Abstract
It is well established that GABA receptors at the central terminals of primary afferent fibers regulate afferent input to the superficial dorsal horn. However, the extent to which peripheral GABA signaling may also regulate afferent input remains to be determined.
The colon was used to explore this issue because of the numerous endogenous sources of GABA that have been described in this tissue. The influence of GABA signaling on colonic afferent excitability was assessed in an ex vivo mouse colorectum pelvic nerve preparation where test compounds were applied to the receptive field. The visceromotor response (VMR) evoked by noxious colorectal distention, was used to assess the impact of GABA signaling on visceral nociception, where test compounds were applied directly to the colon.
Application either GABAA or GABAB receptor agonists attenuated the colonic afferent response to colon stretch. Conversely, GABAA and GABAB receptor antagonists increased the stretch response. However, while the noxious distension-induced VMR was attenuated in presence of GABAA and GABAB receptor agonists, the VMR was only consistently increased by GABAA receptor antagonists.
These results suggest that GABA receptors are present and functional in the peripheral terminals of colonic afferents and activation of these receptors via endogenous GABA release contributes to the establishment of colonic afferent excitability and visceral nociception. These results suggest that increasing peripheral GABA receptor signaling could be used to treat visceral pain.
Keywords: sensory neuron, extrinsic innervation, sensory transduction, sensitization
INTRODUCTION
There is evidence to suggest that GABA receptors are not only present but functional in peripheral terminals of nociceptive afferents (Carlton et al., 1999; Castro et al., 2017). While a low concentration (2 μM) of the GABAA receptor agonist, muscimol, in the skin appears to have no detectable impact of baseline nociceptive threshold, this concentration of agonist attenuates formalin-induced nociceptive behavior. Interestingly, muscimol at a higher concentration (1 mM) not only potentiated formalin-induced nociceptive behavior, but produced heat hypersensitivity (Carlton et al., 1999). The GABAA receptor antagonist bicuculline was shown to block the effects of both low and high concentrations of muscimol. However, the effects of bicuculline alone were not assessed in this study, so it remains to be determined whether endogenous GABA influences the excitability of cutaneous afferents, let alone, whether there are sources of GABA in the skin that could underlie this form of endogenous control of nociceptive signaling.
In contrast to the skin, several sources of endogenous GABA have been identified in the gastro-intestinal (GI) tract (Grider & Makhlouf, 1992; Krantis et al., 1994; Grider, 1998; Wang et al., 2006). Consistent with the suggestion that there may be sufficient levels of GABA in the GI tract to engage GABAA and GABAB receptors in the absence of tissue injury, all the components necessary for GABA signaling have been identified in GI tissue: GABA transporters (GAT1–3, (Fletcher et al., 2002)), GABA synthetic enzymes (GAD65 and GAD67, (Miki et al., 1983; Wang et al., 2006)), GABA catabolic enzyme (GABA transaminase, (Miki et al., 1983; Nichols et al., 1995)) and the release of GABA in colon tissue (Kerr & Krantis, 1983). Nevertheless, the only evidence for endogenous GABAA signaling in this tissue is also in the presence of inflammation, where GABAA receptor activation of colon epithelium was shown to exacerbate inflammatory damage within the colon (Ma et al., 2018). Similarly, while several lines of evidence suggest GABAB receptors are present and functional in the GI tract, where GABAB agonists are not only analgesic but have shown to be an effective strategy for the attenuation of visceral hypersensitivity (Castro et al., 2017; Castro et al., 2018; Sadeghi et al., 2018), these receptors do not appear to be engaged by endogenous GABA. That is, there is not a detectable influence of GABAB antagonists on the excitability of healthy nociceptive afferents (Sengupta et al., 2002; Castro et al., 2017). Thus, it remains to be determined, the extent to which endogenous GABA engages these receptors as a means of controlling the excitability of nociceptive afferents in the absence of tissue injury in a manner comparable to this essential role so well characterized in the central nervous system (CNS).
We sought to further explore this issue in the present study. To do so, we utilized an ex vivo mouse colon rectum–pelvic nerve preparation in combination with an in vivo assay of visceral nociception (the visceromotor response (VMR) evoked with balloon distention of the colon). GABA receptor agonists and antagonists were applied either to the receptive field of functionally identified colonic afferents, or intraluminally. Our results suggest that both receptor subtypes contribute to the establishment of colon afferent excitability and nociception, raising the intriguing possibility that approaches to selectively increase peripheral GABA receptor signaling could be used to treat visceral pain without CNS side effects.
METHODS
Ethical approval
All experiments were approved by and performed in accordance with the guidelines of the University of Pittsburgh Institutional Animal Care and Use Committee. This committee is charged with ensuring that investigators at the University of Pittsburgh follow the policies and guidelines governing the use of animals in research established by the United States Animal Welfare Act, the United States Public Health Service, and the United States Office of Laboratory Animal Welfare. The protocol covering the experiments described in this study was originally approved in 2015 (protocol #15076413) and then reapproved in 2018 (protocol #18063260). This study was performed in accordance with the guidelines established by the by the United States National Institutes of Health for the use of animals in research. The authors understand the ethical principals under which the Journal of Physiology operates, and ensure that this study complies with the animal ethics checklist laid out by Grundy (2015). All attempts were made to both minimize the number of mice used in this study and any pain or distress associated with the procedures performed.
Animals
A total of 188 adult (7–9 week) male and female C57BL/6J mice (Taconic Biosciences, Germantown, NJ) were used in all experiments. Mice were housed by sex in groups of four (males) to five (females), in a temperature and humidity controlled Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facility, with food and water available ad libitum, in rooms on a 12:12:light:dark schedule with lights on at 7:00 AM. No animals were removed from the study.
Ex vivo preparation and single fiber recordings
The ex vivo preparation and the single fiber recordings were done as previously described in detail (Feng & Gebhart, 2015). Mice were killed with isoflurane 5%, and the colorectum in continuity with the pelvic nerve was removed and cleaned of connective tissue. The colorectum was opened and pinned, luminal side up in a Sylgard (Dow Corning Corp., Midland, MI)-lined organ bath consisting of two adjacent chambers. The colorectum was superfused with oxygenated Krebs solution at 30–32̊ C (in mM: 117.9 NaCl, 4.7 KCl, 25 NaH2CO3, 1.3 NaH2PO4, 1.2 MgSO4*7H2O, 2.5 CaCl2, 11.1 D-glucose, 2 sodium butyrate and 20 sodium acetate) to which the L-type calcium channel antagonist, nifedipine (1 μM), and the prostaglandin synthesis inhibitor, indomethacin (3 μM), were added. The pelvic nerve was threaded through a grease gap into a mineral oil filled chamber. Using a dissecting microscope, the nerve sheath was carefully peeled back, and the nerve trunk was split into fine fascicles for subsequent single unit recording. If more than three units were present in a fascicle, it was further divided until one to a maximum of three clearly discriminable units were present.
Characterization of muscular colonic afferents
The preparation was allowed to rest for 60 min before recording was initiated. An electrical stimulus (0.5-ms duration, 0.3 Hz) using a round-tipped concentric electrode (external diameter: 0.55 mm and internal diameter: 0.125 mm, FHC, Bowdoin, ME) perpendicular to the mucosal surface, was used to search for receptive fields. These were localized as the site requiring the lowest stimulus intensity to evoke an action potential. After that, the isolated units were characterized as muscular colonic afferents according to their responses to probing with von Frey-like nylon monofilaments (0.01, 0.4 and 1g) and circumferential stretch (0–170 mN, 58 s) using a servo-controlled force actuator (Aurora Scientific, Aurora, ON, Canada, Figure 1A).
Figure 1. In vitro mouse colon-pelvic nerve preparation.
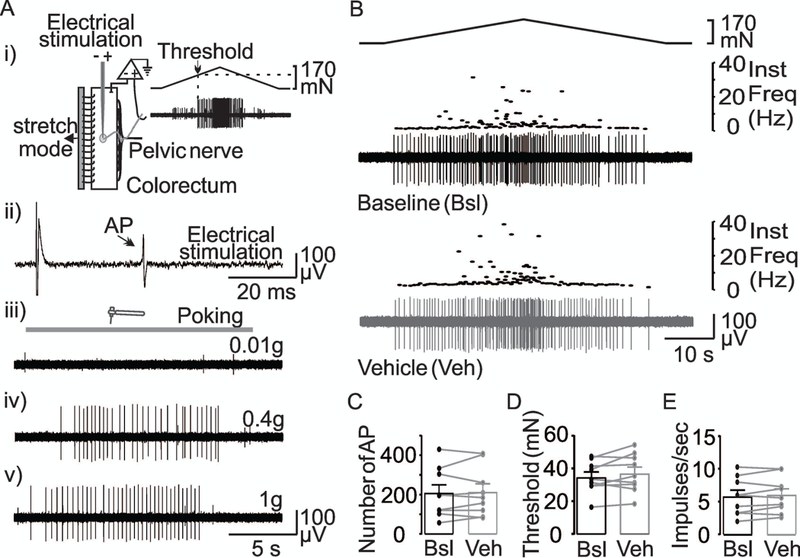
(A) (i) A cartoon of the ex vivo colorectum preparation showing that the receptive field of the colonic afferents was identified by an electrical search stimulus (ii). The unit was further characterized by responses to probing with von Frey-like nylon monofilaments of 0.01,0.4, 1g (iii, iv, and v). Finally, ramped circumferential stretch was applied to the colon (i) to activate muscular afferents (trace in Ai). Colonic muscular afferents were responsive to stretch and von Frey-like nylon monofilaments of 0.4 and 1g. (B) The response of a muscular colonic afferent to stretch (top trace) before (Baseline-Bsl) and after vehicle (DMSO 0.1%) application to the receptive field. Instantaneous firing frequency is plotted above the activity of the isolated unit. The stability of the response to repeated stretch is illustrated in pooled data for the number of action potentials (C), threshold (D), and mean peak firing frequency (E) from units (n = 10) tested before and after the application of vehicle. p>0.05, paired t-Test.
Chemical Application to the Receptive Ending
All the drugs were added directly to the receptive field. The bottom edge of a piece of a brass square chamber (8 mm high and 4 mm along each side) was covered with grease prior to its placement over the receptive field. To be sure that this temporary receptive field isolation chamber encompassed the receptive field, a von Frey-like nylon monofilament (1g) was used to probe inside and outside of the chamber. After establishing a stable baseline to the stretch stimulus, the Krebs solution inside the receptive field chamber was removed and replaced with 150 μL of test solution containing vehicle or test compounds.
Data analysis of single afferent fiber recordings
The stretch stimulus was applied in triplicate every four min before and after the application of test solutions to the receptive field. Action potentials evoked during the stimulus were recorded using a low-noise AC differential amplifier (DAM80; World Precision Instruments). The electrical signals were differentially amplified (10,000X), filtered (0.3 to 10 kHz band pass), sampled at 20 kHz with a 1401 interface (Cambridge Electronic Design, Cambridge, UK), and stored in a PC for analysis off line.
Action potentials were analyzed offline using the Spike 2 (Cambridge Electronic Desing, Cambridge, UK) wave mark function which employs principal component analysis to differentiate action potentials based on spike waveform. It was therefore possible to discriminate single action potentials in fascicles in which more than one unit was present. Nevertheless, to minimize potential errors in action potential discrimination, fascicles were only included in subsequent analysis of no more than two easily discernable units were detectable.
The number of action potentials evoked during the stimulus, the threshold (in mN) to evoke the first action potential (Figure 1Ai) and the mean the firing frequency at the peak stimulus intensity were quantified (Spike 2, Cambridge Electronic Desing, Cambridge, UK) before and after test agents were applied. In some cases, the data were normalized with respect to mean of the baseline response, determined from the last three stimuli applied before test solution application. The instantaneous firing frequency (IF) was calculated for each unit throughout the stimulation period (58 s). The mean firing frequency at the peak stimulus intensity was calculated as the mean IF of action potential generated from 0.5 s before to 0.5 s after the maximal stretch of the colon.
While our intention was to follow units before, during, and after washout of test agents, we ultimately chose not to systematically analyze the reversibility of test agents, this was due to lengthy washout periods (>60 minutes) and we were unable to confidently distinguish the reversal process from time dependent changes in the evoked response.
Visceromotor response (VMR) recordings
Mice were anesthetized with urethane (IP,1.2g/Kg). The depth of anesthesia was monitored with respiration rate, heart rate, and the response to noxious pinch of the hindpaw. A balloon, made from polyethylene (length, 1.5 cm; diameter, 0.9 cm) affixed to the end of PE-60 tubing, was used to distend the colon. The balloon was inserted transanally until the proximal end of the balloon was 0.5 cm from the anal verge (total balloon insertion = 2 cm) and secured to the mouse tail with tape. Balloon distention of the colon to 60 mmHg, referred to as colorectal distention (CRD), was applied for 10 s every four min. The response to three stimuli were collected before and after infusion of test agents. The CRD balloon was designed to enable infusion of the test agents rostral to the balloon using a catheter (PE-10 tubing) running parallel of PE-60 tubing through the balloon and finishing 2 mm outside rostral of the balloon. The volume of each intracolonic infusion was 0.1 ml and was given immediately after the last of the three baseline distensions. We chose not to collect full stimulus response curves to minimize the number of times the colon would have to be stimulated before and after the infusion of test agents, as well as to minimize the possibility of changes in the response to the bolus infusion of a test agent over time.
Abdominal muscle contraction, referred to as the VMR, evoked in response to CRD was measured with EMG electrodes implanted in the abdominal muscle wall. The VMR activity was amplified (10,000X), filtered (0.3 to 10 kHz band pass), sampled at 20 kHz with 1401 interference (Cambridge Electronic Desing, Cambridge, UK) and stored in a PC for analysis off line. Using the Spike 2 software, the EMG waveform was rectified, and the integral of the resting activity subtracted waveform was used to quantify the VMR, where resting activity was the activity in the 10 sec prior to distention. VMR data collected after the infusion of the test compounds were analyzed as a percent change from the average of the three responses just prior to the infusion of test compounds. Following completion of the VMR experiments, mice were killed by decapitation.
Drugs used
GABA (100 – 400 μM) used to activate GABAA and GABAB receptors, was obtained from Sigma-Aldrich (St. Louis, MO). GABAA receptors were selectively activated with muscimol (1–100 μM) and blocked with the antagonist, bicuculline (100 μM), or the blocker, picrotoxin (100 μM). These GABAA related compounds were obtained from Sigma-Aldrich (St. Louis, MO). Likewise, GABAB receptors were activated with baclofen (100 – 200 μM) and blocked with the antagonist, CGP55845 (5 μM). These compounds were obtained from Tocris (Minneapolis, MN). GABA, muscimol, bicuculline and baclofen were dissolved in distilled water at 100 mM. Picrotoxin and CGP55845 were dissolved in DMSO at 100 mM and 5 mM, respectively. Stock solutions were stored at −20 °C. On the day of an experiment, the stock solution was diluted to the final concentration in freshly oxygenated Krebs solution. For picrotoxin and CGP55845 the final concentration of DMSO was 0.1%.
Statistics
Statistical analyses (SigmaStat V 3.5, Systat Software Inc, Chicago IL) of the data included Chi-Square and/or Fisher’s Exact test, t-test/Mann-Whitney Rank Sum test, two-way mixed design ANOVAs, and one-way ANOVAs. The non-parametric tests were used to assess differences in the proportion of units impacted by test agents. The t-test/ Mann-Whitney Rank Sum was used to assess differences between male and female mice with respect to baseline excitability. Two-way mixed design ANOVAs were used to assess influence of sex on the response evoked before and after application of test agents. One-way ANOVAs were used to compare the magnitude of responses to test agents, where the magnitude of the response was calculated as a percent change from baseline. The Holm-Sidak test was used for post-hoc testing; p≤0.05 was considered as statistically significant. Pooled data are plotted as means ± the standard error of the mean (SEM).
RESULTS
In total, 123 mechanosensitive pelvic afferents were studied from male (n = 66) and female (n = 57) mice. All the afferents included in this study were considered muscular afferents (Brierley et al., 2004), because they responded to punctate mechanical stimuli (von Frey-like nylon monofilaments of 0.4g and 1g, Figure 1Aiv-v) as well as to circumferential stretch of the colorectum (Figure 1Ai), but did not respond to brushing or very low intensity punctate stimuli (von Frey-like nylon monofilament of 0.01g) (Figure 1Aiii). There were no differences between male and female mice with respect to any of the measures of baseline excitability used: the number of evoked action potentials was 165 ± 29.7 and 149 ± 29.3 (p = 0.71, Students t-Test), the response threshold was 33.8 ± 2.2 mN and 37.2 ± 2.9 mN in fibers from males and females (p = 0.35, Students t-Test), and the mean firing frequency at peak stimulus intensity was 5.27 ± 0.67 Hz and 5.07 ± 0.72 Hz in males and females, respectively (p = 0.83, Students t-Test).
Because the standard protocol involved repeatedly stimulating colonic afferents, we first sought to determine whether there were time-dependent changes in any of the three measures of excitability assessed in response to stretch of the colon, or changes associated with isolation and/or replacement of solutions of in the receptive field with the brass chamber. Muscular colonic afferents from both male (n = 5) and female (n = 5) mice were studied in this way. Results of a two-way RM ANOVA revealed no significant influence of sex (p = 0.44, 0.47), time/solution exchange (p = 0.85, 0.20), nor a significant interaction (p = 0.80, 0.50) between the two with respect to the number of evoked action potentials, the threshold for activation, or the mean firing frequency at peak stimulus intensity. The mean change in action potential number in response to time/vehicle change over the receptive field was 11 ± 13% (n = 10), that for threshold was 4 ± 4%, while that for mean peak firing frequency was 4 ± 1%. Furthermore, we considered a neuron a “responder” to a solution change, if the change in the number of evoked action potentials, threshold, or mean peak firing frequency was greater than two standard deviations from the average baseline response. Based on this criterion, we would have considered two (one from a male and one from a female) of the 10 muscular colonic afferents treated with vehicle as responders: an increase in the number of evoked action potentials was observed in the male unit and a decrease was observed in the female unit; no changes in threshold or mean peak firing frequency were observed in any vehicle treated units (Figure 1B).
Of note, to maximize the likelihood of detecting a change, the vehicle used for these control experiments contained 0.1% DMSO, the vehicle used for several test agents. As further evidence of the stability of recordings in the colon-nerve preparation, as well as the degree to which the receptive field was isolated by the brass chamber, we collected data from five fascicles as illustrated in Figure 2A, where there were two units, in which one had a receptive field outside the brass chamber. Importantly, despite marked changes in the response properties of the unit with its receptive field inside the brass chamber, pooled data from units with receptive fields outside the brass chamber (n = 5) indicated that there were no time-dependent changes in response to stretch. That is, as a percent change from baseline, the change in: AP number was 2 ± 2%, in threshold was 12 ± 3%, and in mean peak frequency was 1 ± 0.32%. Thus, the response of muscular colonic afferents is relatively stable, enabling us to detect the influence of test agents with accuracy.
Figure 2. Effect of GABA on stretch-sensitive afferents.
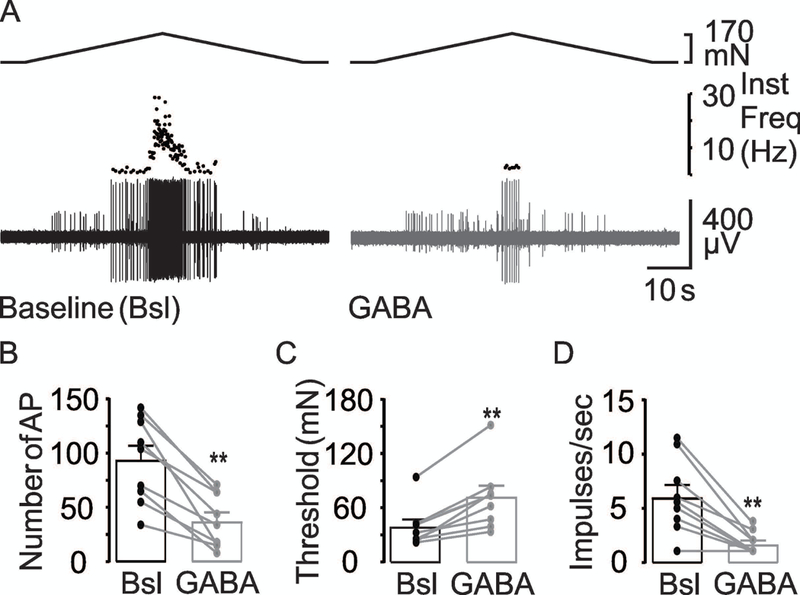
(A) The response of a muscular colonic afferent to stretch before (Baseline-Bsl) and after GABA (GABA 100 μM). The inhibitory influence of GABA on the excitability of colonic afferents is illustrated in pooled action potential number (B), threshold (C) and mean peak firing frequency (D) data from units (n = 9) tested before and after the application of GABA (100 μM). ** is p<0.01, paired t-Test.
GABA decreases the excitability of stretch-sensitive colonic afferents
We first sought to determine whether exogenous application of GABA influenced the excitability of colonic afferents. As seen in the typical response of a colonic afferent to circumferential stretch of the colon, GABA (100 μM) was associated with a decrease in the number of evoked action potentials, an increase in the threshold at which the first action potential was evoked, and a decrease in the mean peak firing frequency compared with their baseline response (Figure 2). Results of a two-way RM ANOVA revealed a significant effect of GABA (p<0.001), but no significant influence of sex (p = 0.67), nor a significant interaction between sex and GABA application (p = 0.51).
Pooled data analyzed as a percent of baseline indicated that GABA was associated with more than a 60% (39.3 ± 5.7 of baseline) decrease in the number of evoked action potential, close to a 100% increase in threshold (192.6 ± 20.6%), and close to an 70% decrease in the mean peak frequency (28.3 ± 5.4%). Nine of 9 fibers tested with GABA demonstrated a decrease in the number of evoked action potentials, an increase in threshold, and a decrease in mean peak frequency. These results suggest GABA receptors are present and functional in the peripheral terminals of colonic afferents.
Baclofen reduces the excitability of stretch-sensitive colonic afferents
Because both GABAA and GABAB receptors appear to be present on primary afferent peripheral terminals (Carlton et al., 1999; Castro et al., 2017), either may have mediated the inhibitory effect of GABA on colonic afferents. We started with GABAB receptors because Castro and colleagues (2017) recently reported that activation of this receptor subtype inhibited the mechanical response of colonic afferents. Our results with the GABAB agonist, baclofen, were consistent with this previous observation (Figure 3). Baclofen (100 μM) decreased the number of action potentials, increased the threshold to produce the first action potential and decreased the mean peak firing frequency in both male and female colonic afferents. Results of a two-way RM ANOVA revealed a significant effect of baclofen on the number of evoked action potentials (p = 0.002), activation threshold (p< 0.001), and mean peak firing frequency (p = 0.009), but no significant influence of sex on any parameter: p = 0.81, 0.79, and 0.87 for AP number, threshold, and mean peak firing frequency, respectively. However, while there was no significant interaction between sex and baclofen application for AP number, (p = 0.91) or peak firing frequency, (p = 0.75), there was a significant interaction between these parameters for threshold (p = 0.04). This latter result was likely due to the relatively larger influence of baclofen on threshold of colonic afferents from females.
Figure 3. Baclofen attenuates muscular colonic afferent excitability.
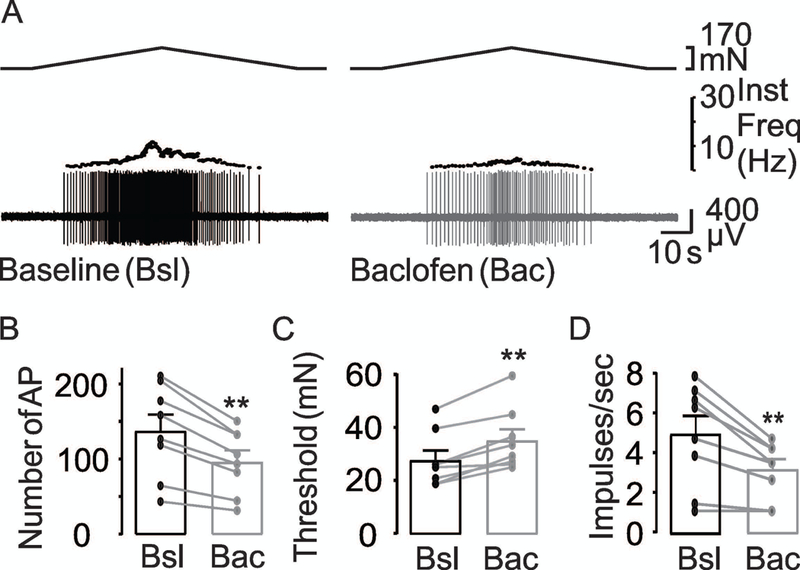
(A) Stretch-evoked response of a muscular colonic afferent before (Baseline, black trace) and after (Baclofen, gray trace) the application of baclofen (100 μM). The inhibitory influence of baclofen on the excitability of colonic afferents is illustrated in pooled action potential number (B), threshold (C) and mean peak firing frequency (D) data units (n = 8) tested before (Bsl) and after application of baclofen (Bac). ** is p<0.01, paired t-Test.
Pooled data analyzed as a percent of baseline indicated that baclofen was associated with close to a 30% (70.7 ± 1.3% of baseline) decrease in the number of evoked action potentials, close to a 30% increase in threshold (128 ± 5.4%), and close to an 35% decrease in the mean peak frequency (64.9 ± 9.9%). Six of 8 fibers tested with baclofen demonstrated an increase in threshold, four of eight fibers exhibited a decrease in action potential number, while six of eight fibers showed a decrease in mean peak firing frequency. Taken together, while these results suggest GABAB receptors may have contributed to the response observed with GABA, it is likely that another receptor subtype (i.e., GABAA) contributed as well.
Muscimol decreases the excitability of stretch-sensitive colonic afferents
We next sought to determine the extent to which GABAA receptor activation might also contribute to the suppression of colonic afferent excitability observed with GABA. Typical examples of the effect of muscimol on stretch-evoked activity in a colonic afferent are shown in Figure 4. Muscimol decreased the number of action potentials, increased the threshold, and decreased the mean peak firing frequency in colonic afferents. Results of a two-way RM ANOVA revealed a significant effect of muscimol (100 μM) on the number of evoked action potentials (p = 0.008), threshold (p < 0.001), and mean peak firing frequency (p < 0.001), but no significant influence of sex (p= 0.56, 0.68 or 0.80 respectively) nor a significant interaction between sex and muscimol application (p = 0.68, 0.46 or 0.36 respectively) on any parameter. Pooled data analyzed as a percent of baseline indicated that muscimol (100 μM) was associated with more than a 50% (47.8 ± 4.4% of baseline) decrease in the number of evoked action potentials, more than a 100% increase in threshold (222.8 ± 15.9%), and close to an 38% decrease in the mean peak frequency (61.3 ± 8%).
Figure 4. Inhibitory effect of muscimol on colonic afferent excitability.
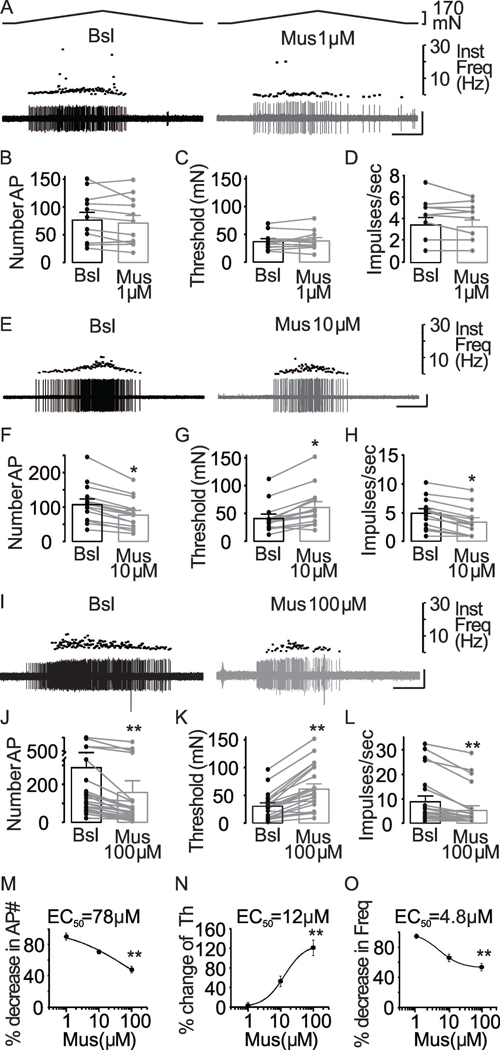
(A) Typical responses of muscular colonic afferents to stretch before (black traces) and after (gray traces) application of muscimol (1μM). Scale in these and subsequent traces is 10 sec by 200 μV. The influence of 1 μM muscimol on the excitability of colonic afferents is illustrated in pooled action potential number (B), threshold (C) and mean peak firing frequency (D) data from units (n = 12) before (Bsl) and after application of muscimol (Mus 1μM). p > 0.05, paired t-Test. (E) Representative responses of muscular colonic afferents to stretch before (black traces) and after (gray traces) application of muscimol (10 μM). The influence of 10 μM muscimol on the excitability of colonic afferents is illustrated in pooled action potential number (F), threshold (G) and mean peak firing frequency (H) data from units (n = 14) before (Bsl) and after application of muscimol (Mus 10 μM). * is p < 0.05, paired t-Test. (I) Typical responses of muscular colonic afferents to stretch before (black traces) and after (gray traces) application of muscimol (100 μM). The influence of 100 μM muscimol on the excitability of colonic afferents is illustrated in pooled action potential number (J), threshold (K) and mean peak firing frequency (L) data from units (n = 23) before (Bsl) and after application of muscimol (Mus 100 μM). ** is p < 0.01, paired t-Test. Each set of traces is from a different mouse. Muscimol concentration-response data for AP number (M), threshold (Th) (N), and mean peak firing frequency (Freq) (O), calculated as percent of baseline, were fitted with modified Hill equations in order to estimate EC50. ** is p < 0.01.
The muscimol-induced suppression of colonic afferent excitability was concentration-dependent. Twenty-one of 23 fibers tested with 100 μM muscimol demonstrated a decrease in the number of evoked action potentials, an increase in threshold, and a decrease in the mean peak firing frequency, while only a decrease in action potential number was observed in the remaining two fibers. In contrast, only one of 12 fibers tested with 1 μM muscimol demonstrated a decrease in AP number, an increase in threshold, and a decrease in mean peak firing frequency, while only a decrease in AP number was observed in one fiber and a decrease in threshold in two with no changes in mean peak frequency. When data were analyzed as a percent decrease in AP number (AP#Drug/AP#Baseline)x100, a percent increase in threshold (ThresholdDrug/ThresholdBaseline)x100, or a percent decrease in mean peak firing frequency (mean peak frequency Drug/mean peak frequency Baseline)x100 data were well fitted with a modified Hill equation, yielding EC50 values of 12 μM, 78 μM and 4.8 μM (Figure 4M-O). Taken together, these data are consistent with the presence of functional GABAA receptors on colonic afferents.
GABA receptor antagonists reveal a role for endogenous GABA in the regulation of colonic afferent excitability
Given evidence of functional of GABAA and GABAB receptors on the peripheral terminals of colonic afferents, we next sought to determine whether either receptor subtype is activated by endogenous GABA to influence baseline excitability of these neurons. To address this question, we used receptor antagonists in a protocol comparable to that used for the agonists (Figure 5). The GABAB receptor antagonist, CGP55845 (CGP), was used at 5 μM (Castro et al., 2017). While there appeared to be a small influence of CGP on AP number (Figure 5Ai vs 5Aii, and 5B) and mean peak firing frequency (Figure 5Ai vs 5Aii, and 5D), the influence on threshold was more apparent (Figure 5Ai, 5Aii, and 5C). Consistent with this impression, results of a two-way RM ANOVA revealed a significant influence of CGP on threshold (p = 0.003), but only a trend with respect to the impact of CGP on AP number (p = 0.09) and mean peak firing frequency (p = 0.12). Furthermore, there was no significant influence of sex on any parameter: p = 0.17, 0.37, and 0.16 for AP number, threshold, and mean peak firing frequency, respectively. Nor was there was a significant interaction between sex and CGP application for AP number (p = 0.91) or mean peak firing frequency (p = 0.42). However, there was a trend toward a significant interaction between sex and CGP application for threshold (p = 0.06). This was likely due to the higher baseline threshold in the group of colonic afferents from female mice, rather than an effect of CGP.
Figure 5. Effect of GABAB receptor antagonist on muscular colonic afferent excitability.
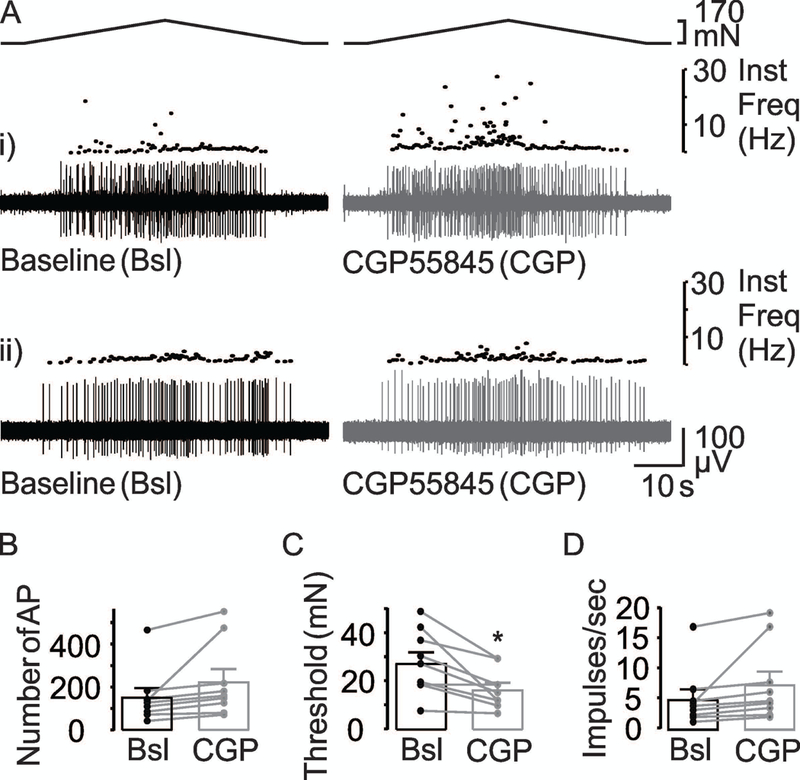
(A) Response of two muscular colonic afferents that responded differently to CGP55845 (CGP, 5 μM) with respect to changes in the number of stretch-evoked AP, threshold, and the mean peak firing frequency: (i) An increase in the stretch-evoked response in presence of CGP and (ii) no change in the stretch-evoked response in the presence of CGP. Baseline (Bsl) responses are shown in black while the response in the presence of CGP is shown in grey. The influence of 5 μM CGP on the excitability of colonic afferents is illustrated in pooled action potential (B), threshold (C) and peak mean firing frequency (D) data from units (n = 8) before (Bsl) and after application of the antagonist. * is p<0.05, paired t-Test.
Pooled data analyzed as a percent of baseline indicated that CGP was associated with close to a 50% (147 ± 37.6% of baseline) increase in the number of evoked action potentials, more than a 35% decrease in threshold (64.9 ± 7.0%), and close to an 46% increase in the mean peak frequency (145 ± 24.3%). Eight of 9 fibers tested with CGP demonstrated an increase in AP number and a decrease in threshold, but only in six fibers an increase in mean peak firing frequency, while no change in any parameter was observed in the remaining fiber. These results suggest that there is constitutive GABAB receptor activation in the colon that is involved in establishing the excitability of colonic afferents.
To assess the involvement of GABAA receptor activation in the regulation of colonic afferent excitability, we evaluated the effects of the GABAA receptor antagonist (bicuculline) and the blocker (picrotoxin). Bicuculline (100 μM) was associated with a dramatic increase in AP number (Figure 6A, B), decrease in threshold (Figure 6C), as well as an increase in mean peak firing frequency (Figure 6D). This was confirmed with two-way RM ANOVA of data from male and female mice, which revealed a significant effect of bicuculline on the number of evoked action potentials (p < 0.001), threshold (p < 0.001), and mean peak firing frequency (p <0.001), but no significant influence of sex (p = 0.87, 0.36, 0.62) nor a significant interaction between sex and bicuculline application (p = 0.93, 0.68, 0.19) on any parameter.
Figure 6. Effect of GABAA receptors antagonists on muscular colonic afferent excitability.
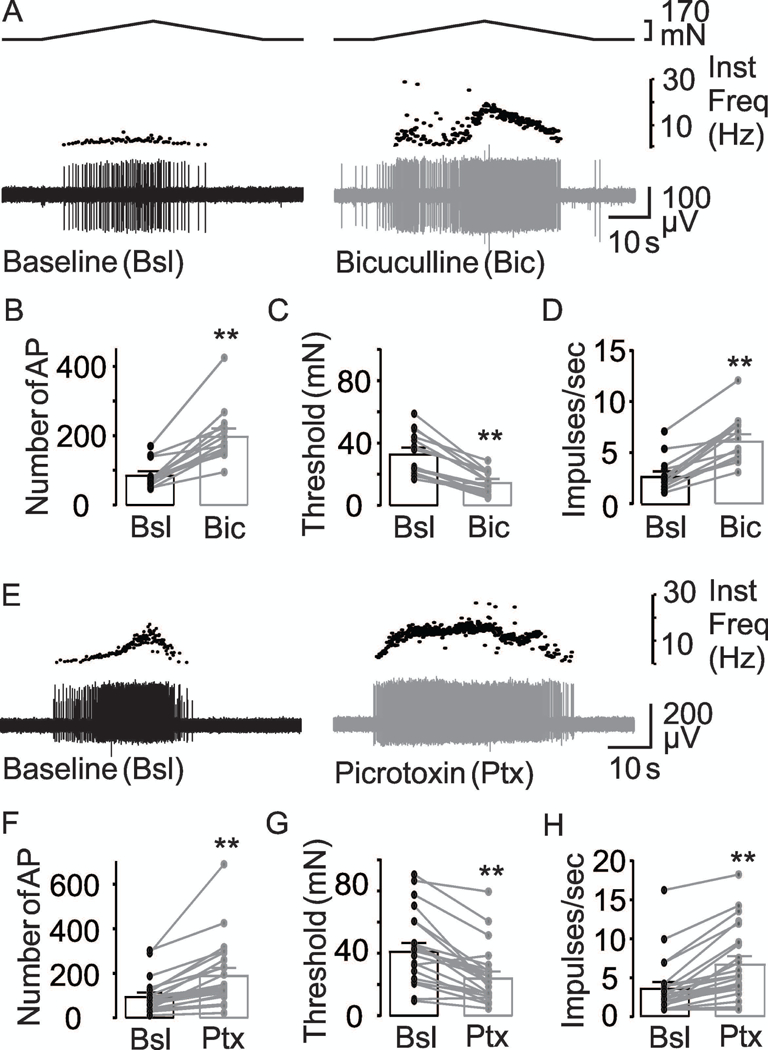
(A) Typical responses of muscular colonic afferents to stretch before (black traces) and after (gray traces) application of the GABAA receptor antagonist, bicuculline, or channel blocker, picrotoxin (E). The influence of 100 μM bicuculline (Bic) on the excitability of colonic afferents is illustrated in pooled action potential number (B), threshold (C) and mean peak firing frequency (D) data from units (n = 14) tested before (Bsl) and after application of the antagonist. ** is p<0.01, paired t-Test (n = 14). The GABAA channel blocker picrotoxin (Ptx) had an influence similar to that of bicuculline on action potential number (F), threshold (G), and mean peak firing frequency (H). ** is p<0.01, paired t-Test (n = 23).
Pooled data analyzed as a percent of baseline indicated that bicuculline (100 μM) was associated with almost a 150% (243.5 ± 20.7% of baseline) increase in the number of evoked action potentials, more than a 50% decrease in threshold (44.9 ± 3.6%), and close to an 128% increase in the mean peak frequency (227.9 ± 15.7%). Thirteen of 14 fibers tested with bicuculline demonstrated an increase in AP number, decrease in threshold, and increase in mean peak firing frequency, while only an increase in AP number was observed in the remaining fiber.
Consistent with the results with bicuculline, picrotoxin (100 μM) was also associated with an increase in AP number (Figure 6E, F), decrease in threshold (Figure 6G), and an increase in mean peak firing frequency (Figure 6H). Pooled data analyzed as a percent of baseline indicated that picrotoxin (100 μM) was associated with more than a 100% (208.2 ± 15.8% of baseline) increase in the number of evoked action potentials, more than a 40% decrease in threshold (59.7 ± 5.0%), and close to an 85% increase in the mean peak frequency (184.04 ± 13%). Eighteen of 23 fibers tested with picrotoxin demonstrated an increase in AP number, a decrease in threshold, and an increase in mean peak firing frequency, while an increase in AP number and increase in mean peak firing frequency only was observed in the remaining five fibers. Together, these results suggest that there is constitutive GABAA receptor activation in the colon that is involved in establishing the excitability of colonic afferents.
Latency of electrically evoked action potential is modulated by GABA receptors
While we focused on the actions of GABA on peripheral terminals, the previous observation that GABA application to the vagus nerve reduces the conduction velocity of the compound action potential (Brown & Marsh, 1978) suggests there are also functional GABA receptors on peripheral axons. To begin to address this possibility in colonic afferents, we evaluated the latency of the electrically evoked action potential recorded in the pelvic nerve following application of GABA or picrotoxin. The latency was defined as the time from stimulus application to peak of the evoked spike. Consistent with the presence of functional channels that are constitutively active, 400 μM of GABA increased the spike latency while picrotoxin 100 μM decreased it (Figure 7A). Pooled data analysis confirmed these changes were significant (Figure 7B).
Figure 7. Influence of GABA and picrotoxin on electrically evoked action potentials.
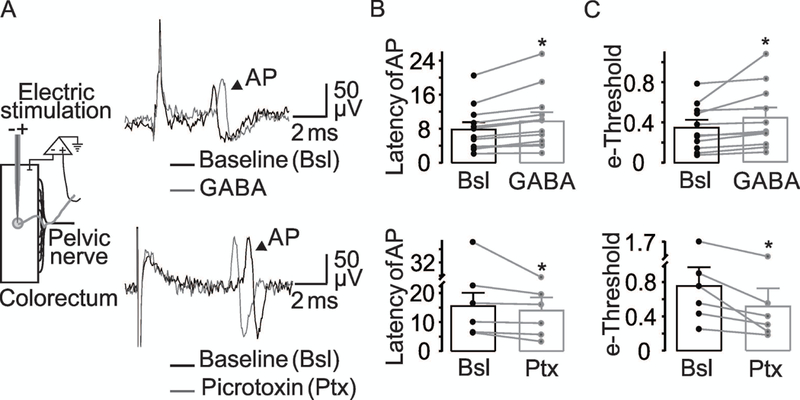
(A) A cartoon of the experimental set-up is shown at left. Voltage traces are examples of an evoked action potential evoked before (black) and after (gray) application of GABA (top traces) or picrotoxin (bottom traces). The arrowhead indicates the action potential (AP). (B) Pooled electrically evoked AP latency data before and after of GABA 400 μM (p = 0.03 paired t-Test, n = 12) or picrotoxin 100 μM (p = 0.03, paired t-Test, n = 6). (C) The electrical threshold was determined with a bipolar electrode placed in the center of the receptor field. The least current (mA) needed to evoke an action potential (e-threshold) was determined before and after application of GABA (p = 0.05, paired t-Test, n = 12) or picrotoxin (B, p = 0.02, paired t-Test, n = 6).
Changes in electrical threshold of colonic afferents induced by GABA receptors agonist and antagonist
To begin to determine whether GABA may act directly on colonic afferents, we evaluated the effect of GABA and picrotoxin on electrical threshold of the evoked action potential. Consistent with the suggestion that the GABA-induced changes in excitability are due to a direct effect on the colonic afferent, GABA (400 μM) applied to the bath solution significantly increased the electrical threshold (Figure 7C), and picrotoxin (100 μM) decreased the electrical threshold (Figure 7C). Together, these results suggest that the bi-directional regulation of colonic afferent excitability is due to the presence of GABAA receptors on colonic afferents.
Effect of peripheral GABA receptors agonists on VMR
To determine the functional consequences of peripheral GABA signaling on visceral nociception, we assessed the impact of intracolonic GABAB or GABAA agonist and antagonist application on noxious colorectal distension (CRD) induced VMR. In total, the VMR was recorded from 30 male and 35 female mice and there were no differences between sex with respect to area of VMR 1.12 ± 0.17 and 1.10 ± 0.15 V*s (p = 0.91, unpaired t-Test, n = 65) from males and females, respectively.
Because the standard protocol involved repeated stimulation of the colon, we first sought to determine whether there were time-dependent changes in the VMR in response to balloon distension, or whether there were changes in the VMR associated with intracolonic application of the vehicle solution. VMR from both male (n = 7) and female (n = 10) mice were studied in this way. Results of a two-way RM ANOVA revealed no significant influence of sex (p = 0.19) or time/vehicle infusion (p = 0.65), nor a significant interaction (p = 0.90) between the two. The average changes in baseline over time/following saline instillation was 1 ± 15 % (100.7 ± 15 %, p = 0.65, paired t-Test, n = 17). Furthermore, as with the single unit data, we defined a response to instillation as a change in the VMR greater than two standard deviations from the average baseline response. Based on this criterion none of the 17 vehicle treated mice would have been considered “responders”. Thus, VMR evoked by noxious distension of the colon was stable, enabling us to detect the influence of test agents with sensitivity.
Baclofen reduces the VMR
We first sought to determine whether exogenous application of GABAB agonist, baclofen influenced the VMR (Figure 8A). Results of a two-way RM ANOVA revealed a significant effect of baclofen 200 μM (p = 0.006), but no significant influence of sex (p = 0.57) nor a significant interaction between sex and baclofen application (p = 0.94). Pooled data analyzed as a percent of baseline indicated that baclofen was associated with more than a 55% (45.6 ± 9% of baseline) decrease in VMR (Figure 8B). Thirteen of 14 mice tested with baclofen demonstrated a decrease in the VMR. The effect of baclofen on VMR suggests that peripheral GABAB receptors influence visceral nociception.
Figure 8. Intracolonic baclofen inhibits the VMR.

(A) Typical VMR to 60 mmHg distension of the colon before (black traces) and after (gray trace) intracolonic instillation baclofen (200 μM). (B) Pooled VMR data from six female and seven male and mice respectively (n=13) before (Bsl) and after intracolonic application of baclofen (Bac). ** is p< 0.01, paired t-Test
Muscimol reduces the VMR
We evaluated the accumulative effect of the GABAA receptor agonist muscimol at 3 different concentrations (1, 10, 100 μM) on the VMR. Intracolonic instillation of muscimol decreased the VMR in a concentration-dependent manner (Figure 9A). Results of a two-way ANOVA revealed a significant effect of muscimol on the VMR (p<0.001), but no significant influence of sex (p= 0.092) nor a significant interaction between sex and muscimol application (p = 0.55). Pooled data analyzed as a percent of baseline indicated that muscimol (100 μM) was associated with more than a 70% (27.7 ± 7.2% of baseline) decrease in the VMR. While the pool data with 10 μM of muscimol showed a trend toward a decrease in the VMR (26 ± 20%) the percentage of decrease was much less (n = 11, p = 0.08) than the changes associated with 100 μM of muscimol. Likewise, treatment with 1 μM muscimol only decreased the VMR by 7 ± 16% (p >0.9). Eight of 11 mice tested with 100 μM muscimol demonstrated a decrease in the VMR. When data were analyzed as fractional decrease in the VMR and fitted a modified Hill equation, they yielded EC50 values of 26 μM (Figure 9B). The effect of muscimol on the VMR suggests that peripheral GABAA receptors influence colon nociception.
Figure 9. Muscimol inhibits the VMR.

Left traces show a representative electromyographic recording of the VMR evoked with a 60 mmHg distension of the colon that was significantly decreased following intracolonic application of muscimol 100 μM, but not 1 μM or 10 μM muscimol. (B) Pooled concentration-response data (n = 11) for muscimol were fitted with modified Hill equation to determine the concentration by which the VMR was inhibited 50 % from baseline (EC50). The VMR was inhibited 73 ± 7.2 % at 100 μM.
GABA receptor antagonists reveal a role for endogenous GABA in the regulation of visceral nociception
Our single unit data with GABA antagonists suggests endogenous GABA contributes to the regulation of visceral nociception. To directly test this suggestion, we assessed the impact of intracolonic GABAB receptor antagonist application on VMR evoked by noxious colorectal distention. Results of a two-way RM ANOVA indicate no significant effect of CGP55845 (CGP) at 5 μM on VMR (p = 0.9), no significant influence of sex (p = 0.36), nor a significant interaction between sex and CGP application (p = 0.45). This was likely due to variability in the impact of CGP between mice. Five mice (3 male and 2 female) met the criteria for a response to CGP with a decrease in the VMR two standard deviations from the average baseline response (Figure 10Ai), three mice (2 male and 1 female) met this criteria for an increase in VMR (Figure 10Aii) and seven mice (3 male and 4 female) would have been considered non-responders (Figure 10Aiii). Pooled data analyzed as a percent of baseline for the opposing effects of CGP indicated that CGP (5 μM) either produced a 35% (64.2 ± 5.6 of baseline, Figure 10Bi) decrease in VMR, or a 30% (129 ± 7.2% of baseline, Figure 10Bii) increase in VMR.
Figure 10. Effect of CGP55845 on the visceromotor responses.
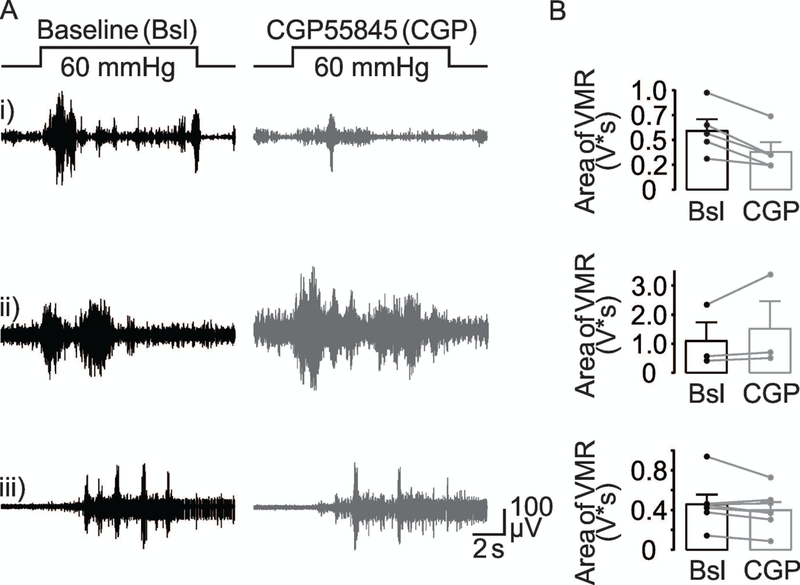
VMR evoked by noxious colorectal distention (60 mmHg) before (black traces) and after (gray trace) intracolonic instillation of CGP55845 (CGP, 5 μM). There were differences between mice with respect to the response to CGP: (Ai) In five mice (3 male and 2 female) the VMR was decreased. (Aii) In three mice (2 male and 1 female), CGP increased the VMR. (Aiii) In seven mice (3 male and 4 female) no change was observed in the VMR. (B) Pooled VMR data for the three groups of mice before (Bsl) and after intracolonic application of CGP55845 (CGP).
To assess the involvement of GABAA receptor activation in the regulation of visceral nociception, we evaluated the effects of the GABAA receptor antagonist (bicuculline) on the VMR. Results of a two-way RM ANOVA revealed a significant effect of bicuculline 100 μM on VMR (p = 0.016), but no significant influence of sex (p= 0.94) nor a significant interaction between sex and bicuculline application (p = 0.16). Pooled data analyzed as a percent of baseline indicated that bicuculline (100 μM) was associated with a 43% (143 ± 34% of baseline) increase in the VMR (Figure 11). Ten of 10 mice tested with bicuculline demonstrated an increase in the VMR. Together, these results suggest that there is constitutive GABAA receptor activation in the colon that is involved in establishing the response to noxious colonic stimulation.
Figure 11. Bicuculline increases the visceromotor responses.
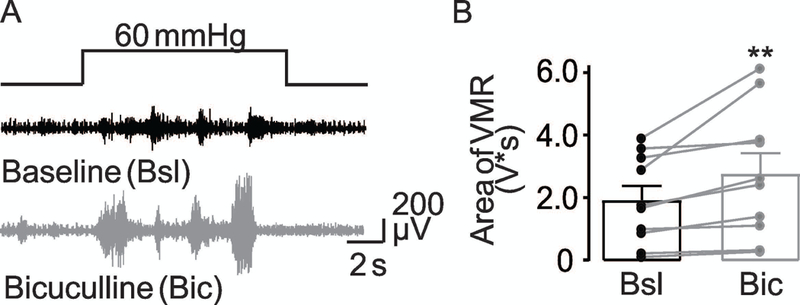
(A) VMR evoked by noxious colorectal distention (60 mmHg) before (black traces) and after (gray trace) intracolonic instillation bicuculline (100 μM). (B) Pooled VMR data from 5 female and 5 male mice respectively (n = 10) before (Bsl) and after intracolonic application of Bicuculline (Bic). ** is p< 0.01, paired t-Test. Emanuel Loeza-Alcocer received his PhD in neurobiology in the laboratory of Professor Rodolfo Delgado-Lezama in Mexico City. His research was focused on characterizing the GABAergic mechanisms underlying the presynaptic inhibition of sensory neurons. At present, he investigates peripheral GABAergic inhibitory mechanisms in the colon in the group of Michael Gold PhD at University of Pittsburgh, USA. His long-term goal is to understand how peripheral GABAergic inhibition modifies sensory perception in health and disease.
DISCUSSION
The aim of this study was to evaluate whether peripheral GABAA and GABAB receptors contribute to the regulation of colon afferent excitability and visceral nociception. We provide evidence that there are functional GABAA and GABAB receptors in the colon. Specifically, peripheral administration of GABA or selective agonists for either receptor subtype decreased colonic afferent excitability and suppressed the VMR to noxious distension of the colon. We also provide evidence that endogenous release of GABA in the colon attenuates the excitability of colonic afferents via activation of both GABAA and GABAB receptor subtypes, and that at least the endogenous activation of GABAA receptors contributes to the suppression of visceral nociception.
Our results with baclofen were consistent with those from previous studies of colonic muscular afferents (Sengupta et al., 2002; Castro et al., 2017) and with vagal afferents in the upper GI track (Smid et al., 2001; Page et al., 2006). Likewise, previous data suggest that GABAB receptors also attenuate colonic nociceptor activity (Castro et al., 2017). Together with our VMR data, these data support the suggestion that GABAB receptor is a viable therapeutic target for the treatment of visceral pain syndromes. Consistent with this suggestion, peripheral administration of Vc1.1, a synthetic version of a GABAB receptor activating peptide, was shown to be anti-nociceptive in a model of chronic visceral hypersensitivity (Castro et al., 2017; Castro et al., 2018; Sadeghi et al., 2018). Similarly, C12AsnGABAOH, a lipopeptide, inhibits visceral hypersensitivity in mice model via activation of GABAB receptors (Perez-Berezo et al., 2017).
The role of GABAB receptors in endogenous GABA signaling in the colon is a little less clear. That is, while our single unit data indicated that activation of GABAB receptors via endogenous GABA results in the suppression of muscular colonic afferent excitability, CGP55845 appeared to have no detectable influence on colonic nociceptor excitability (Castro et al., 2017). Furthermore, there was no net change in the VMR associated with CGP55845 administration, although this appeared to be due to the presence of three distinct effects of CGP55845 on VMR on different groups of mice. That is, there were mice in which the VMR did not change, was potentiated, or was suppressed following CGP55845 administration. And while the “no effect” group would be consistent with the failure of CGP55845 to influence the excitability of colonic nociceptor excitability, and the suggestion that this population of afferents plays a dominant role in driving behavioral responses to noxious colonic distension, the other effects of CGP55845 on VMR in the other groups of mice suggest GABAB signaling is more complex than the simple inhibition of colonic afferent activity. Variability in the relative contribution of colonic afferent types to the VMR between mice could account for the subpopulation of mice in which CGP55845 increased the VMR. This possibility would suggest that muscular afferents or another colonic afferent subtype, with a comparable response to the antagonist, play a dominant role in mediating the VMR in these mice. The group in which the VMR was decreased, however, is more difficult to explain. Given evidence for cells within the colon that appear to influence the activity of colonic afferents (Castro et al., 2013), one possibility is that an indirect action dominates in this third response-type to CGP55845, where GABAB receptor-mediated suppression of a cell releasing an inhibitory signaling molecule like cyclic guanosine-3’,5’-monophosphate (cGMP) (Castro et al., 2013), would account for the inhibitory effects of the GABAB antagonist. Consistent with this possibility, there is evidence that GABAB receptors are not only present in colonic afferents (Castro et al., 2017), but other cells in the colon including epithelial cells (Hyland & Cryan, 2010; Auteri et al., 2015), a primary source of cGMP in the colon (Castro et al., 2013). Thus, blocking the GABAB-mediated inhibition of cGMP release would facilitate cGMP-induced suppression of colonic afferent excitability, and consequently the VMR. That we did not detect a CGP55845-induced decrease in the excitability of muscular afferents would suggest that third type of afferent contributes to the response to CRD in mice where CGP55845 decreased the VMR.
Our results with GABAA receptor agonists and antagonists were not only more consistent, but more robust than those with GABAB antagonists, extending the previous data on GABA signaling in the colon in several important ways. First, and potentially most importantly, our observations suggest that GABAA receptors should also be considered a therapeutic target for the treatment of visceral pain. Second, they suggest GABAA receptors may be an even better target than GABAB receptors. While muscimol had a similar impact on mean peak firing frequency as baclofen (38% vs 35%, respectively), muscimol inhibited a greater percentage of colonic afferents than baclofen (90% vs 50% respectively), produced a greater reduction the number of action potentials (50% vs 30% respectively), and produced a greater increase in mechanical threshold (100% vs 30%). Consistent with these single unit data, the muscimol-induced suppression of the VMR (~70%) was greater than that produced by baclofen (~55%). These latter results may be due to the potency of agonists and/or affinity of the receptors (Chu et al., 1990), rather than their efficacy. However, recent RNAseq data suggests that both GABA receptor types should be present in all colonic afferents (Hockley et al., 2018), even if there are differences between subpopulations of afferents with respect to the subunit composition of GABAA receptors present. This suggests that GABAB receptors in colonic afferents may have a more important role on the regulation of processes other than action potential threshold, spike accommodation, or firing frequency. Consistent with this suggestion are previous data indicating that GABAB receptor activation in DRG neurons results in the inhibition in voltage-gated Ca2+ channels, which may play a more important role in the regulation of transmitter release than excitability per se. Third, GABAA receptor activation via endogenous GABA appears to also play a more important role than GABAB in establishing the baseline excitability of colonic afferents, and consequently visceral sensitivity: Both bicuculline and picrotoxin had a greater impact on the action potential number, mechanical threshold, and mean peak firing frequency in colonic afferents than baclofen, and the effect on the VMR was more consistent and on average greater than in the group of mice in which CGP55845 increased the VMR. This latter observation is in marked contrast to the impact of GABAA receptor signaling in somatic tissue, where the bulk of the limited data available suggests that peripheral GABAA receptors are only engaged in the presence of tissue injury (Cairns et al., 1999; Carlton et al., 1999; Reis et al., 2007; Tan et al., 2014).
The most straight forward explanation for the impact of GABAA receptor activation on colonic afferent excitability and visceral nociception is that the receptors are present on colonic afferents, enabling a direct modulation of excitability. This is consistent with the widespread distribution of GABAA receptor subunits among colonic afferents as noted (Hockley et al., 2018). It is also consistent with the effects of GABAA receptor agonists and antagonists on the electrical threshold for colonic afferent activation (acknowledging that electrical stimulation may also activate an indirect mechanism). More convincing are the latency data, which suggest that GABAA receptors on colonic afferents may not only influence sensory transduction events, but the length constant of the peripheral terminals. This is consistent with a wider distribution of GABAA receptors in the peripheral terminals which could contribute to the apparently greater efficacy of GABAA receptor influence on colonic afferent excitability. However, an indirect mechanism(s) cannot be ruled out either. Like GABAB receptors, GABAA receptors are also present on a number of additional cell types in the colon (Seifi et al., 2014), including a number of types of neurons in the enteric nervous system (Minocha & Galligan, 1993; Krantis et al., 1998).
Given the absence of efficacious therapeutic strategies to treat chronic visceral pain (e.g., inflammatory bowel diseases, irritable bowel syndrome), GABA receptors in the colon make for intriguing therapeutic targets. However, given that GABA, acting through GABAA and GABAB receptors, plays a key role controlling several gastrointestinal functions such as motility (Frigo et al., 1987; Grider & Makhlouf, 1992; Begg et al., 2002), secretion(Goto & Debas, 1983; Tsai et al., 1987; Thirlby et al., 1988; Lin, 1995; Piqueras & Martinez, 2004; Li et al., 2012), and the local immune system (Ma et al., 2018; Seifi et al., 2018), it is important to note that GABA receptor activation in the colon may also be associated with deleterious side effects. Consistent with this suggestion, a recent study shows that activation of GABAA receptors in Colon Epithelium exacerbates acute colitis (Ma et al., 2018). In this context, a detailed characterization of GABA receptor subtypes associated with different functions in the GI tract may suggest a specific target to attenuate visceral pain with a limited impact normal GI function.
Key points summary.
While the presence of GABA receptors on primary afferents has been well described, most functional analyses have focused on the regulation of transmitter release from central terminals and/or signaling in the sensory neuron cell body.
Evidence that GABA receptors are transported to peripheral terminals, and that there are several sources of GABA in the colon raise the possibility that GABA signaling in the periphery may influence colonic afferent excitability.
GABAA and GABAB are present and functional in the colon, where exogenous agonists decrease the excitability of colonic afferents, and suppress visceral nociception.
Endogenous GABA release within the colon is sufficient to establish the resting excitability of colonic afferents as well as the behavioral response to noxious stimulation of the colon, primarily via GABAA receptors.
Peripheral GABA receptors may serve as a viable target for the treatment of visceral pain.
Acknowledgement:
We thank Drs. Gerald F. Gebhart and Jamie Moy for helpful comments in the preparation of the manuscript. Work supported by NIH grant R01 DK10796.
Footnotes
Declaration of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- Auteri M, Zizzo MG & Serio R. (2015). GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93, 11–21. [DOI] [PubMed] [Google Scholar]
- Begg M, Molleman A & Parsons M. (2002). Modulation of the release of endogenous gamma-aminobutyric acid by cannabinoids in the guinea pig ileum. Eur J Pharmacol 434, 87–94. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC 3rd, Gebhart GF & Blackshaw LA. (2004). Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178. [DOI] [PubMed] [Google Scholar]
- Brown DA & Marsh S. (1978). Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res 156, 187–191. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ & Hu JW. (1999). Activation of peripheral GABAA receptors inhibits temporomandibular joint-evoked jaw muscle activity. J Neurophysiol 81, 1966–1969. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S & Coggeshall RE. (1999). Peripheral GABA(A) receptors: evidence for peripheral primary afferent depolarization. Neuroscience 93, 713–722. [DOI] [PubMed] [Google Scholar]
- Castro J, Grundy L, Deiteren A, Harrington AM, O’Donnell T, Maddern J, Moore J, Garcia-Caraballo S, Rychkov GY, Yu R, Kaas Q, Craik DJ, Adams DJ & Brierley SM. (2018). Cyclic analogues of alpha-conotoxin Vc1.1 inhibit colonic nociceptors and provide analgesia in a mouse model of chronic abdominal pain. Br J Pharmacol 175, 2384–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Harrington AM, Garcia-Caraballo S, Maddern J, Grundy L, Zhang J, Page G, Miller PE, Craik DJ, Adams DJ & Brierley SM. (2017). alpha-Conotoxin Vc1.1 inhibits human dorsal root ganglion neuroexcitability and mouse colonic nociception via GABAB receptors. Gut 66, 1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J, Harrington AM, Hughes PA, Martin CM, Ge P, Shea CM, Jin H, Jacobson S, Hannig G, Mann E, Cohen MB, MacDougall JE, Lavins BJ, Kurtz CB, Silos-Santiago I, Johnston JM, Currie MG, Blackshaw LA & Brierley SM. (2013). Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3’,5’-monophosphate. Gastroenterology 145, 1334–1346 e1331–1311. [DOI] [PubMed] [Google Scholar]
- Chu DC, Albin RL, Young AB & Penney JB. (1990). Distribution and kinetics of GABAB binding sites in rat central nervous system: a quantitative autoradiographic study. Neuroscience 34, 341–357. [DOI] [PubMed] [Google Scholar]
- Feng B & Gebhart GF. (2015). In vitro functional characterization of mouse colorectal afferent endings. J Vis Exp, 52310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Clark MJ & Furness JB. (2002). Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res 308, 339–346. [DOI] [PubMed] [Google Scholar]
- Frigo GM, Galli A, Lecchini S & Marcoli M. (1987). A facilitatory effect of bicuculline on the enteric neurones in the guinea-pig isolated colon. Br J Pharmacol 90, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y & Debas HT. (1983). GABA-mimetic effect on gastric acid secretion. Possible significance in central mechanisms. Dig Dis Sci 28, 56–59. [DOI] [PubMed] [Google Scholar]
- Grider JR. (1998). Regulation of excitatory neural input to longitudinal intestinal muscle by myenteric interneurons. Am J Physiol 275, G973–978. [DOI] [PubMed] [Google Scholar]
- Grider JR & Makhlouf GM. (1992). Enteric GABA: mode of action and role in the regulation of the peristaltic reflex. Am J Physiol 262, G690–694. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockley JRF, Taylor TS, Callejo G, Wilbrey AL, Gutteridge A, Bach K, Winchester WJ, Bulmer DC, McMurray G & Smith ESJ. (2018). Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland NP & Cryan JF. (2010). A Gut Feeling about GABA: Focus on GABA(B) Receptors. Front Pharmacol 1, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DI & Krantis A. (1983). Uptake and stimulus-evoked release of [3H]-gamma-aminobutyric acid by myenteric nerves of guinea-pig intestine. Br J Pharmacol 78, 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantis A, Mattar K & Glasgow I. (1998). Rat gastroduodenal motility in vivo: interaction of GABA and VIP in control of spontaneous relaxations. Am J Physiol 275, G897–903. [DOI] [PubMed] [Google Scholar]
- Krantis A, Tufts K, Nichols K & Morris GP. (1994). [3H]GABA uptake and GABA localization in mucosal endocrine cells of the rat stomach and colon. J Auton Nerv Syst 47, 225–232. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiang YY, Lu WY, Liu C & Li J. (2012). A novel role of intestine epithelial GABAergic signaling in regulating intestinal fluid secretion. Am J Physiol Gastrointest Liver Physiol 303, G453–460. [DOI] [PubMed] [Google Scholar]
- Lin WC. (1995). Stimulatory effect of muscimol on gastric acid secretion stimulated by secretagogues in vagotomized rats under anesthesia. Eur J Pharmacol 279, 43–50. [DOI] [PubMed] [Google Scholar]
- Ma X, Sun Q, Sun X, Chen D, Wei C, Yu X, Liu C, Li Y & Li J. (2018). Activation of GABAA Receptors in Colon Epithelium Exacerbates Acute Colitis. Front Immunol 9, 987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Taniyama K, Tanaka C & Tobe T. (1983). GABA, glutamic acid decarboxylase, and GABA transaminase levels in the myenteric plexus in the intestine of humans and other mammals. J Neurochem 40, 861–865. [DOI] [PubMed] [Google Scholar]
- Minocha A & Galligan JJ. (1993). Excitatory and inhibitory responses mediated by GABAA and GABAB receptors in guinea pig distal colon. Eur J Pharmacol 230, 187–193. [DOI] [PubMed] [Google Scholar]
- Nichols K, Staines W, Wu JY & Krantis A. (1995). Immunopositive GABAergic neural sites display nitric oxide synthase-related NADPH diaphorase activity in the human colon. J Auton Nerv Syst 50, 253–262. [DOI] [PubMed] [Google Scholar]
- Page AJ, O’Donnell TA & Blackshaw LA. (2006). Inhibition of mechanosensitivity in visceral primary afferents by GABAB receptors involves calcium and potassium channels. Neuroscience 137, 627–636. [DOI] [PubMed] [Google Scholar]
- Perez-Berezo T, Pujo J, Martin P, Le Faouder P, Galano JM, Guy A, Knauf C, Tabet JC, Tronnet S, Barreau F, Heuillet M, Dietrich G, Bertrand-Michel J, Durand T, Oswald E & Cenac N. (2017). Identification of an analgesic lipopeptide produced by the probiotic Escherichia coli strain Nissle 1917. Nat Commun 8, 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras L & Martinez V. (2004). Peripheral GABAB agonists stimulate gastric acid secretion in mice. Br J Pharmacol 142, 1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G, Pacheco D, Francischi J, Castro M, Perez A & Duarte I. (2007). Involvement of GABA A receptor-associated chloride channels in the peripheral antinociceptive effect induced by GABA A receptor agonist muscimol. Eur J Pharmacol 564, 112–115. [DOI] [PubMed] [Google Scholar]
- Sadeghi M, Carstens BB, Callaghan BP, Daniel JT, Tae HS, O’Donnell T, Castro J, Brierley SM, Adams DJ, Craik DJ & Clark RJ. (2018). Structure-Activity Studies Reveal the Molecular Basis for GABAB-Receptor Mediated Inhibition of High Voltage-Activated Calcium Channels by alpha-Conotoxin Vc1.1. ACS Chem Biol 13, 1577–1587. [DOI] [PubMed] [Google Scholar]
- Seifi M, Brown JF, Mills J, Bhandari P, Belelli D, Lambert JJ, Rudolph U & Swinny JD. (2014). Molecular and functional diversity of GABA-A receptors in the enteric nervous system of the mouse colon. J Neurosci 34, 10361–10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifi M, Rodaway S, Rudolph U & Swinny JD. (2018). GABAA Receptor Subtypes Regulate Stress-Induced Colon Inflammation in Mice. Gastroenterology 155, 852–864 e853. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Medda BK & Shaker R. (2002). Effect of GABA(B) receptor agonist on distension-sensitive pelvic nerve afferent fibers innervating rat colon. Am J Physiol Gastrointest Liver Physiol 283, G1343–1351. [DOI] [PubMed] [Google Scholar]
- Smid SD, Young RL, Cooper NJ & Blackshaw LA. (2001). GABA(B)R expressed on vagal afferent neurones inhibit gastric mechanosensitivity in ferret proximal stomach. Am J Physiol Gastrointest Liver Physiol 281, G1494–1501. [DOI] [PubMed] [Google Scholar]
- Tan SN, Song E, Dong XD, Somvanshi RK & Cairns BE. (2014). Peripheral GABAA receptor activation modulates rat tongue afferent mechanical sensitivity. Arch Oral Biol 59, 251–257. [DOI] [PubMed] [Google Scholar]
- Thirlby RC, Stevens MH, Blair AJ, Petty F, Crawford IL, Taylor IL, Walsh JH & Feldman M. (1988). Effect of GABA on basal and vagally mediated gastric acid secretion and hormone release in dogs. Am J Physiol 254, G723–731. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Taniyama K & Tanaka C. (1987). gamma-Aminobutyric acid stimulates acid secretion from the isolated guinea pig stomach. Am J Physiol 253, G601–606. [DOI] [PubMed] [Google Scholar]
- Wang FY, Zhu RM, Maemura K, Hirata I, Katsu K & Watanabe M. (2006). Expression of gamma-aminobutyric acid and glutamic acid decarboxylases in rat descending colon and their relation to epithelial differentiation. Chin J Dig Dis 7, 103–108. [DOI] [PubMed] [Google Scholar]


