Abstract
Objective
Despite numerous improvements in care, morbidity from heart failure (HF) has remained essentially unchanged in recent years. One potential reason is that depression, which is co-morbid in approximately 40% of hospitalized HF patients and associated with adverse HF outcomes, often goes unrecognized and untreated. The Hopeful Heart Trial is the first study to evaluate whether a widely generalizable telephone-delivered collaborative care program for treating depression in HF patients improves clinical outcomes.
Methods
Hopeful Heart aimed to enroll 750 patients with reduced ejection fraction (HFrEF) (ejection fraction ≤45%) including: (A) 625 patients who screened positive for depression both during their hospitalization (Patient Health Questionnaire (PHQ-2)) and two weeks following discharge (PHQ-9 ≥10); and (B) 125 non-depressed control patients (PHQ-2(−)/PHQ-9 <5). We randomized depressed patients to either their primary care physician’s “usual care” (UC) or to one of two nurse-delivered 12-month collaborative care programs for: (A) depression and HFrEF (“blended”); or (B) HrEFF alone (enhanced usual care (eUC)). Our co-primary hypotheses will test whether “blended” care can improve mental health-related quality of life vs. UC and vs. eUC, respectively, on the Mental Component Summary of the Short-Form 12 Health Survey (SF-12 MCS). Secondary hypotheses will evaluate the effectiveness of our interventions on mood, functional status, hospital readmissions, deaths, provision of evidence-based care for HFrEF and treatment costs.
Results
Not applicable.
Conclusions
The Hopeful Heart Trial will determine whether “blended” collaborative care for depression and HFrEF is more effective at improving patient-relevant outcomes than collaborative care for HFrEF-alone or doctors’ usual care for HFrEF.
Keywords: Heart failure, depression, collaborative care, quality of life, randomized trial
Introduction
Heart failure (HF) is an important and growing public health problem that affects over 6.6 million Americans with over 650,000 newly diagnosed cases and 330,000 deaths annually. Yet despite improvements in cardiac care, HF remains the leading cause for hospitalization among Medicare patients and the only major cardiovascular disease (CVD) whose mortality rate has remained essentially unchanged over the past decade.1
One potential contributor to these persistently poor outcomes is depression. It is co-morbid in approximately 20–40% of patients with HF2,3 and has been strongly linked to reduced health-related quality of life (HRQoL),4–7 poorer adherence with evidence-based HF care, higher levels of health services utilization,8,9 and increased morbidity and mortality independent of disease severity.9–14 In recognition, HF treatment guidelines advocate routine screening for depression,15 but this recommendation is unlikely to be widely adopted without trial-derived evidence that depression care improves patient-relevant outcomes.
Strategies to treat depression in cardiac populations are of great interest given their potential to reduce morbidity. Unfortunately, most interventions evaluated in clinical trials, including some conducted in HF patients,2,16,17 had little or no impact at reducing mood symptoms.16,18–23 Possible explanations include: (1) dependence solely on single antidepressant agents16,19,24 that may be ineffective, untolerated, or discontinued by patients;25 (2) reliance on psychological counseling in medically-ill populations who may be either unwilling or unable to adhere to successive face-to-face encounters with a therapist;20,23 (3) inadequate consideration of patients’ preferences for type and location of treatment;26,27 (4) insufficient attention to adherence with recommended care;28,29 (5) perceived stigma of depression;30 (6) brief duration of treatment and follow-up;16,19,23 and (7) higher than expected spontaneous remission rates for depression.16,20
In an effort to overcome the limitations of earlier interventions, interest has turned towards “collaborative care” strategies for treating depression.31 Numerous trials have proven the effectiveness of this approach at improving the quality of care for cardiovascular disease32–35 and other chronic conditions36–40 as well as treating depression and anxiety in primary care.35,41 Consequently, it has emerged as an integral part of the “patient-centered medical home” model to reorganize and reimburse primary care physicians (PCPs) for providing high-quality chronic illness care.42 Based on Wagner’s Chronic Care Model,43,44 collaborative care involves active follow-up by a non-physician “care manager” who supports patients with the frequency of contacts necessary to educate them about their illness, imparts guideline-based treatment suggestions, proactively monitors their responses to treatment, and activates their self-help potential to promote health behavior changes all in collaboration with patients’ PCPs with specialist back-up as needed.45,46
We earlier demonstrated the clinical-47 and cost-effectiveness48 of telephone-delivered collaborative care for treating depression and improving HRQoL following coronary artery bypass graft (CABG) surgery. Yet, we are unaware of any trial that evaluated the effectiveness of this strategy at treating depression in HF patients. Moreover, as many health care delivery systems are presently using outpatient care managers to promote delivery of guideline-based HF care, it may be impractical for these systems to also provide, and for patients to participate in, separate treatment programs for HF and depression.
Due to the bidirectional adverse impact of depression on co-morbid medical illness,”blended” interventions that focus on treating both psychiatric and physical conditions may produce greater improvements in mood symptoms and adjustment of health behaviors than programs focused solely on treating depression. For example, Katon et al.’s “blended” TEAMcare intervention produced an improvement in mood symptoms (with an effect size [ES] of 0.67) over a usual care control group and better control of CVD risk factors than those produced by physicians’ usual care for depression at 12-months follow-up,35 vs. the 0.20 ES improvement in mood symptoms and no measurable difference in glycosylated hemoglobin observed in their earlier trial of a collaborative care intervention targeting mood symptoms in diabetics.49
Influenced by TEAMcare Trial’s “blended” collaborative care strategy,35,50 we developed the Hopeful Heart Trial to evaluate whether a similar “blended” strategy could also improve treatment outcomes for depressed HF patients. However, unlike TEAMcare or any prior trial of collaborative care for depression only, we included a collaborative care for HF alone arm to serve as an active attention control group. This allowed us to better determine whether any improvements in patient outcomes over physicians’ usual care for treating depression were due to the depression treatment rather than to any additional monitoring, patient expectations, or other factors.51
Methods
Overview
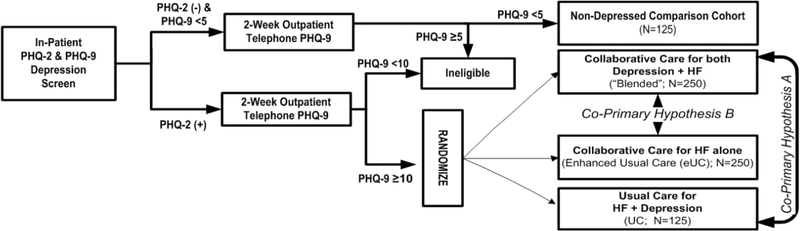
Using a study protocol approved by the University of Pittsburgh Institutional Review Board (IRB) and registered with ClinicalTrials.gov prior to the start of patient enrollment, the Hopeful Heart Trial aimed to enroll 750 patients with reduced left ventricular ejection fraction (EF) ≤45%; HFrEF) from eight Pittsburgh-area hospitals. The study included: (1) 625 patients who expressed mood symptoms before discharge and at 2-weeks post-hospitalization; and (2) 125 patients who served as a nondepressed control cohort to facilitate comparisons with depressed participants (Figure 1).
Figure 1.
Study Design.
The randomized portion of our Trial was designed to test whether a telephone-delivered “blended” collaborative care intervention could produce at 12-months follow-up a: (1) moderate 0.50 ES or greater improvement in mental HRQoL vs. physicians’ usual care (UC); and (2) clinically meaningful 0.30 ES or greater improvement in mental HRQoL vs. collaborative care for HF alone (enhanced usual care (eUC)). Trial secondary hypotheses evaluated the effects of our “blended” intervention on mood, functional status, adherence with guideline-consistent care, incidence of mortality and cardiovascular events, health care utilization, and insurance claims costs between study arms. We applied standardized patient inclusion criteria, random assignment of patients, blinded assessments of clinical outcomes, a centralized intervention protocol, and included patients covered by a variety of insurance plans to maximize both the external and internal validity of our study.
Setting and Hospital Enrollment
Study nurses screened patients hospitalized with HF for protocol-eligibility at two university-affiliated and six community hospitals in the Pittsburgh area. To promote awareness of our Trial prior to the start of recruitment, we: (1) developed IRB-approved posters, brochures, pens, and other marketing materials imprinted with our study logo that we distributed to hospital staff; and (2) delivered in-service and grand rounds presentations about the links between depression and CVD.
Our study nurses utilized several strategies to identify potentially eligible adult patients aged 21 and older with a left ventricular EF ≤ 45% to screen for depression. They included: (1) reviewing the lists of HF patients generated daily for each hospital’s HF care management program; and (2) approaching nurses on cardiac units to inquire about any new admissions for HF who may be appropriate for our study. In keeping with the Health Insurance Portability and Accountability Act (HIPAA), they then asked hospital staff who had routine clinical contact with those identified patients for the patient’s verbal agreement to be approached before entering their room to provide a brief description of our study and seek their verbal consent to undergo our screening procedure.
Inpatient Screening Procedure
As recommended by an American Heart Association Science Advisory52 and others,53 we utilized the two-step Patient Health Questionnaire (PHQ) to efficiently screen patients for depression. First, our study nurses used tablet computers to systematically administer the two-item PHQ-2 screen.54 We programmed our recruitment program to classify the PHQ-2 screen as positive (PHQ-2 (+)) when a patient replied “yes” to at least one PHQ item: “Over the past 2 weeks, have you been bothered by either of the following problems: (1) Little interest or pleasure in doing things; and (2) Feeling down, depressed, or hopeless” (sensitivity and specificity 90% and 69%, respectively).53
If the patient remained protocol-eligible (Table 1), the study nurse then attempted to collect the patient’s signed informed consent to enroll in our study. Finally, we provided all PHQ-2 (+) patients with a copy of the NIMH brochure entitled “Depression and Heart Disease” to raise their awareness of the impact of mood disorders on heart disease regardless of whether they met our other eligibility criteria for our study.
Table 1.
Inclusion/exclusion criteria
| Inclusion Criteria: |
| 1. Systolic heart failure (ejection fraction ≤ 45%). |
| 2. HF symptoms meeting criteria for New York Heart Association (NYHA) classes II, III or IV. |
| 3. Inpatient PHQ-2 screen-positive for depression. |
| 4. PHQ-9 ≥ 10 when reassessed two-weeks following hospital discharge. |
| 5. No cognitive impairment (as documented in the record, use of medications for treating cognitive impairment, or the Montreal Cognitive Assessment79). |
| 6. Able to be evaluated and treated for depression as an outpatient. |
| 7. English speaking, not illiterate, or possessing any other communication barrier. |
| 8. Have a household telephone. |
| Exclusion Criteria: |
| 1. Receiving active treatment for a mood or anxiety disorder from a mental health specialist. |
| 2. Unstable medical condition as indicated by history, physical, and/or laboratory findings. |
| 3. Presence of non-cardiovascular conditions likely to be fatal within 12 months (e.g., cancer). |
| 4. Organic mood syndromes, including those secondary to medical illness or drugs. |
| 5. Active suicidal ideation. |
| 6. Current or history of psychotic illness according to interview by the study nurse and chart review, respectively. |
| 7. Current or history of bipolar illness according to patient self-report, past medical history, and PRIME-MD criteria. |
| 8. Current alcohol or other substance abuse as evidenced by chart review and the AUDIT-C questionnaire. |
| 9. Age ≤ 21 years. |
Two-Week Follow-Up and Baseline Assessment
To confirm the positive PHQ-2 screen, a trained research assessor telephoned all consented patients approximately two weeks after hospital discharge to administer the PHQ-9.55 If the patient scored ≥10 indicating at least a moderate level of mood symptoms and remained protocol-eligible (Table 1), then the research assessor proceeded to administer our baseline assessment battery (Table 2).
Table 2.
Assessment Battery
| Instrument | Baseline | Month 3 | Month 6 | Month 12 |
|---|---|---|---|---|
| Patient Health Questionnaire (PHQ-9)55 | X | |||
| Sociodemographics | X | X | X | X |
| Short-Form 12 Health Survey, Mental & Physical Component Summary scores (SF-12 MCS & PCS57 | X | X | X | X |
| Kansas City Cardiomyopathy Questionnaire (KCCQ-12)61 | X | X | X | X |
| PRIME-MD Mood & Anxiety80 | X | |||
| Mental Health History | X | X | X | X |
| Patient-Reported Outcomes Measurement Information System (PROMIS) Depression & Anxiety scales63 | X | X | X | X |
| Hamilton Rating Scale for Depression (HRS-D)81 | X | X | X | X |
| Perceived Social Support82 | X | X | ||
| Life Orientation Test (LOT)83 | X | X | ||
| Employment | X | X | X | X |
| Health care utilization | X | X | X | X |
| Adherence to heart failure therapy | X | X | ||
| Medications | X | X | X | X |
| Vital status | X | X | X | X |
Randomization
Following completion of our baseline assessment, our data management system automatically randomized depressed participants in a 2:2:1 ratio stratified by hospital type (university or community) and sex using pre-determined permuted blocks of varying size to one of three study arms: (1) collaborative care for both HF and depression (“blended” care); (2) collaborative care for HF only (enhanced usual care (eUC)) or (3) physicians’ usual care (UC) (Figure 1).
To maintain our assessors’ blind as to each patient’s treatment assignment, we programmed our data management system to automatically inform our project coordinator of the patient’s random assignment. She then assigned the patient to a care manager for that intervention arm, or telephoned the patient if they were assigned to UC. Afterwards, they informed the patient’s PCP of their assignment status in a mailed letter (Table 3).
Table 3.
Hopeful Heart Intervention Overview
| TREATMENT COMPONENTS | COLLABORATIVE CARE | ||
|---|---|---|---|
| HF + Depression (“Blended”) | HF-Only (Enhanced Usual Care (eUC)) | Usual Care (UC) | |
| Patients | |||
| Informed of PHQ-2 (+) elevated mood symptom screen during hospitalization for HF | • | • | • |
| Given NIMH brochure on depression and heart disease during hospitalization for HF | • | • | • |
| Informed of randomization status | • | • | • |
| Regularly contacted by nurse care manager X 12 months to discuss depression care | • | ||
| Regularly contacted by nurse care manager X 12 months to discuss HF care | • | • | |
| Systematically screened for suicidal ideation by blinded assessors | • | • | • |
| Nurse Care Managers | |||
| Phones patients at regular intervals X 12 months to provide depression care: (a) psychoeducation and treatment options (e.g., meds, workbook, mental health specialty referral); (b) discuss self-management workbook & review lessons, as applicable; (c) promote adherence/adjust antidepressant pharmacotherapy in concert with PCP; (d) monitor for treatment response and relapse and relay information to PCP; (e) screen for suicidal ideation (f) suggest/facilitate mental health specialty referral. |
• | ||
| Phones patients at regular intervals X 12 months to provide HF care per evidence-based guidelines: (a) basic education re: HF and treatment goals (e.g., 20–30 min aerobic activity 3–5x/wk); (b) encourage tobacco cessation, physical activity, sleep, medication adherence, etc.; (c) confirm use of guideline-recommended treatments and at goal (e.g., ACE-I, beta blocker, spironolactone, statins, BP control (<130/80)); (d) promote adherence/adjustment of HF pharmacotherapy with pt’s PCP/cardiologist; (e) promote outpatient self-monitoring of weight, BP, diet, doctor visits & review results; (f) monitor for treatment response & relapse and relay information to PCP/cardiologist; (g) suggest/facilitate PCP/cardiologist/ER referrals when appropriate. |
• | • | |
| Meets weekly with specialty team to discuss patient progress and treatment recommendations | • | • | |
| Provides case review recommendations to PCP and cardiologist for consideration | • | • | |
| Physicians | |||
| Informed of their Pt’s depression severity and treatment assignment (cardiologist & PCP) | • | • | • |
| Can initiate, adjust, or discontinue depression & HF pharmacotherapy | • | • | • |
| Advised by care manager to start/adjust antidepressant pharmacotherapy | • | ||
| Offered assistance referring their patient to a community mental health specialist | As appropriate | Upon request | Upon request |
| Receive regular progress reports from the care manager | • | • | |
| Informed if medical or psych. condition significantly worsens (e.g., chest pain, suicidality) | • | • | • † |
If detected during a routine blinded follow-up assessment
Non-Depressed Control Cohort
Given the nature of HF, we anticipated many study patients would develop progressive cardiovascular morbidity, become rehospitalized, generate health care costs, and die regardless of treatment assignment. Therefore, as in our earlier trial to treat post-CABG depression,47 we sought to enroll a randomly selected cohort of non-depressed patients to: (1) better evaluate the impact of co-morbid depression on the natural course of HFrEF; (2) determine the benefits derived from our interventions; and (3) control for secular changes in the management of HF over the course of our Trial (e.g., implementation of the Affordable Care Act, adjustments to the Medicare Part D “donut hole”, and use of neprilysin inhibitors).56
We programmed our data management system to randomly sample approximately one non-depressed participant for every 5–10 depressed participants recruited, stratified by study hospital and sex, and oversampled for race. To operationalize this, when a patient screened negative on the PHQ-2, was not using any antidepressants, and met all other inpatient eligibility criteria (Table 1), an automatically generated prompt on the nurse-recruiter’s tablet computer alerted them that this patient was randomly selected as a non-depressed control. If the patient provided their signed informed consent, then one of our trained telephone assessors would contact the patient two weeks after hospital discharge to administer the PHQ-9. We required patients score PHQ-9 <5 to remain in our non-depressed control cohort.
Interventions
Care Managers
Our study care managers were medical nurses with previous cardiac unit experience. At the beginning of their employment with our Trial, each received a week-long training to (1) update their knowledge on HF; (2) re-familiarize themselves with evidence-based pharmacotherapy for HF; (3) acquire motivational interviewing techniques; and (4) learn how to recognize and triage patients with suicidal behavior. In addition, we trained the care managers assigned to our “blended” intervention how to administer and interpret a PHQ-9 score, and about various treatment options for depression that could be provided in primary care. Lastly, our study nurses continued to enhance their knowledge of HF and depression through presenting their patients to our study clinicians at our weekly case review meetings.
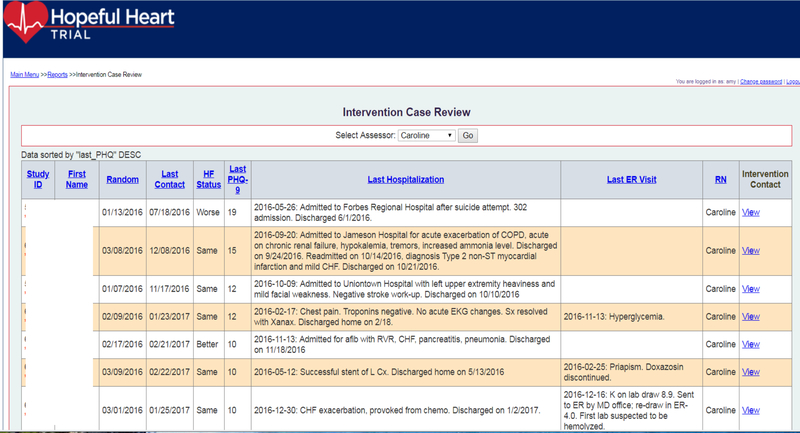
We organized our study nurses into separate eUC and “blended” teams of 2–3 nurses each to minimize contamination between study arms (Table 3). To help standardize nurses’ telephone interactions with study patients and the conduct of our case review sessions, we created an electronic registry that prompted them to systematically inquire about key aspects of their patients’ care and then record this information directly into the computer system for later retrieval (e.g., tobacco use, weight, blood pressure). While only the “blended” team’s registry included prompts about depression care, their registries were otherwise identical (see Figure 2 and Appendix, Supplemental Digital Content).
Figure 2.
Registry Screenshot. See Supplemental Digital Content for more screenshots.
Enhanced Usual Care (eUC)
At first telephone contact, the care manager informed the eUC patient about their randomization assignment and then reviewed their medical history with a particular emphasis on their cardiac history and medications to identify any potential gaps in HF care, medication side effects, and drug interactions. In addition, the nurse also reviewed the patient’s current HF symptoms, weight, blood pressure, diet, physical activity level, smoking status, and plans for follow-up medical appointments.
Based on the patient’s current medical condition, preferences, and clinical status, our eUC care managers typically encouraged: (1) adherence to guideline-recommended HF pharmacologic treatments; (2) limiting their salt intake and promoting physical activity, adequate sleep, and other lifestyle modifications; (3) maintaining their weight within a narrow range; (4) engaging in cardiac rehabilitation; and (5) confirming they have follow-up appointments scheduled with their PCP and cardiologist.
Depending upon the patient’s other comorbidities and treatment preferences, the study nurse made suggestions regarding the patient’s control of their blood pressure, cholesterol, diabetes, and encouraged cessation of tobacco use, and offered basic care coordination. Afterwards, the nurse care manager typically scheduled follow-up telephone calls with the patient approximately every 1–4 weeks for the first 3 months of care depending on the patient’s condition and needs, and then monthly for the remaining duration of our 12-month intervention (Table 3).
Blended Collaborative Care for Heart Failure and Depression
In addition to following our eUC protocol, our “blended” nurse care managers also provided collaborative care for depression using protocols developed for use in our prior studies. Specifically, they: (1) inquired about their patients’ psychiatric history including use of any nonprescription medications or herbal supplements used; (2) provided basic education about depression, its impact on cardiac disease, and various self-management strategies; (3) assessed their treatment preferences for depression; and (4) periodically readministered the PHQ-9 to monitor their mood symptoms.
In keeping with principles of shared decision-making, we offered patients a variety of treatment options depending on their severity and preference. These included: (1) changes in lifestyle (e.g., exercise, social support); (2) referral to a local mental health specialist; (3) initiation or adjustment of antidepressant pharmacotherapy prescribed under their PCPs’ direction and based on the recommendations of our specialty team; (4) a combination of the above; or (5) “watchful-waiting” if the patient’s mood symptoms were only mildly elevated (PHQ-9 score of 10–1455) and they had no prior history of depression (Table 3). They then decided on the goal(s) for both conditions (e.g., medication adherence, regular blood pressure control, physical activity, social support) with the patient, and presented this information at the weekly case review meeting. Our care managers worked closely with the participants’ physicians to convey any treatment recommendations made by the study cardiologist and psychiatrist, alerted them if the patient’s health declined, and sent quarterly progress reports.
Case Review
Our nurse intervention teams held weekly face-to-face case review meetings to review their registry of blended and eUC patients (Table 3). To minimize the potential for contamination between study arms, we: (1) conducted separate case review sessions with each collaborative care team; (2) assigned a separate study internist to each team; and (3) instructed our care managers not to discuss cases with care managers on the other team. Our study cardiologist (RR) and study psychiatrist (JFK; “blended” only) joined these sessions to provide their expert advice.
To facilitate our weekly hour-long review sessions, we developed a password-protected electronic registry in which our nurse care managers documented their contacts with participants. Registry screens enabled the care managers to: (1) organize each patient contact within a systematic and standardized structure; (2) document pertinent information directly into the data base; (3) view key information on all of their patients (e.g., contact dates, clinical notes, treatment(s) accepted, weight, blood pressure, HF pharmacotherapy) and additional quality measures depending upon treatment assignment (PHQ-9 scores, antidepressant pharmacotherapy); and (4) produce formatted notes to send to patients’ physicians that displayed a summary of their patient’s progress and other pertinent information (Figure 2 and eAppendix).
To share information with all case review participants, the presenting nurse utilized an LCD projector connected to a laptop to display their patient registry on a conference room wall. To further enhance the efficiency of our case review meetings, we programmed the registry to enable patients’ records to be sorted by: (1) date of study enrollment; (2) date of last care manager contact; (3) how the patient felt (“better, worse, same”); or (4) most recent PHQ-9 score (blended care only).
Following a discussion, the clinical management team typically formulated one to three treatment recommendations for the study nurse to convey back to the patient and to their physician(s) via the electronic medical record system if possible, or via telephone, fax, or mail, as appropriate, for approval. Patients and PCPs were free to accept or reject our treatment recommendations as well as obtain care outside of study treatment.
Usual Care
Our project coordinator telephoned all participants randomized to UC to inform them of their assignment status, and sent a letter to them and to their PCP indicating their study assignment and encouraging a follow-up appointment to discuss the patient’s elevated level of mood symptoms and HF. Otherwise, UC patients continued to receive care at the discretion of their physicians which could include adjustment of medications for HF and depression and/or referral to specialty cardiac or mental health care.
Concluding the Interventions
Approximately 9 months after randomization, the care manager began to prepare each blended and eUC patient for their 12-month end-of-intervention telephone call. In an effort to increase patient motivation and urgency during the final weeks of care management, the study nurse reminded all intervention patients that their telephone contacts would soon conclude and they should focus their attention on control of any remaining medical problems that might not be under good control (e.g., blood pressure, diabetes, tobacco use). Moreover, among patients randomized to our blended intervention, if the patient’s mood symptoms had completely remitted (PHQ-9 score of <5), then our care managers promoted continued adherence with the current treatment modality. However, if they had not sufficiently remitted (i.e., PHQ-9 score of >10), then our care managers may recommend the initiation or change in pharmacotherapy, or referral to a mental health specialist.
Before the last intervention call, the nurse care manager summarized the patient’s clinical course at the weekly case review session to illicit treatment recommendations beyond the intervention phase. Afterwards, the care manager sent the patient a letter describing their current level of depressive symptoms (if blended), care preferences, and our clinical management team’s final treatment recommendations. To promote adherence and to reduce the risk for miscommunications, we also sent a copy of this letter to the patient’s PCP and cardiologist.
Outcome Measures
To avoid bias, a team of trained research assessors blinded to the participant’s baseline depression status and randomization assignment (Figure 1) attempted to re-contact all study patients via telephone at 3-, 6-, and 12-months after randomization to systematically administer our follow-up assessment battery and inquire about the medical and psychiatric care the patient received (Table 2). To promote adherence, we paid participants $15 for each completed assessment (Total $60).
HRQoL is increasingly recognized as an important outcome, particularly for disorders causing disabling daily symptoms. Therefore we selected the generic and widely used Mental Component Summary of the Short-Form 12 Health Survey (SF-12 MCS)57 as our primary outcome measure and mental HRQoL as our primary outcome since: (1) we47 and others have demonstrated the sensitivity of the SF-12 MCS to changes in mood symptoms58,59; (2) it has been validated for use in patients with HF60; (3) it allowed us to measure the impact of our “blended” intervention on changes in mental HRQoL across both conditions simultaneously; and (4)administering the SF-12 will also provided us with a secondary generic measure of physical HRQoL, the SF-12 Physical Component Summary (SF-12 PCS) without adding to the respondent burden. Still, since a more disease-specific quality of life measure can sometimes provide a more sensitive indicator of program benefit, we also selected the 12-item Kansas City Cardiomyopathy Questionnaire (KCCQ-12)61 and the Patient-Reported Outcomes Measurement Information System (PROMIS) Depression scale62,63 as our secondary outcome measures of HF-related quality of life and mood symptoms, respectively (Table 2).
Adverse Event Monitoring Process
We expected a sizable number of emergency room visits, hospitalizations, and deaths in our study cohort and set-up procedures to collect this information in a timely manner. Specifically, we systematically monitored all study patients’ psychiatric and medical status through periodic telephone assessments, and set-up procedures for discharge summaries to be automatically emailed to our project coordinator within 48 hours of discharge from one of over 20 UPMC acute care hospitals located across western Pennsylvania. Moreover, our study staff advised patients in distress to immediately telephone their PCP and/or cardiologists’ for further advice, present to an emergency room, or call “911” given the seriousness of HF.
Adverse Psychiatric Event Monitoring
We programmed our paperless data management system so that whenever suicidal ideation was uncovered during a routine blinded assessment or care management call and entered into the appropriate electronic form, it would automatically launch of our electronic suicide protocol. It systematically guided our staff through a series of probes to determine the frequency, chronicity, content, and threat level the ideation posed (e.g., access to guns, history of previous attempts).64
If the protocol classified the patient at moderate or high risk, then the staff member immediately contacted the study psychiatrist (J.F.K.) to review the information and to determine the level of attention the symptoms warranted. Depending on the situation, the psychiatrist telephoned the patient directly to elicit further details or provided the care manager with guidance on managing the situation. The psychiatrist or staff member then informed the patient’s PCP about the patient’s suicidal ideation so that he/she could contact the patient and arrange follow-up. Although we did not otherwise directly interact with depressed patients assigned to UC or with their physician(s) to direct treatment, we offered to assist any patient or clinician regardless of intervention status by providing the names and contact information of local mental health professionals and by answering clinician’s questions about their patient’s psychiatric condition. Afterwards, we telephoned patients 1, 3, 7, and 30 days later to monitor their clinical status and confirm follow-up with our recommended treatment plan.
Vital Status
We took several steps to systematically confirm vital status on all study patients. First, when an assessor was unable to reach a patient for their 12-months assessment and we had no prior record of the patient’s death, we searched the electronic medical record for evidence the patient was still alive (e.g., an office visit or lab result after the 12-month date). If we were unable to determine the patient was alive, then the assessor telephoned the secondary contacts and PCP named by the patient at the time of enrollment. If still unsuccessful, the assessor searched obituaries, social media, and other online records to confirm vital status.
Key Events Classifications
We sent all reports of deaths, ER visits, and hospitalizations (“key events”) to a physician-staffed Adjudication Committee for review and classification, and then summaries of these reports to our external Data Safety Monitoring Board (DSMB) for their later review and consideration.
Using a process we developed earlier,65 the project coordinator forwarded documentation of the potential key event (e.g., hospital discharge records, death certificates, obituaries) and our abstraction form to two physician members of our Adjudication Committee who were blinded as to the patient’s baseline depression status, treatment assignment, and other identifiable data. They independently reviewed each potential key event and then: (1) classified the cause of the event as cardiac, cardiovascular, psychiatric, or “other”; and (2) described the event (e.g., decompensated heart failure, stroke, infection). If the two physician-reviewers were in complete agreement, then their classification of the event was automatically entered into our study database. However, if they were not in complete agreement (e.g., “cardiovascular” vs. “other”), then the event was brought to a third physician Committee member for a blinded tie-breaking determination and final adjudication.
Data Management
As with our previous studies, we equipped each nurse recruiter/care manager with a tablet computer installed with a program that guided them through our recruitment process using formatted data entry questionnaires programmed with skip patterns, drop-down menus, check-off boxes, and error checking routines that monitored forms for out-of-range values and missing data.
Our study assessors used similar electronic data entry forms to conduct their blinded assessments on desktop computers (Table 2). Our data management system prompted them with automated reminders at the appropriate time to ensure each assessment was performed according to schedule. It could also produce up-to-date reports on recruitment, assessment completion, and serious adverse events monitoring to enable our research team, local IRB, study sponsor, and DSMB to monitor our Trial’s progress.
We digitally recorded all assessor and care manager calls and conducted spot-checks of these recordings to (1) confirm participants’ responses were rated accurately and corresponded with the information entered into our paperless data-management system; (2) review interactions with suicidal participants; (3) provide feedback to study staff on their performance; and (4) train new staff.
Power and Sample Size
We designed our Trial to compare the effectiveness of “blended” collaborative care to both UC and to eUC, the former to maximize the potential ES difference produced by the “blended” intervention and the latter to control for the non-specific effects of telephone contacts from our care managers and because treating HF may also confer some additional benefit on HRQoL and mood.51 As women with cardiac disease exposed to a depression intervention may experience little benefit and perhaps fare worse than women exposed to a control condition or to men,20,47,66–68 we powered our trial to conduct within sex analyses on our co-primary hypotheses, and assumed a 50:50 sex balance in our study cohort to simplify our power calculations.
For co-primary hypothesis A, we estimated that our “blended” treatment strategy could produce a 0.50 ES or greater improvement on the SF-12 MCS at 12-months follow-up vs. UC (Figure 1). Based on an 80% 12-month assessment completion rate, intent-to-treat (ITT) analyses, and two-sided alpha α = 0.05, 125 “blended” and 62 UC depressed men (or women) would provide: (a) 80% power to detect a ≥ 0.50 ES improvement within sex improvement on the SF-12 MCS or another continuous outcome measures; and (b) >98% power to detect the same ES improvement using our full sample (250 “blended” and 125 UC).
For co-primary hypothesis B to determine whether any observed improvements in mHRQoL among participants receiving the “blended” intervention were due to treatment for depression and not the non-specific effects of additional monitoring, patient expectations, or improved delivery of guideline-based HF care, we estimated that our “blended” intervention would produce a 0.30 ES or greater improvement on the SF-12 MCS at 12-months compared to eUC. Using ITT and the same other assumptions as for co-primary hypothesis A, then 250 patients per treatment arm would provide: (a) 85% power to detect a ≥ 0.30 ES improvement between our “blended” and eUC arms on the SF-12 MCS; and (b) 80% power to detect a ≥ 0.40 ES within gender improvement (N=125 men (or women)/arm).
Statistical Analysis
We will first examine our data by study arm in univariate fashion using cross tabulations, histograms, and box plots applying standard statistical tests to assess covariate balance by randomization status and recruitment site, and adjusting for baseline differences in our multivariable models where appropriate. To handle missing data, we will first investigate the missingness process and investigate the reasons for dropouts, and then use a likelihood-based or multiple imputation-based procedure if “missing at random” is confirmed69 or consider selection models with a logistic dropout process or another model if the missingness (dropout) is nonignorable (missing not at random).70
We will then evaluate the ITT effectiveness of our interventions to address our co-primary hypotheses on HRQoL and a broad variety of other outcomes of interest (e.g., PROMIS Depression, KCCQ) using mixed-effects repeated measures models with time (random) sex, treatment, and interaction terms. We will also evaluate time to first hospitalization, death, and composite major adverse cardiac event (all-cause and cardiovascular-related deaths and rehospitalizations) using Kaplan-Meier survival analyses71 with non-directional 2-tailed log-rank tests to evaluate differences by study arm. Additionally, we will examine differences between treatment arms on various process and quality of care measures (e.g., numbers of care manager contacts, use of renin-angiotensin system inhibitors, beta-blockers, statins, and antidepressant medications), health services utilization, and work outcomes. To better understand the impact of co-morbid depression on HF, we will apply similar ITT techniques to describe and compare our non-depressed study cohort to our depressed study patients. Following submission of the main ITT papers from our Trial, we plan to develop a number of key secondary papers that will describe the impact of patient recovery, patient choice of treatment (e.g., use of and type of antidepressant pharmacotherapy, counseling, cardiac rehabilitation, and use of beta-blockers), neighborhood, and other variables (new vs. recurrent episode of depression) on a variety of patient outcomes to better understand our data and further guide clinicians, policymakers, and inform new avenues of investigation.
Economic Assessment
We obtained signed consent from each patient at the time of study enrollment allowing us to later obtain medical claims data from their insurance carrier. We will use these consents to obtain information regarding the types, dates, and costs of services patients received (e.g., inpatient hospital stay, outpatient physician visit), and Current Procedural Terminology (CPT) codes for each episode of care.
As in our earlier efforts,48 we will model our cost-effectiveness analyses from the perspective of the health insurance payer as this stakeholder is likely to be most influential in supporting future programs that resembe our interventions.50 To facilitate comparisons between the incremental cost-utility ratio of our intervention to other medical interventions, we will transform our SF-12 data into a preference-based utility to calculate quality-adjusted life years (QALYs), and then divide the incremental range of QALYs into our point-estimate of costs to estimate the incremental cost per QALY between intervention arms.72 Finally, we will conduct sensitivity analyses to analyze the robustness of our assumptions (e.g., varying the costs of our intervention strategy) to better establish the “business case” for providing depression care to HF patients.
Discussion
Improving chronic illness care for medically complex patients is one of the major challenges facing medicine today as patients with multiple chronic diseases account for the majority of health care costs. Indeed, 80% of HF patients have four or more chronic conditions, making it impractical to deploy separate collaborative care programs for each.38,73
The Hopeful Heart Trial is the first randomized clinical trial to evaluate the effectiveness of a “blended” model of collaborative care for treating both depression and HF simultaneous in patients with heart failure. If proven effective and cost-effective, our “blended” care approach for treating HF and co-morbid depression may have profound implications for improving chronic illness care and stimulate development of “blended” interventions for other clusters of related medical conditions commonly encountered in routine practice.
At the start, we designed our “blended” collaborative care intervention so it could later be delivered at-scale through existing organized systems of HF care management by: (1) using a simple, validated, two-stage depression screening procedure52,53; (2) delivering our intervention to patients via telephone; (3) scheduling regular follow-up and motivational support calls; (4) promoting delivery of guideline-recommended HF and depression care; (5) considering patients’ treatment preferences and priorities; (6) holding weekly case review sessions with our nurse care managers and study clinicians; and (7) coordinating care across patients’ multiple treating physicians.
Also innovative to our study design, Hopeful Heart is the first collaborative care trial for depression to incorporate an active attention control group in addition to a “usual care” control group. This design feature has been notably lacking in previous trials of collaborative care, including ours, and will help establish whether any improvements in patient outcomes are specifically due to treatment for depression rather than to any additional monitoring, patient expectations, improved medical care, or other factors.51 Furthermore, as in our earlier study to treat post-CABG depression,47 we included a randomly selected cohort of non-depressed HF patients to better evaluate the impact of co-morbid depression on the natural course of HF, the benefits derived from our study interventions, and control for secular changes in the management of HF over the course of our trial.
The Hopeful Heart Trial has several limitations. First, we designed our project to address our co-primary hypotheses on mental HRQoL and not to detect differences in major adverse clinical events (MACE) or mortality that could hasten adoption of our treatment approach if proven effective. Nevertheless, we collected information on MACE to determine whether a larger multi-center trial powered on “hard” outcomes to establish whether treatment for depression can reduce cardiovascular morbidity and mortality remains feasible. Next, we limited enrollment to hospitalized patients with HFrEF rather than restrict enrollment to patients hospitalized for new or worsening HFrEF or take HF patients with preserved ejection fractions (HFpEF). While we may have enrolled patients with just mildly reduced EFs (40–45%), we still required all enrolled patients to have an objectively confirmed EF of ≤45% rather than recruit a mix of HFrEF and HFpEF patients including some who may have been mislabeled as having HF. Next, we required PHQ-2 screen-positive patients to score 10 or higher on the PHQ-9 two-weeks following hospital discharge rather than a formal diagnosis of depression prior to randomization. While a PHQ-9 score may remain elevated in HF patients for reasons other than depression (false positive), this cut-off score has been validated among cardiac versus a gold-standard clinical interview (54% sensitive, 90% specific),53 an American Heart Association Science Advisory recommended use of the instrument to follow-up on a positive PHQ-2 screen,52 and it has been used in a growing number of research studies to evaluate depression. Moreover, unlike a psychiatric interview, the PHQ-9 can be readily administered by non-mental health professionals and is therefore likely to be used in routine clinical practice if we are able to demonstrate the effectiveness and cost-effectiveness of our treatment strategy. Finally, we considered a 2×2 factorial study design that added a “collaborative care for depression” arm. However, a 2×2 design required us to enroll approximately 250 additional depressed HF patients to maintain equivalent study power thereby increasing Project’s costs, and we believe stand-alone depression treatment programs for HF patients are less likely to be deployed than “blended” programs that could be delivered through an existing HF program.
Since Hopeful Heart Trial recruitment commenced in April 2014, there have been several advancements in care that may be worth considering. First, we relied on workbooks74 to inform patients about self-care for depression. However, substituting those workbooks with the use of computerized cognitive behavioral therapy programs provided through a collaborative care program may be a more effective and scalable treatment option.75,76 Next, although our care managers encouraged activities known to improve mood and HRQoL such as cardiac rehabilitation and physical activity,77 they relied on patient self-report and did not objectively monitor their patients’ level of physical activity with a wearable monitoring device, smartphone, or other device.78 Moreover, aside from recommending a heart-healthy low salt diet, we did not provide specific, patient-centered dietary advice. Finally, while we recommended generic medications for HF and depression as much as possible, many study patients were financially challenged to follow all treatment recommendations and we did not provide free samples of any medications.
Conclusion
Improving chronic illness care for medically complex patients is one of the major challenges facing medicine today as patients with multiple chronic diseases account for the majority of health care costs. The Hopeful Heart Trial provides trial-derived evidence on whether a nurse-led and telephone-delivered “blended” collaborative care intervention for treating both depression and HF that could be provided at-scale through existing HF programs is more effective at improving clinical outcomes than either collaborative care for HF alone or doctors’ usual care for these conditions.
Supplementary Material
Acknowledgments
Role of the Sponsor: All work described was supported by a grant from the National Heart Lung and Blood Institute (R01 HL114016). The funding source had no role in the design, conduct, analysis, or reporting of our study or in the preparation, review, or decision to submit this manuscript for publication.
Footnotes
Trial registration: ClinicalTrials.gov identifier NCT02044211. https://www.clinicaltrials.gov/ct2/show/NCT02044211?term=Rollman&rank=1
References
- 1.Heart Disease and Stroke Statistics—2019 Update: A report from the American Heart Association. Circ. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. [DOI] [PubMed] [Google Scholar]
- 3.Freedland KE, Rich MW, Skala JA, Carney RM, Davila-Roman VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–128. [DOI] [PubMed] [Google Scholar]
- 4.Kop WJ, Synowski SJ, Gottlieb SS. Depression in heart failure: biobehavioral mechanisms. Heart Fail Clin. 2011;7:23–38. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38:199–205. [DOI] [PubMed] [Google Scholar]
- 6.Turvey CL, Schultz K, Arndt S, Wallace RB, Herzog R. Prevalence and correlates of depressive symptoms in a community sample of people suffering from heart failure. JAGS. 2003;50:2003–2008. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb SS, Kop WJ, Ellis SJ, Binkley P, Howlett J, O’Connor C, Blumenthal JA, Fletcher G, Swank AM, Cooper L, HF-Action Investigators. Relation of depression to severity of illness in heart failure (from Heart Failure And a Controlled Trial Investigating Outcomes of Exercise Training [HF-ACTION]). Am J Cardiol. 2009;103:1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedland KE, Carney RM, Rich MW. Effect of depression on prognosis in heart failure. Heart Fail Clin. 2011;7:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherwood A, Blumenthal JA, Trivedi R, Johnson KS, O’Connor CM, Adams KF Jr., Dupree CS, Waugh RA, Bensimhon DR, Gaulden L, Christenson RH, Koch GG, Hinderliter Alan L. Relationship of depression to death or hospitalization in patients with heart failure. Arch Intern Med. 2007;167:367–373. [DOI] [PubMed] [Google Scholar]
- 10.Rollman BL, Herbeck Belnap B, Mazumdar S, Houck P, McNamara DM, Alvarez RJ, Schulberg HC, Reynolds CF. A positive depression screen among hospitalized heart failure patients is associated with elevated 12-month mortality. J Cardiac Fail. 2012;18:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams J, Kuchibhatla M, Christopher EJ, Alexander JD, Clary GL, Cuffe MS, Califf RM, Krishnan RR, O’Connor CM, Wei Jiang. Association of depression and survival in patients with chronic heart failure over 12 Years. Psychosomatics. 2012;53:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasure-Smith N, Lesperance F, Habra M, Talajic M, Khairy P, Dorian P, Roy D. Elevated depression symptoms predict long-term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:134–140, 133p following 140. [DOI] [PubMed] [Google Scholar]
- 13.Freedland KE, Hesseler MJ, Carney RM, Steinmeyer BC, Skala JA, Davila-Roman VG, Rich MW. Major Depression and Long-Term Survival of Patients With Heart Failure. Psychosomatic Med. 2016;78:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor CM, Abraham WT, Albert NM, Claire R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2008;156:662–673. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 16.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, Zakhary B, Stough WG, Arias RM, Rivelli SK, Krishnan R. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. JACC. 2010;56:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Echols MR, Jiang W. Clinical trial evidence for treatment of depression in heart failure. Heart Fail Clin. 2011;7:81–88. [DOI] [PubMed] [Google Scholar]
- 18.Thombs BD, de Jonge P, Coyne JC, Whooley MA, Frasure-Smith N, Mitchell AJ, Zuidersma M, Eze-Nliam C, Lima BB, Smith CG, Soderlund K, Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA. 2008;300:2161–2171. [DOI] [PubMed] [Google Scholar]
- 19.Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT Jr., Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, McLvor M. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. [DOI] [PubMed] [Google Scholar]
- 20.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289:3106–3116. [DOI] [PubMed] [Google Scholar]
- 21.Van Melle JP, de Jonge P, Honig A, Schene AH, Kuyper AMG, Crijns HJGM, Schins A, Tulner D, van den Berg MP, Ormel J. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460–466. [DOI] [PubMed] [Google Scholar]
- 22.Strik JJ, Honig A, Lousberg R, Lousberg AH, Cheriex EC, Tuynman-Qua HG, Kuijpers PM, Wellens HJ, Van Praag HM. Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med. 2000;62:783–789. [DOI] [PubMed] [Google Scholar]
- 23.Lesperance F, Frasure-Smith N, Koszycki D, Laliberte MA, van Zyl LT, Baker B, Swenson JR, Ghatavi K, Abramson BL, Dorian P, Guertin MC. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367–379. [DOI] [PubMed] [Google Scholar]
- 24.Angermann CE, Gelbrich G, Störk S, Gunold H, Edelman F, Wachter R, Schunkert H, Graf T, Kindrmann I, Haass M, Blankenberg S, Pankuweit S, Prettin C, Gottwik M, Bohm M, Faller H, Deckert J, Ertl G. Effect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression: The MOOD-HF randomized clinical trial. JAMA. 2016;315:2683–2693. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, West SL, Swindle R, Gilsenan A, Eckert GJ, Dolor R, Stang P, Zhou XH, Hays R, Weinberger M. Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: A randomized trial. JAMA. 2001;286:2947–2955. [DOI] [PubMed] [Google Scholar]
- 26.Cooper-Patrick L, Powe NR, Jenckes MW, Gonzales JJ, Levine DM, Ford DE. Identification of patient attitudes and preferences regarding treatment of depression. J Gen Intern Med. 1997;12:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwight-Johnson M, Sherbourne CD, Liao D, Wells KB. Treatment preferences among depressed primary care patients. J Gen Intern Med. 2000;15:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol. 1995;14:88–90. [DOI] [PubMed] [Google Scholar]
- 29.Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–1823. [DOI] [PubMed] [Google Scholar]
- 30.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Raue P, Friedman SJ, Meyers BS. Perceived stigma as a predictor of treatment discontinuation in young and older outpatients with depression. Am J Psychiatry. 2001;158:479–481. [DOI] [PubMed] [Google Scholar]
- 31.Huffman JC, Adams CN, Celano CM. Collaborative Care and Related Interventions in Patients With Heart Disease: An Update and New Directions. Psychosomatics. 2018;59(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. [DOI] [PubMed] [Google Scholar]
- 33.Fihn SD, Bucher JB, McDonell MM, Diehr P, Rumsfeld JS, Doak M, Doughtery C, Gerrity M, Heidenreich P, Larsen G, Lee PI, Lucas L, McBryde C, Nelson K, Plomondon ME, Stadius M, Bryson C. Collaborative Care Intervention for Stable Ischemic Heart Disease. Arch Intern Med. 2011;171:1471–1479. [DOI] [PubMed] [Google Scholar]
- 34.Lee DS, Stukel TA, Austin PC, Alter DA, Schull MJ, You JJ, Chong A, Henry D, Tu JV. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122:1806–1814. [DOI] [PubMed] [Google Scholar]
- 35.Katon WJ, Lin EHB, Von Korff M, Ciechanowski P, Ludman EJ, Young B, Peterson D, Rutter CM, McGregor M, McCulloch D. Collaborative Care for Patients with Depression and Chronic Illnesses. N Eng J Med. 2010;363:2611–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huffman JC, Mastromauro CA, Beach SR, Celano CM, DuBois CM, Healy BC, Suarez L, Rollman BL, Januzzi JL. Collaborative care for depression and anxiety disorders in patients with recent cardiac events: the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized clinical trial. JAMA Intern Med. 2014;174:927–935. [DOI] [PubMed] [Google Scholar]
- 37.Huffman JC, Mastromauro CA, Sowden G, Fricchione GL, Healy BC, Januzzi JL. Impact of a depression care management program for hospitalized cardiac patients. Circ Cardiovasc Qual Outcomes. 2011;Cardiovascular Quality & Outcomes. 4:198–205. [DOI] [PubMed] [Google Scholar]
- 38.Katon W, Unützer J, Wells K, Jones L. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiat. 2010;32:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroenke K, Krebs EE, Wu J, Yu Z, Chumbler NR, Bair MJ. Telecare collaborative management of chronic pain in primary care: a randomized clinical trial. JAMA. 2014;312:240–248. [DOI] [PubMed] [Google Scholar]
- 40.Aubert R, Herman W, Waters J, Moore W, Sutton D, Peterson BL, Bailey CM, Koplan JP. Nurse case management to improve glycemic control in diabetic patients in a health maintenance organization. A randomized controlled trial. Ann Intern Med. 1998;129:605–612. [DOI] [PubMed] [Google Scholar]
- 41.Rollman BL, Belnap BH, Mazumdar S, Abebe KZ, Karp JF, Lenze EJ, Schulberg HC. Telephone-Delivered Stepped Collaborative Care for Treating Anxiety in Primary Care: A Randomized Controlled Trial. J Gen Intern Med. 2017;32:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katon WJ, Unützer J. Health reform and the Affordable Care Act: The importance of mental health treatment to achieving the triple aim. J Psychosom Res. 2013;74:533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Quarterly. 1996;74:511–544. [PubMed] [Google Scholar]
- 44.Coleman K, Austin BT, Brach C, Wagner EH. Evidence On The Chronic Care Model In The New Millennium. (Millwood). 2009;28:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner EH. More than a case manager. Ann Intern Med. 1998;129(8):654–656. [DOI] [PubMed] [Google Scholar]
- 46.Wagner EH. Deconstructing heart failure disease management. Ann Intern Med. 2004;141:644–646. [DOI] [PubMed] [Google Scholar]
- 47.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, Kapoor WN, Schulberg HC, Reynolds CF III. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302:2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donohue JM, Belnap BH, Men A, He F, Roberts MS, Schulberg HC, Reynolds CF III, Rollman BL. Twelve-month cost-effectiveness of telephone-delivered collaborative care for treating depression following CABG surgery: a randomized controlled trial. Gen Hosp Psychiatry. 2014;36:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. [DOI] [PubMed] [Google Scholar]
- 50.Katon W, Russo J, Lin EH, Schmittdiel J, Ciechanowski P, Ludman E, Peterson D, Young B, Von Korff M. Cost-effectiveness of a Multicondition Collaborative Care Intervention: A Randomized Controlled Trial. Arch Gen Psychiatry. 2012;69:506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and Unusual Care: Existing Practice Control Groups in Randomized Controlled Trials of Behavioral Interventions. Psychosom Med. 2011;73:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lichtman JH, Bigger JT Jr., Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lesperance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. [DOI] [PubMed] [Google Scholar]
- 53.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease. Am J Cardio. 2005;96:1076–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 55.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 57.Ware JE Jr., Kosinski M, Keller SD. A 12-item short-form Health Survey; Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 58.Freedland KE, Carney RM, Rich MW, Steinmeyer BC, Rubin EH. Cognitive behavior therapy for depression and self-care in heart failure patients: A randomized clinical trial. JAMA Internal Med. 2015;175:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Davila-Roman VG, Steinmeyer BC, Hogue CW Jr. Treatment of Depression After Coronary Artery Bypass Surgery: A Randomized Controlled Trial. Arch Gen Psychiatry. 2009;66:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters-Klimm F, Kunz CU, Laux G, Szecsenyi J, Muller-Tasch T. Patient- and provider-related determinants of generic and specific health-related quality of life of patients with chronic systolic heart failure in primary care: a cross-sectional study. Health Qual Life Outcomes. 2010;8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spertus JA, Jones PG. Development and Validation of a Short Version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi SW, Reise SP, Pilkonis PA, Hays RD, Cella D. Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Qual Life Res. 2010;19:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, Riley W, Cella D. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J Clin Epidemiol. 2016;73:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbeck Belnap B, Schulberg HC, He F, Mazumdar S, Reynolds CF III, Rollman BL. Electronic protocol for suicide risk management in research participants. J Psychosom Res. 2015;78:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rollman BL, Belnap Herbeck B, Lemenager M, Mazumdar S, Schulberg HC, Reynolds CF III. The Bypassing the Blues Treatment Protocol: Stepped Collaborative Care for Treating Post-CABG Depression. Psychosom Med. 2009;71:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom Med. 2010;72:842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frasure-Smith N, Lesperance F, Prince RH, Verrier P, Garber RA, Juneau M, Wolfson C, Bourassa MG. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet. 1997;350(9076):473–479. [DOI] [PubMed] [Google Scholar]
- 68.Mittag O, China C, Hoberg E, Juers E, Kolenda K-D, Richardt G, Maurischat C, Raspe H. Outcomes of cardiac rehabilitation with versus without a follow-up intervention rendered by telephone (Luebeck follow-up trial): overall and gender-specific effects. Int J Rehabil Res. 2006;29:295–302. [DOI] [PubMed] [Google Scholar]
- 69.Houck PR, Mazumdar S, Koru-Sengul T, Tang G, Mulsant BH, Pollock BG, Reynolds CF III. Estimating treatment effects from longitudinal clinical trial data with missing values: comparative analyses using different methods. Psychiatry Res. 2004;129:209–215. [DOI] [PubMed] [Google Scholar]
- 70.Mazumdar S, Tang G, Houck PR, Dew MA, Begley AE, Scott J, Mulsant BH, Reynolds CF III. Statistical analysis of longitudinal psychiatric data with dropouts. J Psychiatr Res. 2007;41:1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 72.Hebert PL, Sisk JE, Wang JJ, Tuzzio L, Casabianca JM, Chassin MR, Horowitz C, McLaughlin MA. Cost-Effectiveness of Nurse-Led Disease Management for Heart Failure in an Ethnically Diverse Urban Community. Ann Intern Med. 2008;149:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues in Clinical Neuroscience. 2011;13:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katon W, Ludman E, Simon G. The Depression Helpbook. Boulder, CO: Bull Publishing; 2002. [Google Scholar]
- 75.Rollman BL, Herbeck Belnap B, Abebe KZ, Spring MB, Rotondi AJ, Rothenberger SD, Karp JF. Online Collaborative Care for Treating Mood and Anxiety Disorders in Primary Care: A Randomized Clinical Trial. JAMA Psych. 2018;75:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosenberg T Depressed? Try Therapy Without the Therapist New York Times; June 19, 2015. [Google Scholar]
- 77.Blumenthal JA, Babyak MA, O’Connor C, Keteyian S, Landzberg J, Howlett J, Kraus W, Gottlieb S, Blackburn G, Swank A, Whellan DJ. Effects of exercise training on depressive symptoms in patients with chronic heart failure: the HF-ACTION randomized trial. JAMA. 2012;308:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313. [DOI] [PubMed] [Google Scholar]
- 79.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 80.Spitzer RL, Williams JBW, Kroenke K, Linzer M, Verloin deGruy F III, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 81.Freedland KE, Skala JA, Carney RM, Raczynski JM, Taylor CB, Mendes De Leon CF, Ironson G, Youngblood ME, Rama Krishnan KR, Veith RC. The Depression Interview and Structured Hamilton (DISH): Rationale, Development, Characteristics, and Clinical Validity. Psychosomatic Med. 2002;64:897–905. [DOI] [PubMed] [Google Scholar]
- 82.Sheldon C, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 83.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality & Social Psychology. 1994;67:1063–1078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.