Abstract
Background
Truncating variants in the Titin gene (TTNtvs) are common in individuals with idiopathic dilated cardiomyopathy (DCM). However, a comprehensive genomics-first evaluation of the impact of TTNtvs in different clinical contexts, and evaluation of modifiers such as genetic ancestry, has not been performed.
Methods
We reviewed whole exome sequence data for >71,000 individuals (61,040 from the Geisinger MyCode Community Health Initiative (2007–present) and 10,273 from the PennMedicine BioBank (PMBB; 2013–present) to identify anyone with TTNtvs. We further selected individuals with TTNtvs in exons highly expressed in the heart (proportion spliced in (PSI) >0.9). Using linked electronic health records (EHR), we evaluated associations of TTNtvs with diagnoses and quantitative echocardiographic measures, including sub-analyses for individuals with and without DCM diagnoses. We also reviewed data from the Jackson Heart Study to validate specific analyses for individuals of African ancestry.
Results
1.2% individuals in PMBB and 0.6% at Geisinger were identified with a TTNtv in a highly expressed exon (“hiPSI”). The presence of a hiPSI TTNtv was associated with increased odds of DCM in individuals of European ancestry (odds ratio (OR) [95% confidence interval]: 18.7 [9.1–39.4] (PMBB) and 10.8 [7.0–16.0] (Geisinger). hiPSI TTNtvs were not associated with DCM in individuals of African ancestry, despite a high DCM prevalence (OR: 1.8 [0.2–13.7], p=0.57). Among 244 individuals of European ancestry with DCM in PMBB, hiPSI TTNtv carriers had lower left ventricular ejection fraction (LVEF; β=−12%, p=3×10−7), and increased left ventricular (LV) diameter (β=0.65 cm, p=9×10−3). In the Geisinger cohort, hiPSI TTNtv carriers without a cardiomyopathy diagnosis had more atrial fibrillation (OR: 2.4 [1.6–3.6]) and heart failure (OR: 3.8 [2.4–6.0]), and lower LVEF (β=−3.4%, p=1×10−7).
Conclusions
Individuals of European ancestry with hiPSI TTNtv have an abnormal cardiac phenotype characterized by lower LVEF, irrespective of the clinical manifestation of cardiomyopathy. Associations with arrhythmias, including atrial fibrillation, were observed even when controlling for cardiomyopathy diagnosis. In contrast, no association between hiPSI TTNtvs and DCM was discerned among individuals of African ancestry. Given these findings, clinical identification of hiPSI TTNtv carriers may alter clinical management strategies.
Keywords: genetics, genetic association studies, dilated cardiomyopathy, race, Titin
INTRODUCTION
Titin is a large sarcomeric protein that is vital for cardiac contraction and relaxation.1 Genetic sequencing of large cohorts of patients ascertained based on the diagnosis of familial or idiopathic dilated cardiomyopathy (DCM) has identified heterozygous variants in TTN that are predicted to lead to protein truncation (TTN-truncating variants, or TTNtv) in approximately 15–20% of these cases.2–7 Subsequent work has demonstrated that this association is driven by TTNtvs in exons that are constitutively expressed [proportion spliced in (PSI)>0.9, or “hiPSI”] in the primary cardiac isoforms of TTN (N2BA and N2B).3 However, the prognostic significance of identifying a TTNtv, particularly as an incidental finding, is uncertain because of substantial challenges in variant interpretation and the high frequency of TTNtv in the general population.2,8
A critical step toward enhancing the clinical utility of identifying a TTNtv is to define the phenotypic impact of TTNtvs independent of DCM-based ascertainment.9 This is important as DCM may represent an extreme on a spectrum of TTNtv-related disease that includes milder, but still clinically relevant forms.10 For example, a recent study found that a small group (n=15) of healthy volunteers incidentally identified with TTNtv (PSI>0.15) had mild increases in left ventricular (LV) volumes by cardiac magnetic resonance imaging, suggesting possible deleterious effects of TTNtv in the absence of clinically-recognized DCM.11 Additionally, little is known about associations of TTNtv with disease in non-European populations, despite some suggestion for differential effects in individuals of African ancestry.3
Linking large-scale genomic sequence data to electronic health records (EHR) provides a powerful opportunity to leverage a genomics-first approach to answer such questions, including the possible identification of novel genotype-phenotype associations, and deep characterization of heterogeneous disease based on individual genotype. Therefore, we comprehensively evaluated phenotypes associated with TTNtv in two large biobanks with whole exome sequence data linked to EHRs. These two biobanks represent informative extremes of clinical contexts: the Geisinger MyCode project12 represents a general clinical population with high engagement and continuity; in contrast, the PennMedicine Biobank (PMBB) represents a tertiary care center with a high referral rate, and hence a higher disease prevalence. We hypothesized that individuals with TTNtv would demonstrate a distinct clinical phenotype characterized by associations of clinical diagnoses and differences in ventricular size and function compared with individuals without TTNtv.
METHODS
The data, analytic methods, and study materials from the University of Pennsylvania will be made available to other researchers for purposes of reproducing the results with approval of the University of Pennsylvania institutional review committee (IRB). The data and study materials from Geisinger will not be made available. The study was approved by the University of Pennsylvania IRB and Geisinger IRB, and all subjects gave informed consent.
Cohorts
The MyCode Community Health Initiative at Geisinger is a precision health project in which participants, with opt-in informed consent, provide bio-specimens for broad research use, including permission to link associated data to EHR.12 Exome sequencing was performed through the DiscovEHR collaboration between Geisinger and the Regeneron Genetics Center (RGC; Tarrytown, NY).13–17 This study included the first 92,455 participants who underwent sequencing; this cohort is of predominantly European ancestry.
PMBB recruits patients from throughout the University of Pennsylvania Health System for genomic and precision medicine research. Participants actively consent to allow the linkage of bio-specimens to their longitudinal EHR. Currently over 50,000 participants are enrolled in the PMBB; approximately 23% of these individuals self-identify as having African ancestry. This study included a subset of 10,289 individuals who have undergone whole exome sequencing, performed through a collaboration with the RGC.
The Jackson Heart Study (JHS) is a population-based observational study evaluating the etiology of cardiovascular, renal, and respiratory diseases among African Americans residing in the three counties (Hinds, Madison, and Rankin) that make up the Jackson, Mississippi metropolitan area. Data and biologic materials have been collected from 5,306 participants, aged 35–84 years old. The study population is characterized by a high prevalence of diabetes, hypertension, obesity, and related disorders.
Genetic Sequencing, Variant Calling, and Genotype Assignment
Sample preparation and exome sequencing were completed per the standard RGC methodology, as described by Dewey et al14 and detailed in the Supplemental Methods. Sequence quality was sufficient to provide >20x read depth in >90% of samples for 96% of the bases in TTN for Geisinger and 82% in PMBB (83.6% in individuals of African ancestry and 82.1% in individuals of European ancestry).
Following cohort sequencing, samples showing disagreement between genetically-determined and reported sex, low quality sequence data, genetically-identified sample duplicates, and individuals with closer than 3rd degree relatedness were excluded. After exclusions, 61,040 samples from Geisinger and 10,289 samples from PMBB remained. Samples with quality scores at a given site, normalized by depth (QD) < 3 (< 5 for indels), site read depth (DP) < 7 (< 10 for indels), and alternate allele balance (AB) < 25% (or fewer than 5 alternate reads) were removed. Sufficient positive predictive value for these thresholds was verified by Sanger sequence confirmation in selected samples.
Variant Annotation and Selection
We selected all TTN variants with minor allele frequency < 0.001 and further selected all predicted loss of function, truncating variants (i.e., stop gain, frameshift, splice site) using the Ensembl Variant Effect Predictor (VEP) or ANNOVAR.18,19 Splice site variants included those affecting canonical donor and acceptor splice sites, i.e. two bases flanking either side of each exon. PSI>0.9 (see below) was considered “high”.3
Phenotype Evaluation
For the Geisinger cohort, we collated available demographic (birthdate, age at last encounter, sex, race, vital status), diagnostic (International Classification of Diseases codes from clinical encounters), and quantitative data from the most recent echocardiogram with a physician-reported left ventricular ejection fraction (LVEF) from the Geisinger Phenomic-Initiative database as of April 19, 2018.
Within PMBB genetic ancestry was determined using principal components analysis based on HapMap3 reference populations.13 For individuals of European or African Ancestry quantitative ancestry estimates were performed using ADMIXTURE.20 PMBB phenotype data were directly queried from Penn Data Store, which agglomerates EHR data from across the health system, as of January 18, 2018.
DCM was defined as two or more encounters with the I42.0 (“Dilated cardiomyopathy”) ICD-10 code; or two or more instances of non-specific ICD-9-CM or ICD-10 codes 425.4 (“Other primary cardiomyopathies”), 425.8 (“Cardiomyopathy in other diseases classified elsewhere”), 425.9 (“Secondary cardiomyopathy, unspecified”), I42.8 (“Other cardiomyopathies”), or I42.9 (“Cardiomyopathy, unspecified”) and mention of “dilated” or “DCM” in the underlying diagnosis code or on the left ventricular findings from echocardiography (Geisinger) or the phrases “dilated cardiomyopathy” or “DCM” in the free text encounter notes (PMBB). Individuals with only one occurrence of either specific or non-specific codes, or free text, were excluded from analysis considering DCM as an outcome. Transthoracic echocardiographic parameters were extracted from structured reports (outpatient only for PMBB).
Because phenotype data on DCM was not directly collected in the JHS, DCM was determined based on cardiac morphology derived from echocardiography and cardiac MRI. Non-ischemic DCM was defined based on age, sex, and body surface area standardized reductions in LVEF and increases in left-ventricular end-diastolic dimensions in individuals without a previous history of myocardial infarction or coronary revascularization.3
Statistics
Statistical analyses were performed using R (version 3.5).21 Individuals with hiPSI TTNtv were compared to the rest of their respective sequenced cohorts (less individuals with low PSI TTNtv) using phenome-wide association (PheWAS), adjusted for age, sex, and principal components of ancestry (first 4 components for Geisinger, first 10 for PMBB).22,23 Logistic regression was used to specifically evaluate the association of TTNtv with DCM, adjusting for age, age2, sex, and principal components of ancestry. Due to the high percentage of individuals of African ancestry in PMBB, analyses were performed separately by genetic ancestry and combined with inverse variance weighted meta-analysis. Available echocardiography measurements were compared on a case/control basis, independently for the Geisinger, PMBB, and JHS cohorts, using Welch’s t-test or Wilcoxon test, as appropriate. Comparisons in patient subsets exclusively with (PMBB) and without (Geisinger) DCM were also made using robust linear regression24, adjusting for age, sex, and ancestry. Bootstrapping with the adjusted percentile method (1000 resamples, with replacement) was used to calculate 95% confidence intervals of the regression coefficients.25 Controls were selected for echocardiography analyses in the Geisinger cohort from the sequenced population lacking a TTNtv and matched (5 controls per TTNtv individual) by sex and date of birth to the TTNtv cases.26 In PMBB, the remaining sequenced population without a TTNtv was used for controls for all analyses.
RESULTS
Prevalence and Distribution of TTNtv
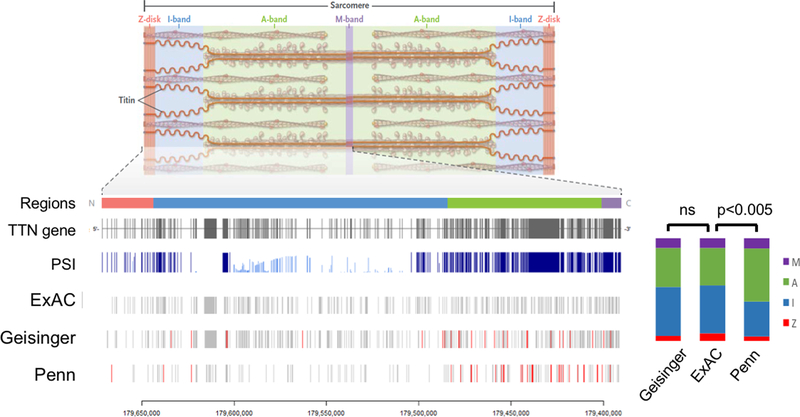
492 truncating variants were identified across TTN in the Geisinger cohort (Supplemental Table 1). Of these, 208 were frameshifting insertions/deletions, 194 were stop gains, and 90 were essential splice site variants. 189 TTNtvs were identified in PMBB, including 80 frameshifts, 66 stops, and 43 splice variants (Supplemental Table 2). Figure 1 shows the distribution of variants within the TTN gene, and compares them to the Exome Aggregation Consortium (ExAC) reference population.27 The variant distribution in the Geisinger cohort was similar to ExAC, whereas the distribution in PMBB strongly biased the A-band and constitutively expressed exons (Figure 1, p<0.005). For Geisinger, 245 TTNtv (49.8%) were located in high PSI (“hiPSI”) exons; while 126 variants in PMBB (75.1%) were hiPSI (Supplemental Table 3).
Figure 1:
Location within the TTN gene of identified variants in the Geisinger and Penn cohorts. Titin, a protein encoded by TTN, is an integral part of the cardiac sarcomere, spanning from Z-disk to M-line. Regions of the TTN gene and protein are designated according to their spatial domain in the sarcomere: Z-disk, I-band, A-band, M-band. The exons of the TTN gene and their associated “proportion spliced in” (PSI) are shown, as well as the distributions of truncating variants in those exons from the Exome Aggregation Consortium (ExAC) reference population and the Geisinger and Penn cohorts. Variants found in patients with dilated cardiomyopathy are designated in red. The relative distribution of variants by TTN region is represented on the right stacked bar plots, showing that A-band variants are over-represented in the Penn cohort compared with ExAC, while the Geisinger and ExAC distributions are comparable.
Individuals and Demographic Details
A total of 501 individuals, representing 0.7% of the combined cohorts, were identified with a hiPSI TTNtv. Demographics are shown in Table 1 for the full cohorts, and shown by ancestry in Supplemental Table 4. The fraction of individuals of African ancestry was 2.8% and 21% in the Geisinger and PMBB cohorts, respectively. The prevalence of hiPSI TTNtvs in PMBB patients of European descent was 1.5%, significantly higher than in ExAC subjects of European descent (0.5%; p <1×10−6). In contrast, the prevalence of hiPSI TTNtvs in PMBB patients of African descent was 0.9%, not significantly different from that in ExAC subjects of African descent (0.7%; p =0.14).
Table 1.
Cohort demographics
| Geisinger | PMBB | |||||
|---|---|---|---|---|---|---|
| Non-hiPSI cohort | hiPSI | p-value | Non-hiPSI cohort | hiPSI | p-value | |
| Participants | 60,681 | 359 | 10,147 | 142 | ||
| Male (%) | 24,538 (40) | 139 (39) | 0.52 | 5,997 (59) | 95 (67) | 0.07 |
| EUR ancestry (%) | 57,371 (95) | 340 (95) | >0.99 | 8,024 (79) | 122 (86) | 0.05 |
| Age at last encounter | 60 (45 – 71) | 57 (43 – 70) | 0.015 | 68 (59 – 77) | 66 (57 – 74) | 0.10 |
| Diabetes (%) | 11,528 (19) | 55 (15) | 0.08 | 3,286 (32) | 53 (37) | 0.24 |
| Hypertension (%) | 32,214 (53) | 172 (48) | 0.06 | 7,505 (74) | 102 (72) | 0.90 |
| CAD (%) | 11,005 (18) | 93 (26) | 3×10−4 | 4,449 (44) | 55 (39) | 0.79 |
| Myocardial infarction (%) | 924 (2) | 10 (3) | 0.08 | 2,103 (21) | 26 (18) | 0.53 |
| Heart failure (%) | 5,515 (9) | 86 (24) | 2×10−17 | 3,947 (39) | 93 (66) | 3×10−10 |
| Non-ischemic DCM (%) | 595 (1) | 27 (8) | 4×10−17 | 570 (5.6) | 43 (30) | 2×10−21 |
| Heart transplant (%) | 20 (<1) | 1 (<1) | 0.12 | 339 (3.3) | 20 (14) | 1×10−7 |
Data are presented as number (%) or median (interquartile range); EUR – European; CAD – Coronary artery disease; DCM – dilated cardiomyopathy; PMBB – PennMedicine Biobank; hiPSI – having variants within exons with “high Proportion Spliced In” to Titin transcripts.
Association with DCM
In the Geisinger cohort, 1.0% of all individuals carried a diagnosis of DCM, in contrast to 5.6% of PMBB, reflecting the large difference in disease prevalence between the cohorts. The distribution of TTNtv associated with DCM diagnosis in both cohorts is indicated in red in Figure 1.
In the Geisinger cohort, 7.5% of individuals with a hiPSI TTNtv had a clinical diagnosis of DCM. The age- and sex-adjusted odds ratio for DCM in individuals with hiPSI TTNtv was 10.8 (95% CI 7.0–16.0; p=2×10−29). Of all sequenced individuals with a DCM diagnosis, 4.2% had a hiPSI TTNtv. The age- and sex-adjusted odds ratio for DCM in individuals with hiPSI TTNtv was 10.8 (95% CI 7.0–16.0; p=2×10−29).
In PMBB, 30% of individuals with a hiPSI TTNtv had DCM, representing 7% of individuals with DCM. Surprisingly, hiPSI TTNtv were more prevalent in DCM patients of European ancestry than in DCM patients of African ancestry (40 [10%] of 397 patients vs 3 [1.4%] of 216 patients, p=2×10−5). The latter prevalence did not differ from the prevalence of hiPSI TTNtv in individuals of African ancestry in ExAC (0.5%, p=0.15). Furthermore, among individuals of European ancestry with TTNtv, the age- and sex-adjusted odds ratio for DCM was 18.7 (95% CI 9.1–39.4, p=4×10−15), whereas the odds ratio among individuals of African ancestry was only 1.8 (0.2–13.7, p=0.57) and was not statistically significant.
To further probe the interaction between ancestry and the association of TTNtv with DCM, we modeled the DCM outcome in the overall PMBB cohort while including both the principal components of genetic ancestry as well as the percentage of African genetic ancestry from ADMIXTURE analysis (Supplemental Figure 1). With this approach, the OR for the association of hiPSI TTNtv variants with DCM was 17 (95% CI 9–31, p=2.5×10−21) in individuals with no African ancestry. Each 10% increase in percentage of African ancestry decreased the magnitude of this association by 22% (OR for interaction between hiPSI and 10% AFR ancestry on ADMIXTURE = 0.78, 95% CI 0.63–0.95, p=0.02, Supplemental Figure 2). The median percentage of African ancestry in African Americans in the PMBB cohort is 85; based on this we calculate the effect of carrying a hiPSI TTNtv on the risk of DCM in the PMBB African American individuals as only 2.2-fold increased. In contrast, the risk of DCM based on ancestry alone from our admixture models is higher, with African Americans in PMBB being estimated to have a 3.4-fold increased risk of DCM than those without any African Ancestry (OR 1.13 per 10% African Ancestry, 95% CI 1.10–1.15, p=0.02).
To validate the observed lack of association between TTNtv and DCM in patients of African ancestry, we turned to the Jackson Heart Study, which conducted WES and extensive phenotypic evaluations of 2974 individuals, all of African descent. Among these individuals, we identified 29 (1%) with echocardiographic parameters specific for DCM (Supplemental Table 5). Zero of these individuals were among the 18 identified with hiPSI TTNtvs (p=0.02, assuming expected prevalence of 12%). There was no association between TTNtv carrier status and any echocardiographic or cardiac MRI parameter or incident heart failure in the participants in the Jackson Heart Study (Supplemental Table 6).
One possible explanation for a weaker/absent association of TTNtvs with DCM in individuals of African ancestry may be an alternate exon splicing pattern. In other words, the exons in TTN that are constitutively expressed (hiPSI) may vary by ancestry, such that the TTNtvs in individuals of African ancestry in our analysis may be occurring in exons with low or intermediate PSI. To test this possibility, we performed RNAseq of TTN mRNAs in 166 post-transplant hearts (89 of Caucasian descent, 77 of African descent), and 155 non-failing discarded donor hearts (122 of Caucasian descent, 33 of African descent). As shown in Figure 2, PSI patterns were nearly identical in all four groups. Alternative splicing patterns thus do not differ between these races.
Figure 2:
Proportion spliced in (PSI) exons in adult hearts from Caucasian (CAU) and African Americans (AA), explanted after cardiac transplantation for dilated cardiomyopathy. No significant differences were noted between groups.
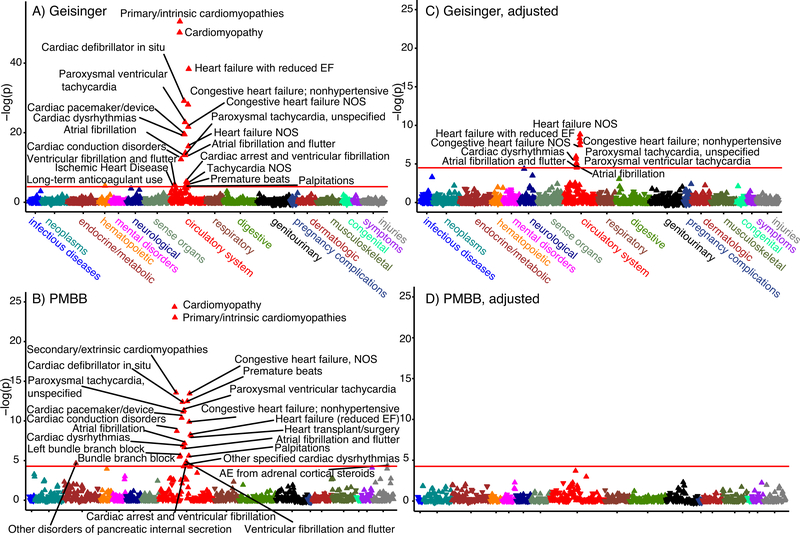
Disease Association
We performed a gene burden PheWAS in the Geisinger and PMBB cohorts to identify associations of broader disease diagnoses with hiPSI TTNtv (Figure 3A and B). In both cohorts, several diagnoses were significantly more common in the group with hiPSI TTNtv. These included cardiomyopathies (OR: 7–9), heart failure (OR: 4–8), arrhythmias (OR up to 13), and need for cardiac pacemaker or defibrillator (OR: 7–12; see Supplemental Table 7 for OR and 95% confidence intervals for individual phenotype codes by cohort). No associations with diagnoses were observed for low or intermediate PSI (<0.9) TTNtv (Supplemental Figure 3), supporting the conclusion that tissue expression level (PSI) is a critical factor in the pathogenesis of TTNtv–related disease.
Figure 3:
Manhattan plots representing the Phenome-wide Association findings from the Geisinger (A) and PennMedicine Biobank (PMBB; B) cohorts demonstrating numerous significant cardiovascular phenotypes associated with TTN truncating variants (TTNtv). After controlling for cardiomyopathy, atrial fibrillation remained significantly associated with TTNtv in the Geisinger cohort (C), while no significant associations were observed in PMBB (D). EF – ejection fraction; NOS – not otherwise specified.
Because many of these phenotypes are likely the downstream consequences of DCM, we repeated the PheWAS adjusting for the lead cardiomyopathy diagnoses. No associations were noted in the PMBB cohort following this adjustment. In the Geisinger cohort, however, several associations remained significant, including atrial fibrillation (OR: 2.4), other dysrhythmias (OR: 2–4), and heart failure (OR: 3–4; Figure 3C and D, Supplemental Table 7). Thus, in the Geisinger cohort, hiPSI TTNtv associate strongly with atrial fibrillation and arrhythmia even after adjustment for DCM, and the implications of hiPSI TTNtv (such as heart failure) exceed the extent of clinically recognized DCM.
Quantitative Phenotype
Measures of cardiac structure and function for individuals with a clinically obtained echocardiogram (n = 200 (56%) for Geisinger, n = 67 (40%) for PMBB) are provided in the Supplement (Supplemental Table 8). Individuals with hiPSI TTNtv in both cohorts had lower LVEF, decreased septal thickness and increased diastolic and systolic LV internal diameters (Supplemental Table 8, Supplemental Figure 4). We also observed that LVEF was statistically different in individuals with hiPSI variants (>0.9) compared with individuals with intermediate (0.16 – 0.9) and low (0 – 0.15) PSI variants (Supplemental Figure 5), further supporting the importance of PSI in determining disease association. Conversely, we did not observe variations in LVEF according to position (“band”) within the TTN gene (Supplemental Figure 6) for variants in hiPSI exons.
To examine the effect of hiPSI TTNtv within the subset of individuals with DCM, we focused on the PMBB cohort, where the number of individuals with DCM and hiPSI TTNtv was high (n=43). We restricted the analysis to DCM patients with available LVEF measurements, and of European ancestry, in light of the low number of hiPSI TTNtv positive patients of African ancestry. Among individuals with DCM, we observed significantly lower LVEF and higher LV systolic diameter in patients with hiPSI TTNtv, compared to those without (Figure 4, Table 2). These differences persisted after adjustments for age, sex, and ancestry (Table 2), and held whether evaluating median, minimum, or most recently measured LVEF (Supplemental Figure 7).
Figure 4:
Density plots comparing left ventricular ejection fraction (LVEF) between hiPSI TTNtv (blue) and controls (red) among patients with (Penn, panel A) and without (Geisinger, panel B) dilated cardiomyopathy (DCM) diagnoses. In both cases, LVEF was significantly lower in the group with TTNtv. hiPSI – having variants within exons with “high Proportion Spliced In” to Titin transcripts; TTNtv – Titin truncating variants.
Table 2.
Echocardiogram measures in hiPSI TTNtv+ individuals and hiPSI TTNtv− controls without (Geisinger) or with (PMBB) DCM diagnosis
| Geisinger (without DCM) | |||||
| hiPSI TTNtv+ | hiPSI TTNtv− | Effect Estimate (95% CI)* | p-value | ||
| LVEF (%) (last) | median | 57 | 57 | −3.4 (−4.7, −2.2) | 1×10−7 |
| IQR | 52 – 58 | 57 – 62 | |||
| n | 123 | 706 | |||
| LVIDd (cm) | median | 4.6 | 4.6 | 0.04 (−0.09, 0.16) | 0.56 |
| IQR | 4.1 – 5.1 | 4.1 – 5.0 | |||
| n | 107 | 604 | |||
| LVIDs (cm) | median | 3.2 | 3.0 | 0.24 (0.10, 0.40) | 1×10−3 |
| IQR | 2.7 – 3.7 | 2.6 – 3.4 | |||
| n | 95 | 501 | |||
| LVPWd (cm) | median | 1.0 | 1.0 | −0.02 (−0.05, 0.02) | 0.44 |
| IQR | 0.9 – 1.2 | 0.9 – 1.2 | |||
| n | 106 | 603 | |||
| IVSd (cm) | median | 1.1 | 1.1 | −0.02 (−0.06, 0.02) | 0.33 |
| IQR | 0.9 – 1.2 | 0.9 – 1.2 | |||
| n | 106 | 603 | |||
| MV E/A | median | 1.0 | 1.0 | −0.02 (−0.10, 0.07) | 0.70 |
| IQR | 0.8 – 1.2 | 0.8 – 1.2 | |||
| n | 78 | 486 | |||
| LA dim† | median | 3.7 | 3.7 | 0.05 (−0.08, 0.19) | 0.44 |
| IQR | 3.2 – 4.2 | 3.2 – 4.1 | |||
| n | 90 | 547 | |||
| PMBB (with DCM; excluding transplant) | |||||
| hiPSI TTNtv+ | hiPSI TTNtv− | Effect Estimate (95% CI)* | p-value | ||
| LVEF (%) (last) | median | 29 | 40 | −12.1 (−16.2, −7.0) | 3×10−07 |
| IQR | 15 | 22.2 | |||
| n | 26 | 218 | |||
| LVIDd (cm) | median | 5.67 | 5.42 | 0.37 (−0.01, 0.77) | 0.07 |
| IQR | 1.22 | 1.17 | |||
| n | 25 | 193 | |||
| LVIDs (cm) | median | 4.82 | 4.12 | 0.65 (0.13, 1.12) | 0.009 |
| IQR | 1.25 | 1.32 | |||
| n | 25 | 192 | |||
| LVPWd (cm) | median | 0.92 | 1.02 | −0.09 (−0.17, 0.01) | 0.07 |
| IQR | 0.22 | 0.24 | |||
| n | 26 | 203 | |||
| IVSd (cm) | median | 0.92 | 1.04 | −0.11 (−0.19, −0.04) | 0.004 |
| IQR | 0.21 | 0.28 | |||
| n | 26 | 203 | |||
| MV E/A | median | 91 | 0.92 | 0.05(−0.18, 0.30) | 0.67 |
| IQR | 0.4 | 0.56 | |||
| n | 16 | 165 | |||
| LA dim† | median | 24.1 | 22.1 | −0.09 (−3.75, 4.72) | 0.97 |
| IQR | 7.2 | 10.5 | |||
| n | 21 | 162 | |||
Effect estimates computed from linear regression, adjusted for age, sex, and ancestry (principal components 1–4 (Geisinger) or 1–10 (PMBB). 95% confidence intervals (CI) defined with bootstrapping with 1000 samples.
LA dimension (dim) represents parasternal diameter for Geisinger and 4-chamber area for PMBB. LVEF – left ventricular ejection fraction; LVID (d/s)– left ventricular internal diameter (diastole/systole); LVPWd – left ventricular posterior wall thickness, diastole; IVSd – interventricular septum thickness, diastole; MV – mitral valve; LA – left atrium; IQR – interquartile range; DCM – dilated cardiomyopathy; hiPSI – having variants within exons with “high Proportion Spliced In” to Titin transcripts; TTNtv – Titin truncating variants; PMBB – PennMedicine Biobank.
To examine the effect of hiPSI TTNtv among individuals without DCM, we focused on the Geisinger cohort, where the number of such individuals is high (n = 132). For this analysis, DCM was defined broadly as any encounter coded with “dilated cardiomyopathy” (I42), “other (primary) cardiomyopathies” (425.4, I42.8), “cardiomyopathy in other diseases classified elsewhere” (425.8), “cardiomyopathy, unspecified” (I42.9), or “secondary cardiomyopathy, unspecified” (425.9). The lack of DCM was further confirmed through blinded physician chart review. After these exclusions, differences in LVEF, but not LV diastolic diameter, persisted adjusted for age, sex, and ancestry (Figure 4, Table 2). We further excluded any individuals with a heart failure diagnosis, and the association with lower LVEF persisted (β = −2.3 [95% CI −3.3 – −1.2], p = 4×10−5).
DISCUSSION
In this genomics-first study, we combined TTN exome sequencing with extensive clinical phenotyping derived from EHR in order to determine genotype-phenotype relationships in individuals bearing TTNtv. We leveraged two cohorts, a general clinical population (Geisinger) and patients from a tertiary referral center (PMBB). In both contexts, we found clear phenotypic differences in individuals with hiPSI TTNtv identified from a genomics-first approach. In a general clinical population (Geisinger), our findings demonstrate that: 1) hiPSI TTNtv convey significantly increased odds for DCM and are also associated with other related cardiac phenotypes, such as systolic heart failure and arrhythmias, some of which may be independent of DCM; and 2) individuals with a hiPSI TTNtv and a clinically-indicated echocardiogram have decreased LV function, even in the absence of a clinical diagnosis of non-ischemic DCM. In a referral population (PMBB), our findings show that: 1) hiPSI TTNtv are strongly associated with cardiac phenotypes, largely driven by DCM; 2) individuals with DCM and hiPSI TTNtv have markedly increased LV size and decreased LV function compared with patients with DCM who lack a TTNtv; and 3) the association between hiPSI TTNtvs and DCM in individuals of African ancestry is much weaker than in individuals of European ancestry.
Yield of Dilated Cardiomyopathy from Genomics-first TTNtv
Overall, 7.5% of Geisinger individuals and 30% of PMBB individuals with hiPSI TTNtv had DCM, corresponding with odds ratios of 10.8 and 18.7, respectively (Europeans only for PMBB). The DCM/TTNtv association has been well-documented through genetic sequencing of patients with DCM,2–4 but not previously documented through a genomics-first approach. The prevalence and OR differences likely reflect patient ascertainment into the respective biobanks. For Geisinger, participants are recruited across a large regional health system, comprising both primary care and specialty clinics, and are thus more representative of the population of central Pennsylvania. For PennMedicine, a tertiary care facility, a large percentage of participants have been referred for specialty care, consistent with the higher observed proportion of advanced disease. Hence, these cohorts represent opposite ends of the clinical spectrum for ascertaining DCM patients, but our findings demonstrate that TTNtv have a meaningful and measurable impact in both settings.
Other Clinical Phenotype Associations
In addition to DCM, we found numerous other significant cardiac phenotype associations of TTNtv (in hiPSI exons) in individuals identified using this genomics-first approach. These included diagnoses of cardiomyopathies; other common clinical manifestations associated with cardiomyopathies, such as heart failure, and ventricular and atrial dysrhythmias; and common interventions for cardiac arrhythmias and dysfunction, such as defibrillator or pacemaker placement. Moreover, the fact that no phenotype is associated with low PSI TTNtv in our PheWAS reinforces the presumed benign nature of variants in low and intermediate PSI exons.
To evaluate whether TTNtv are associated with other diseases independent of DCM, we repeated the phenotype association while conditioning for cardiomyopathy diagnoses. Conditioning on cardiomyopathy in the PMBB cohort removed all other associations, suggesting they were all secondary to cardiomyopathy in this cohort with more advanced disease. However, several associations remained significant after adjustment in the Geisinger cohort, suggesting independence from DCM. These include atrial fibrillation, which is consistent with recent work showing increased early arrhythmic risk in DCM patients with TTNtv,28 as well as genome-wide associations of loci in or near TTN and atrial fibrillation.29–31 Additionally, several systolic heart failure diagnoses remained significant. These findings are unlikely to be independent of DCM, but do suggest a clinical under-recognition of TTN-associated cardiomyopathy.
Quantitative Imaging Phenotype
Little data exist to address the question of whether an incidental finding of a hiPSI TTNtv is associated with structural cardiac dysfunction. The best evidence to date comes from cardiac MRI data in 15 healthy individuals with intermediate or high PSI variants (PSI > 0.15) showing increased ventricular volumes.11 Using our larger cohort of 132 individuals with a hiPSI TTNtv and a clinically indicated echocardiogram but without a diagnosis of non-ischemic DCM, we found that hiPSI TTNtv strongly correlated with lower LVEF, but no difference in diastolic diameter, suggesting a deficit in contractility. While other heart conditions, such as ischemic disease, were present in our clinically-based cohort, the extent to which they are related to or interacting with the TTNtv is unclear. Thus, clinical follow-up of incidental findings of hiPSI TTNtv, such as routine echocardiography, may be warranted to potentially identify underlying heart disease. Prospective studies will be needed to determine if subsequent early intervention prior to the development of overt DCM and/or heart failure, such as neurohormonal blockade, may prevent the development of symptoms in individuals with hiPSI TTNtv.
For patients with DCM, we find that hiPSI TTNtv were strongly associated with cardiac enlargement and as much as a 10% absolute reduction in LVEF, suggesting that hiPSI TTNtv DCM may represent a more severe form of disease. These findings contrast with recent findings in the Netherlands of a “mild” form of DCM associated with hiPSI TTNtv.32 Additionally, a recent United Kingdom longitudinal study found that TTNtv were not associated with worse cardiac parameters in a DCM cohort.33 This contrast between studies may reflect different patient populations, despite our adjustments for age, sex, and ancestry. For example, the mean age of patients with TTNtv in the Dutch cohort was 49 years, compared to 66 years in our cohort. The contrast between studies may also reflect patient ascertainment, acuity of presentation, or comorbidity profile. This heterogeneity between cohorts underscores the challenge of generalizing observations from one clinical population to another.
Ancestry-stratified differences of prevalence of TTNtv in DCM
Among patients with DCM in the PMBB (n=613), the overall prevalence of hiPSI TTNtv (7%) was less than has been reported in ascertained DCM cohorts.2,33 Ancestry specific carrier rates, however, likely explain this difference. Among DCM patients of European ancestry the carrier rate of hiPSI TTNtv was 10%, which is akin to previously reported values.33,34 In striking contrast, the prevalence of TTNtv in individuals of African ancestry with DCM was 1.4%, which is not significantly different from that reported in a general population of African Americans (ExAC). In addition to this, hiPSI TTNtv were not associated with DCM in individuals of African ancestry in the PMBB cohort, nor with echocardiographic or cardiac MRI evidence of DCM in subjects in the Jackson Heart Study. It is possible that we underestimated the true burden of DCM across JHS because some participants with echo or MRI findings close to the normal range might have been treated and had improvement in EF. However, based on the results in the PMBB cohort, we expect this be less likely to occur in DCM patients carrying hiPSI TTNtv than those without variants. Unfortunately, prior DCM cohort studies have not reported on hiPSI TTNtv carrier status by genetic ancestry,2,5 although the presence of TTNtv in women with peripartum cardiomyopathy, a DCM-like disease, does seem to be equivalently high in women of European and African ancestry.7
A number of reasons may explain the lack of observed association between TTNtvs and DCM in patients of African descent. It has been speculated that the development of DCM in the setting of hiPSI TTNtv requires a “second hit” (i.e., extrinsic stress), such as volume overload or chemotherapy.7,11,35 Alternatively, environmental factors may be responsible for the lack of association, including access to health care and social determinants of health, both of which are worse in African American populations.36,37 In addition, patients of African descent have higher rates of DCM in general, such as perhaps higher rates of hypertension-associated DCM, and this high prevalence may dilute the measurable risk conferred by TTNtv. Also, it is possible that referral rates for advanced heart failure differ by race. Finally, it is possible that the genetic background in individuals of African ancestry limits the impact of TTNtv-induced risk of developing DCM. However, one possible such explanation, alternative splicing allowing more TTNtvs to fall in low PSI exons, does not appear to be the case. It will be of future interest to evaluate the TTNtv/DCM relationship in cohorts of other genetic ancestries and with different approaches to ascertainment.
Limitations
The TTN gene is the largest and one of the most complex coding regions of the genome, and still presents technical challenges to next generation sequencing. These technical limits may introduce confounding factors to our analysis, such as a higher than normal false discovery rate. We took steps to mitigate this potential, such as Sanger confirmation for a subset of samples to identify appropriate quality cutoffs, but these steps may not have removed all false positive cases. However, as the likely effect of false positive calls is to introduce noise in the signal, these potential errors are unlikely to explain our findings and may in fact lead to an underestimation of the effects in our observations.
Furthermore, the inability to detect an association between TTNtvs and DCM in African American individuals may be the result of a relatively modest sample size. Although we cannot conclude that there is no association between TTNtv and DCM in African Americans, we can safely exclude an association on the order of magnitude we report in individuals of European Ancestry. Based on our case and control numbers in African Americans, we have a greater than 99% power to detect an OR of 18, that which we report individuals of European ancestry, and greater than 90% power to detect an OR of at least 7, with a 2-sided alpha of 0.05.
In conclusion, our genomics-first evaluation of TTNtv in two disparate clinical populations demonstrates that hiPSI TTNtvs in individuals of European ancestry are associated with worse cardiac function in the presence of DCM and reduced (low-normal) cardiac function in the absence of a DCM diagnosis. Identification of TTNtv carriers of European ancestry may thus alter clinical management strategies, as individuals with hiPSI TTNtv but without clinical evidence of DCM may harbor unrecognized cardiac dysfunction and dysrhythmia, while those patients with hiPSI TTNtv-mediated DCM may be at risk for more severe disease. Conversely, we did not detect any significant association between TTNtvs and DCM among individuals of African ancestry from two independent cohorts, suggesting the odds ratio for DCM in those individuals is much less. Further ancestry-stratified analyses are therefore warranted to confirm this finding and evaluate these trends among other ethnic populations.
Supplementary Material
CLINICAL PERSPECTIVE.
1. What is new?
Genetic variants causing premature truncation of the sarcomere protein, Titin, are highly prevalent in numerous forms of non-ischemic cardiomyopathy, but little is known about the consequences of these Titin variants ascertained independently of cardiomyopathy (i.e., “genomics-first”).
We report that individuals of European ancestry identified through a genomics-first approach have much greater odds of developing dilated cardiomyopathy, and have lower left ventricular function than their peers whether a clinical diagnosis of dilated cardiomyopathy is present or not.
We also find that the association of Titin variants and dilated cardiomyopathy is much weaker in individuals of African ancestry.
2. What are the clinical implications?
Truncating genetic variants in Titin have a measurable effect in large clinical populations, with respect to both strong associations with cardiomyopathy and arrhythmia and quantitative differences in cardiac structure and function, although these associations appear strongest in individuals of European ancestry.
Identification of such variants, either through targeted genetic testing in the setting of cardiomyopathy or screening for secondary findings, may provide clinical value for individualized risk stratification and preventive cardiology.
ACKNOWLEDGEMENTS
We acknowledge all participants in the Geisinger MyCode Community Health Initiative and the PennMedicine Biobank. The authors also wish to thank the staffs and participants of the Jackson Heart Study.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
SOURCES OF FUNDING
ZA was supported by the National Institutes of Health (HL126797) and the Department of Defense (PR171741). CMH was supported by the National Institutes of Health (R01HL141901). SMD was supported by the U.S. Department of Veterans Affairs (IK2-CX001780). This publication does not represent the views of the Department of Veterans Affairs or the United States government. This work was supported in part by the Regeneron Genetics Center. The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). Dr. Wilson is supported by U54GM115428 from the National Institute of General Medical Sciences.
Footnotes
DISCLOSURES
MFM has consulted for Invitae and Merck. BKF is a consultant for the Novartis Cardiovascular Data Science Advisory Board. All other authors have no financial interests to disclose.
REFERENCES
- 1.Linke WA. Titin Gene and Protein Functions in Passive and Active Muscle. Annu Rev Physiol. 2018;80:389–411. [DOI] [PubMed] [Google Scholar]
- 2.Herman DS, Lam L, Taylor MRG, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJR, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of Titin Causing Dilated Cardiomyopathy. N Engl J Med. 2012;366:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts AM, Ware JS, Herman DS, Schafer S, Baksi J, Bick AG, Buchan RJ, Walsh R, John S, Wilkinson S, Mazzarotto F, Felkin LE, Gong S,L. MacArthur JA, Cunningham F, Flannick J, Gabriel SB, Altshuler DM, Macdonald PS, Heinig M, Keogh AM, Hayward CS, Banner NR, Pennell DJ, O’Regan DP, San TR, de Marvao A,W. Dawes TJ, Gulati A, Birks EJ, Yacoub MH, Radke M, Gotthardt M, Wilson JG, O’Donnell CJ, Prasad SK,R. Barton PJ, Fatkin D, Hubner N, Seidman JG, Seidman CE, Cook SA. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci Transl Med. 2015;7:270ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akinrinade O, Alastalo TP, Koskenvuo JW. Relevance of truncating titin mutations in dilated cardiomyopathy. Clin Genet. 2016;90:49–54. [DOI] [PubMed] [Google Scholar]
- 5.Haas J, Frese KS, Peil B, Kloos W, Keller A, Nietsch R, Feng Z, Muller S, Kayvanpour E, Vogel B, Sedaghat-Hamedani F, Lim W-K, Zhao X, Fradkin D, Kohler D, Fischer S, Franke J, Marquart S, Barb I, Li DT, Amr A, Ehlermann P, Mereles D, Weis T, Hassel S, Kremer A, King V, Wirsz E, Isnard R, Komajda M, Serio A, Grasso M, Syrris P, Wicks E, Plagnol V, Lopes L, Gadgaard T, Eiskjaer H, Jorgensen M, Garcia-Giustiniani D, Ortiz-Genga M, Crespo-Leiro MG, Deprez RHLD, Christiaans I, van Rijsingen I a., Wilde a. a., Waldenstrom A, Bolognesi M, Bellazzi R, Morner S, Bermejo JL, Monserrat L, Villard E, Mogensen J, Pinto YM, Charron P, Elliott P, Arbustini E, Katus H a., Meder B. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur Heart J. 2015;36:1123–1135. [DOI] [PubMed] [Google Scholar]
- 6.Akinrinade O, Ollila L, Vattulainen S, Tallila J, Gentile M, Salmenperä P, Koillinen H, Kaartinen M, Nieminen MS, Myllykangas S, Alastalo TP, Koskenvuo JW, Heliö T. Genetics and genotype-phenotype correlations in Finnish patients with dilated cardiomyopathy. Eur Heart J. 2015;36:2327–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F, Cook SA, Halder I, Prasad SK, Pisarcik J, Hanley-Yanez K, Alharethi R, Damp J, Hsich E, Elkayam U, Sheppard R, Kealey A, Alexis J, Ramani G, Safirstein J, Boehmer J, Pauly DF, Wittstein IS, Thohan V, Zucker MJ, Liu P, Gorcsan J, McNamara DM, Seidman CE, Seidman JG, Arany Z. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N Engl J Med. 2016;374:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deo RC. Alternative Splicing, Internal Promoter, Nonsense-Mediated Decay, or All Three: Explaining the Distribution of Truncation Variants in Titin. Circ Cardiovasc Genet. 2016;9:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. [DOI] [PubMed] [Google Scholar]
- 10.Akinrinade O, Koskenvuo JW, Alastalo TP. Prevalence of titin truncating variants in general population. PLoS One. 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer S, de Marvao A, Adami E, Fiedler LR, Ng B, Khin E, Rackham OJL, van Heesch S, Pua CJ, Kui M, Walsh R, Tayal U, Prasad SK, Dawes TJW, Ko NSJ, Sim D, Chan LLH, Chin CWL, Mazzarotto F, Barton PJ, Kreuchwig F, de Kleijn DPV, Totman T, Biffi C, Tee N, Rueckert D, Schneider V, Faber A, Regitz-Zagrosek V, Seidman JG, Seidman CE, Linke WA, Kovalik J, O’Regan D, Ware JS, Hubner N, Cook SA. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat Genet. 2017;49:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, Murray MF, Smelser DT, Gerhard GS, Ledbetter DH. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med. 2016;18:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewey FE, Murray MF, Overton JD, Habegger L, Leader JB, Fetterolf SN, O’Dushlaine C, Van Hout CV., Staples J, Gonzaga-Jauregui C, Metpally R, Pendergrass SA, Giovanni MA, Kirchner HL, Balasubramanian S, Abul-Husn NS, Hartzel DN, Lavage DR, Kost KA, Packer JS, Lopez AE, Penn J, Mukherjee S, Gosalia N, Kanagaraj M, Li AH, Mitnaul LJ, Adams LJ, Person TN, Praveen K, Marcketta A, Lebo MS, Austin-Tse CA, Mason-Suares HM, Bruse S, Mellis S, Phillips R, Stahl N, Murphy A, Economides A, Skelding KA, Still CD, Elmore JR, Borecki IB, Yancopoulos GD, Davis FD, Faucett WA, Gottesman O, Ritchie MD, Shuldiner AR, Reid JG, Ledbetter DH, Baras A, Carey DJ. Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science. 2016;354:aaf6814. [DOI] [PubMed] [Google Scholar]
- 14.Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV., Habegger L, Buckler D, Lai K-MV., Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016;374:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haggerty CM, James CA, Calkins H, Tichnell C, Leader JB, Hartzel DN, Nevius CD, Pendergrass SA, Person TN, Schwartz M, Ritchie MD, Carey DJ, Ledbetter DH, Williams MS, Dewey FE, Lopez A, Penn J, Overton JD, Reid JG, Lebo M, Mason-Suares H, Austin-Tse C, Rehm HL, Delisle BP, Makowski DJ, Mehra VC, Murray MF, Fornwalt BK. Electronic health record phenotype in subjects with genetic variants associated with arrhythmogenic right ventricular cardiomyopathy: a study of 30,716 subjects with exome sequencing. Genet Med. 2017;19:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abul-Husn NS, Manickam K, Jones LK, Wright EA, Hartzel DN, Gonzaga-Jauregui C, O’Dushlaine C, Leader JB, Lester Kirchner H, Lindbuchler DM, Barr ML, Giovanni MA, Ritchie MD, Overton JD, Reid JG, Metpally RPR, Wardeh AH, Borecki IB, Yancopoulos GD, Baras A, Shuldiner AR, Gottesman O, Ledbetter DH, Carey DJ, Dewey FE, Murray MF. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science. 2016;354:aaf7000. [DOI] [PubMed] [Google Scholar]
- 17.Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao W, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee I-T, Liang K-W, Guo X, Rotter JI, Chen Y-DI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjærg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N Engl J Med. 2017;377:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, Flicek P, Cunningham F. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander DH, Novembre J, Lange K. Fast Model-Based Estimation of Ancestry in Unrelated Individuals. Genome Res. 2009;19:1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. 2018;
- 22.Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, Wang D, Masys DR, Roden DM, Crawford DC. PheWAS: Demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carroll RJ, Bastarache L, Denny JC. R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics. 2014;30:2375–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 25.Davison AC, Hinkley DV. Bootstrap Methods and Their Application. New York, NY: Cambridge University Press; 1997. [Google Scholar]
- 26.Ho DE, Imai K, King G, Stuart EA. MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42:8. [Google Scholar]
- 27.Lek M, Karczewski KJ, Minikel E V., Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won H-H, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tayal U, Newsome S, Buchan R, Whiffin N, Walsh R, Barton PJ, Ware JS, Cook SA, Prasad SK. Truncating Variants in Titin Independently Predict Early Arrhythmias in Patients With Dilated Cardiomyopathy. J Am Coll Cardiol. 2017;69:2466–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen JB, Fritsche LG, Zhou W, Teslovich TM, Holmen OL, Gustafsson S, Gabrielsen ME, Schmidt EM, Beaumont R, Wolford BN, Lin M, Brummett CM, Preuss MH, Refsgaard L, Bottinger EP, Graham SE, Surakka I, Chu Y, Skogholt AH, Dalen H, Boyle AP, Oral H, Herron TJ, Kitzman J, Jalife J, Svendsen JH, Olesen MS, Njølstad I, Løchen ML, Baras A, Gottesman O, Marcketta A, O’Dushlaine C, Ritchie MD, Wilsgaard T, Loos RJF, Frayling TM, Boehnke M, Ingelsson E, Carey DJ, Dewey FE, Kang HM, Abecasis GR, Hveem K, Willer CJ. Genome-wide Study of Atrial Fibrillation Identifies Seven Risk Loci and Highlights Biological Pathways and Regulatory Elements Involved in Cardiac Development. Am J Hum Genet. 2018;102:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, Lin H, Arking DE, Smith AV., Albert CM, Chaffin M, Tucker NR, Li M, Klarin D, Bihlmeyer NA, Low SK, Weeke PE, Müller-Nurasyid M, Smith JG, Brody JA, Niemeijer MN, Dörr M, Trompet S, Huffman J, Gustafsson S, Schurmann C, Kleber ME, Lyytikäinen LP, Seppälä I, Malik R, Horimoto ARVR, Perez M, Sinisalo J, Aeschbacher S, Thériault S, Yao J, Radmanesh F, Weiss S, Teumer A, Choi SH, Weng LC, Clauss S, Deo R, Rader DJ, Shah SH, Sun A, Hopewell JC, Debette S, Chauhan G, Yang Q, Worrall BB, Paré G, Kamatani Y, Hagemeijer YP, Verweij N, Siland JE, Kubo M, Smith JD, Van Wagoner DR, Bis JC, Perz S, Psaty BM, Ridker PM, Magnani JW, Harris TB, Launer LJ, Shoemaker MB, Padmanabhan S, Haessler J, Bartz TM, Waldenberger M, Lichtner P, Arendt M, Krieger JE, Kähönen M, Risch L, Mansur AJ, Peters A, Smith BH, Lind L, Scott SA, Lu Y, Bottinger EB, Hernesniemi J, Lindgren CM, Wong JA, Huang J, Eskola M, Morris AP, Ford I, Reiner AP, Delgado G, Chen LY, Chen YDI, Sandhu RK, Li M, Boerwinkle E, Eisele L, Lannfelt L, Rost N, Anderson CD, Taylor KD, Campbell A, Magnusson PK, Porteous D, Hocking LJ, Vlachopoulou E, Pedersen NL, Kjell N, Orho-Melander M, Hamsten A, Heeringa J, Denny JC, Kriebel J, Darbar D, Newton-Cheh C, Shaffer C, Macfarlane PW, Heilmann-Heimback S, Almgren P, Huang PL, Sotoodehnia N, Soliman EZ, Uitterlinden AG, Hofman A, Franco OH, Völker U, Jöckel KH, Sinner MF, Lin HJ, Guo X, METASTROKE Consortium of the ISGC, Neurology Working Group of the CHARGE Consortium, Dichgans M, Ingelsson E, Kooperberg C, Melander O, Loos RJF, Laurikka J, Conen D, Rosand J, van der Harst P, Lokki ML, Kathiresan S, Pereira A, Jukema JW, Hayward C, Rotter JI, März W, Lehtimäki T, Stricker BH, Chung MK, Felix SB, Gudnason V, Alonso A, Roden DM, Kääb S, Chasman DI, Heckbert SR, Benjami EJ, Tanaka T, Lunetta KL, Lubitz SA, Ellinor PT. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49:946–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi SH, Weng L, Roselli C, Lin H, Haggerty CM, Shoemaker MB, Barnard J, Arking DE, Chasman DI, Albert CM, Chaffin M, Tucker NR, Smith JD, Gupta N, Gabriel S, Margolin L, Shea MA, Shaffer CM, Yoneda ZT, Boerwinkle E, Smith NL, Silverman EK, Redline S, Vasan RS, Burchard EG, Gogarten SM, Laurie C, Blackwell TW, Abecasis G, Carey DJ, Fornwalt BK, Smelser DT, Baras A, Dewey FE, Jaquish CE, Papanicolaou GJ, Sotoodehnia N, Van Wagoner DR, Psaty BM, Kathiresan S, Darbar D, Alonso A, Heckbert SR, Chung MK, Roden DM, Benjamin EJ, Murray MF, Lunetta KL, Lubitz SA, Ellinor PT. Association Between Titin Loss-of-Function Variants and Early-Onset Atrial Fibrillation. J Am Med Assoc. 2018;320:2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansweijer JA, Nieuwhof K, Russo F, Hoorntje ET, Jongbloed JDH, Lekanne Deprez RH, Postma AV., Bronk M, van Rijsingen IAW, de Haij S, Biagini E, van Haelst PL, van Wijngaarden J, van den Berg MP, Wilde AAM, Mannens MMAM, de Boer RA, van Spaendonck-Zwarts KY, van Tintelen JP, Pinto YM. Truncating titin mutations are associated with a mild and treatable form of dilated cardiomyopathy. Eur J Heart Fail. 2017;19:512–521. [DOI] [PubMed] [Google Scholar]
- 33.Tayal U, Newsome S, Buchan R, Whiffin N, Halliday B, Lota A, Roberts A, John Baksi A, Voges I, Midwinter W, Wilk A, Govind R, Walsh R, Daubeney P, Jarman JW, Baruah R, Frenneaux M, Barton PJ, Pennell D, Ware JS, Prasad SK, Cook SA. Phenotype and Clinical Outcomes of Titin Cardiomyopathy. J Am Coll Cardiol. 2017;70:2264–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdonschot JAJ, Hazebroek MR, Derks KWJ, Barandiarán Aizpurua A, Merken JJ, Wang P, Bierau J, van den Wijngaard A, Schalla SM, Abdul Hamid MA, van Bilsen M, van Empel VPM, Knackstedt C, Brunner-La Rocca H-P, Brunner HG, Krapels IPC, Heymans SRB. Titin cardiomyopathy leads to altered mitochondrial energetics, increased fibrosis and long-term life-threatening arrhythmias. Eur Heart J. 2018;39:864–873. [DOI] [PubMed] [Google Scholar]
- 35.Linschoten M, Teske AJ, Baas AF, Vink A, Dooijes D, Baars HF, Asselbergs FW. Truncating Titin (TTN) Variants in Chemotherapy-Induced Cardiomyopathy. J Card Fail. 2017;23:476–479. [DOI] [PubMed] [Google Scholar]
- 36.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Ezzati M, Rimm EB, Hajifathalian K, Ueda P, Danaei G. Sick Populations and Sick Subpopulations: Reducing Disparities in Cardiovascular Disease Between Blacks and Whites in the United States. Circulation. 2016;134:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.