Abstract
BACKGROUND
In a prior phase 1 study, the combination of dalantercept, a novel anti-angiogenic targeting activin receptor-like kinase 1 (ALK1), plus axitinib was deemed safe and tolerable with promising efficacy signal in advanced renal cell carcinoma (RCC).
METHODS
In this phase 2, randomized, double-blind, placebo-controlled study, patients with clear-cell RCC treated with one prior angiogenesis inhibitors were randomized (1:1) to receive axitinib plus dalantercept vs. axitinib plus placebo. Randomization was stratified by type of prior therapy. The primary endpoint was progression-free survival (PFS). Secondary endpoints were PFS in patients with two or more prior lines of anticancer therapy, overall survival (OS), and objective response rate (ORR).
RESULTS
Between June 10, 2014 and February 23, 2017, 124 patients were randomly assigned to receive axitinib plus dalantercept (n=59) or placebo (n=65). Median PFS was not significantly different between treatment groups (median 6.8 months vs. 5.6 months; hazard ratio [HR] 1·11 [95% confidence interval (CI) 0·71–1·73], p=0·670). Neither group reached median OS (HR 1·39 [95% CI 0·70–2·77], p=0·349). The ORR was 19·0% (11/58, 95% CI 9·9–31·4) in the dalantercept plus axitinib group and 24·6% (15/61, 95% CI 14·5–37·3) in the placebo plus axitinib group. At least one treatment-emergent adverse event ≥ grade 3 was observed in 59% (34/58) of patients in the dalantercept group and 64% (39/61) of patients in the placebo group. One treatment-related death occurred in the placebo plus axitinib group.
CONCLUSIONS
While well-tolerated, the addition of dalantercept to axitinib did not improve treatment-related outcomes in previously treated patients with advanced RCC.
Keywords: angiogenesis, antiangiogenic agents chemotherapy, drug therapy, randomized controlled trial, renal cell carcinoma
Precis:
In this randomized phase 2 study, patients with clear-cell RCC treated with prior angiogenesis inhibitors were randomized (1:1) to receive axitinib plus dalantercept vs. axitinib plus placebo. While tolerance was comparable, the study did not reach its primary endpoint of improving progression free survival on the investigational vs. the placebo arm.
Introduction
Since 2005, seven antiangiogenic agents targeting vascular endothelial growth factor (VEGF) have been approved for use in renal cell carcinoma (RCC) in the United States;1 however, efficacy is limited with monotherapy, particularly in previously treated patients.1 The efficacy observed with agents co-targeting VEGF receptor 2 (VEGFR2) and other proangiogenic signaling molecules, such as c-MET, AXL, or FGFR, in sunitinib-pretreated patients with RCC suggests that blocking alternative mechanisms of angiogenesis might enhance the efficacy of VEGF inhibition in RCC.2–5
Activin receptor-like kinase 1 (ALK1) is a type I receptor of the transforming growth factor beta (TGF-β) superfamily that is selectively expressed on the surface of activated endothelial cells and binds with high affinity to its ligands bone morphogenetic proteins (BMPs) 9 and 10.6, 7 ALK1 and BMP9 may have functional relevance not only in tumor angiogenesis but also in tumor metastases.8–10
Dalantercept is a soluble ALK1 receptor fusion protein that binds BMP9 and BMP10 with high affinity and acts as a ligand trap. Single-agent efficacy and acceptable tolerance with dalantercept were confirmed in a phase 1 study in patients with advanced cancer.11 In RCC xenograft mouse models (A498, 786-O), dalantercept plus the tyrosine kinase inhibitor (TKI) sunitinib resulted in significantly longer duration of tumor growth inhibition than either agent alone.12–14 Benefit was also observed with this combination following the onset of sunitinib resistance in these models.
Part 1 of the DART Study was a dose-finding trial of 29 patients with TKI-pretreated.15 It determined 0·9 mg/kg dalantercept subcutaneously every three weeks plus 5 mg axitinib orally twice daily (BID) as the recommended combination dose level for further testing. The combination was well tolerated, and efficacy was encouraging.
Methods
Study design
DART was a two-part, multicenter trial; results of Part 1 are published elsewhere.15 Part 2, reported here, was a randomized, double-blind, placebo-controlled phase 2 study of axitinib plus dalantercept in patients with previously treated, advanced RCC. Patients were randomized 1:1 to receive axitinib plus dalantercept vs. axitinib plus placebo. Randomization was stratified by number of prior therapies, prior mTOR inhibitor and prior immune therapy. Patients were identified and enrolled by participating study teams. Once eligibility was confirmed and patients were registered, randomization assignments were generated through a computerized system provided by an interactive web response system. Patients, treating investigators, and study personnel (those administering investigational product, assessing outcomes, and analyzing data) were blinded to group assignment. An unblinded clinical research associate at the clinical research organization ensured the generation of the randomization sequence and enrolled patients, but did not have a role in the rest of the trial. Randomization was concealed by limiting access control within the interactive response technology systems to only those users who had an unblinded role in the trial. The study was conducted across 32 centers in the United States.
Participants
Patients 18 years of age or older with advanced, predominantly clear-cell RCC had demonstrated progression during or after one prior VEGF pathway inhibitor. Advanced RCC was defined as evident metastatic or locally advanced, but surgically unresectable. Additionally, exposure to one approved mammalian target of rapamycin (mTOR) inhibitor and/or immunotherapy was allowed. Subjects had to have adequate performance status (ECOG ≤ 1) and organ function. Prior exposure to axitinib or ALK1-directed therapies was prohibited, as were uncontrolled hypertension, active cardiac disease, or active brain metastases. The study was reviewed and approved by institutional review boards of participating sites and was conducted according to Good Clinical Practice and International Conference on Harmonisation guidelines, the Declaration of Helsinki, and applicable local laws and regulations. All patients provided written informed consent.
Procedures
Patients received continuous oral dosing of axitinib (Pfizer, New York, NY) at a starting dose level of 5 mg BID and either 0·9 mg/kg dalantercept (Acceleron Pharma Inc., Cambridge, MA) or placebo by subcutaneous injection every three weeks. Patients who tolerated axitinib 5 mg BID well for at least four consecutive weeks were permitted to have their axitinib dose increased from 5 to 7 mg BID and, subsequently, from 7 to 10 mg BID using the same tolerability and blood pressure criteria as previously established for axitinib.16 Treatment was continued while tolerated and until progression of disease as defined by RECIST v1·1 with imaging to assess treatment response and disease progression every six weeks. Subsequently, they were contacted approximately every three months (± two weeks) to document survival status.
Outcomes
The primary endpoint was PFS, defined as the time from randomization to radiographic tumor progression (per RECIST v1·1) or death from any cause. The following prespecified variables were examined for association with PFS: number of metastatic sites, prior nephrectomy, ECOG performance status, International Metastatic Renal Cancer Database Consortium (IMDC) and Memorial Sloan Kettering Cancer Center (MSKCC) risk groups.17, 18
A key secondary endpoint was PFS in patients with ≥ 2 prior lines of anticancer therapy. Other secondary endpoints included overall survival (OS; defined as time from randomization to death), overall response rate (ORR; complete response [CR] + partial response [PR]), disease control rate (DCR; CR, PR, or stable disease), and safety/tolerability. Exploratory endpoints included correlation of outcomes with baseline levels and changes in tissue and peripheral blood biomarkers, including circulating VEGFR2 and VEGFR3, as identified as candidate biomarkers in Part 1 of the study.15
Safety was evaluated in all patients who received any study drug, analyzed according to treatment received and included treatment-emergent adverse event (TEAE) and serious adverse event (SAE). A data monitoring committee, independent from the sponsor and comprised of at least three members, reviewed unblinded safety data throughout the study beginning when 25 patients were randomized and at approximately 6-month intervals thereafter.
Statistical analysis
The primary analysis and subgroup analyses of PFS were based on investigator assessment and included any patient who had received study drug. The secondary analysis of PFS in patients who had two or more prior lines of anticancer therapy included the full analysis set (all randomized patients). The intent-to-treat principle was used for this population.
With a projected median PFS (mPFS) of five months in the placebo plus axitinib group, a total of 82 PFS events would provide approximately 80% power to detect a three-month extension from five to eight months in the experimental group when performing a stratified log-rank test (null hypothesis: no difference between treatment groups using a one-sided 10% significance level). Power projections were made under a proportional hazards assumption so that the hazard ratio (HR) for the investigational vs. the placebo arm was equal to 0·625 under the alternative hypothesis. It was estimated that 130 patients needed to be randomized to obtain 82 PFS events. Patients not exhibiting radiographic tumor progression or death were censored on the date of the last tumor assessment on study. Patients missing baseline radiographic tumor assessments were censored on the date of randomization.
A log-rank test stratified by prior mTOR therapy and prior immune therapy was used to test the null hypothesis of no difference between the treatment arms using a one-sided 10% significance level in the primary PFS analysis. For both analyses, Kaplan-Meier analysis was used to compute mPFS and OS with 95% confidence intervals (CIs). The HR was estimated using a stratified Cox proportional hazards model along with the 95% CIs. Descriptive statistics were used to summarize safety and biomarker data.
This trial is registered with ClinicalTrials.gov, number NCT01727336.
Role of funding source
The sponsor of the study was Acceleron Pharma Inc., Cambridge, MA. The sponsor oversaw study design, data collection, data analysis, data interpretation, and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
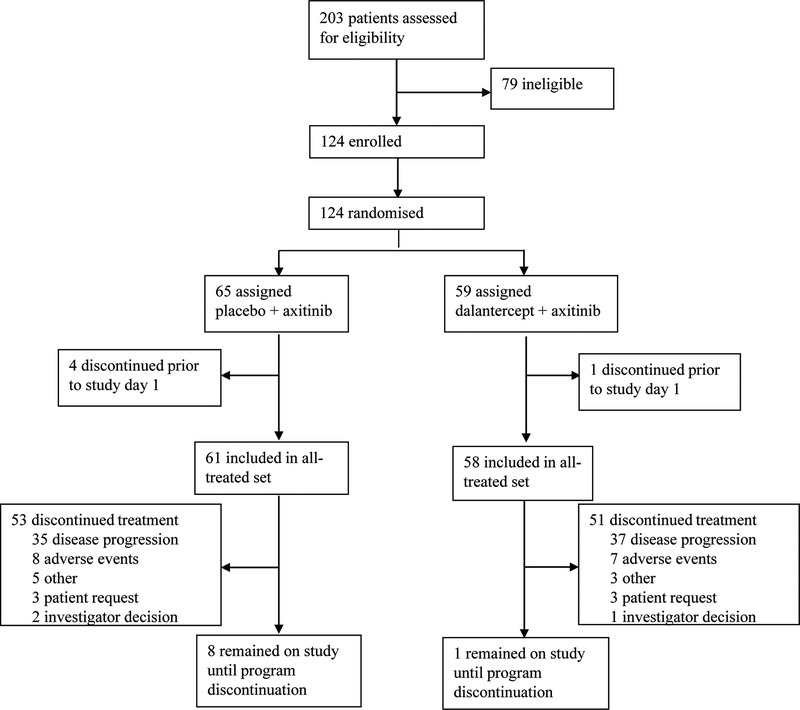
Patients were recruited between June 10, 2014 and February 23, 2017. Of 203 patients screened, 124 were eligible. Of these patients, 59 were randomized to receive dalantercept plus axitinib and 65 were randomized to receive placebo plus axitinib (Figure 1). Of the patients randomized, 58 in the dalantercept plus axitinib group and 61 in the placebo plus axitinib group received study drug and were assessed for the primary endpoint. A similar number of patients in each group discontinued treatment (51 in the dalantercept plus axitinib group and 53 in the placebo plus axitinib group), the majority due to disease progression (62·7% [37/59] in the dalantercept plus axitinib group and 53·8% [35/65] in the placebo plus axitinib group). Eight patients withdrew from the study; three in the placebo plus axitinib group withdrew study consent, one patient in this group was lost to follow up, and three patients in this group and one patient in the dalantercept plus axitinib group discontinued the study for other reasons.
Figure 1: Trial profile.
CONSORT diagram summarizing enrollment and randomization of subjects and discontinuation from study treatment.
Patient characteristics are summarized in Table 1. Overall, treatment groups were well balanced, including baseline performance status, history of prior nephrectomy, and risk distribution per MSKCC as well as IMDC criteria. The majority of patients in both groups had only received one prior therapy (59% in the dalantercept plus axitinib group and 64% in the placebo plus axitinib group).
Table 1:
Demographics and baseline characteristics
| Placebo plus axitinib | Dalantercept plus axitinib | |
|---|---|---|
| n=61 | n=58 | |
| Sex | ||
| Male | 35 (57%) | 38 (66%) |
| Female | 26 (43%) | 20 (35%) |
| Median age in years (range) | 59 (27–75) | 63 (37–81) |
| Prior systemic therapies | ||
| 1 | 39 (64%) | 34 (59%) |
| ≥2 | 22 (36%) | 24 (41%) |
| Prior nephrectomy | 52 (85%) | 56 (97%) |
| ECOG status | ||
| 0 | 31 (51%) | 33 (57%) |
| 1 | 30 (49%) | 25 (43%) |
| MSK risk group | ||
| Favorable | 33 (54%) | 23 (40%) |
| Intermediate | 26 (43%) | 33 (57%) |
| Poor | 2 (3%) | 2 (3%) |
| IMDC risk group | ||
| Favorable | 16 (26%) | 11 (19%) |
| Intermediate | 43 (71%) | 45 (78%) |
| Poor | 2 (3%) | 2 (3%) |
Data are from the all-treated set (all randomized patients who received study drug) and are n (%) unless otherwise noted. ECOG=Eastern Cooperative Oncology Group. IMDC=International Metastatic Renal Cancer Database Consortium. MSK=Memorial Sloan Kettering Cancer Center.
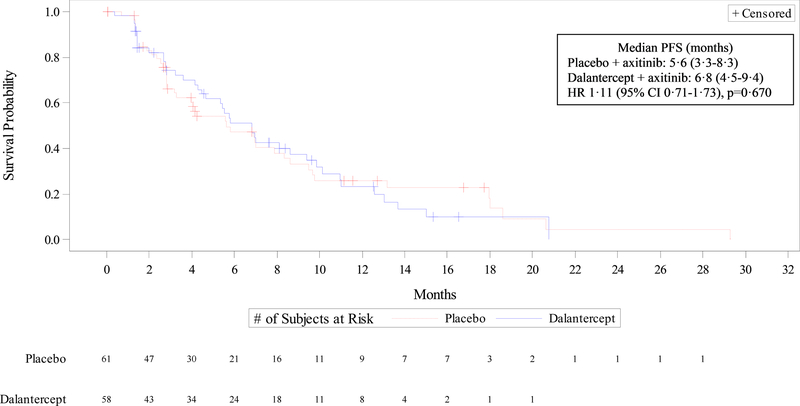
There was no significant difference between the two treatment groups in the primary endpoint analysis for overall PFS (Figure 2). Median PFS was 6·8 months (95% CI 4·5–9·4) in the dalantercept plus axitinib group and 5·6 months (95% CI 3·3–8·3) in the placebo plus axitinib group (Cox proportional HR 1·11 [95% CI 0·71–1·73], p=0·670). Median PFS in patients with two or more prior lines of anticancer therapy was 8·1 months (95% CI 3·6-not estimable) in the dalantercept plus axitinib group and 7·0 months (95% CI 2·8–9·5) in the placebo plus axitinib group (HR 0·78 [95% CI 0·33–1·87], p=0·288).
Figure 2: Overall progression-free survival.
Data are from the all-treated cohort (all randomized patients who received study drug). Kaplan Meier curves depicting progression-free survival for patients treated with axitinib + dalantercept (blue line) vs. axitinib + placebo (red line). CI=confidence interval. HR=hazard ratio. PFS=progression-free survival.
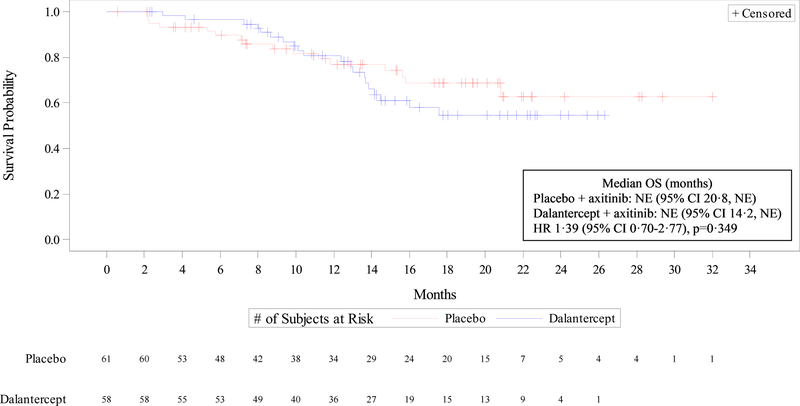
Overall survival did not differ significantly between treatment groups (HR 1·39 [95% CI 0·70–2·77], p=0·349), and with median follow-up of 17.8 months on both arms the median OS was not reached in either treatment group (Figure 3). Across all patients evaluable for response, the ORR was 19% in the dalantercept plus axitinib group and 25% in the placebo plus axitinib group (p=0·432, Table 2). There were no CRs in either treatment group. Progressive disease as best response was recorded in 16% and 17%, respectively; DCR was 83% and 82%, respectively (Table 2).
Figure 3: Overall survival.
Data are from the all-treated set (all randomized patients who received study drug). Kaplan Meier curves depicting overall survival for patients treated with axitinib + dalantercept (blue line) vs. axitinib + placebo (red line). Median follow-up on both arms was 17.8 months at time of analysis. CI=confidence interval. HR=hazard ratio. NE=not estimable. OS=overall survival.
Table 2:
Best radiographic response
| Placebo plus axitinib | Dalantercept plus axitinib | p value | |
|---|---|---|---|
| n=61 | n=58 | ||
| Complete response | 0 | 0 | |
| 95% CI | N/A | N/A | |
| Partial response | 15 (24·6%) | 11 (19·0%) | |
| 95% CI | 14·5–37·3 | 9·9–31·4 | |
| Stable disease | 35 (57·4%) | 37 (63·8%) | |
| 95% CI | 44·1–70·0 | 50·1–76·0 | |
| Progressive disease | 10 (16·4%) | 10 (17·2%) | |
| 95% CI | 8·2–28·1 | 8·6–29·4 | |
| Not evaluable | 1 (1·6%) | 0 | |
| 95% CI | 0·0–8·8 | N/A | |
| Objective response rate | 15 (24·6%) | 11 (19·0%) | 0·432 |
| 95% CI | 14·5–37·3 | 9·9–31·4 | |
| Disease control rate | 50 (82·0) | 48 (82·8) | 0·904 |
| 95% CI | 70·0–90·6 | 70·6–91·4 |
Data are from the all-treated set (all randomized patients who received study drug) and are n (%) unless otherwise noted. p value was calculated using Cochran-Mantel-Haenszel adjusted for randomization strata. Objective response rate was defined as the proportion of patients who achieved either CR or PR. Disease control rate was defined as the proportion of patients evaluable for response who met the criteria for CR, PR, or SD regardless of confirmation of complete response or partial response.
CI=confidence interval. CR=complete response N/A=not applicable. PR=partial response. SD=stable disease.
Treatment-emergent adverse events are summarized in Table 3. Overall, the safety profiles were largely comparable between treatment groups; the frequency of grade 3 or greater TEAEs was 59% in the dalantercept plus axitinib group and 64% in the placebo plus axitinib group. The most common (≥5%) grade 3 or greater TEAEs in either group were hypertension, hyponatremia, diarrhea, hypophosphatemia, increased lipase, anemia, decreased ejection fraction, and fatigue. Of these grade 3 or greater TEAEs, hypophosphatemia, increased lipase, and anemia occurred more frequently in the dalantercept plus axitinib group. Rates of treatment-emergent hypertension were lower in the dalantercept arm (all-grade 22.4% of patients, grade 3+ 10.3%) versus the placebo arm (49.2% and 19.7%, respectively); similarly, the rate of high-grade diarrhea was higher for patients treated on the placebo arm (8.2%, vs. 1.7% on the dalantercept arm; see also table 3). Rates of treatment-emergent palmar-plantar erythrodysaesthesia (PPE) syndrome were similar in the dalantercept plus axitinib group (all-grade 24.1% of patients, grade >3+ 3.4%) versus the placebo plus axitinib group (24.6% and 1.6%, respectively) (Table 3).
Table 3:
Any grade TEAEs in ≥20% of patients in either group or grade ≥3 TEAEs in ≥5% of patients in either group
| Axitinib plus placebo | Axitinib plus dalantercept | |||
|---|---|---|---|---|
| n=61 | n=58 | |||
| Preferred term | All grades | Grade ≥3 | All grades | Grade ≥3 |
| Fatigue | 46 (75·4%) | 3 (4·9%) | 33 (56·9%) | 2 (3·4%) |
| Diarrhoea | 39 (63·9%) | 5 (8·2%) | 36 (62·1%) | 1 (1·7%) |
| Hypertension | 30 (49·2%) | 12 (19·7%) | 13 (22·4%) | 6 (10·3%) |
| Nausea | 28 (45·9%) | 2 (3·3%) | 27 (46·6%) | 1 (1·7%) |
| Constipation | 27 (44·3%) | 1 (1·6%) | 23 (39·7%) | 0 |
| Epistaxis | 7 (11·5%) | 0 | 24 (41·4%) | 0 |
| Oedema peripheral | 14 (23·0%) | 1 (1·6%) | 24 (41·4%) | 0 |
| Decreased appetite | 20 (32·8%) | 1 (1·6%) | 23 (39·7%) | 0 |
| Dysphonia | 24 (39·3%) | 0 | 11 (19·0) | 0 |
| Blood creatinine increased | 11 (18·0%) | 1 (1·6%) | 20 (34·5%) | 1 (1·7%) |
| Headache | 21 (34·4%) | 0 | 14 (24·1%) | 0 |
| Weight decreased | 20 (32·8%) | 2 (3·3%) | 13 (22·4%) | 0 |
| Arthralgia | 19 (31·1%) | 0 | 16 (27·6%) | 0 |
| Vomiting | 17 (27·9%) | 2 (3·3%) | 18 (31·0%) | 0 |
| Palmar-plantar erythrodysaesthesia syndrome | 15 (24·6%) | 1 (1·6%) | 14 (24·1%) | 2 (3·4%) |
| Dyspnoea | 15 (24·6%) | 0 | 13 (22·4%) | 0 |
| Abdominal pain | 15 (24·6%) | 0 | 12 (20·7%) | 1 (1·7%) |
| Stomatitis | 15 (24·6%) | 1 (1·6%) | 8 (13·8%) | 0 |
| Cough | 13 (21·3%) | 0 | 14 (24·1%) | 0 |
| Back pain | 14 (23·0%) | 2 (3·3%) | 9 (15·5%) | 1 (1·7%) |
| Myalgia | 9 (14·8%) | 0 | 13 (22·4%) | 0 |
| Hyponatraemia | 13 (21·3%) | 6 (9·8%) | 5 (8·6%) | 1 (1·7%) |
| Pruritus | 12 (19·7%) | 0 | 4 (6·9%) | 0 |
| Ejection fraction decreased | 9 (14·8%) | 3 (4·9%) | 2 (3·4%) | 1 (1·7%) |
| Anaemia | 4 (6·6%) | 0 | 12 (20·7%) | 3 (5·2%) |
| Hypophosphataemia | 6 (9·8%) | 3 (4·9%) | 7 (12·1%) | 3 (5·2%) |
| Lipase increased | 5 (8·2%) | 1 (1·6%) | 7 (12·1%) | 3 (5·2%) |
Data are from the safety analysis set (all randomized patients who received study drug) and are n (%).
TEAE=treatment-emergent adverse event.
Consistent with prior experience in the development of dalantercept, peripheral edema was common and more frequent in the dalantercept plus axitinib group compared with the placebo plus axitinib group, but no grade ≥3 events occurred (Table 3). Pleural effusions of any grade were observed in 3% of patients in the placebo plus axitinib group versus 10% in the dalantercept plus axitinib group. Only one of these effusions, observed in a patient in the dalantercept plus axitinib group, exceeded grade 2.
In the dalantercept plus axitinib group, 86% of patients had at least one TEAE deemed related to dalantercept, 21% had at least one TEAE related to dalantercept that was grade 3 or higher, and 16% had at least one TEAE leading to dalantercept withdrawal. These rates were similar in the placebo group (90%, 30%, and 15%, respectively). One death occurred in the placebo plus axitinib group (disease progression, unrelated to treatment); there were no grade 5 adverse events in the dalantercept group.
Baseline levels of VEGFR2 and VEGFR3 did not correlate with outcomes in the individual treatment groups. Similarly, there was no difference in PFS between the dalantercept plus axitinib group compared with the placebo plus axitinib group when the analysis was limited to patients with baseline VEGFR2 or VEGFR3 serum levels ≥median or < median (See also Supplemental Figures 1 and 2). Serial serum collections demonstrated greater suppression of VEGFR3 levels after initiation of dalantercept plus axitinib compared with placebo plus axitinib (Supplemental Figure 3); however, this suppression did not correlate with radiographic effect (Supplemental Figure 4). Pre-planned exploratory analyses of archival tumor specimen were ultimately not pursued.
Discussion
Targeting VEGF-mediated tumor angiogenesis is a well-established therapeutic strategy in the management of advanced RCC. While TKI monotherapy leaves other aspects of RCC pathobiology, including non-VEGF aspects of angiogenesis, largely unattended, combination approaches have historically proven challenging.19–23
Here, we paired axitinib with dalantercept, a novel anti-angiogenic agent disrupting vascular maintenance through inhibition of signaling along the ALK1 pathway. This approach was prompted by preclinical RCC data12–14 and supported by encouraging findings during dose escalation, which suggested a promising efficacy signal and reasonable tolerance in 28 evaluable patients with mPFS of 8·3 months and 12-month OS of 75%.15 These results compared favorably to historical data with axitinib monotherapy in patients with RCC who were pretreated with TKI (mPFS of 4·8 months and 12-month PFS and OS of 25% and 60%, respectively).16, 24
In this double-blind, placebo-controlled trial, the addition of dalantercept to axitinib did not result in a significant increase in PFS in TKI-pretreated RCC patients, neither for the overall population (mPFS 6·8 months with dalantercept plus axitinib versus 5·6 months with placebo plus axitinib), nor for the subgroup of more heavily pretreated patients (mPFS of 8·1 months with dalantercept plus axitinib versus 7·0 months with placebo plus axitinib). No difference in ORR or DCR was observed across all evaluable patients.
The efficacy results in the placebo plus axitinib group are comparable to those previously reported with axitinib monotherapy in previously treated patients. In a phase 3 study, mPFS and ORR with axitinib monotherapy in the second-line setting, per investigator-assessment, were 8.3 months and 23%, respectively.24 In the subgroup of patients previously treated with the VEGF TKI sunitinib, mPFS and ORR were 4·6.5 months24 and 11%,25 respectively, with axitinib monotherapy.
Dose escalation of axitinib, permitted on the study for both arms of the study per standard practice, was pursued in more patients on the investigational than the placebo arm (12 vs. 6 patients, respectively), yet the overall small number of patients makes a significant effect on treatment outcomes or tolerance unlikely. While the study’s modest size must be kept in mind when considering the generalizability of our findings, the uniform lack of added benefit across the primary and all secondary analyses, together with the fact that the control arm performed in accordance with historical data ultimately argues against further development of this combination.
Dalantercept plus axitinib was generally well tolerated, and TEAEs were consistent with those observed in dalantercept phase 1 studies.15 Fluid retention events, including peripheral edema and effusions, were observed in a dose-dependent fashion with dalantercept during Part 1 of the trial. In Part 2, peripheral edema was common in the dalantercept plus axitinib group (41%); however, no grade 3 or greater peripheral edema or pericardial effusions were observed. This was likely the result of choosing the 0.9mg/kg dose level as the recommended phase 2 dose (RP2) for Part II, rather that the maximally tolerated dose (MTD) of 1.2mg/kg, for which notably more on-target toxicities were observed during Part I of the trial.
Interestingly, rates of treatment-emergent hypertension were more than two times higher on the placebo- than on the dalantercept arm (49.2 vs. 22.4%) and grade 3+ diarrhea was also observed more frequently on the placebo arm (8.2%, vs. 1.7%). While mechanistically unclear, these findings raise the question whether dalantercept may mitigate some of the on-target toxicities of axitinib (not all, rates of PPE were similar). In a next step, one might ask whether similar opposing effects on tumor cells and/or their microenvironment could account for the lack of added benefit seen with the combination on this randomized trial.
Although larger decreases in VEGFR3 serum levels, potentially reflective of target engagement and damage to vascular beds, were observed in the dalantercept group, these did not appear to correlate with differences in treatment effect. With overall lack of added efficacy observed here, analyses of archival tumor tissue were ultimately not pursued.
In summary, the data reported here suggest that the combination of axitinib plus dalantercept, while well-tolerated, does not increase efficacy over axitinib alone in patients with pretreated RCC. Previous studies of dalantercept in squamous cell cancer of the head and neck, hepatocellular carcinoma, endometrial and ovarian cancer were conducted and did not show sufficient activity to proceed beyond phase 2.26–29 Based on the lack of efficacy observed on this trial, the dalantercept program has been discontinued.
Supplementary Material
Acknowledgements:
The authors are indebted to the patients and their families for their participation and to the clinical teams who facilitated patient coordination as well as sample and data acquisition. We thank the entire DART Study group: Sanjv Agarwala, Daniel Cho, Joseph Clark, Christopher DiSimone, Harry Drabkin, Saby George, Thomas Hutson, Fairooz Kabbinavar, Joshua Lang, Rich Lauer, Lionel Lewis, Jamie Merchan, Donald Richards, Jacob Sands, Ian Schnadig, and Sandy Srinivas for their enthusiastic participation in the trial. We also thank Prometrika, Pharsight, and Certara for providing data and document support as well as Musa Mutyaba, Dawn Wilson, Suchi S. Pandya, and Carrie Barron of Acceleron Pharma Inc. (at the time of the trial) and Holly Capasso-Harris of Synchrogenix. Additionally, we thank Michael Newman at Memorial Sloan Kettering for manuscript assistance. This study was sponsored by Acceleron Pharma Inc, Cambridge, MA.
Funding:
This study was sponsored by Acceleron Pharma Inc, Cambridge, MA. Dr. Voss received support in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of interest:
1. MHV: research funding: BMS and Genentech/Roche; honoraria: Novartis; travel: Eisai, Novartis and Takeda; consulting: Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Natera, Novartis and Pfizer.
2. RSB: research funding: Acceleron; patent: dalantercept
3. NJV: Speaker for BMS, Exelixis, Roche; consultant to Norvartis, Pfizer and Cerulean
4. MF: honoraria/speaking: Exelixis, Pfizer, Prometheus, Ipsen; data safety monitoring board: Immatics; research funding: Acceleron, Alkermes, BMS, Calithera, Eisai, Merck, Nektar, Pfizer, Prometheus; consulting: Alkermes, Eisai, Novartis, Pfizer
5. RSA: honoraria/speaking: AstraZeneca, Astellas, Eisai, Pfizer, Merck, Genentech, Exelexis, Bayer, BMS, Janssen, Sanofi, Amgen; consulting: Eisai, Pfizer, Astellas
6. BIR: research funding and consulting: Pfizer
7. JTB: research funding (institutional): Alexion, Novartis, Lilly, Genentech, AstraZeneca, Calithera, Pfizer, Acceleron, Janssen, BMS, Merck Serono, Vaccinex, Tesaro, IBM
8. MJ: consulting: Sanofi; research grant: AstraZeneca, Pfizer
9. RH: no disclosures
10. MBA: consulting: BMS, Novartis, Merck, Genentech/Roche, Pfizer, Exelixis, Eisai, Alexion; advisory board: Novartis, Merck, X4 Pharma, Arrowhead
11. EB: honoraria/speaking: Roche, Exelixis, Janssen; consulting/advisory board: Roche, Exelixis, Janssen, Takeda; research funding: Astellas, Pfizer
12. TFL: research funding: Acceleron, Abbott Laboratories, Abraxis BioScience, Amgen, Argos, AstraZeneca, Aveo, Biovex, BMS, Eisai, Lilly, GSK, Roche, Immatics, Merck, Novartis, Pfizer, Synta, Threshold Pharmaceuticals, Millenium, Tracon, Cerulean, EMD Serono, Prometheus Laboratories, Macrogenics, Peloton, Iovance Biotherapeutics, Medimmune; Dynavax; consulting: Prometheus Laboratories
13. DS: no disclosures
14. RP: no disclosures
15. NM: no disclosures
16. XZ: employment and equity through Acceleron
17. CG: employment and equity through Acceleron
18. MLS: employment and equity through Acceleron
19. ERP: research funding: Merck, Novartis, Pfizer, Genentech/Roche, AstraZeneca, BMS, Eli Lilly, Pfizer; consulting: Acceleron, AstraZeneca, BMS, Clovis, Exelixis, Genentech/Roche, Horizon Pharma, Incyte, Inovio, Janssen, Merck, Novartis; DSMB: Eli Lilly, Pfizer
Protection of Human and Animal Subjects:
Study reviewed and approved by institutional review boards; study conducted according to the Declaration of Helsinki and applicable local laws and regulations. All patients provided written informed consent.
Clinical trial registry: ClinicalTrials.gov Identifier: NCT01727336
References
- 1.Choueiri TK, Motzer RJ. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N Engl J Med. 2017;376: 354–366. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16: 1473–1482. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Hutson TE, Ren M, Dutcus C, Larkin J. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016;17: e4–e5. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17: 917–927. [DOI] [PubMed] [Google Scholar]
- 6.Oh SP, Seki T, Goss KA, et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93: 682–689. [DOI] [PubMed] [Google Scholar]
- 8.Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J Oncol. 2012;2012: 204946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha SI, Bocci M, Lovrot J, et al. Endothelial ALK1 Is a Therapeutic Target to Block Metastatic Dissemination of Breast Cancer. Cancer Res. 2015;75: 2445–2456. [DOI] [PubMed] [Google Scholar]
- 10.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood. 2011;117: 6999–7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendell JC, Gordon MS, Hurwitz HI, et al. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with advanced cancer. Clin Cancer Res. 2014;20: 480–489. [DOI] [PubMed] [Google Scholar]
- 12.Hu-Lowe DD, Chen E, Zhang L, et al. Targeting activin receptor-like kinase 1 inhibits angiogenesis and tumorigenesis through a mechanism of action complementary to anti-VEGF therapies. Cancer Res. 2011;71: 1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt RS, Atkins MB. Molecular pathways: can activin-like kinase pathway inhibition enhance the limited efficacy of VEGF inhibitors? Clin Cancer Res. 2014;20: 2838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Solban N, Khanna P, et al. Inhibition of ALK1 signaling with dalantercept combined with VEGFR TKI leads to tumor stasis in renal cell carcinoma. Oncotarget. 2016;7: 41857–41869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voss MH, Bhatt RS, Plimack ER, et al. The DART Study: results from the dose-escalation and expansion cohorts evaluating the combination of dalantercept plus axitinib in advanced renal cell carcinoma. Clin Cancer Res. 2017;23: 3557–3565. [DOI] [PubMed] [Google Scholar]
- 16.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378: 1931–1939. [DOI] [PubMed] [Google Scholar]
- 17.Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16: 293–300. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22: 454–463. [DOI] [PubMed] [Google Scholar]
- 19.Atkins MB, Gravis G, Drosik K, et al. Trebananib (AMG 386) in Combination With Sunitinib in Patients With Metastatic Renal Cell Cancer: An Open-Label, Multicenter, Phase II Study. J Clin Oncol. 2015;33: 3431–3438. [DOI] [PubMed] [Google Scholar]
- 20.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27: 1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina AM, Feldman DR, Voss MH, et al. Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2012;118: 1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negrier S, Gravis G, Perol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12: 673–680. [DOI] [PubMed] [Google Scholar]
- 23.Rini BI, Bellmunt J, Clancy J, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. 2014;32: 752–759. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14: 552–562. [DOI] [PubMed] [Google Scholar]
- 25.Research CfDEa. Medical review: INLYTA® (axitinib) tablets for oral administration. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202324Orig1s000MedR.pdf [accessed 11/05/2018, 2018]. [Google Scholar]
- 26.Makker V, Filiaci VL, Chen LM, et al. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study 0229N. Gynecol Oncol. 2015;138: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimeno A, Posner MR, Wirth LJ, et al. A phase 2 study of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Cancer. 2016;122: 3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger RA, Deng W, Makker V, et al. Phase II evaluation of dalantercept in the treatment of persistent or recurrent epithelial ovarian cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2018;150: 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou-Alfa GK, Miksad RA, Tejani MA, et al. A Phase Ib, Open-Label Study of Dalantercept, an Activin Receptor-Like Kinase 1 Ligand Trap, plus Sorafenib in Advanced Hepatocellular Carcinoma. Oncologist. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.