Abstract
Objective:
Evidence suggests damage to brain auditory pathways, rather than inner ear damage, underlies the hearing difficulties HIV+ individuals report. But, anti-retroviral therapy (ART) may affect the hearing system and also lead to hearing complaints.
Design:
Longitudinal study of HIV+ and HIV- individuals in Dar es Salaam Tanzania. A subset of this cohort started ART while in the study allowing the effects of ART to be studied directly.
Methods:
The ability to hear quiet sounds (pure-tone audiometry), cochlear outer hair cell function (distortion-product otoacoustic emissions (DPOAEs), and gaps-in-noise detection thresholds (a central auditory processing test) were assessed at each visit. Visits were scheduled for 6-month intervals, but the number and spacing of visits varied. In the group that started ART while in the study, 107 HIV+ individuals had audiometric thresholds, 98 had DPOAEs, and 98 had gap measurements suitable for analysis. Data were analyzed using a linear mixed model with time and starting ART as fixed effects and individual subject repeated measures as random effects.
Results:
Starting ART did not affect audiometric or gap detection thresholds. The slope of the DPOAE amplitude vs. time relationship was more negative after starting ART but did not differ from the HIV- group. Gap thresholds were higher in the HIV+ group.
Conclusion:
ART did not affect audiometric thresholds significantly suggesting common ART drugs are not major ototoxins. The gap detection results from the study show effects on central auditory processing in HIV+ individuals, supporting the origin of HIV-related hearing complaints in the central auditory system.
Keywords: HIV, Auditory perception, Hearing, Anti-retroviral agents, Neurocognitive Disorders
Background
HIV+ individuals complain of hearing problems (over 25% of a Tanzanian cohort of adults reported some hearing difficulty[1]) and the origin of these complaints seems to be in the central nervous system[1, 2]. But, a contribution from anti-retroviral treatment (ART) has not be ruled out. A major concern for these patients is that lifelong anti-retroviral treatment (ART) may damage the cochlea and lead to difficulty hearing and problems with communication. Some studies have shown a diminished ability to hear soft sounds in HIV+ patients compared to HIV- controls[3, 4], while others have not[1, 5]. To date, there has not been a longitudinal study of patients who started ART to assess whether starting these medications affects hearing.
The evidence for hearing damage in HIV+ infection and treatment has come from cross-sectional studies, where difficulties matching the HIV+ participants with an appropriate control group often exist. Since many factors can affect hearing (such as age, gender, socio-economic status, treatment for TB, noise exposure), balancing the groups on these factors presents challenges. Also, HIV+ individuals may be at more risk for ear infections and serous otitis media which can lead to hearing problems not directly related to either ART or HIV status[6]. In a previous cross-sectional study from our group audiometric thresholds did not differ significantly between the HIV+ and HIV− groups[1], but these groups were not well matched on age, sex, or noise exposure. Luque et al.[5] studied 278 HIV+ adults and compared their results to 120 HIV- controls and showed no major differences in audiometric thresholds between HIV+ and HIV− adults, although those with late stage disease had worse thresholds. The HIV+ and HIV- groups, however, were not well matched in either age, gender, or race. Torre et al.[3] compared 222 HIV+ adults to 174 HIV- controls and showed worse low frequency (250, 500, 1000, and 2000 Hz) and high-frequency (3000, 4000, 6000, 8000 Hz) pure-tone averages (PTA) in the HIV+ group, but the groups differed significantly in age, gender, and race as well.
Distortion product otoacoustic emission (DPOAE) measurements provide a measure of outer hair cell function in the cochlea and can be used to assess ototoxicity[7]. Maro et al. showed that HIV+ adults on ART had significantly lower DPOAE magnitudes compared with HIV− controls, suggesting a potential effect of ART on the cochlea[1]. Torre et al., however, did not detect major effects on DPOAEs in HIV+ adults. The number of HIV+ adults without detectable DPOAEs (DPOAE < −15 SPL or <6 dB above the noise floor) did not differ from the HIV− control group[8]. In a study of HIV+ children, Maro et al. showed reduced DPOAE magnitudes in HIV+ children, but interpretation of these results was complicated by the higher rate of middle ear problems in the HIV+ group[9]. A pediatric study by Torre et al., however, did not show lower DPOAEs in HIV+ compared to HIV- children[10]. The net results from these studies do not provide a conclusive answer on whether HIV treatment affects the inner ear.
We have been running a longitudinal study of hearing function in HIV+ individuals in Tanzania where individuals are scheduled to come for hearing testing every 6 months. During the course of the study, individuals have been enrolled who started ART while they were in the study. The data from these individuals provide a unique opportunity to assess the effects of ART on hearing.
Methods
Study Participants
The research protocol was approved by the institutional review boards of Dartmouth College and the Muhimbili University for Health and Allied Sciences and all participants gave written informed consent. Participants were HIV+ and HIV- adults and children in Dar es Salaam, Tanzania. Patients were eligible to participate if they had a confirmed diagnosis of HIV, had not been noise-exposed prior to testing, and were not taking high doses of aspirin. They were not screened for syphilis.
Study Procedures
The ears were examined otoscopically and cerumen was removed as needed. The hearing testing system has been described in a previous publication from our group[1]. The laptop-based hearing testing system included: (a) questionnaires asking about self-reported hearing and exposure to noise, drugs and toxins, (b) threshold audiometry (either Békésy or a modified Hughson-Westlake protocol) to measure hearing sensitivity, (d) distortion product otoacoustic emission (DPOAE) testing to assess cochlear (outer hair cell) function, and (e) gap detection testing to assess central auditory processing abilities. Participants wore passive noise-attenuating earmuffs over the ears (David Clark Model 19 A, David Clark Company, Worcester, MA or 3M Peltor 97023 Junior Hearing Protector Ear Muffs, St. Paul, MN). Sound levels in the ear canal were measured to establish that sound levels in the ear canal were quiet enough for testing[11]. Data were stored in a relational database management system (Microsoft Access 2010, Microsoft, Redmond, WA).
The Kiswahili-based video questionnaires gathered data on the participants self-reported hearing ability (hearing status questionnaire) and general health (health history questionnaire). The questions covered noise exposure, tinnitus, ear drainage, ear infections, chemical exposure, and balance problems. The questionnaire also asked about past or current tuberculosis (TB) treatment, HIV treatment, gentamicin exposure, and the use of anti-malarials, aspirin, and diuretics. For children who could not answer the question, the test operators completed the questionnaire with the child and the parent or guardian present. The results from the questionnaires have been published previously[1, 9].
A Madsen Otoflex 100 (GN Otometrics, Denmark) was used to perform tympanometry at 226Hz. Measurements of ear canal volume, static admittance, tympanometric peak pressure, tympanometric width, and tympanogram type (A, As, Ad, B, C) were collected. The device classified the tympanogram from the location (pressure and static admittance) of the peak. The type A pressure limits were −100 to +50 daPa, and the static admittance limits were 0.3–1.7 mmho. Tympanograms with static admittance levels less than 0.3 mmho with a discernable peak were classified as type As, those with levels less than 0.3 with no discernable peak were classified as Type B. Those with static admittance levels greater than 1.7 mmho were classified as type Ad. Tympanograms with pressures outside the Type A range, but within the static admittance limits, were classified as Type C. Those with Type B or Type C tympanograms were sent for evaluation and tested on a different day.
Air conduction pure-tone audiometric thresholds were measured at frequencies of 500, 1000, 2000, and 4000 Hz using a Békésy-like tracking procedure described previously[1]. Pulsed tones with a duration of 250 msec, a rise and fall time of 20 msec, and an interstimulus interval of 500 msec were used. When the button was pressed the tone decreased in 4-dB steps until the first reversal, then 2-dB steps were used. Upon releasing the button, the tones increased in 2-dB steps. A total of six good reversals were counted and averaged. The total time for testing was less than five min/ear.
For DPOAEs, emissions were collected at f2 values of 1500, 1700, 2000, 2200, 3000, 3200, 4000, 4200, 6000, 6200, 7800, and 8000 Hz using an f2/f1 ratio of 1.2 and L1/L2 values of 65/55. Data were also collected using L1/L2 values of 70/70, but these were not used due to a higher rate of abnormally high values due to ear canal resonances. The f2-f1 frequency pair were delivered for a minimum of four seconds. After four seconds, if the DPOAE level (DP) and averaged noise floor level (NF) difference was less than 10 dB, the frequency pair continued to be presented until either a DP-NF value of 10 was reached or 10 seconds had elapsed. The operators instructed the participants not to swallow during DPOAE testing, and to remove extraneous noise an adaptive noise-rejection algorithm was used. An in-ear calibration was not used (i.e. the speaker output was not adjusted in the ear canal). In some subjects, likely due to resonances in the ear canal, unrealistically high values for DPOAEs and noise floors were returned. These values were discarded for the data analysis. The level of harmonic distortion for each system was determined using a Brüel and Kjær Type 4157 Ear Simulator/Artificial Ear (Bruel and Kjaer Type, Nærum, Denmark).
Because consistent DPOAE probe placement is important for achieving consistent results over time, a “position check” (frequency sweep) was presented in the ear canal prior to DPOAE testing. Three position checks (500–5000Hz) at 65 dB SPL were averaged, smoothed, and displayed to the operator. A measured level below 20 dB SPL at 500 Hz was used to indicate a bad probe seal. In the case of a bad seal the probe was reseated and the chirps were repeated. If the probe was placed securely in the ear canal and the seal check passed, the results from the position check were saved as a baseline for that subject. On subsequent visits, the baseline position check was displayed so the operator could position the probe to match the frequency sweep within ± 5 dB at each frequency.
To determine an individual’s gap detection threshold, the participant was trained to press a button when a short gap in noise was heard. The gaps were placed randomly in the middle portion of 4.5 s of white noise delivered at 65 dB SPL. The details of the gap detection test have been published previously[1]. The gap test produced a plot of the percentage of time a gap was correctly detected vs. gap length. Previous work has shown that this curve can be fit using the Hill equation to calculate the gap length where 50% of the gaps were detected correctly[12]. These values were used in the analysis.
Statistical Analysis
The study took place over five years and during this time some people dropped out of the study or died. Although the visits were scheduled at six-month intervals, not all participants adhered to that schedule. Additionally, hardware and testing difficulties would arise at particular visits that would produce outliers. A standardized approach was developed to remove these outliers. For both the DPOAE and audiometry tests the output from the tests could be plotted as a result in dB versus frequency. The mean and standard deviation for area under this dB vs. frequency curve (AUC) was calculated for all repeated measurements for each person in the study. From these combined measurements, the standard deviation for the AUC over all subjects was divided into quartiles. Then for each individual subject the repeated measurements were examined to determine if the standard deviation of the AUC for that subject was in the highest quartile. If so, individual measurements were removed in a stepwise fashion and the standard deviation of the AUC was re-calculated. If the person had more than four repeated measurements and removal of a measurement changed the standard deviation of the AUC more than 20%, then that measurement was determined to be an outlier and removed. If the person had only 3 measurements, then a measurement was removed if it changed the standard deviation of the AUC by more than 50%. For the gap data, a different approach was used to remove outliers. The repeated measurements for each subject were fit with a regression line and the residuals were calculated. If the standardized residual was greater than two for a particular measurement it was removed.
The DPOAE, audiometry, and gap data were analyzed using linear mixed effects models. To determine whether group (HIV-, HIV+ART-, ART start) influenced the change in the individual parameters over time, all the groups were included in one model with the interaction of group and time as fixed effects and the individual subject results over time as random effects. For the ART start group, only the points after starting ART were included in this analysis. To assess the effect of starting ART, a new grouping variable was created to label to the data as either prior to or after starting ART (OnART). A linear mixed model on the ART start group was performed using the interaction of OnART and time as fixed effects and individual subject results over time as random effects. To assess the difference between the ART start group after starting ART and the HIV- group a separate linear mixed model with just the HIV- and ART start group values (after starting ART) was also done to determine the differences between these groups and to calculate the slopes used for displaying the results graphically (green lines in Figures 1–3). Authors J.C.B. and J.G. performed the analyses using Matlab 2017b and R.
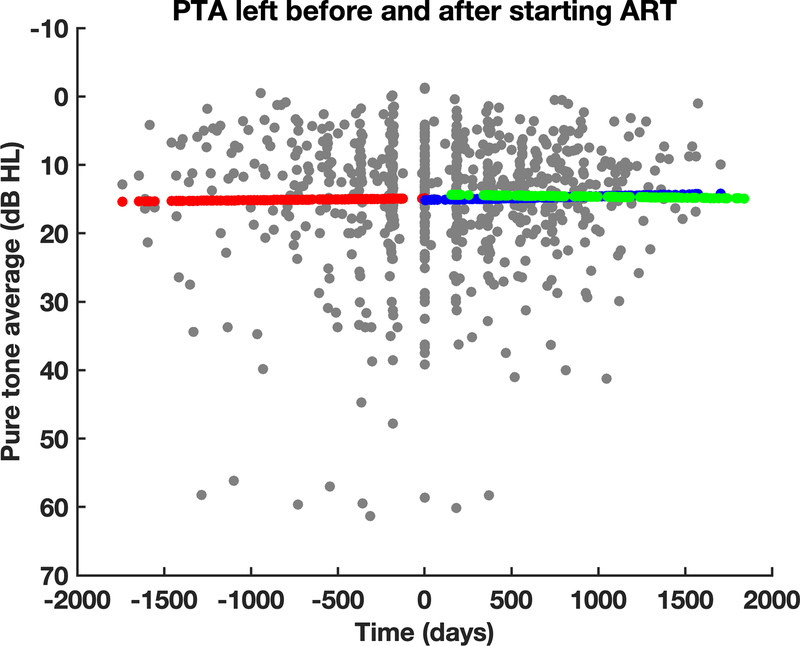
Figure 1:
Audiometric thresholds did not show significant changes after starting ART. Time 0 is the first visit after starting ART. The red line shows the slope prior to starting ART, the blue line is the slope after starting ART, and the green line is the slope of the HIV- group.
Figure 3:
The slope of the gap detection threshold vs. time curve did not change significantly after starting ART (blue) and was not different from the HIV- group (green).
Results
Table I shows the characteristics of the cohorts for the individual analyses. The most common drug regimens in the group starting ART were efavirenz, lamivudine, and zidovudine (34%), efavirenz, lamivudine, and tenofovir (29%), nevirapine, lamivudine, and zidovudine (19%), and efavirenz, emcitribine, and tenofovir (13%). All the HIV+ groups were very similar in age and gender makeup, but in general the HIV- group was significantly younger and more male than the other groups. Table 2 shows the results of the linear mixed model examining the effect of time on the groups. The overall results show no significant differences in slope (i.e. change in parameter/day) between any of the groups. Table 3 shows the results when the data prior to starting ART are compared to the data after starting ART. No significant difference in slope exists for the audiometry or gap results, but the slope of the DPOAE/time relationship is significantly more negative after starting ART. Of note, however, is that the slope of the Pre ART DPOAE/time relationship is positive, which differs from the HIV- group, which shows a gradual reduction in DPOAE amplitude over time.
Table 1.
Characteristics of the groups for each analysis. * indicates the values were significantly different from the HIV- group at the p<0.05 level.
| Audiometry | |||
|---|---|---|---|
| Group | N | Mean age | %Female |
| HIV- | 72 | 27 ± 17.0 | 51 |
| HIV+/ART- | 34 | 37 ± 13.7* | 69* |
| HIV+/ART+ | 250 | 35 ± 15.9* | 71* |
| ART start | 107 | 37 ± 11.5* | 68* |
| Distortion Product Otoacoustic Emissions | |||
| HIV- | 70 | 22 ± 15.1 | 54 |
| HIV+/ART- | 30 | 33 ± 12.6* | 70* |
| HIV+/ART+ | 226 | 33 ± 16.6* | 72* |
| ART start | 98 | 36 ± 11.7* | 67 |
| Gap Detection Threshold | |||
| HIV- | 62 | 27 ± 15.8 | 47 |
| HIV+/ART- | 32 | 37 ± 11.2* | 72* |
| HIV+/ART+ | 222 | 37 ± 14.4* | 68* |
| ART start | 98 | 36 ± 11.3* | 67* |
Table 2.
Change in slope compared to the HIV- group for the different parameters. No significant differences in slope existed between the groups.
| Group | Slope Compared to HIV- | t Statistic | p |
|---|---|---|---|
| Audiometry HIV+ART- | −0.0004 | −0.40 | 0.69 |
| Audiometry HIV+ART+ | −0.0008 | −0.98 | 0.33 |
| Audiometry ARTstart | −0.0008 | −0.93 | 0.35 |
| DPOAE HIV+ART- | 0.006 | 1.26 | 0.21 |
| DPOAE HIV+ART+ | 0.004 | 1.12 | 0.26 |
| DPOAE ARTstart | 0.006 | 1.4 | 0.16 |
| Gap HIV+ART- | 0.00002 | 0.07 | 0.95 |
| Gap HIV+ART+ | −0.0003 | −1.2 | 0.21 |
| Gap ARTstart | −0.0002 | −0.6 | 0.54 |
Table 3.
Change in slope compared to before ART for the different parameters. The slope of the DPOAE/time relationship was significantly lower after starting ART but did not differ from the HIV- group.
| Group | Slope Compared to Pre ART | t Statistic | p |
|---|---|---|---|
| Audiometry | −0.0003 | −0.29 | 0.77 |
| DPOAE | −0.02 | −4.01 | <0.001 |
| Gap | 0.0006 | 1.53 | 0.13 |
Figures 1–3 show the results for the ART start group graphically. In each graph the individual points are in gray. The red line shows the slope of the linear mixed model prior to starting ART, and the blue line show the slope of the model after starting ART. The green line shows the slope from the model for the HIV- group when the HIV- subjects were compared to the ART start subjects after starting ART (i.e. the values prior to starting ART were omitted from this analysis).
Figure 1 shows that starting ART has a minimal effect on audiometric thresholds. Figure 2, however, shows a decrease in slope of the DPOAE/time relationship after starting ART, although the slope does not differ from the slope of the HIV- group (green). Similar to the audiometry results, the gap detection threshold results show no major changes in the slope of the gap threshold vs. time relationship after starting ART.
Figure 2:
The area under the DPOAE amplitude/frequency curve before and after starting ART. The slope of the post ART curve (blue) is significantly different from the pre ART curve (red), but not different from the slope of the HIV- group (green).
The gap detection threshold findings from the previous cross-sectional study on this cohort were confirmed and persisted over time. The intercept of the gap detection vs. time relationship was significantly higher than the HIV- group for the ART start (0.8 msec, p<0.05) and HIV+/ART+ groups (0.9 msec, p<0.01).
Conclusions
The overall results from the study do not show major differences between groups or in audiometric or gap thresholds after starting ART. These data show that over the time period studied, starting ART did not have an effect on the ability to hear soft sounds or affect the change in gap detection thresholds over time. Nevertheless, the gap detection threshold findings were the same as in the previous cross-sectional study from this cohort. The increased gap detection thresholds suggest a central nervous system auditory deficit that was not influenced by starting ART.
For the DPOAE results, however, the findings were less conclusive. The slope of the DPOAE/time relationship did become more negative after starting ART (which would indicate a worsening of outer hair cell function) but the slope of this relationship did not differ significantly from the HIV- group. Despite the significant reduction in slope after starting ART, ART likely did not have a major effect on DPOAEs. Normal aging leads to a decrease in DPOAE amplitude over time and this is apparent in all groups, including the HIV- group (green lines in Figures 1–3). The fact that the slope of the DPOAE/time relationship was positive prior to starting ART does not correspond to the normal effects of aging on DPOAEs. So, it is possible that the slope of the pre ART DPOAE/time relationship was artifactually positive, which led to the significant difference between the pre and post ART measurements. This interpretation is supported by the DPOAE results from the HIV- group. Even though this group was younger, it still showed a decrease in DPOAE amplitude over time that matched the rate of decrease in the post ART group. Additionally, the slope of the DPOAE/time relationship in the HIV+/ART- group was also negative (−0.006).
Part of the reason for the DPOAE findings may be in the difficulties inherent in collecting repeatable DPOAE amplitude measurements over time. Although the study included a frequency sweep “position check” to try to place the DPOAE probe in the same position each time, differences in probe placement from visit to visit can affect the measurements. Also, other technical factors can introduce variability into the DPOAE measurements. Overall, the fact that the audiometric thresholds did not change, and that the pre ART slope was likely artificially high, support the idea that DPOAE amplitudes were not affected dramatically by ARTs.
Although these data do not show major changes in audiometric thresholds after starting ART, this does not mean that HIV- infected individuals do not have hearing complaints. Our previous data from Tanzania have shown that HIV+ adults report hearing problems, particularly difficulty in understanding speech in noise. They also have elevated gap detection thresholds[1]. The gap test is a test of temporal processing in the central auditory pathways. But, completing the test successfully also requires the ability to understanding the task, as well as maintain attention and focus on the task. Results from individuals who could not complete the test successfully are not included in the analysis, which minimizes effects due to lack of understanding or inability to maintain attention. Nevertheless, in addition to central auditory function, the gap test results may also reflect damage in brain areas not strictly involved with auditory processing. This still indicates that the test is reflecting changes likely due to brain injury, but it may not be specific for just the auditory pathways.
Central auditory testing with HIV+ individuals in other settings using tests that don’t require training and are easy to understand (i.e. speech-in-noise testing) has also been performed. Recent results from a study of HIV+ adults in Shanghai China from our group shows that the difficulties HIV+ adults have with understanding speech in noise correlates with cognitive function[2]. Taken together with the gap, DPOAE, and audiometry results, these findings show that the hearing problem in HIV infection is not likely due to an effect from ART on the inner ear. Instead, the hearing difficulties reflect central nervous system dysfunction, which is reflected in difficulty in doing central auditory tasks, such as detecting gaps or understanding speech in background noise.
Acknowledgement
We thank the team at the DarDar clinic in Dar es Salaam,Tanzania who collected these data (Esther Kayichile, Kissa Albert, Safina Sheshe, Claudia Gasana, Mariane Mpessa, Joyce Ghatty, and Riwa Adams). We thank the team at Creare, LLC. that assembled and tested the hearing testing systems. We appreciate the support of Erika Kafwimi and Sabrina Yegela who helped with building the video questionnaire and translating the questions.
Footnotes
Declaration of Interests
The authors have no financial or personal relationships that could inappropriately bias their work.
References
- 1.Maro II, Moshi N, Clavier OH, MacKenzie TA, Kline-Schoder RJ, Wilbur JC, et al. Auditory impairments in HIV-infected individuals in Tanzania. Ear Hear 2014; 35(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan Y, Fellows AM, Qi T, Clavier OH, Soli SD, Shi X, et al. Speech in Noise Perception as a Marker of Cognitive Impairment in HIV Infection. Ear Hear 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre P 3rd, Hoffman HJ, Springer G, Cox C, Young MA, Margolick JB, et al. Hearing Loss Among HIV-Seropositive and HIV-Seronegative Men and Women. JAMA otolaryngology-- head & neck surgery 2015b; 141(3):202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Westhuizen Y, Swanepoel de W, Heinze B, Hofmeyr LM. Auditory and otological manifestations in adults with HIV/AIDS. Int J Audiol 2013; 52(1):37–43. [DOI] [PubMed] [Google Scholar]
- 5.Luque AE, Orlando MS, Leong UC, Allen PD, Guido JJ, Yang H, et al. Hearing function in patients living with HIV/AIDS. Ear Hear 2014; 35(6):e282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taipale A, Pelkonen T, Taipale M, Roine I, Bernardino L, Peltola H, et al. Otorhinolaryngological findings and hearing in HIV-positive and HIV-negative children in a developing country. Eur Arch Otorhinolaryngol 2011; 268(10):1527–1532. [DOI] [PubMed] [Google Scholar]
- 7.Reavis KM, McMillan G, Austin D, Gallun F, Fausti SA, Gordon JS, et al. Distortion-product otoacoustic emission test performance for ototoxicity monitoring. Ear Hear 2011; 32(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torre P 3rd, Hoffman HJ, Springer G, Cox C, Young M, Margolick JB, et al. Cochlear Function Among HIV-Seropositive and HIV-Seronegative Men and Women. Ear Hear 2014; 35(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maro II, Fellows AM, Clavier OH, Gui J, Rieke CC, Wilbur JC, et al. Auditory Impairments in HIV-Infected Children. Ear Hear 2016; 37(4):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torre P 3rd, Yao TJ, Zeldow B, Williams P, Hoffman HJ, Siberry GK, et al. Distortion product otoacoustic emission data in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents in the Pediatric HIV/AIDS Cohort Study. Pediatr Infect Dis J 2015c; 34(3):276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckey JC, Fellows AM, Jastrzembski BG, Maro II, Moshi N, Turk M, et al. Pure-tone audiometric threshold assessment with in-ear monitoring of noise levels. Int J Audiol 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigoryan GG, Fellows A, Musiek FE, Chambers R, Clavier O, Buckey JC. Mathematical Modeling of Gap Detection In: Association for Research in Otolaryngology Midwinter Meeting: Association for Research in Otolaryngology; 2013. [Google Scholar]