OBJECTIVES:
The role of palliative gastrectomy in the management of metastatic gastric cancer remains inadequately clarified.
METHODS:
We analyzed patients with metastatic gastric cancer enrolled in the Surveillance, Epidemiology, and End Results registry from January 2004 to December 2012. Propensity score (PS) analysis with 1:1 matching and the nearest neighbor matching method was performed to ensure well-balanced characteristics of the groups of patients who undergone gastrectomy and those without gastrectomy. Data were analyzed by Kaplan-Meier and Cox proportional hazards regression models to evaluate the overall survival and cancer-specific survival rates with corresponding 95% confidence intervals (CIs).
RESULTS:
In general, receiving any kind of gastrectomy was associated with an improvement in survival in the multivariate analyses (hazard ratio [HR]os = 0.64, 95% CI = 0.59–0.70, HRcss = 0.63, 95% CI = 0.57–0.68) and PS matching (PSM) analyses (HRos = 0.63, 95% CI = 0.56–0.70, HRcss = 0.62, 95% CI = 0.55–0.70). After PSM, palliative gastrectomy was found to be associated with remarkably improved survival for patients with stage M1 with only 1 metastasis but not associated with survival of patients with stage M1 with extensive metastasis (≥2 metastatic sites).
DISCUSSION:
The results obtained from the Surveillance, Epidemiology, and End Results database suggest that patients with metastatic gastric cancer might benefit from palliative gastrectomy on the basis of chemotherapy. However, a PSM cohort study of this kind still has a strong selection bias and cannot replace a properly conducted randomized controlled trial.
INTRODUCTION
Gastric cancer is one of the most fatal cancers. Although its incidence has decreased during the last decade (1,2), it is still the second leading cause of cancer-related mortality worldwide (3). It is often diagnosed at the metastatic stage. Unfortunately, metastatic gastric cancer has a poor prognosis, and the expected overall survival (OS) of metastatic gastric cancer rarely exceeds 1 year, even with new chemotherapy regimens (4–6). These patients are currently not considered as candidates for adequate radical resection, which has currently been considered the only curative treatment modality.
For metastatic gastric cancer, palliative gastrectomy or bypass surgery remains important in relieving symptoms and is generally used in emergencies. It has also been shown that palliative gastrectomy is better than bypass surgery in relieving symptoms such as obstruction, tumor bleeding, or perforation (7–9). Although the REGATTA trial, a phrase III trial, suggested that gastrectomy with chemotherapy could not achieve better survival than chemotherapy alone (10), many other studies found that gastrectomy with chemotherapy improved the survival of patients with stage IV gastric cancer (11–15). The REGATTA trial was terminated early because of futility. Moreover, it has been criticized for the lack of data on quality of life, inclusion of Asian patients only, and poor patients' acceptance of random assignment. In particular, the trial has been criticized for its chemotherapy regimen because the chemotherapy regimen was administered orally and affected by the surgical treatment. In addition, most patients had peritoneal carcinomatosis, which can increase postsurgical problems (10,16,17). Clearly, there is no universally accepted standard of care for metastatic gastric cancer treatment, and uncertainty still exists regarding the role of palliative gastrectomy in this setting.
Thus, we hypothesized that metastatic gastric cancer can benefit from gastrectomy. We tested this hypothesis using the large Surveillance, Epidemiology, and End Results (SEER) registry database.
METHODS
Study population and data sources
SEER encompasses population-based cancer registries covering approximately 28% of the US population and records basic demographics and some clinical characteristics (18). SEER*stat software (version 8.3.4) was used to select patients. Eligible participants were identified from the SEER database if they were pathologically diagnosed with invasive adenocarcinoma of the stomach (International Classification of Diseases for Oncology, third version, codes 8140, 8144, 8211, 8255, 8260, 8323, 8480, and 8481) between January 2004 and December 2012. However, SEER contains metastatic records of bone, liver, brain, and lung at diagnosis only after 2010; therefore, the analyses for metastatic sites were confined to data after 2010. Participants with stage M1, 1 primary only, active follow-up, survival time more than 3 months, and complete data for age, race, and year of diagnosis were included in our study. To increase comparability to other studies, the follow-up time was limited to 5 years. Gastrectomy was defined as surgery in the primary site, including near-total or total gastrectomy and gastrectomy with a resection in continuity with the resection of other organs (SEER RX Summ--Surg Prim Site (1998+), codes 30-33, 40-42, 50-52, 60-63, 80, and 90). As a result, a total of 5,640 patients were identified for this study, including those who received any kind of gastrectomy (cases, n = 4,651) and those who did not receive any kind of gastrectomy (controls, n = 989) (Figure 1).
Figure 1.
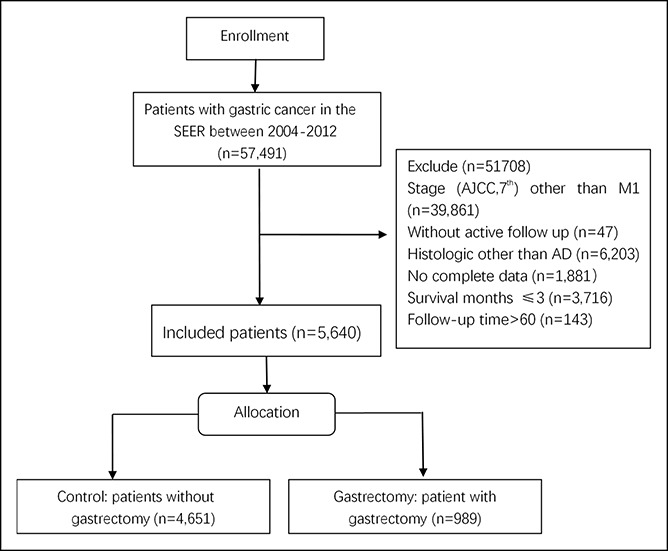
Flowchart of study population selection.
Propensity score matching
Propensity score matching (PSM) is a tool to reduce the selection bias in nonrandomized studies. Propensity 1:1 nearest neighbor matching with propensity score (PS) was performed to reduce possible bias using a 0.02 caliper width (19). χ2 tests were used to examine the covariate balance. A multivariate logistic regression model was used to calculate PSs for each patient in the group. Selected covariates were age, T-stage, N-stage, grade, race, radiotherapy, and chemotherapy, which were selected based on the statistical difference in the univariate logistic regression model and clinical significance.
Statistical analysis
χ2 tests were used to compare the distribution of demographic characteristics. Univariate and multivariate Cox proportional hazards models were constructed to evaluate the hazard ratio (HR) and 95% confidence interval (CI) between patients treated with and without gastrectomy. OS was defined as the time from diagnosis to death of any cause; patients who were alive at the time of the last follow-up were censored. Cause-specific survival (CSS) was defined as the time from diagnosis to death related to gastric cancer only. Patients with any other causes of death or still alive were censored at the time of death or the last follow-up.
To test our hypothesis that OS and CSS are associated with gastrectomy in patients with metastatic gastric cancer at diagnosis, variables were forcefully selected based on clinical significance. Variables were used for propensity scoring and entered into final multivariable Cox regression models. In all multivariable models, the continuous covariates of age and the categorical covariates including sex, region, race, income, surgery, chemotherapy, T-stage, N-stage, and grades stratified as in Table 1 were used.
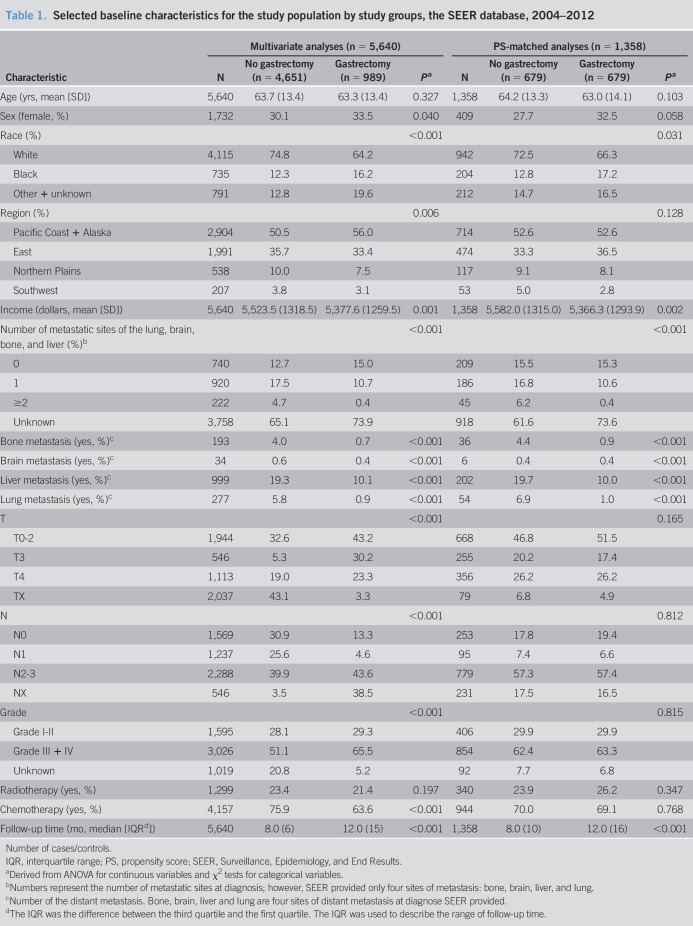
Table 1.
Selected baseline characteristics for the study population by study groups, the SEER database, 2004–2012
Age was used as the time scale for all models, with entry time defined as age at diagnosis and exit time defined as age at death, last follow-up, or December 31, 2014, whichever came first. P values of ≤0.05 (2-sided probability) and P values for interaction of ≤0.10 (2-sided probability) were considered statistically significant. All analyses were conducted using SPSS 22 (IBM Corp, Armonk, NY).
RESULTS
All patients included in the analyses had complete information. In the SEER database, missing data that met our selection criteria were approximately 3% of the total number. We performed sensitivity analyses by inclusion and exclusion of missing data. There was no appreciable difference. Therefore, we excluded missing data in our analysis (Figure 1). Distributions of characteristics are presented in Table 1 for the study groups categorized by receiving any kind of gastrectomy or not. There were 5,114 patients who died of gastric cancer, and 526 patients who were alive or died of other causes for a total to 5,640 patients in the analysis. A total of 989 patients (17.5%) received gastrectomy. Before PSM, cases were more likely to be older, had a lower family income, had received chemotherapy and radiotherapy, and presented a well-defined T-stage, N-stage and grade compared with controls. The distributions of most demographic and clinical factors were well balanced between cases and controls after PSM by 6 factors (age, chemotherapy, radiotherapy, T-stage, N-stage, and grade). In this study, 679 pairs were matched using PSM.
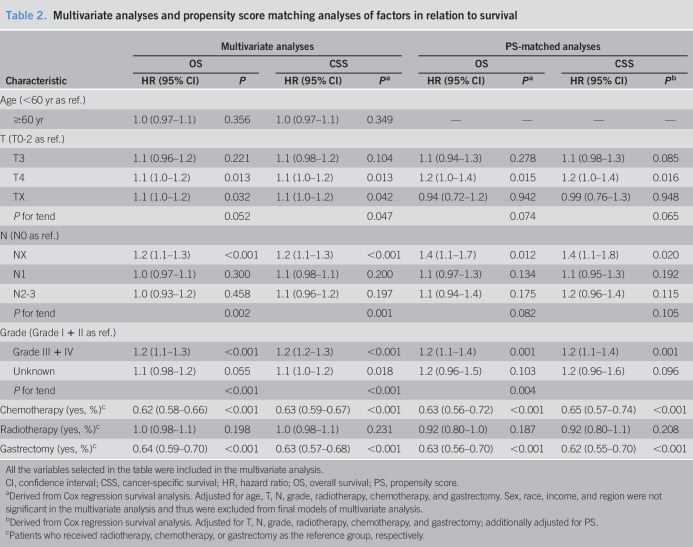
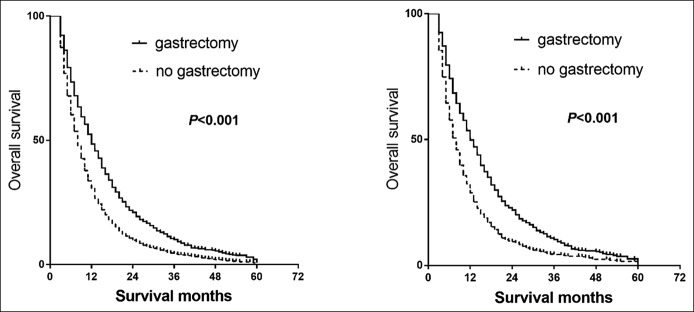
In the multivariate analyses, as expected, patients who received gastrectomy were associated with a better OS (HRos = 0.64, 95% CI = 0.59–0.70, Pos < 0.001) and CSS (HRcss = 0.63, 95% CI = 0.57–0.68, Pcss < 0.001) (Table 2, Figure 2a). Cases with poorer T-stages and N-stages were associated with poorer OS and CSS rates. Receiving chemotherapy was found to be strongly associated with better survival. However, age, sex, race, region, income, histology grade, and radiotherapy were not found to be associated with better survival. In PSM analyses, the results indicated that survival improvement was significantly associated with chemotherapy and gastrectomy (HRos = 0.63, 95% CI = 0.56–0.70, Pcss < 0.001; HRcss = 0.62, 95% CI = 0.55–0.70, Pos < 0.001) (Figure 2b). Radiotherapy was not associated with improved OS or CSS.
Table 2.
Multivariate analyses and propensity score matching analyses of factors in relation to survival
Figure 2.
Effects of any gastrectomy on the OS for patients with metastatic gastric cancer in multivariate analysis and PSM analysis. P values were derived from the log-rank statistics of Kaplan-Meier models in PSM analysis. (a) OS of patients with metastatic gastric cancer in multivariate analysis. (b) OS of patients with metastatic gastric cancer in PSM analysis. OS, overall survival; PSM, propensity score matching.
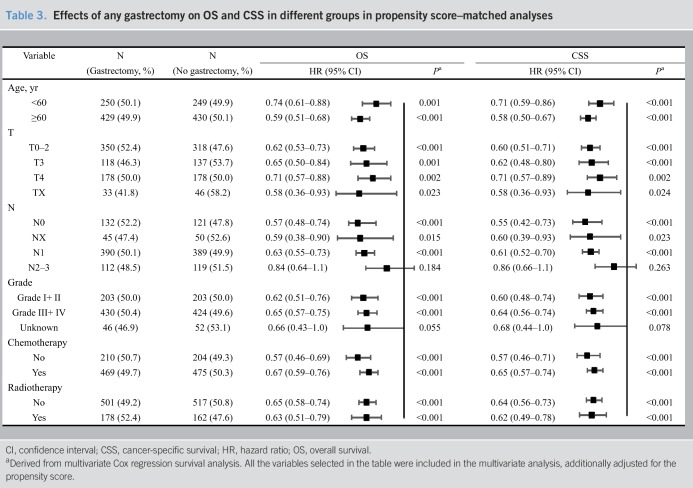
Palliative gastrectomy benefits metastatic gastric cancer patients on OS and CSS by different groups were observed after matching (Table 3). After PSM, numerically, gastrectomy was found to be more associated with survival benefit in patients who were young and with a better T-stage, N-stage, and pathological grade. After PSM, significant heterogeneities for the effect of gastrectomy were observed between patients with chemotherapy and those without (PHeterogeneity for OS or CSS <0.10).
Table 3.
Effects of any gastrectomy on OS and CSS in different groups in propensity score–matched analyses
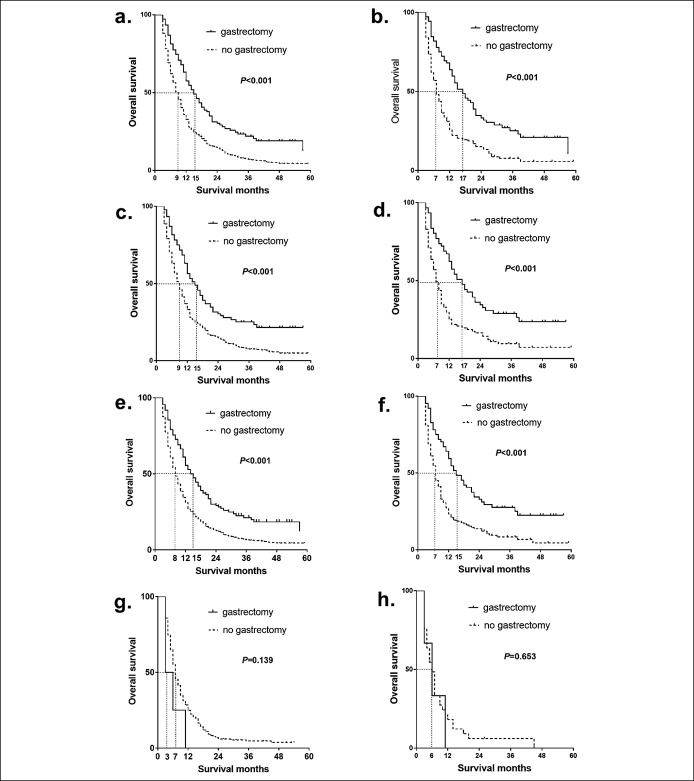
For patients with only 1 metastatic site, the median survival for those who received gastrectomy was 6.0 months longer than those who did not in the multivariate analyses and 10.0 months longer in PSM analyses (Figure 3a,b). For example, the median OS for patients with liver metastasis only was improved from 9.0 months to 15.0 months before PSM (Pos < 0.001) (Figure 3c) and from 7.0 months to 17.0 months in PSM analyses (Pos < 0.001) (Figure 3d). However, the sample size of patients with lung, brain, or bone metastasis only was too small to analyze. In PSM analyses, palliative gastrectomy was associated with survival benefit by 8 months in patients with ≥1 metastatic site(s) (Figure 3e,f). However, there was no association between gastrectomy and survival benefit in patients with metastatic gastric cancer who had more than 2 metastatic sites (Figure 3g,h).
Figure 3.
Effects of any gastrectomy on the overall survival of different metastatic sites for patients with metastatic gastric cancer. (a) OS of patients with metastatic gastric cancer with only 1 metastatic site in multivariate analysis. (b) OS of patients with metastatic gastric cancer with only 1 metastatic site in PSM analysis. (c) OS of patients with metastatic gastric cancer with only liver metastatic in multivariate analysis. (d) OS of patients with metastatic gastric cancer with only liver metastatic in PSM analysis. (e) OS of patients with metastatic gastric cancer with ≥1 metastatic site(s) in multivariate analysis. (f) OS of patients with metastatic gastric cancer with ≥1 metastatic site(s) in PSM analysis. (g) OS of patients with metastatic gastric cancer with ≥2 metastatic sites in multivariate analysis. (h) OS of patients with metastatic gastric cancer with ≥2 metastatic sites in PSM analysis. All P values were derived from the log-rank statistics of Kaplan-Meier models. OS, overall survival; PSM, propensity score matching.
DISCUSSION
To our knowledge, the present study is the first population-based SEER analysis using both multivariate regression analyses and PSM analyses to evaluate the role of palliative gastrectomy in the treatment of metastatic gastric cancer. In this study, we found that gastrectomy, in general, was associated with better survival of metastatic gastric cancer. The beneficial effect of the survival observed using the SEER database highlights the importance of palliative gastrectomy in the management of metastatic gastric cancer.
The 5-year survival rate of patients with metastatic gastric cancer rarely exceeds 5% (20,21). For metastatic gastric cancer, the effectiveness of palliative gastrectomy is controversial (15,22–24). In patients with metastatic gastric cancer, palliative resection of the primary tumor is recommended if obstruction or uncontrolled hemorrhage occurs according to National Comprehensive Cancer Network guidelines (25). In addition, prophylactic gastrectomy in some high-risk patients may not only improve symptom control but also eliminate potential complications (hemorrhage, obstruction, perforation, or debilitating ascites) caused by the primary tumor (26). Thus, metastatic gastric cancer may benefit from palliative gastrectomy.
In the past, gastric resection was considered valueless in treating metastatic disease, partially because of high surgical mortality rates (3,15). Since the 1980s, many studies have reported that patients with metastatic gastric cancer who underwent surgical removal of the gastric primary lesion had greater survival rates than nonoperated patients (14,15,27,28). Saidi et al. (22) (14.4 vs 11.2 months, P = 0.37) and Sarela and Yelluri (29) (8.0 vs 4.0 months, P = 0.30) reported that there was a trend of better survival in patients with gastrectomy in metastatic gastric cancer. Huang et al. (30) reported that the OS of patients who underwent resection was 10.2 months and that of patients without resection was 4.48 months (P < 0.001). Despite previously published cohort studies showing better outcomes, the observational and nonrandomized nature of these studies must be put into perspective. Comparisons between gastrectomy followed by chemotherapy and chemotherapy alone in observational studies were usually confounded by data heterogeneity, selection or publication bias, and by the large variation in the chemotherapy regimens used owing to a lack of treatment standardization across geographical regions. However, the REGATTA trial showed that the median OS was 16.6 months for patients receiving chemotherapy alone and 14.3 months for those assigned to gastrectomy plus chemotherapy (P = 0.70) (10). Nevertheless, the trial was terminated early and had some significant limitations (16). One is that most of the patients, including unselected patients, in the trial underwent total gastrectomy, which is known to have a higher risk of postoperative complications than distal gastrectomy (31). In other words, their results may have been biased considering that approximately 70% of gastric cancer surgeries in Korea and Japan are distal gastrectomies. Second, the REGATTA trial used S-1 and cisplatin for chemotherapy. The administration of oral medications was heavily influenced by postgastrectomy complications, which made it difficult to evaluate the effect of palliative gastrectomy itself. In addition, we believe that palliative gastrectomy should be performed in selected patients, especially in those patients with complications (e.g., hemorrhage, obstruction, or perforation) or with the risk of complications rather than in unselected patients as the REGATTA trial did. Therefore, whether their results can be generalized to patients with metastatic gastric cancer remains unclear. Thus, phase III randomized controlled trials (RCTs) that overcome the flaws of REGATTA are urgently needed to clarify the role of palliative resection in patients with metastatic gastric cancer.
This study showed that gastrectomy was significantly associated with better survival in patients with metastatic gastric cancer with 1 metastasis, but no survival improvement was observed in patients with metastatic sites in 2 or more organs. The consistent findings from this study supported that palliative gastrectomy was related to the survival improvement of patients with metastatic gastric cancer.
Gastrectomy in this study should be considered as palliative resection. Clinically, curative resection is not recommended for patients with metastatic gastric cancer disease (25,32). Gastrectomy was mostly administered to patients with unfavorable metastatic gastric cancer for palliative purposes, such as controlling perforation, or hemorrhage. The prognosis of these unfavorable patients who were treated with gastrectomy may not be as good as patients with metastatic gastric cancer without unfavorable factors if they did not receive gastrectomy. Hence, the survival benefit seen from the current analysis cannot be purely explained by treatment selection biases after adjusting for possible confounding factors and PSM. Although adjusted with PSM, the survival benefits of patients with metastatic gastric cancer observed in this study could be associated with palliative resection of primary gastric tumor lesions or due to confounding from unmeasurable variables.
Nonetheless, metastatic gastric cancer is a systematic disease; palliative gastrectomy might benefit some selected patients, and the benefit should also be conditioned on adquate systemic therapy (33–35). The median OS for patients with and without adjuvant chemotherapy was 12.3 months and 7.7 months, respectively (P = 0.065), as reported by Huang et al. (30). Our results also showed that chemotherapy improved the survival of patients with metastatic gastric cancer.
We acknowledge several limitations to this study. First, as with any observational study, the possibility of bias is a concern. Although the sensitivity analysis showed that the missing data had no effect on the results, the exclusion of the missing data may also be a source of bias. We used the PSM method, which might reduce the bias caused by the imbalanced distribution of measured covariates. Nevertheless, bias from unmeasured factors shall be unavoidable. Unlike RCTs, SEER registry data usually have high completeness and are representative of the real-world patient population. Although our results might be applicable to real-world patients, we acknowledge that patient status, surgical methods, and second-line chemotherapy may all have contributed to study bias. Although it is preferable to obtain more details, we sought to show the survival advantage of any surgery involving the primary lesion for patients with metastatic gastric cancer.
In addition, the SEER registry does not provide any data on risk factors of gastric cancer, which may have an impact on survival. Nevertheless, the study participants were recruited through a representative national database, thus reducing possible selection bias. Multivariable analyses, PSM analyses, and sensitivity analyses were performed, and the results of OS and CSS did not change appreciably and thus seemed stable and valid. Consistent with previous reports (36), we found that the survival benefit of gastrectomy was, to some extent, conditioned on age, T-stage, N-stage, the degree of pathological differentiation, and chemotherapy. Thus, this consistency reinforced our findings. Nevertheless, prospective trials are required to clarify the role of palliative gastrectomy in the treatment of metastatic gastric cancer and to tease out what kind of surgery for specific metastatic gastric cancer patients is most beneficial.
In conclusion, the present study using the SEER database suggests that palliative gastrectomy is associated with survival improvement in patients with metastatic gastric cancer. However, a PSM cohort study of this kind still has a strong selection bias and cannot replace a properly conducted RCT. Therefore, we call for well-designed phase III RCTs to further clarify the survival benefit from palliative gastrectomy for patients with metastatic gastric cancer.
CONFLICTS OF INTEREST
Guarantor of the article: Zhenming Fu, MD, PhD.
Specific author contributions: Qin Li and Jiahua Zou contributed equally to this work. J.Z., M.J., and Z.F. designed and directed the study. Q.L., J.Z., and Z.F. analyzed the data. Q.L., P.L., and Z.F. drafted the manuscript. Z.F. and Q.S. supervised the study. R.Z., J.H., K.H., and Y.Q. helped with the statistical analysis and data cleaning. T.X., R.P., and Q.S. provided clinical insights, performed the literature review, and helped with the drafting of the manuscript. All authors reviewed and approved the final draft. This article is not under consideration for publication elsewhere.
Financial support: This study was supported by Grants 81472971 and 81773555 from the National Science Foundation of China (NSFC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NSFC.
Potential competing interests: The authors have no conflicts of interest.
Study Highlights.
WHAT IS KNOWN
✓ Gastric cancer is often diagnosed at the metastatic stage and has a poor prognosis.
✓ Palliative gastrectomy remains important in relieving symptoms and is generally used in emergencies.
WHAT IS NEW HERE
✓ Palliative gastrectomy is associated with survival improvement in metastatic gastric cancer patients.
✓ Gastrectomy was significantly associated with better survival in one metastasis but no survival improvement in patients with metastatic sites in two or more organs.
TRANSLATIONAL IMPACT
✓ Results of this study may provide some reference for clinicians treating the metastatic gastric cancer patients.
REFERENCES
- 1.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19(8):1893–907. [DOI] [PubMed] [Google Scholar]
- 2.de Martel C, Forman D, Plummer M. Gastric cancer: Epidemiology and risk factors. Gastroenterol Clin North Am 2013;42(2):219–40. [DOI] [PubMed] [Google Scholar]
- 3.Yazıcı O, Özdemir N, Duran AO, et al. The effect of the gastrectomy on survival in patients with metastatic gastric cancer: A study of ASMO. Future Oncol 2016;12(3):343–54. [DOI] [PubMed] [Google Scholar]
- 4.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol 2008;9(3):215–21. [DOI] [PubMed] [Google Scholar]
- 5.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: A randomised phase 3 study. Lancet Oncol 2009;10(11):1063–9. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29(30):3968–76. [DOI] [PubMed] [Google Scholar]
- 7.Dittmar Y, Rauchfuss F, Goetz M, et al. Non-curative gastric resection for patients with stage 4 gastric cancer—A single center experience and current review of literature. Langenbecks Arch Surg 2012;397(5):745–53. [DOI] [PubMed] [Google Scholar]
- 8.Kulig P, Sierzega M, Kowalczyk T, et al. Non-curative gastrectomy for metastatic gastric cancer: Rationale and long-term outcome in multicenter settings. Eur J Surg Oncol 2012;38(6):490–6. [DOI] [PubMed] [Google Scholar]
- 9.Medina-Franco H, Contreras-Saldivar A, Ramos-De La Medina A, et al. Surgery for stage IV gastric cancer. Am J Surg 2004;187(4):543–6. [DOI] [PubMed] [Google Scholar]
- 10.Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol 2016;17(3):309–18. [DOI] [PubMed] [Google Scholar]
- 11.Koga S, Kawaguchi H, Kishimoto H, et al. Therapeutic significance of noncurative gastrectomy for gastric cancer with liver metastasis. Am J Surg 1980;140(3):356–9. [DOI] [PubMed] [Google Scholar]
- 12.Bozzetti F, Bonfanti G, Audisio RA, et al. Prognosis of patients after palliative surgical procedures for carcinoma of the stomach. Surg Gynecol Obstet 1987;164(2):151–4. [PubMed] [Google Scholar]
- 13.Ouchi K, Sugawara T, Ono H, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol 1998;69(1):41–4. [DOI] [PubMed] [Google Scholar]
- 14.Bonenkamp JJ, Sasako M, Hermans J, et al. Tumor load and surgical palliation in gastric cancer. Hepatogastroenterology 2001;48(41):1219–21. [PubMed] [Google Scholar]
- 15.Hartgrink HH, Putter H, Klein Kranenbarg E, et al. Value of palliative resection in gastric cancer. Br J Surg 2002;89(11):1438–43. [DOI] [PubMed] [Google Scholar]
- 16.Oh S-Y, Lee H-J. REGATTA trial: Its achievement and the issues unsolved. Translational Cancer Res 2017;6(Suppl 6):S976–8. [Google Scholar]
- 17.D'Ugo D, Cananzi FC, Persiani R, et al. REGATTA trial: A call for the USA and Europe. Lancet Oncol 2016;17(3):261–2. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Overview of the SEER program (https://seer.cancer.gov/about/). Accessed on February 12, 2019.
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 20.Romano F, Garancini M, Uggeri F, et al. Surgical treatment of liver metastases of gastric cancer: State of the art. World J Surg Oncol 2012;10(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Yan M, Chen J, et al. Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg 2010;14(2):282–8. [DOI] [PubMed] [Google Scholar]
- 22.Saidi R, ReMine S, Dudrick P, et al. Is there a role for palliative gastrectomy in patients with stage IV gastric cancer? World J Surg 2006;30(1):21–7. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Lu H, Huang C, et al. Outcome of palliative total gastrectomy for stage IV proximal gastric cancer. Am J Surg 2011;202(1):91–6. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi KST, Ono H, Fujiya T, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol 1998;69(1):41–4. [DOI] [PubMed] [Google Scholar]
- 25.NCCN Clinical Practice Guidelines in Oncology, Gastric Cancer (Version 1.2018) (https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf). Accessed on March 1, 2018. [Google Scholar]
- 26.Miner TJ, Jaques DP, Karpeh MS, et al. Defining palliative surgery in patients receiving noncurative resections for gastric cancer. J Am Coll Surg 2004;198(6):1013–21. [DOI] [PubMed] [Google Scholar]
- 27.Haugstvedt T, Viste A, Eide G, et al. The survival benefit of resection in patients with advanced stomach cancer the Norwegian multicenter experience. World J Surg 1989;13(5):617–21. [DOI] [PubMed] [Google Scholar]
- 28.Monson DJ, Jr, Mcilrath DC, Farnell MB, et al. Total gastrectomy for advanced cancer: A worthwhile palliative procedure. Cancer 1991;68(9):1863–8. [DOI] [PubMed] [Google Scholar]
- 29.Sarela AI, Yelluri S. Gastric adenocarcinoma with distant metastasis: Is gastrectomy necessary? Arch Surg 2007;142(2):143–9; discussion 149. [DOI] [PubMed] [Google Scholar]
- 30.Huang KH, Wu CW, Fang WL, et al. Palliative resection in noncurative gastric cancer patients. World J Surg 2010;34(5):1015–21. [DOI] [PubMed] [Google Scholar]
- 31.Lee KG, Lee HJ, Yang JY, et al. Risk factors associated with complication following gastrectomy for gastric cancer: Retrospective analysis of prospectively collected data based on the Clavien-Dindo system. J Gastrointest Surg 2014;18(7):1269–77. [DOI] [PubMed] [Google Scholar]
- 32.Blakely A, Miner T. Surgical considerations in the treatment of gastric cancer. Gastroenterol Clin North Am 2013;42(2):337–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu JL, Deng MG, Li W, et al. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol 2013;39(7):694–700. [DOI] [PubMed] [Google Scholar]
- 34.Jeung HC, Rha SY, Jang WI, et al. Treatment of advanced gastric cancer by palliative gastrectomy, cytoreductive therapy and postoperative intraperitoneal chemotherapy. Br J Surg 2002;89(4):460–6. [DOI] [PubMed] [Google Scholar]
- 35.Lin SZ, Tong HF, You T, et al. Palliative gastrectomy and chemotherapy for stage IV gastric cancer. J Cancer Res Clin Oncol 2008;134(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiberio GA, Baiocchi GL, Morgagni P, et al. Gastric cancer and synchronous hepatic metastases: Is it possible to recognize candidates to R0 resection? Ann Surg Oncol 2015;22(2):589–96. [DOI] [PubMed] [Google Scholar]