Abstract
Background:
Deriving novel treatments for alcohol use disorders (AUDs) is of critical importance, as existing treatments are only modestly effective for reducing drinking. Two promising strategies for treating AUDs include cognitive bias modification (CBM) and transcranial direct current stimulation (tDCS). While each strategy has shown positive results in reducing drinking or alcohol related constructs (e.g., craving), initial tests of the combination of CBM and tDCS have shown mixed results. The present study investigated the degree to which combining CBM and tDCS (2.0 mA anodal current over F10) could reduce alcohol approach biases and alcohol consumption.
Methods:
Seventy-nine at-risk drinkers were randomized to one of four conditions in a 2 × 2 factorial design: Verum CBM/Verum tDCS, Verum CBM/Sham tDCS, Sham CBM/Verum tDCS, or Sham CBM/Sham tDCS. Participants completed a baseline assessment of alcohol approach bias and drinking quantity/frequency (i.e., drinks per drinking day (DDD) and percent heavy drinking days (PHDD)), four sessions of combined CBM/tDCS, and follow-up assessments of approach bias and alcohol consumption.
Results:
Results indicated that while participants did demonstrate significant alcohol approach biases at baseline, neither CBM, tDCS, nor the interaction reduced the bias at the follow-up. In addition, there was evidence of a trend towards reducing DDD from baseline to the one-week/one-month follow-ups, but there was no significant effect of intervention on either DDD or PHDD.
Conclusions:
These results partially replicated null results presented in similar CBM/tDCS trials and suggest that this combination, at least with anodal stimulation over dorsolateral or inferior frontal sites, may have limited utility to reduce drinking.
Keywords: transcranial direct current stimulation, alcohol use disorder, cognitive bias modification, implicit bias
Introduction
The 12-month prevalence of alcohol use disorders (AUDs) in the US is 13.9%, with a lifetime prevalence rate of 29.1% (Grant et al., 2015). AUDs cost the US over 249 billion dollars a year in health related costs, loss of productivity, premature death, and legal costs (Sacks et al., 2015). The etiology of AUDs is known to be multifactorial, ranging from genetic influences to psychological and social, making prevention and treatment efforts very difficult. Thus, identifying potential targets for novel treatments is critical for improving options for reducing drinking.
Motivation to drink alcohol is one of the foremost problems in alcohol use disorders, contributing to both the development and maintenance of problematic drinking (Wiers et al., 2007). Evidence from neuroimaging studies has shown enhanced engagement of motivational circuits that is coupled with reduced cognitive control (Hutchison, 2010; Wiers et al., 2007), potentially leading to a greater propensity to use alcohol. Craving during the presentation of alcohol cues is associated with enhanced neural response in ventral and dorsal striatum, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), left dorsolateral prefrontal cortex (DLPFC) and insula compared to neutral non-alcohol related cues (Filbey et al., 2008; Lingford-Hughes et al., 2006; Myrick et al., 2004, 2008; Bragulat et al., 2008; Kareken et al., 2010). As cues associated with alcohol continue to be rewarding, the neural circuits involved in processing those cues and controlling behavior leading to reward are reinforced, leading to attentional biases towards drug related cues as well as a motivational approach tendency in the presence of drug cues (Tiffany, 1990; Wiers et al., 2007; Farris et al., 2010; Palfai, 2001). Many of the behavioral consequences of addiction such as compulsive drug seeking may be the result of reward based associative mechanisms that lead to long-term neuronal adaptations and changes in synaptic plasticity (Kalivas & Volkow, 2011; Hansson et al., 2008). Plasticity in projections from prefrontal cortex to striatal systems occurs at both pre- and postsynaptic glutamate receptors, resulting in an overly sensitive projection that increases motivational drive to ingest drugs of abuse (Everitt & Wolf, 2002; Berridge & Robinson, 1998; Robinson & Berridge, 1993, 2000; Tindell et al., 2004; Kalivas et al., 2005; Koob & Volkow, 2010; Kalivas & Volkow, 2005; Volkow et al., 2004). Thus, behaviors and cues that lead to drug ingestion may become reinforced over time such that other competing, non-drug use behaviors have a lower likelihood emerging (Berke & Hyman, 2000; Hyman et al., 2006).
Studies with individuals with AUDs have revealed attentional and motivational biases towards alcohol (Wiers et al., 2009), further evidence of the long-term influence of alcohol on information processing. However, there is also evidence that these biases may be amenable to change with targeted interventions. For example, studies from Wiers and colleagues have shown that it is possible to change these biases through a variety of cognitive bias modification protocols. To target approach motivations towards alcohol, a cognitive bias modification (CBM) intervention was developed and tested in heavy drinkers (Wiers et al., 2010). In CBM, participants respond to alcohol and non-alcohol pictures by pushing or pulling a joystick, which corresponds to approach and avoid responses, respectively. Unbeknownst to participants, a much larger proportion of alcohol related stimuli require push responses, which may ultimately retrain associations between alcohol cues and automatic response tendencies; by continually pairing alcohol stimuli with avoidance related responses, it may be possible to reduce these automatic biases and reduce drinking (Wiers et al., 2011, 2010). These interventions have shown considerable promise in that participants receiving bias retraining demonstrated reduced implicit approach associations with alcohol and also reduced levels of relapse during subsequent assessments.
Another independent area of research that has shown some initial promise for substance use disorders is neuromodulation (Spagnolo & Goldman, 2017; Feil & Zangen, 2010), using techniques including transcranial direct current stimulation (tDCS). TDCS is a safe technique (Nitsche et al., 2003; Bikson et al., 2009; Iyer et al., 2005) for altering brain activity through application of a low ampere electrical current on the scalp over a brain region of interest. The precise mechanisms by which tDCS works are currently unclear, although there is some data that suggests anodal tDCS increases synchrony of neuronal firing as a result of reduced GABA (Kim et al., 2014) and increased glutamate activity (Clark et al., 2011). Prior research has shown that application of anodal tDCS over dorsolateral prefrontal cortex (DLPFC) reduces craving for alcohol (Boggio et al., 2008; Klauss et al., 2018), cigarettes (Boggio et al., 2009; Falcone et al., 2016), marijuana (Boggio et al., 2010), and food (Fregni et al., 2008). A recent treatment trial in which anodal tDCS was applied over right DLPFC (with cathode placed on left DLPFC) in alcohol dependent patients in a series of ten sessions showed reduced craving and increased 3-month abstinence in the verum tDCS group compared to the sham group (Klauss et al., 2018). In addition to the use of tDCS to reduce craving, this technique has also been used to enhance such cognitive abilities as response inhibition (Hsu et al., 2011; Jacobson et al., 2011) and learning (Clark et al., 2012; Kang & Paik, 2011; Reis et al., 2009). For example, application of 2.0 mA anodal tDCS over right inferior frontal gyrus or right parietal cortex resulted in better task performance at the end of training compared to individuals in the sham control condition receiving only 0.1 mA tDCS (Clark et al., 2012).
Based on previously observed effects, combining tDCS with other techniques could have potential for enhancing the effects of interventions. For example, combining tDCS with CBM could enhance the effects on reducing motivational biases and drinking. Two studies to date have examined the combined effects of anodal tDCS over left DLPFC and CBM in treatment seekers and non-treatment seeking high risk drinkers. In the high risk drinker sample, 1.0 mA was administered over left DLPFC during three sessions of CBM over three to four days, but no effects of CBM condition or tDCS were found on approach biases or alcohol use; however, participants reported reduced subjective craving during a cue reactivity task (den Uyl et al., 2016). In the treatment seeking sample, den Uyl and colleagues administered 2.0 mA over left DLPFC over the course of four training sessions in four consecutive days (den Uyl et al., 2017). Although there were no significant interaction effects for the full sample, there was some indication of a boosting effect of tDCS and CBM, such that relapse was lower in this group at the one year follow-up. This suggests some preliminary evidence that tDCS may influence drinking outcomes.
The goal of the present study was to examine whether anodal tDCS administered over right IFG would enhance the effects of CBM on reducing motivational biases and drinking. We chose right IFG as the stimulation site because this region is known to be involved in overcoming prepotent responses and prior work from our lab suggests reduced engagement of this region in heavy drinkers (Claus et al., 2013). In addition, a preliminary neuroimaging study from our lab of the approach avoidance task showed that right IFG had a greater response when participants were making responses to avoid alcohol stimuli (i.e., pushing a joystick awy from oneself) compared to when they were approaching alcohol stimuli (i.e., pulling a joystick towards oneself) (Claus, unpublished). We used a 2 × 2 design, crossing tDCS condition (verum vs. sham) with CBM condition (active vs. sham), and participants received tDCS while completing four weekly sessions of CBM. We hypothesized that there would be main effects of CBM and tDCS on reducing motivational bias and drinking, such that the active conditions of each would result in a greater reduction in the respective measure. In addition, we hypothesized an interaction effect, such that the combination of verum tDCS and verum CBM would result in greater reductions in motivational bias and drinking than either active condition alone.
Material and Methods
Participants and Procedures
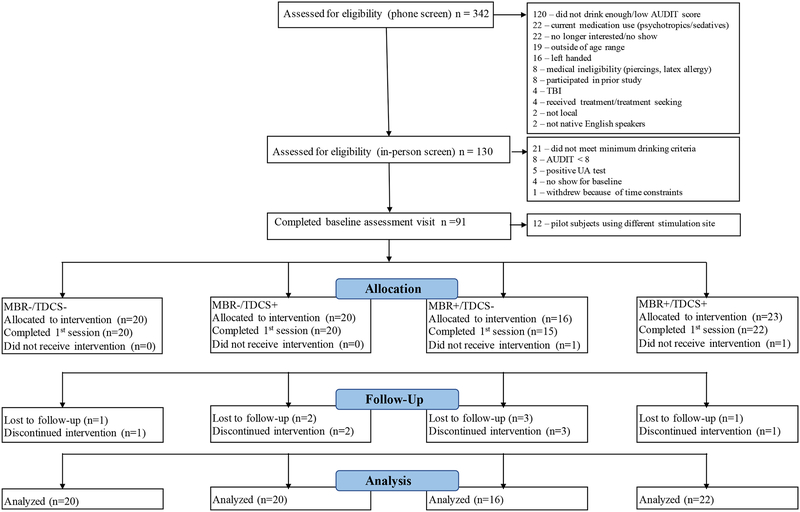
Ninety-one individuals with a recent history of heavy drinking participated in this factorial design (CBM × TDCS) clinical trial from May 2014 to December 2015. Recruitment relied on online postings and flyers placed around the Albuquerque metropolitan area as well as advertisements in local newspapers. To be included in the study, participants had to meet the following inclusion criteria: age 21–30 years; no history of treatment for AUD or desire for treatment; Alcohol Use Disorder Identification Test (AUDIT) (Babor et al., 1992) score greater than eight; no history of alcohol withdrawal; no history of brain injury; not taking psychotropic medications (e.g., antidepressants, antipsychotics); female subjects could not be pregnant; no evidence of recent illicit drug use on a urine screen; right handed; and no contraindications for tDCS (e.g., no ferrous metal anywhere in the body). Individuals were screened over the phone to assess preliminary eligibility and scheduled for an in-person eligibility session. IRB approved informed consent was obtained prior to the commencement of any study related procedures. This trial was registered in clinicaltrials.gov prior to commencing recruitment efforts (trial number NCT02045108). Of note, the first 12 participants that were randomized were pilot participants for which a different stimulation site (i.e., left primary motor cortex) was used; these participants were dropped from all analyses for this reason. The CONSORT diagram showing participant flow through the study is presented in Figure 1.
Figure 1:
CONSORT Diagram
Measures and Materials
Hazardous drinking level was measured with the AUDIT (Babor et al., 2001) and this measure was used to determine eligibility (i.e., AUDIT >8). A Demographics Questionnaire was used to collect information including ethnicity, SES, and education. A Drinking History Questionnaire assessed lifetime drinking behavior (e.g., age of onset, attempts to quit/reduce drinking). A Sensation Questionnaire assessed potential physical sensations that occur during tDCS (i.e., itching, heat, pain, discomfort) on a Likert scale with anchors of 0 (None) to 10 (Unbearable).
Our primary outcome variables were drinks per drinking day and percent heavy drinking days as measured by the Timeline Follow-Back (TLFB) (Sobell & Sobell, 1992). Daily drinking data was collected at baseline, and the follow-up visits at 1-week and 1-month post intervention.
Approach Avoidance Task.
As in prior studies of motivational bias training (Wiers et al., 2010, 2011), the AAT required participants to make avoid or approach responses to alcohol-related or control (non-alcohol containing beverages) pictures based on a perceptual feature of the image. In the current study, approach/avoidance decisions were determined based on whether images were tilted to the right or tilted to the left; tilt direction and relevant instructions were counterbalanced across participants. Participants were instructed to either push (avoid) or pull (approach) a joystick depending on the orientation of the picture; once a response was made, the image shrank into the background (avoidance responses) or increased in size over 2 seconds (approach responses), similar to prior studies that have used the AAT (Wiers et al., 2010, 2011). A 2 (picture type: alcohol vs. control) × 2 (response: approach vs. avoid) design was utilized, with 60 trials of each type presented to participants. Prior to completing the task, participants completed a short practice to learn stimulus-response pairings. Approach bias motivation scores for alcohol stimuli were computed according to the d-score approach described in Lindgren et al (2015) and served as our secondary outcome measure.
Cognitive Bias Modification.
For the CBM sessions, participants completed a variant of the AAT in which the proportion of approach/avoid responses required for alcohol and control stimuli were manipulated. In the Avoid Alcohol Training condition, 90% of the alcohol stimuli required a push/avoid response and 10% required a pull/approach response, and 90% of the control stimuli required a pull/approach response and 10% required a push/avoid response. In the Sham Training condition, both alcohol and control stimuli types required an equal number of push and pull responses. We chose a control condition with equivalent approach and avoid responses rather than flipping the contingencies of the Verum Training condition (i.e., 90% pull alcohol, 10% push alcohol, 10% pull control, 90% push control) to prevent potential enhancement of approach responses towards alcohol, which could lead to increased drinking. Thus, across both conditions, half of the stimuli required approach responses and half required avoid responses. For each training session, participants were presented with 20 alcohol related stimuli and 20 non-alcohol related stimuli, with each stimulus presented 11 times, for a total of 440 trials. As in the baseline AAT, each trial displayed a picture that was tilted to the left or right by 15°, and depending on the instructions given to participants, a push or pull response was required which subsequently shrank or enlarged the picture on the screen. Participants were instructed to respond as quickly and accurately as possible. If a mistake was made, an error message was displayed until the participants corrected his/her response. The total amount of time that was required to complete the task was approximately 18 minutes. Stimulus presentation was controlled by E-Prime (PST). Response time (RT) and accuracy were assessed for each trial and used in subsequent analyses. Each training session utilized a new set of pictures in order to increase generalizability of the stimulus-response pairings.
Procedures
Participants that met eligibility criteria on the phone screen were invited to the laboratory for an eligibility screening session. Participants were breathalyzed to ensure a breath alcohol concentration (BrAC) of 0.0 g% and provided IRB approved informed consent. Participants who met eligibility criteria were scheduled for a baseline session within two weeks of the eligibility session. At the baseline session, the AAT-no bias task was administered, and drinking data was collected.
Upon completion of the eligibility session, participants were randomized to one of four conditions which were a cross of tDCS condition (0.1 mA (Sham) or 2.0 mA (Active)) with CBM condition (Avoid alcohol (Active) vs. No bias (Sham)): Verum CBM/Verum tDCS, Verum CBM/Sham tDCS, Sham CBM/Verum tDCS, or Sham CBM/Sham tDCS. Randomization was stratified by gender; randomization sequences were produced in R by crossing the six blinding box positions for TDCS (3 Verum, 3 Sham) with 4 conditions of CBM (2 Verum, 2 Sham), replicating this sequence 3 times for a total of 72 conditions and randomly selecting without replacement from these conditions to generate separate randomization sequences for male and female participants. The PI of the study (EDC) generated the random sequences, and research staff entered participants into a spreadsheet along with self-reported gender to obtain the randomization position and condition for each participant. Participants and research staff interacting with participants were blind to treatment conditions.
Participants were scheduled to visit the laboratory once a week for 4 consecutive weeks for one-hour sessions. We chose weekly sessions rather than daily sessions for two primary reasons. First, the literature on learning has suggested that spaced training is more effective than blocked training for increasing retention of a variety of domains including motor skill learning (Cepeda et al., 2008; Shea et al., 2000). Based on this prior research, we reasoned that a longer interval between training sessions would allow the CBM to have a more robust effect on changing approach biases, and that completing this training concurrently with the stimulation would produce even greater effects on approach biases and drinking. In addition, because we were recruiting primarily college students, it was assumed that 4 daily sessions would be more difficult for participants to complete given inconsistent schedules across days of the week. During each training session, participants completed a TLFB to assess alcohol use since the last session, and were then prepped for the tDCS administration. Square-shaped, 11 cm2, saline-soaked sponge electrodes were used and the anode was placed on the participants’ scalp over area F10 and the cathode was placed on the contralateral upper arm; electrodes were held in place with a Coban bandage or Surgilast. Our previous experience with an extracranial reference suggests minimal adverse events, which have been primarily been related to skin irritation and skin sensation under the electrode, and no differences between cranial and extracranial reference in other sensations such as headache or other physical or cognitive phenomena. In addition, safety reviews of tDCS (e.g. Bikson et al., 2016) have not found any evidence for safety concerns using an extracranial reference.
Two ActivaDose II tDCS units were connected to a custom blinding box, which allowed current from one of the two units to pass through to the participant. The blinding box had 6 unique positions on a switch, and study staff were instructed to place the switch in the appropriate position (numbered 1–6) and to always set the tDCS unit marked A to 2.0 mA and the unit marked B at 0.1 mA. The units plugged into the blinding box at specified positions, marked A and B, and the anode and cathode were color coded. The same switch position was used during every session for a given participant. Once the electrodes were in place, participants completed a sensation questionnaire in order to establish a baseline sensation rating. Next, study staff started the practice session for the CBM training program, and participants received instructions and practice trials indicating the response required for a given tilt direction. This training required approximately 2 minutes to complete. After 5 minutes, participants rated their sensation again; if any rating had increased to a level above moderate, stimulation was stopped. Upon completion of the rating questionnaire, the training session program was started, and participants completed the training while receiving 20 minutes of stimulation. Approximately 10 minutes into the training, participants were given a short break during which sensation ratings were completed again; stimulation continued through this break. Participants then finished the training, completed another sensation rating, and once the training program completed, the remaining time set on the stimulation device was allowed to complete. Participants completed a final sensation rating, electrodes were removed, and participants were given a mirror to inspect the electrode sites for any signs of irritation.
Upon completion of the 4 week training/stimulation period, participants completed a 1-week followup, during which the no bias AAT was administered a second time in order to assess reaction time measures of alcohol avoidance and approach biases as well as a TLFB. Finally, during the 1-month followup, participants completed a TLFB.
Data Analysis
All reaction time and accuracy distributions from the AAT were examined for outliers and non-normality. Within each participant, outlier reaction times greater than three standard deviations from the mean reaction time were removed from the analysis. Our primary drinking variables were drinks per drinking day and proportion of heavy drinking days at the 1-week and 1-month follow-up visits.
To test whether alcohol training reduced alcohol approach motivations and drinking, reaction time differences between the approach alcohol and avoid alcohol conditions at baseline and the 1 week followup were tested for differences in the context of a 2 (tDCS condition) × 2 (training condition) × 2 (time: baseline vs. 1 wk) linear mixed model implemented in R using the lme4 package. All participants completing the baseline session (n = 77) were included in this analysis; 70 participants also completed the follow-up assessment one week after completing the intervention.
Drinking outcome variables were analyzed using linear mixed models in R with tDCS condition, training condition, and time (i.e., baseline, 1-week, and 1-month), as independent variables. We examined main effects and interactions for each of the independent variables within the linear mixed model. Drinking outcomes were analyzed using all available data from individuals who were randomized (i.e., 79 participants) in an intention to treat (ITT) analysis.
Results
Sample Characterization
Participants were 24.5 (2.7) years old and were drinking 5.1 (2.7) drinks per drinking day on average. In addition, participants reported 26.6 (22.3) heavy drinking days over the 90 days prior to the screening appointment. Finally, although the sample was not treatment seeking, AUDIT scores were fairly high, with a mean of 15.7 (6.7). A total of 113 participants completed baseline visits, and of these, 79 participants met eligibility requirements and were randomized. Of the 79 randomized, 77 completed at least one tDCS/training visit, and 70 completed all four tDCS/training visits. See Table 1 for sample characterization by experimental group.
Table 1:
Sample Characteristics, Approach Bias, and Drinking by Intervention Group
| CBM-/ TDCS- |
CBM-/ TDCS+ |
CBM+/ TDCS- |
CBM+/ TDCS+ |
||
|---|---|---|---|---|---|
| Randomized | 20 | 20 | 16 | 23 | |
| % women | 30 | 30 | 31 | 35 | |
| Attended 1st session | 20 | 20 | 15 | 22 | |
| Age | 22.8 (1.7)* | 25.2 (2.7) | 24.9 (2.8) | 25.5 (2.7)* | |
| AUDIT | 15.6 (6.7) | 15.7 (6.4) | 16.2 (6.9) | 15.3 (6.8) | |
| Alcohol Bias | baseline | 0.10 (0.28) | 0.08 (0.35) | 0.12 (0.28) | 0.21 (0.31) |
| 1-wk FU | 0.13 (0.27) | 0.06 (0.26) | 0.16 (0.37) | 0.10 (0.25) | |
| Control Bias | baseline | −0.13 (0.30) | −0.14 (0.39) | −0.20 (0.29) | −0.31 (0.21) |
| 1-wk FU | −0.13 (0.22) | −0.15 (0.24) | −0.16 (0.33) | −0.12 (0.25) | |
| Alc-Con Bias | baseline | −0.09 (0.24) | 0.01 (0.22) | −0.06 (0.30) | −0.04 (0.20) |
| 1-wk FU | −0.02 (0.26) | 0.0005 (0.26) | −0.10 (0.27) | −0.08 (0.24) | |
| DDD | baseline | 7.3 (3.4) | 6.5 (3.3) | 6.6 (3.4) | 6.2 (4.0) |
| 1-wk FU | 5.1 (3.5) | 5.9 (3.7) | 6.5 (4.4) | 5.7 (1.9) | |
| 1-mo FU | 5.5 (2.2) | 5.7 (3.1) | 6.6 (2.7) | 4.5 (2.5) | |
| PHDD | baseline | 0.33 (0.23) | 0.30 (0.19) | 0.26 (0.09) | 0.25 (0.18) |
| 1-wk FU | 0.21 (0.19) | 0.23 (0.22) | 0.31 (0.30) | 0.29 (0.22) | |
| 1-mo FU | 0.18 (0.16) | 0.16 (0.11) | 0.32 (0.26) | 0.20 (0.17) |
The CBM-/tDCS- and CBM+/tDCS+ groups were different in mean age (p = 0.01). No other baseline variables differed by group.
Approach Biases
At baseline, participants showed evidence of an approach motivational bias for alcohol stimuli, with alcohol bias scores significantly greater than zero (t(76) = 2.86, p = 0.01), and an avoid bias for control stimuli, as indicated by bias scores that were significantly less than zero (t(76) = 4.18, p <0.001.) The comparison of alcohol and control approach biases revealed no overall differences from zero (t(76) = 1.61, p = 0.11). Comparison of mean bias scores across the four groups revealed no significant interactions or main effects, suggesting that all groups were equivalent on approach biases for both alcohol and control stimuli and the difference between alcohol and control biases at baseline. Of the 77 participants that started the training sessions, 70 completed the one week follow-up assessment of approach bias. Examination of changes in alcohol approach bias after the 4-session intervention failed to show any interactive effects of training condition and time (CBM: β = −0.000, p = 1.00, Cohen’s d = 0.02, tDCS: β = 0.11, p = 0.37, Cohen’s d = 0.001, tDCS × CBM interaction: β = −0.10, p = 0.54, Cohen’s d = 0.03). Analysis of approach biases for the control pictures also showed no significant effects (CBM: β = 0.04, p = 0.70, Cohen’s d = 0.02, tDCS: β = −0.12, p = 0.29, Cohen’s d = 0.001, tDCS × CBM interaction: β = 0.19, p = 0.21, Cohen’s d = 0.03). Finally, we found no effects of the difference between alcohol and control approach biases from pre- to post-intervention (CBM: β = 0.04, p = 0.71, Cohen’s d = 0.06, tDCS: β = −0.001, p = 0.98, Cohen’s d = 0.004, tDCS × CBM interaction: β = 0.08, p = 0.65, Cohen’s d = 0.08). Group means for pre- and post-training biases are presented in Table 1.
Effects of TDCS and Training on Drinking
The primary analysis of interest for the current study was the comparison of reductions in drinks per drinking across the four treatment groups at the one-week and one-month post-treatment follow-up visits. Examination of the changes in drinking from baseline to these follow-up visits revealed a non-significant effect in the three-way interaction of tDCS × CBM × time (β = −0.38, p = 0.09, Cohen’s d = −0.13). In addition to the three-way effect, both group × time interactions failed to show any significant effects (CBM × time: (β = 0.06, p = 0.66, Cohen’s d = 0.01; tDCS × time: β = 0.17, p = 0.30, Cohen’s d = 0.12). There was a nonsignificant trend of visit for DDD across all participants (β = −0.18, p = 0.07, Cohen’s d = −0.12), such that participants tended to reduce their drinking over the course of all visits by approximately 1 standard drink per drinking day at the one week follow-up and 1.3 standard drinks at the one month follow-up (see Table 1).
Examination of the proportion of heavy drinking days across the four treatment groups showed similar findings as the drinks per drinking day analysis (see Table 1). The three-way interaction was not significant (tDCS × CBM × time: (β = −0.01, p = 0.37, Cohen’s d = −0.05), nor were either of the two-way group × time interactions (CBM × time: β = −0.016, p = 0.07, Cohen’s d = −0.11; tDCS × time: β = 0.01, p = 0.34, Cohen’s d = 0.05). Finally, there was no significant effect of time (β = −0.00, p = 0.73, Cohen’s d = −0.08), suggesting that the interventions did not change heavy drinking patterns.
Side Effects
For each of the four potential side effects measured for tDCS (i.e., itching, pain, heat, discomfort), we examined group differences across the active and sham tDCS conditions in the context of a mixed model to account for repeated measures. In all cases, the verum tDCS group reported greater severity of symptoms than the sham tDCS group (itch: t(68.8) = 3.23, p = 0.002; pain: t(71.1) = 2.65, p = 0.01; heat: t(70.0) = 2.17, p = 0.03; discomfort: t(70.3) = 2.83, p = 0.006). While there were group differences, it is important to note that the magnitude of these differences was relatively small (itch: 1.3 (1.6) vs. 0.6 (0.9); pain: 0.3 (0.8) vs. 0.1 (0.4); heat: 0.6 (1.2) vs. 0.2 (0.6)); discomfort: 1.0 (1.6) vs. 0.3 (0.7)). We also tested whether these sensation ratings varied over time within visit by group and found no significant interactions.
In addition to the above analyses, we also examined whether individuals who did not complete all four stimulation/training sessions reported more negative sensations compared to those who did complete all sessions, and whether this interacted with tDCS group (verum vs. sham). There was no significant effects of completion status on sensation rating for any of the symptoms (all p’s > 0.20). However, when including tDCS group and the interaction of tDCS group and completion status, we found a significant interaction (t(83.9) = 2.06, p < 0.05) such that indivdiuals in the verum tDCS group that also failed to complete all sessions reported higher itch levels than the other three groups; main effects of tDCS group (t(86.0) = 2.97, p < 0.004; verum tDCS > sham tDCS) and completion status (t(88.0) = 2.60, p = 0.01; completers < non-completers).
Discussion
The current study examined the effects of cognitive bias modification and tDCS on approach biases towards alcohol and on alcohol consumption in a group of at-risk alcohol drinkers. We found that none of our dependent variables of interest (i.e. automatic approach biases, drinks per drinking day, or percent heavy drinking days) showed significant differences as a function of experimental condition. In contrast, there were significant effects of group on sensations experienced during the stimulation sessions, suggesting that our blinding procedure may not have been optimal. Below, we discuss our findings in the context of other cognitive training and brain stimulation studies in heavy drinkers.
The current study provided support for the presence of alcohol approach biases at baseline, but we failed to show any evidence suggesting that these biases may be modified by training. Examination of baseline approach biases largely confirmed prior research (Wiers et al., 2013, 2009), with participants showing a significant approach bias towards alcohol at baseline. Unfortunately, the training sessions did not significantly affect this bias, and in fact, alcohol related approach biases actually increased slightly after four sessions of training. These results are in direct contrast to studies on CBM in alcohol dependent individuals (Wiers et al., 2011, 2010; Eberl et al., 2013) and two tDCS studies that found a significant reduction (den Uyl et al., 2017) and a trend towards reduction (p=0.06; den Uyl et al., 2016) in approach bias after training although there were no group by time differences (den Uyl et al., 2016, 2017). However, our results are consistent with a large study that attempted to replicate CBM findings in two large samples (Lindgren et al., 2015); of note, the sample included here was very similar to that of (Lindgren et al., 2015), who included participants that were non-treatment seeking drinkers reporting at least one binge drinking episode in the past month. Wiers et al (Wiers et al., 2018) have argued that training effects may only be useful in treatment seeking samples, who are highly motivated to change their drinking and also may have greater implicit biases prior to treatment. Participants in the current trial were not informed that the study was an intervention trial to reduce drinking and were excluded from participating if they were seeking treatment, so it seems unlikely that there would be any motivation to change, which could be an important factor in predicting training effects (Lindgren et al., 2015, Boffo et al., 2015). The fact that approach biases for alcohol actually increased, albeit nonsignificantly, suggests that more research into the utility of this treatment is necessary to draw conclusions about potential differences between treatment and non-treatment seeking samples. Further, while one study has suggested that six sessions may be ideal for changing drinking (Eberl et al., 2014), it seems that four sessions should have still produced at least a numerical reduction in alcohol approach bias.
In addition to non-significant changes to alcohol approach bias, we also failed to find any significant effect of either the CBM or tDCS interventions alone or the combination on our primary drinking outcomes of drinks per drinking day and percent heavy drinking days. Again, while these results are in contrast to prior studies of CBM that reported decreased relapse rates in treatment seeking alcohol dependent individuals (Wiers et al., 2011, 2010; Eberl et al., 2013), they do replicate the null results for drinking in the prior study that also tested high risk, non-treatment seeking drinkers (den Uyl et al., 2016) and treatment seekers (den Uyl et al., 2017). The results for the verum CBM conditions are somewhat surprising given initial promising findings for reducing relapse, but given that the current sample was not a treatment seeking sample, the present null finding is not necessarily evidence against using approach bias modification interventions. In addition, because the proposed mediating factor in drinking reductions (i.e., approach bias towards alcohol) did not change, changes in drinking should not be expected (Wiers et al., 2018). While there has been a reasonable number of studies investigating CBM for AUDs, far fewer studies have examined tDCS as a potential treatment. In the three studies that have used tDCS in combination with cognitive bias modification (den Uyl et al., 2016, 2017, 2018), only one found a trend towards the combined intervention decreasing 1-year relapse rates (den Uyl et al., 2017) and none found significant effects on drinks per drinking day, perhaps suggesting that this combined intervention may not hold considerable promise for AUDs. However, before concluding decisively that this is the case, future investigations should test other stimulation protocols including different stimulation sites. While the previous studies targeted dorsolateral prefrontal cortex, the current study placed the anode over F10, which is in close proximity to right inferior frontal gyrus. Frontal cortex stimulation sites are intuitively appealing based on prior neuroimaging literature, but other sites may prove to be more fruitful. In addition, application of tDCS alone may also be beneficial for the treatment of AUDs; as mentioned earlier, one study found that ten sessions of verum tDCS over right DLPFC was associated with reduced craving and increased abstinence rates compared to sham tDCS in a group of patients with an AUD. Future studies that investigate tDCS alone, or in combination with other cognitive treatments may prove useful in the treatment of AUDs.
Some limitations of the study need to be noted. First, our sample size for this trial was rather small, which limited our ability to detect anything but large effects. We recomputed power using simulations of small and medium effect sizes in the context of the linear mixed effects model for drinks per drinking day and found power estimates of .23 (tDCS × time), .29 (MBR × time), and .14 (tDCS × MBR × time) for a small effect size (d = 0.20) and of .87 (tDCS × time), .92 (MBR × time), and .60 (tDCS × MBR × time) for a medium effect size (d = 0.50). Thus, the sample size used in this study was underpowered and unlikely to detect significance with small effect sizes. However, even with a larger sample, the direction of the approach bias results and the lack of any differences in the drinking results suggests that results would have remained nonsignificant. In addition to the small sample size, we may have failed to have a strong control condition for tDCS. Participants in the verum tDCS condition reported greater overall sensations, which could compromise the blind; however, a formal comparison of participant guesses at the tDCS condition suggests that the blind was effective (χ2 = 0.70, p = 0.40). Next, the decision to use weekly training sessions instead of daily sessions of tDCS and CBM may have impacted our ability to reduce approach bias and drinking. To our knowledge, there have not been systematic investigations into the timing of repeated sessions and the delay between sessions for tDCS or CBM. In fact, prior studies with multiple sessions of tDCS in AUD participants have completed 4 sessions within one week (den Uyl et al., 2016, 2017, 2018) or every other day (Klauss et al., 2018). Whether intersession duration influences the efficacy of either CBM or tDCS is unknown and if these techniques continue to show promising effects in reducing drinking, it may be valuable to investigate these timing effects. Finally, as mentioned above, the choice to recruit non-treatment seeking individuals may have hampered our ability to produce significant effects, since the participants in our study were not interested in reducing their drinking. In addition, this reduces our ability to generalize to individuals who are seeking treatment to reduce their alcohol use. Future studies should focus on treatment seeking individuals, particularly when examining intervention effects on drinking outcomes.
In conclusion, we found no evidence that suggests that tDCS over the right inferior frontal gyrus combined with CBM is useful for reducing alcohol-related motivational biases nor drinking related behavior. While this study adds to two others that also found no significant changes in drinking, it may be possible that CBM is only effective on its own with treatment seeking individuals. tDCS needs further exploration to determine whether it may be effective in the treatment of AUDs; some future directions include testing different stimulation sites (e.g., medial prefrontal cortex (Hanlon et al., 2015)), targeting alternative relevant phenotypes (e.g., craving (Klauss et al., 2018)), and testing different phases of treatment (e.g., immediately after detox, during treatment, after treatment). For example, Hanlon et al (2015) has reported that reducing activity in the medial prefrontal cortex may be particularly useful for reducing craving, a response that is known to increase activity in this region (e.g., Schacht et al., 2012) among indivdiuals with an AUD when shown alcohol related cues. Thus, it may be possible to not only enhance engagement of a particular brain region to increase a given cognitive function (e.g., DLPFC for cognitive control over craving responses), but also to directly target those regions implicated in maladaptive behaviors that are observed in AUDs, such as craving. Future stimulation protocols that test these various permutations and others may provide key insights into more effective treatments for AUDs.
Acknowledgments
This work was funded by the National Institutes of Health (R21 AA021201). The funding agency had no involvement in any part of the study design, data collection, analysis, or manuscript preparation. The authors have no conflicts of interest.
References
- Babor T, de la Fuente J, Saunders J, Grant M (1992) The Alcohol Use Disorders Identification Test Guidelines for use in primary health care. World Health Organization. [Google Scholar]
- Babor T, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. [Google Scholar]
- Berke J, Hyman S (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532. [DOI] [PubMed] [Google Scholar]
- Berridge K, Robinson T (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Reviews 28:309–369. [DOI] [PubMed] [Google Scholar]
- Bikson M, Datta A, Elwassif M (2009) Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol 120:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. (2016). Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul 9:641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffo M, Pronk T, Wiers RW, Mannarini S (2015) Combining cognitive bias modification training with motivational support in alcohol dependent outpatients: study protocol for a randomised controlled trial. Trials 16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F (2009) Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett 463:82–86. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, Basaglia A, Fregni F (2008) Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 92:55–60. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F (2010) Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 112:220–225. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor S, Kareken D (2008) Alcohol sensitizes cerebral responses to the odors of alcoholic drinks: an fMRI study. Alcohol Clin Exp Res 32:1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Vul E, Rohrer D, Wixted JT, Pashler H (2008) Spacing Effects in Learning: A Temporal Ridgeline of Optimal Retention. Psych Sci 19: 1095–1102. [DOI] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Mayer AR, Weisend MP, Lane TDR, Calhoun VD, Raybourn EM, Garcia CM, Wassermann EM (2012) TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage 59:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Trumbo MC, Gasparovic C (2011) Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: A 1H magnetic resonance spectroscopy study. Neurosci Lett 500:67–71. [DOI] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE (2013) Behavioral control in alcohol use disorders: relationships with severity. J Stud Alcohol and Drugs 74:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Lindenmeyer J, Wiers RW (2018) A Clinical Trial with Combined Transcranial Direct Current Stimulation and Attentional Bias Modification in Alcohol-Dependent Patients. Alcohol Clin Exp Res 42:1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Rinck M, Lindenmeyer J, Wiers RW (2017) A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict Biol 22:1632–1640. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW (2016) Electrophysiological and Behavioral Effects of Combined Transcranial Direct Current Stimulation and Alcohol Approach Bias Retraining in Hazardous Drinkers. Alcohol Clin Exp Res 40:2124–2133. [DOI] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J (2013) Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci 4:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J (2014) Implementation of approach bias re-training in alcoholism-how many sessions are needed? Alcohol Clin Exp Res 38:587–594. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22:3312–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Bernardo L, Ashare RL, Hamilton R, Faseyitan O, McKee SA, Loughead J, Lerman C (2016) Transcranial Direct Current Brain Stimul Increases Ability to Resist Smoking. Brain Stimul 9:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SR, Ostafin BD, Palfai TP (2010) Distractibility moderates the relation between automatic alcohol motivation and drinking behavior. Psychology of Addict Behav 24:151–156. [DOI] [PubMed] [Google Scholar]
- Feil J, Zangen A (2010) Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev 34:559–574. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE (2008) Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 33:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FAM, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS (2008) Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 51:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS (2015) Epidemiology of DSM-5 Alcohol Use Disorder. JAMA Psychiatry 72:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, DeVries W, Mithoefer O, Badran BW, George MS (2015) What goes up, can come down. Brain Res 1628:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Rimondini R, Neznanova O, Sommer WH, Heilig M (2008) Neuroplasticity in brain reward circuitry following a history of ethanol dependence. European J Neurosci 27:1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TY, Tseng LY, Yu JX, Kuo WJ, Hung DL, Tzeng OJL, Walsh V, Muggleton NG, Juan CH (2011) Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage 1–9. [DOI] [PubMed] [Google Scholar]
- Hutchison KE (2010) Substance Use Disorders: Realizing the Promise of Pharmacogenomics and Personalized Medicine. Annu Rev Clin Psychol 6:577–589. [DOI] [PubMed] [Google Scholar]
- Hyman S, Malenka R, Nestler E (2006) Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM (2005) Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 64:872–875. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Javitt DC, Lavidor M (2011) Activation of Inhibition: Diminishing Impulsive Behavior by Direct Current Stimulation over the Inferior Frontal Gyrus. J Cogn Neurosci. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Volkow N (2005) The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalivas P, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EK, Paik NJ (2011) Effect of a tDCS electrode montage on implicit motor sequence learning in healthy subjects. Experimental & Translational Stroke Medicine 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O’Connor SJ (2010) Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage 50:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG, Jackson SR (2014) tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7T magnetic resonance spectroscopy study. Neuroimage 99:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Anders QS, Felippe LV, Nitsche MA, Nakamura-Palacios EM (2018) Multiple Sessions of Transcranial Direct Current Stimulation (tDCS) Reduced Craving and Relapses for Alcohol Use: A Randomized Placebo-Controlled Trial in Alcohol Use Disorder. Front Pharmacol 9:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Volkow N (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KP, Wiers RW, Teachman BA, Gasser ML, Westgate EC, Cousijn J, Enkema MC, Neighbors C (2015) Attempted Training of Alcohol Approach and Drinking Identity Associations in US Undergraduate Drinkers: Null Results from Two Studies. PLoS One 10:e0134642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Daglish M, Stevenson B, Feeney A, Pandit S, Wilson S, Myles J, Grasby P, Nutt D (2006) Imaging alcohol cue exposure in alcohol dependence using a PET O-15-H2O paradigm: results from a pilot study. Addict Biol 11:107–115. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton R, Li X, Henderson S, Drobes D, Voronin K, George M (2004) Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology 29:393–402. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K (2008) Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry 65:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (2003) Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114:2220–2222. [DOI] [PubMed] [Google Scholar]
- Palfai T (2001) Individual differences in temptation and responses to alcohol cues. J Stud Alcohol 62:657–666. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 106:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Berridge K (1993) The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Reviews 18:247–291. [DOI] [PubMed] [Google Scholar]
- Robinson T, Berridge K (2000) The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction 95:S91–S117. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49:e73–e79. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea CH, Lai Q, Black C, Park J-H (2000) Spacing practice sessions across days benefits the learning of motor skills. Hum Mov Sci 19: 737–760. [Google Scholar]
- Sobell L, Sobell M (1992) Timeline follow-back: a technique for assessing self-reported alcohol consumption in Litten RZ, Allen J, eds., Measuring Alcohol Consumption: Psychosocial and Biochemical Methods, 41–72. Humana Press, Totawa, NJ. [Google Scholar]
- Spagnolo PA, Goldman D (2017) Neuromodulation interventions for addictive disorders: challenges, promise, and roadmap for future research. Brain 18:aww284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany S (1990) A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol Rev 97:147–168. [DOI] [PubMed] [Google Scholar]
- Tindell A, Berridge K, Aldridge J (2004) Ventral pallidal representation of pavlovian cues and reward: Population and rate codes. J Neurosci 24:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang GJ (2004) The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology 47:3–13. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Stelzel C, Park SQ, Gawron CK, Ludwig VU, Gutwinski S, Heinz A, Lindenmeyer J, Wiers RW, Walter H, Bermpohl F (2013) Neural Correlates of Alcohol-Approach Bias in Alcohol Addiction: the Spirit is Willing but the Flesh is Weak for Spirits. Neuropsychopharmacology 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers R, Bartholow B, van den Wildenberg E, Thush C, Engels R, Sher K, Grenard J, Ames S, Stacy A (2007) Automatic and controlled processes and the development of addictive behaviors in adolescents: A review and a model. Pharmacol Biochem Behav 86:263–283. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Boffo M, Field M (2018) What’s in a Trial? On the Importance of Distinguishing Between Experimental Lab Studies and Randomized Controlled Trials: The Case of Cognitive Bias Modification and Alcohol Use Disorders. J Stud Alcohol and Drugs 79:333–343. [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J (2011) Retraining Automatic Action Tendencies Changes Alcoholic Patients’ Approach Bias for Alcohol and Improves Treatment Outcome. Psychol Sci 22:490–497. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E (2009) Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav 8:101–106. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Kordts R, Houben K, Strack F (2010) Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction 105:279–287. [DOI] [PubMed] [Google Scholar]