Abstract
Purpose of review
Knowledge on primary progressive aphasia (PPA) has expanded rapidly in the past few decades. Clinical characteristics, neuroimaging correlates, and neuropathological features of PPA are better delineated. This facilitates scientific studies on the disease pathophysiology and allows speech and language therapy to be more precisely targeted. This review article begins with a summary of the current understanding of PPA and discusses how PPA can serve as a model to promote scientific discovery in neurodegenerative diseases.
Recent findings
Studies on the different variants of PPA have demonstrated the high compatibility between clinical presentations and neuroimaging features, and in turn, enhances the understanding of speech and language neuroanatomy. In addition to the traditional approach of lesion-based or voxel-based mapping, scientists have also adopted functional connectivity and network topology approaches that permits a more multidimensional understanding of neuroanatomy. As a result, pharmacological and cognitive therapeutic strategies can now be better targeted towards specific pathological/molecular and cognitive subtypes.
Summary
Recent scientific advancement in PPA potentiates it to be an optimal model for studying brain network vulnerability, neurodevelopment influences and the effects of nonpharmacological intervention in neurodegenerative diseases.
Keywords: frontotemporal dementia, neurodegenerative disease, neuroimaging, neuropathology, primary progressive aphasia
INTRODUCTION
Primary progressive aphasia (PPA) is a clinical syndrome characterized by speech and language impairment caused by neurodegeneration of language networks. In 1892, Pick [1] reported the case of a 71-year-old man who presented with nonsensical speech, anomia, dyslexia, dysgraphia, personality changes, and memory impairment. Apart from a few reports of Gogi aphasia in Japan in the 1940s [2], discussion of neurodegenerative aphasia remained dormant until the 1970s when British investigators published several cases with progressive semantic loss, semantic dementia [3,4], and Mesulam and colleagues first introduced the term PPA [5]. In 1996, Grossman et al. [6] also introduced the term progressive nonfluent aphasia to describe patients with progressive loss of speech fluency. PPA was initially described as a unitary syndrome with anomia as main symptom but this view did not explain the spectrum of presentations described. Furthermore, many patients did not show the typical features of semantic or nonfluent presentations; it was later clarified that most of these patients have a third PPA subtype, named the logopenic variant of PPA [7]. In 2011, an international group of experts introduced a common framework in which PPA was classified into three different variants, based on specific cognitive and neuroimaging features: semantic (svPPA), nonfluent/agrammatic (nfvPPA), and logopenic variants (lvPPA) [8]. Recent clinicopathological studies demonstrated that each variant is associated with different probabilities of neuropathological changes and, rarely, genetic mutations. Table 1 presents the 2011 PPA classification, including the diagnostic algorithm allowing for three diagnostic levels: clinical, imaging-supported, and definite diagnosis.
Table 1.
Primary progressive aphasia 2011 diagnostic consensus criteria algorithm
| PPA clinical diagnostic criteria | |||
| Inclusion criteria | |||
| (1) Most prominent clinical feature is difficulty with language | |||
| (2) Aphasia should be the most prominent deficit at symptom onset and for the initial phases of the disease | |||
| (3) These deficits are the principal cause of impaired daily living activities | |||
| Exclusion criteria | |||
| (1) Pattern of deficits is better accounted for by other nondegenerative nervous system or medical disorders | |||
| (2) Cognitive disturbance is better accounted for by a psychiatric diagnosis | |||
| (3) Prominent initial episodic memory, visual memory, and visuoperceptual impairments | |||
| (4) Prominent initial behavioural disturbance | |||
| Nonfluent/Agrammatic variant PPA | Semantic variant PPA | Logopenic variant PPA | |
| Clinical diagnosis | Core features: (at least 1) (1) Agrammatism in language production (2) Effortful, halting speech with inconsistent speech sound errors and distortions (apraxia of speech) Supporting features: (at least 2) (1) Impaired comprehension of syntactically complex sentences (2) Spared single-word comprehension (3) Spared object knowledge |
Core features: (both) (1) Impaired confrontation naming (2) Impaired single-word comprehension Supporting features: (at least 3) (1) Impaired object knowledge, particularly for low frequency or low familiarity items (2) Surface dyslexia or dysgraphia (3) Spared repetition (4) Spared speech production (grammar and motor speech) |
Core features: (both) (1) Impaired single-word retrieval in spontaneous speech and naming (2) Impaired repetition of sentences and phrases Supporting features: (at least 3) (1) Speech (phonologic) errors in spontaneous speech and naming (2) Spared single-word comprehension and object knowledge (3) Spared motor speech (4) Absence of frank agrammatism |
| Imaging supported diagnosis (both present) | (1) Clinical diagnosis of nfvPPA | (1) Clinical diagnosis of svPPA | (1) Clinical diagnosis of lvPPA |
| (2) Imaging: (at least 1) | (2) Imaging: (at least 1) | (2) Imaging: (at least 1) | |
| (a) Predominant left posterior frontoinsular atrophy on MRI | (a) Predominant anterior temporal lobe atrophy on MRI | (a) Predominant left posterior perisylvian or parietal atrophy on MRI | |
| (b) Predominant left posterior frontoinsular hypoperfusion or hypometabolism on SPECT or PET | (b) Predominant anterior temporal hypoperfusion or hypometabolism on SPECT or PET | (b) Predominant left posterior perisylvian or parietal hypoperfusion or hypometabolism on SPECT or PET | |
| PPA with definite diagnosis | Clinical diagnosis fulfilled | ||
| AND | |||
| (1) Histopathologic evidence of a specific neurodegenerative disorder (e.g. FTLD-tau, FTLD-TDP, AD, other) | |||
| OR | |||
| (2) Presence of a known pathogenic mutation | |||
Adapted with permission from [8,9]. AD, Alzheimer’s disease; FTLD-tau, frontotemporal lobar degeneration-tau; FTLD-TDP, frontotemporal lobar degeneration–TAR DNA-binding protein; lvPPA, logopenic variant primary progressive aphasia; nfvPPA, nonfluent/agrammatic primary progressive aphasia; PPA, primary progressive aphasia; svPPA, semantic variant primary progressive aphasia.
This article focuses on describing the updated clinical and neuroimaging features of the three main PPA variants. We then summarize pathological and genetic findings and finally discuss the role of PPA in furthering the scientific understanding of neurodegenerative diseases.
SEMANTIC VARIANT PRIMARY PROGRESSIVE APHASIA
The first three cases of this syndrome were described by Warrington [3] in 1975. Patients suffered from loss of word and object knowledge, and deficits were typically most severe for low frequency and low familiarity items [8]. Symptoms included poor performance in confrontational naming, single word comprehension, and object and face identification tasks. Later work by Snowden et al. [4] and Hodges et al. [10] further demonstrated that semantic loss also leads to surface dyslexia, a disorder in which patients can read pseudowords but not exceptionally spelled words. Typical reading errors include ‘regularization’ errors in irregular words, such as ‘yacht’ or ‘island’ are pronounced as ‘yachdt’ and ‘is-land’ [10]. SvPPA patients often produce semantic paraphasias and tend to substitute specific, subordinate (in the semantic hierarchies) words with generalized terms, such as replacing ‘screwdriver’ with ‘thing’ and ‘panther’ with ‘animal.’ For a decade, it was unclear whether semantic dementia and ‘fluent PPA’ were different entities [11]. In 2004, Gorno-Tempini et al. [7,8] included semantic dementia as one of the three PPA variants and the term semantic variant PPA (svPPA) was later adopted by the international workgroup. This new classification of svPPA includes the key classic features of semantic dementia, such as anomia and word comprehension deficits. Supporting diagnostic features include surface dyslexia or dysgraphia, impairment in object or face knowledge, and relatively spared repetition and speech production abilities [8]. Further reports described deficits in different categories of objects and stimuli modalities, such as famous faces [12], voices [12], nonverbal environmental sounds [13], smells [14], and taste stimuli [15], sometimes in relation to greater right ATL damage. In general, the hallmark feature of this disorder is an inability to identify the meaning of stimuli despite preserved perception. As svPPA progresses, behavioural symptoms including lack of empathy, changes in personality, disinhibition, mental inflexibility, and compulsive behaviours commonly occur [16].
In addition to different clinical symptoms, the three main PPA variants show distinct neuroimaging features [8]. The different patterns of neuroanatomical changes in each subtype are determined by the selective vulnerability of certain brain networks to the neurodegenerative disorders that cause neuronal damage [8,17]. Damage to the ATL in svPPA is identified as atrophy on volumetric brain imaging (MRI) or as hypometabolism on fluorodeoxyglucose (FDG)-PET (Fig. 1). The ATL damage is most often greater in the left hemisphere, which is consistent with naming, word comprehension, and reading difficulties [10,18], but in roughly 30% of svPPA cases, atrophy is more severe in the right ATL [19,20]. Individuals with greater right ATL atrophy present initially with difficulty identifying familiar people, recognizing facial expressions, and with behavioural abnormalities, such as loss of empathy and compulsive, repetitive behaviours [19–21]. Structural connectivity analysis demonstrated severe damage to ventral white matter tracts, typically the anterior portion of the inferior longitudinal fasciculi (ILF) and uncinate fasciculi [22,23] (Fig. 2). Task-free fMRI studies show that focal ATL damage causes decreased functional connectivity and functional changes in a widespread semantic network including modality-specific primary and association cortices [24,25]. Magnetoencephalography (MEG) study shows hyposynchrony of alpha and beta frequencies in the left temporoparietal junction, also suggesting that functional disruption areas expand beyond atrophic regions [26].
FIGURE 1.
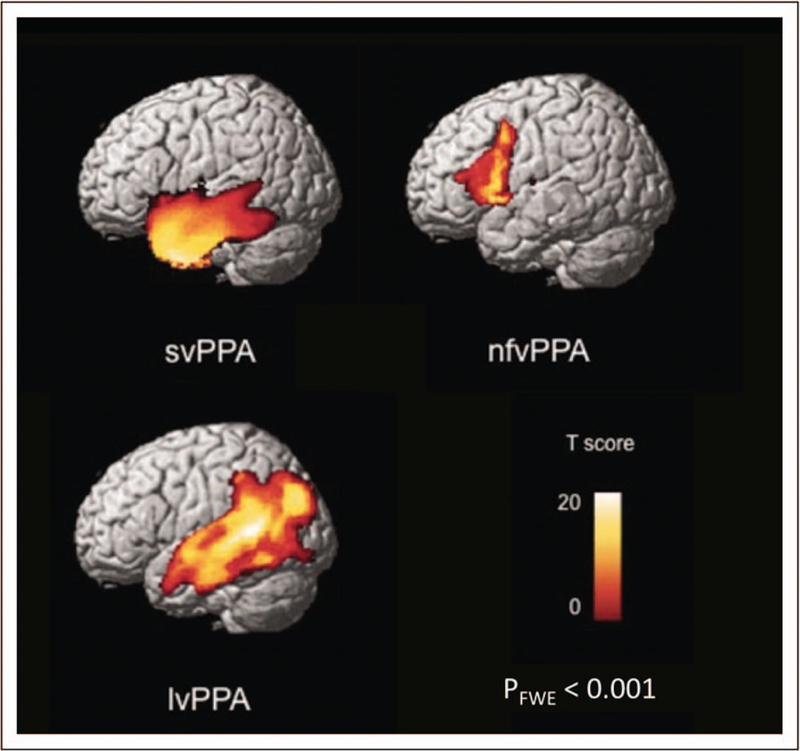
Grey matter atrophy patterns in patients with three main primary progressive aphasia variants versus controls. Presented here are statistical parametric maps that depict the grey matter atrophy patterns in semantic variant PPA (svPPA, n = 58), nonfluent/agrammatic variant PPA (nfvPPA, n = 40), and logopenic variant PPA (lvPPA, n = 24) compared with control groups that are matched for age, sex, scanner and sample size. Voxel-based morphometry results thresholded are set at a family-wise error rate of P < 0.001. FWE, familywise error rate; PPA, primary progressive aphasia. Reproduced with permission from [88].
FIGURE 2.
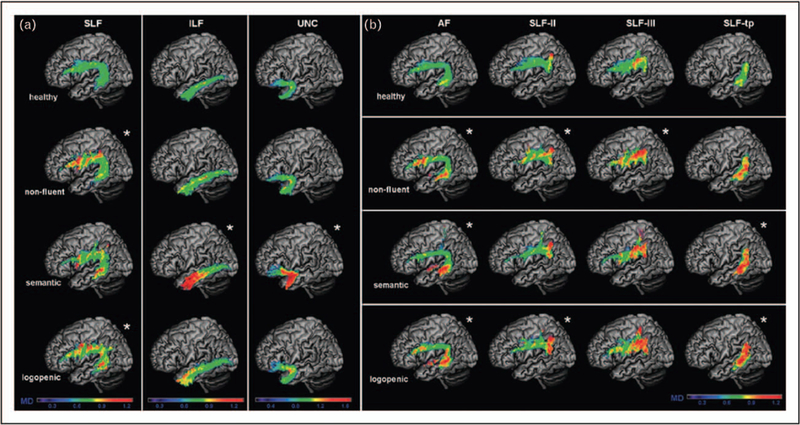
White matter damage in the three main primary progressive aphasia variants versus controls. (a) The average mean diffusivity values for left superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), uncinate fasciculus (UNC) in all three PPA variants when compared with healthy controls on a standard MNI (Montreal Neurological Institute of McGill University Health Centre) brain template. The asterisk symbol (m) indicates statistical difference from normal controls with P value less than 0.05. The colour bar represents the average mean diffusivity values, ranging from low (violet-blue) to high values (yellow-red). Mean diffusivity is measured in 10−3 mm2 s−1. (b) The average mean diffusivity values for arcuate fasciculus (AF), frontoangular SLF (SLF-II), frontosupramarginal SLF (SLF-III), and temporoparietal SLF (SLF-tp) in all three PPA variants when compared with healthy controls on a standard MNI (Montreal Neurological Institute of McGill University Health Centre) brain template. The asterisk (m) indicates statistical difference from normal controls with P value less than 0.05. The colour bar represents the average mean diffusivity values, ranging from low (violet-blue) to high values (yellow-red). Mean diffusivity is measured in 10−3 mm2 s−1. Reproduced with permission from [21].
SvPPA is most often associated with frontotemporal lobar degeneration-TAR DNA-binding protein 43 (FTLD-TDP 43) type C disorder [27]. Consistently, recent molecular PET imaging show that amyloid deposition is uncommon in svPPA especially under 60 years of age [28▪▪] and CSF abeta and tau studies are usually negative [29]. [18F]AV1451 PET designed to specifically target tau shows ATL binding in svPPA, raising concerns regarding the specificity of this ligand to tau [30▪].
As the disease progresses, anatomical damage extends to connected brain regions in the posterior temporal lobes, contralaterally to the initially less involved ATL and anteriorly to orbitofrontal regions [20]. Consistently semantic and language impairments become more generalized, although islands of fluency might remain and can be useful for differential diagnosis later in the disease course. Typically, patients develop more prominent behavioural symptoms, whereas motor functions are usually spared until the final stages, unless svPPA is associated with motor neuron disease [31].
NONFLUENT/AGRAMMATIC VARIANT PRIMARY PROGRESSIVE APHASIA
Key features of the nonfluent/agrammatic variant PPA (nfvPPA) are effortful speech and/or agrammatism with relatively spared semantic knowledge and single word comprehension [8]. Neuroimaging changes occur in a cortical–subcortical network anchored to left posterior frontoinsular region and the disorder is most often associated with FTLD spectrum of the tau subtype (Fig. 1). The cardinal clinical feature in nfvPPA is a motor speech impairment consistent with apraxia of speech (AOS) and often with dysarthria [7,8]. Dysarthria is usually a mixed type with both hypophonic and spastic features [32]. Motor speech planning difficulties cause inconsistent speech sound errors and prosody distortions, sometimes referred to as phonetic and prosodic AOS [33▪]. Motor speech and phoneme selection impairments also cause speech sound substitution, transposition, insertions, and deletions [32,34]. AOS is most frequently elicited when performing multiple repetition of multisyllabic words with initial consonant clusters and various place of articulation, such as ‘artillery’ or ‘catastrophe’ (i.e. repeating ‘artillery’ in rapid succession five times) [32]. Motor speech deficits are almost universally the most salient feature in nfvPPA; when they appear to be isolated from other language symptoms, the term primary progressive apraxia of speech (PPAOS) has been adopted [33▪]. Agrammatism, the other important feature in nfvPPA, mostly manifests as decreased mean length of utterance and simplified grammar in oral and written speech [35]. Omission of grammatical morphemes, incorrect usage of inflection morphology and inaccurate word order in spontaneous speech also can occur. NfvPPA patients produce fewer verbs than nouns as well as fewer function words (e.g. prepositions, pronouns, conjunction etc.) than content words [35,36]. Language testing shows that syntactically complex sentences, such as passive sentences and embedded and object-relative clauses are particularly challenging to produce and understand [37]. When grammatical deficits are most prominent, the term agrammatic PPA(AgPPA) has been used [38]. Patients with AgPPA often show a prominent dysexecutive syndrome, such as working memory, planning, and sequencing deficits [39].
The site of most consistent brain damage in nfvPPA (epicenter) is in the pars opercularis of left inferior frontal gyrus (IFG) and premotor cortex, and atrophy can extend to connected cortical and subcortical regions, such as anterior insula, prefrontal regions, supplementary motor complex, basal ganglia, and supramarginal gyrus [40,41▪]. This network has been named the speech production network (SPN) (Fig. 3) [40]. Differential involvement of specific nodes and connections within this network probably explain the clinical variability within a nonfluent-spectrum disorder, such as pure motoric (premotor cortex), agrammatic (prefrontal), or even dynamic (medial frontal) type of communication disorders [7,41▪,42,43,44▪▪]. In fact, nfvPPA patients with early mutism have cortical volume loss across the entire SPN network [45]. Few studies show significant white matter microstructural damage in the frontal aslant tracts, the fasciculus that connects inferior frontal regions with supplementary motor complex [46,47], frontostriatal, superior longitudinal, and arcuate fasciculi in nfvPPA [43,48] (Fig. 2). White matter integrity in the intrafrontal tracts correlates with the severity of motor speech symptoms [40,47], whereas syntactic processing performance is associated with the integrity of the arcuate and superior longitudinal fasciculi [22,40]. The finding of early white matter damage in nfvPPA is consistent with clinicopathology studies showing FTLD-tau as the most frequent cause of nfvPPA spectrum disorder [49,50]. Task-free fMRI studies in nfvPPA reveal decreased intrinsic functional connectivity within the SPN along with altered network topology [44▪▪], whereas activation fMRI shows decreased recruitment of the frontotemporal syntax network [51]. MEG indicates significant hyposynchrony of alpha and beta frequencies within the left IFG in nfvPPA [26]. Molecular PET studies showed that amyloid positivity is also rare in nfvPPA patients less than 60 years of age [28▪▪,52▪], whereas [18F]AV1451 and [18F]THK5351 PET studies show mild binding in grey matter and white matter within the SPN with unclear specificity to tau [30▪,53▪].
FIGURE 3.
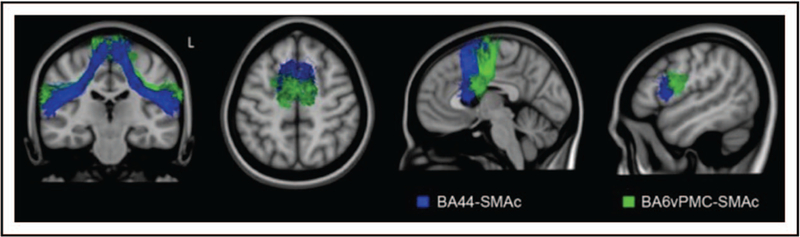
The Aslant tract within the frontal speech production network. Depicted here is the white matter tract reconstruction of Aslant tract within the frontal speech production network (SPN) in healthy controls using MNI brain template. White matter tracts traveling between pre-SMA and SMA to BA44 is shown in blue and to the ventral premotor cortex (BA6) is highlighted in green. Reproduced with permission from [46].
As disease progresses, patients often exhibit motor symptoms, such as parkinsonism and dystonia, orofacial or limb apraxia, frontal lobe dysfunctions, or mood disorders [54]. Clinically, most cases eventually fulfill the diagnostic criteria for progressive supranuclear palsy or corticobasal syndrome [54,55]. Longitudinal neuroimaging shows that atrophy progresses along the predetermined, functionally and structurally connected SPN, supporting the theory of transsynaptic spread of toxic misfolded proteins in neurodegenerative disorders [40,44▪▪]. Longitudinal case series of patients with relatively isolated motor speech deficits, or PPAOS, show that they eventually develop various degrees of aphasic symptoms, suggesting that, in most cases, PPAOS might be an early presentation within a nfvPPA spectrum rather than a separate entity [56]. Pathological studies seem to confirm this hypothesis, as both nfvPPA and PPAOS are mostly caused by FTLD-tau disorder [49,50,56].
LOGOPENIC VARIANT PRIMARY PROGRESSIVE APHASIA
The key clinical feature of lvPPA feature is wordfinding difficulty with pauses in spontaneous speech in the context of spared motor speech and grammatical production [8]. The core cognitive deficit in lvPPA is a phonology and auditory verbal short-term memory disorder [57,58]. These deficits manifest as word-finding pauses, phonological paraphasias in confrontation naming (especially for long words), and difficulty in repeating long, unfamiliar sentences or string of words or digits. The differential diagnosis between nfvPPA and lvPPA is more challenging, as both exhibit varying degrees of impairments in speech fluency and sentence comprehension. In nfvPPA, these deficits are because of motor speech and grammatical problems whereas in lvPPA they are likely secondary to auditory verbal short-term memory deficits [57]. Unlike the effortful pauses in nfvPPA, lvPPA pauses are largely caused by word-finding difficulties, therefore, these patients have islands of fluent speech in between pauses, false starts, and hesitations [35]. Sounds errors in lvPPA are largely phonemic (i.e. existing phonemes being inserted, deleted, or substituted into words) and not phonetic (i.e. errors that produces distorted nonexisting phonetics) in nature [59▪]. Individuals with lvPPA struggle with confrontational naming tasks though to a lesser degree than svPPA individuals [60]. Other features of lvPPA include dyscalculia and ideomotor apraxia [61,62]. Although PPA patients, by definition, do not complain of nonlanguage symptoms, neuropsychological tests assessing visuospatial, memory and executive functions often show differential impairments and can help differentiate lvPPA from nfvPPA [63▪▪,64].
The left inferior parietal lobule (IPL), left posterior temporal lobe and left temporoparietal junction (TPJ) are consistently involved in lvPPA, as demonstrated on volumetric MRI analysis and FDG-PET [49,65] (Fig. 1). Naming difficulties in lvPPA correlate with left middle temporal gyrus atrophy [66], whereas repetition deficits correlate with temporoparietal junction damage [67]. White matter damage in lvPPA is less severe than in nfvPPA and involves left dorsal parietal white matter tracts that connect frontal and posterior temporal regions [22] (Fig. 2). Task-free functional MRI suggest reduced connectivity over the left temporal language and ‘working memory’ network [68]. A portion of these networks is included in the default mode network, the circuit vulnerable to Alzheimer’s disease [71] supporting clinic-pathological and biomarker studies showing that this syndrome is an atypical Alzheimer’s disease variant [49,69]. Indeed, cerebrospinal fluid (CSF) analysis shows a pattern consistent with Alzheimer’s disease [70]. Molecular PET studies also confirmed the presence of amyloid deposition in about 90% of lvPPA patients [28▪▪,52▪,71]. Whereas amyloid deposition is diffuse in lvPPA, [18F]AV1451 binding is high and located in the atrophied language regions [30▪]. A MEG study shows hyposynchrony of high-frequency oscillation bands within the left posterior temporal and occipital cortices but hypersynchrony of low-frequency bands within bilateral frontal and parietal cortices [26].
LvPPA often progress to a global aphasia, with episodic memory impairment, dysexecutive, and visuospatial dysfunction [57,63▪▪,72], resembling the clinical picture of early-onset Alzheimer’s disease patient [65]. Although limb apraxia is common, severe extrapyramidal symptoms, such as dystonia and parkinsonism usually occur later than in nfvPPA. Anxiety, irritability, agitation, and depression have been reported in lvPPA, whereas frank disinhibition and lack of empathy are rare [73]. Longitudinal neuroimaging studies showed progression of grey matter atrophy within the language and default mode networks [72].
PRIMARY PROGRESSIVE APHASIA WITH ATYPICAL PRESENTATIONS
The current clinical classification accurately groups most PPA patients, but cases with mixed or unclassifiable clinical presentations occur, ranging around 6% in one recent large meta-analysis [28▪▪]. The diagnosis of some of the unclassifiable cases sometimes become evident as their disease progresses [8]. A genetic mutation should be considered in ‘atypical’ PPA, as mixed presentations have been reported in cases with autosomal dominant genetic mutations [74].
Generally, less than one-third of PPA cases have a positive family history of FTLD spectrum disorders with less than 10% of cases fulfilling an autosomal dominant inheritance pattern [75] and nfvPPA shows the highest heritability (20–30% having family history). In contrast, only 10– 20% of svPPA and lvPPA individuals have a positive family history of FTLD spectrum disorders [75]. NfvPPA has been reported to be associated with MAPT, GRN, C9ORF72, TARDBP, SQSTM1, TBK1, and CHCHD10 gene mutations [76▪]. In the few cases in which svPPA was associated with a genetic mutation, these individuals were found to have MAPT, C9ORF72, TARDBP, TBK1, TREM 2, or CHCHD10 mutations [76▪]. APP and GRN mutations were also recently discovered in a few lvPPA cases [77▪,78]. Despite often having Alzheimer’s disease neuropathology, patients show variable allele frequency of apolipoprotein e4 allele across different lvPPA cohorts, usually intermediate between amnestic Alzheimer’s disease and FTD-spectrum disorders [79], suggesting the possibility of multiple risk factors.
PRIMARY PROGRESSIVE APHASIA AS A MODEL TO STUDY THE NEUROANATOMY OF SPEECH AND LANGUAGE FUNCTIONS
The distribution of atrophy in PPA is unique and differs from patterns found in stroke aphasia, allowing the study of novel brain– behaviour correlations and providing valuable perspectives for understanding the neural basis of speech and language function in the brain [80▪]. For instance, the functions of the ATL have largely been delineated through studying svPPA [10,18]. The gradual, variable nature of the degeneration of grey and white matter structures in PPA also allowed understanding of the contribution of different anatomical components of speech and language symptomatology. For instance, the association between the degree of damage in the Aslant tract with severity of specific motor speech deficits pointed towards a specific role of this white matter structure in articulatory functions [35,46,47].
Cross-linguistic studies in PPA offer an interesting prospective to the study of language organization in the brain. Studies show unique symptoms in different languages. Japanese speaking svPPA individuals show characteristics of Gogi aphasia, with more severe deficits in kanji (logographic) than kana (alphabetic) script, because kanji strongly relies on semantic knowledge [2]. On the other hand, Italian is a language with a mainly shallow orthography (i.e. mainly composed of regular words) in which stress assignment is arbitrary. Therefore, Italian svPPA would not show surface dyslexia and only show stress assignment errors while reading [e.g. pronouncing ‘ma’cchina’ (nonword) instead of ‘ ‘macchina’ (car)] [81]. Deep dyslexia is instead noted in Chinese-speaking svPPA individuals, likely because Chinese is a logographic language that heavily relies on ATL-supported lexical–semantic memory [82▪]. The unique characteristics of different languages can thus provide interesting perspectives regarding the development, plasticity and cognitive reserve of specific language networks depending on different linguistic context.
PRIMARY PROGRESSIVE APHASIA AS A MODEL OF STUDYING NETWORK SUSCEPTIBILITY TO PATHOGENIC PROTEINS
Three decades of PPA research have demonstrated that by applying the knowledge of basic cognitive and imaging neuroscience to the study of neurodegenerative disease, we can identify specific clinical phenotypes and accurately map these phenotypes to specific brain networks [7]. As each brain network has a selective vulnerability to specific toxic proteins, clinicoanatomical phenotyping improves the prediction of in-vivo neuropathological changes [83]. By combining evidence from clinical, neuroanatomical, genetic, and biomarker studies, we can correctly identify underlying Alzheimer’s disease from FTLD disorder in PPA premortemly. Differentiation between FTLD-subtype disorder is also quite accurate, though slightly less reliable because of the lack of in-vivo biomarkers, such as molecular PET or CSF. Albeit the current PPA consensus classification has greatly increased our ability to predict underlying neuropathology, such prediction will never be perfect, as the selective vulnerability is relative and not absolute. In-vivo pathological prediction is crucial in neurodegenerative diseases because therapeutic pharmacological strategies are, or soon will be, directed towards decreasing or clearing toxic molecules, such as amyloid, tau or TDP.
Different autopsy studies show that the prevalence of FTLD-TDP-43 type C (TDP-C) disorder ranges between 73 and 83% in clinically diagnosed svPPA [28▪▪,49]. The prevalence of TDP-C disorder is higher when svPPA cases are diagnosed prospectively and followed longitudinally [49]. The remaining cases usually have FTLD-tau, including Pick’s disease and globular glial tauopathies (GGT), or, more rarely, Alzheimer’s disease [49]. Consistent with the selective vulnerability theory, nfvPPA shows a very different pattern of pathological changes compared with svPPA, mainly caused by FTLD-tau deposition [49]. In one prospectively diagnosed cohort with 25 nfvPPA patients, nfvPPA was most commonly associated with FTLD-tau disorder (88%), with 72% being four-repeat tauopathies (4Rtau), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP), and 16% having threerepeat tauopathies (3R-tau), such as Pick’s disease [49]. A small portion of nfvPPA cases was found to have FTLD-TDP-43 type A or B disorder, likely because of GRN or C9ORF72 mutations [49]. Sporadic lvPPA clinical syndrome is associated with Alzheimer’s disease biomarkers and Alzheimer’s disease pathological changes in about 85–90% of cases [28▪▪,49,52▪]. Therefore, recent Alzheimer’s disease diagnostic criteria include lvPPA as an atypical earlyonset variant of Alzheimer’s disease [84]. Other causes are Pick’s disease (3R tauopathy) or TDP-43 type A disorder, the latter associated with GRN mutations [28▪▪,78]. A few lvPPA cases also reported Lewy body disorder in isolation or as co-disorder with Alzheimer’s disease [85]. Interestingly, coexisting disorders or biomarkers discordant with the clinical syndrome are more common than predicted in PPA (and FTD), especially in older individuals [28▪▪,52▪]. These studies are very relevant to clinical trials. For instance, given that FTLD-4 repeat (4R) tauopathies are rarely associated with svPPA, svPPA patients should not be considered for clinical trials that target 4R tau that are instead appropriate for nfvPPA.
In-vitro and animal studies have demonstrated that toxic proteins including amyloid, tau, and TDP43 spread transneuronally through connected networks in a prion-like manner [86–88]. Neuroimaging studies in PPA support these findings by showing network-specific damage in each variant. Given the extensive available knowledge on the anatomy and physiology of the speech and language networks, PPA is an ideal model to investigate the intricate relationship between protein deposition patterns and network susceptibility in neurodegenerative diseases. Neurodevelopmental disorders are increasingly associated with neurodegenerative diseases and might contribute to network vulnerability [89,90]. Notably, developmental dyslexia seems overrepresented in PPA, with one study indicating greater association in lvPPA [89,90]. Moreover, nonright handedness dominance is found to be more common in svPPA, at nearly twice the prevalence of the general population [89]. Thus, PPA can potentially be an optimal model to study the neurodevelopment influences in neurodegenerative diseases.
PRIMARY PROGRESSIVE APHASIA AS A MODEL FOR TARGETED SPEECH AND LANGUAGE REHABILITATION STRATEGIES IN NEURODEGENERATIVE DISEASES
There is increasing evidence supporting the effectiveness of targeted speech and language rehabilitation therapy in PPA, with or without the addition of noninvasive brain stimulation. In nfvPPA, motor speech impairment and agrammatism showed positive effects to structured oral reading tasks training and video-implemented script training for aphasia (VISTA) therapy [91▪▪,92]. When coupled with language therapies, transcranial direct current stimulation (tDCS) has demonstrated improvements in various speech and language performances in all variants of PPA [93▪,94,95]. Lexical retrieval treatments are one of the most widely explored nonpharmacological therapies in PPA [96▪]. To maximize its therapeutic effects, it is crucial to build on spared language abilities, such as phonological and autobiographical memory processes in svPPA and semantic memory abilities in lvPPA [96▪]. A recent study shows that intensive naming therapy is associated with increased bilateral activation in functional MRI after treatment [97]. PPA served as a model to show that targeted cognitive therapy can be useful in improving and delaying progression of cognitive symptoms in neurodegenerative disorders.
CONCLUSION
PPA is emerging as a model for understanding the link between clinical, neuroimaging and neurobiological vulnerability in focal neurodegenerative disorders. Research in PPA shows that a multidisciplinary, precision medicine approach is the best strategy towards finding effective pharmacological and cognitive therapies for neurodegenerative diseases.
Supplementary Material
KEY POINTS.
PPA can be largely divided into three variants or subtypes, semantic, nonfluent, and logopenic, each with distinct functional neuroanatomy, underlying proteinopathies and clinical characteristics.
Premortem neuropathological prediction of PPA can be highly accurate by combining the clinical, neuroanatomical, genetic, and biomarker evidence.
PPA provides additional valuable perspectives for understanding the neural basis of speech and language.
PPA is an ideal model to study the link between clinical, neuroimaging, and neurobiological vulnerability in focal neurodegenerative disorders.
Acknowledgements
We would like to thank the participating volunteers, their families, peers, and colleagues working in the field. Special thanks to Dr Michael D. Geschwind for his critical review of the manuscript.
Financial support and sponsorship
The work is supported by Global Brain Health Institute, University of California, San Francisco, National Institutes of Health (NINDS R01 NS050915, NIDCD K24 DC015544, NIDCD R01 DC016291, NIA U01 AG052943, NIA P50 AG023501, NIA P01 AG019724, R01 AG038791, U01 AG045390, U54 NS092089), Alzheimer’s Disease Research Center of California (03-75271 DHS/ADP/ARCC); Larry L. Hillblom Foundation (2013-A-029-SUP and 2005/2T); John Douglas French Alzheimer’s Foundation; Koret Family Foundation; Consortium for Frontotemporal Dementia Research; and McBean Family Foundation.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Pick A Über die Beziehungen der senilen Atrophie zur Aphasie. Prager Medizinische Wochenschrift 1892; 17:165–167. [Google Scholar]
- 2.Imura T Aphasia: characteristic symptoms in Japanese. Psychiatr Neurol Japon 1943; 46:196–218. [Google Scholar]
- 3.Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol 1975; 27:635–657. [DOI] [PubMed] [Google Scholar]
- 4.Snowden J, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 1989; 2:167–182. [Google Scholar]
- 5.Mesulam MM. Primary progressive aphasia–differentiation from Alzheimer’s disease. Ann Neurol 1987; 22:533–534. [DOI] [PubMed] [Google Scholar]
- 6.Grossman M, Mickanin J, Onishi K, et al. Progressive nonfluent aphasia: language, cognitive, and PET measures contrasted with probable Alzheimer’s disease. J Cogn Neurosci 1996; 8:135–154. [DOI] [PubMed] [Google Scholar]
- 7.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004; 55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesulam LMM. Primary progressive aphasia. Ann Neurol 2001; 49: 425–432. [PubMed] [Google Scholar]
- 10.Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 1992; 115(Pt 6):1783–1806. [DOI] [PubMed] [Google Scholar]
- 11.Adlam ALR, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 2006; 129:3066–3080. [DOI] [PubMed] [Google Scholar]
- 12.Luzzi S, Baldinelli S, Ranaldi V, et al. Famous faces and voices: differential profiles in early right and left semantic dementia and in Alzheimer’s disease. Neuropsychologia 2017; 94:118–128. [DOI] [PubMed] [Google Scholar]
- 13.Goll JC, Crutch SJ, Loo JH, et al. Nonverbal sound processing in the primary progressive aphasias. Brain 2010; 133(Pt 1):272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luzzi S, Snowden JS, Neary D, et al. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007; 45:1823–1831. [DOI] [PubMed] [Google Scholar]
- 15.Gorno-Tempini ML, Rankin KP, Woolley JD, et al. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex 2004; 40:631–644. [DOI] [PubMed] [Google Scholar]
- 16.Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology 2005; 64:1384 –1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009; 62:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binney RJ, Embleton KV, Jefferies E, et al. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb Cortex 2010; 20:2728–2738. [DOI] [PubMed] [Google Scholar]
- 19.Kumfor F, Landin-Romero R, Devenney E, et al. On the right side? A longitudinal study of leftversus right-lateralized semantic dementia. Brain 2016; 139(Pt 3):986–998. [DOI] [PubMed] [Google Scholar]
- 20.Brambati SM, Rankin KP, Narvid J, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging 2009; 30:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 2003; 61:1196–1203. [DOI] [PubMed] [Google Scholar]
- 22.Galantucci S, Tartaglia MC, Wilson SM, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 2011; 134(Pt 10):3011–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agosta F, Henry RG, Migliaccio R, et al. Language networks in semantic dementia. Brain 2010; 133(Pt 1):286 –299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo CC, Gorno-Tempini ML, Gesierich B, et al. Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain 2013; 136(Pt 10):2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins JA, Montal V, Hochberg D, et al. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain 2017; 140:457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranasinghe KG, Hinkley LB, Beagle AJ, et al. Distinct spatiotemporal patterns of neuronal functional connectivity in primary progressive aphasia variants. Brain 2017; 140:2737–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohrer JD, Geser F, Zhou J, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology 2010; 75:2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.▪▪.Bergeron D, Gorno-Tempini ML, Rabinovici GD, et al. Prevalence of amyloid-beta pathology in distinct variants of primary progressive aphasia. Ann Neurol 2018; 84:729–740.This is the largest meta-analysis to date studying pattern of amyloid depositions in PPA using CSF analysis, amyloid PET imaging, and autopsy data. It highlights that amyloid deposition is most common in lvPPA; amyloid PET positivity in svPPA, and nfvPPA resemble that of aging healthy controls.
- 29.Kas A, Uspenskaya O, Lamari F, et al. Distinct brain perfusion pattern associated with CSF biomarkers profile in primary progressive aphasia. J Neurol Neurosurg Psychiatry 2012; 83:695–698. [DOI] [PubMed] [Google Scholar]
- 30.▪.Josephs KA, Martin PR, Botha H, et al. [(18) F]AV-1451 tau-PET and primary progressive aphasia. Ann Neurol 2018; 83:599–611.This study investigate [(18)F]AV-1451 PET uptake patterns in all three PPA variants. LvPPA showed strongest uptake especially over temporoparietal region; in contrast, svPPA and AgPPA exhibit mild tracer uptake over anteromedial temporal and prefrontal regions, respectively.
- 31.Le Ber I, Camuzat A, Guillot-Noel L, et al. C9ORF72 repeat expansions in the frontotemporal dementias spectrum of diseases: a flow-chart for genetic testing. J Alzheimer’s Dis 2013; 34:485–499. [DOI] [PubMed] [Google Scholar]
- 32.Ogar JM, Dronkers NF, Brambati SM, et al. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 2007; 21:S23–30. [DOI] [PubMed] [Google Scholar]
- 33.▪.Utianski RL, Duffy JR, Clark HM, et al. Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and language 2018; 184:54–65.This article compared the clinical and neuroimaging data (MRI, DTI, FDG-PET) between prosodic and phonetic of primary progressive apraxia of speech.
- 34.Ash S, McMillan C, Gunawardena D, et al. Speech errors in progressive nonfluent aphasia. Brain Lang 2010; 113:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SM, Henry ML, Besbris M, et al. Connected speech production in three variants of primary progressive aphasia. Brain 2010; 133(Pt 7):2069–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson CK, Lukic S, King MC, et al. Verb and noun deficits in strokeinduced and primary progressive aphasia: the Northwestern Naming Battery. Aphasiology 2012; 26:632–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson CK, Mack JE. Grammatical impairments in PPA. Aphasiology 2014; 28:1018–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesulam M, Wieneke C, Rogalski E, et al. Quantitative template for subtyping primary progressive aphasia. Arch Neurol 2009; 66:1545 –1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butts AM, Machulda MM, Duffy JR, et al. Neuropsychological profiles differ among the three variants of primary progressive aphasia. J Int Neuropsychol Soc 2015; 21:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandelli ML, Vilaplana E, Brown JA, et al. Healthy brain connectivity predicts atrophy progression in nonfluent variant of primary progressive aphasia. Brain 2016; 139(Pt 10):2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.▪.Tetzloff KA, Duffy JR, Clark HM, et al. Longitudinal structural and molecular neuroimaging in agrammatic primary progressive aphasia. Brain 2018; 141:302 –317.This is a longitudinal follow-up study of AgPPA, PPAOS, and matched controls using MRI, DTI, FDG-PET imaging. All imaging modalities indicated that AgPPA tends to have more widespread involvement and PPAOS progression focused around premotor anrd motor regions.
- 42.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012; 135(Pt 5): 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grossman M, Powers J, Ash S, et al. Disruption of large-scale neural networks in nonfluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang 2013; 127:106 –120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.▪▪.Mandelli ML, Welch AE, Vilaplana E, et al. Altered topology of the functional speech production network in nonfluent/agrammatic variant of PPA. Cortex 2018; 108:252–264.This article uses graph theory and longitudinal fMRI data to study the speech production network (SPN) and default mode network (DMN) topology in nfvPPA. It is shown that SPN exhibits network-specific topological alterations, but not DMN.
- 45.Gorno-Tempini ML, Ogar JM, Brambati SM, et al. Anatomical correlates of early mutism in progressive nonfluent aphasia. Neurology 2006; 67: 1849–1851. [DOI] [PubMed] [Google Scholar]
- 46.Catani M, Mesulam MM, Jakobsen E, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 2013; 136(Pt 8):2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandelli ML, Caverzasi E, Binney RJ, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci 2014; 34:9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agosta F, Scola E, Canu E, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex 2012; 22:2705 –2714. [DOI] [PubMed] [Google Scholar]
- 49.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 2017; 81:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006; 129(Pt 6): 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson SM, Dronkers NF, Ogar JM, et al. Neural correlates of syntactic processing in the nonfluent variant of primary progressive aphasia. J Neurosci 2010; 30:16845–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.▪.Santos-Santos MA, Rabinovici GD, Iaccarino L, et al. Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol 2018; 75:342–352.This is a prospective clinical– pathologic case series study that evaluated amyloid PET imaging positivity in all three PPA variants. It is found that 14, 10, and 96% of svPPA, nfvPPA, and lvPPA showed amyloid positive imaging, respectively.
- 53.▪.Schaeverbeke J, Evenepoel C, Declercq L, et al. Distinct [(18)F]THK5351 binding patterns in primary progressive aphasia variants. Eur J Nuclear Med Molecular imaging 2018; 45:2342 –2357.This article studied nfvPPA and lvPPA patients via neurolinguistic assessment, MRI, and [(18)F]THK5351 PET. It revealed that [(18)F]THK5351 tracer-binding topography closely matches the predicted anatomical distribution of tau protein underlying disorder and correlates with neurolinguistical performance.
- 54.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000; 55:1368 –1375. [DOI] [PubMed] [Google Scholar]
- 55.Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology 2010; 74:42–49. [DOI] [PubMed] [Google Scholar]
- 56.Santos-Santos MA, Mandelli ML, Binney RJ, et al. Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol 2016; 73:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008; 71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henry ML, Wilson SM, Babiak MC, et al. Phonological processing in primary progressive aphasia. J Cogn Neurosci 2016; 28:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.▪.Dalton SGH, Shultz C, Henry ML, et al. Describing phonological paraphasias in three variants of primary progressive aphasia. Am J Speech Lang Pathol 2018; 27(1 Suppl):336 –349.This study describe the characteristics of phonological paraphasias in all three variants of PPA and showed unique patterns for each variant.
- 60.Leyton CE, Hodges JR, Piguet O, Ballard KJ. Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer’s disease. Cortex 2017; 86:45–54. [DOI] [PubMed] [Google Scholar]
- 61.Rohrer JD, Ridgway GR, Crutch SJ, et al. Progressive logopenic/ phonological aphasia: erosion of the language network. NeuroImage 2010; 49:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teichmann M, Kas A, Boutet C, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain 2013; 136(Pt 1):3474–3488. [DOI] [PubMed] [Google Scholar]
- 63.▪▪.Watson CL, Possin K, Allen IE, et al. Visuospatial functioning in the primary progressive aphasias. J Int Neuropsychol Soc 2018; 24:259–268.The study investigates the visuospatial cognition profile in the three main PPA variants and showed that PPA variants have varying degree of performance in certain visuospatial composite scores including delayed recall and figure copy performance.
- 64.Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: more than losing words. Neurology 2013; 80:897–903. [DOI] [PubMed] [Google Scholar]
- 65.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology 2009; 73:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Win KT, Pluta J, Yushkevich P, et al. Neural correlates of verbal episodic memory and lexical retrieval in logopenic variant primary progressive aphasia. Front Neurosci 2017; 11:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lukic S, Mandelli M, Welch A, et al. Neurocognitive basis of repetition deficits in primary progressive aphasia. Brain Lang 2018. [DOI] [PMC free article] [PubMed]
- 68.Whitwell JL, Jones DT, Duffy JR, et al. Working memory and language network dysfunctions in logopenic aphasia: a task-free fMRI comparison with Alzheimer’s dementia. Neurobiol Aging 2015; 36:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehmann M, Madison CM, Ghosh PM, et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer’s disease. Proc Natl Acad Sci USA 2013; 110:11606–11611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng E, Yamasaki TR, Tran M, et al. Cerebrospinal fluid biomarkers in clinical subtypes of early-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 2014; 37:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008; 64:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohrer JD, Caso F, Mahoney C, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang 2013; 127:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci 2010; 293:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rohrer JD, Crutch SJ, Warrington EK, Warren JD. Progranulin-associated primary progressive aphasia: a distinct phenotype? Neuropsychologia 2010; 48:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohrer JD, Guerreiro R, Vandrovcova J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology 2009; 73:1451 –1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.▪.Deleon J, Miller BL. Chapter 27 Frontotemporal dementia. In: Geschwind DH, Paulson HL, Klein C, editors. Handbook of clinical neurology, Vol. 148 Elsevier; 2018. pp. 409–430.This is a review article on the genetic mutation reported in FTLD spectrum disorders.
- 77.▪.Van Giau V, Senanarong V, Bagyinszky E, et al. Identification of a novel mutation in APP gene in a Thai subject with early-onset Alzheimer’s disease. Neuropsychiatr Dis Treat 2018; 14:3015 –3023.This is a case report on a lvPPA patient that was found to carry a novel mutation in exon 14 of APP (c.1810C>T, p.V604M).
- 78.Josephs KA, Duffy JR, Strand EA, et al. Progranulin-associated PiB-negative logopenic primary progressive aphasia. J Neurol 2014; 261: 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rogalski EJ, Rademaker A, Harrison TM, et al. ApoE E4 is a susceptibility factor in amnestic but not aphasic dementias. Alzheimer Dis Assoc Disord 2011; 25:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.▪.Grossman M Linguistic aspects of primary progressive aphasia. Annu Rev Linguist 2018; 4:377–403.This is a comprehensive review article on the neurolinguistic aspect of PPA.
- 81.Folegatti A, Pia L, Berti A, Cubelli R. Stress assignment errors in surface dyslexia: evidence from two italian patients with a selective deficit of the orthographic input lexicon. Behav Neurol 2015; 2015:769013. [DOI] [PMC free article] [PubMed]
- 82.▪.Ting SKS, Foo H, Chia PS, et al. Dyslexic characteristics of Chinese-speaking semantic variant of primary progressive aphasia. J Neuropsychiatry Clin Neurosci 2018; 30:31–37.This study illustrated the dyslexic pattern of Chinese speaking svPPA patients.
- 83.Zhou J, Gennatas ED, Kramer JH, et al. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 2012; 73:1216–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014; 13:614–629. [DOI] [PubMed] [Google Scholar]
- 85.Giannini L, Irwin D, McMillan C, et al. Clinicopathological correlations of AD neuropathology in the logopenic variant of primary progressive aphasia (S39.002). Neurology 2016; 86(16 Suppl):S39.002. [Google Scholar]
- 86.Smethurst P, Newcombe J, Troakes C, et al. In vitro prion-like behaviour of TDP-43 in ALS. Neurobiol Dis 2016; 96:236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lewis J, Dickson DW. Propagation of tau pathology: hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol 2016; 131:27–48. [DOI] [PubMed] [Google Scholar]
- 88.Ruiz-Riquelme A, Lau HHC, Stuart E, et al. Prion-like propagation of b-amyloid aggregates in the absence of APP overexpression. Acta Neuropathol Commun 2018; 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain 2013; 136(Pt 11):3461–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rogalski EJ, Rademaker A, Wieneke C, et al. Association between the prevalence of learning disabilities and primary progressive aphasia. JAMA Neurol 2014; 71:1576–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.▪▪.Henry ML, Hubbard HI, Grasso SM, et al. Retraining speech production and fluency in nonfluent/agrammatic primary progressive aphasia. Brain 2018; 141:1799–1814.This is the first study that examined the therapeutic effect of video-implemented script training for aphasia (VISTA) in 10 individuals with nfvPPA and showed improvement in motor speech production and reduction in grammatical errors.
- 92.Henry ML, Meese MV, Truong S, et al. Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behav Neurol 2013; 26:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.▪.Tsapkini K, Webster KT, Ficek BN, et al. Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimers Dement (N Y) 2018; 4:461–472.This article focused on demonstrating the effect of transcranial direct current stimulation (tDCS) coupled with written naming/spelling therapy in PPA.
- 94.Gervits F, Ash S, Coslett HB, et al. Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain Lang 2016; 162:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cotelli M, Manenti R, Petesi M, et al. Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. J Alzheimer’s Dis 2014; 39:799–808. [DOI] [PubMed] [Google Scholar]
- 96.▪.Croot K Treatment for lexical retrieval impairments in primary progressive aphasia: a research update with implications for clinical practice. Semin Speech Lang 2018; 39:242–256.This is a review article that focused on lexical retrieval treatment in PPA and elaborated on different lexical retrieval treatment techniques and clinical guidelines.
- 97.Jokel R, Kielar A, Anderson ND, et al. Behavioural and neuroimaging changes after naming therapy for semantic variant primary progressive aphasia. Neuropsychologia 2016; 89:191–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


