Abstract
Background:
Risk for alcoholism may be enhanced by exposure to early life adversity (ELA) in persons with genetic vulnerabilities. We examined ELA in the presence of a common variant of the gene for the enzyme catechol-o-methyltransferase (COMT, Val158Met, rs4680) in relation to cortisol reactivity, the onset of early drinking, and experimentation with drugs.
Methods:
Saliva cortisol reactivity to speech and mental arithmetic stress was measured in 480 healthy young adults (23.5 years of age, 50% females) who experienced either 0, 1, or ≥ 2 forms of ELA during childhood and adolescence, provided information on use of alcohol and recreational drugs, and were genotyped for the Val158Met polymorphism.
Results:
ELA led to progressively smaller cortisol responses in the Met/Met and Val/Met allele groups but to progressively larger responses in Val homozygotes, F = 3.29, p = .011. ELA independently predicted earlier age at first drink, F = 14.2, p < .0001, with a larger effect in Met allele carriers, F = 13.95, p < .00001 and a smaller effect in Val homozygotes F = 4.14, p = .02. Similar effects were seen in recreational drug use. Cortisol reactivity was unrelated to drinking behavior or drug experimentation.
Conclusions:
ELA leads to blunted stress reactivity and, independently, contributes to potentially risky drinking and drug-use behaviors in persons carrying one or two copies of the COMT 158Met allele. The results reinforce the impact of early experience on the stress axis and on risky behaviors, and they point to the 158Met allele as conveying a vulnerability to the early environment.
Keywords: drinking behavior, cortisol, stress, early life adversity, dopamine, genotype, catechol-O-methyltransferase
Exposure to stress during childhood and adolescence modifies the stress axis in adulthood (Lovallo et al., 2012, Carpenter et al., 2007, Carpenter et al., 2013), illustrating a significant impact of the early environment that may contribute to adverse outcomes, including addictive behaviors (Miller et al., 2009). The degree and direction of this sensitivity to early life adversity (ELA) varies across individuals, determined in part by the actions of catecholamines in the central nervous system. The enzyme catechol-O-methyltransferase (COMT) is responsible for breakdown of catecholamines (Axelrod, 1957, Matsumoto et al., 2003), with particular functional importance in the brain’s prefrontal cortex (Maier and Watkins, 2005, Aston-Jones and Cohen, 2005, Comings and Blum, 2000, Seamans and Yang, 2004, Kaenmaki et al., 2010) relative to other brain regions (Chen et al., 2004). Individual differences in COMT activity may therefore be sensitive to the early environment in ways that affect patterns of stress reactivity and adaptive behavior in adulthood (Lovallo et al., 2017).
A single nucleotide polymorphism of the gene for COMT (Val158Met; rs4680) (Chen et al., 2004, Lotta et al., 1995) is strongly conserved in humans and nonhuman primates. The Val allele results in COMT that is highly stable and more efficient at breakdown of norepinephrine and dopamine in the prefrontal cortex and reward systems (Lotta et al., 1995, Zhu et al., 2004). The greater persistence of dopamine and norepinephrine in Met-allele carriers may plausibly increase sensitivity to the environment relative to their Val/Val counterparts (Radley et al., 2008, Egan et al., 2001, Akil et al., 2003, Alexander et al., 2012). Accordingly, Met-allele carriers may perform better on tests of attention, working memory, and decision-making (Malhotra et al., 2002, Enoch et al., 2009, Schneider et al., 2015, Barnett et al., 2011, Schulz et al., 2012), although not all studies agree (Wardle et al., 2013). Despite cognitive advantages, Met/Met homozygotes are more anxious, sensitive to painful stimuli (Zubieta et al., 2003), reactive to emotional faces (Drabant et al., 2006, Enoch et al., 2008), and they have larger subjective and physiological responses to psychosocial stress (Hernaus et al., 2013, Bouma et al., 2012).
This evidence suggests that Val158- and Met158-allele carriers may also differ in long term consequences of a stressful early environment (Goldman et al., 2005). In the Family Health Patterns cohort, exposure to ELA diminished cortisol and heart rate reactivity to psychological stress and modifies psychological and behavioral characteristics in adulthood (Lovallo, 2013, Sorocco et al., 2015, Lovallo et al., 2012). A growing body of evidence suggests that both ELA and blunted stress reactivity may predict risky behaviors including alcohol and drug abuse (Dawes et al., 1999, Moss et al., 1999, Bibbey et al., 2015, al’Absi, 2006, Evans et al., 2013, Evans et al., 2016, Cook et al., 2012, Brody, 2002). The foregoing suggests further study of sensitivity to the early environment in Met158-allele carriers with potential modifications of the stress axis and an impact on alcohol and drug use. In a prior report on 252 healthy young adults we have shown that ELA resulted in blunted stress reactivity with a strong effect in COMT (rs4680) Met158-allele carriers (Lovallo et al., 2017). We expanded this study sample to 480 persons, and examined ELA exposure, stress reactivity, and their impact on alcohol and drug experimentation.
Methods
Participants
Participants were 480 healthy young adults in the Family Health Patterns (FHP) project (Lovallo et al., 2013) who had been genotyped for the COMT Val158Met polymorphism along with ancestry informative markers (AIMS) and had sufficient background data to compute ELA scores. Each participant signed an informed consent form approved by the Institutional Review Boards of the: University of Oklahoma Health Sciences Center and VA Medical Center, Oklahoma City, OK, University of Texas Health Sciences Center San Antonio, TX, and University of Arkansas for Medical Sciences, Little Rock, AR, USA, and was provided financial compensation.
Study Design and Procedure
Participants passing an initial telephone screen underwent detailed assessments at the laboratory (Lovallo et al., 2010). The lab screening included a diagnostic interview using the computerized diagnostic interview system 4 (C-DIS-4) (Blouin et al., 1988) using Diagnostic and Statistical Manual IV criteria (American_Psychiatric_Association, 1994) that was conducted by a trained interviewer supervised by a clinical psychologist. Participants passing the lab screening then visited the lab twice more for a stress reactivity protocol including a day of public speaking and mental arithmetic challenges followed by a resting control day, as described elsewhere (Lovallo et al., 2013, Lovallo et al., 2010).
Inclusion and exclusion criteria
Participants were 18–30 yr-old men and women recruited from the community who were in self-reported good health, had no reported history of serious medical disorder, had a body mass index < 30, were not taking prescription medications other than hormonal contraceptives, as described earlier (Lovallo et al., 2017). Persons were excluded if they had: a personal history of alcohol or drug dependence; met criteria for current substance abuse within the past 60 days; had a history of any Axis I disorder, other than past depression or dysthymia (> 60 days previous), or if they failed a urine drug screen or breath-alcohol test on days of testing. Smoking and smokeless tobacco use were not exclusionary. Women taking hormonal contraceptives were excluded from the present analysis, if they took part in a morning test session, in light of findings showing significantly elevated cortisol baseline levels and an absence of stress responses during the morning hours in this group relative to women not using hormonal contraceptives (Carr et al., 1979, Kuhl et al., 1993, Lovallo et al., 2019).
ELA assessment
ELA was derived from psychiatric interview items that are closely similar to the adverse life events assessed retrospectively by Caspi and Moffitt (Caspi et al., 2003, Caspi et al., 2002) as follows: Physical or Sexual Adversity (“Have you ever been mugged or threatened with a weapon or experienced a break-in or robbery?” “Have you ever been raped or sexually assaulted by a relative?” “Have you ever been raped or sexually assaulted by someone not related to you?”) and Emotional Adversity (“Before you were 15, was there a time when you did not live with your biological mother for at least 6 months?” “Before you were 15, was there a time when you did not live with your biological father for at least 6 months?”). Each person was assigned to an ELA group based on 0, 1, or ≥ 2 reported forms of adversity they experienced. The levels of ELA were considered nontraumatic because selection criteria excluded persons meeting criteria for posttraumatic stress disorder or current major depression. In a subset of 261 persons, ELA (0–5) scores were significantly correlated with total scores on the Childhood Trauma Questionnaire-SF, r = 0.601, p < .001 (Bernstein et al., 2003).
Alcohol, tobacco, and recreational drug use
During screening, subjects reported on several measures of alcohol, tobacco, and drug use, including the following: the Alcohol Use Disorders Identification Test (Babor et al., 2001); a quantity-frequency index of recent drinking, yielding ounces of pure ethanol consumed in the past month (Babor et al., 1992); listing the age at which they first consumed a full drink of beer, wine or distilled spirits; if they ever began cigarette smoking; and they counted the number of recreational drugs they had “ever tried” one or more times, including: marijuana, hallucinogens, opiates, amphetamine and cocaine, tranquilizers and benzodiazepines, barbiturates, antidepressants, and inhalants.
Stress procedure.
Lab sessions were held at either 9:00 am or 1:00 pm, with the time held constant for each subject. Stress testing lasted 105 min while subjects were seated, including a resting baseline (30 min) followed by simulated public speaking (30 min) and mental arithmetic (15 min) and a 30-min resting recovery period as described elsewhere (Lovallo et al., 2010, Al’Absi et al., 1997). The resting control day involved sitting for 105 min while reading general interest magazines and watching nature videos. Prior reports showed no effect of time of day, smoking, caffeine intake, or menstrual cycle stage on the pattern of cortisol reactivity or group differences (Lovallo et al., 2010). Participants reported their level of perceived activation and distress on a set of 10-point scales at the end of the baseline and stress periods (Lundberg and Frankenhaeuser, 1980).
Saliva samples for cortisol determination were collected using Salivettes at 9 times across each day as shown in Figure 1B. Saliva sampling time points are described in Figure 1B caption. Heart rate was monitored every 2 min along with blood pressure measurements using an automated monitor.
Figure 1.
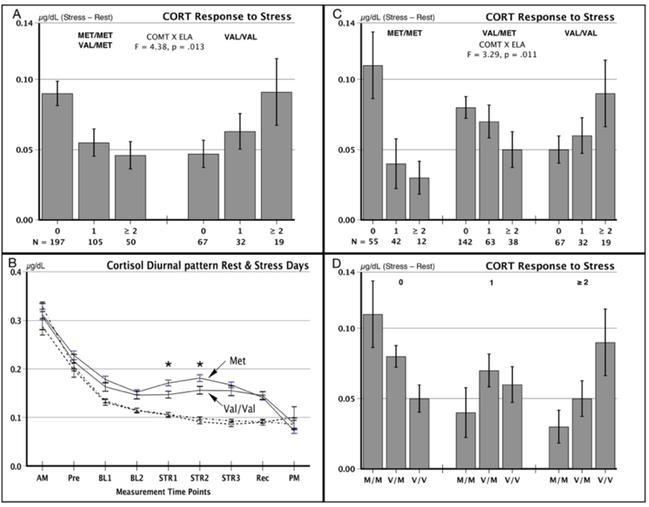
Cortisol saliva measurements at home and during visits to the laboratory. Panel A: Cortisol stress responses in ELA exposure groups (0, 1, ≥ 2) who were Met vs. Val/Val carriers of COMT rs4680 on a stress day referenced to a nonstress control day. Panel B: Cortisol values on a nonstress control day (dashed lines) and on a stress day (solid lines) for Met and Val/Val allele groups. Cortisol measurement time points are: AM, immediately upon awakening; Pre, upon arrival at the lab; BL1, BL2, 30 min and 15 min prior to the onset of the stress procedure or comparable time on the resting control day; STRS1, at the start of the stress protocol, STRS2, following speech presentations, and STRS3, the end of the stress protocol, or the comparable time points on the resting control day; Rec, 45 min after the end of the stress protocol or comparable time point on the resting control day; PM, at bedtime. Panel C: Cortisol stress responses for ELA groups x separate allele groups. Panel D: Data from Panel C rearranged to show the effect of Val158Met genotypes within ELA groups. Figure labels. 0, 1, ≥ 2 indicates the number of childhood adversities experienced in childhood and adolescence. Met and Val refer to single nucleotide polymorphisms of COMT.
Cortisol
Salivettes were centrifuged at 4200 RPM (1500 x g) for 20 min. The saliva was transferred to cryogenic storage tubes and placed into a −70º C freezer until shipping. Saliva free cortisol was quantified by enzyme linked immunosorbent assay by Salimetrics (Carlsbad, CA, USA) (Salimetrics, 2015).
Genotyping
DNA was prepared from saliva collected by passive drool into an Oragene collection and preservation kit (DNA Genotek, Inc., Kanata, Ontario, Canada) and genotyped with the Illumina Human OmniExpressExome-12v1 array. Samples with call rates below 95% were excluded and randomly selected samples showed an average reproducibility of 99.999%.
Assessment of population stratification using ancestry informative markers (AIMS).
Our dataset was predominantly of European origin, with mean (SD) and median European ancestry being 0.89 (0.19) and 0.95 in this sample. Twenty participants had European ancestry scores < 0.50. Of these, 16 were of African ancestry and 4 had Native American ancestry. Genotypes for the COMT Val158Met (rs4680) polymorphism were in Hardy Weinberg Equilibrium in the overall sample (p = 0.57).
Data Analysis
Cortisol responses to stress were calculated as the average of the three samples taken during the stress period on the stress day (STR1, STR2, STR3) minus the equivalent three samples taken on the rest day. Cortisol data were analyzed by a general linear model including: Genotype (Met/Met, Val/Met, Val/Val), ELA (0, 1, ≥ 2), and the G x ELA interaction term. The significant interaction was followed by simple effects tests to compare subgroups. The potential confounders, sex and European and African AIMS scores, were examined as covariates and did not influence the results, as documented elsewhere (Lovallo et al., 2017). The unadjusted F ratios are reported here. Type III sums of squares were used to ensure independence of individual F ratios. Tests were considered statistically significant if p < 0.05. Analyses were conducted using SAS software 9.2 (Copyright, SAS Institute Inc., Cary, NC, USA).
Results
Demographics for the COMT Val158Met genotypes are shown in Table 1. The proportions of Met/Met, Val/Met, and Val/Val genotypes in the present sample (24%, 51%, and 26%, respectively) match the allele distribution in European and Han Chinese populations (Wardle et al., 2013, Li et al., 1997).
Table 1.
Demographics for COMT genotypes
| Genotype | MET/MET | VAL/MET | VAL/VAL | p-value |
|---|---|---|---|---|
| N = 480 (%) | 114 (24%) | 246 (51%) | 120 (25%) | |
| Sex (% F) | 52 | 50 | 50 | 0.93 |
| Age | 24.0 [0.28] | 23.6 [0.22] | 23.6 [0.28] | 0.49 |
| SES | 48.5 [1.15] | 46.4 [0.79] | 47.5 [1.09] | 0.28 |
| Education (yr) | 16.1 [0.19] | 15.7 [0.13] | 15.7 [0.19] | 0.26 |
| ELA (% ≥ 2) | 11 | 16 | 16 | 0.16 |
| FH+ (%) | 37 | 34 | 33 | .81 |
| AIMS (% E) | 91 [1] | 83 [2] | 74 [3] | <.0001 |
Note: Entries show Mean (± SEM) unless otherwise noted. No significant ELA x Genotype interactions were found, and the p-values refer to genotype comparisons only. SES = Socioeconomic Status. ELA = Early Life Adversity. FH = Family History of alcoholism.
AIMS = Ancestry Informative Markers (% with European ancestry markers). p-values refer to F-ratio or Chi-squared comparison across genotype groups.
Genotype x ELA and stress reactivity
Cortisol responses.
Cortisol data are shown in Figure 1. Since responses may be influenced by baseline levels, we examined the cortisol diurnal curves. As shown in Figure 1B, the genotype groups had nearly identical patterns of cortisol secretion across the rest day, indicating equivalent levels of intrinsic HPA axis activity. Cortisol responses during the stress protocol were smaller in the Val/Val homozygotes and larger in the Met carriers, ts > 2.25, ps < .025.
Stress responses differed in the ELA x Genotype groups, as shown in a significant interaction (Figure 1C), F (4, 464) = 3.29, p = .011, partial eta2 = .028, suggesting that the effect of ELA on cortisol stress responses in adulthood differed in the COMT genotype groups. The Met/Met genotype group showed a pronounced diminution of cortisol reactivity to both 1 and ≥ 2 levels of ELA exposure. A similar, less pronounced trend was seen in the Val/Met heterozygotes. In contrast the Val/Val group produced larger cortisol responses in relation to greater ELA exposure.
For visual comparison, Figure 1D shows the genotype data arranged as a function of ELA exposure. Under minimal reported levels of ELA (ELA = 0), the Met/Met group was more reactive than the Val/Val group. In contrast, under high levels of ELA (ELA = 2), the Val/Val carriers had the largest stress responses. The genotype groups reporting a moderate level of ELA (ELA = 1) were relatively similar in cortisol reactivity regardless of genotype.
Given the similar impact of ELA on the Met/Met and Val/Met genotype groups we formed a combined Met allele group as shown in Figure 1A. The graph and the significant interaction indicated clearly opposing effects of ELA exposure in the Val and Met genotype groups, F (2, 464) = 4.38, p = .013, partial eta2 = .019. Neither Genotype nor ELA main effects were significant, Fs > 1.0, ps > .70. The cortisol data from our earlier report on COMT genotypes is provided elsewhere (Lovallo et al., 2017).
Heart rate responses.
HR response to stress (Table S1) was moderately lower in persons with greater levels of ELA exposure, consistent with a prior report (Lovallo et al., 2012), although the ELA effect did not achieve statistical significance in this data set, F = .95, p = .39. Similarly, HR response did not differ across the COMT genotype groups or for the Genotype x ELA interaction, Fs < 1.0, ps > .75. The ELA effects of COMT alleles appear to be confined to the hypothalamic-pituitary-adrenocortical axis and may not affect the autonomic axis.
Subjective states.
As a check on the perceived impact of the stressors, we examined the changes from baseline to poststress in participants’ reports of Activation and Distress as shown in Table S2. Activation and Distress reports for all subgroups increased from the baseline to poststress periods. Change scores did not differ across the ELA or COMT genotype groups (Fs ≤ 2.15, ps ≥ .117) indicating similar experiences of the stress procedure in the respective groups.
Stress reactivity in relation to alcohol and drug use
ELA and COMT genotype effects on alcohol and drug use indicators.
ELA increases the risk for problem intake of alcohol and drugs (Carroll et al., 2017). We explored the relationship between ELA and alcohol and drug use in the COMT genotypes, as shown in Figure 2 and Table S3. Higher levels of ELA led to early use of alcohol and drug experimentation, Fs = 14.20 and 9.08, ps < .0001, respectively. No genotype effects were seen in AUDIT scores or the quantity-frequency index. Cigarette smoking in this population was minimal, and the data did not allow for meaningful comparison among the groups. Exploratory simple effects tests showed that ELA effects on early drinking and drug experimentation were numerically greater in Met/Met persons and smaller in the Val/Met or Val/Val groups as indicated in Figure 2 and shown in Tables S3 and S4.
Figure 2.
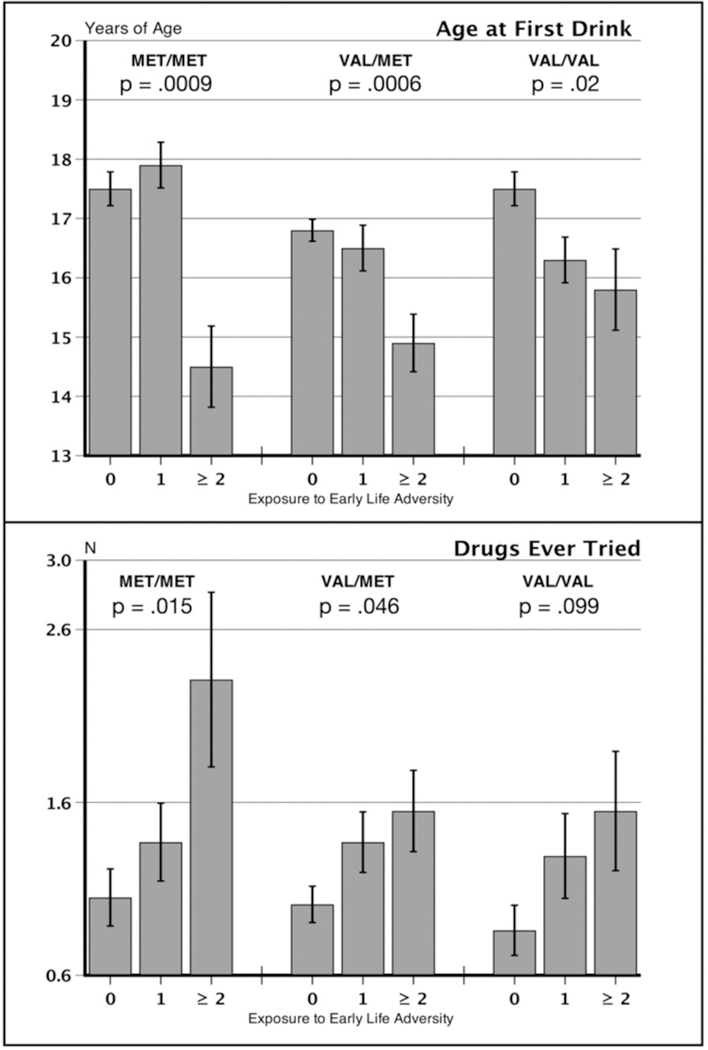
Drinking and drug use variables. Age at first drink and the number of recreational drugs the volunteers reported ever having tried. P-values refer to F ratios of stratified comparisons of ELA groups within genotype. Figure labels as in Figure 1.
Cortisol stress reactivity in relation to drinking and drug use.
Figure 1A indicates that the impact of ELA on cortisol response to stress is not linear; the Met-allele carriers had blunted cortisol responses following ELA while the opposite pattern held true for the Val homozygotes. We then inquired whether cortisol reactivity, would form a predictive bridge from ELA to drinking and drug use. In lieu of complex modeling, we conducted Pearson correlations between cortisol stress responses and age at first drink and the number of drugs ever tried as shown in Table 2. In all cases, the correlations between the cortisol reactivity score and drinking and drug use were ≤ 0.1, accounting for < 1% of the variance. None of the correlations suggested a relationship that would be explained by using more complex models. The indication from these data is that cortisol reactivity and substance use are both affected by a history of ELA, but do not form a direct causal link.
Table 2.
Cortisol reactivity and alcohol and drug use in COMT genotypes
| Age of first drink | Number of drugs tried | |||
|---|---|---|---|---|
| Pearson r | p | Pearson r | p | |
| Overall | − 0.09 | .04 | 0.04 | .43 |
| Mets | − 0.09 | .09 | 0.03 | .58 |
| Val/Val | − 0.10 | .26 | 0.05 | .61 |
Discussion
Stress exposure during critical periods of development can affect health behaviors in adulthood (Dube et al., 2003), and the impact may be greater in persons carrying specific genotypes as illustrated in early G x E studies (Caspi et al., 2002, Caspi et al., 2003). We and others have observed that stress reactivity is diminished in persons exposed to ELA in childhood and adolescence (Lovallo et al., 2012, Carpenter et al., 2007). Adverse childhood experiences and blunted stress reactivity may also signal risk for maladaptive health behaviors and adverse long-term outcomes (Carroll et al., 2017, Anda et al., 2002). ELA may not affect all individuals to the same degree, but instead may be greater in persons carrying specific genetic polymorphisms. We recently reported in a smaller sample of volunteers (N = 252) that the impact of ELA on the stress axis is partially dependent on the individual’s COMT genotype (Lovallo et al., 2017). The present paper extends this finding to an enlarged sample of volunteers (N = 480) with an additional emphasis on alcohol and drug experimentation. These collective results suggest four observations of interest.
First, ELA led to greatly diminished cortisol responses in Met/Met homozygotes with less pronounced effects in Val/Met heterozygotes, consistent with a gene-dose effect of the Met158 allele on cortisol stress reactivity.
Second, homozygous carriers of the Val158 allele had a symmetrically opposite effect; ELA exposure led to increased cortisol responsivity, consistent with a G x E effect of ELA in COMT Val158Met genotypes. These findings bear comparison with a study of COMT genotype and concurrent life stress in children that found diminished cortisol reactivity in those undergoing greater daily stress exposure but with smaller responses in Val/Val carriers, although no G x E interaction was found (Armbruster et al., 2012). We found no effect of Genotype or any G x E effects in basal cortisol levels, heart rate reactivity or reports of subjective responses to the stressor, providing a specific focus on cortisol reactivity relative to ELA and the COMT genotypes.
Third, the individual’s ELA history was related to an earlier age of experimentation with alcohol and a greater number of drugs, although these risky intake variables did not show an overall G x E interaction. Nonetheless, Met homozygotes experiencing ≥ 2 forms of ELA reported taking a first drink at 14.5 years of age in contrast to their 0- and 1-ELA counterparts, who began drinking 3 years later. A similar effect of ELA on the Met158 genotype occurred in experimentation with recreational drugs. Onset of alcohol consumption prior to age 15, is a significant predictor of future dependence. Sensitivity to the early environment appears to be greater in persons carrying the Met allele relative to their Val/Val counterparts, and the present results suggest that this sensitivity may have implications addiction risk.
Fourth, cortisol reactivity was not directly correlated with either alcohol or drug use indicators. This finding may be useful in considering the mechanisms by which ELA exerts its effects on the stress axis on the one hand and on alcohol and drug use on the other hand. In this case, blunted cortisol reactivity may be a consequence of ELA but may not act as a direct contributor to substance abuse. This is a potentially important finding concerning the linkages between early experience, the stress axis, and health behaviors.
Although much attention has been focused on possible health effects of large stress responses, both exaggerated and diminished cortisol reactivity represent departures from normal homeostatic regulation (Lovallo et al., 2010), with possible consequences for long-term health and behavioral adaptation (Carroll et al., 2009). Given cortisol’s role in regulating activity in the central nervous system, normal cortisol responses to stress are considered central to healthy behavioral adaptation relative to demands from the environment (McEwen, 2007, McEwen and Sapolsky, 1995). Several lines of evidence now point to ELA and diminished cortisol responses as being associated with behavioral disorders including disinhibitory behavioral characteristics and vulnerability to alcohol and other substance use disorders (Errico et al., 1993, Lovallo, 2013, Ehrensaft et al., 2004, Biederman et al., 2002, Eaves et al., 2010, Foley et al., 2004, Enoch, 2011). The present results largely agree with this conclusion but suggest that cortisol actions in the central nervous system are not causally related to substance abuse.
The present results and prior analyses of data from the FHP study suggest multiple effects of ELA in adulthood, irrespective of genotype. ELA contributes to globally diminished cortisol and HR reactivity to stress (Lovallo et al., 2012), altered decision-making and cognitive function (Lovallo et al., 2013), and unstable regulation of affect (Sorocco et al., 2015). We show here that the primary effects of ELA are qualified by genotype-driven sensitivities, some of which specifically implicate glucocorticoid mechanisms and some of which contribute to alcohol use and drug experimentation in adolescence. However, the present data do not point to a direct contribution of blunted cortisol responses to risky drinking and drug use in early adulthood. Instead, blunted cortisol reactivity and early adoption of alcohol and drug experimentation may be independent consequences or ELA in COMT Met-allele carriers.
Perspective and limitations
In designing the FHP study, a primary consideration was to avoid possible central nervous system toxicity associated with prolonged heavy use of alcohol and drugs. We therefore excluded persons with a history of substance dependence based on DSM-IV criteria. The strength and magnitude of relationships reported here should now be tested in persons with a wider range of intake history than in this study cohort. Similarly, the reports of ELA were derived from retrospective self-reports, and as such are subject to recall bias. The present findings, nonetheless provide a scaffolding for evaluating studies in persons with a more severe history of substance exposure.
The genotype effects documented here have small effect sizes. Although the present sample is relatively large for studies of this type, due to the modest effect size, we were unable to pursue potentially fruitful G X G interactions on health and behavior. For example, COMT genotypes may interact with other genetic polymorphisms affecting glucocorticoid regulation (Lovallo et al., 2016) or opioid receptor function (Lovallo et al., 2015). Serotonin receptor polymorphisms may cause variations in cortisol response to acute stress (Way et al., 2016), and a polymorphism in the promoter region of the serotonin transporter gene may modify regulation of affect and overt behavior (Lovallo et al., 2014), again having possible interactiosn with COMT Val158Met genotypes.
This study shares limitations with others of its type. The data set is small for a candidate G x E study and limited statistical power raises the possibility of a Type I error, as has been found in a number of such studies (Dick et al., 2015). This concern is mitigated to a degree by two considerations: (a) Existing evidence suggests a high prior probability that the present G x E finding may be true. (b) We followed the recommendations by Moffitt, Caspi, and Rutter in the G x E design and analysis (Moffitt et al., 2006). (c) Moreover, the G x E effect yielded a partial eta2 = .045, accounting for 4.5% of the variance, suggesting a small-to-medium effect size (Cohen, 1988), boosting confidence in this finding. (4) Finally, the larger sample size in the present analysis builds systematically on the smaller sample in our earlier paper. Nevertheless, we emphasize that the present result should be considered provisional prior to replication with independent datasets and in persons with a wider range of alcohol and drug intake. Due to the sample size, we were unable to test for the simultaneous effects of multiple confounders or pursue promising ELA effects in relation to G x G interactions. The sample was selected for an absence of current psychiatric disorders, and the results therefore apply only to otherwise healthy individuals.
Conclusion
Miller and Chen comment that early experience leaves a “biological residue” that persists into adulthood and may affect systems relevant for health and disease (Miller et al., 2009). A goal of the present analysis is to understand how stress in early life affects persons inheriting COMT Val158Met genotypes. ELA exposure leads to diminished cortisol reactivity in Met allele carriers but causes enhanced reactivity in Val/Val carriers. ELA independently contributes to earlier initiation of alcohol intake and greater drug experimentation. Alcohol and drug intake did not appear to depend on cortisol reactivity.
Supplementary Material
Acknowledgments
This work was supported in part by the U.S. Department of Veterans Affairs Medical Research Service; the National Instututes of Health, AA 12207, and the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism. The content is solely the view of the authors and does not necessarily represent the official view of the National Institutes of Health or the Department of Veterans Affairs.
Footnotes
The authors declare no conflicts of interest.
References
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE (2003) Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J. Neurosci 23:2008–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M (2006) Hypothalamic-Pituitary-Adrenocortical responses to psychological stress and risk for smoking relapse. Int. J. Psychophysiol 59:218–227. [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR (1997) Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology 34:266–275. [DOI] [PubMed] [Google Scholar]
- Alexander N, Klucken T, Koppe G, Osinsky R, Walter B, Vaitl D, Sammer G, Stark R, Hennig J (2012) Interaction of the serotonin transporter-linked polymorphic region and environmental adversity: increased amygdala-hypothalamus connectivity as a potential mechanism linking neural and endocrine hyperreactivity. Biol. Psychiatry 72:49–56. [DOI] [PubMed] [Google Scholar]
- American_Psychiatric_Association (1994) Diagnostic and Statistical Manual of Mental Disorders 4th (text rev.) ed., American Psychiatric Association, Washington, D.C. [Google Scholar]
- Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, Williamson DF (2002) Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr. Serv 53:1001–1009. [DOI] [PubMed] [Google Scholar]
- Armbruster D, Mueller A, Strobel A, Lesch KP, Brocke B, Kirschbaum C (2012) Children under stress - COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. Int J Neuropsychopharmacol 15:1229–1239. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28:403–450. [DOI] [PubMed] [Google Scholar]
- Axelrod J (1957) O-methylation of epinephrine and other catechols in vitro and in vivo. Science 126:400–401. [DOI] [PubMed] [Google Scholar]
- Babor TF, De La Fuente JR, Saunders J, Grant M (1992) AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care, World Health Organization, Geneva, Switzerland. [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Montiero MG (2001) The Alcohol Use Disorders Identification Test: Guidelines for use in primary care 2nd ed., World Health Organization: Department of mental health and substance dependence, Geneva, Switzerland. [Google Scholar]
- Barnett JH, Xu K, Heron J, Goldman D, Jones PB (2011) Cognitive effects of genetic variation in monoamine neurotransmitter systems: a population-based study of COMT, MAOA, and 5HTTLPR. Am J Med Genet B Neuropsychiatr Genet 156:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W (2003) Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27:169–190. [DOI] [PubMed] [Google Scholar]
- Bibbey A, Phillips AC, Ginty AT, Carroll D (2015) Problematic Internet use, excessive alcohol consumption, their comorbidity and cardiovascular and cortisol reactions to acute psychological stress in a student population. J Behav Addict 4:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Monuteaux MC (2002) Differential effect of environmental adversity by gender: Rutter’s index of adversity in a group of boys and girls with and without ADHD. Am. J. Psychiatry 159:1556–1562. [DOI] [PubMed] [Google Scholar]
- Blouin AG, Perez EL, Blouin JH (1988) Computerized administration of the Diagnostic Interview Schedule. Psychiatry Res 23:335–344. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Doornbos B, Ormel J, Oldehinkel AJ (2012) Genetically based reduced MAOA and COMT functioning is associated with the cortisol stress response: a replication study. Mol. Psychiatry 17:119–121. [DOI] [PubMed] [Google Scholar]
- Brody S (2002) Age at first intercourse is inversely related to female cortisol stress reactivity. Psychoneuroendocrinology 27:933–943. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Wong AM, Li Z, Noble EP, Heber D (2013) Association of dopamine D2 receptor and leptin receptor genes with clinically severe obesity. Obesity (Silver Spring) 21:E467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH (2007) Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 62:1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr BR, Parker CR Jr., Madden JD, MacDonald PC, Porter JC (1979) Plasma levels of adrenocorticotropin and cortisol in women receiving oral contraceptive steroid treatment. J. Clin. Endocrinol. Metab 49:346–349. [DOI] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR (2017) The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci. Biobehav. Rev 77:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Lovallo WR, Phillips AC (2009) Are large physiological reactions to acute psychological stress always bad for health? Social and Personality Psychology Compass 3:725–743. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R (2002) Role of genotype in the cycle of violence in maltreated children. Science 297:851–854. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet 75:807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences 2nd ed., Lawrence Erlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Comings DE, Blum K (2000) Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog. Brain Res 126:325–341. [DOI] [PubMed] [Google Scholar]
- Cook EC, Chaplin TM, Sinha R, Tebes JK, Mayes LC (2012) The stress response and adolescents’ adjustment: The impact of child maltreatment. Journal of youth and adolescence 41:1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes MA, Dorn LD, Moss HB, Yao JK, Kirisci L, Ammerman RT, Tarter RE (1999) Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug Alcohol Depend 55:165–176. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, Hewitt JK, Kendler KS, Sher KJ (2015) Candidate gene-environment interaction research: reflections and recommendations. Perspect Psychol Sci 10:37–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR (2006) Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch. Gen. Psychiatry 63:1396–1406. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF (2003) The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev. Med 37:268–277. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Prom EC, Silberg JL (2010) The mediating effect of parental neglect on adolescent and young adult anti-sociality: a longitudinal study of twins and their parents. Behav. Genet 40:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A 98:6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrensaft MK, Moffitt TE, Caspi A (2004) Clinically abusive relationships in an unselected birth cohort: men’s and women’s participation and developmental antecedents. J. Abnorm. Psychol 113:258–270. [DOI] [PubMed] [Google Scholar]
- Enoch MA (2011) The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 214:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D (2009) COMT Val158Met and cognition: main effects and interaction with educational attainment. Genes Brain Behav 8:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, White KV, Waheed J, Goldman D (2008) Neurophysiological and genetic distinctions between pure and comorbid anxiety disorders. Depress. Anxiety 25:383–392. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR (1993) Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J. Stud. Alcohol 54:393–398. [DOI] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Franken IH, Huizink AC (2013) Cortisol levels in children of parents with a substance use disorder. Psychoneuroendocrinology 38:2109–2120. [DOI] [PubMed] [Google Scholar]
- Evans BE, Greaves-Lord K, Euser AS, Thissen S, Tulen JH, Franken IH, Huizink AC (2016) Stress Reactivity as a Prospective Predictor of Risky Substance Use During Adolescence. J Stud Alcohol Drugs 77:208–219. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B (2004) Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch. Gen. Psychiatry 61:738–744. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6:521–532. [DOI] [PubMed] [Google Scholar]
- Hernaus D, Collip D, Lataster J, Ceccarini J, Kenis G, Booij L, Pruessner J, Van Laere K, van Winkel R, van Os J, Myin-Germeys I (2013) COMT Val158Met genotype selectively alters prefrontal [18F]fallypride displacement and subjective feelings of stress in response to a psychosocial stress challenge. PLoS One 8:e65662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Mannisto PT (2010) Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J. Neurochem 114:1745–1755. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Jung-Hoffmann C, Weber J, Boehm BO (1993) The effect of a biphasic desogestrel-containing oral contraceptive on carbohydrate metabolism and various hormonal parameters. Contraception 47:55–68. [DOI] [PubMed] [Google Scholar]
- Li T, Vallada H, Curtis D, Arranz M, Xu K, Cai G, Deng H, Liu J, Murray R, Liu X, Collier DA (1997) Catechol-O-methyltransferase Val158Met polymorphism: frequency analysis in Han Chinese subjects and allelic association of the low activity allele with bipolar affective disorder. Pharmacogenetics 7:349–353. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995) Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry (Mosc) 34:4202–4210. [DOI] [PubMed] [Google Scholar]
- Lovallo WR (2013) Early life adversity reduces stress reactivity and enhances impulsive behavior: implications for health behaviors. Int. J. Psychophysiol 90:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Cohoon AJ, Acheson A, Vincent AS, Sorocco KH (2019) Cortisol stress reactivity in women, diurnal variations, and hormonal contraceptives: studies from the Family Health Patterns Project. Stress:1–7. [DOI] [PMC free article] [PubMed]
- Lovallo WR, Enoch MA, Acheson A, Cohoon AJ, Sorocco KH, Hodgkinson CA, Vincent AS, Glahn DC, Goldman D (2015) Cortisol Stress Response in Men and Women Modulated Differentially by the Mu-Opioid Receptor Gene Polymorphism OPRM1 A118G. Neuropsychopharmacology 40:2546–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Acheson A, Cohoon AJ, Sorocco KH, Hodgkinson CA, Vincent AS, Goldman D (2016) Early-Life Adversity Interacts with FKBP5 Genotypes: Altered Working Memory and Cardiac Stress Reactivity in the Oklahoma Family Health Patterns Project. Neuropsychopharmacology 41:1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Sorocco KH, Vincent AS, Acheson A, Cohoon AJ, Hodgkinson CA, Goldman D (2017) Joint Impact of Early Life Adversity and COMT Val158Met (rs4680) Genotypes on the Adult Cortisol Response to Psychological Stress. Psychosom. Med 79:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Yechiam E, Glahn DC, Acheson A, Sorocco KH, Hodgkinson CA, Kim B, Cohoon AJ, Vincent AS, Goldman D (2014) Differential impact of serotonin transporter activity on temperament and behavior in persons with a family history of alcoholism in the Oklahoma Family Health Patterns Project. Alcohol. Clin. Exp. Res 38:1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS (2013) Early life adversity contributes to impaired cognition and impulsive behavior: studies from the Oklahoma Family Health Patterns Project. Alcohol. Clin. Exp. Res 37:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS (2012) Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma family health patterns project. Biol. Psychiatry 71:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Vincent AS (2010) Use of a resting control day in measuring the cortisol response to mental stress: diurnal patterns, time of day, and gender effects. Psychoneuroendocrinology 35:1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M (1980) Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. J. Psychosom. Res 24:125–130. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR (2005) Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev 29:829–841. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D (2002) A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am. J. Psychiatry 159:652–654. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR (2003) Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 116:127–137. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev 87:873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM (1995) Stress and cognitive function. Curr. Opin. Neurobiol 5:205–216. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS (2009) Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U. S. A 106:14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M (2006) Measured gene-environment interactions in psychopathology. Perspectives in Psychological Science 1:5–27. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP (1999) Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol. Psychiatry 45:1293–1299. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE (2008) Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J. Neurosci 28:5806–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimetrics. High sensitivity salivary cortisol enzyme immunoassay kitMay 17, 2015. Available at: https://www.salimetrics.com/assay-kits.
- Schneider KK, Schote AB, Meyer J, Frings C (2015) Genes of the dopaminergic system selectively modulate top-down but not bottom-up attention. Cogn Affect Behav Neurosci 15:104–116. [DOI] [PubMed] [Google Scholar]
- Schulz S, Arning L, Pinnow M, Wascher E, Epplen JT, Beste C (2012) When control fails: influence of the prefrontal but not striatal dopaminergic system on behavioural flexibility in a change detection task. Neuropharmacology 62:1028–1033. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol 74:1–58. [DOI] [PubMed] [Google Scholar]
- Sorocco KH, Carnes NC, Cohoon AJ, Vincent AS, Lovallo WR (2015) Risk factors for alcoholism in the Oklahoma Family Health Patterns project: impact of early life adversity and family history on affect regulation and personality. Drug Alcohol Depend 150:38–45. [DOI] [PubMed] [Google Scholar]
- Wardle MC, de Wit H, Penton-Voak I, Lewis G, Munafo MR (2013) Lack of association between COMT and working memory in a population-based cohort of healthy young adults. Neuropsychopharmacology 38:1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Brown KW, Quaglia J, McCain N, Taylor SE (2016) Nonsynonymous HTR2C polymorphism predicts cortisol response to psychosocial stress II: Evidence from two samples. Psychoneuroendocrinology 70:142–151. [DOI] [PubMed] [Google Scholar]
- Zhu G, Lipsky RH, Xu K, Ali S, Hyde T, Kleinman J, Akhtar LA, Mash DC, Goldman D (2004) Differential expression of human COMT alleles in brain and lymphoblasts detected by RT-coupled 5’ nuclease assay. Psychopharmacology (Berl) 177:178–184. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D (2003) COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science 299:1240–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


