Abstract
Background
Sodium-glucose cotransporter 2 (SGLT2) inhibitors promote glycosuria, resulting in possible effects on calcium, phosphate, and vitamin D homeostasis. Canagliflozin is associated with decreased bone mineral density and a potential increased risk of fracture.
Objective
To estimate the risk of a non-vertebral fracture among patients who newly started canagliflozin compared to a glucagon-like peptide-1 (GLP1)-agonist.
Design
Population-based new-user cohort study.
Setting
Two nationwide US commercial healthcare databases providing coverage to over 70 million patients from March 2013 to October 2015.
Patients
Individuals who had diabetes mellitus type 2 initiating canagliflozin were 1:1 propensity score-matched to individuals initiating a GLP1-agonist.
Measurements
The primary outcome was the composite endpoint of humerus, forearm, pelvis, or hip fracture requiring intervention. A secondary outcome included other sites of fracture. A fixed-effects meta-analysis pooling results from the two databases provided an overall hazard ratio (HR).
Results
We identified 79,964 patients initiating canagliflozin who were 1:1 propensity score matched to 79,964 patients initiating a GLP1-agonist. Mean age was 55 years, 47% were female, average baseline hemoglobin A1C was 8.7%, and 27% were prescribed insulin. The rate of the primary outcome was similar for canagliflozin (2.2 events per 1,000 person-years) compared to GLP1-agonists (2.3 events per 1,000 person-years), with an overall Hr of 0.98 (95%CI 0.75–1.26). The risk of pelvic, hip, humerus, radius, ulna, carpal, metacarpal, metatarsal, or ankle fracture was also similar for canagliflozin (14.5 per 1000 person-years) compared to GLP1-agonists (16.1 per 1000 person-years; overall HR = 0.92, 95% Ci: 0.83,1.02).
Limitations
Unmeasured confounding, measurement error, and low fracture rate.
Conclusion
In this study of middle-aged patients with type 2 diabetes with relatively low fracture risk, canagliflozin use was not associated with an increased risk of fracture compared to GLP1-agonists.
Primary Funding Source
Division of Pharmacoepidemiology and Pharmacoeconomics.
Introduction
Sodium-glucose co-transporter 2 (SGLT2) inhibitors decrease plasma glucose by inhibiting reabsorption of glucose at the proximal tubule and lowering the renal threshold for glucose excretion (1). The resultant osmotic diuresis may also affect calcium, phosphate, and vitamin D homeostasis (2). Canagliflozin, but not other SGLT2 inhibitors, is associated with decreased subsequent bone mineral density (3,4). Since patients with diabetes mellitus are at an increased risk of skeletal fractures, canagliflozin may further exacerbate this risk (5,6).
A recent systematic review of clinical trial data suggested canagliflozin was not associated with increased risk of fractures (7). However, most included trials were small or enrolled patients with relatively mild diabetes. Amongst the trials included in the systematic review, the mean age of patients was about 50 years, most were not prescribed insulin, and diabetes-related complications were uncommon (7).
The CANVAS (CANagliflozin cardioVascular Assessment Study) Program integrated data from two cardiovascular outcome trials: a cardiovascular safety study (CANVAS) and a cardiovascular safety study that also assessed renal outcomes (CANVAS-R) (8,9). Across the two trials that enrolled 10,142 patients, the mean age was 63 years, nearly two-thirds had cardiovascular disease, half were prescribed insulin, and most had diabetes for approximately 13 years. CANVAS identified an increased risk of fracture for patients randomized to canagliflozin compared to placebo (hazard ratio [HR] = 1.56, 95% confidence interval [CI]: 1.18,2.06). Fractures occurred as early as within 12 weeks of treatment and mostly affected the upper (e.g., humerus, radius) or lower (e.g., hip) limbs (9). In CANVAS-R, patients who received canagliflozin did not experience an increased risk of fracture compared to placebo (HR = 0.76, 95% CI: 0.52,1.12).
These conflicting results raise challenges for how patients prescribed canagliflozin should be counselled about the risk of fracture. The primary objective of this study was to assess the risk of fracture (radius, ulna, humerus, hip or pelvis) for patients with diabetes newly prescribed canagliflozin in routine care.
Methods
Data Sources
We conducted a population-based, new-user, longitudinal-cohort study using two commercial insurance claims databases in the United States (US): de-identified Optum© Clinformatics® Data Mart and Truven Health MarketScan (IBM Corp). Both databases capture patient demographics and longitudinal, individual-level data on healthcare utilization, inpatient and outpatient diagnostic tests and procedures, and pharmacy dispensing of drugs for over 70 million people in the US.
The Brigham and Women’s Hospital Institutional Review Board provided ethics approval and a valid data use agreement for Truven MarketScan (“Truven”) and Optum© Clinformatics® Data Mart (“Optum”) databases was in place.
Study Cohort
We compared adults (age > 18 years) with diabetes mellitus type 2 who were newly prescribed canagliflozin or a GLP1 agonist between March 29, 2013 (date of approval of canagliflozin in the US) and October 1st, 2015 (last available data). Patients with diabetes mellitus type 2 were identified using the International Classification of Diseases, Ninth Revision (ICD-9) codes (Data Supplement). New users of canagliflozin or GLP1 agonists were defined as those without a prior prescription for either a SGLT2 inhibitor or a GLP1 agonist in the preceding 180 days (see Appendix 1 for list of eligibility criteria, and Appendix Figure 1). Since GLP1-agonists were approved before SGLT2s, some patients that we identified as new-users of GLP1-agonists may have received these medications long before the cohort entry date (e.g., 2013), discontinued them, and subsequently re-started GLP1-agonists during our study period after a washout period of 180 days without filled prescriptions for either an SGLT2 inhibitor or an GLP1-agonist. Cohort entry date was the date of first prescription during our study period. GLP1-agonists were chosen as the comparator medication because they are a second-line treatment for diabetes, similar to SGLT2 inhibitors, and not associated with an increased risk of fracture (10,11). We did not include other SGLT2 inhibitors since limited data were available for them. We initially considered including dipeptidyl peptidase 4 (DPP4) inhibitors as another comparator, but decided not to since DPP4 inhibitors were used in patients on average 10 years-older than SGLT2 inhibitor users (data not shown), and age is one of the strongest risk factors for fracture.
Patients receiving both canagliflozin and a GLP1-agonist on the cohort entry date were excluded. Patients with any of the following characteristics in the 180 days prior to cohort entry were also excluded: insufficient baseline healthcare data (i.e., less than180 days), HIV, end-stage renal disease (including prior renal transplant), hospitalization for hypoglycemia, cancer, previous fracture (i.e., hip, pelvis, radius, ulna, humerus), and type 1 diabetes.
Cohort Follow-up
Follow-up began on the day after cohort entry and continued until the end of the study period (October 1,2015), end of continuous health plan enrollment, occurrence of a study outcome, discontinuation of the initial medication (or switching to or adding one of the comparator medications), or death. A medication was considered discontinued if 60 days elapsed after the expiration of the last prescription’s supply without the prescription being refilled.
Study Outcomes
The primary outcome was a composite endpoint of pelvic fracture, hip fracture requiring surgery, humerus fracture requiring intervention (surgery, casting, splinting), or radius/ulna fracture requiring intervention (Data Supplement). These fracture definitions based on claims data have been previously validated against hospital records with a positive predictive value (PPV) above 92% (12–14). A post-hoc analysis was performed that excluded pathologic fractures or fractures related to high-impact trauma (Appendix 1). Secondary outcomes included the primary outcome without intervention (e.g., hip fracture without surgery), serious fall injury (15), severe hypoglycemia (PPV = 89%) (16), and including other peripheral sites of fractures reported in the CANVAS trial (carpal, metacarpal, metatarsal, ankle) (9, 17). Serious fall injury and severe hypoglycemia were included to help understand and identify potential triggering events that might lead to fracture.
Covariates
Patient demographics were assessed during the 180 days before cohort entry. Data were collected for each patient based on diagnoses and procedures recorded during health encounters, including chronic medical conditions (e.g., hypertension, coronary artery disease, stroke), diabetes severity (e.g., end-organ damage, insulin use), fracture risk (e.g., osteoporosis, recent steroid use), healthcare utilization (e.g., recent hospitalization, emergency room visit), prescriber information (e.g., endocrinologist, general practitioner), diabetes medications (e.g., metformin), non-diabetes medications (e.g., bisphosphonates, anti-hypertensives), and proxies for frailty (e.g., use of a walker). The frailty indicators have been previously validated and are associated with subsequent mortality, disability, mobility impairment, and recurrent falls (18, 19). All covariates were selected based on previous studies and clinical experience (5, 20, 21).
Statistical analysis
Propensity score (PS) matching methodology was used to adjust for confounding. The probability of initiating canagliflozin versus a GLP1-agonist (control medication) was calculated through a multivariable logistic regression model which contained all potential confounders at baseline except hemoglobin A1C since it was not available for all patients. A hemoglobin A1C value was available for approximately 35% of patients in Optum and 6% of patients in Truven and are summarized in Table 1 as the mean hemoglobin A1C. There were no other missing data in our study. The estimated PS was used to match 1:1 initiators of canagliflozin with initiators of GLP1-agonists in each database separately (i.e., Truven and Optum). Nearest neighbor matching without replacement was performed using a caliper of 0.05 on the PS scale. The propensity score was re-estimated for each subgroup analysis. Covariate balance within the matched cohorts was assessed using standardized differences. A standardized difference of 0.1 or less indicates negligible differences between groups (22).
Table 1.
Baseline patient characteristics after 1:1 PS-matching in Optum and Truven datasets
| Optum | Truven | |||
|---|---|---|---|---|
| GLP1 (N=23,458) | Canagliflozin (N=23,458) | GLP1 (N=56,506) | Canagliflozin (N=56,506) | |
| Male sex | 12,818 (54.6%) | 12,784 (54.5%) | 28,601 (50.6%) | 28,629 (50.7%) |
| Mean age (stdev) | 56.04 (11.47) | 56.31 (10.89) | 54.55 (10.00) | 54.53 (9.79) |
| < 55 years | 10,116 (43.1%) | 9,801 (41.8%) | 25,827 (45.7%) | 26,023 (46.1%) |
| 55 – 64 years | 7,260 (30.9%) | 8,175 (34.8%) | 23,201 (41.1%) | 23,604 (41.8%) |
| 65 – 74 years | 4,967 (21.2%) | 4,385 (18.7%) | 6,044 (10.7%) | 5,495 (9.7%) |
| ≥ 75 years | 1,115 (4.8%) | 1,097 (4.7%) | 1,434 (2.5%) | 1,384 (2.4%) |
| Diabetes-related | ||||
| Hemoglobin A1C, mean (SD) | 8.72 (1.92) | 8.76 (1.79) | 8.60 (1.90) | 8.74 (1.82) |
| Diabetic nephropathy | 1,674 (7.1%) | 1,654 (7.1%) | 2,417 (4.3%) | 2,374 (4.2%) |
| Diabetic neuropathy | 3,164 (13.5%) | 3,163 (13.5%) | 5,550 (9.8%) | 5,534 (9.8%) |
| Recent A1C test ordered | 19,264 (82.1%) | 19,215 (81.9%) | 45,455 (80.4%) | 45,508 (80.5%) |
| Endocrinology visit* | 3,140 (13.4%) | 3,102 (13.2%) | 8,636 (15.3%) | 8,704 (15.4%) |
| Comorbid conditions | ||||
| Hypertension | 17,239 (73.5%) | 17,202 (73.3%) | 36,674 (64.9%) | 36,583 (64.7%) |
| Ischemic heart disease | 2,556 (10.9%) | 2,542 (10.8%) | 5,144 (9.1%) | 5,103 (9.0%) |
| Heart failure | 339 (1.4%) | 326 (1.4%) | 1,167 (2.1%) | 1,170 (2.1%) |
| Stroke or TIA | 344 (1.5%) | 348 (1.5%) | 639 (1.1%) | 647 (1.1%) |
| Renal disease (non-diabetic) | 2,150 (9.2%) | 2,110 (9.0%) | 3,307 (5.9%) | 3,245 (5.7%) |
| Falls or Fracture-related | ||||
| Falls | 177 (0.8%) | 181 (0.8%) | 309 (0.5%) | 302 (0.5%) |
| Osteoporosis | 501 (2.1%) | 512 (2.2%) | 855 (1.5%) | 829 (1.5%) |
| Dementia | 161 (0.7%) | 166 (0.7%) | 259 (0.5%) | 246 (0.4%) |
| Glaucoma or cataract | 3,060 (13.0%) | 3,065 (13.1%) | 6,181 (10.9%) | 6,188 (11.0%) |
| Bone mineral density testing | 497 (2.1%) | 506 (2.2%) | 1,021 (1.8%) | 1,018 (1.8%) |
| Number of frailty indicators18 | ||||
| ...0 | 8,840 (37.7%) | 8,768 (37.4%) | 23,640 (41.8%) | 23,660 (41.9%) |
| ...1 to 2 | 10,655 (45.4%) | 10,662 (45.5%) | 25,338 (44.8%) | 25,397 (44.9%) |
| ...3 or more | 3,963 (16.9%) | 4,028 (17.2%) | 7,528 (13.3%) | 7,449 (13.2%) |
| Medications | ||||
| Oral steroids | 2,077 (8.9%) | 2,063 (8.8%) | 5,193 (9.2%) | 5,114 (9.1%) |
| Bisphosphonate | 214 (0.9%) | 227 (1.0%) | 367 (0.6%) | 349 (0.6%) |
| Calcium channel blocker | 3,515 (15.0%) | 3,482 (14.8%) | 6,890 (12.2%) | 6,855 (12.1%) |
| Statin | 13,883 (59.2%) | 13,910 (59.3%) | 31,960 (56.6%) | 31,881 (56.4%) |
| Beta blocker | 5,270 (22.5%) | 5,281 (22.5%) | 11,937 (21.1%) | 11,932 (21.1%) |
| Anticonvulsant | 2,960 (12.6%) | 3,073 (13.1%) | 6,667 (11.8%) | 6,586 (11.7%) |
| Antidepressant | 5,502 (23.5%) | 5,587 (23.8%) | 13,892 (24.6%) | 13,887 (24.6%) |
| Opioid | 5,583 (23.8%) | 5,639 (24.0%) | 13,456 (23.8%) | 13,294 (23.5%) |
| Benzos, z-drug, anxiolytic | 3,406 (14.5%) | 3,474 (14.8%) | 8,292 (14.7%) | 8,226 (14.6%) |
| Any diuretic | 4,138 (17.6%) | 4,173 (17.8%) | 9,656 (17.1%) | 9,629 (17.0%) |
| Number, mean (stdev)* | 3.94 (3.19) | 3.96 (3.18) | 2.58 (2.37) | 2.57 (2.34) |
| Diabetes-Medications | ||||
| Insulin | 6,625 (28.2%) | 6,622 (28.2%) | 15,321 (27.1%) | 15,348 (27.2%) |
| Metformin | 13,342 (56.9%) | 13,393 (57.1%) | 32,128 (56.9%) | 32,198 (57.0%) |
| Dipeptidyl peptidase-4 inhibitor | 4,386 (18.6%) | 4,412 (18.7%) | 9,545 (16.9%) | 9,485 (16.8%) |
| Sulfonylurea | 8,369 (35.7%) | 8,383 (35.7%) | 18,463 (32.7%) | 18,531 (32.8%) |
| Glitazone | 1,958 (8.3%) | 1,936 (8.3%) | 4,182 (7.4%) | 4,222 (7.5%) |
| Number, mean (stdev) | 1.25 (0.97) | 1.26 (0.95) | 1.16 (0.94) | 1.17 (0.92) |
| Healthcare Utilization | ||||
| ER visit | 2,133 (9.1%) | 2,120 (9.0%) | 6,521 (11.5%) | 6,460 (11.4%) |
| Preventive health measures | 9,157 (39.0%) | 9,144 (39.0%) | 20,945 (37.1%) | 20,984 (37.1%) |
| Outpatient visits, mean (stdev) | 7.23 (6.04) | 7.27 (5.81) | 4.42 (3.46) | 4.40 (3.51) |
Legend - PS = propensity score, differences in baseline characteristics were assessed using standardized differences within each database and all were < 0.1 (supplementary material) after PS matching, stdev = standard deviation, TIA = transient ischemic attack, z-drugs = zolpidem, zaleplon, ER = emergency room
assessed in the preceding 30 days. Hemoglobin A1C data was available for 35% of patients in Optum and 6% of patients in Truven.
After matching, Cox proportional hazards models were used to estimate the HRs and 95% CIs of primary and secondary outcomes without further adjustments. Individual hazard ratios were calculated separately in each database and then combined using a fixed-effects meta-analysis using inverse variance weighting (23). Kaplan-Meier plots with 95% CIs were generated to visualize the risk of outcome events in both groups for each database.
Multiple predefined sensitivity and subgroup analyses were performed including carrying forward the exposure to the first-used medication for 365 days without considering drug discontinuation, switching or augmentation similar to an intention-to-treat analysis, and restricting to patients over 60 years of age (to understand the impact of age on fracture risk). To assess the risk of fracture after longer duration of exposure, we assessed the risk of the primary outcome in a subgroup of patients with at least six months of follow-up with the index medication and started the follow-up at day 181. The statistical analysis was otherwise identical to that performed for the primary outcome. As a positive control, we assessed the risk of first episode of heart failure since previous randomized clinical trials have demonstrated a reduction in heart failure with canagliflozin, but not GLP1-agonists (8). Analyses were conducted using the Aetion platform which has been validated for a range of studies (24) and for predicting clinical trial findings (25,26), R version 3.4.2, and Stata IC version 14.2, StataCorp LP, College Station, Texas, United States of America (Appendix Table 1).
This study was funded by internal resources in the Division of Pharmacoepidemiology and Pharmacoeconomics at the Brigham and Women’s Hospital. The authors had complete control over the design, conduct, analysis and decision to submit the manuscript for publication.
Results
Study Population
Across Optum and Truven we identified 194,581 patients who satisfied study inclusion and exclusion criteria (Appendix Figure 2). Among those, 92,779 patients were newly prescribed canagliflozin and 101,802 patients were newly prescribed a GLP1-agonist (Appendix Table 2). Across both datasets, canagliflozin was prescribed slightly more often in the second half of the study period and patients were more likely to be male and were prescribed slightly more diabetes medications, but less likely to be prescribed a diuretic or an opioid medication. Patients prescribed GLP1-agonists were more likely to have kidney disease and be prescribed insulin, a DPP4 inhibitor, an anti-depressant or an anti-convulsant, or have a measure of frailty (Appendix Table 2). The remaining baseline characteristics including hemoglobin A1C, glaucoma, cataracts, previous fall, osteoporosis, and recent bone mineral density testing were well balanced (standardized differences <0.1) before PS matching.
After 1:1 PS matching, we obtained 23,458 pairs of patients newly prescribed canagliflozin or a GLP-1 agonist in Optum and 56,506 pairs of patients newly prescribed canagliflozin or a GLP-1 agonist in Truven. Overall, the mean age was approximately 55 years, 53% were male, prevalence of metformin or insulin use at baseline was 57% and 27%, respectively. The major risk factors for fracture were rare in both groups (i.e., osteoporosis, dementia, frailty indicators) (Table 1). All covariates were well balanced with standardized differences <0.1. Patients prescribed a GLP1-agonist had a baseline hemoglobin A1C of approximately 8.7% and patients prescribed an SGLT2 inhibitor had a baseline hemoglobin A1C of approximately 8.8%. The mean duration of follow-up was 34 weeks (standard deviation = 27, Appendix Table 3). In CANVAS, an increased risk of fracture was apparent within 12 weeks of initiating treatment with canagliflozin (8, 9).
Primary Outcome
In Truven, we observed 94 events (1.9 events per 1,000 person-years) of pelvic fracture, hip fracture requiring surgery, humerus fracture requiring intervention, or radius/ulna fracture requiring intervention among 66,237 unmatched canagliflozin initiators, compared to 106 events (2.4 events per 1,000 person-years) among 68,729 unmatched GLP1-agonist initiators. In the PS-matched cohort, there were 84 events (2.1 events per 1,000 person-years) among 56,506 PS-matched canagliflozin initiators, compared to 82 events (2.3 events per 1,000 person-years) among 56,506 PS-matched GLP1-agonist initiators. The PS-matched rate of the primary composite endpoint was similar for canagliflozin initiators compared to GLP1-agonist initiators (HR=0.92; 95% CI 0.68,1.25).
In Optum, we observed 40 events (2.7 events per 1,000 person-years) of pelvic fracture, hip fracture requiring surgery, humerus fracture requiring intervention, or radius/ulna fracture requiring intervention among 26,542 unmatched canagliflozin initiators, compared to 58 events (3.2 events per 1,000 person-years) among 33,073 unmatched GLP1-agonist initiators. There were 34 events (2.6 events per 1,000 person-years) among PS-matched 23,458 canagliflozin initiators compared to 30 events (2.3 events per 1,000 person-years) among 23,458 PS-matched initiators of GLPI-agonists. The PS-matched rate of the primary composite endpoint was similar for canagliflozin initiators compared to GLPI-agonist initiators (HR=1.13; 95% CI 0.69,1.84). (Table 2)
Table 2.
Risk of primary outcome with canagliflozin versus GLP1-agonists after propensity-score matching σ2
| Optum | Truven | Combined | ||||
|---|---|---|---|---|---|---|
| GLP1 | Canagliflozin | GLP1 | Canagliflozin | GLP1 | Canagliflozin | |
| No. Patients Mean days of follow-up (Stdev) | 23,458 203 (177) | 23,458 204 (175) | 56,506 233(188) | 56,506 264 (197) | 79,964 224 (185) | 79,964 247(191) |
| Risk of fracture* | ||||||
| No. Events (IR per 1000 person-years) | 30 (2.3) | 34 (2.6) | 82 (2.3) | 84 (2.1) | 112 (2.3) | 118 (2.2) |
| Hazard Ratio (95% CI) | Ref. | 1.13 (0.69,1.84) | Ref. | 0.92 (0.68,1.25) | Ref. | 0.98 (0.75,1.26) |
GLP1: GLP-1 receptor agonist; Stdev: standard deviation; IR: incidence rate; CI: confidence intervals; Ref.: reference; PS: propensity score
pelvic fracture, hip fracture requiring surgery, humerus fracture requiring surgery or casting/splinting, or radius/ulna fracture requiring surgery or casting/splinting
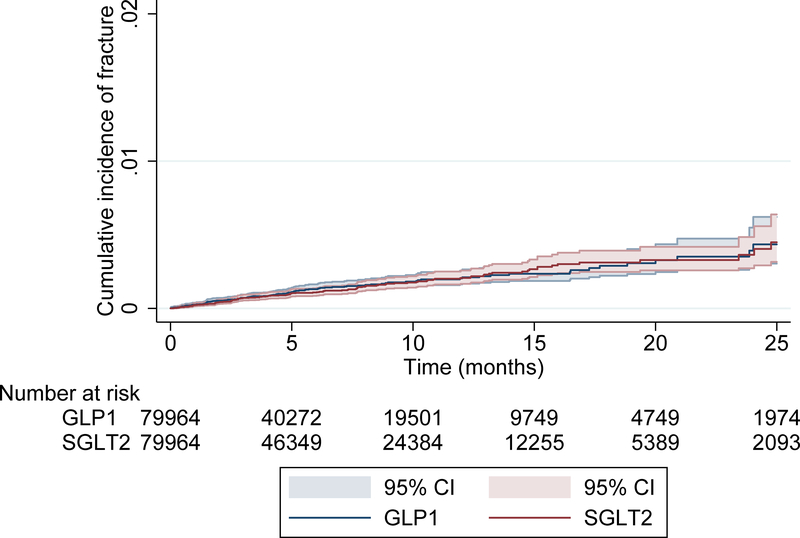
The combined PS-matched rate of the primary outcome across both data sources did not indicate an increased risk of fracture with canagliflozin (HR = 0.98; 95% CI 0.75,1.26). The cumulative incidence of fractures, pooled across both databases, between patients prescribed canagliflozin and GLP1-agonists is depicted in a Kaplan-Meier plot (Figure 1A) and is consistent with these findings. The Kaplan-Meier plot for each individual database showed similar patterns (Appendix Figure 3). In our post-hoc analysis that excluded pathologic fractures or fractures related to recent trauma, our primary outcome was essentially unchanged (HR=0.90, 95% CI 0.70,1.15).
Figure 1A.
Kaplan-Meier plot for the propensity-score matched primary outcome of pelvic fracture, hip fracture requiring intervention (surgery, casting, splinting), humerus fracture requiring intervention, or radius/ulna fracture requiring intervention.
Legend: GLP1 = Glucagon-like peptide-1. SGLT2 = sodium glucose co-transporter 2 and included canagliflozin alone.
Sensitivity analyses
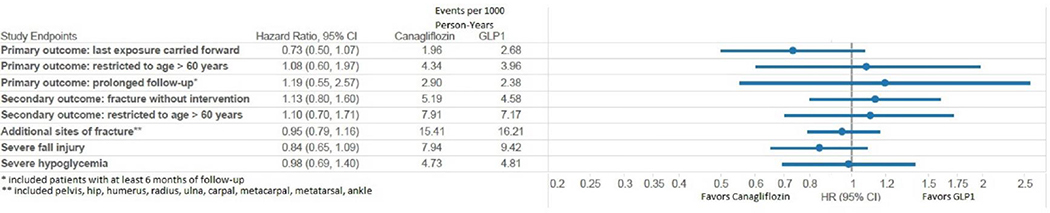
When we carried forward the exposure to the first-used medication for 365 days without considering treatment discontinuation in our intention-to-treat analysis, PS-matched results again showed no increased rate of the primary endpoint (Truven HR = 0.84, 95% CI: 0.59,1.19; Optum HR = 0.73, 95% CI 0.50,1.07; combined HR=0.79, 95% CI: 0.61,1.02) (Figure 2A and 2B). Results were also consistent when we restricted to patients over the age of 60 (Truven HR = 0.77, 95% CI 0.49,1.20; Optum HR = 1.08, 95% CI 0.60,1.97; combined HR=0.87, 95% CI: 0.61,1.24).
Figure 2A.
Forest-plot of sensitivity analyses and secondary analyses for propensity score matched outcomes in Optum.
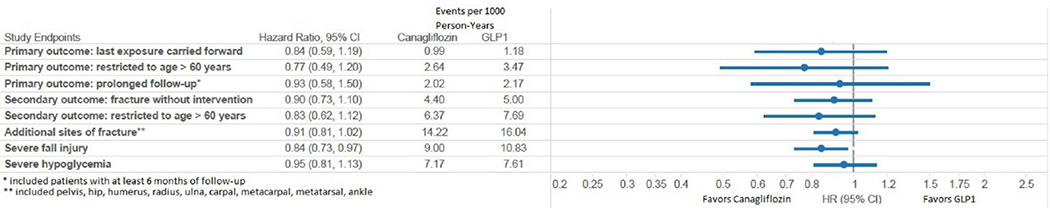
Figure 2B.
Forest-plot of sensitivity analyses and secondary analyses for propensity score matched outcomes in Truven.
Secondary Outcomes
The PS-matched risk for the expanded primary outcome without an associated intervention was similar for control patients and canagliflozin (Truven HR = 0.90, 95% CI: 0.73,1.10; Optum HR = 1.13, 95% CI: 0.80,1.60; combined HR = 0.95; 95% CI: 0.80,1.13). Similar results were observed when this cohort was restricted to patients over the age of 60 (Truven HR = 0.83, 95% CI 0.62,1.12; Optum HR = 1.10, 95% CI 0.70,1.71; combined HR: 0.91 95% CI: 0.71,1.16) (Figure 2).
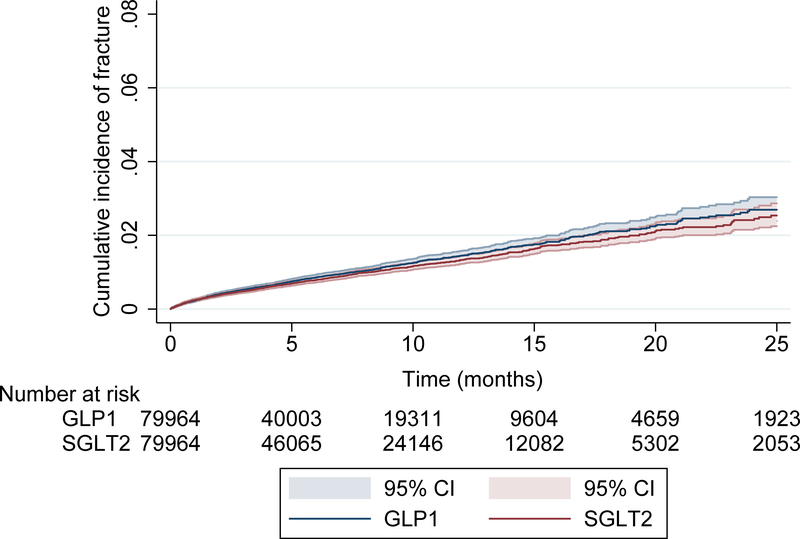
The PS-matched risk of pelvic, hip, humerus, radius, ulna, or other sites of fracture reported in CANVAS (carpal, metacarpal, metatarsal, ankle) was similar for canagliflozin (14.5 per 1000 person-years) compared to GLP1 agonists (16.1 per 1000 person-years) with an overall HR near unity (Truven HR = 0.91, 95% CI: 0.81, 1.02; Optum HR = 0.95, 95% CI 0.79, 1.16; combined HR = 0.92 95% CI: 0.83,1.02). The cumulative incidence of these fractures, pooled across both databases, between patients prescribed canagliflozin and GLP1-agonists is depicted in a Kaplan-Meier plot (Figure 2b) and is consistent with these findings (Appendix Figure 4).
The risk of fracture for patients with a longer duration of exposure as well as the risk of severe fall injury and severe hypoglycemia was not increased with canagliflozin compared to GLP1-agonists (Figure 2).
Validation against a known causal association
The PS-matched risk of first heart failure episode was lower for patients prescribed canagliflozin (Truven HR = 0.80, 95% CI 0.74,0.87; Optum HR = 0.85, 95% CI 0.74, 0.99; combined HR: 0.81, 95% CI: 0.76,0.87) as observed in the CANVAS trial.(8)
Discussion
Using data from two large US healthcare databases, we found that the overall rate of fracture was similarly low among patients with type 2 diabetes who were treated with canagliflozin or GLPI-agonists. Findings were robust across multiple sensitivity and subgroup analyses. Our study population is representative of a meaningful proportion of the commercially insured patients with diabetes in the US population.
Assessing the risk of fracture for patients receiving new diabetes medications is important, since patients with diabetes are prone to fractures (5, 20, 27). Fractures can cause significant morbidity and mortality, with hip fracture associated with a one-year mortality of up to 40% (28). These risks may be particularly relevant for patients prescribed canagliflozin due to effects on phosphate, calcium, vitamin D and parathyroid hormone homeostasis (3, 29). Specifically, the net effect of canagliflozin is that it can increase serum phosphate, reduce serum 1,25-dihydroxyvitamin D, and reduce bone mineral density (3,29). Despite biological mechanisms that might suggest an increased risk of fracture, our study did not detect an increased risk of fracture with canagliflozin as used in routine care.
Our findings support two meta-analyses published prior to the CANVAS program (7,30). In the meta-analysis by Tang et al., fracture with SGLT2s was rare (323 events in 20,264 patients who received an SGLT2) and the overall odds of fracture was 1.02 (95% CI: 0.84,1.23) based on the pooled results of 38 trials (30). These trials were evenly split across the three SGLT2 inhibitors and most (68%) enrolled patients with an average age below 60 years. The meta-analysis by Wu et al. also found that canagliflozin was not associated with an increased risk of fracture and based on regulatory submissions and scientific reports (7). Both meta-analyses were primarily based on clinical trials of relatively young patients, thereby raising questions about whether an increased risk might apply only to older patients. The results of the CANVAS Program may support this hypothesis.
The CANVAS program included 10,142 participants randomized to canagliflozin or placebo. The risk of low-trauma fracture (primary fracture outcome) and all fracture (secondary fracture outcome), were assessed for patients who received one or more dose of canagliflozin or placebo. In CANVAS, the risk of low-trauma fracture was higher for canagliflozin compared to placebo (13 events per 1000 patient-years vs 8 events per 1000 patient-years, respectively; HR 1.56, 95% CI: 1.18,2.06) as was all fractures (17 events per 1000 patient-years vs 11 events per 1000 patient-years, respectively; HR 1.55, 95% CI: 1.21,1.97). Conversely, in CANVAS-R (N=5812), which had nearly identical inclusion and exclusion criteria to CANVAS (N=4330), the risk of a fracture with canagliflozin was not higher than placebo for both low-trauma fracture (HR 0.76, 95% CI: 0.52–1.12) and all fracture (HR 0.86, 95% CI: 0.62, 1.19). It is unknown why the results for CANVAS and CANVAS-R were discordant.
One baseline characteristic that was different between CANVAS and CANVAS-R was the proportion of Asian patients enrolled. In CANVAS, 18% of patients were Asian while only 8% were Asian in CANVAS-R. The meta-analysis by Tang et al. suggested that people of Asian race who received an SGLT2 may have a 2-fold higher odds of fracture (odds ratio = 2.05, 95% CI: 0.86,4.87) compared to patients of Asian race who did not receive an SGLT2. Thus, the discordant risks observed between CANVAS and CANVAS-R might be effect modification by race. We could not test this hypothesis in our study since we did not have access to race information.
Our study had a maximum duration of follow-up of 142 weeks, and a mean duration of follow-up was 34 weeks. Therefore, the Kaplan-Meier curves after 1 year show increasing uncertainty. However, in the CANVAS trial an increased risk of fracture was detected within 12 weeks of treatment initiation, and thus duration of follow-up alone likely does not explain our null findings. An alternate explanation for our findings might be related to a lower baseline risk of fracture for patients in our study compared to participants in the CANVAS Program. The mean age of our patients was relatively young, we excluded individuals with a previous fracture, and most did not have osteoporosis; therefore, our study may not generalize to older patients or those at higher baseline risk of fracture.
When observing null-findings in database analyses, a concern is that biases arising from misclassification and missing data have shrunk the effect size to produce a false null finding. Such an inability to detect known associations (lack of assay sensitivity) can be refuted by demonstrating that the data and methodology reproduced known beneficial or adverse effects (31,32). The fact that our study design was sensitive enough to detect the known causal association between canagliflozin and a 19% decreased risk of heart failure is therefore reassuring that we could have detected a difference in fracture risk if it were present (26, 33). However, we cannot exclude the possibility of an up to 26% increased rate of fracture as defined by our primary analysis considering the upper limit of the 95% CIs (HR=0.98, 95% CI 0.75,1.26). Another limitation is that we did not have access to some relevant data about diabetes severity (e.g., duration of diabetes), risk factors for fracture (e.g., BMI, smoking), and other unmeasured confounders (e.g., race, physical fitness). However, PS matching on the other diabetes-related severity and comorbidity variables has been shown to also balance unmeasured characteristics including duration of diabetes, BMI, and smoking (34). Additionally, the primary outcome in CANVAS was low-trauma fractures as determined by the trial adjudication committee. Our study lacked these granular details and instead we used validated algorithms to identify fractures using claims data, performed a secondary analysis that included other sites of fracture included in the CANVAS trial, and a post-hoc analysis that excluded patients with a pathologic fracture or fractures related to high-impact trauma. Finally, there may be some degree of overlap of patients in Optum and Truven. This means that the confidence intervals of our combined fixed-effect analysis may be slightly narrower than the confidence intervals calculated using a formula that accounts for overlapping patients. A recent pharmacoepidemiologic study of tocilizumab that performed an analysis considering potential overlap between Optum and Truven found that the confidence intervals were slightly wider (i.e., at the level of the second decimal point) than the analysis that did not consider potential overlap of patients between Optum and Truven.25
In this study of nearly 160,000 propensity-score matched patients with diabetes in the US, canagliflozin was not associated with an increased risk of fracture in routine care. Our results are most relevant to patients with diabetes who do not have other risk factors for fracture (e.g., older age, previous fracture). These results should be reassuring to both patients and physicians when considering the potential risks and benefits of canagliflozin. The findings from this study also raise the question of whether the observed increase in fracture risk reported in CANVAS is limited to patients at high baseline risk.
Supplementary Material
Figure 1B.
Kaplan-Meier plot for the propensity-score matched outcome of pelvic, hip, humerus, radius, ulna, or other sites of fracture reported in CANVAS (carpal, metacarpal, metatarsal, ankle).
Legend: CANVAS = CANagliflozin cardioVascular Assessment Study (CANVAS), GLP1 = Glucagon-like peptide-1, SGLT2 = sodium glucose co-transporter 2 and included canagliflozin alone.
Acknowledgments
Funding source: This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Dr. Fralick receives funding from the Eliot Phillipson Clinician-Scientist Training Program at the University of Toronto, the Clinician Investigator Program at the University of Toronto, the Canadian Institutes of Health Research Drug Safety and Effectiveness Cross-Disciplinary Training (DSECT) Program, and from The Detweiler Traveling Fellowship funded by the Royal College of Physicians and Surgeons of Canada.
Dr. Patorno is supported by a career development grant K08AG055670 from the National Institute on Aging. She is investigator on grants to the Brigham and Women’s Hospital from Boehringer Ingelheim and GlaxoSmithKline, not directly related to the topic of this manuscript.
Dr. Schneeweiss is consultant to WHISCON, LLC and to Aetion, Inc., a software manufacturer of which he also owns equity. He is principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Genentech, Bayer and Boehringer Ingelheim not directly related to the topic of this manuscript.
Dr. S Kim has received research grants to the Brigham and Women’s Hospital from Pfizer, Bristol-Myers Squibb, AstraZeneca, Roche, and Merck for unrelated topics.
Dr. D Kim is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropic Society.
Dr. Donald Redelmeier has received funding from a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, and the BrightFocus Foundation
Footnotes
Transparency: MF and EP affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Protocol: posted as data supplement at Annals website.
Statistical code: Interested readers can contact Dr. Mike Fralick: mif823@mail.harvard.edu.
Data: available from data vendors under data use agreement.
References
- 1.Tahrani A, Barnett A, Bailey C. Sglt inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140–151. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Canagliflozin drug label (2017). Accessed at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=204042 on 6 Sep 2017.
- 3.Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljunggren O, Bolinder J, Johansson L, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes, Obes Metab. 2012;14:990–999. [DOI] [PubMed] [Google Scholar]
- 5.Leslie W, Rubin M, Schwartz A, Kanis J. Type 2 diabetes and bone. J Bone Min Res. 2012;27:2231–2237. [DOI] [PubMed] [Google Scholar]
- 6.Lipscombe L, Jamal S, Booth G, Hawker G. The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care. 2007;30:835–841. [DOI] [PubMed] [Google Scholar]
- 7.Wu JHY, Foote C, Blomster J, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. [DOI] [PubMed] [Google Scholar]
- 8.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 9.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo G, Liu H, Lu H. Glucagon-like peptide-1(GLP-1) receptor agonists: potential to reduce fracture risk in diabetic patients? Br J Clin Pharmacol. 2016;81:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driessen JHM, van Onzenoort HAW, Starup-Linde J, et al. Use of glucagon-like-peptide 1 receptor agonists and risk of fracture as compared to use of other anti-hyperglycemic drugs. Calcif Tissue Int. 2015;97:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized medicare files. J Clin Epidemiol. 1992;45:703–714. [DOI] [PubMed] [Google Scholar]
- 13.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high - a systematic review. J Clin Epidemiol. 2013;66:278–285. [DOI] [PubMed] [Google Scholar]
- 14.Schneider AL, Williams EK, Brancati, et al. Diabetes and risk of fracture-related hospitalization the atherosclerosis risk in communities study. Diabetes Care. 2013; 36(5): 1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinetti M, Ling H, Lee D, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginde AA, Blanc PG, Lieberman RM, Camargo CA. Validation of IC9–9-cm coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thur CK, Edgren G, Jansson K-åke, Wretenberg P. Epidemiology of adult ankle fractures in sweden between 1987 and 2004. Acta Orthop. 2012;83:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in medicare data: development and validation of a claims-based frailty index. Journals Gerontol Ser A. 2017;0:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathmann W, Kostev K. Fracture risk in patients with newly diagnosed type 2 diabetes: a retrospective database analysis in primary care. J Diabetes Complications. 2015;29:766–770. [DOI] [PubMed] [Google Scholar]
- 21.Lee RH, Sloane R, Pieper C, et al. Clinical fractures among older men with diabetes are mediated by diabetic complications. J Clin Endocrinol Metab. 2018;103:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dersimonian R, Laird N. Meta-analysis in clinical trials. Stat Med. 1986;188:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Verpillat P, Rassen J, Patrick A, Garry E, Bartels D. Transparency and reproducibility of observational cohort studies using large healthcare databases. Clin Pharm Ther. 2016;99:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis - a multi-database cohort study. Arthritis Rheumatol. 2017;69:1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ. 2018;360:k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert MP, Pratley RE. The impact of diabetes and diabetes medications on bone health. Endocr Rev. 2015;36:194–213. [DOI] [PubMed] [Google Scholar]
- 28.Osteoporosis Canada. Osteoporosis facts & statistics (2017). Accessed at http://www.osteoporosis.ca/osteoporosis-and-you/osteoporosis-facts-and-statistics/ on 31 March 2017).
- 29.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang HL, Li DD, Zhang JJ, et al. Lack of evidence for a harmful effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2016;2:1–8. [DOI] [PubMed] [Google Scholar]
- 31.Temple R, Ellenberg S. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Ann Intern Med. 2000;133:455–463. [DOI] [PubMed] [Google Scholar]
- 32.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of health care databases to support supplemental indications of approved medications. JAMA Intern Med. 2017;178:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an sglt2 inhibitor. N Engl J Med. 2017;376:2300–2302. [DOI] [PubMed] [Google Scholar]
- 34.Patorno E, Gopalakrishnan C, Franklin JM, et al. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes, Obes Metab. 2018;20:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.