OBJECTIVES:
Fecal microbiota transfer (FMT) is suggested as a potential treatment for patients with irritable bowel syndrome (IBS). We aimed to study the effect of allogenic and autologous FMT on IBS symptoms, visceral sensitivity, and compositional changes in fecal and mucosa-adherent microbiota.
METHODS:
Seventeen patients with IBS were randomized either to receive fecal material from a healthy donor (allogenic) or to receive their own fecal material (autologous). The fecal material was administered into the cecum by whole colonoscopy after bowel cleansing.
RESULTS:
No significant differences were found between the allogenic and the autologous FMT regarding symptom scores. However, symptom scores of patients receiving allogenic fecal material significantly decreased after FMT compared with baseline (P = 0.02), which was not the case in the autologous group (P = 0.16). Visceral sensitivity was not affected except for a small beneficial effect on urge scores in the autologous group (P < 0.05). While both fecal and mucosa-adherent microbiota of some patients shifted to their respective donor's fecal microbiota, some patients showed no relevant microbial changes after allogenic FMT. Large compositional shifts in fecal and mucosa-adherent microbiota also occurred in the autologous group.
CONCLUSIONS:
This study showed that a single FMT by colonoscopy may have beneficial effects in IBS; however, the allogenic fecal material was not superior to the autologous fecal material. This suggests that bowel cleansing prior to the colonoscopy and/or processing of the fecal material as part of the FMT routine contribute to symptoms and gut microbiota composition changes in IBS.
INTRODUCTION
Fecal microbiota transfer (FMT) aims at introducing a new gut microbiota to the gastrointestinal system of a patient. It is found to be a safe, highly successful treatment in recurrent Clostridium difficile infection with cure rates of 90% and higher (1,2). Also, other diseases in which the gut microbiota plays a role became of interest for treatment with FMT. Placebo-controlled clinical studies in inflammatory bowel disease have shown that FMT leads to remission in 24%–30% of patients with ulcerative colitis (3–5). Similarly, studies in metabolic syndrome have shown that healthy donor FMT can improve insulin sensitivity (6,7). Due to the increasing evidence that a disturbed gut microbiota also plays a role in the pathophysiology of irritable bowel syndrome (IBS), FMT has been suggested as a potential treatment to improve symptoms in this study population. A recent randomized placebo-controlled clinical trial has studied the effect of FMT administered by colonoscopy on symptoms in 90 patients with IBS (8). More patients in the treatment group (65%) showed a decrease in IBS-severity scoring system (IBS-SSS) score of more than 75 points compared with the placebo group (43%) after 3 months; however, this was not significant anymore after 12 months (56% vs 36%). In an additional recent randomized clinical trial, the effect of FMT administered by capsules in 52 IBS patients was investigated. Although intake of the capsules over 12 days resulted in changes to the gut microbiota of the IBS patients in the treatment group, the symptom improvement after 3 months was larger in the placebo compared with the treatment group (9).
Nowadays, typical characteristics of a healthy gut microbiota are still not known. In IBS patients, butyrate-producing bacteria in fecal samples seem to be reduced compared with healthy subjects (10). Butyrate is a short-chain fatty acid produced in the large intestine by microbial fermentation of undigested dietary carbohydrates. It is the main energy source for colonocytes and has shown to have anti-inflammatory, anticarcinogenic and barrier-protecting properties (11). Butyrate enemas have shown to decrease visceral sensitivity in healthy volunteers and might decrease inflammation in inflammatory bowel disease (12,13). Previous studies demonstrated that the amount of butyrate-producing bacteria could be increased by FMT (7). Here, we describe the outcome of a controlled study investigating FMT in IBS, in which we included donors with a high abundance of butyrate-producing bacteria in their fecal samples and IBS patients with a low abundance of butyrate-producing bacteria. Apart from studying the effect of FMT on the symptoms of IBS patients, also its effect on the patients' visceral sensitivity and on the compositional changes in fecal and mucosa-adherent microbiota were assessed in order to propose mechanistic explanations for the observed responses.
METHODS
The study was conducted according to the principles of the Declaration of Helsinki and its revisions, and ethical approval was obtained from the Central Ethical Review Board of Uppsala, Sweden (registration number 2013/180). The study was performed at Örebro University Hospital in Örebro, Sweden, from May 2014 to April 2016. All participants were recruited in the greater area of Örebro and gave their written informed consent before participation. The trial has been registered at ClinicalTrials.gov (NCT02092402) on March 20, 2014.
Subjects
Patients.
Adult IBS patients that fulfilled the Rome III diagnostic criteria for IBS were included in this study (14). All IBS subtypes, including postinfectious IBS, were eligible for the study. Reasons for exclusion were any other known organic gastrointestinal disease, previous complicated gastrointestinal surgery, nongastrointestinal malignancy, dementia, severe depression, major psychiatric disorder, severe endometriosis, recent diagnosis of lactose intolerance, celiac disease, pregnancy or breast-feeding, antimicrobial treatment within 4 weeks prior to first screening visit, regular consumption of probiotics within 4 weeks prior to randomization, abuse of alcohol and drugs, or any other clinically significant disease/condition which in the investigator's opinion could interfere with the results of the trial. The patients were asked to keep their medication and diet stable over the entire study period. Only IBS patients with a low amount of butyrate-producing bacteria in their fecal samples were included. These bacteria were quantified by quantitative real-time polymerase chain reaction (qPCR) detection of the butyryl-CoA CoA transferase gene, encoding the last step of butyrate formation by gut bacteria (15). IBS patients with less than 50% abundance of this gene in the screening sample were compared to donor 1 qualified for enrolment.
Donors.
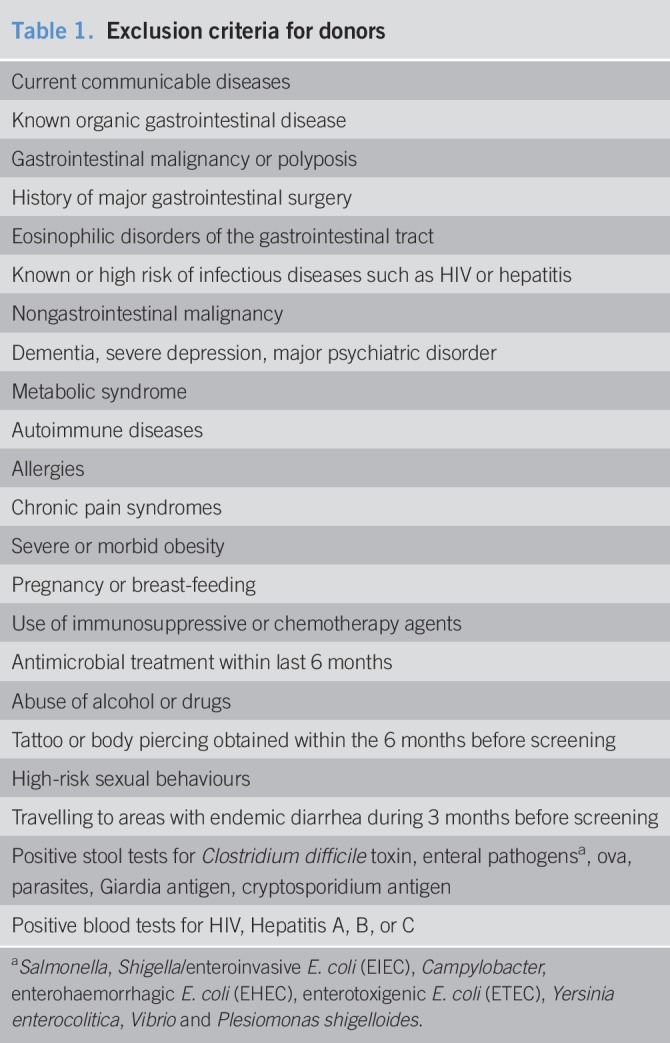
Healthy subjects were carefully screened before inclusion as donors. The exclusion criteria for donors are shown in Table 1 (16). Even though the use of proton pump inhibitors was not a specific exclusion criterion, none of the donors were taking proton pump inhibitors. All potential donors were screened for butyrate-producing bacteria in their fecal samples as described above for the IBS patients (15). The subject with the highest abundance of the butyryl-CoA CoA transferase gene in their fecal sample was selected as donor. After this donor was no longer available, a second donor was selected based on high abundance of this gene. Both donors regularly visited the research facility to donate fresh fecal material and underwent regular blood and stool tests during their participation in the study. The first 3 patients in the treatment group received stool from donor 1, the remaining 5 from donor 2.
Table 1.
Exclusion criteria for donors
Sample size calculations
Sample size calculations showed that to detect 30% difference in symptoms according to the gastrointestinal symptom rating scale (GSRS)-IBS upon allogeneic FMT treatment, compared with 5% difference after autologous treatment, with 80% power, the sample size in each group should be n = 8. As this was a proof-of-concept study using a per-protocol analysis, we aimed at including 8 evaluable patients per group, thus 16 patients in total. The subjects not completing the trial were replaced.
Study design, randomization, and masking
In this randomized controlled double-blinded study, the effect of FMT on IBS patients was studied. The subjects were randomized in a 1:1 manner to either the allogenic group, consisting of infusion of fecal material from a healthy donor, or the autologous group, in which subjects received their own fecal material, processed in the same way as the allogeneic samples. The randomization list was generated by a researcher not involved in the study. Shortly before a scheduled intervention visit, this researcher removed any information about allogenic or autologous treatment from the respective intervention, relabeled it with the day of intervention and the recipient's study code, and relocated the unused transplant in a freezer inaccessible to the researchers involved in the study. All participants and investigators remained blinded until the analyses of the primary outcome and the symptom scale data were completed. Statistical analysis of all the symptom scale data was performed blinded.
Fecal microbiota transfer procedure
The fecal material used for the FMT from the donors and patients was collected directly at the research facility. A total of 30 g of freshly delivered feces were carefully mixed with 0.9% sterile saline solution and then passed through a sterilized stainless steel strainer to remove larger particles. Then, 10% glycerol was added to a final volume of 150 mL (17). The fecal suspension was stored at −80 °C until use, for a maximum of 3 months.
The donor or autologous fecal material was administered into the cecum by colonoscopy. On the day before the colonoscopy, patients underwent a routine bowel cleansing to remove as much as possible of their own commensal gut microbiota and to facilitate the colonoscopy. Patients received 4 mg loperamide before the colonoscopy to retain the transplant. The colonoscopy was performed with support of mild sedation and carbon dioxide inflation in order to avoid oxygen exposure.
Primary and secondary outcomes
The primary outcome was the effect of FMT on IBS symptoms using the IBS version of the GSRS-IBS. The GSRS-IBS is a reliable, validated IBS-specific symptom scale (18). It includes 13 items in 5 symptom clusters (abdominal pain, bloating, constipation, diarrhea, and satiety) and measures symptoms during the past 7 days with a 7-point Likert scale ranging from 1 (no discomfort at all) to 7 (very severe discomfort). The participants completed the GSRS-IBS 2 weeks and 1 day before the FMT, as well as 2 weeks, 4 weeks, 8 weeks, and 6 months after the FMT, respectively.
Secondary outcomes were the effect of FMT on the patients' IBS symptoms using the IBS-SSS (19,20), their general health and quality of life (36-item Short Form Survey (SF-36) (20) and IBS-QoL (21)), as well as their anxiety and depression status using the hospital anxiety and depression scale (22). The IBS-SSS was assessed at the same time points as the GSRS-IBS, while the other scales were completed before as well as 2 and 8 weeks after the FMT. In addition, secondary outcomes included the effect of FMT on the IBS patients' visceral perception (pain, discomfort, and urge scores on a visual analogue scale (VAS) during barostat procedure), as well as the effect on IBS patients' fecal and mucosal microbiota composition (HITChip analysis) (23).
Symptom improvement of 30% was considered clinically relevant, and thus, responders to FMT treatment were defined by a decrease of 30% in the total GSRS-IBS symptom score at one of the assessed time points after FMT.
Adverse event assessment
Adverse events during the first week following the FMT were recorded daily by the participants using a written form that included documentation of body temperature. Subsequently, adverse events were recorded directly by the investigators at every study visit.
Visceral sensitivity assessment using the barostat procedure
Subjects fasted for 12 hours before assessment and were placed in a left lateral position to reduce the influence of adipose tissue mass and abdominal wall tone. The barostat catheter (600 mL; Mui Scientific, Ontario, Canada) was placed 10–15 cm into the rectum. Rectal distensions were performed according to our previous studies using an electronic distension device (Electronic barostat, distender series II; G & J Electronic, Toronto, Ontario, Canada) (24). First, the minimal distension pressure was assessed and set to zero as a reference point during the measurement of visceral sensitivity. For the latter, intermittent semi-random staircase distensions of 60 seconds duration (15, 10, 25, and 20 mm Hg, etc.) were separated by intervals of 30 seconds of baseline pressure. During each distension (after 30 seconds), subjects were asked to report their perception of pain, discomfort, and urge, respectively, using 100 mm VAS. The protocol was stopped when the perceptual threshold for maximal tolerable pain, discomfort, and/or urge was reached, or when the safety value of the maximal pressure of 61 mm Hg was exceeded.
Microbiota profiling
Fecal samples were collected from the patients 2 weeks before FMT, and 2 and 8 weeks after FMT. Samples were collected at home, immediately placed into the home freezer and returned frozen to the research facility where they were stored at −80 °C. Donor fecal samples were directly collected by the investigators from the samples donated for FMT. Mucosal biopsies from the uncleansed sigmoid were collected by sigmoidoscopy at a standardized location (20–25 cm from the anal verge at the crossing with the arteria iliaca communis) from the patients 2 weeks before FMT, and 2 and 8 weeks after FMT, and from the donors at one occasion. Microbial DNA from fecal samples was isolated using repeated bead beating (25) and the QIAamp DNA stool extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNA concentration was assessed using a Qubit spectrometer (Thermo Fisher Scientific, Boston, MA). Microbial DNA from mucosal samples was isolated using repeated bead beating (25) with some adjustments, including a proteinase K incubation prior to the mechanic cell disruption and use of a Maxwell extraction robot (Maxwell 16 Tissue LEV Total RNA Purification Kit; Promega, Madison, WI).
The abundance of the butyryl-CoA CoA transferase gene in fecal sample was assessed by qPCR performed with Applied Biosystems 7900HT Fast Real-Time PCR system (Life Technologies, Carlsbad, CA) using 5x HOT FIREPol EvaGreen qPCR Supermix (Solis BioDyne, Tartu, Estonia) and the primers BCoATscrF (GCI GAI CAT TTC ACI TGG AAY WSI TGG CAY ATG) and BCoATscrR (CCT GCC TTT GCA ATR TCI ACR AAN GC) (TAG Copenhagen A/S, Copenhagen, Denmark). DNA was diluted to 5 ng/μL before PCR amplification. The thermal cycling conditions of the qPCR was set to 95 °C for 15 minutes, followed by 40 cycles of 95 °C for 15 seconds, 53 °C for 20 seconds, 72 °C for 30 seconds, and ended with one cycle of 95 °C for 1 minute and 50 °C for 30 minutes.
Fecal and mucosal microbial composition were separately assessed using the Human Intestinal Tract Chip (HITChip), a customized Agilent microarray, as previously described (23) with minor modifications as described (6). The HITChip is a phylogenetic microarray based on 16S rRNA sequences of 1,033 phylotypes present in the human intestinal tract and allows deep analysis of the bacterial community, as observed by comparisons with 16S rRNA amplicon sequencing and metagenomics (23,26,27). The hybridization signals are normalized and summarized to 130 genus-like phylogenetic groups (level 2, >90% 16S rRNA gene sequence similarity), referred to as species and relatives (23). Since all HITChip data are generated using 2 independent analyses with Cy3 and Cy5 labels (23), it provides a highly reproducible, comparable, and accurate method to assess the bacterial composition and has been used successfully in other FMT studies (5,7,28).
Data analysis
Questionnaire data.
All values were baseline-corrected to account for interindividual differences. For the IBS-SSS and GSRS-IBS scales, the average of the −2 weeks and −1 day values was used as the baseline value. Statistical differences between the allogenic and autologous group were assessed with the Mann-Whitney U-test. Friedman's test was performed on baseline-corrected data to detect significant differences between the time points within the groups. Post hoc testing was performed with the Wilcoxon signed rank test. Descriptive P-values (not adjusted for multiplicity) are reported for all tests comparing questionnaire results.
Barostat data.
The parameters of the logistic functions with intercept of the measured VAS-scores for pain, discomfort, and urge, respectively, at the fixed pressures of 20, 30, 40, 50, and 60 mm Hg, were estimated. The fitted baseline-corrected VAS values were then compared before and after FMT using Wilcoxon signed rank test, and between allogenic and autologous using Mann-Whitney U-test. Descriptive P-values were reported. For details, see Supplemental Methods (see Supplementary Digital Content 1, http://links.lww.com/CTG/A30).
Microbiota data.
HITChip data were normalized to relative abundance by dividing all intensities by the sum of all intensities for each sample. All analyses were performed on genus-like phylogenetic level with the aim to assess whether the FMT had community-level effects on the microbiota. Shannon diversity index was calculated using function “diversity” from the R-package vegan (29,30). Baseline corrections were performed by subtracting the diversity at baseline from values at week 2 and 8, respectively, for each patient. Similarity indices were calculated as pairwise Pearson correlations (function rcorr, from R-package Hmisc). Baseline-corrected diversity and similarity indices were compared before and after FMT using Wilcoxon signed rank test. Descriptive P-values were reported. Redundancy analysis (RDA) was performed (function rda, R-package vegan) as followed: Explanatory variables were (i) time points, coded as dummy variables, and (ii) status of a sample as donor sample or recipient sample. These explanatory variables served to explain microbiota composition in all samples. RDA was applied to both scaled and unscaled microbiota data, where scaling was implemented as standardization, that is, mean value subtraction and division by standard deviation. Principal component analysis was performed on the relative abundance composition data (scaled data) using prcomp-function in R version 3.3.0 with scaled set to true. R version 3.3.0 was used for the analysis (31).
RESULTS
Participants
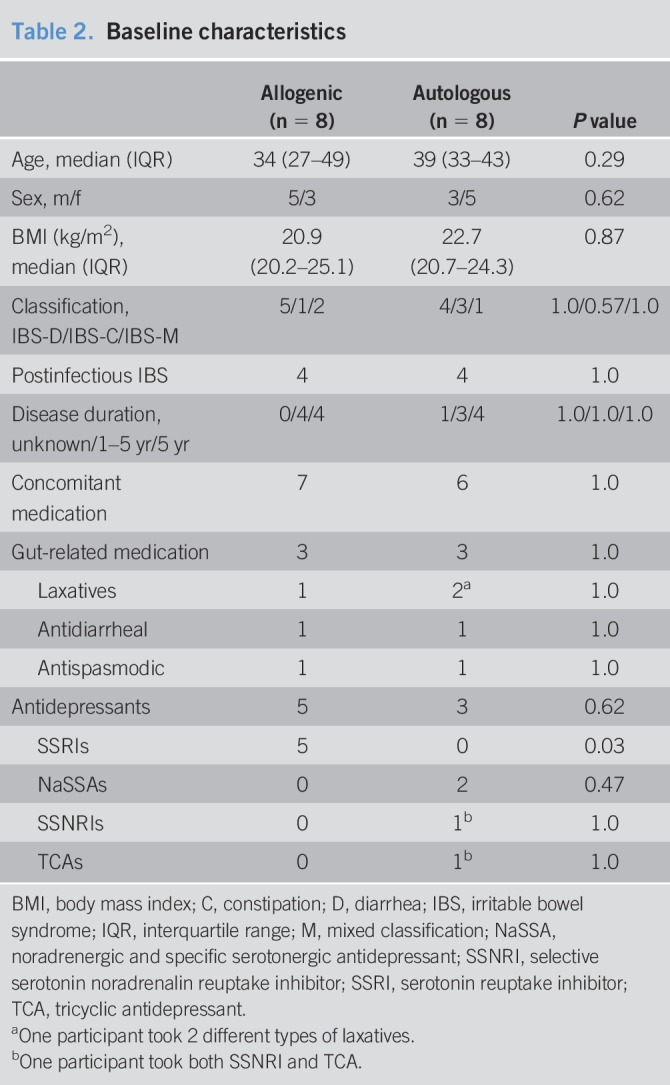
Out of 34 potential participants, 17 IBS patients with a fecal microbiota low in butyrate production capacity were included and randomly assigned to either the allogenic group, receiving fecal material from a healthy donor, or the autologous group, receiving their own fecal material (see Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A30). One participant discontinued the study after the FMT due to discomfort during the procedures (autologous group). This participant was replaced with a new subject who did not continue with the sigmoidoscopies and barostat procedure after the FMT due to discomfort during these procedures, but completed the symptom scales and provided fecal samples. Another participant (autologous group) chose not to undergo the second barostat examination due to discomfort during the previous procedure. Baseline characteristics of the included participants are presented in Table 2 and did not significantly differ between the allogenic and autologous groups. None of the participants were taking proton pump inhibitors during the study. While the overall use of antidepressant drugs was similar in both groups, this use only comprised serotonin reuptake inhibitors in the allogenic FMT group, while the IBS patients in the autologous FMT group used other types of antidepressants. However, this should not affect the results.
Table 2.
Baseline characteristics
Adverse events
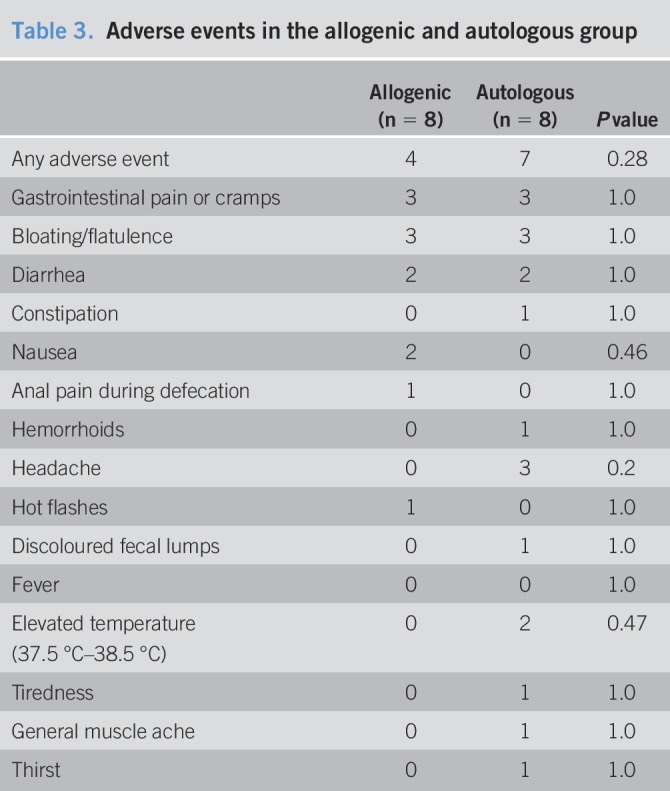
No serious adverse events were reported. Four participants reported adverse events in the allogenic group, and 7 in the autologous group (Table 3).
Table 3.
Adverse events in the allogenic and autologous group
Outcomes
Primary outcome.
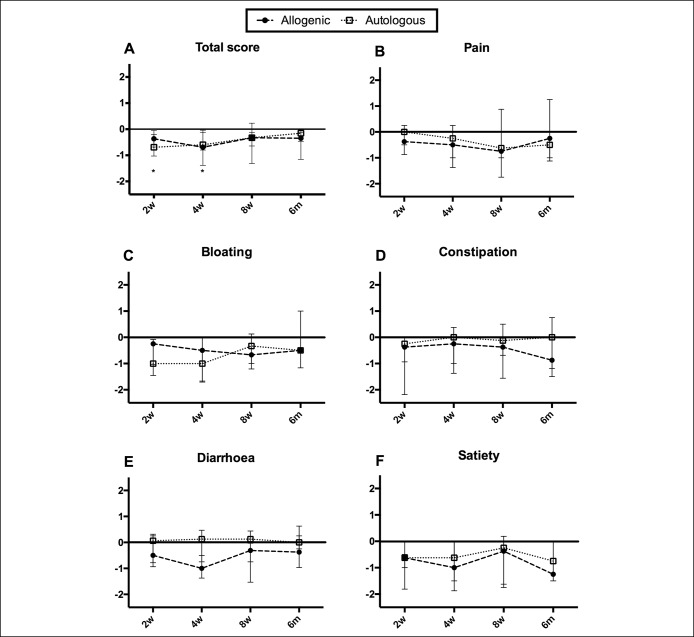
No significant differences in GSRS-IBS scores between the allogenic and autologous groups were found. However, the GSRS-IBS scores of patients receiving donor fecal material decreased significantly after FMT compared to baseline (P < 0.05, Friedman's test). This decrease was not significant in the autologous group (P = 0.16). Baseline-corrected GSRS-IBS scores showed significantly decreased symptoms for the treatment group at 2 weeks after FMT (allogenic: median −0.37, interquartile range (IQR) −0.68 to −0.20, P < 0.05; autologous: −0.69, IQR -1.02 to −0.04) and at 4 weeks after FMT (allogenic: −0.69, IQR −1.39 to −0.12, P < 0.05; autologous: −0.60, IQR −0.80 to 0.04), while this decrease was not significant after 8 weeks and 6 months (Figure 1a). The decreased total symptoms scores seemed to be driven by different symptom subscores in the 2 groups (Figure 1b–f). Numbers in this study are too small to perform subgroup analyses, but symptom improvement did not seem to be related to any specific subgroup.
Figure 1.
Baseline-corrected GSRS-IBS scores at different time points after FMT. Median and interquartile ranges (IQRs) are shown. (a) Total scores. (b–f) Respective subscores. No statistical significance was found between the allogenic and the autologous groups. In the allogenic group, total scores were significantly reduced 2 weeks and 4 weeks after FMT compared with baseline. *P < 0.05. GSRS-IBS, gastrointestinal symptom rating scale, IBS version.
Responders were defined by a decrease of at least 30% in the total GSRS-IBS symptom score after FMT. In the allogenic group, 4 out of 8 patients showed a 30% decrease in GSRS-IBS total score at one of the time points after FMT, while only 1 out of 8 patients reached this symptom reduction in the autologous group. This difference was not significant (P = 0.282, Fisher's exact test). Two out of 3 patients that received material from donor 1 (66%), and 2 out 5 from donor 2 (40%) classified as responders (P = 1.0, Fisher's exact test).
Secondary outcomes.
Questionnaire data.
The IBS-SSS scores of patients receiving donor fecal material decreased after FMT compared to baseline (P = 0.05, Friedman's test). This decrease in symptom score was significant 4 weeks after FMT (median −70.6; IQR −90.1 to −10.5; P < 0.05), 8 weeks after FMT (−69.3; IQR −77.2 to −19.3; P < 0.05), and 6 months after FMT (−36.4; IQR −69.8 to −3.5; P < 0.05) (Figure 2). No significant differences in the autologous group compared with baseline were observed (P = 0.22) and neither between allogenic and autologous treatment.
Figure 2.
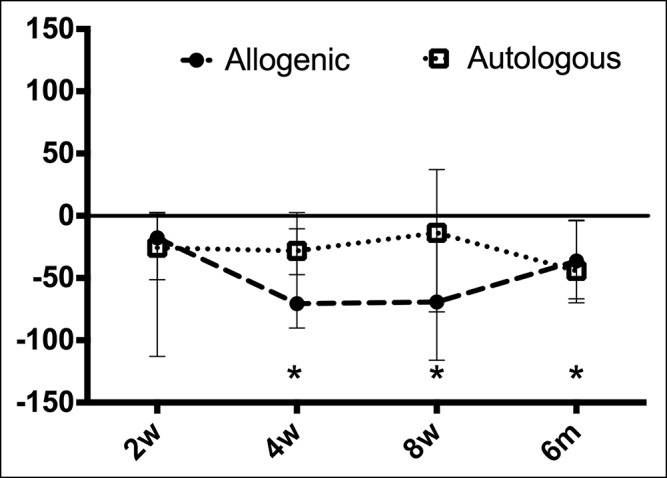
Baseline-corrected IBS-SSS scores at different time points after fecal microbiota transfer (FMT). Median and interquartile ranges (IQRs) are shown. No statistical significant differences were found between the allogenic and the autologous groups. In the allogenic group, total scores were significantly reduced 4 weeks, 8 weeks, and 6 months after FMT compared to baseline. *P < 0.05. IBS-SSS, IBS-severity scoring system.
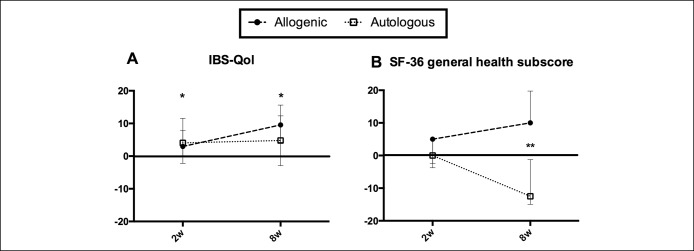
The IBS-QoL total score was significantly increased after FMT compared to baseline in the allogenic group (P < 0.005, Friedman's test), but not in the autologous group (P = 0.50). This increase in the allogenic group was significant 2 weeks and 8 weeks after FMT (2.9; IQR 0.2–7.9; P < 0.05 and 9.6; 4.3, 15.6; P < 0.05, respectively) (Figure 3a). No statistical significance was found between the allogenic and autologous groups.
Figure 3.
Baseline-corrected quality of life (IBS-QoL) and health status (SF-36) scores at different time points after fecal microbiota transfer (FMT). Median and interquartile ranges (IQRs) are shown. (a) IBS-QoL scores. No statistical significant differences were found between the allogenic and the autologous groups. In the allogenic group, total scores were significantly increased 2 weeks and 8 weeks after FMT compared with baseline. *P < 0.05. (b) Short form 36 (SF-36) subscore general health. Scores differed significantly between the allogenic and the autologous groups 8 weeks after FMT. **P < 0.01.
Similar results were observed in 3 out of 8 SF-36 subscores, especially in the “general health” subscore (Figure 3b). This score was significantly increased in the allogenic compared with the autologous group 8 weeks after FMT (10.0; IQR 10.0–19.8 compared to −12.5; IQR −15.0 to −1.3; P < 0.01). No significant differences in hospital anxiety and depression scale scores were found between allogenic and autologous and between before and after FMT (data not shown).
Visceral sensitivity.
Complete barostat data were available from n = 14 participants. The VAS scores at 20, 30, 40, and 50 mm Hg were estimated from the fitted logistic curves, before and 8 weeks after FMT. No significant differences were found before and after FMT in the allogenic (n = 8) and the autologous group (n = 6) for perception of pain and discomfort, respectively. The perception of urge was significantly lower in the autologous group compared with the allogenic group 8 weeks after the FMT at 20 mm Hg (−1.9; IQR −2.3 to −0.7 vs −0.4; IQR −0.6 to 0.2, P < 0.05), 40 mm Hg (−1.6; IQR −2.6 to −0.7 vs 0.3; IQR −0.6 to 1.9, P < 0.05) and 50 mm Hg (−0.8; IQR −1.3 to −0.3 vs 0.3; IQR −0.4 to 2.1, P < 0.05) (Figure 4). The patients with a positive symptom response did not show a decrease in visceral sensitivity, and no differences according to subtypes were noted.
Figure 4.
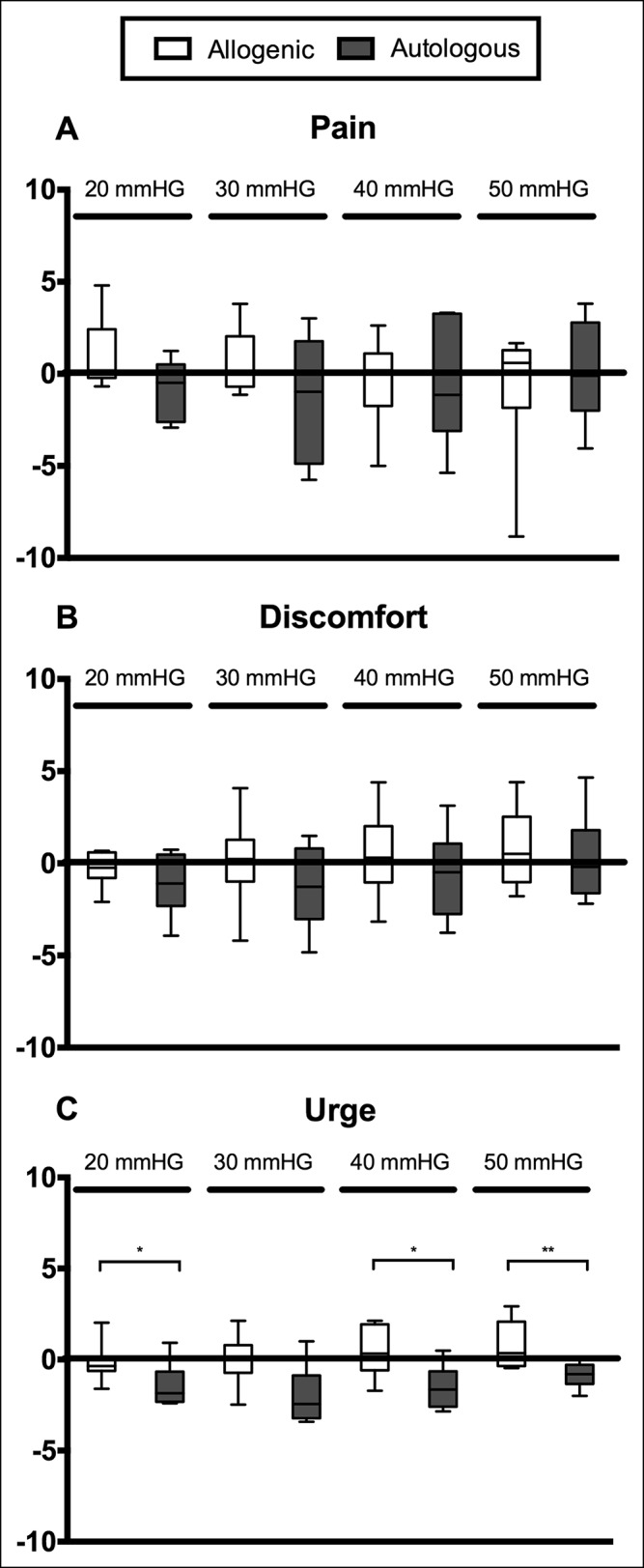
Baseline-corrected VAS scores for visceral perception of pain (a), discomfort (b), and urge (c) 8 weeks after fecal microbiota transfer (FMT) at different pressures. No significant differences were found for perception of pain and discomfort. The perception of urge was significantly lower in the autologous group compared with the allogenic group. VAS, visual analogue scale. *P < 0.05, **P < 0.01.
Microbiota profiling.
Bacterial DNA extracted from fecal samples (n = 54, from 16 patients and 2 donors) and mucosal biopsies (n = 47, from 15 patients and 2 donors) was analyzed by HITChip. Two mucosal samples in the autologous group could not be analyzed due to insufficient amplifiable DNA (n = 1 from 2 weeks after FMT, n = 1 from 8 weeks after FMT).
Redundancy analysis.
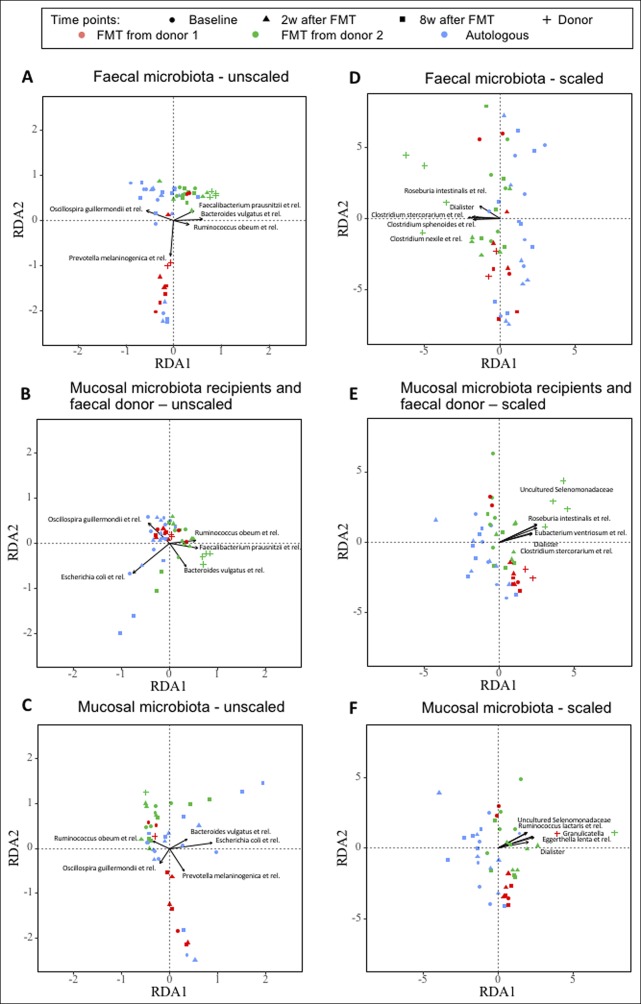
Figure 5 shows RDA biplots of the bacterial composition of fecal and mucosal samples, respectively, with factorization of time points and donor status as response variables, including the most important bacterial genera. Examining the resulting ordinations, fecal samples from the allogenic group 2 and 8 weeks after FMT seemed to cluster closer to their corresponding donor than the baseline samples or the samples from the autologous group, especially for recipients of fecal material from donor 1 (Figure 5a). This pattern was also observed, however to a lesser degree, comparing mucosal microbiota from patients to the donors' fecal microbiota (Figure 5b), or when scaling the raw values to zero mean and unit variance (Figures 5d, e), allowing the representation of bacterial genera with a lower abundance to affect the results in a similar degree as genera with high abundance values.
Figure 5.
Redundancy analysis (RDA) plots of the fecal and mucosal microbiota composition. Explanatory variables were (i) time points, coded as dummy variables, and (ii) status of a sample as donor sample or recipient sample. These explanatory variables served to explain microbiota composition in all samples. Unscaled (a–c) or scaled (d–f) values are shown. Scaled values allow the representation of bacterial genera with a lower abundance to the same degree as genera with high abundance. (a and d) Fecal microbiota of patients and fecal microbiota of donors. (b and e) Mucosal microbiota of patients and fecal microbiota of donors. (c and f) Mucosal microbiota of patients and mucosal microbiota of donors.
The mucosal microbiota of the recipients did not seem to cluster closely to the mucosal microbiota of the donors (Figure 5c, f). The bacterial genera for which the variations explained most of the microbiota's total variation are also depicted in Figure 5. Principal component analysis plots of the bacterial composition are depicted in Supplemental Figure 2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A30). While some of the subjects in the allogenic group shifted toward their corresponding donor, the microbiota in the autologous group also changed over time (see Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A30).
Shannon diversity and Pearson's similarity index.
The Shannon diversity was calculated for both the fecal and the mucosal microbiota samples. The microbial diversity in our study population was not significantly affected by allogenic or autologous FMT, neither in the fecal nor in the mucosal samples (see Figure 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A30). The patients with a positive symptom response did not show increased fecal or mucosal microbiota diversity.
Supplemental Figure 4A (see Supplementary Digital Content, http://links.lww.com/CTG/A30) shows the Pearson correlation for the allogenic group, in which the recipients' fecal microbiota composition was correlated to their corresponding donors' fecal microbiota. Even though the patients' fecal microbiota seemed to become more similar to the donors' microbiota over time, this did not result in significant correlations (see Figure 4A and Table 1, see Supplementary Digital Content, http://links.lww.com/CTG/A30). The responders in the allogenic group generally showed an increased similarity index (see Figure 4A, Supplementary Digital Content, http://links.lww.com/CTG/A30).
Supplemental Figure 4B (see Supplementary Digital Content, http://links.lww.com/CTG/A30) shows Pearson's similarity index for the autologous group, in which the patients' fecal microbiota was correlated to their own fecal microbiota at baseline. The similarity index was significantly reduced 2 and 8 weeks after the autologous FMT compared to baseline both in the fecal and mucosal samples (see Table 2, Supplementary Digital Content, http://links.lww.com/CTG/A30).
There was no significant correlation between the recipient's mucosal microbiota and the donor mucosal microbiota or fecal microbiota in the allogenic group (see Figure 4C, E, Supplementary Digital Content, http://links.lww.com/CTG/A30). In the autologous group, the recipient's own mucosal microbiota after the autologous FMT differed significantly from their mucosal but not significantly from their fecal microbiota at baseline (see Figure 4D, F and Supplemental Table 1, Supplementary Digital Content, http://links.lww.com/CTG/A30).
Butyrate-producing bacteria.
Even though low qPCR counts of butyryl-CoA CoA transferase gene in the screening samples were used as an inclusion criteria for the allogenic group, the abundance of butyrate-producing bacteria in the fecal samples at baseline, as assessed by the HITChip analysis, was not lower in the allogenic group than in the donor group (see Figure 5A, Supplementary Digital Content, http://links.lww.com/CTG/A30). Additionally, no significant change in butyrate-producing bacteria was found after FMT using fecal material from donors with high qPCR counts of the butyryl-CoA CoA transferase gene (see Figure 5B, Supplementary Digital Content, http://links.lww.com/CTG/A30). In the autologous group, the amount of butyrate-producing bacteria in the baseline samples of the patients was lower than in the fecal samples of the donors (P = 0.09; see Supplemental Figure 5A, C, Supplementary Digital Content, http://links.lww.com/CTG/A30). Autologous FMT did not significantly affect the amount of butyrate-producing bacteria (see Figure 5C, Supplementary Digital Content, http://links.lww.com/CTG/A30). Species defined as butyrate producers are listed in Supplemental Table 3 (see Supplementary Digital Content, http://links.lww.com/CTG/A30). Likewise, when analyzing these samples for the counts of butyryl-CoA CoA transferase gene with qPCR, no significant effect of the FMT on the butyrate-producing capacity was found (data not shown).
DISCUSSION
In this double-blinded, randomized controlled study, 17 patients were treated with a single FMT via colonoscopy. No statistical significances were found in the symptoms scores between the allogenic group, which received fecal material from healthy donors, and the autologous group, which received their own autologous fecal material. However, the allogenic group had significantly decreased symptom scores and increased quality-of-life scores compared with baseline, which was not the case in the autologous group. These results are in line with a recently reported placebo-controlled study on the clinical efficacy of FMT in IBS patients (8). In this study, a significantly higher number of responders in the treatment group (FMT administered by colonoscopy) compared with the placebo group (autologous FMT) was found (P = 0.049). Even though 90 IBS patients were included, the differences in symptoms scores between the treatment and the placebo groups also did not reach significance (8). An additional recent study using capsules for administering the FMT in IBS patients also found symptom improvement in both treatment and placebo groups. However, the placebo was significantly favorable to the treatment in this study (9). The difference in results could be due to the different routes of delivery, as administration by these capsules probably led to the release of the transplant into the upper gastrointestinal tract. We chose delivery by the lower gastrointestinal tract as we primarily wanted to achieve colonization of a new microbiota in the colon.
In all studies, the placebo effect was higher than expected. It could be that the bowel cleansing by itself already has a beneficial effect on IBS symptoms. In healthy subjects, bowel cleansing has been shown to have only a momentarily effect on the fecal microbiota that usually recovers within 2 weeks (32). It is unknown how bowel cleansing specifically affects the gut microbiota of IBS patients. In addition, it could be that the handling of the fecal material during preparation for the autologous transplant substantially altered the microbiota composition. The rationale behind using autologous material is to study whether the effect of fecal material from a healthy donor is more effective than the patients' own stool. In our study, the use of bowel cleansing and autologous stool resulted in considerable changes to the fecal and mucosal microbiota 2 and even 8 weeks after the FMT, suggesting that this might not be a true placebo in a pharmacological sense. Future FMT studies in IBS could include a second control arm in which patients only undergo bowel cleansing.
The microbial diversity in fecal samples of IBS patients included in our study was not found to be significantly affected by either the allogenic or autologous FMT. In the FMT in IBS study using capsules, Halkjaer et al. (9) found that the fecal microbiota of IBS patients at baseline was significantly lower in diversity compared with the donors, which was no longer the case after FMT treatment. The diversity in the placebo group was still significantly lower compared with the donors after treatment, indicating that only the intake of FMT capsules resulted in higher diversity. However, diversity did not correlate with symptoms. In our study, the microbial diversity of the IBS patients at baseline was not lower than that of the donors. Only few studies have investigated the microbial diversity in IBS in general, and results are conflicting, reporting both increased and decreased diversity compared with healthy controls (33,34). In general, varying microbial diversity could be explained by different dietary habits (35).
In IBS, it is still unknown which patients are more likely to respond to FMT. In our study, the sample size was too low to perform proper subanalyzes based on IBS subtypes. However, also Halkjaer et al. (9) could not detect subtype-specific effects of FMT on symptoms. In addition, it is important to note that patients classified by the same IBS subtype do not necessarily display the same pathophysiology.
IBS patients in our study were included based on a relatively low amount of butyrate-producing bacteria in their fecal samples using amplification of the butyryl-CoA CoA transferase gene by real-time qPCR (screening samples, often taken several weeks before the intervention baseline samples). Accordingly, donors with a high amount of this enzyme were selected. After randomization into allogenic and autologous groups, microbiota from the fecal samples was analyzed using HITChip. Unexpectedly, the amount of butyrate-producers at baseline in the allogenic group was not lower compared with that of the donors, which is a limitation regarding the outcome of this study. This difference in butyrate producing-bacteria between the screening and baseline samples could be due to technical reasons, for example, differences between HITChip vs butyryl-CoA CoA transferase gene qPCR (36), slightly different protocols used for DNA extraction of screening and baseline samples, and different storage times. Additionally, natural temporal variations of butyrate-producing bacteria in the fecal material of IBS patients could explain the differences observed between the screening and baseline samples. It is not known how the abundance of these and other bacteria vary over time in IBS patients and other study populations. However, it has been suggested that butyrate-producing bacteria are rather sensitive to everyday life variations (37).
Visceral hypersensitivity is common among IBS patients, with a prevalence of 50%–90% (38). A possible role of the gut microbiota in visceral hypersensitivity was suggested by a study that showed that rats had increased rectal sensitivity compared with the control group after colonization with gut microbiota of IBS patients (39). Thus, altering the gut microbiota, for example, by FMT, could hypothetically reduce hypersensitivity in IBS patients. In our study, however, the decrease in symptoms scores did not seem to be mediated by reduced visceral hypersensitivity. Only in the autologous group, a beneficial effect on the perception of urge could be observed, possibly due to the bowel cleansing. That this was not the case in the allogenic group could be due to a reaction of the host mucosa to the introduction of a new, “foreign” microbiota.
A unique approach in this study was that we also collected mucosal biopsies from the sigmoid, from both the patients and the donors, and analyzed the microbiota composition in those samples. We could show that the mucosal microbiota of the recipients was also affected by the newly introduced fecal microbiota, indicating that FMT has the potential to affect the IBS patients' host response. Further research needs to identify the specific effects of FMT for example on the mucosal immune response.
A limitation of this study is the low sample size number. Power calculations were based on detecting 30% difference in symptoms according to the GSRS-IBS upon allogenic FMT treatment, compared with 5% difference after autologous treatment; thus, we clearly underestimated the placebo effect. Nevertheless, we showed similar effects on symptoms as Johnsen et al. (8) who included n = 90 in their study. Patients participating in our study underwent a rather extensive study protocol, including repeated visits for assessment of visceral hypersensitivity (barostat) and collection of biopsies by sigmoidoscopy at several time points before and after the FMT. In addition, it is important to note that our results were not corrected for multiple testing. An additional limitation of this study is that even though participants were asked to keep their diet stable, this was not confirmed by a validated method. Nevertheless, the research reported here gives valuable insight into the physiological changes induced by FMT, a potential treatment for IBS patients.
In conclusion, we showed that a single FMT via colonoscopy could improve symptoms and quality of life in IBS patients, however, not significantly superior to autologous FMT. FMT seemed to affect both the fecal and the mucosa-adherent microbiota, but did not change visceral sensitivity. Future studies are necessary to investigate whether the efficacy of FMT in IBS patients can be improved by repeated FMTs, as often applied in ulcerative colitis (3–5). In addition, future studies should aim at further elucidating the mechanistic properties of FMT to be able to offer effective, individualized treatments for IBS patients.
CONFLICTS OF INTEREST
Guarantor of the article: Robert-Jan Brummer.
Specific author contributions: Julia König, PhD and Robert J. Brummer, MD, PhD should be considered as joint senior authors. R.J.B., J.K., and W.M.d.V. designed the study. S.H., J.K., and R.J.B. collected the data. S.H., J.K., C.M.L., and D.R. analyzed data and performed statistical analysis. S.H. and J.K. drafted the manuscript. A.S., C.M.L., D.R., J.K., R.J.B., S.H., and W.M.d.V. interpreted the data and critically revised the manuscript. All authors have reviewed and approved the final version of the manuscript.
Financial support: W.M. de Vos was supported by SIAM Gravitation Grant (024.002.002) and the Spinoza 2008 Award of the Netherlands Organization for Scientific Research (NWO). Part of this research was funded by the Swedish Nutrition Foundation granted to Savanne Holster in 2016.
Potential competing interest: None.
Study Highlights.
WHAT IS KNOWN
✓ Gut microbiota might play a role in the pathophysiology of IBS.
✓ FMT has been suggested as a potential treatment to improve symptoms in IBS.
WHAT IS NEW HERE
✓ A single FMT by colonoscopy may have beneficial effects in IBS.
✓ Allogenic FMT (stool from healthy donors) does not seem to be significantly superior to autologous FMT (own stool).
✓ FMT has an effect on both fecal as well as mucosal microbiota.
✓ Already bowel cleansing and processing of the fecal material (autologous FMT) have an effect on symptoms and fecal as well as mucosal microbial composition.
TRANSLATIONAL IMPACT
✓ Results of this study may give an insight in the physiological changes induced by FMT in IBS patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the research nurses from the Department of Gastroenterology at University Hospital Örebro, Liza Löfvendahl for helping with DNA extractions, and Jorn Hartman for performing the HITChip experiments.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A30
REFERENCES
- 1.Quraishi MN, Widlak M, Bhala N, et al. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther 2017;46(5):479–93. [DOI] [PubMed] [Google Scholar]
- 2.König J, Siebenhaar A, Högenauer C, et al. Consensus report: Faecal microbiota transfer - clinical applications and procedures. Aliment Pharmacol Ther 2017;45(2):222–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149(1):102–9 e106. [DOI] [PubMed] [Google Scholar]
- 4.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017;389(10075):1218–28. [DOI] [PubMed] [Google Scholar]
- 5.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015;149(1):110–8.e114. [DOI] [PubMed] [Google Scholar]
- 6.Kootte RS, Levin E, Salojarvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab 2017;26(4):611–9.e616. [DOI] [PubMed] [Google Scholar]
- 7.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143(4):913–6.e917. [DOI] [PubMed] [Google Scholar]
- 8.Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 2018;3(1):17–24. [DOI] [PubMed] [Google Scholar]
- 9.Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018;67:2107–15. [DOI] [PubMed] [Google Scholar]
- 10.Pozuelo M, Panda S, Santiago A, et al. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep 2015;5:12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamer HM, Jonkers D, Venema K, et al. Review article: The role of butyrate on colonic function. Aliment Pharmacol Ther 2008;27(2):104–19. [DOI] [PubMed] [Google Scholar]
- 12.Vanhoutvin SA, Troost FJ, Kilkens TO, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil 2009;21(9):952–e976. [DOI] [PubMed] [Google Scholar]
- 13.Hamer HM, Jonkers DM, Vanhoutvin SA, et al. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr 2010;29(6):738–44. [DOI] [PubMed] [Google Scholar]
- 14.Chang L. From Rome to los Angeles-the Rome III criteria for the functional GI disorders. Gastroenterology 2006;130:1480–91.16678561 [Google Scholar]
- 15.Louis P, Flint HJ. Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol 2007;73(6):2009–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakken JS, Borody T, Brandt LJ, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011;9(12):1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satokari R, Mattila E, Kainulainen V, et al. Editorial: A simple faecal preparation for faecal microbiota transplantation—Authors' reply. Aliment Pharmacol Ther 2015;41(3):321. [DOI] [PubMed] [Google Scholar]
- 18.Wiklund IK, Fullerton S, Hawkey CJ, et al. An irritable bowel syndrome-specific symptom questionnaire: Development and validation. Scand J Gastroenterol 2003;38(9):947–54. [DOI] [PubMed] [Google Scholar]
- 19.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11(2):395–402. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Gandek B, Kosinski M, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: Results from the IQOLA project. Int Qual Life Assess J Clin Epidemiol 1998;51(11):1167–70. [DOI] [PubMed] [Google Scholar]
- 21.Wong E, Guyatt GH, Cook DJ, et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl 1998(583):50–6. [DOI] [PubMed] [Google Scholar]
- 22.Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J 1986;292(6516):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajilic-Stojanovic M, Heilig HG, Molenaar D, et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: Analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol 2009;11(7):1736–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilkens TO, Honig A, van Nieuwenhoven MA, et al. Acute tryptophan depletion affects brain-gut responses in irritable bowel syndrome patients and controls. Gut 2004;53(12):1794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salonen A, Nikkilä J, Jalanka-Tuovinen J, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: Effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods 2010;81(2):127–34. [DOI] [PubMed] [Google Scholar]
- 26.Claesson MJ, O'Sullivan O, Wang Q, et al. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One 2009;4(8):e6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature 2011;473(7346):174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368(5):407–15. [DOI] [PubMed] [Google Scholar]
- 29.Fisher RA, Corbet AS, Williams CB. The relation between the number of species and the number of individuals in a random sample of animal population. J Anim Ecol 1943;12(1):42–58. [Google Scholar]
- 30.Hurlbert SH. The nonconcept of species diversity: A critique and Alternative parameters. Ecology 1971;52(4):577–86. [DOI] [PubMed] [Google Scholar]
- 31.Team RC. R: A Language and Environment for Statistical Computing. 2016. (https://www.R-project.org/). Accessed on October 22, 2017. [Google Scholar]
- 32.Jalanka J, Salonen A, Salojärvi J, et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2015;64(10):1562–8. [DOI] [PubMed] [Google Scholar]
- 33.Ponnusamy K, Choi JN, Kim J, et al. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J Med Microbiol 2011;60(Pt 6):817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangel I, Sundin J, Fuentes S, et al. The relationship between faecal-associated and mucosal-associated microbiota in irritable bowel syndrome patients and healthy subjects. Aliment Pharmacol Ther 2015;42(10):1211–21. [DOI] [PubMed] [Google Scholar]
- 35.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016;352(6285):565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuentes S, Rossen NG, van der Spek MJ, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J 2017;11(8):1877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vital M, Karch A, Pieper DH. Colonic butyrate-producing communities in humans: An overview using omics data. mSystems 2017;2(6):e00130–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azpiroz F, Bouin M, Camilleri M, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 2007;19(1 Suppl):62–88. [DOI] [PubMed] [Google Scholar]
- 39.Crouzet L, Gaultier E, Del'Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil 2013;25(4):e272–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.