Abstract
Prostate Cancer (PCa) patients’ mortality is mainly attributed to complications caused by metastasis of the tumor cells to organs critical for survival, such as bone. We hypothesized that prostate cancer cell-bone interactions would promote paracrine signaling. A panel of prostate cancer cell lines were co-cultured with Hydroxyapatite (HA; inorganic component of bone) of different densities. Conditioned media (CM) was collected and analyzed for calcium levels and effect on paracrine signaling, cell migration and viability in vitro and in vivo. Our results showed that calcium levels were elevated in CM from cancer cell-bone co-cultures, compared to media or cancer cells alone, and this could be antagonized by EGTA, a calcium chelator, or knockdown of Snail protein. We also observed increased STAT3 phosphorylation and paracrine cell proliferation and migration in LNCaP cells incubated with CM from various cell lines; this phosphorylation and cell migration could be antagonized by Snail knockdown or various inhibitors including EGTA, STAT3 inhibitor (WP1066) or Cathepsin L inhibitor (Z-FY-CHO). In vivo, higher HA bone density increased tumorigenicity and migration of tumor cells to HA implant. Our study shows that cancer-bone microenvironment interactions lead to calcium-STAT3 signaling, which may present an area for therapeutic targeting of metastatic prostate cancer.
Keywords: STAT3, prostate cancer cells, cancer- bone microenvironmental interactions, Snail, EMT, Hydroxyapatite, cell migration
1 ∣. INTRODUCTION
In Prostate Cancer (PCa), as in many other types of cancer, metastasis of the primary tumor is the main cause of cancer related death.1 PCa has a propensity to metastasize to the bone.2 One elucidation for how PCa cells interact with the bone microenvironment was given by Thalmann et al. Their work showed that the role of osteopontin (OPN), an extracellular matrix glycoprotein, gave PCa cells the ability to grow in an anchorage independent manner, thus providing a potential explanation for why metastasized PCa cells may successfully colonize the new host bone microenvironment.3 Further studies have emphasized the role of the receptor activator of nuclear factor kappa B (RANK) on PCa cells interacting with RANK ligand (RANKL) in the bone microenvironment, which has led to much work using RANKL inhibitors in treatment of bone metastatic cancer.4 However, more work is needed in the determination of what enables PCa cells to successfully adapt within the bone microenvironment.
Snail1 or Snail is a zinc-finger transcription factor that binds to E-box consensus sequence of their target genes.5 Snail transcription factor can either act directly as a repressor or indirectly as an activator of gene expression.5 Snail acts as a suppressor of epithelial genes and an enhancer of mesenchymal genes leading to epithelial-mesenchymal transition (EMT).6 One of the well-studied roles of Snail is its involvement in the EMT process and especially through its role in down-regulating E-cadherin expression, a cell adhesion molecule.7 This leads to loss of cell-cell adhesion and increased cell motility.8,9 Snail protein expression is also increased at the bone metastatic site of PCa, and we have further shown that Snail can promote osteoclastogenesis and bone degradation in vivo.10 Therefore, it is important that new therapeutic targets are designed to down-regulate Snail during tumor progression and metastasis.
Signal transducer and activator of transcription (STAT) family proteins is made up of 7 members namely, STAT1–4, STAT6, STAT5a and 5b.11 STAT1 is known to act as a tumor repressor while STAT3 and 5 are often associated with aggressive tumors.11 Some of the cellular roles of STAT3 and STAT5 show involvement in cell proliferation, resistance to apoptosis, angiogenesis and avoiding immune surveillance.11 As such, STAT3 has been reported to be a good therapeutic target for treatment of cancer.12 Don-Doncow et al., found out that STAT3 and interleukin 6 receptor (IL6R) were present in 95% of the metastases and their expression was high in bone metastases compared to lymph node and visceral ones.13 STAT3 has also been shown to signal in tumor microenvironment where immune cells cooperate with tumor cells to enhance tumor growth and progression.14 This occurs via production of immunosuppressive signal factors that lead to the activation of STAT3 and inhibition of anti-tumor responses.14
Cathepsin L (Cat L) is a lysosomal proteinase that has been reported to be upregulated during tumorigenesis.15 Cat L can degrade collagen type I and IV, fibronectin, and laminin components of the extracellular matrix (ECM).16 Cat L has also been shown to degrade bone associated with rheumatoid arthritis and at the cancer metastatic site.17–19 We have previously shown that Snail overexpression can increase Cat L expression and secreted Cat L activity via STAT3 activation and that Snail-mediated osteoclastogenesis can be abrogated by Z-FY-CHO, a Cat L specific inhibitor, suggesting that Snail promotes osteoclastogeneis via Cat L.20
In an attempt to study cancer-bone microenvironment interactions, we developed an in vitro model where we co-cultured hydroxyapatite (HA), the inorganic bone component, with PCa cells in hopes of elucidating the signaling pathway(s) that mediate tumor progression at the bone metastatic site. We utilized varying bone densities to examine the role of bone density in PCa-bone microenvironment interactions since people have different bone densities and African-American men display higher bone density and more aggressive PCa compared to any other race.21,22 We found that cancer/HA co-cultures increased calcium release that mediated paracrine STAT3 phosphorylation, proliferation and migration, and this could be abrogated by calcium chelation, Snail knockdown, Cat L or STAT3 inhibition. Increasing HA density from 100 mg to 200 mg increased the signaling and biological activity, however, higher density of 240 mg HA was no longer effective. In vivo implantation of PCa cells with HA in immunocompromised mice gave rise to larger tumors and migration to bone implant with higher bone density. Therefore, cancer/HA interactions release calcium that may affect paracrine signaling and promote prostate cancer proliferation and migration.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Reagents and antibodies
RPMI, DMEM media and penicillin/streptomycin were purchased from VWR Int., West Chester, PA. Calcium chloride free media, DMEM, was purchased from Thermo Fisher Scientific, Waltham, MA. The protease inhibitor cocktail was from Roche Molecular Biochemicals, Indianapolis, IN. The calcium assay kit was purchased from Biovision Incorporated, Milpitas, CA. The mouse monoclonal anti-α-tubulin antibody, the STAT3 inhibitor (WP1066), the Cat L inhibitor II (Z-FY-CHO), Hydroxyapatite (HA), Dimethyl sulfoxide (DMSO), and ethylene glycol-bis(2-aminoethylether)N,N,N,`N`-tetraacetic acid (EGTA) were purchased from Millipore-Sigma, Burlington, MA. The phospho-STAT3 (p-STAT3) antibody was a rabbit monoclonal from Abcam, Cambridge, MA. Rabbit monoclonal anti-human Snail antibody, rabbit monoclonal anti-human phospho-AKT, phospho-ERK antibodies and HRP-conjugated goat anti-rat antibody were from Cell Signaling Technology, Inc., Danvers, MA. Mouse monoclonal anti-human total-STAT3, goat polyclonal total-AKT, rabbit polyclonal total-ERK, rabbit polyclonal anti-Snail and donkey anti-goat secondary antibodies were from Santa Cruz biotechnology, Inc., Dallas, Texas. Mouse monoclonal anti-E-cadherin antibody was from BD Biosciences, San Jose, CA. Mouse monoclonal anti-luciferase antibody was from Novus Biological, Littleton, CO. HRP-conjugated sheep anti-mouse antibody and HRP-conjugated donkey anti-rabbit were purchased from Amersham Biosciences, Buckingham, England. Enhanced chemiluminescence (ECL) prime western blotting detection reagent was purchased from Thermo Fisher Scientific Inc., Waltham, MA. Fetal bovine serum (FBS) was from Atlanta biologicals Inc., Flowery Branch, GA.
2.2 ∣. Cell culture
The human prostate cancer cell line, LNCaP, and human embryonic kidney cell line, HEK-293, were obtained from ATCC, Manassas, VA. C4–2 cells were a kind gift from Dr. Leland Chung (Cedar Sinai Medical Center, Los Angeles, CA). C4–2 cells with stable knockdown of Snail using shRNA were previously generated.23 E006AA and E006AA-hT were generated as published 24 and obtained as a gift by Dr. Shahriar Koochekpour, Roswell Cancer Institute, New York. Cell lines have been authenticated by ATCC and we tested for mycoplasma before use. Cells were grown in either RPMI or DMEM (for E006AA and E006AA-hT cell lines) media supplemented with 10 % fetal bovine serum and 1X penicillin-streptomycin at 37 °C in a 5 % CO2 humidified incubator.
2.3 ∣. Co-culture with hydroxyapatite
100–240 mg hydroxyapatite (HA), the inorganic bone component, was added to 6-well plates in calcium-free media and allowed to harden in media overnight. Subsequently, various cancer cells were added and cultured for 6 days, changing half the media after 3 days. As a control, we included wells with HA alone. We collected conditioned media (CM) after 6 days. We subsequently used the CM to 1) treat LNCaP cells for various time points followed by western blot analysis, or 2) add to LNCaP cells to perform paracrine viability assays, or 3) use as a chemoattractant to perform paracrine cell migration assays.
2.4 ∣. Luciferase transduction
C4–2 NS and C4–2 Snail shRNA were plated in a 6 well plate at a 5 × 105 cells per well and allowed to grow overnight at 37°C. The next day, the lentivirus stock (ready-to-use lentivirus marker supernatant from Biogenova, Rockville, MD) was diluted at 1:10 multiplicity of infection (MOI) with fresh complete media containing 8 μg/ml polybrane and added to the cells. At day three post-transduction, the media containing the virus was replaced with fresh complete media. We allowed the cells to grow for about a month and a half while changing the complete media every three days, using 5 μg/ml blasticidin for selection. Cells were tested for luciferase activity using the Dual-Glo luciferase assay kit from Promega, Madison, WI.
2.5 ∣. Western blot analysis
Western blot analysis was performed as previously described.25 The membranes were blocked in TBS-T (Tris Buffer Saline with 0.05 % Tween-20) containing 3% milk, and subsequently incubated with diluted antibody in blocking buffer. Cell signaling antibodies were diluted in 3% bovine serum albumin (BSA) with TBS-T buffer. After washing, the membranes were incubated in peroxidase-conjugated sheep anti-mouse, donkey anti-rabbit, or goat anti-rat IgG, then washed, and visualized using ECL prime reagent. The membranes were stripped using stripping buffer prior to re-probing with a different antibody.
2.6 ∣. Calcium assay
Calcium assay was done using the BioVision Incorporated kit # K380–250 according to manufacturer instructions in 96-well plate using 50 μl media alone as control or conditioned media, plus chromogenic reagent and calcium assay buffer. The assay plate was then incubated at room temperature for 10 min before reading the absorbance at 575 nm. Calcium concentration was calculated using the formula given in the kit, C=Sa/Sv, where Sa is the calcium sample amount and Sv is sample volume (μl) added into the well.
2.7 ∣. Inhibitor treatment
1X106 cells were plated overnight at 37°C in 10 cm dishes followed by serum starvation for 5 h in phenol red-free RPMI. STAT3 inhibitor, WP1066 (20 µM) or Cat L inhibitor II (Z-FY-CHO, 5 µM) was utilized for various time points, or controls treated with 0.005% DMSO. EGTA was utilized at 0.2 mM. All treatments were performed using DMEM calcium chloride-free media.
2.8 ∣. In vitro cell migration assay
We utilized costar 24-well plates containing a polycarbonate filter insert (BD Biosciences, Franklin Lakes, NJ) with an 8-μm pore size, coated with 4.46 µg/µl rat tail collagen I on the outside for migration assays. 10,000 LNCaP cells were plated in the upper chamber containing cells in calcium-free media, DMEM, while the lower chamber had CM from co-culture of various PCa cells with HA, or controls. 24 h later, cells that had migrated to the bottom of the insert was fixed with 10% formalin, stained with crystal violet, and counted to obtain the relative cell migration.
2.9 ∣. Cell viability assay
Cell viability was assayed using MTS assay as per manufacturer instructions. Briefly, 2X103 cells were plated to each well of a 96 well plate and incubated overnight at 37°C. The next day, media was removed and 100 μl conditioned media from the co-culture of various PCa cells with HA was added to the appropriate well. Absorbance was read at 490 nm wavelength.
2.10 ∣. Animal studies
Male athymic Balb/c nu/nu mice, aged 4–6 weeks old were utilized. The animal protocol was approved by Institutional animal care and use committee of Morehouse School of Medicine/ Atlanta University Center. The left flank of the mouse was implanted subcutaneously with 40 mg or 100 mg of HA plus human bone marrow stromal cells to mimic bone. Two weeks later, 2X106 C4–2 NS or C4–2 Snail shRNA that stably express luciferase gene were mixed 1:1 with matrigel and 500 µl injected subcutaneously into the nude mice 2 cm from HA implantation site. Mice weights and tumor volumes were recorded weekly with a caliper. After 8–12 weeks, all the mice were sacrificed and tumor and HA bone implants removed, sectioned, and processed for H&E staining. IHC was done to examine Snail, E-cadherin and luciferase expression.
2.11 ∣. Immunohistochemistry (IHC)
IHC study was done on paraffin embedded tissues as follows: antigen retrieval was performed using aqua depar and reveal decloaker solutions (Biocare Medical, Concord, CA.), followed by antibody incubations with the Snail (1:200, Santa Cruz, CA), E-cadherin (1:500, BD Biosciences, San Jose, CA) and Luciferase (1:5000, Novus Biological, Littleton, CO) antibodies. Secondary antibodies were used at dilutions ranging between 1:500–1:1000. The DAB detection system used was the LSAB2 kit from Agilent, Santa Clara, CA. Processed slides were imaged using the Aperio Scanner, Leica Biosystems, Inc., Buffalo, IL.
2.12 ∣. Immunofluorescence (IF)
IF was performed as previously 26 described, but additionally, True Black lipofuscin autofluorescence quencher was used at 1X concentration in 70% ethanol (Biotium, Fremont, CA) following antigen retrieval. Anti-Luciferase antibody was used at 1:5000. The secondary antibody used was alexa rabbit anti-goat at 1:1000 (Thermo Fisher Scientific, Waltham, MA). Fluor gel was used for mounting the slides Electron Microscopy Sciences, Hatfield, PA.
2.13 ∣. Statistical analysis
Results are reported as mean + SD from 2–3 experiments. Statistical significance was assessed using GraphPad Prism software by paired ANOVA1 and considered significant at p < 0.05 or less.
3 ∣. RESULTS
3.1 ∣. Co-culture of various cancer cells with hydroxyapatite promotes paracrine phosphorylation of STAT-3 in LNCaP cells
Most studies on cancer/bone microenvironmental interactions focus on cancer cell interaction with osteoblasts/osteoclasts, where it has been shown that cancer cells can promote osteoblastogenesis and osteoclastogenesis in PCa. 3 We were interested in cancer/bone microenvironment interactions, particularly if cancer cell interaction with the inorganic component of bone can also alter signaling. We began our study by assessing the basal levels of signaling proteins that have been identified as regulating or being regulated by Snail. These proteins included Snail, p-STAT3, p-AKT, and p-ERK. As shown in Supplemental Fig. 1, all cell lines being used displayed constitutive p-STAT3, p-AKT and p-ERK, with the African American cell lines showing the highest levels of p-STAT3 and p-ERK, associated with higher cell viability and migration. Next, LNCaP, C4–2, E006AA and E006AA-hT cell lines were co-cultured with hydroxyapatite (HA), the inorganic bone component that was first allowed to harden in media overnight. We tested two different HA densities, 200 and 240 mg. After 6 days, we collected conditioned media (CM) from the co-culture. We subsequently used the CM to treat LNCaP cells for various time points followed by collection of protein lysates and western blotting to determine the levels of p-STAT3, p-AKT, p-ERK and total protein levels. Interestingly, the most significant and consistent results showed that CM from different cancer cells/HA (200 and 240 mg) co-culture led to elevated p-STAT3 protein levels in LNCaP cells within 5 min to 1 h (Fig. 1A, B, C, D). Although p-AKT was unaffected, p-ERK was also increased within 5 min for LNCaP/C4–2 co-culture with 200 mg HA or E006AA/E006AA-hT co-culture with 240 mg HA. As a control, we performed the HA co-cultures with a non-cancer cell line, HEK-293, and observed no upregulation of p-STAT3 with HEK-293/HA co-cultures (Supplemental Fig. 2). Therefore, the STAT3 and ERK signaling pathway may be essential for PCa/HA paracrine cell signaling.
FIGURE 1. Cancer/HA interaction increases paracrine STAT3 phosphorylation.
We first collected conditioned media (CM) from LNCaP, C4-2, E006AA and E006AA-hT cell lines co-cultured with HA for six days. We then used the CM to treat LNCaP cells at various time- points followed by collection of protein lysates and western blotting to determine the levels of p-STAT3, p-AKT, p-ERK and total proteins. Alpha-tubulin was utilized as a loading control. Results are representative of 3 independent experiments.
3.2 ∣. PCa/HA co-culture increases calcium levels in CM as well as paracrine cell migration and viability dependent on calcium
We hypothesized that PCa cells co-cultured with HA may degrade it to release calcium, similar to what happens during osteoclastogenesis where PCa cells have been shown to directly degrade bone through secretion of proteases.27 Therefore, we analyzed calcium levels in the Day 6 CM after co-culturing LNCaP, C4–2, E006AA or E006AA-hT cell lines with HA. In media alone or PCa cells alone without HA, relative calcium levels were low, but increased significantly upon co-culture with HA (Fig. 2A). To start to address the functional consequences of PCa/HA interactions, we then assessed the chemotactic cell migratory potential of LNCaP cells across collagen, towards CM from various PCa/HA co-cultures, using the boyden chamber. Our results showed that the LNCaP cells have a significantly higher migratory potential towards CM from PCa cells co-cultured with HA as compared to CM from PCa cells alone or media control (Fig. 2B). Furthermore, addition of EGTA to CM prior to cell migration assay abrogated LNCaP migration towards CM from LNCaP/HA co-culture (Fig. 2B), suggesting that calcium plays a role in this paracrine cell migration.
FIGURE 2. Increased calcium levels correspond to increased paracrine cell migration and viability.
(A) We analyzed the calcium levels in the Day 6 CM from LNCaP, C4-2, E006 AA or E006 AA HT cell lines co-cultured with 200 or 240 mg hydroxyapatite (HA) bone component. (B) We then assessed the cell migratory potential of LNCaP cells plated in the top well and CM from co-cultures as chemoattractant in the bottom well. We analyzed the proliferation levels in LNCaP cells grown in the presence of Day 6 CM from HA co-cultured with (C) LNCaP, (D) C4-2, (E) E006AA or E006AA-hT cells. EGTA was utilized to chelate calcium while Z-FY-CHO was utilized as a Cat L specific inhibitor. Results are representative of 2 independent experiments done in quadruplicates. Statistical significance was assessed using GraphPad Prism software by one- way ANOVA (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001).
We have previously shown that PCa induces osteoclastogenesis via Cat L protease activation, and that this could be inhibited by Z-FY-CHO, a Cat L specific inhibitor.20 We hypothesized that the PCa/HA signaling may involve Cat L protease. We therefore examined paracrine cell proliferation of LNCaP cells in response to CM from various PCa cells/HA co-culture, in the presence or absence of Z-FY-CHO or EGTA. We observed higher cell proliferation of LNCaP cells cultured in CM from the four PCa cell lines plus HA, which was higher with higher bone density; this could be abrogated by Z-FY-CHO or EGTA (Fig. 2 C, D, E). We also utilized E006AA cells to treat with CM from E006AA or E006AA-hT/HA co-cultures prior to paracrine cell migration and viability assays and observed similar results (data not shown). We therefore conclude that increased calcium levels from PCa/HA co-cultures leads to increased paracrine cell migratory capability, as well as paracrine cell proliferation, and this is mediated by Cat L activity.
3.3 ∣. Snail may mediate PCa/HA interactions
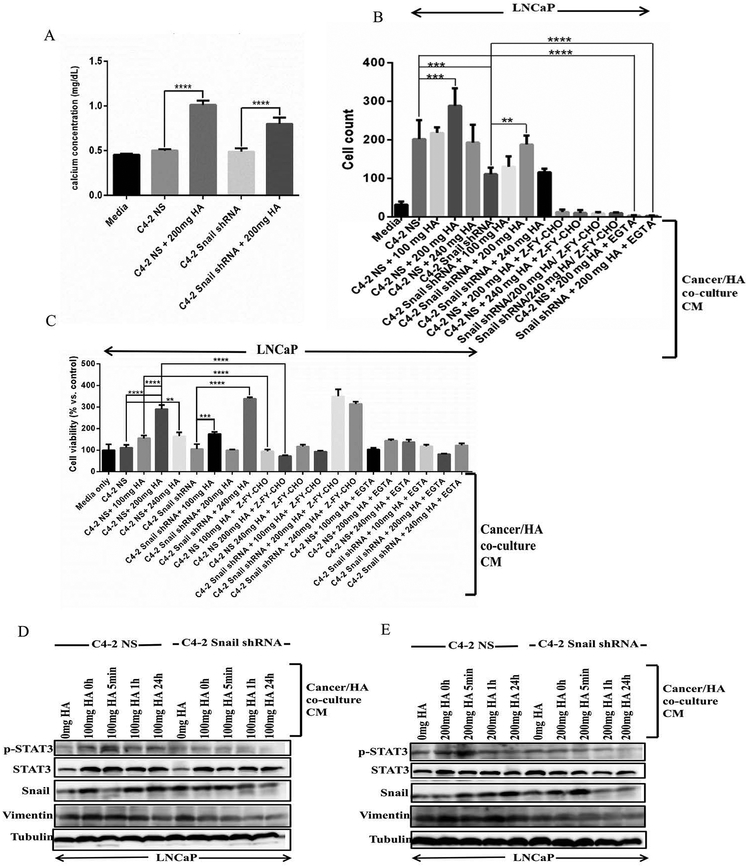
Next, we wanted to determine whether Snail plays a role in the PCa/HA interactions, since we have previously shown that Snail induces osteoclastogenesis via active Cat L.20 To begin, we performed a calcium colorimetric assay to determine the calcium levels in cells co-cultured with HA. We utilized C4–2 cells stably transfected with either non-silencing control (NS) or Snail shRNA that we have previously shown to express decreased Snail and Cat L activity.20 We observed that both C4–2 NS and C4–2 Snail shRNA CM had low levels of calcium comparable to media alone, however, calcium levels increased significantly upon co-culture with HA, with C4–2 NS/HA CM displaying higher calcium levels as compared to C4–2 Snail shRNA/HA CM (Fig. 3A). We then performed a paracrine chemotactic cell migration assay using CM from C4–2 NS/HA or C4–2 Snail shRNA/HA co-cultures in the bottom well, and LNCaP cells in the top well of a boyden chamber. We observed higher cell migration of LNCaP cells towards CM from co-culture of C4–2 NS with either 100 or 200 mg HA (but not 240 mg HA) as compared to C4–2 NS CM alone, which could be abrogated by Z-FY-CHO (Cat L inhibitor) or EGTA (calcium chelator) (Fig. 3B). CM from Snail knockdown cells (C4–2 Snail shRNA) showed a similar trend, however, migratory potential was significantly lower as compared to CM from C4–2 NS cells co-cultured with HA (Fig. 3B). Similar results were obtained for paracrine cell viability except for higher cell viability observed with the highest HA (240 mg) for LNCaP cells cultured with C4–2 Snail shRNA CM (Fig. 3C). Western blot analysis revealed that p-STAT3 was increased in LNCaP cells treated with CM from C4–2 NS/HA co-cultures, but not when Snail was knocked down (Fig. 3D, E). Interestingly, although Snail and vimentin expression increased in LNCaP cells upon exposure to C4–2 NS/HA CM, it was still elevated upon exposure to C4–2 Snail shRNA/HA CM (Fig. 3D, E), suggesting this paracrine signaling may partially involve EMT. Therefore, calcium in HA contributes to increased paracrine cell migration and viability, especially in combination with high Snail expression levels.
FIGURE 3. Snail contributes to increased paracrine cell migration and viability.
(A) We analyzed the calcium in CM following C4-2 non-silencing (NS) control or C4-2 cells with stable Snail knockdown (C4-2 Snail shRNA) co-cultured with hydroxyapatite (HA) bone component. CM from these cells was added to LNCaP cells to test (B) paracrine chemotactic cell migration across collagen and (C) paracrine cell proliferation. EGTA was utilized to chelate calcium while Z-FY-CHO was utilized to inhibit Cat L. CM from C4-2 NS or Snail shRNA co-cultured with either (D) 100 mg HA or (E) 200 mg HA for six days was used to treat LNCaP cells at various time- points followed by collection of protein lysates and western blotting to determine the levels of p-STAT3, total STAT3, Snail and vimentin. Tubulin was utilized as a loading control. Results are representative of 3 independent experiments done in triplicates or quadruplicates. Statistical significance was assessed using GraphPad Prism software by one- way ANOVA (**p<0.01, ***p<0.001, and ****p<0.0001).
3.4 ∣. STAT3 inhibition abrogates cancer/HA paracrine cell migration that is promoted by Snail
Since we had found that cancer/HA co-culture promotes paracrine STAT3 phosphorylation and that Snail promotes cancer/HA paracrine cell migration and viability, we wanted to ask whether STAT3 plays a direct role in the Snail paracrine signaling following PCa/HA co-culture, by using STAT3 inhibitor, WP1066. CM was first collected from C4–2 NS/HA co-culture and used to treat LNCaP parental cells with or without WP1066 from 5 min up to 48 h; we utilized 200 mg HA as a representative density that had consistently shown increased signaling upon co-culture with cancer cells. We then performed western blot analysis to examine p-STAT3 and Snail expression. We also included Z-FY-CHO and EGTA as controls. We observed that p-STAT3 expression increased in cells treated with C4–2 NS/HA CM within 5 min, as compared to CM from C4–2 NS cells with DMSO control or cells alone (0 mg HA) (Fig. 4A). This STAT3 phosphorylation could be inhibited by WP1066 and EGTA, but not Z-FY-CHO (Fig. 4A), suggesting that calcium signaling, but not Cat L activation, is upstream of STAT3 activation by Snail. Snail expression increased upon treatment with CM from C4–2 NS/HA within 1h up to 48 h, and was inhibited by WP1066, Z-FY-CHO or EGTA, except at 24 h when we did not achieve inhibition of p-STAT3 (Fig. 4A, B). Therefore, this suggests that Snail-expressing PCa cells interact with HA bone to stimulate paracrine STAT3 activation, in addition to increased paracrine Snail expression in naïve cancer cells that previously expressed low Snail.
FIGURE 4. STAT3 pathway plays a crucial role in the cancer/HA signaling.
C4-2 NS cells were co-cultured with 200 mg HA, and CM used to treat LNCaP cells in the presence or absence of STAT3 specific inhibitor (WP1066), Cat L inhibitor (Z-FY-CHO) or EGTA to chelate calcium for (A) 5 min, 1 h or (B) 24-48 h. Protein lysate collected was used for western blot analysis for p-STAT3, total STAT3, as well as Snail expression. (C) Paracrine cell migration was also examined in the presence or absence of WP1066 STAT3 inhibitor. Results are representative of 3 independent experiments. Statistical significance was assessed using GraphPad Prism software by one- way ANOVA (**p<0.01 and ****p<0.0001).
To determine if STAT3 pathway played a crucial role in paracrine cell migration promoted by Snail-expressing cancer cells co-cultured with HA, C4–2 NS was co-cultured with 200 mg HA, and the CM utilized for paracrine cell migration in the presence or absence of WP1066, STAT3 inhibitor. Our results showed that addition of CM from C4–2 NS/HA co-culture led to increased cell migration which was abrogated by addition of WP1066 to the CM (Fig. 4C). This indicates that STAT3 activation is essential for paracrine cell migration following cancer/HA interaction.
3.5 ∣. Increased bone density promotes tumor growth in vivo in C4–2 cells with Snail knockdown
We were interested in studying cancer/bone interaction in vivo using a simple model. Initially we examined the effect of tumor growth in the presence of HA bone using subcutaneous implantation of cancer and HA into nude mice. We surgically implanted either 40mg HA or 100mg HA mixed with human bone marrow stromal cells in the hind-leg position of the mice (Fig. 5A). The size of the mice did not allow us to implant 200 mg HA so we chose 100 mg HA and a lower density of 40 mg HA to examine the role of bone density. 2 weeks later, C4–2 NS or Snail shRNA cells were injected 2 cm away from the surgical HA implant site (Supplemental Fig. 3). Tumor size and weight was determined weekly and recorded. Mice were sacrificed after 5 weeks, and a representative mouse is shown that subcutaneously shows the position of tumor and HA that has calcified into bone (Fig. 5A). At week 2 and 3, Snail shRNA cells injected mice showed larger tumors than C4–2 NS injected mice when comparing same bone density (Fig. 5B). In vitro, cell viability was significantly higher in C4–2 Snail shRNA as compared to C4–2 NS (Supplemental Fig. 4A). Surprisingly, while C4–2 NS tumor cells in mice with 40 mg HA continued to grow over 5 weeks, C4–2 NS tumors in mice with 100 mg HA initially grew but then regressed such that after 3 weeks, we could no longer detect tumor (Fig. 5B). However, C4–2 Snail shRNA tumors in mice with 100 mg HA were significantly larger than tumors in mice with 40 mg HA (Fig. 5B). We also confirmed by IHC that C4–2 NS tumors expressed higher Snail than C4–2 Snail shRNA (Fig. 5C). Therefore, although there was no statistically difference in tumor size between C4–2 NS and C4–2 NS Snail shRNA in the presence of 40 mg HA, there was significantly larger tumor for C4–2 Snail shRNA in presence of 100 mg HA as compared to 40 mg HA. Therefore, bone may promote more tumor growth in tumor cells expressing less Snail. Interestingly, this was supported by an in vitro assay that showed that C4–2 Snail shRNA/HA CM led to higher parental C4–2 proliferation, as compared to C4–2 NS/HA (Supplemental Fig. 4A), suggesting that C4–2 cells with high Snail decrease autocrine cell proliferation, but still have higher cell migration (Supplemental Fig. 4B) as compared to Snail knockdown cells. Indeed, Snail has been shown to decrease cell proliferation in LNCaP cells overexpressing Snail yet is still able to increase paracrine cell proliferation.28
FIGURE 5. Higher bone density leads to larger tumors in mice in C4–2 Snail shRNA xenografts and possibly more cell migration to HA implants in vivo.
We performed in vivo animal studies using nude mice. 40 or 100 mg HA mixed with human bone marrow stromal cells was surgically implanted in the hind-leg position of the mice (n=12) and injecting C4-2 NS or C4-2 Snail shRNA tumor cells subcutaneously near HA implant 2 weeks later. (A) A representative mouse that has been sacrificed after 5 weeks is shown. (B) Tumor size was determined weekly and graphed. Statistical significance was assessed using Excel two-tailed t-test (*p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001). (C) Immunohistochemistry (IHC) was done using the Snail antibody on tumor xenografts recovered at the end of the study. We performed IHC and Immunofluorescence (IF) and stained the tumor tissue and HA implants taken from mice implanted with either (D, F) 40 mg HA or (E, G) 100 mg HA, with E-cadherin or luciferase antibody for IHC and luciferase antibody for IF to detect cancer cells. We also performed H&E to determine the morphology of the tumor and the bone tissues. DAPI was used for nuclear staining. Results are representative of 6 independent experiments.
Tumor and bone tissue were examined by H&E to see morphology, and IHC or IF for various markers to identify whether any tumor cells could be found in the bone implant. As shown in H&E staining, the tumors were well organized and were surrounded by several blood vessels indicated by the red punctate structures (Fig. 5D, E). IHC done using the luciferase antibody (as the cells had been transduced with a lenti- luciferase vector prior to being injected in the mice) showed that there was brown staining within bone implanted next to tumor for both C4–2 NS and C4–2 Snail shRNA that was higher than mice containing HA bone alone (Fig. 5D, E). We did observe some non-specific background staining within lacunae (Fig. 5D, E). We attempted to stain for E-cadherin as well since prostate cells are epithelial and express E-cadherin. We observed that the C4–2 Snail shRNA tumor cells had a higher expression of E-cadherin than the C4–2 NS control tumors (Fig. 5D, E), possibly due to the decreased Snail expression in these tumors hence the de-repression of E-cadherin gene. Again, in the bone implant alone E-cadherin staining appeared to be non-specific (Fig. 5D, E). We performed IF with the luciferase antibody to confirm IHC data, and observed luciferase staining to be located in the cytoplasm of cells as well as around the blood vessel clusters (Fig. 5F, 5G). Although the luciferase IF appeared higher in 100 mg bone implanted next to tumor, compared to 40 mg, again, there was some non-specific staining lining lacunae-like structures (Fig. 5F, G). Therefore, cancer cells injected next to bone may migrate to bone implant.
4 ∣. DISCUSSION
4.1 ∣. Bone microenvironment and prostate cancer progression
PCa has a propensity to metastasize to the bone. It is therefore critical to understand how the bone microenvironment provides an ideal site for prostate tumor progression. Adult bone is made up of about 30% collagen which provides flexibility. 65% of bone comprises the mineral hydroxyapatite which is an insoluble salt of calcium and phosphorus, and the rest is made up of water.
4.2 ∣. STAT3 pathway is important to the cancer/bone interaction
To elucidate the role of the bone microenvironment on cancer cells, more specifically, the inorganic component of bone, we began by investigating which signaling pathways were induced following co-culture of PCa cells with HA. We found that co-culture of PCa cells with HA increased calcium levels in CM. We propose that the increased calcium may arise from interaction between PCa cells and HA that releases calcium similar to osteoclastogenesis. The CM was used to treat LNCaP cells to examine paracrine signaling, cell migration and viability. Our results showed that p-STAT3 protein levels were increased, and this was associated with increased cell migration and cell viability. We therefore concluded that the STAT3 pathway is critical to the PCa/HA interactions and cell migration/viability. We also showed that this signaling was dependent on calcium as chelation with EGTA abolished the migration and proliferation induced by HA. Another study that was studying breast cancer similarly found that HA and calcium could increase mammary cell migration and concluded that HA in the tumor microenvironment may increase migration of surrounding tumor cells and promote breast cancer progression.29 However, the mechanism was not provided except that HA may promote secretion of MMPs that may degrade the basement membrane allowing for migration and metastasis.29 In their model, they were looking at how mammary cells can themselves lead to formation of HA at the primary site which leads to mineralisation. However, our model is examining it from the point of view of the bone microenvironment, but, we cannot exclude that it may also have a role at the primary site. We further propose that Cat L protease may play a role in this signaling since Z-FY-CHO, a Cat L specific inhibitor, was able to abrogate the paracrine cell migration and viability. We also found that increasing the amount of HA from 100 mg to 200 mg could generally increase calcium levels, p-STAT3, as well as paracrine cell migration and viability, however, 240 mg was no longer stimulatory. It is unclear why the highest bone density was not stimulatory. Since people have different bone densities, this could have some clinical relevance in terms of cancer aggressiveness. Indeed, an epidemiological study found that increased bone mineral density was associated with PCa in older Afro-Caribbean population vs the Caucasian population. 30 Interestingly, African-American men have higher bone density and more aggressive prostate PCa compared to any other race.21,22. However, another study found an inverse relationship between bone mineral content and PCa risk in a cohort that consisted of 89% Caucasians.31 Therefore, the interplay between bone and PCa appears complex, but we hope that our preliminary findings may start to tease out the signaling going on between bone and PCa cells.
4.3 ∣. Snail plays a role in the STAT3 signaling pathway induced by high calcium levels
We have also shown that Snail promotes osteoclastogenesis, in part, via Cat L protease.20 We were therefore interested to test whether Snail may play a role in the PCa/HA signaling. When we co-cultured HA with C4–2 cells manipulated to express different levels of Snail, we observed that p-STAT3 expression was higher in LNCaP cells exposed to CM from C4–2 NS (non-silencing control)/HA co-culture, but not CM from C4–2 Snail shRNA(Snail knockdown)/HA co-cultures. This was associated with increased paracrine cell migration that could be abrogated by STAT3 inhibitor (WP1066), EGTA or Z-FY-CHO. Therefore, we conclude that Snail is critical to the STAT3 signaling pathway activated by increased calcium levels. It would appear that cells with Snail that come in contact with bone may lead to calcium release that signals to neighboring cells to similarly increase STAT3 signaling, Snail and possibly partial EMT. We did observe increased paracrine Snail levels but vimentin and E-cadherin (data not shown) were not significantly affected.
4.4 ∣. In vivo studies showed that mice injected with Snail knockdown PCa cells tend to have larger tumors compared to control
For our in vivo studies, instead of direct co-culture, we injected cancer cells in close proximity to the HA implant. Even then, it was interesting to note that Snail knockdown cells showed larger tumor volumes than C4–2 NS in the presence of 40 mg HA. Unexpectedly, the C4–2 NS tumors in mice with 100 mg HA started to grow then regressed, while those in mice with 40 mg HA continued to grow. It is possible that similar to our findings that 240 mg HA was not stimulatory in vitro, 100 mg HA in vivo was inhibitory; more so in cancer cells expressing Snail. This may give credence to studies that show that higher bone mineral content inversely correlates with PCa risk. Alternatively, decreased tumor growth may suggest increased metastasis, which agrees with higher luciferase staining with 100 mg HA suggesting more cancer cells had migrated to bone implant. Conversely, C4–2 cells with Snail knockdown actually grew much larger in mice with 100 mg vs 40 mg HA. This agreed with in vitro studies in which C4–2 Snail shRNA displayed higher cell viability but lower cell migration as compared to C4–2 NS, even after co-culture with HA. This autocrine effect is supported by previous data where we showed that Snail overexpression in LNCaP cells decreased cell proliferation, but CM from these cells increased paracrine cell proliferation via secretion of neuropeptides.28 Therefore, it is important to note that the difference between in vitro and in vivo studies; most of our in vitro studies were focused on paracrine assays, whereas in vivo we were looking at the direct effect of HA on cancer cells next to them. To look more at paracrine assays in vivo similar to what was performed in vitro, we would need to mix cancer cells with HA before implanting them together and injecting “naïve” cancer cells nearby. Our current preliminary in vivo studies help us to look initially at the direct cancer cell/bone interaction before employing more complex studies. Using IHC assays we confirmed that Snail levels were higher and E-cadherin lower in xenografts from mice injected with C4–2 NS cells as compared to C4–2 Snail shRNA xenografts. IF was also performed with the luciferase antibody and also found to be located in the cytoplasm and around the blood vessel clusters. Although there was non-specific luciferase staining seen in mice implanted only with bone, and also within spaces in bone, there was still higher staining in bone from mice implanted with cancer cells, suggesting the cancer cells may have started to migrate towards the HA implant.
4.5 ∣. STAT3 signaling and PCa progression model
Overall, we propose that HA inorganic component of bone is not an innocent bystander; its interaction with PCa cells, particularly cancer cells containing Snail that may secrete more Cat L protease that may lead to HA degradation, thus releasing calcium (Fig. 6). This calcium may signal to neighboring cancer cells to increase STAT3 activity leading to increased Snail expression, paracrine cell viability and migration. Thus this vicious cycle may contribute to PCa progression and metastasis.
FIGURE 6. Overview of cancer/bone signaling.
Our data supports a signaling pathway in which prostate cancer cells co-cultured in the presence of HA bone component results in increased calcium levels which leads to increased paracrine p-STAT3 activation, increased Snail and increased cell proliferation and migration that is mediated by Cat L activity. This signaling can be antagonized by calcium chelation with EGTA or inhibition of Cat L protease activity with Z-FY-CHO. Snail-expressing cancer cells and higher bone density may increase this signaling.
Supplementary Material
Acknowledgments
Funding
This work was supported by: NIH grants 1R15CA169899–01A1 (VOM), 1P20MD002285 (VOM), G12RR003062–22 (VOM)
Abbreviations:
- EMT
epithelial to mesenchymal transition
- PCa
prostate cancer
- STAT3
signal transducer and activator of transcription 3
- PI3K/AKT
Phosphoinositide 3-kinases/ Protein Kinase B
- ERK
Extracellular signal-regulated kinases
- p-ERK
phospho-ERK
- p-AKT
phospho-AKT
Footnotes
Conflict of Interest
The authors declare that there are no competing interests, financial and non-financial, in relation to the work described.
REFERENCES
- 1.Wallerand H, Robert G, Pasticier G, et al. The epithelial-mesenchymal transition-inducing factor TWIST is an attractive target in advanced and/or metastatic bladder and prostate cancers. Urologic oncology. 2010;28(5):473–479. [DOI] [PubMed] [Google Scholar]
- 2.Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian journal of andrology. 2009;11(1):57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thalmann GN, Sikes RA, Devoll RE, et al. Osteopontin: possible role in prostate cancer progression. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5(8):2271–2277. [PubMed] [Google Scholar]
- 4.Deng X, He G, Liu J, et al. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40(6):730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nature reviews Molecular cell biology. 2002;3(3):155–166. [DOI] [PubMed] [Google Scholar]
- 6.De Craene B, Berx G. Snail in the frame of malignant tumor recurrence. Breast cancer research : BCR. 2006;8(4):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. [DOI] [PubMed] [Google Scholar]
- 8.Christofori G New signals from the invasive front. Nature. 2006;441(7092):444–450. [DOI] [PubMed] [Google Scholar]
- 9.Heeboll S, Borre M, Ottosen PD, Dyrskjot L, Orntoft TF, Torring N. Snail1 is over-expressed in prostate cancer. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2009;117(3):196–204. [DOI] [PubMed] [Google Scholar]
- 10.Odero-Marah VA, Wang R, Chu G, et al. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res. 2008;18(8):858–870. [DOI] [PubMed] [Google Scholar]
- 11.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nature reviews Cancer. 2004;4(2):97–105. [DOI] [PubMed] [Google Scholar]
- 12.Siveen KS, Sikka S, Surana R, et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochimica et biophysica acta. 2014;1845(2):136–154. [DOI] [PubMed] [Google Scholar]
- 13.Don-Doncow N, Marginean F, Coleman I, et al. Expression of STAT3 in Prostate Cancer Metastases. European urology. 2017;71(3):313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature reviews Immunology. 2007;7(1):41–51. [DOI] [PubMed] [Google Scholar]
- 15.Nomura T, Katunuma N. Involvement of cathepsins in the invasion, metastasis and proliferation of cancer cells. The journal of medical investigation : JMI. 2005;52(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 16.Katunuma N. Mechanisms and regulation of lysosomal proteolysis. Revisiones sobre biologia celular : RBC. 1989;20:35–61. [PubMed] [Google Scholar]
- 17.Trabandt A, Aicher WK, Gay RE, et al. Expression of the collagenolytic and Ras-induced cysteine proteinase cathepsin L and proliferation-associated oncogenes in synovial cells of MRL/I mice and patients with rheumatoid arthritis. Matrix. 1990;10(6):349–361. [DOI] [PubMed] [Google Scholar]
- 18.Katunuma N, Tsuge H, Nukatsuka M, Asao T, Fukushima M. Structure-based design of specific cathepsin inhibitors and their application to protection of bone metastases of cancer cells. Arch Biochem Biophys. 2002;397(2):305–311. [DOI] [PubMed] [Google Scholar]
- 19.Leto G, Sepporta MV, Crescimanno M, Flandina C, Tumminello FM. Cathepsin L in metastatic bone disease: therapeutic implications. Biol Chem. 2010;391(6):655–664. [DOI] [PubMed] [Google Scholar]
- 20.Burton LJ, Smith BA, Smith BN, et al. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis. 2015;36(9):1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308. [DOI] [PubMed] [Google Scholar]
- 22.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8(5):468–489. [DOI] [PubMed] [Google Scholar]
- 23.Neal CL, McKeithen D, Odero-Marah VA. Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell Adh Migr. 2011;5(3):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koochekpour S, Willard SS, Shourideh M, et al. Establishment and characterization of a highly tumorigenic African American prostate cancer cell line, E006AA-hT. International journal of biological sciences. 2014;10(8):834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton LJ, Smith BA, Smith BN, et al. Muscadine grape skin extract can antagonize Snail-cathepsin L-mediated invasion, migration and osteoclastogenesis in prostate and breast cancer cells. Carcinogenesis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BN, Burton LJ, Henderson V, et al. Snail promotes epithelial mesenchymal transition in breast cancer cells in part via activation of nuclear ERK2. PLoS One. 2014;9(8):e104987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Sweatman OH, Orr FW, Singh G. Human metastatic prostate PC3 cell lines degrade bone using matrix metalloproteinases. Invasion Metastasis. 1998;18(5–6):297–305. [DOI] [PubMed] [Google Scholar]
- 28.McKeithen D, Graham T, Chung LW, Odero-Marah V. Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate. 2010;70(9):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox RF, Hernandez-Santana A, Ramdass S, McMahon G, Harmey JH, Morgan MP. Microcalcifications in breast cancer: novel insights into the molecular mechanism and functional consequence of mammary mineralisation. British Journal Of Cancer. 2012;106:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bunker CH, Zmuda JM, Patrick AL, et al. High bone density is associated with prostate cancer in older Afro-Caribbean men: Tobago prostate survey. Cancer Causes Control. 2006;17(8):1083–1089. [DOI] [PubMed] [Google Scholar]
- 31.Farhat GN, Taioli E, Cauley JA, et al. The association of bone mineral density with prostate cancer risk in the Osteoporotic Fractures in Men (MrOS) Study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.