confounding effects. There have been 3 nested case-control studies within prospective cohorts, 2 in the United States (i.e., the Women's Health Study (18) among women only and the Physician's Health Study (19) among men only) and 1 in Chinese women (20). No association was found between telomere length and risk of colorectal cancer overall or by subtype (colon and rectal cancer) in the 2 US studies. The third nested case-control study in Chinese women (20) found that leukocyte telomere length at baseline was higher in rectal cancer cases, but similar in colon or colorectal cancer cases combined compared with controls. Further analysis revealed that both shortest and longest quartiles of telomere lengths were associated with a statistically nonsignificant higher risk of colorectal cancer. There were several reasons for the lack of statistically significant association in nested case-control studies within prospective cohorts. Two of these studies were women only, which were consistent with ours. The small sample size (n = 191 cases) resulted in underpowered in at least 1 study (19). Different characteristics of study populations (i.e., prevalence of smoking, men, diabetes, and obesity) may also contribute variations to the different results observed. Furthermore, only 1 study (19) reported separately the results for colon and rectal cancer; telomere length was higher in rectal cancer cases than controls, and the difference reached statistically borderline significance. Our study was the first using a prospective study design with sufficient power and long-term follow-up, thus providing definitive evidence in support of longer telomeres to be associated with an increased risk of colorectal cancer, especially rectal cancer.
The telomere length dynamic pattern before cancer onset and during cancer progression may partially explain the different results between studies with retrospective design and those with prospective design. Accordingly, the telomere lengths measured in peripheral leukocytes collected after cancer diagnosis and treatment may be influenced by the disease progression, diagnostic procedures, or treatment. Even in the prospective studies in which blood samples are collected before cancer diagnosis, the follow-up duration would influence the association between telomere length and colorectal cancer risk because of the difference in the attrition rate of telomere length in individuals who subsequently develop cancer compared with that in cancer-free individuals. A longitudinal study that measured leukocyte telomere length at multiple time points demonstrating that the age-related attrition rate of telomere length in subjects who subsequently developed cancer was significantly higher than that in cancer-free persons during the 14 years of follow-up after blood draw (40). More importantly, longer telomeres were observed among persons who developed cancer within the first 7 years after blood collection than cancer-free individuals because of the reverse trend in age-related telomere length attrition in relation to the time to diagnosis. These data support our findings that longer telomeres were significantly associated with a higher risk of colorectal cancer within the first 7 years of follow-up after blood draw, but not associated with the risk of colorectal cancer beyond 7 years after blood draw.
In stratified analysis, although we found that the highest quartile of telomere length was significantly associated with an elevated HR of colorectal or rectal cancer for men, older individuals (≥62 years of median age), never smokers, or subjects without a history of diabetes; none of these factors were effect modifiers that achieved statistical significance in the interaction models. Our result on the association between longer telomeres and higher risk of colorectal cancer in persons aged 62 years or older, however, was inconsistent with a previous study by Boardman et al. (16) who reported that longer telomeres were associated with a higher risk of colorectal cancer in younger persons (<50 years), but lower risk in older persons (≥50 years). Difference in the study design between ours (i.e., prospective study) and theirs (retrospective case-control study) might explain this inconsistency.
Telomere lengths differ in different tissues, depending on their proliferative capacities and activities. Prior studies have shown that telomere lengths in different organs and tissue types of the same individual are highly correlated with each other (41) reported that leukocyte telomere length was strongly correlated with telomere lengths in the skin cells, muscle cells, and fat cells (all P values < 0.0001). A study by Valls-Bautista et al. (42) found that there was a strong correlation between telomere repeat length factors of colonic mucosa and that of their blood cells among control subjects. Taken together, the leukocyte telomere length may reflect telomere length in colonic cells despite their different proliferative rates.
Our data clearly show a stronger association for telomere length with rectal cancer than colon cancer, an expected finding. Our early analysis demonstrated that smoking and alcohol intake were associated with rectal cancer risk only (30), whereas BMI with colon cancer only (43) in this cohort. Although there is nonexistence of biological explanation for such a differential association with telomere length, it seems that the effect of telomere length on rectal cancer is stronger, so we can observe a significant association with a smaller sample size. The current evidence cannot rule out that telomere length has no effect on colon cancer. More studies are, therefore, needed to confirm whether telomere length has any impact on colon cancer risk.
The current study has several strengths. The measurement of telomere length in the entire cohort of more than 26,000 participants in a prospective study provided strong statistical power, resulting in robust risk estimates. A comprehensive baseline questionnaire collected lifestyle factors such as smoking, alcohol use, dietary factors, physical activity, and BMI and history of diseases that may have an impact on leukocyte telomere length. The simultaneous adjustment for these potential confounders minimized their confounding effects on the association between telomere length and colorectal cancer risk. The long-term follow-up of the cohort diminished the potential impact of disease (i.e., colorectal cancer) progression and treatment on telomere length, thus reduced the likelihood of possible reverse causality for telomere length and colorectal cancer risk.
Our study also has some limitations. First, telomere length was measured in leukocytes rather than in target tissue. Second, telomere length was measured one time point, which may not be representative for true telomere length over a long period. Furthermore, the one time-point measurement precluded our ability to examine the telomere length attrition rate and the risk of colorectal cancer development over time. It may be concerned that multiplex qPCR used in our study measured the relative telomere length only. This qPCR measurement accounted for 84.4% of variation in telomere length measured by the Southern blot method (28,44,45). Using the flow cytometric method (46), we also quantified telomere length in peripheral lymphocytes collected from approximately 1,000 participants of the Singapore Chinese Health Study, approximately 13.7 years after initial blood collection used in our qPCR measurement. The overall Spearman correlation coefficient between the qPCR- and the flow cytometry–measured telomere lengths was 0.32 (P < 0.0001) after adjustment for age at first blood draw, suggesting that the baseline qPCR-measured telomere length was informative for long-term classification of individuals into long and short telomere categories (unpublished data).
In conclusion, we found that longer telomeres in white blood cells were significantly associated with a higher risk of colorectal cancer and particularly rectal cancer. The increasing trend of developing colorectal cancer associated with longer leukocyte telomeres is more apparent among men, never smokers, and individuals without diabetes. Our findings support a potential role of longer telomeres in the pathogenesis of colorectal cancer and thus might have a clinical relevance to be used as a part of aging and other biomarkers for an eventual risk prediction model.
CONFLICTS OF INTEREST
Guarantor of the article: Jian-Min Yuan, MD, PhD.
Specific author contributions: Planning and/or conducting the study: J.-M.Y., R.W., J.A.-H., and W.-P.K. Collecting and/or interpreting data: H.N.L., M.Q., R.W., J.A.-H., I.M., P.L.O., A.J., W.-P. K., and J.-M.Y. Drafting the manuscript: H.N.L., M.Q., and J.-M.Y. All authors approved the final submitted manuscript.
Financial support: The Singapore Chinese Health Study was supported by the National Institutes of Health (NIH) of the United States (grants R01 CA144034 and UM1 CA182876). W.-P. K. is supported by the National Medical Research Council, Singapore (NMRC/CSA/0055/2013). H.N.L. is partially supported by the United States NIH Grant 1P20CA210300-01 and the University of Pittsburgh Medical Center Hillman Cancer Center start-up grant.
Potential competing interests: None.
Study Highlights.
WHAT IS KNOWN
✓ Telomeres and telomerase play important roles in maintaining chromosome integrity and genomic stability.
✓ There are inconsistent results with the association between leukocyte telomere length and colorectal cancer risk.
WHAT IS NEW HERE
✓ Longer leukocyte telomere length is associated with increased risk of colorectal cancer, particularly rectal cancer.
TRANSLATIONAL IMPACT
✓ Longer telomere length might have a clinical relevance to be used as a part of aging and other biomarkers for an eventual risk prediction model.
ACKNOWLEDGEMENTS
We thank Kenneth Beckman, Shalane Porter, and Dinesha Walek of the University of Minnesota Genetic Center for measuring telomere length using the qPCR method and the Singapore Cancer Registry for the identification of incident cancer cases among participants of the Singapore Chinese Health Study. We also thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study.
REFERENCES
- 1.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers 2015;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ries LAG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review: 1975–2004. National Cancer Institute: Bethesda, MD, 2004. [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- 5.Ma X, Zhang B, Zheng W. Genetic variants associated with colorectal cancer risk: Comprehensive research synopsis, meta-analysis, and epidemiological evidence. Gut 2014;63:326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters U, Bien S, Zubair N. Genetic architecture of colorectal cancer. Gut 2015;64:1623–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Z, Pan X, Liu L, et al. Telomere length maintenance, shortening, and lengthening. J Cel Physiol 2014;229:1323–9. [DOI] [PubMed] [Google Scholar]
- 8.Liu YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev 2002;66:407–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart SA, Weinberg RA. Telomeres: Cancer to human aging. Annu Rev Cel Dev Biol 2006;22:531–57. [DOI] [PubMed] [Google Scholar]
- 10.Blackburn EH. Telomeres and telomerase: The means to the end (Nobel lecture). Angew Chem Int Ed Engl 2010;49:7405–21. [DOI] [PubMed] [Google Scholar]
- 11.Harley CB. Human ageing and telomeres. Ciba Found Symp 1997;211:129–44;discussion 139–44. [DOI] [PubMed] [Google Scholar]
- 12.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: A twin study of three age groups. Am J Hum Genet 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 13.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005;366:662–4. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 15.Aviv A, Anderson JJ, Shay JW. Mutations, cancer and the telomere length paradox. Trends Cancer 2017;3:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boardman LA, Litzelman K, Seo S, et al. The association of telomere length with colorectal cancer differs by the age of cancer onset. Clin Transl Gastroenterol 2014;5:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellatt AJ, Wolff RK, Lundgreen A, et al. Genetic and lifestyle influence on telomere length and subsequent risk of colon cancer in a case control study. Int J Mol Epidemiol Genet 2012;3:184–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IM, Lin J, Castonguay AJ, et al. Mean leukocyte telomere length and risk of incident colorectal carcinoma in women: A prospective, nested case-control study. Clin Chem Lab Med 2010;48:259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zee RYL, Castonguay AJ, Barton NS, et al. Mean telomere length and risk of incident colorectal carcinoma: A prospective, nested case-control approach. Cancer Epidemiol Biomark Prev 2009;18:2280–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui Y, Cai Q, Qu S, et al. Association of leukocyte telomere length with colorectal cancer risk: Nested case-control findings from the Shanghai Women's Health study. Cancer Epidemiol Biomark Prev 2012;21:1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Q, Sun J, Yin J, et al. Telomere length in peripheral blood leukocytes is associated with risk of colorectal cancer in Chinese population. PLoS One 2014;9:e88135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan JM, Stram DO, Arakawa K, et al. Dietary cryptoxanthin and reduced risk of lung cancer: The Singapore Chinese Health study. Cancer Epidemiol Biomark Prev 2003;12:890–8. [PubMed] [Google Scholar]
- 23.Seow A, Shi CY, Chung FL, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: Relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomark Prev 1998;7:775–81. [PubMed] [Google Scholar]
- 24.Seow A, Shi CY, Franke AA, et al. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomark Prev 1998;7:135–40. [PubMed] [Google Scholar]
- 25.Hankin JH, Stram DO, Arakawa K, et al. Singapore Chinese Health study: Development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001;39:187–95. [DOI] [PubMed] [Google Scholar]
- 26.Parkin DM, Whelan S, Ferlay J, et al. (eds). Cancer incidence in five continents. Vol. 8 In: Cancer Incidence in Five Continents. Lyon, France: IARC Press, 2002. [Google Scholar]
- 27.Yuan JM, Beckman KB, Wang R, et al. Leukocyte telomere length in relation to risk of lung adenocarcinoma incidence: Findings from the Singapore Chinese Health Study. Int J Cancer 2018;142:2234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009;37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 30.Tsong WH, Koh WP, Yuan JM, et al. Cigarettes and alcohol in relation to colorectal cancer: The Singapore Chinese Health study. Br J Cancer 2007;96:821–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman DG, Bland JM. Interaction revisited: The difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seow A, Yuan JM, Koh WP, et al. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst 2006;98:135–8. [DOI] [PubMed] [Google Scholar]
- 33.Eaglehouse YL, Koh WP, Wang R, et al. Physical activity, sedentary time, and risk of colorectal cancer: The Singapore Chinese Health study. Eur J Cancer Prev 2017;26:469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Koh WP, Jin A, et al. Telomere length and risk of developing gastric adenocarcinoma: The Singapore Chinese Health Study. Gastric Cancer 2018;21:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luu HN, Long J, Wen W, et al. Association between genetic risk score for telomere length and risk of breast cancer. Cancer Causes Control 2016;27:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Espiridion B, Chen M, Chang JY, et al. Telomere length in peripheral blood leukocytes and lung cancer risk: A large case-control study in Caucasians. Cancer Res 2014;74:2476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seow WJ, Cawthon RM, Purdue MP, et al. Telomere length in white blood cell DNA and lung cancer: A pooled analysis of three prospective cohorts. Cancer Res 2014;74:4090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doherty JA, Grieshober L, Houck JR, et al. Nested case-control study of telomere length and lung cancer risk among heavy smokers in the β-Carotene and Retinol Efficacy Trial. Br J Cancer 2018;118:1513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rode L, Nordestgaard BG, Bojesen SE. Long telomeres and cancer risk among 95,568 individuals from the general population. Int J Epidemiol 2016;45:1634–43. [DOI] [PubMed] [Google Scholar]
- 40.Hou L, Joyce BT, Gao T, et al. Blood telomere length attrition and cancer development in the normative aging study cohort. EBioMedicine 2015;2:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 2013;4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valls-Bautista C, Piñol-Felis C, Reñé-Espinet JM, et al. In colon cancer, normal colon tissue and blood cells have altered telomere lengths. J Surg Oncol 2015;111:899–904. [DOI] [PubMed] [Google Scholar]
- 43.Odegaard AO, Koh WP, Yu MC, et al. Body mass index and risk of colorectal cancer in Chinese Singaporeans: The Singapore Chinese Health study. Cancer 2011;117:3841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan M, Stone RC, Hunt SC, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc 2010;5:1596–607. [DOI] [PubMed] [Google Scholar]
- 45.Aviv A, Hunt SC, Lin J, et al. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res 2011;39:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bull CF, O'Callaghan NJ, Mayrhofer G, et al. Telomere length in lymphocytes of older South Australian men may be inversely associated with plasma homocysteine. Rejuvenation Res 2009;12:341–9. [DOI] [PubMed] [Google Scholar]