Abstract
Background:
Older patients with advanced cancer who are 100% certain they will be cured pose unique challenges for clinical decision-making, but the prevalence and correlates of absolute certainty about curability (ACC) are unknown.
Methods:
Cross-sectional data were collected in a geriatric assessment trial. ACC was assessed by asking patients: “What do you believe are the chances that your cancer will go away and never come back with treatment?” Response options were 100% (coded ACC), >50%, 50/50, <50%, 0%, and uncertain. Willingness to bear adversity in exchange for longevity was assessed by asking patients to consider trade-offs between survival and two clinical outcomes that varied in abstractness: a) maintaining quality of life (QoL; an abstract outcome) and b) specific treatment-related toxicities (e.g., nausea/vomiting, worsening memory). Logistic regression was used to assess the independent associations of willingness to bear adversity with ACC.
Results:
Of the 524 patients (age range: 70-96), 5.3% reported that there was a 100% chance that their cancer will be cured (ACC). ACC was not significantly associated with willingness to bear treatment-related toxicities, but was more common among patients who were willing to trade QoL for survival (Adjusted Odds Ratio: 4.08, 95% Confidence Interval 1.17-14.26).
Conclusions:
Patients who were more willing to bear adversity in the form of an abstract state, decreased QoL, were more likely to demonstrate ACC. Although conversations about prognosis should be conducted with all patients, those who are willing to trade QoL for survival may especially benefit from conversations that focus on values and emotions.
Keywords: Survival, quality of life, treatment-related toxicity, trade-offs, beliefs about curability, absolute certainty about curability
Precis:
Patients who were more willing to bear adversity in the form of an abstract state, decreased quality of life, were more likely to believe, with 100% certainty, that they could be cured. Although conversations about prognosis should be conducted with all patients, those who are willing to trade quality of life for survival may especially benefit from conversations that focus on values and emotions.
Introduction
Many patients with advanced cancer are older adults who also have significant comorbidities and functional impairments1 which increase the complexity of care and contribute to poorer outcomes. To allow informed decision-making about treatment, it is important to ensure that older patients have an accurate understanding of the curability of their cancer. Unfortunately, it has proven difficult to help patients understand that their disease is incurable, 2 and recent research has begun to offer explanations.3 Disease understanding has been shown to be affected by patients’ abstract beliefs, values, and worldviews,4 and some patients privilege those perspectives over scientific data5 in absolutist ways, leaving no room for uncertainty. Studies evaluating patients’ perceptions of the curability of advanced cancer generally have patients report their responses in the form of percentages.6,7 We are aware of no studies that have examined the prevalence of absolute certainty about curability (ACC), believing with 100% certainty that one will be cured.
Estimating the prevalence of ACC and identifying its correlates are crucial, as patients who are 100% certain they will be cured pose unique challenges for physicians. For example, it may be more difficult to encourage a patient to accept symptom-directed care or limit aggressive medical interventions if the patient is absolutely certain they will be cured. It is likely that these patients have higher healthcare needs,8 utilize more resources,9 and require more time.
Many medical interventions are invasive, dangerous, and potentially harmful, but patients may be more willing to bear these consequences in exchange for the prospect of a longer life. Trade-off preferences are presumed to vary widely, with some older patients perceiving outcomes such as compromised function, impaired cognition, adverse medication reactions, and poor quality of life (QoL) as fates “worse than death.”10 In contrast, other patients are willing to trade these adversities in exchange for living longer. Although it has been shown that beliefs about curability may be affected by patients’ abstract beliefs, values, and worldviews,4 it is unknown whether patients’ trade-off preferences, which proxy abstract beliefs,11 are similarly associated with beliefs about curability. In this study, we examine the association between patients’ willingness to bear adversity in exchange for longevity and ACC.
Our premise is that patients’ trade-off preferences reflect the extent to which they want to be cured, and this desire for cure will be reflected in their numeric responses to questions about the perceived curability of their cancer. We hypothesized that patients who report greater willingness to bear adversity (treatment-related toxicity and decreased QoL) in exchange for longevity would be more likely to report that there was a 100% chance that their cancer will be cured, thereby demonstrating ACC. To this end, we estimate the prevalence of ACC and examine the relationships between trade-off preferences and ACC.
Methods
Study design
This is a cross-sectional analysis of baseline data from a national cluster-randomized controlled trial evaluating the effect of a standardized geriatric assessment (GA) on communication between older patients with advanced solid tumors or lymphoma, oncologists, and caregivers [University of Rochester Cancer Center (URCC); NCT02107443]. The study was conducted within the University of Rochester National Cancer Institute Community Oncology Research Program (NCORP) and enrolled patients and caregivers from 31 community oncology sites between October 2014 and April 2017.12 Prior to enrollment, the study was approved by the Research Subjects Review Board at University of Rochester and Institutional Review Boards at all of the individual NCORP affiliate sites. All patients and caregivers provided informed consent from to enrollment.
Participants and brief description of the primary study
URCC 13070 included patients who were aged ≥70 years, had a diagnosis of stage III/IV solid tumor or lymphoma that was considered by their treating oncologists to be incurable, were considering or receiving any kind of cancer treatment (any line), and had ≥1 impaired domain on GA (other than polypharmacy risk).13–16 Once informed consent was obtained, patients completed demographics and underwent a GA. Patients who met eligibility criteria completed additional measures as described below, which were also conducted at baseline. The unplanned secondary analyses reported here focus on data collected before patients were exposed to the standardized GA intervention or usual care.
Measures
Outcome variable: Beliefs about curability
Patients were asked about their beliefs regarding the curability of their cancer: “What do you believe are the chances that your cancer will go away and never come back with treatment?” Options were 100%, >50%, 50/50, <50%, 0%, or uncertain. This question was adapted from a prior study. 17 Those who did not provide any response were excluded from our analysis. The remaining patients were categorized into two groups: ACC (100%) versus “Other”.
Independent variables: trade-off preferences
We examined two types of trade-offs. The first type concerned trade-offs between non-abstract treatment-related toxicities and survival. Patients were asked to provide their responses to the following five scenarios: I would like to try treatments for my cancer if they could make me live longer, even if it is very likely they would a) have a high level of side effects (such as nausea/vomiting), b) make me bedbound and unable to use the bathroom without assistance, c) make me require more assistance from family and friends with completing daily activities (such as shopping and managing money), d) make my memory worse, e) cause me to become confused often so that I am not aware of my surroundings. For each of these five scenarios, response options were strongly agree, agree, neutral, disagree, and strongly disagree. We assigned a score of 1 if patients selected strongly agree or agree. The scores were summed to a total score that ranged from 0 to 5. A higher score indicated that patients were more likely to try treatments despite toxicity if the treatments would make them live longer.
The second type of trade-off focused on an abstract outcome, maintaining QoL, with no mention of cancer treatments. Patients responded to the following statement: “Maintaining my quality of life is more important to me than living longer.” Response options were strongly agree, agree, neutral, disagree, and strongly disagree. These responses were collapsed into three categories: strongly agree or agree, neutral, and disagree or strongly disagree.
Other covariates
Covariates included age, gender, race, education level, marital status, annual household income (≤$50,000 vs. >$50,000 vs. decline to answer; in US dollars), cancer type (breast, gastrointestinal, genitourinary, lung, other; extracted from the medical records by a Clinical Research Associate at each site), polypharmacy risks,18 and the presence (vs. absence) of impairments in the following seven GA domains: physical performance, functional status, comorbidity, cognition, nutrition, instrumental social support (e.g. someone to take you to the doctor if needed), and psychological health.19,20 GA impairments were defined using validated cut-points (Supplemental Table 1). GA domains were included as potential covariates because patients’ underlying medical, psychological, and social health may affect how patients process and understand information about their disease.
Two communication variables, the Perceived Efficacy in Patient-Physician Interactions (PEPPI) scale and patient’s recalled prognostic discussion with their oncologist, were included as potential covariates because the perceived quality and content of doctor-patient communication may influence prognostic understanding. The PEPPI is a 5-item questionnaire that assesses a patient’s confidence in his or her ability to communicate with his or her oncologist.21 Each question is rated on a 5-point Likert scale, 1 being not confident and 5 being very confident. Higher score indicates higher communication self-efficacy. For recalled prognostic discussion, patients were asked the following question: “To what extent have you discussed your prognosis with your doctor?” Options were completely, mostly, a little, or not at all.22 These options were further collapsed into two groups: completely or mostly vs. a little or not at all.
Statistical analyses
We used descriptive analyses to summarize the data. Bivariate analyses were initially used to evaluate the associations of patient trade-off preferences with ACC. We also explored the bivariate relationships of demographic, clinical, GA, and communication covariates with ACC. All variables in the bivariate analyses with a p-value ≤0.20 were entered into a multivariate logistic regression. A two-sided p value of <0.05 was considered statistically significant.
For sensitivity analyses, we removed patients who were uncertain about curability and performed a separate logistic regression to evaluate the association of trade-off preferences with ACC. In addition to conducting analyses on binary outcomes (ACC present vs. absent), we treated beliefs about curability as a 5-level ordinal variable (i.e. 0%, <50%, 50/50, >50%, and 100%) and used ordinal regression. All analyses were conducted using the SAS software (version 9.3).
Results
URCC 13070 enrolled 541 patients, 17 of whom did not respond to the question “What do you believe are the chances that your cancer will go away and never come back with treatment?” leaving a sample of 524 patients for this analysis.
The mean age was 76.6 [range: 70-96, standard deviation (SD): 5.2] and 51.3% were male. Most were married (64.5%), white (89.7%), and had some college education (51.9%). Over 49% had an annual household income of ≤$50,000. Approximately 71% recalled discussing their prognosis “completely” or “mostly” with their oncologist. Detailed description of the cohort is shown in Table 1.
Table 1:
Characteristics of the study sample
| All patients | ACC | Bivariate analyses with ACC | ||||
|---|---|---|---|---|---|---|
| N=524 | Yes (N=28) | No (N=496) | OR (95% CI) | P-value | ||
| Age, mean (SD) | 76.6 (5.2) | 77.8 (6.2) | 76.6 (5.2) | 1.04 (0.98-1.12) | 0.23 | |
| Gender | Male | 269 (51.3) | 12 (42.9) | 256 (51.6) | Reference | |
| Female | 255 (48.7) | 16 (57.1) | 240 (48.4) | 1.43 (0.67-3.09) | 0.36 | |
| Marital Status | Married | 338 (64.5) | 15 (53.6) | 322 (64.9) | Reference | |
| Other | 186 (35.6) | 13 (46.4) | 174 (35.1) | 1.62 (0.75-3.48) | 0.22 | |
| Race | White | 470 (89.7) | 21 (75.0) | 449 (90.5) | Reference | |
| Non-white | 54 (10.3) | 7 (25.0) | 47 (9.5) | 3.18 (1.29-7.89) | 0.01 | |
| Education | Some college or above | 272 (51.9) | 10 (35.7) | 262 (52.8) | Reference | 0.02d |
| High school graduate | 189 (36.1) | 10 (35.7) | 179 (36.1) | 1.46 (0.60-3.59) | 0.41 | |
| <High school | 63 (12.0) | 8 (28.6) | 55 (11.1) | 3.81 (1.44-10.09) | 0.007 | |
| Annual household incomea | >$50,000 | 163 (31.2) | 2 (7.1) | 160 (32.4) | Reference | 0.05d |
| ≤$50,000 | 257 (49.2) | 18 (64.3) | 239 (48.4) | 6.06 (1.39-26.48) | 0.02 | |
| Decline to answer | 102 (19.5) | 8 (28.6) | 95 (19.2) | 6.85 (1.43-32.93) | 0.02 | |
| Cancer typea | Breast | 68 (13.0) | 3 (10.7) | 65 (13.2) | Reference | 0.49d |
| Gastrointestinal | 123 (23.5) | 6 (21.4) | 117 (23.7) | 1.11 (0.27-4.59) | 0.88 | |
| Genitourinary | 69 (13.2) | 2 (7.1) | 67 (13.6) | 0.65 (0.11-4.00) | 0.64 | |
| Lung | 132 (25.2) | 6 (21.4) | 126 (25.5) | 1.03 (0.25-4.26) | 0.97 | |
| Other | 131 (25.1) | 11 (39.3) | 119 (24.1) | 1.99 (0.54-7.37) | 0.31 | |
| Physical performance | Impaired | 490 (93.3) | 26 (92.9) | 463 (93.4) | 0.89 (0.20-3.95) | 0.89 |
| Functional status | Impaired | 309 (59.0) | 12 (42.9) | 297 (59.9) | 0.50 (0.23-1.09) | 0.08 |
| Comorbidity | Impaired | 335 (64.0) | 17 (60.7) | 318 (64.1) | 0.87 (0.40-1.89) | 0.72 |
| Cognition | Impaired | 174 (33.2) | 7 (25.0) | 167 (33.7) | 0.66 (0.27-1.58) | 0.35 |
| Nutrition | Impaired | 315 (60.1) | 15 (53.6) | 300 (60.5) | 0.75 (0.35-1.62) | 0.47 |
| Instrumental social support | Impaired | 150 (28.6) | 15 (53.6) | 135 (27.2) | 3.09 (1.43-6.66) | 0.004 |
| Polypharmacy | Screen positive | 437 (83.4) | 20 (71.4) | 418 (84.3) | 0.47 (0.20-1.11) | 0.09 |
| Psychological health | Impaired | 131 (25.0) | 5 (17.9) | 126 (25.4) | 0.64 (0.24-1.72) | 0.37 |
| Recalled prognosisc | Completely/Mostly | 371 (71.2) | 22 (81.5) | 349 (70.7) | Reference | |
| A little/Not at all | 150 (28.8) | 5 (18.5) | 145 (29.4) | 0.55 (0.20-1.47) | 0.23 | |
| PEPPI, mean (SD) | 21.4 (3.4) | 21.7 (3.7) | 21.3 (3.4) | 1.03 (0.92-1.16) | 0.62 | |
| Adverse reaction vs. survival trade-off, mean (SD) | 2.4 (1.6) | 2.3 (1.3) | 2.3 (1.5) | 0.94 (0.73-1.22) | 0.64 | |
| Quality of life vs. survival trade-offb | Strongly agree/agree | 381 (72.9) | 18 (64.3) | 364 (73.5) | Reference | 0.39d |
| Neutral | 101 (19.3) | 6 (21.4) | 95 (19.2) | 1.27 (0.49-3.30) | 0.62 | |
| Strongly disagree/disagree | 41 (7.8) | 4 (14.3) | 36 (7.3) | 2.18 (0.70-6.78) | 0.18 | |
2 patients had missing data
1 patient had missing data
3 patients had missing data
Overall P-value
Abbreviations: ACC, absolute certainty about curability; OR, Odds Ratio; CI, Confidence Interval; PEPPI, Perceived Efficacy in Patient-Physician Interactions
Beliefs about curability and prevalence of ACC
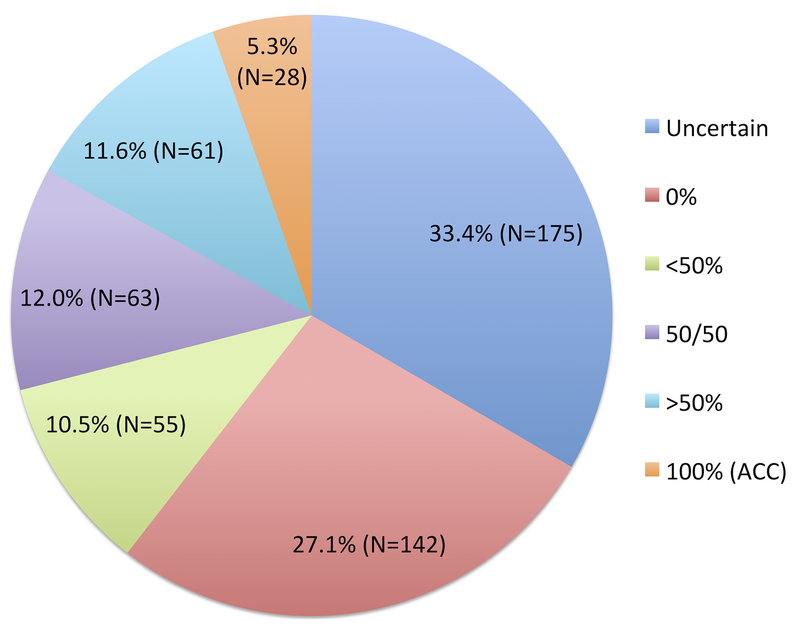
Figure 1 shows the distribution of beliefs about curability. When asked, “What do you believe are the chances that your cancer will go away and never come back with treatment”, 28 (5.3%) patients responded with 100%, demonstrating ACC.
Figure 1:
Distribution of beliefs about curability among older patients with advanced cancer who expressed beliefs (Question: What do you believe are the chances the cancer will go away and never come back with treatment?)
Multivariate analyses
Forty-one respondents (7.8%) disagreed or strongly disagreed with the statement “maintaining my quality of life is more important than living longer.” On multivariate analysis, those who disagreed/strongly disagreed with this statement were more likely to believe that there was a 100% chance that their cancer could be cured [Adjusted Odds Ratio (AOR) 4.08, 95% Confidence Interval (CI) 1.17-14.26] (Table 2). The association between trade-off preference in the form of treatment-related toxicity and ACC did not meet the pre-specified cut-off in bivariate analysis (Odds Ratio (OR) 0.94, 95% CI 0.73-1.22; P=0.64) for inclusion in the multivariate analysis (Table 1).
Table 2:
Multivariate model evaluating the associations of trade-off preferences and other covariates with absolute certainty about curability
| Multivariate analyses with ACC (N=521) | |||
|---|---|---|---|
| AOR (95% CI) | P-value | ||
| Race | White | Reference | - |
| Non-white | 3.40 (1.22-9.49) | 0.02 | |
| Education | Some college or above | Reference | 0.12a |
| High school graduate | 1.27 (0.49-3.32) | 0.62 | |
| <High School | 3.03 (1.03-8.94) | 0.04 | |
| Annual household income | >$50,000 | Reference | 0.09a |
| ≤$50,000 | 4.64 (0.99-21.72) | 0.05 | |
| Decline to answer | 6.41 (1.22-33.77) | 0.03 | |
| Functional status | Not impaired | Reference | - |
| Impaired | 0.41 (0.18-0.94) | 0.04 | |
| Social support | Not impaired | Reference | - |
| Impaired | 3.43 (1.49-7.91) | 0.004 | |
| Polypharmacy | Screen negative | Reference | - |
| Screen positive | 0.39 (0.15-1.01) | 0.05 | |
| Quality of life vs. survival trade-off | Strongly agree/agree | Reference | 0.06a |
| Neutral | 1.95 (0.69-5.52) | 0.21 | |
| Strongly disagree/disagree | 4.08 (1.17-14.26) | 0.03 | |
Overall P-value
Abbreviations: ACC, absolute certainty about curability; AOR, adjusted odds ratio; CI, confidence interval
ACC was more common among patients who were non-white vs. white (AOR 3.40, 95% CI 1.22-9.49), had <high school education vs. some college or above (AOR 3.03, 95% CI 1.03-8.94), and had impaired instrumental social support vs. not (AOR 3.43 95% CI 1.49-7.91). ACC was less common among patients who had impaired functional status vs. not (AOR 0.41, 95% CI 0.18-0.94) (Table 2).
Sensitivity analyses
In the logistic regression model excluding patients who were uncertain about curability, patients who were willing to trade QoL for survival were more likely to demonstrate ACC (AOR 4.27, 95% CI 1.11-16.46). In the ordinal regression model, patients who were willing to trade QoL for survival were more likely to respond with increasing certainty of curability (ordinal: AOR 2.32, 95% CI 1.10-4.91). Consistent with the primary findings, trade-off preferences between treatment-related toxicity and survival were not statistically significant in the ordinal and logistic regression models.
Discussion
In this cross-sectional analysis among older patients with advanced cancer who expressed beliefs about curability, we showed that a minority (5%) believed that there was a 100% chance that their cancer will go away and never come back with treatment. Patients who were more willing to trade QoL (an abstract concept) for survival were more likely to hold this absolutist belief, but there was no relationship between willingness to bear specific treatment-related toxicity and ACC.
Our findings help elucidate the intricate relationship between patients’ willingness to trade off QoL for survival and their beliefs about the curability of their cancer. More broadly, the results are inconsistent with the idea that disease understanding is the product of a dispassionate, rationale assessment by the patient of his or her situation. Rather, disease understanding is affected by patients’ beliefs, values, and worldview, as well as information provided by their oncologist. However, merely sharing prognostic information will not be sufficient to ensure that patients with an incurable cancer develop an accurate understanding of their prognosis.
Whereas responses to the abstract trade-off item about QoL were associated with beliefs about curability, responses to specific items about willingness to bear concrete, easily envisioned symptoms and side effects (nausea/vomiting, assistance with activities, bedbound state, confusion, worsening memory) were not. We thus found mixed support for the hypotheses. The findings are consistent with the premise that patients’ trade-off preferences reflect the extent to which they hope to be cured, and this hope for cure will be reflected in their responses to items about the perceived curability of their cancer. However, it appears that this effect is confined to trade-off items involving abstract constructs (such as QoL) and is not observed when the trade-off items pertain to concrete, easily imagined symptoms and states (e.g., being bedbound). It seems that some patients, when asked to trade-off something abstract (like not maintaining QoL) in exchange for survival will respond in absolutist terms, perhaps reflecting a particular worldview, and this will manifest in ACC.
Prior studies23,24 have shown that patients’ incomplete understanding of the curability of their disease could lead to the use of aggressive care that may confer little survival benefit. There is an increasing focus on improving patients’ disease understanding by enhancing provider-patient communication,17 introducing educational aids to facilitate patients’ communication,25 or including palliative care services early in the treatment process.26 Nevertheless, for patients who believe with 100% certainty that they could be cured, our findings raised the question of whether existing interventions might improve their understanding of their cancer status. One concern is that these patients may value survival to the exclusion of QoL in which case they may discount any information to the contrary irrespective of the interventions deployed. Interventions that focus only on providing prognostic information will likely prove ineffective, if attention is not also paid to why patients have specific preferences and beliefs.
Although thoughtful and in-depth conversations about prognosis should be conducted with all patients, those who are willing to trade QoL for survival may especially benefit from conversations that focus on values and emotions. One technique that may be helpful is using “wish” statements27 (e.g., “I wish I could say that your cancer can be cured. Would you make a different decision if you thought that the disease could not be cured?”). If a patient appears ready to engage in the discussion, then it would be reasonable to provide further information about their prognosis. Another technique would be the use of the “ask-tell-ask” approach (e.g., “What is your understanding of your cancer prognosis?”). If the patient’s response suggests near-certainty of cure, then the oncologist should explore the emotions behind this conviction (e.g., “What are you most afraid of?”). Involvement of palliative care providers may also help with prognostic discussions, even though patients who demonstrate ACC may be more likely to resist palliative care referrals. These strategies and techniques may be used together or in isolation, depending on a particular patient’s beliefs and status, allowing for personalized and multimodal interventions targeting disease comprehension. However, if a patient’s beliefs about ACC appear fixed despite these multimodal interventions, then persistent attempts to discuss prognosis may cause distress in some patients and could affect the patient-physician relationship. It is important to acknowledge that these fixed beliefs may serve a beneficial psychological role for patients who want themselves or their families to feel that they had done everything they could for their cancer.28
Patients with impaired function were less likely to demonstrate ACC. Given that these patients have poorer underlying functional health, it is not surprising that they are less likely to believe that their disease is curable. On the other hand, ACC was more common among patients with lower education levels, non-white patients, and patients with low levels of instrumental social support. Education is an indicator of socioeconomic status and health literacy level. Studies have shown that patients with lower socioeconomic status and health literacy are more likely to receive aggressive care at the end-of-life.29–32 It is possible that these disparities in healthcare utilization are partially due to beliefs about curability,4 though other explanations are plausible, such as distrust of physicians.33 Similarly, non-white patients have been shown to be more likely to believe they could be cured4 and may partially explain the higher utilization of aggressive care in advanced stage cancer.29,34 We also showed that older patients who lacked instrumental social support were more likely to demonstrate ACC. The lack of “social capital” that allows for further discussion of prognosis outside the clinic visits may contribute to beliefs about curability.35 Communication with patients lacking social support warrants greater attention, as patients who lack instrumental support may be particularly dependent on their oncologist and afraid that the oncologist will “give up” on them due to the lack of social support. Respondents who screened positive for cognitive impairment were not more likely to believe, with absolute certainty, than others that they would be cured. Perhaps the potential cognitive impairment identified in these brief screens were not sufficiently severe to affect patients’ prognostic understanding. The sample only included patients who were considering or receiving active treatments, so older patients with severe cognitive impairment were not offered an opportunity to participate in our study. Another limitation of our study is that we did not ask respondents how they understand the term QoL. In particular, when respondents failed to respond affirmatively to the item “maintaining my QoL is more important to me than living longer”, we do not know how they interpreted or imagined “not maintaining” QoL.
Several other limitations should be noted. First, given our cross-sectional study design, causation cannot be determined. Second, our study only included a small percentage of non-white patients (10%). Third, we did not measure literacy, numeracy, religiosity or worldviews, such as fatalism. Fourth, we did not ask oncologists if they had discussed the prognosis with their patients.
There are a number of strengths to our study. To our knowledge, this is the first study to document the prevalence of ACC in older patients with advanced cancer, and the first to demonstrate a positive relationship between patients’ willingness to trade off QoL for survival and beliefs about curability. Our study also included a large number of older patients treated at community oncology practices.
In conclusion, patients who were more willing to bear adversity in the form of decreased QoL were more likely to believe that there was a 100% chance that their cancer will go away and never come back. In order to help older patients with advanced cancer understand their prognosis, it is imperative that physicians elicit their preferences, values, emotions, and fears.
Supplementary Material
Acknowledgments
The work was funded through a Patient-Centered Outcomes Research Institute (PCORI) Program contract (4634; Mohile), UG1 CA189961, R01 CA177592 (Mohile) and R01CA168387 (Duberstein) from the National Cancer Institute, and K24 AG056589 from the National Institute of Aging (Mohile). This work was made possible by the generous donors to the Wilmot Cancer Institute (WCI) geriatric oncology philanthropy fund. All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies, and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest: No relevant conflicts of interest to report.
References
- 1.Mohile SG, Fan L, Reeve E, et al. : Association of Cancer With Geriatric Syndromes in Older Medicare Beneficiaries. Journal of Clinical Oncology 29:1458–1464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagerty RG, Butow PN, Ellis PM, et al. : Communicating prognosis in cancer care: a systematic review of the literature. Ann Oncol 16:1005–53, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Admane S, et al. : Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 29:2319–26, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Duberstein PR, Chen M, Chapman BP, et al. : Fatalism and educational disparities in beliefs about the curability of advanced cancer. Patient Educ Couns 101:113–118, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevino KM, Zhang B, Shen MJ, et al. : Accuracy of advanced cancer patients’ life expectancy estimates: The role of race and source of life expectancy information. Cancer 122:1905–12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin DW, Cho J, Kim SY, et al. : Patients’ and family caregivers’ understanding of the cancer stage, treatment goal, and chance of cure: A study with patient-caregiver-physician triad. Psychooncology 27:106–113, 2018 [DOI] [PubMed] [Google Scholar]
- 7.El-Jawahri A, Traeger L, Kuzmuk K, et al. : Prognostic understanding, quality of life and mood in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 50:1119–24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Wright AA, Huskamp HA, et al. : Health care costs in the last week of life: associations with end-of-life conversations. Arch Intern Med 169:480–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeks JC, Catalano PJ, Cronin A, et al. : Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med 367:1616–25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried TR, Bradley EH, Towle VR, et al. : Understanding the treatment preferences of seriously ill patients. The New England Journal of Medicine 346:1061–1066, 2002 [DOI] [PubMed] [Google Scholar]
- 11.McCaul KD, Peters E, Nelson W, et al. : Linking decision-making research and cancer prevention and control: important themes. Health Psychol 24:S106–10, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Mohile SG, Epstein RM, Hurria A, et al. : Improving communication with older patients with cancer using geriatric assessment (GA): A University of Rochester NCI Community Oncology Research Program (NCORP) cluster randomized controlled trial (CRCT). Journal of Clinical Oncology 36:LBA10003–LBA10003, 2018 [Google Scholar]
- 13.Mohile SG, Velarde C, Hurria A, et al. : Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. Journal of the National Comprehensive Cancer Network: JNCCN 13:1120–1130, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina-Garrido MJ, Guillen-Ponce C, Blanco R, et al. : Delphi consensus of an expert committee in oncogeriatrics regarding comprehensive geriatric assessment in seniors with cancer in Spain. J Geriatr Oncol 9:337–345, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Fagard K, Casaer J, Wolthuis A, et al. : Value of geriatric screening and assessment in predicting postoperative complications in patients older than 70 years undergoing surgery for colorectal cancer. J Geriatr Oncol 8:320–327, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Owusu C, Margevicius S, Schluchter M, et al. : Short Physical Performance Battery, usual gait speed, grip strength and Vulnerable Elders Survey each predict functional decline among older women with breast cancer. J Geriatr Oncol 8:356–362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein RM, Duberstein PR, Fenton JJ, et al. : Effect of a Patient-Centered Communication Intervention on Oncologist-Patient Communication, Quality of Life, and Health Care Utilization in Advanced Cancer: The VOICE Randomized Clinical Trial. JAMA Oncol 3:92–100, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R, Chen L, Fan L, et al. : Incidence and Effects of Polypharmacy on Clinical Outcome among Patients Aged 80+: A Five-Year Follow-Up Study. PLoS One 10:e0142123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurria A, Togawa K, Mohile SG, et al. : Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 29:3457–3465, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol:Jco2018788687, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maly RC, Frank JC, Marshall GN, et al. : Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc 46:889–94, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Gramling R, Fiscella K, Xing G, et al. : Determinants of Patient-Oncologist Prognostic Discordance in Advanced Cancer. JAMA Oncol 2:1421–1426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mack JW, Walling A, Dy S, et al. : Patient beliefs that chemotherapy may be curative and care received at the end of life among patients with metastatic lung and colorectal cancer. Cancer 121:1891–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuyama R, Reddy S, Smith TJ: Why do patients choose chemotherapy near the end of life? A review of the perspective of those facing death from cancer. J Clin Oncol 24:3490–6, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Whelan T, Sawka C, Levine M, et al. : Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer. J Natl Cancer Inst 95:581–7, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. The New England Journal of Medicine 363:733–742, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Quill TE, Arnold RM, Platt F: “I wish things were different”: expressing wishes in response to loss, futility, and unrealistic hopes. Ann Intern Med 135:551–5, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Norton SA, Wittink MN, Duberstein PR, et al. : Family caregiver descriptions of stopping chemotherapy and end-of-life transitions. Support Care Cancer, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loh KP, Kansagra A, Shieh MS, et al. : Predictors of the Use of Specific Critical Care Therapies in Patients With Metastatic Cancer. J Natl Compr Canc Netw 15:22–30, 2017 [DOI] [PubMed] [Google Scholar]
- 30.Givens JL, Tjia J, Zhou C, et al. : Racial and ethnic differences in hospice use among patients with heart failure. Arch Intern Med 170:427–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CY, Hung YT, Chang CM, et al. : The association between individual income and aggressive end-of-life treatment in older cancer decedents in Taiwan. PLoS One 10:e0116913, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volandes AE, Paasche-Orlow M, Gillick MR, et al. : Health literacy not race predicts end-of-life care preferences. J Palliat Med 11:754–62, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Armstrong K, Ravenell KL, McMurphy S, et al. : Racial/ethnic differences in physician distrust in the United States. Am J Public Health 97:1283–9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps E, True G, Harris D, et al. : Approaching the end of life: attitudes, preferences, and behaviors of African-American and white patients and their family caregivers. J Clin Oncol 21:549–54, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Pinquart M, Duberstein PR: Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol 75:122–37, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.