Abstract
We used antiretroviral (ARV) drug testing to evaluate the accuracy of self-reported data for HIV status and antiretroviral treatment (ART) among people who inject drugs (PWID) enrolled in an HIV prevention trial. ARV drugs were detected in enrollment samples from 72/482=14.9% HIV-infected participants (39/52=75.0% who reported being on ART; 33/430=7.7% who reported not being on ART). Overall, 213/482=44.2% participants indicated that they were not aware of their HIV-positive status prior to study entry; of those, 30 had ARV drugs detected at enrollment, including 15 who also had ARV drugs detected at the screening visit. These participants were likely aware of their HIV-positive status at study entry but did not report this to study staff. This study shows that self-reported data on HIV testing history and ART may not be accurate and that ARV drug testing can help identify persons who are aware of their HIV-positive status and are on ART.
Keywords: HIV, ART, self-report, PWID, Indonesia, Ukraine, Vietnam
INTRODUCTION
People who inject drugs (PWID) are at high risk of HIV infection, since sharing contaminated needles and other injection equipment provides a direct route for HIV transmission. Poor access to health services, social stigma, and criminalization also contribute to the high risk of HIV infection among PWID [1]. HIV prevalence among PWID is more than 20 times that of the general population [1-3]. Among an estimated 15.6 million PWID globally, approximately 17.8% are estimated to be living with HIV [2]. Reliable estimates of the number of PWID who are aware of their HIV status and are on antiretroviral treatment (ART), as well as the proportion of HIV-infected PWID who are unaware of their HIV status, are needed to optimize strategies for HIV prevention and treatment among PWID.
Most studies rely on self-reported data to estimate the proportion of HIV-infected people who are aware of their HIV status and are on treatment, and to identify individuals who are newly diagnosed [4-6]. However, self-reported data for HIV status and ART in clinical and research settings can be unreliable [7-14]. As with other risk groups, HIV-infected PWID may choose not to report a prior HIV diagnosis or that they are on ART for several reasons (e.g., concerns that this information might be shared, or exclusion from a research study that does not enroll those who are previously diagnosed or on ART). Also, some people may not understand or may misinterpret questions about HIV testing and care.
Antiretroviral (ARV) drug testing can be used to obtain more accurate estimates of the prevalence of ART [8, 9, 15, 16] and to evaluate the accuracy of self-reported data on HIV testing history and ARV drug use (by identifying those who are on ART, but do not report being aware of their HIV-positive status [9-14] or do not report being on ART [7-9]). ARV drug testing can also be used to identify individuals who report that they are on ART, but do not have ARV drugs in their study samples, indicating issues with medication adherence [9, 17]. In this study, we used ARV drug testing to evaluate the accuracy of self-reported data on HIV testing history and ART obtained in a multi-national trial that evaluated use of an integrated intervention to reduce HIV transmission from HIV-infected PWID to their injection partners (HIV Prevention Trials Network [HPTN] 074) [18, 19].
METHODS
Study cohort
HPTN 074 (NCT02935296) was a randomized, controlled clinical trial conducted at three study sites (Jakarta, Indonesia; Kyiv, Ukraine; Thai Nguyen, Vietnam; enrollment: 2015-2016). The study enrolled HIV-infected PWID (index participants) and their HIV-uninfected injection partners (ages 18-60 years old; men and women) [18, 19]. In HPTN 074, active injection drug use was initially defined as self-report of injecting drugs ≥2 times per week for the previous three months and self-identification of the anatomical location of the most recent injection site, with confirmation by study staff. Eight months after the study started, the definition of active drug use was updated to self-report of injecting drugs ≥12 times in the past three months and at least six times in the past month and the participant had to be considered a PWID according to study staff. Eligibility criteria for index participants included: active injection drug use; viral load ≥1,000 copies/mL and CD4 cell count >50 cells/mm3 at the screening visit; and the ability to identify and enroll at least one HIV-uninfected drug injection partner. Index participants in the intervention arm received counselling and systems navigation with facilitated referral for ART and substance use treatment [19]; participants in the control arm received standard-of-care services for HIV and substance use treatment. This report includes analysis of samples and data collected from index participants at HPTN 074 enrollment (baseline data); a subset of participants had additional testing performed using samples collected at the screening visit.
Participants were tested for HIV infection at the screening visit; this occurred a median of 26 days prior to the enrollment visit (range 1-69 days) for the HIV-infected index participants included in this report. At the enrollment visit, index participants had face-to-face interviews with study staff. Participants were asked, “Prior to participating in this study, did you ever have an HIV test?” If they answered “No”, no further questions were asked about HIV status. If they answered “Yes”, they were also asked, “How many times have you had an HIV test in your lifetime?”, “When was your most recent HIV test?”, and “What was the result of that test?” Participants were also asked about prior and current ART. Current ART did not exclude participants from enrolling in the study. The study aimed to enroll at least 50% of the index participants who were ART-naïve at study entry (based on self-report); this was not disclosed to participants during recruitment.
Laboratory testing
HIV testing, HIV viral load testing, and CD4 cell count testing were performed at study sites using locally-available tests. With the exception of CD4 cell count data, all laboratory data analyzed in this report were from testing performed retrospectively at the HPTN Laboratory Center (Baltimore, MD). HIV viral load testing was performed with the RealTime HIV-1 Viral Load Assay (Abbott Molecular, Abbott Park, IL; lower limit of quantification of 40 copies/mL). ARV drug testing was performed using samples collected at the enrollment visit for all index participants; ARV drug testing was also performed using samples collected at the screening visit for a subset of index participants. This testing was performed using a qualitative assay based on high-performance liquid chromatography coupled with high-resolution, accurate-mass mass spectrometry [20]. The assay detects 20 ARV drugs (three non-nucleoside reverse transcriptase inhibitors [NNRTIs], six nucleoside/nucleotide reverse transcriptase inhibitors [NRTIs], nine protease inhibitors [Pls], and raltegravir and maraviroc). The limit of detection for the assay is 2 or 20 ng/mL depending on the drug [15, 20, 21]. Based on the lower limits of detection of the assay and the clearance kinetics of ARV drugs in the assay, NNRTIs should be detected for approximately one week after the last dose and NRTIs should be detected for 2-3 days after the last dose. Testing was repeated if unusual combinations of ARV drugs were detected. ART regimens recommended in Indonesia, Ukraine, and Vietnam at the time of the study included an NNRTI or PI with two NRTIs. In this study, participants were considered to be on ART if an NNRTI or PI was detected, with or without NRTIs.
Statistical analysis
The chi-square test was used to compare the frequency of ARV drug detection among participants who did or did not report that they were on ART. Chi-square and Fisher’s exact tests were used to evaluate associations between categorical variables in those groups; two-sample t-tests were used to assess associations with continuous variables. The mid-p McNemar’s test was used to compare the proportion of participants who were classified as newly diagnosed by self-report only and the proportion of participants who were classified as newly diagnosed by self-report combined with ARV drug testing.
Ethical considerations
Written informed consent was obtained from all participants enrolled in HPTN 074. The study was approved by institutional review boards and ethics committees at each participating institution.
RESULTS
Analysis of ARV drug use
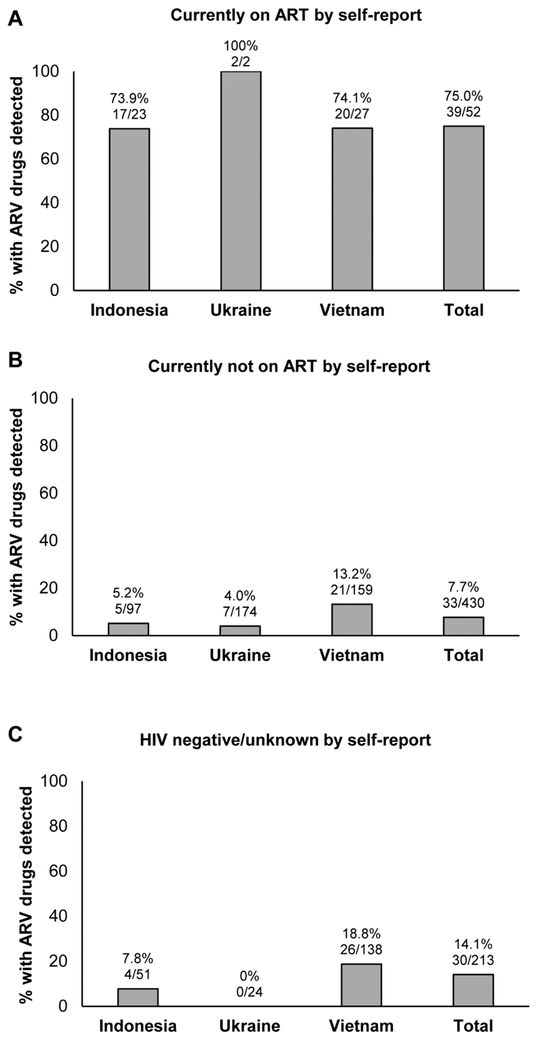
HPTN 074 enrolled 502 HIV-infected PWID. ARV drug testing was performed using enrollment samples from 482 (96.0%) of the 502 participants (20 did not have a sample available for testing); the 482 participants included 52 who reported that they were on ART at enrollment and 430 who reported that they were not on ART at enrollment. ARV drugs were detected in 72 (14.9%) of the 482 samples (18.3% in Indonesia; 5.1% in Ukraine; 22.0% in Vietnam). This included 39 (75.0%) of the participants who reported that they were on ART (Figure 1 A) and 33 (7.7%) of the participants who reported that they were not on ART (Figure 1B, p<0.0001). Overall, almost one half of those with ARV drugs detected (33/72 [45.8%]) did not report that they were on ART.
Figure 1. Antiretroviral drugs detected in samples from HIV-infected participants.
The figure shows the number and percentage of participants who had antiretroviral (ARV) drugs detected in samples collected at study enrollment. Results are shown for each study site and for the three sites combined (Total). (A) Results obtained for participants who reported that they were on antiretroviral treatment (ART). (B) Results obtained for participants who did not report that they were on ART. (C) Results obtained for participants who did not report having a positive HIV test prior to study entry.
The patterns of drugs detected were consistent with ART and were similar among those who did vs. did not report being on treatment. Among those who reported being on ART, an NNRTI or boosted PI with one or two NRTIs was detected in 34 (87.2%) of the 39 cases; the other cases had an NNRTI alone (n=4) or the unusual combination of two NNRTIs with one NRTI (n=1). Among those who did not report being on ART, an NNRTI or boosted PI with one or two NRTIs was detected in 23 (69.7%) of the 33 cases; the other cases had an NNRTI alone (n=8); a boosted-PI alone (n=1), or the unusual combination of two NNRTIs with one NRTI (n=1). The mean viral load was also similar among those who had ARV drugs detected who did vs. did not report being on treatment (Table 1).
Table 1.
Factors associated with reporting being on antiretroviral treatment among the participants with antiretroviral drugs detected.
| Variables | Reported being on ART |
|||
|---|---|---|---|---|
| Total (N=72) |
Yes (N=39) |
No (N=33) |
P valuea | |
| Mean (SD) HIV RNA, log10 copies/mL | 72 | 3.40 (1.04) | 3.28 (1.16) | 0.63 |
| Mean (SD) CD4 cell count, cells/mm3 | 72 | 2.50 (2.32) | 3.02 (1.80) | 0.30 |
| Study site | 0.012 | |||
| Indonesia | 22 | 17 (77.3%) | 5 (22.7%) | |
| Ukraine | 9 | 2 (22.2%) | 7 (77.8%) | |
| Vietnam | 41 | 20 (48.8%) | 21 (51.2%) | |
| Sex | 1.00 | |||
| Male | 68 | 37 (54.4%) | 31 (45.6%) | |
| Female | 4 | 2 (50.0%) | 2 (50.0%) | |
| Age | 0.05 | |||
| 18-29 | 7 | 5 (71.4%) | 2 (28.6%) | |
| 30-39 | 51 | 23 (45.1%) | 28 (54.9%) | |
| 40-60 | 14 | 11 (78.6%) | 3 (21.4%) | |
| Education | 0.003 | |||
| No education or primary school | 9 | 7 (77.8%) | 2 (22.2%) | |
| Some or completed secondary school | 39 | 14 (35.9%) | 25 (64.1%) | |
| Higher educationb | 24 | 18 (75.0%) | 6 (25.0%) | |
| Marital status | 0.98 | |||
| Married/Have partner but not married | 37 | 20 (54.1%) | 17 (45.9%) | |
| Single/Divorced/Separated/Widowed | 35 | 19 (54.3%) | 16 (45.7%) | |
| Employment | 0.84 | |||
| Employed full-time | 20 | 10 (50.0%) | 10 (50.0%) | |
| Employed part-time | 21 | 11 (52.4%) | 10 (47.6%) | |
| Unemployed/retired | 31 | 18 (58.1%) | 13 (41.9%) | |
| Hazardous alcohol usec | 0.61 | |||
| Yes | 15 | 9 (60.0%) | 6 (40.0%) | |
| No | 57 | 30 (52.6%) | 27 (47.4%) | |
| Injected amphetamines | 0.09 | |||
| Yes | 3 | 0 (0%) | 3 (100%) | |
| No | 69 | 39 (56.5%) | 30 (43.5%) | |
| Non-injection drug use: Opiates | 1.00 | |||
| Yes | 8 | 4 (50.0%) | 4 (50.0%) | |
| No | 64 | 35 (54.7%) | 29 (45.3%) | |
| Non-injection drug use: Stimulants (cocaine, methamphetamines) | 0.15 | |||
| Yes | 26 | 17 (65.4%) | 9 (34.6%) | |
| No | 46 | 22 (47.8%) | 24 (52.2%) | |
| Ever participated in medication assisted treatment for substance use | 1.00 | |||
| Yes | 62 | 34 (54.8%) | 28 (45.2%) | |
| No | 10 | 5 (50.0%) | 5 (50.0%) | |
| Number of sexual partnersd | 0.68 | |||
| 0 | 37 | 22 (59.5%) | 15 (40.5%) | |
| 1 | 29 | 14 (48.3%) | 15 (51.7%) | |
| ≥2 | 6 | 3 (50.0%) | 3 (50.0%) | |
| Number of injection partnerse | 0.09 | |||
| 1 | 4 | 0 (0%) | 4 (100%) | |
| 2-4 | 55 | 31 (56.4%) | 24 (43.6%) | |
| ≥5 | 13 | 8 (61.5%) | 5 (38.5%) | |
| Jail/Prisond | ||||
| Yes | 4 | 3 (75.0%) | 1 (25.0%) | 0.62 |
| No | 68 | 36 (52.9%) | 32 (47.1%) | |
Chi-square and Fisher’s exact tests were used to evaluate associations between categorical variables; two-sample t-tests were used to assess associations with continuous variables. P-values <0.05 are considered significant (bolded).
Higher education indicates some or completed technical training or college/university.
Hazardous alcohol use was determined as an AUDIT-C score of ≥4 among males and ≥3 among females.
Assessed for the period one month prior to study enrollment.
Assessed for the period three months prior to study enrollment.
Abbreviations: ARV: antiretroviral; N: number; SD: standard deviation.
We compared demographic and other characteristics in two groups of participants who had ARV drugs detected: the 39 participants who reported that they were on ART and the 33 participants who reported that they were not on ART (Table 1). The proportion of participants who reported that they were on ART differed by study site and educational level.
Accuracy of self-reported data on HIV testing history
We next used the results from ARV drug testing to analyze the accuracy of self-reported data on prior HIV testing. Among the 482 participants included in this report, 269 (55.8%) indicated that they were aware of their HIV-positive status prior to study entry (i.e., they reported that their last HIV test prior to study entry was positive). Self-reported data from the remaining 213 participants indicated that they were not aware of their HIV-positive status prior to study entry (36 reported that their last HIV test was negative, 26 reported that they did not know the result of their last HIV test, and 151 reported that they never had an HIV test prior to participating in the study).
We compared ARV test results to self-reported data to identify participants who were on ART (and therefore aware that they were HIV infected), but did not report having a prior HIV diagnosis to study staff. Among the 213 participants who did not report having a positive HIV test prior to study entry, 30 (14.1%) had ARV drugs detected at the enrollment visit (Figure 1C). To assess whether these participants were aware of their HIV status prior to study participation (before the screening visit), we tested the screening samples from these 30 participants for the presence of ARV drugs; 15 of the 30 participants had ARV drugs detected at the screening visit. This indicates that at least half of the 30 participants were aware of their HIV-positive status at screening, but did not report this to study staff.
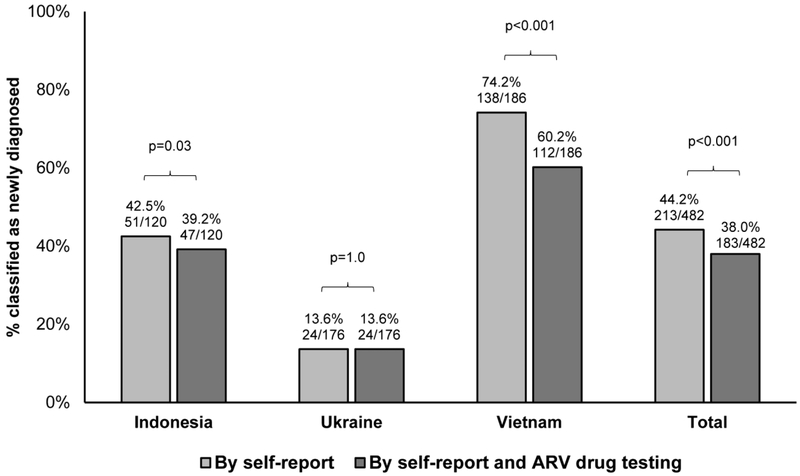
In research studies, participants who test positive for HIV infection at study entry and do not report a prior diagnosis are usually classified as newly diagnosed; these people would not be expected to be on ART at study entry. In this study, the proportion of persons classified as newly diagnosed by self-report only was higher than the proportion of persons classified as newly diagnosed based on self-report plus ARV drug testing (213/482 [44.2%] vs. 183/482 [38.0%], p<0.001, Figure 2). A significant difference between the proportion of individuals classified as newly diagnosed based on self-report alone vs. self-report plus ARV drug testing was observed in Indonesia and Vietnam, but not in Ukraine (Figure 2).
Figure 2. Proportion of participants classified as newly diagnosed by self-report only vs. self-report plus antiretroviral drug testing.
Study participants who test positive for HIV infection can be classified as newly diagnosed based on self-report only (e.g., if they do not report having a prior positive HIV test) or based on both self-report and antiretroviral (ARV) drug testing (e.g., if they do not report having a prior positive HIV test and also do not have ARV drugs detected in study samples). The figure compares the portion of HPTN 074 participants classified as newly diagnosed using these two approaches using the mid-p McNemar’s test; p<0.05 is considered significant. Results are shown for each study site and for the three sites combined (Total).
DISCUSSION
This study assessed the accuracy of self-reported HIV status in a clinical trial of PWID in Eastern Europe and Asia. The study has several key findings. First, almost half of the participants with ARV drugs detected did not report that they were on ART. Second, 25% of the participants who reported that they were on ART had no ARV drugs detected. Most of the participants who had drugs detected were on NNRTI-based ART. Because NNRTIs have relatively long half-lives, participants who did not have ARV drugs detected were most likely not on ART, or were on ART but had not taken their medication for a week or more. Third, 8% of participants who reported that they were not on ART had ARV drugs detected. Fourth, 14% of the participants who did not report having a positive HIV test prior to study entry were taking ARV drugs at the time of study enrollment, indicating that they were likely aware of their HIV-positive status.
ARV drugs are used for indications other than treatment of HIV infection (e.g., pre-exposure prophylaxis [PrEP] or treatment of hepatitis); in addition, some people use ARV drugs off-label for recreational purposes [22, 23]. In this study, most of the participants with ARV drugs detected had an NNRTI or boosted PI detected with two NRTIs, which indicates that they were on ART. Some participants had an NNRTI or boosted PI detected alone or with one NRTI; this pattern of ARV drugs is also consistent with ART, since NRTIs have shorter half-lives than NNRTIs and Pis and may not be detected, especially if adherence to an ART regimen is suboptimal. In HPTN 074, all index participants were required to have a viral load ≥1,000 copies/mL at the screening visit and few were virally suppressed at the enrollment visit. Most likely, these people with detectable ARV drugs were not adherent to their ART regimen, or were failing ART due to HIV drug resistance. None of the participants appeared to be taking ARV drugs for PrEP or hepatitis treatment, which might be seen in persons who were HIV infected, but not aware of their status.
In HPTN 074, the proportion of PWID with ARV drugs detected (14.9%) was lower than the prevalence of ART reported in other studies of HIV-infected PWID in these regions [24, 25]. This may reflect the design of the HPTN 074 study, which only enrolled those with viral loads ≥1,000 copies/mL at screening and included efforts to recruit HIV-infected PWID who were not on ART. For these reasons, the proportion of PWID with ARV drugs detected in the HPTN 074 cohort should be lower than the prevalence of ART among the general population of HIV-infected PWID at the HPTN 074 study sites. Previous studies have used ARV drug testing to determine if participants with low or undetectable HIV viral loads were on ART; those studies reported higher frequencies of undisclosed ART than what we observed in the HPTN 074 cohort where participants were not virally suppressed [10, 12, 13].
In HPTN 074, we were not able to determine why some participants did not disclose their prior HIV testing history or that they were on ART. Participants may have been concerned about social harms if their injection partners or others became aware that they knew they were HIV infected before joining the study. It is also possible that some participants misunderstood questions during the interview. In HPTN 074, most of the participants who had ARV drugs detected and did not report having a prior positive HIV test or that they were on ART were from Vietnam. Participants from Vietnam had less education than the participants from the other study sites. In the HPTN 074 cohort, only 28% of those from Vietnam had completed secondary school or higher education compared to 82% in Indonesia and 92% in Ukraine [18]. Educational level may have influenced participants’ ability to understand questions asked about HIV testing history and ART. A previous study among PWID in Thai Nguyen, Vietnam showed that HIV knowledge was higher among those with greater levels of education [26]. Among those with ARV drugs detected in this study, those who reported being on ART had a higher education than those who did not disclose ART.
CONCLUSIONS
Accurate information on ARV drug use and knowledge of HIV status is needed to optimize delivery of HIV prevention and treatment services, and for the design and interpretation of clinical trials and cohort studies. ARV drug testing provides an objective, direct measure of ARV drug use, and can be combined with data from self-report to obtain more accurate information on HIV status and ART. In this study, nearly half of the HIV-infected participants who had ARV drugs detected at enrollment did not report being on ART, and one quarter of the participants who did report being on ART did not have ARV drugs detected. ARV drugs were also detected in some participants who did not report having had a positive HIV test prior to joining the study. The accuracy of self-reported data on ARV drug use and knowledge of HIV status may vary in different populations and risk groups, and may also be impacted by factors such as study enrolment criteria. Therefore, it is important to interpret results from ARV drug testing in the context of the study population and other factors.
Acknowledgements
The authors thank the HPTN 074 study team and participants for providing the samples and data used in this study. We also thank the laboratory staff who helped with sample management and testing. This work was supported by the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse (NIDA), and Office of AIDS Research, of the National Institutes of Health (NIH) [UM1-AI068613 (Eshleman); UM1-AI068617 (Donnell); and UM1-AI068619 (Cohen/El-Sadr)].
Footnotes
Conflicts of Interest
None of the authors has a financial or personal relationship with other people or organizations that could inappropriately influence (bias) their work, with the following exceptions: Susan Eshleman has collaborated on research studies with investigators from Abbott; Abbott has provided reagents for collaborative research studies.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
REFERENCES
- 1.UNAIDS. The gap report 2014. Available at: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf. Accessed: 1 Feb 2018.
- 2.Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global health observatiory (GHO) data. Available at: http://www.who.int/gho/hiv/en/. Accessed: 1 Feb , 2018.
- 4.Habecker P, Abadie R, Welch-Lazoritz M, Reyes JC, Khan B, Dombrowski K. Injection partners, HCV, and HIV status among rural persons who inject drugs in Puerto Rico. Subst Use Misuse. 2017:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pares-Badell O, Espelt A, Folch C, Majo X, Gonzalez V, Casabona J, et al. Undiagnosed HIV and Hepatitis C infection in people who inject drugs: From new evidence to better practice. J Subst Abuse Treat. 2017;77:13–20. [DOI] [PubMed] [Google Scholar]
- 6.Zelaya CE, Le Minh N, Lau B, Latkin CA, Viet Ha T, Minh Quan V, et al. The effect of a multi-level intervention on the initiation of antiretroviral therapy (ART) among HIV-Infected men who inject drugs and were diagnosed late in Thai Nguyen, Vietnam. PLoS One. 2016;11(8):e0161718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogel JM, Wang L, Parsons TL, Ou SS, Piwowar-Manning E, Chen Y, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV Prevention Trials Network 052). J Infect Dis. 2013;208(10):1624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabowski MK, Reynolds SJ, Kagaayi J, Gray RH, Clarke W, Chang LW, et al. The validity of self-reported antiretroviral use in persons living with HIV: a population-based study. AIDS. 2018;32(3):363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim AA, Mukui I, Young PW, Mirjahangir J, Mwanyumba S, Wamicwe J, et al. Undisclosed HIV infection and antiretroviral therapy use in the Kenya AIDS indicator survey 2012: relevance to national targets for HIV diagnosis and treatment. AIDS. 2016;30(17):2685–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan AK, Savage EJ, Lowndes CM, Paul G, Murphy G, Carne S, et al. Non-disclosure of HIV status in UK sexual health clinics--a pilot study to identify non-disclosure within a national unlinked anonymous seroprevalence survey. Sex Transm Infect. 2013;89(2):120–1. [DOI] [PubMed] [Google Scholar]
- 11.Savage EJ, Lowndes CM, Sullivan AK, Back DJ, Else LJ, Murphy G, et al. Effect of nondisclosure of HIV status in sexual health clinics on unlinked anonymous HIV prevalence estimates in England, 2005-2009. AIDS. 2016;30(1):145–9. [DOI] [PubMed] [Google Scholar]
- 12.Marzinke MA, Clarke W, Wang L, Cummings V, Liu TY, Piwowar-Manning E, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis. 2014;58(1):117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel JM, Zhang Y, Guo X, Clarke W, Breaud A, Cummings V, et al. Accuracy of self-reported HIV status among African men and transgender women who have sex with men who were screened for participation in a research study: HPTN 075. AIDS Behav. 2018; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoots B, Wejnert C, Martin A, Haaland R, Masciotra S, Sionean C, et al. Undisclosed HIV infection among MSM in national HIV behavioral surveillance. 25th Conference on Retroviruses and Opportunistic Infections, Boston, MA, March 4-7, 2018. Abstract #910. [Google Scholar]
- 15.Fogel JM, Clarke W, Kulich M, Piwowar-Manning E, Breaud A, Olson MT, et al. Antiretroviral drug use in a cross-sectional population survey in Africa: NIMH Project Accept (HPTN 043). J Acquir Immune Defic Syndr. 2017;74(2):158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen I, Connor MB, Clarke W, Marzinke MA, Cummings V, Breaud A, et al. Antiretroviral drug use and HIV drug resistance among HIV-infected Black men who have sex with men: HIV Prevention Trials Network 061. J Acquir Immune Defic Syndr. 2015;69(4):446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Mancilla JR, Haberer JE. Adherence measurements in HIV: new advancements in pharmacologic methods and real-time monitoring. Curr HIV/AIDS Rep. 2018;15(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancaster KE, Hoffman IF, Hanscom B, Ha TV, Dumchev K, Susami H, et al. Regional differences between people who inject drugs in an HIV prevention trial integrating treatment and prevention: HPTN 074. J Int AIDS Soc. 2018; 21(10):e25195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller WC, Hoffman IF, Hanscom BS, Ha TV, Dumchev K, Djoerban Z, et al. A scalable, integrated intervention to engage people who inject drugs in HIV care and medication-assisted treatment: A randomized, controlled vanguard trial (HPTN 074). Lancet. 2018; 392: 747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzinke MA, Breaud A, Parsons TL, Cohen MS, Piwowar-Manning E, Eshleman SH, et al. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta. 2014;433:157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Clarke W, Marzinke MA, Piwowar-Manning E, Beauchamp G, Breaud A, et al. Evaluation of a multidrug assay for monitoring adherence to a regimen for HIV preexposure prophylaxis in a clinical study, HIV Prevention Trials Network 073. Antimicrob Agents Chemother. 2017;61(7):e02743–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis GP, Surratt HL, Levin FR, Blanco C. Antiretroviral medication: an emerging category of prescription drug misuse. Am J Addict. 2014;23(6):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grelotti DJ, Closson EF, Smit JA, Mabude Z, Matthews LT, Safren SA, et al. Whoonga: potential recreational use of HIV antiretroviral medication in South Africa. AIDS Behav. 2014;18(3):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larney S, Peacock A, Leung J, Colledge S, Hickman M, Vickerman P, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5(12):e1208–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iskandar S, de Jong CA, Hidayat T, Siregar IM, Achmad TH, van Crevel R, et al. Successful testing and treating of HIV/AIDS in Indonesia depends on the addiction treatment modality. J Multidiscip Healthc. 2012;5:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim TW, Davis WW, Quan VM, Frangakis C, Viet T, Minh NL, et al. Association between HIV knowledge and risk behavior in persons who inject drugs in Thai Nguyen, Vietnam. Southeast Asian J Trop Med Public Health. 2014;45(6):1425–36. [PMC free article] [PubMed] [Google Scholar]